Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ruthenium-catalyzed “open-loop” recycling of polyethylene via tandem isomerization-metathesis (ISOMET)†
Vajk
Farkas
 abd,
Pascal
Albrecht
abd,
Pascal
Albrecht
 d,
Ádám
Erdélyi
d,
Ádám
Erdélyi
 ac,
Márton
Nagyházi
ac,
Márton
Nagyházi
 ac,
Beatrix
Csutorás
ac,
Beatrix
Csutorás
 c,
Gábor
Turczel
c,
Gábor
Turczel
 a,
Norbert
Miskolczi
a,
Norbert
Miskolczi
 c,
Janka
Bobek-Nagy
c,
Janka
Bobek-Nagy
 c,
Ole
Osterthun
c,
Ole
Osterthun
 d,
Jürgen
Klankermayer
d,
Jürgen
Klankermayer
 *d and
Robert
Tuba
*d and
Robert
Tuba
 *ac
*ac
aInstitute of Materials and Environmental Chemistry, Hungarian Research Network, Research Centre for Natural Sciences, Magyar tudósok körútja 2., Budapest H-1519, Hungary. E-mail: tuba.robert@ttk.hu
bDepartment of Organic Chemistry and Technology, Budapest University of Technology and Economics, Szent Gellért tér 4., H-1111 Budapest, Hungary
cResearch Centre for Biochemical, Environmental and Chemical Engineering, Department of MOL Hydrocarbon and Coal Processing, University of Pannonia, Egyetem u. 10., Veszprém H-8210, Hungary. E-mail: tuba.robert@mk.uni-pannon.hu
dInstitut für Technische und Makromolekulare Chemie, RWTH Aachen University, Worringerweg 2, D-52074 Aachen, Germany. E-mail: jklankermayer@itmc.rwth-aachen.de
First published on 16th August 2024
Abstract
As a model of a chemical upcycling process, we have developed a single-metal homogeneous catalytic system to break down persistent polyethylene waste into valuable chemical intermediates. This could ultimately be used to produce important chemical products, including environmentally friendly, biodegradable plastics. In the first step, a slow pyrolysis of polyolefin waste yields oils, containing long-chain olefins as the major components. Then, for the next transformation step, tailored bicyclic (alkyl)(amino)carbene (BICAAC)-Ru olefin metathesis catalysts were used in combination with an alkene isomerization catalyst (RuHCl(CO)(PPh3)3) for the transformation of the pyrolysis oil to propylene via isomerization-metathesis (ISOMET) reaction in ethylene atmosphere. Eventually, translation of the highly efficient single-metal catalyst system enabled ISOMET reaction to a 900 mL reactor setup and repetitive batch experiments could prove the long-term stability of the catalyst system and the highest total turnover number (tTON = 2788 mol propylene per mol olefin metathesis catalyst) reported so far using post-consumer polyethylene waste feedstock.
Introduction
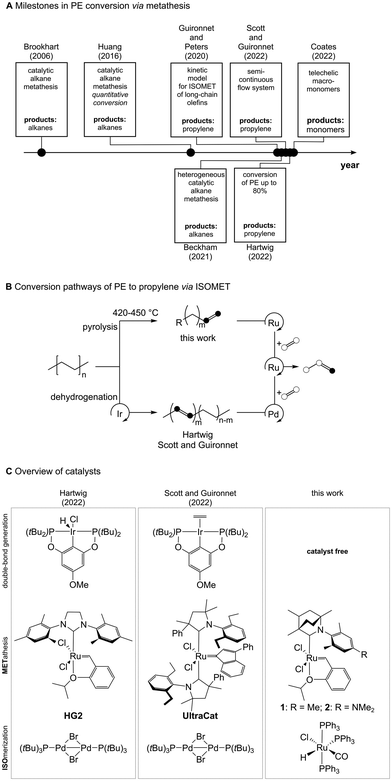
In 2022 the global plastics production reached an annual value of 400 Mt and this is expected to rise to 1000 Mt by 2050.1,2 Currently, only limited recycling strategies exist to convert end-of-life plastics. The Geyer Group estimated in 2017 that only 10% of the plastic waste ever generated was recycled, and only 14% of that was recycled multiple times.2 While the share of mechanically recycled plastics is increasing year by year (8.3% of annual production in 2022), chemical recycling technologies currently account for less than 0.1% of annual plastic production.3–6Polyolefins dominated the production of plastics with a combined share of 45% in 20221 and currently lack efficient end-of-life treatment.7–11 In this context, polyethylene (PE) conversion via olefin metathesis has been investigated since 2006 (Fig. 1A). Brookhart's work applied catalytic alkane metathesis to PE, based on consecutive dehydrogenation, isomerization, metathesis, and hydrogenation reactions, ultimately resulting in liquid saturated hydrocarbons.12 Huang's group extended this concept in 2016, and demonstrated for the first time quantitative PE conversion by this method using a heterogeneous, Re2O7/Al2O3 olefin metathesis catalyst system.13 Alkanes can be re-integrated into the chemical industry, nevertheless, energy intensive steps are necessary to convert them to products of higher value. In contrast, the use of a tandem isomerization-metathesis reaction with ethylene (ISOMET; Fig. 1B) will result in the selective production of propylene, which can directly be fed into existing processes in the chemical industry. Converting PE to propylene would therefore establish an open-loop recycling process. Theoretical studies by Guironnet and Peters revealed the feasibility of the PE conversion to propylene through cascade reactions including dehydrogenation, and ISOMET reaction with ethylene.14,15 Later on, the iridium-catalyzed post-consumer PE dehydrogenation followed by palladium-based isomerization and the UltraCat metathesis catalyst-induced ISOMET reaction was demonstrated by Guironnet and Scott (Fig. 1C, middle). In the same year, Hartwig reported a similar reaction cascade using Hoveyda–Grubbs 2nd generation (HG2, Fig. 1C, left) olefin metathesis catalyst reaching up to 80% propylene yield.16 However, both concepts currently work only on a small scale and in the presence of high catalyst loadings (over 1 mol%). In addition, three different transition metal-based catalyst systems were used, which makes the recovery of the transition metals difficult. In 2022, our group systematically investigated the ISOMET reaction of long-chain olefins using a single metal (ruthenium)-based catalyst system including tailored olefin metathesis catalysts (1 and 2, Fig. 1C, right) at as low as 10 ppm olefin metathesis and 200 ppm isomerization catalyst loadings. The observed highest turnover number was 55000, which correlates to an expected production of 3.7 t of propylene from 1.4 t of long-chain olefin (e.g. 1-octadecene) using 1 kg of metathesis catalyst (2).17,18 In order to make post-consumer PE “upcycling” economically feasible, a simpler PE pre-treatment and a high-activity single-metal ISOMET catalyst system need to be used. Thus, the development of a pyrolysis process19–23 for the production of olefin-rich pyrolysis oil blends from PE waste, combined with the use of high-efficiency single-metal ISOMET catalyst systems, is an obvious choice and represents an important next development.
Results and discussion
To produce the pyrolysis oils for the ISOMET reactions, slow pyrolysis of PE was performed in electrically heated stainless-steel reactors (V = 1250 cm3) at 420–450 °C in a nitrogen gas stream for 3 hours (see ESI† for detailed information). The condensed hydrocarbons were separated from the gaseous products in a phase separator resulting in the crude pyrolysis oils. The pyrolysis was separately performed on virgin HDPE granules (TIPELIN® 6010B, a bimodal polyethylene copolymer with 1-butene; see ESI† for details) and post-consumer HDPE (Fig. 2) to investigate the influence of additives on the pyrolysis and the subsequent ISOMET transformation.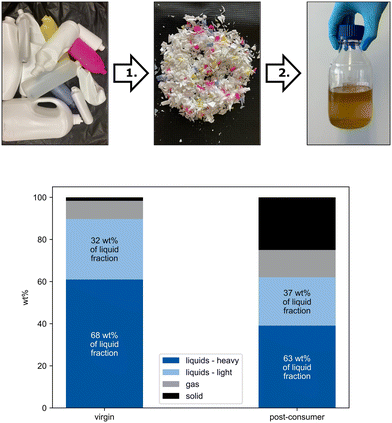 | ||
| Fig. 2 Top: conversion of assorted post-consumer HDPE plastic waste to pyrolysis oil. The post-consumer HDPE was initially shredded (1) and then subjected to slow pyrolysis (2) resulting in pyrolysis oils (see ESI† for further information). Bottom: composition of pyrolysis products of virgin and post-consumer HDPE. | ||
During pyrolysis of virgin HDPE granules 90 wt% of HDPE was transformed into pyrolysis oil, while the remaining HDPE was converted to gaseous (9 wt%) and solid products (1 wt%; Fig. 2). When subjecting the post-consumer HDPE to the pyrolysis, the share of solid residue increased to 25 wt%, while 62 wt% of the HDPE was recovered as pyrolysis oil with the remaining 13 wt% being collected as gaseous products (Fig. 2). This shift in the product distribution is attributed to the more heterogeneous composition of consumer plastics (e.g. colorant and other additive contaminations). Each of the liquid fractions from virgin and post-consumer HDPE were further fractionated at 100 °C and reduced pressure (0.2 mbar) yielding the respective light (≤C10) and heavy fractions (m.p. 25–30 °C, ≥C10, see Fig. 2 and Fig. S3–5†). GC-MS analysis of the hydrocarbon mixture revealed that each homolog structure consists of three signals: the corresponding alkane, alkene and diene species (Fig. S4,† alkane and alkene species are the major, dienes are the minor components). For the pyrolysis oil composition from post-consumer HDPE, 55 wt% alkanes, 40 wt% alkenes and 5 wt% dienes were calculated based on quantitative GC-MS (see chapter 2.2 in the ESI†). An olefin composition of 75% terminal and 25% internal olefins was determined via1H-NMR spectroscopy (Fig. S7†). GC measurements of the gaseous products from pyrolysis showed propylene (26 vol%) and ethylene (12 vol%) as the major products besides saturated C1–C5 hydrocarbons (Fig. S3†).
To investigate whether the pyrolysis oils from virgin and post-consumer HDPE exhibit different reactivities in ISOMET reactions, the untreated (crude) pyrolysis oils were tested in lab-scale experiments using RuH and 2 as catalysts, resulting in TONs for propylene of 800 and 350, respectively, after 3 hours (entries 1 and 4, Table 1). It was presumed that low-boiling contaminants could be formed during the pyrolysis inhibiting the activity of the metathesis catalyst, so the heavy (C ≥ 10) and the light (C ≤ 10) fractions were investigated one by one. As expected, the heavy fraction of both the virgin and post-consumer HDPE pyrolysis oils resulted in significantly higher TONs of 4800 and 900 for propylene, respectively, compared to the corresponding crude oils (entries 2 and 5, Table 1). While the light fraction of both virgin and post-consumer HDPE pyrolysis oils yielded significantly lower TON values (500 and 250, respectively, entries 3 and 6, Table 1). Interestingly, the differences between TONs are much less pronounced during reactions with post-consumer HDPE pyrolysis oils, suggesting an inhibitory effect of impurities potentially formed during the pyrolysis of post-consumer HDPE waste.
| Entry | HDPE pyrolysis oil feed | TON at 3 h | TON at 24 h |
|---|---|---|---|
| a 2.5 mg (4.1 μmol) catalyst 2 and 100 mg (105 μmol) catalyst RuH, 3.2 g HDPE pyrolysis oil in 5.5 mL toluene. (ND: no data). | |||
| 1 | Virgin crude | 800 | ND |
| 2 | Virgin heavy | 4800 | 6800 |
| 3 | Virgin light | 500 | ND |
| 4 | Post-consumer crude | 350 | ND |
| 5a | Post-consumer heavy | 900 | 1400 |
| 6 | Post-consumer light | 250 | ND |
| 7a | Post-consumer heavy (purified) | 1900 | 3900 |
Thus, the heavy fraction of post-consumer HDPE pyrolysis oil was purified on a short alumina column and then used in ISOMET reaction. Surprisingly, the purification resulted in significantly improved TONs for propylene of 1900 (entry 7, Table 1). In the reaction with the purified heavy fraction of the post-consumer HDPE pyrolysis oil, a gas phase composition of 11 vol% propylene after 3 hours of reaction time and over 23 vol% propylene after 24 hours was measured via GC. The concentration of propylene in the gas phase of 23 vol% is indicative of reaching the chemical equilibrium of the reaction in a closed reactor system.
The investigation of the reaction mixture and the mass balance at approximately 30% olefin conversion revealed a significant shift in the chain length distribution of the olefins (Fig. 3). Prominently, the C19–C25 olefins were converted to shorter-chain olefins, which were not present in the pyrolysis oil before the reaction. The mass differences between the blue and red curves turned predominantly to propylene (Fig. 3) which has been indicated by quantitative GC-FID (gas phase) and GC-MS (liquid phase) analysis. These results confirm that the applied catalyst system does not have a kinetic preference for the degradation of a particular olefin component but can convert all olefines in the mixture.
 | ||
| Fig. 3 Analysis of communal heavy pyrolysis oil at appr. 30% olefin conversion. Composition before (blue) and after (red) the ISOMET reaction. ISOMET conditions: n(2) = 4.1 μmol, n(RuH) = 105 μmol, m(pyrolysis oil) = 0.8 g, V(toluene) = 5.5 mL, pethylene = 10 bar, T = 75 °C, t = 24 h. (See ESI†). | ||
To verify that the reaction is limited by reaching the equilibrium in the gas phase and to gain insight into the catalyst deactivation, repetitive batch experiments were performed on laboratory scale with 1 mL (750 mg) of the heavy fraction of post-consumer HDPE pyrolysis oil (Fig. 4). At each sampling point of the gas phase the atmosphere in the Fisher–Porter bottle was fully exchanged by purging with ethylene three times. After 24 hours, no more propylene formation could be detected. To remove possibly deactivated catalysts, the reaction mixture was purified over a short silica column and subjected to a new run with fresh catalyst (Fig. 4A). Interestingly, further conversion of the remaining pyrolysis oil was observed, confirming the presence of catalyst deactivation during the reaction. Overall, cumulative yields for propylene and butene of 66% and 21%, respectively, could be obtained – corresponding to a tTON of 1030 for propylene. GC analysis of the liquid fraction after the second run revealed traces of remaining olefins, while the majority of the components could be identified as the saturated hydrocarbons (see chapter 3.4 in the ESI†).
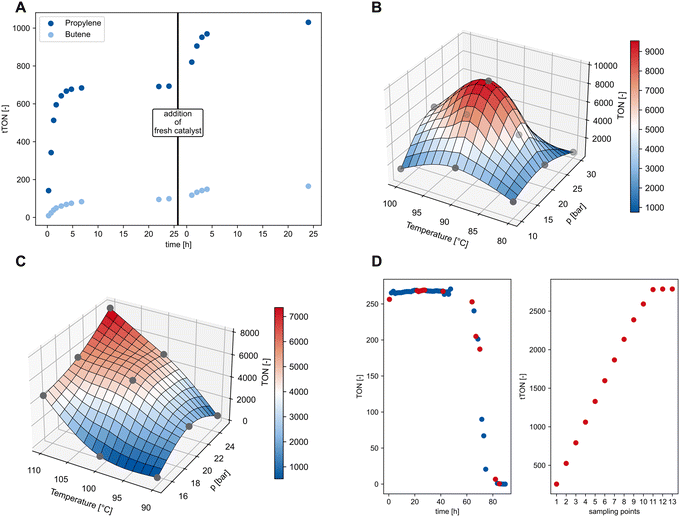 | ||
| Fig. 4 (A) Reaching full conversion of the heavy fraction from post-consumer HDPE pyrolysis oil by repetitive exchange of the gas phase at each sampling point. After 24 h reaction time, the reaction mixture was purified over a short silica column and fresh catalysts were added. Reaction conditions first run: n(2) = 11.8 μmol, n(RuH) = 96 μmmol, 2/RuH = 1/8.1, m(pyrolysis oil) ISOMET conditions: n(2) = 4.1 μmol, n(RuH) = 105 μmol, m(pyrolysis oil) = 0.8 g, V(toluene) = 5.5 mL, pethylene = 10 bar, T = 75 °C, t = 24 h (see ESI†). (B) Interpolated response surface of statistical experimental design in 20 mL reactors (full details see ESI†). (C) Interpolated response surface of statistical experimental design in 50 mL reactors (for full details, see ESI†). (D) Left: TON-time profile in the large-scale high-pressure reactor for the conversion of the heavy fraction of post-consumer HDPE pyrolysis oil to propylene. Red marked points denote the times after which measurement the gas phase was fully exchanged. Right: total TON (tTON) as function of the sampling points in the presence of catalyst 2 for 85 h time-on-stream (see ESI chapter 3.7†). | ||
Metathesis catalysts are known to undergo a number of different deactivation pathways, which have been studied in detail by the group of Fogg for NHC and CAAC-based ruthenium metathesis catalysts.24,25
In order to better elucidate the catalyst deactivation in the ISOMET reaction of pyrolysis oils, the effect of possible contaminants – which could form during pyrolysis – on the overall propylene yield were investigated (Table 2). As a baseline, a TON of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 for propylene was obtained with pure 1-octadecene in the presence of 2 and RuH (entry 1, Table 2). Spiking the reaction mixture with 50 ppm 1-dodecanol resulted in a decreased TON for propylene of 9200 (entry 2, Table 2). The presence of ethanethiol has also a negative impact on the catalyst performance (TON: 4000, entry 3, Table 2). As expected, the presence of primary amine (1-dodecylamine), resulted in a significant decrease, as well (TON of 2700 for propylene, entry 4, Table 2). In fact, the inhibitory effect of amines has previously been reported by the group of Fogg.26 However, the addition of hydrochloric acid to the reaction mixture spiked with 1-dodecylamine resulted in higher TON (6000, entry 5, Table 2), indicating that the inhibiting effect of the amine impurities can possibly be suppressed by protonation. One of the least explored areas in ruthenium-catalyzed olefin metathesis chemistry is the role of (conjugated) dienes on catalyst performance. Therefore, the influence of dienes on the overall ISOMET activity was systematically investigated. Indeed, it was found that the addition of traces (50 ppm) of either 1,5-hexadiene or 1,7-octadiene resulted in a decreased TON of 5700 and 6000 (entries 6 and 7, Table 2). The inhibitory effect of 1,7-octadiene content was demonstrated at the same level as the catalyst concentration (10 ppm). It was also shown that increasing the concentration of 1,7-octadiene resulted in further gradual deactivation of the catalyst (Fig. S8 and Table S4†).
400 for propylene was obtained with pure 1-octadecene in the presence of 2 and RuH (entry 1, Table 2). Spiking the reaction mixture with 50 ppm 1-dodecanol resulted in a decreased TON for propylene of 9200 (entry 2, Table 2). The presence of ethanethiol has also a negative impact on the catalyst performance (TON: 4000, entry 3, Table 2). As expected, the presence of primary amine (1-dodecylamine), resulted in a significant decrease, as well (TON of 2700 for propylene, entry 4, Table 2). In fact, the inhibitory effect of amines has previously been reported by the group of Fogg.26 However, the addition of hydrochloric acid to the reaction mixture spiked with 1-dodecylamine resulted in higher TON (6000, entry 5, Table 2), indicating that the inhibiting effect of the amine impurities can possibly be suppressed by protonation. One of the least explored areas in ruthenium-catalyzed olefin metathesis chemistry is the role of (conjugated) dienes on catalyst performance. Therefore, the influence of dienes on the overall ISOMET activity was systematically investigated. Indeed, it was found that the addition of traces (50 ppm) of either 1,5-hexadiene or 1,7-octadiene resulted in a decreased TON of 5700 and 6000 (entries 6 and 7, Table 2). The inhibitory effect of 1,7-octadiene content was demonstrated at the same level as the catalyst concentration (10 ppm). It was also shown that increasing the concentration of 1,7-octadiene resulted in further gradual deactivation of the catalyst (Fig. S8 and Table S4†).
| Entry | Contaminants | TON at 3 h |
|---|---|---|
| 1 | None (blank) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| 2 | 1-Dodecanol | 9200 |
| 3 | Ethanethiol | 4000 |
| 4 | 1-Dodecylamine | 2700 |
| 5 | 1-Dodecylamine·HCl | 6000 |
| 6 | 1,5-Hexadiene | 5700 |
| 7 | 1,7-Octadiene | 6000 |
This effect may be explained by the in situ formation of conjugated species, which can inhibit the metathesis catalyst activity, resulting in faster decomposition and thus shortening the catalyst lifetime.27,28
To demonstrate the ISOMET of the heavy fraction of post-consumer HDPE pyrolysis oil on a large scale, the reaction setup was changed from Fisher–Porter bottles to high-pressure steel autoclaves and stepwise scaled up from 20 to 900 mL reactors (see Fig. S13†). To avoid disturbance of the reaction by contaminants in the pyrolysis oil, the initial scale up was performed on 1-octadecene with catalyst 1. A systematic optimization of the reaction temperature and ethylene pressure was performed for each new reactor volume (see ESI, Tables S7 and S8†).
Changing the reactor setup initially from glass-ware Fisher–Porter bottles to 20 mL stainless steel reactors revealed significant influence of the reaction temperature and ethylene pressure on the catalyst performance. This stark difference is expected, since the heat transfer and the ratio of liquid to gas volume are not comparable between the two reactors. Nevertheless, a maximum TON of 9613 for propylene was detected at 90 °C and 20 bar ethylene pressure (Fig. 4B). Switching to 50 mL reactors comes again with a change in the overall geometry of the reactor and the stirrer. While in all experiments before standard magnetic stirring bars are used, the 50 mL reactors are equipped with gas-intake stirrers. Again, a systematic optimization was performed, revealing best TONs for propylene at 110 °C and 25 bar ethylene of 7733 (Fig. 4C). The need for higher temperatures can be explained by the lower surface to liquid ratio, i.e. worse heat transfer from the heat source to the reaction medium inside the reactor. At this stage, the reaction was switched from the model substrate 1-octadecene to the heavy fraction of post-consumer HDPE pyrolysis oil. To this end, the amount of metathesis catalyst was increased by a factor of 5, while the isomerization catalyst amount was increased by a factor of 7. The benefit of higher isomerization catalyst loadings has been studied before.17 Furthermore, the volume of olefinic substrate was reduced from 12.5 mL to 6.25 mL while keeping the overall liquid volume constant. These changes were made according to the results obtained in earlier experiments in the Fisher–Porter bottles. Interestingly, within 3 hours a TON of 1576 for propylene was obtained at 20 bar ethylene pressure, whereas with 10 bar ethylene pressure the TON decreased to 52. Finally, the ISOMET of the heavy fraction of post-consumer HDPE pyrolysis oil was converted in the 900 mL reactor system. Since the increased temperatures in the 50 mL reactor were attributed to the insufficient heat transfer, the significantly improved heating of the 900 mL reactor (heating jacket vs. aluminum cone) led us to lower the reaction temperatures to 90 °C based on the results from the 20 mL reactors. In the first experiment, the reactor was operated in semi-batch configuration, i.e. a back-pressure valve ensured that if the pressure in the reactor dropped ethylene is back-filled to the initial pressure. A constant propylene production was observed for 15 hours resulting in a tTON of 314 for propylene. The system was then changed into a repetitive semi-batch system, in which the gas-phase was fully exchanged after pre-defined times (Fig. 4D, red dots). In addition, the catalyst was changed from 1 to the more active and stable catalyst 2. Surprisingly, it was found that the equilibrium concentration of propylene in the gas-phase is reached within 60 minutes after initial pressurization, resulting in a TON of 256 for propylene. However, the ISOMET catalyst system activity remained constant after exchanging the gas-phase eleven times (Fig. 4D right), highlighting the robustness and high activity of the catalyst system in the presence of post-consumer HDPE pyrolysis oil – ultimately, reaching an overall tTON of 2788 for propylene. While these TONs are the highest reported so far for polyethylene conversion, the detailed investigations indicated that the current setup has limitations including the untapped potential of rapidly reaching equilibrium concentrations of propylene under selected conditions. These observations highlighted the potential of the process if the reactor is changed to a continuous flow system, where the formed propylene is removed with a constant gas flow, so the equilibrium can be shifted in the direction of propylene formation. Build-up and investigations in a continuous flow reactor setup are currently ongoing.
Conclusions
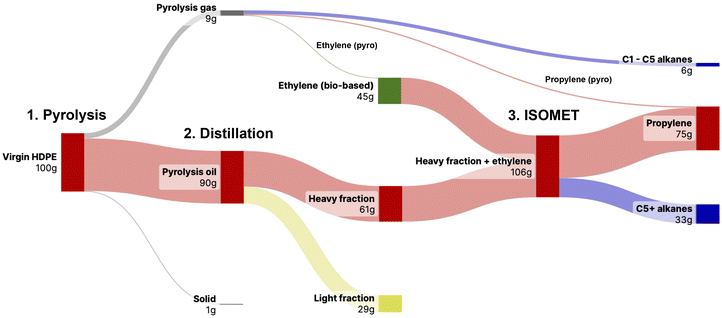
Efficient conversion of PE waste to propylene using slow pyrolysis followed by a single-metal ruthenium-catalyzed ISOMET reaction was demonstrated. The conversion of post-consumer PE waste was demonstrated in detail, which underlines the effectiveness and robustness of the process against impurities and additives. Although reduced catalyst activities were observed when switching from virgin HDPE pyrolysis oil to the post-consumer HDPE pyrolysis oil, larger-scale reactions demonstrate exceptional stability of the homogeneous catalyst system. Ultimately, one can imagine that 75 g of propylene can be produced from 100 g of PE waste and 45 g of (preferably bio-based) ethylene (Fig. 5). The remaining alkane fraction can be reprocessed by recently reported tandem dehydrogenation/metathesis reactions13 or fed into established conversion processes such as steam cracking or FCC. In reactions performed in Fisher–Porter bottles, a TON of 3900 for propylene could be achieved (entry 7, Table 1), scaling to a 900 mL reactor and repetitive batch experiments showed the long-term stability of the catalyst system with a tTON of 2788 after 82 hours of operation. This is the highest TON reported for post-consumer HDPE polyethylene ISOMET systems so far. Moreover, as it is a single metal catalytic system, the recovery of the ruthenium content should be feasible. It should also be noted that the overall propylene yield and TONs depend more on the quality of the pyrolysis oil than on the activity of the ISOMET catalyst systems. Based on these results, work is currently underway to optimize the slow pyrolysis conditions as well as to develop a continuous system enabling the large-scale applicability of the ISOMET reaction.Author contributions
Conceptualization: Róbert Tuba, Jürgen Klankermayer. Methodology: Vajk Farkas, Pascal Albrecht, Ádám Erdélyi, Márton Nagyházi, Beatrix Csutorás, Gábor Turczel, Norbert Miskolczi, Janka Bobek-Nagy, Ole Osterthun. Visualization: Róbert Tuba, Ole Osterthun Writing-original draft: Róbert Tuba, Jürgen Klankermayer. Writing-review & editing: Vajk Farkas, Márton Nagyházi, Pascal Albrecht, Gábor Turczel, Ole Osterthun.Data availability
The data supporting this article have been included as part of the ESI.† The processed data used for the visualizations in this study can be accessed at https://doi.org/10.5281/zenodo.13350938.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. Klankermayer and R. Tuba are grateful for funding by the BMBF through the Alexander von Humboldt Research Group Linkage. This research has been supported by the National Research, Development and Innovation Office through the project nr. 2022-1.1.1-KK-2022-00002, titled “Establishment of a waste management competence center at the University of Pannonia”. R. Tuba thanks the Hungarian National Research, Development and Innovation Office – NKFIH under the Grant TKP2021-EGA-31. The project has been implemented with the support provided by the National Research, Development and Innovation Fund of Hungary, financed under the 2021-1.2.4-TÉT-2021-00021 funding scheme. Financial support from NKFIH grant K-147172 is gratefully acknowledged. Funding by the Werner Siemens Foundation within the WSS project of the century “catalaix” is acknowledged. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence StrategyExzellenzcluster 2186 “The Fuel Science Center” ID: 390919832. We thank Pooja Dubey for her assistance in conducting some preliminary experiments.References
- Plastics – the fast Facts 2023, https://plasticseurope.org/de/knowledge-hub/plastics-the-fast-facts-2023/, (accessed March 12, 2024).
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- S. T. Schwab, M. Baur, T. F. Nelson and S. Mecking, Chem. Rev., 2024, 124, 2327–2351 CrossRef CAS PubMed.
- H. Lv, F. Huang and F. Zhang, Langmuir, 2024, 40, 5077–5089 CrossRef CAS PubMed.
- H. Ran, S. Zhang, W. Ni and Y. Jing, Chem. Sci., 2024, 15, 795–831 RSC.
- K. Faust, P. Denifl and M. Hapke, ChemCatChem, 2023, 15, e202300310 CrossRef CAS.
- Z. Akdogan and B. Guven, Environ. Pollut., 2019, 254, 113011 CrossRef CAS PubMed.
- L. D. Ellis, N. A. Rorrer, K. P. Sullivan, M. Otto, J. E. McGeehan, Y. Román-Leshkov, N. Wierckx and G. T. Beckham, Nat. Catal., 2021, 4, 539–556 CrossRef CAS.
- M. Baur, F. Lin, T. O. Morgen, L. Odenwald and S. Mecking, Science, 2021, 374, 604–607 CrossRef CAS PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, ACS Sustainable Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- A. H. Westlie, E. Y.-X. Chen, C. M. Holland, S. S. Stahl, M. Doyle, S. R. Trenor and K. M. Knauer, Macromol. Rapid Commun., 2022, 43, 2200492 CrossRef CAS PubMed.
- A. S. Goldman, A. H. Roy, Z. Huang, R. Ahuja, W. Schinski and M. Brookhart, Science, 2006, 312, 257–261 CrossRef CAS PubMed.
- X. Jia, C. Qin, T. Friedberger, Z. Guan and Z. Huang, Sci. Adv., 2016, 2, e1501591 CrossRef PubMed.
- D. Guironnet and B. Peters, J. Phys. Chem. A, 2020, 124, 3935–3942 CrossRef CAS PubMed.
- N. M. Wang, G. Strong, V. DaSilva, L. Gao, R. Huacuja, I. A. Konstantinov, M. S. Rosen, A. J. Nett, S. Ewart, R. Geyer, S. L. Scott and D. Guironnet, J. Am. Chem. Soc., 2022, 144, 18526–18531 CrossRef CAS PubMed.
- R. J. Conk, S. Hanna, J. X. Shi, J. Yang, N. R. Ciccia, L. Qi, B. J. Bloomer, S. Heuvel, T. Wills, J. Su, A. T. Bell and J. F. Hartwig, Science, 2022, 377, 1561–1566 CrossRef CAS PubMed.
- M. Nagyházi, Á. Lukács, G. Turczel, J. Hancsók, J. Valyon, A. Bényei, S. Kéki and R. Tuba, Angew. Chem., Int. Ed., 2022, 61, e202204413 CrossRef PubMed.
- V. Farkas, D. Csókás, Á. Erdélyi, G. Turczel, A. Bényei, T. Nagy, S. Kéki, I. Pápai and R. Tuba, Adv. Sci., 2024, 11, 2400118 CrossRef CAS PubMed.
- N. Miskolczi, A. Angyal, L. Bartha and I. Valkai, Fuel Process. Technol., 2009, 90, 1032–1040 CrossRef CAS.
- D. Zhao, X. Wang, J. B. Miller and G. W. Huber, ChemSusChem, 2020, 13, 1764–1774 CrossRef CAS PubMed.
- I. Ahmad, M. I. Khan, H. Khan, M. Ishaq, R. Tariq, K. Gul and W. Ahmad, Int. J. Green Energy, 2015, 12, 663–671 CrossRef CAS.
- S. Kumar and R. K. Singh, J. Pet. Eng., 2013, 2013, e987568 Search PubMed.
- J. A. Onwudili, N. Insura and P. T. Williams, J. Anal. Appl. Pyrolysis, 2009, 86, 293–303 CrossRef CAS.
- D. L. Nascimento and D. E. Fogg, J. Am. Chem. Soc., 2019, 141, 19236–19240 CrossRef CAS PubMed.
- D. L. Nascimento, M. Foscato, G. Occhipinti, V. R. Jensen and D. E. Fogg, J. Am. Chem. Soc., 2021, 143, 11072–11079 CrossRef CAS PubMed.
- S. K. Cormier and D. E. Fogg, ACS Catal., 2023, 13, 11834–11840 CrossRef CAS PubMed.
- Á. Balla, M. Al-Hashimi, A. Hlil, H. S. Bazzi and R. Tuba, ChemCatChem, 2016, 8, 2865–2875 CrossRef.
- E. Kovács, P. Sághy, G. Turczel, I. Tóth, G. Lendvay, A. Domján, P. T. Anastas and R. Tuba, J. Organomet. Chem., 2017, 847, 213–217 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc03912b |
| This journal is © The Royal Society of Chemistry 2024 |