Benzotrifuran-based donor–acceptor covalent organic frameworks for enhanced photocatalytic hydrogen generation†
Chuanmeng
Yang‡
a,
Zhenwei
Zhang‡
a,
Jiali
Li
a,
Yuxin
Hou
a,
Qi
Zhang
*b,
Zhongping
Li
 d,
Huijuan
Yue
d,
Huijuan
Yue
 c and
Xiaoming
Liu
c and
Xiaoming
Liu
 *a
*a
aCollege of Chemistry, Jilin University, Changchun, 130012, P.R. China. E-mail: xm_liu@jlu.edu.cn
bSchool of Engineering, University of Warwick, Coventry CV4 7AL, UK. E-mail: 1033012429@qq.com
cState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P.R. China
dSchool of Energy and Chemical Engineering/Center for Dimension-Controllable Organic Frameworks, Ulsan National Institute of Science and Technology (UNIST), Ulsan, 44919, Republic of Korea
First published on 19th January 2024
Abstract
Visible-light-driven hydrogen evolution from water represents a green and sustainable technology to convert solar energy into chemical energy. Covalent organic frameworks (COFs) are the most competitive platforms among the variety of photocatalysts. Herein, a novel benzotrifuran-based donor–acceptor material (COF-JLU42) was constructed using a Schiff base polycondensation reaction by introducing electron-rich benzotrifuran and deficient triazine moieties into the framework. The new COF features high crystallinity and permanent porosity with a large surface area and proves to be a photoactive semiconductor with efficient visible-light harvesting capacity. Importantly, COF-JLU42 exhibits a superior photocatalytic activity with a hydrogen evolution rate of over 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μmol g−1 h−1 under visible-light illumination, which is 2.5 times higher than that of the isomorphic benzotrithiophene-based COFs under the same conditions. These findings suggest the huge application prospect of benzotrifuran-based framework materials as metal-free and solid photocatalysts in solar-energy conversion and storage.
000 μmol g−1 h−1 under visible-light illumination, which is 2.5 times higher than that of the isomorphic benzotrithiophene-based COFs under the same conditions. These findings suggest the huge application prospect of benzotrifuran-based framework materials as metal-free and solid photocatalysts in solar-energy conversion and storage.
Introduction
Sunlight-driven photocatalytic hydrogen production from water is a green and sustainable technology for the conversion of solar energy into chemical energy to alleviate the serious energy crisis and environmental pollution problems.1–4 Semiconductor photocatalysts are the key aspect in the whole catalytic process because they are responsible for visible-light harvesting, charge carrier transport and separation, as well as surface redox reactions.5 Therefore, over the past decades, great efforts have been devoted to constructing highly effective semiconductor photocatalysts, including inorganic metal oxides6,7 and organic polymers such as graphite carbon nitride (g-C3N4),8–13 to realize sustainable light-driven hydrogen evolution. However, inefficient visible-light absorption, expensive metals, and rapid charge recombination seriously hinder their practical applications.8Covalent organic frameworks (COFs) have emerged as a new class of porous crystalline polymers by integrating functional organic building units into periodic network architectures through strong covalent bonds under reticular chemistry.12–18 Considering structural aspects, their inherent ordered pores and large specific surface areas can provide large spaces for matter diffusion and transformation. Two-dimensional (2D) COFs offer columnar π-arrays and afford an ideal channel for photoinduced charge carrier transmission owing to the orbital overlap of stacked aromatic blocks.17–23 As desirable alternatives to inorganic photocatalysts, metal-free 2D-COFs have recently received much attention in various heterogeneous photocatalysis, including photocatalytic hydrogen production.24–34 In particular, the most important advantage of 2D-COFs is that it is easy to regulate their structure and functionality by selecting special building blocks as co-monomers and different linkages. For example, Lotsch et al. were the first to utilize a hydrazone-linked triazine-based COF26 for photocatalytic hydrogen evolution under visible-light illumination. In 2018, Cooper and co-workers successfully prepared a fused-sulfone-based COF,25 which provided a superior hydrogen evolution rate (HER) of 16.3 mmol g−1 h−1 when dye-sensitized. Subsequently, highly crystalline covalent triazine frameworks (CTFs)25–37 and keto-enamine-like 2D-COFs38,39 were proven to have outstanding hydrogen production performances with high photocatalytic activity. In addition, a series of functional organic building units such as triazine, pyrene, porphyrin, and phthalocyanine has been introduced into the frameworks to functionalise them.17,18,21 However, designing and synthesizing novel organic structures as monomers to construct metal-free, high-performance COF-based photocatalysts with small exciton binding energy (Eb) and high photocatalytic activity are still highly desired.
It is well known that benzodifuran compounds with electron-rich properties are widely utilized as hole-transfer materials in organic light-emitting diodes and photovoltaic cells.40,41 Recently, a few COFs containing benzodifuran moiety have emerged and demonstrated excellent photocatalytic properties.42,43 Benzo[1,2-b:3,4-b′:5,6-b′′]trifuran is a fused trifuran with an oxygen-rich, C3h symmetry, planar and extended π-system, which has not been explored in the field of organic porous polymers.17,18,21,44 In this context, we have successfully constructed a novel benzotrifuran-based COF material (named COF-JLU42) with an electron donor–acceptor (DA) character for photocatalytic hydrogen evolution via water splitting. Due to its high crystallinity, large porosity, and inherent built-in electric field with small Eb, COF-JLU42 showcases a superior photocatalytic activity with hydrogen evolution rate (HER) of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 μmol g−1 h−1 under visible-light illumination, which is 2.5 times higher than that of the isomorphic benzotrithiophene-based COF under the same conditions.
430 μmol g−1 h−1 under visible-light illumination, which is 2.5 times higher than that of the isomorphic benzotrithiophene-based COF under the same conditions.
Results and discussion
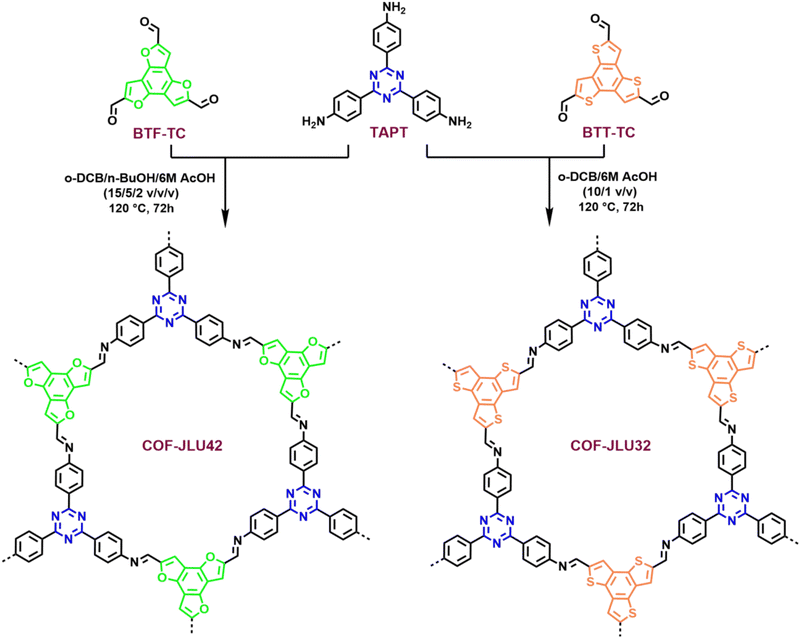
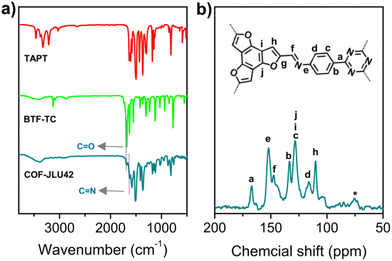
A novel benzotrifuran-based imine-linked DA framework material COF-JLU42 was synthesized by the condensation of benzo[1,2-b:3,4-b′:5,6-b′′]trifuran-2,5,8-tricarbaldehyde (BTF-TC) as an electron donor and 2,4,6-tris(4-aminophenyl)-1,3,5-triazine (TAPT) as an electron acceptor in o-dichlorobenzene (o-DCB), 1-butanol and acetic acid (AcOH, 6 M) at 120 °C for 72 h (Fig. 1, for details see the ESI†), which produced a yellow powder at 90% yield. In order to compare the structure and properties, an isomorphic benzotrithiophene-based COF (COF-JLU32) was also synthesized according to the previously reported process (Fig. 1).20 The COF-JLU42 was confirmed by Fourier transform infrared (FT-IR) spectroscopy and solid-state 13C cross-polarization magic angle spinning (CP/MAS) NMR spectroscopy. Compared with the monomer BTF-TC, the characteristic vibration peak at 1686 cm−1 almost disappeared in the FT-IR spectrum of COF-JLU42. A strong stretching signal at 1626 cm−1 originating from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group for COF-JLU42 was obtained, proving the formation of imine linkage (Fig. 2a). Furthermore, the chemical structure of COF-JLU42 at the molecular level was also researched by 13C CP/MAS NMR analysis. As shown in Fig. 2b, the low-field signal at 167.2 ppm can be assigned to the carbon atom of the triazine ring. The peak at about 147.7 ppm is attributed to the imine group. In addition, other signals at 152.3, 133.2, 128.1, 116.4, 109.8, and 103.8 ppm are ascribed to the carbons in the aromatic rings. Those findings matched the carbon species in the structure of COF-JLU42.
N group for COF-JLU42 was obtained, proving the formation of imine linkage (Fig. 2a). Furthermore, the chemical structure of COF-JLU42 at the molecular level was also researched by 13C CP/MAS NMR analysis. As shown in Fig. 2b, the low-field signal at 167.2 ppm can be assigned to the carbon atom of the triazine ring. The peak at about 147.7 ppm is attributed to the imine group. In addition, other signals at 152.3, 133.2, 128.1, 116.4, 109.8, and 103.8 ppm are ascribed to the carbons in the aromatic rings. Those findings matched the carbon species in the structure of COF-JLU42.
The permanent porosity of COF-JLU42 was evaluated by utilizing the nitrogen (N2) sorption isotherms at 77 K. As shown in Fig. 3a, the sorption curve of COF-JLU42 exhibited a typical type I reversible isotherm featured by a rapid N2 uptake under a low relative pressure at p/p0 < 0.05, which is a characteristic of microporous materials. Based on the adsorption curve, the Brunauer–Emmett–Teller (BET) surface area of COF-JLU42 was calculated to be 1375 m2 g−1, which is smaller than that of isomorphic COF-JLU32 (1501 m2 g−1).20 The pore volume was 0.817 cm3 g−1 at p/p0 = 0.99. In addition, the nonlocal density functional theory (NLDFT) model indicated that the pore size distribution was concentrated at approximately 1.85 nm (Fig. 3b), which is reasonably close to its theoretical value of 1.9 nm.
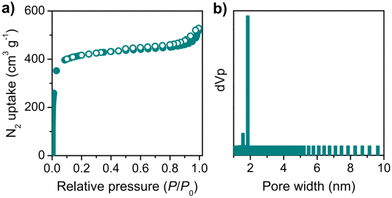 | ||
| Fig. 3 (a) Nitrogen adsorption–desorption isotherm of COF-JLU42 measured at 77 K (adsorption: filled circle; desorption: open circle). (b) Pore size distribution of COF-JLU42. | ||
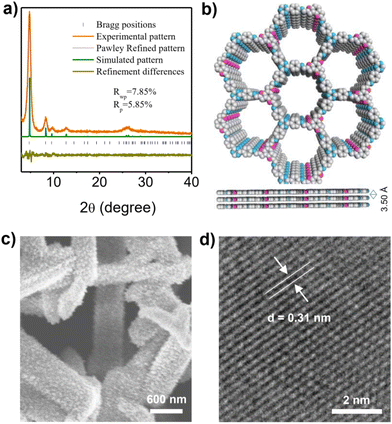
The crystal structure of COF-JLU42 was investigated using powder X-ray diffraction (PXRD) analysis coupled with the Materials Studio simulations. The experimental PXRD pattern presents a strong peak at about 4.80°, and other relatively weak signals at 8.28°, 9.76°, 12.7°, and 26.1°, which are assignable to the (100), (200), (210), (220), and (001) reflections of the hexagonal lattice (Fig. 4a, yellow line), respectively. The full width at half maximum (FWHM) of the peak at 2θ = 1.80° for COF-JLU42 was calculated as 0.46° (Fig. S1a†), which is larger than that of COF-JLU32 (0.37°, Fig. S1b†), implying that COF-JLU42 has slightly weaker crystallinity than the latter.20 The theoretical simulation of the crystal structure demonstrated that the COF-JLU42 tended to stack in the AA model (Fig. 4a, green line). Pawley refinement showed that the XRD curve was coincident with the obtained pattern, as evidence of negligible distinction (Fig. 4a, yellow-green line; Rp = 5.85% and Rwp = 7.85%). In addition, field-emission scanning electron microscopy (FE-TEM) images indicated that the COF-JLU42 is a pure solid phase with ribbon morphology (Fig. 4c). High-resolution transmission electron microscopy (HR-TEM) of COF-JLU42 displayed long-range ordered channels, further illustrating its high crystallinity (Fig. 4d and S2†). The lattice stripe spacing in the measured TEM photographs essentially matches the layer spacing of the AA stacking, which corresponds to the (001) crystal surface.
The thermal durability of COF-JLU42 was estimated via thermogravimetric analysis (TGA), which demonstrated a high thermal decomposition temperature of up to 520 °C under an N2 atmosphere (Fig. S3†). Additionally, we also researched the chemical stability and photochemical durability of the new COF material. The COF-JLU42 sample was treated with methanol (MeOH), tetrahydrofuran (THF), H2O, HCl (6 M), and NaOH (6 M) aqueous solution for 72 h at room temperature. Then samples were collected by centrifugation, washed, and dried in a vacuum. Pleasantly, all obtained samples maintained high crystallinity and skeleton connection as demonstrated by PXRD and FT-IR spectra (Fig. S4†), ever under irradiation with an OLED lamp. The results of the control experiment indicated the excellent durability of COF-JLU42.
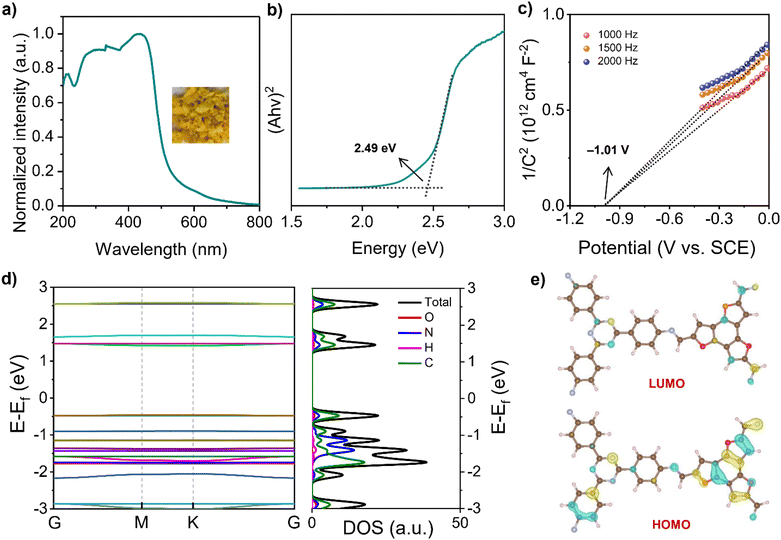
The ultraviolet-visible diffuse reflectance (UV-vis DR) spectrum of COF-JLU42 showed a wide visible-light harvesting extending into 800 nm with maximum peaks at 439 nm (Fig. 5a), which is consistent with its color (Fig. 5a, inset). Based on the absorption band using the Tauc plot analysis, the optical band gap energy (Eg) was calculated to be 2.49 eV. Furthermore, the Mott–Schottky measurements were conducted and we evaluated the conduction band minimum (CBM) of COF-JLU42 to be −1.01 V vs. SCE (Fig. 5b). Therefore, the valence band maximum (VBM) can be estimated to be 1.48 V (vs. SCE) for COF-JLU42 according to the equation Eg = EVB − ECB, and the band structure alignment is displayed in Fig. S5d.† Furthermore, the density functional theory (DFT) calculations were still performed for both COF-JLUs. Their projected density of states (PDOS) and the band structures are presented in Fig. 5d and S6a.† We found that the CB mainly originated from TAPT and VB came from BTF-TC for COF-JLU42 or BTT-TC for COF-JLU32, which is consistent with the molecular orbital contributions, as displayed in Fig. 5e and S6b,† where the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) mainly resulted from the acceptor TAPT and the donor BTF-TC or BTT-TC, respectively. These findings indicated that the two isomorphic COF-JLUs have similar absorption, Eg, and energy levels, which meet the thermodynamical requirement of photocatalytic proton reduction.
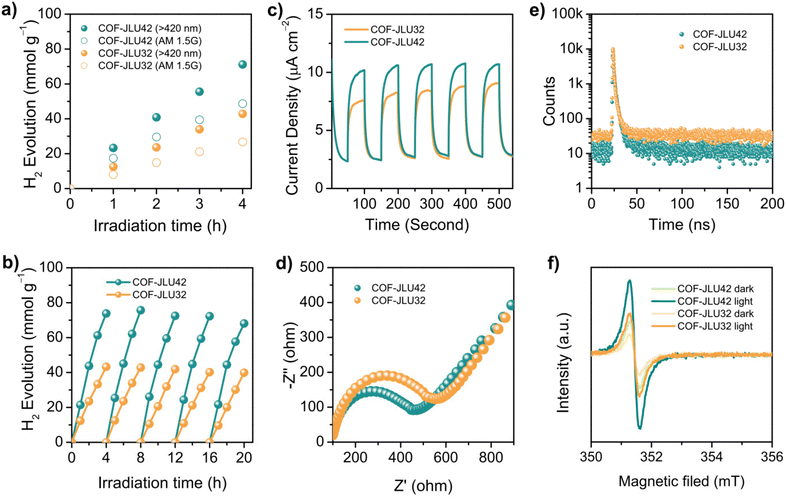
Subsequently, the photocatalytic hydrogen generation of COF-JLU42 via water splitting was explored to evaluate the inherent photoactivity of benzotrifuran-based framework materials. For the COF-JLU42-based photocatalytic system, ascorbic acid (AA) and platinum (Pt) nanoparticles were employed as the sacrificial electron donor (SED) and co-catalyst to facilitate hydrogen evolution.22,23 The results of the control experiment showed that the photo-deposited Pt content seriously affected the hydrogen production rate of the framework (Fig. S7†). For instance, 5 mg of COF sample with 3 wt% Pt was dispersed in 50 mL of an aqueous solution and displayed an average hydrogen evolution rate (HER) of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 μmol g−1 h−1 under visible-light illumination (420 < λ < 780 nm), which is higher than that of isomorphic COF-JLU32 (10
790 μmol g−1 h−1 under visible-light illumination (420 < λ < 780 nm), which is higher than that of isomorphic COF-JLU32 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 μmol g−1 h−1) under the same conditions (Fig. 6a). The average HER of COF-JLU42 improved to 28
710 μmol g−1 h−1) under the same conditions (Fig. 6a). The average HER of COF-JLU42 improved to 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 μmol g−1 h−1 and 3 mg of the polymer with 3 wt% Pt was utilized (Fig. S8†). Furthermore, reducing the mass of COF to 1 mg, surprisingly, a superior HER of 40
650 μmol g−1 h−1 and 3 mg of the polymer with 3 wt% Pt was utilized (Fig. S8†). Furthermore, reducing the mass of COF to 1 mg, surprisingly, a superior HER of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 μmol g−1 h−1 was obtained (Fig. S7†), which exceeded that of the state-of-the-art COF-based photocatalysts (Table S2†). In sharp contrast, the photocatalytic activity of COF-JLU32 (16
430 μmol g−1 h−1 was obtained (Fig. S7†), which exceeded that of the state-of-the-art COF-based photocatalysts (Table S2†). In sharp contrast, the photocatalytic activity of COF-JLU32 (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 μmol g−1 h−1) was less than half that of COF-JLU42 under the same conditions, although the former had a larger surface area (Fig. S9) and higher crystallinity (Fig. S1†).
080 μmol g−1 h−1) was less than half that of COF-JLU42 under the same conditions, although the former had a larger surface area (Fig. S9) and higher crystallinity (Fig. S1†).
Furthermore, the apparent quantum yield (AQY) of COF-JLU42 and COF-JLU32 were also conducted under monochromatic incident light. As depicted in Fig. S10a,† the highest AQY of 1.18% at 450 nm for COF-JLU42 was obtained, which surpassed those of the well-known photocatalysts such as N3-COF (AQY450 nm = 0.44%),47 sp2c-COFERDN (AQY490 nm = 0.48%),46 and g-C40N3-COF (AQY420 nm = 1.10%).40 Moreover, the AQYs were estimated to be 0.85%, 0.47%, and 0.26% at 420, 500, and 550 nm, respectively. In addition, the AQY of COF-JLU32 also showed a similar wavelength-dependent trend, with the highest value of 0.63% at 450 nm, which is smaller than that of the isomorphic COF-JLU42 (Fig. S10b†). Next, the stability of both COF-JLUs as photocatalysts for hydrogen evolution via water splitting was evaluated by repeated experiments. No significant loss of photocatalytic activity was obtained during the measurement period, even after five cycles of at least 20 h, and the FT-IR, PXRD, UV-Vis spectrum, surface area, and FE-SEM data were comparable with those of the original COF samples (Fig. S11†).
In order to study the difference in the photocatalytic activity of the two isomorphic COFs for hydrogen evolution, a series of photoelectric experiments were performed. It is well known that the highly efficient generation, separation, and transport of the photogenerated charge carriers in the semiconductor photocatalyst are crucial for improving photocatalytic activity. Accordingly, transient photocurrent response and electrochemical impedance spectroscopy (EIS) were first conducted. Under light illumination, two materials exhibited fast photocurrent responses with a few reduplicative cycles of intermittent on–off irradiation, which provided clear evidence for the photogenerated carrier transport (Fig. 6c). Obviously, COF-JLU42 showed a stronger transient photocurrent than that of COF-JLU32, suggesting the more convenient dissociation of photoinduced electron/hole pairs. As shown in Fig. 6d, furthermore, the EIS Nyquist plots exhibited that the semicircle diameter of COF-JLU42 is smaller than that of COF-JLU32, indicating more effectively photogenerated charge carrier transport and separation in COF-JLU42, thus restricting the electron–hole recombination more effectively.48,49 In addition, time-resolved fluorescence decay spectroscopy of COF-JLUs was recorded. As displayed in Fig. 6e, the average lifetime was calculated to be 1.85 ns for COF-JLU42 and 1.62 ns for COF-JLU32, respectively. The longer lifetime of COF-JLU42 implies stronger separation of the charge carriers compared with that of COF-JLU32, which also corresponds to better photocatalytic activity. Then, electron spin-spectroscopy was carried out to further explore the charge separation efficiency of the two COFs. As shown in Fig. 6f, both COF-JLUs represented an isotropic peak with a Lorentzian-type pattern at g = 2.003 under light illumination and dark, which follows the electrons in the π-delocalized aromatic frameworks.50 COF-JLU42 revealed a much stronger signal intensity than the isomorphic COF-JLU32 in the dark or under light irradiation, suggesting a large charge separation efficiency of COF-JLU42.
We further investigated the temperature-dependent photoluminescence (PL) spectra to evaluate the exciton dynamics properties. As shown in Fig. 7 insets, the PL intensity of both COF-JLUs increased monotonically as the temperature decreased, indicating the thermal quenching phenomenon of PL emission in the research temperature scope.45,51–54 By fitting the temperature dependence of the integrated PL intensities as a function of temperature according to the Arrhenius equation: I(T) = I0/[1 + A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Eb/kBT)] (where I0 is the intensity at 0 K, T is the temperature, and kB is the Boltzmann constant), the Eb of both COFs can be obtained. As displayed in Fig. 7, the Eb values for COF-JLU42 and COF-JLU32 were calculated to be 81.7 and 128.5 meV, respectively. The smaller Eb value indicates that the charge separation is more likely to occur in COF-JLU42. All these observations provided the structural advantages of a benzotrifuran-based donor–acceptor framework for improving photocatalytic activity.
exp(−Eb/kBT)] (where I0 is the intensity at 0 K, T is the temperature, and kB is the Boltzmann constant), the Eb of both COFs can be obtained. As displayed in Fig. 7, the Eb values for COF-JLU42 and COF-JLU32 were calculated to be 81.7 and 128.5 meV, respectively. The smaller Eb value indicates that the charge separation is more likely to occur in COF-JLU42. All these observations provided the structural advantages of a benzotrifuran-based donor–acceptor framework for improving photocatalytic activity.
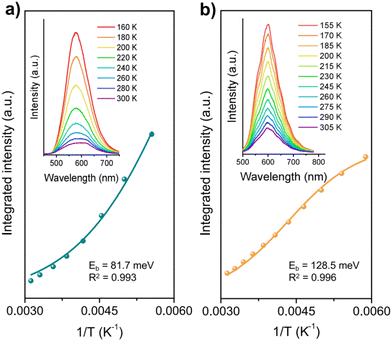 | ||
| Fig. 7 Integrated PL emission intensity as a function of the temperature of COF-JLU42 (a) and COF-JLU32 (b), respectively. Inset: the corresponding temperature-dependent PL spectra. | ||
Conclusions
In summary, a novel benzotrifuran-based covalent organic framework (COF-JLU42) was successfully designed and constructed via the imine condensation reaction under solvothermal conditions. Experimental and theoretical studies indicated that the new COF material had strong crystallinity, a high surface area, superior durability, and good photoelectric properties with small exciton binding energy. More significantly, the benzotrifuran-based COF exhibited a high hydrogen evolution rate of up to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 μmol g−1 h−1 under visible-light illumination with outstanding reusability, which is higher than the isomorphic benzotrithiophene-based COF (COF-JLU32). We believe that this work will open up a new avenue for the preparation of benzotrifuran-based porous functional materials at the molecular level. Currently, such studies are underway in our laboratory.
430 μmol g−1 h−1 under visible-light illumination with outstanding reusability, which is higher than the isomorphic benzotrithiophene-based COF (COF-JLU32). We believe that this work will open up a new avenue for the preparation of benzotrifuran-based porous functional materials at the molecular level. Currently, such studies are underway in our laboratory.
Author contributions
X. L. conceived the project, designed experiments, and provided funding. C. Y. and Z. Z. conducted the experiments and analyzed the data. Q. Z. performed the theoretical data calculations and analyzed the results. J. L., Y. H., H. Y., and Z. L. provided experimental testing support and gave useful help during the experiments. X. L. and C. Y. wrote the manuscript and discussed the results with all authors.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the project of the National Natural Science Foundation of China (No. 52073119, 52373210), Natural Science Foundation of Jilin Province (No. 20230101029JC), and State Key Laboratory of Inorganic Synthesis and Preparative Chemistry (No. 2024-7).References
- Y. Wang, A. Vogel, M. Sachs, R. S. Sprick, L. Wilbraham, S. J. A. Moniz, R. Godin, M. A. Zwijnenburg, J. R. Durrant, A. I. Cooper and J. Tang, Nat. Energy, 2019, 4, 746–760 CrossRef CAS.
- B. Shao, Z. Liu, L. Tang, Y. Liu, Q. Liang, T. Wu, Y. Pan, X. Zhang, X. Tan and J. Yu, J. Hazard. Mater., 2022, 435, 129067 CrossRef CAS PubMed.
- B. Shao, L. Shen, Z. Liu, L. Tang, X. Tan, D. Wang, W. Zeng, T. Wu, Y. Pan, X. Zhang, L. Ge and M. He, Chem. Eng. J., 2023, 455, 140476 CrossRef CAS.
- Z. Liu, M. He, L. Tang, B. Shao, Q. Liang, T. Wu, Y. Pan, X. Zhang, S. Luo, Q. He and L. Ge, J. Colloid Interface Sci., 2023, 634, 255–267 CrossRef CAS PubMed.
- T. Banerjee, F. Podjaski, J. Kröger, B. P. Biswal and B. V. Lotsch, Nat. Rev. Mater., 2020, 6, 168–190 CrossRef.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- T. Takata, J. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Nature, 2020, 581, 411–414 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- W. Ting, Z. F. Liu, B. B. Shao, Q. H. Liang, Q. Y. He, P. Yuan, X. S. Zhang, Y. Liu, J. W. Sun and S. X. Gong, Chem. Eng. J., 2022, 447, 137332 CrossRef.
- T. Wu, Q. Liang, L. Tang, J. Tang, J. Wang, B. Shao, S. Gong, Q. He, Y. Pan and Z. Liu, J. Hazard. Mater., 2023, 443, 130251 CrossRef CAS PubMed.
- Y. Pan, Z. Peng, Z. Liu, B. Shao, Q. Liang, Q. He, T. Wu, X. Zhang, C. Zhao, Y. Liu, L. Ge and M. He, J. Environ. Chem. Eng., 2022, 10, 107366 CrossRef CAS.
- T. Wu, Q. He, Z. Liu, B. Shao, Q. Liang, Y. Pan, J. Huang, Z. Peng, Y. Liu, C. Zhao, X. Yuan, L. Tang and S. Gong, J. Hazard. Mater., 2022, 424, 127177 CrossRef CAS PubMed.
- X. Zhang, S. Tong, D. Huang, Z. Liu, B. Shao, Q. Liang, T. Wu, Y. Pan, J. Huang, Y. Liu, M. Cheng and M. Chen, Coord. Chem. Rev., 2021, 448, 214177 CrossRef CAS.
- P. J. Waller, F. Gándara and O. M. Yaghi, Acc. Chem. Res., 2015, 48, 3053–3063 CrossRef CAS PubMed.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- Y. Song, Q. Sun, B. Aguila and S. Ma, Adv. Sci., 2018, 6, 1801410 CrossRef PubMed.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- T. Li, Y. Pan, B. Shao, X. Zhang, T. Wu, Q. He, M. He, L. Ge, L. Zhou, S. Liu, X. Zheng, J. Ye and Z. Liu, Adv. Funct. Mater., 2023, 33, 2304990 CrossRef CAS.
- R. Liu, K. T. Tan, Y. Gong, Y. Chen, Z. Li, S. Xie, T. He, Z. Lu, H. Yang and D. Jiang, Chem. Soc. Rev., 2021, 50, 120–242 RSC.
- T. He and Y. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202303086 CrossRef CAS PubMed.
- H. Chen, H. S. Jena, X. Feng, K. Leus and P. V. D. Voort, Angew. Chem., Int. Ed., 2022, 61, e202204938 CrossRef CAS PubMed.
- Z. Li, J. Wang, S. Ma, Z. Zhang, Y. Zhi, F. Zhang, H. Xia, G. Henkeim and X. Liu, Appl. Catal., B, 2022, 310, 121335 CrossRef CAS.
- Z. Zhang, J. Jia, Y. Zhi, S. Ma and X. Liu, Chem. Soc. Rev., 2022, 51, 2444–2490 RSC.
- Z. Li, T. Deng, S. Ma, Z. Zhang, G. Wu, J. Wang, Q. Li, H. Xia, S. Yang and X. Liu, J. Am. Chem. Soc., 2023, 145, 8364–8374 CAS.
- S. Ma, T. Deng, Z. Li, Z. Zhang, J. Jia, G. Wu, H. Xia, S. Yang and X. Liu, Angew. Chem., Int. Ed., 2022, 61, e202208919 CrossRef CAS PubMed.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, Chem. Sci., 2014, 5, 2789–2793 RSC.
- X. Wang, L. Chen, S. Y. Chong, M. A. Little, Y. Wu, W.-H. Zhu, R. Clowes, Y. Yan, M. A. Zwijnenburg, R. S. Sprick and A. I. Cooper, Nat. Chem., 2018, 10, 1180–1189 CrossRef CAS PubMed.
- J. Yang, A. Acharjya, M. Y. Ye, J. Rabeah, S. Li, Z. Kochovski, S. Youk, J. Roeser, J. Grüneberg, C. Penschke, M. Schwarze, T. Wang, Y. Lu, R. Krol, M. Oschatz, R. Schomäcker, P. Saalfrank and A. Thomas, Angew. Chem., Int. Ed., 2021, 60, 19797–19803 CrossRef CAS PubMed.
- R. Chen, Y. Wang, Y. Ma, A. Mal, X. Y. Gao, L. Gao, L. Qiao, X. B. Li, L. Z. Wu and C. Wang, Nat. Commun., 2021, 12, 1354 CrossRef CAS PubMed.
- Y. Li, L. Yang, H. He, L. Sun, H. Wang, X. Fang, Y. Zhao, D. Zheng, Y. Qi, Z. Li and W. Deng, Nat. Commun., 2022, 13, 1355 CrossRef CAS PubMed.
- S. Wei, F. Zhang, W. Zhang, P. Qiang, K. Yu, X. Fu, D. Wu, S. Bi and F. Zhang, J. Am. Chem. Soc., 2019, 141, 14272–14279 CrossRef CAS PubMed.
- Y. Wang, W. Hao, H. Liu, R. Chen, Q. Pan, Z. Li and Y. Zhao, Nat. Commun., 2022, 13, 100 CrossRef CAS PubMed.
- W. Dong, Z. Qin, K. Wang, Y. Xiao, X. Liu, S. Ren and L. Li, Angew. Chem., Int. Ed., 2023, 62, e2022160 Search PubMed.
- S. Li, R. Ma, S. Xu, T. Zheng, G. Fu, Y. Wu, Z. Liao, Y. Kuang, Y. Hou, D. Wang, P. S. Petkov, K. Simeonova, X. Feng, L. Wu, X. Li and T. Zhang, J. Am. Chem. Soc., 2022, 144, 13953–13960 CrossRef CAS PubMed.
- S. Ghosh, A. Nakada, M. A. Springer, T. Kawaguchi, K. Suzuki, H. Kaji, I. Baburin, A. Kuc, T. Heine, H. Suzuki, R. Abe and S. Seki, J. Am. Chem. Soc., 2020, 142, 9752–9762 CAS.
- W. Chen, L. Wang, D. Mo, F. He, Z. Wen, X. Wu, H. Xu and L. Chen, Angew. Chem., Int. Ed., 2020, 59, 16902–16909 CrossRef CAS PubMed.
- S. Zhang, G. Cheng, L. Guo, N. Wang, B. Tan and S. Jin, Angew. Chem., Int. Ed., 2020, 59, 6007–6014 CrossRef CAS PubMed.
- M. Liu, Q. Huang, S. Wang, Z. Li, B. Li, S. Jin and B. Tan, Angew. Chem., Int. Ed., 2018, 57, 11968–11972 CrossRef CAS PubMed.
- Z. Xie, X. Yang, P. Zhang, X. Ke, X. Yuan, L. Zhai, W. Wang, N. Qin, H. Cui, L. Qu and X. Chen, J. Chin. Catal., 2023, 47, 171–180 CrossRef CAS.
- C. Li, J. Liu, H. Li, K. Wu, J. Wang and Q. Yang, Nat. Commun., 2022, 13, 2357 CrossRef CAS PubMed.
- W. Weng and J. Guo, Nat. Commun., 2022, 13, 5768 CrossRef CAS PubMed.
- H. Tsuji, C. Mitsui, L. Ilies, Y. Sato and E. Nakamura, J. Am. Chem. Soc., 2007, 129, 11902–11903 CrossRef CAS PubMed.
- Z. Li, H. Li, S. Chen, T. Froehlich, C. Yi, C. Schönenberger, M. Calame, S. Decurtins, S. Liu and E. Borguet, J. Am. Chem. Soc., 2014, 136, 8867–8870 CrossRef CAS PubMed.
- W. An, S. Zheng, X. Xu, L. Liu, J. Ren, L. Fan, Z. Yang, Y. Ren and C. Xu, Appl. Catal., B, 2022, 316, 121630 CrossRef CAS.
- G. Wang, F. Zhu, Q. Lin, J. Kan, K. Xie, S. Li, Y. Geng and Y. Dong, Chem. Commun., 2021, 57, 4464–4467 RSC.
- J. Li, Z. Zhang, J. Jia and X. Liu, Chem. Res. Chin. Univ., 2022, 38, 275–289 CrossRef CAS.
- V. S. Vyas, F. Haase, L. Stegbaur, G. Savasci, F. Podjaski, C. Ochsenfeld and B. V. Lotsch, Nat. Commun., 2015, 6, 8508 CrossRef CAS PubMed.
- E. Jin, Z. Lan, Q. Jiang, K. Geng, G. Li, X. Wang and D. Jiang, Chem, 2019, 5, 1632–1647 CAS.
- S. Bi, C. Yang, W. Zhang, J. Xu, L. Liu, D. Wu, X. Wang, Y. Han, Q. Liang and F. Zhang, Nat. Commun., 2019, 10, 2467 CrossRef PubMed.
- H. Liu, X. Zheng, J. Xu, X. Jia, M. Chao, D. Wang and Y. Zhao, ACS Appl. Mater. Interfaces, 2023, 15, 16794–16800 CrossRef CAS PubMed.
- K. Huang, J. Bai, R. Shen, X. Li, C. Qin, P. Zhang and X. Li, Adv. Funct. Mater., 2023, 2307300 CrossRef CAS.
- Z. Lan, M. Wu, Z. Fang, Y. Zhang, X. Chen, G. Zhang and X. Wang, Angew. Chem., Int. Ed., 2022, 61, e202201482 CrossRef CAS PubMed.
- Z. Xie, X. Yang, P. Zhang, X. Ke, X. Yuan, L. Zhai, W. Wang, N. Qin, C. Cui, L. Qu and X. Chen, Chin. J. Catal., 2023, 47, 171–180 CrossRef CAS.
- S. Chai, X. Chen, X. Zhang, Y. Fang, R. S. Sprick and X. Chen, Environ. Sci.: Nano, 2022, 9, 2464–2469 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, full characterisation and spectroscopic data. See DOI: https://doi.org/10.1039/d3gc04972h |
| ‡ These authors contributed equally to manuscript. |
| This journal is © The Royal Society of Chemistry 2024 |