Selective separation of dye/salt mixtures via a tannic acid-polyethyleneimine-modified hollow fiber membrane with high flux†
Received
7th April 2024
, Accepted 4th July 2024
First published on 17th July 2024
Abstract
In this study, a loose nanofiltration (NF) membrane with excellent perm-selectivity was fabricated via co-depositing tannic acid (TA) and polyethyleneimine (PEI) on a PVDF hollow fiber substrate. The performance of the TA-PEI-modified hollow fiber membrane (HFM) was optimized by tuning certain parameters (e.g., TA concentration and co-deposition time). The tailored membrane TA-PEI/PVDF exhibited competitive water permeability (32 L m−2 h−1 per bar) while maintaining an excellent dye rejection performance (i.e., 99% for CR). Furthermore, it presented low retention for inorganic salts (i.e., 2.3% for NaCl and 6.4% Na2SO4). The as-fabricated TA-PEI/PVDF loose NF HFM was proved to have good stability and antifouling performance for treating simulated textile wastewater. In summary, we believe that the as-prepared loose NF HFM has great application prospects for practical wastewater treatment.
Water impact
The direct discharge of large amounts of textile wastewater containing dyes and salts has become a global concern as many of the dyes are toxic and non-biodegradable, and thus can cause serious damage to natural water systems and human health. Furthermore, the existence of inorganic salts greatly limits the fractionation and purification efficiency. Nanofiltration (NF), with energy-saving and environmentally friendly advantages, has shown great merits in dye purification and wastewater reclamation. However, conventional NF membranes not only exhibit high retention for dyes, but also for salts, which leads to low separation efficiency. Recently, increasing attention has been drawn to the preparation of loose NF membranes for the fractionation of dye/salt mixtures. However, most membranes reported prior involved intricate synthesis procedures and expensive raw materials, which largely restricted their practical application in textile wastewater. Recently, the surface co-deposition of polyphenol monomers, also called bioinspired deposition, has been widely explored for the preparation of loosely structured NF membranes owing to the advantages of simple operation and a green process. Dopamine (DA) is the most-commonly used polyphenol-inspired monomer for preparing modified membranes. However, it is expensive, which has largely restricted its large-scale application in loose NF membranes. In contrast, tannic acid (TA) is a cheaper polyphenol, which exists widely in plants, and is used to prepare loose NF membranes. Despite the promising results, it is worth noting that the reported membranes are loose NF flat-sheet membranes. Loose NF membranes in the hollow fiber configuration have unique advantages, such as a self-supporting structure, high surface area per fiber, and high packing density. Therefore, it is essential to develop high-performance loose NF hollow fiber membranes via a simple and economic route. In this study, TA and PEI were co-deposited on a PVDF hollow fiber substrate to prepare a loose NF hollow fiber membrane for treating aqueous dye/salt mixtures. The effects of coating parameters (e.g., TA concentration and co-deposition time) on the properties of the modified membrane were investigated. The tailored membrane TA-PEI/PVDF exhibited competitive water permeability (32 L m−2 h−1 per bar) while maintaining an excellent dye rejection performance (i.e., 99% for CR). It also presented low retention for inorganic salts (i.e., 2.3% for NaCl and 6.4% Na2SO4). Furthermore, the as-fabricated TA-PEI/PVDF loose NF HFM was proven to have good stability and antifouling performance for treating simulated textile wastewater. Moreover, the preparation method is simple, green, and cost-effective. To sum up, we believe that the prepared TA-PEI/PVDF loose NF HFM has great application prospects for the treatment and reuse of textile wastewater.
|
1. Introduction
The direct discharge of textile wastewater poses a threat to natural water systems and human health as synthetic dyes in wastewater are toxic and non-biodegradable.1,2 Apart from dyes, a large number of inorganic salts (e.g. 6% NaCl and 5.6% Na2SO4) are also found in wastewater as they are used to enhance fabric dyeing efficiency.3 However, the presence of such inorganic salts in dye wastewater greatly limits the fractionation and purification efficiency for the wastewater. Traditional technologies, including advanced oxidation,4 adsorption,5 precipitation,6 and biological degradation,7 mainly aim to remove dyes from wastewater; however, these technologies are not suitable for recycling valuable dyes and salts.
Nanofiltration (NF), as an energy-saving and high-efficiency separation technology, has been considered to have great merits in dye/salt separation.8 However, conventional NF membranes not only exhibit high retention for dyes, but also for salts, which leads to a low separation efficiency.9 Compared to conventional NF membranes, loose NF membranes with a larger pore size can achieve high salt permeation and dye retention, making them more advantageous for treating dye wastewater.10,11 Many attempts, such as interfacial polymerization,12,13in situ self-assembly,14,15 and blending-phase inversion,16,17 have been attempted for the fabrication of loose NF membranes. It has been found that these modified membranes demonstrate improved water permeability and good dye/salt selectivity. Nevertheless, membranes fabricated via the above-mentioned methods typically need multiple steps and toxic ingredients, which greatly restrict the large-scale manufacturing of loose NF membranes. Therefore, it is very urgent to seek for a simple and green method for the fabrication of highly permeable and highly selective loose NF membranes.
Recently, surface deposition using polyphenol monomers has been reported as an efficient method to prepare loose NF membranes owing to its simplicity and eco-friendliness.18,19 Dopamine (DA) is the most-commonly used polyphenol-inspired monomer for preparing high-performance loose NF membranes. However, it is expensive, which greatly restricts its industrial application. Tannic acid (TA), which exists abundantly in plants, is a cheap alternative for membrane preparation. More and more attention has been focused on the fabrication of loose NF membranes via utilizing TA-PEI.20,21 Despite the promising results, most reported loose NF membranes are in the form of flat sheets due to their easy fabrication. Compared with flat-sheet membranes, loose NF hollow fiber membranes (HFMs) can provide distinct advantages, such as a large surface area per unit volume, high loading density, self-supporting characteristics, and good flexibility in operation, and are more suitable for the purification of dye wastewater and industrial applications.22,23
In order to improve the perm-selectivity of the membrane and utilize the advantages of its hollow fiber configuration, a novel high-flux loose NF HFM with superior dye/salt selectivity was developed in this work via the co-deposition of TA and PEI on a PVDF hollow fiber support. The proposed membrane modification process is simple, cost-effective, and green, which can favor its use for the large-scale production of loose NF HFM for the purification of dye wastewater. The loose NF separation layer was constructed via a Michael addition/Schiff base reaction between TA and PEI (see Fig. S1†). The formed TA/PEI coating layer endowed the membrane with improved water permeability, a better antifouling ability, and performance stability because of the inherent adhesivity and hydrophilicity of TA/PEI. The chemical structure, morphology, and surface properties of the resultant loose NF HFMs were investigated, and the coating parameters, including the TA content and co-deposition time, were optimized for achieving a high water flux and dye/salt selectivity. We believe that the as-fabricated TA-PEI-modified HFM has great application prospects for the treatment and recovery of textile wastewater.
2. Experimental section
2.1. Materials
Polyvinylidene fluoride (PVDF) HFM (pore radius ≈ 45 nm) prepared via thermally induced phase separation (TIPS) was obtained from Tianjin Motimo Co. (China). Eriochrome black T (EBT, purity: >99%), rhodamine B (RhB, purity: >99%), and Congo red (CR, purity: >99%) were purchased from Macklin Reagent (China). Table S1† presents information on the four dyes. The four inorganic salts for testing, namely NaCl (≥99.5%), MgCl2 (≥98%), MgSO4 (≥99%), and Na2SO4 (≥99%), were acquired from Tianjin Guangfu Fine Chemical Research Institute (China). Polyethylenimine (PEI, Mw = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 50 wt%, aqueous solution), tannic acid (TA, purity: 98%) and polyethylene glycol (PEG) were supplied from Macklin Reagent (China). K2HPO4·3H2O (purity: ≥99%) and KH2PO4 (purity: ≥99.5%) were acquired from Aladdin Industrial Co.
000, 50 wt%, aqueous solution), tannic acid (TA, purity: 98%) and polyethylene glycol (PEG) were supplied from Macklin Reagent (China). K2HPO4·3H2O (purity: ≥99%) and KH2PO4 (purity: ≥99.5%) were acquired from Aladdin Industrial Co.
2.2. Fabrication of the TA-PEI/PVDF loose NF HFM
First, phosphate buffer solution (pH = 7.8) was prepared, which provided alkaline conditions for the Michael addition/Schiff base reactions between TA and PEI. Second, PEI was dissolved in the alkaline buffer solution and then TA powder was added to the well-mixed solution under magnetic stirring. Fresh coating solutions were prepared with phosphate buffer containing PEI (2 g L−1) and different contents of TA (ranging from 0.1–0.8 g L−1). Third, the PVDF hollow fibers after sealing were immersed in the above mixture, with deposition times of 1, 2, 5, 10, 15, 20 min, respectively. The co-deposition process for the TA-PEI-modified HFM is shown in Fig. 1. Finally, the deposited hollow fibers were taken out and rinsed with deionized (DI) water to remove residual solution.
 |
| | Fig. 1 Green co-deposition process for obtaining the TA-PEI/PVDF loose NF HFM. | |
2.3. Characterizations of the TA-PEI/PVDF loose NF HFM
The surface chemistry of the prepared loose NF HFM was characterized by X-ray photoelectron spectroscopy (XPS, Thermo Fisher, USA) and Fourier transform infrared spectroscopy (FTIR, Thermo Fisher, USA). Prior to the characterizations, all the membrane samples were heated for 8 h at 60 °C. The morphologies of the modified membranes were obtained by scanning electron microscopy (SEM, Regulus 8100, Japan). The membrane samples were freeze-dried and then fractured in liquid nitrogen. Prior to testing, the membranes for the SEM analysis were sprayed with gold. The surface charge was analyzed by zeta potential measurements using an electrokinetic analyzer (SurPASS, Anton Paar, Austria). The pore size and molecular weight cut-off (MWCO) of the resultant membrane were determined via solute rejection experiments using PEG with various molecular weights ranging from 200–5000 Da. The concentrations of PEG were obtained using a total organic carbon analyzer. The PEG rejection R (%) was calculated from eqn (1).| | 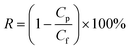 | (1) |
The solute Stokes radius (rs) could be calculated viaeqn (2), where Mw is the molecular weight of the solute PEG.24
| | rs = 0.01673 × M0.557w, (Mw ≤ 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) | (2) |
The pore-size distribution of the HFM was fitted based on eqn (3). The MWCO of the HFM is defined as the molecular weight of PEG when the HFM retained PEG with a rejection rate of 90%.25
| | 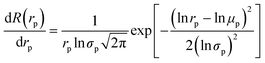 | (3) |
where
rp stands for the pore size (nm), and
μp (nm) is the mean effective pore size when the HFM retained PEG with a rejection rate of 50%. The geometric standard deviation (
σp) was calculated by ratio of
rp at
R = 84.13% over that at 50%.
2.4. Performance evaluation of the TA-PEI-modified HFM
Fig. 2 shows the cross-flow filtration device used to estimate the separation performance of the TA-PEI-modified HFM. Filtration modules were assembled from five individual hollow fibers with an effective length of about 15 cm. Prior to the tests, each HFM module was filtered for 60 min at 2 bar to get a stable permeation flux. The flux (J, L m−2 h−1 per bar) was calculated using eqn (4).| | 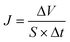 | (4) |
where ΔV (L) represents the permeate solution volume, S (m2) represents the effective membrane area, and Δt (h) stands for the time interval.
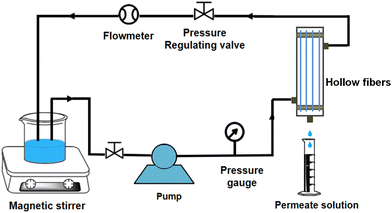 |
| | Fig. 2 Cross-flow filtration apparatus. | |
The dye concentrations were determined using a UV-vis spectrophotometer, while the salt concentrations were obtained by measuring the conductivity of the solution using a conductivity meter. The solute rejection R (%) was obtained using eqn (5).
| |  | (5) |
where the concentrations of the feed and permeate solute are represented by
Cf (mg L
−1) and
Cp (mg L
−1), respectively.
2.5. Stability and anti-dye fouling measurement
The stability of the TA-PEI-modified HFM was assessed by performing measurements with CR/NaCl mixtures. The NF process could operate continuously at 2 bar for 48 h. The antifouling property of the TA-PEI-modified HFM was studied by testing model foulants (CR, 50 mg L−1). The feed solution for the testing was pure water for the first 60 min, which was then replaced by dye solution for the next 60 min. The pure water flux (J1) and the permeability (Jp) were calculated every 10 min. After washing with water for 30 min, the pure water flux (J2) of the membrane was recorded again. The antifouling capability of the loose NF HFM was evaluated by considering the following indices:12| | 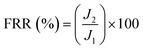 | (6) |
| | 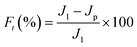 | (7) |
| | 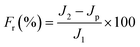 | (8) |
| | 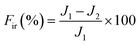 | (9) |
where Ft, FRR, Fir, and Fr represent the total pollution, flux recovery rate, irreversible pollution, and reversible pollution, respectively.
3. Results and discussion
3.1. Optimization of the membrane performance
The impact of the TA concentration on the membrane performance is shown in Fig. 3a. As expected, increasing the TA concentration from 0.1 to 0.6 g L−1 resulted in an increase in permeability and rejection of CR (97.1% to 99%). Upon further increasing the TA content, there was slight increase in dye rejection, while the water flux declined considerably. On the one hand, with the increasing TA content in the coating solution, more hydrophilic hydroxyl groups were found on the membrane surface, thus helping to improve the membrane permeability. On the other hand, an excessively high TA concentration would enhance the co-deposition reaction and form a denser and thicker coated layer, resulting in a decline in the water flux. These results were in agreement with the information from the SEM images (see Fig. 6 and S2†).
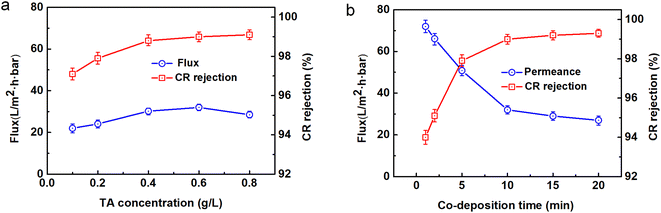 |
| | Fig. 3 Influence of the fabrication conditions on the performance of the TA-PEI-modified membranes: (a) TA content; (b) co-deposition time. | |
The influence of co-deposition time on the membrane performance is shown in Fig. 3b. With the increase in deposition time from 1 to 10 min, the water flux declined from 72 to 32 L m−2 h−1 per bar, while the CR retention improved from 94% to 99%. As the deposition time increased, the chemical reaction was more complete and the crosslinking density of the coating increased, leading to a decrease in water flux and an increase in dye retention. With further increasing the coating time, the dye rejection rate slightly increased, while the water flux decreased significantly. To balance the perm-selectivity, the TA-PEI-modified membrane fabricated with optimized parameters (TA content of 0.6 g L−1 and co-deposition time of 10 min) was selected for the subsequent characterizations and study.
3.2. Chemical structures of the loose NF HFM
The chemical structures of the original HFM and TA-PEI-modified membranes were determined by FTIR, as shown in Fig. 4a. Compared with the PVDF HFM, two distinct peaks at 1730 and 1505 cm−1 emerged in the loose NF membrane spectrum, corresponding to the ester groups of TA and N–H of PEI, respectively.26,27 These results indicated that TA and PEI existed on the surface of the support HFM. Most importantly, a new peak at 1645 cm−1 only appeared in the TA-PEI-modified HFM spectrum, which was attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds formed via the crosslinking between PEI and TA.28 In addition, a broad peak was observed at 3500 cm−1 in the loose NF membrane spectrum, representing an –OH stretching vibration. The XPS survey spectra for the original HFM and TA-PEI-modified HFM are shown in Fig. 4b. The typical peaks for C1s and F1s could be seen in the PVDF membrane spectrum, derived from the C and F elements of the PVDF membrane. Compared with the PVDF HFM, two new peaks for O1s and N1s emerged in the resultant loose NF membrane spectrum, which could be explained by the O and N elements in the TA-PEI-coated layer obtained by co-deposition. Due to the coatings, the F1s peak was significantly weakened in the XPS spectrum for the TA-PEI-modified HFM. These results demonstrated that the TA-PEI layer was successfully constructed on the original membrane surface via co-deposition. Fig. 4c and d show the high-resolution C1s spectra of the PVDF HFM and loose NF HFM. The C1s spectrum of the original HFM could be convoluted into two characteristic peaks: C–H at 286.1 eV and C–F at 290.6 eV. For the TA/PEI loose NF HFM, the C1 s spectrum could also be divided into other peaks: C–N bond at 285.5 eV, C
N bonds formed via the crosslinking between PEI and TA.28 In addition, a broad peak was observed at 3500 cm−1 in the loose NF membrane spectrum, representing an –OH stretching vibration. The XPS survey spectra for the original HFM and TA-PEI-modified HFM are shown in Fig. 4b. The typical peaks for C1s and F1s could be seen in the PVDF membrane spectrum, derived from the C and F elements of the PVDF membrane. Compared with the PVDF HFM, two new peaks for O1s and N1s emerged in the resultant loose NF membrane spectrum, which could be explained by the O and N elements in the TA-PEI-coated layer obtained by co-deposition. Due to the coatings, the F1s peak was significantly weakened in the XPS spectrum for the TA-PEI-modified HFM. These results demonstrated that the TA-PEI layer was successfully constructed on the original membrane surface via co-deposition. Fig. 4c and d show the high-resolution C1s spectra of the PVDF HFM and loose NF HFM. The C1s spectrum of the original HFM could be convoluted into two characteristic peaks: C–H at 286.1 eV and C–F at 290.6 eV. For the TA/PEI loose NF HFM, the C1 s spectrum could also be divided into other peaks: C–N bond at 285.5 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond at 286.3 eV, C
N bond at 286.3 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond at 288.3 eV, and C–C bond at 284.6 eV, which further indicated the formation of the TA/PEI-coating layer.20
O bond at 288.3 eV, and C–C bond at 284.6 eV, which further indicated the formation of the TA/PEI-coating layer.20
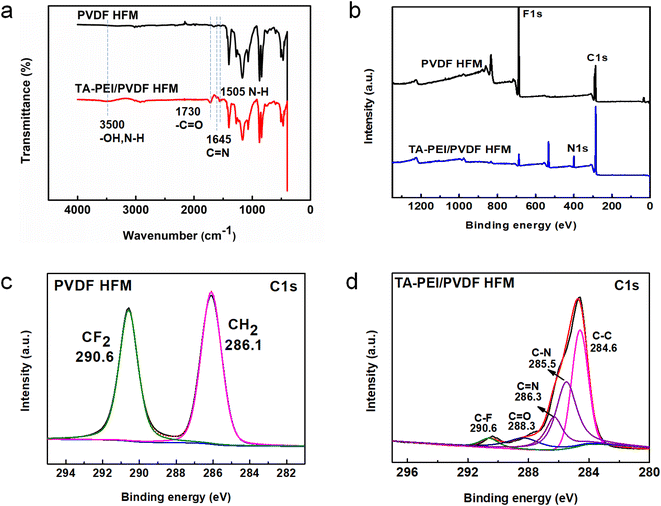 |
| | Fig. 4 FTIR (a) and XPS (b) images of the pristine membrane and the optimized TA-PEI-modified HFM; C1s core level spectra of the pristine membrane (c) and the optimized modified membrane (d). | |
3.3. Membrane morphology
Fig. 5a and c show the morphologies from surface and cross-section images of the pristine membrane, while Fig. 5b and d present the surface and cross-sectional structures of the TA/PEI-modified membrane. It could be observed that there was an obvious variation in the TA/PEI-modified membrane surface (Fig. 5b) compared with the pristine PVDF membrane (Fig. 5a). After coating with the TA-PEI solution, the membrane surface became rougher, and small nodular structures appeared on the membrane surface. These results indicated the formation of new skin layer on the pristine membrane surface via coating. The cross-sectional morphologies of the two membranes were also compared. For the pristine membrane, no additional skin layer was observed, as shown in Fig. 5c. As expected, after modification with TA-PEI solution, the membrane was endowed with a new skin layer, as shown in Fig. 5d. Furthermore, it could be clearly observed that the formed TA-PEI coating layer fitted closely with the underlying PVDF membrane. These results proved that the selective TA/PEI coating layer was successfully formed on the PVDF membrane.
 |
| | Fig. 5 Outer surfaces of (a) the pristine membrane and (b) the optimized TA-PEI-modified HFM. Enlarged cross-sections of (c) the pristine membrane and (d) the modified HFM. | |
Fig. 6 shows the surface morphologies of the modified membranes fabricated with different TA concentrations, where the co-deposition time was fixed at 10 min. It could be observed that the TA/PEI layer became denser and more aggregates appeared on the surface of the membrane with the increase in the TA concentration, which was mainly due to the extent of the reaction between TA and PET being enhanced. Fig. 7 shows the surface morphologies of the modified HFMs fabricated via different coating times but with the TA content fixed at 0.6 g L−1. As the deposition time increased, the deposited layer became more uniform and dense. This result was ascribed to the more complete chemical reactions and increased crosslinking density between TA and PEI.
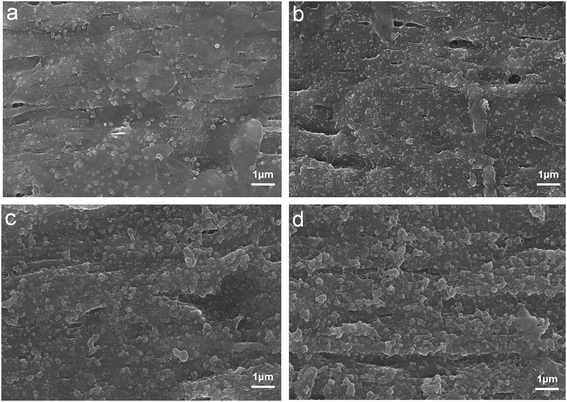 |
| | Fig. 6 Surface morphologies of the TA-PEI-modified membranes prepared with different TA concentrations: (a) 0.2, (b) 0.4, (c) 0.6, and (d) 0.8 g L−1. | |
 |
| | Fig. 7 Surface morphologies of the TA-PEI-modified membranes prepared at different deposition times: (a) 2, (b) 10, and (c) 20 min. | |
3.4. Surface charge, MWCO, and pore sizes of the modified membrane
The surface charge characteristics of the original membrane and the modified membrane were studied, as shown in Fig. 8a. The pristine HFM presented a negative charge property in a wide range of pH values. After coating with PEI-TA solution, the membrane surface was positively charged over the pH range from 2.0 to 6.1, which could be explained by the existence of amino groups from PEI on the membrane surface. When the pH value was above 6.1, the modified membrane showed negatively charged characteristics. Fig. 8b present the MWCO and pore-size distribution of the TA-PEI-modified membrane. The commercial PVDF HFM had a large pore size of 45 nm. After modification with TA/PEI, the pore size of the membrane was reduced to 1.37 nm. This result indicates that the membrane became denser with the formation of the TA/PEI-coated layer.
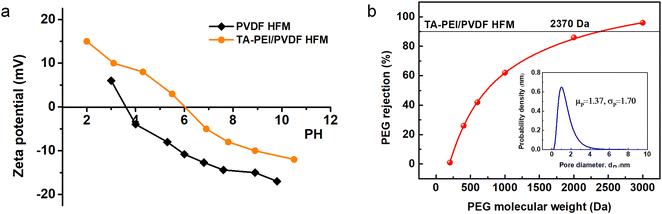 |
| | Fig. 8 (a) Surface ζ-potentials and (b) MWCO and pore-size distributions of the pristine membrane and the optimized TA-PEI-modified membrane. | |
3.5. Filtration performance
3.5.1. NF performance.
Three single dyes and four types of inorganic salts were selected to estimate the NF performance of the TA-PEI-modified HFM, as shown in Fig. 9. The resultant loose NF HFM could reject the CR efficiently due to the effects of steric hindrance and Donnan exclusion (see Fig. S3†). Compared with CR with a large molecular weight, the membrane showed relatively low retentions for such low molecular-weight dyes as EBT and RhB. This result could be mainly ascribed to the steric hindrance effect. Furthermore, the prepared membrane presented low retention for the four inorganic salts tested, including NaCl (2.3%) and Na2SO4 (6.4%), which was also ascribed to a size exclusion effect and Donnan exclusion effect.29 When processing dye/salt mixtures, it also exhibited good perm-selectivity (Table S2†). The separation results is what we expected because it favors the efficient purification of dyes and salts.
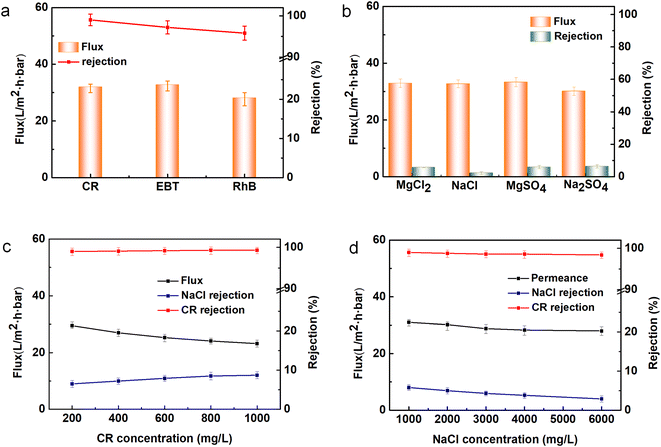 |
| | Fig. 9 Membrane filtration performance: (a) different dyes, (b) various salts, (c) different CR concentrations, and (d) different NaCl concentrations. | |
The impacts of the dye (i.e., CR) concentration and salt (i.e., NaCl) concentration on the performance of the resultant modified membrane are shown in Fig. 9c and d. As the CR concentration increased, there was no significant change in dye rejection, whereas the water flux significantly declined. This was due to the fact that with the dye concentration increasing, more dye molecules accumulated on the membrane surface and formed a filter cake layer with resistance.30 In contrast, the salt rejection increased with increasing the dye concentration, which was due to CR being a negatively charged dye, and so the membrane surface charge would change after adsorption of the dye.31 With the NaCl concentration increasing, the salt retention declined, whereas the CR rejection kept stable. This result was mainly due to the increase in salt content, which enhanced the electrostatic shielding effect and weakened the electrostatic repulsion.32
3.5.2. Stability and anti-dye fouling.
Fig. 10 presents the performance stability of the TA-PEI-modified membrane for processing aqueous CR/salt mixtures. The water flux presented a significantly declining trend at the initial stage of filtration, while CR and NaCl rejection kept constant throughout the continuous filtration process. On the one hand, the applied pressure could lead to a compaction of the membrane, making it denser, which leads to a decreased water flux. On the other hand, the dye molecules could adsorb on the membrane surface or pores to form a dense cake layer, which increased the transmembrane resistance, leading to a decreased water flux. Excitingly, the prepared loose NF membrane maintained an over 99% rejection of CR but a low NaCl retention rate (<7.5%) during continuous filtration for 24 h, which indicated that the prepared membrane had good performance stability for treating simulated textile wastewater. In addition, other dyes (e.g., EBT) and salts (e.g., Na2SO4) were also selected to further examine the stability of the modified membrane. Simulated textile wastewater was prepared by mixing 50 ppm of EBT and 1000 ppm of Na2SO4. As shown in Fig. S5,† the prepared loose NF membrane still maintained a stable retention of the small molecule dye EBT and a low retention of the divalent salt (Na2SO4), which indicated the potential of the TA-PEI-modified membrane developed in this work for practical textile wastewater application. To further demonstrate the stability of the TA/PEI coating layer, the morphological changes of the membrane before and after long-term separation were studied. As shown in Fig. S4,† the formed TA-PEI coating layer could still fit tightly onto the surface of the underlying PVDF membrane after long-term separation. This result demonstrated that the interactions between the TA-PEI coating layer and the PVDF-support layer were strong enough and indicated that the TA/PEI coating layer had good stability.
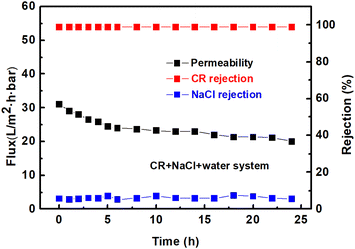 |
| | Fig. 10 Stability of the TA-PEI-modified membranes for processing aqueous dye/salt mixtures. | |
CR solution as the fouling source was used to evaluate the anti-dye fouling capacity of the modified membrane, as shown in Fig. 11. It could be observed that the permeation flux of the fouled membrane could be greatly restored with periodic rinsing using DI water. After two fouling cycles, the FRR value of the modified membrane for the CR solution still reached 92.86%, and its Rir value was less than 5.5%. This good antifouling capacity was mainly ascribed to the enhanced membrane surface hydrophilicity caused by introducing hydrophilic –OH groups in to TA. In addition, the as-fabricated modified membrane had a relatively dense coated layer, which was beneficial for preventing dye molecules from entering the membrane pores. In addition, EBT (Mw = 461 Da) as the model foulant was also used to examine the fouling resistance of the modified membrane, as shown in Fig. S6.† For the EBT foulant, the FRR was 88.7% and the corresponding Rir was 9.7%. The antifouling capacity for EBT was lower than that of CR, which could be mainly attributed to the EBT molecule having a smaller molecular weight, which meant it could easily enter the membrane pores or even some blind alleys, resulting in weak antifouling properties. To sum up, the TA-PEI-modified membrane exhibited a huge potential for processing aqueous dye/salt mixtures.
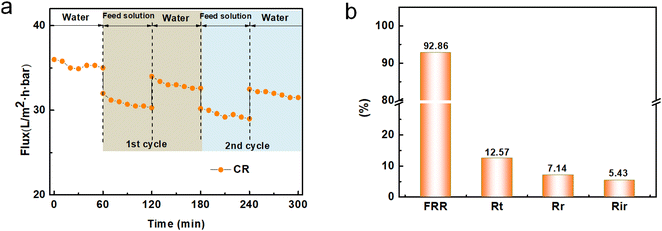 |
| | Fig. 11 (a) Time-dependent flux of the TA-PEI-modified HFM before and after the filtration of CR solution and (b) the single values of Ft, FRR, Fir, and Fr of the modified HFM after two cycles of fouling and cleaning. | |
3.5.3. Performance comparison.
A performance comparison of the resultant TA-PEI-modified HFM with other reported loose NF membranes is shown in Fig. 12. For the commercially available or lab-made loose NF membranes, the water fluxes were in the range of 7.2–41.1 L m−2 h−1 per bar. The selectivity for dyes/salts ranged from 2.3–33.7. It should be noted that the TA-PEI-modified HFM in this work exhibited excellent selectivity for dyes and salts up to 43.0, while the water flux was also as high as 32.0 L m−2 h−1 per bar, which makes them very attractive for dyes and salts recovery. Compared with other membrane modification methods (e.g., interfacial polymerization (IP), in situ self-assembly, and blending), our preparation procedure is simple, green, and economic. In addition, it is worth noting that these reported loose NF membranes were mainly based on a flat-sheet configuration. The TA-PEI/PVDF loose NF HFM prepared in this work takes advantage of a hollow fiber configuration and thus has great potential for water purification. On combining the above aspects, our work may provide a simple and economical strategy for engineering high-performance loose NF HFMs.
 |
| | Fig. 12 Comparison of membranes in terms of dye/NaCl selectivity and water flux (references are listed in Table 1). | |
Table 1 Performance comparison of the resultant TA-PEI-modified membrane with some reported loose NF membranes for dye/salt separation. The dye/NaCl selectivity was calculated by α = (Rdye)/(RNaCl)29
| Membrane |
Manufacture |
Flux (L m−2 h−1 per bar) |
R
NaCl (%) |
Dye |
R
Dye (%) |
Selectivity (α) |
Ref. |
| Sepro 2A |
— |
10.2 |
21.8 |
CR |
99.96 |
4.6 |
33
|
| Sepro 6 |
— |
13.7 |
10 |
CR |
99.96 |
10.0 |
33
|
| SPEI/TMC |
IP |
41.1 |
3.2 |
CR |
97 |
30.3 |
29
|
| PEA/TMC |
IP |
16.6 |
16.4 |
MB |
95.4 |
5.8 |
34
|
| ZDNMA/TMC |
IP |
10.7 |
14.3 |
CR |
99.9 |
7.0 |
35
|
| Fe3+-PEI@HPAN |
Self-assembly |
6.0 |
7.46 |
CR |
99.53 |
13.3 |
15
|
| Ag+-PEI@HPAN |
Self-assembly |
10.6 |
9.6 |
CV |
99.80 |
10.4 |
36
|
| TiO2-HMDI-PES |
Self-assembly |
26.0 |
12.4 |
CR |
97.4 |
33.7 |
37
|
| Chitosan@HPAN |
Self-assembly |
7.2 |
12.5 |
CR |
99.6 |
8.0 |
14
|
| CeO2/PES |
Incorporation |
26.24 |
3.3 |
CR |
99.36 |
30.1 |
38
|
| GO/MoS2 |
Incorporation |
10.2 |
43.2 |
MB |
97.4 |
2.3 |
39
|
| PRGO/HNTs-PSS |
Incorporation |
8.8 |
6.8 |
RB5 |
97.9 |
14.4 |
17
|
| TA-PEI |
Co-deposition |
40.6 |
6.1 |
CR |
99.8 |
16.4 |
21
|
| Catechol/PEI |
Co-deposition |
24.5 |
7.0 |
CR |
99.75 |
14.3 |
18
|
| TA-PEI |
Co-deposition |
32 |
2.3 |
CR |
99.0 |
43.0 |
This work |
4. Conclusion
In this study, TA and PEI were co-deposited on a PVDF hollow fiber substrate to prepare a loose NF hollow fiber membrane for treating aqueous dye/salt mixtures. The impacts of the coating conditions (i.e., TA concentration and co-deposition time) on the properties of the as-fabricated HFMs were studied. The tailored TA-PEI/PVDF HFM exhibited a high water flux of 32 L m−2 h−1 per bar while also demonstrating a satisfying CR retention of 99%. In addition, the as-fabricated loose NF membrane had low retention (below 6.5%) toward common inorganic salts. Moreover, the resultant membrane also presented a stable performance and good flux recovery rate for treating dye wastewater. To sum up, we believe that the prepared TA-PEI/PVDF loose NF HFM has great application prospects in the treatment and recovery of dye wastewater.
Author contributions
Chuanfeng Wang: conceptualization, methodology, validation, formal analysis, data curation, writing – original draft preparation; Qian Chen: data curation, software, formal analysis; Jiapeng Yang: resources, investigation; Lina Ge: investigation, visualization.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the financial support from Postgraduate Education Reform and Quality Improvement Project of Henan Province (No. YJS2023JD65).
References
- Y. Liu, G. Van Eygen, S. Yuan, H. Wang, M. Chi, D. Xu, Q. Gao, G. Li, J. Zheng and B. Van der Bruggen, Facile and novel fabrication of high-performance loose nanofiltration membranes for textile wastewater recovery, Sep. Purif. Technol., 2023, 308, 122867 CrossRef CAS.
- W. Sun, N. Zhang, Q. Li, X. Li, S. Chen, L. Zong, Y. Baikeli, E. Lv, H. Deng, X. Zhang and H. Baqiah, Bioinspired lignin-based loose nanofiltration membrane with excellent acid, fouling, and chlorine resistances toward dye/salt separation, J. Membr. Sci., 2023, 670, 121372 CrossRef CAS.
- Y. Liu, J. Mo, H. Ding, Y. Cheng, Z. Zhang, C. Wang and X. Li, Ultrafast loose nanofiltration membrane intercalated by in-situ grown nanoparticles for dye purification and reuse, Desalination, 2023, 551, 116439 CrossRef CAS.
- H. Song, J.-A. You, C. Chen, H. Zhang, X. Z. Ji, C. Li, Y. Yang, N. Xu and J. Huang, Manganese functionalized mesoporous molecular sieves Ti-HMS as a Fenton-like catalyst for dyes wastewater purification by advanced oxidation processes, J. Environ. Chem. Eng., 2016, 4(4), 4653–4660 CrossRef CAS.
- P. Ilgin, H. Ozay and O. Ozay, Selective adsorption of cationic dyes from colored noxious effluent using a novel N-tert-butylmaleamic acid based hydrogels, React. Funct. Polym., 2019, 142, 189–198 CrossRef CAS.
- S. Asha, C. Hentry, M. R. Bindhu, A. M. Al-Mohaimeed, M. R. AbdelGawwad and M. S. Elshikh, Improved photocatalytic activity for degradation of textile dyeing waste water and thiazine dyes using PbWO(4) nanoparticles synthesized by co-precipitation method, Environ. Res., 2021, 200, 111721 CrossRef CAS PubMed.
- D. Daâssi, S. Rodríguez-Couto, M. Nasri and T. Mechichi, Biodegradation of textile dyes by immobilized laccase from Coriolopsis gallica into Ca-alginate beads, Int. Biodeterior. Biodegrad., 2014, 90, 71–78 CrossRef.
- G. Han, T. S. Chung, M. Weber and C. Maletzko, Low-Pressure Nanofiltration Hollow Fiber Membranes for Effective Fractionation of Dyes and Inorganic Salts in Textile Wastewater, Environ. Sci. Technol., 2018, 52(6), 3676–3684 CrossRef CAS PubMed.
- W. Ye, J. Lin, R. Borrego, D. Chen, A. Sotto, P. Luis, M. Liu, S. Zhao, C. Y. Tang and B. Van der Bruggen, Advanced desalination of dye/NaCl mixtures by a loose nanofiltration membrane for digital ink-jet printing, Sep. Purif. Technol., 2018, 197, 27–35 CrossRef CAS.
- X. Feng, D. Peng, J. Zhu, Y. Wang and Y. Zhang, Recent advances of loose nanofiltration membranes for dye/salt separation, Sep. Purif. Technol., 2022, 285, 120228 CrossRef CAS.
- S. Guo, Y. Wan, X. Chen and J. Luo, Loose nanofiltration membrane custom-tailored for resource recovery, Chem. Eng. J., 2021, 409, 127376 CrossRef CAS.
- P. Jin, J. Zhu, S. Yuan, G. Zhang, A. Volodine, M. Tian, J. Wang, P. Luis and B. Van der Bruggen, Erythritol-based polyester loose nanofiltration membrane with fast water transport for efficient dye/salt separation, Chem. Eng. J., 2021, 406, 126796 CrossRef CAS.
- L. Liu, L. Yu, B. Borjigin, Q. Liu, C. Zhao and D. Hou, Fabrication of thin-film composite nanofiltration membranes with improved performance using β-cyclodextrin as monomer for efficient separation of dye/salt mixtures, Appl. Surf. Sci., 2021, 539, 148284 CrossRef CAS.
- S. Liu, Z. Wang and P. Song, Free radical graft copolymerization strategy to prepare catechin-modified chitosan loose nanofiltration (NF) membrane for dye desalination, ACS Sustainable Chem. Eng., 2018, 6, 4253–4263 CrossRef CAS.
- P. Li, Z. Wang, L. Yang, S. Zhao, P. Song and B. Khan, A novel loose-NF membrane based on the phosphorylation and cross-linking of polyethyleneimine layer on porous PAN UF membranes, J. Membr. Sci., 2018, 555, 56–68 CrossRef CAS.
- X. Liang, P. Wang, J. Wang, Y. Zhang, W. Wu, J. Liu and B. Van der Bruggen, Zwitterionic functionalized MoS2 nanosheets for a novel composite membrane with effective salt/dye separation performance, J. Membr. Sci., 2019, 573, 270–279 CrossRef CAS.
- L. Zhu, H. Wang, J. Bai, J. Liu and Y. Zhang, A porous graphene composite membrane intercalated by halloysite nanotubes for efficient dye desalination, Desalination, 2017, 420, 145–157 CrossRef CAS.
- J. Wang, R. He, X. Han, D. Jiao, J. Zhu, F. Lai, X. Liu, J. Liu, Y. Zhang and B. Van der Bruggen, High performance loose nanofiltration membranes obtained by a catechol-based route for efficient dye/salt separation, Chem. Eng. J., 2019, 375, 121982 CrossRef CAS.
- Y. Cao, H. Zhang, S. Guo, J. Luo and Y. Wan, A robust dually charged membrane prepared via catechol-amine chemistry for highly efficient dye/salt separation, J. Membr. Sci., 2021, 629, 119287 CrossRef CAS.
- X. Chen, Y. He, Y. Fan, G. Zeng and L. Zhang, Nature-inspired polyphenol chemistry to fabricate halloysite nanotubes decorated PVDF membrane for the removal of wastewater, Sep. Purif. Technol., 2019, 212, 326–336 CrossRef CAS.
- Q. Li, Z. Liao, X. Fang, D. Wang, J. Xie, X. Sun, L. Wang and J. Li, Tannic acid-polyethyleneimine crosslinked loose nanofiltration membrane for dye/salt mixture separation, J. Membr. Sci., 2019, 584, 324–332 CrossRef CAS.
- J. Gao, Z. Thong, K. Y. Wang and T.-S. Chung, Fabrication of loose inner-selective polyethersulfone (PES) hollow fibers by one-step spinning process for nanofiltration (NF) of textile dyes, J. Membr. Sci., 2017, 541, 413–424 CrossRef CAS.
- T. Wang, X. He, Y. Li and J. Li, Novel poly(piperazine-amide) (PA) nanofiltration membrane based poly(m-phenylene isophthalamide) (PMIA) hollow fiber substrate for treatment of dye solutions, Chem. Eng. J., 2018, 351, 1013–1026 CrossRef CAS.
- J. Lin, W. Ye, M.-C. Baltaru, Y. P. Tang, N. J. Bernstein, P. Gao, S. Balta, M. Vlad, A. Volodin, A. Sotto, P. Luis, A. L. Zydney and B. Van der Bruggen, Tight ultrafiltration membranes for enhanced separation of dyes and Na2SO4 during textile wastewater treatment, J. Membr. Sci., 2016, 514, 217–228 CrossRef CAS.
- G. Han, Y. Feng, T. S. Chung, M. Weber and C. Maletzko, Phase Inversion Directly Induced Tight Ultrafiltration (UF) Hollow Fiber Membranes for Effective Removal of Textile Dyes, Environ. Sci. Technol., 2017, 51(24), 14254–14261 CrossRef CAS PubMed.
- Y. Xu, Z. Li, K. Su, T. Fan and L. Cao, Mussel-inspired modification of PPS membrane to separate and remove the dyes from the wastewater, Chem. Eng. J., 2018, 341, 371–382 CrossRef CAS.
- Y.-L. Ji, Q.-F. An, Q. Zhao, W.-D. Sun, K.-R. Lee, H.-L. Chen and C.-J. Gao, Novel composite nanofiltration membranes containing zwitterions with high permeate flux and improved anti-fouling performance, J. Membr. Sci., 2012, 390–391, 243–253 CrossRef CAS.
- J. Zhao, C. Fang, Y. Zhu, G. He, F. Pan, Z. Jiang, P. Zhang, X. Cao and B. Wang, Manipulating the interfacial interactions of composite membranes via a mussel-inspired approach for enhanced separation selectivity, J. Mater. Chem. A, 2015, 3(39), 19980–19988 RSC.
- J. Ding, H. Wu and P. Wu, Preparation of highly permeable loose nanofiltration membranes using sulfonated polyethylenimine for effective dye/salt fractionation, Chem. Eng. J., 2020, 396, 125199 CrossRef CAS.
- A. K. An, J. Guo, S. Jeong, E. J. Lee, S. A. A. Tabatabai and T. Leiknes, High flux and antifouling properties of negatively charged membrane for dyeing wastewater treatment by membrane distillation, Water Res., 2016, 103, 362–371 CrossRef CAS PubMed.
- C. Wang, Y. Chen, X. Hu and X. Feng, In-situ synthesis of PA/PVDF composite hollow fiber membranes with an outer selective structure for efficient fractionation of low-molecular-weight dyes-salts, Desalination, 2021, 503, 114957 CrossRef CAS.
- Y. Xu, J. Lin, C. Gao, B. Van der Bruggen, Q. Shen, H. Shao and J. Shen, Preparation of High-Flux Nanoporous Solvent Resistant Polyacrylonitrile Membrane with Potential Fractionation of Dyes and Na2SO4, Ind. Eng. Chem. Res., 2017, 56(41), 11967–11976 CrossRef CAS.
- J. Lin, W. Ye, H. Zeng, H. Yang, J. Shen, S. Darvishmanesh, P. Luis, A. Sotto and B. Van der Bruggen, Fractionation of direct dyes and salts in aqueous solution using loose nanofiltration membranes, J. Membr. Sci., 2015, 477, 183–193 CrossRef CAS.
- Y. F. Mi, N. Wang, Q. Qi, B. Yu, X. D. Peng and Z. H. Cao, A loose polyamide nanofiltration membrane prepared by polyether amine interfacial polymerization for dye desalination, Sep. Purif. Technol., 2020, 248, 117079 CrossRef CAS.
- Y.-F. Mi, G. Xu, Y.-S. Guo, B. Wu and Q.-F. An, Development of antifouling nanofiltration membrane with zwitterionic functionalized monomer for efficient dye/salt selective separation, J. Membr. Sci., 2020, 601, 117795 CrossRef CAS.
- S. Liu, Z. Wang, M. Ban, P. Song, X. Song and B. Khan, Chelation–assisted in situ self-assembly route to prepare the loose PAN–based nanocomposite membrane for dye desalination, J. Membr. Sci., 2018, 566, 168–180 CrossRef CAS.
- L. Zhang, H. Guan, N. Zhang, B. Jiang, Y. Sun and N. Yang, A loose NF membrane by grafting TiO2-HMDI nanoparticles on PES/β-CD substrate for dye/salt separation, Sep. Purif. Technol., 2019, 218, 8–19 CrossRef CAS.
- T. Tavangar, M. Karimi, M. Rezakazemi, K. R. Reddy and T. M. Aminabhavi, Textile waste, dyes/inorganic salts separation of cerium oxide-loaded loose nanofiltration polyethersulfone membranes, Chem. Eng. J., 2020, 385, 123787 CrossRef CAS.
- P. Zhang, J.-L. Gong, G.-M. Zeng, B. Song, W. Cao, H.-Y. Liu, S.-Y. Huan and P. Peng, Novel “loose” GO/MoS2 composites membranes with enhanced permeability for effective salts and dyes rejection at low pressure, J. Membr. Sci., 2019, 574, 112–123 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  *,
Qian
Chen
,
Jiapeng
Yang
and
Lina
Ge
*,
Qian
Chen
,
Jiapeng
Yang
and
Lina
Ge
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 50 wt%, aqueous solution), tannic acid (TA, purity: 98%) and polyethylene glycol (PEG) were supplied from Macklin Reagent (China). K2HPO4·3H2O (purity: ≥99%) and KH2PO4 (purity: ≥99.5%) were acquired from Aladdin Industrial Co.
000, 50 wt%, aqueous solution), tannic acid (TA, purity: 98%) and polyethylene glycol (PEG) were supplied from Macklin Reagent (China). K2HPO4·3H2O (purity: ≥99%) and KH2PO4 (purity: ≥99.5%) were acquired from Aladdin Industrial Co.
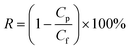
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)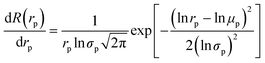
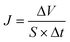

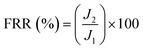
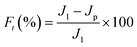
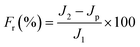
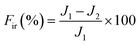

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds formed via the crosslinking between PEI and TA.28 In addition, a broad peak was observed at 3500 cm−1 in the loose NF membrane spectrum, representing an –OH stretching vibration. The XPS survey spectra for the original HFM and TA-PEI-modified HFM are shown in Fig. 4b. The typical peaks for C1s and F1s could be seen in the PVDF membrane spectrum, derived from the C and F elements of the PVDF membrane. Compared with the PVDF HFM, two new peaks for O1s and N1s emerged in the resultant loose NF membrane spectrum, which could be explained by the O and N elements in the TA-PEI-coated layer obtained by co-deposition. Due to the coatings, the F1s peak was significantly weakened in the XPS spectrum for the TA-PEI-modified HFM. These results demonstrated that the TA-PEI layer was successfully constructed on the original membrane surface via co-deposition. Fig. 4c and d show the high-resolution C1s spectra of the PVDF HFM and loose NF HFM. The C1s spectrum of the original HFM could be convoluted into two characteristic peaks: C–H at 286.1 eV and C–F at 290.6 eV. For the TA/PEI loose NF HFM, the C1 s spectrum could also be divided into other peaks: C–N bond at 285.5 eV, C
N bonds formed via the crosslinking between PEI and TA.28 In addition, a broad peak was observed at 3500 cm−1 in the loose NF membrane spectrum, representing an –OH stretching vibration. The XPS survey spectra for the original HFM and TA-PEI-modified HFM are shown in Fig. 4b. The typical peaks for C1s and F1s could be seen in the PVDF membrane spectrum, derived from the C and F elements of the PVDF membrane. Compared with the PVDF HFM, two new peaks for O1s and N1s emerged in the resultant loose NF membrane spectrum, which could be explained by the O and N elements in the TA-PEI-coated layer obtained by co-deposition. Due to the coatings, the F1s peak was significantly weakened in the XPS spectrum for the TA-PEI-modified HFM. These results demonstrated that the TA-PEI layer was successfully constructed on the original membrane surface via co-deposition. Fig. 4c and d show the high-resolution C1s spectra of the PVDF HFM and loose NF HFM. The C1s spectrum of the original HFM could be convoluted into two characteristic peaks: C–H at 286.1 eV and C–F at 290.6 eV. For the TA/PEI loose NF HFM, the C1 s spectrum could also be divided into other peaks: C–N bond at 285.5 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond at 286.3 eV, C
N bond at 286.3 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond at 288.3 eV, and C–C bond at 284.6 eV, which further indicated the formation of the TA/PEI-coating layer.20
O bond at 288.3 eV, and C–C bond at 284.6 eV, which further indicated the formation of the TA/PEI-coating layer.20











