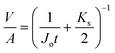Ultrafiltration behavior of CuO particles synthesized without and with different surfactants using PAN and PES membranes†
Olabimpe Genevieve
Badru
ab and
Ime
Akanyeti
 *bc
*bc
aEnvironmental Science, Institute of Graduate Studies and Research, Cyprus International University, Haspolat, Via Mersin 10, Nicosia, North Cyprus, Turkey. E-mail: smallbadru@gmail.com
bEnvironmental Research Centre, Cyprus International University, Haspolat, Via Mersin 10, Nicosia, North Cyprus, Turkey
cEnvironmental Engineering, Faculty of Engineering, Cyprus International University, Haspolat, Via Mersin 10, Nicosia, North Cyprus, Turkey. E-mail: iakanyeti@ciu.edu.tr; Tel: +90 6711111 2451
First published on 20th September 2024
Abstract
Four different CuO particles were synthesized, with no surfactant (CuO/NS) and with three surfactants: Triton X-100 (CuO/TX100), cetyltrimethylammonium bromide (CuO/CTAB) and sodium dodecyl sulfate (CuO/SDS). The filtration behavior of these particles at different concentrations with two UF membranes (PAN and PES), was studied. More than 99% CuO removal was obtained in all experiments, while the membrane fluxes showed variations. At 50 and 100 mg L−1 CuO concentrations, the normalized flux values were either 1.0, indicating no change, or were greater than 1.0, suggesting that the filtration of CuO particles improved the membrane flux. However, at 50 mg L−1, CuO/TX100 showed a significant flux decline for PES by 10%, while at 100 mg L−1 a 15% flux decline was observed for CuO/CTAB with PAN. At lower CuO/NS particle concentrations (<50 mg L−1), PAN showed a much larger flux decline of up to 27%, whereas the PES performance was more stable with a maximum decline of 5%. When the Cu2+ mass distribution was studied in the membrane system, the copper mass within the membrane for all types of particles was considerably larger for the PAN membrane than for the PES membrane. FT-IR results confirmed the appearance of new functional groups on PAN after the filtration of CuO/NS, indicating a possible interaction between Cu2+ and the membrane.
Water impactThe extensive use of metal oxides in industrial applications has environmental consequences when released into the environment. Detailed investigations on ultrafiltration of metal oxides and removal technology are limited. In particular, the behavior of metal oxides during ultrafiltration when particles are prepared using different surfactants is required to be elucidated. For the first time, we investigated the ultrafiltration performance when four different CuO particles prepared with and without surfactants were filtered at different concentrations. We concluded that although the CuO removal percentage was very high (>99%) and did not vary under the tested conditions, the membrane flux was considerably influenced by the surfactant type, particle concentration, and membrane type. Our process provides a dependable and sustainable strategy to protecting water quality by eliminating CuO. This immediately contributes to the provision of safe and sanitary drinking water for people around the globe. By creating measure to limit the discharge of CuO into natural water bodies, our study contributes to the preservation of aquatic ecosystems and the biodiversity. We believe that our manuscript will provide new insight into the ultrafiltration behavior of metal oxides prepared with and without different surfactants and the importance of membrane type/material selection for industrial wastewater treatment applications. |
1. Introduction
Industrialization, increase in population, and other worldwide trends have reduced the supply and availability of safe water.1,2 Hence, water reuse and recovery have become very important in meeting global water demands.3,4 The detection of various industrial by-products in wastewaters has emphasized the need to improve and develop new wastewater treatment methods, to reduce environmental pollution.1,5,6 Nanotechnology is an area of emerging interest in many industrial applications and is used to manufacture products related to health, paints, electronics, cosmetics and many others.7 Despite the benefits of using nanoscale processes and products in commercial applications, the related environmental risks should not be underestimated.8 The release of nanomaterials into the environment through various pathways results in serious environmental and public health problems.9 Metal oxide nanoparticles are one of the most widely employed nanomaterials,10 and their occurrence in industrial wastewater has previously been reported.11,12 Metal oxide nanoparticles, once released, have the ability to accumulate in the environment and have harmful impacts on both the ecosystem and human health.13–16 Metallic nanostructures, which are made up of just one metal element, are frequently stable and do not easily dissolve.17 When exposed to a biological environment, metal oxide and metal alloy-based nanomaterials typically display a lower level of stability and are more prone to dissolution and ion release, which causes the generation of reactive oxygen species and oxidative stress to cells.14,18 Amongst the numerous heavy metal oxide nanoparticles, copper oxide (CuO) have been characterized as soluble under certain conditions, because of their distinctive electrical and biocidal qualities.19 CuO nanoparticles are also reported to be more hazardous than their ionic form Cu2+, because of their oxidative nature.14In the past decade, the removal of heavy metals from water has been elucidated extensively, with different treatment methods20,21 such as ion exchange,22,23 chemical precipitation,24 adsorption25 and complexation ultrafiltration.26,27 However, the studies investigating the removal of metal oxides from wastewater streams are limited.28,29 A few studies which investigated the removal of CuO and other metal oxide particles from the water focused on coagulation,30 foam flotation,31 ultrafiltration32 and other hybrid methods.33–35 In contrast, CuO particles have been employed in membrane production to increase the permeate flux and reduce fouling.36 However, understanding how metal oxide particles synthesized with different starting materials and methods influence the fouling of membranes remains a major challenge.37 Various surfactants can be used in the synthesis of CuO particles due to different industrial applications.38–40 However, it is not well known how the type of surfactants used in the particle synthesis will influence the membrane performance during CuO filtration.
Previous studies showed that the addition of surfactants in the same solution with the surfactant free particles can influence the filtration and separation performance of UF membranes for various contaminants. Zhu et al.,41 reported that the presence of sodium dodecyl sulphate (SDS) and sodium dodecyl benzene sulfonate (LAS) surfactants in water containing algae can enhance the pore blockage which reduces the membrane flux. In some studies, surfactants are used to enhance the removal performance of the UF membranes. In the study of Aryanti et al.,42 where, sapindus rarak extract was used as a surfactant, the rejection percentage of remazol dye molecules was enhanced by 2%, while membrane flux was reduced. When surfactants are present, the critical micelle concentration (CMC) was shown to play an important role in the fouling behavior of the UF membranes.42,43 Mizoguchi et al.,43 reported that when the concentration of the surfactant was slightly lower than the CMC, the surfactant formed pre-micelles by concentration polarization on the surface of the UF membrane, which resulted in severe fouling.43 Similarly, in the study of Marques et al.,44 the use of polyvinyl alcohol (PVA) as a surfactant resulted in a decline in UF flux due to the accumulation of the loaded solution on the membrane surface. Depending on the ionic properties of the surfactant used, interaction between the membrane and the surfactant can vary. According to the findings of Fernández et al.,45 depending on the surfactant type, electrostatic or hydrophobic interactions can play a role, influencing both surfactant retention and membrane flux. Trzaskus et al.32 studied the influence of surfactant type and concentration on the retention and fouling behavior of silica nanoparticles with UF membrane and showed significant variations. Most of the studies reported above investigated the influence of different surfactant types and surfactant concentrations added to the feed solution on the UF membrane performance. Up to the authors' knowledge, the influence of metal oxide particles synthesized without and with different surfactants on membrane filtration performance has not been studied in detail.
In this study, four different CuO particles were synthesized without and with three different surfactants; TX-100 (non-ionic), CTAB (cationic), and SDS (anionic) and characterized extensively. The selection of surfactants was based on their notable particle stability effect, ability to regulate size and charge, and their widespread use in particle synthesis.38 The influence of the four different CuO particles on the ultrafiltration performance was elucidated for two distinct types of UF membrane made of different polymeric materials and molecular weight cut-off. In addition, the filtration behavior of the same UF membranes were studied with CuO particles synthesized with no surfactant at varying particle concentrations.
2. Materials and methodology
2.1 Chemicals
Analytical grade copper sulphate pentahydrate (CuSO4·5H2O), 65% nitric acid (HNO3), sodium hydroxide (NaOH), diethyl ether (C2H5OC2H5) and hydroxylammonium chloride (NH2OH·HCl) were purchased from Merck Millipore (Massachusetts, USA). 95% ethanol (C2H5OH) was obtained from a local supplier, while the surfactants; TritonX-100 (TX-100; C14H22O(C2H4O)n) in liquid form, cetyltrimethylammonium bromide (CTAB; C19H42BrN) and sodium dodecyl sulfate (SDS; NaC12H25SO4), all in powder form were purchased from Sigma-Aldrich (St. Louis, USA), Carl Roth GmbH (Karlsruhe, Germany) and Bio Shop (Burlington, Canada), respectively. The chemical characteristics of the surfactants have been provided on Table S1.† All synthetic solutions were prepared using distilled water produced with a Sartorius Stedim Arium 61316 water purification system (Göttingen, Germany).2.2 Membranes
The MW series 50 kDa polyacrylonitrile, PAN (GE Osmonics) and LY series 100 kDa polyethersulfone, PES (Snyder) flat sheet UF membranes were purchased from Sterlich (Auburn, USA). The membranes utilized were of distinct characteristics to investigate particle–membrane interactions under the same conditions.2.3 Synthesis of CuO particles
Four types of copper oxide particles, with 1) no surfactant (CuO–NS), 2) TX-100, 3) CTAB and 4) SDS were synthesized using the simple precipitation method adapted from previous work.46,47 For the synthesis of CuO–NS, 20 g of copper sulphate pentahydrate was mixed with 9 g of hydroxyl ammonium chloride in 45 mL of distilled water. The mixture was then allowed to cool down in a water bath shaking at 1000 rpm and 35 °C. Afterwards 222 mL of 0.75 M sodium hydroxide was added into the solution stirred at 700 rpm for 1 hour. For the particles with surfactants, 3% (w/v) CTAB, 3% (w/v) SDS and 3% (v/v) TritonX-100 were added to the mixture under constant stirring. When the muddy brown color was observed the stirring was stopped and the precipitation took place. The supernatant free residue was added into 100 mL of distilled water and the mixture was shaken vigorously. The content was washed several times to separate the chloride content and the free surfactant from the dispersed solution. 95% ethanol and ether were used as the final washing solutions. The residue was filtered and dried at 220 °C for 24 hours. Finally, the samples were further dried at 250 °C for 12 hours. Dried CuO particles were mixed with 1 L distilled water to prepare experimental feed solutions. The presence of free surfactant in the synthesized particle solutions was checked with UV-VIS spectrophotometer and it was confirmed that the free surfactant concentration was negligible (Fig. S1 in the ESI†).2.4 Characterization of CuO particles and membranes
The crystallinity of all CuO particles were determined using an XRD (X-ray diffraction) Ultima IV powder X-ray (Rigaku, Japan) diffractometer, using a CuKα as the radiation source of 40 kV/30 mA, with a scanning range of 20–70° (2θ) and a scan step of 2° min−1. Data analyses were performed using PC-APD diffraction software and Match! software. The average crystalline size of CuO particles was calculated using the Derby–Scherrer eqn (1),3,39,48 where D is the size of the crystal, λ is the X-ray wavelength (0.15405 nm), β is the full width half maximum (FWHM) in radians, k is the crystallite shape factor (0.9), and θ is the diffraction angle in degrees. | (1) |
The size distribution and average size of the aggregated particles were analyzed using a Mastersizer 2000 ver. 5.60 particle size analyzer equipped with Mie light scattering with a measuring range of 0.02–2000 μm, absorption coefficient of 0.1 and a light refractive index of 2.6 and 1.33 for the particle and the dispersant phase (water), respectively. 1 mL of sample was transferred into a Hydro 2000G dispersion unit, with a pump speed and stirring speed of 850 rpm.
The zeta potential and diffusion coefficient of all particles were analyzed using a Zetasizer Ultra-pro (Malvern Analytical, Netherlands) with a dynamic light scattering with mixed-mode measurement phase analysis light scattering (M3-PALS), maximum sample conductivity of 260 mS cm−1 and a size range of 3.8 nm to 100 μm diameter. The particle solutions for size, zeta potential and distribution analysis were prepared by adding 2 mg of particle into 50 mL distilled water. The solution was sonicated for 30 minutes at 25 ± 5 °C. For zeta potential analysis, the sample was filtered with a 0.45 μm syringe filter and left to stand for 48 hours before the analysis. The results of zeta potential, size and particle distribution analysis have been presented as mean values (± standard deviation errors) of three repeats.
The effective surface areas, total pore volumes, adsorption average pore diameter, micropore volume and BJH desorption average pore size of both PAN and PES membranes were determined based on the Brunauer, Emmett and Teller (BET) and Joyner and Halenda (BJH) models. Prior to the analysis both PAN and PES were degassed at 65 °C and 50 °C respectively. MicroActive Tristar II plus 2.0.2 software was used to analyze the results. Contact angle measurements of virgin membranes and membranes with particle deposits (50 mg L−1 particle concentration) were conducted by the static contact angle measurement (sessile drop). Samples were dried prior to analysis to ensure the removal of any trace of water content on all samples to be analyzed. The samples were measured by a liquid drop optical tensiometer (Biolin Scientific, China Beijing) and recorded with the One Attension Software. SEM images of virgin membranes and membranes with their deposit layers formed after particle filtration (CuO/NS, CuO/TX-100, CuO/CTAB and CuO/SDS) were examined using a GAIA3 + Oxford XMax 150 EDS Focused Ion Beam-Scanning Electron Microscopy (FIB-SEM) (Tescan, Czech Republic). BET, contact angle and SEM analysis were conducted at Hacettepe University Advanced Technologies Research and Application Center (HUNITEK).
2.5 Stirred cell and membrane filtration protocol
All UF experiments were conducted using a stirred dead-end system equipped with a 300 mL stainless-steel cell and a membrane holder with an active membrane surface area of 0.0008042 m2. The cell contained a magnetic stirrer assembly (Sterlich, Auburn U.S.A.) operated at 300 rpm, whilst placed on a Velp Scientifica magnetic stirrer (New York, U.S.A.) at room temperature (25 ± 5 °C). The permeate was collected in an Erlenmeyer flask placed on a Radwag PS 1000.R2 precision balance (Torunska, Poland). The flux was calculated using the permeate mass collected every minute with a data acquisition software (Pomiar Win 4.0.4, Väddö, Sweden) connected to the balance.Prior to each experiment, PAN and PES membranes were pre-conditioned. PAN membranes were first soaked in distilled water for 24 hours while PES membranes were soaked in 50% (v/v) isopropyl alcohol for 15 min. Both membranes were finally rinsed with distilled water. After pre-conditioning, the membranes were compacted and pre-fluxes were determined with distilled water at 14.50 psi (1 bar) and 43.51 psi (3 bar) for PAN and PES membranes, respectively.
Synthetic particle solutions at different concentrations were prepared by diluting the stock solutions of 1000 mg L−1 and sonicating at 25 ± 2 °C for 1 h prior to membrane filtration. Particle concentration experiments were conducted at 1–100 mg L−1 with PAN and PES membranes using only CuO–NS particles. The influence of surfactants used to synthesize the particles on the membrane performance was evaluated, conducting experiments at 50 mg L−1 and 100 mg L−1 with all four particles using both PAN and PES membranes. The experimental particle concentrations were selected based on the concentration range (5–100 mg L−1) of CuO detected in industrial wastewater.49 For the filtration experiments, 180 mL of particle feed solution was fed to the system and four permeate samples were collected until no solution was left in the cell and all the particles were deposited on the membrane. Following the particle filtration, post flux data were collected using distilled water to maintain the experimental conditions. Finally, the membrane was removed from the holder and various samples were collected using 1 M HNO3 for the Cu2+ mass balance calculations. The surface of the membrane was spray washed with 1 M HNO3 solution and the washing solution was collected and weighed on the analytical balance. The Cu2+ mass was calculated multiplying the washing solution volume with the Cu2+ concentration detected in the solution and named as “membrane ON”. Afterwards, the membrane was soaked in 10 mL 1 M HNO3 solution for 24 hours, to recover the Cu2+ mass that could not be recovered by surface spraying. The Cu2+ mass was calculated by multiplying 10 mL with the detected Cu2+ concentration in the soaking solution and named as “membrane IN”. The Cu2+ concentration in feed, permeate, membrane ON and membrane IN, samples were analyzed.
2.6 Atomic absorption spectrophotometer analysis of Cu
The concentration of Cu2+ in all samples was determined with Flame Furnace system – AA-700, Shimadzu atomic absorption spectrophotometer (AAS) (Perkin Elmer, USA). For the preparation of the calibration curve, copper standard solutions were prepared by diluting the stock solution of 1000 mg L−1 with 1 N HNO3. The calibration curve with an R2 > 0.99 was obtained for a concentration range of 0.5–2 mg L−1. The required dilutions were made for the samples with a concentration above the calibration range and the samples were filtered with a 0.45 μm syringe filter, prior to the analysis. All analysis were done in 3 replicates. The averages of representative samples were taken to calculate the mass distribution percentages.2.7 Fouling models
Fouling models (Table 1) were applied in non-linear form to the experimental data using Origin Pro 2019b.2.8 Data analysis
The Cu2+ mass balance was checked using the following eqn (2) where, Vf, Vpi, Vmi and Vmo are the volume (L) of feed, permeate, membrane IN and membrane ON, respectively and Cf, Cpi, Cmi and Cmo are the Cu2+ concentration (mg L−1) of feed, permeate, membrane IN and membrane ON, respectively and Mun is the Cu2+ mass unaccounted in the system. | (2) |
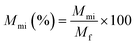 | (3) |
 | (4) |
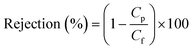 | (5) |
 | (6) |
3. Result and discussion
3.1 Membrane characteristics
The membrane characteristics and the filtration conditions have been presented on Table 2. Considering the contact angle and the water content obtained in this study, PES is a less hydrophilic membrane when compared to PAN, which shows more hydrophilic characteristics. Both membranes were expected to be negatively charged, over a wide range of solution pH, having zeta potential values around −40 and −50 mV at neutral pH. The negative charge of the membranes could be attributed to the presence of sulfonic group (SOO−) and carboxyl group (COO−) on PES and PAN polymers, respectively.| Polymer | PAN (50 kDa) | PES (100 kDa) |
|---|---|---|
| a Not specific for GE Osmonics. b Specific for PES 50 kDa. | ||
| Average pure water flux (L m−2 h) | 216.3 ± 24.7 | 456.4 ± 16.5 |
| Pure water permeability (L m−2 h bar) | 216.3 ± 24.7 | 152.2 ± 5.5 |
| Contact angle (°) | 37.56 ± 0.43 | 45.74 ± 0.11 |
| Zeta potential (mV) | Negatively charged at pH > 3.5, −50 mV at pH 7 (ref. 51 and 52)a | Negatively charged at pH > 4, −40 mV at pH 7 (ref. 53)b |
| ∼−40 mV at pH 7 (ref. 51)a | ||
| Water content (%) | 58.4 | 18.7 |
| BET surface area (m2 g−1) | 3.05 | 14.03 |
| Total pore volume (cm3 g−1) | 0.009 | 0.031 |
| Adsorption average pore diameter (nm) | 12.1 | 8.83 |
| Micropore volume (cm3 g−1) | 0.003 | 0.001 |
| BJH desorption average pore diameter (DBJH) (nm) | 28.0 | 36.5 |
The BET results showed that the average mesopore diameter obtained with BJH desorption was larger for PES membrane (36.5 nm) when compared to that of PAN (28.0 nm). While the pore volume of PES was almost three times the volume for PAN, the available micropore volume for PAN membrane was twice as much as that for PES indicating the presence of more micropores. This was expected considering that PAN has a smaller MWCO compared to PES. With a larger total pore volume, the BET surface area of the PES (14.0 m2 g−1) was larger than that of PAN (3.05 m2 g−1). Although pore volume was larger for PES, water absorption capacity was lower due to its less hydrophilic nature. N2 adsorption–desorption isotherms presented in Fig. 1 conforms to the type IV model based on the IUPAC classification. Both membranes display characteristic hysteresis loops appearing in the multilayer region (Fig. 1a and b). The hysteresis loops of PAN (Fig. 1c) and PES (Fig. 1d) membranes have resemblances to the shapes observed in H1 and H2(b) hysteresis (Fig. S2 in ESI†), respectively.54,55 Based on this resemblance, the pores of PAN may exhibit a cylindrical shape with uniformity, while the pores of PES possibly possess a shape resembling that of an ink bottle. The pore shapes confirm PAN membrane having a larger average pore size of 12.1 nm as compared to PES membrane that has an average pore size of 8.83 nm, regardless of their MWCO. PES membrane has a wider range of pore sizes (5.28–81.3 nm) compared to that of PAN membrane (6.66–103 nm) as presented in the pore size distribution Fig. S3.†
 | ||
| Fig. 1 Nitrogen adsorption–desorption isotherm of (a) PAN, (b) PES membranes; hysteresis loops of (c) PAN, (d) PES. | ||
3.2 Particle characteristics
The phase compositions and crystallinity of all synthesized particles (CuO/NS, CuO/TX-100, CuO/CTAB, and CuO/SDS) were determined by broad-angle XRD as presented in Fig. 2. Observed diffraction peaks within the 2θ values of a 25° to 70° range for CuO/NS, CuO/TX-100, CuO/CTAB, and CuO/SDS had similar angles of 32.72 (1, 1, 0), 35.56 (0, 0, 2), 38.8 (1, 1, 1) (JCPDS: 048-1548), 48.96 (1, 1, −2) (JCPDS: 089-5899), 54.68 (2, 0, −2), 58.82 (2, 0, 2), 61.86 (1, 1, −3), 66.94 (0, 2, 2), and 68.42 (1, 1, 3) degrees, indicating the formation of CuO.48 The additional 2θ values of 30.4, 36.5 (JCPDS: 079-2301) and 42.58 degrees for CuO/SDS showed the presence of Cu2O.56 During the particle synthesis, SDS was expected to undergo complete ionisation into dodecyl sulphate ion (DS−) in the presence of water. The DS− ions were expected to interact with the Cu2+ and form a DS−[Cu−(OH)4]2− complex which will contribute to the formation of both CuO and Cu2O (ref. 57) explaining the presence of additional Cu2O peaks in the XRD results (Fig. 2). | ||
| Fig. 2 XRD patterns of all synthesized particles (CuO/NS, CuO/TX-100, CuO/CTAB & CuO/SDS); CuO (*), Cu2O (#). | ||
The crystallite size of the synthesized particles is presented in Table 3. The unit cell parameters for all particles were a = 4.67 Å, b = 3.43 Å and c = 5.12 Å, where a ≠ b ≠ c, suggesting all particles synthesized have orthorhombic crystal shapes.58
| Particles | Crystallite size (XRD) (nm) | Zeta potential (Zetasizer) (mV) | Aggregated average size (Mastersizer) (nm) | Diffusion coefficient (cm2 s−1) |
|---|---|---|---|---|
| CuO/NS | 28.15 ± 13.34 | −41.53 ± 3.558 | 415 ± 109.7 | 7.169 × 10−8 |
| CuO/TX-100 | 29.52 ± 19.19 | −60.34 ± 11.12 | 373 ± 89.63 | 1.773 × 10−7 |
| CuO/CTAB | 26.00 ± 7.66 | −34.46 ± 0.7502 | 732 ± 45.57 | 4.558 × 10−8 |
| CuO/SDS | 31.85 ± 27.47 | −41.81 ± 14.39 | 775 ± 64.93 | 7.167 × 10−8 |
The results shown in Table 3 indicated that the crystallite size of the particles in powder form varied between 28.1 nm to 31.8 nm, CuO/CTAB (26 nm) being slightly smaller and CuO/SDS (∼32 nm) being slightly larger than the others. When the size of the particles was measured in solution, larger values were obtained. Lim and Matusiak59,60 reported that colloidal particles in solution can overcome repulsive forces leading to flocculation59 and cause colloidal instability,60 which supports the aggregation behavior of the particles as observed in this study. When the aggregated sizes are considered (Table 3), CuO/NS and CuO/TX100 showed much smaller sizes as 415 nm and 373 nm, respectively, almost half of the size of that for CuO/CTAB (732 nm) and CuO/SDS (775 nm). The size distributions for all the particles are provided in Fig. S4.†
As shown in Table 3, the zeta potential of CuO/NS was −41.53 mV, which agrees with the findings of Aparna et al.,61 suggesting that the particles are negatively charged and moderately stable. The surface of CuO nuclei is usually positive or negative, and dependent on the amount of OH− and Cu2+ ions present.39 In this research, due to the use of NH2OH·HCl and NaOH during synthesis, OH− was the dominant ion hence allowing for the surface charge of CuO/NS to be negatively charged. While the CuO particles were formed in the presence of TX-100 the surfactant adsorbs on the particle.62 Hence, the OH− tail on the surfactant increases the number of negatively charged functional groups on the particles resulting in a reduction of the zeta potential as observed in Table 3. For CuO/TX-100 the zeta potential is the smallest (−60.34 mV) suggesting that the particles became more stable. More stable particles tend to aggregate less forming smaller clusters as can be observed from the aggregated size of 373 nm for CuO/TX-100. Zeta potential was measured as −34.46 mV for CuO/CTAB, which was less than that of CuO/NS. The reduced zeta potential was attributed to the electrostatic interaction between the negatively charged CuO particle (Table 1) and the positively charged CTAB (29.84 mV) (Table S1†) agreeing with the previous study of Li et al.63 The reduction in the charge is likely to be caused by complete ionization of CTAB, where the Br− ion is dissociated from the CTAB molecule, leaving a cationic CTA+ that is adsorbed on the surface of the CuO particle through electrostatic interactions. Lower zeta potential value for CuO/CTAB is an indication for less stability inducing more aggregation which can explain the larger aggregated size of 732 nm for CuO/CTAB.
CuO/SDS particles had a similar zeta potential of −41.81 mV to that for CuO/NS indicating a little or no effect of the SDS on particle charge. SDS micelles, due to their low diffusion coefficient (Table 3), are not expected to adsorb on the surface of CuO particles explaining why SDS did not influence the charge of the particle. Similarly in the study of Trzaskus,32 the charge of silica particles was not influenced by the presence of SDS indicating minimal interaction between the surfactant and the particles. Due to the limited interaction between the SDS and CuO particles, agglomeration was observed leading to an increased aggregated particles size (Table 3). Another potential explanation for the lack of influence of SDS on particle charge during particle synthesis could be the low concentration of SDS used during particle synthesis, aligning with the research conducted by Liao et al.64
3.3 The influence of concentration and type of CuO particles on PAN and PES flux
The influence of four types of particles on UF behavior was investigated at 50 and 100 mg L−1 concentrations with PAN and PES membranes and the results are presented in Fig. 3. | ||
| Fig. 3 Influence of particle type on PAN and PES membrane flux at the particle concentrations a) 50 mg L−1 and b) 100 mg L−1. | ||
Normalized flux presented in Fig. 3a clearly showed that the flux of PAN membrane was enhanced when 50 mg L−1 particle concentration was filtered regardless the type of the particle. With PES, at the same concentration (50 mg L−1), normalized flux increased to above 1.0 with CuO/NS and CuO/CTAB, while it did not change with CuO/SDS and reduced by 7% with CuO/TX100. In order to investigate the change in membrane flux, alterations of the hydrophilicity of the membranes after particle filtration was investigated. Contact angle measurements of the virgin membranes and the particle deposits on the membrane were conducted for the experiments with 50 mg L−1 particle concentration and results are demonstrated in Fig. 4. For both PAN and PES membranes, when CuO/NS was filtered their contact angle values were reduced by 24% and 53%, respectively, showing an increased hydrophilicity. Increased hydrophilicity can explain the slightly increased normalized flux values for both membranes after CuO/NS filtration (Fig. 3a). With CuO/TX-100, the hydrophilicity of PAN membrane did not significantly change while the contact angle increased by 34% for PES membranes. The considerable increase in the PAN flux with CuO/TX-100 cannot be attributed to the change in the hydrophilicity. However, the increased hydrophobicity (34%) of the PES membrane can be the reason of the reduced normalized flux after CuO/TX-100 particles were filtered. Both membranes showed increased contact angles after the filtration of CuO/CTAB and CuO/SDS; CuO/SDS had the highest increase. In contrast, normalized flux values showed an increase or no change.
 | ||
| Fig. 4 Contact angle of virgin PAN and PES membranes and membranes with particle (CuO/NS, CuO/TX-100, CuO-CTAB, CuO/SDS) deposits. | ||
When 100 mg L−1 particle concentrations were considered instead of 50 mg L−1 (Fig. 3b), flux enhancement was not as obvious for most of the particle types (Fig. 3a). These reduced flux values at higher particle concentration can be attributed to varied factors. Several studies suggested that metal oxide particles may result in improvement of the membrane hydrophilicity when an optimum amount of particles is used; however above this optimum amount, flux tends to decline.65 Another explanation could be increased deposit thickness at higher concentrations resulting in increased resistance hence membrane flux decline.66
It is important to discuss the influence of each particle type on UF flux separately. With CuO/NS particles, similar normalized flux values indicate that under the tested experimental conditions, membrane performances were not influenced by the particle concentration when particles were prepared with no surfactant. Regardless of the concentration of the particles, the normalized flux did not change considerably for CuO/NS.
For CuO/TX-100 with PAN membrane, the normalized flux declined by 10% when the particle concentration was increased from 50 to 100 mg L−1, while it increased by 14% with PES membrane. During the filtration of CuO/CTAB with PAN and PES membranes, the normalized flux declined by 37% and 15%, respectively, when the particle concentration was increased from 50 to 100 mg L−1. Similar normalized flux values were obtained with PAN when CuO/SDS concentration was increased from 50 mg L−1 to 100 mg L−1, while a 10% increase was observed with PES membranes. The findings clearly showed that particles prepared without or with different surfactants behaved differently during UF. To explain these differences, the particle and membrane characteristics were considered in the following discussion. The membranes were imaged before and after particle deposition at 50 mg L−1 concentration and the images are presented in Fig. S5.† Moreover, the porosity of the particle deposit layers (50 mg L−1) on the membranes were elucidated with SEM analysis (Fig. 5). In addition, the distribution of Cu2+ mass in the system was investigated at 50 mg L−1 and 100 mg L−1 concentrations with both membranes for all particles (Fig. 6 and S6†).
 | ||
| Fig. 5 SEM images of virgin membranes and cake layer of all particles on membrane at 2 μm magnification (50 mg L−1 particle concentration). | ||
 | ||
| Fig. 6 The % Cu2+ mass distribution in membrane filtration systems for all particles a) PAN: 50 mg L−1 CuO b) PES: 50 mg L−1 CuO c) PAN: 100 mg L−1 CuO d) PES: 100 mg L−1 CuO. | ||
One of the important findings presented in Fig. 6 was that the percentage of Cu2+ mass washed from the membrane surface (membrane ON) was higher with PES membrane (Fig. 6b and d and S6b and d†) for all types of particles compared to those obtained with PAN membrane (Fig. 6a and c and S6a and c†). In contrast, the percentage of Cu2+ mass that represents the “membrane IN” was obviously higher with PAN membrane for all types of particles compared to those with PES. Images in Fig. S5† confirm these results with much less particles being deposited on PAN membrane in comparison to PES. This difference could be related to the slightly more hydrophilic nature of the PAN membrane and the enhanced diffusion of the CuO particles into the pores of the hydrophilic membrane.
For all the experiments presented in Fig. 6, the removal percentages of the CuO particles were above 99% and the permeate Cu2+ concentrations were below the detection limit (0.029 mg L−1) of the AAS complying with the drinking water standards of World Health Organization (2 mg L−1)67 and United States Protection Agency (USEPA) (1.3 mg L−1).68 When the Cu2+ mass distribution was compared for the different particles, the percentage “membrane IN” followed the order of CuO/TX100 > CuO/NS ≃ CuO/SDS > CuO/CTAB as shown in Fig. 6a and S6a.† This order was similar to the order of the diffusion coefficients of the particles as presented in Table 3. CuO/TX-100 had the highest diffusion coefficient value of 1.773 × 10−7 cm2 s−1 and showed the highest “membrane IN”, with 60% for PAN membrane at 50 mg L−1 particle concentration. Diffusion of CuO particles into the porous structure of the PAN membrane can be a reason for the improved flux for this membrane although there is no direct relationship between the diffusion coefficient and membrane flux. This improved flux values were observed in Fig. 3 with normalized flux values larger than 1.0 for all types of particles with PAN compared to the ones with PES at 50 mg L−1. In Fig. 5, it was also observed that the porosity of the deposit layers on PAN for all particles are more than that obtained with PES membrane, resulting in reduced deposit resistance and higher flux values.
At 100 mg L−1, the normalized flux was comparable for PAN and PES with all particles except CuO/CTAB. Khezraqa et al.69 reported that when 2 wt% CuO particles was added to PVC membranes, the porosity and hydrophilicity declined, compared to the lower percentages due to the agglomeration of the particles. Similarly in this study, lower normalized flux values obtained for PAN with CuO/TX100 and CuO/CTAB at 100 mg L−1, compared to the ones at 50 mg L−1, can be attributed to the agglomeration of the particles and hence, reduced porosity and/or hydrophilicity.
For PES, the same diffusion effect was not observed due to the less hydrophilic nature of the membrane at 50 mg L−1. The “membrane IN” for CuO/TX-100 was considerably larger than those obtained for the other particles with PES at 50 mg L−1. The large value of “membrane IN” may indicate CuO/TX-100 particles penetrating the voids of PES hence causing pore blockage or stronger physical/chemical interaction with the membrane material, both of which may explain the reduced flux value for this specific experiment. CuO/TX-100, with a zeta potential value of −60 mV, was the most stable particle amongst all four particles and was expected to aggregate less, compared to the other particles. The aggregated size of the CuO/TX-100 particles was measured as 373 nm (Table 3) being the smallest among all particles. At lower concentrations it is more likely that particles stay individually or in smaller aggregated sizes, causing several types of pore blockage, especially for PES membranes with a larger pore volume (Table 3). In addition, the smaller size aggregates can form less porous deposits with larger resistance, causing a reduced flux. In Fig. 5, less porous deposit layer obtained with CuO/TX-100 filtered with PES was observed in comparison to that of PAN. However, at higher concentrations the aggregated sizes can get larger, and the deposit resistance may be reduced, due to the reduced porosity, which can explain the increased flux at 100 mg L−1 for CuO/TX-100.
For CuO/CTAB, a considerable flux decline was observed when the particle concentration increased from 50 to 100 mg L−1 with PAN. For this specific experiment, the “membrane IN” percentage was more than double the amount obtained at 50 mg L−1. Pore blockage or possible interaction with the membrane material could be the reason for this enhanced flux decline. If the stability of the particles is considered CuO/CTAB is the least stable particle among the four with the largest zeta potential value (−34 mV) (Table 3) and expected to form the least porous aggregates. According to deposit filtration theory70 deposit resistance is directly proportional to the deposit thickness and inversely proportional to the deposit porosity. Reduced normalized flux with CuO/CTAB at 100 mg L−1 can be attributed to the increased resistance due to the less porous aggregates forming the deposit and increased deposit thickness at a larger concentration.
3.4 The influence of CuO/NS particle concentration on membrane flux for PAN and PES membranes
To investigate the behavior of the two distinct types of UF membrane for the filtration of particles at various concentrations, CuO/NS was filtered at five more different concentrations ranging between 1–20 mg L−1 in addition to 50 and 100 mg L−1 (Fig. 7).Considering at 50 and 100 mg L−1 filtration of CuO/NS particles improved the flux with a normalized flux value above 1.0, for both PAN and PES membranes, at lower concentrations the membrane fluxes differed considerably. With PAN membrane, between 1–20 mg L−1, as the CuO/NS particle concentration decreased, the flux decline increased (Fig. 7a). With PES membrane, at the particle concentration of 1 and 2 mg L−1, their normalized fluxes were remarkably close to 1.0, indicating no decline in the flux at those concentrations. However, when the concentration increased to 5 and 10 mg L−1, their normalized fluxes declined to 0.98 and 0.95 respectively, representing a decrease in membrane performance (Fig. 7b). At 20 mg L−1, the normalized flux increased to 1.1 and became comparable to the values obtained at 50 and 100 mg L−1. CuO/NS particles showed an improvement of the fluxes for both polymeric membranes at an optimized concentration which agrees with literature.65,66,71
It was interesting to observe that at lower CuO/NS concentrations the membrane fouling was more severe with PAN membrane in comparison to PES. Although, an increased contact due to a higher surface area and more interaction could be expected between the particles and the PES membrane in comparison to the PAN membrane, as seen in Table 2,72 but in this study, this was not observed. Also, as the BET surface area increased, the material was expected to have a more porous structure and likely to be more permeable.73 However, in this study, PES was a more porous membrane with almost five times higher SBET compared to that of PAN. It was expected that particles would penetrate the pores of PES and cause membrane fouling due to its larger BJH average pore diameter and more porous structure. However, it was observed that PAN membrane experienced more fouling than PES membranes at lower CuO/NS concentrations.
To understand the fouling mechanisms further, membrane fouling models for constant pressure filtration were applied to the data for the experiments, where flux declines were experienced. The model fitting coefficients, and their constants are presented in Table 4. Cake layer formation with high regression coefficient (R2) and the least residual sum of squares (SSR) showed to be the best fit model for both membranes. The particle deposits on both membranes were also observed (Fig. S5†) after the experiments. The aggregated size of the CuO/NS was comparably larger than the pore size range of the membranes (Fig. S4†) and particles clearly formed deposit layers on membrane surface. However, looking at the relatively close R2 and SSR values, other fouling mechanisms can also contribute to the fouling. The second dominating model for PAN membrane was the intermediate blocking, indicating particles have settled on the previously settled particles. For PES membrane, both intermediate and standard blocking had relatively close values for R2 and SSR, suggesting that particles accumulated inside the membrane, on the pore walls. PES membrane having a larger pore volume may have induced more particles penetrating the pores, thereon causing pore constriction.
| Samples | Cake layer formation | Intermediate blocking | Standard blocking | Complete blocking | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R 2 | SSR | K c (s m−2) | R 2 | SSR | k i (m−1) | R 2 | SSR | K s (m−1) | R 2 | SSR | K b (s−1) | |
| PAN CuO/NS 1 mg L−1 | 0.9991 | 2.97 × 10−4 | 4.28 × 104 | 0.9987 | 4.15 × 10−4 | 2.29 | 0.9985 | 4.88 × 10−4 | 2.15 | 0.9983 | 5.73 × 10−4 | 1.22 × 10−4 |
| PAN CuO/NS 2 mg L−1 | 0.9999 | 2.29 × 10−5 | 1.97 × 104 | 0.9999 | 2.31 × 10−5 | 1.13 | 0.9999 | 2.41 × 10−5 | 1.08 | 0.9999 | 2.58 × 10−5 | 6.28 × 10−5 |
| PAN CuO/NS 5 mg L−1 | 0.9967 | 1.06 × 10−3 | 4.89 × 104 | 0.9960 | 1.31 × 10−3 | 2.57 | 0.9955 | 1.46 × 10−3 | 2.40 | 0.9998 | 3.41 × 10−4 | 1.39 × 10−4 |
| PAN CuO/NS 10 mg L−1 | 0.9984 | 5.84 × 10−4 | 3.23 × 104 | 0.9981 | 6.87 × 10−4 | 1.76 | 0.9979 | 7.47 × 10−4 | 1.68 | 0.9978 | 8.12 × 10−4 | 9.6 × 10−5 |
| PAN CuO/NS 20 mg L−1 | 0.9979 | 6.94 × 10−4 | 3.18 × 104 | 0.9977 | 7.92 × 10−4 | 1.75 | 0.9975 | 8.46 × 10−4 | 1.66 | 0.9973 | 9.49 × 10−4 | 9.56 × 10−5 |
| PES CuO/NS 5 mg L−1 | 0.9906 | 1.65 × 10−3 | 2.94 × 104 | 0.9849 | 2.07 × 10−3 | 3.06 | 0.9882 | 2.33 × 10−3 | 2.77 | 0.9868 | 2.64 × 10−3 | 3.17 × 10−4 |
| PES CuO/NS 10 mg L−1 | 0.9979 | 3.06 × 10−4 | 1.10 × 104 | 0.9977 | 3.38 × 10−4 | 1.29 | 0.9976 | 3.36 × 10−4 | 1.23 | 0.9975 | 3.75 × 10−4 | 1.49 × 10−4 |
FT-IR analysis of the virgin and fouled membranes was also studied to clarify whether there was any interaction between CuO/NS and the polymeric membranes. The FT-IR spectra of virgin and CuO/NS filtered membranes are presented in Fig. 8a and b for PAN and PES, respectively. For PAN membrane (Fig. 8a), the absorption peaks at 3404 cm−1 and 1737 cm−1 correspond to hydroxyl (–OH), carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively.74 There were also distinct peaks at 2247 cm−1, 1658 cm−1, and 1242 cm−1, corresponding to the stretching of the triple, double and single bond between carbon and nitrogen (–C
O), respectively.74 There were also distinct peaks at 2247 cm−1, 1658 cm−1, and 1242 cm−1, corresponding to the stretching of the triple, double and single bond between carbon and nitrogen (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, –C
N, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, –CN).75 In addition, the peaks at 2924 cm−1, 1448 cm−1 and 1373 cm−1 were attributed to –CH stretch, –CH2 and CH3 bends, respectively. For PES membranes, the peak at 719 cm−1 was attributed to the –CH stretching bond, while the ones at 1487 cm−1 and 1587 cm−1 were corresponding to the C
N, –CN).75 In addition, the peaks at 2924 cm−1, 1448 cm−1 and 1373 cm−1 were attributed to –CH stretch, –CH2 and CH3 bends, respectively. For PES membranes, the peak at 719 cm−1 was attributed to the –CH stretching bond, while the ones at 1487 cm−1 and 1587 cm−1 were corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations on the aromatic ring structure for the phenyl and bisphenyl groups. Additionally, for PES membranes, there were peaks at 3116 cm−1 attributed to the –CH stretching vibrations. According to the literature, the peak at 1109 cm−1 is associated with the sulfone group (O
C stretching vibrations on the aromatic ring structure for the phenyl and bisphenyl groups. Additionally, for PES membranes, there were peaks at 3116 cm−1 attributed to the –CH stretching vibrations. According to the literature, the peak at 1109 cm−1 is associated with the sulfone group (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and the peak at 1246 cm−1 is associated with the aromatic ether group (C–O–C).76,77
O), and the peak at 1246 cm−1 is associated with the aromatic ether group (C–O–C).76,77
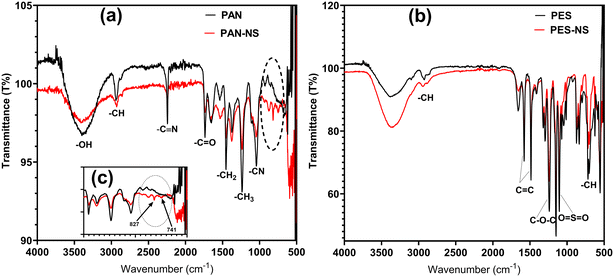 | ||
| Fig. 8 The FT-IR of virgin and CuO/NS filtered membrane a) PAN and b) PES and c) focus on 500–1500 nm for PAN. | ||
In Fig. 8b, no change was observed in the peaks for PES between the virgin and particle filtered membrane. However, for PAN membrane, in Fig. 8a, two new peaks at 741 cm−1 and 827 cm−1 appeared after the filtration of CuO/NS particles indicating a change in the functional groups on PAN.
It is possible that CuO dissolves in water in the absence of the surfactant. With the dissolution of CuO in the water leading to the formation of ionic Cu2+ and OH−. Chen et al.78 stated that nitrile groups (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) on PAN fibers can easily be transformed into the >N– and the –NH– groups and allow for the chelation of the ionic Cu2+ present in the solution on the PAN fiber. Similarly in this study, hydrolyzed nitrile groups on PAN membrane observed at 2241 cm−1 can undergo such a transformation. They could cause the nitrile groups to collide with the Cu2+ present in the solution, leading to the possible formation of the C
N) on PAN fibers can easily be transformed into the >N– and the –NH– groups and allow for the chelation of the ionic Cu2+ present in the solution on the PAN fiber. Similarly in this study, hydrolyzed nitrile groups on PAN membrane observed at 2241 cm−1 can undergo such a transformation. They could cause the nitrile groups to collide with the Cu2+ present in the solution, leading to the possible formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Cu and N–O–Cu band.78,79 Two new peaks appeared at 741 cm−1 and 827 cm−1 are likely to be attributed to C
N–Cu and N–O–Cu band.78,79 Two new peaks appeared at 741 cm−1 and 827 cm−1 are likely to be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Cu and N–O–Cu as expected since metal and metal oxide peaks appear below 1000 cm−1.80
N–Cu and N–O–Cu as expected since metal and metal oxide peaks appear below 1000 cm−1.80
3.5 Filtration mechanisms of the particles
A schematic predicting the filtration mechanisms of all particles at 50 mg L−1 with PAN and PES membranes was illustrated in Fig. 9. Regardless the particle type, with or without surfactant, particles have penetrated into the voids of the PAN membrane much more than those of the PES membrane. In addition, the porosity of the deposit layers formed with all types of particles on PAN membrane was larger than those on PES membrane, which may explain why normalized flux values of PAN were larger than those of PES after particle filtration. Only CuO/NS particles caused an increase in the hydrophilicity of both membranes which may contribute to the increased flux data. CuO/TX-100 is the most stable particle expected to stay more individually or form smaller aggregates. Reduction in PES flux with CuO/TX-100 was attributed to the blockage of the larger pore volume of PES with the individual particles or small aggregates in comparison to PAN with a smaller pore volume. The largest normalized flux values obtained with CuO/CTAB could not be explained by any of the characterization data and further investigation is required. CuO/SDS being the largest particle among all formed the largest aggregates leading to largest deposit porosity. Larger porosity of CuO/SDS deposit on PAN may lead to larger flux compared to PES with less porous particle deposit. However, the largest contact angles values were also obtained with both membranes with CuO/SDS which contradicts with the flux data. Two new FTIR peaks attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Cu and N–O–Cu appeared on PAN membrane after the filtration of CuO/NS particles, while PES did not show any new peak. The appearance of the new peaks on PAN showed an interaction such as adsorption of copper on the membrane material which may result in changes in membrane characteristics leading to increased flux.
N–Cu and N–O–Cu appeared on PAN membrane after the filtration of CuO/NS particles, while PES did not show any new peak. The appearance of the new peaks on PAN showed an interaction such as adsorption of copper on the membrane material which may result in changes in membrane characteristics leading to increased flux.
Although the particles penetrated the voids of the membranes, the normalized flux increased especially for PAN. Information of hydrophilicity and porosity membranes with particles deposits after filtration was not sufficient to explain the reasons behind the flux enhancement. Therefore, further investigation of the pore morphology and hydrophilicity within the voids of the membranes may help to shed more light on the changes in the water flux in further studies.
4. Conclusions
In this study, the influence of four different CuO particles (CuO/NS, CuO/TX-100, CuO/CTAB, CuO/SDS) on the retention and fouling of two different UF (PAN, PES) membranes was elucidated. More than 99% CuO removal efficiency was obtained in all experiments. The membrane flux was increased for all CuO particles, particularly for ones filtered with the PAN membrane, at a 50 mg L−1 particle concentration. However, the membrane flux decreased considerably at 100 mg L−1. Two considerable membrane flux declines were recorded with CuO/TX100 at 50 mg L−1 for PES and CuO/CTAB at 100 mg L−1 for the PAN membrane.The influence of surfactant type and particle concentration on UF behavior was clearly observed in this study. In addition, membrane performance was strongly influenced by the membrane type and material. The membrane flux of the lower MWCO UF membrane, PAN, 50 kDa was more declined than that of the PES 100 kDa at CuO/NS particle concentrations less than 50 mg L−1. New FT-IR peaks appeared for the PAN membrane after filtration of the CuO/NS particles, indicating an interaction between the particles and the membrane material, whereas such an interaction was not observed for the PES membrane. The importance of membrane type and material selection for the removal of metal oxides from industrial wastewater was clearly demonstrated.
Data availability
The data supporting this article have been included in the figures/tables of the manuscript and as part of the ESI.†Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Olabimpe Genevieve Badru. The first draft of the manuscript was written by Olabimpe Genevieve Badru and Ime Akanyeti and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.Acknowledgements
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. Nurten Aşina and Sylvia Beryl Kpange from Cyprus International University are acknowledged for helping with the XRD analysis. Sterlitech Corporation was thanked for kindly supplying 100 kDa PES membranes.References
- A. J. Hamilton, V. L. Versace, F. Stagnitti, P. Li, W. Yin and P. Maher, et al., Balancing environmental impacts and benefits of wastewater reuse, WSEAS Trans. Environ. Dev., 2006, 2(2), 117–129 CAS , available from: http://www.deakin.edu.au/dro/view/DU:30003627.
- M. Khalifa and S. Bidaisee, The Importance of Clean Water, Sch. J. Appl. Sci. Res., 2018, 1(7), 17–20 Search PubMed.
- M. Suleiman, M. Mousa and A. I. A. Hussein, Wastewater disinfection by synthesized copper oxide nanoparticles stabilized with surfactant, J. Mater. Environ. Sci., 2015, 6(7), 1924–1937 CAS.
- C. Maquet, Wastewater reuse: A solution with a future, Field Actions Science Reports, 2020, 22, 64–69 Search PubMed , available from: http://journals.openedition.org/factsreports/6341.
- C. Chahal, B. Van der Akker, F. Young, C. Franco, J. Blackbeard and P. Monis, Pathogen and Particle Associations in Wastewater : Significance and Implications for Treatment and Disinfection Processes, Adv. Appl. Microbiol., 2016, 97, 63–119 CrossRef CAS PubMed.
- J. Conny, R. J. Hamers, P. Kamat, A. Lazarides, E. A. Lilleskov, J. Liu, M. Salit, W. Shih, W. Trogler and M. Zachariah, Nanotechnology and the Environment, Report of a National Nanotechnology Initiative Workshop, May 8–9 2003, Arlington, VA, available from: http://www.nano.gov/sites/default/files/pub_resource/nanotechnology_and_the_environment_app_imp.pdf Search PubMed.
- S. F. Hansen, E. S. Michelson, A. Kamper, P. Borling, F. Stuer-Lauridsen and A. Baun, Categorization framework to aid exposure assessment of nanomaterials in consumer products, Ecotoxicology, 2008, 17(5), 438–447 CrossRef CAS PubMed.
- W. Hannah and P. B. Thompson, Nanotechnology, risk and the environment: A review, J. Environ. Monit., 2008, 10(3), 291–300 RSC.
- I. Bhatt and B. N. Tripathi, Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment, Chemosphere, 2011, 82(3), 308–317, DOI:10.1016/j.chemosphere.2010.10.011.
- X. Wang, J. Yang, L. Shi and M. Gao, Surfactant-free Synthesis of CuO with Controllable Morphologies and Enhanced Photocatalytic Property, Nanoscale Res. Lett., 2016, 11, 125 CrossRef PubMed.
- Z. Alhalili, Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment, Molecules, 2023, 28(7), 3086 CrossRef CAS PubMed.
- L. E. Barton, Fate and Transformation of Metal- (Oxide) Nanoparticles in Wastewater Treatment, PhD. Dissertation, Duke University, 2014 Search PubMed.
- S. Attarilar, J. Yang, M. Ebrahimi, Q. Wang, J. Liu and Y. Tang, et al., The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review From the Biomedical Perspective, Front. Bioeng. Biotechnol., 2020, 8, 822 CrossRef PubMed.
- A. Rastogi, M. Zivcak, O. Sytar, H. M. Kalaji, X. He and S. Mbarki, et al., Impact of metal and metal oxide nanoparticles on plant: A critical review, Front. Chem., 2017, 5, 1–16 Search PubMed.
- B. Robert and E. B. Brown, Environmental Behavior, Potential Phytotoxicity, andAccumulation of Copper Oxide Nanoparticles and Arsenic inRice Plants, Environ. Toxicol. Chem., 2018, 37(1), 11–20 CrossRef PubMed.
- E. V. Soares and H. M. V. M. Soares, Harmful effects of metal(loid) oxide nanoparticles, Appl. Microbiol. Biotechnol., 2021, 105(4), 1379–1394 CrossRef CAS PubMed.
- J. Hedberg, E. Blomberg and W. I. Odnevall, In the Search for Nanospecific Effects of Dissolution of Metallic Nanoparticles at Freshwater-Like Conditions: A Critical Review, Environ. Sci. Technol., 2019, 53(8), 4030–4044 CrossRef CAS PubMed.
- A. Manuja, B. Kumar, R. Kumar, D. Chhabra, M. Ghosh and M. Manuja, et al., Metal/metal oxide nanoparticles: Toxicity concerns associated with their physical state and remediation for biomedical applications, Toxicol. Rep., 2021, 8, 1970–1978, DOI:10.1016/j.toxrep.2021.11.020.
- A. Boyadzhiev, M. L. Avramescu, D. Wu, A. Williams, P. Rasmussen and S. Halappanavar, Impact of copper oxide particle dissolution on lung epithelial cell toxicity: response characterization using global transcriptional analysis, Nanotoxicology, 2021, 15(3), 380–399, DOI:10.1080/17435390.2021.1872114.
- N. Ghosh, S. Das, G. Biswas and P. K. Haldar, Review on some metal oxide nanoparticles as effective adsorbent in wastewater treatment, Water Sci. Technol., 2022, 85(12), 3370–3395 CrossRef CAS PubMed.
- M. Von Sperling, Wastewater Characteristics, Treatment and Disposal, Biological Wastewater Treatment Series, IWA Publishing, 2007, vol. 1, pp. 163–215 Search PubMed.
- M. S. Chauhan, A. K. Rahul, S. Shekhar and S. Kumar, Removal of heavy metal from wastewater using ion exchange with membrane filtration from Swarnamukhi river in Tirupati, Mater. Today: Proc., 2023, 78, 1–6 CAS.
- Z. J. Fu, S. K. Jiang, X. Y. Chao, C. X. Zhang, Q. Shi and Z. Y. Wang, et al., Removing miscellaneous heavy metals by all-in-one ion exchange-nanofiltration membrane, Water Res., 2022, 222, 118888 CrossRef CAS PubMed.
- M. C. Benalia, L. Youcef, M. G. Bouaziz, S. Achour and H. Menasra, Removal of Heavy Metals from Industrial Wastewater by Chemical Precipitation: Mechanisms and Sludge Characterization, Arabian J. Sci. Eng., 2022, 47, 5587–5599 CrossRef CAS.
- T. M. A. Babeker and Q. Chen, Heavy Metal Removal from Wastewater by Adsorption with Hydrochar Derived from Biomass: Current Applications and Research Trends, Curr. Pollut. Rep., 2021, 7, 54–71 CrossRef CAS.
- W. Guo, R. Guo, H. Pei, B. Wang, N. Liu and Z. Mo, PAN/PEI Nanofiber Membrane for Effective Removal of Heavy Metal Ions and Oil–Water Separation, J. Polym. Environ., 2022, 30, 4835–4847 CrossRef CAS.
- K. Trivunac and S. Stevanovic, Removal of heavy metal ions from water by complexation-assisted ultrafiltration, Chemosphere, 2006, 64, 486–491 CrossRef CAS PubMed.
- R. F. Wildeer, P. J. Barrett, L. W. Henslee and D. Arpi, Recovery of Metal Oxides from Fly Ash, Oakland, California, 1984 Search PubMed.
- P. P. Prabhu and B. Prabhu, A Review on Removal of Heavy Metal Ions from Waste Water using Natural/ Modified Bentonite, MATEC Web Conf., 2018, 144, 1–13 CrossRef.
- R. J. Honda, V. Keene, L. Daniels and S. L. Walker, Removal of TiO2 nanoparticles during primary water treatment: Role of coagulant type, dose, and nanoparticle concentration, Environ. Eng. Sci., 2014, 31(3), 127–134 CrossRef CAS PubMed.
- N. Hu, R. Li, Z. L. Wu, D. Huang and H. Z. Li, Intensification of the separation of CuO nanoparticles from their highly diluted suspension using a foam flotation column with S type internal, J. Nanopart. Res., 2015, 17, 401 CrossRef.
- K. W. Trzaskus, S. L. Lee, W. M. de Vos, A. Kemperman and K. Nijmeijer, Fouling behavior of silica nanoparticle-surfactant mixtures during constant flux dead-end ultrafiltration, J. Colloid Interface Sci., 2017, 506, 308–318 CrossRef CAS PubMed.
- R. Khan, M. Ali Inam, D. R. Park, S. Khan, M. Akram and I. T. Yeom, The removal of CuO nanoparticles from water by conventional treatment C/F/S: The effect of pH and natural organic matter, Molecules, 2019, 24(5), 2–15 CrossRef PubMed.
- A. H. Konsowa, M. G. Eloffy, W. A. Ibrahim, Y. A. El-Taweel and O. E. Abdelwahab, Removal of copper oxide nanoparticles from aquatic mediums by coagulation-ultrafiltration membrane hybrid continuous system, Desalin. Water Treat., 2019, 171, 78–92 CrossRef CAS.
- S. Surawanvijit, M. Kim and Y. Cohen, Analysis of membrane filtration efficiency for the removal of metal oxide nanoparticles from aqueous nanoparticle suspension with feed coagulation pretreatment, in NSTI-Nanotech 2010, 2010, pp. 591–593 Search PubMed.
- J. María Arsuaga, A. Sotto, G. del Rosario, A. Martínez, S. Molina and S. B. Teli, et al., Influence of the type, size, and distribution of metal oxide particles on the properties of nanocomposite ultrafiltration membranes, J. Membr. Sci., 2013, 428, 131–141 CrossRef.
- M. Pakan, M. Mirabi and A. Valipour, Effectiveness of different CuO morphologies nanomaterials on the permeability, antifouling, and mechanical properties of PVDF/PVP/CuO ultrafiltration membrane for water treatment, Chemosphere, 2023, 337, 139333 CrossRef CAS PubMed.
- C. Dong, X. Xiao, G. Chen, H. Guan and Y. Wang, Morphology control of porous CuO by surfactant using combustion method, Appl. Surf. Sci., 2015, 349, 844–848 CrossRef CAS.
- H. Siddiqui, M. S. Qureshi and F. Z. Haque, Surfactant assisted wet chemical synthesis of copper oxide (CuO) nanostructures and their spectroscopic analysis, Optik, 2016, 127, 2740–2747 CrossRef CAS.
- P. Samarasekara, C. Karunarathna, B. M. C. M. Bandaranayake and C. A. N. Fernando, Enhancing the photocurrent of spin coated CuO thin films using TX-100 Surfactant, Georgian Electronic Scientific Journals: Physics, 2020, 1–21 Search PubMed.
- T. Zhu, Z. Zhou, F. Qu, B. Liu and B. Van der Bruggen, Separation performance of ultrafiltration during the treatment of algae-laden water in the presence of an anionic surfactant, Sep. Purif. Technol., 2022, 281, 119894 CrossRef CAS.
- N. Aryanti, A. Nafiunisa, T. D. Kusworo and D. H. Wardhani, Micellar-enhanced ultrafiltration using a plant-derived surfactant for dye separation in wastewater treatment, Membranes, 2020, 10(9), 1–16 CrossRef PubMed.
- K. Mizoguchi, K. Fukui, H. Yanagishita, T. Nakane and T. Nakata, Ultrafiltration behavior of a new type of non-ionic surfactant around the CMC, J. Membr. Sci., 2002, 208(1–2), 285–288 CrossRef CAS.
- S. S. Marques, I. I. Ramos, S. R. Fernandes, L. Barreiros, S. A. C. Lima and S. Reis, et al., Insights on ultrafiltration-based separation for the purification and quantification of methotrexate in nanocarriers, Molecules, 2020, 25(8), 1879 CrossRef CAS PubMed.
- E. Fernández, J. M. Benito, C. Pazos and J. Coca, Ceramic membrane ultrafiltration of anionic and nonionic surfactant solutions, J. Membr. Sci., 2005, 246(1), 1–6 CrossRef.
- M. Ghulam, T. Hajira, S. Muhammad and A. Nasir, Synthesis and characterization of cupric oxide (CuO) nanoparticles and their application for the removal of dyes, Afr. J. Biotechnol., 2013, 12(47), 6650–6660 CrossRef.
- G. Savita, Engineering Chemistry, I.K. International Publishing House Pvt. Ltd., New Delhi, 2017, p. 304, available from: https://books.google.com.cy/books?id=QFylEAAAQBAJ Search PubMed.
- D. Manyasree, K. M. Peddi and R. Ravikumar, CuO nanoparticles: Synthesis, characterization and their bactericidal efficacy, Int. J. Appl. Pharm., 2017, 9(6), 71–74 CrossRef CAS.
- L. Otero-González, J. A. Field and R. Sierra-Alvarez, Inhibition of anaerobic wastewater treatment after long-term exposure to low levels of CuO nanoparticles, Water Res., 2014, 58, 160–168 CrossRef PubMed.
- G. Bolton, D. LaCasse and R. Kuriyel, Combined models of membrane fouling: Development and application to microfiltration and ultrafiltration of biological fluids, J. Membr. Sci., 2006, 277(1–2), 75–84 CrossRef CAS.
- C. Nurra, E. Clavero, J. Salvadó and C. Torras, Vibrating membrane filtration as improved technology for microalgae dewatering, Bioresour. Technol., 2014, 157, 247–253 CrossRef CAS PubMed.
- A. Swierczynska, J. Bohdziewicz, G. Kaminska and K. Wojciechowski, Influence of the Type of Membrane-Forming Polymer on the Membrane Fouling, Environ. Prot. Eng., 2016, 42(2), 197–210 Search PubMed.
- I. R. Marques, C. Silveira, M. J. L. Leite, A. M. Piacentini, C. Binder and M. E. R. Dotto, et al., Simple approach for the plasma treatment of polymeric membranes and investigation of the aging effect, J. Appl. Polym. Sci., 2021, 138(24), 1–10 CrossRef.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso and J. Rouquerol, et al., Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report), Pure Appl. Chem., 2015, 87(9–10), 1051–1069 CrossRef CAS.
- K. A. Cychosz and M. Thommes, Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials, Engineering, 2018, 4(4), 559–566, DOI:10.1016/j.eng.2018.06.001.
- Y. Yang, D. Xu, Q. Wu and P. Diao, Cu2O/CuO bilayered composite as a high-efficiency photocathode for photoelectrochemical hydrogen evolution reaction, Sci. Rep., 2016, 6, 1–10 CrossRef CAS PubMed.
- M. Guzman, M. Arcos, J. Dille, C. Rousse, S. Godet and L. Malet, Effect of the Concentration and the Type of Dispersant on the Synthesis of Copper Oxide Nanoparticles and Their Potential Antimicrobial Applications, ACS Omega, 2021, 6(29), 18576–18590 CrossRef CAS PubMed.
- M. I. Said, A. A. Othman and E. M. Abd Elhakeem, Structural, optical and photocatalytic properties of mesoporous CuO nanoparticles with tunable size and different morphologies, RSC Adv., 2021, 11(60), 37801–37813 RSC.
- V. H. Lim and Y. Adachi, Analysis of initial stage of colloidal particles flocculation induced by different degree branching polyelectrolytes, Colloids Surf., A, 2021, 625, 126–986 CrossRef.
- J. Matusiak and E. Grządka, Stability of colloidal systems - a review of the stability measurements methods, Ann. Univ. Mariae Curie-Sklodowska, Sect. AA: Chem., 2017, 72(1), 33–45 Search PubMed.
- Y. Aparna, K. V. Rao and P. S. Subbarao, Preparation and Characterization of CuO Nanoparticles by Novel Sol-Gel Technique, J. Nano- Electron. Phys., 2012, 4(3), 4–7 Search PubMed.
- S. Dikmen, B. Ersoy and Z. Dikmen, Adsorption Behaviour of Ionic and Non-Ionic Surfactants onto Talc A Naturally Hydrophobic Mineral-A Comparative Study, Eskişehir Technical University Journal of Science and Technology A - Applied Sciences and Engineering, 2020, 21, 139–152 CrossRef.
- J. Li, F. L. Kwong, J. Zhu and D. H. L. Ng, Synthesis of biomorphic ZnO nanostructures by using the cetyltrimethylammonium bromide modified silk templates, J. Am. Ceram. Soc., 2010, 93(11), 3726–3731 CrossRef CAS.
- D. L. Liao, G. S. Wu and B. Q. Liao, Zeta potential of shape-controlled TiO2 nanoparticles with surfactants, Colloids Surf., A, 2009, 348, 270–275 CrossRef CAS.
- N. Nasrollahi, S. Aber, V. Vatanpour and N. M. Mahmoodi, Development of hydrophilic microporous PES ultrafiltration membrane containing CuO nanoparticles with improved antifouling and separation performance, Mater. Chem. Phys., 2019, 222, 338–350 CrossRef CAS.
- C. Hackett, M. Abolhassani, L. F. Greenlee and A. K. Thompson, Ultrafiltration Membranes Functionalized with Copper Oxide and Zwitterions for Fouling Resistance, Membranes, 2022, 12(5), 544 CrossRef CAS PubMed.
- WHO, Guidelines for drinkin-water quality: fourth edition incorporating the first and second addenda, World Health Organization, Geneva, 2022, pp. 367–370 Search PubMed.
- EPA, Aquatic life ambient freshwater quality criteria-Copper, United States Environmental Protection Agency, Washington, DC, 2007, p. 204 Search PubMed.
- H. Khezraqa, A. Samiei, H. Etemadi and M. Salami-Kalajahi, Effect of Copper Oxide Nanoparticles on the Performance of Polyvinyl Chloride Membranes, Chem. Eng. Technol., 2023, 46, 1–12 CrossRef.
- W. R. Bowen and F. Jenner, Theoretical descriptions of membrane filtration of colloids and fine particles: An assessment and review, Adv. Colloid Interface Sci., 1995, 56, 141–200 CrossRef CAS.
- L. Y. Ng, A. W. Mohammad, C. P. Leo and N. Hilal, Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review, Desalination, 2013, 308, 15–33 CrossRef CAS.
- M. Said, A. Ahmad, A. W. Mohammad, M. T. M. Nor and S. R. Sheikh Abdullah, Blocking mechanism of PES membrane during ultrafiltration of POME, J. Ind. Eng. Chem., 2015, 21, 182–188 CrossRef CAS.
- Z. Wang, J. Ma and Q. Liu, Pure sponge-like membranes bearing both high water permeability and high retention capacity, Desalination, 2011, 278(1–3), 141–149 CrossRef CAS.
- H. Guo, Y. Ma, P. Sun, S. Cui, Z. Qin and Y. Liang, Self-cleaning and antifouling nanofiltration membranes - Superhydrophilic multilayered polyelectrolyte/CSH composite films towards rejection of dyes, RSC Adv., 2015, 5(78), 63429–63438, 10.1039/C5RA11438A.
- N. Mohammad and Y. Atassi, Enhancement of removal efficiency of heavy metal ions by polyaniline deposition on electrospun polyacrylonitrile membranes, Water Sci. Eng., 2021, 14(2), 129–138 CrossRef.
- Y. Gao, L. Liang, S. Zhao, Y. Qi, W. Zhang and X. Sun, et al., Hydrophilic and antimicrobial core-shell nanoparticles containing guanidine groups for ultrafiltration membrane modification, RSC Adv., 2018, 8(44), 24690–24700 RSC.
- I. G. Sandoval-olvera, L. Villafaña-lópez and J. A. Reyes-aguilera, Surface modification of polyethersulfone membranes with goethite through self-assembly, Desalin. Water Treat., 2017, 65, 199–207 CrossRef CAS.
- C. Chen, F. Li, Z. Guo, X. Qu, J. Wang and J. Zhang, Preparation and performance of aminated polyacrylonitrile nanofibers for highly efficient copper ion removal, Colloids Surf., A, 2019, 568, 334–344, DOI:10.1016/j.colsurfa.2019.02.020.
- S. Mohabey and H. Mohabey, IR Spectra, Magnetic and Thermal Studies of Copper (II) Complex of N-Hydroxy-N-(4-Chloro) Phenyl N'(4-Fluoro) Phenyl Benzamidine Hydrochloride, Mater. Sci. Res. India, 2014, 11(1), 63–65 Search PubMed.
- I. E. Wachs, Infrared spectroscopy of supported metal oxide catalysts, Colloids Surf., A, 1995, 105(1), 143–149 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ew00462k |
| This journal is © The Royal Society of Chemistry 2024 |