Open Access Article
Open Access ArticleAdvancing sustainable end-of-life strategies for photovoltaic modules with silicon reclamation for lithium-ion battery anodes
Owen
Wang†
 a,
Zhuowen
Chen†
a,
Zhuowen
Chen†
 b and
Xiaotu
Ma
b and
Xiaotu
Ma
 *c
*c
aActon-Boxborough Regional High School, 36 Charter Road, Acton, MA, USA
bSchool of Business, Worcester Polytechnic Institute, 100 Institute Road, Worcester, MA, USA
cDepartment of Mechanical and Materials Engineering, Worcester Polytechnic Institute, 100 Institute Road, Worcester, MA, USA. E-mail: xma3@wpi.edu
First published on 12th February 2024
Abstract
Solar panels are an ever-growing solution to generate clean energy. Lots of solar panels are popping up on rooftops, next to highways, and in massive solar farms. Unfortunately, all of these solar panels degrade over time and many need to be disposed of once as they reach their 25-year lifespan. However, they are tightly constructed in order to have such a long life, which makes recycling difficult. In addition, the recovered silicon is limited by its purity and cannot be directly reused in solar cells unless it goes through a costly purification process. Thus, it is necessary to explore new applications for recovered silicon, like its use as anode materials for lithium-ion batteries (LIBs). Although this alternative avenue has garnered interest, comprehensive studies assessing its feasibility, environmental implications, and influence on the economy and supply chain are sparse. In this study, we offer a holistic overview of the current state of solar panel recycling, critically examine its technical viability, and provide an in-depth analysis of the associated environmental impact and economic and supply chain ramifications. This serves as a foundational guide for shaping future research in solar panel recycling.
Introduction
To achieve net zero by 2050, coal, gas, and oil-fired power plants are being replaced by renewable energy sources to reduce carbon emissions.1 Among the renewable energy sources, photovoltaic (PV) energy has emerged as a reliable and widely used renewable energy source. It has helped reduce greenhouse gas emissions and provided low-cost electricity. From 2000 to 2020, the global PV capacity has grown from 1.4 GW to 760 GW.2 Currently, it generates almost 4% of global electricity, and it is projected to continue growing in the future.2 However, at the end of their lives, solar panels bring the challenge of disposal: the cumulative amount of solar panel waste is predicted to be 80 million tons in 2050.3 Four types of solar modules are currently used commercially: crystalline silicon (c-Si), cadmium telluride (CdTe), copper indium gallium selenide (CuInxGa1−xSe2 or CIGS), and amorphous silicon (a-Si).4 Among these types, c-Si solar modules compose more than 90% of the global PV market.3 Therefore, c-Si module recycling is the most pressing.However, since solar panels are designed to last for around 25 years,5 they are not constructed for easy dismantling.2,3 Currently, the main disassembly method is a mechanical process. After removing aluminum frames and junction boxes, recyclers often simply shred the rest and then separate and sell them as low-value products, which can recover up to 85% of the mass of a panel, including aluminum, glass, and copper.2 However, the solar wafers, including solar grade silicon and other metals, are discarded because of their low added value and the high cost of complicated purification processes, presenting a significant hurdle in their recycling process.5 In particular, the purity of waste silicon is insufficient for reuse in solar cells, which necessitates a staggering 99.9999% (6 N) purity. However, depending on the type of solar cell, they may contain boron (B), phosphorus (P), silver (Ag), aluminum (Al) or silicon nitride (SiNx). As a result, the recovered silicon must undergo the costly Siemens method, adding to the expense of the recycling process to reach the quality and purity of solar-grade silicon.6,7 Finally, the landfill is an even worse disposal method. The presence of lead (Pb) and tin (Sn) will cause environmental concerns without proper disposal.
A potential solution to this problem is to find innovative applications for the recovered silicon. Silicon is incredibly versatile, yet its high-value applications, such as semiconductors, generally demand the same stringent purity levels.7 However, a promising avenue appears to be its use as an anode material in lithium-ion batteries (LIBs), which doesn't stipulate such high purity requirements. Moreover, the impurities found in discarded solar cells, such as B, P, and Ag, have been confirmed to enhance the stability of the silicon anode.8–10 So, the complexity of the purification process could be diminished, paving the way for a more simplified, cost-effective, and less energy-intensive recycling process. Therefore, this approach, which academia has shown interest in, presents a feasible and economically viable pathway for harnessing recuperated silicon.6,7,11
Herein, we advocate for a series of research and development practices that could feasibly incorporate recovered Si into LIBs. A comprehensive overview of the existing status and obstacles associated with solar panel recycling will be provided. The technical feasibility of reusing waste solar panel Si in LIBs will be investigated. The potential ramifications for the supply chain, along with a detailed evaluation of the possible economic advantages and environmental impacts, will be assessed. The purpose of this paper is to inspire innovative thought and provoke fresh ideas within the area of solar panel recycling, potentially laying the groundwork for more sustainable patterns of energy generation and utilization.
Status and challenges of recycling solar panels
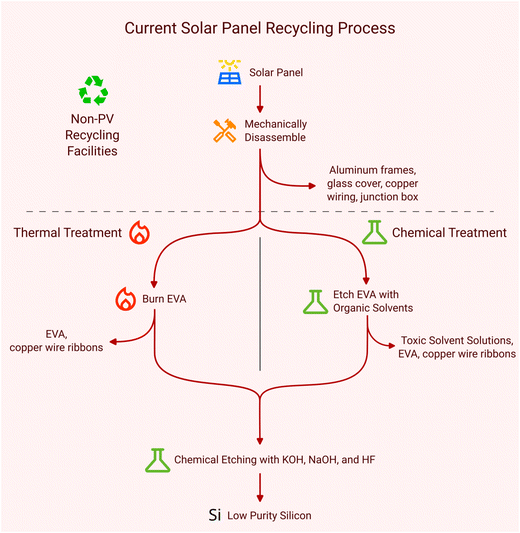
Currently, PV recycling mainly involves two steps: disassembly and purification. Although there are thousands of models of Si PV panels, they generally share the same basic design. The sandwich structure solar cells, composed of aluminum, silicon and silver, are connected into modules by copper wires soldered with Pb and Sn. Then, these modules are sandwiched between two layers of poly(ethylene-co-vinyl acetate) (EVA) to ensure a weatherproof seal. Finally, it is sandwiched again between a sheet of glass on top, polyethylene terephthalate (PET) and poly(vinyl fluoride) (PVF) behind, and surrounded with Al frames. This sturdy structure provides an extensive lifespan, but it results in challenges during disassembly. In the disassembly process, removing the junction box and Al frame is the easiest part and most recycling facilities stop there. Removing the glass cover is the most challenging part since the EVA is hard to remove or decompose.Currently, there are three main disassembly methods to tease off the glass cover, including mechanical, chemical, and thermal treatments. The different disassembly methods will lead to different difficulties in the purification step. Mechanical processes dominate the recycling industry because of their simplicity and low cost. After the aluminum frame and junction box are removed, many PV recycling facilities shred or incinerate the rest together and conduct simple separation by size or density.2,4 Then, the glass cullet and low purity silicon mixed with silver are sold as low value recycled products due to complicated refining processes. However, the value of Ag and Si is over 50% of the value of a typical Si PV cell,2 which means that the main reason for an unprofitable PV recycling process is unrecyclable Si and Ag.
Therefore, alternative methods, such as pyrolysis12 and chemical treatments,12 are designed to gently peel off glass and solar cells for recycling Si and Ag in solar cells by removing or extracting EVA. EVA is a substance frequently used to encapsulate solar cells, protecting the PV panels.13 This encapsulation complicates the separation of the glass cover, back sheet, and the recycling of the solar cell. Consequently, most PV recycling facilities typically only recycle easy-to-remove components. As shown in Fig. 1, chemical treatments employ solvents to extract the EVA.12 EVA can swell, dissolve and separate in most organic solvents, but due to the limited contact areas, the reaction time is longer than expected. Kumar Trivedi et al.14 investigated ten organic solvents and conducted a comparative study of EVA swelling during an interaction between organic solvents and EVA. Prasad et al.15 studied the optimization of parameters, including efficient solvent investigation, the best position for dissolution, temperature effects on solubility and saturation studies of EVA. However, these methods face cost and safety concerns. The solvents, while effective, come at a high price and pose significant threats to both human health and the environment, making them less desirable.
On the other hand, thermal treatments involve heating the panels in an oven for a specific duration.16 Upon completion, the encapsulant, copper wire ribbons, and the actual cell can be separated and processed.16 Although thermal methods prove to be more economically viable and safer when executed correctly, they have a high energy cost17 and produce CO2 emissions18 and may result in harmful gas generation because of the decomposition of the back sheet.19 Dobra et al.16 claimed that it took only 33 minutes to remove EVA at 600 °C. Fiandra et al.20 removed EVA after milling off the back sheet at 500 °C for 1 h. In addition, based on thermal treatment, researchers developed many interesting but efficient processes. NPC Japan has developed commercial equipment using a heated blade to melt the EVA layer to separate glass from other materials.21 Li et al.5 utilized a laser to weaken the adhesive strength of the back EVA and remove the back sheet. However, these pyrosis methods are still limited by their high energy cost and the possibility of harmful gas generation.
As mentioned at the beginning, the purification step faces different challenges with different disassembly methods. Similar to the PV panel structure, the solar cell is also a sandwich structure: the top is an antireflection layer of SiNx with front contact of Ag and Cu ribbons (Cu ribbons always contain some Pb and Sn, which are harmful to the environment), the middle is a silicon wafer and part of it with P or B doped, and the bottom is a passivation layer of SiO2 or SiNx and rear contact of Al.22 However, during the regeneration process of solar cells, the antireflection layer, front contact, passivation layer and rear contact need to be removed. The dopants of P and B have to be removed from the Si wafer as well. Therefore, thermal and chemical treatments, including hot blade and laser methods, are more welcome because they can obtain intact solar cells. In this case, the surface contact, anti-reflective coating, passivation layer, and rear contact can be removed layer by layer, which is usually done by chemical etching. Ag, with or without Cu ribbons, can be etched with inorganic acids, like nitric acid (HNO3)23 or a combination of acids of HNO3 and hydrofluoric acid (HF).24 The antireflective layer is always removed by harmful acids, such as HF25 or phosphoric acid (H3PO4).26 Sodium hydroxide (NaOH),23,25 potassium hydroxide (KOH)23 and HF26,27 are utilized to remove the p–n junction, which is B or P doped Si. The removal of doped B or P is very difficult so the layer is always etched. The Al from the back contact can be etched with the same chemicals as the front contact or antireflective layer.17
However, there are still some concerns regarding this method. The recycling rate of the etching process is low, which was mostly reported to be around 85%.17 This is because Si will react with HF and alkali and each layer is very thin, so to avoid loss of Si, the etching process must be strictly controlled. Secondly, HF is very harmful to humans and the environment, leading to fluoride wastewater and possible secondary pollution. Third, there are many different types of solar cells with different structure and composition, and one process can not fit all of them perfectly. Thus, the etching conditions, etching solution and the order of etching parts need to be optimized case by case. In addition, the parts in the etching solution are not considered to be recycled again, which means that 6–10 wt% of solar cells cannot be recycled. Finally, although many studies mentioned that they can obtain the intact spent solar cell after thermal or chemical treatment, microcracks have not been investigated and may occur, which makes Si wafer defective. When removing EVA by chemical or thermal methods, the volume of EVA will expand first, leading to pressure on solar cells. After etching, the thickness of the Si wafer will be thinner, which is more brittle.
On the other hand, the mechanical process will bring the biggest challenge to solar cell recycling since it shreds all these parts together into powders to produce metallurgical-level silicon. Therefore, Si will be exposed to the etching solution and dissolve in the solution if applying the etching method to the mixture powder for purification. Although it has potential to serve as the feedstock of metallurgical-grade Si in the supply chain of Si wafer production, impurities, such as SiNx, Ag, B and P, are not common in regular feedstock, leading to concerns about the refining process. Thus, most current recycling companies stop here and sell it as a low value product. However, it is still possible to recycle Ag as a valuable product by using HNO3, which will not react with Si. Above all, many refining and purification works are necessary and needed in the mechanical recycling process after shredding.
In summary, compared to the mechanical recycling process, chemical and thermal recycling processes are more feasible to re-introduce solar cells back into the supply chain. In particular, the aim is to reuse recycled Si wafers in solar cell production directly, which can gain more benefits from chemical and thermal treatments. However, the recovered Si may have defects, which is more likely to be re-introduced in crystal growth or ingot production. Considering the long recycling path and harmful chemicals utilization, solar panel recycling may not be profitable and environment friendly. Therefore, upcycling solar panel silicon for an application, where purity is not paramount, could be a better choice.
Technical challenges of upcycling solar panel silicon
Exploring new applications with lower purity requirements and high value is a feasible solution for solar panel recycling. Si anodes, as a high value application of Si, can be manufactured by using recycled Si from solar panel to reduce cost. Silicon anodes in next generation LIBs deliver an ultrahigh capacity of 4200 mA h g−1.7 However, the massive volume changes (around 300%) during the lithiation and delithiation processes cause fragmentation and disruption of electrical contact between particles, leading to rapid degradation and capacity fading.28–31 Thus, to introduce recycled solar panel Si into LIB manufacturing, two main challenges must be addressed to reach the performance quality and suppress the volume change.The first challenge is purity. Although the purity requirement of the Si anode is only over 2 N,32 much lower than that of solar cells (>6 N), Si found in solar panel waste typically maintains an exceptional purity level, around 90 wt%, even without further purification.6 Hence, a straightforward purification process is necessary. However, it is worth noting that certain impurities may actually enhance the performance and stability of the Si anode and suppress its volume change, potentially reducing the need for extensive purification. For instance, elements like B33,34 and P35 are challenging to be removed from Si wafers but they have been shown to improve the initial coulombic efficiency and stability of the Si anode.29–31 Additionally, SiOx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 and SiO2,37 from the oxidation of Si wafer causing solar cell failure, have demonstrated potential as anode candidates. SiNx has also been explored as an anode material for LIBs38–40 suggesting the possibility of retaining it as part of the silicon anode. Moreover, while metal impurities in cathodes are generally viewed negatively,41 they can alloy with Si to form anodes of LIBs,42 such as Cu–Si,43 Sn–Si,44 and Al–Si.45 Although Pb is not commonly reported as an alloying anode material with Si, it can serve as an anode in LIBs.42 Lastly, silver can improve the electrochemical conductivity of Si. Consequently, the purification process for upcycling solar panel Si into LIB anodes primarily aims to control the quantity and formation of these “impurities” to achieve better performance of the Si anode while keeping the purification simple, cost-effective, and environment-friendly.
36 and SiO2,37 from the oxidation of Si wafer causing solar cell failure, have demonstrated potential as anode candidates. SiNx has also been explored as an anode material for LIBs38–40 suggesting the possibility of retaining it as part of the silicon anode. Moreover, while metal impurities in cathodes are generally viewed negatively,41 they can alloy with Si to form anodes of LIBs,42 such as Cu–Si,43 Sn–Si,44 and Al–Si.45 Although Pb is not commonly reported as an alloying anode material with Si, it can serve as an anode in LIBs.42 Lastly, silver can improve the electrochemical conductivity of Si. Consequently, the purification process for upcycling solar panel Si into LIB anodes primarily aims to control the quantity and formation of these “impurities” to achieve better performance of the Si anode while keeping the purification simple, cost-effective, and environment-friendly.
Morphology is another key parameter for the Si anode to suppress the volume change and improve performance. The high lithium stoichiometry of Si, accommodating 3.75 Li atoms per Si atom, results in significant volume changes during battery cycling,46 causing particle fracture and limiting cycle life. Lithiation/delithiation induces two types of cracks: anisotropic expansion47–49 and stress reversal50 because of different lithiation rates, leading to pulverization and capacity decay. Studies show that Si particles can endure stress if below 150 nm,51 driving research into using nanosize Si as a viable anode material,52 such as nanoparticles,53 nanolayers,54 nanowires,55,56 and nanotubes.57 However, due to a low tap density, poor coulombic efficiency and intricate synthesis of nanosize Si, the development of microstructures that possess the properties of nanoscale Si holds great significance and practical value, like core–shell,58 yolk–shell,59,60 and porous.61,62 In addition, to alleviate the foregoing challenges, the incorporation of silicon active materials in carbonaceous products (e.g., carbon nanotubes, graphite and graphene) is considered as a widely applied process for anode enhancement.59
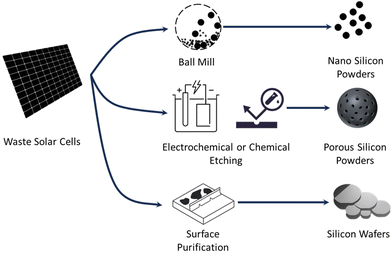
While various morphological design strategies have been explored to address the challenges associated with Si anodes, a definitive conclusion regarding the optimal morphology is yet to be reached. Notably, when dealing with recycled Si sourced from Si wafers with a fixed morphology, the process of morphology conversion becomes pivotal in the upcycling endeavor. In light of this, we recommend the conversion of Si wafers into nano-sized particles or porous structures. These particular morphologies are deemed more feasible for conversion when compared to other options, presenting a promising avenue for enhancing the Si anode performance in lithium-ion batteries.
Technical feasibility of upcycling solar panel silicon
To date, silicon cutting waste63 and metallurgical Si with low purity64 have been successfully utilized to synthesize low-cost Si anodes. The synthesis of Si anodes using Si ingot sawing ash65 and waste PV panels66 has also been reported, indicating the feasibility of upcycling Si into materials for LIBs. Therefore, this section focuses on the technical feasibility of upcycling Si from waste PV panels into anode materials for LIBs by summarizing reported work and proposing potential work.Although many research studies have reported the upcycling of Si from waste solar panels into LIB anodes, they are still using the etching method to increase the purity of recovered Si. Liao et al.67 used a combined method of chemical and thermal treatment to obtain waste solar cells, and HCl, HNO3 and HF were utilized to remove impurities. The purity of the recovered Si was 99.91 wt%. Then, the particle size of purified Si was reduced and mixed with graphite by ball milling. The obtained Si/C anode presented better rate and cycling performances compared to unpurified Si and unpurified Si/C anodes. Sim et al.68 used the combination of H3PO3, HNO3, and KOH to replace HF in the purification process and gained nanoparticles of recycled Si with 99.5 wt% purity via ball milling. However, above methods reported similar purification methods in recycling Si back to solar with similar chemical usage, so that the process, cost, energy cost and environmental impact are not prompted.
As we expected, the upcycling process will use less chemicals and a short purification process, especially eliminating the utilization of HF. Hence, the above examples are not preferred. The process, like Boon Tay et al.11 reported, only used HCl for purification and obtained Si with a purity of 91.94 wt%. The Si-graphite anode containing 10 wt% of recovered Si attained 87.5 wt% retention after 200 cycles at a high charging rate of 500 mA g−1. Rahman et al.7 reported a purification process with KOH only and the recovered nanoparticle Si-graphite anode was obtained by ball milling. Liu et al.66 reported that the ball milled solar cells could be directly reused in LIBs as anode materials without any purification process. In this work, the purity of the recovered Si is less than 90 wt% and Al was converted to Al2O3, which plays a significant role in inhibiting the volume change. These works indicate that the upcycling strategy can effectively reduce the complexity, cost, energy cost and environmental impact of solar panel recycling and enhance its profitability and efficiency.
On the other hand, morphology conversion must be considered in the upcycling process.69 Zhang et al.70 utilized nano-metal catalyzed HF acid etching to recover porous Si/carbon anode materials after a simple purification process. Because of the properties of Si, HF is always used to make pores on Si,63,70 while it is not expected in a sustainable process. Fortunately, there are many ways to obtain a porous structure for Si. Zhang et al.71 successfully recovered a porous Si anode by employing the molten-salt electrolysis method. Sreenarayanan et al.72 reported an atomization process after ball milling purified solar and semiconductor-grade silicon scrap to synthesize the spherical jackfruit-like structured Si anode.
Therefore, HF is replaceable in both purification and morphology modification processes, leading to a greener and more sustainable recycling process. Fig. 2 presents three potential methods to upcycle waste solar cells to anodes of Li-based batteries. Nanoparticles and porous Si have been discussed. The silicon wafer can also be reused in all-solid-state batteries (ASSBs). Na et al.73 reported an interesting study where grooved Si wafer presented better performance as an anode in ASSBs, paving a new way for next-generation high-energy ASSBs. Inspired by this work, waste solar cells have the potential to be directly reused in ASSBs with or without a simple surface purification process, avoiding the morphological modification process in the proposed upcycling strategy. In summary, repurposing Si from waste PV in LIB anodes is a more efficient, cost-effective, and environment-friendly upcycling process compared to the conventional recycling process.
Triple bottom line assessment of solar panel silicon
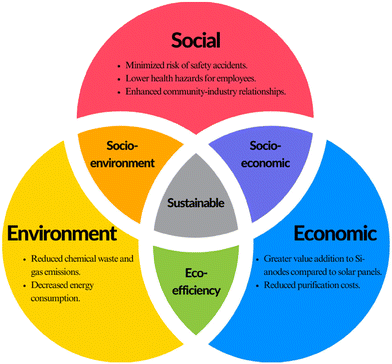
The integration of recovered solar panel silicon into LIB anodes is not just a technical enhancement—it is a paradigm shift in green chemistry and sustainability. According to Roger A. Sheldon,74 a technology is considered sustainable when it meets three dimensions of sustainability, which are called the “triple bottom line”. To comprehensively examine the advantages of this method over others, this study conducted an investigation from environmental, social, and economic perspectives (as shown in Fig. 3).Environmental
Recovered silicon from used solar panels offers multifaceted environmental advantages. Firstly, the ultrahigh capacity of Si significantly boosts the LIB performance, especially in terms of energy density. This indirectly contributes to the proliferation of renewable energy storage solutions, reducing the reliance on fossil fuels and promoting the transition to a cleaner energy ecosystem.Secondly, since this strategy does not require high purity for the recovered Si, the energy consumption for purification can be significantly reduced by over 50% compared to the route for high purity silicon production.32 While the water consumption aspect is not explicitly detailed in previous research, it is plausible that the reduced complexity of the purification process could also lead to lower water usage.
The substantial decrease in energy usage is directly correlated with a marked reduction in greenhouse gas emissions. A study by Riahi et al.75 analyzed a thermal treatment recycling process with detailed data on energy consumption and emissions. Based on their proposed process, the emission of carbon dioxide equivalent (CO2e) is less than 1/3 of conventional Si production. In the face of the high energy consumption of the pyrolysis process and complex purification processes for the recovery of solar panel Si, the proposed strategy in this work may lead to even lower CO2e.
Additionally, our proposed strategy reduces reliance on hazardous chemicals, such as HF, in the recycling process, curtailing the risks of secondary pollution and waste generation. Although HF can react with most Si-related materials to obtain a higher purity of the recovered product, it is a highly corrosive liquid and powerful contact poison, leading to safety and environmental issues. By avoiding processes that release harmful chemicals into the atmosphere, this proposed strategy can contribute to reducing smog formation and ozone depletion; besides, the specific impact would need further quantification. In summary, upcycling silicon in LIBs holds the potential to alleviate certain environmental issues by eliminating chemical waste and gas emissions and decreasing energy and water consumption.
Social
Social sustainability such as workplace safety is another important advantage of this strategy. This advanced method could significantly lower the risk of accidents in workplaces and health concerns for workers, by reducing the reliance on toxic and hazardous chemicals. Workers are less likely to be exposed to toxic gases, flammable materials, and other dangerous substances during purification, leading to a safer and healthier work environment. Such improvements in occupational safety are crucial, as they directly affect the well-being of employees and reduce the likelihood of work-related injuries and illnesses. The reduction in toxic exposure limits the risk of long-term health concerns among workers, such as respiratory problems, skin conditions, or more severe chronic diseases.In addition, this is particularly important for communities close to recycling facilities, as it decreases the likelihood of environmental contamination that can affect public health. Furthermore, this strategy of adopting a process that prioritizes health and occupational safety can enhance the public perception of the recycling industry. By demonstrating a commitment to social responsibility and employee welfare, the industry can build trust and goodwill within communities and amongst stakeholders. This positive perception can lead to stronger community–industry relationships and potentially garner public support for recycling initiatives and bring further economic benefits.
Economical
The role of economic indicators in ensuring sustainability cannot be underestimated. Firstly, this advanced strategy of upcycling waste silicon holds the potential to decrease costs by simplifying purification processes compared with recycling Si back to solar panels. This led to low energy and chemical costs – acid for etching.In addition, though recycling solar panels is often seen as unfavorable due to the expense of around $15–$45 to recycle a silicon PV module in the US76 compared to just $1–$5 for landfill disposal,2,76 other potential costs—such as those tied to waste processing—are frequently disregarded in the analysis. This strategy saves costs for waste processes by generating less waste and secondary pollution.
Furthermore, this strategy can generate more valuable reclaimed silicon for their use in LIB anodes than in solar panels. Presently, the price of Si-based anodes ranges between $14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and $17
000 and $17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton, while the cost of LIB Si-based anodes surpasses that of graphite anodes, which are priced between $4880 and $10
000 per ton, while the cost of LIB Si-based anodes surpasses that of graphite anodes, which are priced between $4880 and $10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 458 per ton,75 and their ultrahigh capacity brings a notably higher energy density. Therefore, the need to explore and develop new methodologies for producing cost-effective Si-based anodes is imperative. Additionally, the price of solar-grade silicon is around $10 per kg ($10
458 per ton,75 and their ultrahigh capacity brings a notably higher energy density. Therefore, the need to explore and develop new methodologies for producing cost-effective Si-based anodes is imperative. Additionally, the price of solar-grade silicon is around $10 per kg ($10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton),75 which is lower than that of silicon anodes. Therefore, this variance could lead to potentially higher profits for recyclers.
000 per ton),75 which is lower than that of silicon anodes. Therefore, this variance could lead to potentially higher profits for recyclers.
In summary, upcycling silicon from discarded solar panels for incorporation into LIBs not only streamlines the recycling procedure and benefits the environment and society, but also culminates in a more economically efficient approach.
Supply chain analysis of upcycling solar panel silicon
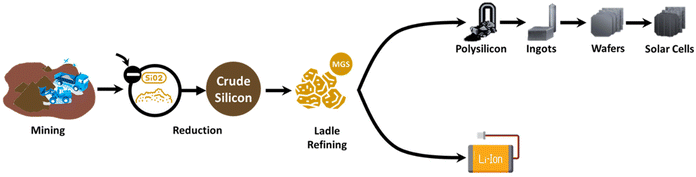
Supply chains are receiving increasing attention as green chemistry and sustainability are considered. Although there have been studies focusing on repurposing waste Si from solar panels to LIBs, studies from a supply chain perspective are insufficient. A thorough investigation into the advantages of this strategy from the supply chain perspective, especially focused on demand, is executed in this study.Firstly, diversifying silicon supplies for Si-based anodes by adopting recycling from solar panels could enhance the flexibility of the supply chain. The Li–Si batteries and solar panels are intricate since both are dependent on the supply of silicon, as shown in Fig. 4. The increasing demand for LIBs and solar panels is leading to an increased demand for Si at an increasing price. In 2017, the global demand for metallurgical silicon (MGS) stood at 29.59 million tons, 13.59% of which was attributable to solar applications.5
Predictions indicate that this demand will escalate to 44.80 million tons by 2030,75 with the solar industry's consumption increasing from 4.02 million to 9.45 million tons over the same period.5
Simultaneously, the growing electric vehicle market, and the consequent boom in LIB production, have amplified the demand for silicon, an integral element of Li–Si battery anodes.75 It is estimated that by 2030, there will be a demand for 0.94 million tons of Si anodes for LIBs, accounting for 10% of the projected Si demand, which may pose challenges to the Si supply chain.77 As of August 2021, the average spot market price for silicon metal was nearly 50% higher than the annual average price in 2020.73 Generally, the strategy of upcycling silicon from solar panels to Si-based anodes enriches the secondary use scenarios of silicon, making the solar panel and LIB supply chains more intertwined. Therefore, this strategy can effectively enhance the flexibility and efficiency of silicon supply chains.
In addition, the fragility of silicon supply chains is amplified by a multitude of regional and geopolitical influences. A staggering 68% of the world's silicon production emanates from China. This inherent instability in supply chains is particularly pronounced due to the uneven distribution of MGS production across the globe. In order to establish a more resilient supply chain, manufacturers in specific nations have initiated explorations into diversifying their sources of silicon or even advancing non-Si perovskite technologies to remove dependence on other countries. Consequently, the strategy of upcycling silicon from solar panels for LIB applications arises as a viable strategy to mitigate regional limitations and augment supply diversity in the long term.
In conclusion, upcycling silicon from waste solar panels to produce LIBs can not only improve the flexibility and efficiency of supply chains, but also enhance the stability and diversity of the silicon supply.
Perspectives and conclusions
It is evident that the world is entering a new epoch in energy generation and storage, with PV and Li-based batteries playing pivotal roles. The coming mass of waste PV and immature recycling technologies have led us to rethink the methods employed for PV recycling and disposal. As described above, existing PV recycling methods fail to capture the full material value. The recycled materials are of low purity or are not completely harvested, and the multi-step purification processes are complicated and accompanied by harmful chemicals. These challenges make PV recycling unfeasible in the economy. This perspective suggests a solution that bridges these challenges with LIBs: repurpose Si from waste solar panels into LIBs. This strategy has the potential to not only improve the effectiveness of PV recycling, but also to lower the costs of Si-based anodes and increase the energy density of LIBs. This process opens up avenues for a more efficient, cost-effective, and environmentally friendly use of silicon.Despite the promise of the proposed resource recovery strategy, several challenges must be addressed to realize its full potential. One crucial obstacle is characterizing the types, concentrations, and functional roles of impurities in waste silicon from solar panels. Currently, the literature offers limited insights into the profile of these impurities, thereby complicating the downstream recycling processes. Given that impurity management remains a high-priority issue across various recycling processes, it often necessitates complex and potentially hazardous purification steps that could give rise to secondary pollution. Therefore, a comprehensive understanding of the impurity profile is essential for the development of targeted purification methods.
Furthermore, the purity requirements for Si anodes in batteries are notably more lenient than those specified for solar-grade Si. Although impurities are conventionally considered to be deleterious to the material performance, intriguingly, certain types of impurities in waste solar panel silicon may confer beneficial properties to Si anodes. This difference introduces innovative perspectives and necessitates alternative processing methods for managing impurity concentration and composition. Thus, in-depth investigations are needed to elucidate the specific concentrations and roles of these impurities to optimize the material performance.
Another complex issue centers on the necessity for morphology conversion in Si anodes. Due to their substantial volumetric changes during charge and discharge cycles, Si anodes require specialized morphological designs, such as nanoparticles or porous particles, to mitigate these effects. Various methods exist for achieving morphology conversion, including ball milling, as well as chemical and electrochemical etching techniques. Notably, ball milling has demonstrated its efficacy in strategies aimed at repurposing silicon. However, there remains a pressing need to develop innovative, cost-effective, and efficient techniques. One potential avenue for optimization involves the concurrent processing of impurity refinement and morphology conversion. Such an integrated approach could streamline the upcycling process, thereby enhancing its economic viability. Although this integrated solution has been sparsely reported in the literature, insights may be gleaned from existing research on the Si morphology for diverse applications, which often employ acid and alkaline solutions capable of impurity refinement.
Moreover, traditional chemical etching processes that yield the desired porous structures frequently involve the use of HF, a substance at odds with the environmental and social objectives of this strategy. This highlights the imperative to explore alternative methods for morphology conversion devoid of HF, such as acid-assisted ball milling, alkaline etching, and HF-free electrochemical etching techniques.
In addition, the imperative to develop next-generation recycling technologies, particularly those inspired by silicon anode manufacturing, cannot be overstated. The silicon wafer featured in state-of-the-art all-solid-state batteries serves as a seminal example36 that has the potential to revolutionize the field of solar panel recycling. Building on this foundation, researchers have the opportunity to investigate techniques for the meticulous removal of surface metals and SiNx to produce high-performance silicon wafer anodes. It is essential to maintain structural integrity by preventing cracks during the purification and resizing stages, as such flaws could compromise performance. This multidimensional approach will pave the way for a new era of performance, efficiency, and environmental stewardship.
Additionally, some valuable metals, like Ag, which are not necessary for Si anodes, should be considered to be extracted by a simple pre-purification process to enhance the supply chain of solar cells and promote a circular economy of solar panel recycling.
This study examines the advantages of this strategy from the perspective of the “triple bottom line”. However, the existing literature and studies provide insufficient quantitative analysis of these environmental, social, and economic advantages. Therefore, we suggest that future studies provide an in-depth assessment of the sustainability of this strategy through methods such as techno-economic assessment (TEA) and life cycle assessment (LCA) modelling.
The environmental advantages inherent to this innovative approach are of paramount importance. As previously elucidated, the recycling of silicon sourced from PVs in LIBs not only diversifies the silicon supply chain but also simplifies the purification processes, thus resulting in a substantial reduction in energy consumption and waste emission. Consequently, this strategy aligns seamlessly with the aspirations of achieving net-zero emissions. Given the increasing emphasis on environmental advantages in investment decisions, we contend that this technology possesses the potential to draw substantial interest from ethical investors, environmental, social, and governance (ESG) investors, and green investors.
Recycling silicon extracted from PVs and repurposing it for use in LIBs is a promising approach to bolster supply chain flexibility, efficiency, and stability. As we look ahead to the future development of this supply chain, it becomes increasingly imperative to transition from laboratory experiments to industrial implementation, thereby fortifying the practical application of this strategy and securing the longevity of supply chain improvements.
The upcycling of waste solar panel silicon for LIBs has the potential to intertwine the supply chains of solar cells and LIBs. Consequently, it is imperative to enhance collaboration among stakeholders to facilitate the industrialization and scalability of this strategy. Government entities and official organizations are poised to play pivotal roles in the regulation and oversight of this emerging field. The diversification of recycling methods represents an inevitable trajectory for the future of the recycling industry.
In conclusion, as global energy needs escalate alongside mounting environmental concerns, the imperative for sustainable and efficient energy solutions is increasingly urgent. The innovative upcycling of waste solar panel silicon for lithium-ion batteries (LIBs) presents a compelling avenue to address these multifaceted challenges, highlighting the critical role of interdisciplinary collaboration and technological ingenuity in steering society toward a more sustainable trajectory. This work further emphasizes the indispensability of robust governmental oversight, including the development and continual refinement of legislative frameworks, as well as the allocation of financial resources to facilitate cleaner, more efficient recycling technologies. Such a multi-pronged approach, combining technological advancements with policy support, has the potential to catalyze transformative changes in both energy storage and waste management, thereby contributing significantly to global sustainability objectives.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The TOC is generated by ChatGPT and the authors revised it.References
- R. Gahlot, S. Mir and N. Dhawan, Energy Fuels, 2022, 36, 14554–14572 CrossRef CAS.
- M. Peplow, ACS Cent. Sci., 2022, 8, 299–302 CrossRef CAS PubMed.
- G. A. Heath, T. J. Silverman, M. Kempe, M. Deceglie, D. Ravikumar, T. Remo, H. Cui, P. Sinha, C. Libby, S. Shaw, K. Komoto, K. Wambach, E. Butler, T. Barnes and A. Wade, Nat. Energy, 2020, 5, 502–510 CrossRef CAS.
- M. Tao, V. Fthenakis, B. Ebin, B. Steenari, E. Butler, P. Sinha, R. Corkish, K. Wambach and E. S. Simon, Prog. Photovoltaics Res. Appl., 2020, 28, 1077–1088 CrossRef CAS.
- X. Li, H. Liu, J. You, H. Diao, L. Zhao and W. Wang, Waste Manag., 2022, 137, 312–318 CrossRef CAS PubMed.
- N. Eshraghi, L. Berardo, A. Schrijnemakers, V. Delaval, M. Shaibani, M. Majumder, R. Cloots, B. Vertruyen, F. Boschini and A. Mahmoud, ACS Sustainable Chem. Eng., 2020, 8, 5868–5879 CrossRef CAS.
- M. M. Rahman, S. Mateti, I. Sultana, C. Hou, A. Falin, P. Cizek, A. M. Glushenkov and Y. Chen, Adv. Energy Sustainability Res., 2021, 2, 2100081 CrossRef CAS.
- P. Li, J.-Y. Hwang and Y.-K. Sun, ACS Nano, 2019, 13(2), 2624–2633 CAS.
- J. Im, J. Kwon, D. Kim, S. Yoon and K. Y. Cho, Small Methods, 2022, 6, 2101052 CrossRef CAS PubMed.
- F. Xi, Z. Zhang, Y. Hu, S. Li, W. Ma, X. Chen, X. Wan, C. Chong, B. Luo and L. Wang, J. Hazard. Mater., 2021, 414, 125480 CrossRef CAS PubMed.
- Y. Boon Tay, Y. Sim, J. Ang Koon Keong, M. Iszaki Bin Patdillah, H. Min Chua, E. Tang Jun Jie, M. Srinivasan and N. Mathews, ChemSusChem, 2022, 15, e202200978 CrossRef CAS.
- Md. S. Chowdhury, K. S. Rahman, T. Chowdhury, N. Nuthammachot, K. Techato, Md. Akhtaruzzaman, S. K. Tiong, K. Sopian and N. Amin, Energy Strategy Rev., 2020, 27, 100431 CrossRef.
- S. K. Gaddam, R. Pothu and R. Boddula, J. Materiomics, 2021, 7, 920–928 CrossRef.
- H. Kumar Trivedi, A. Meshram and R. Gupta, Mater. Today: Proc., 2023 DOI:10.1016/j.matpr.2023.08.109.
- D. S. Prasad, B. Sanjana, D. S. Kiran, P. P. Srinivasa Kumar and R. Ratheesh, Sol. Energy Mater. Sol. Cells, 2022, 245, 111850 CrossRef CAS.
- T. Dobra, D. Vollprecht and R. Pomberger, Waste Manage. Res., 2022, 40, 96–103 CrossRef CAS PubMed.
- R. Deng, Y. Zhuo and Y. Shen, Resour., Conserv. Recycl., 2022, 187, 106612 CrossRef.
- M. Tao, T. Chen, N. Click and R. Adcock, Curr. Opin. Green Sustainable Chem., 2023, 44, 100863 CrossRef CAS.
- V. Aryan, M. Font-Brucart and D. Maga, Prog. Photovoltaics Res. Appl., 2018, 26, 443–459 CrossRef CAS.
- V. Fiandra, L. Sannino, C. Andreozzi, F. Corcelli and G. Graditi, Waste Manag., 2019, 87, 97–107 CrossRef CAS PubMed.
- Solar Panel Recycling Service | NPC incorporated, https://www.npcgroup.net/eng/solarpower/reuse-recycle/recycle-service, (accessed January 7, 2024).
- L. V. Mercaldo and P. D. Veneri, In: Enrichi F, Righini GC, editors. Solar Cells and Light Management, Elsevier, 2020, pp. 35–57 Search PubMed.
- W.-H. Huang, W. J. Shin, L. Wang, W.-C. Sun and M. Tao, Sol. Energy, 2017, 144, 22–31 CrossRef CAS.
- E. Klugmann-Radziemska and P. Ostrowski, Renewable Energy, 2010, 35, 1751–1759 CrossRef CAS.
- T.-Y. Wang, J.-C. Hsiao and C.-H. Du, in 2012 38th IEEE Photovoltaic Specialists Conference, 2012, pp. 002355–002358.
- J. Park and N. Park, RSC Adv., 2014, 4, 34823–34829 RSC.
- E. Klugmann-Radziemska, P. Ostrowski, K. Drabczyk, P. Panek and M. Szkodo, Sol. Energy Mater. Sol. Cells, 2010, 94, 2275–2282 CrossRef CAS.
- R. Guo, S. Zhang, H. Ying, W. Yang, J. Wang and W.-Q. Han, ACS Appl. Mater. Interfaces, 2019, 11, 14051–14058 CrossRef CAS PubMed.
- Z. Bitew, M. Tesemma, Y. Beyene and M. Amare, Sustainable Energy Fuels, 2022, 6, 1014–1050 RSC.
- C. Zhang, F. Wang, J. Han, S. Bai, J. Tan, J. Liu and F. Li, Small Struct., 2021, 2, 2100009 CrossRef CAS.
- W. U. Rehman, H. Wang, R. Z. A. Manj, W. Luo and J. Yang, Small, 2021, 17, 1904508 CrossRef CAS.
- X. Li and R. B. Wehrspohn, Joule, 2019, 3, 1172–1175 CrossRef.
- M. Chen, B. Li, X. Liu, L. Zhou, L. Yao, J. Zai, X. Qian and X. Yu, J. Mater. Chem. A, 2018, 6, 3022–3027 RSC.
- S. Cho, W. Jung, G. Y. Jung and K. Eom, J. Power Sources, 2020, 454, 227931 CrossRef CAS.
- I. P. Gordon, W. Xu, S. Randak, T. R. Jow and N. P. Stadie, Chem. Mater., 2023, 35, 549–557 CrossRef CAS.
- X. Zhu, B. Liu, J. Shao, Q. Zhang, Y. Wan, C. Zhong and J. Lu, Adv. Funct. Mater., 2023, 33, 2213363 CrossRef CAS.
- G. Mu, D. Mu, B. Wu, C. Ma, J. Bi, L. Zhang, H. Yang and F. Wu, Small, 2020, 16, 1905430 CrossRef CAS.
- A. Ulvestad, J. P. Mæhlen and M. Kirkengen, J. Power Sources, 2018, 399, 414–421 CrossRef CAS.
- Substoichiometric Silicon Nitride – An Anode Material for Li-ion Batteries Promising High Stability and High Capacity | Scientific Reports, https://www.nature.com/articles/s41598-018-26769-8, (accessed July 14, 2023).
- H. Lee, K.-B. Kim and J.-W. Choi, Chem. Eng. J., 2020, 401, 126086 CrossRef CAS.
- R. Zhang, Z. Meng, X. Ma, M. Chen, B. Chen, Y. Zheng, Z. Yao, P. Vanaphuti, S. Bong, Z. Yang and Y. Wang, Nano Energy, 2020, 78, 105214 CrossRef CAS.
- B. T. Heligman and A. Manthiram, ACS Energy Lett., 2021, 6, 2666–2672 CrossRef CAS.
- H. Zeng, Y. He and M. Chamas, Front. Energy Res., 2022, 10 DOI:10.3389/fenrg.2022.968259.
- Z. Dong, W. Du, C. Yan, C. Zhang, G. Chen, J. Chen, W. Sun, Y. Jiang, Y. Liu, M. Gao, J. Gan, Y. Yang and H. Pan, ACS Appl. Mater. Interfaces, 2021, 13, 45578–45588 CrossRef CAS PubMed.
- S. S. Sharma, P. J. Crowley and A. Manthiram, ACS Sustainable Chem. Eng., 2021, 9, 14515–14524 CrossRef CAS.
- 25th Anniversary Article: Understanding the Lithiation of Silicon and Other Alloying Anodes for Lithium–Ion Batteries - McDowell - 2013 - Advanced Materials - Wiley Online Library, https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.201301795, (accessed January 19, 2024).
- I. Ryu, S. W. Lee, H. Gao, Y. Cui and W. D. Nix, J. Power Sources, 2014, 255, 274–282 CrossRef CAS.
- M. K. Y. Chan, B. R. Long, A. A. Gewirth and J. P. Greeley, J. Phys. Chem. Lett., 2011, 2, 3092–3095 CrossRef CAS.
- S. W. Lee, M. T. McDowell, J. W. Choi and Y. Cui, Nano Lett., 2011, 11, 3034–3039 CrossRef CAS PubMed.
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 1–16 Search PubMed.
- X. H. Liu, L. Zhong, S. Huang, S. X. Mao, T. Zhu and J. Y. Huang, ACS Nano, 2012, 6, 1522–1531 CrossRef CAS PubMed.
- M. Je, D.-Y. Han, J. Ryu and S. Park, Acc. Chem. Res., 2023, 56, 2213–2224 CrossRef CAS PubMed.
- L. Li, C. Fang, W. Wei, L. Zhang, Z. Ye, G. He and Y. Huang, Nano Energy, 2020, 72, 104651 CrossRef CAS.
- Y. Son, J. Ma, N. Kim, T. Lee, Y. Lee, J. Sung, S.-H. Choi, G. Nam, H. Cho, Y. Yoo and J. Cho, Adv. Energy Mater., 2019, 9, 1803480 CrossRef.
- W. Kang, J.-C. Kim and D.-W. Kim, J. Power Sources, 2020, 468, 228407 CrossRef CAS.
- S. Cangaz, F. Hippauf, F. S. Reuter, S. Doerfler, T. Abendroth, H. Althues and S. Kaskel, Adv. Energy Mater., 2020, 10, 2001320 CrossRef CAS.
- X. Zhang, W.-L. Song, Z. Liu, H.-S. Chen, T. Li, Y. Wei and D. Fang, J. Mater. Chem. A, 2017, 5, 12793–12802 RSC.
- B. Jiang, S. Zeng, H. Wang, D. Liu, J. Qian, Y. Cao, H. Yang and X. Ai, ACS Appl. Mater. Interfaces, 2016, 8, 31611–31616 CrossRef CAS PubMed.
- Y. Li, K. Yan, H.-W. Lee, Z. Lu, N. Liu and Y. Cui, Nat. Energy, 2016, 1, 1–9 Search PubMed.
- Y. Jin, S. Li, A. Kushima, X. Zheng, Y. Sun, J. Xie, J. Sun, W. Xue, G. Zhou, J. Wu, F. Shi, R. Zhang, Z. Zhu, K. So, Y. Cui and J. Li, Energy Environ. Sci., 2017, 10, 580–592 RSC.
- Y. Yu, L. Gu, C. Zhu, S. Tsukimoto, P. A. van Aken and J. Maier, Adv. Mater., 2010, 22, 2247–2250 CrossRef CAS PubMed.
- W. An, B. Gao, S. Mei, B. Xiang, J. Fu, L. Wang, Q. Zhang, P. K. Chu and K. Huo, Nat. Commun., 2019, 10, 1447 CrossRef PubMed.
- J. Zhang, S. Li, F. Xi, X. Wan, Z. Ding, Z. Chen, W. Ma and R. Deng, Chem. Eng. J., 2022, 447, 137563 CrossRef CAS.
- Y. Chen, L. Liu, J. Xiong, T. Yang, Y. Qin and C. Yan, Adv. Funct. Mater., 2015, 25, 6701–6709 CrossRef CAS.
- K. Wang, Y. Tan, P. Li and Y. Wang, J. Hazard. Mater., 2021, 407, 124778 CrossRef CAS PubMed.
- Y.-H. Liu, Y.-L. Chen, Y.-S. Chen, S.-M. Huang, H.-M. Huang, S.-J. Lin and C.-Y. Yang, Green Chem., 2022, 24, 5151–5161 RSC.
- Q. Liao, S. Li, F. Xi, Z. Tong, X. Chen, X. Wan, W. Ma and R. Deng, Energy, 2023, 281, 128345 CrossRef CAS.
- Y. Sim, Y. B. Tay, Ankit, X. Lin and N. Mathews, Sol. Energy Mater. Sol. Cells, 2023, 257, 112394 CrossRef CAS.
- K. Wang, Y. Tan, P. Li and J. Sun, Electrochim. Acta, 2020, 353, 136538 CrossRef CAS.
- Z. Zhang, N. Yang, F. Xi, X. Chen, S. Li, W. Ma, Y. Lei and R. Deng, New J. Chem., 2022, 46, 11788–11796 RSC.
- C. Zhang, Q. Ma, M. Cai, Z. Zhao, H. Xie, Z. Ning, D. Wang and H. Yin, Waste Manag., 2021, 135, 182–189 CrossRef CAS PubMed.
- B. Sreenarayanan, M. Vicencio, S. Bai, B. Lu, O. Mao, S. Adireddy, W. Bao and Y. S. Meng, J. Power Sources, 2023, 578, 233245 CrossRef CAS.
- I. Na, H. Kim, S. Kunze, C. Nam, S. Jo, H. Choi, S. Oh, E. Choi, Y. B. Song, Y. S. Jung, Y. S. Lee and J. Lim, ACS Energy Lett., 2023, 8, 1936–1943 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- S. Riahi, J. A. Mckenzie, S. Sandhu and P. Majewski, Sustainable Mater. Technol., 2023, 36, e00646 CrossRef CAS.
- J. Walzberg, A. Carpenter and G. A. Heath, Nat. Energy, 2021, 6, 913–924 CrossRef.
- U.S. Department of Energy, Solar Photovoltaics Supply Chain Deep Dive Assessment, U.S. Department of Energy, 2022 Search PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |