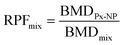Open Access Article
Open Access ArticleEstrogenic activity of plastic nanoparticle mixtures under in vitro settings†
Lucija
Božičević
 a,
Korinna
Altmann
b,
Jana
Hildebrandt
a,
Korinna
Altmann
b,
Jana
Hildebrandt
 b,
Xenia
Knigge
b,
Valerije
Vrček
b,
Xenia
Knigge
b,
Valerije
Vrček
 c,
Nikolina
Peranić
a,
Nikolina
Kalčec
a and
Ivana
Vinković Vrček
c,
Nikolina
Peranić
a,
Nikolina
Kalčec
a and
Ivana
Vinković Vrček
 *a
*a
aInstitute for Medical Research and Occupational Health, Ksaverska cesta 2, 10001 Zagreb, Croatia. E-mail: ivinkovic@imi.hr
bBundesanstalt für Materialforschung und –prüfung, Unter den Eichen 87, 12205 Berlin, Germany
cFaculty of Pharmacy and Biochemistry, University of Zagreb, Ante Kovačića 1, Zagreb, Croatia
First published on 19th March 2024
Abstract
The plastic value chain, a central part of modern living, causes environmental pollution and bioaccumulation of plastic nanoparticles (PNPs). Their ubiquitous presence in different environmental and biological compartments has become a serious threat to human health and ecosystems. Frequently used plastic materials such as polypropylene (PP), polystyrene (PS) and polyethylene (PE) have been detected in the form of PNPs in the food chain, soil, water and air, as well as in human feces and blood. In this study, we aimed to provide novel insights into the endocrine disrupting properties of PNPs using in vitro estrogen receptor (ER) transactivation assay. The effects of PP-NPs, PE-NPs and PS-NPs and their mixture on the T47D-KBluc cell line stably transfected with luciferase as a reporter enzyme were evaluated by means of cytotoxicity, cellular uptake and ER activation. The tested dose range for PNPs was 0.001–10 mg L−1. Both cellular uptake and cytotoxicity for all PNPs were found to be dose-dependent. Only the highest dose of PP-NPs and PE-NPs induced apoptosis and cell death, while PS-NPs were not cytotoxic in the tested dose range. For tested concentrations, PP-NPs and PE-NPs showed significant agonistic activity on the ER, while PS-NPs cannot be considered ER active. When applied as a mixture, PNPs demonstrated additive toxicity effects compared to the effect of each individual PNP. Additivity was also observed for the ER agonistic effect of the PNP mixture according to the benchmark dose-addition modelling approach. This study provides missing science-based evidence on endocrine disrupting effects of PE-NPs, PP-NPs, PS-NPs and their mixtures and highlights the importance of considering unintentional, aggregate and combined exposure to different PNPs in risk management.
Environmental significanceGlobal production of single-use plastic items, inappropriate plastic waste management and resistance of plastic materials to degradation have become issues of great concern for both human and ecosystem health due to their ubiquitous presence in the environment, foodstuff and even in the plastic value chain. Once released into the environment, plastic materials undergo slow chemical, physical and biological degradation and fragmentation to macro-, micro and nanoparticles. These particles may further contaminate different environmental compartments, plants, aquatic and terrestrial organisms, as well as humans. Whole risk management of unintentional exposure to plastics, either human or environmental, is further complicated by a high number of different polymers in industrial applications and use. Combined and aggregated exposure to plastics from different sources may have more pronounced adverse effects on human health and may trigger stronger adverse outcomes than exposure to individual polymer types alone, even at concentrations considered as safe. This study is particularly focused on the estrogenic activity of different plastic nanoparticles (PNP), namely polypropylene (PP), polystyrene (PS) and polyethylene (PE) nanoparticles and their mixtures. Interaction with and activation of estrogen receptors may result in endocrine disruption that may cause developmental, reproductive, neurological and immune adverse effects. Testing was performed according to the OECD test guideline No. 455. Results presented here provide the first evidence of endocrine disrupting properties of different plastic nanoparticles (PE-NPs, PP-NPs and PS-NPs) and their mixtures. Both PE-NPs and PP-NPs can be considered positive for the agonistic effect towards the ER, which was not the case for PS-NPs. Furthermore, the mixture of all three PNPs shows higher agonistic affinity towards the ER in comparison to individual components of the mixture, which highlights the importance of investigating environmentally present contaminants not as individual agents, but rather as parts of complex mixtures. |
Introduction
Plastics, mainly consisting of polymers, are ubiquitous and unavoidable in industrial applications as a cheap, multifunctional, resistant, easy-to-process and affordable material. Due to these unique properties, the plastic value chain is central to modern living and constitutes a vital source for innovation-driven growth.1–6 A 200-fold increase has been estimated for the production of plastics in the last 50 years.7 Plastics hold similar promise for the future with production expected to double again over the next 20 years in a business-as-usual scenario.8–10 However, global production of single-use plastic items, inappropriate plastic waste management and resistance of plastic materials to degradation cause environmental contamination with plastic micro- and nanoparticles.11 Plastics in the environment undergo slow photo-, chemical, physical and biological degradation, which leads to fragmentation into pieces smaller than 5 mm, which are further degraded to plastic nanoparticles (PNPs) with sizes varying from 1 to 1000 nm. Polymeric particles have been detected in oceans, seas, rivers and lakes, while a surface contamination of up to 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles per km2 has been estimated for open oceans.12 Their ubiquitous presence in the environment, foodstuffs and even in the plastic value chain has become an issue of great concern for both human and ecosystem health. A recent literature survey from 26 different studies and in combination with US dietary data estimated total annual exposure to 81
000 particles per km2 has been estimated for open oceans.12 Their ubiquitous presence in the environment, foodstuffs and even in the plastic value chain has become an issue of great concern for both human and ecosystem health. A recent literature survey from 26 different studies and in combination with US dietary data estimated total annual exposure to 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 121
000, 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 74
000, 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 98
000, and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plastic particles for male children, male adults, female children, and female adults, respectively.13 A recent study14 revealed that humans are exposed to the abundance of plastic micro- and nanoparticles from bottled water with particle count reaching up to 300
000 plastic particles for male children, male adults, female children, and female adults, respectively.13 A recent study14 revealed that humans are exposed to the abundance of plastic micro- and nanoparticles from bottled water with particle count reaching up to 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles per bottle, while this was previously estimated to be the number of ca. 300 particles. As a support of these exposure-based findings, several research groups detected particles of different polymeric types in human faeces15–17 and in human blood at concentrations up to 10 mg L−1.18 These data revealed the presence of plastic particles in all stool samples, with polypropylene (PP), polyethylene terephthalate (PET), polystyrene (PS) and polyethylene (PE) as the most frequently found polymer types. Three polymers used in this study (PE, PP and PS) are among the most frequently detected polymers not only in human samples and foodstuffs, but also in the environment.19–21 The dose range was selected based on the data obtained from several review papers that discuss the toxicological effects and human health impact of plastic micro- and nanoparticles present in the environment.22–24 Unfortunately, the risk that plastic particles might pose to human health is largely unknown due to the lack of science-based data. Almost neglected ten years ago, this is today one of the most active research topics in the field of toxicology and risk assessment in the European Union and worldwide.25,26 Environmental contamination with plastic particles can occur along the whole life cycle of plastic-based products, from their manufacturing to their disposal, in their original, aged or transformed form. Whole risk management of unintentional exposure to plastics, either human or environmental, is further complicated by a high number of different polymers in industrial applications and use. The health risks posed by the unwanted presence of specific plastics and their degradation products in different environmental compartments cannot be clearly distinguished from the total risk related to the exposure due to a combination of different plastic types, e.g. multilayer packages, or other types of additives, which typically exceeds the risk related to the exposure to each of the individual components in the mixture. Such combined and aggregated exposure to plastics from different sources may have more pronounced adverse effects on human health and may trigger stronger adverse outcomes (AOs) than exposure to individual polymer types alone, even at concentrations considered as safe (i.e. where no effects are expected). In the European Union (EU), the evaluation of human exposure to mixtures was set as a research priority already in the White Paper for a future chemicals policy from 2001. In 2019, the EU set out the European Green Deal program with the zero pollution ambition for a toxic-free environment aiming to make the EU a sustainable climate neutral and circular economy by 2050. However, there is a huge lack of science-based and regulatory-relevant data for proper risk management of plastic particle mixtures. Moreover, human health impacts of exposure to plastic micro- and nanoparticles have been discussed significantly less in available scientific literature compared to their environmental impact despite indications for casual links between such exposure and increased incidence of immune disorders, neurodegenerative disease and cancers.27,28 Most studies on the toxicity effects of micro- and nanoplastics are focused on cytotoxicity, oxidative stress response, immunotoxicity and genotoxicity,9,24,29,30 while there is also some evidence on endocrine disrupting (ED) activities of plastic products and plastic microparticles.31–35 Endocrine disruptors may mimic the effects of natural hormones by interaction with various hormone receptors or they can alter the metabolism of natural hormones. They interfere with the body's endocrine system and may cause developmental, reproductive, neurological and immune adverse effects.36 Thus, any ED activity of substances and materials represents a global challenge and a source of concern for many EU citizens.37–39 The screening and testing of potential ED chemicals was initiated by the Organisation for Economic Co-operation and Development (OECD) as a high-priority activity back in 1998. One of the regulatory relevant targets for ED adverse effects is estrogenic activity and interference with normal estrogen signalling mediated by estrogen receptors (ERs). To this end, the OECD provides performance-based test guideline (PBTG) No. 455 (ref. 40) for screening and prioritization purposes of ED chemicals. This PBTG describes protocols to identify chemicals that may activate (i.e. act as agonists) and also suppress (i.e. act as antagonists) ER-dependent transcription.
000 particles per bottle, while this was previously estimated to be the number of ca. 300 particles. As a support of these exposure-based findings, several research groups detected particles of different polymeric types in human faeces15–17 and in human blood at concentrations up to 10 mg L−1.18 These data revealed the presence of plastic particles in all stool samples, with polypropylene (PP), polyethylene terephthalate (PET), polystyrene (PS) and polyethylene (PE) as the most frequently found polymer types. Three polymers used in this study (PE, PP and PS) are among the most frequently detected polymers not only in human samples and foodstuffs, but also in the environment.19–21 The dose range was selected based on the data obtained from several review papers that discuss the toxicological effects and human health impact of plastic micro- and nanoparticles present in the environment.22–24 Unfortunately, the risk that plastic particles might pose to human health is largely unknown due to the lack of science-based data. Almost neglected ten years ago, this is today one of the most active research topics in the field of toxicology and risk assessment in the European Union and worldwide.25,26 Environmental contamination with plastic particles can occur along the whole life cycle of plastic-based products, from their manufacturing to their disposal, in their original, aged or transformed form. Whole risk management of unintentional exposure to plastics, either human or environmental, is further complicated by a high number of different polymers in industrial applications and use. The health risks posed by the unwanted presence of specific plastics and their degradation products in different environmental compartments cannot be clearly distinguished from the total risk related to the exposure due to a combination of different plastic types, e.g. multilayer packages, or other types of additives, which typically exceeds the risk related to the exposure to each of the individual components in the mixture. Such combined and aggregated exposure to plastics from different sources may have more pronounced adverse effects on human health and may trigger stronger adverse outcomes (AOs) than exposure to individual polymer types alone, even at concentrations considered as safe (i.e. where no effects are expected). In the European Union (EU), the evaluation of human exposure to mixtures was set as a research priority already in the White Paper for a future chemicals policy from 2001. In 2019, the EU set out the European Green Deal program with the zero pollution ambition for a toxic-free environment aiming to make the EU a sustainable climate neutral and circular economy by 2050. However, there is a huge lack of science-based and regulatory-relevant data for proper risk management of plastic particle mixtures. Moreover, human health impacts of exposure to plastic micro- and nanoparticles have been discussed significantly less in available scientific literature compared to their environmental impact despite indications for casual links between such exposure and increased incidence of immune disorders, neurodegenerative disease and cancers.27,28 Most studies on the toxicity effects of micro- and nanoplastics are focused on cytotoxicity, oxidative stress response, immunotoxicity and genotoxicity,9,24,29,30 while there is also some evidence on endocrine disrupting (ED) activities of plastic products and plastic microparticles.31–35 Endocrine disruptors may mimic the effects of natural hormones by interaction with various hormone receptors or they can alter the metabolism of natural hormones. They interfere with the body's endocrine system and may cause developmental, reproductive, neurological and immune adverse effects.36 Thus, any ED activity of substances and materials represents a global challenge and a source of concern for many EU citizens.37–39 The screening and testing of potential ED chemicals was initiated by the Organisation for Economic Co-operation and Development (OECD) as a high-priority activity back in 1998. One of the regulatory relevant targets for ED adverse effects is estrogenic activity and interference with normal estrogen signalling mediated by estrogen receptors (ERs). To this end, the OECD provides performance-based test guideline (PBTG) No. 455 (ref. 40) for screening and prioritization purposes of ED chemicals. This PBTG describes protocols to identify chemicals that may activate (i.e. act as agonists) and also suppress (i.e. act as antagonists) ER-dependent transcription.
Our ambition was to contribute to overcome knowledge and data gaps on ED-related effects of various PNPs given individually or in mixtures. A literature search performed in the Web of Science database in May 2023 using keywords micro* OR nano* AND plast* AND endocr* AND disrupt* resulted in 834 papers (Fig. 1). Refinement of this search by additional keyword mix* showed only 71 papers on ED effects of plastic particles, out of which 24 papers reported their interaction with the ER. Most studies were focused on PS-based particles, while studies on PE and PP showed results for chemicals released from PE- or PP-based products.
 | ||
| Fig. 1 Results of the literature search on ED effects of plastic micro- and nanoparticles including the numbers of scientific papers found for each keyword's combination. | ||
Here, we present the first study on the interaction of individual PS-, PE- and PP-based PNPs and their mixture with the ER. Our work has been conducted in accordance with regulatory guidelines for the ED screening programs recommended by the OECD40 and the United States Environmental Protection Agency (US EPA).41 Testing was performed using human-derived cell line T47D-KBluc. As we aimed to reach regulatory relevant results that are unbiased by lack of data on PNP characteristics, we used PNPs with defined properties, as scientific cases. The PS-NPs were obtained commercially, while PE-NPs and PP-NPs were developed and prepared by the team of Bundesanstalt für Materialforschung und –prüfung (BAM, Berlin, Germany) within the project PlasticsFatE (No. 965367), https://www.plasticsfate.eu), granted under the EU Horizon 2020 program. Finally, the obtained results are discussed in the context of the adverse outcome pathways (AOPs) concept that has been launched by the OECD to support risk assessment using mechanistic and causative knowledge on adverse health effects of chemicals and materials.42–44
Materials and methods
Characterization of nanoparticles
Polystyrene nanoparticles (PS-NPs, 25 nm in size and stock concentration of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mg L−1) were purchased from Phosphorex (Hopkinton, MA, USA). Polyethylene nanoparticles (PE-NPs, 350 nm in size and stock concentration of 82 mg L−1) and polypropylene nanoparticles (PP-NPs, 180 nm in size and stock concentration of 41 mg L−1) were prepared at the Bundesanstalt für Materialforschung und –prüfung (BAM, Berlin, Germany) according to the published procedure.45 No modification was done for PE-NPs' production.
500 mg L−1) were purchased from Phosphorex (Hopkinton, MA, USA). Polyethylene nanoparticles (PE-NPs, 350 nm in size and stock concentration of 82 mg L−1) and polypropylene nanoparticles (PP-NPs, 180 nm in size and stock concentration of 41 mg L−1) were prepared at the Bundesanstalt für Materialforschung und –prüfung (BAM, Berlin, Germany) according to the published procedure.45 No modification was done for PE-NPs' production.
Visualization of PNPs by transmission electron microscopy (TEM) was used to determine their shape and primary size (d, nm). For that purpose, PNP suspensions were prepared at a concentration of 1 mg L−1 in the medium used for in vitro testing, i.e. RPMI-1640 medium without phenol red (Sigma Aldrich, Steinheim, Germany) supplemented with 5% charcoal-stripped fetal bovine serum (CS-FBS) (Sigma Aldrich, Steinheim, Germany). Then, a drop of each suspension was deposited on a Formvar®-coated copper grid (SPI Supplies, West Chester, PA, USA) and left overnight to dry at room temperature. The TEM instrument (JEOL JEM 1010, JEOL, Tokyo, Japan) was operated in a bright field mode at an accelerating voltage of 80 kV and images were taken with a Canon PowerShot S50 Camera (Canon, Tokyo, Japan). The primary size was obtained by the analysis of 60 particles per nanoparticle type using the ImageJ software (LOCI, University of Wisconsin, Madison, WI, USA). Results are reported as mean values with standard deviations obtained from measurements of 60 particles.
The size distribution and surface charge of PNPs were examined in the medium used for in vitro experiments for 24 h by determination of the hydrodynamic diameter (dH) and ζ potential using dynamic light scattering (DLS) and electrophoretic light scattering (ELS) methods, respectively. Measurements were done on the Zetasizer Ultra instrument (Malvern Panalytical, Malvern, UK). The dH values were obtained as the average of six measurements and expressed as intensity-weighed size distribution. Surface charge was obtained by determining the ζ potential from the Henry equation with the Smoluchowski approximation by using mean values from three replicated ELS measurements. Data was processed in ZS Xplorer 3.21 (Malvern Panalytical, Malvern, UK). Results are shown as mean values with standard deviations obtained from six measurements for dH values and three measurements for surface charge values.
Surface characterization according to ageing status and oxidized functionality was conducted by X-ray photoelectron spectroscopy (XPS). The measurements were performed with a lab-based ULVAC-PHI “Quantes” spectrometer (Chanhassen, USA) that is equipped with two X-ray sources: a monochromatic Al Kα-source at 1486.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV for XPS and a monochromatic Cr Kα-source at 5414.8
eV for XPS and a monochromatic Cr Kα-source at 5414.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV for HAXPES. The starting material granules were directly prepared with double-adhesive tape on a stainless-steel sample holder. The PP-NPs were prepared by putting a droplet on an Au surface and evaporation of the solvent. The particle residue on the Au surface was measured directly. The measuring spot was 100 μm and the photoelectrons were collected at an emission angle of 45°. The pressure within the measuring chamber was lower than 10−6 Pa during the whole measurement. The spectra were corrected to a binding energy of 285 eV for the C 1s peak. The XPS spectra were collected as survey spectra with a step size of 1
eV for HAXPES. The starting material granules were directly prepared with double-adhesive tape on a stainless-steel sample holder. The PP-NPs were prepared by putting a droplet on an Au surface and evaporation of the solvent. The particle residue on the Au surface was measured directly. The measuring spot was 100 μm and the photoelectrons were collected at an emission angle of 45°. The pressure within the measuring chamber was lower than 10−6 Pa during the whole measurement. The spectra were corrected to a binding energy of 285 eV for the C 1s peak. The XPS spectra were collected as survey spectra with a step size of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV at a pass energy of 280
eV at a pass energy of 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV and a time per step of 200
eV and a time per step of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ms. The measurements were repeated with 2 sweeps for XPS at an X-ray power of 25
ms. The measurements were repeated with 2 sweeps for XPS at an X-ray power of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W at 15
W at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kV. Here the binding energy ranged from 0
kV. Here the binding energy ranged from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV to 1100
eV to 1100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV. High-resolution spectra were detected with a pass energy of 54 eV and a step size of 0.1 eV for XPS.
eV. High-resolution spectra were detected with a pass energy of 54 eV and a step size of 0.1 eV for XPS.
Cell line T47D-KBluc
T47D-KBluc (American Type Culture Collection (ATCC), Manassas, VA, USA) is a human epithelial cell line derived from ductal breast carcinoma. This reporter-labelled cell line was developed for screening of estrogenic or anti-estrogenic activity of chemicals46 by transfection with a triplet construct: estrogen-responsive elements (ERE)–promoter–luciferase reporter gene.Cells were cultured in tissue culture (TC) treated T75 flasks (Sarstedt, Nümbrecht, Germany) in RPMI-1640 cell culture medium supplemented with 10% (v/v) CS-FBS and 1% (v/v) antibiotic–antimycotic solution (Sigma Aldrich, Steinheim, Germany). Cells were grown at 37 °C and 5% CO2 until they reached a density of approximately 1 × 106 cells per mL (90–95% confluency) at which point they were ready to be used in experiments.
Flow cytometry analysis of cell viability and apoptosis
Cells were seeded in TC-treated 12-well plates (Sarstedt, Nümbrecht, Germany) at density of 1 × 105 cells per well in 1 mL of complete cell culture medium and were left to attach for 24 hours in the incubator at 37 °C and 5% CO2. On the following day, the cell culture medium was replaced and cells were treated with different concentrations of NPs alone and their mixture in the range of 1 × 10−4–10 mg L−1. Negative controls were non-treated cells, while cells treated with 10% v/v of DMSO (Sigma Aldrich, Steinheim, Germany) were used as positive controls. In addition, cytotoxic effects of Tween and sodium azide were also tested as PS-NPs were obtained as a suspension in deionized water containing 0.1% (v/v) Tween and 2 mM sodium azide. The original suspension of 10 g L−1 of PS-NPs was diluted 1000 times prior to the experiments and final contents of Tween and sodium azide in the CCM were 0.0001% and 2 μM, respectively, which were tested as a vehicle control (VC) and showed no differences compared to non-treated cells (Ctrl).Treated cells were incubated for 48 hours at 37 °C and 5% CO2. Then, the cell culture medium was removed, and was acquired in 2 mL tubes (Eppendorf, Hamburg, Germany) and the remaining cells were washed three times with sterile phosphate-buffered saline (PBS). After washing, cells were detached by adding the trypsin–EDTA solution (Sigma Aldrich, Steinheim, Germany) and incubation at 37 °C and 5% CO2 for 5–7 minutes until the complete detachment of cells was observed under the microscope. Detached cells were added to 2 mL tubes containing the cell culture medium previously collected. Cells were prepared for measurement by staining with annexin V–FITC and propidium iodide (PI) using annexin V kit (Bio-Rad, Hercules, California, USA) according to the manufacturer's instructions.
Measurements and analysis were done using the Cytoflex SRT instrument and software (Beckman Coulter Life Sciences, Indianapolis, Indiana, USA). Cells stained with annexin V–FITC dye were considered apoptotic, PI positive cells were categorized as dead and cells stained with both annexin V–FITC and PI were considered late apoptotic. Cells that were not stained were considered live intact cells. Results are expressed as mean % values of apoptotic, dead or live cells compared to negative controls and were obtained from 3 independent experiments by performing 3 replicates in each experiment.
Flow cytometry analysis of NP cellular uptake
The prerequisite step to follow the cellular uptake of plastic NPs by flow cytometry was due to the determination of their fluorescence characteristics. All three types of NPs used in this study are characterized by fluorescence emission maxima in the green part of the visible electromagnetic spectrum. While this was a known property for PS-NPs, we determined the emission spectrum for PE-NPs and PP-NPs with a Cary Eclipse fluorescence spectrometer (Agilent, Melbourne, Australia) using a 10 mm path length quartz cuvette (Fig. S1†).Cellular uptake was then analyzed using a Cytoflex SRT device and software by comparing median fluorescence intensity (MFI) on a 525/40 (FITC) detector of treated and negative control cells. These measurements were done only for NP concentrations that did not affect cellular viability, i.e. non-toxic concentrations. Results are presented as % of MFI on the FITC detector in treated cells compared to non-treated control cells. To ensure that the detected fluorescence signals originate from NPs that entered the cells, the experimental set-up (PMT voltage for forward and side scatter light) was adjusted so that it was possible to clearly distinguish debris from cell population using forward versus side scatter gating. Only the cell population, excluding the debris, was chosen for further analysis.
Determination of estrogen receptor activity by luciferase assay
One week prior to the experiment, cells were kept in the cell culture medium in which 10% FBS was exchanged with 10% charcoal-stripped FBS to diminish interferences from serum hormones. After 7 days, cells were seeded in white opaque flat-bottom Nunc™ MicroWell™ 96-well microplates (Thermo Fisher Scientific, Waltham, Massachusetts, USA) at a density of 2 × 104 cells per well in 100 μL per well of complete cell culture medium where 10% (v/v) charcoal-stripped FBS was exchanged for 5% (v/v) charcoal-stripped FBS.Cells were incubated for 24 hours at 37 °C and 5% CO2 to attach properly and then treated for 48 hours with different concentrations of plastic NPs alone or their mixture in a range of 1 × 10−4−10 mg L−1. Non-treated cells were used as a negative control, while cells treated with diethylstilbestrol (DES) were considered as a positive control. After the treatment, cells were prepared for measurement using a Promega luciferase assay system (Cat. No.: E1500 and E1501, Promega, Madison, Wisconsin, USA) according to the manufacturer's instructions. Briefly, the cell culture medium was discarded and cells were washed thoroughly with PBS. Then, the cell lysate was prepared by adding 20 μL of cell culture lysis reagent (included in the kit, diluted 5 times with distilled water as instructed) to each well followed by centrifugation for 20 minutes at 25 °C and 300 rpm using an Eppendorf 5810R centrifuge (Eppendorf, Hamburg, Germany) to achieve complete cell lysis and equilibrate lysates to the temperature optimal for the assay. Then, measurements were done using a SpectraMax iD3 microplate reader (Molecular Devices, San Jose, California, USA), which was prepared by priming the injector system to inject 100 μL of the freshly prepared luciferase assay reagent into each well at the time and performing a 2-second measurement delay followed by a 10-second luminescence measurement read.
The Promega luciferase assay system is based on activated ER binding to the estrogen-responsive element (ERE) which is part of cells' DNA sequence. This binding enables gene transcription which results in the production of luciferase enzyme. The produced luciferase enzyme converts assay reagent beetle luciferin to the luminescent product oxyluciferin which produces light at all wavelengths and the measurements are done with all open channels. Before any experiments, the responsiveness of the test system was examined with two positive control substances, 17β-estradiol and diethylstilbestrol (DES), and one negative control substance, fulvestrant (Fig. S2†). In addition, the system was tested for any interferences (Fig. S3 in the ESI†). Diethylstilbestrol was chosen due to better stability and easier handling, after it was confirmed that it produces ER activity comparable to 17β-estradiol. According to the OECD PBTG No. 455,40 quality control of the assay requires that the mean luciferase activity of the positive control should be at least 4-fold that of the mean of the negative/vehicle control on each plate. This criterion was met and confirmed by satisfactory differences between positive and negative controls in each run of the assay. During setting up the protocol, interferences of each PNP and their mixtures with the assay components and readouts were also carefully checked and all testing proved the absence of any interferences. For this set of experiments, results were expressed in two different ways as recommended by the aforementioned OECD test guideline No. 455 – as fold inductions of the luminescent signal compared to non-treated cells and % of fold induction of the luminescent signal compared to positive control cells (treated with 10 nM DES).
Identification of potential adverse outcome pathways (AOPs) in AOP-Wiki
The freely accessible web-based tool AOP-Wiki (https://aopwiki.org/) has been used to identify AOPs related to agonistic activity towards the ER, the main adverse effect examined in our study. The search was set up to find the AOPs in which agonism towards the ER is defined as a molecular initiating event (MIE) or key event (KE). After their retrieval, an analysis of AOPs linked to this specific MIE/KE was conducted to ensure that they were relevant and applicable to the results of this study.Statistical analysis
Statistical analysis of all the data acquired from the experiments was done using GraphPad Prism6 (GraphPad Software, San Diego, California, USA). Statistical significance was determined by one-way ANOVA followed by Dunnett's multiple comparison test where all the treatment values were compared to negative control values. The threshold for statistical significance for all experimental data was set at P < 0.05. Statistically significant results were denoted with an asterisk (*).Results and discussion
Considering the ubiquitous presence of different PNPs in different environmental, food and biological matrices it is of utmost importance to determine any possible AO related to human exposure not just to individual PNPs but also to their mixtures.10,47–49 Many studies already demonstrated the endocrine-disrupting potency of plastics, especially the negative effects of plastic materials on the steroid hormone homeostasis.50–52 From this perspective, the interaction of a specific substance with the ER has been considered as KE, even as MIE, in the steroid hormone homeostasis.53 Therefore, our main aim is to reveal for the first time the individual and joint effects of three different PNPs (PE-NPs, PP-NPs and PS-NPs) on the modulation of the ER under in vitro settings.Physico-chemical characteristics and stability of plastic nanoparticles and their mixture
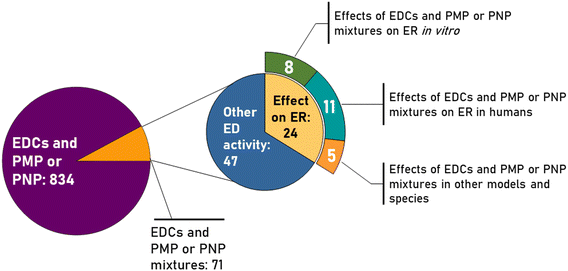
The shape of PS-NPs, PP-NPs and PE-NPs, as evaluated by TEM (Fig. 2), was spherical, while their primary diameters (dTEM) were 25.3, 187.5 and 344.9 nm, respectively (Table 1). It should be highlighted here that PE-NPs and PP-NPs were prepared top-down and their shape should be irregular. After preparation they were characterized as irregularly shaped by scanning electron microscopy (SEM) as given in a previous study.45 The differences in shape seen in SEM compared to TEM images can arise from the limitations of SEM and TEM. TEM is often used for NPs and works quite well. However, PNPs are polymer-based and suffer from TEM irradiation, which may change their origin shape. Both images are in a way correct just showing the limitations. | ||
| Fig. 2 Transmission electron micrographs (TEM) of polyethylene (a), polypropylene (b) and polystyrene (c) nanoparticles. | ||
| Particle type | d TEM (nm) | Parameter | Measurement conditions (medium, incubation time) | ||
|---|---|---|---|---|---|
| UPW, t = 0 h | CCM, t = 0 h | CCM, t = 48 h | |||
| PS-NPs | 25.3 ± 2.9 | d H (nm) | 27.0 ± 1.8 | 53.9 ± 5.7 | 87.5 ± 15.3 |
| ζ potential (mV) | −26.2 ± 3.3 | −8.5 ± 1.3 | −18.5 ± 1.8 | ||
| PP-NPs | 187.5 ± 28.7 | d H (nm) | 208.5 ± 6.3 | 345.8 ± 16.3 | 497.9 ± 37.9 |
| ζ potential (mV) | −31.1 ± 0.5 | −15.3 ± 2.9 | −21.8 ± 1.3 | ||
| PE-NPs | 344.9 ± 18.9 | d H (nm) | 372.6 ± 16.9 | 565.9 ± 72.4 | 649.2 ± 90.7 |
| ζ potential (mV) | −32.6 ± 1.6 | −15.8 ± 1.3 | −22.3 ± 0.6 | ||
Additional important information can be obtained from the surface of the particles, which was analysed by X-ray photoelectron spectroscopy (XPS). It can be assumed that, due to the different production processes, PE and PP are aged on the surface and have oxygen-containing groups, whereas this should not be the case with PS. As an example, the C 1s peak of the starting material (PP pellets) and PP-NPs is shown. It is obvious that there is a further peak at 288.4 eV indicating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (Fig. S4†). Such ketone groups can be also detected in thermo extraction desorption-gas chromatography/mass spectrometry (TED-GC/MS) measurements not presented in this manuscript. The fluorescence of PE-NPs and PP-NPs themselves can be caused by these ketone groups. No labelling dye was used for PE-NPs and PP-NPs.54
O groups (Fig. S4†). Such ketone groups can be also detected in thermo extraction desorption-gas chromatography/mass spectrometry (TED-GC/MS) measurements not presented in this manuscript. The fluorescence of PE-NPs and PP-NPs themselves can be caused by these ketone groups. No labelling dye was used for PE-NPs and PP-NPs.54
Furthermore, hydrodynamic diameter (dH) and ζ potential values (Table 1) were measured in both ultrapure-water, the medium in which they are dispersed, and in the cell culture medium (RPMI-1640 supplemented with 5% CS-FBS). The main aim was to evaluate the colloidal stability of different PNPs in the media used for cell experiments. As both parameters are extrinsic properties, thus medium dependent, and all PNP types were dispersed in water after production, the values obtained in the water can be considered as the initial or starting values. Measurements in cell culture medium were done immediately after the addition of PNPs into the medium and after 48 hours of incubation at 37 °C to gain data about PNP behavior during cell experiments. As expected, an increase in dH values and less negative ζ potential values were observed for all PNP types in the cell culture medium compared to water due to the formation of the hydration shell and protein corona on the nanosurface as most proteins from the cell culture medium may be of size between 1–20 nm and characterized by lower ζ potential values compared to the tested PNPs. The increase of hydrodynamic diameter of PNPs in the cell culture medium is also an indication of aggregation due to the increased ionic strength of the medium. However, results indicate that the fate for all tested PNPs in the cell culture medium was similar as their dH values doubled after transferring them from water to the cell culture medium (CCM). Moreover, the similar ζ potential values observed in this medium after 48 h (Table 1) indicate a similar “aging” process for different PNP types in particular media. Indeed, the presence of CS-FBS in the CCM led to protein corona formation on the PNP surface. Additionally, the colloidal stability and behavior of PNPs given in mixture were also investigated in the CCM at time t = 0 h and t = 48 h. However, such results should be taken with care. Although there were PNPs with sizes of 25 nm, 187 nm and 345 nm in the mixture, only one peak was visible for the mixture containing 10 mg L−1 of each PNP type (261.2 ± 9.4 nm). Indeed, DLS techniques cannot distinguish particles of different sizes and provide only the average size distribution. Moreover, larger particles can “mask” smaller particles. To carefully characterize the size distribution of mixtures containing nanoparticles of very different sizes like in our study other techniques such as particle tracking analysis and electrospray-scanning mobility particle sizer should be used.55 However, such characterization was beyond the scope of this preliminary study as the main aim was to evaluate ER activity of PNPs given individually or in mixtures.
Cytotoxic effect of plastic nanoparticles and their mixtures
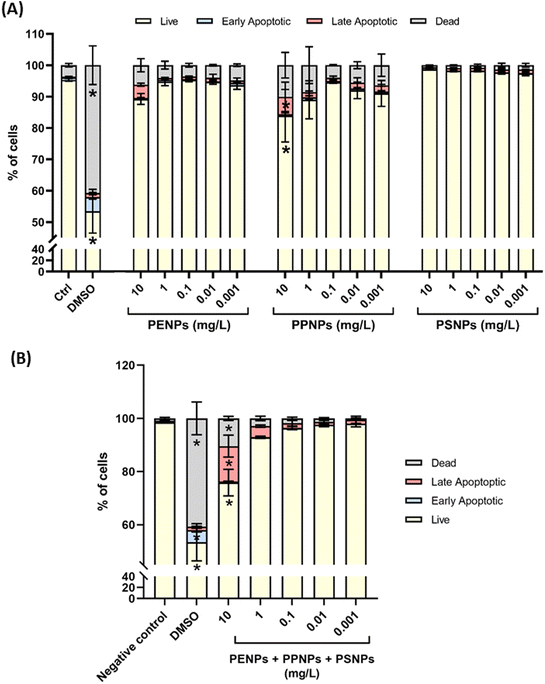
Cytotoxic effects of PNPs and their mixtures were evaluated prior to any other experiments to determine the safe doses that will not kill or damage the cells. This was the pivotal step to find the dose range in which interaction with and the effect on the ER can be studied in viable T47D-KBluc cells. Therefore, experiments started employing a wide range of 0.001–10 mg L−1 for each PNP type administered individually or in mixtures containing all three PNP types at equal concentrations. Results showed that none of the tested PNPs in the given dose range induced significant damage in T47D-KBluc cells, either by means of the % of apoptotic or dead cells (Fig. 3A). The highest number of dead cells was observed after treatment with PP-NPs (around 5% for doses below 0.1 mg L−1 and 9% and 10% for 1 and 10 mg L−1, respectively). All tested doses of PE-NPs killed less than 5% of cells, while no cytotoxicity was observed for PS-NPs. Even the treatment with the highest dose of 10 mg L−1 led to only 10% or less of dead cells and ca. 10% of apoptotic cells after treatments with PE-NPs and PP-NPs.The treatment with the highest concentration of PNPs (10 mg L−1) resulted in more than 80% of live cells (84% for PP-NPs, 89% for PE-NPs and 98% for PS-NPs). When applied as a mixture consisting of the three PNP types with the same concentration, significant toxicity was observed only for the mixture at 10 mg L−1 (Fig. 3B) which induced apoptosis in 14% of cells and killed 11% of cells, which may account for the additive effect of each PNP type in the mixture. Therefore, only a dose range between 0.001 and 1 mg L−1 was used in subsequent experiments to skip any biased results that may arise from dead, unviable or damaged cells.
Cellular uptake of plastic nanoparticles
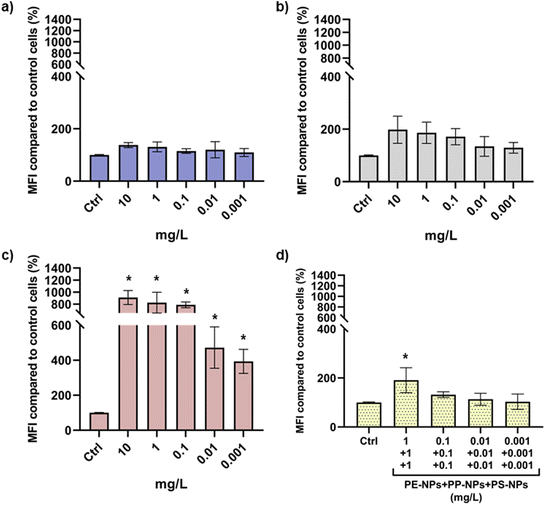
The uptake of PE-NPs, PP-NPs, PS-NPs and their mixtures was analyzed by flow cytometry employing their emission maxima in the green part of the spectrum. Thus, the changes in the median fluorescence intensity detected on the 525/40 (FITC) detector indicated internalization of PNPs. Dose–response in cellular uptake was only observed for PS-NPs that also demonstrated significantly higher cellular uptake compared to the other two tested PNPs (Fig. 4).These results are probably caused by the particle size differences as all three PNPs had similar surface charges. The smallest type, PS-NPs, less than 100 nm in diameter even after agglomeration in the cell culture medium, was more easily internalized by human cells compared to PP-NPs and PE-NPs (Table 1). The cellular uptake of NPs is heavily dependent on their size. This has been discussed in several studies which demonstrated that NPs of smaller size are internalized faster and more extensively under both in vitro and in vivo conditions.56–60
Uptake of the PNP mixture followed a dose–response curve and significantly higher MFI values were observed only for mixtures containing more than 1 mg L−1 PNPs (Fig. 4d). However, the obtained results for mixtures indicate that the uptake of PS-NPs was significantly inhibited when combined with PE-NPs and PP-NPs, probably due to the presence of large agglomerates that obstructed contact and interaction of PS-NPs with the cell surface.
ER activity of plastic nanoparticles and their mixture
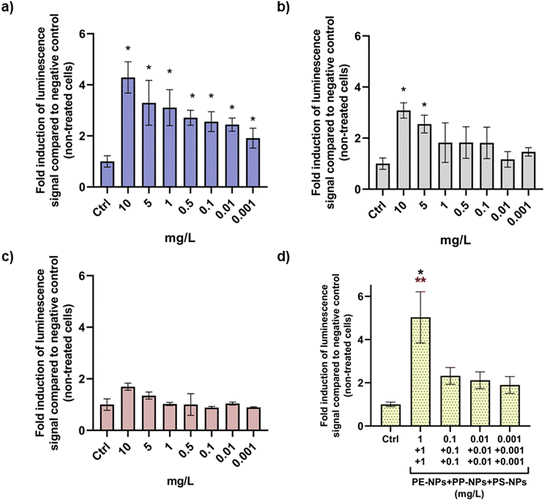
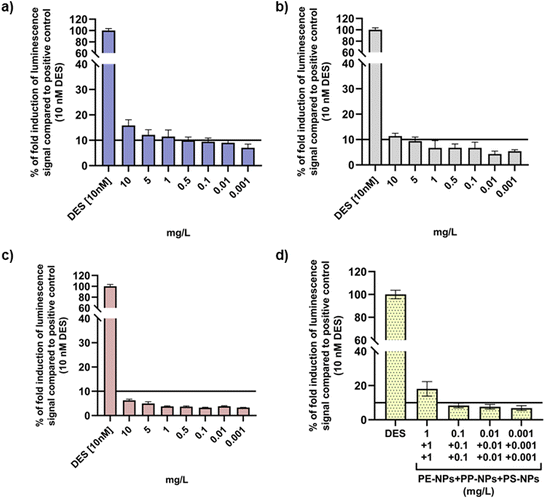
For the ER agonist assay, decision criteria dictate that the substance can be considered positive if the maximal ER response produced by the treatment with the test substance is equal to or exceeds 10% of the ER response observed in T47D-KBluc cells treated with the positive control. OECD TG No. 455 recommends analysis of results as both fold induction compared to the negative control (non-treated cells) and % of ER induction compared to the positive control (cells treated with DES). Both types of analyses were therefore applied to results obtained by performing luciferase assay as shown in Fig. 5 and 6. Evaluation of ER activity in T47D-KBluc cells after treatment revealed the highest agonistic effect of PE-NPs on ER activity that exhibited significant fold induction of luminescence signals compared to the negative control at all tested concentrations except at the lowest one. Significant ER induction was observed for PP-NPs only at the highest concentration (Fig. 5b). Interestingly, PS-NPs did not affect the ER activity significantly despite their highest cellular uptake compared to the other two PNP types. When T47D-KBluc cells were treated with a PNP mixture, a significant increase in fold induction values compared to the negative control was observed for all applied doses starting from 0.001 mg L−1 in a dose response manner (Fig. 5d). At the highest tested mixture dose of 1 mg L−1 of each PNP a 5 times higher luminescent signal was observed than the signal of the negative control.In the case of results for % of ER induction compared to cells treated with DES (Fig. 6), only the highest doses (10 mg L−1) of PE-NPs and PP-NPs (10 mg L−1) can be considered as positive ER agonists when applied alone, while no positive ER response was observed for PS-NPs. However, the PNP mixture showed a positive ER response at 1 mg L−1 which was observed only insignificantly for PE-NPs (Fig. 6d).
Considering that the modulation of ER activity is not correlated with the uptake of individual NPs, these results could point towards the effect on the ER being affected by plastic materials (polymer) properties. According to the European Food Safety Authority (EFSA), there are several different approaches to calculate the potency of a mixture compared to the individual chemical.61 As results obtained for the response of T47D-KBluc cells to PE-NPs, PP-NPs, PS-NPs and their mixtures indicate additivity, dose addition modelling62 was used. Additivity can be assumed when chemicals act in the same or similar mode of action and their joint effect is cumulative compared to individual chemicals. Thus, the relative potency factor (RPF) model was applied to compare the relative potency of the PNP mixture with each of its individual components (PE-NPs, PP-NPs and PS-NPs). This approach was chosen because RPF is calculated from benchmark doses (BMD), defined as doses of a substance that result in a pre-specified level of benchmark response (BMR). In our study, data corresponding to the % of ER activation compared to the positive control was used as BMD, and BMR was set to 10% in accordance with the OECD PBTG criteria for labelling chemicals as positive for agonistic activity towards the ER.40 The advantage of this method is that BMD are equipotent doses for each chemical and are therefore applicable throughout the whole dose–response range even when it differs between substances.63,64
Dose–response curves are generated to obtain BMDmix and BMDPx-NP for each type of used PNP using the freely available software PROAST.65 Generated curves are shown in the ESI (Fig. S4–S7†). The BMD of both the PNP mixture and each individual PNPs were calculated as the mean value between the highest and the lowest BMD doses. BMDmix was estimated to be 0.6345 and the BMD of each PNP type are shown in Table 2. Then, these data were used to calculate the RPFmix according to the equation:
| Type of PNPs | BMDPx-NP | RPFmix |
|---|---|---|
| PE-NPs | 4.6845 | 7.38 |
| PP-NPs | 8.7677 | 13.82 |
| PS-NPs | 13.4850 | 21.25 |
AOPs related to agonistic activity towards the ER
Results of the search for potential AOs related to interaction with the ER, performed by using AOP-Wiki, are listed in Table 3. In this search, ER activation was defined either as a key event (KE) or molecular initiating event (MIE).| AOP number | AOP title | Role of ER agonism in the AOP | AO | Status |
|---|---|---|---|---|
| 200 | Estrogen receptor activation leading to breast cancer | MIE | ER + breast cancer | Open for adoption |
| 167 | Early-life estrogen receptor activity leading to endometrial carcinoma in the mouse | MIE/KE | Increased adenosquamous carcinomas of the endometrium | Under development |
| 445 | Estrogen receptor alpha agonism leads to impaired reproduction | MIE | Impaired reproduction | Under development |
| 29 | Estrogen receptor agonism leads to reproductive dysfunction | MIE | Decrease in population, altered reproductive behavior and larval development, impaired development of reproductive organs (in oviparous vertebrates) | Under development |
| 52 | ER agonism leading to skewed sex ratios due to altered sexual differentiation in males | MIE | Skewed sex ratio | Under development |
| 53 | ER agonism leading to reduced survival due to renal failure | MIE | Reduced survival | Under development |
| 314 | Binding to estrogen receptor (ER)-α in immune cells leading to exacerbation of systemic lupus erythematosus (SLE) | MIE | Exacerbation of SLE | Under development |
| 112 | Increased dopaminergic activity leading to endometrial adenocarcinomas (in Wistar rat) | KE | Endometrial adenocarcinoma | Under development |
| 465 | Alcohol dehydrogenase leading to reproductive dysfunction | KE | Reproductive dysfunction | Under development |
The AOP-Wiki search showed that activation of ER leads to the development of various cancers such as breast or ovarian cancer and affects the reproductive capability of different organisms with high-level levels of evidence in various organisms (from invertebrates to mammals). Apart from the effects on the reproductive system, ER activation is also linked to autoimmune diseases such as systemic lupus erythematosus due to the presence of ER on immune cells. Another important cognition is that ER activity is intertwined with other hormonal and enzymatic activities (e.g. dopamine and alcohol dehydrogenase activity). Development of cancers after ER activation as an MIE is mediated through various KEs such as increased proliferation and migration of cells, oxidative stress, non-genomic signalling and inflammatory response through activation of fibroblasts, macrophages and angiogenesis.
This insight in AOPs shows that the interplay of reproductive (and other endocrine) hormones with other organ systems is complex and intricate. Evidence of in vivo reproductive toxicity of individual PNPs was already provided in previous studies23,66–70 and the number of studies on health hazards following aggregate human exposure to complex mixtures is increasing. For example, we showed the effects of mixtures on human breast cells, human lymphocytes and human monocytes using in vitro models.71–73 However, there is no scientific data on the reproductive toxicity of complex mixtures containing different PNP types. Our pioneering effort to provide scientific evidence of combined PNP effects on ER activity as one of the crucial MIEs/KEs involved in AOs that may lead to severe pathogenesis (Table 3) is fundamental for proper risk assessment that should be implemented in the circular economy for the plastic value chain. Based on the data collected in Table 3, the AOP network has been constructed (Fig. 7), which shows different possible modes of action of PNPs, either given individually or in mixture.
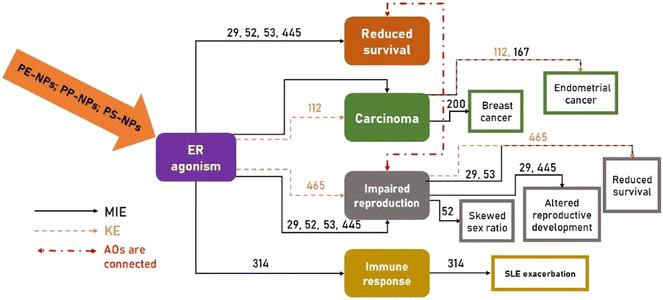 | ||
| Fig. 7 Schematic summary of the network of different adverse outcome pathways (AOPs) (see Table 3) associated with the agonistic activity of PE-NPs, PP-NPs and PS-NPs (given individually or in mixture) towards the estrogen receptor (ER), where the ER agonistic effect was found to be either a molecular initiating event (MIE) or key event (KE). | ||
Finally, the main limitation of this study should be discussed as well. The study provides first and preliminary evidence on the ER agonistic action of different PNPs under in vitro settings and given individually or in mixture. However, it is not possible to reveal at this stage which physico-chemical characteristics were the most critical for observed biological effects and cellular uptake, as PE-NPs, PP-NPs and PS-NPs were of different sizes ranging from 25 nm to 345 nm. Moreover, they had also different surface chemistry, i.e. ketones on the surface of PE-NPs and PP-NPs, while the surface chemistry was not declared by the supplier of fluorescently labelled PS-NPs. All these properties may significantly impact PNP behavior in biological media and their interaction with cells including cellular uptake, cytotoxicity and interaction with receptors. Following studies should consider specifically the specific effects for different PNPs' physico-chemical characteristics.
Conclusion
Results presented in this paper provide the first evidence of endocrine disrupting properties of plastic nanoparticles (PE-NPs, PP-NPs and PS-NPs) to the in vitro model for the detection of ER agonists and antagonists. Both PE-NPs and PP-NPs can be considered positive for the agonistic effect towards the ER in the T47D-KBluc cell line. Furthermore, the mixture of all three PNPs shows higher agonistic affinity towards ER in comparison to individual components of the mixture. Cytotoxicity of individual PNPs was also significantly lower compared to their mixtures. Some ambiguity may result from inherent features of different PNPs (e.g. hydrodynamic diameter), but this issue is out of the scope of the current study and will be resolved shortly. All this proves that environmentally present contaminants should not be investigated exclusively as individual agents, but rather as parts of complex mixtures. Therefore, future research on environmentally relevant contaminants, especially plastic nanoparticles, should take into account significant differences between the toxicological profiles of individual components versus their mixtures with other nanoparticles or different chemicals.Author contributions
Lucija Božičević (data curation; formal analysis; investigation; methodology; visualization; writing – original draft; writing – review & editing), Korinna Altmann (formal analysis; methodology, funding acquisition; writing – review & editing), Jana Hildebrandt (investigation; formal analysis), Xenia Knigge (methodology; formal analysis), Valerije Vrček (supervision; writing – review & editing), Nikolina Peranić (data curation; formal analysis; investigation; methodology), Nikolina Kalčec (data curation; formal analysis; investigation; methodology), Ivana Vinković Vrček (conceptualization; methodology; funding acquisition; project administration; resources; supervision; writing – review & editing).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was performed using the facilities and equipment funded within the European Regional Development Fund project KK.01.1.1.02.0007 “Research and Education Centre of Environmental Health and Radiation Protection – Reconstruction and Expansion of the Institute for Medical Research and Occupational Health”. The authors thank the European Commission for funding the PlasticsFatE joint research project (965367) as part of the Horizon 2020 cluster.References
- Y. L. Zhang, S. C. Kang and T. G. Gao, Microplastics have light-absorbing ability to enhance cryospheric melting, Adv. Clim. Change Res., 2022, 13(4), 455–458, DOI:10.1016/j.accre.2022.06.005.
- A. Amobonye, P. Bhagwat, S. Raveendran, S. Singh and S. Pillai, Environmental Impacts of Microplastics and Nanoplastics: A Current Overview, Front. Microbiol., 2021, 12, 768297, DOI:10.3389/fmicb.2021.768297.
- J. P. da Costa, P. S. M. Santos, A. C. Duarte and T. Rocha-Santos, (Nano)plastics in the environment - Sources, fates and effects, Sci. Total Environ., 2016, 566–567, 15–26, DOI:10.1016/j.scitotenv.2016.05.041.
- L. M. Rios Mendoza, D. Leon Vargas and M. Balcer, Microplastics occurrence and fate in the environment, Curr. Opin. Green Sustainable Chem., 2021, 32, 100523, DOI:10.1016/j.cogsc.2021.100523.
- D. M. Mitrano, P. Wick and B. Nowack, Placing nanoplastics in the context of global plastic pollution, Nat. Nanotechnol., 2021, 16(5), 491–500, DOI:10.1038/s41565-021-00888-2.
- S. S. Patil, R. V. Bhagwat, V. Kumar and T. Durugkar, Megaplastics to Nanoplastics: Emerging Environmental Pollutants and Their Environmental Impacts, 2019, pp. 205–235, DOI:10.1007/978-981-13-7904-8_10.
- K. Kik, B. Bukowska and P. Sicińska, Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms, Environ. Pollut., 2020, 262, 114297, DOI:10.1016/j.envpol.2020.114297.
- Z. Sobhani, Y. Lei, Y. Tang, L. Wu, X. Zhang, R. Naidu, M. Megharaj and C. Fang, Microplastics generated when opening plastic packaging, Sci. Rep., 2020, 10(1), 1–7, DOI:10.1038/s41598-020-61146-4.
- M. Shen, Y. Zhang, Y. Zhu, B. Song, G. Zeng, D. Hu, X. Wen and X. Ren, Recent advances in toxicological research of nanoplastics in the environment: A review, Environ. Pollut., 2019, 252, 511–521, DOI:10.1016/j.envpol.2019.05.102.
- L. Wang, W. M. Wu, N. S. Bolan, D. C. W. Tsang, Y. Li, M. Qin and D. Hou, Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives, J. Hazard. Mater., 2021, 401, 123415, DOI:10.1016/j.jhazmat.2020.123415.
- R. Lehner, C. Weder, A. Petri-Fink and B. Rothen-Rutishauser, Emergence of Nanoplastic in the Environment and Possible Impact on Human Health, Environ. Sci. Technol., 2019, 53(4), 1748–1765, DOI:10.1021/acs.est.8b05512.
- J.-Q. Jiang, Occurrence of microplastics and its pollution in the environment: A review, Sustain. Prod. Consum., 2018, 13, 16–23, DOI:10.1016/j.spc.2017.11.003.
- K. D. Cox, G. A. Covernton, H. L. Davies, J. F. Dower, F. Juanes and S. E. Dudas, Human Consumption of Microplastics, Environ. Sci. Technol., 2019, 53(12), 7068–7074, DOI:10.1021/acs.est.9b01517.
- N. Qian, X. Gao, X. Lang, H. Deng, T. M. Bratu, Q. Chen, P. Stapleton, B. Yan and W. Min, Rapid single-particle chemical imaging of nanoplastics by SRS microscopy, Proc. Natl. Acad. Sci. U. S. A., 2024, 121(3), e2300582121, DOI:10.1073/pnas.2300582121.
- Z. Yan, Y. Liu, T. Zhang, F. Zhang, H. Ren and Y. Zhang, Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status, Environ. Sci. Technol., 2022, 56(1), 414–421, DOI:10.1021/acs.est.1c03924.
- Z. Yan, H. Zhao, Y. Zhao, Q. Zhu, R. Qiao, H. Ren and Y. Zhang, An efficient method for extracting microplastics from feces of different species, J. Hazard. Mater., 2020, 384, 121489, DOI:10.1016/j.jhazmat.2019.121489.
- N. Zhang, Y. Bin Li, H. R. He, J. F. Zhang and G. S. Ma, You are what you eat: Microplastics in the feces of young men living in Beijing, Sci. Total Environ., 2021, 767, 144345, DOI:10.1016/j.scitotenv.2020.144345.
- H. A. Leslie, M. J. M. van Velzen, S. H. Brandsma, A. D. Vethaak, J. J. Garcia-Vallejo and M. H. Lamoree, Discovery and quantification of plastic particle pollution in human blood, Environ. Int., 2022, 163, 107199, DOI:10.1016/j.envint.2022.107199.
- Y. Feng, C. Tu, R. Li, D. Wu, J. Yang, Y. Xia, W. J. G. M. Peijnenburg and Y. Luo, A systematic review of the impacts of exposure to micro- and nano-plastics on human tissue accumulation and health, Eco-Environment & Health, 2023, 2(4), 195–207, DOI:10.1016/j.eehl.2023.08.002.
- P. Schwabl, S. Koppel, P. Konigshofer, T. Bucsics, M. Trauner, T. Reiberger and B. Liebmann, Detection of various microplastics in human stool: A prospective case series, Ann. Intern. Med., 2019, 171(7), 453–457, DOI:10.7326/M19-0618.
- D. Lithner, A. Larsson and G. Dave, Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition, Sci. Total Environ., 2011, 409(18), 3309–3324, DOI:10.1016/j.scitotenv.2011.04.038.
- M. Shen, Y. Zhang, Y. Zhu, B. Song, G. Zeng, D. Hu, X. Wen and X. Ren, Recent advances in toxicological research of nanoplastics in the environment: A review, Environ. Pollut., 2019, 252, 511–521, DOI:10.1016/j.envpol.2019.05.102.
- I. Ali, Q. Cheng, T. Ding, Q. Yiguang, Z. Yuechao, H. Sun, C. Peng, I. Naz, J. Li and J. Liu, Micro- and nanoplastics in the environment: Occurrence, detection, characterization and toxicity – A critical review, J. Cleaner Prod., 2021, 313, 127863, DOI:10.1016/j.jclepro.2021.127863.
- J. L. Xu, X. Lin, J. J. Wang and A. A. Gowen, A review of potential human health impacts of micro- and nanoplastics exposure, Sci. Total Environ., 2022, 851, 158111, DOI:10.1016/j.scitotenv.2022.158111.
- European Commission, The European Green Deal. European Commission, 2019, vol. 53(9), p. 24 Search PubMed.
- European Commission, Chemicals Strategy for Sustainability Towards a Toxic-Free Environment, https://eur-lex.europa.eu/resource.html?uri=cellar:f815479a-0f01-11eb-bc07-01aa75ed71a1.0003.02/DOC_1&format=PDF Search PubMed.
- S. Allen, D. Allen, S. Karbalaei, V. Maselli and T. R. Walker, Micro(nano)plastics sources, fate, and effects: What we know after ten years of research, J. Hazard. Mater. Adv., 2022, 6, 100057, DOI:10.1016/j.hazadv.2022.100057.
- J. C. Prata, J. P. da Costa, I. Lopes, A. C. Duarte and T. Rocha-Santos, Environmental exposure to microplastics: An overview on possible human health effects, Sci. Total Environ., 2020, 702, 134455, DOI:10.1016/j.scitotenv.2019.134455.
- S. S. Sana, L. K. Dogiparthi, L. Gangadhar, A. Chakravorty and N. Abhishek, Effects of microplastics and nanoplastics on marine environment and human health, Environ. Sci. Pollut. Res., 2020, 27(36), 44743–44756, DOI:10.1007/s11356-020-10573-x.
- K. Yin, Y. Wang, H. Zhao, D. Wang, M. Guo, M. Mu, Y. Liu, X. Nie, B. Li, J. Li and M. Xing, A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system, Sci. Total Environ., 2021, 774, 145758, DOI:10.1016/j.scitotenv.2021.145758.
- C. Z. Yang, S. I. Yaniger, V. C. Jordan, D. J. Klein and G. D. Bittner, Most Plastic Products Release Estrogenic Chemicals: A Potential Health Problem That Can Be Solved, Environ. Health Perspect., 2011, 119(7), 989–996, DOI:10.1289/ehp.1003220.
- J. H. Park, S. Hong, O.-H. Kim, C.-H. Kim, J. Kim, J.-W. Kim, S. Hong and H. J. Lee, Polypropylene microplastics promote metastatic features in human breast cancer, Sci. Rep., 2023, 13(1), 6252, DOI:10.1038/s41598-023-33393-8.
- E. Drakvik, R. Altenburger, Y. Aoki, T. Backhaus, T. Bahadori, R. Barouki, W. Brack, M. T. D. Cronin, B. Demeneix, S. H. Bennekou, J. van Klaveren, C. Kneuer, M. Kolossa-Gehring, E. Lebret, L. Posthuma, L. Reiber, C. Rider, J. Rüegg, G. Testa, B. van der Burg, H. van der Voet, A. M. Warhurst, B. van de Water, K. Yamazaki, M. Öberg and Å. Bergman, Statement on advancing the assessment of chemical mixtures and their risks for human health and the environment, Environ. Int., 2020, 134, 105267, DOI:10.1016/j.envint.2019.105267.
- A. F. Hernandez, A. Buha, C. Constantin, D. R. Wallace, D. Sarigiannis, M. Neagu, B. Antonijevic, A. Wallace Hayes, M. F. Wilks and A. Tsatsakis, Critical assessment and integration of separate lines of evidence for risk assessment of chemical mixtures, Arch. Toxicol., 2019, 93(10), 2741–2757, DOI:10.1007/s00204-019-02547-x.
- S. K. Bopp, A. Kienzler, A.-N. Richarz, S. C. van der Linden, A. Paini, N. Parissis and A. P. Worth, Regulatory assessment and risk management of chemical mixtures: challenges and ways forward, Crit. Rev. Toxicol., 2019, 49(2), 174–189, DOI:10.1080/10408444.2019.1579169.
- E. Diamanti-Kandarakis, J.-P. Bourguignon, L. C. Giudice, R. Hauser, G. S. Prins, A. M. Soto, R. T. Zoeller and A. C. Gore, Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement, Endocr. Rev., 2009, 30(4), 293–342, DOI:10.1210/er.2009-0002.
- M. Batke, G. Damm, H. Foth, A. Freyberger, T. Gebel, U. Gundert-Remy, J. Hengstler, A. Mangerich, F. Partosch, C. Röhl, T. Schupp and K. M. Wollin, The EU chemicals strategy for sustainability: critical reflections on proposed regulatory changes for endocrine disruptors and mixture toxicity, Arch. Toxicol., 2022, 96(4), 1133–1135, DOI:10.1007/s00204-022-03227-z.
- J. Ahlers, B. Schwarz-Schulz and H.-C. Stolzenberg, Strategie für eine zukünftige chemikalienpolitik, Umweltwiss. Schadst.-Forsch., 2001, 13(2), 75–78, DOI:10.1007/BF03038641.
- R. N. Carvalho, A. Arukwe, S. Ait-Aissa, A. Bado-Nilles, S. Balzamo, A. Baun, S. Belkin, L. Blaha, F. Brion, D. Conti, N. Creusot, Y. Essig, V. E. Ferrero, V. Flander-Putrle, M. Fürhacker, R. Grillari-Voglauer, C. Hogstrand, A. Jonáš, J. B. Kharlyngdoh, R. Loos, A. K. Lundebye, C. Modig, P. E. Olsson, S. Pillai, N. Polak, M. Potalivo, W. Sanchez, A. Schifferli, K. Schirmer, S. Sforzini, S. R. Stürzenbaum, L. Søfteland, V. Turk, A. Viarengo, I. Werner, S. Yagur-Kroll, R. Zounková and T. Lettieri, Mixtures of Chemical Pollutants at European Legislation Safety Concentrations: How Safe Are They?, Toxicol. Sci., 2014, 141(1), 218–233, DOI:10.1093/toxsci/kfu118.
- OECD Test Guideline No. 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonists; OECD Guidelines for the Testing of Chemicals, Section 4, OECD, 2021, DOI:10.1787/9789264265295-en.
- U.S. EPA, Office of Prevention, Endocrine Disruptor Screening Program Test Guidelines - OPPTS 890.1300, Estrogen Receptor Transcriptional Activation, 2009, EPA-HQ-OPPT-2009-0576-0006.
- Y. Sakuratani, M. Horie and E. Leinala, Integrated Approaches to Testing and Assessment: OECD Activities on the Development and Use of Adverse Outcome Pathways and Case Studies, Basic Clin. Pharmacol. Toxicol., 2018, 123, 20–28, DOI:10.1111/bcpt.12955.
- N. Delrue, M. Sachana, Y. Sakuratani, A. Gourmelon, E. Leinala and R. Diderich, The Adverse Outcome Pathway Concept: A Basis for Developing Regulatory Decision-making Tools, ATLA, Altern. Lab. Anim., 2016, 44(5), 417–429, DOI:10.1177/026119291604400504.
- M. Vinken, The adverse outcome pathway concept: A pragmatic tool in toxicology, Toxicology, 2013, 312, 158–165, DOI:10.1016/j.tox.2013.08.011.
- J. Hildebrandt and A. F. Thünemann, Aqueous Dispersions of Polypropylene: Toward Reference Materials for Characterizing Nanoplastics, Macromol. Rapid Commun., 2023, 44(6), 2200874, DOI:10.1002/marc.202200874.
- V. S. Wilson, Development and Characterization of a Cell Line That Stably Expresses an Estrogen-Responsive Luciferase Reporter for the Detection of Estrogen Receptor Agonist and Antagonists, Toxicol. Sci., 2004, 81(1), 69–77, DOI:10.1093/toxsci/kfh180.
- J. C. Prata, J. L. Castro, J. P. da Costa, A. C. Duarte, M. Cerqueira and T. Rocha-Santos, An easy method for processing and identification of natural and synthetic microfibers and microplastics in indoor and outdoor air, MethodsX, 2020, 7, 1–9, DOI:10.1016/j.mex.2019.11.032.
- J. Q. Jiang, Occurrence of microplastics and its pollution in the environment: A review, Sustain. Prod. Consum., 2018, 13, 16–23, DOI:10.1016/j.spc.2017.11.003.
- Q. Yu, X. Hu, B. Yang, G. Zhang, J. Wang and W. Ling, Distribution, abundance and risks of microplastics in the environment, Chemosphere, 2020, 249, 126059, DOI:10.1016/j.chemosphere.2020.126059.
- F. Amereh, M. Babaei, A. Eslami, S. Fazelipour and M. Rafiee, The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge, Environ. Pollut., 2020, 261, 114158, DOI:10.1016/j.envpol.2020.114158.
- C. Duan, Y. Fang, J. Sun, Z. Li, Q. Wang, J. Bai, H. Peng, J. Liang and Z. Gao, Effects of fast food packaging plasticizers and their metabolites on steroid hormone synthesis in H295R cells, Sci. Total Environ., 2020, 726, 138500, DOI:10.1016/j.scitotenv.2020.138500.
- S. Ullah, S. Ahmad, X. Guo, S. Ullah, S. Ullah, G. Nabi and K. Wanghe, A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals, Front. Endocrinol., 2023, 13, 1084236, DOI:10.3389/fendo.2022.1084236.
- N. Fuentes and P. Silveyra, Estrogen receptor signaling mechanisms, Adv. Protein Chem. Struct. Biol., 2019, 116, 135–170, DOI:10.1016/bs.apcsb.2019.01.001.
- M. P. O'Sullivan and A. C. Testa, Fluorescence of aliphatic ketones, J. Am. Chem. Soc., 1970, 92(20), 5842–5844, DOI:10.1021/ja00723a005.
- H. Lee, D.-B. Kwak, S. C. Kim and D. Y. H. Pui, Characterization of colloidal nanoparticles in mixtures with polydisperse and multimodal size distributions using a particle tracking analysis and electrospray-scanning mobility particle sizer, Powder Technol., 2019, 355, 18–25, DOI:10.1016/j.powtec.2019.07.029.
- R. Firdessa, T. A. Oelschlaeger and H. Moll, Identification of multiple cellular uptake pathways of polystyrene nanoparticles and factors affecting the uptake: Relevance for drug delivery systems, Eur. J. Cell Biol., 2014, 93(8–9), 323–337, DOI:10.1016/j.ejcb.2014.08.001.
- T. Wang, L. Wang, X. Li, X. Hu, Y. Han, Y. Luo, Z. Wang, Q. Li, A. Aldalbahi, L. Wang, S. Song, C. Fan, Y. Zhao, M. Wang and N. Chen, Size-Dependent Regulation of Intracellular Trafficking of Polystyrene Nanoparticle-Based Drug-Delivery Systems, ACS Appl. Mater. Interfaces, 2017, 9(22), 18619–18625, DOI:10.1021/acsami.7b05383.
- A. Banerjee, L. O. Billey and W. L. Shelver, Uptake and toxicity of polystyrene micro/nanoplastics in gastric cells: Effects of particle size and surface functionalization, PLoS One, 2021, 16(12), 1–25, DOI:10.1371/journal.pone.0260803.
- H. J. Johnston, M. Semmler-Behnke, D. M. Brown, W. Kreyling, L. Tran and V. Stone, Evaluating the uptake and intracellular fate of polystyrene nanoparticles by primary and hepatocyte cell lines in vitro, Toxicol. Appl. Pharmacol., 2010, 242(1), 66–78, DOI:10.1016/j.taap.2009.09.015.
- M. Forte, G. Iachetta, M. Tussellino, R. Carotenuto, M. Prisco, M. De Falco, V. Laforgia and S. Valiante, Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells, Toxicol. In Vitro, 2016, 31, 126–136, DOI:10.1016/j.tiv.2015.11.006.
- S. J. More, V. Bampidis, D. Benford, C. Bragard, T. I. Halldorsson, A. F. Hernández-Jerez, S. H. Bennekou, K. Koutsoumanis, C. Lambré, K. Machera, E. Mullins, S. S. Nielsen, J. Schlatter, D. Schrenk, D. Turck and M. Younes, Guidance on the use of the benchmark dose approach in risk assessment, EFSA J., 2022, 20(10), e07584, DOI:10.2903/j.efsa.2022.7584.
- A. Hardy, D. Benford, T. Halldorsson, M. J. Jeger, K. H. Knutsen, S. More, A. Mortensen, H. Naegeli, H. Noteborn, C. Ockleford, A. Ricci, G. Rychen, V. Silano, R. Solecki, D. Turck, M. Aerts, L. Bodin, A. Davis, L. Edler, U. Gundert-Remy, S. Sand, W. Slob, B. Bottex, J. Cortiñas Abrahantes, D. Court Marques, G. Kass and J. R. Schlatter, Update: use of the benchmark dose approach in risk assessment, EFSA J., 2017, 15(1), e04658, DOI:10.2903/j.efsa.2017.4658.
- Scientific Committee on Consumer Safety (SCCS), Toxicity and Asessment of Chemical Mixtures, 2005, pp. 1–50 Search PubMed.
- M. J. Zeilmaker, S. Fragki, E. M. J. Verbruggen, B. G. H. Bokkers and J. P. A. Lijzen, Mixture exposure to PFAS: A Relative Potency Factor approach, National Institute for Public Health and the Environment (RIVM) Report number: 2018-0070, 2018, DOI:10.21945/RIVM-2018-0070.
- National Institute for Public Health and the Environment, ProastWeb, https://proastweb.rivm.nl/ Search PubMed.
- L. Sun, K. Liao and D. Wang, Comparison of transgenerational reproductive toxicity induced by pristine and amino modified nanoplastics in Caenorhabditis elegans, Sci. Total Environ., 2021, 768, 144362, DOI:10.1016/j.scitotenv.2020.144362.
- R. C. Marcelino, R. M. Cardoso, E. L. B. C. Domingues, R. V. Gonçalves, G. D. A. Lima and R. D. Novaes, The emerging risk of microplastics and nanoplastics on the microstructure and function of reproductive organs in mammals: A systematic review of preclinical evidence, Life Sci., 2022, 295, 120404, DOI:10.1016/j.lfs.2022.120404.
- X. Lin, Y. Wang, X. Yang, P. Watson, F. Yang and H. Liu, Endocrine disrupting effect and reproductive toxicity of the separate exposure and co-exposure of nano-polystyrene and diethylstilbestrol to zebrafish, Sci. Total Environ., 2023, 865, 161100, DOI:10.1016/j.scitotenv.2022.161100.
- B. Jeong, J. Y. Baek, J. Koo, S. Park, Y.-K. Ryu, K.-S. Kim, S. Zhang, C. Chung, R. Dogan, H. S. Choi, D. Um, T. K. Kim, W. S. Lee, J. Jeong, W. H. Shin, J. R. Lee, N. S. Kim and D. Y. Lee, Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny, J. Hazard. Mater., 2022, 426, 127815, DOI:10.1016/j.jhazmat.2021.127815.
- E.-J. Park, J.-S. Han, E.-J. Park, E. Seong, G.-H. Lee, D.-W. Kim, H. Y. Son, H. Y. Han and B. S. Lee, Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation, Toxicol. Lett., 2020, 324, 75–85, DOI:10.1016/j.toxlet.2020.01.008.
- Ž. Roje, K. Ilić, E. Galić, I. Pavičić, P. Turčić, Z. Stanec and I. Vinković Vrček, Synergistic effects of parabens and plastic nanoparticles on proliferation of human breast cancer cells, Arh. Hig. Rada Toksikol., 2019, 70(4), 310–314, DOI:10.2478/aiht-2019-70-3372.
- K. Ilić, L. Krce, J. Rodriguez-Ramos, F. Rico, N. Kalčec, I. Aviani, P. Turčić, I. Pavičić and I. Vinković Vrček, Cytotoxicity of nanomixture: Combined action of silver and plastic nanoparticles on immortalized human lymphocytes, J. Trace Elem. Med. Biol., 2022, 73, 127004, DOI:10.1016/j.jtemb.2022.127004.
- K. Ilić, N. Kalčec, L. Krce, I. Aviani, P. Turčić, I. Pavičić and I. Vinković Vrček, Toxicity of nanomixtures to human macrophages: Joint action of silver and polystyrene nanoparticles, Chem.-Biol. Interact., 2022, 368, 110225, DOI:10.1016/j.cbi.2022.110225.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3en00883e |
| This journal is © The Royal Society of Chemistry 2024 |