Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Principles and practice of greener ionic liquid–nanoparticles biosystem†
Joanna
Feder-Kubis
 *ab,
Anna
Wirwis
*ab,
Anna
Wirwis
 a,
Małgorzata
Policht
a,
Małgorzata
Policht
 a,
Jagpreet
Singh
a,
Jagpreet
Singh
 c and
Ki-Hyun
Kim
*d
c and
Ki-Hyun
Kim
*d
aWrocław University of Science and Technology, Faculty of Chemistry, Wybrzeże Wyspiańskiego 27, 50-370 Wrocław, Poland. E-mail: joanna.feder-kubis@pwr.edu.pl
bDepartment of Inorganic Chemistry, Technische Universität Dresden, 01069 Dresden, Germany
cDepartment of Chemistry, University Centre for Research and Development, Chandigarh University, Gharuan – Mohali-140413, Punjab, India
dDepartment of Civil & Environmental Engineering, Hanyang University, 222 Wangsimni-Ro, Seoul 04763, Korea. E-mail: kkim61@hanyang.ac.kr
First published on 31st January 2024
Abstract
The growing demand for advanced nanomaterials (NMs) has led to the development of safer, cost-effective, and bio-compliant synthesis methods for the nanoparticles (NPs) in compliance with stringent quality and environmental standards, particularly in the field of healthcare section. Conventional NPs preparation methods often fall short in terms of safety and environmental concerns, hindering their compatibility with modern biomedicine and biotechnology. In this context, ionic liquids (ILs) can play a pivotal role in the fabrication of NPs with precise morphologies and bioapplicability. The incorporation of ILs offers more efficient and environmentally friendly routes for the synthesis of NPs with deliberate control over their fundamental properties (e.g., in terms of surface morphology, functionalities, dispersibility, and size distribution). The use of ILs in bioactive NPs is often in line with the principles of green chemistry to help establish a fundamental strategy for biological applications. Here, a comprehensive review is offered to describe the perspectives of ILs-NPs (bio)systems with special emphasis on biotechnology and biomedicine. The design of useful and applicable ILs-NPs (bio)systems is proposed under the umbrella concept of green principles. Furthermore, the six principles of green ILs-NPs biosystems are outlined to meet the requirements of green chemistry. Finally, our discussions are extended to cover research gaps and future directions of ILs-NPs within the framework of sustainable development. This review highlights the promising role of ILs in advancing green chemistry practices with regard to the synthesis and application of NPs for the betterment of biotechnology and biomedicine.
1. Introduction
As one of the most popular areas of contemporary research, nanotechnology has flourished in multiple disciplines with the advent of synthesis methods to develop new, cost-effective, and environmentally friendly nanomaterials (NMs). There have been numerous quests for highly innovative and effective fabrication methods for nanoparticles (NPs) to meet quality demands, especially in biomedical applications. In this regard, ionic liquids (ILs) have raised hopes as a class of compounds with high tailorability for specific functions. ILs are low-melting salts (e.g., below 100 °C) that are composed entirely of ions.1 They can serve as alternative media in the preparation of tunable NPs to open up novel technological avenues for diverse applications. Although these salts have already been employed for the synthesis of numerous NPs, relatively little is known about their utility with regard to the production of biologically active NPs.2–5 As such, it is very important to adequately describe IL-NPs systems (ILs and NPs working as a hybrid) in the context of green chemistry and bioapplications from a sustainable development perspective. A more in-depth discussion of this topic is therefore justified, given the potential of such nanostructures in a broad spectrum of bioapplications.One of the main aims of this work is to highlight the utility of ILs in the synthesis of bioactive NPs along with tunable control of their properties for a wide spectrum of bioapplications (e.g., production of biomaterials for biomedicine and biochemical/medical diagnostics) (Scheme 1).
 | ||
| Scheme 1 Schematic of the topics covered in this review: comprehensive bioapplications of IL-NPs (bio)system followed by sustainable development.6–8 | ||
Despite numerous reports made of hybrid ILs-NPs systems to date, their utility has rarely been examined with respect to biomedical applications (cancer diagnosis and therapy,9 advanced nanomedical applications,10 or antiseptic and disinfectant properties,11 bioapplications,12 and biomedical applications of ILs with a few examples of ILs-NPs13). More research efforts are thus required to promote biomedical applications of hybrid ILs-NPs systems as marked in green font on the timelines in Fig. 1 and 2. The historical timeline in Fig. 1 includes the most important topics related to the discovery of NPs such as research on their initial properties and the first instances of their hybrid usage with ILs. The first report,14 widely recognized as the benchmark of the nanoscience era, dates back to 1857 when Michael Faraday reported the presence of metallic colloidal gold NPs in solutions with different shades of red depending on the light.
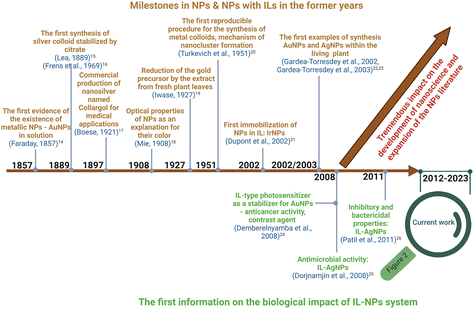 | ||
| Fig. 1 Timeline of first important achievements in the development of NPs and ILs-NPs systems.14–26 | ||
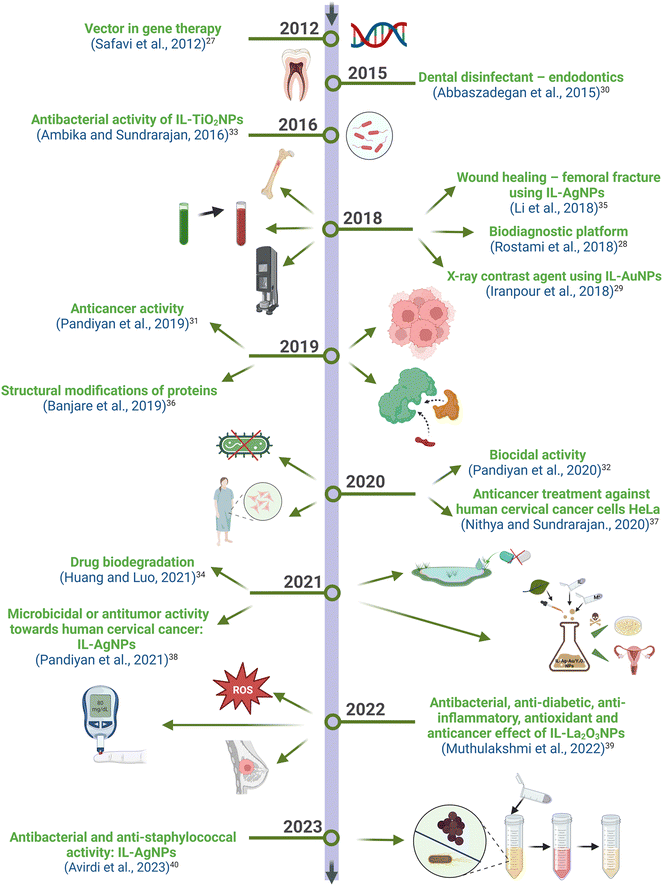 | ||
| Fig. 2 A short chronological survey of the timeline for IL-NPs milestones in biotechnological applications.27–40 | ||
The initial attempts to identify nanosilver were traced back to 1889. During that period, Carey Lea introduced a protocol for the synthesis of colloidal silver NPs using various salts and acids with the citrate and ferrous sulfate components as the stabilizer and reducing agent, respectively.15,17 Interestingly, the commercial synthesis of silver NPs with a size of 10 nm, marketed under the name “Collargol”, began in 1897 for medical purposes, particularly for gynecological and surgical applications.9 Michael Faraday's revelation of the existence of gold NPs sparked further investigation to advance synthesis techniques and to enhance the potential for property manipulation. In 1908, Mie20 introduced his theory on the optical properties of gold NPs to explain the color variations in solutions. Furthermore, the utilization of plant material was first reported in 1927 in the form of an extract for the reduction of metal precursor, when Eiichi Iwase produced dispersoid gold using fresh plant leaf extracts as a reducing agent.24 Additionally, there are cases where a living plant organism serves as an environment for the formation of NPs. Undoubtedly, a fascinating aspect in the history of metal NPs synthesis is the use of whole plants or their components as sources of natural reducing agents. Turkevich and others21 reported in 1951 a methodology of obtaining metal colloids and the mechanism of their formation based on nucleation and growth stages. In 2002, Dupont and co-authors16 presented the initial instance of immobilization transition metal NPs in an IL containing an imidazolium cation. The first experimental evidence for the formation of gold and silver NPs within the structural components of living plants was presented by J. L. Gardea-Torresdey and co-authors in 2002 and 2003.25,26 Intensive efforts were made to describe the biological applications of ILs-NPs systems with respect to their anticancer activity18 to subsequently encompass antibacterial therapies.22,23Fig. 2 depicts the recent progress made in the biomedical applications of ILs-NPs systems since the year 2012, which covers a range of research areas such as gene therapy,27 diagnostics for neurodegenerative diseases or diabetes,28 X-ray tomography,29 endodontics,30 and anticancer therapy.31 Certain ILs-NPs were also reported to serve as antibacterial reagents,32,33 while others contributed to the degradation of pharmaceuticals in the environment.34
This review has been organized to offer a comprehensive description of ILs-NPs (bio)systems with particular emphasis on the greener concepts and techniques. We discuss the identification and assessment of research gaps in this research field, which will help stimulate advances in the incorporating ILs into NPs for the development of highly biologically active NMs for practical bioapplications, notably in terms of enhanced biocompatibility and increased bioavailability. The novelty of this review lies in identifying and highlighting actual instances that can promote the synergistic interactions between the fabrication of precisely engineered NPs and the enhancement of their biofunctionality, all within the framework of sustainable development. Thus, this alignment should thus be indispensable for the effective realization of such biosystems. This review is expected to provide a new avenue for the sustainable development of IL-assisted bioactive NPs in the field of biotechnology.
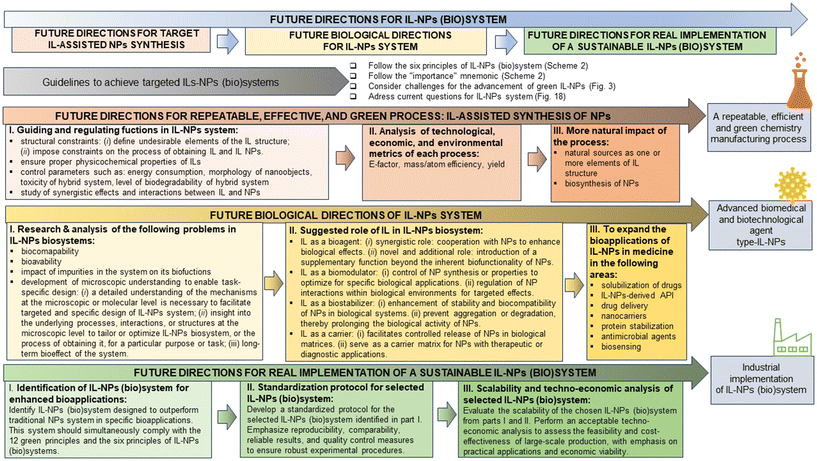
2. Design of six principles of IL-NPs (bio)system
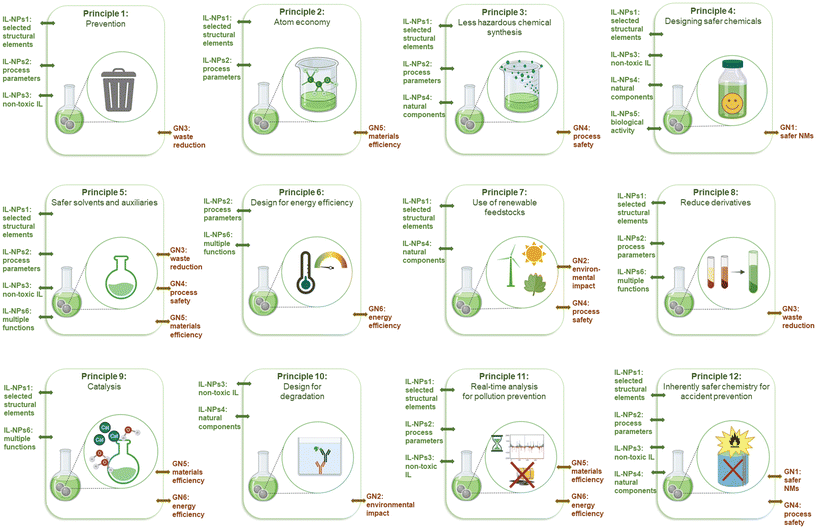
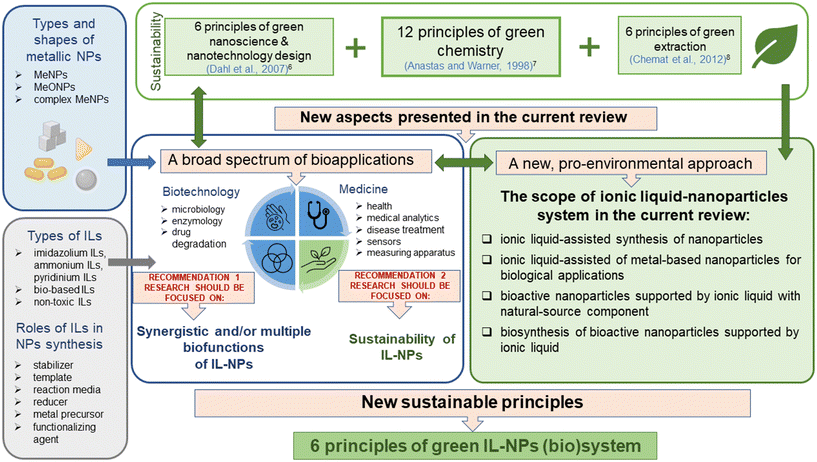
Although nanotechnology has made significant contributions to various biotechnological sectors, its application in clinical and biomedical fields has been limited (e.g., due to hazardous side effects). To develop a strategy for selecting the most suitable IL-NPs (bio)system as a viable and sustainable solution both for biological and for many other applications, we formulated six green principles describing this hybrid system (Scheme 2).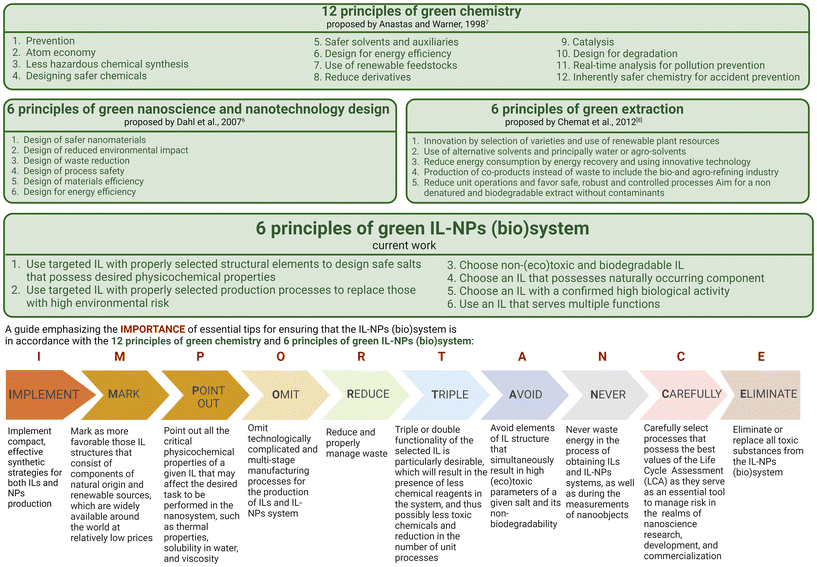 | ||
| Scheme 2 Principles of green chemistry (GC),7 green nanoscience & nanotechnology (GN),6 and green extraction (GE),8 the new principles of green IL-NPs (bio)system (IL-NPs) and the mnemonic called “importance”. | ||
Principle 1: Use targeted IL with properly selected structural elements to design safe salts that possess desired physicochemical properties
The objective is to achieve environmentally ‘safe-and-sustainable-by-design’ (SSbD) as well as biocompatible ionic compounds through the careful combination of suitable cations and anions in targeted ILs. These salts should possess specific physicochemical and surface properties to respond to the challenges posed by NP-assisted substances. To achieve this goal, key structural elements such as the type of amine core, the optimal length of the hydrocarbon chain, important structural linker, and the selection of an appropriate substituent in the anion part of the intended salt need to be examined.41
Principle 2: Use targeted IL with properly selected production processes to replace those with high environmental risk
Strategic process elements for obtaining ILs and IL-NPs should be considered as alternatives to environmentally unfavorable methods: (i) quantitative measurement of IL process efficiency through green chemistry matrices as indicators, (ii) synthesis of ILs under solvent-free conditions or using safe solvents, (iii) atom economy (AE)-based design of IL synthesis to simplify product purification without a separation step, (iv) the use of alternative energy sources to reduce overall energy consumption in IL production and IL-assisted synthesis of NPs, and (v) a reduction of unit operations. All these operations can collectively promote safe, robust, and controlled processes in an economically viable manner and achieve material efficiency in the production of environmentally friendly and high-performance IL-NPs (bio)system.
Principle 3: Choose non-(eco)toxic and biodegradable IL
To mitigate the environmental impact of conventionally used chemicals, an alternative approach is to replace them with deliberately designed ILs. It is important to evaluate the potential toxicological and environmental impact of the chosen salts in order to create advanced and inherently safer NMs with well-established rules of structure selection.42 For enhanced biodegradability, these guidelines encompass the use of cations such as ammonium and pyridinium, moderately long alkyl chains, and bio-based and alkyl sulfate anions.
Principle 4: Choose an IL that possesses naturally occurring component
To develop safe IL-NPs (bio)system, especially with low or negligible toxicity and biodegradability, it is important to consider structural modifications of ILs using derivatives sourced from natural and renewable materials.43 Incorporation of various natural moieties (such as amino acids, amino acid esters, organic acids, alcohols, or sugars) into the desired salt for safe IL well-suited for the synthesis of IL-NPs with (bio)functionality. The majority of naturally-derived ILs are synthesized by means of naturally occurring compounds such as choline, L-carnitine, betaine, and monoterpene-based cations. Derivatives of fatty acids and carboxylic acids such as acetic or formic acid are used as the anionic components of ILs.44,45
Principle 5: Choose an IL with a confirmed high biological activity
Careful selection of ion pairs with specific biofeatures is needed to design an advanced and highly selective IL-NPs biosystem. This approach is important in the context of combating antibiotic-resistant pathogens or malignant cell lines.
The specific biological activity of an IL can be determined by structural modifications (e.g., the type of biomoiety incorporated into the cation and/or anion pair, the hydrophilicity of the molecule, the length of the alkyl chain attached to the cationic core, and even the presence of polar functional groups). Recent literature has focused mainly on the synthesis and characterization active pharmaceutical ingredient (API)-ILs constructed with biocomponents derived from lidocaine, docusate, ibuprofen, salicylic acid, and alendronic acid.13 The concept of combining the biological activity of NPs with the bioactivity provided by selected IL can maintain the synergistic effect of NPs and an IL.23,46–49 A range of bioactivity of such an IL-NPs biosystem can be expanded in the control of pathogenic microorganisms, and a system with dual biological properties derived from both NPs and ILs may be possible.
Principle 6: Use an IL that serves multiple functions
A combination of appropriate ionic components within an IL can provide for multifunctionality. A SSbD IL-NPs system can be constructed by incorporating two or more substituents with different functions into one of the counterions.50 This approach promotes green and sustainable practices of IL-NPs by minimizing the number of required reagents, reducing waste generation, and ensuring energy efficiency.
In addition to introducing green principles for the IL-NPs (bio)system, we employ the “importance” mnemonic (Scheme 2) to delve into various facets of the design and process treatment that are critical for compliance with sustainable development. This mnemonic also ensures that a created IL-NPs (bio)system is seamlessly aligned with the 12 principles of green chemistry and the six principles of a green IL-NPs (bio)system (Scheme 2). We believe that the “importance” we have presented is crucial to the meticulous construction of the IL-NPs (bio)system, starting with the design process, with a special emphasis on the use of environmentally benign materials. This emphasis further extends to the sustainable planning of syntheses, the precise selection of chemicals, and finally to the preparation for industrial implementation.
The six principles for IL-NPs (bio)system presented here are aligned with the principles of sustainable development and green chemistry. Our guidelines apply to all three categories of the major principles such as (i) “12 principles of green chemistry” according to Anastas and Warner,7 (ii) the “six principles of green nanoscience and nanotechnology design” according to Dahl et al.,6 and (iii) the “six principles of green extraction” according to Chemat et al.8
3. Navigating greener nanotechnology of IL-NPs (bio)system: “concerns”, “design thinking”, and “what if” analysis
This chapter explores three aspects of navigating greener nanotechnology of IL-NPs (bio)system. First, it addresses concerns related to ILs-NPs (bio)systems, emphasizing sustainable and responsible nanotechnology practices. Second, it delves into the “design thinking” approach, providing insights into innovative design strategies for these (bio)systems. Finally, a “what-if” analysis is conducted based on the existing literature to explore the most environmentally sustainable ILs-NPs biosystems.The key questions and directions in this section are related to the creation of a green IL-NPs (bio)system as follows:
1. What are the specific characteristics of ILs that pose particular challenges in the development of green nanotechnology based on IL-NPs (bio)system? How can green chemistry principles be applied to effectively address these challenges?
2. What specific characteristics of NPs production strategies may pose challenges in the development of environmentally friendly green nanotechnology based on ILs-NPs (bio)systems? How can green chemistry principles be applied to effectively address these challenges in the synthesis methods?
3.1. Addressing concerns about IL-NPs (bio)system: towards sustainable and responsible nanotechnology
In the 21st century, nanotechnology has emerged as a transformative technology that can respond to societal, environmental, and planetary challenges. It can help foster cross-disciplinary collaboration among diverse scientific and engineering communities by exerting positive impacts on key research areas such as health care, energy, environmental conservation, and resource management through the development of smart materials and networked devices.51 Greener nanoscience, based on principles of green chemistry, aims to maximize the benefits of nanotechnology while minimizing negative impacts on health and the planet. The synthesis and application of nanostructures should ensure responsible development.In nanotechnologies, the concerns about health, environmental and economic constraints include, but are not limited to: (i) the presence of toxic elements and/or solvents as NMs may contain toxic elements or solvents that pose health and environmental risks during their production, usage, and disposal, (ii) the generation of waste during the synthesis and application of NMs could cause pollution and ecological damage to the environment, (iii) NMs could disrupt ecosystems and affect the food chain through bioaccumulation in living organisms, (iv) some NMs may remain persistently in the environment to exert long-term effects due to the lack of efficient biodegradation pathways, (v) the production of NMs is not environmentally sustainable because it consumes significant quantities of resources, water, and energy, (vi) the presence of limited or unreliable processes in NMs synthesis has the potential to hinder the feasibility of large-scale applications and commercialization, (vii) production of NMs without adequate plans for end-of-life management (e.g., reuse, repurposing, or degradation) may cause waste and environmental problems, (viii) AE, yield, and techno-economic analysis (TEA) results in the inadequately improved analysis of atom economics, yields, and techno-economic aspects may reduce the cost-effectiveness of the final NM.
Research for the development of ILs-NPs confronts various concerns that can be grouped into several categories such as the design of an appropriate IL structure to perform the intended function(s) (refer to Fig. 3). There are also technical and engineering concerns related to the conditions and progress of the synthesis process, such as a range of process-engineering limitations, operational scalability challenges, and the lack of techno-economic and life cycle assessments (LCA). The assessment of the potential adverse effects on the natural environment or human health is also equally important in evaluating the chosen synthesis strategy. All these categories of concerns are closely related, and an appropriate approach to address them will enable the development of an optimal IL-NPs (bio)system that meets both environmental and functional requirements.
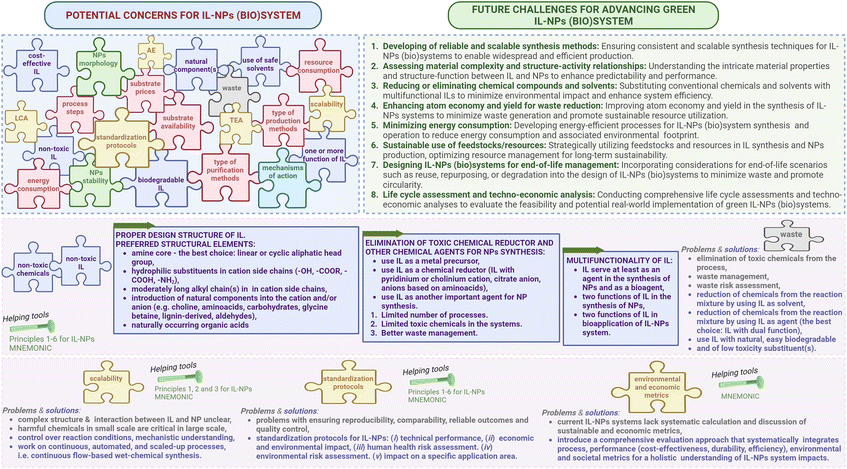 | ||
| Fig. 3 Optimal IL-NPs (bio)system: addressing concerns and overcoming challenges for green chemistry integration. | ||
Apart from the aspects of concerns, Fig. 3 also outlines distinctive challenges associated with troubleshooting for ILs-NPs as a guide for an optimal IL-NPs (bio)system in alignment with the principles of green chemistry. To solve the given concerns and environmental challenges for IL-NPs (bio)system, it is important to analyze the significance of the following aspects (Fig. 3), which can be grouped as follow: (i) analysis of the IL structure includes being non-toxic, readily biodegradable, having naturally occurring component(s) and being commercially available and economically feasible with the possibility of performing more than one function. (ii) Suitable processes are required to obtain IL and IL-NPs (bio)system through an analysis of (a) the number of unit processes, (b) energy, water, and resource consumption, (c) production and purification methods, (d) type of equipment required and availability, and (e) substrate prices and availability for (bio)synthesis. (iii) Control over NPs morphologies and physicochemical properties of the obtained IL-NPs system is an issue. (iv) The mechanisms linking IL and NPs are often presented as probabilities rather than with conclusive evidence. Insufficient comprehension of the transport and interaction mechanisms between IL and NPs and target biological structures may lead to the bioaccumulation of NMs in human tissues and cells, resulting in damage. (v) Environmental and economic indexes, standardization protocols and operational scalability are also important. Relatively little is known in the current literature about process scalability, LCA, techno-economic considerations, as well as standardization protocols for IL and IL-NPs (bio)system. (vi) Waste management needs to be addressed by reducing the generation of hazardous waste during IL synthesis and IL-NPs (bio)system development, which may result from several factors, including non-compliance with environmental regulations. Finally, in terms of effective resource management, it is important to develop strategies for the recycling of wastes generated during IL synthesis and IL-NPs (bio)system production.
Given the stringent nature of the socio-environmental requirements, certain parameters, the analysis and effective management of which have a direct impact on the challenges for IL-NPs discussed above, warrant special attention. These parameters are outlined below and are shown in Fig. 3.
The well-designed structure of IL serves a critical success indicator and prevents the introduction of toxic and difficult-to-biodegrade chemical compounds into the system. This also contributes to the replacement of classical chemicals with high toxicity factors commonly used in NP synthesis. In this context, our recommendations are consistent with Beil et al. (2021)42 on the design of proactive environmentally friendly ILs (Fig. 3). A properly designed structure, incorporating additional natural and/or bioactive elements and ensuring the multifunctionality of this salt, reduces toxic substances in the system and the number of unit processes and also facilitates waste management and potential recycling of the IL-based agent. We encourage researchers to adopt our approach to structural analysis prior to preparing a targeted IL because it may be the key to achieving an effective and environmentally friendly IL-NPs system.
The predominant approach for conducting an environmental impact assessment of (nano)products or processes is LCA.52–54 LCA assesses of the environmental impacts of a given (nano)product or process considering the entire life cycle of the (nano)product. Conducting LCA for IL-NPs system, supported by robust data and decision-making tools tailored to the circular economy is crucial to accurately assess resource use and use footprints. This initiative aims to develop tools based on multi-criteria indices specifically designed to measure the benefits of transitioning IL-NPs system to the circular economy. The ultimate goal is to provide established best practices for management in this context, but first, we recommend starting analyses of this metric for IL-NPs system.
TEA has gained recent recognition as a methodological framework that seamlessly integrates technologically-informed evaluations with economic assessment.54 TEA is still rarely addressed in the context of NP production. For example, Wrasman et al. (2022)55 present a solvent distillation-based protocol that facilitates the recycling of surfactants and solvents in colloidal NP synthesis over more than 10 consecutive cycles. Through a TEA, the study illustrates the significant potential of this methodology to significantly reduce solvent-related costs in the synthesis of colloidal metal NPs. This has implications for improving its feasibility leading to wider (even commercial scale) adoption.
Key parameters for TEA calculations are primarily based on market demand, resource consumption and scalability factors. TEA inherently includes sensitivity analysis of these key parameters and then establishes a framework for economic feasibility that considers resource costs, production cost elements, and environmental impacts. It is important to note that production costs at this stage are very preliminary and provide initial insights for a sensitivity analysis. Potential solutions may include heuristic process optimization, use of environmentally friendly IL-NPs-based NMs, and implement efficient production strategies to improve economic viability. TEA helps identify cost-effective pathways and guides decision making in the development and implementation of IL-NP technologies, particularly at the conceptual and laboratory or bench process scale, with the ultimate goal of developing a full-scale process. It is important to recognize that TEA is not intended to replace a comprehensive economic analysis, which becomes critical during the optimization phase when process and site investments are being considered.
There is a scarcity of publications addressing TEA aspects of IL-NPs systems. Karadaghi et al. (2021)56 used an imidazolium IL, specifically 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, as a solvent for the synthesis of platinum NPs. They demonstrated that the adopted imidazolium salt can be recovered and reused in successive reactions, thus maintaining its potency in both the original and recycled forms. Recycling of imidazolium-based IL does not affect the quality of the final product and does not alter the morphology of NPs. TEA of this synthesis method showed that by recycling IL, the cost of NPs preparation using an imidazolium-based salt can be competitive and even potentially lower than that of the conventional organic solvent 1-octadecene. In other work, Karadaghi et al. (2023)57 conducted an experimentally-guided preliminary techno-economic analysis of a model PtNP synthesis using six different ILs as reaction solvents. The study used a continuous-flow membrane separation system for IL purification using acidified water—this allowed recycling of both water-immiscible and water-miscible ILs. Each IL exhibited a different bulk price. However, this synthesis-oriented economic analysis revealed the effects of manipulating the IL solvent system. These included variations in NP yields and different solvent recoveries depending on the water miscibility of the ILs.
When considering the upscaling of ILs-NPs (bio)systems, the potential for real scale-up seems feasible, although it has not yet been realized in either a laboratory or industrial setting. Table S2† provides comprehensive data on the scale of production of ILs-NPs biosystems. Our calculations indicate that the most extensive scale achieved for obtaining such hybrid systems presented here was at the gram scale (Table S2†). There is also a lack of data related to process upscaling.
3.1.4.1. Scalability perspective for IL-NPs. Large-scale production of NMs is challenged by current laboratory syntheses, which yield limited quantities and suffer from batch-to-batch variability. These limitations not only hinder the scale-up of NPs, but also result in inconsistencies in critical properties such as size and shape.59 Therefore, a critical challenge for the practical application and industrialization of NPs, but also for ILs-NPs, is the development of scalable synthesis methods with a particular emphasis on continuous, automated, and scale-up processes (Fig. 3). The prospective direction for achieving large-scale production of NPs leans towards continuous flow-based wet-chemical synthesis under steady-state conditions. This method allows intelligent management of reactant addition, reactant mixing, and simultaneous operation of multiple reactors. However, implementing continuous synthesis presents challenges including controlling flow rate, managing surface tension and viscosity, selecting surfactants/ILs that affect fluid miscibility and residence time, controlling mixing time and reactant position, and preventing backmixing of products with reactants. Current methods for large-scale production are limited by expensive reagents and stringent reaction conditions. Continuous flow systems offer a solution by allowing the recycling of costly solvents and surfactants/ILs through well-designed separation and purification schemes. This capability positions continuous flow systems for low-cost and high-throughput commercial production of various NMs. We believe that this continuous flow system will also be suitable for large-scale production of hybrid ILs-NPs systems.
To ensure reproducibility, comparability, and reliable results and quality control, we have divided the standardization protocols for IL-NPs synthesis into five main areas (Fig. 3): (1) standardization protocol of technical performance (repeatability of synthesis in terms of efficiency, physicochemical properties of IL-NPs system, morphology of nanoobjects); (2) standardization protocol of economic and environmental impact (economic metrics, environmental metrics); (3) standardization protocol of human health risk assessment; (4) standardization protocol of environmental risk assessment; and (5) specific (bio)application standardization protocol. These protocols provide professional standardization for ILs-NPs (bio)systems and contribute to a comprehensive understanding of the criteria for their sustainability assessment.
The success of proper standardization procedures depends on the precise engineering protocol for IL-NPs, which involves systematic variation of physicochemical properties and comprehensive characterization. Subsequently, it is critical to subject this precise protocol of IL-NPs to biological evaluation using a sensitive, high-content, and high-throughput assay. This assay is designed to improve our understanding of how both dose–response and uptake-elimination affect nano/bio interactions.
By considering these aspects and aligning them with green chemistry principles, it is possible to pave the way for the construction of more sustainable and efficient ILs-NPs (bio)systems with reduced environmental impact and enhanced applicability.
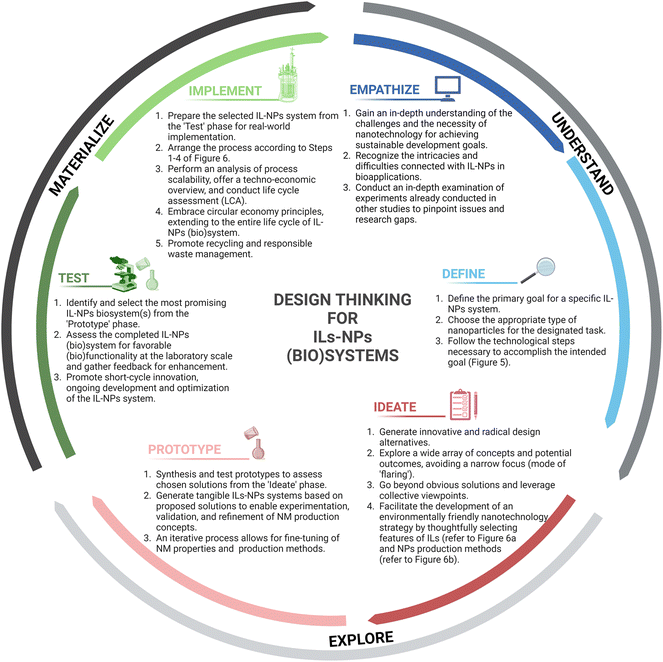
3.2. “Design thinking” approach
The adoption of “design thinking” can be a valuable approach in finding optimal solutions for the development route of IL-NPs from design to implementation. The concept “design thinking” has a rich history dating back to the 1960s and has been shaped by significant contributions from numerous researchers. In the 1980s, Peter Rowe published a seminal paper on “design thinking”63 to emphasize its role as a catalyst for innovation in product and service development. Over the past decade, the term “design thinking” has gained widespread popularity, becoming synonymous with a novel problem-solving approach applicable to any business or organization.The traditional flow of “design thinking” consists of the following parts: understand–explore–materialize and phases: empathize–define–ideate–prototype–test–implement, which is well in line with our ideology. Fig. 4 illustrates this process along with corresponding descriptions related to the creation of an IL-NPs (bio)system according to the principles of green chemistry.
During the “empathize” phase of the “understand” part, shown in Fig. 4, the focus is on understanding and identifying the challenges and needs encountered in nanotechnology, particularly in the area of NPs applications. Furthermore, this stage involves exploring the role that IL can play in the preparation and applications of NPs.
In the “define” phase, the primary focus is on the precise specification of the specific problems to be solved within the field of hybrid ILs-NPs. In Fig. 5, we present 4 steps that need to be considered from the “define” phase through the subsequent phases of the “design thinking” approach to achieve the target IL-NPs (bio)system.
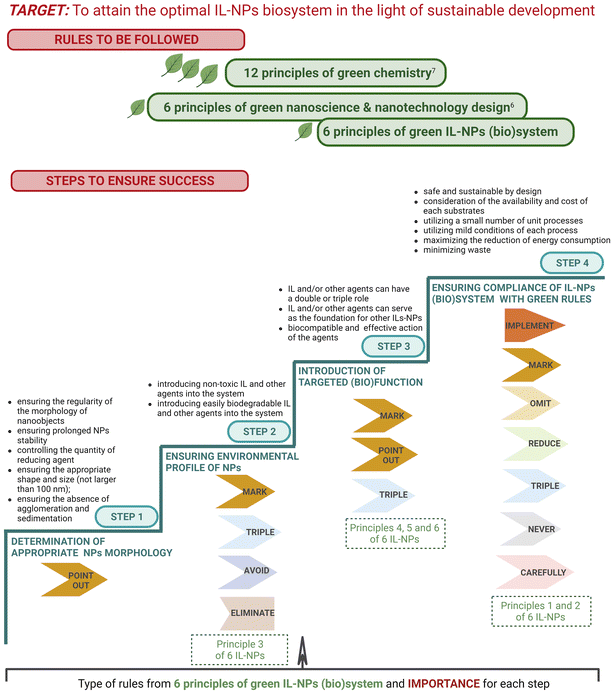 | ||
| Fig. 5 Steps to be followed to obtain targeted IL-NPs (bio)system in light of sustainable development. | ||
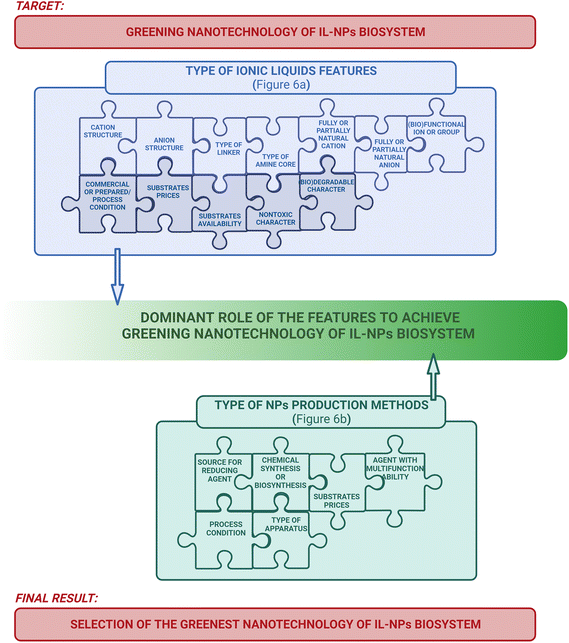
The “ideate” phase of “explore” part of the “design thinking” approach (Fig. 4) encourages the generation of diverse and innovative design alternatives by exploring a wide range of concepts and leveraging collective perspectives for IL-NPs biosystem. The primary goal is to move beyond obvious solutions and leverage collective perspectives. By acting in the “ideate” phase, a green nanotechnology strategy can be realized through the careful selection of appropriate ILs features (Fig. 6a) and appropriate production methods for NPs (Fig. 6b).
The primary objective of the “prototype” phase (Fig. 4) is to synthesize and test a prototype or a series of prototypes in order to verify all or part of the solutions that were selected in the previous phases, especially in the “ideate” phase. This involves the implementation of tangible ILs-NPs (bio)systems of the proposed solutions to allow the experimentation, validation, and refinement of the NM production concepts.
In the “test” phase of the “materialize” part, the primary objective is to identify and select the most promising IL-NPs biosystem(s) that have been synthesized and tested in the “prototype” phase. The focus is on evaluating the completed IL-NPs (bio)system with positive functionality, at least at the laboratory scale, and to gather valuable feedback for further improvement.
In the “implement” phase, the selected IL-NPs (bio)system from the previous “test” phase is prepared for the real-world implementation.
3.3. Prospects for greener nanotechnology of IL-NPs (bio)system – “what if” analysis
In conducting a comprehensive “what-if” analysis for the IL-NPs (bio)system, several scenarios were explored, each envisioning different applications and outcomes of ILs in NPs syntheses and applications. Table 1 serves as a detailed description of these scenarios, highlighting the critical parameters that influence the environmental sustainability of nanotechnology applications of IL-NPs. The “what-if” analysis presented here provides a framework for informed decision making, emphasizing the complex interplay between scientific advancement, environmental responsibility, and economic viability in the field of nanotechnology.| Case | Factors | Level of positive impact for the greener nanotechnology of the IL-NPs (bio)system | Comments/recommendations | ||||
|---|---|---|---|---|---|---|---|
| IL structure | IL functionality | Process safety | Environmental impact | Cost and/or scalability (e.g. TEA, LCA) | |||
| ✓ – an indication of a positive and/or fulfilled factor; × – an indication of a negative and/or unfulfilled factor; n.a. – not applicable, an indicator of a factor that is not taken into consideration in a given case. | |||||||
| 1. What if ILs with an inherently environmentally compatible structure enable multifunctional performance and ensure safe, environmentally benign processes in ILs-NPs (bio)systems? In addition, the entire system is designed to be inherently scalable, taking into account process conditions as well as techno-economic and LCA analyses | ✓ | ✓ | ✓ | ✓ | ✓ | Very high | This situation represents the most desirable scenario and deserves special attention. Such a system could be considered as the optimal IL-NPs (bio)system. |
| 2. What if the IL's eco-friendly structure enables multifunctionality in the IL-NPs system without hazards, but uncertain scalability and high synthesis costs pose challenges? | ✓ | ✓ | ✓ | ✓ | n.a. | High–moderate–low | While the proposed IL-NPs (bio)system in Case 2 has commendable environmental attributes and scalability, it is important to recognize that cost considerations may influence the rejection of cutting-edge nanotechnology. In many cases, the higher cost of implementing nanotechnology may be the most important selection indicator. This may lead to the preference of less environmentally friendly or less effective but more budget-friendly options. Striking a balance between environmental benefits, process effectiveness and economic viability will be a key challenge for widespread adoption and sustainability. |
| 3. What if the IL's specific functional groups enhance multifunctionality within the IL-NPs (bio)system? | ✓ | ✓ | n.a. | n.a. | n.a. | Very high–high | The scenario shown in Case 3 represents a promising option for the IL-NPs (bio)system to comply with green principles. This would significantly reduce the number of unit processes and minimize or potentially eliminate the need for numerous chemical reagents. Most importantly, materials of natural origin should be used. It would be essential to scale up the selected (bio)system before considering its potential implementation. Furthermore, the strong recommendation for the implementation of this system in other bio-applications remains unchanged. It would be essential to scale up the selected (bio)system before considering its potential implementation. Additionally, the strong recommendation for the implementation of this system in other bioapplications remains unchanged. |
| 4. What if a system is established wherein an IL incorporates natural components, and the biosynthesis process involves the reduction of NPs utilizing abundant natural reducing agents found in plant extracts? | ✓ | ✓ | n.a. | n.a. | n.a. | Very high–high | An approach presented in Case 4 is well in line with the principles of green chemistry. However, when applying this solution, it is crucial to consider the availability of natural substances used on a large scale and to thoroughly analyze the biosynthesis process, e.g. in terms of energy consumption. In some cases, processes such as calcination at very high temperatures are commonly used. An IL containing natural components should effectively perform its intended functions in the (bio)system, at least at the same level as a counterpart without natural components. Finally, one should be aware of the potential challenges associated with scale up the process, which can be particularly arduous and complex in biosynthetic processes. |
| 5. What if an IL with a substantial biological element is used to enhance the properties of the nanoobjects, positively impacting both the biology of the IL-NPs system and the morphology of the nanoobjects, but at the same time posing challenges in terms of environmental factors (such as increased energy consumption) and economic factors (such as the high cost of producing such an IL)? | ✓ | n.a. | n.a. | × | × | High–moderate–low | The question in Case 5 explores the positive impact of using a biologically active IL on the biology and nanostructure morphology of the IL-NPs system. While this unique IL composition may improve performance, it raises concerns about increased energy consumption, requiring consideration of alternative, environmentally friendly processes. However, changing the process can affect the morphology of the (bio)system, requiring a careful balance between environmental impact and morphology. Economic challenges arise due to the high production cost of IL, which may limit its practicality in nanotechnology. Addressing the cost challenge may require research into more efficient production methods. |
| 6. What if the IL, highly effective in enhancing both nanostructure morphology and biofunction, exhibits ecotoxicity? | ✓✓ | ✓ | n.a. | × | n.a. | Low–does not affect | In Case 6, IL shows promise in improving the morphology of nanostructures and the biofunctionality of IL-NPs. However, its significant ecotoxicity raises concerns about its environmental impact, potentially hindering its use in green or biocompatible nanotechnology solutions. Achieving a balance between performance benefits and environmental considerations is essential. Evaluating the feasibility and ethical aspects of using such an IL in nanotechnology is crucial. In addition, rigorous research and collaborative efforts are needed to mitigate ecotoxicity while preserving desired functionalities, thereby promoting responsible and sustainable nanotechnology practices. Addressing these concerns paves the way for safer, environmentally friendly applications of IL-NPs in various fields. |
4. IL-assisted synthesis of NPs
The application of IL-assisted synthesis of NPs is crucial because IL can play a multifaceted role, ensuring precise control over crucial factors such as morphology, size, and agglomeration. This is of great importance for the successful implementation of nanobiotechnology applications.The key questions and directions towards the creation of a green ionic liquid–nanoparticles (bio)system are described in this section as follows:
1. The selection of the key IL structural components and processes to create an IL-NPs system should be consistent with the 12 principles of green chemistry and our proposed six principles of the IL-NPs (bio)system to ensure consistency with sustainable development and future implementation considerations.
2. Do certain characteristics of an IL ensure its substantial role in NPs synthesis while being consistent with sustainability goals, and can these characteristics be replicated in future systems?
Worldwide problems associated with environmental contamination, especially high energy consumption and the use of large amounts of hazardous organic solvents, are driving the need for “greener” and more environmentally friendly processes in chemistry and chemical engineering. Collins’ pioneering work in the early 1990s64 introduced the term “green chemistry”. It led industry and laboratories to a new initiative that supported and promoted the importance of sustainability in the design of both processes and products. Anastas and Warnersoon delineated the currently well-known 12 principles of green chemistry with a broad description that facilitated the determination of the usefulness and importance to the world of the innovative concept given by Collins. Since then, chemists and chemical engineers have been developing less hazardous chemical syntheses to achieve these goals. Materials produced by environmentally friendly and biocompatible reagents could significantly reduce the toxicity of the final materials and the negative environmental impact of the by-products and various wastes. Subsequently, these principles have become the subject of intense consideration in the context of various usable materials, including NMs. The appropriate development of methods for the production of NPs is of enormous importance. UV irradiation, laser ablation, ultrasonication, aerosol technologies, lithography, and photochemical reduction methods are well recognized, high-quality techniques for the effective production of NPs; however, they remain costly and involve the use of hazardous substances. Hence, there is a great need to develop environmentally friendly methods for the fabrication of NPs that achieve the principles of green chemistry. Various solutions have been proposed on that account, such as the use of non-toxic or relatively low-toxic solvents (preferably water), “greener” production techniques without contact with reaction media and air (among others: ultrasound, microwave (MW), magnetic, hydrothermal, biological methods), closed reactor systems, and reduction of energy consumption in the processes by applying low temperatures of each unit process. The production of metal, metal oxide, and salt NPs via relatively greener routes can also involve plant extracts65 and other natural products. This approach has become increasing important and has been a focus of research for several years, making it a very promising area of nanotechnology.
Another alternative to the production of NPs in accordance with the green rules is the implementation of ILs into these nanosystems. ILs have been widely described in many scientific papers as green solvents66,67 and as compounds with green components designed for targeted functionality.68–70 The utilization of ILs in the synthesis of NPs is highly beneficial and can often increase the efficiency of the process of obtaining them and/or significantly influence the structure of the intended nanoobjects. The physicochemical properties of ILs, which include parameters such as melting and decomposition temperatures, polarity, density, transport properties, surface and interfacial tensions, and surface activity, exert a significant influence on the properties of NPs.71 Together, these parameters dictate the interactions and behaviors that govern NP synthesis and morphology in the IL environment, as broadly described in ESI (see paragraph S3†). The morphological improvements in the modern NPs (e.g., in terms of shape, size, surface charge, and limited agglomeration) have helped to further expand their bioapplications through the precise fabrication of tunable NPs using ILs (Fig. 7). ILs can be used during and after the synthesis of NPs as stabilizing agents,27,72–74 templates,75 reducing agents,76–78 functionalizing agents,79 reaction media,80–88 and metal precursors.89 The potent role(s) of ILs in the synthesis of NPs has been widely recognized in the literature. A detailed discussion of the importance, often versatile function of ILs is provided in the ESI (see paragraph S1†). The IL-assisted role provided in NPs synthesis also determines various molecular interactions between ILs and NPs and sheds light on the complexity of the involvement of these ionic compounds in nanoobject production. Furthermore, several mechanisms describe the stabilization of NPs by IL incorporation, a visual representation of which is shown in Fig. S1.† Given the paramount importance of size and shape control achieved by IL-assisted NP synthesis in ensuring NP stability, a detailed description of these phenomena is provided in the ESI, specifically in section S3.†
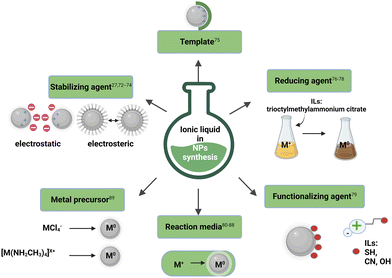 | ||
| Fig. 7 Smart roles of IL in NPs synthesis.27,72–89 | ||
Due to the many positive and promising results in this manner, scientists have extensively studied the role of ILs in the fabrication of NPs with tunable morphology based on the IL-assisted synthesis for the assembly of functionalized and highly specialized NPs.71,90–95
ILs offer many possibilities when employed for the synthesis of NPs. Table 2 shows that ILs can have a significant impact on the production of targeted NPs, regardless of the synthesis method, chemical composition, or degree of complexity of the reaction system.
| Major benefits offered by an IL in the synthesis of NPs | Relevant green chemistry rules (GC)a | Relevant nanoscience & nanotechnology design rules (GN)b | Comments to green chemistry rules and green nanoscience & nanotechnology design rules | Relevant green IL-NPs (bio)system rules (IL-NPs)c | Recommendations for designing and conducting NPs syntheses with IL assistance |
|---|---|---|---|---|---|
| a Principles of green chemistry7 (GC). b Green nanoscience & nanotechnology6 (GN). c The current review principles of green IL-NPs (bio)system (IL-NPs). Details for a,b,c are given in Scheme 2. | |||||
| Specific structural features of IL favor the formation of NPs with defined size, morphology, surface characteristics, and water solubility.96 | 4 | 1 | Using a SSbD IL with specific structural elements can modify the surface area of NPs, giving them the desired morphology, appropriate solubility, and attractive activity. | 1 | Structural elements of the IL like specific moieties, the presence and suitable length of the alkyl chain, as well as the size and structure of the anion, should be carefully considered to obtain small and monodisperse NPs.41,97 |
| There is a tendency to test mainly imidazolium ILs with high toxicity index. Therefore, it is recommended to prioritize the exploration of cations that are less environmentally harmful and readily biodegradable, such as those containing linear aliphatic ammonium, pyrrolidinium, or pyridinium cores.42,98 Several examples exist related to their successful utilization as effective stabilizers for NPs.23,46 | |||||
| IL provides sufficient steric or electrostatic stabilization that effectively prevents aggregation and growth of NPs dispersed in different media.76 | 4 | 1 | By applying properly designed IL according to green chemistry rules, it is possible to achieve long-term stability of NPs, thereby enhancing their functionality and expanding their potential applications, such as improving biological effectiveness. | 1, 5 | The synthesis of NPs should be conducted in the presence of an IL, which guarantees its direct impact on their stability from the very first stages of creation.97 This leads to enhanced stability compared to unstabilized NPs, which would require dispersion in selected media after synthesis and centrifugation. Additionally, conducting the synthesis in the presence of IL reduces the number of steps involved in ensuring that stable NPs are obtained efficiently and effectively. |
| The syntheses of NPs that require high temperatures and a vacuum can be safely carried out in non-flammable and low-volatile IL.99 Nevertheless, it is also possible to conduct the synthesis at sub-ambient, ambient, or moderately elevated temperatures because ILs remain in a liquid state over a wide range of thermal conditions.71 | 3, 5, 6 | 1, 3, 4, 5, 6 | The safety of NM synthesis can be enhanced by replacing flammable reagents or solvents with an IL, resulting in reduced energy consumption and costs. Opting for a bottom-up approach in designing a near-ambient green process is preferable. | 2 | The need for specific safety measures associated with flammable reagents or solvents can be eliminated by replacing the organic solvent with the desired IL. This makes the resulting NMs safer for biological applications. However, we encourage conducting NPs synthesis at a moderate temperature, preferably close to ambient temperature, to reduce energy consumption, synthesis costs, and the need for additional specialized laboratory equipment.100 To compensate for the extended synthesis time at low temperatures, alternative energy sources are recommended.100 |
| The use of an IL is advantageous in MW-assisted processes. It effectively absorbs MW irradiation, allowing for high reaction and heating rates.76 | 5, 6 | 3, 4, 5 | The use of IL as a solvent, with the ability to absorb MW for NPs synthesis leads to the elimination of organic solvents, which makes the synthetic procedure much safer. Additionally, it reduces the number of steps involved in NPs synthesis as there is no need to remove the solvent from the system. Furthermore, IL often contributes to increased selectivity in the synthesis process. | 2 | We recommend using alternative energy sources such as MW or ultrasound instead of conventional heating, which is associated with reducing energy losses, shortening the duration of the process by increasing its speed and obtaining the NPs in ambient temperature conditions.59,97 The combination of MW with a carefully chosen IL is highly recommended as it ensures an optimal solution in terms of process effectiveness and efficiency.101,102 |
| The IL employed in the synthesis of NPs can be effectively recovered and reused depending on the specific process and the role performed by IL.103 | 3, 6 | 4, 6 | Advanced and environmentally-friendly methods of NPs production should be developed, ideally aligning with the principles of the circular economy. The use of a recyclable and reusable IL104 is highly desirable in this context and is commonly employed in scientific research.105 | 2 | When an IL is used solely as a solvent for NPs synthesis, it should be effectively recycled and reused once the NPs are formed. Special consideration should be given to ILs that remain in liquid form at room temperature (Room Temperature Ionic Liquids, RTILs) in this context. However, it is even more crucial and sustainable to synthesize stable metal NPs using a stabilizing agent such as an IL, which can maintain their stability over an extended period and prevent agglomeration even after recycling and reuse.106 |
| The IL can be prepared with complete utilization of all reactants (100% AE), thus by-products are not generated (e.g., the preparation of protic IL through the protonation reaction of a suitable amine with a strong Brønsted acid).107,108 This approach promotes multifunctional efficiency for the comprehensive IL-NPs system. | 2, 8 | 3, 5 | AE, one of the 12 Principles of Green Chemistry, strives for optimal efficiency in chemical synthesis. It is a measure of the ratio between the mass of the final product and the combined masses of all the reactants. The concept of multifunctional efficiency, derived from the principles of AE in green chemistry, aims to enhance the functionality of manufactured nanomaterials (MNMs) by minimizing the presence of inactive components.109 Simultaneously, it promotes the development of a synthesis process that is both straightforward and sustainable. | 2 | The IL synthesis should be carefully designed and carried out to utilize all the substrates effectively, with a focus on avoiding the formation of by-products.66 Furthermore, the NPs should also be manufactured in stoichiometric reactions.100 This ensures that the reactants are combined in precise proportions, leading to the desired composition and minimizing the formation of undesired by-products. The adoption of stoichiometric reactions enhances the efficiency and selectivity of IL-NPs synthesis, contributing to a more sustainable and controlled manufacturing process. |
| When the AE is 100%, the efficiency and selectivity of NMs synthesis are increased compared to systems where by-products are generated and additional separation steps are required after synthesis. | |||||
| In this context, it is crucial to select an appropriate IL to obtain the IL-NPs system, ensuring a process with an AE of 100% for the production of the salt. | |||||
| The IL's physicochemical and surface properties (viscosity, density, surface tension, and hydrophilicity) can be tailored for a given task or specific application set for the hybrid IL-NPs system. | 4, 9 | 2 | The physicochemical properties of an IL play a crucial role in influencing the hybrid system of IL-NPs. Several endogenous and exogenous variables, such as NPs size and concentration, physicochemical properties of IL, temperature, and pressure, simultaneously affect different intermolecular interactions within the hybrid system.94 | 1 | The physicochemical properties of an IL, combined with NPs size and concentration, have a significant impact on the behavior and stability of the IL-NPs hybrid system. Understanding and controlling these variables are essential for optimizing the performance and (bio)applications of such systems. |
| IL can be designed to be environmentally friendly, characterized by attributes such as easy biodegradability, minimal or negligible (eco)toxicity, and sourcing main structural components from renewable materials.99 | 3, 4, 5, 7, 10 | 1, 2, 3, 4 | Designing NMs using an IL that incorporates subunits of natural origin in its structure may facilitate its easy biodegradation. Naturally occurring structural elements present in the IL can also enhance or increase the biological activity of the created IL-NPs system. | 3, 4, 5 | We recommend incorporating simple linear aliphatic amines and/or bio-based amines in the design of the cation of the IL. These alternatives have lower toxicity compared to imidazolium or pyridinium derivatives.42 For the IL anion, it is preferable to use structures derived from natural sources, such as amino acids, which have confirmed biological compatibility. This approach is more environmentally preferable than using anions like BF4− or PF6−.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42,110 42,110 |
| The IL can be designed as a reaction media and/or reactive agents, such as precursors or reductors.76 | 3, 5 | 3, 4, 5 | The synthesis of NPs in the presence of a specific IL, which, thanks to a carefully chosen moiety in its structure, can serve as a reaction medium and/or metal precursor and/or reducing agent, is safer due to the elimination of the need to introduce additional reagents. It also enables a wider range of functions during the process and reduces the number of unit processes, ultimately leading to a reduced negative impact on the environment. | 1, 2, 6 | The presence of an IL with reducing properties is an important and recommended factor in the design of environmentally friendly NPs synthesis. This functionality can be achieved, for example, by introducing acid or amino acid residues, especially in the anion structure.27 One of the significant advantages of using an IL as a reducing agent for NPs preparation is the elimination of toxic conventional reductants like NaBH4 or hydrazine. |
| Fewer reactants and auxiliaries are required because the IL can serve multiple functions during the synthesis of NPs.71 | 2, 3, 4, 5, 6, 8 | 1, 2, 3, 4, 5, 6 | The use of an IL that simultaneously performs multiple functions in NPs synthesis leads to the design of a safer process with reduced waste or even waste-free production if the IL is considered as an integral part of the system and not removed after synthesis. This approach minimizes the negative environmental impact. The multifunctional IL enables NPs synthesis to be less resource-intensive in terms of substrates or reagents usage compared to the conventional methods. Additionally, it often allows for the synthesis of NMs under mild conditions. | 2, 6 | We recommend designing ILs in such a way that appropriately selected structural components can influence the formation of a multifunctional salt. It is also possible to introduce only one key element whose presence will provide versatility, such as a natural component that acts as a reductant and also impacts the biological activity of the newly formed IL-NPs system, such as acid or amino acid residues.42 One should keep in mind the relative lack of toxicity and ease of biodegradability of the chosen ionic structures. |
Although there are good examples of successful industrial implementations of NPs, further research on the application of these nanotechnologies according to the rules of sustainable development is still needed. For this reason, several major challenges must be addressed, including how to assemble nanoobjects into macroscale structures with high accuracy, how to control the performance of NPs, how to optimize the process of their fabrication, and how to produce them cost-effectively while maintaining high efficiency. Some of these tasks for NPs scale-up and development of industrial applications could be facilitated by ILs without contravening sustainable development requirements presented in Table 2.
5. Bioapplication of IL-NPs hybrid system
This section provides an in-depth exploration of scientific research related to ILs-NPs systems with recognizable biological properties. It highlights notable examples from the literature that demonstrate their potential applications in various fields of biotechnology and medicine. Special emphasis is placed on those examples that show the most promise for practical implementation, both in terms of functionality and environmental impact.The key questions and directions in this section towards the creation of green ionic liquid–nanoparticles biosystem are as follows:
1. The structure of the IL should affect both the morphology of the tested nanoobjects and the type of bioapplication of the IL-NPs biosystem and must comply with the 12 principles of green chemistry and our proposed six principles of the IL-NPs (bio)system.
2. What are the benefits of an IL with bioactive properties in the context of using these biofunctions for the IL-NPs biosystem? Does the biologically active IL give clear advantages to this hybrid system compared to NPs formed without IL or with IL but not having biological features?
3. Is it possible to distinguish types of bioactive ILs that will guarantee their significant contribution to the biofunctionality of ILs-NPs biosystems while meeting the requirements of sustainable development? Can these ILs also be incorporated into other biosystems and scaled-up systems?
Metal-based NPs such as silver, copper, or gold NPs are among the most extensively used NMs because of their outstanding bactericidal and bacteriostatic properties. These metal-based nanoobjects are effective against numerous pathogens, including multidrug-resistant bacterial strains such as methicillin-resistant Staphylococcus aureus.111–113 While the precise mechanism of toxicity of metallic NPs towards bacteria remains under discussion, the most plausible modes of their action are: (i) generation of reactive oxygen species such as H2O2, ˙OH, and ˙O2− that damage cellular structures and disrupt the cell's biological processes, (ii) interactions with the cell membrane to which NPs adhere to cause changes in its permeability and transport properties, and (iii) entry of released metal ions into the cell and their binding to biomolecules (typically DNA or proteins), which inhibits cellular processes.114,115
Metal-based NPs act non-specifically (i.e., they do not bind exclusively to specific receptors), which explains their wide activity spectrum. Therefore, metallic NPs are commonly used in the manufacture of wound dressings,116,117 pharmaceuticals,118 crop protection products,119 disinfectants,120 and other antimicrobial agents.121,122 In addition to their antibacterial properties, certain metallic NPs also exhibit other useful features including anti-inflammatory (e.g., AgNPs), antifungal (e.g., CuNPs), antitumor (e.g., AuNPs), and anti-diabetic (e.g., ZnONPs)114,123 effects.
The requirements for the functional properties of a given NM vary depending on its intended use. In biology and medicine, in addition to the ability to interact with selected biological systems, NPs should also be biocompatible, selective towards target biostructures, degradable in a controlled manner, stable in biologically relevant media (e.g., blood plasma, cell culture media, high ionic strength buffers, protein-rich media), and have low or no toxicity to non-target biostructures at intended doses.124
While the use of metal NPs in medicine and related biotechnological fields holds great promise, there is also justified concerns about their biocompatibility and toxicity. Legal regulations differ greatly in the context of a region.125 For example, in the European Union, the European Medicines Agency (EMA) is responsible for ensuring safety and controlling the quality of newly introduced medicines, including those containing metal NPs. In the United States, the Food and Drug Administration (FDA) controls the nanomedicine market, while other parts of the world are implementing their own regulations. These agencies and institutions do not have a common standpoint on this issue.
Regulatory aspects and challenges regarding the introduction of nanomedicines into clinical practice were summarized and discussed by Foulkes et al.125 starting from (i) the lack of a unified set of global regulations, (ii) different definitions and classifications of NMs around the world, and (iii) the use of safety data for bulk materials as a comparison for nanomedicines (which is not representative due to different pharmacokinetic and pharmacodynamic activities), through biological concerns such as (iv) increased permeation and mobility (leading to crossing a blood–brain barrier crossing by smaller particles), (iv) genotoxicity, (v) accumulation of NPs in the organs, (vi) problems in comparing the in vitro tests of nanotoxicology due to the complex nature of an organism, ending with problems of (vii) stability of nanomedicines when their manufacturing is scaled up and (viii) possible environmental impact.
In parallel, the application of ILs in medicine requires careful consideration of crucial factors. Key among these considerations are (i) the toxicity and biocompatibility of ILs, (ii) establishing safe dosage levels for ILs when used as main APIs or as additives to other drugs, including nanodrugs. In addition, (iii) research into the detailed mechanism of action is essential for a full understanding of the potential applications of ILs in medicine.126,127 These factors collectively help to shape the design of ILs integration into medical practice, consistent with the broader challenges and regulatory frameworks discussed in the context of nanomedicines.
Biocompatibility is a crucial aspect to consider when designing novel ILs-NPs systems—especially for medical applications. Both NPs and ILs can interact with biological systems in a variety of ways, and thus it is important to ensure that they do not cause adverse effects in living organisms. There are several key aspects to consider here: (i) material composition of NPs. Composition includes the type of the metal/metal oxide core and depends on preferred properties, low toxicity, and compatibility with biological media. Noble metals such as gold and silver are preferred. (ii) NP morphology and surface characteristics. Larger metal NPs are more toxic than smaller ones. The regularity of NP shape ensures their homogenous properties and action. The interaction of NPs with different biological structures can also be affected by their surface charge; therefore, neutral or slightly negative values of surface charge of metal NPs are preferred. (iii) Stability in biological media. Besides being stable in water dispersion under controlled, laboratory conditions, the IL-NPs system should also be prone to aggregation and sedimentation in buffer solutions similar to biological fluids such as blood, plasma, gastric fluid or lymph. Each of these solvents has its specific chemical composition, pH, ionic strength, etc., and IL-NPs should thus be designed to retain their properties in these media. (iv) Solubility in water and biological media. ILs-NPs systems that act in biological systems must be highly soluble in biological fluids. Poor solubility directly affects the NPs’ tendency to aggregate and reduces their efficacy. (v) Immunogenicity. Like any other drug or agent introduced into an organism, IL-NPs should not induce an unwanted response from the immune system, such as inflammation. This can be ensured by using biocompatible ILs with anions of biological origin, e.g. derivatives of amino acids or sugars, as well as by modifying or functionalizing the NPs surface with an IL of appropriate structure. (vi) Interaction with macromolecules. Any possible correlation of the introduced IL-NPs system with structures such as proteins and enzymes should be considered and tested. (vii) Cellular uptake and distribution. One should ask how the IL-NPs system is taken up by cells and distributed within tissues? (viii) Toxicity and cytotoxicity studies. This includes the effect of the IL-NPs system on organs, tissues, and overall physiological functions. (ix) Degradation pathway. One should determine the conditions under which the IL-NPs system degrades as well as the by-products. Safety and toxicity of the by-products should be assessed. Applying such considerations along with detailed testing of the biological properties of the IL-NPs system can ensure its proper and safe application.
The translational advancement of the IL-NPs biosystem towards clinical applications requires the innovation of safe, straightforward, environmentally sustainable, and economically viable methodologies for the synthesis of specific entities including hybrid systems. It also requires a comprehensive understanding of the physicochemical intricacies involved. This includes a thorough exploration of the in vitro and in vivo effects, biodistribution patterns, safety control mechanisms, and pharmacokinetic and pharmacodynamic properties inherent to the IL-NP biosystem.
Table 3 shows that ILs can be involved in the production of a wide variety of bioactive NPs that meet at least some of the requirements for use in biomedical areas. A comparison between conventional NPs fabrication routes and IL-mediated processes demonstrates the significant advantages offered by ILs in terms of physicochemical and biological properties, in line with the principles of green chemistry. Numerous interdependent factors describe the influence of ILs on the morphology of NPs. Additionally, environmental and economic aspects should be carefully considered to minimize the negative impact on the environment, reduce energy consumption, and lower process costs. Current research efforts should be directed towards the development of multifunctional ionic compounds that both modify the surface of NPs and exhibit effective biological or therapeutic activity. The introduction of derivatives of natural components into the structure of IL (especially those with confirmed biological activity) in the cation, anion, or ideally in both components of the IL, would decrease (eco)toxicity and ensure biodegradability, biocompatibility, and significant bioactivity of the proposed IL-NPs biosystem.
| Type of requirements to achieve bioactive NPs | Agreement between IL and requirements for bioactive NPs | Relevant green chemistry rules (GC)a | Relevant green nanoscience & nanotechnology design rules (GN)b | Relevant green IL-NPs (bio)system rules (IL-NPs)c | Ref. |
|---|---|---|---|---|---|
| a Principles of green chemistry7 (GC). b Green nanoscience & nanotechnology6 (GN). c The current review principles of green IL-NPs (bio)system (IL-NPs). Details for a,b,c are given in Scheme 2. | |||||
| Stability in biologically-relevant media | – IL is capable of stabilizing NPs in different media through a number of mechanisms (steric, electrostatic, etc.) | 4 | 1 | 1 | 76 |
| – The stabilizing properties of an IL can be tuned to a medium of choice | 4, 10 | 1, 2 | 1 | 99 | |
| Preferentially small size | – IL was reported to favor the formation of smaller particles (particularly metal NPs) because of its low surface tension | 4 | 1 | 1 | 76 |
| – IL can effectively stabilize nanodispersions, thus preventing agglomeration and growth of NPs | 4 | 1 | 1 | 71 | |
| No susceptibility to unintended protein adsorption (formation of so-called proteincorona) | – NPs can be surface modified with IL having a low ability to solvate proteins (e.g., cholinium hexenoate) to prevent protein adsorption | 10 | 2 | 1 | 128 |
| Controllable degradability in biological settings | – NPs synthesized or functionalized using IL retain their biodegradability | 10 | 2 | 3 | 128 |
| – It is possible to use biodegradable IL, e.g., when the IL is bound to NPs or to address concerns regarding IL residues in the final product | 10 | 2 | 3 | 78 | |
| High biocompatibility and non-toxic (or minimally toxic) to non-target biostructures | – IL can be used to modify the properties of synthesized NPs (morphology, surface charge, size, etc.) and consequently alter their biological action | 4, 5, 10 | 1, 2, 3, 4, 5 | 1, 4, 5, 6 | 129 |
| – IL may serve as coatings (either biologically active or inert), preventing NPs from unintended interactions with biostructures | 4, 5 | 1, 3, 4, 5 | 1, 4, 5, 6 | 128 | |
| – Biocompatible IL (e.g., amino acid-derived) can be used for protective coatings | 3, 4, 5, 7 | 1, 2, 3, 4 | 3 | 128 | |
| Susceptibility to functionalization | – NPs can be functionalized in multiple ways with IL depending on their chemical composition. An IL can be incorporated into the core of NPs or can be covalently grafted or physically adsorbed onto the NPs surface | 5 | 3, 4, 5 | 1 | 96 |
| – IL may serve as a solvent for functionalization procedures and facilitate ongoing chemical reactions | 5 | 3, 4, 5 | 1, 2 | 71 | |
| Ability to target (passively or actively) specific biostructures | – IL bound to the surface of NPs may serve as ligands for specific recognition of biostructures | 10 | 2 | 1 | 96 |
Fig. 8a–e show a few excellent examples that we have selected from the literature to illustrate ILs-NPs biosystems, where ILs play a crucial role in enhancing the biological functionality of specific types of NPs. We have conducted a detailed study of these selected examples to assess whether the structural characteristics of the salts chosen for experimentation, along with the process conditions used to obtain the hybrid system, are in line with the recommendations for promoting an environmentally sustainable approach.
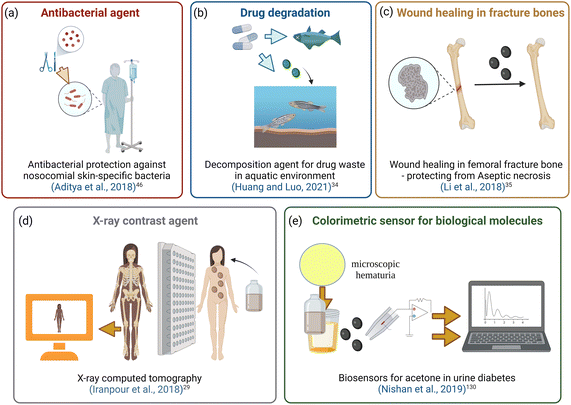 | ||
| Fig. 8 Biomedical application of IL-NPs system.29,34,35,46,130 | ||
Fig. 8a shows the antibacterial effect of IL-ZnONPs, which were tested as an alternative to surgical treatment of postoperative infections caused by nosocomial bacteria.46 Two ILs were used to achieve high dispersibility of ZnONPs: 1-butyl-3-methylimidazolium chloride and cholinium acetate. A structural comparison is difficult due to the use of two ILs with fundamentally different structures, both with different ammonium backbones and containing other anions. However, it is evident that imidazolium salts have a much higher (eco)toxicity than cholinium salts or (more generally) salts with an aliphatic group head.42 Therefore, the higher toxicity towards pathogenic microorganisms seems obvious in this case. However, when considering green principles for IL-NPs, the choice of choline salt is more environmentally friendly and safer (the principle 3 for IL-NPs). The presented synthetic route has several advantages, including the use of readily available and cost-effective zinc precursors, and the ability to conduct the process under ambient conditions. Attention is drawn to the multi-stage nature of the process in the case of the formed IL-NPs biosystem. Centrifugation can cause agglomeration of NPs, thereby affecting their stability and increasing the energy input required for the process.131 Furthermore, it is worth noting that the use of vacuum drying in the subsequent step of the procedure results in significant energy consumption.59 In the process of obtaining NPs, we recommend replacing the proposed solvent, methanol, with a less toxic alternative such as ethanol. The authors employed ILs after the synthesis of ZnONPs to enhance the dispersion of nanoobjects and as stabilizers before measuring the antimicrobial efficiency. We wonder whether it would be feasible, within this specific methodology, to introduce each IL directly into the synthesis of NPs, as this technique could reduce the number of synthesis steps (according to the principles 2 of IL-NPs). However, such an approach may have a high impact on the formation and morphology of the nanoobjects due to the influence of the ionic compound. In their work, the authors reported better antimicrobial efficiency in the case of imidazolium-based IL-ZnONPs compared to cholinium-based IL-ZnONPs, attributing this to the inherently superior antibacterial efficacy of imidazolium IL. They also highlighted that the better dispersion of ZnONPs in imidazolium-based salt led to improved surface interactions of the NPs, which subjected the bacteria to mechanical stress. Furthermore, the Im-IL-ZnONPs system exhibited better biocompatibility with skin cells, specifically human keratinocytes, as well as under coculture conditions. However, it remains unknown whether similar results of excellent dispersion of ZnONPs and antimicrobial effectiveness could be achieved through ionic compounds such as cholinium salts (which are environmentally neutral) by introducing appropriate structural modifications, such as replacing one of the methyl groups with a long alkyl chain or introducing a highly bioactive anion.
NPs have also found application in the degradation of drug waste in water reservoirs, as illustrated in Fig. 8b.34 Shape-controlled Cu2ONPs were synthesized using 1-butyl-3-methylimidazolium bromide, and these nanoobjects exhibited good stability. The IL-Cu2ONPs system demonstrated effective degradation of diclofenac drugs over a wide pH range. Considering the organic salt chosen by the authors, it is important to explore the applicability of another salt that is equally effective but easily biodegradable, which will be in the line with the principle 3 of IL-NPs green rules. In this regard, we believe it would be worthwhile to investigate ILs with fully mineralizable cation head groups, such as aliphatic alicyclic, or cyclic cations with bio-based or alkyl sulfates anions.42 Other aspects related to green principles should also be considered in terms of the choice of a process and the choice of reagents (according to the principle 2 of IL-NPs). For instance, the synthesis of IL-Cu2ONPs involves several steps, including centrifugation and drying, which are performed at a relatively high temperature of 80 °C. We suggest exploring the use of alternative energy sources, such as ultrasounds, which would reduce energy consumption, lower the reaction temperature, and increase the reaction rate.59
Fig. 8c graphically describes the use of IL-AgNPs biosystem for the preparation of materials with which blood vessels are reconstructed, with the expectation of accelerating the healing of fractures, such as those of the femur.35 The synthesis of AgNPs was performed using a MW-hydrothermal method employing 1-dodecyl-3-methylimidazolium chloride and an aqueous solution of sodium borohydride. Considering 12 principles of green chemistry and six principles of IL-NPs system, alternatives to both of these toxic chemicals should be explored. It is important to note that besides the known toxicity of NaBH4, the imidazolium cation is also recognized as one of the most (eco)toxic cation headgroups among ILs.42 Therefore, it is crucial to make efforts to replace both of these compounds, which will be in the line with the principles 1 and 3 of IL-NPs. It may be possible to replace both chemicals with a single IL that can effectively support the synthesis of AgNPs, thereby eliminating the need for an additional reducing agent (according to the principle 6 of IL-NPs). In this context, we suggest testing morpholinium and cholinium ILs, which are recognized as having low (eco)toxicity compared to others. Additionally, we propose to modify the structure of the selected IL from these proposed ILs and incorporate a special group that can be a reducing agent for NPs.77,78 Additionally, the production of AgNPs was carried out through a combination of MW heating and the hydrothermal method. While MW heating enhances the reaction efficiency and rate, it makes process control and scale-up difficult, making ultrasound a potentially better alternative. The increase in energy consumption is noticeable during the isolation of NPs from the aqueous medium through centrifugation. This may lead to the formation of agglomerates. Furthermore, drying (which occurs at elevated temperatures for a relatively long duration) can also contribute to agglomeration.
It has been determined that carefully selected IL-NPs biosystem can be used as contrast agents in medical imaging29 employing a particularly environmentally friendly approach (Fig. 8d). Notably, AuNPs were synthesized using glucosammonium formate as a non-toxic reducing agent and arabic gum as a coating agent, which aligns with the principles 1 and 3 of IL-NPs. The choice of these chemical compounds highlights their environmental friendliness, setting this IL-NPs biosystem apart from the cases previously discussed in relation to Fig. 8, which often fall short in terms of ecological considerations. The mild reaction conditions, including a temperature of 60 °C and a short reaction time of 5–20 min are also commendable. However, further efforts should be made to reduce the reaction temperature, for instance, by incorporating ultrasound-assisted techniques.
It is being explored whether ILs-NPs systems can support the treatment and diagnosis of lifestyle-related diseases, which are increasing. The IL-TiO2NPs system can act as a detector of acetone in diabetes urine by dispersing the previously obtained TiO2NPs in 1-H-3-methylimidazolium acetate (Fig. 8e).130 The IL chosen by the authors is a protic ionic liquid (PIL) and exhibits aromaticity in both the imidazolium cation and carboxylate anion. This aromaticity helps to create an electron-rich conductive environment that facilitates the reaction, unlike non-aromatic PILs, which lack such resonance structures. Furthermore, PILs are preferred over aprotic ILs due to the suspected involvement of the PIL proton in the generation of free radicals when TiO2NPs are present. These free radicals are believed to initiate the reaction between eosin dye and acetone, which justifies using this specifically designed PIL for the intended task. However, the hydrothermal procedure proposed by the authors for TiO2NPs preparation necessitates the use of high temperatures, which is an undesirable feature of green syntheses. This approach requires a significant amount of energy, which violates the 6th principle of green chemistry.7 The subsequent calcination stage further demands a significant amount of energy and the use of additional equipment (i.e., a furnace).59
Tables 4 and 5 in this study are based on the same examples, highlighting the diverse applications of NPs in various fields of biotechnology and medicine with the assistance of ILs. Specifically, Table 4 shows the structure and preparation methods of ILs utilized in the greening nanotechnology of the IL-NPs biosystem, while Table 5 provides insights into the types of NPs production methods employed for the same purpose.
| Type of NPs | Type of IL anion | Source of IL/type of the process for IL synthesis | Role of IL | Biological properties | Ref. | Comments | Recommendations |
|---|---|---|---|---|---|---|---|
| Imidazolium IL-assisted synthesis of various NPs for biological applications | |||||||
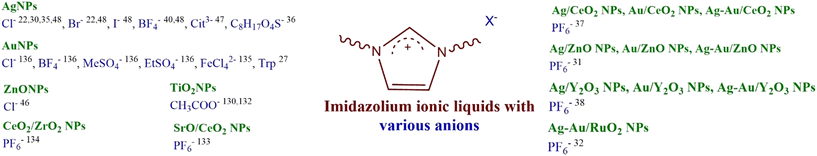
|
|||||||
| Au | Tryptophan | Two ILs were synthesized by the authors. 3 steps: (1) bromide salts production – no additional solvent for reaction, 70 °C, purification by diethyl ether, vacuum; (2) hydroxides production – THF, vacuum; (3) tryptophan ILs production – purification using acetonitrile–methanol mixture, vacuum | Reductor, capping agent | Vectors in gene therapies | 27 | Numerous examples involve imidazolium ILs (Im-ILs) that contribute to the synthesis of bioactive NPs. Surprisingly, rather toxic anions, such as hexafluorophosphates and tetrafluoroborates, are also often selected for ILs intended for such purposes. Only a limited number of studies focus on the structures of ionic compounds containing functional components with biotechnological or medical effects. For instance, there are works on imidazolium salts with tryptophan anion27 or ILs based on a metronidazole core.47 | Imidazolium salts with carefully selected functional groups warrant further investigation, particularly regarding their synergistic influence on the morphology of nanoobjects and the biological activity of the Im-IL-NPs biosystem under study. Such an approach would be in line with the principle 6 of IL-NPs. Additionally, it is advisable to focus on amine cores that are less toxic and more readily biodegradable, such as linear aliphatic ammonium salts (according to the principles 1 and 3 of IL-NPs). |
| In the majority of the cases discussed, the ILs were procured from suppliers. The availability of imidazolium salts in the supplier's offerings is significant, and we believe that this aspect often influences the choice of these salts. However, there are instances where original recipes for compound synthesis or modified methods of existing methodologies are presented. These processes are generally straightforward and effective. Unfortunately, in certain cases, the use of harmful solvents, such as THF, was observed. | From a biological perspective, the use of bioelements within the imidazolium ionic structure, such as salicylate or docusate, appears reasonable and promising (according to the principle 4 of IL-NPs). Considering the undeniable advantages of the citrate-based IL reducing agent47 synthesized by the authors, which we believe is an exemplary model from the Im-ILs-NPs biosystems in terms of functionality and environmental aspects, we strongly recommend further research into similar reducing agents based on this IL structure. For instance, exploring salts with the same cation derived from metronidazole but different naturally occurring anions that are derivatives of carboxylic acid. This approach will contribute to the creation of a comprehensive database of compounds that offer advantages for the advancement of ILs-NPs biosystems, and will be in the line with the principles 4 and 5 of IL-NPs. | ||||||
| Imidazolium-based ILs play a crucial role in the synthesis of NPs, influencing their morphology, and since they are an integral part of the tested Im-IL-NPs biosystem they simultaneously participate in the determination of various biological properties, including antimicrobial treatments, anticancer therapies, and gene therapy. | The strategy towards a more functionalized IL structure often requires its synthesis rather than relying on commercially available compounds. In this regard, we strongly recommend an assessment of the process conditions for IL synthesis and purification, with a focus on promoting sustainability (according to the principle 2 of IL-NPs). Special attention should be given to modifying procedures to eliminate harmful solvents and minimize excessive energy consumption. | ||||||
| Ag | Citrate (metronidazole derivative) | One IL was synthesized by the authors. 3 steps: (1) iodide salt production – ethanol, 50 °C, vacuum; (2) chloride salt production – vacuum, re-crystalized in deionized water; (3) 50 °C, vacuum, re-crystalized in deionized water | Reductor, capping agent | Antimicrobial treatment | 47 | ||
| Ag | Octylsulfate | Commercial supplier | Capping agent | Structural modification of globular proteins | 36 | ||
| Ag | Chloride | The origin of IL is not given in the work | Capping agent | Antibacterial agent, dental disinfection | 30 | ||
| Ag | Chloride, Bromide | Three hydroxyl functionalized ILs were synthesized by the authors. 2 steps: (1) 1-alkylimidazoles production – ethanol, vacuum; (2) chloride and bromide salts production – acetonitrile, extraction with hexane and recrystallized from acetonitrile | Capping agent, reductor | Antimicrobial treatment | 22 | ||
| Ag | Chloride, Bromide, Iodide, Tetrafluoroborate | Four ILs were synthesized by the authors. 1 step for halide salts: (1) halide production – no additional solvent for reaction, 70 °C, washed several times with ethyl acetate under nitrogen atmosphere, vacuum. 2 steps for tetrafluoroborate salt: (1) bromide salt production – the procedure is given above; (2) tetrafluoroborate production – solvent: acetone, purification: filtered off, washed several times with dichloromethane, vacuum | Solvent, stabilizing agent | Antibacterial agent | 48 | ||
| Ag | Chloride | Commercial supplier | Template | Wound healing, bone fracture treatment | 35 | ||
| Ag | Tetrafluoroborate | Commercial supplier | Stabilizing agent | Antibacterial activity | 40 | ||
| Ag–Au/CeO2 | Hexafluorophosphate | Commercial supplier | Templating agent, co-solvent | Anticancer and antibacterial effect | 37 | ||
| Ag/CeO2 | |||||||
| Au/CeO2 | |||||||
| Ag–Au/ZnO | Hexafluorophosphate | Commercial supplier | Templating agent, co-solvent | Anticancer and antibacterial | 31 | ||
| Ag/ZnO | |||||||
| Au/ZnO | |||||||
| Ag–Au/RuO2 | Hexafluorophosphate | Commercial supplier | Templating agent, co-solvent | Anticancer and antibacterial | 32 | ||
| Ag–Au/Y2O3 | Hexafluorophosphate | Commercial supplier | Capping and stabilizing agent | Antibacterial and anticancer | 38 | ||
| Ag/Y2O3 | |||||||
| Au/Y2O3 | |||||||
| ZnO | Chloride | Commercial supplier | Stabilizing agent | Antibacterial agent | 46 | ||
| TiO2 | Acetate | One protic-based IL was synthesized by the authors. 1 step: (1) under cooling, vacuum | Capping agent | Biodiagnostic platform | 130 and 132 | ||
| SrO/CeO2 | Hexafluorophosphate | Commercial supplier | Capping agent | Antioxidant and antibacterial effect | 133 | ||
| CeO2/ZrO2 | Hexafluorophosphate | Commercial supplier | Capping agent | Antioxidant antibacterial, and anti-biofilm | 134 | ||
| Au | Ferric tetrachloride | The origin of IL is not given in the work | Stabilizing agent | Enhancing surface-enhanced Raman scattering signal | 135 | ||
| Au | Tetrafluoroborate, Chloride, Methylsulfate, Ethylsulfate | Commercial supplier | Stabilizing agent, template | Enzyme immobilization, antimicrobial activity | 136 | ||
| Ammonium IL-assisted synthesis of various NPs for biological applications | |||||||
|
|||||||
| ZnO | Acetate (cholinium-based IL) | Commercial supplier | Stabilizing agent | Antibacterial agent | 46 | Assays of cholinium-based ILs have proven to be highly suitable,46 due to their low toxicity, affordability, and widespread availability. Some of these salts occur naturally, such as chloride and hydroxide, while many of them are commercially available. The biological properties of cholinium salts are also highly recognized.137 In the study examining the microbiological properties of ZnONPs,46 a cholinium salt with an acetate anion was utilized, which, in our opinion, both achieves the desired effect and aligns exceptionally well with the principles of sustainable development. | Considering the principles 1 and 3 of IL-NPs, it seems appropriate to focus on ammonium salts derived from linear aliphatic amines due to their significantly lower toxicity and easier biodegradability. It is strongly recommended to explore more examples of these salts, particularly those that belong to APIs (according to the principles 1 and 5 of IL-NPs). This approach allows for achieving a bifunctional effect, influencing both the morphology and biological activity of the system, such as a synergistic bioeffect while maintaining favorable environmental parameters of those ILs (according to the principle 6 of IL-NPs). Furthermore, there is a wide range of ammonium salts available for purchase. |
| Another example is ammonium salts with an ibuprofen anion,138 which, in our opinion, is a highly appropriate choice. The best combination was achieved by selecting salts with positive environmental attributes (non-toxicity, easy biodegradability) and combining them with cation-anions that possess biological significance. | The suggestion of combining the cholinium cation with an anion possessing strong pharmaceutical139 or other biological properties appears to be the most suitable. Among various types of aliphatic salts, cholinium salts have been recognized for their exceptionally low toxicity,42 making them an ideal choice. Selecting a suitable bioanion provides the perfect means to achieve a dual-function IL-NPs biosystem without any adverse environmental impact, including during the IL manufacturing process. | ||||||
| Ag | Ibuprofenate (lidocaine derivative) | Commercial supplier | Capping agent | Anesthetic effect | 138 | ||
| Pyridinium IL-assisted synthesis of various NPs for biological applications | |||||||
|
|||||||
| Ag | Trifluoroacetate | One protic IL was synthesized by the authors. The procedure is not available | Functionalizing agent | Biodiagnostic platform | 28 | A few examples are based on pyridinium salts. However, it should be emphasized that in other applications, they are also used less frequently. The presented examples23,28 demonstrate the potential of these ILs in creating effective ILs-NPs biosystems. | Further investigation into pyridinium salts appears to be justified. In our opinion, the research on nicotinamide (vitamin B3) derivatives140,141 would be particularly important and could yield two functions of such quaternary compounds, namely, influencing both the morphology and bioeffect of NPs (according to the principles 1, 4, 5 and 6 of IL-NPs). In addition, pyridinium salts are considered to be easily biodegradable,42 which is consistent with the principle 3 of IL-NPs. |
| Ag | Bromide | One IL was synthesized by the authors. 1 step: (1) bromide salt production – ethanol, 60–65 °C, washed with ethyl acetate, filtration, vacuum | Stabilizing agent and solvent | Antibacterial | 23 | ||
| Type of NPs | Biosynthesis method | Process conditions | Type of apparatus | Biological properties | Ref. | Comments | Recommendations |
|---|---|---|---|---|---|---|---|
| The scale of the processes described in Table 5 (amount of metal precursor and IL used) is detailed in the ESI, Table S2.† In general, the processes fell within the following numerical ranges: (i) the smallest production scale of NPs involved the use of 0.34 mg (ref. 28) of metal precursor and 0.4 mg (ref. 28) or 0.3 mL (ref. 135) of an IL; (ii) the largest production scale of NPs also employed 3.4 g (ref. 136) of metal precursor and 100 mg (ref. 23) or 30 mL (ref. 48) of an IL. | |||||||
| Imidazolium IL-assisted synthesis of NPs for biological applications | |||||||
| Au | — | Stirred at 50 °C | Stirrer, conventional heating equipment | Vectors in gene therapies | 27 | Presented procedures27,30,36,47 are easy and require only basic laboratory equipment. | More attention should be devoted to replacing toxic chemical reducers with less or non-toxic alternatives, such as ILs that possess dedicated moieties capable of acting as reducers (according to the principles 1 and 3 of IL-NPs). It is particularly desirable if these components are derived from natural sources, such as amino acids (according to the principle 4 of IL-NPs). A promising example in this regard is the utilization of an IL with a citrate anion, which eliminates the need for an additional reducing agent.47 |
| Ag | — | Stirred at 50 °C, then stirred for 24 h under the reflux, purified | Stirrer, conventional heating equipment, centrifuge | Antimicrobial treatment | 47 | The use of a conventional heating method in the synthesis process leads to heightened energy consumption. | Additionally, exploring the use of ultrasound as a substitute for conventional heating in reaction conditions presents an opportunity to achieve more environmentally friendly synthesis.142 Sonochemists embrace this technique due to its remarkable benefits of enhanced reactivity and acceleration. This substitution has the potential to significantly reduce energy consumption while maintaining comparable synthesis efficiency, even at room temperature. Ultrasound also enables the production of cleaner products with minimal or no by-products. |
| Ag | — | Stirred at room temp., purified | Stirrer, centrifuge, incubator | Structural modification of globular proteins | 36 | Unfortunately in some cases,27 even though the amino acid-based anion facilitated the double functionality of the IL, it was still necessary to employ a chemical reducing agent (NaBH4) to obtain the desired morphology effect of the produced nanoobjects, indicating the need for additional reducing procedures. | It is worth noting that centrifugation is not always advantageous, as it can increase the likelihood of NPs aggregation.131 While it may not be feasible to replace this process in many cases, it is crucial to control and optimize the centrifugation procedure effectively. |
| Ag | — | Stirred at room temp., purified | Stirrer, centrifuge | Antibacterial agent, dental disinfection | 30 | Other proposed procedures of NPs synthesis30,36 also involved a chemical reducer with a high degree of toxicity – NaBH4. | |
| In most of the cases discussed30,36,47 centrifugation was applied as final product isolation, which is a frequent cause of NPs agglomeration.131 | |||||||
| Ag | — | Stirred for 2 h at 60 °C, purified, vacuum dried | Stirrer, conventional heating equipment, centrifuge, vacuum oven | Antimicrobial treatment | 22 | The main advantage of the procedure is that the IL was used both as a reducer and a capping agent for AgNPs.22 However, large amounts of energy were used due to the use of elevated temperature. The downside to using centrifugation has already been mentioned above. | The described case22 involves process design aligned with sustainable development. Various process elements have been implemented, such as the utilization of ultrasound, which leads to reduced temperature and synthesis time. Furthermore, the use of ILs as dual-function agents eliminates the need for additional chemical reducers (according to the principles 1 and 6 of IL-NPs). |
| Ag | — | Stirred for 14 h under N2 and H2, 16 bar, room temp. | Stirrer, vacuum line with N2 and H2 installation, Parr reactor, vacuum pump, fume hood | Antibacterial agent | 48 | The use of N2 and H2 gases poses safety risks to the synthesis process, requiring proper storage and secure handling of gas vessels and installations. Additionally, H2 is a flammable gas that can potentially explode upon contact with oxygen in air under certain conditions. Moreover, it is important to note that in numerous industrial processes, a pressure of 16 bar is considered relatively high. | In our view, a captivating aspect of the process would be to investigate the synthesis of planned AgNPs with microbiological properties, involving the presence of ILs selected by the authors while utilizing ultrasounds142 or MWs143 techniques. Such an approach would allow the study of differences between various process paths and the use of different apparatus, considering the morphology of the resulting NM, as well as aspects of sustainable development, including the number of unit processes and energy consumption. |
| The noteworthy aspect of the reaction is that it occurs at room temperature, which is a significant process factor deserving attention. | Although a safer reducer would be applied instead of H2, yield of the reduction should be considered. Maybe it would be possible to implement an IL with reducing properties in this role. | ||||||
| Ag | — | Stirred for 30 min in an open system, then autoclaved at 120 °C, purified, dried for 12 h | Magnetic stirrer, stainless-steel autoclave, hot air oven, centrifuge | Wound healing, bone fracture treatment | 35 | The procedure requires the introduction of specialized laboratory equipment and the use of high temperatures, which leads to increased energy consumption. The use of NaBH4 does not qualify it as environmentally friendly. The purification of NPs was carried out using a widely recognized method involving centrifugation and drying. | The chemical reducer could be replaced with a more environmentally friendly alternative, such as a natural extract. However, it should be emphasized that such consideration is purely hypothetical, as its use would completely alter the process, the types of reagents used, and even the morphology of the resulting nanoobjects |
| The use of apparatus and synthetic steps should be minimized to reduce costs and energy consumption in the process. Alternative methods for producing NPs could be suggested, such as the ones mentioned in the previous example48 (ultrasound or MW) by us. | |||||||
| Ag | — | Stirred for 48 h at 95 °C and for 4.5 h at 40 °C, pH 8 | Stirrer, heating equipment, centrifuge, freeze-dryer | Antibacterial activity | 40 | An environmentally friendly reducer agent, sodium citrate has been used in the procedure. The process necessitates the application of relatively high temperatures for over 48 h and requires specific pH conditions. Centrifugation was performed to obtain the final NM. | It is captivating that the IL (imidazolium salt with tetrafluoroborate anion; Table 4) used in the procedure served solely as a stabilizing agent. It suggests that an IL with additional functionalities could be employed to enhance the NPs production process (according to the principle 6 of IL-NPs). Additionally, one could consider process modifications that have the potential to significantly alter the energy consumption of the system. For instance, incorporating techniques like ultrasound or MW irradiation, as previously mentioned, could be worth exploring. |
| Ag–Au/CeO2 | Hydrothermal, plant-extract assisted | Stirred for 1 h at room temp., then autoclaved for 24 h at 120 °C, purified, dried for 6 h at 100 °C, calcinated for 6 h at 600 °C | Stirrer, stainless-steel autoclave, centrifuge, hot air oven, furnace | Anticancer and antibacterial effect | 37 | The proposed synthetic procedures are highly complex, time- and energy-consuming. They necessitate the use of specialized equipment, including an autoclave, oven, and furnace, which results in substantial energy consumption. The total synthesis time exceeds one day. | Regrettably, the reliance on plants as an exclusive source for obtaining a reducing agent fails to address the comprehensive tenets of green chemistry. These problems are related to: frequent lack of global coverage for a given natural source, problems with the stability of NPs, lack of process repeatability taking into account other bioprocesses occurring during targeted biosynthesis, and therefore problems with transfer of scale. Moreover, the conducted works31,32,37,38 encompassed procedures demanding exceedingly high temperatures. |
| Ag/CeO2 | An inherent advantage of the proposed method31,32,37,38 lies in the utilization of plant extracts as a rich source of bioactive reducing agents. | To enhance the synthetic procedure and mitigate energy expenditure, it would be advantageous to adopt sophisticated heating methodologies such as ultrasound or MW techniques, enabling process execution at ambient or marginally elevated temperatures.59,144 This would facilitate shorter reaction times by increasing the reaction rate. | |||||
| Au/CeO2 | |||||||
| Ag–Au/ZnO | 31 | ||||||
| Ag/ZnO | |||||||
| Au/ZnO | |||||||
| Ag–Au/RuO2 | 32 | ||||||
| Ag/RuO2 | |||||||
| Au/RuO2 | |||||||
| Ag–Au/Y2O3 | 38 | ||||||
| Ag/Y2O3 | |||||||
| Au/Y2O3 | |||||||
| ZnO | — | 30 min under the reflux, room temp., purified, vacuum dried for 3 h, NPs dispersed in IL/phosphate buffered saline by sonication | Centrifuge, vacuum dryer, sonicator | Antibacterial agent | 46 | The implemented procedure is executed under ambient conditions, exhibiting minimal energy consumption. Additionally, ultrasounds were employed as a source of energy. | To enhance the environmental sustainability of the procedure, we recommend employing an alternative reducing agent (according to principle the 1 of IL-NPs). Further investigation into potential natural-origin alternative reducing agents would be advantageous (according to the principle 4 of IL-NPs). In the current process, IL serves as a stabilizer. It is worth exploring the possibility of selecting a salt that can fulfill multiple roles (according to the principle 6 of IL-NPs). Additionally, introducing such a salt during the NPs synthesis stage should not increase the number of unit processes. |
| Nevertheless, it is important to acknowledge that this process involves chemical reduction utilizing NaOH. Furthermore, the introduction of the stabilizing agent occurs after the synthesis of NPs, necessitating the inclusion of an additional process stage. | |||||||
| TiO2 | — | 3 h, 180 °C, pH = 2.62, purified, calcinated for 2 h at 600 °C;130 40 min, 100 °C, pH = 2.62, purified, calcinated for 2 h at 600 °C (ref. 132) | Heating equipment, furnace, pestle and mortar | Biodiagnostic platform | 130 and 132 | The utilization of oxalic acid as a reducing agent entails inherent risks due to its harmful nature. The synthesis method necessitates highly stringent reaction conditions. The requirement for maintaining a specific pH value further emphasizes this aspect. Additionally, the process involves exceptionally high temperatures, particularly during the calcination step. | In the context of the discussed case,130,132 it is crucial to explicitly propose procedures that eliminate the need for excessively high temperatures. Such an approach would effectively mitigate the environmental costs associated with the procedures and reduce substantial energy consumption. The utilization of alternative energy sources, such as ultrasound59,144 can play a significant role in achieving this objective. |
| SrO/CeO2 | Co-precipitation, plant-extract assisted | Stirred for 6 h, room temp., purified, dried for 6 h at 100 °C, calcinated for 4 h at 700 °C | Stirrer, centrifuge, hot air oven, furnace | Antioxidant and antibacterial effect | 133 | The utilization of plant extracts as a source of metal precursor reducer eradicates the negative environmental impact associated with traditional agents. However, a drawback of the procedures133,134 lies in the complexity of the final product formation, which entails multiple unit processes, including the utilization of high-temperature procedures. | The primary challenge posed by the procedures under consideration pertains to the exceedingly high temperatures, especially during the calcination step. Given the significant potential of the natural reducing factor employed in the given examples,133,134 it would be advantageous to refine the procedures and, ideally, eliminate the need for the calcination process. |
| CeO2/ZrO2 | Co-precipitation, plant-extract assisted | Stirred for 6 h, room temp., purified, dried at 100 °C, calcinated for 4 h at 700 °C | Stirrer, centrifuge, hot air oven, mortar and pestle, furnace | Antioxidant antibacterial, and anti-biofilm | 134 | ||
| Au | — | MW-assisted, 7 min, 100 °C, 600 W | Magnetic stirrer, MW oven | Enhancing surface-enhanced Raman scattering signal | 135 | In NPs production, the adoption of MW heating as an alternative heat source alongside a reduced synthesis time has been validated. Sodium citrate served as the employed chemical reducing agent. | Further advancements are warranted to refine the proposed method, building upon the already promising results, particularly concerning energy consumption and the significantly reduced process duration. The use of sodium citrate as a reducing agent in this study appears to be a good idea, considering its generally recognized safety as a food additive and pharmaceutical ingredient. However, it is important to note that further investigations are needed to fully assess its suitability for the production of IL-NPs biosystem. In conclusion, this study represents a positive example of compliance with the principles of sustainable development. Based on our assessment, we recommend expanding the investigation to explore alternative, readily available reducing agents, that are consistent with the principle 1 of IL-NPs. This exploration may lead to the identification of an optimal system for the production of ILs-NPs biosystems. |
| Au | — | Stirred for 4 h, room temp. | Magnetic stirrer | Enzyme immobilization, antimicrobial activity | 136 | The proposed synthesis method exhibits notable characteristics of low energy consumption, primarily attributed to its operation at room temperature. NaBH4 has been employed as the chemical-reducing agent in the process. | The minimal energy consumption and relatively short process times associated with the proposed method present significant advantages in terms of sustainable development. Therefore, it would be worth exploring the possibility of substituting the current reducing agent with a more ecologically friendly alternative (according to the principle 1 of IL-NPs), provided that it does not adversely impact the process conditions while effectively fulfilling its intended role. |
| Ammonium ILs-assisted synthesis of NPs for biological applications | |||||||
| Ag | — | Stirred for 30 min, room temp., pH 10, purified, vacuum dried at 60 °C | Stirrer, centrifuge, vacuum oven | Anesthetic effect | 138 | The synthesis process exhibited a rapid rate of progression at ambient temperature. The surge in energy consumption was solely attributed to the utilization of centrifugation and vacuum drying steps. NaBH4, a chemical reducer, was employed for the reduction process. | The planned research processes (process conditions and apparatus)138 in our opinion align with the principles of sustainable development and are not associated with excessively high energy consumption |
| However, it is worth considering whether the modification of the process and its related factors would be a worthwhile endeavor in the pursuit of an exceptional NM produced in accordance with the principles of green chemistry. This includes but is not limited to: (i) exploring and identifying a suitable reducing agent with environmentally favorable structural characteristics (according to the principle 1 of IL-NPs); (ii) investigating whether the omission of the centrifugation process would significantly affect the tested biological properties of the obtained IL-NPs biosystem. It is necessary to determine the justification and potential impact of such an alteration; (iii) assessing the validity of drying the obtained nanoobject solutions, considering the feasibility of conducting direct application testing without the requirement of drying the resulting IL-NPs solution system. | |||||||
| Pyridinium IL-assisted synthesis of NPs for biological applications | |||||||
| Ag | — | Stirred, room temp. | Stirrer | Biodiagnostic platform | 28 | The synthesis method demonstrates characteristics of being both time-efficiency and low in energy consumption. However, the usage of NaBH4 as the chemical reductant diminishes the eco-friendliness of the process. | Conducting a comprehensive investigation into the behavior of an environmentally favorable IL with structurally positive elements, serving as a dual-function agent encompassing functionalization and reduction capabilities, would be a highly suitable improvement to the existing procedure (according to the principle 6 of IL-NPs). |
| Ag | — | Stirred for 2 h, room temp., purified | Stirrer, centrifuge | Antibacterial | 23 | The synthesis procedure exhibits a substantial toxicity profile attributed to the utilization of hydrazine as a chemical-reducing agent. The inclusion of centrifugation as an additional step could potentially contribute to the agglomeration of NPs, leading to an increase in energy consumption. | We highly recommend replacing the current reducing agent with a more ecologically friendly alternative for this procedure (according to the principle 1 of IL-NPs), as it represents the most significant environmental concern within the process. |
5.1. Bioactive metal-based NPs supported by IL
Biologically stable AuNPs were proposed as novel Au-based contrast agents for X-ray computed tomography (Fig. 2 and 8d).29 An ammonium IL with formate anion (a type of D-glucosamine derivative) was used to reduce gold ions, resulting in precipitation. Gum arabic can improve dispersibility when applied as a capping agent. The shapes and sizes of the AuNPs were strongly dependent on the amount of the capping agent in the solution. AuNPs prepared in this way had no cytotoxicity to human hepatocyte cells and formed stable dispersions in a NaCl solution, phosphate buffer, and human blood serum. These AuNPs had a higher X-ray absorption capability than commercial iodine-based contrast media. The sheath from gum arabic prevented absorption of standard test proteins (human and bovine serum albumin) on their surface, thus allowing the contrast agents to pass through the body unmodified. Importantly, both IL and gum arabic are biocompatible and non-toxic, making them suitable for biomedical applications.
The biosynthesis of AuNPs has been performed using oil palm kernel extract and imidazolium IL with acetate anion in two steps.149 Phytochemicals from the plant were first extracted with IL and then used for MW-assisted gold reduction. The presence of IL resulted in more efficient MW absorption and steric and electrostatic stabilization of NPs. Compared to the aqueous plant extract, the IL-assisted route allowed higher extraction efficiency and the formation of uniform AuNPs with a narrow particle size distribution. The stability of the gold dispersion increased from 14 to 122 days with the IL in the reaction system (Fig. 9a).
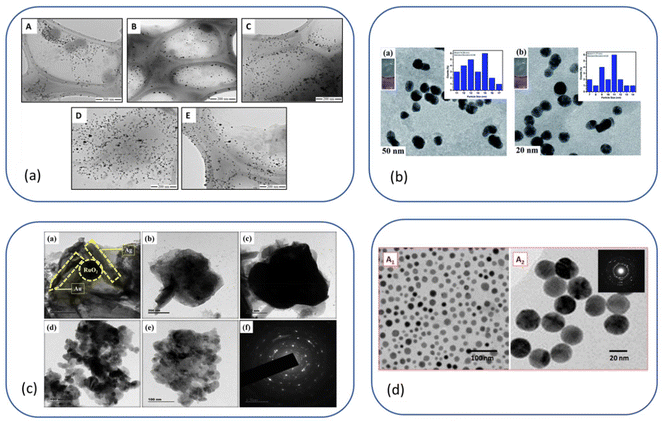 | ||
| Fig. 9 Morphologies of NPs in the presence of ILs: (a) TEM images of AuNPs synthesized with oil palm kernel extract and imidazolium IL with acetate anion over time (120 days), reproduced from ref. 149 with permission from Elsevier, copyright 2020. (b) TEM images of (a) AuNPs and (b) bromide-based pyridinium IL-AuNPs. Reproduced from ref. 150 with permission from Royal Society of Chemistry, copyright 2020. (c) TEM images of Ag–Au loaded Ru2O NPs in imidazolium IL with hexafluorophosphate anion. Reproduced from ref. 32 with permission from Elsevier, copyright 2020. (d) TEM images of AgNPs synthesized with imidazolium IL with chloride anion. Reproduced from ref. 35 with permission from Elsevier, copyright 2018. | ||
Understanding the mechanism of interaction between the IL-NPs system and the bioactive substance leads to selective and highly sensitive IL-NPs biosensors. Synthesis with low toxicity and biodegradable ILs is also important. These sensors were used with monodisperse IL-AuNPs as a fluorescent glutamine detector based on the interaction between IL-AuNPs and human serum albumin (HSA). The protein is composed of amino acid residues and the sulfhydryl groups present in human blood plasma. HSA is active in the transport of biological compounds and was shown to regulate osmotic pressure.150 This study demonstrated the high detection capability of the IL-AuNPs-HSA system (Fig. 10a and b), especially at a temperature of 32 °C, which facilitated the design of a sensor for amino acids. The synthesis of IL-AuNPs focused on the chemical reduction in HAuCl4 by NaBH4 accompanied by pyridinium IL with bromide anion. This IL played two roles as a colorimetric detector film and a reagent. Pyridinium quaternary salt protects AuNPs against aggregation, as confirmed by a decrease in the nanogold diameter from 14.59 to 11.77 nm (Fig. 9b).
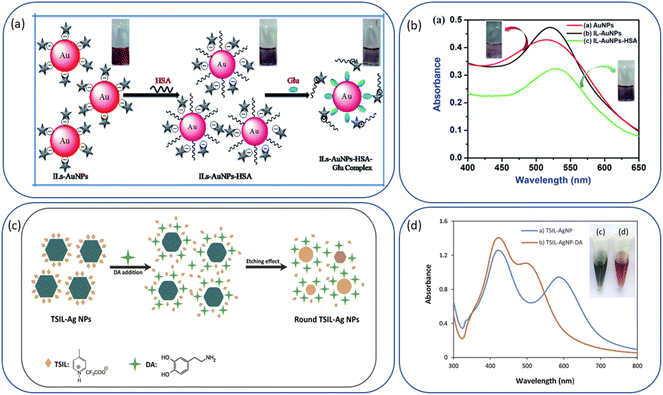 | ||
| Fig. 10 ILs-NPs systems acting as biosensors. (a) Schematic representation for the interaction of IL-AuNPs with HSA for selective sensing of glutamine. (b) UV-Vis spectra of IL-AuNPs and IL-AuNPs with HSA. Reproduced from ref. 150 with permission from Royal Society of Chemistry, copyright 2020. (c) A probable mechanism of dopamine detection by TSIL-AgNPs. (d) UV–Vis spectra of dopamine incubated TSIL-AgNPs solutions: (a) TSIL-AgNPs, (b) etched TSIL-AgNPs in the presence of dopamine. Reproduced from ref. 28 with permission from Elsevier, copyright 2018. | ||
Imidazolium IL can be used as a soft template for the MW-assisted hydrothermal synthesis of AgNPs and promotes blood vessel growth. It can be utilized for the fabrication of bone fraction implants (Fig. 2 and 8c).35 The IL template produced crystalline and spherical NPs with high internal order and narrow size distribution (Fig. 9d). Medical tests showed that the well-defined morphology translated into favorable biocompatibility and better therapeutic efficacy of AgNPs versus control groups.
The antimicrobial effect could be enhanced when combined with an antibiotic imidazolium IL (i.e., citrate-based metronidazole derivative). In this case, IL is an antibiotic as well as a reducing and stabilizing agent (Tables 4 and 5).47 The strategy used in the synthesis of IL and its stabilizing effect on the obtained AgNPs with full description of interaction analysis between them is shown in ESI, Fig. S2.† The resulting IL-capped NPs were potent antibacterial agents even at low concentrations (i.e., 3 mg mL−1) during microbiological assays. They had results comparable to standard antibiotic therapy for S. aureus and improved efficacy of Pseudomonas aeruginosa bacterial strains (greater than 120% with respect to lomefloxacin for [IL-capped NPs] = 400 mg mL−1).
The alkyl chain length of the IL cation used as the coating agent affects the core size and hydrophilicity of AgNPs, which is important for their antimicrobial activity.152–155
To confirm this, the action of IL-AgNPs was compared with that of ILs based on imidazolium or pyridinium cations with 12 or 18 atoms of carbon in the alkyl chain and chloride anions. Their cytotoxicity against Escherichia coli and Enterococcus faecalis biofilms was examined along with their antimicrobial effectiveness against microorganisms such as Candida albicans, E. coli, Bacillus subtilis, and Salmonella typhi.155 Antimicrobial activity is strongly dependent on the hydrophobic–lipophilic balance, which explains the higher activity of IL-AgNPs when the alkyl chain contains at least 12 carbon atoms, regardless of the IL cation structure.153,154
Interestingly, nanosilver is also being used to produce biosensors for bioactive compounds, thus leading to cheaper and faster alternatives to common analytical methods such as high-performance liquid chromatography.156 Hexagonal plate AgNPs stabilized by pyridinium task-specific IL (TSIL) with the trifluoroacetate anion were synthesized (Fig. 10c and d) and applied as colorimetric sensors for the measurement of dopamine in human blood serum (Tables 4, 5 and Fig. 2).28 The synthesis of AgNPs was carried out using a simple procedure.28 An aqueous solution containing AgNO3, trisodium citrate, and polyvinylpyrrolidone was stirred, and then NaBH4 was added drop by drop until the solution turned light yellow. The prepared silver nanoclusters with ascorbic acid were added (with stirring) to an aqueous solution containing AgNO3, trisodium citrate, polyvinylpyrrolidone, and pyridinium IL with trifluoroacetate anion. A dark green color change of the solution indicated the formation of TSIL-AgNPs. The biosensitivity of TSIL-AgNPs was conditioned via dopamine and identified by placing the NPs with human blood serum in an Eppendorf tube. The samples were incubated at room temperature, and their interactions were confirmed by the color change of the mixture from green to red. The functionality of TSIL-AgNPs is worth confirming because dopamine is a neurotransmitter responsible for the proper functioning of the nervous system. Pathological changes in dopamine concentrations can lead to many serious neurodegenerative diseases or depression.156 Direct contact between dopamine and TSIL-AgNPs reduces the NPs’ diameters, thus implying that they can be used as an efficient biodiagnostic platform for dopamine, even at low concentrations.28
AgNPs have also been stabilized with an IL in the preparation of a colorimetric biosensor for hydrogen peroxide.157 The assessment of the hydrogen peroxide content is important industrially (textiles, food) and in biomedicine. This compound is a by-product of oxidative metabolism in the human body and can lead to cardiovascular disease. Its concentration is maintained at an appropriate level by the enzyme peroxidase, and metal bionanosensors mimic the behavior of this enzyme. The system of imidazolium IL with acetate anion-stabilized lignin-doped AgNPs was prepared through a green synthetic route using Longan fruit extract. The appropriate amount of lignin-doped AgNPs was dispersed in the IL by grinding it in a mortar until a greyish mixture was formed. The fabricated biosensor was successfully used to determine the hydrogen peroxide level of in the blood of hypertensive patients. As the H2O2 concentration increased, the sample changed from colorless to an increasingly intense green.
An interesting example of the dual functionality of an IL as a solvent and stabilizing reagent was presented for the synthesis of AgNPs with confirmed antibacterial properties.48 As an illustration, imidazolium ILs with halide and tetrafluoroborate anions were synthesized as the reaction medium. The AgNO3 as silver precursor was initially incubated in a selected IL under a nitrogen atmosphere for half an hour, and then the reduction was performed with a constant flow of 16 bar of hydrogen gas for over 14 hours in a closed reactor. The characteristics of the resulting colloidal AgNPs showed that their size decreased according to the anion of the halide in the following order: Cl− > Br− > I−. A prior study,48 the antibacterial activity of the prepared systems was evaluated against Gram-positive bacteria Bacillus cereus and Gram-negative bacteria E. coli (Fig. 11c). The results showed that the antibacterial activity is the highest for colloidal AgNPs prepared with iodide using imidazolium IL with iodide anion. The antimicrobial activity of the colloidal AgNPs with selected IL exceeds that of pure ILs.
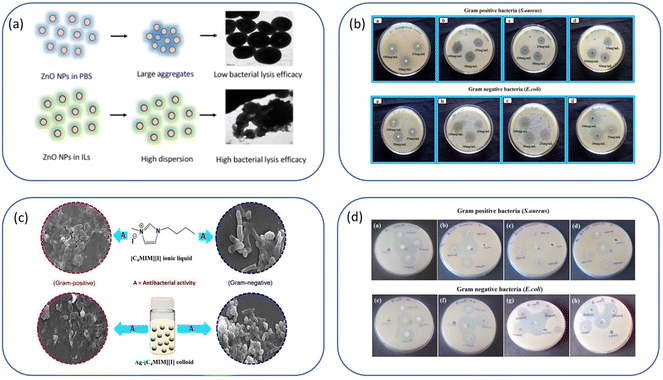 | ||
| Fig. 11 The antibacterial activity of NPs synthesized in the presence of ILs: (a) ZnONPs dispersed in cholinium IL with acetate anion and imidazolium IL with chloride anion. Reproduced from ref. 46 with permission from American Chemical Society, copyright 2018. (b) a. CeO2NPs, b. Ag loaded CeO2 NPs, c. Au loaded CeO2 NPs and d. Ag–Au loaded CeO2 NPs synthesized in the presence of imidazolium IL with hexafluorophosphate anion. Reproduced from ref. 37 with permission from Elsevier, copyright 2020. (c) AgNPs synthesized in imidazolium ILs with various halide anions. Reproduced from ref. 48 with permission from Elsevier, copyright 2020. (d) Ag–Au loaded RuO2 NPs synthesized in the presence of imidazolium IL with hexafluorophosphate anion. Reproduced from ref. 32 with permission from Elsevier, copyright 2020. | ||
Patil et al.23 described the IL-AgNPs system formed via a pyridinium IL with a bromide anion as a stabilizing agent and solvent for AgNPs nanoobjects and as antibacterial active agents against E. coli, S. aureus, and P. aeruginosa (Tables 4 and 5). The procedure proposed by the authors for the synthesis of NPs proposed was very simple and fast. Hydrazine hydrate was added after the aqueous solutions of pyridinium salt and the silver nitrate had been mixed for 10 minutes. The color then changed from yellow to yellow-brown. The resulting IL-AgNPs were 2–20 nm and mostly spherical with a small number of nanorods. There were no changes in NPs size, and agglomeration symptoms were not observed after a 10-month stability test. The IL-AgNPs had the highest bactericidal effect against Gram-positive bacteria – namely S. aureus.
Inbasekar and Fathima162 assume that IL-coated cerium(IV) oxide NPs can be applied as stabilizers in collagen-based biomaterials. CeO2NPs promote cell differentiation, induce conformational changes in proteins, and even stimulate collagen production, making them valuable in tissue engineering. Unfortunately, conventional polymeric capping agents for nanoceria rarely have the biocompatibility required for long-term contact with animal tissues (e.g., scaffolds and implants).160 For this reason, cholinium-based amino acid IL (CAAIL) can be used as an alternative NPs coating.162 Collagen samples treated with IL-capped NPs show improved thermal and dimensional stability compared to the native protein. The use of CAAIL promotes the formation of cross-links between collagen side groups. The dimensional stability of the treated collagen is also satisfactory. Although CeO2NPs induce some conformational changes, they are not sufficiently profound to alter the functionality of the protein. CAAIL acted as an NPs capping agent and played a key role in collagen stabilization.
ZnONPs can absorb UV and infrared radiation and can show semiconductor and magnetic features, which makes them useful in optical materials, solar cells, electrodes, and cosmetics. They can also be applied as biosensors or diagnostic imaging devices.163,164 However, the ease of aggregation of ZnONPs causes a loss in antibacterial functionality, which underscores the need for morphological and surface control.46,165 Therefore, it is imperative to develop stable IL-ZnONPs. The effects of two ILs, cholinium acetate and imidazolium IL with chloride anion, were compared using nosocomial skin-specific bacteria: Staphylococcus epidermidis and Klebsiella pneumoniae (Tables 4, 5 and Fig. 8a).46 In this study, ZnONPs with a diameter of 60 nm were produced at ambient temperature. The antibacterial activity was assessed after the incorporation of nanozinc into each IL (Fig. 11a). The application of imidazolium IL-ZnONPs yielded a highly effective antibacterial agent against S. epidermidis, which is responsible for infections occurring during implantation or in cases that require catheterization. This IL-ZnONPs system is cheap, easily available, and non-destructive against healthy skin cells. It can eliminate the need for surgical removal of the infection, and the destructive mechanism against bacterial cells is based on an increase in reactive oxygen species caused by the spontaneous oxidation of ZnONPs to Zn2+.
A bioactive IL-Cu2ONPs system including imidazolium IL with bromide anion was proposed as a potential decomposition agent for the antipyretic and analgesic drug diclofenac. The waste products of this drug can be harmful to fish and other freshwater aquatic species (Fig. 2 and 4b).34,166 IL-Cu2ONPs were prepared by adding various concentrations of imidazolium IL with bromide anion. The degradation of IL-Cu2ONPs was observed over the entire pH range, which confirmed that the concentrations of reactive oxygen species and the sulfate radical resulting from the cleavage of the inter-oxide bond to sodium persulfate are responsible for drug decomposition. The degradation rate of diclofenac at neutral pH reached 0.89 at a persulfate dosage of 0.25 g L−1 (Fig. 12). The active surface of the citrate nanocopper was controlled, and an equal octagonal shape was maintained by the IL present in the system.
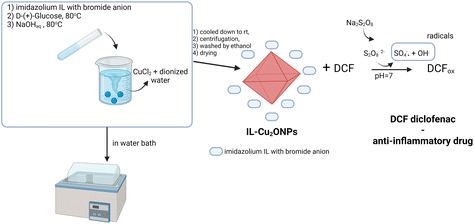 | ||
| Fig. 12 Synthesis procedure and biodegradation effect of IL-Cu2ONPs.34 | ||
IL-TiO2 nanostructures can be used as metal oxide NPs-based biodetectors, although the preparation of TiO2NPs requires several rounds of surface titanium plate purification (Tables 4 and 5).132 Important factors in selecting TiO2NPs for this purpose are high absorption capacity, stability at high temperatures, and structural features such as a large specific surface area or distances between particles. The NM is readily available and has low toxicity. IL-TiO2NPs biosensors were prepared by grinding TiO2NPs in a mortar in imidazolium IL with acetate anion, which resulted in a pinkish-grey powder (Fig. 13).132 The average size of IL-TiO2NPs was 26.5 nm. The biosensitive reaction was prepared by mixing dopamine and capped TiO2NPs in the presence of NaOH at various pH values (including physiologically-relevant pH values). The working principle of this detector is related to the attainment of a reddish-brown color derived from dopamine quinone. The detector was formed by hydroxyl radicals located on the surface of titanium oxide nanostructures reacting with dopamine. The same detector was successfully used as an acetone biomarker in the urine of diabetics (Tables 4, 5 and Fig. 8e).130 The IL stabilizes TiO2NPs against aggregation in biological fluids and increases the effectiveness of TiO2NPs as a simulated enzyme. The activity of this detection platform is strongly dependent on the type of interactions created between the nanostructures of the metal oxide being studied and the IL – as well as any changes in their size.167,168
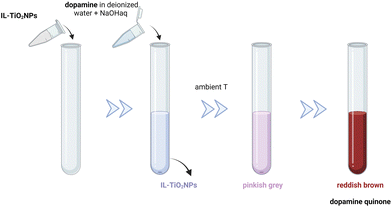 | ||
| Fig. 13 Biosensor activity test of IL-TiO2NPs.132 | ||
| Type of NPs | Type of reducing agent | Type of ILa | Role of IL | Process conditions | NPs shape | NPs average size [nm] | Bioapplication | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Bio-derived ions are shown in cursive. The scale of the processes described in Table 6 (amount of metal precursor and IL used) is detailed in the ESI, Table S2.† In general, the processes fell within the following numerical ranges: (i) the smallest production scale of NPs involved the use of 0.17 mg (ref. 49) of metal precursor and 5.0 mg (ref. 49) or 2.0 mL (ref. 162) of an IL; (ii) the largest production scale of NPs also employed 1.98 g (ref. 46) of metal precursor and 1.0 g (ref. 49) or 3.0 mL (ref. 16) of an IL. | ||||||||
| Naturally-sourced ILs assistance in performing bioactive NPs via chemical reduction | ||||||||
| Au | IL (D-glucosamine derivative) | Ammonium IL with formate anion (D-glucosamine derivative) | Reducing agent | Stirred for 5–20 min at 60 °C | Spherical | 25 | Contrast agent for X-ray tomography | 29 |
| Au | IL (tryptophan derivate) | Ammonium IL with tryptophanate anion (tetraethylammonium based-IL) | Reducing and stabilizing agent | Stirred at 50 °C, [Au+]/IL = 1/2 | Multiple-twinned | 18–20 | Inhibition of erythrocytes hemolysis; promising agent for drug delivery engineering | 175 |
| Au | IL (tryptophan derivate) | Ammonium IL with tryptophanate anion (cholinium-based IL) | Reducing and stabilizing agent | Stirred at 50 °C, [Au+]/IL = 1/3 | 5-Fold twinned | 32–34 | Inhibition of erythrocytes hemolysis; promising agent for drug delivery engineering | 175 |
| Au | IL (cholinium derivate) | Ammonium IL with purpurin-18 anion (cholinium-based IL) | Reducing and stabilizing agent | Stirred for 2 h at room temp., purified | Pentagonal bipyramid | 8–25 | Photosensitizer in photodynamic therapy, contrast agents | 18 |
| CeO2 | Sonication/alkaline process environment | Ammonium IL with serinate anion (cholinium-based IL) | Solvent, capping, and functionalizing agent | Stirred for 45 min, ultrasound bath for 12 h (ambient conditions), purified, dried overnight at 80 °C | — | 192.3 | Crosslinking agent for collagen | 162 |
| ZnO | NaOH | Ammonium IL with acetate anion (cholinium IL) | Dispersing agent | 30 min under the reflux, room temp., purified, vacuum dried for 3 h, NPs dispersed in IL/phosphate buffered saline by sonication | — | 64.4 | Antibacterial agent | 46 |
| Ag | Tetrabutylammonium borohydride | Pyridinium IL with salicylate anion | Dispersing agent and endowing bactericidal activity of NPs | Stirred for 30 min at room temp. | Spherical | >10 | Antimicrobial agent | 49 |
5.2. Bioactive metal-based NPs supported by IL with naturally-sourced component
The key questions and directions in this section related to the creation of green ionic liquid–nanoparticles biosystem include:1. An IL with a naturally-sourced component is expected to influence both the morphology of the tested nanoobjects and the type of bioapplication of the IL-NPs biosystem. The natural component should be widely available and inexpensive, and its incorporation into the IL structure should adhere to the 12 principles of green chemistry.
2. Does the use of an IL containing a natural component, as opposed to a structurally similar IL without a natural component, enhance the effectiveness of creating an appropriate IL-NPs biosystem? Can such an IL serve multiple functions and effectively address the morphology, stabilization, and bioapplication requirements of the investigated NM?
3. Is it feasible to develop IL-NPs biosystem wherein the structure of the IL or part of it incorporates the identical natural compound that is also used as a reducing agent for the biosynthesis of the NPs?
4. Can specific types of ILs with naturally-sourced components significantly contribute to the biofunctionality of ILs-NPs biosystems while meeting sustainable development criteria? Are these ILs promising candidates for advancing the development of ideal ILs-NPs biosystems, potentially replacing current ILs-NPs systems?
5. Can naturally-sourced IL-NPs biosystem be easily scaled-up?
The current trend seen in natural products has sparked interest in bio-based molecules as building blocks for ILs, which have been designed to be harmless to users and the environment, cost-effective, and consistent with sustainable development.176,177 Naturally occurring L-carnitine, choline, or betaine cations seem to be the most commonly used for the synthesis of the partially or fully naturally-sourced ILs.178–181 Derivatives of fatty acids, carboxylic acids, and amino acids occupy a special place in this group and are often utilized as the source of the anion.182,183 The most common of these groups are acetic, formic, lactic, succinic, or tartaric derivatives, but valine, alanine, and isoleucine are also used. Recently, there has been a growing body of research focused on ILs derived from sugars.184,185 Another captivating alternative are terpene-based ILs, especially those containing a monoterpene component, e.g., (−)-menthol, (−)-borneol, (+)-fenchol. Attention should be paid to their multifunctional biological applications that often surpass conventional standards, including antimicrobial agents,44,186,187 wood preservatives,188 plant resistance inducers,189,190 and even enzyme stabilizers and activators.191 Considering the advantages of natural components as fundamental building blocks of ILs, and the extensive array of potential bioapplications, we highly recommend employing these functionalized ILs to fabricate hybrid ILs-NPs biosystems.
Meticulous care is required to obtain the best possible naturally-sourced IL-NPs biosystem. Fig. 14 illustrates the essential factors involved in establishing the optimal naturally-sourced IL-NPs biosystem. First, a comprehensive exploration of diverse structural variations is necessary to determine the most effective approach for incorporating a natural element into the ionic compound's structure. Simultaneously, it is essential to establish guidelines to ensure that the entire process and the resulting NMs meet sustainability criteria. Furthermore, a well-defined procedure or set of steps should be devised to enhance the likelihood of success in creating the ideal IL-NPs biosystem using naturally-sourced IL. Factors presented include the structural aspects of ILs containing naturally-derived components, the applicable green guidelines to be adhered to, and the sequential steps required for achieving an effective hybrid system.
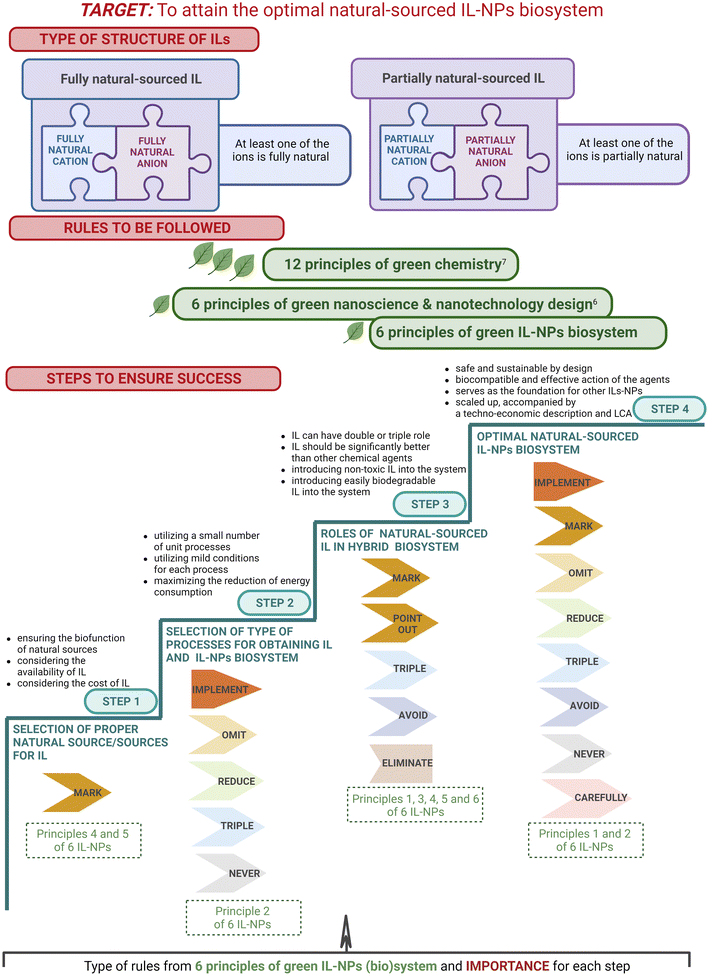 | ||
| Fig. 14 Elements of an optimal naturally-sourced IL-NPs biosystem: types of naturally-sourced IL structures, green rules to be followed, and steps to be taken to obtain an effective system. | ||
Utilizing ILs with naturally-sourced components in the fabrication, stabilization, and biofunctionalization of NPs deserves extensive exploration. However, the number of examples employing ILs derived from natural resources remains relatively limited, as evidenced by the literature (Table 6).29,46,49,162,175 This observation is rather unexpected, considering the substantial growth of research on ILs incorporating natural elements.192,193 In our opinion, partially or fully naturally-sourced ILs can serve as a solid foundation for establishing a comprehensive platform for ILs-NPs biosystems and may pave the way for future directions in expanding the environmentally friendly development of nanotechnology.
Ammonium ILs containing an amino sugar-derived cation29 or an amino acid-based anion175 acting as reducing agents for AuNPs production were effectively implemented (Table 6 and Fig. 8d). These examples demonstrate remarkable attributes in terms of sustainability. First, the utilization of ILs with a natural component as a reducing29,175 and even stabilizing agent175 enhances the biodegradability of the resulting salts. The use of aliphatic amine cations in the salt ensures low toxicity or non-toxicity.42 The processes employed for producing AuNPs,29,175 as outlined by the authors, required only mild conditions. This is evident in the minimal number of unit processes involved, the absence of high process temperatures (within the range of 50–60 °C for both cases), and the achievement of highly satisfactory morphological and bioapplication effects. The results for AuNPs indicate that the ratio of the precursor to IL plays a substantial role in determining the size of the resulting IL-NPs biosystem.29,175 When considering the practical bioapplication of ILs-NPs hybrid systems, alternative energy sources might be incorporated to replace conventional heating methods, thereby reducing energy consumption as demonstrated in the previously mentioned examples.29,175 Such an approach would allow discussion of whether changes in the process method significantly affect the resulting biosystem in terms of sustainability and environmental impact,142,143 and provide guidelines for optimizing such systems.
A captivating application of cholinium-based IL is its utilization in the synthesis of the IL-AuNPs system (Table 6 and Fig. 1).18 The authors have synthesized water-soluble cholinium–purpurin-18-carboxylate salt, which belongs to quaternary ammonium compounds consisting of the cholinium cation and the purpurin-18-carboxylate anion derived from dihydroporphyrin. When considering the use of natural components, it is important to note that the aforementioned ionic compound consists of two unmodified components derived from natural sources and from the cationic and anionic parts of the compounds. Purpurin-18, a naturally occurring compound found in Spirulina maxima algae is particularly known for its photosensitizing properties. The synthesis for cholinium–purpurin-18-carboxylate is relatively straightforward, involving a two-step pathway that does not require extreme process conditions. First, cholinium hydroxide is obtained through the reaction of cholinium chloride with KOH. Subsequently, the hydroxide salt is combined with purpurin-18 in an aqueous solution at elevated temperature. The final product is purified by extraction with water-dichloromethane, followed by evaporation and crystallization from cold acetone or acetonitrile. The resulting cholinium-based IL is employed for the synthesis and stabilization of AuNPs, serving a dual function. This feature confers an additional advantage to the naturally-sourced IL-NPs biosystem since as it eliminates the need to introduce additional reducing and stabilizing chemicals. Consequently, the number of unit processes is significantly reduced, enhancing the overall environmental sustainability of the biosystem. The diameter of the resulting NPs ranged from 8 to 25 nm, exhibiting no agglomeration. The authors emphasize that these IL-purpurin derivative-stabilized NPs can be effectively employed as contrast agents or in photothermal therapy. In this specific example, two natural components were employed to synthesize TSIL, wherein the incorporated salt serves a dual role as both a reducing agent and a stabilizer for the generated nanoobjects within the hybrid system. All the processes involved in producing IL and IL-NPs are characterized by mild conditions and do not require any demanding operating conditions. Therefore, the recommendations are in line with these previous examples29,175 and are largely based on process optimization and the search for an ideal naturally-sourced IL-NPs biosystem. This could include the introduction of modifications aimed at reducing energy consumption.
Another example revolves around an IL consisting of a naturally-derived cation and anion, specifically cholinium serinate, for the synthesis of TSIL (Table 6).162 The synthesis involved the functionalization of CeO2NPs with the support of cholinium-based IL through ultrasonic treatment of a mixture containing the salt precursor (Ce(NO3)3·6H2O), sodium hydroxide, and cholinium serinate under ambient conditions. The presence of the desired quaternary ammonium salt on the NPs surface was confirmed through variations in the zeta potential (negative value), hydrodynamic diameter (increase), and polydispersity index in comparison to organic salt-free CeO2NPs. Structural changes were observed in the collagen isolated from the tail tendons of laboratory rats. The application of IL-CeO2NPs resulted in an increase of the stability of collagen at temperatures ranging from 67 to 80 °C, utilizing NPs concentration of 0.5% (v/v). The low toxicity and likely facile biodegradability exhibited by the selected TSIL unquestionably aligned with the principles of green chemistry. A significant advantage lies in the fact that the employed salt simultaneously serves as a solvent, functionalizing agent, and capping agent, thereby reducing the reliance on multiple chemical reagents during the synthesis of NPs. The inclusion of ultrasonic treatment and ambient conditions rendered the process more environmentally friendly. The information provided by the authors that the NPs obtained are larger than 192 nm makes it advisable to introduce certain modifications in the conditions of NPs production. These include adjustments in temperature, reaction time, and duration of ultrasound exposure. Changes in these three parameters could be sufficient to produce nanoobjects with sizes below 100 nm. Nevertheless, the IL-CeO2NPs system might be applied as a collagen crosslinking agent in producing NMs for tissue engineering purposes.
The implementation of a TSIL containing an acetate anion derived from naturally occurring choline resulted in the formation of an IL-ZnONPs system,46 which exhibited remarkable antibacterial properties. This example has been comprehensively discussed in the preceding sections of our study, highlighting the bioapplication advantages of this hybrid system (Fig. 8a and 11). The structure and production of the chosen IL for the system (Table 4), as well as the procedure employed to produce the hybrid IL-ZnONPs system (Table 5), have been thoroughly discussed in accordance with the principles of green chemistry.
Another example with a distinct cation structure based on pyridine that possesses a biomoiety has been proposed for the development of a naturally-sourced IL-NPs biosystem (Table 6).49 The authors of this research demonstrated that the combination of pyridinium ILs and the resulting NPs exhibited a broader spectrum of antimicrobial effects. In this case, a pyridinium salt with a salicylate anion was synthesized and utilized to produce AgNPs, concurrently serving as a dispersing agent and an enhancer of their antibacterial capacity by utilizing the well-established disinfecting properties of salicylic acid. The simplicity of the synthetic procedure, the use of aqueous reaction media, short reaction time, and ambient conditions for NPs synthesis are indisputable advantages of this approach. The NPs obtained through this method displayed small-size characteristics. It is advisable to consider alternative solutions in future studies, as the use of a chemical reducer (e.g. tetrabutylammonium borohydride, TBABH4) is not recommended. The modification of the IL composition to enable the salt to also act as a reducing agent or exploring the utilization of a natural reducer that has minimal environmental impact are both worth considering.
The examples presented in this section frequently depict IL structures consisting of even two natural components,18,29,49,175 (according to the principle 4 of IL-NPs) and many cases demonstrate the presence of multiple organic salt functionalities in ILs-NPs hybrid systems (according to the principle 6 of IL-NPs).18,49,162,175 The manufacturing processes employed for these ILs-NPs systems are often gentle and do not require complex laboratory techniques or difficult-to-access equipment (according to the principle 2 of IL-NPs). The previously advantages mentioned above provide clear directions for future efforts. Notable progress has already been made concerning the morphology and bioactivity of the systems. The focus should now shift towards optimizing the naturally-sourced ILs-NPs biosystems to maximize their potential, including significant energy-saving measures, and exploring possibilities for scalability. It is worth considering the implementation of well-established naturally-sourced ILs-NPs biosystems for various other bioapplications.
One of the inquiries we presented at the beginning of this section remained unresolved. It concerns the creation of a system in which the IL structure (or a component of it) comprises the same natural compound that is utilized as a reducing agent for NPs biosynthesis. For instance, it is worth considering the possibility of developing an IL-NPs biosystem that incorporates an IL with a (−)-menthol component, while utilizing mint leaves as the reducing agent, which serves as the source of obtaining the optically active form of (−)-menthol. We believe that this is represents an important avenue that can be explored, where a single material of natural origin performs multiple roles within designated processes.
5.3 Biosynthesized metal-based NPs supported by IL
The key questions and directions in this section towards the creation of green ionic liquid–nanoparticle biosystems include:1. The use of naturally-sourced reducers in the metallic NPs synthesis represents a notable advancement towards obtaining environmentally friendly and SSbD IL-NPs biosystem. These “green” reducing agents should possess characteristics such as ready accessibility, low procurement cost, effectiveness at least comparable to conventional counterparts, and the ability to ensure high synthesis efficiency and replicability of results. By adhering to these criteria, the overall process of obtaining the IL-NPs biosystem will align with the 12 principles of green chemistry.
2. Are plant materials as a source of reducing substances for NPs formation more suitable than a non-natural reducing factor in terms of fulfilling multiple functions and effectively addressing the morphology, stabilization, and bioapplication requirements of the investigated nano–micro IL-NPs biosystem?
3. What are the potential implications and prospects of employing a combination of plant extract and ILs in the synthesis of ILs-NPs biosystems, particularly with respect to their application in the design of industrially significant NMs?
The techniques involving various types of naturally occurring reagents, mainly plant extracts, vitamins, sugars, microorganisms, and biodegradable polymers as chelating/reducing and capping agents for obtaining NPs are currently considered attractive in nanotechnology. The biosynthesis of NPs through these methods is both rapid and environmentally friendly. It involves the utilization of various microorganisms as viable nanofactories including bacteria,194–196 fungi,197–199 viruses,200 algae,201,202 and enzymes.203,204 Despite the numerous examples of the biosynthesis of NPs using a variety of microorganisms, the use of plant material extraction products has recently received attention. A major advantage of promoting the use of plant extracts is the significant reduction in preparation time and the increase in safety by eliminating the need to cultivate microbes that often act as pathogens. The presence of polyphenols in plant material often plays a pivotal role in these bioprocesses. The techniques employed in these processes are straightforward, ecologically friendly, and typically involve one-pot methodologies.205
Indeed, substances present in extracts derived from leaves, flowers, fruits, and various plant materials have the ability to reduce metals and form nanostructures. Green-synthesized metallic NPs have comparable properties to their chemically synthesized counterparts. The reports indicate a lack of consistency in the size and shape of the synthesized NPs, and in most cases the production has not been quantified.206 Despite the widespread popularity of green-synthesized NPs using plant-derived resources, this method currently faces several limitations. Overcoming these limitations would be a significant milestone toward developing a fully environmentally friendly protocol for large-scale NPs production and subsequent commercialization. Several requirements have been identified for the synthesis of NPs based on plant-derived reducers. First, it is necessary to establish conditions that ensure the effective progress of the synthesis and its reproducibility. In addition, appropriate stabilizers must be employed to regulate and prolong NPs stability. Understanding the mechanism by which plant-derived extracts reduce metallic precursors and rely on specific bioactive substances poses a major challenge. Hence, it is crucial to develop a simple procedure to isolate individual components of the extract and evaluate their impact on NPs synthesis. The variety of plant compounds with reducing capacity, such as compounds with phenolic, amino, carboxyl, and hydroxyl groups, proteins, and amino acids, leads to variations in the synthesized materials under different production conditions. Many reports do not clearly specify the reducing agent used, and stoichiometric calculations are lacking.206 A comprehensive description of the resulting NPs under each extraction condition could enable fine-tuning of their properties. The aforementioned issues raise important questions, such as the quantity of reducing agent that can be obtained from a specific plant and the amount of biomass required for sustainable NPs production.
From our perspective, using renewable sources as reduction agents in conjunction with ILs aligns with the 12 principles of green chemistry, offering a notable technological solution for producing sustainable NMs. Several factors need to be carefully considered prior to achieving the optimal IL-NPs biosystem through reduction based on plant extract produced by green extraction methods. These factors, as depicted in Fig. 15, illustrate the essential elements involved in establishing the ideal IL-NPs biosystem obtained via green synthesis with the support of ILs. First, it is necessary to conduct a comprehensive analysis of diverse natural reducing sources to determine the most suitable option. Second, the selection of structural components for the IL should be carefully selected to ensure that the resulting salt meets the desired objectives. Simultaneously, adherence to the principles of green chemistry is crucial to ensure that the entire process and the resulting NM meet sustainability criteria. A well-defined procedure or a set of steps need to be devised to increase the probability of successfully producing the ideal IL-NPs biosystem through green synthesis for NPs production. Factors to be considered include the type of natural reducing agent, the structural aspects of ILs, the applicable green guidelines, and the sequential steps required for achieving an effective hybrid system.
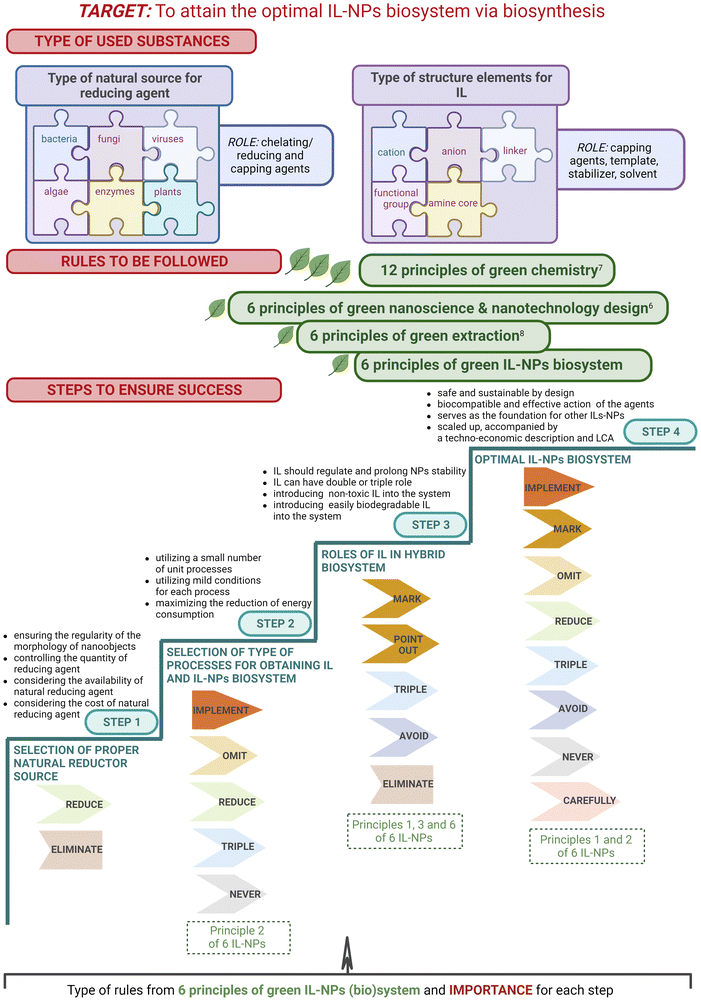 | ||
| Fig. 15 Elements of an optimal IL-NPs biosystem: types of natural sources of reductors, types of IL structures, green rules to be followed, and steps to be taken to develop an effective system. | ||
Table 7 illustrates the synthesis of NPs through biological methods utilizing plant extracts as raw materials. The table provides examples of bioactive metallic NPs, including metal oxides and metal-based complexes, and highlights the significant influence of ILs in these processes. An imidazolium IL with a hydrogen sulfonate anion was applied as the extraction solvent to obtain an extract rich in polyphenolic compounds from Polygonum minus leaves. This extract was subsequently used for the biosynthesis of CuNPs (Table 7).207 Employing the MW technique resulted in a shortened reduction process time, producing CuNPs with an average size of 20–30 nm. The biological activity of NPs was demonstrated through their highly inhibitory effect on three bacteria, namely E. coli, S. aureus, and Aeromonas hydrophilia. The reduction of CuSO4 by the polyphenolic compounds present in the plant extract was supported by the IL. However, it is worth considering testing an IL with a lower toxicity profile in the same procedure.
| Type of NPs | Type of natural reducing agent | Type of IL | Role of IL | Process conditions | NPs shape | NPs average size [nm] | Bioapplication | Ref. |
|---|---|---|---|---|---|---|---|---|
| The scale of the processes described in Table 7 (amount of metal precursor and IL used) is detailed in the ESI, Table S2.† In general, the processes included 1.9 mg (ref. 207) of metal precursor and 1.0 mL (ref. 31–33, 37–39, 133 and 208–212) of an IL. | ||||||||
| CeO2, SrO | Pedalium murex leaf extract | Imidazolium IL with hexafluorophosphate anion | Capping agent | Stirred for 6 h, room temp., purified, dried for 6 h at 100 °C, calcinated for 4 h at 700 °C | – Nanorod | 5 | Antimicrobial treatment | 133 |
| Yb2O3 | Andrographis paniculata leaf extract | Imidazolium IL with hexafluorophosphate anion | Stabilizing agent | Stirred, room temp., then autoclaved for 12 h at 120 °C, purified, dried at 80 °C and calcinated for 6 h at 600 °C | – Hexagonal | 25–75 | Antibacterial, anti-inflammatory and anti-diabetic action | 208 |
| – Circular | ||||||||
| – Rectangular | ||||||||
| TiO2 | Vitex negundo linn leaf extract | Imidazolium IL with tetrafluoroborate anion | Template, stabilizing agent | Stirred for 2 h, room temp., purified, dried at 100 °C and calcinated for 3 h at 500 °C | – Spherical | 15–26 | Antibacterial treatment | 33 |
| – Nanorod | ||||||||
| Cu | Polygonum minus leaf extract | Imidazolium IL with hydrogen sulfate anion | Extracting solvent, stabilizing agent | MWs for 5 min at 60 °C, 400 W, purified, vacuum dried for 3 h at 60 °C | – Spherical | 20–30 | Antibacterial treatment | 207 |
| La2O3 | A. paniculata leaf extract | Imidazolium IL with hexafluorophosphate anion | Template, stabilizing agent | Stirred for 3–5 h, room temp., purified, dried at 80–120 °C, calcinated for 5–6 h at 600–650 °C | – Cubic | 20–80 | Antibacterial, antioxidant, anti-inflammatory and anti-diabetic agent | 209 |
| Sm2O3 | 210 | |||||||
| Nd2O3 | 211 | |||||||
| La2O3 | Couroupita guianensis abul leaf extract | Imidazolium IL with tetrafluoroborate anion | Stabilizing agent | Stirred for 4 h at room temp., then autoclaved for 6 h at 120 °C, purified, dried for 4 h at 80 °C, calcinated for 6 h at 600 °C | – Spherical | 15–70 | Antibacterial, antioxidant, anti-inflammatory, anti-diabetic and anticancer agent | 39 |
| – Tetrahedral | ||||||||
| – Hexagonal | ||||||||
| Nd2O3 | Stirred for 4 h at room temp., purified, dried at 80 °C, calcinated for 5 h at 550 °C | – Hexagonal | 50–75 | 212 | ||||
| – Spherical | ||||||||
| – Oval | ||||||||
| Ag–Au/ZnO | Justicia adhatoda leaf extract | Imidazolium IL with hexafluorophosphate anion | Template | Stirred for 1 h at room temp., then autoclaved at 120 °C, 24 h, purified, dried for 6 h at 100 °C, calcinated for 6 h at 600 °C | – Nanoneedle | 25 | Antibacterial and anticancer treatment | 31 |
| Ag/ZnO | – Nanostick | 34 | ||||||
| Au/ZnO | – Nanostick | 32 | ||||||
| Ag–Au/CeO2 | – Spherical | 28 | 37 | |||||
| Ag/CeO2 | – Hexagonal | 33 | ||||||
| Au/CeO2 | – Nanoneedle | 31 | ||||||
| Ag–Au/RuO2 | – Spherical | 24 | 32 | |||||
| Ag/RuO2 | ||||||||
| Au/RuO2 | ||||||||
| Ag–Au/Y2O3 | Capping and stabilizing agent | – Face-center cubic | 30 | 38 | ||||
| Ag/Y2O3 | ||||||||
| Au/Y2O3 | ||||||||
The stabilizing effect of imidazolium-based IL with hexafluorophosphate or tetrafluoroborate anions was observed in the synthesis of lanthanide oxide NPs using plant leaf extract at room temperature (Table 7). A specific example is the preparation of samarium oxide NPs (Sm2O3NPs) based on an ethanolic extract of the leaves of Andrographis paniculata in the presence of hexafluorophosphate IL.210 Regrettably, the anions of both ILs are highly toxic, which significantly compromises the green aspects of the IL-NPs system. The process conditions, including the high energy input required for purification, drying, and calcination, pose significant drawbacks in terms of green chemistry guidelines. The resulting IL-stabilized Sm2O3NPs exhibited excellent stability, with an average diameter of 40–57 nm and a cubic structure. The NPs exhibited potent antibacterial activity against E. coli and S. aureus, leading to the disruption of their cell membranes. These NPs exhibited a stronger antioxidant effect than that of vitamin C and a more robust anti-inflammatory effect compared to the standard anti-inflammation drug – diclofenac.
The synthesis of ytterbium oxide NPs (Yb2O3NPs) was conducted with the same stabilizer (imidazolium IL with a hexafluorophosphate anion) and a reducing agent (A. paniculata leaves extract) (Table 7).208 The resulting IL-Yb2O3NPs had a size range of 25 to 75 nm. This nanosystem showed high biomedical capacity due to its antioxidant and antibacterial properties. Their anti-diabetic and anticancer activities against breast cancer neoplastic cells were confirmed. The influence of the imidazolium IL with a tetrafluoroborate anion on the morphology of lanthanum oxide NPs was validated during the preparation of nanoobjects via a route based on Couroupita guianensis leaf extract.39 Dynamic light scattering analysis revealed that the IL-La2O3NPs obtained through this approach exhibited sizes ranging from 15 to 70 nm, with a tendency to form agglomerates of approximately 52 nm in diameter. The considerable toxicity associated with the anions of the proposed ILs raises concerns regarding the suitability of this system for drug delivery, despite the demonstrated anti-diabetic, antibacterial, anti-inflammatory, and anticancer activities of IL-Yb2O3NPs.
The templating effect of ILs can also be observed in the synthesis of complex metal-based NPs. Pandiyan et al.32 conducted a biogenic synthesis procedure to obtain IL-Ag–Au/RuO2 NPs, which exhibited high activity against microorganisms such as E. coli and S. aureus, as well as activity against cervical cancer cells (Table 7 and Fig. 16a). The plant extract was derived from the leaves of Justicia adhatoda, while an imidazolium IL with hexafluorophosphate anions served as the reaction medium. The resulting IL-Ag–Au/RuO2 NPs exhibited a small diameter of 24 nm.
 | ||
| Fig. 16 Schematic representation of the green synthesis of IL-NPs systems: (a) RuO2, Ag loaded RuO2, Au loaded RuO2, and Ag–Au loaded RuO2 NPs. Reproduced from ref. 32 with permission from Elsevier, copyright 2020. (b) ZnO, Ag-doped ZnO, Au-doped ZnO, and Ag–Au-doped ZnO NPs synthesized by Justicia adhatoda leaf extract in an imidazolium IL medium. Reproduced from ref. 31 with permission from Elsevier, copyright 2019. | ||
Pandiyan et al.31 utilized the same imidazolium-based IL as both a stabilizing agent and a template in the green synthesis of ZnO, Ag-doped ZnO, Au-doped ZnO, and Ag–Au-doped ZnO NPs through reduction with an ethanolic extract obtained from J. adhatoda leaves (Table 7 and Fig. 16b). The Ag–Au/ZnO NPs had a size range of 20–25 nm and displayed promising antibacterial activity as well as high toxicity against human cervical cancer cells. These findings led the authors to speculate that the IL-Ag–Au/ZnO NPs could be applied as novel NMs with desired properties for biomedical applications.
The analysis of the examples presented in Table 7 of IL-assisted biosynthesis of metal and metal oxide NPs using plant extracts led to the conclusion that there are relatively few syntheses reported in this research area. These syntheses are often based on the use of salts with inherent toxicity, which contradicts the principles 1 and 3 of IL-NPs. Furthermore, an additional drawback is the requirement for demanding conditions in the production processes of these systems, which is inconsistent with the principle 2 of IL-NPs. Although the initial mixing of reagents is typically done at room temperature, subsequent steps in the synthesis required elevated temperatures and advanced laboratory equipment.
We have prepared a graphical representation (Fig. 17) that combines the limitations associated with this biosynthesis approach supported by ILs, along with recommendations and solutions to enhance its efficacy and greening aspects in the future. This figure is based on the detailed analysis of the data collected by us (Table 7) and considers the advantages of using hybrid systems obtained via biosynthesis. Our literature search revealed that the current testing of ILs has focused primarily on imidazolium cations paired with BF4− and PF6− anions. However, these salts are considered environmentally unfriendly,42 and such an approach is completely inconsistent with the principles we have adopted for IL-NPs, especially those numbered 1 through 4. Further investigations should prioritize the use of ILs with lower toxicity indices, considering the structural recommendations outlined in Paragraphs 2 and 3 and Table 4, to at least meet the principles 1 and 3 for IL-NPs. The design of ILs for this purpose should include a selection of structural elements (according to the principles 1 and 4 of IL-NPs) that both fulfill the desired function(s) (according to the principles 5 and 6 of IL-NPs) and facilitate their biodegradation in the environment without introducing of highly toxic elements (according to the principle 3 of IL-NPs).
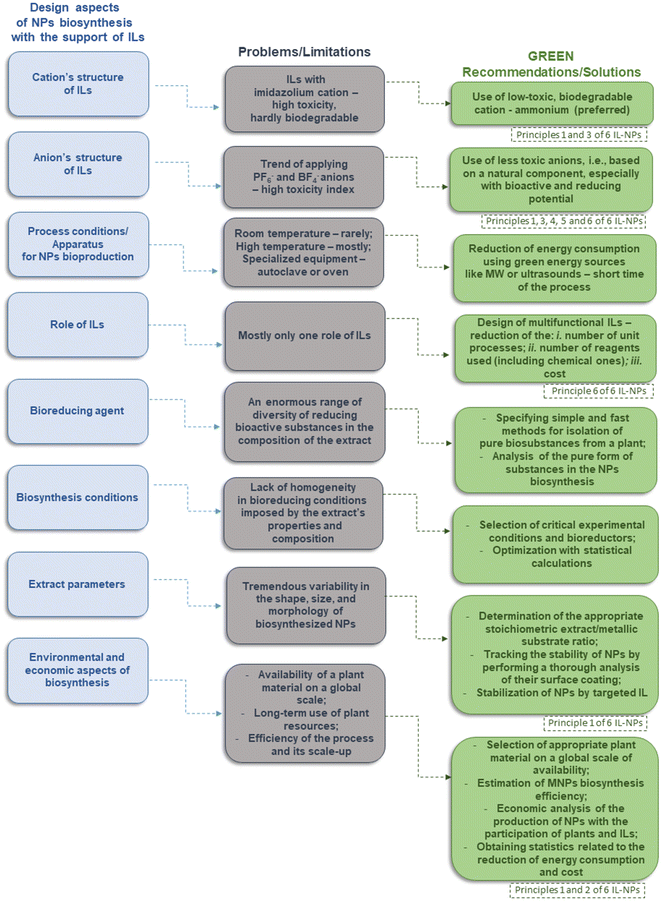 | ||
| Fig. 17 Problems and recommended approaches leading to improving the biosynthesis efficiency of IL-NPs biosystem. | ||
An attractive solution appears to be the production of ILs with linear amines in the cation core, such as cholinium-based ILs (according to the principles 1 and 4 of IL-NPs), due to their significantly lower toxicity and improved biodegradability,42 which is in the line with the principle 3 of IL-NPs. Another important aspect of the SSbD approach is to minimize the number of reagents required to obtain both IL (according to the principles 1 and 2 of IL-NPs), and NPs. In the literature, ILs often serve a single role in biosynthesis. Considering the diverse characteristics of ILs documented in the literature, it is crucial to design IL structures that can simultaneously perform multiple functions simultaneously. Based on the examples presented in Table 7, special attention should be given to ILs that can act as stabilizers, templates, extraction solvents, and media for NPs synthesis, which is in the line with the principle 6 of IL-NPs.
An innovative approach to achieve an optimal system would be to incorporate a naturally occurring structural element into the IL (according to the principle 4 of IL-NPs). This element can also serve as a bioreducing agent, such as leaves, which are rich in this substance. We have presented this solution and a detailed proposal in the previous section on the example of the use of monocyclic, natural alcohol (−)-menthol.
Consideration of the biosynthesis of NPs production in the presence of ILs requires optimization of the biosynthesis conditions, including pH value, temperature, time, and the stoichiometric ratio of extract/metallic precursor/ILs. These factors, along with the composition of the extract, have a substantial impact on the morphology, size, shape, and stability of the resulting NPs.
Our analysis of the conditions used in the biosynthetic processes from Table 7 indicates that the temperature is high and time required is long, especially for processes that involve advanced techniques, such as the synthesis of NPs in an autoclave or using calcination furnaces. This approach is strongly discouraged due to the increased energy consumption. As we emphasized repeatedly (especially in Table 5), it is important to consider more definitively greener alternative energy sources such as ultrasound or MWs when proposing a synthesis path that aligns with sustainable development. This is further supported by the example of CuNPs synthesis207 (Table 7), where the reduction was successfully achieved using P. minus leaf extract in only 5 minutes using MWs, eliminating the need for the autoclave technique.207
The development of an environmentally friendly system of (bio)IL-NPs (Fig. 15), while eliminating existing disadvantages (Fig. 17), requires a careful balance between all parameters affecting the process. This will be possible by understanding the components within the extract and the elements within the implemented IL that are responsible for the reduction mechanism. Insufficient knowledge in this regard, along with the diverse functionality of compounds present in the plant source, contributes to the formation of poor quality NPs.213 As a result, obtaining reproducible shapes and sizes is challenging. It is imperative to pay special attention to exploring new green solutions for the industrial-scale application of biosynthesis. From a commercial perspective, further research should also focus on estimating the cost-effectiveness of the process. This includes evaluating the efficiency of (bio)IL-NPs synthesis, analyzing statistics related to the cost of preparing all the necessary reagents, assessing the required energy input, and considering the availability of the plant material.
To summarize, our strategy aims, on the one hand, to demonstrate the construction of an optimal IL-NPs biosystem when NPs are obtained through biosynthesis with the assistance of IL (Fig. 15). On the other hand, we aim to address the challenges and limitations associated with implementing green technology in the IL-NPs biosystem, accompanied by relevant recommendations (Fig. 17). We sincerely hope that this approach will prove valuable both in the design stage and in the modification and optimization of the process for the production of the final nano–micro hybrid system.
6. Correlation analysis of green principles for ILs-NPs hybrid systems
In 2007, Dahl et al.6 proposed a translation of the 12 principles of green chemistry for application to nanoscience, presenting the six green nanoscience and nanotechnology design principles. These principles serve as a guiding framework for nanotechnology practitioners to ensure safer and more sustainable designs of nanostructures.One of the aims of this review was to establish a clear correlation between the six principles of green IL-NPs (bio)system, the 12 principles of green chemistry, and the six principles of green nanoscience and nanotechnology. To achieve this goal, Scheme 3 is presented, as it combines the proposal of Dahl et al. with the addition of our own principles, thereby providing a comprehensive and illustrative representation. A correlation matrix is introduced to find the connections between these principles and to guide practitioners in the development of safer and more sustainable nanostructures assisted by ILs.
Based on the extensive analysis of the literature on ILs-NPs objects conducted in this review, we observed that all the principles of green chemistry can be readily applied to the design of environmentally safe IL-NPs products. In all 12 cases, two or more of the six principles of green IL-NPs (bio)system can be applied simultaneously to optimize the design and solution.
Throughout this review, we have explored how these principles apply to the design of the IL structure and other substances necessary for obtaining targeted IL-NPs (bio)system, the design of the processes involved, and the biofunctionality of IL-NPs materials.
To conclude this section, we pose an open question—Is it possible to find at least one IL-NPs (bio)system that successfully combines all 12 principles of green chemistry with the six principles of green nanoscience and nanotechnology design and the six principles of green IL-NPs (bio)system? This challenge remains a subject of ongoing research and exploration in the field of sustainable and eco-friendly NMs.
7. Conclusions and outlook
NPs applied in biology, medicine, and related fields must have desired biological activity, excellent physicochemical features, and a favorable safety profile. ILs employed as reagents, auxiliaries, or surface-modifying agents in NPs synthesis can satisfy these requirements. The majority of NPs derived from IL-mediated routes exhibit superior performance characteristics compared to products from conventional synthesis methods. The versatility and tunable nature of ILs provide numerous possibilities to control crucial factors such as surface morphology, dispersibility, and size distribution of growing nanostructures. The enhanced homogeneity of these NPs facilitates a more accurate assessment of their interactions with biological systems, thereby improving their safety profile. When used as functionalizing agents, ILs can modulate nano-bio interactions by enhancing the selectivity, biocompatibility, or biodistribution of NPs.The applications of ILs-NPs systems have demonstrated relevance, effectiveness, and sufficient biocompatibility to warrant further exploration for biological and medical needs. Satisfactory results have been obtained in several areas, including antibacterial, antioxidant, and anticancer activities, as well as in wound healing and bone fracture treatment. ILs-NPs systems have also found utility in biodiagnostic platforms, X-ray contrast agents, colorimetric sensors for biological molecules, and even as suitable agents for drug biodegradation.
Remarkably, imidazolium cation-based ILs have emerged as highly effective assistants in the synthesis of biologically active NPs for a wide range of biological applications, as extensively documented in Tables 4–6. Particularly noteworthy from our perspective are salts derived from natural compounds, such as cholinium ILs, as well as ILs with anions possessing inherent biological activity, such as salicylate ILs. Although less frequently described in the literature (Tables 4–6), these ILs with specific structural elements deserve special attention due to their favorable environmental parameters and significant biological activity. Concurrently, the utilization of natural sources as reducing agents in NPs biosynthesis supported by ILs (Table 7 and Fig. 15) offers significant advantages, including waste prevention and efficient utilization of precursors. This combination of safe natural reducing agents and compatible ILs to both contributes to environmental safety and enhances the overall sustainability of the synthesis.
Regardless of the specific IL-NPs (bio)system structure, a careful analysis of both IL preparation and IL-NPs processes is crucial prior to their implementation. Energy efficiency can be achieved by striving for favorable conditions, such as normal pressure and room temperature, which can be planned in advance and easily applied during experiments. Other benefits include contamination prevention, safety precautions, cost-effectiveness, and compliance with regulations.
From our perspective, ILs-NPs biosystems offer unique combinations of biotechnologically attractive functionalities that are often unattainable with classical nanostructures. While there are notable successful applications of these biosystems, overcoming several significant challenges is crucial for their further translation into practical applications. These challenges include controlling and optimizing the performance of IL-NPs assemblies and developing cost-effective yet highly efficient methods for producing these nano–micro systems. Consequently, intensive investigation, understanding, and the development of new fabrication protocols for IL-assisted NMs are essential in the near future, as the current review emphasizes. In order to facilitate the intensive development of the field of IL-NPs, it is crucial to establish appropriate guidelines that promote the production of nanostructures with the desired morphology and accelerate the production of desired (bio)systems, all in line with sustainable development rules. In this regard, at the beginning of this work, we proposed a set of six principles of green IL-NPs (bio)system (Scheme 2) which consider the overarching 12 principles of green chemistry. These standards introduce novel green guidelines specifically tailored to the IL-NPs (bio)system.
Furthermore, we provide a comprehensive guide that underscores the “importance” of essential elements. The purpose of this guide is twofold: to ensure compliance of the 12 principles of green chemistry and with the 6 principles of the IL-NPs (bio)system, and to complement in detail the six principles of the IL-NPs (bio)system, as illustrated in Scheme 2.
Research on the IL-NPs topic is relatively new, and there are still certain issues that need to be addressed (Fig. 18). First, our understanding of the molecular interactions between ILs and NPs is currently limited. Achieving precise control over the synthesis and characteristics of NPs in the presence of IL will remain a challenge until we gain a deeper understanding of the mechanism of action of IL. Only through this understanding can we effectively control the morphology of biologically active NPs and prevent the agglomeration of nanoscale entities (Fig. 18).
Another important issue pertains to the characterization of nanoproduct properties for industrial applications. The analyses conducted to date are limited and quite often too selective to be considered decisive in evaluating commercial applications. Extensive and advanced studies are needed to determine whether the performance of the NPs is indeed satisfactory. This is particularly critical in the field of biomedicine, where safety and efficacy are paramount. Therefore, essential tasks arise for the biological function of the IL-NPs (bio)system. It is important to select ILs that fulfill two different roles of (Fig. 18): (i) stabilizing NPs (or even affecting the morphology of these nanoobjects) and (ii) influencing the biocompatibility and bioavailability of biologically-active NPs. The greatest success seems to be the possibility of creating an IL-NPs system in which the IL has antimicrobial activity and acts synergistically with NPs against bacteria. Surprisingly, there has been limited exploration of bioactive NPs incorporating ILs with excellent biological properties. Adopting a “dual-function of ILs” approach could significantly impact the overall biological outcomes of the IL-NPs system. Finally, it is essential to provide evidence of a properly selected IL-NPs system that demonstrates effective bioactivity for industrial needs and can be successfully implemented within a relatively short timeframe. Such evidence would validate the viability and effectiveness of such a biosystem.
Also, the literature has largely overlooked the matter of scaling up IL-mediated synthesis routes. However, for the proposed solutions to be successfully implemented in an industrial setting while maintaining competitiveness, it is crucial to ensure high-quality and cost-effective nanoproducts. Factors such as the availability and price of ILs, other required substances, raw materials (in the case of biosynthesis), energy consumption, and waste generation at the laboratory scale are important to assess. We believe that a TEA should be performed for certain ILs-NPs systems with high potential biological applications. Unfortunately, the literature usually omits techno-economic approaches. TEA bridges the gap between research and development, engineering, and business, providing an assessment of the economic feasibility and technological potential of the processes analyzed to achieve complete descriptions of these hybrid systems. When one is conducting a LCA, which evaluates the environmental impacts of a product or process throughout its entire life cycle, the incorporation of TEA can provide valuable insights for IL-NPs system (Fig. 18). This integration allows for the consideration of both economic and environmental aspects to be considered, facilitating the identification of opportunities to improve cost-effectiveness and environmental performance of the proposed IL-NPs biosystem.
Despite the challenges, ILs certainly have great potential for the fabrication of safe and potent biologically active NPs. To address the challenges faced by the described IL-NPs (bio)system, we propose the adoption of the six principles of green IL-NPs (bio)system that we have developed. Based on the presented studies, it is evident that ILs incorporated into the biologically-active NPs play a significant role in controlling the morphology of these nanoobjects. This, in turn, can lead to the production of high-efficiency NMs that are both safe and economically desirable and exhibit additional and supporting biological functions.
Fig. 19 outlines a comprehensive roadmap for the advancement of IL-NPs technology. It includes three key facets: future directions for IL-NPs synthesis, innovative applications in the biological domain, and strategic pathways for real-world implementation of IL-NPs. This graphical representation provides a holistic view and guides researchers toward a more sustainable and effective integration of ILs and NPs.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
J. F.-K., A. W., and M. P. are thankful for the financial support given by the H2020 project (grant agreement no. 953206). Open innovation test bed for developing safe nano-enabled bio-based materials and polymer bionanocomposites for multifunctional and new advanced applications “BIONANOPOLYS” (2021–2024). J. F.-K. expresses her sincere gratitude to the Eleonore Trefftz Programme in Dresden, Germany, for facilitating a long-term academic visit to TUD. K.-H. K. acknowledges the support provided by a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (MSIT) of the Korean Government (Grant No: 2021R1A3B1068304).References
- A. Stark and K. R. Seddon, Kirk-Othmer Encyclopedia of Chemical Technology, 2007, vol. 26, pp. 836–920 Search PubMed.
- A. Wirwis, J. Feder-Kubis and A. M. Trzeciak, Mol. Catal., 2018, 445, 61–72 CrossRef CAS.
- J. Pinto, E. Fabre and S. M. S. Murshed, Heat Mass Transf., 2022, 1, 1–7 Search PubMed.
- X. Zhang, H. Sun, C. Pan, X. Wu, L. Lin and S. Lou, Ceram. Int., 2023, 49, 4733–4750 CrossRef CAS.
- X. Ou, Q. Liu, F. Wei, C. Sun, Y. Liao, Y. Zhou and F. Yan, Chem. Eng. J., 2023, 451, 139037 CrossRef CAS.
- J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228–2269 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998 Search PubMed.
- F. Chemat, M. A. Vian and G. Cravotto, Int. J. Mol. Sci., 2012, 13, 8615–8627 CrossRef CAS.
- M. Hassanpour, M. H. Shahavi, G. Heidari, A. Kumar, M. Nodehi, F. D. Moghaddam, M. Mohammadi, N. Nikfarjam, E. Sharifi, P. Makvandi, H. K. Male and E. N. Zare, J. Ionic Liq., 2022, 2, 100033 CrossRef.
- R. A. Khan, H. A. Mohammed, G. M. Sulaiman, A. Al Subaiyel, A. Karuppaiah, H. Rahman, S. Makhathini, P. Ramburrun and Y. E. Choonara, Int. J. Mol. Sci., 2022, 23, 14346 CrossRef CAS PubMed.
- F. Faísca, L. Filipe, Z. Petrovski, M. M. Santos, S. Gago and L. C. Branco, Surfaces, 2021, 4, 169–190 CrossRef.
- C. Verma, E. E. Ebenso and M. A. Quraishi, J. Mol. Liq., 2019, 276, 826–849 CrossRef CAS.
- A. M. Curreri, S. Mitragotri and E. E. L. Tanner, Adv. Sci., 2021, 8, 2004819 CrossRef CAS PubMed.
- M. Faraday, Philos. Trans. R. Soc. London, 1857, 147, 145–181 CrossRef.
- G. Frens and J. T. G. Overbeek, Kolloid Z. Z. Polym., 1969, 233, 922–929 CrossRef CAS.
- J. Dupont, G. S. Fonseca, A. P. Umpierre, P. F. P. Fichtner and S. R. Teixeira, J. Am. Chem. Soc., 2002, 124, 4228–4229 CrossRef CAS.
- M. C. Lea, Am. J. Sci., 1889, s3–37, 476–491 CrossRef.
- D. Demberelnyamba, M. Ariunaa and Y. K. Shim, Int. J. Mol. Sci., 2008, 9, 864–871 CrossRef CAS PubMed.
- K. Boese, Dtsch. Z. Chir., 1921, 163, 62–84 CrossRef.
- G. Mie, Ann. Phys., 1908, 330, 377–445 CrossRef.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55 RSC.
- D. Dorjnamjin, M. Ariunaa and Y. K. Shim, Int. J. Mol. Sci., 2008, 9, 807–820 CrossRef CAS.
- R. S. Patil, M. R. Kokate, P. P. Salvi and S. S. Kolekar, C. R. Chim., 2011, 14, 1122–1127 CrossRef CAS.
- E. Iwase, Bull. Chem. Soc. Jpn., 1927, 2, 187–191 CrossRef.
- J. L. Gardea-Torresdey, E. Gomez, J. R. Peralta-Videa, J. G. Parsons, H. Troiani and M. Jose-Yacaman, Langmuir, 2003, 19, 1357–1361 CrossRef CAS.
- J. L. Gardea-Torresdey, J. G. Parsons, E. Gomez, J. Peralta-Videa, H. E. Troiani, P. Santiago and M. J. Yacaman, Nano Lett., 2002, 2, 397–401 CrossRef CAS.
- A. Safavi, S. Zeinali and M. Yazdani, Amino Acids, 2012, 43, 1323–1330 CrossRef CAS PubMed.
- S. Rostami, A. Mehdinia, A. Jabbari, E. Kowsari, R. Niroumand and T. J. Booth, Sens. Actuators, B, 2018, 271, 64–72 CrossRef CAS.
- P. Iranpour, M. Ajamian, A. Safavi, N. Iranpoor, A. Abbaspour and S. Javanmardi, J. Mater. Sci. Mater. Med., 2018, 29, 1–9 CrossRef CAS PubMed.
- A. Abbaszadegan, M. Nabavizadeh, A. Gholami, Z. S. Aleyasin, S. Dorostkar, M. Saliminasab, Y. Ghasemi, B. Hemmateenejad and H. Sharghi, Int. Endod. J., 2015, 48, 790–800 CrossRef CAS.
- N. Pandiyan, B. Murugesan, M. Arumugam, J. Sonamuthu, S. Samayanan and S. Mahalingam, J. Photochem. Photobiol., B, 2019, 198, 111559 CrossRef CAS PubMed.
- N. Pandiyan, B. Murugesan, M. Arumugam, J. Sonamuthu, S. Samayanan and S. Mahalingam, J. Mol. Liq., 2020, 312, 113245 CrossRef CAS.
- S. Ambika and M. Sundrarajan, J. Mol. Liq., 2016, 221, 986–992 CrossRef.
- J. Huang and Y. Luo, Water Sci. Technol., 2021, 84, 1930–1942 CrossRef CAS.
- X. Li, M. Liu, H. Cheng, Q. Wang, C. Miao, S. Ju and F. Liu, J. Photochem. Photobiol., B, 2018, 183, 385–390 CrossRef CAS PubMed.
- M. K. Banjare, K. Behera, R. K. Banjare, R. Sahu, S. Sharma, S. Pandey, M. L. Satnami and K. K. Ghosh, ACS Sustainable Chem. Eng., 2019, 7, 11088–11100 CrossRef CAS.
- P. Nithya and M. Sundrarajan, J. Photochem. Photobiol., B, 2020, 202, 111706 CrossRef CAS PubMed.
- N. Pandiyan, B. Murugesan, M. Arumugam, D. Chinnaalagu, S. Samayanan and S. Mahalingam, Adv. Powder Technol., 2021, 32, 2213–2225 CrossRef CAS.
- V. Muthulakshmi, C. Dhilip Kumar and M. Sundrarajan, Anal. Biochem., 2022, 638, 114482 CrossRef CAS PubMed.
- E. Avirdi, H. K. Paumo, B. P. Kamdem, M. B. Singh, K. Kumari, L. M. Katata-Seru and I. Bahadur, J. Mol. Liq., 2023, 369, 120935 CrossRef CAS.
- S. Wegner and C. Janiak, Top. Curr. Chem., 2017, 375, 65 CrossRef PubMed.
- S. Beil, M. Markiewicz, C. S. Pereira, P. Stepnowski, J. Thöming and S. Stolte, Chem. Rev., 2021, 121, 13132–13173 CrossRef CAS PubMed.
- S. P. F. Costa, A. M. O. Azevedo, P. C. A. G. Pinto and M. L. M. F. S. Saraiva, ChemSusChem, 2017, 10, 2321–2347 CrossRef CAS PubMed.
- J. Feder-Kubis, A. Wnętrzak and A. Chachaj-Brekiesz, ACS Biomater. Sci. Eng., 2020, 6, 3832–3842 CrossRef CAS PubMed.
- M. Niemczak, Ł. Sobiech and M. Grzanka, J. Agric. Food Chem., 2020, 68, 13661–13671 CrossRef CAS.
- A. Aditya, S. Chattopadhyay, D. Jha, H. K. Gautam, S. Maiti and M. Ganguli, ACS Appl. Mater. Interfaces, 2018, 10, 15401–15411 CrossRef CAS.
- F. Farjadian, A. R. Akbarizadeh and L. Tayebi, Heliyon, 2020, 6, e04747 CrossRef PubMed.
- V. Patil, S. Mahajan, M. Kulkarni, K. Patil, C. Rode, A. Coronas and G. R. Yi, Chemosphere, 2020, 243, 125302 CrossRef CAS PubMed.
- L. T. Silveira, A. M. A. Liberatore, I. H. J. Koh, M. A. Bizeto and F. F. Camilo, J. Nanopart. Res., 2015, 17, 1–10 CrossRef CAS.
- Y. Zhang, Y. Shen, J. Yuan, D. Han, Z. Wang, Q. Zhang and L. Niu, Angew. Chem., Int. Ed., 2006, 45, 5867–5870 CrossRef CAS PubMed.
- I. Iavicoli, V. Leso, W. Ricciardi, L. L. Hodson and M. D. Hoover, Environ. Health, 2014, 13, 78 CrossRef.
- L. Pokrajac, A. Abbas, W. Chrzanowski, G. M. Dias, B. J. Eggleton, S. Maguire, E. Maine, T. Malloy, J. Nathwani, L. Nazar, A. Sips, J. Sone, A. van den Berg, P. S. Weiss and S. Mitra, ACS Nano, 2021, 15, 18608–18623 CrossRef CAS PubMed.
- M. Finkbeiner, A. Inaba, R. Tan, K. Christiansen and H.-J. Klüppel, Int. J. Life Cycle Assess., 2006, 11, 80–85 CrossRef.
- G. Thomassen, M. Van Dael, S. Van Passel and F. You, Green Chem., 2019, 21, 4868–4886 RSC.
- C. J. Wrasman, C. Zhou, A. Aitbekova, E. D. Goodman and M. Cargnello, J. Am. Chem. Soc., 2022, 144, 11646–11655 CrossRef CAS PubMed.
- L. R. Karadaghi, N. Malmstadt, K. M. Van Allsburg and R. L. Brutchey, ACS Sustainable Chem. Eng., 2021, 9, 246–253 CrossRef CAS.
- L. R. Karadaghi, B. Pan, F. G. Baddour, N. Malmstadt and R. L. Brutchey, RSC Sustainability, 2023, 1, 1861–1873 RSC.
- J. E. Hutchison, ACS Sustainable Chem. Eng., 2016, 4, 5907–5914 CrossRef CAS.
- H. Duan, D. Wang and Y. Li, Chem. Soc. Rev., 2015, 44, 5778–5792 RSC.
- J. A. Gerbec, D. Magana, A. Washington and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 15791–15800 CrossRef CAS PubMed.
- G. Glaspell, L. Fuoco and M. S. El-Shall, J. Phys. Chem. B, 2005, 109, 17350–17355 CrossRef CAS PubMed.
- J. Park, K. An, Y. Hwang, J.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891–895 CrossRef CAS.
- P. Rowe, Design Thinking, MIT Press, 1987 Search PubMed.
- T. J. Collins, J. Chem. Educ., 1995, 72, 965–966 CrossRef CAS.
- P. P. Gan and S. F. Y. Li, Rev. Environ. Sci. Biotechnol., 2012, 11, 169–206 CrossRef CAS.
- M. Deetlefs and K. R. Seddon, Green Chem., 2010, 12, 17–30 RSC.
- A. A. Quintana, A. M. Sztapka, V. de C. Santos Ebinuma and C. Agatemor, Angew. Chem., Int. Ed., 2022, 61, e202205609 CrossRef CAS PubMed.
- J. Feder-Kubis, J. Mol. Liq., 2017, 226, 63–70 CrossRef CAS.
- M. Niemczak, Ł. Chrzanowski, T. Praczyk and J. Pernak, New J. Chem., 2017, 41, 8066–8077 RSC.
- A. T. Silva, I. S. Oliveira, J. Gomes, L. Aguiar, D. Fontinha, D. Duarte, F. Nogueira, M. Prudêncio, E. F. Marques, C. Teixeira, R. Ferraz and P. Gomes, ChemMedChem, 2022, 17, e202100650 CrossRef CAS.
- J. Łuczak, M. Paszkiewicz, A. Krukowska, A. Malankowska and A. Zaleska-Medynska, Adv. Colloid Interface Sci., 2016, 230, 13–28 CrossRef.
- H. S. Schrekker, M. A. Gelesky, M. P. Stracke, C. M. L. Schrekker, G. Machado, S. R. Teixeira, J. C. Rubim and J. Dupont, J. Colloid Interface Sci., 2007, 316, 189–195 CrossRef CAS PubMed.
- M. H. G. Prechtl, J. D. Scholten and J. Dupont, J. Mol. Catal. A: Chem., 2009, 313, 74–78 CrossRef CAS.
- R. Venkatesan, M. H. G. Prechtl, J. D. Scholten, R. P. Pezzi, G. MacHado and J. Dupont, J. Mater. Chem., 2011, 21, 3030–3036 RSC.
- M. Mokhtary, in Progress and Developments in Ionic Liquids, IntechOpen, 2017 Search PubMed.
- K. Richter, P. S. Campbell, T. Baecker, A. Schimitzek, D. Yaprak and A. V. Mudring, Phys. Status Solidi B, 2013, 250, 1152–1164 CrossRef CAS.
- E. Husanu, C. Chiappe, A. Bernardini, V. Cappello and M. Gemmi, Colloids Surf., A, 2018, 538, 506–512 CrossRef CAS.
- A. Tzani, S. Koutsoukos, D. Koukouzelis and A. Detsi, J. Mol. Liq., 2017, 243, 212–218 CrossRef CAS.
- S. Rabieh and M. Bagheri, Mater. Lett., 2014, 122, 190–192 CrossRef CAS.
- L. Tong, Y. Liu, H. Rong and L. Gong, Mater. Lett., 2013, 112, 5–7 CrossRef CAS.
- J. Jiang, S.-H. Yu, W.-T. Yao, H. Ge and G.-Z. Zhang, Chem. Mater., 2005, 17, 6094–6100 CrossRef CAS.
- S. W. Cao and Y. J. Zhu, Acta Mater., 2009, 57, 2154–2165 CrossRef CAS.
- G. S. Fonseca, G. Machado, S. R. Teixeira, G. H. Fecher, J. Morais, M. C. M. Alves and J. Dupont, J. Colloid Interface Sci., 2006, 301, 193–204 CrossRef CAS PubMed.
- M. H. G. Prechtl, M. Scariot, J. D. Scholten, G. Machado, S. R. Teixeira and J. Dupont, Inorg. Chem., 2008, 47, 8995–9001 CrossRef CAS PubMed.
- C. W. Scheeren, G. Machado, J. Dupont, P. F. P. Fichtner and S. R. Texeira, Inorg. Chem., 2003, 42, 4738–4742 CrossRef CAS PubMed.
- C. W. Scheeren, G. Machado, S. R. Teixeira, J. Morais, J. B. Domingos and J. Dupont, J. Phys. Chem. B, 2006, 110, 13011–13020 CrossRef CAS.
- M. Zhao, L. Zheng, X. Bai, N. Li and L. Yu, Colloids Surf., A, 2009, 346, 229–236 CrossRef CAS.
- J. Xia, H. Li, Z. Luo, H. Shi, K. Wang, H. Shu and Y. Yan, J. Phys. Chem. Solids, 2009, 70, 1461–1464 CrossRef CAS.
- M. Iida, C. Baba, M. Inoue, H. Yoshida, E. Taguchi and H. Furusho, Chem. – Eur. J., 2008, 14, 5047–5056 CrossRef CAS PubMed.
- H. T. Phan and A. J. Haes, J. Phys. Chem. C, 2019, 123, 16495–16507 CrossRef CAS PubMed.
- L. Xu, H. W. Liang, Y. Yang and S. H. Yu, Chem. Rev., 2018, 118, 3209–3250 CrossRef CAS PubMed.
- E. Joseph and G. Singhvi, in Nanomaterials for Drug Delivery and Therapy, William Andrew Publishing, 2019, pp. 91–116 Search PubMed.
- C. Janiak, Z. Naturforsch., B: J. Chem. Sci., 2013, 68, 1059–1089 CrossRef CAS.
- Z. He and P. Alexandridis, Phys. Chem. Chem. Phys., 2015, 17, 18238–18261 RSC.
- J. Łuczak, M. Paszkiewicz, A. Krukowska, A. Malankowska and A. Zaleska-Medynska, Adv. Colloid Interface Sci., 2016, 227, 1–52 CrossRef.
- T. S. De Almeida, A. Júlio, J. P. Mota, P. Rijo and C. P. Reis, Ther. Delivery, 2017, 8, 461–473 CrossRef PubMed.
- O. S. Hammond and A.-V. Mudring, Chem. Commun., 2022, 58, 3865–3892 RSC.
- M. Petkovic, K. R. Seddon, L. P. N. Rebelo and C. Silva Pereira, Chem. Soc. Rev., 2011, 40, 1383–1403 RSC.
- M. G. Montalbán, G. Carissimi, A. A. Lozano-Pérez, J. L. Cenis, J. M. Coburn, D. L. Kaplan and G. Víllora, in Recent Advances in Ionic Liquids, IntechOpen, 2018 Search PubMed.
- L. M. Gilbertson, J. B. Zimmerman, D. L. Plata, J. E. Hutchison and P. T. Anastas, Chem. Soc. Rev., 2015, 44, 5758–5777 RSC.
- C. Vollmer, E. Redel, K. Abu-Shandi, R. Thomann, H. Manyar, C. Hardacre and C. Janiak, Chem. – Eur. J., 2010, 16, 3849–3858 CrossRef CAS PubMed.
- H. Jang, J. R. Lee, S. J. Kim, H. Jeong, S. Jung, J.-H. Lee, J.-C. Park and T.-W. Kim, J. Colloid Interface Sci., 2021, 599, 828–836 CrossRef CAS PubMed.
- A. A. Lozano-Pérez, M. G. Montalbán, S. D. Aznar-Cervantes, F. Cragnolini, J. L. Cenis and G. Víllora, J. Appl. Polym. Sci., 2014, 132, 41702 CrossRef.
- K. E. Gutowski, G. A. Broker, H. D. Willauer, J. G. Huddleston, R. P. Swatloski, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2003, 125, 6632–6633 CrossRef CAS PubMed.
- F. C. C. Oliveira, F. B. Effenberger, M. H. Sousa, R. F. Jardim, P. K. Kiyohara, J. Dupont, J. C. Rubim and L. M. Rossi, Phys. Chem. Chem. Phys., 2011, 13, 13558 RSC.
- K. Manojkumar, A. Sivaramakrishna and K. Vijayakrishna, J. Nanopart. Res., 2016, 18, 103 CrossRef.
- M. Anouti and J. Jacquemin, Colloids Surf., A, 2014, 445, 1–11 CrossRef CAS.
- M. Anouti, A. Mirghani, J. Jacquemin, L. Timperman and H. Galiano, Ionics, 2013, 19, 1783–1790 CrossRef CAS.
- R. Freund, U. Lächelt, T. Gruber, B. Rühle and S. Wuttke, ACS Nano, 2018, 12, 2094–2105 CrossRef CAS PubMed.
- K. Kuroda, New J. Chem., 2022, 46, 20047–20052 RSC.
- T. Kruk, K. Szczepanowicz, J. Stefańska, R. P. Socha and P. Warszyński, Colloids Surf., B, 2015, 128, 17–22 CrossRef CAS PubMed.
- M. Kasithevar, M. Saravanan, P. Prakash, H. Kumar, M. Ovais, H. Barabadi and Z. K. Shinwari, J. Interdiscip. Nanomed., 2017, 2, 131–141 CrossRef CAS.
- D. Hu, H. Li, B. Wang, Z. Ye, W. Lei, F. Jia, Q. Jin, K. F. Ren and J. Ji, ACS Nano, 2017, 11, 9330–9339 CrossRef CAS PubMed.
- E. Sánchez-López, D. Gomes, G. Esteruelas, L. Bonilla, A. L. Lopez-Machado, R. Galindo, A. Cano, M. Espina, M. Ettcheto, A. Camins, A. M. Silva, A. Durazzo, A. Santini, M. L. Garcia and E. B. Souto, Nanomaterials, 2020, 10, 1–39 Search PubMed.
- S. Shahzadi, N. Zafar and R. Sharif, in Bacterial Pathogenesis and Antibacterial Control, IntechOpen, 2018, ch. 3 Search PubMed.
- R. Singh and D. Singh, Int. Wound J., 2014, 11, 264–268 CrossRef PubMed.
- R. Augustine, A. Augustine, N. Kalarikkal and S. Thomas, Prog. Biomater., 2016, 5, 223–235 CrossRef CAS PubMed.
- M. Binandeh, S. Rostamnia and F. Karimi, Biochem. Biophys. Rep., 2021, 28, 101159 CAS.
- D. Lopez-Lima, A. I. Mtz-Enriquez, G. Carrión, S. Basurto-Cereceda and N. Pariona, Sci. Hortic., 2021, 277, 109810 CrossRef CAS.
- M. Zhang, K. Lian, L. Ai, W. Kang and T. Zhao, RSC Adv., 2020, 10, 37473–37481 RSC.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS PubMed.
- E. Z. Gomaa, J. Gen. Appl. Microbiol., 2017, 63, 36–43 CrossRef CAS PubMed.
- I. A. Mamonova, I. V. Babushkina, I. A. Norkin, E. V. Gladkova, M. D. Matasov and D. M. Puchin'yan, Nanotechnol. Russ., 2015, 10, 128–134 CrossRef CAS.
- R. Martínez, M. F. Navarro Poupard, A. Álvarez, E. Soprano, M. Migliavacca, C. Carrillo-Carrión, E. Polo, B. Pelaz and P. Del Pino, in Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications, 2019, pp. 5–18 Search PubMed.
- R. Foulkes, E. Man, J. Thind, S. Yeung, A. Joy and C. Hoskins, Biomater. Sci., 2020, 8, 4653–4664 RSC.
- Y. Lu, J. Qi and W. Wu, Pharm. Res., 2022, 39, 2329–2334 CrossRef CAS PubMed.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Chem. Rev., 2017, 117, 7132–7189 CrossRef CAS PubMed.
- C. M. Hamadani, M. J. Goetz, S. Mitragotri and E. E. L. Tanner, Sci. Adv., 2020, 6, eabd7563 CrossRef CAS PubMed.
- J. Wegener, Measuring Biological Impacts of Nanomaterials, Springer International Publishing, Cham, 2016, vol. 5 Search PubMed.
- U. Nishan, F. Bashir, N. Muhammad, N. Khan, A. Rahim, M. Shah, R. Nazir and M. Sayed, J. Mol. Liq., 2019, 294, 111681 CrossRef.
- V. Soni, P. Raizada, P. Singh, H. N. Cuong, R. S, A. Saini, R. V. Saini, Q. Van Le, A. K. Nadda, T.-T. Le and V.-H. Nguyen, Environ. Res., 2021, 202, 111622 CrossRef CAS PubMed.
- U. Nishan, U. Sabba, A. Rahim, M. Asad, M. Shah, A. Iqbal, J. Iqbal and N. Muhammad, Mater. Chem. Phys., 2021, 262, 124289 CrossRef CAS.
- N. Pandiyan, B. Murugesan, J. Sonamuthu, S. Samayanan and S. Mahalingam, Ceram. Int., 2019, 45, 12138–12148 CrossRef CAS.
- N. Pandiyan, B. Murugesan, J. Sonamuthu, S. Samayanan and S. Mahalingam, J. Photochem. Photobiol., B, 2018, 178, 481–488 CrossRef CAS PubMed.
- H. Li, X. Yue, N. Gao, J. Tang, X. Lv and J. Hou, Anal. Bioanal. Chem., 2020, 412, 3063–3071 CrossRef CAS PubMed.
- S. Kumar, A. Sindhu and P. Venkatesu, ACS Appl. Nano Mater., 2021, 4, 3185–3196 CrossRef CAS.
- B. L. Gadilohar and G. S. Shankarling, J. Mol. Liq., 2017, 227, 234–261 CrossRef CAS.
- Q. Jiang, S. Yu, X. Li, C. Ma and A. Li, J. Photochem. Photobiol., B, 2018, 178, 367–370 CrossRef CAS.
- J. M. M. Araújo, C. Florindo, A. B. Pereiro, N. S. M. Vieira, A. A. Matias, C. M. M. Duarte, L. P. N. Rebelo and I. M. Marrucho, RSC Adv., 2014, 4, 28126–28132 RSC.
- J. Pernak and J. Feder-Kubis, Tetrahedron: Asymmetry, 2006, 17, 1728–1737 CrossRef CAS.
- W. Stachowiak, D. K. Kaczmarek, T. Rzemieniecki and M. Niemczak, J. Agric. Food Chem., 2022, 70, 8222–8232 CrossRef CAS PubMed.
- P. Cintas, Ultrason. Sonochem., 2016, 28, 257–258 CrossRef CAS PubMed.
- N. Kaur, A. Singh and W. Ahmad, J. Inorg. Organomet. Polym. Mater., 2023, 33, 663–672 CrossRef CAS.
- O. V. Kharissova, B. I. Kharisov, C. M. Oliva González, Y. P. Méndez and I. López, R. Soc. Open Sci., 2019, 6, 191378 CrossRef PubMed.
- K. F. Lei and Y. K. C. Butt, Microfluid. Nanofluid., 2010, 8, 131–137 CrossRef CAS.
- A. Yahia-Ammar, D. Sierra, F. Mérola, N. Hildebrandt and X. Le Guével, ACS Nano, 2016, 10, 2591–2599 CrossRef CAS PubMed.
- J. Song, J. Zhou and H. Duan, J. Am. Chem. Soc., 2012, 134, 13458–13469 CrossRef CAS PubMed.
- I. H. El-Sayed, X. Huang and M. A. El-Sayed, Nano Lett., 2005, 5, 829–834 CrossRef CAS PubMed.
- M. Irfan, T. Ahmad, M. Moniruzzaman, S. Bhattacharjee and B. Abdullah, Arabian J. Chem., 2020, 13, 75–85 CrossRef CAS.
- S. Sahu, Reshma, S. Sharma, I. Karbhal and K. K. Ghosh, RSC Adv., 2020, 10, 31400–31410 RSC.
- A. C. Burduşel, O. Gherasim, A. M. Grumezescu, L. Mogoantă, A. Ficai and E. Andronescu, Nanomaterials, 2018, 8, 1–25 CrossRef PubMed.
- A. Abbaszadegan, A. Gholami, S. Abbaszadegan, Z. S. Aleyasin, Y. Ghahramani, S. Dorostkar, B. Hemmateenejad, Y. Ghasemi and H. Sharghi, Iran. Endod. J., 2017, 12, 481–487 CAS.
- Y. Ghahramani, F. Yaghoobi, R. Motamedi, A. Jamshidzadeh and A. Abbaszadegan, Iran. Endod. J., 2018, 13, 559–564 CAS.
- A. Adl, A. Abbaszadegan, A. Gholami, F. Parvizi and Y. Ghahramani, Iran. Endod. J., 2019, 14, 122–125 Search PubMed.
- A. Gholami, M. S. Shams, A. Abbaszadegan and M. Nabavizadeh, Green Process. Synth., 2021, 10, 585–593 CrossRef CAS.
- T. Kienast and A. Heinz, CNS Neurol. Disord. Drug Targets, 2008, 5, 109–131 CrossRef PubMed.
- U. Nishan, A. Niaz, N. Muhammad, M. Asad, A.-ul.-H. A. Shah, N. Khan, M. Khan, S. Shujah and A. Rahim, Arabian J. Chem., 2021, 14, 103164 CrossRef CAS.
- Z. Sadowski and A. Pawlowska, Nanotechnology Applied To Pharmaceutical Technology, 2017, pp. 91–111 Search PubMed.
- V. O. Fasiku, S. John Owonubi, N. M. Malima, D. Hassan and N. Revaprasadu, Metal Oxide Nanoparticles: A Welcome Development for Targeting Bacteria, Elsevier Inc., 2020 Search PubMed.
- M. P. Nikolova and M. S. Chavali, Biomimetics, 2020, 5, 27 CrossRef CAS.
- M. Hong, Z. Miao, X. Xu and Q. Zhang, ACS Appl. Bio Mater., 2020, 3, 3664–3672 CrossRef CAS PubMed.
- C. Inbasekar and N. N. Fathima, Int. J. Biol. Macromol., 2020, 147, 24–28 CrossRef CAS.
- Y. Zhang, T. Nayak, H. Hong and W. Cai, Curr. Mol. Med., 2013, 13, 1633–1645 CrossRef CAS PubMed.
- Y. Wang, S. Song, J. Liu, D. Liu and H. Zhang, Angew. Chem., 2015, 127, 546–550 CrossRef.
- K. Rajathi and A. Rajendran, Indo Am. J. Pharm. Sci., 2017, 4, 4699–4704 CAS.
- K. Kołecka, M. Gajewska, P. Stepnowski and M. Caban, Sci. Total Environ., 2019, 647, 149–157 CrossRef PubMed.
- S. Rauf, M. A. H. Nawaz, N. Muhammad, R. Raza, S. A. Shahid, J. L. Marty and A. Hayat, J. Mol. Liq., 2017, 243, 333–340 CrossRef CAS.
- F. Zarif, S. Rauf, M. Z. Qureshi, N. S. Shah, A. Hayat, N. Muhammad, A. Rahim, M. H. Nawaz and M. Nasir, Microchim. Acta, 2018, 185, 1–9 CrossRef CAS PubMed.
- N. Basavegowda and K.-H. Baek, Molecules, 2021, 26, 912 CrossRef CAS PubMed.
- P. Srinoi, Y. T. Chen, V. Vittur, M. D. Marquez and T. R. Lee, Appl. Sci., 2018, 8, 1106 CrossRef.
- M. A. Kazakova, D. M. Morales, C. Andronescu, K. Elumeeva, A. G. Selyutin, A. V. Ishchenko, G. V. Golubtsov, S. Dieckhöfer, W. Schuhmann and J. Masa, Catal. Today, 2020, 357, 259–268 CrossRef CAS.
- C. Kan, W. Cai, C. Li, L. Zhang and H. Hofmeister, J. Phys. D: Appl. Phys., 2003, 36, 1609 CrossRef CAS.
- M. Khawaji and D. Chadwick, Catal. Sci. Technol., 2018, 8, 2529–2539 RSC.
- V. Mazumder, M. Chi, K. L. More and S. Sun, Angew. Chem., Int. Ed., 2010, 49, 9368–9372 CrossRef CAS PubMed.
- S. Kumar, I. Jha, N. K. Mogha and P. Venkatesu, Appl. Surf. Sci., 2020, 512, 145573 CrossRef CAS.
- L. Gontrani, E. Scarpellini, R. Caminiti and M. Campetella, RSC Adv., 2017, 7, 19338–19344 RSC.
- Y. Fukaya, Y. Iizuka, K. Sekikawa and H. Ohno, Green Chem., 2007, 9, 1155–1157 RSC.
- M. Sharma, D. Mondal, C. Mukesh and K. Prasad, RSC Adv., 2014, 4, 42197–42201 RSC.
- Q. P. Liu, X. D. Hou, N. Li and M. H. Zong, Green Chem., 2012, 14, 304–307 RSC.
- J. Pernak, M. Niemczak, Ł. Chrzanowski, Ł. Ławniczak, P. Fochtman, K. Marcinkowska and T. Praczyk, Chem. – Eur. J., 2016, 22, 12012–12021 CrossRef CAS PubMed.
- K. Häckl, A. Mühlbauer, J. F. Ontiveros, S. Marinkovic, B. Estrine, W. Kunz and V. Nardello-Rataj, J. Colloid Interface Sci., 2018, 511, 165–173 CrossRef PubMed.
- J. C. Plaquevent, J. Levillain, F. Guillen, C. Malhiac and A. C. Gaumont, Chem. Rev., 2008, 108, 5035–5060 CrossRef CAS PubMed.
- A. Jordan, A. Haiß, M. Spulak, Y. Karpichev, K. Kümmerer and N. Gathergood, Green Chem., 2016, 18, 4374–4392 RSC.
- P. Garg and S. R. Reddy, Asian J. Org. Chem., 2022, 11, e202200322 CrossRef CAS.
- P. Muthukuru, K. P., J. Rayadurgam and S. Rajasekhara Reddy, New J. Chem., 2021, 45, 20075–20090 RSC.
- J. Feder-Kubis and K. Tomczuk, Tetrahedron, 2013, 69, 4190–4198 CrossRef CAS.
- J. Suchodolski, J. Feder-Kubis and A. Krasowska, Int. J. Mol. Sci., 2021, 22, 7543 CrossRef CAS PubMed.
- J. Feder-Kubis, J. Zabielska-Matejuk, A. Stangierska, P. Przybylski, J. Jacquemin and M. Geppert-Rybczyńska, ACS Sustainable Chem. Eng., 2019, 7, 15628–15639 CrossRef CAS.
- J. Feder-Kubis, P. Czerwoniec, P. Lewandowski, H. Pospieszny and M. Smiglak, ACS Sustainable Chem. Eng., 2020, 8, 842–852 CrossRef CAS.
- M. Markiewicz, P. Lewandowski, M. Spychalski, R. Kukawka, J. Feder-Kubis, S. Beil, M. Smiglak and S. Stolte, Green Chem., 2021, 23, 5138–5149 RSC.
- J. Feder-Kubis and J. Bryjak, Acta Biochim. Pol., 2013, 60, 741–745 CrossRef PubMed.
- K. Kawai, K. Kaneko, H. Kawakami and T. Yonezawa, Langmuir, 2011, 27, 9671–9675 CrossRef CAS PubMed.
- Z. Zhang, R. Zhao, S. Wang and J. Meng, Front. Bioeng. Biotechnol., 2023, 11, 1117944 CrossRef PubMed.
- J. A. Baranska and Z. Sadowski, Physicochem. Probl. Miner. Process., 2013, 49, 71–79 CAS.
- R. L. Kimber, E. A. Lewis, F. Parmeggiani, K. Smith, H. Bagshaw, T. Starborg, N. Joshi, A. I. Figueroa, G. van der Laan, G. Cibin, D. Gianolio, S. J. Haigh, R. A. D. Pattrick, N. J. Turner and J. R. Lloyd, Small, 2018, 14, 1703145 CrossRef PubMed.
- M. Saravanan, S. K. Barik, D. MubarakAli, P. Prakash and A. Pugazhendhi, Microb. Pathog., 2018, 116, 221–226 CrossRef CAS PubMed.
- I. Maliszewska and Z. Sadowski, J. Phys.: Conf. Ser., 2009, 146, 012024 CrossRef.
- P. Mukherjee, S. Senapati, D. Mandal, A. Ahmad, M. I. Khan, R. Kumar and M. Sastry, ChemBioChem, 2002, 3, 461–463 CrossRef CAS PubMed.
- K. AbdelRahim, S. Y. Mahmoud, A. M. Ali, K. S. Almaary, A. E. Z. M. A. Mustafa and S. M. Husseiny, Saudi J. Biol. Sci., 2017, 24, 208–216 CrossRef CAS PubMed.
- S. S. Ahiwale, A. V. Bankar, S. Tagunde and B. P. Kapadnis, Indian J. Microbiol., 2017, 57, 188–194 CrossRef CAS PubMed.
- K. S. Uma Suganya, K. Govindaraju, V. Ganesh Kumar, T. Stalin Dhas, V. Karthick, G. Singaravelu and M. Elanchezhiyan, Mater. Sci. Eng., C, 2015, 47, 351–356 CrossRef CAS PubMed.
- A. Moshfegh, A. Jalali, A. Salehzadeh and A. S. Jozani, Micro Nano Lett., 2019, 14, 581–584 CrossRef CAS.
- C. Arib, J. Spadavecchia and M. L. de la Chapelle, Sci. Rep., 2021, 11, 1–8 CrossRef PubMed.
- J. M. Palomo, Chem. Commun., 2019, 55, 9583–9589 RSC.
- O. V. Kharissova, H. V. R. Dias, B. I. Kharisov, B. O. Pérez and V. M. J. Pérez, Trends Biotechnol., 2013, 31, 240–248 CrossRef CAS PubMed.
- J. R. Peralta-Videa, Y. Huang, J. G. Parsons, L. Zhao, L. Lopez-Moreno, J. A. Hernandez-Viezcas and J. L. Gardea-Torresdey, Nanotechnol. Environ. Eng., 2016, 1, 4 CrossRef.
- H. Ullah, C. D. Wilfred and M. S. Shaharun, Environ. Technol., 2018, 40, 3705–3712 CrossRef PubMed.
- V. Muthulakshmi, P. Kumar and M. Sundrarajan, J. Environ. Chem. Eng., 2021, 9, 105270 CrossRef CAS.
- M. Veerasingam, B. Murugesan and S. Mahalingam, J. Rare Earths, 2020, 38, 281–291 CrossRef CAS.
- V. Muthulakshmi, M. Balaji and M. Sundrarajan, Mater. Chem. Phys., 2020, 242, 122483 CrossRef CAS.
- M. Sundrarajan and V. Muthulakshmi, J. Environ. Chem. Eng., 2021, 9, 104716 CrossRef CAS.
- V. Muthulakshmi, C. Dhilip Kumar and M. Sundrarajan, J. Biomater. Sci., Polym. Ed., 2022, 33, 1063–1082 CrossRef CAS PubMed.
- K. Pal, S. Chakroborty and N. Nath, Green Process. Synth., 2022, 11, 951–964 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc04387h |
| This journal is © The Royal Society of Chemistry 2024 |