Open Access Article
Open Access ArticleSuperstructure optimization for management of low-density polyethylene plastic waste†
Borja
Hernández
 ab,
Dionisios G.
Vlachos
ab,
Dionisios G.
Vlachos
 ab and
Marianthi G.
Ierapetritou
*ab
ab and
Marianthi G.
Ierapetritou
*ab
aDepartment of Chemical and Biomolecular Engineering, 150 Academy Street, Suite 250, Newark, Delaware 19716, USA. E-mail: mgi@udel.edu
bCenter for Plastics Innovation, University of Delaware, 221 Academy Street, Suite 250, Newark, Delaware 19716, USA
First published on 14th August 2024
Abstract
We introduce a systematic framework centered on superstructure optimization to identify the most efficient economic and environmentally friendly approach for managing plastic waste. Applying the proposed framework to low-density-polyethylene (LDPE) plastic waste, we determine that pyrolysis is the most profitable technology followed by hydroformylation to C4–C8 olefins, and the oligomerization of higher carbon olefins. Coupling the results with geographical information, the selected superstructure has the potential to improve the economics of plastic waste management by approximately $3 per kgLDPE in countries like the United States. On the other hand, the lowest CO2 emission plastic waste management uses solvent-based recycling only when there is significant degradation during mechanical recycling. When plastic waste can be recycled mechanically more than five times, the emissions in mechanical recycling are lower. These technologies collectively contribute to emissions reductions ranging from 1.5 and 3 kgCO2eq. per kgLDPE, for mechanical and solvent-based recycling, respectively.
1. Introduction
Global plastic production is estimated to be around 400 million tons per year worldwide, and it is projected to triple by 2060.1,2 Plastics, in particular single use ones, are utilized for a short time and cannot be converted or reused, and recycling is limited. In Europe, 38% of the total plastics are recycled,3 and in the United States (U.S.), recycling is below 10%.4 Increasing these rates to 80% by 2040 has been recommended by the United Nations5 due to the environmental problems like microplastics,6 CO2 emissions in their life cycle (104 MMtonCO2e per year in the US) and embodied energy required (3.4 EJ per year in US).7 Plastic waste disposed into landfills is associated with a loss opportunity of $7.2 billion per year of market value in the US.7 Among all polymers, low density polyethylene (LDPE) is the most used, around 24% of the market,8 and it is responsible for the majority of the emissions and energy consumption.9 LDPE is also the easiest to separate through mechanical methods since its density is lower than other polymers (e.g. high density polyethylene (HDPE), poly-vinyl chloride (PVC)).10Mechanical recycling stands as the prevailing technology for treating plastic waste, mitigating both economic and environmental impacts. This technology has low processing costs, ∼$0.5 per kg for LDPE,11 but it results in a degraded polymer that cannot be infinitely recycled.12 Alternatively, chemical recycling methods combining solvents and anti-solvents can recover polymers with nearly intact properties.13,14 The process can recover polyethylene terephthalate (PET) at prices of $0.7 per kg,15 but its implementation at industrial scale is limited by the low diffusivity of polymers in solvents, and the solvents employed (e.g. toluene) are toxic, for the plastic to be commercialized latter. Thermochemical technologies are easier to implement at industrial scale.16,17 Pyrolysis possesses environmental and economic advantages and can handle multiple plastic wastes.16,18 The naphtha produced in pyrolysis consists of paraffins, olefins, and aromatics. Olefins can be processed into ethylene and propylene monomers for chemical recycling,19 or higher value products (e.g., fuel oils, diesel,20 lubricants18), known as upcycling. Comparison of the two approaches has shown that upcycling is more competitive than chemical recycling when selling naphtha to refineries without any other processing.21 However, no comparative analysis of alternative products that can be generated from naphtha exists. Olefins and aromatics are the most valuable fractions of naphtha.22 In particular, olefins can be transformed into multiple end-products like plastics, aldehydes, alcohols, or solvents. Gasification is another thermochemical technology widely explored. It produces synthesis gas23 that can be transformed into multiple end products like hydrogen,24 ammonia, or methanol.25 Another conventional technology for plastic depolymerization is hydrothermal liquefaction (HTL),26 which results in gasoline and diesel fuels. Beyond these conventional thermochemical technologies, novel catalytic processes have been demonstrated efficient in depolymerizing plastic waste at mild temperatures. Hydrocracking over platinum catalysts produces gasoline and diesel fuels,27 and hydrogenolysis over ruthenium catalyst depolymerizes the polyolefins into naphtha that can be separated into lube oil and fuel (gasoline and diesel) fractions.28,29
Comparison of all these technologies from economic and environmental perspectives is challenging due to the extensive set of alternative products and technologies. Most studies typically compare a single new technology to a conventional one, using an end-product. These studies have employed different methodologies. Technoeconomic analyses based on process design demonstrated pyrolysis to be preferred over conventional30 and novel catalytic technologies.18 On the other hand, environmental assessments have found that the combination of mechanical recycling and pyrolysis is necessary for the sustainable management of plastic waste.31 Mechanical recycling is economically superior to solvent-based recycling when plastic degradation is low.32 Recent works have included novel upcycling technologies. A process analysis comparison based on fixed products determined hydrogenolysis as the thermochemical technology with the lowest CO2 emissions.18 Meanwhile, a material flow analysis for Europe recently proposed that sustainable management of plastic waste will require mechanical recycling as a main management technology, with hydrocracking and hydrogenolysis contributing less than 5%.33
An integrated supply and process selection framework suggests that novel technologies (in particular hydrocracking) will treat all plastic waste that cannot be managed by mechanical recycling in order to minimize CO2 emissions.34 For all approaches, the processes have been compared for a fixed product in each technology. However, the naphtha from pyrolysis can yield a diverse range of products. The most promising products have recently been identified using a superstructure based on process yields from literature.35 However, the lack of considering mass and energy balances in the separations can lead into improper selection of products. For HDPE, a superstructure optimization study highlighted this role of separation, determining the optimal fractions to be obtained from different pyrolysis technologies.36 However, as remarked by other techno-economic and LCA studies, new technologies have been developed in the last years that can compete with pyrolysis and there is a need on upcycling its naphtha to get more valuable products as recently highlighted by Li et al.37 With the aim of determining the optimal trade-offs this work provides a superstructure study expanding the number of technologies considered.
The formulated superstructure includes the selection of the depolymerization processes and the processing of naphtha. This allows the systematic comparison of the products obtained from all alternative plastic waste upcycling and recycling processes. The selection of the optimal processes and products is performed based on multiple objectives including profit maximization, minimization of global warming potential (GWP) commonly used in other works, and minimization of the Impact on the Ecosystems, defined in Recipe.38 This comparison determines the sensitivity of the technologies selected under multiple environmental indicators, contributing to a nuanced understanding of the decision making in plastic waste management.
2. Methodology
2.1 General description of the methodology
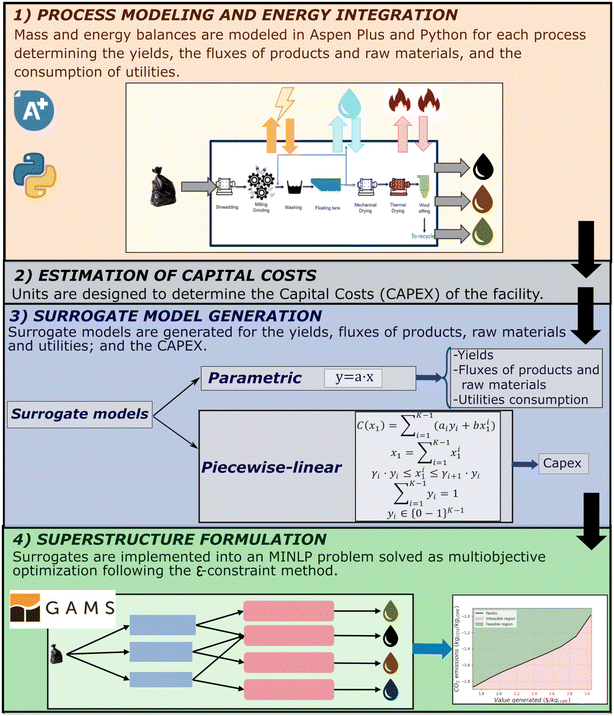
The most profitable or sustainable route is determined using a superstructure implemented as a Mixed Integer Nonlinear Programming problem (MINLP). This requires evaluating and addressing all mass and energy balances and, economic and environmental evaluation for each process. The steps are summarized in Fig. 1.First, a literature search is carried out for the depolymerization and upcycling to determine the processes and products. The search is extended from depolymerization products (naphtha, syngas) into final products. The superstructure's selection of alternative end products is confined to processes where the primary raw material is a constituent of either naphtha or syngas (e.g., styrene is not considered since benzene is not a product obtained in naphtha from the pyrolysis considered). Once the alternatives have been identified, process models are generated (blocks of Fig. 2 and 3). Thermochemical processes are modeled in Aspen Plus® with specifications given in the ESI.† When unit operations are unavailable in Aspen Plus®, processes are modeled with custom equations in Python. Since recycling streams are difficult to handle in superstructure optimization, processes including them are defined as integrated blocks. For example, hydrogenolysis and hydrocracking blocks recycle hydrogen upon separation from the products. Once the processes are modeled, energy integration is performed following the pinch method in each block of Fig. 2 and 3. After heat integration, the fluxes of raw materials, products, and utilities are determined per amount of main raw material, generating a parametric model. Technoeconomic analysis evaluating multiple scales of a plant is performed to determine the CAPEX. The surrogate models for each process and the assumptions for the estimation of the CAPEX are given in the ESI.†
All parametric models for the raw materials, utilities, and fluxes of components are implemented for each block of the optimization problem. The problem is non-convex, and it is formulated as a MINLP optimization problem. Non-linearities are due to the splitters used for dividing the mass flow rates between multiple technologies. For example, as shown in Fig. 2, up to 8 technologies can co-exist for depolymerization, and in Fig. 3, 7 technologies can co-exist for treating the propylene (C3-fraction) from pyrolysis. The components in each of the streams, defined after the depolymerization technology as parameters, are tracked downstream to determine the fluxes of each component in each downstream process. This allows to track the Olefin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
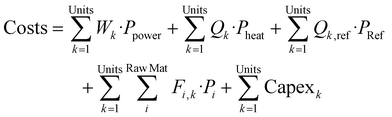
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Paraffin ratio in the downstream processing of the naphtha obtained in pyrolysis. The MINLP problem is solved maximizing the plant profitability, which is determined as the Income generated by selling the products minus the Cost, see eqn (1). The Cost includes the OPEX for the raw materials and utilities and the amortization of the CAPEX. This CAPEX is introduced in the MINLP optimization using a piecewise linear approximation of the cost function, see details of the formulation in the ESI.†
Paraffin ratio in the downstream processing of the naphtha obtained in pyrolysis. The MINLP problem is solved maximizing the plant profitability, which is determined as the Income generated by selling the products minus the Cost, see eqn (1). The Cost includes the OPEX for the raw materials and utilities and the amortization of the CAPEX. This CAPEX is introduced in the MINLP optimization using a piecewise linear approximation of the cost function, see details of the formulation in the ESI.†
| max(Profit) = max(Income − Cost) | (1) |
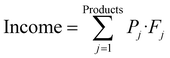 | (2) |
 | (3) |
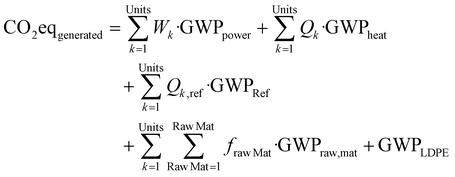
In a similar way to the economic analysis, Life Cycle Assessment (LCA) is performed following a bin-to-gate approach. A second objective function defined in eqn (4) is employed for determining the superstructure that minimizes the Global Warming Potential (GWP). The LCA follows a system expansion approach using Traci v.2.0 method and Ecoinvent® database. In the emissions estimation, only the utilities and materials involved in the continuous operation of the processes are considered, see eqn (5). Eqn (4) also has a credits term that discounts the emissions that the product would generate if it was produced from fossil-based sources. This Credits term is computed as in eqn (6). Details regarding the boundaries of the system and all assumptions involved are given in the ESI.†
| min(GWP) = min(CO2eqgenerated − Credits) | (4) |
 | (5) |
 | (6) |
2.2. Evaluation of alternatives for LDPE waste management
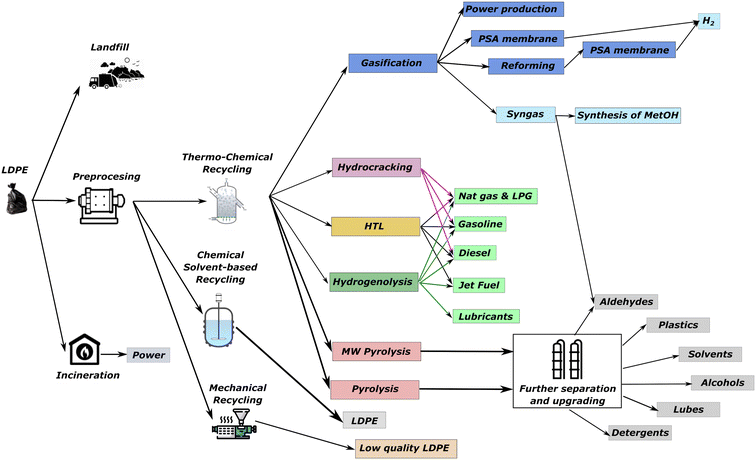
The methodology is applied to determine the optimal management strategy for the LDPE waste. LDPE has lower density than other plastic wastes, being its selective separation easier from mixed plastic waste.11 A summary of technologies is given in Fig. 2 and described in more detail in the ESI.† The model comprises 2504 single variables, 658 binary variables, and 2455 equations and is solved in GAMS with Baron as solver.Conventional treatments (landfill, incineration, and mechanical recycling) are also considered in the superstructure. Thermochemical technologies include pre-treatment (cleaning and sorting) to remove organic compounds. In mechanical recycling, the LDPE recovered cannot be infinitely recycled. Thus, as a base case, a maximum of two recycling loops are considered as a conservative assumption.12 In line with this assumption, the LDPE recovered mechanically has a price of one-half of the virgin LDPE, and the credits obtained for CO2 emissions are also one-half of the ones required in producing virgin LDPE.
Thermochemical depolymerization alternatives include: (1) gasification with steam for syngas production.23 Syngas can be upgraded to hydrogen, ethanol, methanol, or fuels by Fischer–Tropsch synthesis. In this work, we consider hydrogen and methanol as the main products. Methanol requires a H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio of ∼2, requiring the use of a water–gas-shift reactor to adequate the syngas stream from gasification. (2) Hydrothermal liquefaction of the plastic at high pressure and mild temperatures (∼400 °C) to produce naphtha involving a mix of hydrocarbons (mostly paraffins and smaller fraction of olefins and aromatics) that are later separated by distillation into different fractions (gasoline, diesel, and jet fuel). (3) Hydrocracking and (4) hydrogenolysis. Both employ H2 at high pressure and mild temperatures. Hydrocracking uses platinum group catalysts supported by zeolites. The polymer deposits first on the platinum and it cracks subsequently on the acid sites of the zeolite. The naphtha generated is mostly composed of paraffins that are separated in Liquid Petrol Gas (LPG), gasoline, and diesel fractions.18,27 Hydrogenolysis employs Ruthenium catalysts over tungstated zirconia. On the catalyst, the polymer deposits onto ruthenium catalysts, triggering a cascade of scissions along the C–C bonds. As a result, shorter chains of polymers generate a naphtha with a wide distribution of components that are separated in LPG, gasoline, diesel, and lube-oils. These lube oils require to be sent into an oligomerization stage for producing alpha olefins.39,40
CO ratio of ∼2, requiring the use of a water–gas-shift reactor to adequate the syngas stream from gasification. (2) Hydrothermal liquefaction of the plastic at high pressure and mild temperatures (∼400 °C) to produce naphtha involving a mix of hydrocarbons (mostly paraffins and smaller fraction of olefins and aromatics) that are later separated by distillation into different fractions (gasoline, diesel, and jet fuel). (3) Hydrocracking and (4) hydrogenolysis. Both employ H2 at high pressure and mild temperatures. Hydrocracking uses platinum group catalysts supported by zeolites. The polymer deposits first on the platinum and it cracks subsequently on the acid sites of the zeolite. The naphtha generated is mostly composed of paraffins that are separated in Liquid Petrol Gas (LPG), gasoline, and diesel fractions.18,27 Hydrogenolysis employs Ruthenium catalysts over tungstated zirconia. On the catalyst, the polymer deposits onto ruthenium catalysts, triggering a cascade of scissions along the C–C bonds. As a result, shorter chains of polymers generate a naphtha with a wide distribution of components that are separated in LPG, gasoline, diesel, and lube-oils. These lube oils require to be sent into an oligomerization stage for producing alpha olefins.39,40
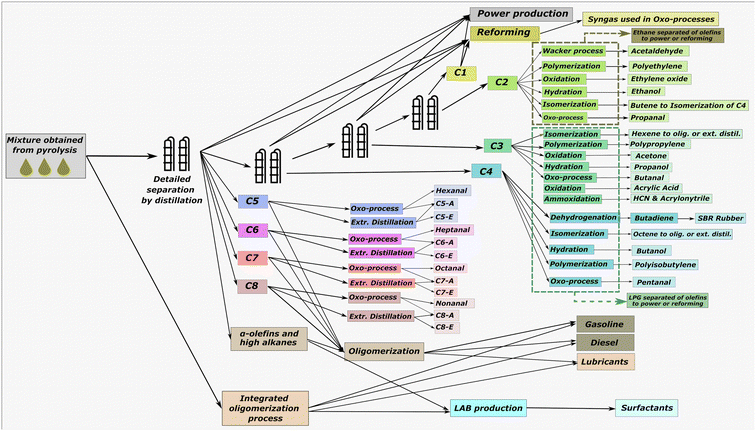
Pyrolysis is also considered for producing naphtha with a high concentration of olefins. Among reactor types,16 we consider a non-catalytic fluidized bed reactor heated by employing N2 as the carrier gas at 550 °C and a microwave (MW) heated reactor with alumina as catalysts that operates at 350 °C.41 The first one presents a stable solution (Technology Readiness Level (TRL) above 7) that can be implemented nowadays, and the second one is a novel process intensification technology with a TRL of 4, which allows us to determine the improvements that could be achieved in the near future. The second reactor has a greater yield to olefins since it has an electric heating that allows of better control of the temperature on the catalyst where LDPE reacts. The alumina catalyst supported on SBA-15 zeolites features acid sites where polymers deposit. Beta-scission reactions occur due to microwave-generated heating concentrated on the catalyst. As the heating primarily affects the catalyst, it reduces the desorption of polymer radicals, thereby limiting the formation of alkanes. The naphtha obtained from both types of pyrolysis is separated into C1 to C8 fractions and one containing all the >C8 compounds, as given in Fig. 3. A sequence of fractional distillation columns can do this separation with a debutanizer followed possibly by a depropanizer, a de-ethanizer, and a demethanizer. After separating the products, multiple alternatives are considered. The alternatives for some fractions like C2 and C3 are extensive. For simplicity, we reduced the large set to those processes where the main contributor to the costs and emissions is one olefin of the naphtha. The description of all processes considered for treating each fraction and a summary of models and costs are given in the ESI.†
2.3. Case studies
The following case studies are considered to explore the effects of different objectives and different scenarios.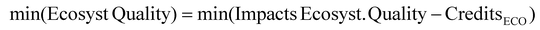 | (7) |
3. Results
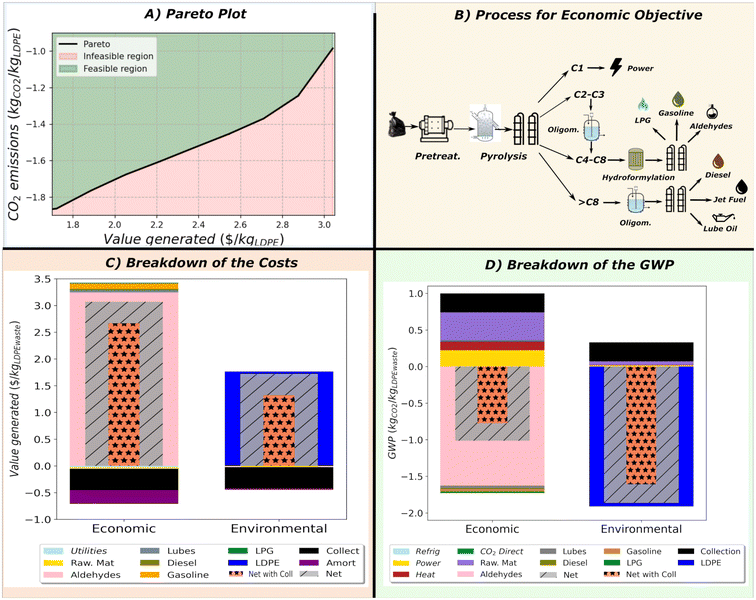
3.1 CASE 1: Base case
Multi-objective optimization with economic and environmental criteria determines the Pareto front, see Fig. 4A. Intensified MW pyrolysis is the most profitable alternative. The resulting naphtha is separated into the fractions of Fig. 4B. Methane is burnt for energy. Ethylene and propylene are oligomerized to alpha olefins. This oligomerization stage is carried out at high pressure, ∼70 bar, employing acid zeolites like HZSM-5.40 Since the cost only lies in the power consumption and the amortization of the CAPEX, the process is selected for generating more valuable fractions, alpha olefins. Butylene and alpha olefins (C5–C8) are converted into aldehydes by hydroformylation. Olefins with more than 8 carbons are oligomerized to lubricant base oils. A breakdown of the costs and emissions is shown in Fig. 4C and D. The selected product highly governs profitability. Aldehydes are more profitable than diesel and gasoline. As recently demonstrated,18 the most relevant cost contributor is plastic waste collection. Regarding emissions, aldehydes significantly contribute to the Raw Materials term mainly produced by the syngas. Hydroformylation on cobalt oxide catalysts requires H2 and CO that react with the alkenes. Hydrogen production is very energy intensive and increases emissions. Apart from the drawbacks due to energy requirements, it is important to note that aldehydes, in particular those with large chains, can have multiple structures depending on the location of the double bound of the olefins.Solvent-antisolvent-based recycling minimizes the GWP and is preferred over mechanical recycling since it does not degrade the plastic waste, allowing infinite recycling. Like pyrolysis, the most relevant cost contributor is the collection of plastic waste. The emissions are low since solvent-based recycling does not depolymerize plastic waste thermally. The emissions in the process stem from the production of solvents and their recovery by distillation. However, distillation can be integrated energetically with the pinch, saving energy. Furthermore, the plastic obtained is of the same quality as the virgin one with an almost 100% of yield, which results in higher credits than aldehydes.
Apart from determining the most environmentally friendly and sustainable solutions, the Pareto front also provides intermediate solutions. In most of the Pareto curve, MW-pyrolysis the preferred technology. As one moves from most profitable to most sustainable solution, the conversion of propylene to acetone replaces the oligomerization to higher olefins and the introduction of the Wacker process for converting ethylene to acetaldehyde. Next, the C4 olefins is converted directly pentanal instead of oligomerizing it, and extractive distillation for the C8 fraction instead of hydroformylation and partially selling the C1 fraction as Natural Gas instead of burning it for power. These changes take place with a gradual increase of the plastic sent to solvent-based based recovery, which gradually increases the environmental performance. This main change of technologies in the Pareto front is highly different from the one determined by Zhao and You. In our work we observe that the use of plastic waste is mostly driven by the choice of more sustainable depolymerization techniques (solvent-based recycling) and the use given to olefins. In the work of Zhao and You, only two process flowsheets where determined with the only change in the use paraffins (more diesel produced for the best environmental solution),36 which generate less profit than olefins, see breakdown in Fig. 4(C) and (D). More details about the superstructure results and the fractions sent to each technology are given in the ESI.†
3.2 CASE 2: Novel versus conventional processes in comparison with current management in different countries
A Pareto front with only conventional technologies selects conventional pyrolysis as the preferred option in most cases. Despite pyrolysis does not provide large olefins content, its product is more valuable than other technologies. Comparing the Pareto curve with the one of novel technologies, the profitability drops nearly by one-half, and the GWP of best environmental objective is reduced by ∼40%, see Fig. 5A and B. The best points achieved with conventional and novel process intensification technologies are also compared with the current use of plastic waste in several regions of the world. The GWP and economic performance in these regions have been computed as given in eqn (8) and (9). The mix of technologies has been taken from the Organization for Economic Cooperation and Development (OECD) and the inventory reported in the ESI† has been employed for each technology. It is important to note that for the GWP results, the data reported for each country corresponds to the diversion term that could be discounted in a system expansion LCA.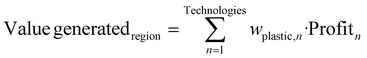 | (8) |
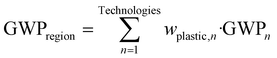 | (9) |
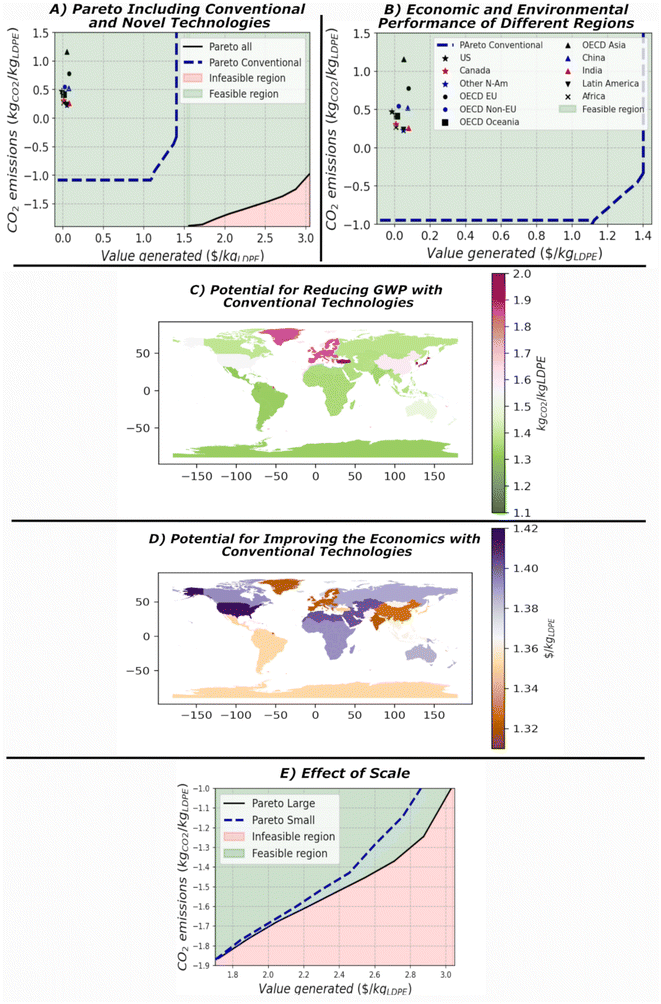 | ||
| Fig. 5 (A) Comparative results for the Pareto frontier versus conventional technologies. (B) Comparison of different regions of the emissions and economic performance of plastic waste management in different regions of the world versus the best economic and environmental solutions determined with conventional technologies.47 (C) Potential that each of the regions has for reducing the GWP if they adopt the best environmental solution. (D) Potential that each of the regions has for generating more money by managing the plastic waste with the optimal superstructure determined here with conventional technologies. (E) Effect of the scale on the Pareto frontier. | ||
Analyzing the GWP, the regions, that perform the worst are those with a high fraction of waste sent to incineration like the OECD countries in Asia and the European Union. Incineration of plastic recovers energy, but it increases the GWP if the CO2 is not captured. The best management for decarbonization requires solvent-based and mechanical recycling. Solvent-based recycling can be hard to scale-up due to the low diffusivity of long chain polymers,44 but mechanical recycling is affordable. In Fig. 5C we present the decarbonization potential in each region showing that the OECD countries in Asia, the emissions can be reduced up to 2 kgCO2 per kgLDPE. This reduction in the emissions does not consider the emissions the variability in the emissions from one country to another. This is a critical factor that has been recently studied in other work and that can result in the selection of different processing technologies in each of the regions.34
In a similar way, the economics can also be improved, see Fig. 5D. OECD countries of the European Union and China, are the ones with more value generated from plastic waste since it is mostly recycled. On the other hand, those regions that landfill the plastic, like the United States, are the ones with the highest economic improvement. They could make $1.44 per kgLDPEwaste by producing aldehydes and lubricant base oil. The superstructure of conventional pyrolysis and Microwave (MW) pyrolysis are different. For conventional pyrolysis, methane burnt, C2 and C3 olefins are oligomerized and alpha olefins are converted to aldehydes, except C7, which is recommended to separate heptane from heptene by extraction to use it as solvent. Fractions higher than C8 should be oligomerized to lube base oils. The differences between the downstream processing of MW pyrolysis and conventional pyrolysis underscore the importance of the work developed here. Recent works have focused on hydroformylation for upgrading the naphtha obtained in pyrolysis.45 Our work shows that a combination of different technologies driven by the composition of naphtha stream can improve economics. If the naphtha does not have a higher content of olefins, it is suggested to separate the olefins and paraffins and sell the olefins directly as solvent. Processing olefins by hydroformylation requires a new facility with significant CAPEX if the amount processed is small. In addition, hydroformylation is also affected by the cost of H2. Mechanical recycling minimizes emissions and the combination of pyrolysis with mechanical recycling provides intermediate trade-offs. For more sustainable options the fraction sent to mechanical recycling should increase. More details about the technologies selected at intermediate points of the Pareto curve, and the breakdown of the costs and emissions are given in the ESI.†
3.3 CASE 3 Degradation in mechanical recycling
Solvent-based recycling is preferred over mechanical recycling since the polymer recovered is not degraded in the base case (CASE 1). However, mechanical recycling was assumed under its most pessimistic scenario. Increasing the number of loops that LDPE can be recycled mechanically, at 6 loops we obtain that mechanical recycling is preferred against solvent-based recycling for minimizing the GWP. More detailed results of the Pareto front are given in the ESI,† with similar results to the base case. Economically, mechanical recycling is more competitive than solvent based recycling. Easier to scale-up and does not involve toxic compounds. Solvent based recycling employs toluene as a solvent, which may limit the selling potential of the recycled polymer in some countries, like the European Union.463.4 CASE 4: Effects of scale-up
The Pareto curves for different scales are given in Fig. 5E, illustrating that smaller scales reduce profitability. In particular, this reduction is more significant in cases on the right-hand side of the Pareto, where profit is larger. The difference between both scales is due to the CAPEX, which smaller amortization contribution at large scales.3.5 CASE 5: Alternative environmental metrics
Apart from the GWP, plastic waste has other environmental impacts, such as the eutrophication and photo-oxidation potential, due to the chemicals used in washing the plastic waste and downstream processes. With the aim of accounting for additional environmental impacts, the Ecosystems Quality End-point indicator from Recipe has been employed.38 By optimizing the superstructure, the process that minimizes the impact on the ecosystem is surprisingly the same that maximizes profit. The superstructure obtained uses MW pyrolysis is preferred followed by hydroformylation and oligomerization, Fig. 4B.4. Conclusions
This paper has presented a superstructure optimization approach for determining the optimal management of low-density polyethylene waste. The superstructure considers all the thermochemical, chemical, and conventional technologies and those downstream technologies for processing the naphtha obtained from thermochemical depolymerization into final refinery products. With sufficient information, future works can expand the superstructure including final transformation into consumer products, e.g., aldehydes to perfumes or adhesives. The work also considers the potential for improving the management of plastic waste in terms of economic and environmental objectives for different regions of the world. From these analyses, the following conclusions are obtained:HIGH valuable products can be generated from plastic waste facilitating the introduction of management technologies to solve the plastic waste problem. The most profitable route suggests using pyrolysis as desired technology, where the naphtha generated is utilized to produce C5–C9 aldehydes that can be used in the perfume industry. The fraction with a carbon number higher than C8 is suggested to be oligomerized to produce lube oil. Increasing the olefins to paraffins ratio is critical to achieving a higher value from plastic waste. The development of novel technologies that increase this ratio can significantly increase the benefits obtained from plastic waste and subsequently increase the percentage of plastic treated. The use of pyrolysis is not only limited to the production of aldehydes but as it is found for different points of Pareto frontier. The use of pyrolysis in the Pareto set under different objectives including environmental metrics, remarks the importance of upcycling by this technology in the upcoming years.
The composition of the naphtha obtained from pyrolysis is highly correlated with the downstream technologies: The downstream technologies selected when plastic waste is treated by conventional pyrolysis differ from those selected for MW pyrolysis. The composition in olefins is critical for determining which downstream technologies must be employed. Very valuable products like aldehydes are suggested for all the alpha olefins when the Olefin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Paraffin ratio is high. However, if this ratio is low simpler processing like extractive distillation is recommended. The superstructure presented suggests these products as the most profitable ones with the current market prices. However, the plastic waste transformed to aldehydes can be limited in a specific region. In future works, region specific conditions (e.g. collection system and product consumption) can be addressed if sufficient data is available about the market size of each of the products generated in the superstructure. This will provide more insights on what should be done with plastic waste, which is a global problem but with regional specificness as reported in other works.33
Paraffin ratio is high. However, if this ratio is low simpler processing like extractive distillation is recommended. The superstructure presented suggests these products as the most profitable ones with the current market prices. However, the plastic waste transformed to aldehydes can be limited in a specific region. In future works, region specific conditions (e.g. collection system and product consumption) can be addressed if sufficient data is available about the market size of each of the products generated in the superstructure. This will provide more insights on what should be done with plastic waste, which is a global problem but with regional specificness as reported in other works.33
NOVEL process technologies have the potential to double profitability and cut down emissions by at least 40%. Introducing novel reactors that increase the olefins ratio is critical to improve the performance of plastic waste management. We demonstrate that scaling-up MW pyrolysis leads to economically competitive and more environmentally friendly solution than conventional plastic waste pyrolysis since it provides better control of the temperature in the reactor. The better control of temperature makes the process more efficient for value-added products that can double the value generated per every kg of plastic waste treated. The choice of catalyst is also an important consideration. Determining the optimal catalyst for pyrolysis of every plastic waste is challenging. First, catalysts should be robust to deal with mixed streams, and/or novel separations processes should precede the reaction to handle impurities as proposed in recent work of Kots et al.48 for the case of poly-vinyl-chloride (PVC).
Solvent–antisolvent chemical recycling is critical for the sustainable management of plastic waste. Chemical solvent-based recycling is the most environmentally friendly technology. Solvent-based recycling is preferred over mechanical recycling when the number of recycling loops for low-density polyethylene in mechanical recycling is below 6. However, mechanical recycling is cheaper. Reducing the degradation of plastic in mechanical recycling must be studied. Similarly, it is important to track plastic properties in chemical recycling. Smaller particles can facilitate mass transfer, and therefore scale-up of solvent based recycling. However, shredding to very fine particles can result in significant degradation of the polymer. Determining the extend of this degradation is critical for evaluating if the process is easily scalable. Another barrier to solvent-based recycling is the utilization of toxic compounds like toluene that can limit the commercialization of the recovered polyethylene for food packaging in some regions like Europe. Finding alternative solvents is necessary to ensure full commercialization of the products from plastic waste.
Regions must modify their treatment technologies for reducing the GWP for plastic waste management. Regions like the OECD countries of the European Union and Asia have lower landfilling rate, but the fraction sent to incineration results in higher CO2 emissions than other regions that landfill the plastic. Implementing sustainable technologies for plastic waste management is required in countries that landfill or incinerate plastic waste. Minimizing the fraction sent to incineration is challenging since it depends on developing an adequate infrastructure with the required separation techniques. Education and consumer responsibility are also necessary since plastics waste must be adequately placed in the proper bin to avoid diversion to organic waste incineration plants. An alternative solution calls for increasing the percent of bio-based plastics since they are CO2 neutral although there are obvious limitations due to the high plastics demand. It is thus necessary that those countries implement more ambitious programs for separating the plastic waste from the remaining waste streams and increase the rate of plastic waste sent to mechanical recycling or solvent-based recycling.
Countries can unveil a tremendous economic opportunity in plastic waste upcycling. Apart from the reduction of the GWP, investing in upcycling technologies can be a great economic opportunity. In this context, countries like the United States, where plastic is mainly landfilled, can increase the value of plastic waste by ∼$1.4 per kgLDPE. This solution will require coordination between multiple institutions since upcycling can also be seen as a great opportunity for fossil fuel companies that can divert plastic waste from recycling to upcycling. Although upcycling technologies are more profitable, recycling is more environmentally friendly. Defining new policies that determine the suitable trade-off between upcycling and recycling technologies is a challenge to be considered in the upcoming years.
Abbreviations
| CAPEX | Capital costs |
| GWP | Global warming potential |
| HDPE | High density polyethylene |
| HTL | Hydrothermal liquefaction |
| LCA | Life cycle assessment |
| LDPE | Low density poly-ethylene |
| LPG | Liquid petrol gases |
| MINLP | Mix-integer non-linear programming |
| MW | Micro-wave |
| OECD | Organization for economic cooperation and development |
| OPEX | Operating costs |
| PET | Poly-ethylene-terephthalate |
| PVC | Poly-vinyl-chloride |
| TRL | Technology readiness level |
| US | United States |
Symbols
| Capexk | Capital cost of every process block l of the superstructure |
| Costs | Overall cost of the system |
| Co2eqgenerated | CO2 equivalent emissions generated in all the processes |
| Credits | CO2 equivalent credits generated by products substitution |
| CreditsECO | Ecosystem Quality credits generated by products substitution |
| Ecosyst Quality | Overall value of the Ecosystems Quality indicator |
| F j | Flux of product j produced in the system |
| F i,k | Flux of raw material i consumed in a process k |
| GWP | Global warming potential |
| GWPheat | Global warming potential indicator for heat |
| GWPj | Global warming potential indicator of a product j |
| GWPLDPE | Global warming potential indicator of LDPE |
| GWPn | Global warming potential of every technology n |
| GWPpower | Global warming potential indicator for power |
| GWPraw,mat | Global warming potential indicator of a raw material |
| GWPRef | Global warming potential indicator for refrigeration |
| GWPregion | Global warming potential estimated in every region of the OECD |
| Impacts Ecosyst. Quality | Positive impacts generated in the processes in the Ecosystem Quality indicator |
| Income | Overall income of the system |
| Profit | Overall profit of the system |
| Profitn | Profit of every technology n |
| P heat | Price of heat |
| P i | Price of each raw material (i) |
| P j | Price of each product j. |
| P power | Price of power |
| P Ref | Price of refrigeration |
| Q k | Heat consumption in each process block k |
| Q k,ref | Heat of refrigeration in each process block k |
| Value generatedregion | Value generated in every region per kg of plastic waste treated |
| w plastic,n | Fraction of plastic waste sent to every technology n |
| W k | Power consumption in a process k |
Subscripts
| Heat | Referred to heat |
| i | Set of raw materials |
| j | Set of products |
| k | Set of process blocks and units |
| LDPE | Referred to LDPE |
| n | Set of technologies involved as alternatives in the OECD reports |
| Power | Referred to power |
| Raw Mat | Referred to raw materials |
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Science Foundation, award numbers OIA – 2119754 and CBET-2217472.References
- Ellen-Macarthur-Foundation, available in:https://ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed 1st December 2023).
- C. M. Rochman, M. A. Browne, B. S. Halpern, B. T. Hentschel, E. Hoh, H. K. Karapanagioti, L. M. Rios-Mendoza, H. Takada, S. Teh and R. C. Thompson, Nature, 2013, 494, 169–171 CrossRef CAS PubMed.
- Eurostat, Plastic packaging waste: 38% recycled in 2020 – Products Eurostat News – Eurostat, https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20221020-1, (accessed 29 July 2023).
- U.S. EPA, Plastics: Material-Specific Data | US EPA, https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data, (accessed 29 July 2023).
- United Nations News, New UN ‘roadmap’ shows how to drastically slash plastic pollution | UN News, https://news.un.org/en/story/2023/05/1136702, (accessed 29 July 2023).
- X. Fei, Y. Guo, Y. Wang, M. Fang, K. Yin and H. He, Nat. Rev. Earth Environ., 2022, 3, 733–735 CrossRef.
- A. Milbrandt, K. Coney, A. Badgett and G. T. Beckham, Resour., Conserv. Recycl., 2022, 183, 106363 CrossRef.
- J. D. Chea, K. M. Yenkie, J. F. Stanzione and G. J. Ruiz-Mercado, AIChE Annu. Meet., Conf. Proc., 2021 Search PubMed.
- S. R. Nicholson, N. A. Rorrer, A. C. Carpenter and G. T. Beckham, Joule, 2021, 5, 673–686 CrossRef CAS.
- D. Civancik-Uslu, T. T. Nhu, B. Van Gorp, U. Kresovic, M. Larrain, P. Billen, K. Ragaert, S. De Meester, J. Dewulf and S. Huysveld, Resour., Conserv. Recycl., 2021, 171, 105633 CrossRef CAS.
- M. Larrain, S. Van Passel, G. Thomassen, B. Van Gorp, T. T. Nhu, S. Huysveld, K. M. Van Geem, S. De Meester and P. Billen, Resour., Conserv. Recycl., 2021, 170, 105607 CrossRef CAS.
- O. Dogu, M. Pelucchi, R. Van de Vijver, P. H. M. Van Steenberge, D. R. D′hooge, A. Cuoci, M. Mehl, A. Frassoldati, T. Faravelli and K. M. Van Geem, Prog. Energy Combust. Sci., 2021, 84, 100901 CrossRef.
- T. W. Walker, N. Frelka, Z. Shen, A. K. Chew, J. Banick, S. Grey, M. S. Kim, J. A. Dumesic, R. C. Van Lehn and G. W. Huber, Sci. Adv., 2020, 6, eaba7599 CrossRef PubMed.
- J. G. Poulakis and C. D. Papaspyrides, Resour., Conserv. Recycl., 1997, 20, 31–41 CrossRef.
- A. del C. Munguía-López, D. Göreke, K. L. Sánchez-Rivera, H. A. Aguirre-Villegas, S. Avraamidou, G. W. Huber and V. M. Zavala, Green Chem., 2023, 25, 1611–1625 RSC.
- H. Li, H. A. Aguirre-Villegas, R. D. Allen, X. Bai, C. H. Benson, G. T. Beckham, S. L. Bradshaw, J. L. Brown, R. C. Brown, V. S. Cecon, J. B. Curley, G. W. Curtzwiler, S. Dong, S. Gaddameedi, J. E. García, I. Hermans, M. S. Kim, J. Ma, L. O. Mark, M. Mavrikakis, O. O. Olafasakin, T. A. Osswald, K. G. Papanikolaou, H. Radhakrishnan, M. A. Sanchez Castillo, K. L. Sánchez-Rivera, K. N. Tumu, R. C. Van Lehn, K. L. Vorst, M. M. Wright, J. Wu, V. M. Zavala, P. Zhou and G. W. Huber, Green Chem., 2022, 24, 8899–9002 RSC.
- Plastic-Energy, available in:https://plasticenergy.com/plastic-energy-completes-e145-million-fundraise-to-accelerate-global-expansion-of-recycling-technology-and-plants/(accessed: 31st December 2022).
- B. Hernández, P. Kots, E. Selvam, D. G. Vlachos and M. G. Ierapetritou, ACS Sustainable Chem. Eng., 2023, 11, 7170–7181 CrossRef.
- U. R. Gracida-Alvarez, O. Winjobi, J. C. Sacramento-Rivero and D. R. Shonnard, ACS Sustainable Chem. Eng., 2019, 7, 18254–18266 CrossRef CAS.
- A. Fivga and I. Dimitriou, Energy, 2018, 149, 865–874 CrossRef CAS.
- M. Larrain, S. Van Passel, G. Thomassen, U. Kresovic, N. Alderweireldt, E. Moerman and P. Billen, J. Cleaner Prod., 2020, 270, 122442 CrossRef CAS.
- G. Yadav, A. Singh, A. Dutta, T. Uekert, J. S. DesVeaux, S. R. Nicholson, E. C. D. Tan, C. Mukarakate, J. A. Schaidle, C. J. Wrasman, A. C. Carpenter, R. M. Baldwin, Y. Román-Leshkov and G. T. Beckham, Energy Environ. Sci., 2023, 16, 3638–3653 RSC.
- G. Lopez, M. Artetxe, M. Amutio, J. Alvarez, J. Bilbao and M. Olazar, Renewable Sustainable Energy Rev., 2018, 82, 576–596 CrossRef CAS.
- A. A. Al-Qadri, U. Ahmed, A. G. A. Jameel, U. Zahid, M. Usman and N. Ahmad, Polymers, 2022, 14, 2056 CrossRef CAS PubMed.
- K. Prifti, A. Galeazzi and F. Manenti, Ind. Eng. Chem. Res., 2022, 62, 6175 Search PubMed.
- K. Jin, P. Vozka, G. Kilaz, W. T. Chen and N. H. L. Wang, Fuel, 2020, 273, 117726 CrossRef CAS.
- S. Liu, P. A. Kots, B. C. Vance, A. Danielson and D. G. Vlachos, Sci. Adv., 2021, 7, 8283–8304 CrossRef PubMed.
- J. E. Rorrer, C. Troyano-Valls, G. T. Beckham and Y. Román-Leshkov, ACS Sustainable Chem. Eng., 2021, 9, 11661–11666 CrossRef CAS.
- C. Wang, K. Yu, B. Sheludko, T. Xie, P. A. Kots, B. C. Vance, P. Kumar, E. A. Stach, W. Zheng and D. G. Vlachos, Appl. Catal., B, 2022, 319, 121899 CrossRef CAS.
- R. R. Bora, R. Wang and F. You, ACS Sustainable Chem. Eng., 2020, 8, 16350–16363 CrossRef CAS.
- M. Bachmann, C. Zibunas, J. Hartmann, V. Tulus, S. Suh, G. Guillén-Gosálbez and A. Bardow, Nat. Sustain., 2023, 6, 599–610 CrossRef.
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, ACS Sustainable Chem. Eng., 2023, 11, 965–978 CrossRef CAS.
- I. S. Lase, D. Tonini, D. Caro, P. F. Albizzati, J. Cristóbal, M. Roosen, M. Kusenberg, K. Ragaert, K. M. Van Geem, J. Dewulf and S. De Meester, Resour., Conserv. Recycl., 2023, 192, 106916 CrossRef CAS.
- O. Badejo, B. Hernández, D. G. Vlachos and M. G. Ierapetritou, Sustain. Prod. Consum., 2024, 47, 460–473 CrossRef.
- Z. Chen, Y. Kimura and D. T. Allen, ACS Sustainable Chem. Eng., 2023, 11, 9394–9402 CrossRef CAS.
- X. Zhao and F. You, AIChE J., 2021, 67, e17127 CrossRef CAS.
- H. Li, J. Wu, Z. Jiang, J. Ma, V. M. Zavala, C. R. Landis, M. Mavrikakis and G. W. Huber, Science, 2023, 381, 660–666 CrossRef CAS PubMed.
- M. Goedkoop, R. Heijungs, A. De Schryver, J. Struijs and R. Van Zelm, ReCiPe, 2008 Search PubMed.
- C. Wang, T. Xie, P. A. Kots, B. C. Vance, K. Yu, P. Kumar, J. Fu, S. Liu, G. Tsilomelekis, E. A. Stach, W. Zheng and D. G. Vlachos, J. Am. Chem. Soc., 2021, 1, 1422–1434 CAS.
- C. S. Hsia Chen, B. Heights and S. A. Tabak, US Pat., US4568786A, 1985.
- E. Selvam, P. A. Kots, B. Hernandez, A. Malhotra, W. Chen, J. M. Catala-Civera, J. Santamaria, M. Ierapetritou and D. G. Vlachos, Chem. Eng. J., 2023, 454, 140332 CrossRef CAS.
- V. Dua and E. N. Pistikopoulos, Ann. Oper. Res., 2000, 99, 123–139 CrossRef.
- National Institute for Public Health and the Environment, LCIA: the ReCiPe model | RIVM, https://www.rivm.nl/en/life-cycle-assessment-lca/recipe, (accessed 3 August 2023).
- R. Kol, R. Denolf, G. Bernaert, D. Manhaeghe, E. Bar-Ziv, G. W. Huber, N. Niessner, M. Verswyvel, A. Lemonidou, D. S. Achilias and S. De Meester, ACS Sustainable Chem. Eng., 2024, 12, 4619–4630 CrossRef CAS PubMed.
- H. Li, J. Wu, Z. Jiang, J. Ma, V. M. Zavala, C. R. Landis, M. Mavrikakis and G. W. Huber, Science, 2023, 381, 660–666 CrossRef CAS PubMed.
- European Union, Commission Regulation (EU) 2022/1616 of 15 September 2022 on recycled plastic materials and articles intended to come into contact with foods, and repealing Regulation (EC) No 282/2008, https://eur-lex.europa.eu/eli/reg/2022/1616/oj, (accessed 17 August 2023).
- OECD, The current plastics lifecycle is far from circular, https://www.oecd.org/environment/plastics/plastics-lifecycle-is-far-from-circular.htm, (accessed 15 August 2023).
- P. A. Kots, B. C. Vance, C. M. Quinn, C. Wang and D. G. Vlachos, Nat. Sustain., 2023, 6, 1258–1267 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc00339j |
| This journal is © The Royal Society of Chemistry 2024 |