Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhancement of the texture and microstructure of faba bean-based meat analogues with brewers' spent grain through enzymatic treatments
Yue
Fan
 a,
Shiyu
Zheng
a,
Shiyu
Zheng
 a,
Pratheep K.
Annamalai
a,
Pratheep K.
Annamalai
 abc,
Bhesh
Bhandari
a and
Sangeeta
Prakash
abc,
Bhesh
Bhandari
a and
Sangeeta
Prakash
 *a
*a
aSchool of Agriculture and Food Sustainability, The University of Queensland, St Lucia QLD 4072, Australia. E-mail: s.prakash@uq.edu.au
bCentre for Future Materials, University of Southern Queensland, Toowoomba QLD 4350, Australia
cSchool of Agriculture and Environmental Sciences, University of Southern Queensland, Toowoomba QLD 4350, Australia
First published on 24th April 2024
Abstract
The meat industry significantly contributes to environmental issues, and the high consumption of animal-based foods is a growing concern. As a response, there has been a rise in the development and commercialization of plant-based meat alternatives. However, producing these alternatives often involves processing additives and complex techniques to achieve the desired texture and sensory properties. To overcome this challenge, this study proposes using enzymatic crosslinking treatment with cost-effective processing methods to enhance the textural and structural properties of faba bean protein-based meat analogues with minimal additives. This study explores the use of enzymes such as transglutaminase (TG) and laccase (LC) to promote crosslinking reactions of amino acids through different mechanisms. The study uses various techniques to demonstrate the effectiveness of transglutaminase and laccase in improving the microstructure, texture, and water-holding capacity of formulated meat analogues. Transglutaminase has led to excellent replication of a commercial luncheon meat product. At the same time, laccase has shown potential in making the designed meat analogues more comparable to animal meat products. This study provides valuable insights into the role of transglutaminase and laccase in cross-linking plant proteins and offers a more accurate formulation of meat analogues that closely resemble authentic meat in the future.
Sustainability spotlightIn the face of the environmental toll caused by global meat consumption, the priority to create sustainable alternatives intensifies. This study breaks from using sophisticated technologies, opting for a low-energy enzymatic treatment to produce plant-based meat from faba bean protein and brewers' spent grain, successfully replicating the structural and textural properties of commercial animal meat. By repurposing industrial waste or by-products (brewers' spent grain), it not only signifies a breakthrough in sustainable food production but also aligns with the United Nations' Sustainable Development Goals (SDGs), especially Goal 2 (sustainable agriculture) and Goal 13 (climate action). |
1. Introduction
As a more sustainable and less controversial alternative to animal meat, plant-based meat has gained increasing attention in recent years. A plant-based meat, as the name suggests, is a meat analogue product derived from plant materials, which simulates the sensory perception and nutritional value of animal meat. Numerous studies have demonstrated that plant-based meats have a smaller ecological footprint than conventional meat.1–4 As opposed to animal meat, plant-based meats are not associated with any ethical or religious issues. Moreover, plant-based meats are low in saturated fats and cholesterol-free, providing a path towards a healthier diet.The low sensory appeal of plant-based meat alternatives has been identified as one of the major obstacles contributing to their low consumption rate. For meat analogues to attain the desired sensory properties, modern production is heavily dependent on additives and sophisticated techniques (e.g., extrusion, shear cell technology, 3D printing, spinning processes, and freezing structuring) that require a substantial amount of mechanical energy. It is possible to further enhance the sustainability benefits of meat alternatives by incorporating low-energy processing methods and environmentally friendly ingredients (e.g., industrial wastes and by-products). Accordingly, this study sought to process plant-based meat analogues (PBMAs) in a sustainable manner using classical mixing and heating techniques combined with enzymatic treatment to mimic an animal meat product.
The food structure is undeniably an important element, since it directly affects the texture and perception of food by consumers. There are numerous ways to tailor the structure of food, one of which is through the creation of covalent cross-links between food biopolymers.5 Protein cross-linking is an effective method of improving the physical characteristics of meat products, such as their texture and stability. It is the process of linking amino acid residues within the protein polypeptide chain through intra- or inter-molecular covalent bonds, which lead to the formation of a protein network.6 Several methods can be employed to induce cross-linking reactions in food matrixes, including chemical, physical, and enzymatic approaches. Among the major advantages of enzymatic treatment over other methods are its specificity and the mild conditions required to initiate the reaction.7
Proteins contain several reactive groups that can be cross-linked by enzymes, including cysteine, glutamine, lysine, and tyrosine. Based on the enzyme type, the accessibility of the target reactive groups within the biopolymer, and the process conditions, the type of reaction initiated will vary.7
It has been demonstrated that transglutaminase and laccase act as effective crosslinking agents for the generation of new textures or the modulation of formulations without compromising the intended mouthfeel of food products, particularly protein-stabilized emulsions and gels.6,8 Transglutaminase (EC 2.3.2.13, γ-glutamyl-peptide, amine-γ-glutamyl transferase) is a natural enzyme that catalyses the formation of a covalent bond (i.e., ε-(γ-glutamyl)lysine isopeptide bond) between the glutamine residue side chains and lysine residue side chains, leading to polymerization.9 Laccase (EC 1.10.3.2, benzenediol: oxygen oxidoreductase) is another potential food protein cross-linker that has been proposed as an alternative to transglutaminase in the development of plant-based meat.10 Mechanistically, transglutaminase catalyses cross-link formation directly, whereas laccase which is an oxidative enzyme, catalyses the oxidation of phenolic compounds through a single electron, generating a reactive aromatic radical that can attack proteins and cause them to crosslink via tyrosine residues.8 The potential of laccase to enhance the quality of plant-based meat has been however investigated only in a limited number of studies.
For most studies, soy or pea proteins were used as the primary proteins for creating meat analogues owing to their excellent emulsifying and gelling properties.11–15 It is necessary to diversify and utilize plant protein sources that have not previously been examined to prevent overdependence on soy and pea proteins. It is also noteworthy that most studies focus on the fabrication of meat analogues with desirable sensory properties using sophisticated technologies that require a considerable amount of mechanical energy. Further to the application of low-energy processing methods (i.e., enzymatic treatment), this study aims to incorporate environmentally friendly materials to maximize the sustainability of plant-based meats.
Faba is a nitrogen-fixing crop capable of fixing atmospheric nitrogen and depositing it in the soil, thereby fertilising the soil.16 The cultivation of faba beans requires only a small amount of fertilizer. Nutritionally, faba beans are considered as a high-quality protein source due to their complete and well-balanced amino acid profile and the presence of multiple bioactive compounds (e.g., bioactive peptides and phenolic compounds).17 Moreover, faba bean protein has excellent gelling properties, which allow it to create a desirable texture with minimal additives.18,19 For these reasons, faba bean protein is used as a matrix material for constructing meat analogues in this study.
The brewers' spent grain (BSG), a by-product of the brewing industry, is used as a binding agent in this work. This by-product is also sustainable and nutrient-rich, containing a large number of phenolic compounds (i.e., hydroxycinnamic acids), which possess multiple functional properties. The high fibre (approximately 50%) and protein (approximately 20%) contents of BSG allow it to act as a binding agent (a physical additive), inducing structural changes in the protein matrix system. There is considerable potential for fibres to enhance the texture and structure of meat analogues by entrapping free water that would otherwise be located between proteinaceous structures.11,20 Further, BSG can supplement the dietary fibre content in food products, which promotes cholesterol and fat excretion and prevents constipation by increasing stool weight and accelerating intestinal transit. The growing concern regarding the reuse of industrial wastes or by-products has led to BSG becoming an increasingly popular ingredient in human foods. However, the incorporation of BSG into meat substitute formulations has not been reported.
This study aims to develop a plant-based meat product with enhanced textural and structural properties using a low-energy processing method (i.e., enzymatic treatment in combination with classical mixing and heating techniques) and environmentally friendly materials (i.e., faba bean protein isolate and BSG flour) to maximize sustainability. Building upon cost-effective processing, enzymatic cross-linking treatment (performed with transglutaminase or laccase) was chosen to improve meat analogue properties. Researchers have demonstrated that transglutaminase can form cross-links with plant proteins, including soybean, pea, potato, and peanut protein, for the construction of meat analogues.21–25 Even fewer studies have assessed laccase's potential for improving meat analogues than transglutaminase, and they were limited to soybean protein.26,27 By reporting the analysis and detailed discussion, this study demonstrates that faba bean protein is a promising ingredient in building meat analogues and provides evidence for the cross-linking ability of transglutaminase and laccase to enhance the texture and structure of meat analogue products. Through the combination of faba bean protein and transglutaminase or laccase, a uniform texture animal meat product was successfully replicated. This information will be useful in the development of meat analogue formulations that are more closely aligned with the desired physicochemical properties and sensory characteristics of authentic meat.
2. Materials and methods
2.1. Materials
A luncheon meat product (Hans, Australia) available from a local market was selected as the target product. This product consists of the following ingredients: pork, water, seasoning [potato starch, tapioca starch, salt, modified starch (1442 maize or tapioca), soy protein, mineral salts (451 and 341), dextrose (maize or tapioca), spices, antioxidant (316), preservative (250), red fermented rice], and acidity regulators (326, 325, and 262). Faba bean protein isolate was purchased from Australia Plant Proteins Pty Ltd, Australia, which contains 88% protein, 5–6% fat, 0.5% carbohydrates, and 2–3% ash. Brewers' premium flour was provided by Grainstone Pty Ltd, Australia, containing 50% dietary fibre, 26% protein, 9% fat, 14% carbohydrates and 0.2% ash. Sunflower oil, purchased from Moi International Pty Ltd, Australia, was 100% pure. Two enzymes were used: transglutaminase and laccase, which were purchased from Enzymes Bio, New Zealand.2.2. Methods
A steam oven (Unox, Australia) with a humidity level of 10% was used to heat the mixed dough for 20 minutes at 100 °C. To verify that enzyme reactions were terminated, samples were monitored after heating to ensure that 80 °C had been reached. Three samples were prepared, an enzyme-free sample, a transglutaminase-treated sample, and a laccase-treated sample, which were denoted as S-EF, S-TG and S-LC, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.28 The mixed solution was heated at 90 °C for 4 min and then centrifuged (Labco mini centrifuge, Australian Scientific Ltd., NSW, Australia) at 4000 g for 10 min at 25 °C.28 A supernatant containing 4 μg protein per μL was loaded into the gel (Mini-PROTEIN TGX precast gel 4–20%, 15-well comb) together with a protein marker (Precision Plus Protein™ Dual Xtra Protein Standard).28 The gels were run at 100 V. Following electrophoresis, Coomassie Brilliant Blue R-250 was used to stain the gel, followed by Millipore water to rinse and destain it. Finally, the gel was scanned with a GS-900 calibrated densitometer (Bio-Rad Laboratories, Inc., USA). A quantitative analysis of band intensity was performed using ImageJ.JS (version 0.5.8) to determine the relative composition of the three meat analogue pastes.
1 ratio.28 The mixed solution was heated at 90 °C for 4 min and then centrifuged (Labco mini centrifuge, Australian Scientific Ltd., NSW, Australia) at 4000 g for 10 min at 25 °C.28 A supernatant containing 4 μg protein per μL was loaded into the gel (Mini-PROTEIN TGX precast gel 4–20%, 15-well comb) together with a protein marker (Precision Plus Protein™ Dual Xtra Protein Standard).28 The gels were run at 100 V. Following electrophoresis, Coomassie Brilliant Blue R-250 was used to stain the gel, followed by Millipore water to rinse and destain it. Finally, the gel was scanned with a GS-900 calibrated densitometer (Bio-Rad Laboratories, Inc., USA). A quantitative analysis of band intensity was performed using ImageJ.JS (version 0.5.8) to determine the relative composition of the three meat analogue pastes.
| VE (%) = (H2 − H1)/H1 × 100 | (1) |
| WHC (%) = W2/W1 | (2) |
3. Results and discussion
3.1. Characterisation of uncooked meat analogue pastes
To investigate the effectiveness of the two enzymes in initiating cross-linking reactions before heating, enzyme-free and enzyme-treated meat analogue pastes were evaluated. SDS-PAGE (sodium dodecyl-sulfate polyacrylamide gel electrophoresis) and rheological measurements determined the effectiveness of the enzymes in cross-linking. SDS-PAGE separated proteins based on size and was used to determine the extent to which the enzymes were able to cross-link proteins in uncooked meat analogue pastes. Rheological properties were assessed over time to gain a deeper understanding of how the cross-linking effects of the two enzymes developed. | ||
| Fig. 1 SDS-PAGE analysis of the enzyme-free sample (EF) and the samples treated with transglutaminase (TG) or laccase (LC) before heating. Lane M: standard protein markers. | ||
According to the relative composition table, the LC sample contained lower levels of convicilin and vicilin than the enzyme-free sample, but there was no difference in legumin percentages between the two samples. The differences between LC and EF samples are not as significant as those between TG and EF samples, indicating that laccase is less efficient at forming large protein–protein compounds or cross-linking proteins than transglutaminase.
Fig. 2 depicts the mechanisms of transglutaminase- and laccase-mediated cross-linking in food proteins. Transglutaminase is also known as γ-glutamyl-peptide, amine-γ-glutamyl transferase, which catalyses the formation of a covalent bond (i.e., ε-(γ-glutamyl)lysine isopeptide bond) between the peptide-bound lysine (acyl acceptors) and peptide-bound glutamine (acyl donors).5 Unlike transglutaminase, laccase is an oxidative enzyme, or more specifically, it is a multi-copper enzyme that catalyses the oxidation of phenolic compounds through a single electron, generating an unstable reactive aromatic radical that can attack proteins, causing them to cross-link via tyrosine residues.8 A possible explanation for transglutaminase causing larger protein complexes than laccase may be due to the fact that transglutaminase gives priority to the faba bean protein in the covalent cross-linking reaction.32 In particular, faba bean protein contains a high level of lysine residues,17 which provide an optimal substrate for transglutaminase to form cross-linked networks through intermolecular or intramolecular covalent bonds.32
 | ||
| Fig. 3 Time-sweep test of uncooked meat analogue pastes showing the influence of enzymatic treatment on elastic properties (storage modulus, G′). | ||
It was found that S-TG experienced an apparent increase in G′ over time, except for a brief reduction at the beginning. The brief decline at the beginning can be attributed to the change in the paste structure. The paste had an intact structure at the start, yielding high values for the storage modulus. As the oscillation proceeded, the initial structure of the sample was damaged, resulting in a decrease in G′. The substantial increase in G′ for S-TG paste over time indicates the ability of transglutaminase to crosslink structures. A similar finding was reported by Song et al.,36 stating that the storage modulus increases with the duration of cross-linking due to an increase in the formation of protein networks.
Unlike S-TG, S-LC remained steady over time after a brief decrease in the beginning, which followed a similar trend to S-EF (control sample). In comparison to S-EF (control sample), S-TG had significantly higher G′ values, while S-LC had slightly lower values. In other words, transglutaminase (TG) increased the storage modulus in a considerable manner, whereas laccase (LC) somewhat decreased it. As illustrated in Fig. 2, laccase induces a cross-linking reaction that generates water as a by-product,37 which is not the case with transglutaminase.6 Based on the findings of the study conducted by Upadhyay et al.,38G′ decreased as the water content increased. The rheological results indicate that laccase was unable to cross-link the structure effectively during the test period of 5 minutes. However, there is a possibility that the increased water content may obscure the effect of laccase cross-linking, preventing an increase in G′. Despite laccase reducing the storage modulus, it may still indicate that it initiates the cross-linking reaction due to the possibility of additional water present.
3.2. Influence of enzymatic treatment on cooked meat analogues
| S-EF | S-TG | S-LC | |
|---|---|---|---|
| a Different lowercase letters within rows indicate a significant difference (p < 0.05). | |||
| Height-BH (mm) | 19.5 ± 0.5c | 21.8 ± 0.3a | 20.7 ± 0.3b |
| Height-AH (mm) | 26.0 ± 0.9a | 27.2 ± 1.0a | 26.5 ± 0.5a |
| Volume expansion (%) | 33.3 ± 2.3a | 24.4 ± 3.2b | 28.2 ± 1.3ab |
Since equal weight samples were placed in equal volume containers (see Section 2.2.4. Volume expansion), the height of the uncooked paste before heating (height-BH) can provide an indication of its density. The greater the height-BH value, the lower the density of an uncooked paste. The heights of the three samples before heating are listed in the decreasing order as follows: S-TG (21.8 mm), S-LC (20.7 mm) and S-EF (19.5 mm). It suggests that both enzymes caused the sample pastes to swell without the use of heat. As mentioned earlier, transglutaminase catalyses acyl-transfer reactions between glutamyl residues and lysyl residues in proteins, leading to polymerization or amine incorporation.5 During this reaction, one molecule of ammonia (NH3) is generated per crosslink (Fig. 2a). Gas generated by transglutaminase-induced cross-linking may cause swelling of the sample paste due to increased internal pressure.39
As depicted in Fig. 2b, laccase catalyses the oxidation of phenolic compounds via a single-electron removal mechanism, resulting in the generation of free radicals and a concurrent reduction of molecular oxygen to water (H2O). The water released during cross-linking may be responsible for swelling in the laccase-catalysed paste. Meat analogue pastes are primarily composed of protein, dietary fibre and starch, all of which are hydrated upon contact with water, allowing the molecular chains to move more freely and the food matrix to swell.40 An increase in water content will inevitably result in a greater swelling. Height-BH results indicate that both enzymes initiated the reaction, which is in line with SDS-PAGE results.
The volume expansion of a sample is the degree to which it expands from an uncooked state to a cooked state. It provides information on the degree of cross-linking. Cross-linking involves the formation of covalent bonds between portions of several polymer chains. Through extensive cross-linking, a three-dimensional network of interconnected chains is formed (Fig. 2), which causes the chains to be less mobile and thus impedes the expansion of the sample during heating.36 Therefore, a lower volume expansion indicates a greater degree of cross-linking.
As revealed in Table 1, enzyme-free samples experienced the greatest volume expansion (33.3%), followed by LC-treated samples (28.2%) and TG-treated samples (24.4%). Accordingly, both laccase and transglutaminase inhibited the expansion of the sample during heating; however, transglutaminase inhibited the expansion in a more significant manner. The volume expansion test confirmed that both transglutaminase and laccase can establish cross-linking interactions, which is consistent with the results obtained from the SDS-PAGE analysis. Even though laccase does not appear to cross-link proteins as effectively as transglutaminase, it still shows promise in initiating cross-linking reactions within faba bean proteins.
 | ||
| Fig. 4 Fourier transform infrared spectroscopy (FT-IR) spectra of enzyme-free (EF), transglutaminase-treated (TG) and laccase-treated (LC) samples. | ||
There is a strong and wide absorbance band observed in the wavenumber region of 3500–3200 cm−1, which is attributed to the O–H and N–H symmetric stretching vibration of alcohols and amides in proteins.42 The EF-sample exhibited higher absorbance than the enzymatically treated samples (S-TG and S-LC). A lower absorbance for S-TG and S-LC can be attributed to the consumption of O–H and N–H functional groups upon cross-linking reactions as shown in Fig. 2. The TG-sample is due to the generation of ammonia (NH3) as a result of transglutaminase-initiated cross-linking, which readily volatilizes,5 thus reducing the N–H bond. It is a different scenario in laccase. A possible explanation for the lower absorption in the LC-sample could be that the additional water (H2O) generated during the laccase-mediated cross-linking evaporated upon heating, resulting in the diminution of the O–H bond. This supports the occurrence of crosslinking reactions initiated by laccase and transglutaminase enzymes.
In Fig. 4, the band between 2950 cm−1 and 2850 cm−1 is related to the C–H asymmetric and symmetric stretching vibrations of lipids.43 The band at approximately 1780 cm−1 is associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of phospholipids.43 The sample treated with transglutaminase (S-TG) displayed the highest absorbance bands, followed by the sample treated with laccase (S-LC) and the sample not treated with enzymes (S-EF). This may be due to the fact that enzyme-mediated cross-linking may provide opportunities for lipidated proteins to be synthesized.44
O stretching of phospholipids.43 The sample treated with transglutaminase (S-TG) displayed the highest absorbance bands, followed by the sample treated with laccase (S-LC) and the sample not treated with enzymes (S-EF). This may be due to the fact that enzyme-mediated cross-linking may provide opportunities for lipidated proteins to be synthesized.44
Moreover, the FTIR technique has been shown to be sensitive to the secondary structure of proteins, making it a useful tool for investigating the formation of protein aggregates.45 There are two prominent features in the FTIR spectra of proteins: the amide I (∼1650 cm−1) and the amide II (∼1540 cm−1) bands, the former attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and the latter attributed to the combined vibration of N–H bending with C–N stretching.42,43,45 There was a significant difference between the intensities of amide I and II bands in enzymatically treated samples compared to the enzyme-treated samples. It implies that both transglutaminase and laccase altered the secondary structures of faba bean protein. According to Gui et al.,32 this enzyme-induced alteration in secondary structures may result in changes in α-helices, β-sheet, β-turn and random coils.
O stretching vibration and the latter attributed to the combined vibration of N–H bending with C–N stretching.42,43,45 There was a significant difference between the intensities of amide I and II bands in enzymatically treated samples compared to the enzyme-treated samples. It implies that both transglutaminase and laccase altered the secondary structures of faba bean protein. According to Gui et al.,32 this enzyme-induced alteration in secondary structures may result in changes in α-helices, β-sheet, β-turn and random coils.
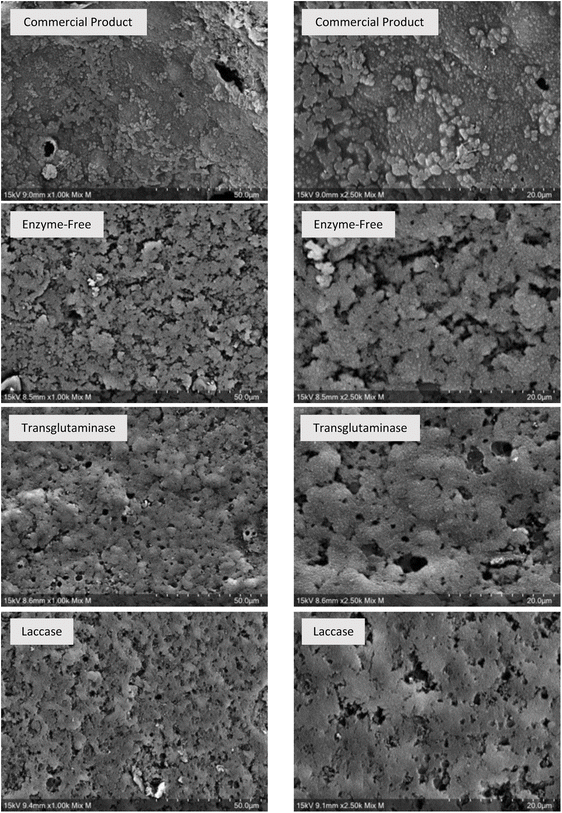
The microstructure is undoubtedly an important attribute of a food product, as it is closely correlated with the key characteristics of food perceived by consumers. The cross-section microscopy images of the microstructures of the commercial meat product and the prepared samples are revealed in Fig. 6. Although the four samples had similar appearances or macrostructures (Fig. 5), their microstructures were distinct. The reference meat product exhibited a compact structure with a few holes embedded within it. In contrast, the enzyme-free sample displayed a loose structure consisting of numerous clusters of granules. Compared to the enzyme-treated samples, the enzyme-free sample exhibited a significantly greater distribution of holes and smaller clusters. This suggests that both transglutaminase and laccase contributed to aggregate formation, making them comparable to the structure of the commercial meat product. However, the transglutaminase-treated sample displayed a lumpier structure or larger clusters than the laccase-treated sample. The reason for this may be related to the formation of larger protein complexes by transglutaminase due to a more compact network of interconnected chains, as evidenced by SDS-PAGE analysis (Section 3.1.1).
Table 2 compares the textural properties of the three samples prepared with those of the commercial product. While enzyme-free samples (S-EF) appeared similar to the commercial product (Fig. 5), their textural properties differed significantly. The addition of transglutaminase significantly enhanced the texture, with the hardness, cohesiveness, and chewiness increasing from 7.32 to 14.68 N, 0.62 to 0.79, and 4.55 to 11.63 N, respectively. In the presence of transglutaminase, the sample (S-TG) exhibited textural properties that closely resembled those of the commercial product. The treatment with laccase (S-LC) also resulted in an improved texture, with hardness, cohesiveness, and chewiness, reaching 12.38 N, 0.75 and 9.32 N, respectively. It is notable that laccase showed less improvement in texture than transglutaminase, with only cohesiveness being comparable to the commercial product.
| S-EF | S-TG | S-LC | Commercial product | |
|---|---|---|---|---|
| a Different lowercase letters within rows indicate a significant difference (p < 0.05). | ||||
| Hardness (N) | 7.32 ± 0.12c | 14.68 ± 0.28a | 12.38 ± 0.05b | 14.92 ± 0.98a |
| Cohesiveness | 0.62 ± 0.04b | 0.79 ± 0.01a | 0.75 ± 0.02a | 0.80 ± 0.03a |
| Chewiness (N) | 4.55 ± 0.29c | 11.63 ± 0.29a | 9.32 ± 0.23b | 11.94 ± 1.00a |
The substantial variation in hardness can be attributed to differences in microstructures. Generally, a more compact or aggregated structure yields a texture that is more resistant to force, leading to a higher value for hardness.48 The absence of enzymes resulted in a loose structure, transglutaminase-mediated cross-linking produced a compact structure most similar to the commercial product, and laccase-mediated cross-linking produced a structure that was somewhere in between (Fig. 6). Consequently, S-TG had the hardest texture, followed by S-LC and S-EF.
In the case of cohesiveness, it varies depending on the degree of cross-linking. Food cohesiveness is determined by the strength of the internal bonds that hold it together.47 As cross-linking occurs as a consequence of strong interactions between molecules, intermolecular interactions and cross-linking are closely related.49 Thus, a higher level of cross-linkage corresponds to a greater intermolecular force, which in turn translates to a greater cohesiveness value. Accordingly, the highest cohesiveness was observed for S-TG, followed by S-LC and S-EF.
For chewiness, it is determined by the amount of work required to chew the product.48 Clearly, the sample containing the larger protein complexes will be more difficult to chew than the sample containing the smaller protein complexes. According to the microstructures (Fig. 6), the cluster sizes in decreasing order are S-TG, S-LC, and S-EF. Consequently, S-TG ranked first in chewiness, followed by S-LC and S-EF.
A comparison of the water-holding capacity of the three samples prepared in this study and the commercial product is provided in Table 3. The four samples ranked in the decreasing order of their water-holding capacity are the transglutaminase sample (95.57%), enzyme-free sample (93.73%), laccase sample (91.30%), and commercial product (83.72%). It is interesting to note that transglutaminase increased the ability to retain water, whereas laccase decreased it.
| S-EF | S-TG | S-LC | Commercial product | |
|---|---|---|---|---|
| a Different lowercase letters within rows indicate a significant difference (p < 0.05). | ||||
| WHC (%) | 93.73 ± 0.35b | 95.57 ± 0.25a | 91.30 ± 0.79c | 83.72 ± 0.64d |
The cross-linking of proteins by transglutaminase results in the formation of polymers with a greater capacity for retaining water. This is in agreement with the findings of Wang et al.,52 in which it was demonstrated that transglutaminase treatment of wheat gluten induced the formation of protein polymers with a greater ability to retain water. As presented in SDS-PAGE profiles (Fig. 1, Section 3.1.1), the major proteins in the meat analogue samples are vicilin and legumin, which are 7S and 11S globulins, respectively. Motoki et al. proved that the treatment of transglutaminase can improve the water-holding capacity of 7S and 11S globulins by increasing their ability to swell and bind water.53 Additionally, a study conducted by Moon and Cho,54 which examined the improvement in the functional properties of mung bean protein isolate treated with transglutaminase, revealed that the cross-linking created by transglutaminase altered the tertiary structure of the protein, allowing the protein matrix to physically retain water.
The reduction of water retention caused by laccase from 93.73% to 91.30% may be related to the fact that the meat analogue sample treated with laccase contains more water than the other two samples (see Section 3.2.1. Volume expansion). In aqueous solutions, protein molecules are bound to water by hydrogen bonds. A greater solid content in the sample will allow it to better bind water, thereby facilitating the retention of water.55 In the laccase-catalysed reaction, additional water was generated (Fig. 2b), causing a decrease in the solid content of the sample, which in turn reduced its ability to retain water. Even though laccase ranked last among the three samples prepared in this study, it is still significantly superior to the commercial meat product. The results demonstrate that the formulation designed in this work can create a stable plant-based meat product with a water-holding capacity even greater than that of the reference meat product.
Fig. 7 presents a schematic comparison between the three samples and a reference product (a commercial luncheon meat) in terms of appearance, microstructure, texture, and water-holding capacity. Despite the difference in colour, all three samples closely resembled the reference product, suggesting that the formulation had accurately replicated the commercial product even without the use of enzymes. However, their microstructures and textures were distinct. It was found that both enzymes contributed to the formation of a more aggregated structure, making them more comparable to the reference product. Consequently, the enzymatically treated samples had an improved texture due to the improved structure. However, transglutaminase is evidently capable of replicating the texture of the reference product with high accuracy. Laccase improved the texture to a lesser extent than transglutaminase, but this improvement was nonetheless significant. As for the water-holding capacity, it should be noted that although laccase did not enhance of meat analogue samples in the same manner as transglutaminase, it was still substantially greater than that of the commercial product.
4. Conclusions
Transglutaminase displayed a superior ability to cross-link faba bean protein based on rheology measurements and SDS-PAGE analysis. This enables the meat analogue sample to closely resemble a commercial animal meat product in terms of both the microstructure and the texture. Though laccase does not crosslink proteins as effectively as transglutaminase, it nevertheless shows promise. The laccase-treated sample exhibited a closer resemblance in structure and texture to the reference product than the control sample (enzyme-free sample). Overall, the formulation designed in this study successfully replicated the appearance and water-holding capacity of the reference animal meat product using sustainable ingredients (i.e., plant protein, vegetable oil and food-grain by-products). Upon enzymatic cross-linking, significant improvements in the texture and the microstructure were achieved, allowing the meat analogue samples to be compared with animal meat products.This research advances our knowledge of protein cross-linking and provides a practical approach to improving plant-based meat alternatives. By utilizing transglutaminase and exploring the potential of laccase, this study contributes to the evolution of the food industry and offers the possibility of creating plant-based products that are comparable to animal-based products. Further, the successful integration of BSG into meat analogues is a significant achievement for this study, which represents an important advance toward sustainable food production.
Author contributions
Yue Fan (conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review & editing, and visualization), Shiyu Zheng (validation and investigation), Pratheep K. Annamalai (conceptualization, resources, writing – review & editing, and supervision), Bhesh Bhandari (conceptualization and supervision), Sangeeta Prakash (conceptualization, resources, writing – review & editing, supervision, and funding acquisition).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.References
- K. Van Mierlo, S. Rohmer and J. C. Gerdessen, J. Clean. Prod., 2017, 165, 930–950 CrossRef CAS.
- C. Van der Weele, P. Feindt, A. J. van der Goot, B. van Mierlo and M. van Boekel, Trends Food Sci. Technol., 2019, 88, 505–512 CrossRef CAS.
- M. Neacsu, D. McBey and A. Johnstone, Sustainable Protein Sources, 2017, 359–375 Search PubMed.
- J. De Boer and H. Aiking, Ecol. Econ., 2011, 70, 1259–1265 CrossRef.
- J. Buchert, D. Ercili Cura, H. Ma, C. Gasparetti, E. Monogioudi, G. Faccio, M. Mattinen, H. Boer, R. Partanen and E. Selinheimo, Annu. Rev. Food Sci. Technol., 2010, 1, 113–138 CrossRef CAS.
- N. Sulaiman, M. Sintang, H. Zaini, E. Munsu, P. Matanjun and W. Pindi, Int. Food Res. J., 2022, 29, 723–739 CAS.
- N. Karaki, A. Aljawish, C. Humeau, L. Muniglia and J. Jasniewski, Enzyme Microb. Technol., 2016, 90, 1–18 CrossRef CAS.
- S. Isaschar-Ovdat and A. Fishman, Trends Food Sci. Technol., 2018, 72, 134–143 CrossRef CAS.
- M. P. Savoca, E. Tonoli, A. G. Atobatele and E. A. Verderio, Micromachines, 2018, 9, 562 CrossRef.
- K. Sakai, Y. Sato, M. Okada and S. Yamaguchi, Sci. Rep., 2021, 11, 1–10 CrossRef.
- J. R. Diaz, K. Kantanen, J. Edelmann, H. Suhonen, T. Sontag-Strohm, K. Jouppila and V. Piironen, Innovat. Food Sci. Emerg. Technol., 2022, 77, 102954 CrossRef.
- O. Yuliarti, T. J. K. Kovis and N. J. Yi, J. Food Eng., 2021, 288, 110138 CrossRef.
- F. K. Schreuders, B. L. Dekkers, I. Bodnár, P. Erni, R. M. Boom and A. J. van der Goot, J. Food Eng., 2019, 261, 32–39 CrossRef CAS.
- I. Zahari, F. Ferawati, A. Helstad, C. Ahlström, K. Östbring, M. Rayner and J. K. Purhagen, Foods, 2020, 9, 772 CrossRef CAS PubMed.
- K. J. Grabowska, S. Tekidou, R. M. Boom and A.-J. van der Goot, Food Res. Int., 2014, 64, 743–751 CrossRef CAS PubMed.
- E. S. Jensen, M. B. Peoples and H. Hauggaard-Nielsen, Field Crops Res., 2010, 115, 203–216 CrossRef.
- D. Martineau-Côté, A. Achouri, S. Karboune and L. L'Hocine, Nutrients, 2022, 14, 1541 CrossRef PubMed.
- O. Nivala, E. Nordlund, K. Kruus and D. Ercili-Cura, LWT--Food Sci. Technol., 2021, 139, 110517 CrossRef CAS.
- V. Raikos, M. Neacsu, W. Russell and G. Duthie, Food Sci. Nutr., 2014, 2, 802–810 CrossRef CAS PubMed.
- Z. Zhang, L. Zhang, S. He, X. Li, R. Jin, Q. Liu, S. Chen and H. Sun, Food Rev. Int., 2022, 1–36 Search PubMed.
- H. Kim, M.-Y. Lee, J. Lee, Y.-J. Jo and M.-J. Choi, Foods, 2022, 11, 3337 CrossRef CAS PubMed.
- H. Zhou, X. Hu, X. Xiang and D. J. McClements, Food Hydrocolloids, 2023, 108909 CrossRef CAS.
- M. Schlangen, M. A. Ribberink, S. T. Dinani, L. M. Sagis and A. J. van der Goot, Food Hydrocolloids, 2023, 136, 108261 CrossRef CAS.
- E. Herz, T. Kinne, N. Terjung, M. Gibis and J. Weiss, Future Foods, 2023, 7, 100235 CrossRef CAS.
- M.-L. Shen, J.-Y. Ciou, L.-S. Hsieh and C.-L. Hsu, World J. Microbiol. Biotechnol., 2023, 39, 200 CrossRef CAS.
- K. Sakai, Y. Sato, M. Okada and S. Yamaguchi, Sci. Rep., 2021, 11, 16631 CrossRef CAS PubMed.
- P. Moll, H. Salminen, L. Stadtmueller, C. Schmitt and J. Weiss, Foods, 2023, 12, 85 CrossRef CAS PubMed.
- N. Shahbal, X. Jing, B. Bhandari, B. Dayananda and S. Prakash, Food Biosci., 2023, 53, 102515 CrossRef CAS.
- H. Liu, Q. Li, D. Zhao, M. Zhang, S. Jiang and C. Li, Food Chem., 2021, 363, 130284 CrossRef CAS PubMed.
- M. Sreejith, S. Prashant, S. Benny and T. Aneesh, in Microscopic Techniques for the Non-expert, Springer, 2022, pp. 227–241 Search PubMed.
- A. Dick, B. Bhandari and S. Prakash, Future Foods, 2021, 3, 100006 CrossRef CAS.
- Y. Gui, J. Li, Y. Zhu and L. Guo, LWT--Food Sci. Technol., 2020, 123, 109116 CrossRef CAS.
- I. Ansari, G. Gupta, J. Ramkumar and K. Kar, Handbook of Fly Ash, ed. K. K. Kar, Butterworth-Heinemann, Oxford, UK, 2022, pp. 681–713 Search PubMed.
- G. Kimbell and M. A. Azad, in Bioinspired and Biomimetic Materials for Drug Delivery, Elsevier, 2021, pp. 295–318 Search PubMed.
- J. Wemmer, S. Holtgrave, L. Wiest, M. Michel, M. E. Leser and E. J. Windhab, Food Funct., 2020, 11, 2040–2047 RSC.
- K. Song, B. Ren, Y. Zhai, W. Chai and Y. Huang, Biofabrication, 2021, 14, 015014 CrossRef.
- G. Renzone, S. Arena and A. Scaloni, Mass Spectrom. Rev., 2022, 41, 861–898 CrossRef CAS PubMed.
- R. Upadhyay, D. Ghosal and A. Mehra, J. Food Eng., 2012, 109, 104–113 CrossRef.
- Y. Wen, H. W. Kim and H. J. Park, Innovat. Food Sci. Emerg. Technol., 2022, 81, 103114 CrossRef CAS.
- M. Schopf and K. A. Scherf, Foods, 2021, 10, 228 CrossRef CAS PubMed.
- G. Jung, S. G. Jung and J. M. Cole, Chem. Sci., 2023, 14, 3600–3609 RSC.
- V. J. Sinanoglou, D. Cavouras, D. Xenogiannopoulos, C. Proestos and P. Zoumpoulakis, Foods, 2018, 7, 152 CrossRef PubMed.
- A. Dashti, Y. Weesepoel, J. Müller-Maatsch, H. Parastar, F. Kobarfard, B. Daraei and H. Yazdanpanah, Microchem. J., 2022, 181, 107735 CrossRef CAS.
- C. C. Hanna, J. Kriegesmann, L. J. Dowman, C. F. Becker and R. J. Payne, Angew. Chem., Int. Ed., 2022, 61, e202111266 CrossRef CAS PubMed.
- L. M. Miller, M. W. Bourassa and R. J. Smith, Biochim. Biophys. Acta, 2013, 1828, 2339–2346 CrossRef CAS PubMed.
- N. Jonkers, J. van Dommelen and M. Geers, Mechanics of Time-dependent Materials, 2021, pp. 1–24 Search PubMed.
- A. J. Rosenthal and P. Thompson, J. Texture Stud., 2021, 52, 294–302 CrossRef PubMed.
- L. Godschalk-Broers, G. Sala and E. Scholten, Foods, 2022, 11, 2227 CrossRef CAS PubMed.
- I. Ando, M. Kobayashi, M. Kanekiyo, S. Kuroki, S. Ando, S. Matsukawa, H. Kurosu, H. Yasunaga and S. Amiya, in Experimental Methods in Polymer Science: Modern Methods in Polymer Research and Technology, Academic Press, London, 2000, pp. 261–493 Search PubMed.
- R. D. Warner, in Lawrie's Meat Science, Elsevier, 2023, pp. 457–508 Search PubMed.
- B. Bowker, in Poultry Quality Evaluation, Elsevier, 2017, pp. 77–113 Search PubMed.
- J.-S. Wang, M.-M. Zhao, X.-Q. Yang, Y.-M. Jiang and C. Chun, Food Hydrocolloids, 2007, 21, 174–179 CrossRef CAS.
- M. Motoki and K. Seguro, Trends Food Sci. Technol., 1998, 9, 204–210 CrossRef CAS.
- S.-H. Moon and S.-J. Cho, Foods, 2023, 12, 1998 CrossRef CAS PubMed.
- W. Rawls, Y. A. Pachepsky, J. Ritchie, T. Sobecki and H. Bloodworth, Geoderma, 2003, 116, 61–76 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |