Fabrication of Fe3O4 based cellulose acetate mixed matrix membranes for As(III) removal from wastewater
Sidra
Liaquat
 a,
Sarah
Farrukh
a,
Sarah
Farrukh
 *ab,
Nasir
Ahmad
a,
Syed Shujaat
Karim
*ab,
Nasir
Ahmad
a,
Syed Shujaat
Karim
 a,
Erum
Pervaiz
a,
Erum
Pervaiz
 a,
Ayesha
Sultan
a and
Subhan
Ali
a
a,
Ayesha
Sultan
a and
Subhan
Ali
a
aDepartment of Chemical Engineering, School of Chemical and Materials Engineering (SCME), National University of Sciences and Technology (NUST), Islamabad, Pakistan
bInstitute of Materials and Processes. School of Engineering, The University of Edinburgh, EH9 3FB, Scotland, UK
First published on 13th May 2024
Abstract
Water poisoning due to arsenic is getting worse worldwide because of its serious health hazards and carcinogenic nature. A productive method is required to remove it from water to protect the environment and human life. In this direction, iron oxide (Fe3O4)/cellulose acetate (CA)-based mixed matrix membranes (MMMs) were fabricated by varying the concentration of Fe3O4 nanoparticles from 0–2 wt% using the phase inversion method for efficient As(III) removal. The impact of Fe3O4 on the membranes' surface morphology and mechanical properties was analyzed through scanning electron microscopy (SEM) and ultimate tensile strength (UTS). Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) were performed for chemical functionalities and phase structure analysis. Atomic Adsorption Spectrophotometry (AAS) is used to detect the As(III) concentration in water samples. The As(III) adsorption experiments were performed at different concentrations with varying time intervals, and the coefficient of determination and sum of square error function were used to conduct the analysis. The results were best fitted into the Langmuir isotherm model (R2 > 0.99) with a maximum adsorption capacity of 90.3 mg g−1. The pseudo-second-order and Weber-Morris models were used to examine intra-particle diffusion as a rate-limiting step. According to membrane performance tests, the nanoparticles' addition increased the hydrophilicity and water flux, improving the membranes' permeability, wettability, and porosity. It was found that a 2 wt% loading of Fe3O4 nanoparticles in the MMM achieved a maximum percentage As(III) removal efficiency of 93%. This study shows that these membranes can efficiently remove As(III) from contaminated water because of their adsorption and filtration properties.
Water impactDue to the growing risk that arsenic poses to the environment, this research focused heavily on the most economical and practical approach for removing arsenic from wastewater ensuring availability and sustainable management of water. The fabricated membranes are generally good candidates for removing arsenic from wastewater and have potential for other heavy metal removal and water purification applications. |
1. Introduction
Every human needs water, and recent reports indicate that water usage is under significant stress, putting more pressure on the water treatment sector to keep up with the rising water demand. According to the 2023 Report on Sustainable Development Goals (SDGs), billions of people still suffer from recurring water shortages and accessibility issues in safe water for drinking purposes.1 According to the United Nations, 80% of wastewater enters rivers without being treated first.2 Water is a limited resource, and wastewater is a crucial resource for clean water supply. Industrial wastewater contains pollutants and heavy metals, posing threats to human health and aquatic life.3,4Arsenic (As) has been identified as the most dangerous heavy metal contaminating water bodies nowadays. Based on the oxidation potential and water pH, As is present in both organic and inorganic forms, which exist in natural and urban wastewater in various oxyanions, such as trivalent arsenite, As(III), and pentavalent arsenate, As(V). As(III) is considered much more poisonous and harder to remove in comparison to arsenate. In particular, long-term exposure to it significantly threatens human health, leading to chronic disorders, high blood pressure, skin problems, and cancer, even in small amounts.5 Groundwater comprises As(III) concentrations of 0.50 to 2.50 mg L−1, and industrial wastewater is reported to contain more than 100 mg L−1. Therefore, there is a need to meet the maximum contaminant level (MCL) of acceptable As(III) developed by the World Health Organization (WHO) and the US Environmental Agency Protection (US-EAP) for drinking water in the range of 50 to 10 μg L−1.6 Due to this strict standard of arsenic contaminant concentration, several conventional terminologies like adsorption, ion exchange, electrocoagulation, flocculation, chemical precipitation, electro-kinetic technique, phytoremediation, and photocatalysis processes are employed for its removal from water. Significant issues with these previous techniques included generating massive amounts of harmful sludge, using huge volumes of chemicals, a high operational area and cost, and long time durations.7
Membrane separation processes have gained significance over other classical methods over the years to overcome the shortcomings detailed above due to their distinct selectivity, simplicity, reusability, high removal efficiency, ease of scaling up, small footprints, and lower energy consumption. Polymeric and ceramic membranes are commonly utilized in industry, but researchers are currently considering the new hybrid or mixed matrix membranes (MMMs), which combine the features of both membranes.8,9 However, with the advent of MMMs, inserting the adsorbents inside the membrane bulk results in improved mechanical, thermal, magnetic, and electrostatic properties to remove the target contaminant.10–12 Mohan and Pittman identified different kinds of adsorbents for arsenic removal, such as metal oxides like iron oxides, activated alumina, magnesia, titania, hydrotalcites, and phosphates, which have been mainly used because of their cheapness, smaller size, green nature, recyclability, and specific adsorption capacities for arsenic species.13,14
Earlier literature explicitly declared that iron oxide-based nanoparticles were preferred because of their low cost, smaller size, and high reactivity. Various forms of iron oxide applied for As(III) removal are magnetite, maghemite, and hematite, but magnetite (Fe3O4) tested so far provides higher removal efficiencies, improved strength and stiffness, and the capability to build strong interactions with As(III).15–17 Feng et al. successfully utilized Fe3O4 magnetic nanoparticles for removing As(III) on account of their capability to build inner sphere complexes with As(III) because of their high adsorption capacity, excellent thermal stability, specific surface area and chemical resistance, antifouling property, suitable anti-bacterial property, and biodegradability.18,19 However, nanoparticles are unsuitable for adsorbents because their separation after treatment is a complicated process. Secondly, in flow through systems, more pressure drops and inter-particle forces will aggregate particles, causing a reduction in surface area.20,21 Thus, to overcome these deficiencies, nanoparticles are added to the polymer membranes for the practical usage of nanoparticles in wastewater remediation.
Many polymers have been explored and used further for membrane synthesis in water purification.22 Cellulose acetate (CA) is the most favoured polymer in membranes. It is preferred because of the formation of a hydrogen bond as it consists of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –OH groups, as well as for its ease of accessibility, low cost, higher hydrophilicity, adsorption property, non-toxic nature, and biocompatibility. Saranya et al. and Rajeswari et al. developed different adsorbents and nanoparticle-based CA MMMs for environmental amendments and heavy metal removals from textile industry effluents.23,24 Durthi and Rajulapati studied As(III) removal using CA-incorporated zinc oxide (ZnO) nanoparticle-based MMMs with 58.77% removal efficiency.25 Further research was made by Mithun Kumar using CA and its derivatives as additives in membranes with zirconium oxide (ZrO2) and binary zinc–magnesium oxide, with 70–87% and 78–81% As(III) removal from the contaminated water, respectively.26–28 For the polyethersulfone (PES) membrane, using graphene oxide-polyvinylpyrrolidone (GO-PVP) and nano zerovalent iron-kaolin (nZVI-Kaol) as nanocomposites for removing As(III) from synthetic wastewater results in an efficiency of 88.6 and 50%.29,30 Another study observed 50% removal of As(III) using Fe2O3 in a polyvinylidene fluoride (PVDF) polymer matrix.31 There is a need to innovate a new membrane for high As(III) removal efficiency. This research deals with the influence of Fe3O4 in the CA membrane and its affinity towards As(III) removal from wastewater.
O and –OH groups, as well as for its ease of accessibility, low cost, higher hydrophilicity, adsorption property, non-toxic nature, and biocompatibility. Saranya et al. and Rajeswari et al. developed different adsorbents and nanoparticle-based CA MMMs for environmental amendments and heavy metal removals from textile industry effluents.23,24 Durthi and Rajulapati studied As(III) removal using CA-incorporated zinc oxide (ZnO) nanoparticle-based MMMs with 58.77% removal efficiency.25 Further research was made by Mithun Kumar using CA and its derivatives as additives in membranes with zirconium oxide (ZrO2) and binary zinc–magnesium oxide, with 70–87% and 78–81% As(III) removal from the contaminated water, respectively.26–28 For the polyethersulfone (PES) membrane, using graphene oxide-polyvinylpyrrolidone (GO-PVP) and nano zerovalent iron-kaolin (nZVI-Kaol) as nanocomposites for removing As(III) from synthetic wastewater results in an efficiency of 88.6 and 50%.29,30 Another study observed 50% removal of As(III) using Fe2O3 in a polyvinylidene fluoride (PVDF) polymer matrix.31 There is a need to innovate a new membrane for high As(III) removal efficiency. This research deals with the influence of Fe3O4 in the CA membrane and its affinity towards As(III) removal from wastewater.
The impregnation of Fe3O4 as a potential As(III) nanoadsorbent in the CA polymer matrix improves the adsorptive properties and the membrane's selectivity, permeability, hydrophilicity, porosity, and removal efficiency. According to our study, no prior research has been reported regarding the fabrication of Fe3O4/CA MMMs, batch adsorption experiments for non-linear adsorption kinetic models and isotherms utilizing a solver add-in of Microsoft Excel, and filtration experiments for removal efficiency of As(III) in the previous literature. These membranes offer potential for both As(III) removal and high water flux, simultaneously paving the way for new practical applications. The objectives of this work are (i) fabrication of Fe3O4/CA MMMs using the phase inversion method; (ii) characterization of Fe3O4 and MMMs by several techniques like SEM, FTIR, XRD, and UTS; (iii) testing the membrane's performance in terms of water flux, permeability, wettability, and porosity; (iv) non-linear analysis of adsorption kinetics and isotherms to find out the adsorption mechanism and best fit model; (v) filtration experiments performed to calculate As(III) removal efficiency. As far as we know, Fe3O4-incorporated CA membranes were never fabricated before and used for removing As(III) from contaminated water.
2. Materials and methods
2.1. Materials
Cellulose acetate (CA, Mw = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), tetrahydrofuran (THF, purity ≥ 99.9%), iron oxide nanoparticles (Fe3O4, size = 50–100 nm), arsenic trioxide (As2O3, Mw = 197.84 g.mol−1) and deionized water (DI water) were bought from Sigma Aldrich, USA. Paradise Gases Private Ltd., Pakistan, provided lab scale nitrogen (N2 gas, purity ≥ 99.95%). There is no need for any additional purification technique, and all chemical reagents purchased are utilized directly.
000 g mol−1), tetrahydrofuran (THF, purity ≥ 99.9%), iron oxide nanoparticles (Fe3O4, size = 50–100 nm), arsenic trioxide (As2O3, Mw = 197.84 g.mol−1) and deionized water (DI water) were bought from Sigma Aldrich, USA. Paradise Gases Private Ltd., Pakistan, provided lab scale nitrogen (N2 gas, purity ≥ 99.95%). There is no need for any additional purification technique, and all chemical reagents purchased are utilized directly.
2.2. Membrane preparation
The Fe3O4 nanoparticles embedded in CA MMMs were synthesized using the phase inversion method.32 The concentration of Fe3O4 nanoparticles varied by 0–2 wt% in the membrane's casting solution. The names and compositions of fabricated membranes are provided in Table 1.| Membrane name | CA (wt%) | THF (wt%) | Fe3O4 (wt%) |
|---|---|---|---|
| M0 | 10 | 90 | 0 |
| M0.5 | 10 | 89.5 | 0.5 |
| M1 | 10 | 89 | 1 |
| M1.5 | 10 | 88.5 | 1.5 |
| M2 | 10 | 88 | 2 |
1 g CA was added into 7 mL THF under constant stirring conditions at 350 rpm overnight at an ambient temperature. Due to the magnetic nature of Fe3O4, it was impossible to use a magnetic stirrer. Therefore, the nanoparticles were dispersed in the remaining amount of solvent with the help of ultra-sonication for 30 min and then mixed with the already prepared solution. This solution is again placed on a magnetic stirrer until a considerable diffusion of nanoparticles occurs. After that, the mixture was ultra-sonicated for 45 min and degassed. The resultant homogenous mixture is then cast on a glass plate and, after a 30 s delay, immersed for 24 h into a non-solvent DI water coagulation bath. Membrane preparations were made at room temperature and kept in DI water before use. The synthesized membranes were cut to the required size for filtration experiments.
3. Characterization
3.1. Particle characterization
The shape, size, structure and uniform distribution of metal elements of Fe3O4 nanoparticles were observed by high resolution SEM. The XRD pattern was obtained on a diffraction system with Cu Kα radiation at a 2θ angle varying from 0 to 80° for the phase characterization. In order to verify the existence of different kinds of functional groups, the FTIR spectrum was collected.3.2. Membrane characterization
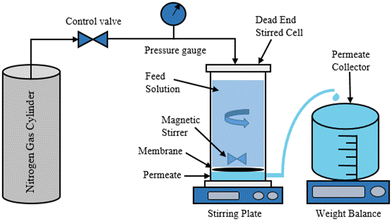
3.3. Membrane performance tests
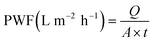 | (1) |
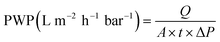 | (2) |
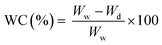 | (3) |
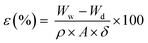 | (4) |
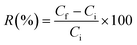 | (5) |
3.3.8.1. Adsorption kinetics. In the batch adsorption experiment, a volume of 100 mL solution containing As(III) was used to dissolve the membranes with a weight quantity of 0.10 g. After that, the mixture was shaken for 560 min at 300 rpm in a rotary shaker because the membrane required more time to adsorb before reaching equilibrium. Using AAS, an aliquot (5 mL) was examined at various time intervals to determine the arsenic content. The experimental data was applied to the pseudo-first-order (PFO) and pseudo-second-order (PSO) kinetic models to determine the adsorption kinetics. The adsorption study using the non-linear type model of PFO is shown in eqn (6):
| Qt = Qe[1 − exp(−K1t)] | (6) |
The nonlinear form of the PSO model is described as eqn (7):
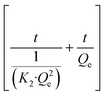 | (7) |
The kinetic model is also depicted by Web–Morris which is used to determine the rate-controlling step. According to this model, if a straight line passes through the origin after plotting the graph, intraparticle diffusion is the rate-limiting step; otherwise film diffusion is considered a rate-rate-limited step of adsorption kinetics.47 The intraparticle diffusion is given by eqn (8):
| qt = kpt1/2 + C | (8) |
3.3.8.2. Adsorption isotherms. To analyze the adsorption isotherms, 100 mg/100 mL of all fabricated membranes was used to adsorb the initial concentration of As(III) solution (0–100 mg L−1) at room temperature. With the help of 0.1 M HCL/NaOH, the pH level of the solutions was brought to 7.0 ± 0.1. The membrane samples were submerged in that solution and continuously agitated with a rotatory shaker at 300 rpm. The membranes were removed from the solution after 560 min, and AAS was used to determine As(III)'s equilibrium concentration. The experimental adsorption data were fitted into Freundlich and Langmuir isotherm models to determine each membrane's ability to bind As(III).
The relationship between the initial concentration Co and the equilibrium concentration Ce of As(III) can be visualized using a mathematical isotherm model. Eqn (9)49 was used to calculate Qe.
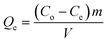 | (9) |
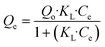 | (10) |
The Freundlich isotherm in non-linear form is given by eqn (11):
| Qe = KF·C1/ne | (11) |
4. Results and discussion
4.1. Analysis of Fe3O4 nanoparticles
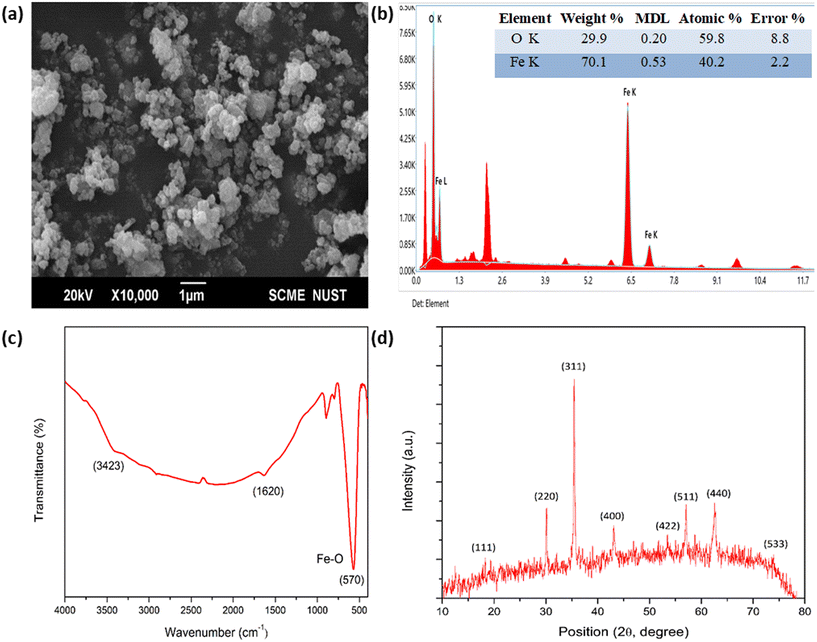
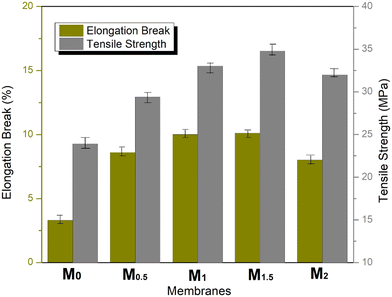
Fig. 2a depicts the characterization results of Fe3O4 nanoparticles in which the SEM image is at ×10000 and 20 kV voltage magnification. The highly agglomerative form may be due to nanoparticles' highly reactive nature and their particle size varying from 50–100 nm. Elemental analysis shows atomic % of Fe as 40.2 and O as 59.8, respectively (Fig. 2b). In the FTIR spectrum (Fig. 2c), the characteristic peak at 570 cm−1 is because of the metal oxide Fe–O functional group and the broad bands aside this correlate with the stretching vibrations of Fe–O bonds, specifically those of the crystalline lattice of Fe3O4 nanoparticles. The present peaks at 3423 cm−1 and 1620 cm−1 are because of the water molecule lattice.51The XRD spectrum of Fe3O4 (Fig. 2d) shows that the reflection peaks at 2θ equal to 18.29, 30.24, 35.64, 43.38, 53.84, 57.52, 63.02, and 74.53 are attributed to diffraction from the (111), (220), (311), (400), (422), (511), (440), and (533) planes of Fe3O4 cubic inverse spinel, respectively. These characteristic peaks best correspond with the standard pattern of Fe3O4 (JCPDS 19-629). The absence of any additional peak represents the purity of nanoparticles.51,52
4.2. Membrane characterization
The viscosity of the casting dope solution increased with an increase in the composition of Fe3O4 nanoparticles. They tend to agglomerate on the membrane surface because of the delayed demixing rate. This phenomenon is observed on the top surface of M2 (2 wt% Fe3O4 nanoparticles), where nanoparticles aggregate because of poor interactions and improper distribution in the polymer solution, causing the breakage of polymer chains.54 The cross-sectional images consist of the top skin layer, which is in accordance with the spinodal decomposition mechanism that ensures selectivity and rejection. The middle layer is responsible for productivity, and the bottom layer provides mechanical support to the MMMs.55 The creation of a large number of pores in the middle layer can be associated with the increase in the contents of hydrophilic nanoparticles that accelerate the de-mixing rate of the polymer solution with the non-solvent during phase separation.56
The presence of macro-voids indicates faster solvent and non-solvent exchange during the phase inversion process, thus improving the overall membrane porosity.57 With excessive loading of nanoparticles, the casting solution becomes more viscous and thermodynamically unstable, forming thicker membranes from M0 to M2. The bottom layer is smooth and dense, with no pores.58
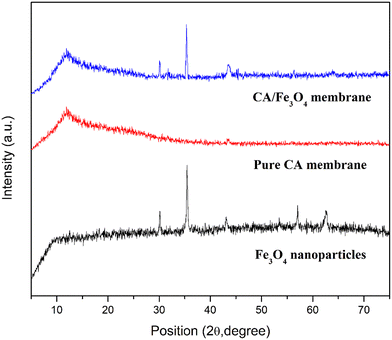
The XRD spectrum of the pure CA membrane shows a sharp peak at 2θ = 12° that corresponds to the (001) plane, showing the semi-crystalline nature of the polymer. CA has low crystallinity because of its significant intermolecular attraction to hydrogen bonding between acetyl and hydroxyl groups.33 However, the Fe3O4/CA mixed matrix membrane shows the signature peaks at 2θ = 12°, 30.24°, 35.64°, and 43.38°, which is in accordance with the CA polymer and the Fe3O4 nanoparticle characteristic peaks. It ensures that Fe3O4 is inserted into the CA polymer matrix.59
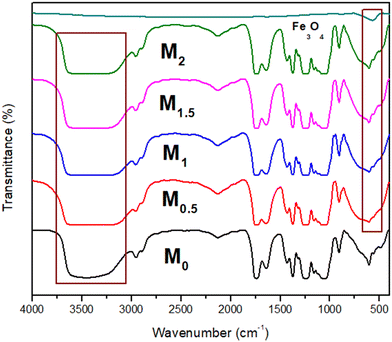
In the case of Fe3O4 nanoparticles, the decisive peak was at 570 cm−1 assigned to the Fe–O group. In pure membrane M0, the broadband at 3600–3200 cm−1 is the characteristic of O–H stretching. The 1753 cm−1 band was attributed to the functional group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of CA. Furthermore, peaks at 1251, 1373, 2128, and 2940 cm−1 were assigned to the C–O (stretching), CH3 (symmetric deformation), carbonyl C
O of CA. Furthermore, peaks at 1251, 1373, 2128, and 2940 cm−1 were assigned to the C–O (stretching), CH3 (symmetric deformation), carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, and CH3 (asymmetric stretching). In all other membranes M0.5, M1, M1.5, and M2, there is no additional peak present in membranes.60,61
O stretching vibrations, and CH3 (asymmetric stretching). In all other membranes M0.5, M1, M1.5, and M2, there is no additional peak present in membranes.60,61
4.3. Membrane performance
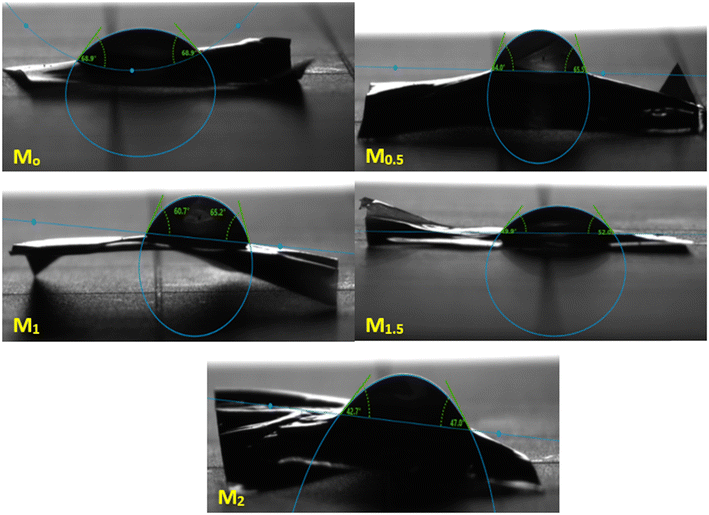
| Membranes | Water content (%) | Porosity (%) | Contact angle (°) |
|---|---|---|---|
| M0 | 44 | 66 | 68.9 ± 0 |
| M0.5 | 49 | 68 | 65.5 ± 2 |
| M1 | 55 | 71 | 60.7 ± 4 |
| M1.5 | 61 | 73 | 52 ± 3 |
| M2 | 68 | 74 | 47 ± 4 |
4.3.6.1. Adsorption kinetics. The kinetic investigation is an important parameter to find out the impact of As(III) adsorption on the membranes. The adsorption kinetic curves for each Fe3O4/CA membrane are drawn in Fig. 10. The results demonstrated that in the first 120 min, there was a quick uptake of As(III), which was then followed by a slower adsorption phase and equilibrium was reached within 560 min. The kinetic study parameters and the experimentally calculated adsorption capacity are tabulated in Table 3.
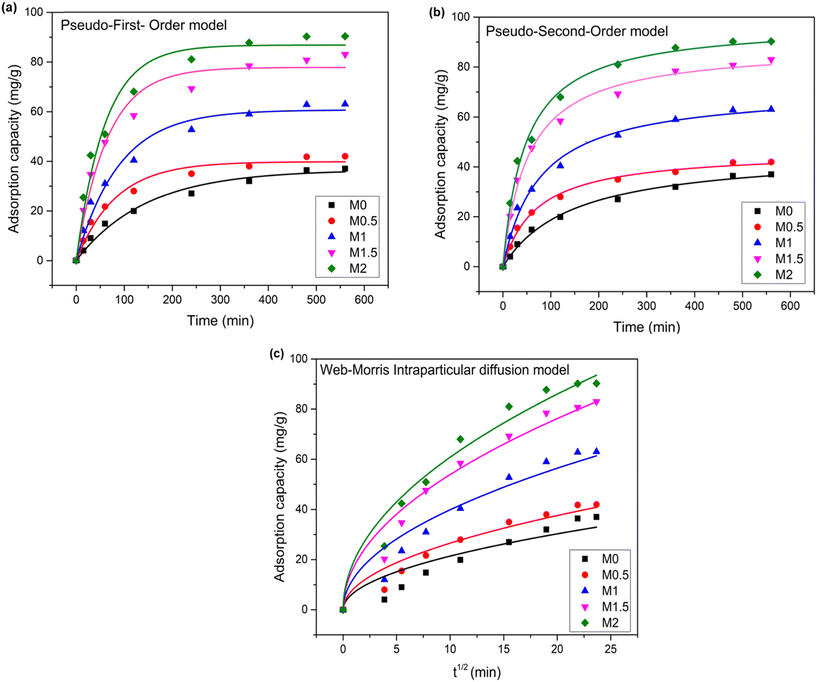 | ||
| Fig. 10 Adsorption kinetic models: (a) pseudo-first-order (b), pseudo-second-order and (c) Web–Morris intraparticle diffusion plots of As(III) removal from Fe3O4/CA membranes. | ||
| Membrane | Adsorption capacity at equilibrium | First-order model | Second-order model | Intraparticle diffusion model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q e,exp | Q e1 | K 1 | R 2 | Q e2 | K 2 | R 2 | K 1 | C | R 2 | |
| mg g−1 | mg g−1 | min−1 | mg g−1 | g mg−1 min−1 | mg g−1 min1/2 | |||||
| M0 | 37 | 36.4 | 0.006 | 0.98 | 46.0 | 0.0001 | 0.99 | 6.77 | 9.98 | 0.85 |
| M0.5 | 42 | 39.8 | 0.012 | 0.97 | 46.4 | 0.0003 | 0.99 | 8.40 | 9.98 | 0.93 |
| M1 | 63 | 60.65 | 0.011 | 0.97 | 71.5 | 0.0001 | 0.99 | 12.6 | 9.98 | 0.93 |
| M1.5 | 83 | 77.7 | 0.015 | 0.96 | 88.5 | 0.0002 | 0.99 | 17.0 | 1.80 | 0.96 |
| M2 | 90.3 | 86.7 | 0.016 | 0.97 | 97.5 | 0.0002 | 0.99 | 19.2 | 1.55 | 0.96 |
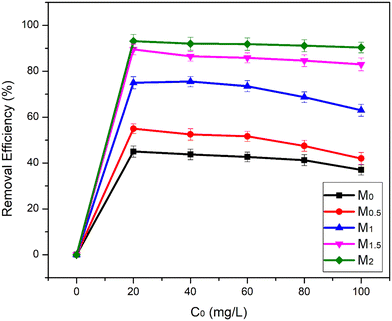
The result depicts that the experimental value is a little bit closer to the PFO, but the kinetic model is best fit to the PSO kinetic model, as supported by the high regression coefficient (R2 > 0.99) value because R2 is one of the significant factors in determining the kinetic model. The Fe3O4/CA membrane was thought to chemically adsorb As(III) species, according to the PSO kinetic model. It is recorded that as the amount of Fe3O4 increases from 0, 0.5, 1, and 1.5 to 2 wt%, the adsorption capacity values increase from 37, 42, 63, 83, and 90.3 mg g−1.
Fig. 10 demonstrates the deviation of the curve from the origin, which indicates that intraparticle diffusion is not only the rate-limiting step; but in this film, diffusion is also involved.64 The high value of the intercept is directly proportional to the boundary layer effect and suggests that surface adsorption is a rate-determining step. In the present study, initially, the intercept value is very high, which indicates the presence of external mass transfer resistance in the thick boundary layer, and when the dosage of Fe3O4 nanoparticles increases (M1.5 and M2), the intercept value is low, which signifies a minimum resistance to mass transfer and enhances the adsorption rate.65,66 The result also depicts that the increase in the dosage value of Fe3O4 nanoparticles and the rate constant of intra-diffusion increase indicates a faster rate of diffusion, and a high value (19.22) is obtained at membrane M2, which shows that adsorbate molecules readily move through the pores, which causes a faster adsorption process. The present study also shows that the rate constant value decreases when the boundary layer effect increases.67
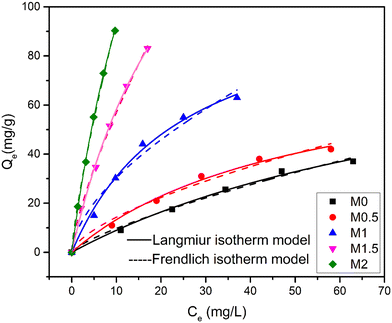
4.3.6.2. Adsorption isotherms. As(III) adsorption isotherm experiments on Fe3O4/CA membranes were done at various concentrations of As(III) (0, 20, 40, 60, 80, 100 mg L−1) at different intervals of time (0, 15, 30, 60, 120, 240, 360, 480, 560 min). As indicated in Fig. 11, the results of the experiment were both fitted into the Freundlich and Langmuir isotherm models, and Table 4 lists the adsorption isotherm model parameters. The findings revealed that all the membranes' adsorption capacities increased along with the As(III) initial concentration. In comparison to the Freundlich isotherm model, the values of the experiments were successfully fitted into the Langmuir isotherm model based on the regression coefficient.
| Isotherm model | Parameters | Membranes | ||||
|---|---|---|---|---|---|---|
| M0 | M0.5 | M1 | M1.5 | M2 | ||
| Langmuir | Q o | 97.65 | 81.49 | 108.01 | 210.48 | 279.44 |
| K L | 0.010 | 0.01952 | 0.0399 | 0.038 | 0.0494 | |
| R 2 | 0.994 | 0.992 | 0.992 | 0.999 | 0.999 | |
| Freundlich | N | 1.7308 | 3.26 | 0.596 | 0.736 | 0.7907 |
| K F | 0.749 | 0.6419 | 7.68 | 10.42 | 15.19 | |
| R 2 | 0.975 | 0.95381 | 0.946 | 0.997 | 0.996 | |
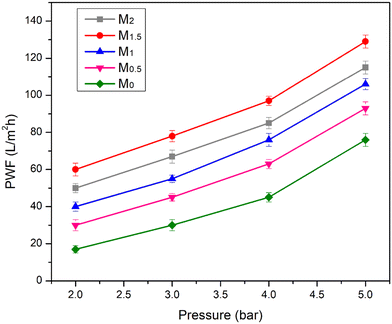
Theoretically, As(III) adsorption was assumed to be on the single layer of the Fe3O4/CA membranes rather than the multi-layer when the results of the experiments of adsorption isotherms fitted the Langmuir model. M2 performed better than the other membranes in an adsorption capacity comparison using an adsorption kinetic and isotherm analysis. The pure water flux and permeability values reduced as the Fe3O4 contents increased from 1.5 to 2.0 wt%, which could be caused by the agglomeration of excess Fe3O4 nanoparticle loading within the membrane, but this contributed to increased adsorption capacity because of the availability of more adsorption sites, as seen from the SEM image.
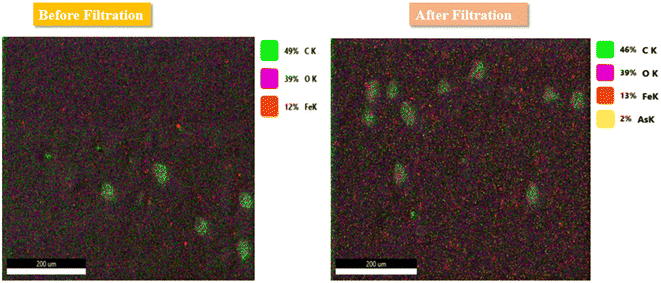
As(III) removal through membranes involves two main mechanisms: adsorption and size exclusion. Whenever the As(III)-polluted water diffuses into the membrane's cross section, Fe3O4 nanoparticles can effectively adsorb arsenic via inner sphere complexation and produce an arsenic-free permeate.68,69Fig. 13 revealed SEM–EDX mapping of the membrane done before and after the filtration experiment.
Thus, As(III) is adsorbed on the surface of Fe3O4 by forming As–Fe complexes through a ligand–exchange mechanism. These interactions occur due to the electropositive charge on the As metal ion that acts as an electron acceptor and the electronegative charge on the oxygen atoms attached to Fe3O4 having a lone pair of electrons acting as an electron donor.70 The As(III) removal percentage increased from 37% to 93%, with the percentage increase in Fe3O4 nanoparticles from 0–2 wt%. These values are associated with the more available active sites for the adsorbent Fe3O4 nanoparticles distributed on the membrane bulk, which provides a large surface area for incoming As(III) ions. In the case of Fe3O4, they have hydroxyl groups that when exposed to water bind or release H+ ions, which causes adsorptive removal behaviour because of the OH2+, OH−, and O− functional groups. As(III) exhibits both metallic (As) and ligand (O) characteristics for binding to Fe3O4. This is the reason why As(III) and Fe3O4 will form a tight internal spherical complex.71Table 5 shows all the fabricated adsorptive membranes that have been used for As(III) adsorption. These membranes have varying concentration of nanoparticles and showed As(III) removal efficiencies from 50 to 96.9%.29–31,36,49,72–75 In the case of adsorption capacity, it can be concluded that the M2 membrane, among all the other fabricated membranes, shows the highest As(III) adsorption capacity in wastewater.
| Membranes | Nanoparticles (wt%) | As(III) feed concentration (mg L−1) | Adsorption isotherm model | As(III) adsorption capacity (mg g−1) | As(III) removal efficiency (%) | References |
|---|---|---|---|---|---|---|
| PVDF/zirconia | 20.0 | 100 | Langmuir | 21.50 | 96.9 | 72 |
| PES/Fe-Mn binary oxide | 22.5 | 100 | Langmuir | 73.50 | 87.5 | 73 |
| PSF/iron ore slimes | 10.0 | 100 | Freundlich | 7.50 | 90 | 74 |
| PSF/HINM | 1.5 | 100 | Freundlich | 41.09 | 90.8 | 36 |
| CA/MnO2 | 1.5 | 100 | Langmuir | 50.34 | — | 49 |
| PVDF/TiO2-HNT | 0.5 | 0.25 | Langmuir | 0.18 | 94 | 75 |
| PES/GO-PVP | 0.5 | 100 | Langmuir | 88.6 | 88.6 | 29 |
| PES/nZVI-Kaol | 0.75 | 75 | — | 50 | 50 | 30 |
| PVDF/Fe2O3 | 15 | 0.25 | — | 23 | 50 | 31 |
| CA/Fe3O4 | 2.0 | 100 | Langmuir | 90.3 | 93 | This study |
5. Conclusion
In this research work, Fe3O4/CA MMMs were fabricated by varying the amount of Fe3O4 nanoparticles from 0 to 2 wt% using a phase inversion process and investigated for As(III) removal from contaminated water. The fabricated membranes were characterized by several techniques, like SEM, FTIR, XRD, and UTS, for membrane surface morphology, functional group detection, phase structure, and mechanical strength. The incorporation of Fe3O4 nanoparticles endows the polymeric membranes with enhanced hydrophilicity, optimized porosity, improved water flux and permeation in comparison to the pure CA membrane. The adsorption experiments showed that at 100 mg L−1 As(III) concentration with 0.1 g of M2 at 560 min, a maximum adsorption capacity of 90.3 mg g−1 was attained. This system confirms the monolayer adsorption of As(III) on the Fe3O4/CA membrane, as depicted by non-linear analysis. The data was best fitted into the Langmuir adsorption isotherm and the Weber–Morris intraparticle diffusion model following pseudo-second-order kinetics. These membranes efficiently removed As(III) from water with better removal efficiency, comparable with the literature. It was observed that among all the membranes, the membrane with 2 wt% Fe3O4 nanoparticles exhibited the highest As(III) removal efficiency of 93%. In short, the addition of Fe3O4 nanoparticles can be a simple modification strategy that enhances the membrane's performance parameters with promising potential and can successfully treat As(III) contaminated water as defined by the WHO and USEPA to fulfill the MCL, i.e. 0.01 mg L−1.Abbreviations
| CA | Cellulose acetate |
| THF | Tetrahydrofuran |
| MMMs | Mixed Matrix Membranes |
| As | Arsenic |
| As(III) | Arsenite |
| As(V) | Arsenate |
| As2O3 | Arsenic trioxide |
| N2 | Nitrogen |
| HCL | Hydrochloric acid |
| NaOH | Sodium hydroxide |
| DI Water | De-ionized water |
| Fe | Iron |
| C | Carbon |
| O | Oxygen |
| Cu | Copper |
| Fe3O4 | Iron oxide |
| ZnO | Zinc oxide |
| ZrO2 | Zirconium oxide |
| TiO2 | Titanium oxide |
| MnO2 | Manganese oxide |
| GO | Graphene oxide |
| nZVI | nano Zero-Valent Iron |
| PSF | Polysulfone |
| PES | Polyethersulfone |
| PVP | Polyvinylpyrrolidone |
| PVDF | Polyvinylidene fluoride |
| pH | Potential of hydrogen |
| OH | Hydroxyl group |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O | Carbonyl group |
| CH3 | Methyl group |
| WHO | World Health Organization |
| US-EPA | Environmental Agency Protection |
| SDGs | Sustainable Development Goals |
| SEM | Scanning Electron Microscopy |
| EDX | Energy Dispersive X-ray |
| FTIR | Fourier Transform Infrared Spectroscopy |
| XRD | X-ray Diffraction |
| UTS | Ultimate Tensile Strength |
| AAS | Atomic Absorption Spectroscopy |
| PWF | Pure Water Flux |
| PWP | Pure Water Permeability |
| PFO | Pseudo-First-Order |
| PSO | Pseudo-Second-Order |
| MCL | Maximum contaminant level |
| % | Percentage |
| Fig | Figure |
| Eq. | Equation |
| g | Gram |
| μg | Microgram |
| mg | Milligram |
| mA | Milliampere |
| mm | Millimeter |
| cm | Centimeter |
| L | Liter |
| m | Meter |
| nm | Nano Meter |
| kV | Kilovolt |
| ° | Degree |
| kW | Kilowatt |
| s | Second |
| min | Minute |
| h | Hour |
| kN | Kilonewton |
| wt | Weight |
| °C | Degree Celsius |
| Δ | Differential |
| Q e | Adsorption capacity at equilibrium (mg g−1) |
| Q t | Relevant contact time (mg g−1) |
| K 1 | Rate constant of Pseudo-First-Order (PFO) (min−1) |
| Q o | Constant associated with membrane's maximum adsorption capacity (mg g−1) |
| K L | Adsorption constant (L mg−1) |
| K F | Adsorption capability (L mg−1) |
| n | Adsorbent degree of saturation constant |
| K 2 | Pseudo-Second-Order rate constant (g (mg−1 min−1)) |
| W w | Wet weight (g) |
| W d | Dry weight of the membrane (g) |
| Q | Permeate's volume (L) |
| T | Time taken for collection of permeate (h) |
| A | Membrane's active area (m2) |
| ΔP | Difference of pressure (bar) |
| K p | Intra-particle diffusion rate constant (mg g−1 min1/2) |
| C | Constant |
Author contributions
Sidra Liaquat (investigation, acquisition of data, writing original draft), Sarrah Farrukh (project administration, supervision), Nasir Ahmad (resources analysis tool), Syed Shujaat Karim (review and editing), Erum Pervaiz (formal analysis), Ayesha Sultan and Subhan Ali (visualization).Conflicts of interest
There are no conflicts to declare.Acknowledgements
In order to perform this research, we gratefully acknowledge the fastest-growing Membranes for Applied Research (MEMAR) lab at the School of Chemical and Materials Engineering (SCME), National University of Sciences and Technology (NUST), Islamabad, Pakistan.References
-
U. Water, Sustainable Development Goal 6 synthesis report on water and sanitation, Published by the United Nations New York, New York, 2018, vol. 10017 Search PubMed
.
- W. H. Organization, 1 in 3 people globally do not have access to safe drinking water, UNICEF, WHO, Available from: https://www.who.int/news/item/18-06-2019-1-in-3-people-globally-do-not-have-access-to-safe-drinking-water-unicef-who Search PubMed.
- A. Azimi, A. Azari, M. Rezakazemi and M. Ansarpour, Removal of heavy metals from industrial wastewaters: a review, ChemBioEng Rev., 2017, 4, 37–59 CrossRef
.
- L. Joseph, B.-M. Jun, J. R. Flora, C. M. Park and Y. Yoon, Removal of heavy metals from water sources in the developing world using low-cost materials: A review, Chemosphere, 2019, 229, 142–159 CrossRef CAS PubMed
.
- W. Altowayti, N. Othman, S. Shahir, A. Alshalif, A. Al-Gheethi, F. Al-Towayti, Z. Saleh and S. Haris, Removal of arsenic from wastewater by using different technologies and adsorbents: a review, Int. J. Environ. Sci. Technol., 2021, 1–24 Search PubMed
.
- W. H. Organization, A global overview of national regulations and standards for drinking-water quality, 2021.
- S. Alka, S. Shahir, N. Ibrahim, M. J. Ndejiko, D.-V. N. Vo and F. Abd Manan, Arsenic removal technologies and future trends: a mini review, J. Cleaner Prod., 2021, 278, 123805 CrossRef CAS
.
- D. Qadir, H. Mukhtar and L. K. Keong, Mixed matrix membranes for water purification applications, Sep. Purif. Rev., 2017, 46, 62–80 CrossRef
.
- I. Salahshoori, A. Seyfaee and A. Babapoor, Recent advances in synthesis and applications of mixed matrix membranes, Synthesis and Sintering, 2021, 1, 1–27 CrossRef
.
- T. Siddique, N. K. Dutta and N. R. Choudhury, Mixed-matrix membrane fabrication for water treatment, Membranes, 2021, 11, 557 CrossRef CAS PubMed
.
- M.-C. Shih, An overview of arsenic removal by pressure-drivenmembrane processes, Desalination, 2005, 172, 85–97 CrossRef CAS
.
- C. M. Nguyen, S. Bang, J. Cho and K.-W. Kim, Performance and mechanism of arsenic removal from water by a nanofiltration membrane, Desalination, 2009, 245, 82–94 CrossRef CAS
.
- Y. J. Lim, S. M. Lee, R. Wang and J. Lee, Emerging materials to prepare mixed matrix membranes for pollutant removal in water, Membranes, 2021, 11, 508 CrossRef CAS PubMed
.
- D. Mohan and C. U. Pittman Jr, Arsenic removal from water/wastewater using adsorbents—a critical review, J. Hazard. Mater., 2007, 142, 1–53 CrossRef CAS PubMed
.
- A. Chiavola, E. Amato, M. Stoller, A. Chianese and M. Boni, Application of iron based nanoparticles as adsorbents for arsenic removal from water, Chem. Eng. Trans., 2016, 47, 325–330 Search PubMed
.
- P. Sabbatini, F. Rossi, G. Thern, A. Marajofsky and M. F. de Cortalezzi, Iron oxide adsorbers for arsenic removal: a low cost treatment for rural areas and mobile applications, Desalination, 2009, 248, 184–192 CrossRef CAS
.
- S. I. Siddiqui and S. A. Chaudhry, Iron oxide and its modified forms as an adsorbent for arsenic removal: a comprehensive recent advancement, Process Saf. Environ. Prot., 2017, 111, 592–626 CrossRef CAS
.
- S. Lunge, S. Singh and A. Sinha, Magnetic iron oxide (Fe3O4) nanoparticles from tea waste for arsenic removal, J. Magn. Magn. Mater., 2014, 356, 21–31 CrossRef CAS
.
- L. Feng, M. Cao, X. Ma, Y. Zhu and C. Hu, Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal, J. Hazard. Mater., 2012, 217, 439–446 CrossRef PubMed
.
- X. Yu, S. Tong, M. Ge, J. Zuo, C. Cao and W. Song, One-step synthesis of magnetic composites of cellulose@ iron oxide nanoparticles for arsenic removal, J. Mater. Chem. A, 2013, 1, 959–965 RSC
.
- X. Zhang, Y. Wang, X. Chang, P. Wang and B. Pan, Iron oxide nanoparticles confined in mesoporous silicates for arsenic sequestration: effect of the host pore structure, Environ. Sci.: Nano, 2017, 4, 679–688 RSC
.
- A. Rabajczyk, M. Zielecka, K. Cygańczuk, Ł. Pastuszka and L. Jurecki, Nanometals-containing polymeric membranes for purification processes, Materials, 2021, 14, 513 CrossRef CAS PubMed
.
- A. Rajeswari, E. J. S. Christy, G. I. C. Mary, K. Jayaraj and A. Pius, Cellulose acetate based biopolymeric mixed matrix membranes with various nanoparticles for environmental remediation-A comparative study, J. Environ. Chem. Eng., 2019, 7, 103278 CrossRef CAS
.
- R. Saranya, Y. L. Thuyavan and G. Arthanareeswaran, Development of Adsorbents-based Cellulose Acetate Mixed Matrix Membranes for Removal of Pollutants from Textile Industry Effluent, J. Teknol. Lab., 2014, 70, 1–5 Search PubMed
.
- C. P. Durthi, S. B. Rajulapati, A. A. Palliparambi, K. K. Anand and S. H. Sonawane, Studies on removal of arsenic using cellulose acetate–zinc oxide nanoparticle mixed matrix membrane, Int. Nano Lett., 2018, 8, 201–211 CrossRef
.
- M. Kumar, S. RaoT, A. M. Isloor, G. S. Ibrahim, N. Ismail, A. F. Ismail and A. M. Asiri, Use of cellulose acetate/polyphenylsulfone derivatives to fabricate ultrafiltration hollow fiber membranes for the removal of arsenic from drinking water, Int. J. Biol. Macromol., 2019, 129, 715–727 CrossRef CAS PubMed
.
- M. Kumar, A. M. Isloor, T. S. Rao, A. F. Ismail, R. Farnood and P. Nambissan, Removal of toxic arsenic from aqueous media using polyphenylsulfone/cellulose acetate hollow fiber membranes containing zirconium oxide, Chem. Eng. J., 2020, 393, 124367 CrossRef CAS
.
- M. Kumar, A. M. Isloor, S. R. Todeti, H. Nagaraja, A. F. Ismail and R. Susanti, Effect of binary zinc-magnesium oxides on polyphenylsulfone/cellulose acetate derivatives hollow fiber membranes for the decontamination of arsenic from drinking water, Chem. Eng. J., 2021, 405, 126809 CrossRef CAS
.
- N. R. N. Abdul Ghani, S. S. Sulaiman, A. Tahreen and M. S. Jami, Polyether Sulfone-Graphene Oxide-Polyvinyl Pyrrolidone Nanocomposite Adsorptive Membrane for Arsenic Removal from Wastewater, J. Water Environ. Nanotechnol., 2021, 6, 121–137 Search PubMed
.
- B. K. Selvan, K. Thiyagarajan, S. Das, N. Jaya, S. A. Jabasingh, P. Saravanan, M. Rajasimman and Y. Vasseghian, Synthesis and characterization of nano zerovalent iron-kaolin clay (nZVI-Kaol) composite polyethersulfone (PES) membrane for the efficacious As2O3 removal from potable water samples, Chemosphere, 2022, 288, 132405 CrossRef CAS PubMed
.
- Y.-A. Boussouga, F. Tantish and A. I. Schäfer, Microporous Hematite-Loaded Composite Membrane for Arsenic(III) and Arsenic(V) Removal, ACS Appl. Eng. Mater., 2023, 1, 1164–1175 CrossRef CAS
.
-
L. Rozelle, J. Cadotte, R. Corneliussen, E. Erickson, K. Cobian and C. Kopp Jr, Phase inversion membranes, Encyclopedia of Separation Science, ed. M. Mulder, Academic Press, Cambridge, MA, USA, 2000, pp. 3331–3346 Search PubMed
.
- R. Abedini, S. M. Mousavi and R. Aminzadeh, A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: preparation, characterization and permeation study, Desalination, 2011, 277, 40–45 CrossRef CAS
.
- T. Marino, F. Russo, L. Rezzouk, A. Bouzid and A. Figoli, PES-kaolin mixed matrix membranes for arsenic removal from water, Membranes, 2017, 7, 57 CrossRef PubMed
.
- A. Gholami, A. Moghadassi, S. Hosseini, S. Shabani and F. Gholami, Preparation and characterization of polyvinyl chloride based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water, J. Ind. Eng. Chem., 2014, 20, 1517–1522 CrossRef CAS
.
- A. M. Nasir, P. S. Goh and A. F. Ismail, Highly adsorptive polysulfone/hydrous iron-nickel-manganese (PSF/HINM) nanocomposite hollow fiber membrane for synergistic arsenic removal, Sep. Purif. Technol., 2019, 213, 162–175 CrossRef CAS
.
- M. Batool, A. Shafeeq, B. Haider and N. M. Ahmad, TiO2 nanoparticle filler-based mixed-matrix PES/CA Nanofiltration membranes for enhanced desalination, Membranes, 2021, 11, 433 CrossRef CAS PubMed
.
- A. Chand, P. Chand, G. Khatri and D. Paudel, Enhanced Removal Efficiency of Arsenic and Copper from Aqueous Solution Using Activated Acorus calamus Based Adsorbent, Chem. Biochem. Eng. Q., 2021, 35, 279–293 CAS
.
- R. Rezaee, S. Nasseri, A. H. Mahvi, R. Nabizadeh, S. A. Mousavi, A. Rashidi, A. Jafari and S. Nazmara, Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water, J. Environ. Health Sci. Eng., 2015, 13, 1–11 CrossRef CAS PubMed
.
- J. C. Hsu, C. J. Lin, C. H. Liao and S. T. Chen, Removal of As(V) and As(III) by reclaimed iron-oxide coated sands, J. Hazard. Mater., 2008, 153, 817–826 CrossRef CAS PubMed
.
- S. Dixit and J. G. Hering, Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility, Environ. Sci. Technol., 2003, 37, 4182–4189 CrossRef CAS PubMed
.
- X. Huang, Y. Liu, X. Wang, L. Zeng, T. Xiao, D. Luo, J. Jiang, H. Zhang, Y. Huang and M. Ye, Removal of arsenic from wastewater by using nano Fe3O4/zinc organic frameworks, Int. J. Environ. Res. Public Health, 2022, 19, 10897 CrossRef CAS PubMed
.
- C. H. Liu, Y. H. Chuang, T. Y. Chen, Y. Tian, H. Li, M. K. Wang and W. Zhang, Mechanism of Arsenic Adsorption on Magnetite Nanoparticles from Water: Thermodynamic and Spectroscopic Studies, Environ. Sci. Technol., 2015, 49, 7726–7734 CrossRef CAS PubMed
.
- J. Perendija, Z. S. Veličković, I. Cvijetić, J. D. Rusmirović, V. Ugrinović, A. D. Marinkovic and A. Onjia, Batch and column adsorption of cations, oxyanions and dyes on a magnetite modified cellulose-based membrane, Cellulose, 2020, 27, 8215–8235 CrossRef CAS
.
- S. Mandal, M. K. Sahu and R. K. Patel, Adsorption studies of arsenic(III) removal from water by zirconium polyacrylamide hybrid material (ZrPACM-43), Water Resour. Ind., 2013, 4, 51–67 CrossRef
.
- A. A. Khan, S. R. Naqvi, I. Ali, M. Arshad, H. AlMohamadi and U. Sikandar, Algal-derived biochar as an efficient adsorbent for removal of Cr (VI) in textile industry wastewater: Non-linear isotherm, kinetics and ANN studies, Chemosphere, 2023, 316, 137826 CrossRef CAS PubMed
.
- Ş. Yilmaz, A. Zengin, T. Şahan and Ö. Selçuk Zorer, Utilization of a novel polymer-clay material for high elimination of hazardous radioactive contamination uranium(VI) from aqueous environments, Environ. Technol. Innovation, 2021, 23, 101631 CrossRef
.
- U. Ecer, Ş. Yilmaz and T. Şahan, Investigation of Mercury(II) and Arsenic(V) adsorption onto sulphur functionalised pumice: a response surface approach for optimisation and modelling, Int. J. Environ. Anal. Chem., 2020, 102, 1–21 Search PubMed
.
- Z. Qiu, H. Chen, Z. Wang, T. Zhang, D. Yang and F. Qiu, Efficient removal of As (III) via the synergistic effect of oxidation and absorption by FeOOH@ MnO2@ CAM nano-hybrid adsorption membrane, Chemosphere, 2020, 258, 127329 CrossRef CAS PubMed
.
- J. He, Y. Song and J. P. Chen, Development of a novel biochar/PSF mixed matrix membrane and study of key parameters in treatment of copper and lead contaminated water, Chemosphere, 2017, 186, 1033–1045 CrossRef CAS PubMed
.
- V. Srivastava, P. Singh, C. Weng and Y. Sharma, Economically viable synthesis of FeO nanoparticles and their characterization, Pol. J. Chem. Technol., 2011, 13, 1–5 CrossRef
.
- P. Daraei, S. S. Madaeni, N. Ghaemi, E. Salehi, M. A. Khadivi, R. Moradian and B. Astinchap, Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu (II) removal from water, J. Membr. Sci., 2012, 415, 250–259 CrossRef
.
- C. Evangeline, V. Pragasam, K. Rambabu, S. Velu, P. Monash, G. Arthanareeswaran and F. Banat, Iron oxide modified polyethersulfone/cellulose acetate blend membrane for enhanced defluoridation application, Desalin. Water Treat., 2019, 156, 177–188 CrossRef CAS
.
- R. Saranya, G. Arthanareeswaran, A. Ismail, D. D. Dionysiou and D. Paul, Zero-valent iron impregnated cellulose acetate mixed matrix membranes for the treatment of textile industry effluent, RSC Adv., 2015, 5, 62486–62497 RSC
.
- P. Sherugar, N. S. Naik, M. Padaki, V. Nayak, A. Gangadharan, A. R. Nadig and S. Déon, Fabrication of zinc doped aluminium oxide/polysulfone mixed matrix membranes for enhanced antifouling property and heavy metal removal, Chemosphere, 2021, 275, 130024 CrossRef CAS PubMed
.
- X. Zhang, X. Fang, J. Li, S. Pan, X. Sun, J. Shen, W. Han, L. Wang and S. Zhao, Developing new adsorptive membrane by modification of support layer with iron oxide microspheres for arsenic removal, J. Colloid Interface Sci., 2018, 514, 760–768 CrossRef CAS PubMed
.
- M. B. M. Y. Ang, K. P. O. Devanadera, A. N. R. Duena, Z.-Y. Luo, Y.-H. Chiao, J. C. Millare, R. R. Aquino, S.-H. Huang and K.-R. Lee, Modifying cellulose acetate mixed-matrix membranes for improved oil–water separation: Comparison between sodium and organo-montmorillonite as particle additives, Membranes, 2021, 11, 80 CrossRef CAS PubMed
.
- A. S. M. Ali, M. M. Soliman, S. H. Kandil and M. Khalil, Emerging mixed matrix membranes based on zeolite nanoparticles and cellulose acetate for water desalination, Cellulose, 2021, 28, 6417–6426 CrossRef CAS
.
- I. Shakeel, A. Hussain and S. Farrukh, Effect analysis of nickel ferrite (NiFe2O4) and titanium dioxide (TiO2) nanoparticles on CH4/CO2 gas permeation properties of cellulose acetate based mixed matrix membranes, J. Polym. Environ., 2019, 27, 1449–1464 CrossRef CAS
.
- M. M. Ibrahim, T. Y. Fahmy, E. I. Salaheldin, F. Mobarak, M. A. Youssef and M. R. Mabrook, Role of tosyl cellulose acetate as potential carrier for controlled drug release, Life Sci. J., 2015, 10, 127–133 Search PubMed
.
- S. H. Sonawane, A. Terrien, A. S. Figueiredo, M. Clara Goncalves and M. N. De Pinho, The role of silver nanoparticles on mixed matrix Ag/cellulose acetate asymmetric membranes, Polym. Compos., 2017, 38, 32–39 CrossRef CAS
.
- R. Sahraei, T. Shahalizade, M. Ghaemy and H. Mahdavi, Fabrication of cellulose acetate/Fe3O4@ GO-APTS-poly (AMPS-co-MA) mixed matrix membrane and its evaluation on anionic dyes removal, Cellulose, 2018, 25, 3519–3532 CrossRef CAS
.
- N. Tanne, R. Xu, M. Zhou, P. Zhang, X. Wang and X. Wen, Influence of pore size and membrane surface properties on arsenic removal by nanofiltration membranes, Front. Environ. Sci. Eng., 2019, 13, 19 CrossRef CAS
.
- A. V. Vitela-Rodriguez and J. R. Rangel-Mendez, Arsenic removal by modified activated carbons with iron hydro(oxide) nanoparticles, J. Environ. Manage., 2013, 114, 225–231 CrossRef CAS PubMed
.
- J. Wang and X. Guo, Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications, Chemosphere, 2022, 309, 136732 CrossRef CAS PubMed
.
- F.-C. Wu, R.-L. Tseng and R.-S. Juang, Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics, Chem. Eng. J., 2009, 153, 1–8 CrossRef CAS
.
- A. E. Ofomaja, E. B. Naidoo and A. Pholosi, Intraparticle diffusion of Cr (VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study, S. Afr. J. Chem. Eng., 2020, 32, 39–55 Search PubMed
.
- R. Jain, Recent advances of magnetite nanomaterials to remove arsenic from water, RSC Adv., 2022, 12, 32197–32209 RSC
.
- W. Cheng, W. Zhang, L. Hu, W. Ding, F. Wu and J. Li, Etching synthesis of iron oxide nanoparticles for adsorption of arsenic from water, RSC Adv., 2016, 6, 15900–15910 RSC
.
- M. Ahmaruzzaman, Magnetic nanocomposite adsorbents for abatement of arsenic species from water and wastewater, Environ. Sci. Pollut. Res., 2022, 29, 82681–82708 CrossRef CAS PubMed
.
- S. Yean, L. Cong, C. T. Yavuz, J. Mayo, W. Yu, A. Kan, V. Colvin and M. Tomson, Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate, J. Mater. Res., 2005, 20, 3255–3264 CrossRef CAS
.
- Y.-M. Zheng, S.-W. Zou, K. N. Nanayakkara, T. Matsuura and J. P. Chen, Adsorptive removal of arsenic from aqueous solution by a PVDF/zirconia blend flat sheet membrane, J. Membr. Sci., 2011, 374, 1–11 CrossRef CAS
.
- R. J. Gohari, W. Lau, T. Matsuura and A. Ismail, Fabrication and characterization of novel PES/Fe–Mn binary oxide UF mixed matrix membrane for adsorptive removal of As (III) from contaminated water solution, Sep. Purif. Technol., 2013, 118, 64–72 CrossRef
.
- S. Chatterjee and S. De, Adsorptive removal of arsenic from groundwater using chemically treated iron ore slime incorporated mixed matrix hollow fiber membrane, Sep. Purif. Technol., 2017, 179, 357–368 CrossRef CAS
.
- A. Moslehyani, R. Farnood, S. Tabe, T. Matsuura and A. F. Ismail, Novel Nanocomposite HNT-TiO2/PVDF Adsorptive Nanofiber Membranes for Arsenic (III) Removal, J. Membr. Sci. Res., 2020, 6, 416–423 CAS
.
| This journal is © The Royal Society of Chemistry 2024 |