Open Access Article
Open Access ArticleAn assessment of spent coffee grounds as a replacement for peat in the production of Scotch whisky: chemical extraction and pyrolysis studies†
Kacper P.
Krakowiak
 ,
Ruaraidh D.
McIntosh
,
Ruaraidh D.
McIntosh
 * and
David
Ellis
* and
David
Ellis
 *
*
Institute of Chemical Sciences, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh EH14 4AS, UK. E-mail: r.mcintosh@hw.ac.uk; d.ellis@hw.ac.uk
First published on 13th November 2023
Abstract
The potential of spent coffee grounds (SCG) to act as a replacement fuel material for the malt-drying process during whisky production was evaluated. The extracts of both materials, and the smoke they produced through burning, were subjected to analysis by high resolution 1H NMR spectroscopy. Malts infused with the smokes were similarly studied to gain an understanding of the transfer of chemical species from smoke to grain. In addition, the thermal degradation of peat and SCG were investigated using thermogravimetric analysis and pyrolysis – GCMS. Our studies revealed that, despite some chemical differences between the source materials, the composition of the smoke produced by both is remarkably similar. It may be concluded that the aroma and flavour of the spirit, resulting from substitution of peat by SCG is also likely to be similar, however the presence of additional congeners in the SCG-derived spirit, including furans and methylpyridines, could introduce undesirable off notes.
Sustainability spotlightThis work contributes to UN's Sustainable Development Goals 12 (responsible consumption and production), 13 (climate action) and 15 (life on land). This is achieved through the usage of spent coffee grounds in place of peat, a limited natural resource, during the whisky production process. Continuous exploitation of peatlands has led to their severe degradation, inhibiting their ability to both store and absorb carbon dioxide. For restoration of peatlands to be successful, peat harvesting needs to be limited and therefore current major users of peat, such as the scotch whisky industry must look to utilising alternative, more sustainable materials. Furthermore, wetlands act as habitat for a variety of unique plant and animal life, which can be preserved by limiting peat usage. |
1. Introduction
Scotch Whisky is Scotland's most famous export, generating £6.2 billion worth of export sales in 2022.1 This represents a 37% increase compared to the previous year and confirming the continued increasing appeal of the distilled spirit to consumers across the world. Scotch Whisky is produced in several regions across Scotland but perhaps the most distinctive, in terms of flavour and aroma, are those from the island of Islay. These spirits are immediately recognizable due to smoky characteristics which are created during the kilning process.2,3 Burning peat is used to dry the malt, and the resulting smoke infuses into the grain. The malt is converted to liquid wort transferring many of the compounds from the smoke along with the fermentable sugars and species responsible for the ultimate flavour of the mature spirit. The geographical origin of the peat and depth from which it was extracted (and thus its composition, e.g. content of carbohydrates in peat decreases with depth), kilning process (e.g., the length of the process, and burning temperature) are all important elements that can significantly influence the nature of the final spirit.4–6 The scope of this work is a proof-of-concept determination, with only single samples of peat and SCG examined. Future studies will investigate multiple samples of both, enabling a full statistical analysis of the potential of SCG to act in place of peat in the manufacture of Scotch.Traditionally, the peat used in whisky production is harvested (cut) from Scottish peatlands and dried as a raw material before burning. Continuous exploitation of these peatlands (including harvesting peat for compost or fuel), combined with historic drainage of wetlands has resulted in many areas of peatland becoming depleted.7,8 As a result, restrictions have been placed on exploitation of peat and actions have been undertaken by the UK and Scottish Governments to stop and potentially reverse the damage done to the peatlands.9,10 Preservation of peatlands is crucial because they act as carbon sinks and stores due to the slow rate of decomposition of organic material and active CO2 absorption.11 It has been estimated that one third of the world's carbon is stored within peatlands, which also continue to absorb carbon dioxide from the atmosphere, providing that they are in a healthy condition.12 Additionally, peat-containing bogs are natural habitats for a variety of unique plant and animal life.13 While the efforts undertaken so far, help the restoration process, it will likely still take many decades before these areas can be restored due to the slow rate at which the dead plant matter accumulates to produce peat, with natural accumulation rates usually reported below 1 mm per year.14,15 The combination of the aforementioned factors presents the Scotch Whisky industry with a challenge to a process which has remained unchanged for hundreds of years. As a result, novel solutions, which previously would have been unthinkable, may have to be considered. Consequently, the Scotch Whisky Association have made the decision to support peatland restoration efforts and mitigate the use of peat by investigating ways in which it can be used more efficiently.16 Alternatively, peat, in principle, could be sourced from different parts of the world, where it is more abundant though the long supply chains involved would be highly undesirable. A more innovative solution to the issue may be to replace peat altogether, or complement its use, with another more sustainable material. To find a suitable replacement, the chemical composition of peat, and the relationship that has to the desired flavour compounds in the final spirit, needs to be investigated. Extensive research in the area of whisky aroma compounds has indicated that aromatic compounds, such as phenols, cresols and guaiacols are the primary species associated with smoky flavour and odour (for examples of such small molecules, see Fig. 1), though a detailed investigation is required, as whisky (smoked or otherwise) is a complex mixture of hundreds of compounds.3,17–19
 | ||
| Fig. 1 Congeners associated with ‘smoky’ odour in peated whisky.18 | ||
The principal components of peat are cellulose, hemicellulose and lignin.20,21 Lignin is of particular interest in being a polymer found within most plant matter and consisting of phenolic building blocks, the most common units being guaiacyl and syringyl units. This polyaromatic biomolecule decomposes on burning in the kilning process, the resulting smoke containing the desired groups of flavour compounds (often referred to as congeners) which are then imparted into the malt and eventually into the spirit, though the exact composition of the smoke and how much of each congener is transferred has not been studied thoroughly.2,4 As lignin is the main source of many of the congeners any potential replacement material should contain significant amounts of it in terms of overall weight percentage. Additionally, free small molecules present within peat and the replacement material need to be considered, as they can have a strong impact, depending on their nature. A potential candidate that fulfils this requirement is coffee waste (spent coffee grounds, SCG).
SCG are solid residues left after preparation of coffee. The worldwide popularity of this beverage has resulted in the annual generation of 6 million tons of SCG.22 The vast majority is disposed of through landfill, albeit efforts have been made in recent years to find other downstream uses for SCGs, including use as fillers and additives for polymer composites,23,24 animal feed25 and fertilizers.26,27 Additionally, due to presence of phenolic compounds, the antioxidant properties of SCGs have been studied.28 Ballesteros et al., reported that SCG are, like peat, composed principally of lignin, cellulose and hemicellulose29 thus making it an attractive option for peat replacement as it would be expected that the chemical profile of smoke produced by burning SCGs would be similar to peat smoke. However, coffee, and the waste arising from its production, has a high content of nitrogen containing compounds (for example proteins constitute nearly 20% of SCG dry mass) which upon decomposition could produce harmful chemicals, such as ethyl carbamate or nitrogen oxides.30,31 The presence of the latter in the emissions is one of the main deterrents to using coffee waste as a solid fuel. Additionally, even small amounts of undesirable compounds could have a significant effect on the overall aroma and taste of the spirit produced. To address these issues, the chemical composition of peat, SCG, their smokes and infused malts were investigated using a variety of techniques, including nuclear magnetic resonance (NMR) spectroscopy and pyrolysis gas chromatography/mass spectrometry (py-GCMS). CHN analysis of peat and SCG samples was also performed to compare their nitrogen content and therefore approximate the potential for formation of harmful N-compounds (though more detailed toxicological and atmospheric studies would have to be performed before the proposed material could be used as a widescale peat replacement). Additionally, the thermal degradation profiles of SCGs and peat were established using thermal gravimetric analysis (TGA).
A combination of the above methods offers the potential to conduct a thorough analysis and comparison of peat and SCG. NMR is a technique widely used in chemical analysis, most commonly to determine structures of small molecules, it can also be used to identify components of, and differentiate between, complex mixtures, having been successfully used in the analysis of whisky,32 beer,33 wine and other food products.34 Sample preparation for NMR analysis is quick and simple, usually only requiring the sample to be dissolved or diluted in deuterated solvent, used in place of a conventional protonated solvent to prevent intense signals from the relatively large quantity of solvent present from dominating the spectrum. The spectra show signals from all of the compounds present in the sample (non-selective), making it possible to establish a chemical profile of the complete mixture from a single experiment. In contrast, techniques such as gas chromatography (GC), may require multiple different methods to observe the complete mixture if several classes of compounds are present in the mixture. More recent developments in NMR allow experiments to be performed on fully protonated solutions. In order to observe the relatively minor mixture constituents from the dominating solvent, the solvent signal/s must be suppressed using appropriate pulse sequences.
While the potential benefits are clear, one drawback is that NMR suffers from a relative lack of sensitivity when compared with other commonly used analytical techniques. It is therefore advantageous to combine NMR analysis with other techniques to build a more complete picture of the mixture. As combustion plays a key role in the whisky production process, pyrolysis GC-MS (py-GC/MS) is an ideal technique to use in this work as the samples are pyrolysed followed by separation of the compounds in the mixtures before MS analysis. As indicated previously, it can be challenging for GC to analyse multiple compound groups but this study will primarily focus on aromatic compounds, e.g., phenols, cresols and guaiacols.
2. Experimental
2.1 Samples
SCG samples were obtained from a local coffee retail outlet, a peat sample (surface peat, 0–5 cm depth, chosen to best represent the fraction of peat which would be used for peated whisky production) was collected from the island of Islay, UK and the malt samples were provided by the Scotch Whisky Research Institute (SWRI). A series of analyses were carried out upon; chemical extracts of spent coffee grounds (SCG), spent coffee grounds from decaffeinated coffee (DSCG) and peat (P), trapped smoke obtained by burning the SCGs (SCGS), decaffeinated coffee waste (DSCGS) and peat (PS), and extracts of industrially peated malt (PM) and malt infused with SCG smoke (CPM).Several extraction solvents combined with different extraction conditions were tested and methanol solid/liquid extraction for three days at room temperature was found to provide best results for SCG and DSCG samples (based on the number and intensity of NMR signals), while ethanol/water (90/10% v/v) solution under the same conditions was optimal for PM and CPM samples. As we are focused on identifying the maximum possible number of compounds in the samples, the most effective solvent combinations, which don't bias the results, were chosen, rather than defaulting to an alcoholic matrix (ethanol/water 40/60% v/v) that reflects the bulk composition of whisky. In any event, the latter only becomes relevant when considering the migration of compounds into the evolving wort during the mashing process, where the precise composition of the liquid at any given time is unclear. For full details on the extraction methodology, see ESI.† SCG and peat smoke was generated using a commercial smoking device (Sage Smoking Gun™ Pro GSM 700) and passed through 5 mL of solvent thereby trapping soluble components of the smoke. Following evaluation, methanol solvent was determined to give best results for all samples tested. It should also be noted that there is currently a vigorous debate on the relative advantages and disadvantages of the various extraction methods available. Some of these methods may result in chemical transformations of the analytes. Examples include the alkaline extraction designed to obtain a significant amount of natural organic matter (NOM), one of the recognized fractions of peat, which was first carried out in 1786.35 Organic solvents have also been used previously, including combinations of benzene/alcohol36,37 and DMSO/sulphuric acid.38 For this work, we decided on a less disruptive approach designed to avoid problematic chemical transformations during the extraction process that would be inconsistent with the methods used in the production of Scotch Whisky.
2.2 NMR spectroscopy
NMR samples were prepared by adding a 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TSP) standard solution (0.1 mL) to the relevant sample (0.5 mL) producing a total volume of approximately 0.6 mL. For ethanol/water extracts the TSP standard solution was prepared using TSP (0.0107 g, 6.211 × 10−5 mol) and D2O in a 10 mL volumetric flask, giving a final concentration of 1.035 mM in a standard 5 mm NMR tube. The TSP standard solution for methanol extracts and smoke samples was prepared using TSP (0.0103 g, 5.979 × 10−5 mol) and methanol in a 10 mL volumetric flask, giving a final concentration of 0.9965 mM in a standard 5 mm NMR tube. Each sample was analysed in triplicate.All NMR experiments were performed on a Bruker AVIII HD 400 MHz spectrometer (1H spectra acquired at 400.03 MHz). NMR data obtained was processed using TopSpin and Microsoft Excel software. Spectra were acquired using a multiple solvent suppression sequence based on the Bruker pulse programme lc1pndcgpps. This sequence features presaturation during both the relaxation delay and the mixing time. Off-resonance presaturation is achieved with a Bruker defined shaped pulse, Squa100.1000 and cp-13C decoupling through a Garp.p31 element. Number of scans = 64 and the relaxation delay (d1) = 3.0 s. Suppression is necessary to reduce the intensity of the strong solvent signals of methanol, ethanol and water, which would otherwise make observation of minor mixture components impossible. As the intensity of the 13C satellites of methyl and methylene resonances of the alcohols (at 0.5% vs. that of the main signals) might be comparable to that of compounds of interest, these also should be suppressed, hence the inclusion of 13C decoupling. The assignment of signals was performed through comparison with online databases and journal articles,39–42 literature,32,43,44 spectra of pure compounds, and spiking experiments, in which the pure chemical of interest is added to the mixture.
2.3 Thermal decomposition analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Oven temperature ramp: 40 °C with 1 min hold time, 40 to 165 °C at 3 °C with 1 min hold time, 165 to 310 °C at 5 °C min−1 with 10 min hold time. MS temperature: source 230 °C, quadrupole 150 °C, transfer line 300 °C. Data analysis was performed via Agilent Masshunter Unknowns and NIST14 library.
1. Oven temperature ramp: 40 °C with 1 min hold time, 40 to 165 °C at 3 °C with 1 min hold time, 165 to 310 °C at 5 °C min−1 with 10 min hold time. MS temperature: source 230 °C, quadrupole 150 °C, transfer line 300 °C. Data analysis was performed via Agilent Masshunter Unknowns and NIST14 library.
3. Results and discussion
3.1 CHN analysis
A general overview of the chemical composition of peat and spent coffee grounds was established through determination of carbon, hydrogen and nitrogen content. The Islay peat sample showed 51.52% carbon, 6.81% hydrogen and 1.85% nitrogen content. SCG sample showed 46.29% carbon, 6.63% hydrogen, 1.70% nitrogen content, while the results for decaffeinated SCG were 48.60% carbon, 6.81% hydrogen and 1.85% nitrogen. The first observation that can be made is that, despite what might be expected, the nitrogen contents for peat and spent coffee grounds are very similar. This suggests that combustion of either of these materials would lead to release of similar quantities of nitrogenous species into the atmosphere. The carbon and hydrogen content of the two samples is also comparable. The carbon content of both SCG samples is slightly lower than that of peat, potentially due to lower biopolymer presence in coffee waste, compared to peat. Comparison of the two SCG samples also reveals that the decaffeinated sample has a higher nitrogen content, which is contrary to what would be expected given the lack of caffeine, which is the most abundant nitrogenous species in coffee. That said, numerous other nitrogen containing compounds are also found in coffee grounds and the results for nitrogen content are close enough that the difference could be explained by variance between the samples, caused by differences in origin of the coffee beans used or coffee preparation process.3.2 NMR analysis of peat extract
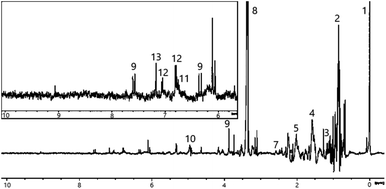
Initial 1H NMR analysis revealed that all extracts contained many compounds, producing complex spectra. Multiple overlapping signals appear over the entire length of the spectral profiles. To aid analysis, the spectrum was divided into three regions: low frequency region (0–3 ppm), middle region (3–5.5 ppm) and high frequency region (5.5–10 ppm). The latter is expected to be of particular importance as the aromatic compounds of interest for this study would be expected to show 1H NMR signals in this region. It is noted that certain areas of some of the spectra show significant but unavoidable baseline distortions resulting from the use of solvent suppression pulse sequences. As a result, the data was deemed unsuitable for quantitative analysis.For the peat extract (see Fig. 2), the low frequency region of the 1H NMR spectrum displays signals in the 0.8–1.8 ppm range, arising from long, aliphatic chain compounds, fatty acids, alcohols and their esters, a cluster of signals around 0.9 ppm stems from the terminal methyl group-protons of these species while another group at 1.5 ppm originates from the corresponding methylene group-protons. Additional signals in this region likely arise from methyl groups of other simple molecules, e.g., ethyl acetate or 2- and 4-ethyl phenol, since some triplet multiplicities can be observed (e.g., that at 1.17 ppm). Signals at higher frequency indicate the presence of small organic molecules, whose chemical shifts are well documented e.g., acetic acid (2.01 ppm) and acetone (2.14 ppm) together with methylene groups from larger carbohydrates containing heteroatoms, namely organic acids, aldehydes and ketones. It should be noted that slight deviations from the chemical shifts of the pure compounds are to be expected in the complex mixtures described herein. Furthermore, assignments of signals are often hampered by signal overlap, e.g., the multiplet centred at 2.5 ppm can be attributed to overlapping singlets of the O-methyl groups of ortho- and para-cresol or analogues.
The middle region of the spectrum obtained from peat-extract shows relatively few signals. Cellulose and other carbohydrates might be expected to feature strongly here, failure to observe this may be due to the poor solubility of cellulose into the solution during extraction. The resonances that are present arise from compounds with CHx groups connected directly to oxygen, including guaiacols and syringols. This indicates that these important congeners are already present in peat and do not exclusively arise as a result of burning. A distinct, strong singlet at 3.88 ppm can be assigned to the methyl group of ferulic acid. This compound is produced through the decay of plants and subsequent absorption into the peat. The presence of ferulic acid could signify that other phenolic acids derived from plant material may be found in peat though at concentrations too low to be detected by NMR.
The high frequency region of the spectrum features additional signals associated with ferulic acid, including characteristic doublets with large 3J couplings (6.34 ppm, 3J = 15.6 Hz; 7.59 ppm, 3J = 15.8 Hz) arising from the trans-alkene moiety. Signals around 6 ppm are likely to arise from other unsaturated compounds. Other compounds expected to show signals at higher frequencies are o- and p-cresols, guaiacols, syringols and phenolic acids. While signals corresponding to some of these can be identified, others appear to be absent. This may arise as a result of their low concentration however such congeners may be identifiable through the signals from the more intense uncoupled signals attributed to the methyl groups.
3.3 NMR analysis of SCG extract and a comparison with peat extract
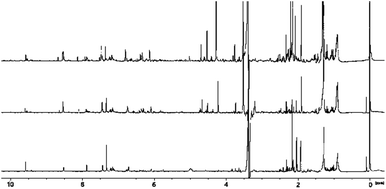
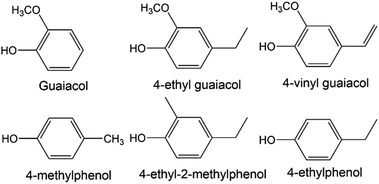
Initial comparison of the spectra from SCG and peat reveals significant commonality in several regions but there are some important differences (see Fig. 3).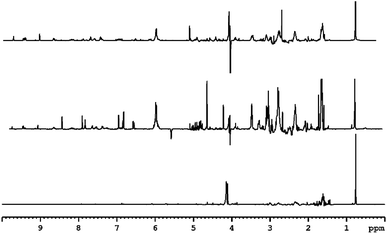 | ||
| Fig. 3 Comparison of 1H NMR (recorded at 400 MHz) spectra of methanol extracts of peat (bottom), spent coffee grounds (middle) and SCG from decaffeinated coffee (top). | ||
The spectrum of decaffeinated SCG extract was also recorded to confirm the absence of caffeine and confirm similarities, therefore allowing benchmarking of results from smoke and spirit analysis for the two varieties later (see Fig. 3). Spectra for both caffeinated and decaffeinated SCG are otherwise similar, except for the expected, additional signals relating to caffeine in the ‘normal’ SCG (s, 3.52 ppm; s, 3.96 ppm; s, 7.85 ppm; the signal at 3.4 ppm is not observed due to proximity to the partially suppressed methyl group resonance from methanol).
The low frequency regions of all spectra show similarities, displaying multiplets in the 0.8 to 1.8 ppm range, correlating to long chain aliphatic compounds, e.g., fatty acids. The signals present in the SCG spectra appear to have greater intensity possibly indicating a more efficient transfer of compounds into the solution, rather than a difference in concentration of substances in the materials themselves. More in depth investigation of the concentration lies beyond the scope of this work as its objective was to identify compounds rather than establish exact concentrations of each species. Several resonances above 1.8 ppm are only present in the coffee waste spectra; quinic acid, previously identified in roasted coffee beans,43,44 and its esters are associated with the obvious multiplet at 2.05 ppm, the doublet at 2.46 ppm and a second multiplet at 2.56 ppm. Other signals of interest in this region include those of methyl and methylene groups from ethyl and methyl derivatives of aromatic molecules, such as cresols (2.18–2.30 ppm), which are also present in the peat extract, though at significantly lower concentration.
The middle region of the SCG extract spectrum contains multiple signals, (in contrast to the peat spectrum), including the strong caffeine-derived singlets at 3.52 ppm and 3.96 ppm (which also appear in the decaffeinated sample, though at much lower intensity). A cluster of signals above 4 ppm are assigned to various methoxy groups in species including ferulic acid (3.88 ppm, also found in peat), guaiacols and N-methyl groups of trigonelline and methylpyridines/pyridinium ions. Additionally, signals of mono- and disaccharides are likely to be present in this region, which could explain the cluster of signals around 5.2 ppm, characteristic for the anomeric protons of carbohydrates. The presence of simple carbohydrates in the SCG sample can be explained by partial hydrolysis of cellulose and hemicellulose leading to production of glucose and arabinose. Finally, esters of various short and long chain organic acids may contribute resonances here, the latter group displaying matching resonances in the low frequency region of the spectrum.
Multiple deviations can be observed in the aromatic regions of the spectra, in particular in terms of the relative intensities of the signals, which are greater for SCG compared to peat. This suggests that the aromatic compounds within coffee waste are more easily transferred into the solution (potentially due to partial degradation of lignin during the coffee making process) or present in higher quantities, which could be connected to higher abundance of lignin within SCG, compared to peat. Some of the aromatic compounds identified in the SCG spectra include those found within peat, such as phenol, 2-ethylphenol and cresols (which appear as a cluster of signals in the region 6.8–7.3 ppm). Additionally, doublets with large 3J values (3J = 16 Hz), characteristic of trans-alkenes, are identified at 6.34 ppm, 6.40 ppm, 7.58 ppm and 7.59 ppm. These are attributed to ferulic and cinnamic acids, which have previously been reported in roasted coffee beans.44 Ferulic acid was also identified in the spectrum of peat extract, however this is likely to arise through a different pathway to that in SCG, because ferulic acid is commonly associated with decaying plant matter. Signals at 8.06 ppm, 8.89 ppm and 9.19 ppm are due to trigonelline, the second most abundant nitrogen containing species in caffeinated coffee (after caffeine itself). Other resonances above 8 ppm are ascribed to the aromatic protons of thermal degradation products of trigonelline, such as methylpyridinium and methylpyridines, which form during the coffee roasting process.45 The presence of these compounds, in significant quantities, is undesirable due to their unpleasant aromas, which could potentially be imparted into the spirit if SCG were to be used for malt drying.
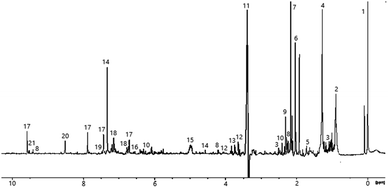
3.4 NMR analysis of peat smoke
The low frequency region of the spectrum derived from peat-smoke is dominated by signals of long chain fatty acids and hydrocarbons, similar to the spectrum of the unburned peat extract (see Fig. 4).The spectra from extract and smoke are similar, however some additional methyl groups associated with products of thermal decomposition can be identified, including 5-methylfurfural at 2.41 ppm and hydroxyacetone at 2.19 ppm, both of which originate from cellulose.46 Furthermore, compounds originating from the decomposition of lignin, including p-cresol (2.27 ppm) and 4-ethylphenol (2.50 ppm) also appear. A greater number of signals are present in the 2–2.5 ppm region, compared to the peat extract, which indicates the presence of a greater quantity of compounds containing protons in electron rich chemical environments, e.g., aromatic rings. The identification of specific compounds is challenging due to signal overlap, however it appears to be the case that the smoke produced by burning peat contains small aromatic congeners in noticeably larger quantities than does peat itself.
The middle region of the spectrum contains signals of levoglucosan (3.60 ppm, 3.64 ppm, 3.74 ppm, 4.06 ppm), an anhydrous form of glucose, which is the primary product of cellulose decomposition. The intensity of the resonances are comparatively low, which indicates further degradation of levoglucosan into smaller molecules e.g., hydroxyacetaldehyde, hydroxyacetone, pyruvaldehyde or various furans (most notably 5-hydroxymethylfurfural and furfural). Signals arising from the methylene group of hydroxyacetone can be seen at 4.21 ppm, further suggesting decomposition of cellulose and levoglucosan. The methoxy derivatives of phenol would also be expected to show in this region, more precisely in the range 3.5–4 ppm. Certainty over the identification of guaiacol, syringol and similar species is made difficult due to the abundance of signal in this region of the spectrum, however these are likely to be present, based on the known composition of peat.20,21
Among the more easily identifiable signals in the high frequency region of the spectrum are those of furfural at 6.71 ppm, 7.44 ppm, 7.88 ppm and 9.58 ppm. This compound is characteristic of aged whisky and is often used as an indicator for the length of maturation time. Interestingly, it is also found in peated malt, so its presence in the smoke is expected. Several other related compounds, such as 5-hydroxymethylfurfural (5-HMF) and 5-methylfurfural can also be identified. None of these congeners have been detected in the extract of peat, consistent with the fact that they are usually formed as a result of thermal decomposition of carbohydrates, in the case of peat; cellulose and levoglucosan. Signals of multiple aromatic congeners, traces of which were present in peat extract, are apparent around 6.7 ppm and 7.1 ppm. These originate from phenolic compounds mentioned previously, but in much greater abundance (both in terms of variation and concentration) than in peat itself, again suggesting the increase in the amount of these congeners is a result of burning. Additionally, these findings align well with previous research into the origin of smoky notes in peated whisky.4,18,47 Finally, the presence of signals above 9 ppm is attributed to pyridine (specifically the relatively strong resonance at 8.52 ppm) and structurally related compounds.
3.5 NMR analysis of SCG smoke and a comparison with peat smoke
The low frequency region of the spectra appear to be very similar in all three samples, the largest difference being the relative intensity of the signals, noticeably greater in the SCG spectra (see Fig. 5).A similar observation was made for the peat- and SCG extract samples, evidencing that relatively larger quantities of long chain hydrocarbons are present in the SCG, compared to peat. While alkanes and alkenes are unlikely to have an impact on the overall aroma of the final spirit, their functionalised derivatives: alcohols, aldehydes, acids and esters have been reported to produce detectable smells even at concentrations as low as 0.02 parts per million.48 it should also be understood that the transfer of ‘larger’ hydrocarbons into aqueous media is likely to be limited due to their low solubility, even for functionalised derivatives. SCG spectra contain several weak signals around 1.4–1.5 ppm, which are not present in the peat smoke, which could signify the presence of a distinct group of compounds, such as cyclic hydrocarbons, which tend to display slight shifts to higher frequencies, compared to aliphatic chains. Signals of several common compounds can be identified around 2–2.5 ppm, however additional signals are observed in the SCG spectra, which could arise from methyl pyridines – the products of thermal decomposition of trigonelline and N-methylpyridinium compounds. The overall similarity of the spectra in this region suggests that both SCG smoke and peat smoke contain the desired congeners for the production of smoky whisky.
The middle region of the spectra is where the greatest differences between peat smoke and SCG smoke can be observed. The most noticeable difference is the presence of several strong signals in the SCG spectra, arising from protons of methylene and methine groups, connected directly to an oxygen (or nitrogen) atom and an aromatic ring, such as those in furfuryl and benzyl alcohols. Multiplets observed in the region are assigned to carbohydrates or their degradation products, while singlets observed at lower chemical shifts are associated with guaiacols. It should be noted that common low frequency signals are observed in all samples, suggesting that the phenolic congeners of interest are present in all samples (though some resonances are observed exclusively in the peat smoke sample, possibly indicating multiple isomers). The presence of strong signals, potentially arising from functionalised aromatics could make a significant impact on the flavour and aroma that the smoke would impart into the malt and therefore the spirit, as these compound classes possess distinct odours (e.g. furfuryl alcohol which has been described as alcoholic, caramellic and bready).
The aromatic regions of these spectra are remarkably similar, showing that despite significant differences in chemical composition of the original materials, the decomposition products (from burning) are similar. This includes phenolic species, such as cresols, guaiacols and syringols (clustered together around 6.7 ppm and 7.1 ppm) and some furan derivatives, such as 5-methylfurfural, 5-HMF and furfural. Together these compounds are likely to produce the desired smoky flavour and aroma, though the strength of these sensory effects is likely to depend on their concentration, which can differ significantly based on methodology and conditions used for burning, as well as the origin of the material. These results are encouraging however, it should be noted that several strong signals are present only in the coffee waste smoke samples. Resonances at 6.30 ppm and 6.36 ppm are connected to 2-methylfuran, which is the most abundant furan derivative in coffee and is often described as having a chocolate aroma and imparting a coffee taste.49 Other signals present in the 6–7 ppm region are likely to be other, less abundant furan derivatives and enol forms of cyclic ketones. These compounds have been connected to the presence of meaty and burnt notes, especially at higher concentrations.50 Another group of compounds which would be expected at high frequency are pyridine derivatives, referred to earlier. Indeed, several signals are observed at approximately 8.5 ppm in the SCG smoke spectra and are associated with various methylpyridines and methylpyrazines, some of the same resonances are also observed in the peat smoke sample, though they are generally of lower intensity.
3.6 Thermal gravimetric analysis
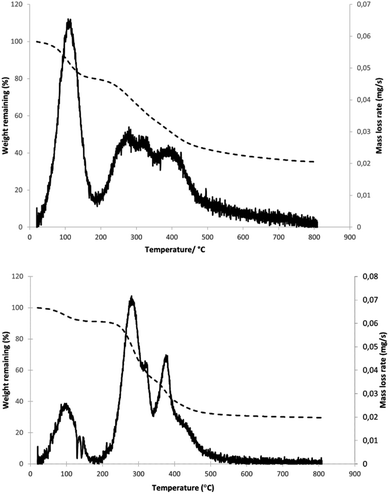
To establish a basic profile for thermal decomposition of the materials under study, thermal gravimetric analysis was performed on samples of peat and coffee waste (see Fig. 6). For peat, an initial drop in mass was observed, resulting from evaporation of water and possibly other low boiling point (e.g. short chain alcohols, aldehydes). It should be noted that the TG study was performed on the peat sample as received, without additional drying. Following a short plateau, another, much larger drop in mass is observed, beginning at approximately 200 °C with a nearly constant rate of reduction until 480 °C, at which point the mass drops very slowly until it reaches a constant value at 700 °C. Plotting the rate of mass loss against temperature reveals three maxima (aside from one resulting from water evaporation) in the 200–400 °C range, the first, at 280 °C resulting from thermal decomposition of hemicellulose,51 second at 325 °C, from cellulose,46 and third at 400 °C. The last peak is correlated to degradation of polyphenolic species, e.g., lignin.52 These results are consistent with TG data previously reported for peat.53 Ignoring the contribution of water, hemicellulose, cellulose and lignin were seen to produce overall mass losses of 17%, 11% and 19%, respectively. The relatively high value of the last figure is unexpected, as the lignin content of peat is reported to be lower than cellulose. This may be understood in the context of the known wide variation in peat composition or alternatively could reflect the presence of other polyaromatic polymers which decompose at a similar temperature.SCG presents a similar TG profile with an initial mass drop resulting from water evaporation followed by a large mass decrease from 250 °C to 500 °C. A decrease in gradient can be observed around 340 °C, which becomes much more pronounced once mass loss rate is plotted against temperature. In doing so, three distinct maxima (ignoring water) are revealed, with a clear separation of the second and third. The peaks are positioned at 285 °C, 325 °C and 380 °C, which correlates closely to those observed for peat. In the case of SCG, however, the corresponding mass loss values are 40%, 7% and 24% respectively. This clearly shows that hemicellulose is the major component, followed by lignin, reflecting the reported composition of SCG (30–40% hemicellulose, 5–10% cellulose, 20–30% lignin).25 Aside from the sharp maximum, a tail can be observed for lignin decomposition at temperatures above 400 °C, arising from the initial breaking of the bonds within the polymer, followed by decomposition of smaller aromatics over a wide temperature range. The remaining mass after heating to 800 °C constitutes approximately 30% of the original mass, while for peat the corresponding value is 35%. If water is not taken into account, the percentages of the original mass are 33% and 44% respectively. This observation that a larger proportion of peat remains after pyrolysis could be explained by the presence of significant amounts of ash containing inorganic compounds within peat, which does not decompose under the conditions used in this experiment.
3.7 Pyrolysis GC/MS analysis
Analysis of peat and SCG using pyrolysis coupled with gas chromatography/mass spectrometry (py-GCMS) revealed approximately two hundred different small molecules in peat smoke and three hundred in SCG smoke (considering those above 0.5% abundance). The most abundant and otherwise deemed important compounds found are summarized in Table 1, while the full list of identified compounds with error analysis for quantitation can be seen in the ESI (Tables S29 and S30†). The majority of these compounds are long chain alkanes or their various functionalised derivatives (including acids, alcohols, esters, ketones, aldehydes). Of the compounds identified, seventy one were found in both peat and SCG. In terms of odour and flavour, several compounds of interest were found in both samples, including phenol, guaiacol, furfural, o-cresol, p-cresol, 2-acetylfuran, 3,4-xylenol, 4-ethylphenol and 4-ethylguaiacol. Several of these congeners were identified in the smoke samples from NMR analysis, further reinforcing the conclusions drawn earlier. The relative percentage content of each compound was also determined, revealing that most congeners were present at higher concentration in the peat samples. In some cases, the difference was highly pronounced (e.g., the percentage value for p-cresol was over 15 times higher in peat than for SCG), while others were very similar (2,5-dimethylfuran, 2-acetylfuran). Compounds common to both peat and SCG samples also include nitrogen containing aromatics, some of which were more abundant in peat (3-methylpyridazine), others more prevalent in SCG (pyridine) and some similar for both (2-methylpyridine). This similarity is somewhat unexpected, as in SCG, these compounds are known to result from thermal degradation of trigonelline, while no such pathways exist for peat. It must therefore be the case, that peat contains different precursors to these compounds. Other compounds found in both peat and SCG samples are mostly small, functionalised molecules and aromatics, such as toluene, styrene, naphthalene and its derivatives. While predicting the flavour and aroma contribution of every single identifiable species is not possible (due to the lack of sensory data), some general predictions can be made. The presence of phenolic congeners in both materials suggests that a smoky character would be imparted into the spirit from SCG-kilning, however, the reduced content of these compounds in SCG might necessitate the use of larger amount of raw material in order to obtain the same levels of ‘peatiness’. Such practice could result in high levels of undesirable compounds, found only within coffee waste, being transferred into the spirit.| Compound | Compound (%) | Type of compound | |
|---|---|---|---|
| SCG | Peat | ||
| Common compounds | |||
| Palmitic acid | 4.37 | 0.41 | Fatty acid |
| Stearic acid | 0.90 | 0.14 | Fatty acid |
| Arachidic acid | 0.57 | 0.09 | Fatty acid |
| Heptadecane | 0.11 | 0.15 | Alkane |
| Tetradecane | 0.07 | 0.08 | Alkane |
| Tridecane | 0.06 | 0.08 | Alkane |
| 1-Tetradecene | 0.08 | 0.08 | Alkene |
| 1-Pentadecene | 0.05 | 0.07 | Alkene |
| Toluene | 0.31 | 1.10 | Aromatic |
| Styrene | 0.06 | 0.33 | Aromatic |
| Ethylbenzene | 0.05 | 0.10 | Aromatic |
| m-Xylene | 0.04 | 0.17 | Aromatic |
| Phenol | 0.27 | 0.58 | Phenolic |
| Guaiacol | 0.07 | 0.22 | Phenolic |
| o-Cresol | 0.05 | 0.21 | Phenolic |
| p-Cresol | 0.04 | 0.67 | Phenolic |
| 3,4-Xylenol | 0.03 | 0.12 | Phenolic |
| 4-Ethylphenol | 0.03 | 0.16 | Phenolic |
| 3-Methylpyridazine | 0.19 | 1.10 | N aromatic |
| Pyridine | 0.16 | 0.07 | N aromatic |
| 2-Methylpyridine | 0.02 | 0.02 | N aromatic |
| Furfural | 0.07 | 0.69 | Furan |
| 2,5-Dimethylfuran | 0.08 | 0.11 | Furan |
| 2-Acetylfuran | 0.04 | 0.02 | Furan |
| Levoglucosan | 0.09 | 0.98 | Anhydro sugar |
| Squalene | 0.99 | 1.17 | Steroid precursor |
| Compound | Compound (%) | Type of compound |
|---|---|---|
| SCG only compounds | ||
| Linoleic acid | 2.49 | Fatty acid |
| Pregnenolone | 2.44 | Steroid precursor |
| 2-Methylbicyclo[3.2.1]octane | 2.17 | Polycyclic |
| Caffeine | 1.06 | Alkaloid |
| 2-Furanmethanol | 0.43 | Furan |
| 2(5H)-furanone | 0.12 | Furan |
| 3-Methylfuran | 0.04 | Furan |
| 5-Methyl-2(5H)-furanone | 0.01 | Furan |
| 2,3-Dihydrofuran | 0.007 | Furan |
| 2,5-Dihydro-3,5-dimethyl-2-furanone | 0.006 | Furan |
| 1,2-Cyclopentanedione | 0.25 | Cyclic dione |
| 3-Methyl-1,2-cyclopentanedione | 0.21 | Cyclic dione |
| 2-Hydroxy-2-cyclopenten-1-one | 0.18 | Enol form of cyclic dione |
| 2-Hydroxy-3-methyl-2-cyclopenten-1-one | 0.12 | Enol form of cyclic dione |
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 0.06 | Enol form of cyclic dione |
| 3-Methyl-2-cyclopenten-1-one | 0.04 | Cyclic ketone |
| 1-Methyl-1H-pyrrole | 0.06 | Pyrrole |
| 2-Methyl-1H-pyrrole | 0.04 | Pyrrole |
| 3-Methylpyridine | 0.04 | N aromatic |
| Pyrazine | 0.02 | N aromatic |
| 2,5-Dimethylpyridine | 0.02 | N aromatic |
| 2-Ethylpyridine | 0.006 | N aromatic |
| Butanoic acid | 0.16 | Short chain fatty acid |
| Valeric acid | 0.08 | Short chain fatty acid |
| Limonene | 0.04 | Terpene |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Peat only compounds | ||
| Stigmasta-3,5-diene | 1.22 | Polycyclic |
| 1-Heneicosanol | 0.53 | Long chain alcohol |
| Behenic alcohol | 0.49 | Long chain alcohol |
| 1-Octadecanol | 0.40 | Long chain alcohol |
| 1-Eicosanol | 0.39 | Long chain alcohol |
| 1-Docosene | 0.46 | Long chain alkene |
| 1-Nonadecene | 0.40 | Long chain alkene |
| Tricosane | 0.43 | Long chain alkane |
| Tetracosane | 0.28 | Long chain alkane |
| Heneicosane | 0.26 | Long chain alkane |
| Octacosane | 0.26 | Long chain alkane |
| 5-Methylguaiacol | 0.36 | Phenolic |
| 4-Methylguaiacol | 0.31 | Phenolic |
| Syringol | 0.11 | Phenolic |
| 2-Isopropylphenol | 0.02 | Phenolic |
| 5-Methyl-2-furancarboxaldehyde | 0.24 | Furan |
| 2-Methylfuran | 0.07 | Furan |
| Isoacetovanillone | 0.07 | Aromatic |
| Mesitylene | 0.07 | Aromatic |
| 4,7-Dimethylbenzofuran | 0.04 | Aromatic |
| Aniline | 0.03 | N aromatic |
| 2,3-Dimethylpyridine | 0.01 | N aromatic |
| Acetic acid | 0.16 | Organic acid |
A variety of different classes of compounds were found exclusively in spent coffee grounds, though the three most abundant were all relatively large molecules: linoleic acid (fatty acid) at 2.49% total mass content, pregnenolone (steroid precursor) at 2.44% and 2-methylbicyclo[3.2.1]octane (polycyclic hydrocarbon) at 2.17%. The fourth most abundant compound is caffeine itself, which interestingly was not detected in the SCG smoke. This indicates that the conditions of thermal degradation can have a strong impact on the products generated and that not all of the products formed are transferred within the smoke dependent on their volatility. In broader terms, two groups of compounds with significant potential odour impacts can be identified among the pyrolysis products: furans and cyclic ketones. Compounds from both of these groups have been correlated to caramellic and maple odours and flavour, as well as burnt, especially at higher concentrations. Some congeners in these groups have also been noted as contributing significantly to the distinct coffee taste and smell. Other compounds located include pyrroles and their derivatives, (generally associated with the Maillard reaction), methylpyridine derivatives mentioned earlier, some organic acids including butanoic and valeric acid and a variety of other compounds which couldn't be identified in the GCMS.
Among the compounds found exclusively within peat the principal groups are once again long chain alcohols, alkanes, alkenes and other functionalised hydrocarbons. Smaller compounds of interest include aromatics, e.g., isomers of guaiacol, syringol and phenol derivatives. These compounds are usually related to phenolic and smoky aromas and as such are expected to play an important role in imparting these into peated whisky. The absence of some of the characteristic compounds in SCG raises an interesting question of the potential impact on the overall odour/flavour profile. Judging the impact on aroma and taste of other compounds where sensory data is unavailable, is more difficult, though their contribution cannot be ignored. Even if they do not contribute directly to ‘smokiness’ of the spirit they may influence the sensory response in other ways. The contribution of long chain organic compounds depends mostly on their functionalization.
3.8 NMR analysis of malt extract
The overall spectral profiles of extracts from malt that had been exposed to peat- and SCG smoke appear very similar (Fig. 7). This is because only a small portion of compounds found within the smoke is effectively transferred into the malt during the drying process. Signals in the low frequency region are very weak, compared to those observed in the raw material extracts and smokes. This may be explained by the change in solvent from methanol to a mixture of ethanol and water at 10% v/v (chosen initially to reflect the predistillation alcohol matrix of the fermented grain), which led to an increased transfer of aromatic compounds of interest but was less effective for those in this region (for spectra obtained using methanol, see ESI†). The largest signal in the region is that of the partially suppressed methyl group from ethanol. The remaining resonances correspond to small polar molecules, such as carboxylic acids and esters as well as alkyl groups of phenolic species. These include o-cresol (2.19 ppm) and p-cresol (2.37 ppm) which were previously found in smoke samples, strongly suggesting that this class of compounds is transferred into the malt during the drying process, irrespective of whether peat or SCG is used in the kiln. Other compounds showing identifiable signals in the region include acetic acid (1.94 ppm) and acetaldehyde (2.24 ppm), which are present in untreated malt extracts and are not expected to have originated from the smoke.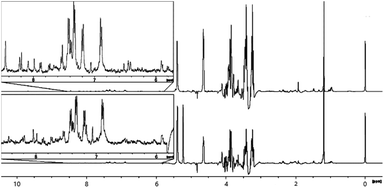 | ||
| Fig. 7 Comparison of 1H NMR spectra (recorded at 400 MHz) of ethanol/water (10% v/v) extracts of industrially peated malt (bottom) and malt smoked using spent coffee grounds (top). | ||
The middle region contains the most intense signals, these being representative of various carbohydrates, principally glucose and sucrose, easily identifiable by characteristic resonances at 4.64 ppm (b-glucose anomeric proton), 5.22 ppm (a-glucose anomeric proton) and 5.39 ppm (sucrose glycosidic linkage). The appearance of these signals in the spectra (two overlapping doublets rather than a doublet) suggests presence of another carbohydrate in the mixture, possibly maltose – a product of starch hydrolysis, commonly found in malt. For both peat- and SCG spectra, signal integrals are near-identical, unsurprising as these species are native to the malt itself, rather than derived from the smoke. The presence of these strong sugar resonances masks evidence of other, less abundant, compounds coming from the smoke. As a result, this part of the spectrum provides minimal information on the effect of smoke exposure to malt. The SCG-malt spectrum lacks the intense doublet at 5.22 ppm due to inadvertent suppression by the suppression scouting sequence, but this did not impact on the assignments.
The aromatic region of the spectrum is of most interest in this study, as signals appearing here are unlikely to originate from the malt and therefore should reflect the suitability of SCG as a peat replacement for imparting smoky flavour and aroma. The most notable differences between the two samples is the presence of signals at 6.41 ppm and 6.44 ppm in the SCG malt, both of which arise from aromatic protons of 2-methylfuran. This compound is the most abundant furan derivative found in coffee, explaining its presence in the treated malt. Based on this observation it is likely that other coffee-derived furans could be transferred into the malt, though at lower, potentially unobservable concentrations. The other significant difference is the intensity of signals above 8 ppm in the SCG malt sample, reflecting the increased abundance of pyridine and methylpyridines. There is commonality in the presence of other strong signals in this region, associated with phenolic congeners, in addition to other unidentified aromatics. These observations suggest that the chemical composition of the spirit produced using malt dried using both peat and SCG would be similar, based on the results of NMR investigation. The principal differences are related to the presence of furans and pyridines in the case of SCG-derived spirit, which may lead to undesirable off-notes, though it is uncertain whether those compounds are carried over into the final aged spirit.
4. Conclusions
The chemical composition of peat and spent coffee grounds were investigated through analysis of their extracts, smoke and treated malt. The suitability of spent coffee grounds as a replacement of peat in the malt drying process was assessed based on the presence of key flavour compounds. The extracts of raw materials showed vastly different chemical profiles, though some of the congeners of interest, e.g., o-cresol, p-cresol and phenol were identified in both. Others, e.g., guaiacol and its derivatives were only present in peat. Following burning, the smoke produced was analysed, the resulting spectra now presenting many similarities. Such observations confirm that thermal degradation of biopolymers present in coffee waste and peat can generate common products, potentially resulting in similar flavour profiles in the final spirit. This finding was further confirmed through the analysis of the extract of malt dried using SCG in place of peat, the 1H NMR spectrum being similar to that from industrially peated malt extract. Despite these promising results, the presence of higher concentrations of pyridine derivatives at all stages of SCG processing could indicate that presence of nitrogen containing species in coffee waste could lead to release of harmful chemicals, which aligns with previous studies investigating combustion of SCG. Additionally, the presence of furan derivatives, e.g., 2-methylfuran may alter the aroma/taste profile. Results obtained from py-GCMS analysis revealed that while many compounds produced by thermal degradation of SCG and peat are the same (including the desired congeners), there is variability between peat and SCG samples in the composition of other small molecules present at low concentration (undetectable by NMR). The impact on flavour and aroma is difficult to predict, due to the number of species involved the and lack of available sensory data.Overall, coffee waste can produce smoke, which will impart flavour to the spirit in a similar way to peat. The methodology presented here provides an insight into the composition of materials studied and has the potential to identify other suitable peat replacements. It should be noted again however, that the work herein provides a snapshot of the comparison between the nature of peat and SCG. Only one sample of each has been examined and further work is required to evaluate the impact of the known wide variability of peat and coffee on the characterization of the smoke and malts.
Author contributions
Kacper P. Krakowiak: data curation, formal analysis, funding acquisition, investigation, writing – original draft. Ruaraidh D. McIntosh – conceptualization, funding acquisition, methodology, project administration, supervision, writing – review and editing. David Ellis – conceptualization, funding acquisition, methodology, project administration, supervision, writing – review and editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to thank the Carnegie Trust for Universities of Scotland for funding, Impact Solutions for performing py-GC/MS analysis, Barry Harrison and Nicholas Pitts of The Scotch Whisky Research Institute for providing the malt samples, and James Anderson for providing the peat samples.Notes and references
- Scotch Whisky Association, https://www.scotch-whisky.org.uk/newsroom/scotch-whisky-exports-2022/, accessed March 2023.
- T. A. Bringhurst and J. Brosnan, in Whisky, Elsevier, London, 2015, vol. 6, pp. 49–122 Search PubMed.
- B. Guthrie, J. Beauchamp, A. Buettner, S. Toth and M. Qian, Sex, Smoke, and Spirits the Role of Chemistry, American Chemical Society, Washington D. C., 2019 Search PubMed.
- G. N. Bathgate, J. Inst. Brew., 2019, 125(2), 200 CrossRef CAS.
- B. Harrison, J. Ellis, D. Broadhurst, K. Reid, R. Goodacre and F. G. Priest, J. Inst. Brew., 2006, 112(4), 333 CrossRef.
- B. Harrison and F. G. Priest, J. Agric. Food Chem., 2009, 57(6), 2385–2391 CrossRef CAS PubMed.
- H. Rydin and J. K. Jeglum, in The Biology of Peatlands, Oxford University Press, Oxford, 2013, pp. 274–295 Search PubMed.
- H. Rydin and J. K. Jeglum, in The Biology of Peatlands, Oxford University Press, Oxford, 2013, pp. 230–253 Search PubMed.
- UK, I. Peatland Programme, https://www.iucn-uk-peatlandprogramme.org/, accessed October 2022 Search PubMed.
- S. Yamulki, R. Anderson, A. Peace and J. I. L. Morison, Biogeosciences, 2013, 10(2), 1051 CrossRef.
- H. Vasander and A. Kettunen, in Boreal Peatland Ecosystems, ed. R. K. Wieder and D. H. Vitt, Springer Berlin, Heidelberg, 2006, pp. 165–194 Search PubMed.
- G. Hambley, R. Andersen, P. Levy, M. Saunders, N. R. Cowie, Y. A. Teh and T. C. Hill, Mires Peat, 2018, 23(5), 1 Search PubMed.
- H. Rydin and J. K. Jeglum, in The Biology of Peatlands, Oxford University Press, Oxford, 2013, pp. 21–47 Search PubMed.
- Y. Arlen-Pouliot and N. Bhiry, Holocene, 2005, 15(3), 408 CrossRef.
- P. Kuhry and J. H. Turunen, in Boreal Peatland Ecosystems, ed. R. K. Wieder and D. H., Vitt, Springer Berlin, Heidelberg, 2006, pp. 25–46 Search PubMed.
- Scotch Whisky Association, https://www.scotch-whisky.org.uk/insights/sustainability/caring-for-the-land/, accessed November 2022.
- G. N. Bathgate and A. G. Taylor, J. Inst. Brew., 1977, 83(3), 163 CrossRef CAS.
- H. H. Jeleń, M. Majcher and A. Szwengiel, Food Sci. Technol., 2019, 107, 56 Search PubMed.
- K. Y. M. Lee, A. Paterson, J. R. Piggott and G. D. Richardson, J. Inst. Brew., 2001, 107(5), 287 CrossRef.
- J. Schellekens, P. Buurman and T. W. Kuyper, Soil Biol. Biochem., 2012, 53, 32 CrossRef CAS.
- G. Vinci, P. Mazzei, M. Drosos, C. Zaccone and A. Piccolo, Chem. Biol. Technol. Agric., 2020, 7(1), 1 CrossRef.
- T. Tokimoto, N. Kawasaki, T. Nakamura, J. Akutagawa and S. Tanada, J. Colloid Interface Sci., 2005, 281(1), 56 CrossRef CAS PubMed.
- H. Moustafa, C. Guizani and A. Dufresne, J. Appl. Polym. Sci., 2017, 134(8), 44498 CrossRef.
- H. Moustafa, C. Guizani, C. Dupont, V. Martin, M. Jeguirim and A. Dufresne, ACS Sustainable Chem. Eng., 2017, 5(2), 1906 CrossRef CAS.
- A. Kovalcik, S. Obruca and I. Marova, Food Bioprod. Process., 2018, 110, 104 CrossRef CAS.
- R. Cruz, S. Morais, E. Mendes, J. A. Pereira, P. Baptista and S. Casal, Food Chem., 2014, 148, 294 CrossRef CAS PubMed.
- J. P. Ribeiro, E. D. Vicente, A. P. Gomes, M. I. Nunes, C. Alves and A. L. C. Tarelho, Environ. Sci. Pollut. Res., 2017, 24(18), 15270 CrossRef CAS PubMed.
- S. I. Mussatto, L. F. Ballesteros, S. Martins and J. A. Teixeira, Sep. Purif. Technol., 2011, 83(1), 173 CrossRef.
- L. F. Ballesteros, J. A. Teixeira and S. I. Mussatto, Food Bioprocess Technol., 2014, 7(12), 3493 CrossRef CAS.
- J. McNutt and Q. He, J. Ind. Eng. Chem., 2019, 71, 78–88 CrossRef CAS.
- S. B. Kang, H. Y. Oh, J. J. Kim and K. S. Choi, Renew. Energy, 2017, 113, 1208–1214 CrossRef CAS.
- W. Kew, W. I. Goodall and D. Uhrin, Food Chem., 2019, 298, 8 CrossRef PubMed.
- C. Almeida, I. F. Duarte, A. Barros, J. Rodrigues, M. Spraul and A. M. Gil, J. Agric. Food Chem., 2006, 54(3), 700 CrossRef CAS PubMed.
- L. Viggiani and M. A. C. Morelli, J. Agric. Food Chem., 2008, 56(18), 8273–8279 CrossRef CAS PubMed.
- F. K. Achard, Crell’s Chem. Ann., 1786, 2, 391 Search PubMed.
- I. W. Tjurin, Arbeiten der jubiläumstagung Anlässlich des 100 Geburtstages von W. W. Dokutschajew, 1st A. N. SSSR, Moscow, 1949 Search PubMed.
- J. Kosaka, C. Honda and A. Izeki, Soil Sci. Plant Nutr., 1961, 7, 48 CrossRef.
- G. Song, E. H. Novotny and M. H. B. Hayes, J. Soils Sediments, 2023, 23, 1146 CrossRef CAS.
- ChemicalBook, https://www.chemicalbook.com/, accessed December 2022 Search PubMed.
- John Wiley & Sons, I. SpectraBase, https://spectrabase.com/, accessed December 2022 Search PubMed.
- T. Saito, K. Hayamizu, M. Yanagisawa and O. Yamamoto, Spectral Database for Organic Compounds SDBS, https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=hnmr%26sdbsno=1898, accessed February 2022 Search PubMed.
- D. S. Wishart, A. Guo, E. Oler, F. Wang, A. Anjum, A. H. Peters and V. Gautam, Nucleic Acids Res., 2022, 50(D1), D622 CrossRef CAS PubMed.
- H. E. Gottlieb, V. Kotlyar and A. Nudelman, J. Org. Chem., 1997, 62(21), 7512 CrossRef CAS PubMed.
- F. Wei, K. Furihata, M. Koda, F. Hu, T. Miyakawa and M. Tanokura, J. Agric. Food Chem., 2012, 60(4), 1005 CrossRef CAS PubMed.
- R. H Stadler, N. Varga, J. Hau, F. A. Vera and D. H. Welti, J. Agric. Food Chem., 2002, 50(5), 1192 CrossRef PubMed.
- D. K. Shen and S. Gu, Bioresour. Technol., 2009, 100(24), 6496–6504 CrossRef CAS PubMed.
- B. Harrison, Peat Source and Its Impact on the Flavour of Scotch Whisky, ProQuest Dissertations Publishing, 2007 Search PubMed.
- M. Zarzo, Sensors, 2012, 12(4), 4105 CrossRef CAS PubMed.
- A. Rahn and C. Yeretzian, Food Chem., 2019, 272, 514 CrossRef CAS PubMed.
- B. Wailzer, J. Klocker, P. Wolschann and G. Buchbauer, Nat. Prod. Commun., 2016, 11(10), 1475 CrossRef PubMed.
- C. Di Blasi, Prog. Energy Combust. Sci., 2008, 34(1), 47 CrossRef CAS.
- M. Asmadi, H. Kawamoto and S. Saka, J. Anal. Appl. Pyrolysis, 2011, 92(2), 417 CrossRef CAS.
- J. Yang, H. Chen, W. Zhao and J. Zhou, J. Anal. Appl. Pyrolysis, 2016, 117, 296 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fb00088e |
| This journal is © The Royal Society of Chemistry 2024 |