Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
NHC/B(OH)3-mediated C3-selective acylation of unprotected monosaccharides: mechanistic insights and toward simpler/greener solutions†
Jiamiao
Jin
ab,
Jiangtao
Guo
bc,
Feng
Xu
bc,
Ya
Lv
a,
Guanjie
Wang
b,
Jia
Song
a,
Wen-Xin
Lv
 a,
Tingting
Li
a,
Tingting
Li
 *a and
Yonggui Robin
Chi
*a and
Yonggui Robin
Chi
 *ab
*ab
aState Key Laboratory of Green Pesticides, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, 550025, China. E-mail: litt8293@163.com; robinchi@ntu.edu.sg
bSchool of Chemistry, Chemical Engineering, and Biotechnology, Nanyang Technological University, Singapore, 637371, Singapore
cSchool of Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang, China
First published on 15th April 2024
Abstract
Selective acylation of saccharides is an important basic transformation in saccharide chemistry. Synthetic strategies and protocols for saccharides can reach broad users when reagents are simple (inexpensive and readily available), green, and easy to handle. Herein, we disclose that the simplest boric acid, B(OH)3, can work as an effective promoter for C3–OH selective acylation of unprotected monosaccharides under the catalysis of a readily available N-heterocyclic carbene catalyst. Solvents such as THF from untreated commercial sources can be directly used. Mechanistically, the boric acid plays multiple roles in benefiting reaction yields and regioselectivities, including solubilizing the saccharide substrates by forming borate esters with the saccharides, shielding the C6–OH unit of the saccharides from acylation, and enhancing the selectivity between C3–OH and C2–OH reactions. We also found that the solvent has a profounder effect on the reaction yield and selectivity than the base. Selectively acylated saccharide products can be easily obtained with good-to-excellent yields and gram scales. Our study will encourage the further development of new synthetic strategies for saccharides that can reach beyond laboratory scales and to scientists not necessarily with expertise in organic synthesis. Compared to previous methods using aryl and alkyl boronic acids as modulating reagents, the use of boric acid provides a greener solution economically and environmentally.
Introduction
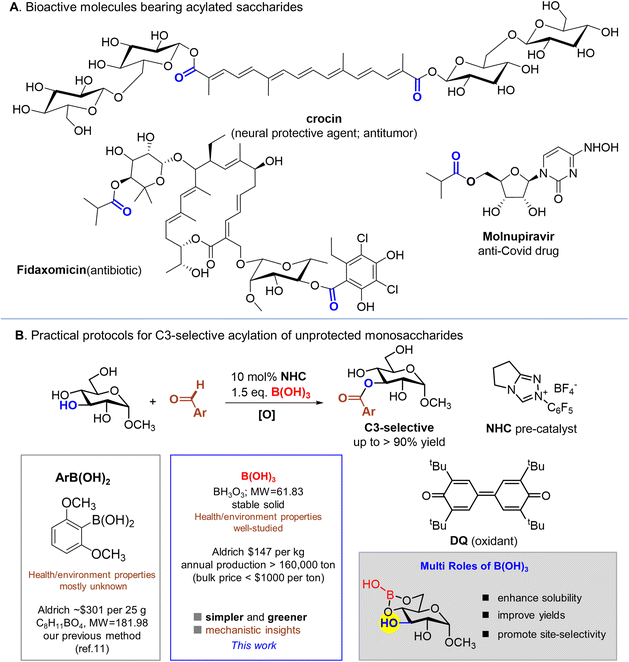
Monosaccharides are fundamental structural units in complex polysaccharides and saccharide-containing molecules (Fig. 1A).1 They also function as basic building blocks for assembling various functional molecules, including medicines2 and natural products.3 A crucial category of reactions involved in transforming saccharide building blocks into useful molecules is acylation, which converts hydroxyl groups into corresponding carboxylic esters.4 These acylation processes can serve as protection strategies in saccharide transforming chemistry4a,c,5 or as critical steps in linking saccharides with other structures for various purposes, such as preparing prodrugs.6 Traditional methods for acylation typically require pre-protection of specific hydroxyl groups of the saccharides.7 In recent years, direct transformation (including acylation) of unprotected saccharides via site-selective strategies has received increasing attention, as reported by Hanessian, Taylor, Blaszczyk, Tang, Miller, Kawabata, and Studer.8 Drawing on our longstanding interests in developing catalysis for selective reactions,9 we recently reported a site-selective acylation of unprotected saccharides mediated by N-heterocyclic carbene (NHC) organic catalysts10 and aryl boronic acids (Fig. 1B).11 To achieve optimal site-selectivities and reaction yields, we employed NHC catalysts and relatively complex aryl boronic acids (such as the one illustrated in Fig. 1B) in our previous report.11 However, relatively complex aryl boronic acids and their large molecular weights present certain barriers for broad (and large-scale) application of our method for operational and economic reasons. In addition, the reactions in our previous report were mostly carried out under an inert atmosphere with argon.To develop simpler and greener methods that can be used beyond laboratory scales and by players who are not necessarily organic chemists, here we decided to move from aryl boronic acids to boric acid, B(OH)3, the simplest member of this boronic acid family (Fig. 1B). Boric acid is produced in quantities of several hundred thousand metric tons per year (with a cost of less than a dollar per kilogram). It is also more environmentally benign compared to most other boronic acids, with its well documented physical and chemical properties. From a practical standpoint, boric acid is the best choice when compared to any of the aryl or alkyl boronic acids. Unlike in our previous report,11 where the reaction was carried out under N2, in our present new protocol with boric acid, the reaction is performed without exclusion of air. Solvents from commercial sources without further dehydration can be used directly in our present protocol. Regarding mechanistic aspects, we found that boric acid plays two major roles in our selective acylation reactions (Fig. 1B). One role is to shield the hydroxyl groups on C4 and C6-carbon atoms from acylation and the other is to enhance the solubility of the saccharides, thereby significantly improving the reaction yield. The NHC catalyst used in this transformation is also readily available and relatively simple. Our present work will encourage further explorations of simple reagents for important complex transformations.
Results and discussion
The reaction between unprotected glucoside 1a (0.1 mmol) and p-chlorobenzyl aldehyde 2a (0.15 mmol) was chosen as the model to form corresponding C3, C2, and C6-acylation products (3a, 4a, and 5a, respectively) (Fig. 2). Conditions including 10 mol% NHC pre-catalyst, 10 mol% of base, 1.5 equiv. of B(OH)3, 1.5 equiv. of DQ and 2 mL of solvent were used to study the effect of different solvents and bases. Key results from extensive studies of various solvents and bases are summarized in Fig. 2.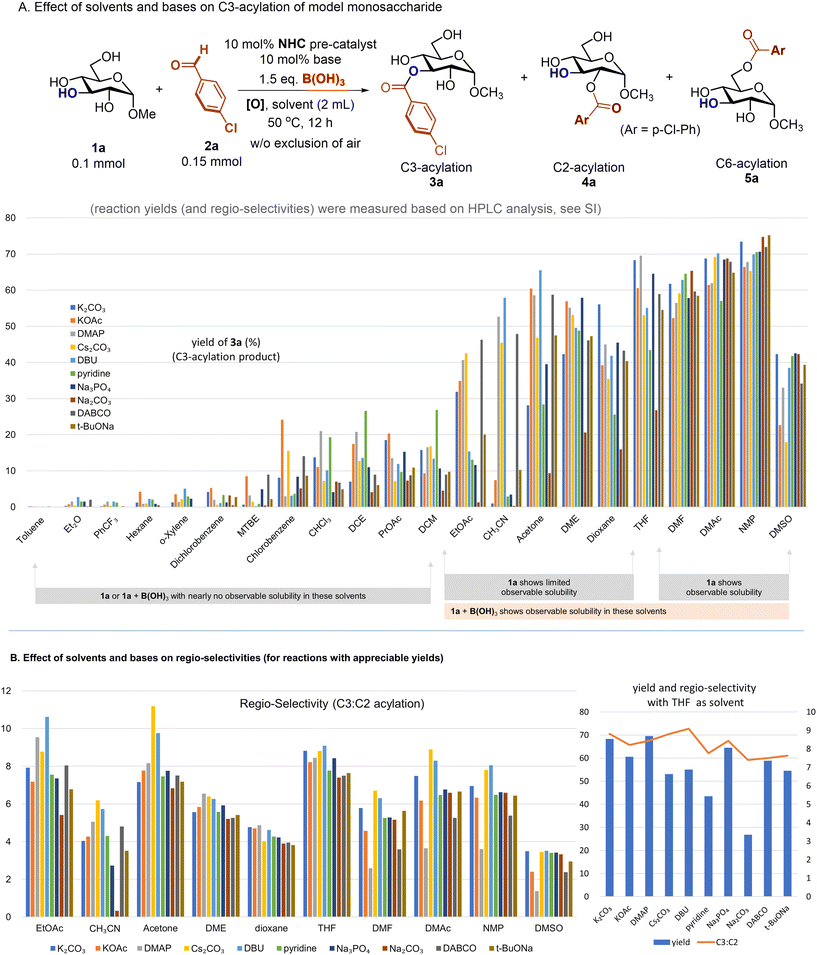 | ||
| Fig. 2 A detailed study on the effect of different solvents and bases on the yield (C3-acylation) and selectivity (C3:C2 acylation) of a model reaction (see ESI† for experimental details). | ||
Solvents with various polarities and abilities to dissolve the saccharide substrate were evaluated. As important technical information, among twenty-two solvents included in Fig. 2A, the glucoside 1a alone or in combination with B(OH)3 showed nearly no observable solubility in twelve solvents (including toluene, diethyl ether, and dichloroethane). In these “poor” solvents, the acylation reaction did not proceed well, and C3-acylation products were observed in very low yields (e.g., from nearly no product to around 20% or lower). In six of these solvents (EtOAc, CH3CN, acetone, dimethyl glycol, dioxane, and THF), the glucoside 1a alone showed low but observable solubility. When boric acid was added, the glucoside (and boric acid) was completely dissolved except with EtOAc as the solvent. The last few solvents (DMF, DMAc, NMP and DMSO) could dissolve 1a well. In the latter two scenarios (e.g., when 1a was dissolved alone or in the presence of boric acid), acylation proceeded smoothly with the C3-acylation product (3a) formed in moderate-to-good yields in most cases (with different bases). Notably, in the presence of boric acid, main acylation products were from C3–OH acylation (e.g., 3a) and C2–OH acylation (4a). The C6–OH acylation product (5a) was nearly undetectable (less than 1% or around 1–2% as estimated via HPLC analysis in most cases).
Many inorganic and organic bases common in the laboratory were evaluated. However, the bases did not exhibit effects as dramatic as those of the solvents. In most of the solvents that could dissolve the saccharide substrate well, all of these bases appeared to be effective. In particular, the bases did not show a clear difference with respect to the C3-acylation yield when DMF, DMAC, NMP, or DMSO was used as the solvent. However, with acetonitrile or acetone as the solvent, the effect of different bases on the C3-acylation yield did appear to be significant. Common solvents that lead to optimal yields of C3–OH acylation under these conditions include THF, DMF, DMAc, and NMP (Fig. 2A).
The effects of different solvents and bases on the reaction regio-selectivity (C3–OH:C2–OH acylation) are included in Fig. 2B. We chose reactions where 3a and 4a were formed in appreciable yields to estimate regio-selectivity values. Although the solvents and bases showed effects, the observed ratios of 3a and 4a were more affected by the solvents than the bases. Remarkably, we were very fortunate to find that when THF was used as the solvent, all of the bases (used directly from commercial sources) gave very consistent performances with ratios of 3a and 4a between 7 and 8.
Several further experiments to study the effect of different bases for reactions in THF were then performed (see ESI Table S3†). The bases K3PO4 and Li2CO3 were found to perform slightly better than the bases shown in Fig. 2B. Specifically, the use of K3PO4 in THF led to 3a in 75% yield with a ratio of C3–OH to C2–OH acylation of 8.2. The use of Li2CO3 led to 3a in 77% yield with a ratio of the two acylation products (3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a) of 8.1.
4a) of 8.1.
We also studied the reaction progress using the combination of Li2CO3/THF (Fig. 3). Considering experimental errors in measuring the relatively low yields of the C2-acylation product, the ratios of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a did not change significantly during the reaction. As a technical note, the ratios measured during the first two hours were not accurate due to the very low yield of 4a. The reaction nears completion at around 8 hours with the formation of 3a in close to 80% yield. The yield of 4a was less than 10%, and the formation of C6-acylation adduct 5a was minimal during the reaction (less than 1% yield). Although the presence of water was believed to affect the reaction yield and selectivity value based on our earlier studies,11 under our present protocol we found that rigorous drying of the solvents is not necessary. The use of AR grade THF from a commercial source without any treatment could give nearly the same reaction outcome. Technically, although many solvents (such as CH3CN and DMF) that show good solubilities for saccharide substrates can be potentially optimized to offer acceptable results, THF stands out as a practical choice as it (without special treatment) worked well under a broad range of conditions (such as with the use of different bases). For example, although CH3CN could also become a good choice under certain conditions, careful control of the conditions is necessary, and the reaction outcomes can vary significantly (Fig. 2). In particular, the presence of air and choice of base could have significant effects on the reaction outcome. The use of CH3CN from different sources (presumably with different water contents) could also affect the reaction yield.
4a did not change significantly during the reaction. As a technical note, the ratios measured during the first two hours were not accurate due to the very low yield of 4a. The reaction nears completion at around 8 hours with the formation of 3a in close to 80% yield. The yield of 4a was less than 10%, and the formation of C6-acylation adduct 5a was minimal during the reaction (less than 1% yield). Although the presence of water was believed to affect the reaction yield and selectivity value based on our earlier studies,11 under our present protocol we found that rigorous drying of the solvents is not necessary. The use of AR grade THF from a commercial source without any treatment could give nearly the same reaction outcome. Technically, although many solvents (such as CH3CN and DMF) that show good solubilities for saccharide substrates can be potentially optimized to offer acceptable results, THF stands out as a practical choice as it (without special treatment) worked well under a broad range of conditions (such as with the use of different bases). For example, although CH3CN could also become a good choice under certain conditions, careful control of the conditions is necessary, and the reaction outcomes can vary significantly (Fig. 2). In particular, the presence of air and choice of base could have significant effects on the reaction outcome. The use of CH3CN from different sources (presumably with different water contents) could also affect the reaction yield.
The exact origins of regioselectivity are a complex issue. For reactions affording appreciable yields (Fig. 2B), in the same solvent, the effect of different bases on the regioselectivity is relatively small. For example, with THF as the solvent, the regioselectivity ranges from 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 9
1 to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 when using different bases.
1 when using different bases.
The effects of different solvents on the reaction yield and regioselectivity value are quite significant. For reactions with appreciable yields (Fig. 2B), highly polar solvents (e.g., DMSO and DMF) that can dissolve the saccharide substrate well (without the assistance of boric acid) often lead to lower regioselectivity. This is presumably due to the “background” acylation reactions of saccharides unmodified with boric acid (that yield lower regioselectivity values). In contrast, with less polar solvents (e.g., THF and EtOAc) that do not dissolve saccharides well (without the assistance of boric acid), the reaction gives high regioselectivity. This is because in such less polar solvents, the reaction of “free saccharides” with boric acid is essential to bring the saccharide substrate (as corresponding boronic esters) into the solution for subsequent NHC-catalyzed acylation reactions (with higher regio-selectivity). Therefore, although solvents may play multiple roles (such as forming non-covalent interactions with the substrates and catalysts, as in most organic reactions), the major roles of the solvents in our present system are associated with the solubility of the saccharide substrates (with and without the presence of boric acid).
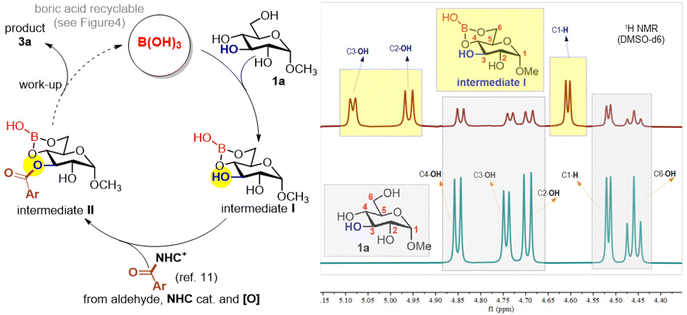
A plausible reaction pathway is illustrated in Scheme 1. Reaction of the saccharide substrate (1a) with boric acid forms corresponding shielded boric ester C4,6–OH groups (intermediate I), as evidenced by 1H NMR studies (Scheme 1, see ESI† for details). This observation is similar to our earlier studies using aryl boronic acids in related NHC-mediated reactions of saccharides.11
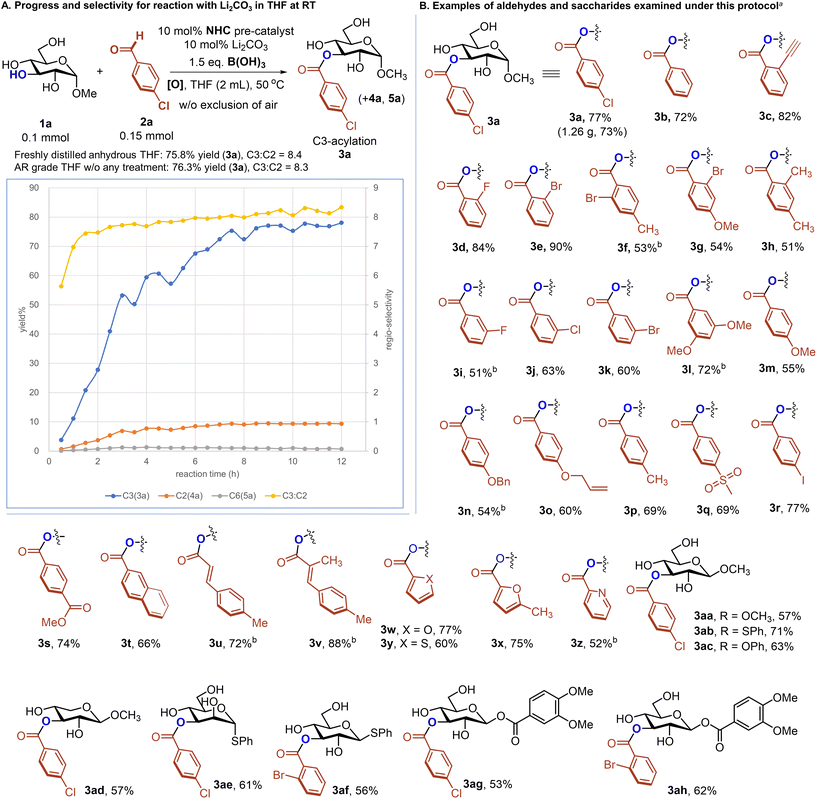
With a practical protocol and detailed understanding of the reaction process, we moved to evaluate the generality of this NHC/B(OH)3-mediated C3–OH acylation protocol by looking into various aldehydes as acylation partners and several common monosaccharides (Fig. 3B). Electron-withdrawing substituents and halogen atoms could be installed on the o-position of the phenyl group of 2a, with target products afforded in excellent yields (3b to 3e). When bromine was introduced at the o-position of aldehyde and electron-donating groups were present at the p-position, the corresponding C3-acylation products were obtained in moderate yields (3f and 3g). However, the presence of a methyl group at the ortho and para position of the aldehyde was beneficial to enhancing the yield of desired products (3h). Electron-withdrawing groups existing at the 3-position of the benzene ring resulted in decreased product yields (3i to 3k), while electron-donating groups at this position could give 3l in a 72% yield. Aromatic aldehyde substrates bearing various p-substituents on the aryl group worked well in this acylation reaction (3m to 3t). The phenyl group of 2a could be switched to a 2-naphthalene group, with the desired product afforded in a 66% yield. The C3–OH acylation could be achieved with an α,β-unsaturated aldehyde (3u and 3v). Good results could also be obtained with aldehydes of other aromatic heterocycles (3w to 3z). Moreover, various kinds of monosaccharides and their analogs have the potential for selective C3–OH acylation (3aa to 3ah). As a technical note, alkyl aldehyde substrates do not work well as the acylation partners under oxidative NHC catalysis. This limitation is associated with the capability of NHC catalysis itself and side reactions of alkyl aldehydes under basic conditions used here.
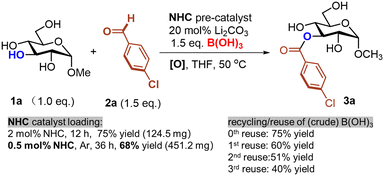
We also performed preliminary studies regarding NHC catalyst loading and possible recycling/reuse of the catalyst and boric acid. For instance, the use of 0.5 mol% NHC catalyst is sufficient to provide acylation product 3a in 68% yield (Fig. 4). The boric acid can be recycled (via simple extraction, see ESI†) as a crude mixture and re-used several times with reasonable performance (Fig. 4). Purification of product 3avia SiO2 chromatography is easily achievable due to the significant polarity difference of 3a and the other component left in the reaction mixture.
Conclusions
In summary, we have developed a simple and greener protocol for C3–OH selective acylation of unprotected monosaccharides. Our method employs a readily available NHC catalyst and the smallest (and most inexpensive and most understood) boric acid to achieve desired reactions with good-to-excellent yields and regio-selectivities. The utilization of boric acid is particularly effective, especially for the C3-acylation of monosaccharides, as it offers transient shielding comparable to that of more intricate (aryl) boronic acids. Our protocol, with simple reagents and easy operation, will reach beyond organic chemists for the preparation of acylated monosaccharides and related building blocks for various applications. On the mechanistic side, we conclude that the solvent has a profound effects on the reaction yield and selectivity. Different bases can also affect the reactions, but on a much smaller scale than what used to be believed. We expect the present study to open new windows in developing practical and green solutions for selective reactions of saccharides and to establish a comprehensive understanding of mechanisms for reactions involving saccharides and other complex molecules.Author contributions
J. Jin: writing – original draft, data curation, methodology; J. Guo, F. Xu, Y. Lv, J. Song: investigation; G. Wang, W. Lv: supervision, formal analysis; T. Li: writing – review & editing, funding acquisition, validation; Y. R. Chi: writing – review & editing, funding acquisition, project administration, validation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding support from the National Key Research and Development Program of China (2022YFD1700300), the National Natural Science Foundation of China (U23A20201, 22071036), the Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules, Department of Education, Guizhou Province [Qianjiaohe KY number (2020)004], the Natural Science Foundation of Guizhou University [Guida Tegang Hezi (2023)23], the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023) at Guizhou University, the Singapore National Research Foundation under its NRF Competitive Research Program (NRF-CRP22-2019-0002), the Singapore Ministry of Education under its MOE AcRF Tier 1 Award (RG70/21, RG84/22), MOE AcRF Tier 2 Award (MOE-T2EP10222-0006), and MOE AcRF Tier 3 Award (MOE2018-T3-1-003), a Chair Professorship Grant, and Nanyang Technological University.Notes and references
- (a) C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357–2364 CrossRef CAS PubMed; (b) H. M. I. Osborn, P. G. Evans and K. Bezouska, in Glycoscience: Chemistry and Chemical Biology, ed. B. O. Fraser-Reid, K. Tatsuta and J. Thiem, Springer Berlin Heidelberg, Berlin, Heidelberg, 2008, pp. 2399–2444 Search PubMed; (c) X. Cao, X. Du, H. Jiao, Q. An, R. Chen, P. Fang, J. Wang and B. Yu, Acta Pharm. Sin. B, 2022, 12, 3783–3821 CrossRef CAS PubMed.
- (a) P. H. Seeberger and D. B. Werz, Nature, 2007, 446, 1046–1051 CrossRef CAS PubMed; (b) B. Ernst and J. L. Magnani, Nat. Rev. Drug Discovery, 2009, 8, 661–677 CrossRef CAS PubMed; (c) P. Sosicka, B. G. Ng and H. H. Freeze, Biochemistry, 2020, 59, 3064–3077 CrossRef CAS PubMed; (d) S. S. Shivatare, V. S. Shivatare and C.-H. Wong, Chem. Rev., 2022, 122, 15603–15671 CrossRef CAS PubMed.
- (a) K. C. Nicolaou, W. M. Dai and R. K. Guy, Angew. Chem., Int. Ed., 2003, 33, 15–44 CrossRef; (b) S. Quideau, D. Deffieux, C. Douat-Casassus and L. Pouységu, Angew. Chem., Int. Ed., 2011, 50, 586–621 CrossRef CAS PubMed; (c) S. I. Elshahawi, K. A. Shaaban, M. K. Kharel and J. S. Thorson, Chem. Soc. Rev., 2015, 44, 7591–7697 RSC; (d) K. Singh and S. S. Kulkarni, J. Med. Chem., 2022, 65, 8525–8549 CrossRef CAS PubMed.
- (a) T. Kawabata and T. Furuta, Chem. Lett., 2009, 38, 640–647 CrossRef CAS; (b) D. Lee and M. S. Taylor, J. Am. Chem. Soc., 2011, 133, 3724–3727 CrossRef CAS PubMed; (c) D. Lee, C. L. Williamson, L. Chan and M. S. Taylor, J. Am. Chem. Soc., 2012, 134, 8260–8267 CrossRef CAS PubMed; (d) H. Takeuchi, K. Mishiro, Y. Ueda, Y. Fujimori, T. Furuta and T. Kawabata, Angew. Chem., Int. Ed., 2015, 54, 6177–6180 CrossRef CAS PubMed; (e) P. Peng, M. Linseis, R. F. Winter and R. R. Schmidt, J. Am. Chem. Soc., 2016, 138, 6002–6009 CrossRef CAS PubMed; (f) G. Xiao, G. A. Cintron-Rosado, D. A. Glazier, B. M. Xi, C. Liu, P. Liu and W. Tang, J. Am. Chem. Soc., 2017, 139, 4346–4349 CrossRef CAS PubMed; (g) S. A. Blaszczyk, G. Xiao, P. Wen, H. Hao, J. Wu, B. Wang, F. Carattino, Z. Li, D. A. Glazier, B. J. McCarty, P. Liu and W. Tang, Angew. Chem., Int. Ed., 2019, 58, 9542–9546 CrossRef CAS PubMed; (h) H. Shibayama, Y. Ueda, T. Tanaka and T. Kawabata, J. Am. Chem. Soc., 2021, 143, 1428–1434 CrossRef CAS PubMed.
- J. Koviach-Cote and A. L. Pirinelli, Chem. Rev., 2018, 118, 7986–8004 CrossRef CAS PubMed.
- (a) C. S. Dias, B. S. Anand and A. K. Mitra, J. Pharm. Sci., 2002, 91, 660–668 CrossRef CAS PubMed; (b) J. Quan, Z. Chen, C. Han and X. Lin, Bioorg. Med. Chem., 2007, 15, 1741–1748 CrossRef CAS PubMed; (c) Q. Wu, A. Xia and X. Lin, J. Mol. Catal. B: Enzym., 2008, 54, 76–82 CrossRef CAS; (d) C. Wu, J. Quan, J. Xie, C. Branford-White, L. Zhu, Y. Yu and Y. Wang, Polym. Bull., 2010, 67, 593–608 CrossRef.
- (a) K. Yamatsugu and M. Kanai, Chem. Rev., 2023, 123, 6793–6838 CrossRef CAS PubMed; (b) P. P. Deshpande and S. J. Danishefsky, Nature, 1997, 387, 164–166 CrossRef CAS PubMed; (c) P. Sears and C. H. Wong, Science, 2001, 291, 2344–2350 CrossRef CAS PubMed; (d) D. A. Griffith and S. J. Danishefsky, J. Am. Chem. Soc., 2002, 112, 5811–5819 CrossRef; (e) C.-W. Cheng, Y. Zhou, W.-H. Pan, S. Dey, C.-Y. Wu, W.-L. Hsu and C.-H. Wong, Nat. Commun., 2018, 9, 5202 CrossRef CAS PubMed; (f) J. A. Rose, S. Mahapatra, X. Li, C. Wang, L. Chen, S. M. Swick and S. B. Herzon, Chem. Sci., 2020, 11, 7462–7467 RSC; (g) K. M. Hoang, N. R. Lees and S. B. Herzon, J. Am. Chem. Soc., 2021, 143, 2777–2783 CrossRef CAS PubMed; (h) M. Kaneko, Z. Li, M. Burk, L. Colis and S. B. Herzon, J. Am. Chem. Soc., 2021, 143, 1126–1132 CrossRef CAS PubMed.
- (a) S. David and S. Hanessian, Tetrahedron, 1985, 41, 643–663 CrossRef CAS; (b) M. Amoretti, C. Amsler, G. Bonomi, A. Bouchta, P. Bowe, C. Carraro, C. L. Cesar, M. Charlton, M. J. T. Collier, M. Doser, V. Filippini, K. S. Fine, A. Fontana, M. C. Fujiwara, R. Funakoshi, P. Genova, J. S. Hangst, R. S. Hayano, M. H. Holzscheiter, L. V. Jørgensen, V. Lagomarsino, R. Landua, D. Lindelöf, E. L. Rizzini, M. Macrì, N. Madsen, G. Manuzio, M. Marchesotti, P. Montagna, H. Pruys, C. Regenfus, P. Riedler, J. Rochet, A. Rotondi, G. Rouleau, G. Testera, A. Variola, T. L. Watson and D. P. van der Werf, Nature, 2002, 419, 456–459 CrossRef CAS PubMed; (c) D. Lee and M. S. Taylor, J. Am. Chem. Soc., 2011, 133, 3724–3727 CrossRef CAS PubMed; (d) D. Lee, C. L. Williamson, L. Chan and M. S. Taylor, J. Am. Chem. Soc., 2012, 134, 8260–8267 CrossRef CAS PubMed; (e) H. Takeuchi, K. Mishiro, Y. Ueda, Y. Fujimori, T. Furuta and T. Kawabata, Angew. Chem., Int. Ed., 2015, 54, 6177–6180 CrossRef CAS PubMed; (f) D. L. Cramer, S. Bera and A. Studer, Chem. – Eur. J., 2016, 22, 7403–7407 CrossRef CAS PubMed; (g) G. Xiao, G. A. Cintron-Rosado, D. A. Glazier, B.-m. Xi, C. Liu, P. Liu and W. Tang, J. Am. Chem. Soc., 2017, 139, 4346–4349 CrossRef CAS PubMed; (h) S. A. Blaszczyk, G. Xiao, P. Wen, H. Hao, J. Wu, B. Wang, F. Carattino, Z. Li, D. A. Glazier, B. J. McCarty, P. Liu and W. Tang, Angew. Chem., Int. Ed., 2019, 58, 9542–9546 CrossRef CAS PubMed; (i) A. L. Featherston, Y. Kwon, M. M. Pompeo, O. D. Engl, D. K. Leahy and S. J. Miller, Science, 2021, 371, 702–707 CrossRef CAS PubMed; (j) H. Shibayama, Y. Ueda, T. Tanaka and T. Kawabata, J. Am. Chem. Soc., 2021, 143, 1428–1434 CrossRef CAS PubMed.
- (a) Z. Huang, X. Huang, B. Li, C. Mou, S. Yang, B. A. Song and Y. R. Chi, J. Am. Chem. Soc., 2016, 138, 7524–7527 CrossRef CAS PubMed; (b) B. S. Li, Y. Wang, R. S. Proctor, Z. Jin and Y. R. Chi, Chem. Commun., 2016, 52, 8313–8316 RSC; (c) B. Liu, J. Yan, R. Huang, W. Wang, Z. Jin, G. Zanoni, P. Zheng, S. Yang and Y. R. Chi, Org. Lett., 2018, 20, 3447–3450 CrossRef CAS PubMed; (d) Y. Liu, Q. Chen, C. Mou, L. Pan, X. Duan, X. Chen, H. Chen, Y. Zhao, Y. Lu, Z. Jin and Y. R. Chi, Nat. Commun., 2019, 10, 1675 CrossRef PubMed; (e) S. Zhuo, T. Zhu, L. Zhou, C. Mou, H. Chai, Y. Lu, L. Pan, Z. Jin and Y. R. Chi, Angew. Chem., Int. Ed., 2019, 58, 1784–1788 CrossRef CAS PubMed; (f) X. Peng, J. Xu, T. Li, Y. R. Chi and Z. Jin, Chem. Sci., 2020, 11, 12533–12539 RSC; (g) X. Lv, J. Xu, C. Sun, F. Su, Y. Cai, Z. Jin and Y. R. Chi, ACS Catal., 2022, 12, 2706–2713 CrossRef CAS; (h) G. Fan, Q. Wang, J. Xu, P. Zheng and Y. R. Chi, Nat. Commun., 2023, 14, 4243 CrossRef CAS PubMed; (i) J. Lv, J. Zou, Y. Nong, J. Song, T. Shen, H. Cai, C. Mou, W. Lyu, Z. Jin and Y. R. Chi, ACS Catal., 2023, 13, 9567–9576 CrossRef CAS.
- (a) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307–9387 CrossRef CAS PubMed; (b) R. S. Menon, A. T. Biju and V. Nair, Chem. Soc. Rev., 2015, 44, 5040–5052 RSC; (c) M. H. Wang and K. A. Scheidt, Angew. Chem., Int. Ed., 2016, 55, 14912–14922 CrossRef CAS PubMed; (d) C. Zhang, J. F. Hooper and D. W. Lupton, ACS Catal., 2017, 7, 2583–2596 CrossRef CAS; (e) K. J. R. Murauski, A. A. Jaworski and K. A. Scheidt, Chem. Soc. Rev., 2018, 47, 1773–1782 RSC; (f) X. K. Chen, H. L. Wang, Z. C. Jin and Y. R. Chi, Chin. J. Chem., 2020, 38, 1167–1202 CrossRef CAS; (g) T. Li, Z. Jin and Y. R. Chi, Sci. China: Chem., 2021, 65, 210–223 CrossRef; (h) J. Lv, Z. Jin, H. Wang and Y. R. Chi, ACS Sustainable Chem. Eng., 2022, 10, 13901–13916 CrossRef CAS; (i) Y. Zhang, H. Cai, X. Gan and Z. Jin, Sci. China: Chem., 2023, 67, 482–511 CrossRef.
- W.-X. Lv, H. Chen, X. Zhang, C. C. Ho, Y. Liu, S. Q. Wu, H. Wang, Z. Jin and Y. R. Chi, Chem, 2022, 8, 1518–1534 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc05005j |
| This journal is © The Royal Society of Chemistry 2024 |