Evaluation and comparison of UV/H2O2 and adsorption on active carbon as a tertiary wastewater treatment for pharmaceutical removal within a small WWTP: a pilot study†
Vladislav
Knytl
ab,
Pavel
Mašín
b,
Věra
Vlčková
d,
Jaroslav
Semerád
 c,
Klára
Michalíková
c,
Petra
Najmanová
b and
Tomáš
Cajthaml
c,
Klára
Michalíková
c,
Petra
Najmanová
b and
Tomáš
Cajthaml
 *ac
*ac
aInstitute for Environmental Studies, Faculty of Science, Charles University, Benátská 2, CZ-128 01, Prague 2, Czech Republic
bDekonta a.s, Dřetovice 109, CZ-273 42, Stehelčeves, Czech Republic
cInstitute of Microbiology of the Czech Academy of Sciences, Vídeňská 1083, CZ-142 00, Prague 4, Czech Republic. E-mail: cajthaml@biomed.cas.cz
dDepartment of Biotechnology UCT Prague, Technická 5, CZ-166 28, Prague 6, Czech Republic
First published on 13th October 2023
Abstract
Pharmaceuticals and their metabolites are ubiquitous in the environment and represent typical anthropogenic micropollutants. Due to their low diffusive concentrations and often recalcitrant nature, the compounds are not completely removed by conventional biological wastewater treatment technologies, which emphasizes the need for tertiary treatment steps. This study was performed to evaluate the feasibility of photooxidation UV/H2O2 technology for the removal of selected pharmaceuticals (carbamazepine, diclofenac, hydrochlorothiazide, sulfamethoxazole, and tramadol) as a tertiary treatment step within the wastewater treatment plant (WWTP) process. The UV/H2O2 technology was compared with the more common treatment method of adsorption on granulated activated carbon (AC) in short-term and long-term tests. Both treatment systems were installed as pilot-scale units at a WWTP in a small village (equivalent of about 900 people) where a psychiatric hospital is located in the Czech Republic. The short-term tests highlighted several important aspects that need to be addressed within full-scale operations (e.g., mechanical pretreatment of wastewater, relation between H2O2 dose and UV dose). The initial concentration of tramadol was up to 5000 ng l−1, and that of carbamazepine and hydrochlorothiazide was up to 3000 ng l−1 in the WWTP outflow. The results showed that both units were capable of removing more than 95% of the pharmaceuticals during the long-term tests. As oxidation processes can generate transformation products (TPs), the ecotoxicity evaluation was addressed. Ecotoxicity using the bioluminescence bacterium Vibrio fischeri and the rainbow trout gill cell line (RTgill-W1) did not indicate any increase in ecotoxicity parameters in comparison to the inflow water samples for both units. Both processes were finally evaluated from an economical point of view, and the pilot-scale AC unit was more favorable in this context; however, estimations for a full-scale system suggest that the UV/H2O2 system is more economically feasible in terms of operational costs.
Water impactMicropollutants, including pharmaceuticals and their metabolites, often persist in effluent discharged from wastewater treatment plants. Here, two pilot-scale tertiary treatment approaches—UV/H2O2 photooxidation and adsorption on granulated activated carbon—were compared for their efficiency to degrade selected pharmaceuticals. Both units removed >95% of pharmaceuticals from real wastewater. The methods were also evaluated in terms of cost-effectiveness for full-scale application. |
1 Introduction
Emerging micropollutants represent synthetic or natural compounds released from point and nonpoint sources that are present in the aquatic environment at low concentrations. Micropollutants are not commonly monitored, and these substances have raised concerns due to their effects on living organisms and the aquatic environment. Recently, improvements and new possibilities in analytical techniques have led to a better understanding of their presence in wastewater, surface or groundwater. The most typical and monitored micropollutants include personal care products, pesticides, flame retardants, X-ray contrast compounds, steroid hormones and pharmaceuticals and their residues.1 Many of the substances from these groups are difficult to remove from wastewater by conventional treatment processes. They are commonly found in aquatic environments at low concentrations in the range of μg l−1 or ng l−1; nevertheless, ecotoxicity, bioaccumulation and chronic exposure effects have been investigated and documented in recent years.2Pharmaceuticals and their metabolites are ubiquitous in the environment.3 Their occurrence in wastewater has been confirmed in many countries around the world.4–6 Despite a significant reduction in concentrations, the majority of current wastewater treatment plants (WWTPs) are unable to completely eliminate pharmaceuticals from treated water. Some of the most persistent compounds can be detected even in groundwater hundreds of meters from the place of infiltration.7
To improve the removal of micropollutants, various technologies have been investigated in recent years as a possible final treatment step.8,9 Despite the vast number of laboratory-scale studies, only a few pilot- and full-scale studies are available regarding this problem.8 Most advanced legislative regulations came into effect in Switzerland, requiring approximately 100 municipal wastewater treatment plants in the country to be upgraded within the next 20 years to remove micropollutants from the final effluent.10
Advanced oxidation processes (AOPs) have been shown to be promising methods for the removal of various organic micropollutants from drinking water and wastewater. AOPs can be categorized according to the mechanism of oxidation into ozone-based, UV-based, electrochemical, catalytic, and physical AOPs.11 Recently, the combination of AOP implementations has been favored in the literature as an efficient solution in addressing the issue of global environmental waste management.12
Among those UV-based AOPs, UV-oxidation with hydrogen peroxide (H2O2), usually referred to as UV/H2O2, has attracted great attention, and numerous advantages have been documented for micropollutant control. Although simple UV light application does not yield satisfactory results, amendment of oxidizing agents (H2O2, S2O82−) together with the UV light potential for generating radicals has shown significant improvements.13,14
One of the major concerns regarding AOP-based technologies for water purification is that most organic pollutants are not completely mineralized but are partially oxidized into transformation products (TPs), adding chemical composition complexity to the treated water and posing risks to humans, ecological systems, and the environment.15 One parent compound can be oxidized into several TPs.16 Analytical identification of the whole spectrum of possible TPs is often beyond analytical capabilities. Up to hundreds of new TP chemical formulas with an increased degree of oxidation and decreased aromaticity were obtained by using ultrahigh-resolution mass spectrometry after drinking water treatment by the UV/H2O2 process.17 Several toxicological parameters were used as markers of possible higher toxicity of TPs. Vom Eyser et al. (2013) detected several TPs in ozonation and UV/H2O2 treatment processes.18 Accompanying analysis showed no genotoxic, cytotoxic or estrogenic potential for the investigated compounds after oxidative treatment of real wastewaters. Another finding was published by Han et al. (2018), who documented increased cytotoxicity and chromosome damage effects caused by TPs generated during ozonation.19 The fate of the generated TPs, their accumulation and chronic toxicity are under continual research.
According to available review articles, UV/H2O2 was confirmed as a suitable solution for the removal of various pharmaceuticals, but mostly at the laboratory scale.11 Although there have been a few documented attempts, studies employing UV/H2O2 as a final clean-up step (tertiary degree) within the WWTP process are scarce. Baresel et al. (2019) used UV/H2O2 for the removal of pharmaceutical residues from municipal wastewater, documenting satisfactory removal efficiencies for pharmaceuticals detected in the effluent of Stockholm's largest WWTP.20 Earlier, another attempt was well documented by Martijn et al. (2007), who implemented the UV/H2O2 process within a drinking water treatment plant.21 Sarathy et al. (2011) tested oxidation processes on natural organic matter when using a pilot-scale UV/H2O2 unit.22 Lhotský et al. (2017) successfully used UV/H2O2 for groundwater treatment containing a commingled plume of pharmaceuticals and aromatic compounds.23 Nevertheless, it is always necessary to compare novel treatment approaches with more commonly available technologies, such as adsorption on active carbon, or to consider their possible combination, which can increase their efficiency and usability.
In this study, we focused on the field evaluation of UV/H2O2 and its comparison to a more commonly used technology – adsorption on active carbon (AC) particles. Both pilot-scale units were designed as final (tertiary) treatment steps at a WWTP for the removal of pharmaceuticals from wastewater. As mentioned before, the lack of pilot-scale or full-scale studies on sites treating real wastewater limits the evaluation of UV/H2O2 technology for tertiary treatment within the WWTP process. Furthermore, the comparison of both technologies in the current study was performed on a site representing a village with a psychiatric hospital (see below). The efficiency was monitored using appropriate analytical methods, ecotoxicology tests and other parameters that can be used as surrogates in practice. Finally, both units were economically evaluated in terms of operating costs.
2 Materials and methods
2.1 Site description
The WWTP facility was chosen in the village located in the Ústí nad Labem Region, Czech Republic. The village has approximately 900 equivalent people. One of the main reasons why this locality was chosen is the presence of a psychiatric hospital with a capacity of up to 500 patients. Previous research on this site showed a limited removal of pharmaceutical compounds from wastewater and even confirmed the presence of pharmaceuticals in the groundwater.7,24 All previously described reasons supported considering this WWTP as a suitable site for pilot-scale experimental setup installation. The particular WWTP is designed for 3000 equivalent inhabitants. Inlet wastewater flows through hand coarse screens and a pair of pumping stations to the area of the biological reactor stage. The biological WWTP consists of a circular concrete tank divided into nitrification, denitrification and sedimentation units. Sewage sludge is gravitationally sedimented into a sludge pit and further processed. Treated water flows into the recipient, creating a stream. The stream ends in 3 infiltration ponds at a distance of 700 m. The treated wastewater is therefore in direct contact with the soil and groundwater.2.2 Photochemical oxidation unit
The photochemical oxidation unit built for on-site pilot experiments consisted of two cylindrical reactors made from quartz glass (120 cm length, 15 cm in diameter). The treated water flowed through each of the reactors installed in parallel; nevertheless, the system could also be employed in serial connection. Each reactor was surrounded by a circle consisting of twenty low-pressure 36 W UV-C 254 nm lamps (T8 G13 Philips Netherlands), 720 W per photoreactor in total. The reactor was covered by an aluminum cover, which acted as a protective shield against UV light emission. The actual radiation of each reactor was measured by a UV radiometer (HD 2302, Delta Ohm, Italy) equipped with a UV-C probe. The average values inside each of the reactors without wastewater reached 2500 μW cm−2. Hydrogen peroxide (w/w 35% technical grade, Penta, Czech Republic) was stored in a sealed plastic container and pumped into the water stream by a dosing pump (EWN-B11VCERA, Iwaki, Japan). It was replenished periodically to ensure optimal reactivity. The unit was equipped with a cylindrical AC trap to remove the residual H2O2 concentration (see below). The operational parameters of the UV/H2O2 unit could be adjusted by regulating the water flow and changing the dose of the H2O2 solution. The UV/H2O2 unit was installed in a specially adapted container. The scheme of the unit is depicted in Fig. 1B.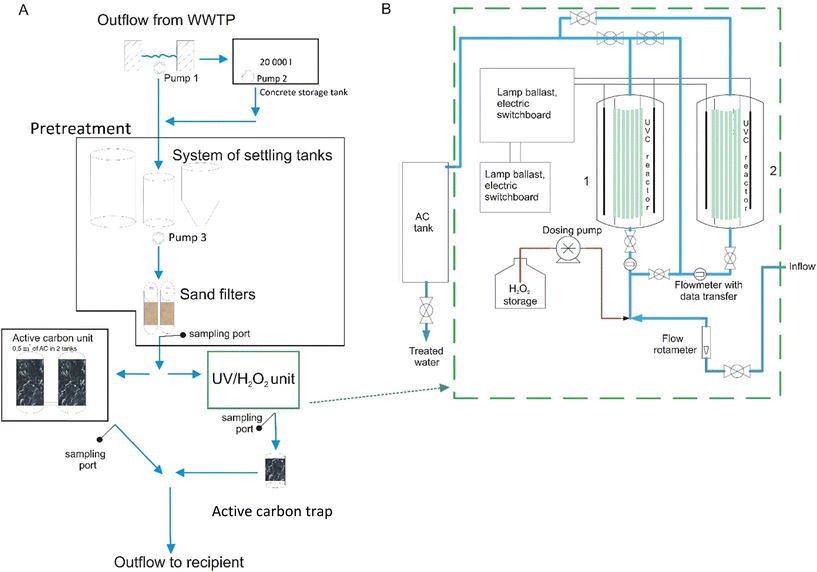 | ||
| Fig. 1 A) A general scheme of the pilot unit setup including both AC and UV/H2O2 units and B) a more detailed scheme of the UV/H2O2 pilot unit. | ||
2.3 Adsorption unit
The AC unit was installed in another container (Fig. 1A). This system consisted of two adsorption tanks with a conical bottom filled with commercially available granular AC (Aquasorb 5005, Jacobi, Germany) with a mean particle size of 1.7 mm and a specific surface area of 1200 m2 g−1. The amount of AC in each tank was equal to 0.4 m3 (150 kg) and pressed between two adapted stainless-steel grates. Each tank was further equipped with a degassing valve, manometer and electronic flowmeter. The flow rate through the AC and UV/H2O2 units could be adjusted by operating a mechanical valve.2.4 Pretreatment system
A separated pretreatment system was installed before the AC and UV/H2O2 units to remove sludge particles present in wastewater. This part consisted of settling tanks, a distribution pump (pump 3) and mechanical sand filters and was used for both units within the experimental setup.2.5 Experimental setup
| UV Dose = I × t | (1) |
I = average irradiance
t = time of exposure
The average UV irradiance in the empty reactor was measured by a portable radiometer equipped with a UVC probe. The average value inside the quartz glass cylinder was 2500 μW cm−2. Three flow rates were tested, specifically, 4, 8 and 16 L min−1, and the average UV dose for each flow rate according to the abovementioned equation was 1750, 3500, and 7000 J m−2, respectively. The last flow rate (16 l min−1) represented the upper limit for feasible operation of the unit regarding the mechanical pretreatment of the wastewater.
The calculated UV doses do not represent the actual values due to the turbidity of the real wastewater and the presence of H2O2 in the treated water, which can significantly influence ultraviolet transmission (UVT). To achieve more precise values, the (real/active) UV doses were calculated using the following simplified equations:25
UV dose for the perfect collimated beam:
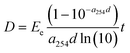 | (2) |
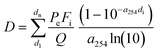 | (3) |
D = UV dose (mJ cm−2)
E e = UV irradiance entering the water
F i = the fraction of UV with path length di
P f = the Petri factor
P e = the total radiant power entering the water spread over area A
R = reflectance at the air–water interface
d = path length (cm); d1, dn = multiple path lengths in a non-uniform reactor
a 254 = UV absorption coefficient (cm−1) at 254 nm
t = exposure time(s)
Q = flow rate
The UV absorption coefficient was determined in a water sample collected from the sampling port before the mechanical pretreatment step. Based on calculations according to eqn (3), the operating effective UV doses for 4, 8 and 16 l min−1 were determined to be 930, 465 and 233 J m−2, respectively. It is noteworthy that these calculations are simplifications and do not account for hydraulic efficiency parameters.25 The effect of H2O2 concentration on the absorption coefficient was assessed by spectrophotometric measurements (see section 2.6.3). The differences between average UV doses (eqn (1)) and real/active UV doses (eqn (3)) document that the geometry of the reactor, water parameters and especially its turbidity play crucial roles in UV/H2O2 efficiency. The rising complexity of the wastewater matrix can negatively affect the UV fluence in the photoreactor and the treatment efficiency.26
The treated water flowing from the UV/H2O2 unit contained a residual concentration of H2O2. To remove this residue, an open AC trap was installed at the unit outflow. The function of this granular AC bed (20 kg) was either to catalyze H2O2 decomposition27 or to remove possible oxidation by-products or degradation products of pharmaceuticals.
2.5.2.1 Short-term test. The purpose of this test was to find the relationship between UV doses and concentrations of H2O2. The intensity of UVC light inside the reactors was constant. Therefore, the dose of UVC light was proportional to the water flow rate through the system. Throughout the short-term test, three different flow rates were tested, which represented three different theoretical UV doses – 7000, 3500 and 1750 J m−2 (representing 930, 465 and 232 J m−2 effective UV doses, respectively) and three different doses of H2O2 solution (35%): 0.5, 0.75, and 1.5 ml min−1. The actual concentration of H2O2 in the treated wastewater was therefore dependent on the flow rate. The unit operated under 9 different conditions.
2.5.2.2 Long-term test. A long-term test was performed to evaluate the removal efficiency of pharmaceuticals and other parameters of both UV/H2O2 and AC units. In this case, a part of the outflow from the WWTP was pumped continuously to the storage tank. Before starting, new sand filters with increased capacity (100 l) were installed in the pretreatment section. The wastewater flow rate was set to 5 l min−1 for each unit and kept at this value for the whole testing period, resulting in a theoretical UV dose of 5600 J m−2 for the UV/H2O2 unit. This flow rate was selected according to the results of the short-term test and feasibility regarding the maintenance of the mechanical pretreatment system and the maximum capacity of the AC trap. A limitation of the UV/H2O2 technology could be the insufficient removal of the residual H2O2 concentration in the treated water. Furthermore, the purpose of this long-term test was to find the upper limit of the H2O2 dose to ensure sustainable removal of the H2O2 residue.
2.6 Analytical methods
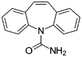
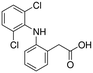
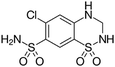
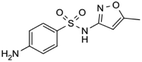
| Chemical structure | |||||
|---|---|---|---|---|---|
| Compound | Carbamazepine | Diclofenac | Hydrochlorothiazide | Sulfamethoxazole | Tramadol |
| Usage | Anticonvulsant, antiepileptic | Anti-inflammatory | Diuretic medication | Antibiotic | Opioid pain medication |
| Formula | C15H12N2O | C14H11Cl2NO2 | C7H8ClN3O4S2 | C10H11N3O3S | C16H25NO2 |
| CAS number | 298-46-4 | 15307-86-5 | 58-93-5 | 723-46-6 | 27203-92-5 |
| MW (g mol−1) | 236.269 | 296.148 | 297.74 | 253.279 | 263.381 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow Kow |
2.5 | 0.7 | 0.89 | 3.01 |
The samples were collected at the outflow of the AC and UV/H2O2 units (sampling ports in Fig. 1). The samples were filtered using a glass filter (0.45 μm) and extracted using solid-phase extraction (SPE) using SPE columns (LiChrolut® EN, Merck KGaA). The columns were conditioned with 3 ml of methanol and then 3 mL of deionized water. The columns were loaded with 500 ml of a water sample. Afterward, the SPE columns were dried to remove the remaining water. The sample extracts were eluted with 3 ml of acetonitrile/methanol (50/50) and evaporated under a stream of nitrogen. The samples were redissolved in 1 ml acetonitrile and analyzed using HPLC-MS/MS (Accela 1250) equipped with PAL Accela Open AS and TSQ Quantum Access Max (Thermo Scientific, USA). For the separation, the column Luna Omega 1.6 μm polar C18, 50 × 2.1 mm was used equipped with precolumn C18, 2.1 mm (Phenomenex, USA). The gradient elution method was used, using A – water + 0.1 v/v% HCOOH and B – methanol + 0.1 v/v% HCOOH. The gradient program was as follows: 0 min, 20% B; 0.5 min to 2 min, a linear gradient to 95% B; and 2 min to 5 min, 95% B was maintained.
A TOC analyzer (Liqiu TOC II, Elementar, Germany) was employed using catalytic combustion with infrared spectrophotometry detection. For determination of the UVA254 parameter, the collected water samples were filtered with a 0.45 μm membrane filter and analyzed using a Shimadzu UV 1800 UV/VIS spectrophotometer (Japan) onsite. Attention was given to the immediate processing of the samples due to the influence of residual H2O2 on organic compounds. The residual H2O2 concentration was determined by a titration method using potassium iodide in the presence of the catalyst [(NH4)6Mo7O24·4H2O].32
3 Results and discussion
3.1 Initial monitoring
The initial monitoring of the wastewater was performed before installing the pilot unit setup. Samples were collected from the inflow to the WWTP and from the outflow, and the filtered activated sludge particles floating in the outflow wastewater were analyzed. The samples were analyzed for the presence of the five selected pharmaceuticals (see Table 2). The pharmaceuticals were also chosen according to their recalcitrance. The complete initial monitoring included 17 other pharmaceuticals, and their concentrations are shown in ESI† Table S1.| Pharmaceutical (ng L−1) | Inflow to WWTP | Outflow from WWTP | Activated sludge in outflow (ng g−1) | |||
|---|---|---|---|---|---|---|
| Average | RSD [%] | Average | RSD [%] | Average | RSD [%] | |
| Tramadol | 2102.9 | 7.4 | 2234.5 | 3.3 | 122.8 | 8.9 |
| Carbamazepine | 2453.6 | 2.8 | 2526.2 | 5.7 | 135.1 | 4.7 |
| Sulfamethoxazole | 111.0 | 1.4 | 819.6 | 6.6 | 30.9 | 7.6 |
| Hydrochlorothiazide | 3135.0 | 7.9 | 2837.3 | 8.9 | 121.5 | 4.6 |
| Diclofenac | 2233.7 | 7.7 | 1368.6 | 6.2 | 77.2 | 2.2 |
The results in Table 2 show that the common treatment process within the WWTP is not sufficient to efficiently remove any of these compounds. Moreover, the concentrations of some of the compounds (carbamazepine, tramadol) were not affected at all. Traces of these pharmaceuticals were also detected in groundwater samples extracted from historic wells downstream from the WWTP.24 These findings are in agreement with the literature when carbamazepine is well documented for its persistence caused by generally low biodegradability.33,34
The treated water from the WWTP contained a large amount of activated sludge particles. The analytical results of the pharmaceuticals in the particles are also summarized in Table 2. All the pharmaceuticals were detected at concentrations up to hundreds of ng g−1. Removal of these particles is desirable not only to increase the efficiency of the treatment process but also to prevent their accumulation in the recipient sediments, where they can represent a secondary pollution risk.
The additional parameters pH, TOC, UVA254, COD (Cr) (chemical oxygen demand) and BOD (biochemical oxygen demand) of the wastewater from the outflow are summarized in Table 3.
| Units | Wastewater inflow parameters | Wastewater outflow parameters | |||
|---|---|---|---|---|---|
| Average | RSD [%] | Average | RSD [%] | ||
| TOC | mg l−1 | 202.4 | 24.7 | 10.1 | 8.4 |
| UVA254 nm | cm−1 | 2.8 | 9.6 | 0.2 | 5 |
| COD (Cr) | mg l−1 | 500.8 | 19.4 | 40.8 | 10.4 |
| BOD | mg l−1 | 422.8 | 17.3 | 2.6 | 7.9 |
| pH | 7.1 | 13.1 | 7.7 | 8.1 | |
The pH value of wastewater flowing into the experimental setup was between 7 and 8 during the whole test. The TOC parameter was 10.1 mg l−1, and this value was also stable during the experiment. The BOD/COD ratio was under the value of 0.1, which documents advanced previous biodegradation in the treated water due to the WWTP treatment processes.
3.2 Removal of pharmaceuticals
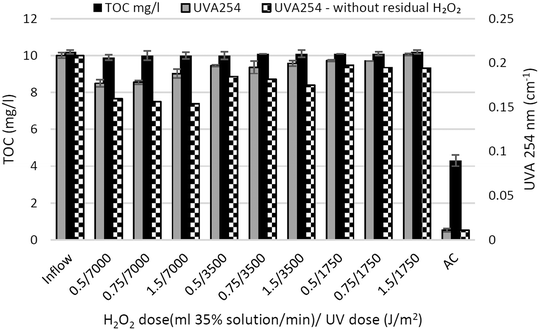
The data in Fig. 2 show similar TOC concentrations in all the UV/H2O2 variants of the short-term test. The explanation lies in only partial decomposition of macromolecular organic compounds.35,36 The data document that the TOC parameter is not suitable for monitoring the removal efficiency of micropollutants in real wastewater via the UV/H2O2 treatment process. The AC unit caused a reduction in the TOC concentration of more than 50% to 4.3 mg l−1.
The UVA254 parameter representing the absorption of UV light at 254 nm is also proportional to the content of organic matter in water with respect to the presence of an aromatic moiety. Contrary to TOC, the parameter UVA254 showed a decrease in comparison to the inflow values (Fig. 2). The reduction in UVA254 indicates a loss of aromatic and conjugated double bond structures of the effluent organic matter (EfOM).35 Such partial oxidation typically leads to the opening of phenolic rings and the degradation of organic compounds in the treated water.37 Residual H2O2 also has an influence on the absorbance of the samples. The final UVA254 value without residual H2O2 was obtained by subtracting the molar absorption coefficient of the residual H2O2 from the recorded value. The variant with the highest UV dose and the highest dose of 35% H2O2 (0.5 ml min−1 – 7000 J m−2) showed a 24% decrease in UVA254 after subtracting the residual H2O2 absorbance.
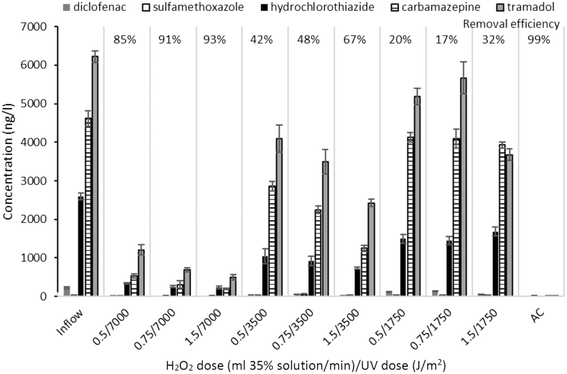
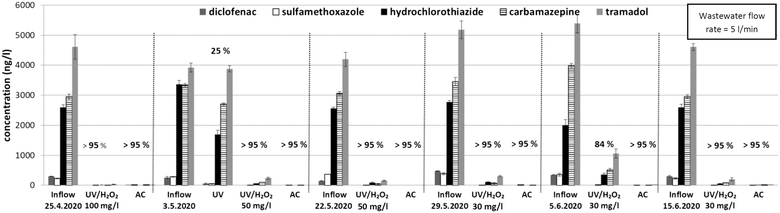
Concentrations of the pharmaceuticals in the wastewater treated by each of the tested UV/H2O2 variants and adsorption on AC are summarized in Fig. 3.
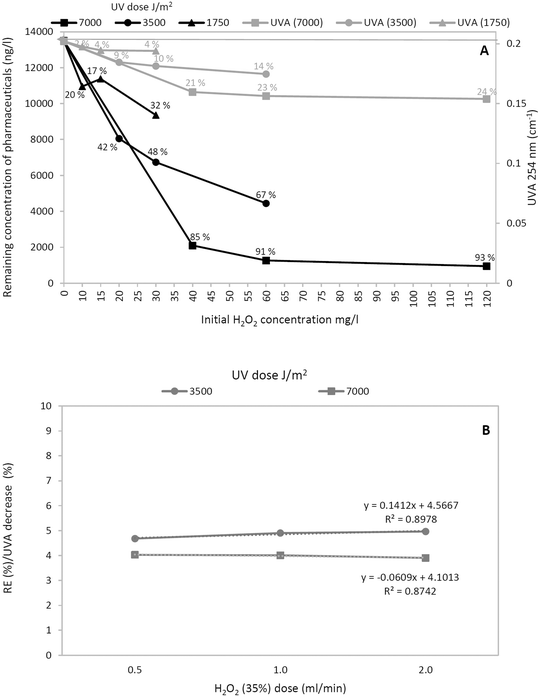
The highest removal efficiency was found with the highest UV dose (85–93% removal efficiencies). Higher efficiencies were generally observed with increasing H2O2 dose for the same UV dose level. This corresponds to the highest decrease in the UVA254 parameter (Fig. 2). No preferential oxidation of the individual pharmaceuticals in the wastewater (tramadol, carbamazepine, hydrochlorothiazide, and diclofenac) was observed. Although sulfamethoxazole was reported in previous studies at this site7,24 as well as during the long-term test in this study (see below), its concentrations reached only a maximum of tens of ng l−1 during the short-term test. The AC unit reached up to 99% removal efficiency. As discussed above, the regulation of the flow rate through the system, while maintaining the constant H2O2 dose pump, had an effect on the concentration of H2O2 in the treated water. The initial H2O2 concentrations in all the variants and UV dose effects on the removal of the pharmaceuticals are shown in Fig. 4A.
The trends of the curves (black line) show proportionality between the removal efficiency of pharmaceuticals and the increasing UV dose and concentration of H2O2 (Fig. 4A). For instance, the curve representing 7000 J m−2 clearly shows a decreasing proportional trend depending on the rising concentration of H2O2 (only an 8% rise between variants for 40 mg l−1 and 120 mg l−1 H2O2). Higher concentrations of H2O2 produce increasing recombination of OH radicals and therefore substantially decrease the removal of pharmaceuticals. A similar finding was observed by Somathilake et al. (2018) under laboratory conditions38 and was confirmed in a real pilot wastewater treatment.20 Lower UV doses showed a more considerable increase in the removal efficiency with respect to increasing concentrations of H2O2. Nevertheless, the overall removal efficiency was lower (max. 32% for 1750 J m−2 and max. 67% for 3500 J m−2). The discrepancy (10 and 15 mg l−1 of H2O2) in the case of the lowest UV dose was caused by a temporary accidental decrease in the efficiency of the mechanical water pretreatment, which was observed during testing of this UV dose.
The decrease in UVA254 after subtracting the residual H2O2 absorbance (gray line) is also depicted in the graph (Fig. 4A). Similarly, the decrease was dependent on the UV dose and the H2O2 concentration. The maximum decrease (24%) was observed for the variant with the highest UV dose (7000 J m−2) and H2O2 concentration. For better visualization (Fig. 4B), the correlations between the ratio of the removal efficiency and UVA254 (Y axis) with increasing doses of H2O2 (X axis) were depicted. The variants with UV doses of 7000 and 3500 J m−2 showed similar horizontal curves with correlation coefficients (R2) of 0.87 and 0.90, respectively. The combination with the UV dose 1750 J m−2 is not shown due to the low removal results and a temporary accidental decrease in the efficiency of the mechanical water pretreatment.
The flow rate was set to 5 l min−1 throughout the whole test, and the theoretical UV dose was then 5600 J m−2. Between Apr 25 and May 22, the dose of H2O2 was set to 50 mg l−1. The removal efficiency for UV/H2O2 was above 95% for both sampling events. The sample from May 3 was also analyzed without any addition of H2O2 (labeled as UV). The results confirmed that only UV photolysis (254 nm) without any other amendments is not sufficient to achieve satisfactory results regarding pharmaceutical removal. Similar results were shown, for example, by Chowdhury et al. (2020).39
It was observed that the AC trap capacity was not sufficient to remove the residual H2O2. For this reason, the H2O2 dose was lowered to 30 mg l−1. The results from May 22 to 29 still showed very good removal efficiency over 95%. Interestingly, for UV/H2O2, the removal efficiency was even higher in comparison to short-term testing (Fig. 4A – 40 mg l−1 H2O2 and theoretical UV dose 7000 J m−2). The explanation lies in the installation of new sand filters with higher capacity and therefore more efficient pretreatment of the wastewater. The removal efficiency dropped to 84% on June 5 due to an accidental inflow of water with higher turbidity from the WWTP. The pretreatment system was not capable of sufficient removal of the activated sludge particles.
The concentrations of the pharmaceuticals in the samples from the AC unit were below the respective detection limits throughout the whole long-term test, documenting that there were no breakthroughs due to insufficient AC adsorption capacity. The long-term operation of the AC unit was prolonged to monitor the breakthrough capacity of the AC bed. The flow rate was alleviated to 12 l min−1. Samples of water flowing from the AC unit were collected every 14 days. The breakthrough was noted in November 2020 as the removal of the pharmaceuticals dropped to 75%. All previous samples showed over 95% removal efficiency. The total amount of water flowing through the AC unit was approximately 2000 m3, this amount also represented the breakthrough limit for the AC unit. It is important to note that this value was influenced by time periods where the pretreatment system was not functioning properly. Otherwise, the breakthrough limit of AC columns could have been higher.
3.3 Ecotoxicity
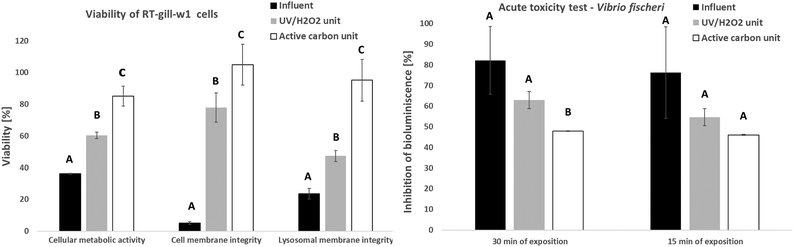
The sample taken on May 22 containing 30 mg l−1 H2O2 was subjected to ecotoxicological testing. The results of the acute toxicity test using V. fischeri revealed a decrease in toxicity of the effluent from both the UV/H2O2 and AC units compared to the untreated influent (Fig. 6 left). A similar decrease in toxicity (an increase in viability) was observed using the toxicological test employing the fish cell line (Fig. 6 right). The results of three different approaches for quantification of cytotoxicity toward the fish cell line and the standardized bioluminescence test proved that the use of both technologies (photooxidation and adsorption) did not increase the toxicity of the wastewater. These results correspond to findings by Andreozzi et al. (2004), who tested AOP processes on a mixture of pharmaceuticals using the alga Synechococcus leopoliensis and the rotifer Brachyonus calyciflorus.40 More recently, Angeles et al. (2020) studied the behavioral effects in larval zebrafish exposed to advanced treatment technologies, including ozonation installed on 7 WWTPs.41 None of the final effluents caused any negative effects. The authors pointed out that it was important to assess the long-term effect and chronicity of possible TPs generated by AOP technologies.3.4 Economic evaluation
The long-term operation of both units was evaluated in terms of the operating costs. The construction costs were not taken into account. The main relevant parameters resulting from the pilot-scale testing accounted for in the cost calculations are summarized in Table 4.| Parameter | UV/H2O2 | AC adsorption |
|---|---|---|
| Pretreatment | - Mechanical pretreatment system | - Mechanical pretreatment system |
| Energy consumption | - Pumps for water flow | - Pumps for water flow |
| - UVC light lamps | ||
| - H2O2 dosing pump | ||
| Agents | - H2O2 solution (needed periodical replenishment) | - AC particles – including regeneration of AC or not |
| - AC particles for AC carbon trap | ||
| Additional materials | - UVC light lamps change (1× per year) | |
| Maintenance | - Pretreatment system | - Pretreatment system |
| - UV dose measurements | - AC tanks maintenance | |
| - H2O2 dosing | ||
| - Cleaning quartz glass |
The data show that the costs for the treatment of 1 m3 wastewater based on the mentioned parameters are 0.95 € per m3 for the AC unit and 1.15 € per m3 for the UV/H2O2 unit. These values are substantially higher in comparison to available estimations for UV/H2O2 between 0.08 and 0.13 € per m3 by Karl and Dolling (2018)42 or even <0.08 € per m3 by Baresel et al. (2019).20 These estimations are for full-scale application to much larger WWTPs with wastewater flow rates over 1000 m3 h−1. Estimations based on this study are 0 and 3 m3 h−1 pilot tests. It can be assumed that with a larger scale, the price per m3 will be significantly lower.
It is important to note that full-scale operation on the site might be different. The annual flow rate through this WWTP is 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 (approximately 13 m3 h−1), which means the consumption of 24 t of granulated AC. In fact, the AC amount could be lower with a more robust pretreatment system, which could increase the estimated breakthrough limit. If the full-scale operation included regeneration of granulated AC, it can be generally assumed that the environmental impact (in the sense of life cycle assessment) and even economical parameters of the full-scale process could be more favorable. At the same time, it is noteworthy that the possibility of AC regeneration in Central Europe is still scarce. Generally, the consumption of AC in the case of full-scale treatment might cause the UV/H2O2 technology to be more favorable, especially in the case of decreasing costs of UV/H2O2 due to more efficient reactor setups with protected UVC lamps inside the reactors, and therefore better utilization of UVC light.25 On the other hand, a construction of the full-scale UV/H2O2 unit will be more expensive in comparison to the AC technology. Finally, the removal of residual H2O2 by an AC trap installed at the outflow from a UV/H2O2 full-scale technology should be more robust to achieve sufficient removal of residual H2O2.
000 m3 (approximately 13 m3 h−1), which means the consumption of 24 t of granulated AC. In fact, the AC amount could be lower with a more robust pretreatment system, which could increase the estimated breakthrough limit. If the full-scale operation included regeneration of granulated AC, it can be generally assumed that the environmental impact (in the sense of life cycle assessment) and even economical parameters of the full-scale process could be more favorable. At the same time, it is noteworthy that the possibility of AC regeneration in Central Europe is still scarce. Generally, the consumption of AC in the case of full-scale treatment might cause the UV/H2O2 technology to be more favorable, especially in the case of decreasing costs of UV/H2O2 due to more efficient reactor setups with protected UVC lamps inside the reactors, and therefore better utilization of UVC light.25 On the other hand, a construction of the full-scale UV/H2O2 unit will be more expensive in comparison to the AC technology. Finally, the removal of residual H2O2 by an AC trap installed at the outflow from a UV/H2O2 full-scale technology should be more robust to achieve sufficient removal of residual H2O2.
4 Conclusion
This study evaluated the feasibility of removal of selected pharmaceuticals (carbamazepine, diclofenac, hydrochlorothiazide, sulfamethoxazole, and tramadol) from WWTP wastewater using a UV/H2O2 pilot-scale unit and an AC pilot-scale unit. Pilot testing has revealed some aspects that are crucial to the proper use of wastewater treatment systems based on UV/H2O2. Designing a robust mechanical pretreatment system is important to ensure a constant flow rate of water with stable quality parameters. Activated sludge, often present in treated wastewater, contains up to hundreds of ng g−1 pharmaceuticals, which further accumulate in the recipient sediment. Turbidity can dramatically lower the effective UV dose and the absorbance of wastewater and should be evaluated before installing the treatment setup. In our case, we calculated only 13% of the theoretical UV dose value to be considered the operating effective UV dose. Emphasis should also be placed on designing the geometry of the reactor. Otherwise, the usability of the technology is reduced, as the light transmission through the column of treated water is restricted.The removal efficiency of the monitored pharmaceuticals is dependent mainly on the concentration of H2O2 in treated water and the UV dose. It was found that high concentrations of H2O2 together with high UV doses can inhibit the removal of pharmaceuticals, probably due to OH− recombination processes. Furthermore, the production of residual concentrations of H2O2 in wastewater could represent a problem within continuous and large-scale operation, as releasing the oxidizing agent to a recipient is undesired. To monitor the treatment efficiency, UVA254 was selected as a reliable surrogate parameter. Higher UV doses (3500 and 7000 J m−2) showed a good correlation between UVA254 and the removal efficiency of the pharmaceuticals. The TOC parameter was not sensitive enough for real wastewater containing a rich spectrum of various organic compounds. Adsorption on AC particles showed over 95% removal efficiency even in the prolonged period (up to 2000 m3).
The toxicity of the treated wastewater regarding possible incomplete oxidation and generation of intermediate products was evaluated by an acute toxicity test using Vibrio fischeri and cytotoxicity toward RT-gill-w1 cells. It was confirmed that the samples taken from UV/H2O2 did not show any increase in the ecotoxicity parameters in comparison to the inflow water samples.
Both technologies were proven within this pilot study to be suitable for tertiary wastewater treatment with respect to the removal of pharmaceuticals. The importance for proper setting of the operation parameters is crucial and should be evaluated before using each technology. Economical evaluation of both systems during long-term operations showed better results for the AC carbon unit. This might change if we took into account full-scale application for the whole WWTP (120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per year).
000 m3 per year).
The authors emphasize the need for more pilot-scale and full-scale studies to evaluate the efficiency and economic feasibility for comparison with other technologies e.g., adsorption on active carbon, ozonation, etc.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Ministry of Industry and Trade of the Czech Republic (OP PIK 2014-2020) under project ID CZ.01.1.02/0.0/0.0/15_019/0004571: the technology using photochemical oxidation with sorption for the elimination of micropollutants nature of pharmaceutical substances from wastewater. This work was also supported by the Technology Agency of the Czech Republic (project No. SS02030008, program: Prostředí pro život) and by the Center for Geosphere Dynamics (UNCE/SCI/006).References
- J. Margot, L. Rossi, D. A. Barry and C. Holliger, A review of the fate of micropollutants in wastewater treatment plants, Wiley Interdiscip. Rev.: Water, 2015, 2, 457–487 CrossRef CAS.
- M. Arslan, I. Ullah, J. A. Müller, N. Shahid and M. Afzal, in Enhancing Cleanup of Environmental Pollutants, Springer International Publishing, 2017, vol. 1, pp. 65–99 Search PubMed.
- K. Kümmerer, in Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks, Springer Berlin, Heidelberg, Berlin, Heidelberg, 2008, pp. 1–521 Search PubMed.
- S. Mohapatra, C. H. Huang, S. Mukherji and L. P. Padhye, Occurrence and fate of pharmaceuticals in WWTPs in India and comparison with a similar study in the United States, Chemosphere, 2016, 159, 526–535 CrossRef CAS PubMed.
- E. Gracia-Lor, J. V. Sancho, R. Serrano and F. Hernández, Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia, Chemosphere, 2012, 87, 453–462 CrossRef CAS PubMed.
- A. Rojjanapinun, S. Pagsuyoin and J. Luo, in Systems and Information Engineering Design Symposium, SIEDS 2019, Institute of Electrical and Electronics Engineers Inc., 2019 Search PubMed.
- D. Rozman, Z. Hrkal, P. Eckhardt, E. Novotná and Z. Boukalová, Pharmaceuticals in groundwaters: a case study of the psychiatric hospital at Horní Beřkovice, Czech Republic, Environ. Earth Sci., 2015, 73, 3775–3784 CrossRef CAS.
- X. T. Bui, T. P. T. Vo, H. H. Ngo, W. S. Guo and T. T. Nguyen, Multicriteria assessment of advanced treatment technologies for micropollutants removal at large-scale applications, Sci. Total Environ., 2016, 563–564, 1050–1067 CrossRef CAS.
- D. K. Kanaujiya, T. Paul, A. Sinharoy and K. Pakshirajan, Biological Treatment Processes for the Removal of Organic Micropollutants from Wastewater: a Review, Curr. Pollut. Rep., 2019, 5, 112–128 CrossRef.
- M. Bourgin, B. Beck, M. Boehler, E. Borowska, J. Fleiner, E. Salhi, R. Teichler, U. von Gunten, H. Siegrist and C. S. McArdell, Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products, Water Res., 2018, 129, 486–498 CrossRef CAS PubMed.
- D. B. Miklos, C. Remy, M. Jekel, K. G. Linden, J. E. Drewes and U. Hübner, Evaluation of advanced oxidation processes for water and wastewater treatment – A critical review, Water Res., 2018, 139, 118–131 CrossRef CAS PubMed.
- P. K. Pandis, C. Kalogirou, E. Kanellou, C. Vaitsis, M. G. Savvidou, G. Sourkouni, A. A. Zorpas and C. Argirusis, Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review, ChemEngineering, 2022, 6, 8 CrossRef CAS.
- H. Bielak, A. Boergers, J. Raab, J. Tuerk and E. Dopp, in Disinfection By-products in Drinking Water, Royal Society of Chemistry, 2015, vol. 352, pp. 180–188 Search PubMed.
- Y. Q. Gao, N. Y. Gao, Y. Deng, Y. Q. Yang and Y. Ma, Ultraviolet (UV) light-activated persulfate oxidation of sulfamethazine in water, Chem. Eng. J., 2012, 195–196, 248–253 CrossRef CAS.
- W. L. Wang, Q. Y. Wu, N. Huang, Z. Bin Xu, M. Y. Lee and H. Y. Hu, Potential risks from UV/H2O2 oxidation and UV photocatalysis: A review of toxic, assimilable, and sensory-unpleasant transformation products, Water Res., 2018, 141, 109–125 CrossRef CAS PubMed.
- X. S. Miao, J. J. Yang and C. D. Metcalfe, Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant, Environ. Sci. Technol., 2005, 39, 7469–7475 CrossRef CAS PubMed.
- Y. Xiang, M. Gonsior, P. Schmitt-Kopplin and C. Shang, Influence of the UV/H2O2 Advanced Oxidation Process on Dissolved Organic Matter and the Connection between Elemental Composition and Disinfection Byproduct Formation, Environ. Sci. Technol., 2020, 54, 14964–14973 CrossRef CAS PubMed.
- C. Vom Eyser, A. Börgers, J. Richard, E. Dopp, N. Janzen, K. Bester and J. Tuerk, Chemical and toxicological evaluation of transformation products during advanced oxidation processes, Water Sci. Technol., 2013, 68, 1976–1983 CrossRef CAS.
- Y. Han, M. Ma, N. Li, R. Hou, C. Huang, Y. Oda and Z. Wang, Chlorination, chloramination and ozonation of carbamazepine enhance cytotoxicity and genotoxicity: Multi-endpoint evaluation and identification of its genotoxic transformation products, J. Hazard. Mater., 2018, 342, 679–688 CrossRef CAS PubMed.
- C. Baresel, M. Harding and C. Junestedt, Removal of pharmaceutical residues from municipal wastewater using UV/H2O2; B report; B2354, IVL Swedish Environmental Research Institute, 2019 Search PubMed.
- B. J. Martijn, J. C. Kruithof and L. P. M. Rosenthal, in Proceedings of the Water Environment Federation, Water Environment Federation, 2007, pp. 134–153 Search PubMed.
- S. R. Sarathy, M. I. Stefan, A. Royce and M. Mohseni, Pilot-scale UV/H2O2 advanced oxidation process for surface water treatment and downstream biological treatment: effects on natural organic matter characteristics and DBP formation potential, Environ. Technol., 2011, 32, 1709–1718 CrossRef CAS.
- O. Lhotský, E. Krákorová, P. Mašín, R. Žebrák, L. Linhartová, Z. Křesinová, J. Kašlík, J. Steinová, T. Rødsand, A. Filipová, K. Petrů, K. Kroupová and T. Cajthaml, Pharmaceuticals, benzene, toluene and chlorobenzene removal from contaminated groundwater by combined UV/H2O2 photo-oxidation and aeration, Water Res., 2017, 120, 245–255 CrossRef PubMed.
- D. Rozman, Z. Hrkal, M. Váňa, J. Vymazal and Z. Boukalová, Occurrence of Pharmaceuticals in Wastewater and Their Interaction with Shallow Aquifers: A Case Study of Horní Beřkovice, Czech Republic, Water, 2017, 9, 218 CrossRef.
- K. Bircher, Calculating UV Dose for UV/AOP Reactors Using Dose/Log as a Water-Quality Metric, IUVA News, 2015, vol. 17, pp. 19–24 Search PubMed.
- A. Cibati, R. Gonzalez-Olmos, S. Rodriguez-Mozaz and G. Buttiglieri, Unravelling the performance of UV/H2O2 on the removal of pharmaceuticals in real industrial, hospital, grey and urban wastewaters, Chemosphere, 2022, 290, 133315 CrossRef CAS PubMed.
- J. Li, A. Zamyadi and R. Hofmann, Effect of granular activated carbon type and age on quenching H2O2 residuals after UV/H2O2 drinking water treatment, J. Water Supply: Res. Technol.--AQUA, 2016, 65, 28–36 Search PubMed.
- PubChem, https://pubchem.ncbi.nlm.nih.gov/, (accessed 6 April 2023).
- J. O'Brien, I. Wilson, T. Orton and F. Pognan, Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity, Eur. J. Biochem., 2000, 267, 5421–5426 CrossRef PubMed.
- K. Schirmer, A. G. J. Chan, B. M. Greenberg, D. G. Dixon and N. C. Bols, Methodology for demonstrating and measuring the photocytotoxicity of fluoranthene to fish cells in culture, Toxicol. In Vitro, 1997, 11, 107–113 CrossRef CAS.
- K. Tanneberger, M. Knöbel, F. J. M. Busser, T. L. Sinnige, J. L. M. Hermens and K. Schirmer, Predicting fish acute toxicity using a fish gill cell line-based toxicity assay, Environ. Sci. Technol., 2013, 47, 1110–1119 CrossRef CAS PubMed.
- R. J. Kieber and G. R. Helz, Two-Method Verification of Hydrogen Peroxide Determinations in Natural Waters, Anal. Chem., 1986, 58, 2312–2315 CrossRef CAS.
- A. Bahlmann, W. Brack, R. J. Schneider and M. Krauss, Carbamazepine and its metabolites in wastewater: Analytical pitfalls and occurrence in Germany and Portugal, Water Res., 2014, 57, 104–114 CrossRef CAS PubMed.
- D. P. Mohapatra, S. K. Brar, R. D. Tyagi, P. Picard and R. Y. Surampalli, Analysis and advanced oxidation treatment of a persistent pharmaceutical compound in wastewater and wastewater sludge-carbamazepine, Sci. Total Environ., 2014, 470–471, 58–75 CrossRef CAS.
- H. W. Yu, T. Anumol, M. Park, I. Pepper, J. Scheideler and S. A. Snyder, On-line sensor monitoring for chemical contaminant attenuation during UV/H2O2 advanced oxidation process, Water Res., 2015, 81, 250–260 CrossRef CAS PubMed.
- J. Molnar, J. Agbaba, A. Tubić, M. Watson, M. Kragulj, S. Rončević and B. Dalmacija, The effects of ultraviolet/H2O2 advanced oxidation on the content and characteristics of groundwater natural organic matter, Water Sci. Technol.: Water Supply, 2015, 15, 34–41 CAS.
- S. R. Sarathy and M. Mohseni, The impact of UV/H2O2 advanced oxidation on molecular size distribution of chromophoric natural organic matter, Environ. Sci. Technol., 2007, 41, 8315–8320 CrossRef CAS PubMed.
- P. Somathilake, J. A. Dominic, G. Achari, C. H. Langford and J. H. Tay, Influence of UV dose on the UV/H2O2 process for the degradation of carbamazepine in wastewater, Environ. Technol., 2018, 40, 3031–3039 CrossRef PubMed.
- P. Chowdhury, S. R. Sarathy, S. Das, J. Li, A. K. Ray and M. B. Ray, Direct UV photolysis of pharmaceutical compounds: Determination of pH-dependent quantum yield and full-scale performance, Chem. Eng. J., 2020, 380, 122460 CrossRef CAS.
- R. Andreozzi, L. Campanella, B. Fraysse, J. Garric, A. Gonnella, R. Lo Giudice, R. Marotta, G. Pinto and A. Pollio, Effects of advanced oxidation processes (AOPs) on the toxicity of a mixture of pharmaceuticals, Water Sci. Technol., 2004, 50, 23–28 CrossRef CAS.
- L. F. Angeles, R. A. Mullen, I. J. Huang, C. Wilson, W. Khunjar, H. I. Sirotkin, A. E. McElroy and D. S. Aga, Assessing pharmaceutical removal and reduction in toxicity provided by advanced wastewater treatment systems, Environ. Sci.: Water Res. Technol., 2020, 6, 62–77 RSC.
- P. Karl and E. Dolling, Weitergehende Abwasserreinigungmit der Aktivkoks-Festbett-Biologie undnachfolgender UV-Behandlungmit H2O2-Oxidation, KW--Korresp. Wasserwirtsch., 2018, 5, 398–412 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ew00258f |
| This journal is © The Royal Society of Chemistry 2024 |