Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
E-Dakin reaction: oxidation of hydroxybenzaldehydes to phenols with electrochemically generated peroxodicarbonate as sustainable ex-cell oxidizer†
Fiona
Sprang
a,
Niclas
Schupp
a,
Philipp J.
Kohlpaintner
a,
Lukas J.
Gooßen
b and
Siegfried R.
Waldvogel
 *acd
*acd
aJohannes Gutenberg-University Mainz, Department of Chemistry, Duesbergweg 10-14 55128 Mainz, Germany. E-mail: siegfried.waldvogel@cec.mpg.de
bEvonik Chair of Organic Chemistry, Ruhr-University Bochum Universitätsstraße 150, 44901 Bochum, Germany
cInstitute of Biological and Chemical, Systems – Functional Molecular Systems (IBCS-FMS), Kaiserstraße 12, 76131 Karlsruhe, Germany
dMax Planck Institute for Chemical Energy Conversion, Stiftstrasse 34-36, 45470 Mülheim an der Ruhr, Germany
First published on 8th February 2024
Abstract
Electrochemically generated peroxodicarbonate solution efficiently oxidizes hydroxybenzaldehydes to valuable phenols. The reaction is carried out in aqueous media without additional solvents or additives. The reaction gives high yields of up to 97% and tolerates a broad variety of substituents, as demonstrated by 20 diverse examples. It was successfully scaled to multi-gram range. This transformation further expands the synthetic applicability of the green oxidizer peroxodicarbonate, which can be generated on demand from ecologically safe carbonate solutions and has a high oxidation potential at mildly basic conditions.
Introduction
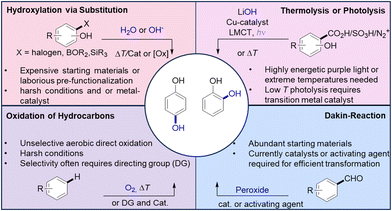
Phenols represent building blocks with fundamental importance to industry and prominently feature in pharmaceutical active ingredients and natural products.1–5 Within this heterogeneous class of molecules, the dihydric members catechols and hydroquinones represent in particular valuable moieties due to their broad fields of applications (Scheme 1).6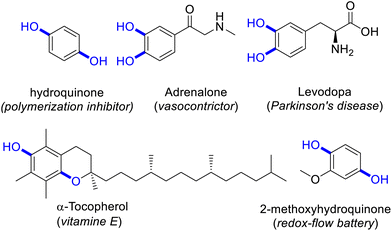 | ||
| Scheme 1 Selection of catechol and hydroquinone containing compounds with relevance in pharmaceutical and technical industry. | ||
Hydroquinones exhibit remarkable redox features.7 They can be reversibly oxidized into their corresponding quinone congener and subsequently be reduced again without harming their structural integrity.8 The reversible redox properties open up a plethora of applications, including energy storage concepts. Successful use of all organic hydroquinone–quinone redox flow batteries has been reported.9 Moreover, 2-methoxyhydroquinone was investigated as a simple lignin-derived electrolyte for sustainable future energy storage units.10 Other examples of the application as redox-active molecule include the use as an inhibitor of polymerization and oxidation within the food and cosmetic industry.11 Hydroquinone is used as a reducing agent in photography due to its water soluble nature.11
Aside from that, hydroxyphenols are useful monomers for the synthesis of complex molecules.3 Catechol for example is used as a starting material for the synthesis of the vasoconstrictor adrenalone while 2,3,5-trimethylhydroquinone is a precursor for the synthesis of α-tocopherol (vitamin E).6,12
Due to their tremendous importance numerous strategies for the synthesis of phenols and hydroxyphenols have emerged (Scheme 2).
The aerobic C–H oxidation of non-functionalized fossil hydrocarbons arguably is the most desirable entry to phenols but suffers from a lack of selectivity. Various directing groups have been developed that enable regiospecific interaction with transition metal catalysts.13–17 Still, most synthetic entries are based on functional group interconversions. Common substrates are haloarenes,18–21 aryl boronic acids,22–24 and silylarenes.25,26 In most cases, these starting materials are of non-natural origin and need to be synthesized via additional pre-functionalization steps, increasing their production cost and ecological footprints. One-pot transformations combining the extrusion of a volatile leaving group with hydroxylation steps allow the synthesis of phenols from benzoic acids, sulfonic acids, or diazonium salts.6,27,28 The activating energy barrier is either overcome via photolysis or thermal energy.29 The previously mentioned strategies all contradict one or more of the principles of green chemistry.30 Some are limited by the problematic use of extreme reaction conditions and others by the dependence of scarce resources, the production of excessive waste in pre-functionalization steps, the use of expensive catalysts, or of ecologically questionable reagents or solvents.
An advantageous synthetic entry to phenols was already reported in the early 20th century by Dakin,31,32 who demonstrated that hydroxybenzaldehydes can be converted to hydroxyphenols by treatment with hydrogen peroxide in alkaline media. However, the initial protocol calls for harsh conditions, i.e., the use of excess of hydrogen peroxide at elevated temperatures, and in many cases, only modest yields were achieved.31 This approach has gained renewed interest in the context of the ongoing change from fossil to renewable resources in chemical value creation, since hydroxybenzaldehydes are abundant in biogenic feedstocks.33–36 The wide availability of these starting materials has prompted the development of numerous improved variants of Dakin's original method. All these variations have in common that they require activation agents, e.g., boric acid, and sometimes also catalysts to ensure sufficient reactivity. Some of them require problematic or inaccessible solvents and are questionable in terms of reproducibility.6,37–44
Our goal was to develop a particularly sustainable reaction variant that involves an electrogenerated oxidizer and proceeds without organic solvents and additives.
The tremendous potential of electrochemistry in the context of green chemistry has meanwhile been widely recognized.45,46 Important aspects are the replacement of stoichiometric reagents by continuously administered electric current and the resulting process safety.47–49 This has paved the way to a renaissance of electrosynthesis.50,51 Recently, we reported an electrosynthetic method to transform 4-hydroxybenzaldehydes into para-benzoquinones via direct anodic oxidation.52 Although hydroquinones are formed as intermediates in this process, a galvanostatic electrolysis will always proceed all the way to benzoquinones. After all, the hydroquinone oxidation has a lower oxidation potential than the first step. We, thus, sought alternative pathways to gain access to valuable hydroxyphenols based on electrochemical methods. Recently, our labs established a novel protocol for efficient generation of peroxodicarbonate (PODIC) by flow electrolysis of aqueous carbonate.53 This approach provides the highest peroxodicarbonate concentration so far. It is noteworthy that peroxodicarbonate is distinctly different from percarbonate in its structure and reactivity and is only accessible electrochemically.54 PODIC can serve as platform oxidizer originating from alternative anodic reaction for water splitting. The use of environmentally benign alkali carbonates is a tremendous advantage over the well-established periodates.55–61 Besides the advantage that PODIC is made from inexpensive and safe to store carbonate salts it does not generate toxic or problematic waste. Moreover, it is also a powerful reagent for the synthesis of various important products. Previous investigations demonstrated its unique features for the epoxidation of double bonds, the hydroxylation of boronic acids, N-oxidation and valorisation of lignin.53,62–64
Here, PODIC functions as an electrochemically generated ex-cell oxidant which already features the required mild base equivalent for the transformation of benzaldehydes into phenols (Scheme 3).
Results and discussion
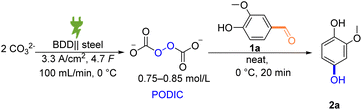
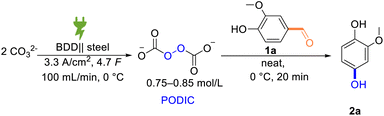
We commenced with vanillin (1a) as a model substrate to investigate the feasibility of a Dakin-type of reaction with electrochemically generated peroxodicarbonate in an ex-cell set-up. Vanillin is a natural product that can also be derived from the renewable feedstock lignin via electrochemical depolymerization.64–67 Therefore, further conversion of this compound into valuable commodities is highly desirable. The resulting product of this particular Dakin reaction, 2-methoxyhydroquinone, was recently reported as a promising active electrolyte for organic flow batteries.10Initially, various solvents were tested for their potential to facilitate the desired reaction of concentrated peroxodicarbonate solution with vanillin, starting from those that have been reported to be suitable for the Dakin reaction or to be compatible with PODIC (Table 1).37,40,41,53,62,68,69 However, neither methanol or ethanol nor acetonitrile mixtures provided the desired product (Table 1, entries 1–3). In contrast, tetrahydrofuran directly gave considerable yields of 2a (Table 1, entry 4). It is noteworthy that the reaction tolerates the presence of the stabilizer butylhydroxytoluene, which is supplemented to commercially tetrahydrofuran. After identifying a solvent which allows the desired reaction to proceed, we increased the equivalents of added PODIC to promote higher conversion to 2a. An increase of the PODIC equivalents from 1 to 2 improved the yield from 26% to 72% (Table 1, entries 4 and 5). Driven by our intent to develop a sustainable alternative to conventional protocols, we searched for conditions without organic solvents. This was also fortified by safety concerns that arise from the combination of peroxide and cyclic ethers. Consequently, we explored different aqueous options. The use of water or carbonate solutions in place of THF gave 2a only in lower yields (Table 1, entries 6–8). However, when treating neat 1a with concentrated peroxodicarbonate, a satisfactory yield of 62% was achieved (Table 1, entry 9).
We next optimized all reaction parameters, such as the amount of oxidant, the reaction temperature, and the mode of addition (Table 2). Lowering of the PODIC amount from 2 eq. to 1.5 eq. led to an increase in selectivity without decreasing the conversion of 1a (Table 2, entries 2 and 3). At even lower excess, the conversion and the overall yield decreased (Table 2, entry 4). Next, the slow addition of the oxidizer in equal portions every 5 minutes was tested (Table 2, entry 5). However, the yield was slightly lower in comparison to the original protocol in which the whole amount of PODIC is added at once (Table 2, entry 3) Moreover, a diluted peroxodicarbonate concentration was used which only gave the desired compound in 66% yield (Table 2, entry 6). This control experiment clearly highlights the importance of the high concentration of peroxodicarbonate, which is currently only achievable with our previously developed protocol and flow-electrolyzer (Table 2, entry 6).53 We observed that a short reaction time of 20 minutes is sufficient for the reaction to reach completion, whereas shorter reaction times led to incomplete consumption of starting material (Table 2, entries 3 and 7). It is known, that peroxodicarbonate has a decreased half-life at elevated temperatures.70 Combined with the fact that an incomplete mass balance was observed, we tested a lowered temperature of 0 °C to circumvent this (Table 2, entry 8). Both the selectivity and the yield of desired product 2a reached an optimum with the addition of 1.75 eq. PODIC at 0 °C (Table 2, entry 9; for further optimization experiments and data see ESI†).
| Entry | PODIC/eq. | T/°C | Deviation | Conv.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a/% a/% |
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a/% a/% |
|---|---|---|---|---|---|
| a Yield determined via1H NMR with 1,3,5-trimethoxybenzene as internal standard. Yield in brackets refers to isolated yield. | |||||
| 1 | 2 | rt | THF | 97 | 72 |
| 2 | 2 | rt | — | 99 | 62 |
| 3 | 1.5 | rt | — | 97 | 80 |
| 4 | 1 | rt | — | 77 | 58 |
| 5 | 1.5 | rt | Continuous addition | 98 | 72 |
| 6 | 1.5 | rt | c(PODIC) = 450 mM | 97 | 66 |
| 7 | 1.5 | rt | 15 min | 82 | 63 |
| 8 | 1.5 | 0 °C | — | 81 | 71 |
| 9 | 1.75 | 0 °C | — | 1 | 92 (91) |
With an optimized yield of 91% for the test substrate vanillin, we strived to explore the scope of our newly developed protocol. For this, we subjected a broad variety of substituted hydroxybenzaldehydes to our reaction protocol (Scheme 4).
 | ||
Scheme 4 Scope of the Dakin reaction with peroxodicarbonate as oxidizer. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Substrate dissolved MeOH. b Substrate dissolved MeOH. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5. eq PODIC. 3.5. eq PODIC. | ||
Initially, we tested different alkoxylated 4-hydroxybenzaldehydes. Similar to our test substrate 1a, ethylvanillin (1b) was transformed into the corresponding phenol 2b in excellent yield. Additional combinations of substituents with dissimilar electronic effects (1c–d) were also well suited for our protocol and gave the phenols in 85% and 62% yield, respectively.
Next, a variety of halogenated 4-hydroxybenzaldehydes was explored including mono- and disubstituted substates (1e–h). Initially, we observed very low conversion even at prolonged reaction times. This can be attributed to a poor solubility of the halogenated substrates in aqueous PODIC solution. In order to react, dissolution of the hydroxybenzaldehydes in the PODIC solution has to occur, which is then followed by deprotonation. To achieve this for compounds 1e–h, a minor amount of methanol was employed as co-solvent.
Consequently, the conversion was significantly improved and mitigated the need for prolonged reaction times (2e–f). In comparison to the alkoxylated substrates, we observed slightly lower yields in the range of 69% and 79%. Usually, electron-withdrawing moieties on the substrate are detrimental and often cause diminished yields.31 Nevertheless, the reaction occurred selectively under the reaction conditions established and the remaining non-converted starting material was easily recovered. The reaction can be pushed to a higher conversion by a slight increase of equivalents of PODIC, as could be demonstrated for substrates 1e and 1f. There, the yield could be improved from 69% and 62% to 91% and 94%, respectively.
The unsubstituted 4-hydroxybenzaldehyde was also suitable for our protocol resulting in 92% of hydroquinone (2i). Afterwards we explored a selection of alkyl substituted 4-hydroxybenzaldehydes. First, we applied benzaldehydes with low steric hinderance as provided by different amounts of methyl groups. The methylated phenols 2j–l were obtained in yields of 74%, 85% and 94% yield, respectively. Subsequently, we moved to alkyl groups with a higher rate of steric hinderance such as tert-butyl and isopropyl substituents. A set of non-polar substituents again caused the need of a minor amount of methanol to aid their dissolution. Following this, the mono-tert-butylated phenol 2m was obtained in 82% yield, the phenol with an additional methyl group 2n was isolated in 77%, and with two isopropyl groups provided 2o in 70% yield. Lastly, 2-hydroxylated benzaldehydes were also investigated as potential substrates. For the conventional Dakin reaction it is known that 2-hydroxybenzaldehydes are more reactive than its 4-hydroxy congeners.71 Therefore, we were interested to see how these substrates perform within the conditions of our E-Dakin reaction. Initially, the ortho-isomer 1p of our test substrate vanillin 1a was subjected to our established conditions. The resulting catechol 2p was obtained in 95% yield. Moreover, the ethoxylated benzaldehyde 2q, another methoxylated isomer 2r, as well as the tert-butylated 2s, and the mono-halo derivative 2t were successfully synthesized in 82%, 94%, and 97% yield, respectively.
Mechanistically, we propose that the reaction sequence is initiated by mono decarboxylation of the peroxodicarbonate. Subsequently, the very nucleophilic peroxide anion interacts with the carbaldehyde function. The formed tetrahedral intermediate expels a carbonate and undergoes a [1,2]-aryl shift. Finally, hydrolysis liberates phenol and formate underlining the necessity of base equivalents along with the oxidizer (Scheme 5).32
The mechanistic proposal also underlines the interesting chemoselectivity of the reaction. The electrochemically generated peroxodicarbonate shows high functional group compatibility and the resulting hydroxy groups remain unaffected by it. As a result of the innovative ex-cell approach, hydroquinones are exclusively obtained. In contrast to that the direct electrochemical conversion inevitably yields the benzoquinones due to the galvanostatic conditions.52,72 Therefore, this novel protocol complements our previous efforts by expanding the space of obtainable products. Ultimately, we tested the scalability of our established protocol. For this, we performed the oxidation of our test substrate vanillin on 10 mmol and 20 mmol scale (Table 3).
On gram scale, the reaction proceeded smoothly in comparable yields, further underlining the synthetic utility of the E-Dakin reaction.
Since this research was initially motivated by the aspiration to develop a greener approach to hydroquinones and catechols via a Dakin-type reaction, a quantitative comparison to previous protocols was conducted (Fig. 1). To evaluate the sustainability of the reaction, we considered different established green chemistry metrics.73,74 Additionally, high importance is also attributed to the economic factors, synthetic utility and safety of a reaction. Therefore, we also included such criteria in our assessment. Synthetic utility, as evidenced by the chemical yield, of all three protocols is comparably high with almost quantitative yields. Nevertheless, in times of depletion of resources and demand for more sustainable chemical transformations this single factor is no longer sufficient to rate the importance of a chemical protocol alone. Therefore, an attempt to quantify the sustainability of the protocols was undertaken. First, with the atom efficiency the molecular mass of the desired product is brought into relation to the overall mass of the formed products. Since the transformation occurs with high selectivity, but the nature of the mechanism inevitably leads to the co-generation of formate the atom economy is high but never 100%. Next, the reaction mass efficiency was calculated. This factor puts the chemical yield and atom economy of a reaction into context with the excess reagent factor. Furthermore, the mass efficient yield, incorporating the mass of non-benign reagents, was taken into account. This is an especially important factor given the environmental problems that arise from the use of such chemicals. As is evident by Fig. 1, our protocol introduces significant advancements in this regard, since it eliminates the need for problematic organic solvents and only uses a benign carbonate-based oxidant which comes along with a mild base equivalent. Apart from this, the cost of a chemical transformation is also an important factor to consider, especially for large scale synthesis. Therefore, we also considered the generated value of the product in contrast to the cost of the used reagents. Again, an improvement to previous protocols was made, due to the inexpensive nature of the applied reagents. Finally, the safety of the protocols was evaluated based on the hazards originating from the applied chemical reagents (for details and calculation see ESI†).74,75 In summary, this quantification demonstrates that our novel protocol has introduced significant advances in regards of minimization of problematic reagents while effectively lowering the cost of the transformation and improving the reaction safety.
 | ||
Fig. 1 Comparison of PODIC protocol (green) and published conventional methods (blue) for the oxidation of 1a to 2a. Left: Comparison to oxidation with percarbonate.76 Right: Comparison organo-catalysed oxidation. For details on conventional methods and calculation of depicted values see ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 | ||
Conclusions
An efficient and sustainable protocol for the conversion of widely accessible hydroxybenzaldehydes into valuable hydroquinones and catechols was established. This transformation is enabled through the powerful electrochemical platform oxidizer peroxodicarbonate (PODIC), which also provides the mildly basic conditions required for the oxidation step. PODIC can be synthesized on demand via electrolysis of ecologically safe aqueous carbonate solutions, circumventing the storage of potentially dangerous peroxides. The process safety is further improved due to the self-decomposition of peroxodicarbonate at room temperature preventing the build-up of peroxides in the reaction products. The reaction proceeds swiftly without an organic solvent and in the absence of any catalysts or activating agents. This simplifies the reaction work-up and minimizes waste generation. The broad applicability of the protocol was demonstrated for 20 examples with yields up to 97%. A broad variety of substituents was compatible, and the synthetic utility was proven through synthesis on a multi-gram scale.Author contributions
F. S. planned and conducted the optimization study and isolated the scope. N. S. and P. K synthesized the ex-cell oxidant and were involved in the early stages of the optimization study. S. W. supervised the project and provided continuous input. L. G. planned and constructed the electrolysis cell. L. G and S. W. acquired funding for the project. F. S. and S. W. wrote the initial manuscript, and all authors revised it. All authors listed have made a substantial, direct, and intellectual contribution to the work and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC-2033-390677874 – RESOLV, and Wa1276/23-2 – UNODE and by the Federal Ministry of Food and Agriculture (BMEL) in frame of FORESTII (2220HV053C).References
- K. A. Scott, P. B. Cox and J. T. Njardarson, J. Med. Chem., 2022, 65, 7044–7072 CrossRef CAS PubMed.
- W.-Y. Huang, Y.-Z. Cai and Y. Zhang, Nutr. Cancer, 2010, 62, 1–20 CAS.
- D.-P. Yang, H.-F. Ji, G.-Y. Tang, W. Ren and H.-Y. Zhang, Molecules, 2007, 12, 878–884 CrossRef CAS PubMed.
- R. Bodoira and D. Maestri, J. Agric. Food Chem., 2020, 68, 927–942 CrossRef CAS.
- S. Molloy, Nat. Rev. Microbiol., 2006, 4, 880–881 CrossRef CAS.
- R. Bernini, A. Coratti, G. Provenzano, G. Fabrizi and D. Tofani, Tetrahedron, 2005, 61, 1821–1825 CrossRef CAS.
- Y. Guo, D. He, A. Xie, W. Qu, Y. Tang, L. Zhou and R. Zhu, Polymers, 2019, 11, 1907 CrossRef CAS PubMed.
- S. Patai, The chemistry of the quinonoid compounds, Wiley, London, 1974–1988 Search PubMed.
- X. Yang, S. N. Garcia, T. Janoschka, D. Kónya, M. D. Hager and U. S. Schubert, Molecules, 2021, 26, 3823 CrossRef CAS PubMed.
- W. Schlemmer, P. Nothdurft, A. Petzold, G. Riess, P. Frühwirt, M. Schmallegger, G. Gescheidt-Demner, R. Fischer, S. A. Freunberger, W. Kern and S. Spirk, Angew. Chem., 2020, 132, 23143–23146 CrossRef.
- B. Assoah, L. F. Veiros, C. A. M. Afonso and N. R. Candeias, Eur. J. Org. Chem., 2016, 3856–3861 CrossRef CAS.
- Z. Ye, B. Shi, Y. Huang, T. Ma, Z. Xiang, B. Hu, Z. Kuang, M. Huang, X. Lin, Z. Tian, Z. Deng, K. Shen and T. Liu, Innovation, 2022, 3, 100228 CAS.
- L. Massignan, X. Tan, T. H. Meyer, R. Kuniyil, A. M. Messinis and L. Ackermann, Angew. Chem., Int. Ed., 2020, 59, 3184–3189 CrossRef CAS PubMed.
- Y.-H. Zhang and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 14654–14655 CrossRef CAS PubMed.
- T. Jintoku, K. Nishimura, K. Takaki and Y. Fujiwara, Chem. Lett., 1990, 19, 1687–1688 CrossRef.
- Y. Yan, P. Feng, Q.-Z. Zheng, Y.-F. Liang, J.-F. Lu, Y. Cui and N. Jiao, Angew. Chem., Int. Ed., 2013, 52, 5827–5831 CrossRef CAS PubMed.
- T. Yamaguchi, E. Yamaguchi, N. Tada and A. Itoh, Adv. Synth. Catal., 2015, 357, 2017–2021 CrossRef CAS.
- K. W. Anderson, T. Ikawa, R. E. Tundel and S. L. Buchwald, J. Am. Chem. Soc., 2006, 128, 10694–10695 CrossRef CAS PubMed.
- T. Schulz, C. Torborg, B. Schäffner, J. Huang, A. Zapf, R. Kadyrov, A. Börner and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 918–921 CrossRef CAS PubMed.
- A. Tlili, N. Xia, F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 8725–8728 CrossRef CAS PubMed.
- S. Xia, L. Gan, K. Wang, Z. Li and D. Ma, J. Am. Chem. Soc., 2016, 138, 13493–13496 CrossRef CAS PubMed.
- R. E. Maleczka, F. Shi, D. Holmes and M. R. Smith, J. Am. Chem. Soc., 2003, 125, 7792–7793 CrossRef CAS PubMed.
- J. Xu, X. Wang, C. Shao, D. Su, G. Cheng and Y. Hu, Org. Lett., 2010, 12, 1964–1967 CrossRef CAS PubMed.
- H.-L. Qi, D.-S. Chen, J.-S. Ye and J.-M. Huang, J. Org. Chem., 2013, 78, 7482–7487 CrossRef CAS PubMed.
- E. J. Rayment, N. Summerhill and E. A. Anderson, J. Org. Chem., 2012, 77, 7052–7060 CrossRef CAS PubMed.
- J. D. Sunderhaus, H. Lam and G. B. Dudley, Org. Lett., 2003, 5, 4571–4573 CrossRef CAS PubMed.
- T. Cohen, A. G. Dietz and J. R. Miser, J. Org. Chem., 1977, 42, 2053–2058 CrossRef CAS.
- A. A. Núñez Magro, G. R. Eastham and D. J. Cole-Hamilton, Dalton Trans., 2009, 4683–4688 RSC.
- W. Su, P. Xu and T. Ritter, Angew. Chem., Int. Ed., 2021, 60, 24012–24017 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green chemistry. Theory and practice, Oxford University Press, Oxford, 1st edn, 2000 Search PubMed.
- H. D. Dakin, Am. Chem. J., 1909, 42, 477–498 Search PubMed.
- Z. Wang, Comprehensive organic name reactions and reagents, John Wiley, Hoboken (N.J.), 2009 Search PubMed.
- V. E. Tarabanko and N. Tarabanko, Int. J. Mol. Sci., 2017, 18, 2421 CrossRef PubMed.
- N. Zhou, W. P. D. W. Thilakarathna, Q. S. He and H. P. V. Rupasinghe, Front. Energy Res., 2022, 9, 758744 CrossRef.
- X. Liu, F. P. Bouxin, J. Fan, V. L. Budarin, C. Hu and J. H. Clark, ChemSusChem, 2020, 13, 4296–4317 CrossRef CAS PubMed.
- Z. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef CAS PubMed.
- S. Chen and F. W. Foss, Org. Lett., 2012, 14, 5150–5153 CrossRef CAS PubMed.
- W. Sun, L. Gao, X. Sun, H. Yang and G. Zheng, Adv. Synth. Catal., 2019, 361, 5210–5216 CrossRef CAS.
- B. Saikia, P. Borah and N. C. Barua, Green Chem., 2015, 17, 4533–4536 RSC.
- G. W. Kabalka, N. K. Reddy and C. Narayana, Tetrahedron Lett., 1992, 33, 865–866 CrossRef CAS.
- A. Roy, K. R. Reddy, P. K. Mohanta, H. Ila and H. Junjappat, Synth. Commun., 1999, 29, 3781–3791 CrossRef CAS.
- E. T. da Silva, C. A. Câmara, O. A. C. Antunes, E. J. Barreiro and C. A. M. Fraga, Synth. Commun., 2008, 38, 784–788 CrossRef.
- R. S. Varma and K. P. Naicker, Org. Lett., 1999, 1, 189–192 CrossRef CAS.
- B. Saikia and P. Borah, RSC Adv., 2015, 5, 105583–105586 RSC.
- C. Schotten, T. P. Nicholls, R. A. Bourne, N. Kapur, B. N. Nguyen and C. E. Willans, Green Chem., 2020, 22, 3358–3375 RSC.
- Y. Yuan and A. Lei, Nat. Commun., 2020, 11, 802 CrossRef CAS PubMed.
- D. Pollok and S. R. Waldvogel, Chem. Sci., 2020, 11, 12386–12400 RSC.
- A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 5594–5619 CrossRef CAS PubMed.
- S. Möhle, M. Zirbes, E. Rodrigo, T. Gieshoff, A. Wiebe and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 6018–6041 CrossRef PubMed.
- S. R. Waldvogel and B. Janza, Angew. Chem., Int. Ed., 2014, 53, 7122–7123 CrossRef CAS PubMed.
- M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed.
- F. Sprang, J. Klein and S. R. Waldvogel, ACS Sustainable Chem. Eng., 2023, 11, 7755–7764 CrossRef CAS.
- A.-K. Seitz, P. J. Kohlpaintner, T. van Lingen, M. Dyga, F. Sprang, M. Zirbes, S. R. Waldvogel and L. J. Gooßen, Angew. Chem., Int. Ed., 2022, 61, e202117563 CrossRef CAS PubMed.
- R. E. Dinnebier, S. Vensky, P. W. Stephens and M. Jansen, Angew. Chem., Int. Ed., 2002, 41, 1922–1924 CrossRef CAS PubMed.
- S. Arndt, P. J. Kohlpaintner, K. Donsbach and S. R. Waldvogel, Org. Process Res. Dev., 2022, 26, 2564–2613 CrossRef CAS.
- S. Arndt, D. Weis, K. Donsbach and S. R. Waldvogel, Angew. Chem., Int. Ed., 2020, 59, 8036–8041 CrossRef CAS PubMed.
- S. Arndt, R. Rücker, A. Stenglein and S. R. Waldvogel, Org. Process Res. Dev., 2022, 26, 2447–2455 CrossRef CAS.
- C. M. Kisukuri, R. J.-R. Bednarz, C. Kampf, S. Arndt and S. R. Waldvogel, ChemSusChem, 2022, 15, e202200874 CrossRef CAS PubMed.
- J. Klein, K. Alt and S. R. Waldvogel, Adv. Sustainable Syst., 2022, 6, 2100391 CrossRef CAS.
- J. Klein, R. Kupec, M. Stöckl and S. R. Waldvogel, Adv. Sustainable Syst., 2022, 10, 2200431 Search PubMed.
- S. Arndt, B. Grill, H. Schwab, G. Steinkellner, U. Pogorevčnik, D. Weis, A. M. Nauth, K. Gruber, T. Opatz, K. Donsbach, S. R. Waldvogel and M. Winkler, Green Chem., 2021, 23, 388–395 RSC.
- P. J. Kohlpaintner, L. Marquart, L. J. Gooßen and S. R. Waldvogel, Eur. J. Org. Chem., 2023, e202300220 CrossRef CAS.
- A.-K. Seitz, T. van Lingen, M. Dyga, P. J. Kohlpaintner, S. R. Waldvogel and L. J. Gooßen, Synlett, 2022, 1527–1531 CAS.
- M. Zirbes, T. Graßl, R. Neuber and S. R. Waldvogel, Angew. Chem., Int. Ed., 2023, 62, e202219217 CrossRef CAS PubMed.
- M. Breiner, M. Zirbes and S. R. Waldvogel, Green Chem., 2021, 23, 6449–6455 RSC.
- M. Zirbes, L. L. Quadri, M. Breiner, A. Stenglein, A. Bomm, W. Schade and S. R. Waldvogel, ACS Sustainable Chem. Eng., 2020, 8, 7300–7307 CrossRef CAS.
- M. Zirbes and S. R. Waldvogel, Curr. Opin. Green Sustainable Chem., 2018, 14, 19–25 CrossRef.
- S. Chen, M. S. Hossain and F. W. Foss, Org. Lett., 2012, 14, 2806–2809 CrossRef CAS PubMed.
- M. Klein, D. L. Troglauer and S. R. Waldvogel, JACS Au, 2023, 3, 575–583 CrossRef CAS PubMed.
- C. P. Chardon, T. Matthée, R. Neuber, M. Fryda and C. Comninellis, ChemistrySelect, 2017, 2, 1037–1040 CrossRef CAS.
- M. B. Hocking, K. Bhandari, B. Shell and T. A. Smyth, J. Org. Chem., 1982, 47, 4208–4215 CrossRef CAS.
- F. Sprang, J. D. Herszman and S. R. Waldvogel, Green Chem., 2022, 24, 5116–5124 RSC.
- A. P. Dicks and A. Hent, Green chemistry metrics. A guide to determining and evaluating process greenness, Springer, Cham, 2015 Search PubMed.
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- L. Goclik, H. Walschus, A. Bordet and W. Leitner, Green Chem., 2022, 24, 2937–2945 RSC.
- M. Fache, E. Darroman, V. Besse, R. Auvergne, S. Caillol and B. Boutevin, Green Chem., 2014, 16, 1987–1998 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc04597h |
| This journal is © The Royal Society of Chemistry 2024 |