Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible photons for the regioselective nucleophilic ring opening of epoxides†
Beatrice
Bernardoni‡
a,
Lorenzo
Di Terlizzi‡
 *a,
Eirini M.
Galathri
*a,
Eirini M.
Galathri
 b,
Christoforos G.
Kokotos
b,
Christoforos G.
Kokotos
 b,
Maurizio
Fagnoni
b,
Maurizio
Fagnoni
 *a and
Stefano
Protti
*a and
Stefano
Protti
 a
a
aPhotoGreen Lab, Department of Chemistry, University of Pavia, Viale Taramelli 12, 27100 Pavia, Italy. E-mail: lorenzo.diterlizzi01@universitadipavia.it; maurizio.fagnoni@unipv.it
bLaboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Athens 15771, Greece
First published on 20th August 2024
Abstract
Arylazo sulfones were used as Photoacid Generators (PAGs) for the visible-light photorelease of strong sulfonic acids to promote the ring opening of epoxides in benign media (DMC/water mixtures) or under neat conditions. Water, alcohols, azide and thiocyanate anions, as well as electron-rich aromatics were used in the role of the nucleophile. The resulting 1,2-disubstituted adducts were formed mostly in >99% yield in a high regioselective fashion.
Introduction
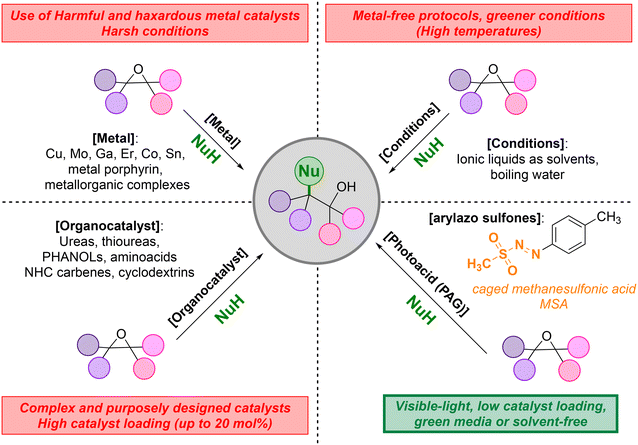
Epoxides are key building blocks in organic synthesis1 exploited for the construction of natural bioactive chemical frameworks,2 of benzo-fused heterocycles,3 of polycyclic ethers via a cascade-ring opening reaction,4 as well as in the preparation of 1,2-difunctionalized compounds.5 As an example, the ring opening of epoxides with alcohols6 provides access to β-alkoxyalcohols which structural motif has found wide applications in immunosuppressive and anti-tumoral pharmaceuticals.7 Moreover, the hydrolysis of epoxides is adopted to synthesize 1,2-diols, which in turn find applications as coolants and as co-monomers in the production of polyester fibres and resins.8 In particular, the synthesis of ethylene glycol from ethylene oxide is performed industrially in the presence of high excess of water, for the sake of chemoselectivity,9 and the final product must be recovered from the aqueous crude mixture by distillation. In this context, the use of metal-based Lewis acids, such as cobalt10 or tin11 can be helpful to lower the water/ethylene oxide ratio in the procedure. At any rate, the ring opening of epoxides by weak nucleophiles preferably takes place under catalytic conditions, and metal catalysis is the preferred option12 (even making use of expensive catalysts with a rather limited availability, such as molybdenium,12i,j erbium12k and gallium derivatives12l), along with the use of metal porphyrins12m or other organometallic species12n–p (Scheme 1). A particular case is the homolytic ring opening of epoxides by the Nugent–RajanBabu Reagent (Cp2TiCl).13 Nonetheless, chemists have proposed several metal-free approaches to trigger the ring-opening reaction of epoxides,14 including, among others, the use of cyclodextrins,15 metal-free boron-based frustrated Lewis pairs,16 N-heterocyclic carbenes17 or graphene oxide as the catalysts18 (Scheme 1). The procedures have been performed in different media, including ionic liquids,19 hot water20 or under continuous flow conditions.21 Given the impressive range of chemical structures accessible from epoxides, the design and the development of increasingly sustainable, versatile and efficient approaches for their opening is still a challenge.Recently, there is a growing interest in the development of PhotoAcid Generators (PAG) known to release an acid species upon irradiation. These derivatives (whether non-ionic or ionic) may be used as an acid surrogate since their slow release in solution could be beneficial for the reaction to catalyze.22 We recently used arylazo sulfones due to their sulfonic acid photorelease (under aerated conditions) to promote the protection of alcohols,23a ketones23b and the functionalization of indoles.23c,d We then envisaged that the visible light photoreactivity of these sulfones,24,25 may be helpful to induce the mild ring opening of epoxides (Scheme 1).
Results and discussion
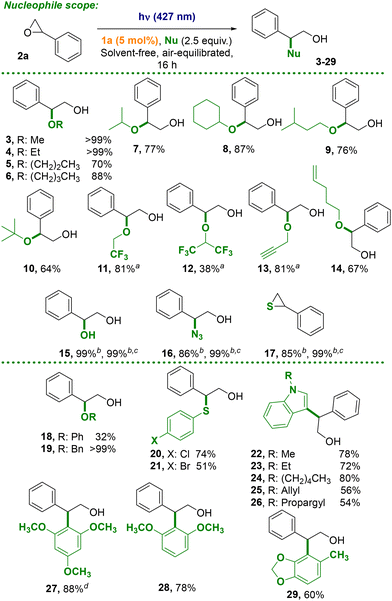
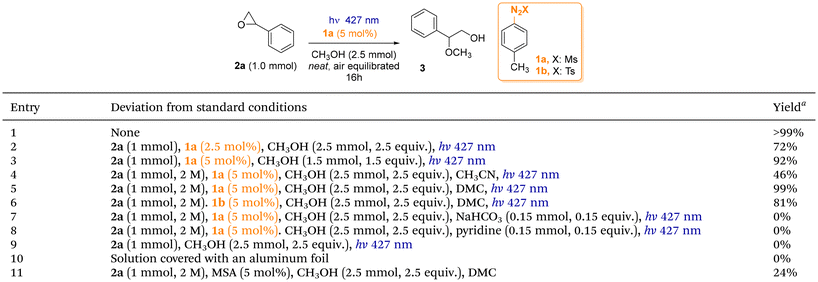
The feasibility of our proposal was tested on the ring opening of styrene oxide 2a (1 mmol) by using methanol (2.5 mmol, 2.5 equiv.) in the presence of arylazo sulfone 1a (5 mol%, precursor of methanesulfonic acid, MSA) in an air-equilibrated neat conditions upon visible light irradiation (for the spectrum in the visible range of 1a see Fig. S2†). To our delight, methoxy alcohol 3 was obtained quantitatively with a complete regioselectivity by irradiation with a 427 nm lamp for 16 h (Table 1, entry 1). Several conditions were tested (for instance nucleophile-epoxide ratio, solvents, irradiation wavelengths etc., see Table S1† for further details). Decreasing the amount of 1a (2.5 mol%) or of MeOH (1.5 equiv.) caused a yield drop (entries 2 and 3). The effect of a cosolvent (mandatory when the nucleophile is not soluble in the reaction mixture) was tested. Thus, the presence of MeCN affected the outcome of the reaction (46% yield, entry 4), contrary to DMC (a benign cosolvent26) where compound 3 was again formed in quantitative yield (entry 5). When arylazo sulfone 1b (5 mol%) was tested as alternative PAG (as a photochemical precursor of PTSA) by maintaining DMC as the solvent, compound 3 was obtained in a slightly lower yield (81%, entry 6). Finally, some control experiments were carried out (entries 7–10). The presence of an inorganic (NaHCO3, 0.15 equiv., entry 7) or an organic base (pyridine, 0.15 equiv., entry 8) completely suppressed the reaction (the epoxide was recovered unaltered). The presence of an arylazo sulfone (entry 9), as well as of the light (entry 10), was required to promote the ring opening. Moreover, the addition of MSA (5 mol%) in one time to the starting reaction mixture again formed 3, but unsatisfactorily (ca. 24% yield, entry 11). Kinetic analysis of the reaction (Fig. S7†) showed that the product was formed very fast at the initial stage of the reaction (60% yield after 1 h), but the complete conversion of the epoxide required 16 h irradiation. With the optimised conditions in our hand, we explored the scope of the protocol by using a series of alcohols in the role of the nucleophile (Scheme 2). The reaction has been performed mostly under neat conditions or, in alternative, by adopting DMC or in DMC/water mixtures as reaction media. The reaction with unbranched primary alcohols such as ethanol, propanol or butanol afforded the desired products 4–6 in satisfactory yields (>70%). Branched alcohols, such as iso-propanol, cyclohexanol, iso-pentanol or even tert-butanol served likewise as suitable nucleophiles (>64% yield, compounds 7–10). Less nucleophilic fluorinated alcohols gave, however, products 11 (81% yield) and 12 (38% yield) from 2,2,2-trifluoroethanol (TFE) and hexafluoroisopropanol (HFIP), respectively. Unsaturated alcohols (propargyl alcohol and pent-4-en-1-ol) yielded 13 and 14 in a satisfying yield (81% and 67%).| a Yields determined by GC analysis. |
|---|
|
Testing other types of nucleophiles (e.g. water, sodium azide or potassium thiocyanate) led to the quantitative formation of the corresponding glycol 15, the azido alcohol 16, or the thiirane 17 (Scheme 2). The latter reactions were carried out in DMC/water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and have been repeated on a 5 mmol scale maintaining the same excellent results (Fig. S4†).
1 and have been repeated on a 5 mmol scale maintaining the same excellent results (Fig. S4†).
More complex nucleophiles having an aromatic ring were then investigated and the reaction with phenol, benzylic alcohol, and substituted thiols gave the 1,2-disubstituted products 18–21 in variable yields. Finally, some electron-rich aromatics were explored to mimic a Friedel Crafts-like reaction. To our pleasure, our protocol proved to be robust and afforded the regioselective arylation of N-substituted indoles (products 22–26) and of electron-rich benzenes (products 27–29) in good yields (Scheme 2).
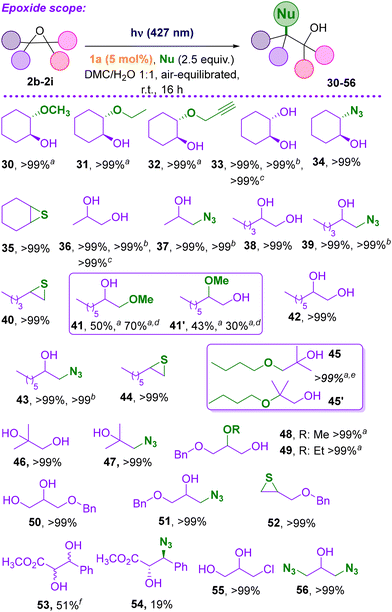
Other epoxides were employed to investigate the versatility of this protocol, starting from cyclohexene oxide (2b, Scheme 3). The organocatalyzed reaction of 2b with selected alcohols gave trans-derivatives 30–32 in almost quantitative yields. The formation of glycol 33 was quantitative in batch, as well even on a 5 mmol scale 2b and under flow conditions (Fig. S5 and S6†), where 20 mmol of epoxide were processed after 6 h reaction, reaching a 9.3 g day−1 productivity of 33 (see ESI, section 4†). Compound 2b was found an excellent starting material for the conversion into azido alcohol 34 and thiirane 35. We then tested related epoxides of terminal alkenes. Propylene glycol 36 (extensively used as solvent carrier in e-cigarettes liquids and in various industrial applications27) was easily formed from propylene oxide 2c in up to 5 mmol scale in batch or in 20 mmol scale in flow (productivity of 6.1 g day−1). The reaction of 2c with azide anion led to the regioselective formation of 37 (>99% yields on 5 mmol scale). Good results have been obtained from 1,2-hexene oxide 2d, (compounds 38–40). The reaction is still regioselective, leading to the more substituted alcohol (e.g. in compound 39). In the ring-opening of 1,2-octene oxide 2e, a single product was obtained, except from the reaction with MeOH that afforded two regioisomers (41 and 41′) in a 93% overall yield. Interestingly, when the irradiation was carried out by using 1b (a caged PTSA), the yield increased to quantitative, along with the change of the regioisomeric ratio (41 became by far the more abundant isomer, 70% instead of 50%). The ring opening reaction was also performed using isobutene oxide 2f. The addition of n-butanol onto this epoxide caused the quantitative formation of the 1,2-adducts 45 and 45′ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. A single product was however formed, when using water and azide anion as the nucleophiles (compounds 46 and 47, quantitative yield) by using a DMC/water mixture as the reaction media. 2-((Benzyloxy)methyl)oxirane 2g was next investigated and the functionalization with methanol and ethanol afforded 48 and 49 quantitatively, as in the synthesis of glycol 50, azido alcohol 51 and thiirane 52 (Scheme 3). The reaction on electron-poor epoxides such as methyl 3-phenyloxirane-2-carboxylate 2h, proved to be less efficient. Glycol 53 was obtained as a mixture of diastereoisomers (1
1 ratio. A single product was however formed, when using water and azide anion as the nucleophiles (compounds 46 and 47, quantitative yield) by using a DMC/water mixture as the reaction media. 2-((Benzyloxy)methyl)oxirane 2g was next investigated and the functionalization with methanol and ethanol afforded 48 and 49 quantitatively, as in the synthesis of glycol 50, azido alcohol 51 and thiirane 52 (Scheme 3). The reaction on electron-poor epoxides such as methyl 3-phenyloxirane-2-carboxylate 2h, proved to be less efficient. Glycol 53 was obtained as a mixture of diastereoisomers (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) with an overall yield of 51%, meanwhile the azido alcohol 54 was obtained in only 19% yield as the trans isomer.
1 ratio) with an overall yield of 51%, meanwhile the azido alcohol 54 was obtained in only 19% yield as the trans isomer.
Finally, the reactivity on epichlorohydrin 2i was tested making use of water and sodium azide as nucleophiles. In the first case, chlorine containing glycol 55 was formed, whereas in the latter 1,3-diazidopropan-2-ol 56 (resulting from the nucleophilic substitution on the C–Cl bond and by the nucleophilic ring opening reaction) was prepared both in quantitative fashion.
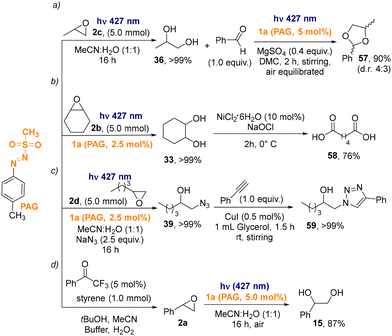
The appeal of our protocol was further highlighted by performing selected telescopic reactions involving the present ring opening procedure. Propylene oxide 2c (5.0 mmol) was opened to form the corresponding glycol 36 that it was converted into acetal 57 by reaction with benzaldehyde, exploiting again arylazo sulfone 1a as PAG (90% yield, dr 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (Scheme 4a).23b In another case, adipic acid 58 was obtained in two steps, starting from cyclohexene oxide by the Ni(II)-catalyzed oxidation of glycol 33 (Scheme 4b).28 The adduct formed between 2d and sodium azide was successfully converted into 1,2,3-triazole 59, following a green procedure in glycerol in just 1.5 h (Scheme 4c).29 Noteworthy, all of these telescopic reactions were carried out with no need to purify the ring-opened products and diacid 58 was isolated upon a simple recrystallization of the crude product. Finally, epoxide 2a was prepared starting from styrene30 and then photochemically opened to diol 15.
3) (Scheme 4a).23b In another case, adipic acid 58 was obtained in two steps, starting from cyclohexene oxide by the Ni(II)-catalyzed oxidation of glycol 33 (Scheme 4b).28 The adduct formed between 2d and sodium azide was successfully converted into 1,2,3-triazole 59, following a green procedure in glycerol in just 1.5 h (Scheme 4c).29 Noteworthy, all of these telescopic reactions were carried out with no need to purify the ring-opened products and diacid 58 was isolated upon a simple recrystallization of the crude product. Finally, epoxide 2a was prepared starting from styrene30 and then photochemically opened to diol 15.
The mild ring-opening of epoxides described herein occurs at room temperature under neat conditions or in an eco-sustainable solvent mixture (DMC/water or neat DMC26). The protocol exploits the tuneable release of methanesulfonic acid from a readily prepared PAG (compound 1a) that leaves only traces of toluene as byproduct in the reaction mixture.23 In some cases, a simple work-up consisting in the addition of MgSO4, removal of the drying agent and the solvent afforded the desired product in high yield and satisfactory purity. The low values of PMI calculated for the preparation of compounds 16 and 33 (2.86 and 3.53 kg kg−1, respectively) is an indication of the tiny amounts of waste produced in the reaction (see ESI, section 4† for further details). In the synthesis of 33, a further reduction of the PMI value (from 3.53 to 1.86 kg kg−1) resulted, when the process was scaled up to a 20 mmol scale in flow (irradiation time 6 h vs. 16 h). With our method, uncharged weak nucleophiles, such as water and alcohols, were easily added to epoxides. In the first case, 1,2-diols, including the wide used propylene glycol 36, were formed, while in the latter case valuable 1,2-alkoxyalcohols are accessed, despite a low regioselectivity was observed in selected cases (see for instance products 41 and 41′). The addition of SCN− to epoxide is efficiently catalyzed by 1a (see compounds 17, 35, 40, 44, 52 in Schemes 2 and 3). This reaction led to the formation of the corresponding thiiranes via the oxathiolan-2-imine intermediate31 as previously observed under catalyzed conditions12a,32a,b and under catalyst-free conditions32c using PEG32d or ionic liquids32e as the reaction media.
Conclusions
Summing up, we would point out that the simplicity of the method presented herein well met most of the photo-click chemistry criteria,33 since it allows a process with a significantly high thermodynamic driving force,34 it is wide in scope and affords the desired products in very high yields. Furthermore, in several cases, isolation of the end products took place with no need of separation upon column chromatography and products were obtained (at least starting from 2b and 2h) in a satisfactory diasteroselectivity.33 The ring-opening of epoxides may be easily included in telescopic transformations as illustrated in Scheme 4. Noteworthy, the synthesis of 57 was carried out by two consecutive acid catalyzed reactions promoted by the same arylazo sulfone as PAG.Author contributions
Beatrice Bernardoni: Investigation; Lorenzo Di Terlizzi: conceptualization, investigation and writing – original draft; Eirini M. Galathri: investigation. Christoforos G. Kokotos: funding acquisition, writing – review and editing. Maurizio Fagnoni: conceptualization, supervision, writing – original draft, writing – review and editing. Stefano Protti: funding acquisition, writing – review and editing.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge support from UniPV and MUR through the program ‘Departments of Excellence’ (2023–2027). SP thanks the Italian Ministry for Universities and Research, PRIN-PNRR, project P2022HSF3R for financial support. EMG and CGK gratefully acknowledge the Hellenic Foundation for Research and Innovation (HFRI) for financial support through a grant, which is financed by 1st Call for H. F. R. I. Research Projects to Support Faculty Members & Researchers and the procurement of high-cost research equipment grant (grant number 655).References
- (a) J. G. Smith, Synthesis, 1984, 629–656 CrossRef CAS; (b) J. He, J. Ling and P. Chiu, Chem. Rev., 2014, 114, 8037–8128 CrossRef CAS PubMed; (c) S. Meninno and A. Lattanzi, ACS Org. Inorg. Au, 2022, 2, 289–305 CrossRef CAS PubMed; (d) D. Tang, K. Dang, J. Wang, C. Chen, J. Zhao and Y. Zhang, Sci. China: Chem., 2023, 66, 3415–3425 CrossRef CAS; (e) T. Yan, H. Liu, Z. X. Zeng and W. G. Pan, J. CO2 Util., 2023, 68, 102355 CrossRef CAS.
- R. R. Rodríguez-Berríos, S. R. Isbel and A. Bugarin, Int. J. Mol. Sci., 2023, 24, 6195–6229 CrossRef PubMed.
- A. J. Das and S. K. Das, Eur. J. Org. Chem., 2022, e202101034 Search PubMed.
- I. Vilotijevic and T. F. Jamison, Angew. Chem., Int. Ed., 2009, 48, 5250–5281 CrossRef CAS PubMed.
- (a) D. M. Hodgson and E. Gras, Synthesis, 2002, 1625–1642 CrossRef CAS; (b) F. Moschona, I. Savvopoulou, M. Tsitopoulou, D. Tataraki and G. Rassias, Catalysts, 2020, 10, 1117 CrossRef CAS; (c) M. Thirumalaikumar, Org. Prep. Proced. Int., 2022, 54, 1–39 CrossRef CAS; (d) B. R. Moser, S. C. Cermak, K. M. Doll, J. A. Kenar and B. K. Sharma, J. Am. Oil Chem. Soc., 2022, 99, 801–842 CrossRef CAS.
- (a) A. S. Rao, S. K. Paknikar and J. G. Kirtane, Tetrahedron, 1983, 39, 2323–2367 CrossRef CAS; (b) S. S. Kahandal, S. R. Kale, S. T. Disale and R. V. Jayaram, Catal. Sci. Technol., 2012, 2, 1493–1499 RSC; (c) A. J. L. Leitão, J. A. R. Salvador, R. M. A. Pinto and M. L. Sá e Melo, Tetrahedron Lett., 2008, 49, 1694–1697 CrossRef; (d) J. Koopmeiners, C. Diederich, J. Solarczek, H. Voß, J. Mayer, W. Blankenfeldt and A. Schallmey, ACS Catal., 2017, 7, 6877–6886 CrossRef CAS; (e) A. Rostami, A. Ebrahimi, J. Husband, M. U. Anwar, R. Csuk and A. Al-Harrasi, Eur. J. Org. Chem., 2020, 1881–1895 CrossRef CAS; (f) M. Mirza-Aghayan, M. Alizadeh, M. Molaee Tavana and R. Boukherroub, Tetrahedron Lett., 2014, 55, 6694–6697 CrossRef CAS.
- (a) R. J. Jones and H. Rapoport, J. Org. Chem., 1990, 55, 1144–1146 CrossRef CAS; (b) C. Jaramillo, J. L. Chiara and M. Martinlomas, J. Org. Chem., 1994, 59, 3135–3141 CrossRef CAS; (c) P. Stead, H. Marley, M. Mahmoudian, G. Webb, D. Noble, Y. T. Ip, E. Piga, T. Rossi, S. Roberts and M. J. Dawson, Tetrahedron: Asymmetry, 1996, 7, 2247–2250 CrossRef CAS.
- (a) J. Zheng, L. Huang, C. Cui, Z. Chen, X. Liu, X. Duan, X. Cao, T. Yang, H. Zhu, K. Shi, P. Du, S. Ying, C. Zhu, Y. Yao, G. Guo, Y. Yuan, S. Xie and L. Zheng, Science, 2022, 376, 288–292 CrossRef CAS PubMed; (b) Z. Yan, C. Du, G. Luo and J. Deng, React. Chem. Eng., 2021, 6, 2159–2169 RSC; (c) A. Theodorou, I. Triandafillidi and C. G. Kokotos, Eur. J. Org. Chem., 2017, 1502–1509 CrossRef CAS.
- Q. Su, X. Tan, X. Yao, T. Ying, L. Dong, M. Fu, W. Cheng and S. Zhang, Green Chem., 2021, 23, 2992–3000 RSC.
- B. Li, S. Bai, X. Wang, M. Zhong, Q. Yang and C. Li, Angew. Chem., Int. Ed., 2012, 51, 11517–11521 CrossRef CAS PubMed.
- B. Tang, W. Dai, G. Wu, N. Guan, L. Li and M. Hunger, ACS Catal., 2014, 4, 2801–2810 CrossRef CAS.
- (a) N. Iranpoor, H. Firouzabadi and M. Shekarize, Org. Biomol. Chem., 2003, 1, 724–727 RSC; (b) D. B. G. Williams and M. Lawton, Org. Biomol. Chem., 2005, 3, 3269–3272 RSC; (c) K. Lam and I. E. Markó, Chem. Commun., 2009, 95–97 RSC; (d) J. Barluenga, H. Vázquez-Villa, A. Ballesteros and J. M. González, Org. Lett., 2002, 4, 2817–2819 CrossRef CAS PubMed; (e) K. Lam and I. E. Markó, Tetrahedron, 2009, 65, 10930–10940 CrossRef CAS; (f) J. G. Flores, J. L. Obeso, V. Martínez-Jiménez, N. Martín-Guaregua, A. Islas-Jácome, E. González-Zamora, H. Serrano-Espejel, B. Mondragón-Rodríguez, C. Leyva, D. A. Solís-Casados, I. A. Ibarra, R. A. Peralta, J. Aguilar-Pliego and J. A. de los Reyes, RSC Adv., 2023, 13, 27174–27179 RSC; (g) J.-Y. Li, C.-L. Tan, M.-Y. Qi, Z.-R. Tang and Y.-J. Xu, Angew. Chem., Int. Ed., 2023, 62, e202303054 CrossRef CAS PubMed; (h) J. F. Blandez, A. Santiago-Portillo, S. Navalón, M. Giménez-Marqués, M. Álvaro, P. Horcajada and H. García, J. Mol. Catal. A: Chem., 2016, 425, 332–339 CrossRef CAS; (i) J. Kandasamy and D. K. Chand, Synthesis, 2008, 807–819 Search PubMed; (j) S. Budhi, C. Peeraphatdit, S. Pylypenko, V. H. T. Nguyen, E. A. Smith and B. G. Trewyn, Appl. Catal., A, 2014, 475, 469–476 CrossRef CAS; (k) R. Dalpozzo, M. Nardi, M. Oliverio, R. Paonessa and A. Procopio, Synthesis, 2009, 3433–3438 CAS; (l) C. P. Krap, R. Newby, A. Dhakshinamoorthy, H. García, I. Cebula, T. L. Easun, M. Savage, J. E. Eyley, S. Gao, A. J. Blake, W. Lewis, P. H. Beton, M. R. Warren, D. R. Allan, M. D. Frogley, C. C. Tang, G. Cinque, S. Yang and M. Schröder, Inorg. Chem., 2016, 55, 1076–1088 CrossRef CAS PubMed; (m) A. Lidskog, Y. Li and K. Wärnmark, Catalysts, 2020, 10, 705–770 CrossRef CAS; (n) A. A. Tehrani, S. Abedi, A. Morsali, J. Wang and P. C. Junk, J. Mater. Chem. A, 2015, 3, 20408–20415 RSC; (o) S. Banerjee, D. P. Kumar, S. Bandyopadhay, N. N. Adarsh and P. Dastidar, Cryst. Growth Des., 2012, 12, 5546–5554 CrossRef CAS; (p) J. L. Obeso, J. G. Flores, C. V. Flores, R. Rios-Escobedo, J. Aguilar-Pliego, A. K. Inge, J. A. de los Reyes, R. A. Peralta, I. A. Ibarra and C. Leyva, ChemCatChem, 2023, 15, e202300471 CrossRef CAS.
- (a) A. Rosales, I. Rodríguez-García, J. Muñoz-Bascón, E. Roldan-Molina, N. M. Padial, L. Pozo Morales, M. García-Ocaña and J. E. Oltra, Eur. J. Org. Chem., 2015, 4567–4591 CrossRef CAS; (b) J. Gordon, S. Hildebrandt, K. R. Dewese, S. Klare, A. Gansäuer, T. V. RajanBabu and W. A. Nugent, Organometallics, 2018, 37, 4801–4809 CrossRef CAS PubMed; (c) T. McCallum, X. Wu and S. Lin, J. Org. Chem., 2019, 84, 14369–14380 CrossRef CAS PubMed.
- (a) V. Kumar and S. S. Chimni, ChemistrySelect, 2023, 8, e202301963 CrossRef CAS; (b) S. Meninno and A. Lattanzi, Chem. – Eur. J., 2016, 22, 3632–3642 CrossRef CAS PubMed; (c) D. C. Braddock, I. D. MacGilp and B. G. Perrya, Adv. Synth. Catal., 2004, 346, 1117–1130 CrossRef CAS; (d) C. M. Kleiner and P. R. Schreiner, Chem. Commun., 2006, 41, 4315–4317 RSC; (e) E. M. Fleming, C. Quigley, I. Rozas and S. J. Connen, J. Org. Chem., 2008, 73, 948–956 CrossRef CAS PubMed; (f) F. Fringuelli, O. Piermatti, F. Pizzo and L. Vaccaro, J. Org. Chem., 1999, 64, 6094–6096 CrossRef CAS.
- (a) R. Sridhar, B. Srinivas, K. Surendra, N. S. Krishnaveni and K. R. Rao, Tetrahedron Lett., 2005, 46, 8837–8839 CrossRef CAS; (b) B. Srinivas, V. P. Kumar, R. Sridhar, K. Surendra, Y. V. D. Nageswar and K. R. Rao, J. Mol. Catal. A, 2007, 261, 1–5 CrossRef CAS; (c) M. A. Reddy, N. Bhanumathi and K. Rama Rao, Tetrahedron Lett., 2002, 43, 3237–3238 CrossRef.
- (a) T. Krachko, E. Nicolas, A. W. Ehlers, M. Nieger and J. C. Slootweg, Chem. – Eur. J., 2018, 24, 12669–12677 CrossRef CAS PubMed; (b) M. Pineschi, F. Bertolini, R. M. Haak, P. Crotti and F. Macchia, Chem. Commun., 2005, 1426–1428 RSC; (c) C. Baylon, G. Prestat, M.-P. Heck and C. Mioskowski, Tetrahedron Lett., 2000, 41, 3833–3835 CrossRef CAS.
- (a) K. Y.-K. Chow and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 8126–8127 CrossRef CAS PubMed; (b) G.-L. Zhao and A. Cardova, Tetrahedron Lett., 2007, 48, 5976–5980 CrossRef CAS; (c) H. Jiang, B. Gschwend, L. Albrecht and K. A. Jørgensen, Org. Lett., 2010, 12, 5052–5055 CrossRef CAS PubMed; (d) J. Qi, X. Xie, J. He, L. Zhang, D. Ma and X. She, Org. Biomol. Chem., 2011, 9, 5948–5950 RSC.
- A. Dhakshinamoorthy, M. Alvaro, P. Concepción, V. Fornésa and H. Garcia, Chem. Commun., 2012, 48, 5443–5445 RSC.
- (a) F. Fringuelli, O. Piermatti, F. Pizzo and L. Vaccaro, J. Org. Chem., 1999, 64, 6094–6097 CrossRef CAS; (b) F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, Green Chem., 2003, 5, 436–440 RSC; (c) F. Fringuelli, F. Pizzo and L. Vaccaro, J. Org. Chem., 2004, 69, 2315–2321 CrossRef CAS PubMed; (d) F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, J. Org. Chem., 2004, 69, 8780–8785 CrossRef CAS PubMed; (e) J. S. Yadav, B. V. S. Reddy, B. Jyothi and M. S. R. Murty, Tetrahedron Lett., 2005, 46, 6559–6562 CrossRef CAS; (f) J. Chen, C. Jin, X. Zhang and W. Su, Green Chem., 2006, 8, 330–332 RSC.
- Z. Wang, Y.-T. Cui, Z.-B. Xu and J. Qu, J. Org. Chem., 2008, 73, 2270–2274 CrossRef CAS PubMed.
- S. A. Abbas, L. Cao, D. Seo, A. O. Farah, P. H.-Y. Cheong, J. K. Park and K. M. Nam, ChemCatChem, 2023, 15, e202201138 CrossRef CAS.
- For selected examples: (a) P. Wan and D. Shukla, Chem. Rev., 1993, 93, 571–584 CrossRef CAS; (b) M. Tsunooka, K. Suyama, H. Okumura and M. Shirai, J. Photopolym. Sci. Technol., 2006, 19, 65–71 CrossRef CAS; (c) M. Shirai and M. Tsunooka, Bull. Chem. Soc. Jpn., 1998, 71, 2483–2507 CrossRef CAS; (d) J. V. J. Crivello, Polym. Sci., Part A: Polym. Chem., 1999, 37, 4241–4254 CrossRef CAS; (e) C. J. Martin, G. Rapenne, T. Nakashima and T. Kawai, J. Photochem. Photobiol., C, 2018, 34, 41–51 CrossRef CAS; (f) N. Spiliopoulou, N. F. Nikitas and C. G. Kokotos, Green Chem., 2020, 22, 3539–3545 RSC; (g) N. A. Kuznetsova, G. V. Malkov and G. G. Gribov, Russ. Chem. Rev., 2020, 89, 173–190 CrossRef CAS; (h) J. Saway, Z. M. Salem and J. J. Badillo, Synthesis, 2021, 489–497 CAS.
- (a) L. Di Terlizzi, A. Martinelli, D. Merli, S. Protti and M. Fagnoni, J. Org. Chem., 2023, 88, 6313–6321 CrossRef CAS PubMed; (b) L. Di Terlizzi, E. M. Galathri, S. Protti, C. G. Kokotos and M. Fagnoni, ChemSusChem, 2023, 16, e202201998 CrossRef CAS PubMed; (c) E. M. Galathri, L. Di Terlizzi, M. Fagnoni, S. Protti and C. G. Kokotos, Org. Biomol. Chem., 2023, 21, 365–369 RSC; (d) E. M. Galathri, L. Di Terlizzi, M. Fagnoni, S. Protti and C. G. Kokotos, ChemCatChem, 2024, e202400686 CrossRef , in press.
- (a) D. Qiu, C. Lian, J. Mao, M. Fagnoni and S. Protti, J. Org. Chem., 2020, 85, 12813–12822 CrossRef CAS PubMed; (b) L. Di Terlizzi, L. Nicchio, S. Protti and M. Fagnoni, Chem. Soc. Rev., 2024, 53, 4926–4975 RSC; (c) R. Chawla, A. K. Singh and P. K. Dutta, Org. Biomol. Chem., 2024, 22, 869–893 RSC.
- For selected examples on arylazo sulfone photochemistry see: (a) L. Di Terlizzi, I. Cola, C. Raviola, M. Fagnoni and S. Protti, ACS Org. Inorg. Au, 2021, 1, 68–71 CrossRef PubMed; (b) L. Di Terlizzi, S. Scaringi, C. Raviola, R. Pedrazzani, M. Bandini, M. Fagnoni and S. Protti, J. Org. Chem., 2022, 87, 4863–4872 CrossRef CAS PubMed; (c) Y. Gao, S. Sparascio, L. Di Terlizzi, M. Serra, G. Yue, Y. Lu, M. Fagnoni, X. Zhao and S. Protti, Adv. Synth. Catal., 2023, 365, 1082–1087 CrossRef CAS; (d) L. Di Terlizzi, L. Nicchio, C. Callegari, S. Scaringi, L. Neuville, M. Fagnoni, S. Protti and G. Masson, Org. Lett., 2023, 25, 9047–9052 CrossRef CAS PubMed.
- P. Tundo, F. Aricò, E. A. Rosamilia, S. Grego and L. Rossi, in Green Chemical Reactions, ed. P. Tundo and V. Esposito, Springer Science + Business Media B.V., Dordrecht, 2008, pp. 213–232 Search PubMed.
- (a) J. A. Okolie, iScience, 2022, 25, 104903 CrossRef CAS PubMed; (b) L. Li, E. S. Lee, C. Nguyen and Y. Zhu, Aerosol Sci. Technol., 2020, 54, 1270–1281 CrossRef CAS PubMed; (c) M. Sara, T. Rouissi, S. K. Brar and J. F. Blais, in Platform Chemical Biorefinery, ed. S. Kaur Brar, S. Jyoti Sarma and K. Pakshirajan, Elsevier, ch. 5, 2016, pp. 77–100 Search PubMed.
- J. M. Grill, J. W. Ogle and S. A. Miller, J. Org. Chem., 2006, 71, 9291–9296 CrossRef CAS PubMed.
- C. Vidal and J. García-Álvarez, Green Chem., 2014, 16, 3515–3521 RSC.
- (a) D. Limnios and C. G. Kokotos, J. Org. Chem., 2014, 10, 4270–4276 CrossRef PubMed; (b) I. Triandafillidi, M. G. Kokotou, D. Lotter, C. Sparr and C. G. Kokotos, Chem. Sci., 2021, 30, 10191–10196 RSC; (c) E. Poursaitidis, P. L. Gkizis, I. Triandafillidi and C. G. Kokotos, Chem. Sci., 2024, 15, 1177–1203 RSC; (d) E. Poursaitidis, F. Trigka, C. Mantzourani, M. G. Kokotou, I. Triandafillidi and C. G. Kokotos, Eur. J. Org. Chem., 2024, e202400082 CrossRef CAS.
- C. M. Kleiner, L. Horst, C. Wurtele, R. Wendea and P. R. Schreiner, Org. Biomol. Chem., 2009, 7, 1397–1403 RSC.
- (a) N. Iranpoor and F. Kazemi, Tetrahedron, 1997, 53, 11377–11382 CrossRef CAS; (b) N. S. Krishnaveni, K. Surendra, M. S. Reddy, Y. V. D. Nageswar and K. R. Rao, Adv. Synth. Catal., 2004, 346, 395–397 CrossRef CAS; (c) A. Tsoukaki, E. Skolia, I. Triandafillidi and C. G. Kokotos, Eur. J. Org. Chem., 2022, e202200463 CrossRef CAS; (d) B. Das, V. S. Reddy and M. Krishnaiah, Tetrahedron, 2006, 47, 8471–8473 CrossRef CAS; (e) J. S. Yadav, B. V. S. Reddy, C. S. Reddy and K. Rajasekhar, J. Org. Chem., 2003, 68, 2525–2527 CrossRef CAS PubMed.
- (a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS; (b) B. D. Fairbanks, L. J. Macdougall, S. Mavila, J. Sinha, B. E. Kirkpatrick, K. S. Anseth and C. N. Bowman, Chem. Rev., 2021, 121, 6915–6990 CrossRef CAS PubMed; (c) G. S. Kumar and Q. Lin, Chem. Rev., 2021, 121, 6991–7031 CrossRef CAS PubMed.
- K. M. Morgan, J. A. Ellis, J. Lee, A. Fulton, S. L. Wilson, P. S. Dupart and R. Dastoori, J. Org. Chem., 2013, 78, 4303–4311 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods, detailed synthetic procedures, 1H and 13C NMR spectra of all compounds. See DOI: https://doi.org/10.1039/d4gc02612h |
| ‡ The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |