Open Access Article
Open Access ArticleA comparative life cycle assessment of the synthesis of mesoporous silica materials on a small and a large scale†
Jose Vicente
Ros-Lis
 *a,
Sylvia
Vetter
b and
Pete
Smith
b
*a,
Sylvia
Vetter
b and
Pete
Smith
b
aREDOLí research group. Instituto Interuniversitario de Investigación de Reconocimiento Molecular y Desarrollo Tecnológico (IDM), Universitat de València, Doctor Moliner 50, Burjassot, Valencia 46100, Spain. E-mail: J.Vicente.Ros@uv.es
bInstitute of Biological and Environmental Sciences, School of Biological Sciences, University of Aberdeen, Aberdeen, UK
First published on 8th August 2024
Abstract
Silica mesoporous materials have been the subject of wide scientific interest with various applications. However, the environmental impacts associated with their preparation have scarcely been studied. In the present work, we applied the Life Cycle Assessment (LCA) methodology to the materials MCM-41, MCM-48, UVM-7, mesoporous Stober particles, SBA-15, SBA-16, HMS, KIT-5, KIT-6, MSU, FDU, nano-MCM-41 and nano-MCM-48 for small- (grams) and large-scale (several kilograms) production. Furthermore, various improvements are proposed, and the impact associated with each of them is quantified. The results show that the values of a single score, a normalized and weighed combination of the damage categories, and net greenhouse gas emissions (NGHGE) are highly dependent on the synthesis procedures. On a small scale, the main impact is due to the use of energy and solvents. By contrast on a large scale, the use of solvents, tetraethylorthosilicate and the structure directing agent are the main determinants. From the values obtained for the different materials and scenarios, we estimate that the preparation of this class of materials could have an NGHGE of 54 ± 30 and 31 ± 18 kg CO2 eq. per kg of mesoporous material for small- and large-scale production, respectively. The use of calcination versus extraction, the incorporation of renewable energy and distillation/rectification are initiatives that can contribute to a significant reduction of the environmental impact.
Introduction
Nanomaterials have received enormous attention in recent decades due to their novel properties and potential applications in fields as diverse as energy, health, catalysis, sensors, and others. Among the different classes of nanomaterials, one of the most relevant families is that of mesoporous silica materials (MSM) due to their chemical possibilities and numerous applications in catalysis, biomedicine, sensors, controlled release, etc.1 They have a large pore size (between 1 and 50 nm in diameter) and an ordered pore structure. The first example was reported in the preparation of the material MCM-41 in 19922 in which the strategy of employing a structure-directing agent (typically a surfactant) in combination with the sol–gel process was described. The structure directing agent forms, or assists in the formation of, a three-dimensional structure around which condensation processes and silica formation occur. Following this approach, other materials with different pore sizes or three-dimensional structures have been reported, such as MCM-48, SBA-15, SBA-16, HMS, KIT-5, KIT-6, MSU, FDU-1 or UVM-7 (the references for the synthesis and structure of these materials can be found in the methodology section).Despite the large number of studies on silica mesoporous materials (more than 3000 studies per year can be found in Web of Science using mesoporous AND silica as keywords), they are centered mainly on novel methods of preparation and application of these kinds of materials from a technical point of view and only a few of them focus on achieving more sustainable processes. A few, such as the study of Gérardin et al., examine the possibility of applying eco-design to ordered mesoporous silica materials, and some recent examples use silica-rich waste as a source of silicon, an eco-friendly template, or supercritical fluids in diverse steps of the synthesis.3 The use of these materials in products more sustainable than current solutions, such as nanopesticides, has also been explored.4 However, these studies do not report a complete evaluation to determine whether the new solutions have a lower environmental impact than the alternatives. The principles of Green Chemistry can be applied to compare different types of materials with tools such as DOZN 2.0, via which it is observed that materials such as MCM-41, SBA-15 or HMS have a much greater impact than other silicas such as precipitated silica, and therefore greater possibilities exist for improvement.5
According to the International Organization for Standardization, Life Cycle Assessment (LCA) is a tool used for the determination of the environmental impacts (for example water use or toxicity) of resources and processes applied in the preparation, use and disposal of products (ISO 14040:2006). It allows the identification of opportunities to improve the environmental performance of products. Strategies to reduce environmental impacts may involve using renewable energy sources, optimizing production processes, implementing water recycling systems, or designing products for easier recycling or biodegradation.6
Greenhouse gas emissions (GHGEs) are a critical focus in LCA due to their contribution to climate change. Throughout a product's life cycle, activities like energy consumption, transportation, and manufacturing processes release carbon dioxide (CO2), methane (CH4), and others.7 These emissions amplify the greenhouse effect, leading to global warming and climate instability.8 Water usage is another crucial factor in LCA.9 Industries often withdraw and consume vast amounts of water, impacting local ecosystems and potentially leading to water scarcity.10,11 Additionally, the discharge of pollutants into water bodies during manufacturing and waste disposal stages can severely degrade water quality, affecting aquatic life and human health.11
Despite the interest in this technique to quantify the impact and design strategies towards more sustainable synthesis of nanomaterials, the application of LCA to these types of materials shows certain limitations.12 LCA has been applied to silica based or silica containing materials such as in the preparation of coated magnetic nanoparticles13 or the functionalization of non-porous silica or magnetite.14 In the case of silica material obtained from rice residues for the removal of nitrates from water, the LCA proved that the innovative process could contribute to reducing the environmental impact of water-treatment technologies, resulting in a lower environmental impact with respect to the use of bottled water.15 The inclusion of silica nanoparticles in power capacitors reduces the impact by 20%, but it is a minor variation since it does not mitigate the main impacts on capacitors.16 LCA of the preparation of hollow silica nanospheres used as isolators revealed that the main impact is from the silica coating step, while the CO2 emission from polymer template combustion has a minor contribution.17 The impact analysis of silica aerogels and amorphous silica concluded that the most critical stage is the production of raw materials mostly due to the high energy consumption.18 Also, in a mesoporous aerogel functionalized with amines for lead removal, it was observed that the main impacts were energy consumption and greenhouse gas emissions and that solvents were the origin of the main impact among the reactants.19 Although most of the studies on silica refer to small-scale processes, some studies report information on large scale processes.20
In the case of mesoporous silica materials, there are a few LCA studies. An evaluation of the impact of including MSM into insulating panels considered only the mixing process and did not include the elimination of the structure directing agent, a key aspect of the impact of this class of materials.21 A full process LCA was carried out for MCM-41 modified with Sn and In for the conversion of sugar into methyl lactate, demonstrating that the inclusion of this type of material improved performance compared to the biochemical process, but had a greater environmental impact.22 The evaluation of UVM-7 material prepared with a microwave assisted process revealed a strong reduction of the impact after scale-up.23 Finally, SBA-15 was studied indirectly in combination with magnetite as catalysts in the Photo-Fenton process,24 showing a considerably greater impact than other materials such as magnetite coated with organic polymers, but similar to that of ZnO and lower than that of TiO2.
To our knowledge, LCA has not been applied previously with a comparative purpose to the preparation of a wide set of silica mesoporous materials. We hypothesize that LCA can be an effective tool for the identification of opportunities for innovation towards more sustainable synthesis procedures of silica mesoporous materials and we offer a comparison of the impact of the most studied materials within this family.
Methodology
Goal and scope
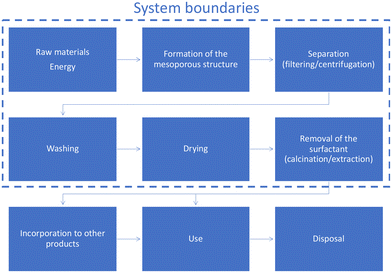
In agreement with the Life Cycle Assessment (ISO 14040:2006), the first elements to be defined are goal and scope. In our case, the goal addresses the evaluation of the environmental impact associated with the preparation of mesoporous silica materials, with the determination of the main factors contributing to that impact and with the designing strategies to reduce the impact. A compilation of 13 of the most common mesoporous silica materials was selected for the present study. Since our interest is focused on the preparation of materials, the functional unit was defined as the preparation of 1 kg of material. A cradle-to-gate basis was used as the system boundary (see Fig. 1). The incorporation into other materials, and the use and disposal phases are out of the scope of the study, as well as the emissions associated with these. Initially, no cut-off criteria were defined. Although the synthesis steps diverge among the materials, all of them can be summarized in the first phase of formation of the mesoporous structure, in which the sol–gel process produces the hydrolysis and condensation characteristics; followed by separation, washing and drying steps to obtain the as-made product; and finally, the removal from the structure directing agent.Life cycle inventory
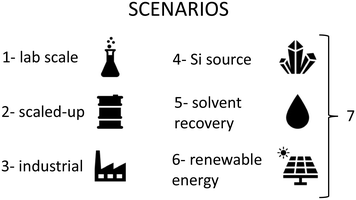
The inventory was calculated from published synthesis procedures for MCM-41,25 MCM-48,26 UVM-7,27 mesoporous Stober particles (MSP),27 SBA-15,28 SBA-16,29 HMS,30 KIT-5,31 KIT-6,32 MSU,33 FDU,34 and two nanoparticulated materials: nano-MCM-41 and nano-MCM-48.35 Although all the syntheses have similarities, for example the use of tetraethyl orthosilicate (TEOS), we can find some differences in the nature of the surfactant (anionic: MCM-41, MCM-48, UVM-7, MSP, HMS, and nano-MCM-41; non-ionic: SBA-15, SBA-16, KIT-5, KIT-6, MSU, and FDU; both: HMS and nano-MCM-48), pH synthesis media (acidic: SBA-15, SBA-16, KIT-5, KIT-6, and FDU; basic: MCM-41, MCM-48, UVM-7, MSP, HMS, nano-MCM-41, and nano-MCM-48), synthesis solvent (ethanol: MSP, HMS, and nano-MCM-48), other reagents (triethanolamine: UVM-7 and MSP; H2SO4: SBA-15; butanol: SBA-16 and KIT-6; NaF: MSU), heating during the synthesis (MCM-48, UVM-7, MSP, SBA-15, SBA-16, KIT-5, KIT-6, FDU, and nano-MCM-41), solvent used in the washing process (ethanol: MSP, HMS, and nano-MCM-41; acetone: SBA-15), concentration (diluted: MSN, nano-MCM-41, and nano-MCM-48), drying in the oven (MCM-41, UVM-7, MSP, SBA-15, SBA-16, KIT-5, KIT-6, MSU, FDU, and nano-MCM-48), and surfactant removal method (calcination: MCM-41, MCM-48, UVM-7, MSP, SBA-15, SBA-16, KIT-5, KIT-6, MSU, FDU, and MCM-48; extraction: HMS and nano-MCM-41).The life cycle inventory of each material has been compiled in Table S1 (ESI†). A compilation of the scenarios studied in this work can be found in Fig. 2. In the first scenario, the energy consumption was determined from the laboratory operation described in the procedures and energy consumption measurements executed in our lab, unless otherwise specified. In those cases, in which the procedure did not define a concrete amount of chemical or time, it was estimated according to our experience in the preparation of silica mesoporous materials and the habitual practices in the laboratory. Thus, the first scenario represents the impact of the synthesis at the lab scale (around 1 gram of silica). Next, scenarios to study the influence of a larger-scale synthesis were defined (scenarios 2 and 3). Scenario 2 assesses the impact of a scale-up synthesis at the laboratory level. This includes working with a 2 L batch during synthesis, centrifugation in batches of 270 mL or 9 g, extraction in batches of 10 g, or washing, filtering, drying or calcination in batches of 30 g of the final material.
In scenario 3, the impact of a scale-up synthesis at the industrial level is evaluated (kilogram scale), and energy costs have been estimated from the data extracted from the literature.20 Finally, scenarios to identify the impact reduction associated with process improvements have been evaluated (scenarios 4 to 7). Scenario 4 evaluates the effect of replacing TEOS with an alternative silicon source, sodium silicate, usually exploited as a more sustainable solution. The procedures for the preparation of the materials departing from sodium silicate are similar to those using TEOS, except for the basic characteristic of sodium silicate. Thus, in those syntheses that need a slightly acidic media, it is necessary to correct the pH. By contrast, it avoids the necessity of bases such as ammonia or sodium hydroxide during the synthesis step. Scenario 5 includes a reduction in solvent use through distillation recovery. A solvent recovery of 95% has been estimated in each cycle. It implies a reduction in the consumption of reagents, but an increase in energy use. Scenario 6 assesses the impact of using renewable energy instead of conventional sources. Finally, scenario 7 includes a combination of innovations propositioned in scenarios 4 through 6.
Life cycle impact assessment
The impact was calculated using the Ecoinvent 3 database and the ReCiPe 2016 Midpoint (H) V1.08/World (2010) or the ReCiPe 2016 Endpoint (H) V1.08/World (2010) H A−1 models available in the SimaPro software. If available, data from Spain or Europe have been used. The method includes global warming, stratospheric ozone depletion, ionizing radiation, ozone formation (human health), fine particulate matter formation, ozone formation (terrestrial ecosystems), terrestrial acidification, freshwater eutrophication, marine eutrophication, terrestrial ecotoxicity, freshwater ecotoxicity, marine ecotoxicity, human carcinogenic toxicity, human non-carcinogenic toxicity, land use, mineral resource scarcity, fossil resource scarcity and water consumption as impact categories. Some of the reagents were not available in the database, so analogous compounds were used for the analysis: CTABr/estequat, Pluronic F127/ethoxylated alcohol AE > 20, poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (POL1)/ethoxylated alcohol AE > 20, Pluronic P123/ethoxylated alcohol AE > 20, tergitol/non-ionic surfactant, B50-6600/ethoxylated alcohol AE > 20. A compilation of the values of single score and net greenhouse gas emissions (NGHGE) for the diverse materials and scenarios can be found in Tables 1 and 2.| Material | Single score (Pt) scenario | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| FDU-1 | 125.3 | 8.6 | 0.5 | 0.2 | 8.6 | 3.2 | 2.9 |
| HMS | 52.5 | 26.0 | 16.0 | 15.8 | 13.2 | 19.4 | 4.9 |
| KIT-5 | 29.6 | 3.8 | 0.4 | 0.1 | 3.7 | 1.6 | 1.2 |
| KIT-6 | 23.6 | 3.9 | 0.7 | 0.4 | 3.9 | 1.7 | 1.5 |
| MCM-41 | 15.1 | 2.4 | 0.6 | 0.1 | 2.3 | 1.3 | 0.8 |
| MCM-48 | 49.6 | 3.6 | 0.7 | 0.4 | 3.5 | 1.7 | 1.3 |
| MSP | 14.5 | 4.5 | 2.4 | 2.1 | 3.6 | 3.2 | 1.9 |
| MSU | 137.7 | 4.5 | 0.6 | 0.4 | 4.5 | 1.9 | 1.6 |
| Nano-MCM-41 | 92.6 | 17.9 | 5.4 | 5.1 | 13.4 | 9.8 | 4.6 |
| Nano-MCM-48 | 100.3 | 4.7 | 3.0 | 2.7 | 3.0 | 3.6 | 1.5 |
| SBA-15 | 66.4 | 13.7 | 5.9 | 5.6 | 13.6 | 8.5 | 8.1 |
| SBA-16 | 26.7 | 3.3 | 0.8 | 0.6 | 3.2 | 1.7 | 1.3 |
| UVM-7 | 17.8 | 3.1 | 1.3 | 1.0 | 3.0 | 1.9 | 1.5 |
| Material | Net greenhouse gas emission (kg CO2 eq.) scenario | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| FDU-1 | 3.851 | 265 | 18 | 8 | 264 | 76 | 66 |
| HMS | 1.533 | 687 | 384 | 375 | 400 | 457 | 111 |
| KIT-5 | 912 | 117 | 14 | 5 | 115 | 40 | 28 |
| KIT-6 | 727 | 121 | 24 | 15 | 121 | 46 | 37 |
| MCM-41 | 468 | 79 | 22 | 4 | 76 | 37 | 19 |
| MCM-48 | 1.524 | 109 | 20 | 11 | 107 | 43 | 31 |
| MSP | 416 | 135 | 72 | 63 | 116 | 88 | 57 |
| MSU | 4.238 | 140 | 22 | 13 | 140 | 48 | 39 |
| Nano-MCM-41 | 2.229 | 510 | 139 | 129 | 410 | 229 | 102 |
| Nano-MCM-48 | 3.073 | 135 | 82 | 73 | 97 | 96 | 43 |
| SBA-15 | 1.939 | 285 | 45 | 36 | 282 | 102 | 90 |
| SBA-16 | 822 | 102 | 27 | 18 | 100 | 46 | 34 |
| UVM-7 | 550 | 100 | 43 | 34 | 98 | 58 | 46 |
Results and discussion
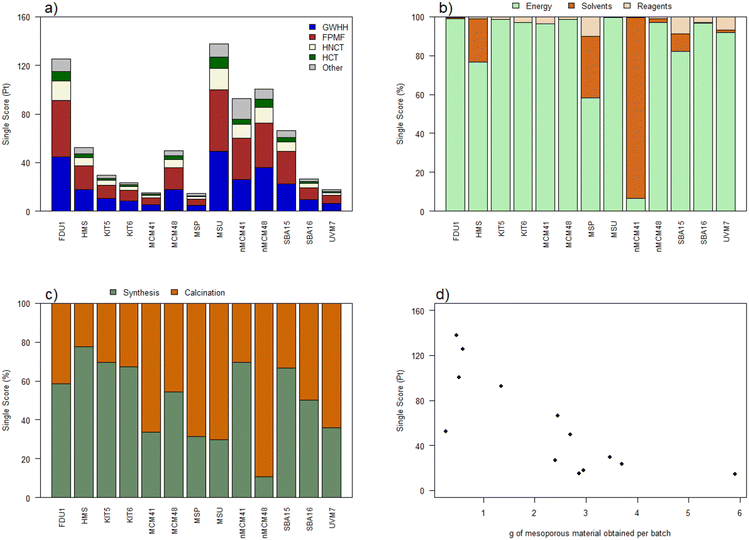
LCA is a methodology that allows the identification of those processes that have the greatest environmental impact. It covers not only global warming, but many other parameters of great importance for the sustainability of human actions. Although at first glance it may seem that one process is more sustainable than another, this methodology allows those reagents or processes with the greatest impact to be identified and the impact is quantified so that it can be reduced.As an initial step towards the objective of having a comparison of the impact of the synthesis of different mesoporous silica materials and identifying opportunities for improvement, the synthesis procedures were studied simply by replicating the number of syntheses necessary to obtain the functional unit (1 kg of final material) (scenario 1). As an example, it would be necessary to prepare 340 or 408 batches for the synthesis of 1 kg of MSM-41 or SBA-15, respectively. A compilation of the results for scenario 1 can be found in Fig. 3. As can be seen, there are important differences between the materials, with values ranging from about 130 Pt for FDU-1 or MSU to less than 20 for materials such as MCM-41, MSP or UVM-7 (Fig. 3a). The main categories of impact, by far from the others, are global warming, fine particulate matter formation, human non-carcinogenic toxicity and human carcinogenic toxicity, counting in most of the materials with more than 90% of the total score. The midpoint indicator net greenhouse gas emissions (NGHGE) follows a trend similar to that of the single score (Table 2) with emission values between 400 and 4000 kg CO2 equivalent for the preparation of 1 kg of mesoporous material with several batches in the gram scale. Thus, the preparation of mesoporous materials at a laboratory scale, which requires a large number of batches and inefficient use of resources, has a significant environmental impact, and there are significant opportunities for improvement.
To better understand those factors with greater influence, in a first approximation resources were classified as energy, solvents or reagents. As can be seen in Fig. 3b, energy consumption is by far the most important element, followed by the use of solvents in some materials such as MSP, nano-MCM-41 or SBA-15. Thus, the procedures carried out in the different stages of synthesis are the key elements to define the impact of synthesis at the laboratory scale. Of this energy, although it varies according to the material, approximately half is consumed in obtaining the material “as made” (until the drying process) and the other half in the removal of the structure directing agent from the interior of the pores (Fig. 3c). Both FDU-1 and MSP are the materials that present smaller batches, while the syntheses of MCM-41, MSP or UVM-7 are the ones that produce more material. This effect is visible in Fig. 3d in which a clear inverse correlation between the batch size and the single score value can be observed. Reducing the number of batches means a more efficient use of laboratory equipment and therefore lower energy consumption. Therefore, whenever possible it will be advisable to prepare batches of the largest possible size in the laboratory.
In view of these results, a new scenario was defined at the laboratory scale (scenario 2) in which the equipment was used to the maximum of its capacity. We are aware that scaling up a chemical reaction, particularly in material preparation, is not immediate, but a move to this scale of work should be feasible for most materials. As an example, reaction mixtures would be produced in batches of 2 liters and the muffle for calcination would only be used to the maximum of its capacity. Standard washing processes were also defined for comparing different materials. As can be seen in Tables 1 and 2, by using this strategy a reduction in the single score and NGHGE between scenario 1 and scenario 2 of up to 95% is achieved and allows us to better study the factors that influence the impact of the preparation of the different materials. This sharp reduction also suggests that the direct use of synthesis in small quantities may lead to an overestimation of impact values.
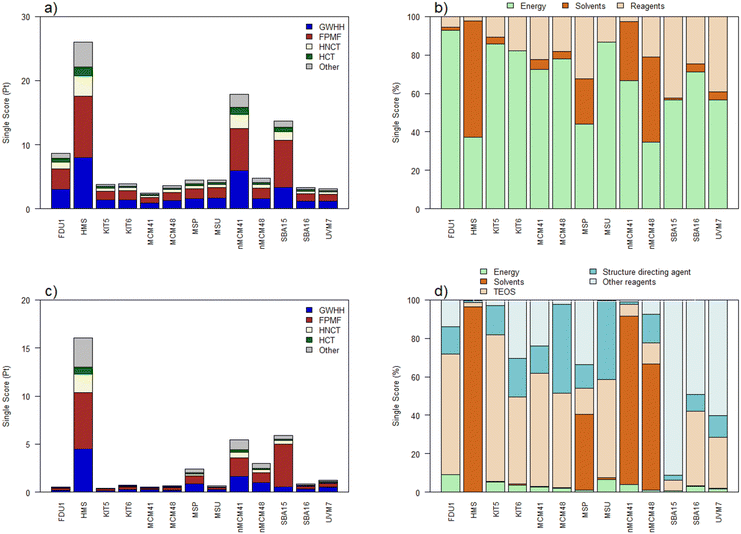
In scenario 2, the same impact categories are maintained as those in the main ones, but we can observe two groups of materials according to their level of impact (Fig. 4a). On the one hand, we have the materials that offer a high value: HMS, nano-MCM-41 and SBA-15, and on the other, the remaining materials. Again, energy is the main factor in determining the impact (Fig. 4b). Most of the materials include calcination processes, so we can rule it out as the origin of the differences. In the case of HMS, the greatest contribution comes from ethanol, which is used in the synthesis (33% as solvent), in the successive washes and in the extraction of the structure directing agent. The preparation of nano-MCM-41 works under conditions of high dilution, washing with large amounts of ethanol and extraction of the directing agent from structure to reflux. SBA-15 presents a prolonged aging process in solution at high temperatures and various drying processes, apart from the usual calcination. In addition, one of the processes uses sulfuric acid which has a large environmental impact.
From this scenario, it can be concluded that, for laboratory syntheses, once scaled up, in general, all mesoporous materials would present a similar level of impact. Even so, it should be considered in the design of a procedure, as far as possible, to minimize the periods of heating in solution, eliminate the use of organic solvents in washes, promote the use of calcination to eliminate the structure directing agent against extraction, and replace high impact acids such as sulfuric acid with more sustainable ones.
Given that energy continues to be the main origin of impact in scenario 2, and being aware that the efficiency of laboratory processes is much lower than what could be obtained at an industrial level, scenario 3 is proposed in which the energy cost of operations is calculated for large-scale synthesis (several kilograms scale).20 We believe that this scenario may give a more reliable illustration than previous ones of the real impact of the preparation of large-scale mesoporous silica materials. The single score decreases considerably for all materials (Table 1 and Fig. 4c), which is in accordance with the importance of energy in the previous scenario and the significant reduction it experiences when considering processes at an industrial level. Net greenhouse gas emissions also decrease considerably to 31 ± 18 kg CO2 eq. per kg of mesoporous material in a large-scale cradle-to-gate system, if we discount HMS and the two nanomaterials. For clarity, in this case, the processes have been grouped into 5 impact sources: energy, solvents, the silicon source (TEOS), the structure directing agent, and others. If we evaluate which are the main contributors to the single score, we see that they are strongly dependent on the type of nanomaterial (Fig. 4d) and therefore the improvement processes must be adapted to each of them. In half of the materials, the objective should be reducing the impact of TEOS (FDU-1, KIT-5, KIT-6, MCM-41, MCM-48, MSU). In others, reagents or solvents are an intrinsic part of the synthesis process, thus they are difficult to replace or reduce. This is the case for triethanolamine in the preparation of materials through the atrane route (MSP and UVM-7) or butanol (SBA-16 and KIT-6). In any case, the industrial scenario offers single score values much lower than those found at laboratory scales.
Going a step further, various improvement scenarios were proposed both for the laboratory and at an industrial level. In the latter case, the effect of replacing TEOS as a source of silicon with another material was evaluated (scenario 4). In the literature, there are numerous examples of materials prepared from sodium silicate, rice husk ash and other materials of biological origin, or recycled glass. The last two require collection and transport and treatments that include various processes of mechanical treatment, washing, drying, chemical treatment, etc. that require a specific analysis and exceed the objective of this work. Thus, we propose the replacement of TEOS by sodium silicate, which also has the indirect effect, because of its basic character, in dispensing with the bases used in the synthesis of some materials. As can be seen in Table 1, the incorporation of sodium silicate has a reduction effect of 0.3 points of the single score, with a relative importance in the reduction that depends on each material and varies between 1.7% (HMS) and 65.3% (KIT-5) depending on the relevance of TEOS in scenario 3. We consider that the use of sodium silicate as a source of silicon is a minor improvement compared to the rest of the possibilities for improvement, but it has a non-negligible contribution to large-scale processes, not only at the level of environmental impact but also regarding the economic cost of the raw material.
At the laboratory level, the main impacts are the use of energy and solvents. The incorporation of distillation or rectification for solvent recovery (scenario 5) is especially advantageous for those materials that use large amounts of ethanol (HMS and the nanomaterials MSP, nano-MCM-41 and nano-MCM-48) despite the cost of evaporation (see Table 2). However, the energy generated from the combustion of ethanol could give a negative result. The replacement of the usual energy mix by electricity generated by photovoltaic panels (scenario 6) offers a significant reduction in the single score in all materials and varies between 1 and 8 points (23 to 63%). It has the advantage that the synthesis methods do not need to be modified; however, it is a difficult improvement to implement in laboratories or small-scale production centers due to space limitations. Finally, we have proposed a scenario (scenario 7) in which we combine the previous improvements: use of sodium silicate, solvent recovery by distillation or rectification, and use of renewable energies on a small scale (scenario 2). The single score values are lower than in the rest of the small-scale scenarios (scenarios 1, 2, 5 and 6) but are still higher than those of industrial-level preparations (scenarios 3 and 4) (see Table 1). The main reductions are due, in this order, to the use of renewable energy, distillation and finally the source of silicon. If we review the effect of improvements on net greenhouse gas emissions (see Table 2), we can see how the small-scale synthesis of mesoporous materials can reach values of 54 ± 30 kg CO2 eq. per kg of mesoporous material. The effect of the different improvements proposed in scenario 7 on the reduction of greenhouse gas emissions would follow the same trend as in the case of the single score, with the smallest effect due to the change of the silicon source.
Energy sources play a pivotal role in the LCA of producing mesoporous materials. The selection of energy sources significantly influences the environmental impact and the amounts of GHGs that are emitted. Fossil fuels, predominantly coal, oil, and natural gas, have been primary energy sources for decades. However, their combustion releases vast amounts of GHGs contributing significantly to global warming.7,8 Additionally, the extraction and refining processes of these fuels harm ecosystems, pollute air and water, and pose health risks to communities living nearby.36 Alternatively, renewable energy sources, such as solar, wind, hydroelectric, and geothermal, offer options with lower environmental impacts.37 Solar energy harnesses sunlight through photovoltaic cells,38 wind energy uses turbines to convert wind power into electricity,6 hydroelectric power relies on water flow,39 and hydrogen fuels are produced through various processes, with water electrolysis using renewable electricity being a particularly environmentally friendly method and geothermal energy taps into Earth's internal heat.40 These sources emit much lower amounts of GHGEs during operation, reducing the overall carbon footprint.7 Transitioning towards cleaner, more sustainable energy sources is imperative to mitigate climate change and reduce environmental degradation. Investing in research, innovation, and infrastructure for renewable energies, coupled with efficient use, can significantly reduce GHGEs and minimize environmental impacts, ensuring a more sustainable production of silica mesoporous materials.
Conclusions
The LCA application to a wide variety of silica mesoporous materials has allowed us to calculate the environmental impact (as a single score) and NGHGE of the diverse materials prepared on a small and large scale, determine the factors of greatest influence, and suggest proposals for improvement. To be able to compare materials/procedures with each other, it is key not only to define a common functional unit (1 kg of material) but also to standardize the procedures and use of the equipment. Once this is considered, calcination seems to be a more sustainable procedure for the removal of the structure directing agent compared to extraction. From a procedural point of view, other improvement strategies include working in larger batches or reducing drying and washing processes. The use of sodium silicate as a source of silicon would be recommended only at an industrial level due to its lower effect. In the comparison between materials, a clear difference can be observed between nanoparticulate and non-nanoparticulate materials, in favor of the latter. Most non-nanoparticulate materials have a similar level of impact, yet stand out for their low impact: MCM-41, UVM-7 and SBA-16.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was carried out thanks to the grant PID2021-126304OB-C43 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”; the Ministry of Universities grant PRX22/00157; and the AGROALNEXT programme supported by MCIN with funding from European Union NextGenerationEU (PRTR-C17.I1) and by Generalitat grant number EUAGROALNEXT/2022/065.References
- V. C. Niculescu, Front. Mater., 2020, 7, 36 CrossRef CAS; B. Singh, J. Na, M. Konarova, T. Wakihara, Y. Yamauchi, C. Salomon and M. B. Gawande, Bull. Chem. Soc. Jpn., 2020, 93(12), 1459 CrossRef.
- C. Kresge, M. Leonowicz, W. Roth, J. Vartuli and J. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- C. Gérardin, J. Reboul, M. Bonne and B. Lebeau, Chem. Soc. Rev., 2013, 42, 4217 RSC; J. A. S. Costa and C. M. Paranhos, Microporous Mesoporous Mater., 2020, 309, 110570 CrossRef CAS; J. Chun, Y. M. Gu, J. Hwang, K. K. Oh and J. H. Lee, J. Ind. Eng. Chem., 2020, 81, 135 CrossRef; H. Dai, J. Yang, J. Ma, F. Chen, Z. Fei and M. Zhong, Microporous Mesoporous Mater., 2012, 147(1), 281 CrossRef; E. Prouzet, A. Kacheff, G. Aubert, A. Bentaleb, R. Backov and C. Aymonier, J. Supercrit. Fluids, 2019, 143, 139 CrossRef.
- G. Wang, X. Xu, Q. Cheng, J. Hu, X. Xu, Y. Zhang, S. Guo, Y. Ji, C. Zhou, F. Gao, L. Yang, Y. Yanxue Liu, S. Shuyan Yin and C. Chenyu Su, Sustainable Mater. Technol., 2023, 35, e00538 CrossRef CAS.
- C. Brambila, P. Boyd, A. Keegan, P. Sharma, C. Vetter, E. Ponnusamy and S. V. Patwardhan, ACS Sustainable Chem. Eng., 2022, 10(16), 5288 CrossRef CAS PubMed.
- N. Y. Amponsah, M. Troldborg, B. Kington, I. Aalders and R. L. Hough, Renewable Sustainable Energy Rev., 2014, 39, 461 CrossRef CAS; R. Paul, S. Kenway and P. Mukheibir, J. Cleaner Prod., 2019, 215, 1457 CrossRef; T. A. Kurniawan, M. H. Dzarfan Othman, G. H. Hwang and P. Gikas, J. Cleaner Prod., 2022, 357, 131911 CrossRef.
- R. Turconi, A. Boldrin and T. Astrup, Renewable Sustainable Energy Rev., 2013, 28, 555 CrossRef CAS; W. F. Lamb, T. Wiedmann, J. Pongratz, R. Andrew, M. Crippa and J. G. J. Olivier, Environ. Res. Lett., 2021, 16(7), 073005 CrossRef.
- IPCC, 2023: Sections, in Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. Core Writing Team, H. Lee and J. Romero, IPCC, Geneva, Switzerland, 2023, pp. 35–115, DOI:10.59327/IPCC/AR6-9789291691647.
- L. Milà i Canals, J. Chenoweth, A. Chapagain, S. Orr, A. Antón and R. Clift, Int. J. Life Cycle Assess., 2009, 14, 28 CrossRef CAS; S. Pfister, A. Koehler and S. Hellweg, Environ. Sci. Technol., 2009, 43(11), 4098 CrossRef PubMed.
- A. Y. Hoekstra, Chapter 7 - The water footprint of industry, in Assessing and Measuring Environmental Impact and Sustainability, ed. J. J. Klemeš, Butterworth-Heinemann, Oxford, 2015, p. 221 Search PubMed.
- P. Chowdhary, R. N. Bharagava, S. Mishra and N. Khan, Role of Industries in Water Scarcity and Its Adverse Effects on Environment and Human Health, in Environmental Concerns and Sustainable Development, ed. V. Shukla and N. Kumar, Springer, Singapore, 2020 Search PubMed.
- B. Salieri, D. A. Turner, B. Nowack and R. Hischier, Nanoimpact, 2018, 10, 108 CrossRef.
- S. Feijoo, S. González-García, Y. Moldes-Diz, C. Vazquez-Vazquez, G. Feijoo and M. T. Moreira, J. Cleaner Prod., 2017, 143, 528 CrossRef CAS.
- G. Barjoveanu, C. Teodosiu, I. Morosanu, R. Ciobanu, F. Bucatariu and M. Mihai, Nanomaterials, 2023, 13, 840 CrossRef CAS PubMed; O. P. Fuentes and J. F. Osma, Polymers, 2023, 15, 1457 CrossRef PubMed.
- M. Mazzoccoli, E. Arato and C. Moliner, Energies, 2022, 15, 2605 CrossRef CAS.
- T. Alaviitala and T. J. Mattila, J. Cleaner Prod., 2015, 93, 247–353 CrossRef.
- R. D. Schlanbusch, B. P. Jelle, L. I. C. Sandberg, S. M. Fufa and T. Gao, Build. Environ., 2014, 80, 115–124 CrossRef.
- I. Pinto, J. D. Silvestre, J. de Brito and M. F. Júlio, J. Cleaner Prod., 2020, 252, 119696 CrossRef CAS; E. Errington, M. Guo and J. Y. Y. Heng, Green Chem., 2023, 25, 4244 RSC.
- Y. Zhang, L. Magagnin, K. Yuan, Z. Wei, X. Wu, Z. Jiang and W. Wang, Microporous Mesoporous Mater., 2022, 345, 112280 CrossRef CAS.
- S. N. Joglekar, R. A. Kharkar, S. A. Mandavgane and B. D. Kulkarni, Environ. Sci. Pollut. Res., 2019, 26, 492 CrossRef CAS PubMed; O. Karatum, Md. Mainul, H. Bhuiya, M. K. Carroll, A. M. Anderson and D. L. Plata, J. Ind. Ecol., 2018, 22(6), 1365 CrossRef.
- K. Shanmugam, S. Jansson, V. Gadhamshetty, L. Matsakas, U. Rova, M. Tysklind, P. Christakopoulos and V. K. K. Upadhyayula, ACS Sustainable Chem. Eng., 2019, 7(24), 20000–20012 CrossRef CAS.
- O. de la Iglesia, M. Sarango, M. Munárriz, M. Malankowska, A. Navajas, L. M. Gandía, J. Coronas and C. Téllez, ACS Sustainable Chem. Eng., 2022, 10(9), 2868 CrossRef CAS PubMed.
- M. Benítez, C. Rodríguez-Carrillo, S. Sánchez-Artero, J. El Haskouri, P. Amorós and J. V. Ros-Lis, Green Chem., 2024, 26, 785–793 RSC.
- S. Feijoo, J. González-Rodríguez, L. Fernández, C. Vázquez-Vázquez, G. Feijoo and M. T. Moreira, Catalysts, 2020, 10, 23 CrossRef CAS.
- M. Grün, K. K. Unger, A. Matsumoto and K. Tsutsumi, Microporous Mesoporous Mater., 1999, 27, 207 CrossRef.
- J. Xu, Z. Luan, H. He, W. Zhou and L. Kevan, Chem. Mater., 1998, 10, 3690 CrossRef CAS.
- S. Muñoz-Pina, P. Amorós, J. El Haskouri, A. Andrés and J. V. Ros-Lis, Nanomaterials, 2020, 10, 1927 CrossRef PubMed.
- H. Zhao and H. Han, J. Solid State Chem., 2020, 282, 121074 CrossRef CAS.
- Z. Cao, P. Du, A. Duan, R. Guo, Z. Zhao, H. Zhang, P. Zheng, C. Xu and Z. Chen, Chem. Eng. Sci., 2016, 155, 141 CrossRef CAS.
- Sh. Ghasemi, Z. Jomeh Farsangi, A. Beitollahi, M. Mirkazemi, S. M. Rezayat and S. Sarkar, Ceram. Int., 2017, 43, 11225 CrossRef CAS.
- D. N. Peter, R. Pushpakumar, E. Jayaseelan and N. Ananthi, Mater. Today: Proc., 2021, 47, 739 CAS.
- B. Zhou, C. Y. Li, N. Qi, M. Jiang, B. Wanga and Z. Q. Chen, Appl. Surf. Sci., 2018, 450, 31 CrossRef CAS.
- E. Prouzet, F. Cot, C. Boissière, P. J. Kooyman and A. Larbot, J. Mater. Chem., 2002, 12, 1553 RSC.
- C. Yu, Y. Yu, L. Miao and D. Zhao, Microporous Mesoporous Mater., 2001, 44–45, 65 CrossRef CAS.
- R. R. Castillo, L. de la Torre, F. García-Ochoa, M. Ladero and M. Vallet-Regí, Int. J. Mol. Sci., 2020, 21, 7899 CrossRef CAS PubMed; T.-W. Kim, P.-W. Chung and V. S.-Y. Lin, Chem. Mater., 2010, 22, 5093 CrossRef.
- M.A. Abdelkareem, K. Elsaid, T. Wilberforce, M. Kamil, E.T. Sayed and A. Olabi, Sci. Total Environ., 2021, 752, 141803 CrossRef CAS PubMed; F. Perera and K. Nadeau, N. Engl. J. Med., 2022, 386(24), 2303–2314 CrossRef PubMed.
- A. Rahman, O. Farrok and M. M. Haque, Renewable Sustainable Energy Rev., 2022, 161, 112279 CrossRef; G. Bölük and M. Mert, Energy, 2014, 74, 439 CrossRef.
- M. Tawalbeh, A. Al-Othman, F. Kafiah, E. Abdelsalam, F. Almomani and M. Alkasrawi, Sci. Total Environ., 2021, 759, 143528 CrossRef CAS PubMed.
- A. Kuriqi, A. N. Pinheiro, A. Sordo-Ward and L. Garrote, J. Cleaner Prod., 2019, 232, 1028 CrossRef.
- M. K. Singla, P. Nijhawan and A. S. Oberoi, Environ. Sci. Pollut. Res., 2021, 28, 15607 CrossRef CAS PubMed; A. Coskun Avci, O. Kaygusuz and K. Kaygusuz, J. Eng. Res. Appl. Sci., 2020, 9(1), 1414 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc02347a |
| This journal is © The Royal Society of Chemistry 2024 |