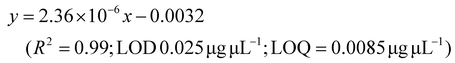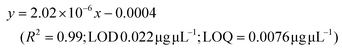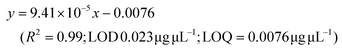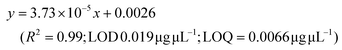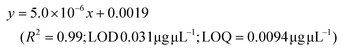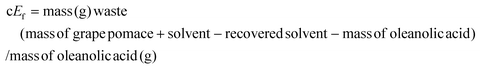Open Access Article
Open Access ArticleEfficient and selective extraction of oleanolic acid from grape pomace with dimethyl carbonate†
Francesco
Errichiello
a,
Raffaele
Cucciniello
 *b,
Michele
Tomasini
b,
Laura
Falivene
*b,
Michele
Tomasini
b,
Laura
Falivene
 b,
Angelita
Gambuti
a,
Chiara
Cassiano
c and
Martino
Forino
a
b,
Angelita
Gambuti
a,
Chiara
Cassiano
c and
Martino
Forino
a
aDepartment of Agricultural Sciences, Grape and Wine Science Division, University of Napoli ‘Federico II’, Viale Italia, 83100 Avellino, Italy
bDepartment of Chemistry and Biology ‘Adolfo Zambelli’, University of Salerno, Via Giovanni Paolo II, 132, 84084 Fisciano, SA, Italy. E-mail: rcucciniello@unisa.it
cDepartment of Pharmacy, School of Medicine and Surgery, University of Napoli ‘Federico II’, Via D. Montesano 49, 80131 Napoli, Italy
First published on 2nd September 2024
Abstract
Grape pomace is a major winery solid residue and several tons are annually produced worldwide. Since it is a valuable source of high value-added compounds, many strategies have been implemented for its valorization. The extraction of bioactive molecules with a broad range of applications is certainly the most investigated topics. In this context, oleanolic acid, a triterpenoid with a relevant biological activity, has been recently detected in grape pomace in remarkable quantities (0.45 mg per gram of fresh pomace). Herein, we report on a selective extraction of oleanolic acid from grape pomace by using dimethyl carbonate (DMC), a recommended green solvent as a better alternative to fossil-based solvents. Chemical–physical properties, Hildebrand's solubility and Kamlet–Abboud–Taft parameters have been considered to select a greener alternative to fossil-based solvents and theoretical calculations have been performed to determine the interaction between DMC and the oleanolic acid. The obtained grape pomace extracts were characterized by means of NMR and LC-MS. DMC allows the recovery of oleanolic acid from grape pomace, due to its weak polarity and poor ability to form H-bonds, with a molar selectivity of 61%, thus promoting the adoption of alternative green and sustainable technologies for biomass residue valorization. Also, DMC was recycled and reused in three consecutive extractions and no significant losses in terms of oleanolic acid extraction yield were detected.
Introduction
According to some studies conducted by the Food and Agricultural Organization,1 grapes are among the most produced fruit across the planet with an estimated amount of about 80 million tons per year. Given that grapes are mostly employed for winemaking, a large volume of by-products is inevitably accumulated in wineries over a short period of time in concomitance with the harvest. The consequential environmental and economic implications are enormous.2,3 Winery by-products include wine lees, grape stalks, vine shoots and grape pomace. The latter accounts for some 45% of the total wine-derived waste.4 Grape pomace, even called marc, originates from pressing and fermentation processes and its composition is highly variable in terms of texture and metabolites depending upon many factors such as the cultivar, fruit maturity, soil and winemaking procedures. Generally, it is composed of erratic quantities of skins, seeds, pulps and stalks,4 among which skins account approximately for half the grape pomace weight.5 Major metabolites contained in pomaces include fibres and sugars, with a typical fibre content of over 50% in red grapes and of about 20–30% in white ones.6 Also, red grape skins, which are qualitatively and quantitatively richer in metabolite composition than white ones, in addition to tannins and other phenolics, contain remarkable quantities of anthocyanins.7 Seeds that constitute another 25% of weight of grape pomace are a source of saturated and unsaturated fatty acids, besides oligosaccharides and phenolics.8 Finally, stalks accounting for the remaining 25% of the weight of grape pomace are constituted essentially by fibres (cellulose) and lignin.5So far, in the frame of circular economy, grape pomace has been mainly reused for the distillation of spirits,9 for animal feeding and for soil fertilization. Unfortunately, the latter two reuses are not devoid of drawbacks. In fact, livestock health can be impaired by the high content of fibres, tannins and anthocyanins that as a whole negatively affect the animal digestion process.10 On the other hand, the spreading of pomace across open areas can inhibit germination as a consequence of the phytotoxic and antimicrobial properties of some metabolites such as polyphenols.11 Alternatively, on account of its rich selection of nutrients and healthy compounds, grape pomace has been valorised by manufacturing food supplements, nutraceuticals, cosmeceuticals and even medical devices.12,13 So far, much effort has been made principally to extract polyphenols, due to their interesting health-related bioactivities and relatively high abundancy in pomaces.14,15 The procedures developed for the recovery of polyphenols have been mainly based on solid/liquid extractions and optimized either by modulating the concentrations of the used solvents or by varying the acidity of the extraction mixtures. Often, such methodologies, besides being time-consuming and costly, do not provide high extraction yields.13 Better results have been obtained by assisting the solid/liquid extraction with other green technologies including ultrasound, microwaves, supercritical or pressurized fluids. These methodologies, especially if the treated biomass is ground, reduce the extraction time and avoid a detrimental long exposure of phytochemicals to high temperatures.10 Also, eutectic solvents have been lately proven to reduce extraction times especially if coupled with other methodologies. As a way of example, the same amount of proanthocyanidins from grape pomace can be extracted in just one hour by using a combination of eutectic solvents with microwaves, while at least four hours are generally needed by classical extraction protocols.16
Given the numerous health-related effects shown by grape pomace extracts, including antioxidant, anti-inflammatory, antidiabetic and antimicrobial, among others, it is evident that all these beneficial properties can hardly be ascribed to a single class of polyphenols.15 Moreover, plant secondary metabolism usually leads to the synthesis of several molecules that significantly differ from one another by chemical structure and are thus expected to be responsible for different bioactivities. Based on the above considerations, we have recently analysed samples of Aglianico (Vitis vinifera L.) red grape pomace with the purpose of identifying potential bioactive compounds in addition to polyphenols.17 In this study we have indeed detected and quantified a triterpenoid, oleanolic acid, in the order of 0.45 mg per gram of fresh pomace (Fig. 1). Because of oleanolic acid's poor solubility in the wine aqueous solution, it was not surprising to discover such high residual quantities in the analysed matrix.
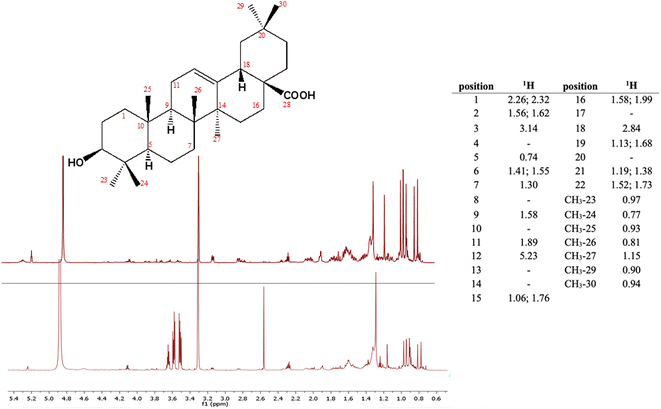 | ||
| Fig. 1 Stereostructure of oleanolic acid and its chemical shifts in CD3OD; 1H NMR spectrum of oleanolic acid (top) and of the extract in DMC of Aglianico grape pomace from Campania, Italy (bottom). | ||
Triterpenoids are a class of specialized metabolites widely spread across the plant kingdom and, of late, they have become a topical research focus because of their many biological properties including antibiotic, antiviral and anticancer properties.18 In more detail, oleanolic acid is a pentacyclic triterpenoid that is a major component of the grape berry cuticle along with cutin polymer and other aliphatic waxes. Berry cuticle plays a strategic role in protecting grapes from both biotic and abiotic environmental stresses. In this regard, VanderWeide et al.19 have reported that climate changes and more specifically heatwaves stimulate a greater production of total waxes and triterpenoids to limit cuticular transpiration. Thus, as heatwaves are predicted to intensify in terms of frequency and duration in the future, it is reasonable to assume that the recoverable amount of oleanolic acid from grape pomace is expected to be increasing. It follows that by setting up sustainable extraction protocols to recover it will be strategic not only to reduce the environmental impact of winemaking, but also to assist the transition of wineries towards the circular economy by favouring their competitiveness and resilience.20 An issue that is worth investigating is the possible reuse of oleanolic acid in winemaking itself. In fact, oleanolic acid is reported to be an inhibitor of tyrosinases, enzymes that are naturally contained in fruit including grapes.21 Enzyme activity leads to undesirable color changes particularly of white wines, referred to as oxidative browning that negatively affects the quality of the finished product.22 Therefore, by inhibiting the tyrosinase activity early in winemaking, it would be possible to minimize its detrimental effects while preserving the phenolic composition and thus the quality of the wine.
Over the past few decades, several conventional methods to extract oleanolic acid from natural sources have been proposed, including heat reflux or Soxhlet.23 Yet, these techniques are not totally convenient as they are either solvent- or time-consuming.24 Additionally, they are poorly selective and cause the degradation of thermolabile compounds. However, in the case of triterpenoids temperature seems to be not a key factor. In fact, the yield of oleanolic acid was not significantly modified by varying the extraction temperature from 40 to 70 °C.23
With regard to the extracting solvents, as mentioned above, triterpenoids are slightly polar compounds.25 High extraction yields of oleanolic acid (Hildebrand's solubility parameter of 10.2) using n-butanol, ethyl ether, chloroform, methanol and ethanol have been reported.26 However, all of the above solvents hardly address the increasing demand for more sustainable extraction methods that need to be based on the substitution of fossil-based and toxic solvents, including chlorinated ones.
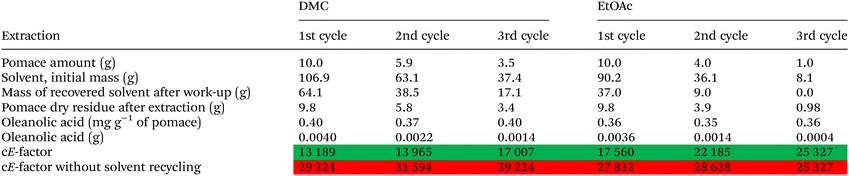
The development of new protocols able to selectively extract oleanolic acid from grape pomace by relying on bio-based and non-toxic solvents, while maximizing the extraction yield by reducing the E-factor, is a challenging task. Moreover, the possibility of recovering the used solvent for consecutive extraction cycles would be a significant advancement for the wine industry in the light of the green chemistry principles (the 2nd, 5th, and 7th, and indirectly referred to in almost every other case).
In this frame, we have tested the extraction efficiency of a set of solvents for selective recovery of oleanolic acid from grape pomace. In particular, three conventional organic solvents (ethyl acetate, acetone, n-butanol) were used to compare their efficiency and selectivity with two alternative promising biobased and low-toxic solvents, namely dimethyl carbonate (DMC) and 2-methyltetrahydrofuran (2-MeTHF). The two latter solvents have not been tested for oleanolic acid extraction from biomasses. DMC is a weakly polar, aprotic, non-toxic and easily biodegradable solvent obtained from the valorisation of CO2,27 whereas 2-MeTHF is a high-value solvent obtained from both furfural and levulinic acid biorefinery and it has recently emerged as a valuable solvent for the extraction of bioactive compounds from biomasses.28,29 The extraction efficiencies of these two solvents have been discussed on the basis of their polarity, Hildebrand's solubility parameter and Kamlet–Abboud–Taft (KAT) parameters.
Also, density functional theory (DFT) calculations have been employed to visualize the interplay between the solvent and the substrate, which is responsible for the extraction mechanism. Furthermore, in order to evaluate their potential reuse for consecutive extraction cycles, recycling experiments were carried out for DMC and ethyl acetate (EtOAc) that were proved to be the two solvents with the highest oleanolic acid extraction yield. In conclusion, E-factor (Ef) was used to evaluate the impacts in terms of produced waste and to drive the choice toward greener alternatives.
Materials and methods
Chemicals and reagents
All of the solvents used in this study (acetonitrile, ethanol, methanol, n-butanol, EtOAc, acetone, DMC, 2-MeTHF, acetic acid and pyridine) were of HPLC grade or higher. Malvidin-3-O-glucoside, (+)-catechin, (–)-epicatechin, quercetin, trans-resveratrol, and oleanolic acid were used as standards for HPLC analyses. The above solvents and standards were purchased from Sigma-Aldrich (Milan, Italy). Aqueous solutions were prepared with Milli-Q water from Millipore (Bedford, MA, USA).Biological samples and extraction procedures
Red grape pomaces were obtained from two different cultivars, namely Aglianico (Vitis vinifera L. cv) and Cabernet (Vitis vinifera L. cv) grapes, collected in the autumn of 2021 from three different areas located in Southern Italy: Calabria, Irpinia and Sannio. Hence, six biological samples from grape pomaces were obtained.In order to evaluate the content of oleanolic acid in each of the six analysed grape pomaces, 5-g lyophilized samples, after being homogenized by a laboratory blender, were extracted twice and in triplicate with 30.0 mL of an ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (8
water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture under stirring overnight at room temperature. 1 mL from each extract was analysed by means of LC-MS/MS, as reported below.
2) mixture under stirring overnight at room temperature. 1 mL from each extract was analysed by means of LC-MS/MS, as reported below.
Aglianico grape pomaces collected from Sannio (AS) turned out to be the richest in oleanolic acid content (see Results and discussion) and thus were selected to test the following five extracting solvents: acetone (H319, safety score = 5, health score = 3, env. score = 5), EtOAc (H319, safety score = 5, health score = 3, env. score = 3), n-butanol (H318, safety score = 3, health score = 4, env. score = 3), DMC (none, safety score = 4, health score = 1, env. score = 3), and 2-MeTHF (H318, safety score = 6, health score = 5, env. score = 3). The Chem21 solvent selection guide of the American Chemical Society – Green Chemistry Roundtable, assesses one Safety, one Health and one Environment criterion, each scored from 1 to 10, with 10 representing the highest hazard in each category. 1-g samples of lyophilized and ground AS were twice extracted with 10.0 mL (solid/liquid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) of each of the above listed solvents in triplicate, under stirring, overnight at room temperature to compare their efficiencies and selectivity to extract oleanolic acid. After filtration through 0.45 μm Durapore membrane filters (Millipore–Ireland), solvents were removed under vacuum. In Table 2, dry weights of the obtained extracts are displayed.
10) of each of the above listed solvents in triplicate, under stirring, overnight at room temperature to compare their efficiencies and selectivity to extract oleanolic acid. After filtration through 0.45 μm Durapore membrane filters (Millipore–Ireland), solvents were removed under vacuum. In Table 2, dry weights of the obtained extracts are displayed.
The dry extracts were fully solubilized in 5.0 mL of methanol, of which 1.0 mL was subjected as such to LC-MS/MS analysis and 2.0 mL were again vacuum dried. The so obtained solid residues were analysed by NMR as reported below.
NMR analyses
Dry residues coming from 2 ml of each extract, obtained by using acetone, EtOAc, n-butanol, DMC and 2-MeTHF were, respectively, solubilized in 500 μL of CD3OD (δH 3.31; δC 49.3) (99.95% Sigma-Aldrich) and transferred into Norell Select Series 5 mm NMR tubes. 1H NMR spectra (700 MHz) were obtained on a Bruker spectrometer (BioSpin GmBH, Rheinstetten, Germany). The NMR data were processed using TopSpin 3.2 software. NMR spectra were acquired at 300 K by the zg pulse sequence. D1 (relaxation delay) was set at 1.0 s, 80 number scans were used and data were collected into 64 k data points. Each free induction decay (FID) was zero-filled to 128 k data points. Spectra were processed and analysed using MestreNova 10 software.For the NMR-based quantitation of the identified metabolites, 5.0 μL of anhydrous pyridine were added to each NMR tube as an internal standard. 1H NMR spectra were again acquired by setting the d1 value at 7.0 s to allow a complete relaxation of nuclei to equilibrium. The inversion-recovery T1 relaxation experiment was run to assess the pyridine T1 value (0.80 s). NMR signals of each metabolite to quantitate were chosen on the basis of resolution, their areas were integrated, divided by the number of the protons generating them, and finally converted into the relative number of moles by comparing the obtained area with that of the pyridine protons, for which the number of moles was known.
LC-MS/MS analyses
LC-MS/MS analyses were performed on LTQ-XL coupled with an Ultimate 3000 HPLC (Thermo Fisher Scientific, Waltham, MA, USA). Chromatographic separation was obtained on a Kinetex 2.6 μm C18 (100 × 2.1) column (Phenomenex, Torrance, CA, USA) by means of a 13 min linear gradient from 60 to 95% of B (Buffer A: H2O and 0.01% formic acid and Buffer B: CH3CN and 0.02% formic acid). The ESI source was operated in negative ion mode and the analyser was operated in SRM mode. Source parameters and the analyser were optimized by injecting a solution of oleanolic acid standard. The selected reaction monitoring (SRM) transition 455.3 → 407.3, obtained with a normalized collision energy of 30 AU, was chosen and a five-point calibration curve (15.6–250 ng mL−1) was used for quantification (y = 28.215x + 102.98; R2 = 0.9973; LOD 0.028 μg μL−1; LOQ = 0.0090 μg μL−1). For quantitation of the other detected polyphenols, extracted ion chromatograms (XICs) relative to each compound to be quantified with a 5 ppm mass tolerance were analysed. As standards, (+)-catechin, (–)-epicatechin, quercetin and trans-resveratrol were used. For each of these standards, a calibration curve was plotted on the basis of peak areas (triplicate injections) obtained by using six different concentrations (0.1, 0.5, 1.0, 5.0, 10.0 and 20.0 mg L−1). The following calibration curve equations were obtained:(+)-Catechin:
(−)-Epicatechin:
Quercetin:
trans-Resveratrol:
Malvidin-3-O-glucoside:
For quantification purposes, procyanidins, myricetin, resveratrol dimer and anthocyanin coumaroyl derivatives were assumed to possess the same molar response as (+)-catechin, quercetin, trans-resveratrol and malvidin-3-O-glucoside, respectively.
Recycling experiments and E-factor calculation
Solvent recycling experiments were carried out for DMC and EtOAc. Solvents were recovered after the first extraction cycle by using a rotary evaporator. The recovered solvents were then utilized for a second extraction cycle with fresh grape pomace while maintaining the initial fixed solid–liquid ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Likewise, a third extraction cycle was conducted.
10). Likewise, a third extraction cycle was conducted.
After each extraction cycle, the recovered solvents were analysed by means of 1H NMR and 13C NMR spectroscopy (see the ESI†).
The extraction efficiency of the recovered solvents was evaluated by determining the oleanolic acid content by LC-MS analyses as previously described.
Concerning the extraction of oleanolic acid and to enlarge the discussion about the process's greenness, cEf calculations were carried out.30,31
DFT calculations
All density functional theory (DFT) calculations were performed at the long range corrected hybrid ωB97X-D functional with the Gaussian16 software. The geometric optimizations were performed with the single-ζ SVP basis set for all atoms. Next, the reported electronic energies were built through single point energy calculations on the ωB97X-D/SVP geometries using the same functional and the triple-ζ TZVP basis set on all atoms.Statistical analyses
Quantitative data were compared by Tukey's method for the evaluation of significant differences (p < 0.05). These analyses were performed using IBM SPSS (version 29.0.1.0; Armonk, NY: IBM Corp.). All data are means of three values.Results and discussion
With the purpose of selecting the richest biomass in terms of oleanolic acid content, we initially determined the triterpenoid (Fig. 1) concentration in six samples of red grape pomaces already available in our laboratory. The pomaces had been obtained from two different red grape varieties: Aglianico and Cabernet (Vitis vinifera) harvested in the autumn of 2021 from three different geographical areas of Southern Italy (Calabria, Sannio and Irpinia), where these two vines are traditionally grown to produce high-quality wines.Grape pomaces were all extracted with a hydroethanolic mixture. In each of the obtained extracts the content in oleanolic acid was initially ascertained by means of NMR, by searching for diagnostic 1H resonances of the triterpenoid under investigation.17 Successively, the concentration of oleanolic acid (expressed as mg mL−1) in the extracts was assessed by LC-MS/MS in SRM mode by choosing the transition 455.3 → 407.3 (Table 1).
| Red cultivars | Oleanolic acid (mg g−1) |
|---|---|
| All the data are expressed as mean ± standard deviation. Different letters indicate a statistically significant difference among the samples, according to Tukey's analysis (p < 0.05). Capital letters express the significance of the two cultivars, whereas lower case letters express the significance of the samples obtained from the same cultivar. | |
| Aglianico Sannio | 0.40 ± 0.01Aa |
| Aglianico Irpinia | 0.30 ± 0.01Db |
| Aglianico Calabria | 0.27 ± 0.01Fc |
| Cabernet Sannio | 0.39 ± 0.01Ad |
| Cabernet Irpinia | 0.35 ± 0.01Ce |
| Cabernet Calabria | 0.33 ± 0.01Ef |
Variations in the oleanolic acid content among grape cultivars have been documented in studies focusing on both wine32 and table grapes.33 In our investigation, while the two grape cultivars analysed did not exhibit remarkable differences in terms of oleanolic acid concentration, a clear range of variation was detected among the samples of the same variety depending upon the geographical provenance. Our data are consistent with previous studies that have shown how various environmental factors, such as UV-B and UV-C irradiation and heatwaves,19,34,35 can influence the total berry wax composition. This issue is an interesting focus for viticulturers and geneticists to investigate the adaptation mechanisms of vines especially in times characterized by rapidly changing climatic conditions.36
Considering that oleanolic acid is located solely on the berry skins, it is important to bear in mind that pomaces from red grapes, unlike those from white grapes, undergo fermentation processes through the maceration phase, during which a percentage of oleanolic acid, even if quite low, gets solubilized in the ensuing wines.37 Conversely, the production of white wines requires that white grape skins are rapidly separated from musts. This prevents a significant solubilization of all of the metabolites present in the berry skins into the wines. Hence, it would be of some interest to investigate also the triterpenoid content in pomaces produced from white grape varieties in order to explore the suitability of its recovery from these matrices as well.
On the basis of our data, we decided to resort to use the Aglianico pomace from Sannio (AS), as it had turned out to be the one with the highest content of oleanolic acid, in order to test the efficiency and selectivity of the five chosen extracting solvents. Initially, the AS lyophilized pomace was ground by a lab blender in order to obtain homogeneous samples. This was a necessary step as pomace itself is quite a heterogeneous mixture of skins, stalks, pulp and seeds. Additionally, by grinding the samples we intended to increase the surface-area-to-volume ratio and thus the rate of the metabolite solubilization. As previously mentioned, two sets of solvents were tested: fossil-based and biobased ones. Fossil-based solvents included n-butanol, EtOAc and acetone. Even if n-butanol can be produced also from renewables (acetone–butanol–ethanol (ABE) process and the Guerbet reaction starting from ethanol), to date its production is mainly based on propene hydroformylation.38 The dry weights of extracts from 1-g samples of AS obtained with the selected solvents – n-butanol, acetone, EtOAc, DMC and 2-MeTHF – are listed in Table 2.
| Solvent | Extract dry weight (mg) |
|---|---|
| All the data are expressed as means ± standard deviation. Different letters indicate a statistically significant difference among the samples, according to Tukey's analysis (p < 0.05). | |
| n-Butanol | 116.23 ± 2.10A |
| Acetone | 42.17 ± 1.85B |
| EtOAc | 22.33 ± 0.71D |
| DMC | 17.23 ± 0.75E |
| 2-MeTHF | 36.50 ± 0.46C |
Previous studies have reported that the best extracting solvents are those with Hildebrand's parameters falling in the range of 10–12, close to that calculated for oleanolic acid (δ 10.2).39–41 In these studies, the authors suggested that high yields of oleanolic acid were obtained by using n-butanol, which has a solubility parameter of 11.3 (δ). Even alcohols including methanol (δ 14.5) and ethanol (δ 13) provided good extraction yields, whilst non-polar solvents such as n-hexane or toluene were not as good. Accordingly, we decided to test n-butanol under our experimental conditions to compare, along with other solvents with lower Hildebrand's parameters such as acetone (δ 9.8) and ethyl acetate (δ 8.9) to explore their extraction efficiencies towards oleanolic acid.
As bioderived solvents we selected DMC and 2-MeTHF due to their excellent properties as solvents and to address the green chemistry community's quest for sustainable alternatives to fossil-based solvents. Following the 5th principle of the green chemistry, the substitution of harmful solvents with greener ones has become one of the main factors to favour a sustainable transition in both academic and industrial laboratories. In detail, DMC (C3H6O3, MW 90.08 g mol−1, boiling point 90.3 °C, melting point 4.6 °C, flash point 21.7 °C, and density 1.069 g cm−3) has attracted increasing interest as an environmentally sustainable compound and also as an alternative solvent in chromatography.42 DMC is a weakly polar aprotic solvent with good miscibility with water (139 g L−1) and can be produced from both waste and bio-based molecules (i.e. CO2 and methanol, glycerol).43 To date, it has been mainly used in organic chemistry as methylating agent27 and as solvent for electrolyte formulations in ion batteries.43 DMC shows a Hildebrand's parameter of 20.3 (δ),44 low toxicity and low boiling point, a critical parameter in terms of purification procedures after extraction. These properties are associated with a good availability on the market and, also, with a low cost (72.5 € per liter, ReagentPlus® 99%, Merck KGaA, Darmstadt, Germany).45 It is also recommended by the Chem21 solvent selection guide of the American Chemical Society – Green Chemistry Roundtable.46 2-MeTHF is a promising value-added solvent obtained from biomasses (furfural or levulinic acid as starting materials) with a Hildebrand solubility parameter of 16.9 (δ).47 It is a volatile cyclic ether with the following chemical–physical characteristics (C5H10O, MW 86.13 g mol−1, boiling point 78 °C, melting point −136 °C, flash point −10 °C at 101.3 kPa, and density 0.86 g cm−3; 60.6 € per liter, Emplura® 99%, Merck KGaA, Darmstadt, Germany). It has been used as a green solvent for the preparation of high-efficiency bulk heterojunction organic solar cells48 and cross-coupling of aryl chlorides and tosylates.49
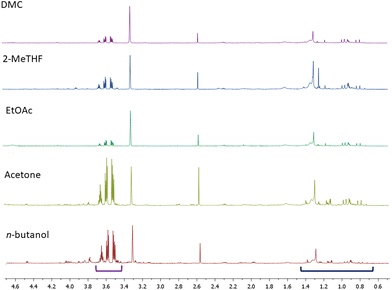
NMR-based characterization of extracts from Aglianico grape pomace
The extracts obtained from AS by using each of the five selected solvents, once lyophilized, were analysed by means of both NMR and LC-MS/MS techniques. In the 1H NMR spectra of each extract we could detect resonances unambiguously assignable to glycerol and oleanolic acid (Fig. 2 and Table 3).| Oleanolic acid | Glycerol | 9(Z),11(E) CLA | Malic acid | Citric acid | Glucose and fructose | Proline | |
|---|---|---|---|---|---|---|---|
| All the data are expressed as mean ± standard deviation. Capital letters indicate a significant difference between the individual molecules extracted by each solvent, while lowercase letters indicate significant differences among the compounds extracted by the same solvent, according to Tukey's analysis (p < 0.05). | |||||||
| EtOAc | 0.34 ± 0.01Bb | 0.38 ± 0.01Da | 0.06 ± 0.01Ac | 0.06 ± 0.00Cc | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| DMC | 0.38 ± 0.03Ab | 0.45 ± 0.01Ca | 0.02 ± 0.00Bc | 0.04 ± 0.00Ec | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Acetone | 0.11 ± 0.01Dc | 0.60 ± 0.03Aa | 0.01 ± 0.00Cd | 0.08 ± 0.00Bc | 0.02 ± 0.00Bd | 0.17 ± 0.01Bb | 0.00 ± 0.00 |
| 2-MeTHF | 0.18 ± 0.01Cb | 0.47 ± 0.01Bca | 0.03 ± 0.01Be | 0.09 ± 0.00Ad | 0.03 ± 0.00Ae | 0.12 ± 0.02Cc | 0.00 ± 0.00 |
| Butanol | 0.04 ± 0.00Ede | 0.51 ± 0.02Ba | 0.01 ± 0.00Ce | 0.05 ± 0.00Dd | 0.02 ± 0.00Bde | 0.25 ± 0.02Ab | 0.10 ± 0.01Ac |
As mentioned above, the presence of glycerol was not surprising as we were analysing red grape pomaces that had undergone fermentation. Additionally, among organic acids we essentially identified malic and citric acids. In the acetone, 2-MeTHF and n-butanol extracts carbohydrates (mainly glucose and fructose) were also detected while in the sole n-butanol extract even proline appeared to be quite abundant. Typical NMR signals of polyphenols including flavan-3-ols, coumaroyl anthocyanins and both gallic and syringic acids were present in traces in all of the extracts. Additionally, resonances assigned to a 9(Z),11(E) conjugated linoleic acid (CLA) derivative were unambiguously identified in all of the NMR spectra of the extracts by comparison with NMR data reported in the literature50 (Fig. 3).
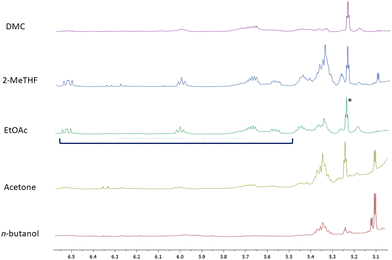 | ||
| Fig. 3 Signals resonating between 5.4 and 6.5 ppm are assigned to 9(Z),11(E) conjugated linoleic acid (CLA); asterisk indicates the signal relative to olefinic H-12 of oleanolic acid. | ||
The chemical structure of the detected CLA-derivative was determined by MS-based analysis. An ion peak [M − H]+ at m/z 309.20 (Rt = 15.90 min) was assigned to the ethyl ester CLA on the basis of its fragmentation pattern (major fragment ions were detected at m/z 263.24 and 245.23) superimposable with MS data reported in the literature.51 CLA generally refers to a mixture of positional and geometric isomers of linoleic acid with conjugated double bonds that are quite common in ruminants and dairy products. The occurrence of the ethyl ester of CLA in grape pomaces is likely the product of yeast reactions similar to those leading to the esterification of carboxylic moieties with ethanol quite common in wines. CLA has recently attracted the attention of scientists on account of its many health-promoting activities, including anti-obesity, anti-cardiovascular, anti-diabetic, anti-inflammation and anti-cancer.52 In regard to such bioactivities, it needs to be underlined that specific CLA isomers are endowed with distinct healthy benefits. In particular, the 9(Z),11(E) CLA isomer seems to be useful in cancer prevention52 and as a dietary health supplement.53 Given their different bioactivities, it is clear that the obtainment of specific CLA isomers is certainly a prerequisite. This is a quite challenging task, since the availability of pure CLA isomers in natural sources is poor and a chemical synthesis yielding high-purity CLAs is complex. It follows that red grape pomace could be a good natural candidate for developing efficient extraction protocols for the recovery of the 9(Z),11(E) CLA isomer. In this context, EtOAc turned out to be the best extracting solvent (Table 3). Additionally, it would be of some interest investigating the origin of CLA in grape pomace as well as its specific localization in the grape compartments. We can suggest that it is likely the product of the metabolism of lactic acid bacteria that are part of the microorganism pool involved in the winemaking processes.54
In addition to the qualitative chemical characterization of the five extracts under investigation, we also quantified the most abundant metabolites by using a known amount of pyridine as an internal standard. Pyridine was selected as its NMR resonances (δH 7.42, 7.69 and 8.61 ppm) did not overlap with those of the target metabolites. Concentrations of the identified metabolites are listed in Table 3. By referring to the relative amounts of the quantified metabolites in 1.0 mg of extract (dry weight), we could conclude that the highest amounts of oleanolic acid were recovered by using both EtOAc and DMC; roughly half of that amount was present in the acetone and 2-MeTHF extracts, while the lowest amount was detected in n-butanol (Table 3). This datum was quite surprising as in the literature it had been suggested that n-butanol was the best solvent for the solubilization of oleanolic acid. Our experimental observations led us to speculate now on the key factors affecting the extraction of oleanolic acid.
Firstly, we analysed the Kamlet–Abboud–Taft (KAT) parameters, i.e. α (hydrogen bond donating ability), β (hydrogen bond accepting ability) and Π* (dipolarity). Similar Π* values are reported for EtOAc, DMC and 2-MeTHF (∼0.5),55 whereas a lower value and a higher value are reported for n-butanol and acetone, 0.47 and 0.7, respectively. Moreover, β values are similar for EtOAc/acetone/2-MeTHF (∼0.55), while DMC shows a lower value of ∼0.3 and n-butanol shows β = 0.84.56
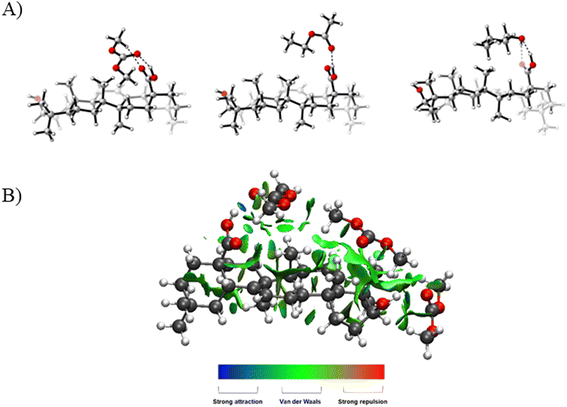
Additionally, we have investigated the interactions between the solvents and oleanolic acid by means of DFT calculations (see the ESI† for Computational details). The electronic energies of the possible adducts that oleanolic acid can form with DMC, EtOAc and n-butanol molecules were calculated, and non-covalent interaction maps were plotted. The electronic energies of these different complexes were calculated at the ωB97X-D/TZVP//ωB97X-D/SVP level of theory.
In all cases, stronger interactions occurred between the solvent and the carboxylic group on the substrate rather than with its alcoholic moiety. Particularly, the most stable adducts (ΔE = −11.3 (DMC–oleanolic acid), −12.6 (EtOAc–oleanolic acid) and −15.3 kcal mol−1 (n-butanol–oleanolic acid)) were formed through a hydrogen bond between the carboxylic group of oleanolic acid and the DMC/EtOAc carbonyl group or the alcoholic group of n-butanol. The lowest energy of these adducts is ascribed to the presence of a second hydrogen bond, as shown in Fig. 4. Complexes bearing only van der Waals interactions were also analysed to investigate the effect of hydrophobic interplays; in average their ΔE fell in a range between −3.1 and −7.6 kcal mol−1, thus contributing to the solubilization of oleanolic acid to a lesser extent.
Lastly, to simulate better a solvent solution, a complex between oleanolic acid and up to three molecules of DMC were characterized by means of a 3D-NCI plot as an example. As shown in Fig. 4b, for the most favourable interactions, two DMC molecules interact through hydrogen bonds with both the carboxylic and the alcohol moieties, whereas the third one forms van der Waals interactions with the condensed rings.
Overall, the calculated energies seem to follow the trend of the β parameters and suggest n-butanol as the solvent that should better extract oleanolic acid based on the strength of the established interactions.
As a consequence, we can reasonably hypothesize that, in addition to the solubility parameters, even the matrix is a key factor in the recovery of the triterpenoid from grape pomaces.
In fact, it is worth highlighting that both EtOAc and DMC extracts appear to be less rich in further extracted metabolites in comparison to the other solvents. Indeed, besides glycerol occurring more or less at the same level of concentration in each extract, organic acids and carbohydrates were more abundant in acetone, 2-MeTHF and n-butanol, which in turn extracted even good quantities of proline. These results can be attributed to the high dielectric constants of acetone (ε = 20.7 at 20 °C), 2-MeTHF (ε = 7.4 at 20 °C), and n-butanol (ε = 17.8 at 20 °C), which favour the extraction of hydrophilic compounds rather than hydrophobic ones like oleanolic acid. Therefore, solvents with both high β values and polarity favour the extraction of carbohydrates and hydrophilic compounds, as highlighted in Table 2.
In conclusion, EtOAc (ε = 6.0 at 20 °C) and DMC(ε = 3.1 at 20 °C) emerged as the most suitable solvents to selectively recover oleanolic acid from red grape pomace due to their weak polarity and hydrogen bond accepting capability (β). By comparing EtOAc and DMC extracts from a quali-quantitative standpoint, we could refer to DMC as the solvent that best extracted the relatively highest amount of oleanolic acid (molar selectivity of 61% vs. 40% in EtOAc) with the lowest concentrations of other co-extracted metabolites (39%).
LC-MS/MS-based characterization of extracts from Aglianico grape pomaces
The NMR-based analysis of the extract led us to characterize major extracted metabolites from both qualitative and quantitative standpoints, but prevented us from identifying and quantifying metabolites occurring at concentrations below the quantitation or even the detection limits of NMR. Thus, we decided to investigate our extracts also by means of a targeted LC-MS/MS analysis, with the purpose of searching for all of the typical classes of polyphenols occurring either in grapes or wines and that were identified in the Aglianico grape pomace investigated in a previous study.17 In each of the five extracts (EtOAc, n-butanol, acetone, 2-MeTHF and DMC) we could ascertain the occurrence only of some of the metabolites previously characterized; even fewer could be quantified (Table 4).| Compounds | m/z | m/z fragments | Solvent | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EtOAc mg g−1 Rt | Acetone mg g−1 Rt | n-Butanol mg g−1 Rt | 2-MeTHF mg g−1 Rt | DMC mg g−1 Rt | ||||||||
| R t: retention time (min). Pn-3-c-p-coumglc: peonidin-3-O-6-O-cis-p-coumaryl-β-glucoside. Pn-3-t-p-coumglc: peonidin-3-O-6-O-trans-p-coumaryl-β-glucoside. Mv-3-c-p-coumglc: malvidin-3-O-6-O-cis-p-coumaryl-β-glucoside. Mv-3-t-p-coumglc: malvidin-3-O-6-O-trans-p-coumaryl-β-glucoside. Quantitative data are expressed as mean ± standard deviation. Lower case letters indicate a significant difference between the individual molecules extracted by each solvent, according to Tukey's analysis (p < 0.05). | ||||||||||||
| Phenolic acids | ||||||||||||
| Gallic acid | 171.03 | 127.50 | Trace | 0.34 | Trace | 0.32 | Trace | 0.48 | Trace | 0.35 | Trace | 0.37 |
| Coutaric acid | 297.17 | 279.23; 251.20; 183.14 | Trace | 14.29 | Trace | 14.62 | Trace | 14.37 | Trace | 14.27 | Trace | 14.31 |
| Syringic acid | 199.06 | 155.49 | Trace | 3.38 | Trace | 3.31 | Trace | 3.20 | Trace | 3.58 | Trace | 3.60 |
| Flavonols | ||||||||||||
| Myricetin | 319.04 | 301.03 | 0.007 ± 0.001a | 13.97 | 0.006 ± 0.001a | 13.77 | 0.007 ± 0.002a | 13.66 | 0.003 ± 0.000b | 13.78 | 0.004 ± 0.001c | 13.76 |
| Quercetin | 303.04 | 285.24 | 0.007 ± 0.001a | 14.92 | 0.005 ± 0.001b | 14.89 | 0.008 ± 0.002c | 14.89 | 0.003 ± 0.000d | 14.91 | 0.003 ± 0.000d | 14.90 |
| Stilbens | ||||||||||||
| Resveratrol dimer | 455.35 | 437.34 | 0.005 ± 0.001a | 15.10 | 0.005 ± 0.001a | 15.07 | 0.003 ± 0.000b | 15.09 | 0.003 ± 0.000b | 15.06 | 0.007 ± 0.001c | 15.09 |
| Flavan-3-ols | ||||||||||||
| (+)-Catechin | 291.08 | 127.04; 273.08 | Trace | 7.85 | Trace | 7.75 | Trace | 7.82 | Trace | 7.80 | Trace | 7.80 |
| (−)-Epicatechin | 291.08 | 181.05; 273.08 | Trace | 10.01 | Trace | 10.02 | Trace | 10.03 | Trace | 10.03 | Trace | 10.02 |
| Procyanidin dimer B | 579.14 | 289.08; 409.09; 453.12; 561.09 | Trace | 12.32 | Trace | 12.34 | 0.003 ± 0.000 | 12.35 | Trace | 12.34 | Trace | 12.33 |
| Anthocyanins | ||||||||||||
| Pn-3-c-p-coumglc | 609.16 | 301.07 | Trace | 16.56 | Trace | 16.59 | 0.003 ± 0.000 | 16.56 | Trace | 16.58 | Trace | 16.56 |
| Mv-3-c-p-coumglc | 639.17 | 331.08 | Trace | 17.65 | Trace | 17.60 | Trace | 17.63 | Trace | 17.62 | Trace | 17.63 |
| Pn-3-t-p-coumglc | 609.16 | 301.07 | Trace | 16.93 | 0.007 ± 0.002a | 16.93 | 0.008 ± 0.002a | 16.93 | Trace | 16.95 | Trace | 16.92 |
| Mv-3-t-p-coumglc | 639.17 | 331.08 | Trace | 17.85 | 0.005 ± 0.001a | 17.84 | 0.005 ± 0.001a | 17.84 | Trace | 17.80 | Trace | 17.82 |
Such apparent discrepancy with data reported earlier by us17 can be explained by taking into account that, while in our previous study an exhaustive extraction of metabolites from pomace was conducted by using an ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (8
water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture, in the present work extractions were carried out by selecting some solvents on the basis of their affinity with triterpenoids, as discussed above. This caused compounds with a higher degree of hydrophilicity to be not completely solubilized. Consistent with the NMR analysis, with regard to anthocyanins only n-butanol turned out to be able to recover peonidin-3-O-(6-O-p-coumaryl)glucoside and malvidin-3-O-(6-O-p-coumaryl)glucoside from pomace, along with acetone even if to a lesser extent. The only flavan-3-ols that we could unambiguously identify were (+)-catechin, (−)-epicatechin and a single isomer of a procyanidin dimer B. Among these three compounds only the procyanidin isomer occurred above the quantification limit in the n-butanol extract. Also, n-butanol and EtOAc proved to be more efficient in extracting flavonols, namely quercetin and myricetin, especially in comparison with 2-MeTHF and DMC. Conversely, DMC was the best solvent to extract a resveratrol dimer, the only identified stilbene.
2) mixture, in the present work extractions were carried out by selecting some solvents on the basis of their affinity with triterpenoids, as discussed above. This caused compounds with a higher degree of hydrophilicity to be not completely solubilized. Consistent with the NMR analysis, with regard to anthocyanins only n-butanol turned out to be able to recover peonidin-3-O-(6-O-p-coumaryl)glucoside and malvidin-3-O-(6-O-p-coumaryl)glucoside from pomace, along with acetone even if to a lesser extent. The only flavan-3-ols that we could unambiguously identify were (+)-catechin, (−)-epicatechin and a single isomer of a procyanidin dimer B. Among these three compounds only the procyanidin isomer occurred above the quantification limit in the n-butanol extract. Also, n-butanol and EtOAc proved to be more efficient in extracting flavonols, namely quercetin and myricetin, especially in comparison with 2-MeTHF and DMC. Conversely, DMC was the best solvent to extract a resveratrol dimer, the only identified stilbene.
Recovery of DMC and EtOAc and E-factor calculation
As recently reported in the literature, the recovery of the solvent, its reuse in a new extraction cycle and the retention of its extraction efficiency are all aspects that need to be considered as a whole to define the real sustainability of an extraction process.57Therefore, we decided to investigate the potential recycling of both solvents for DMC and EtOAc, as these two proved to be the best solvents in extracting oleanolic acid. To this aim, we used 10.0 g of homogenised grape pomace (dry weight) and 100.0 mL of solvent (106.9 g of DMC and 90.2 g of EtOAc) to reproduce the same solid/liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 adopted to initially test their extraction efficiencies. Different from the first extraction experiments, we scaled-up the solvent volume to 100.0 mL instead of 10.0 mL, in order to make possible consecutive extraction cycles. The solid/liquid ratio was maintained constant throughout the subsequent cycles. Hence, the amount of grape pomace was calculated by taking into account the solvent loss during the extraction process (Table 5). Before reusing the recovered solvents in subsequent extractions, their volumes were evaluated, and their purity checked by NMR spectroscopy (Fig. S1†). The extraction process was repeated for three consecutive cycles. The results in terms of solvent recovery (reported as the mass recovered over mass used) and oleanolic acid yield for each extraction cycle are reported in Table 5.
10 adopted to initially test their extraction efficiencies. Different from the first extraction experiments, we scaled-up the solvent volume to 100.0 mL instead of 10.0 mL, in order to make possible consecutive extraction cycles. The solid/liquid ratio was maintained constant throughout the subsequent cycles. Hence, the amount of grape pomace was calculated by taking into account the solvent loss during the extraction process (Table 5). Before reusing the recovered solvents in subsequent extractions, their volumes were evaluated, and their purity checked by NMR spectroscopy (Fig. S1†). The extraction process was repeated for three consecutive cycles. The results in terms of solvent recovery (reported as the mass recovered over mass used) and oleanolic acid yield for each extraction cycle are reported in Table 5.
Our analyses ascertained that the oleanolic acid extraction yields remained basically constant after three extraction cycles likely due to the high purity degree of the recovered solvents (Fig. S1–S4†).
In conclusion, DMC and EtOAc can be both recycled without any losses in terms of extraction efficiencies.
Recoveries of either solvent were not quantitative mainly due to the evaporation during the work-up procedure. However, DMC showed better results in terms of recovered amount than EtOAc. This datum can be explained by the fact that DMC features a lower volatility than EtOAc (DMC vapor pressure = 18 mm Hg at 20 °C; EtOAc vapor pressure = 73 mm Hg at 20 °C), thus further corroborating the greenness of this new extraction procedure. This latter issue also justifies the lower Ef values in the case of DMC due to the possibility of recovering a higher amount of DMC in comparison to EtOAc.
With the purpose of reducing the Ef values, the potential valorisation of the grape pomace residues will be investigated in the future to produce for instance hydrochars,58 among other alternatives. In addition, considering the reduction of the liquid volumes (from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solid ratios) consequent to the recycling experiments, we could observe a reduction in terms of oleanolic acid yield (−20% in DMC and EtOAc) in spite of the selectivity that remained constant.
1 solid ratios) consequent to the recycling experiments, we could observe a reduction in terms of oleanolic acid yield (−20% in DMC and EtOAc) in spite of the selectivity that remained constant.
Therefore, we can infer that the liquid–solid ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 seems to be the best compromise to extract oleanolic acid from grape pomace. Nonetheless, the obtained results in terms of Ef are similar to those available in the literature when the focus is the extraction of high value-added chemicals from biomass.59
1 seems to be the best compromise to extract oleanolic acid from grape pomace. Nonetheless, the obtained results in terms of Ef are similar to those available in the literature when the focus is the extraction of high value-added chemicals from biomass.59
In conclusion, among the tested solvents, DMC is the best choice to extract selectively oleanolic acid from grape pomace as it guarantees high selectivity and, at the same time, low environmental impacts (i.e. lower Ef value in comparison to EtOAc).
Conclusions
In the present study, the efficiency of three conventional organic solvents (n-butanol, acetone and ethyl acetate) to recover oleanolic acid from red grape pomaces was tested and compared with that of two green solvents, namely dimethyl carbonate and 2-methyltetrahydrofuran. The extractions were carried out under fixed conditions (biomass![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvents of 1
solvents of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, overnight and at room temperature). A comprehensive NMR- and LC-MS-based analysis of the obtained extracts led us to identify ethyl acetate and dimethyl carbonate as the most efficient solvents for the recovery of the triterpenoid. Also, dimethyl carbonate was the most selective solvent to afford an extract more enriched in oleanolic acid (61% as molar selectivity) than the other tested solvents. Considering all the possible interactions and the chemical–physical properties of the solvents responsible for oleanolic acid extraction from grape pomace, the ability of DMC to selectively extract oleanolic acid can be mainly attributed to the weak polarity of this solvent compared to the other tested solvents. The higher selectivity observed with DMC is also related to its lower ability to form hydrogen bonds (β = 0.3) with other abundant analytes in grape pomace, such as carbohydrates, which were extracted in high yield with other solvents capable of forming hydrogen bonds, such as n-butanol. Solvent recyclability was investigated for dimethyl carbonate and ethyl acetate, and in both cases, the extraction efficiency of oleanolic acid was retained over three subsequent extractions. In addition to its higher selectivity toward oleanolic acid, dimethyl carbonate was more favourably recovered after three consecutive extraction cycles due to its lower volatility compared to ethyl acetate. This aspect is also highlighted by the cEf calculations, which suggest that dimethyl carbonate is the best alternative for oleanolic acid extraction according to green chemistry principles.
10, overnight and at room temperature). A comprehensive NMR- and LC-MS-based analysis of the obtained extracts led us to identify ethyl acetate and dimethyl carbonate as the most efficient solvents for the recovery of the triterpenoid. Also, dimethyl carbonate was the most selective solvent to afford an extract more enriched in oleanolic acid (61% as molar selectivity) than the other tested solvents. Considering all the possible interactions and the chemical–physical properties of the solvents responsible for oleanolic acid extraction from grape pomace, the ability of DMC to selectively extract oleanolic acid can be mainly attributed to the weak polarity of this solvent compared to the other tested solvents. The higher selectivity observed with DMC is also related to its lower ability to form hydrogen bonds (β = 0.3) with other abundant analytes in grape pomace, such as carbohydrates, which were extracted in high yield with other solvents capable of forming hydrogen bonds, such as n-butanol. Solvent recyclability was investigated for dimethyl carbonate and ethyl acetate, and in both cases, the extraction efficiency of oleanolic acid was retained over three subsequent extractions. In addition to its higher selectivity toward oleanolic acid, dimethyl carbonate was more favourably recovered after three consecutive extraction cycles due to its lower volatility compared to ethyl acetate. This aspect is also highlighted by the cEf calculations, which suggest that dimethyl carbonate is the best alternative for oleanolic acid extraction according to green chemistry principles.
We contend that the outcome of our work can contribute to pursue the aim of setting up ever more efficient and sustainable strategies to approximate the zero-waste objective by an appropriate management of agro-industrial by-products according to the principles of the circular economy and following the 5th principle of green chemistry.
As a future perspective, dimethyl carbonate could be tested also with the purpose of valorising other grapevine waste products taking into account its efficiency and selectivity in recovering oleanolic acid. As a way of example, dimethyl carbonate could be used for the extraction of the triterpenoid from thinned grapes. Thinning is a green pruning technique employed to enhance the size and composition of grapes, specifically for the production of high-quality red wines.60 Traditionally, thinned grapes are abandoned in the field to decompose. Pensec et al.34 reported a decrease in oleanolic acid content during fruit maturation, thus the utilization of dimethyl carbonate for the extraction of oleanolic acid from this viticultural waste could be regarded as a potential sustainable practice.
Another relevant aspect would be the possible use of the dimethyl carbonate extracts rich in oleanolic acid within the same winemaking cycle. In fact, oleanolic acid is a survival factor for yeast cells under conditions characterized by a lack of oxygen, without mentioning that the presence of oleanolic acid has been also associated with an augmentation in the biomass of yeast cells.61
Finally, as discussed in the Introduction, it would be of great interest to enologists the evaluation of the oleanolic acid inhibitory activity against grape tyrosinases that are responsible for detrimental oxidation reactions occurring during the first stages of winemaking.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was carried out within the Agritech National Research Center and received funding from the European Union Next- GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 D.D. 1032 17/06/2022022, CN00000022). The authors are grateful to the University of Salerno (ORSA234311). The technical assistance of Alessandra Sessa (University of Salerno) is gratefully acknowledged.References
- FAO, The State of Food and Agriculture 2019: Moving forward on food loss and waste reduction, FAO, Rome, Italy, 2019 Search PubMed.
- E. Kalli, I. Lappa, P. Bouchagier, P. A. Tarantilis and E. Skotti, Bioresour. Bioprocess., 2018, 5, 46 CrossRef.
- I. Drevelegka and A. M. Goula, Chem. Eng. Process., 2020, 149, 107845 CrossRef CAS.
- J. Broome and K. Warner, Calif. Agric., 2008, 62, 133–141 CrossRef.
- Q. Jin, A. P. Neilson, A. C. Stewart, S. F. O'Keefe, Y.-T. Kim, M. McGuire, G. Wilder and H. Huang, ACS Sustainable Chem. Eng., 2018, 6, 16279–16286 CrossRef CAS.
- Q. Deng, M. H. Penner and Y. Zhao, Food Res. Int., 2011, 44, 2712–2720 CrossRef CAS.
- I. Pugajeva, I. Perkons and P. Górnaś, J. Food Compos. Anal., 2018, 74, 44–52 CrossRef CAS.
- N. Unusan, J. Funct. Foods, 2020, 67, 103861 CrossRef CAS.
- J. García-Lomillo and M. L. González-SanJosé, Compr. Rev. Food Sci. Food Saf., 2017, 16, 3–22 CrossRef PubMed.
- M. Perra, G. Bacchetta, A. Muntoni, G. De Gioannis, I. Castangia, H. N. Rajha, M. L. Manca and M. Manconi, J. Funct. Foods, 2022, 98, 105276 CrossRef CAS.
- M. A. Olszewska, A. Gędas and M. Simões, Food Res. Int., 2020, 134, 109214 CrossRef CAS PubMed.
- I. Hoss, H. N. Rajha, R. El Khoury, S. Youssef, M. L. Manca, M. Manconi, N. Louka and R. G. Maroun, Cosmetics, 2021, 8, 109 CrossRef CAS.
- R. Sirohi, A. Tarafdar, S. Singh, T. Negi, V. K. Gaur, E. Gnansounou and B. Bharathiraja, Bioresour. Technol., 2020, 314, 123771 CrossRef CAS PubMed.
- K. Dwyer, F. Hosseinian and M. Rod, J. Food Res., 2014, 3, 91 CrossRef.
- G. R. Caponio, F. Minervini, G. Tamma, G. Gambacorta and M. De Angelis, Sustainability, 2023, 15, 9075 CrossRef CAS.
- R. T. Neto, S. A. O. Santos, J. Oliveira and A. J. D. Silvestre, Molecules, 2021, 27, 246 CrossRef PubMed.
- F. Errichiello, M. D'Amato, A. Gambuti, L. Moio, A. Pastore, H. Al-Hmadi, M. Stornaiuolo, E. Serino, O. Taglialatela-Scafati and M. Forino, J. Funct. Foods, 2023, 104, 105548 CrossRef CAS.
- G. F. Tolufashe, M. M. Lawal, K. K. Govender, F. O. Shode and T. Singh, J. Mol. Graphics Modell., 2021, 105, 107900 CrossRef CAS PubMed.
- J. VanderWeide, Y. Yan, W. F. Zandberg and S. D. Castellarin, Environ. Exp. Bot., 2022, 202, 105036 CrossRef CAS.
- M. Arshadi, T. M. Attard, R. M. Lukasik, M. Brncic, A. M. da C. Lopes, M. Finell, P. Geladi, L. N. Gerschenson, F. Gogus, M. Herrero, A. J. Hunt, E. Ibáñez, B. Kamm, I. Mateos-Aparicio, A. Matias, N. E. Mavroudis, E. Montoneri, A. R. C. Morais, C. Nilsson, E. H. Papaioannou, A. Richel, P. Rupérez, B. Škrbić, M. B. Solarov, J. Švarc-Gajić, K. W. Waldron and F. J. Yuste-Córdoba, Green Chem., 2016, 18, 6160–6204 RSC.
- S. K. Han, Y. G. Kim, H. C. Kang, J. R. Huh, J. Y. Kim, N.-I. Baek, D.-K. Lee and D.-G. Lee, J. Korean Soc. Appl. Biol. Chem., 2014, 57, 735–742 CrossRef CAS.
- H. Li, A. Guo and H. Wang, Food Chem., 2008, 108, 1–13 CrossRef CAS.
- C. S. D. Oliveira, P. Moreira, M. T. Cruz, C. M. F. Pereira, A. Gaspar, C. Pascoal Neto, P. C. R. O. Pinto, P. Costa Branco, A. M. S. Silva, S. A. O. Santos and A. J. D. Silvestre, RSC Sustainability, 2023, 1, 1016–1024 RSC.
- F. Chemat, M. A. Vian, A.-S. Fabiano-Tixier, M. Nutrizio, A. R. Jambrak, P. E. S. Munekata, J. M. Lorenzo, F. J. Barba, A. Binello and G. Cravotto, Green Chem., 2020, 22, 2325–2353 RSC.
- J. Wang, S. Li, Y. Liu, W. Li, Y. Wang and J. Wu, J. Chem. Eng. Data, 2023, 68, 236–250 CrossRef CAS.
- J. M. Castellano, S. Ramos-Romero and J. S. Perona, Nutrients, 2022, 14, 623 CrossRef CAS PubMed.
- G. Fiorani, A. Perosa and M. Selva, Green Chem., 2018, 20, 288–322 RSC.
- S. Santoro, F. Ferlin, L. Luciani, L. Ackermann and L. Vaccaro, Green Chem., 2017, 19, 1601–1612 RSC.
- Á. Morón-Ortiz, P. Mapelli-Brahm, A. León-Vaz, A. M. Benitez-González, R. León and A. J. Meléndez-Martínez, Food Chem., 2024, 434, 137437 CrossRef PubMed.
- M. Itatani, N. Német, N. Valletti, G. Schuszter, P. Prete, P. Lo Nostro, R. Cucciniello, F. Rossi and I. Lagzi, ACS Sustainable Chem. Eng., 2023, 11, 13043–13049 CrossRef CAS PubMed.
- R. Arthur Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- J. Pérez-Navarro, A. Da Ros, D. Masuero, P. M. Izquierdo-Cañas, I. Hermosín-Gutiérrez, S. Gómez-Alonso, F. Mattivi and U. Vrhovsek, Food Res. Int., 2019, 125, 108556 CrossRef PubMed.
- M. Yang, Z. Luo, S. Gao, T. Belwal, L. Wang, M. Qi, Z. Ban, B. Wu, F. Wang and L. Li, Postharvest Biol. Technol., 2021, 173, 111430 CrossRef CAS.
- F. Pensec, C. Pączkowski, M. Grabarczyk, A. Woźniak, M. Bénard-Gellon, C. Bertsch, J. Chong and A. Szakiel, J. Agric. Food Chem., 2014, 62, 7998–8007 CrossRef CAS PubMed.
- P. Trivedi, L. Klavins, A. L. Hykkerud, J. Kviesis, D. Elferts, I. Martinussen, M. Klavins, K. Karppinen, H. Häggman and L. Jaakola, Front. Plant Sci., 2022, 13, 980427 CrossRef PubMed.
- M. Iorizzo, A. Sicilia, E. Nicolosi, M. Forino, L. Picariello, A. R. Lo Piero, A. Vitale, E. Monaco, F. Ferlito, M. Succi, P. Tremonte, A. Gambuti, C. Villano, A. Bonfante, R. Aversano and R. Coppola, Front. Plant Sci., 2023, 14, 1250208 CrossRef PubMed.
- M. Forino, A. Gambuti and L. Moio, Food Chem., 2019, 278, 497–501 CrossRef CAS PubMed.
- N.-P.-T. Nguyen, C. Raynaud, I. Meynial-Salles and P. Soucaille, Nat. Commun., 2018, 9, 3682 CrossRef PubMed.
- I. J. Jin, Y. I. Ko, Y. M. Kim and S. K. Han, Arch. Pharmacal Res., 1997, 20, 269–274 CrossRef CAS PubMed.
- S. Jäger, K. Winkler, U. Pfüller and A. Scheffler, Planta Med., 2007, 73, 157–162 CrossRef PubMed.
- P. Schneider, S. S. Hosseiny, M. Szczotka, V. Jordan and K. Schlitter, Phytochem. Lett., 2009, 2, 85–87 CrossRef CAS.
- S. Felletti, M. Spedicato, D. Bozza, C. De Luca, F. Presini, P. P. Giovannini, M. Carraro, M. Macis, A. Cavazzini, M. Catani, A. Ricci and W. Cabri, J. Chromatogr. A, 2023, 1712, 464477 CrossRef CAS PubMed.
- S.-H. Pyo, J. H. Park, T.-S. Chang and R. Hatti-Kaul, Curr. Opin. Green Sustain. Chem., 2017, 5, 61–66 CrossRef.
- C. F. Huang and Y. T. Tsai, 20170107169A1, 2017.
- P. Tundo and M. Selva, Acc. Chem. Res., 2002, 35, 706–716 CrossRef CAS PubMed.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. Robert McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- D. F. Aycock, Org. Process Res. Dev., 2007, 11, 156–159 CrossRef CAS.
- X. Chen, X. Liu, M. A. Burgers, Y. Huang and G. C. Bazan, Angew. Chem., Int. Ed., 2014, 53, 14378–14381 CrossRef CAS PubMed.
- E. Bisz and M. Szostak, ChemSusChem, 2018, 11, 1290–1294 CrossRef CAS PubMed.
- D. Prema, J. L. Pilfold, J. Krauchi, J. S. Church, K. K. Donkor and B. Cinel, J. Agric. Food Chem., 2013, 61, 9915–9921 CrossRef CAS PubMed.
- M. Gong, W. Wei, Y. Hu, Q. Jin and X. Wang, J. Chromatogr. B, 2020, 1153, 122292 CrossRef CAS PubMed.
- B. Kim, H. R. Lim, H. Lee, H. Lee, W. Kang and E. Kim, J. Funct. Foods, 2016, 25, 588–598 CrossRef CAS.
- B. Yang, H. Gao, C. Stanton, R. P. Ross, H. Zhang, Y. Q. Chen, H. Chen and W. Chen, Prog. Lipid Res., 2017, 68, 26–36 CrossRef CAS PubMed.
- S. O. Lee, C. S. Kim, S. K. Cho, H. J. Choi, G. E. Ji and D.-K. Oh, Biotechnol. Lett., 2003, 25, 935–938 CrossRef CAS PubMed.
- S. Jin, F. Byrne, C. R. McElroy, J. Sherwood, J. H. Clark and A. J. Hunt, Faraday Discuss., 2017, 202, 157–173 RSC.
- P. G. Jessop, D. A. Jessop, D. Fu and L. Phan, Green Chem., 2012, 14, 1245–1259 RSC.
- A. Mero, A. Mezzetta, M. D. Leo, A. Braca and L. Guazzelli, Green Chem., 2024, 26, 6109–6123 RSC.
- M. Pala, I. C. Kantarli, H. B. Buyukisik and J. Yanik, Bioresour. Technol., 2014, 161, 255–262 CrossRef CAS PubMed.
- A. Dall'armellina, M. Letan, C. Duval and C. Contino-Pépin, Green Chem., 2021, 23, 8891–8900 RSC.
- G. Fia, G. Bucalossi, C. Proserpio and S. Vincenzi, Aust. J. Grape Wine Res., 2022, 28, 8–26 CrossRef CAS.
- P. Ribéreau-Gayon, D. Dubourdieu, B. Donèche and A. Lonvaud, Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications, John Wiley & Sons, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc03624g |
| This journal is © The Royal Society of Chemistry 2024 |