Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Where is chemistry's moon? Highlights from the 1st conference for the Center of the Transformation of Chemistry (CTC) at Ringberg Castle 2023
Matthew B.
Plutschack
 *ab and
Peter H.
Seeberger
*ab and
Peter H.
Seeberger
 abc
abc
aDepartment of Biomolecular Systems, Max-Planck Institute of Colloids and Interfaces, Am Mühlenberg 1, 14476 Potsdam, Germany. E-mail: matthew.plutschack@mpikg.mpg.de
bCenter for the Transformation of Chemistry (CTC), Richard-Wagner-Straße 7 A, 04509 Delitzsch, Germany
cDepartment of Chemistry and Biochemistry, Freie Universität Berlin, Arnimallee 22, 14195 Berlin, Germany
First published on 19th March 2024
Abstract
In 2023, the Center for the Transformation of Chemistry (CTC) began a three-year start-up phase, aiming to draft its research program. The CTC-Conference at Ringberg brought scientists together to discuss their research and brainstorm a “moon-shot” program to align efforts, enable rapid discoveries, and move towards a sustainable circular economy.
I. Background
On August 14th, 2020, the “Act for Structural Change in Coal Mining Areas” (StStG) came into force as a measure to structurally aid the regions affected by the coal phase-out in Germany. In order to create new perspectives for the coal regions, a strategic decision was made to “establish one new institutionally funded large-scale research centre in each region in the Saxonian part of Lusatia and the Central German mining region (Mitteldeutsches Revier) on the basis of a competitive procedure”. In January 2021, the joint initiative of the Federal Government, represented by the Federal Ministry of Education and Research, the Free State of Saxony and the State of Saxony-Anhalt (Fig. 1), “Knowledge Creates Perspectives for the Region!” began and around 100 proposals were submitted by the end of April.1 In July 2021, a commission selected the best six proposals and recommended them to be funded for further development into a detailed concept. Starting in November 2021, these six initiatives, including the CTC, worked out their visions for a potential large-scale research centre and conceptualized its integration in and potential effects on the region. In July 2022, the CTC Expert pool defended the concept before a subject-specific Scientific Commission. Then, in August 2022, additional CTC Experts explained the CTC’s vision before a Commission for Transfer and Structural Impact in terms of technology transfer, organizational structures and expected economic impact on the region. The twelve members of this Expert pool contributed their expertise in creating institutions, entrepreneurship, start-ups, investment, innovation and transfer as well as regional development. At the end of September 2022, the CTC was notified that its concept was selected to be implemented. This strategic decision by the selection committee is significant for the green chemistry community as it aligns with efforts to transition away from fossil fuels and invest in sustainable technologies. The CTC has the potential to catalyse innovation and research aimed at developing environmentally friendly solutions, thereby contributing to the advancement of green chemistry practices and new perspectives in the Mitteldeutsches Revier.II. Introduction: pioneering a new era in chemistry
January 1st, 2023 marked the beginning of a three-year “start-up phase”. In addition to setting up the organizational structures and preparing for the construction of the research centre, the main task is to work out a strategy to unify the field of chemistry. The CTC-Conference at Ringberg Castle in September 2023 kicked off long awaited international, interdisciplinary discussions to this effect. A total of 40 participants represented 15 countries, 15 research institutions and 10 companies. Their diverse input was important for aligning incentives and transforming the field together.The Apollo Program, the Human Genome Project, and CERN (European Organization for Nuclear Research) have each made significant contributions to their respective fields of space exploration, genetics, and particle physics. Their “moon-shot” programs have had a profound impact on aligning scientists’ efforts and advancing their respective fields.2 While chemistry is a critical scientific discipline for all sectors of the economy and numerous fields of science, it has not had a single unifying project on the scale of the major initiatives listed above. This could be partly because chemistry is a diverse field with applications in various domains, from materials science to drug development, and lacks a singular, overarching goal. It could also be in part because the field of chemistry is incredibly diverse and complex, continuously evolving out of necessity like any enabling field for diverse applications.
The goal of the CTC kick-off conference in Ringberg was to bring together enthusiastic scientists from all over the world, to learn about each other's passions and brainstorm what chemistry's moon-shot program could look like. Peter Seeberger set the stage by introducing the “Center for the Transformation of Chemistry (CTC)”, and the team's vision to establish a circular economy with resilient chemical processes as its foundation. He articulated the research centre's desire to unite the field of chemistry, with local, national, international, academic and private interests all aligning to overcome one of chemistry's most formidable challenges: creating resilient, eco-friendly supply chains for all pillars of society.
The CTC holds particular significance for the green chemistry community, underscoring the commitment of the German chemical industry, one of the world's largest, to prioritize the development of sustainable chemical processes and supply chains. By emphasizing sustainability as a primary consideration, the industry demonstrates its dedication to minimizing environmental impact while fostering innovation and economic prosperity within the chemical sector.
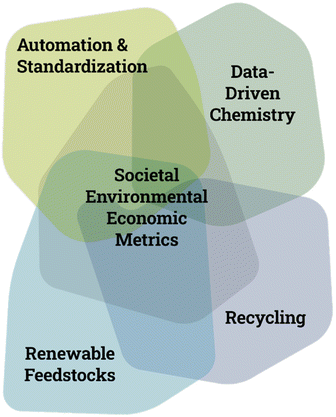
Peter Seeberger wrapped up by explaining how over the next three years the CTC will develop its research program with the core research domains seated within the thematic areas of Automation & Standardization, Data- & Computer-Driven Chemistry, Recycling, Renewable Feedstocks and Societal, Environmental and Economic (SEE) Metrics (Fig. 2). He explained the centre's commitment to fostering interdisciplinary collaboration and innovation. Ringberg 2023’s participants presented their work in organic synthesis, automated methodologies, analytics, data-driven approaches, sustainable innovations, and cloud-based collaborative endeavours. Their works epitomize the diverse, dynamic frontier of chemistry.
III. Transformation through synthesis: advancing enabling technologies
Chemical synthesis lies at the heart of chemical research. Our ability to make new molecules, polymers or materials enables us to generate new levels of activity and/or function, which in turn broadens or enables our understanding of a much more diverse palette of scientific fields. Several presentations at the CTC 2023 conference highlighted cutting-edge approaches to synthesis, as well as demonstrating advancements beyond chemical complexities via their implementation of enabling technologies.Lewis Mcghie of the Molloy group at the MPICI presented his work “Illuminating Routes to Complex Organoboron Scaffolds”, while Hannah Kortman described her efforts in the Molloy group to gain “Expedient Access to 3D Organoboron Heterocycles via Energy Transfer Catalysis”. Their efforts in new method development, in particular utilizing light as a mediator, is enabling the synthesis of new molecules with complex organoboron structures.
Arne Thomas from the Technical University of Berlin discussed how “Unifying Systems in Catalysis (UniSysCat)” is essential for transforming the chemical industry. UniSysCat is a collaborative consortium comprising over 300 researchers from four universities and four research institutes situated in the Berlin and Potsdam region. Together, they are dedicated to addressing contemporary challenges in the field of catalysis.3 He underscored the importance of catalysis with the statistic that catalysts facilitate over 85% of all industrial processes. The covalent organic frameworks (COFs) developed by his group demonstrate versatile applications, serving not only in photocatalysts,4 but also as novel materials for producing potable water (Fig. 3).5 He also pointed to other work within UniSysCat, such as the use of new technologies like operando spectroscopy, which is improving our understanding of and facilitating the development of new heterogeneous catalytic systems.6
 | ||
| Fig. 3 New porous crystalline COFs with acridine moieties exhibit high catalytic activity in metallaphotocatalytic C–N cross-couplings under green light. Taken from Traxler et al.4 and reused under CC BY 4.0. | ||
Siegfried Waldvogel from Johannes Gutenberg-University Mainz shared how his team is “Electrifying Organic Synthesis”, implementing new electrolysis reactor technologies. His group aims to activate chemical bonds electrochemically to generate reactive intermediates used in synthesis, with potential applications in lignosulfonate processing,7 production of biofuels,8 production of fine and commodity chemicals,9 and the synthesis of high-value fragrances.10 His group's work developing Laboratory Automation and Batch Scheduling (LABS) is also promoting the implementation of enabling technologies by developing a way to simultaneously control multiple devices, such as those needed for electrochemical flow setups capable of single-pass and cyclic electrolysis.11
The department of Biomolecular Systems at the MPICI is also no stranger to implementing enabling technologies like flow chemistry to facilitate synthesis. Dimitrije Djukanovic shared his work on the “Synthesis of Polyfunctional Amides, Ketones and Pyridines using Organometallic Reagents in Continuous Flow and Batch Methodologies”, which highlighted his work in the Knochel group using flow approaches to generate reactive organometallic species, such as dicarbamoylzincs12 and aryllithiums,13 as well as 3,4-pyridynes,14 and react them with high functional-group tolerance. His approach using continuous flow chemistry offers numerous advantages, including the safer utilization of reactive, pyrophoric reagents, precise generation of sensitive intermediates through residence time control, straightforward scale-up (on the lab scale), and a wider window for process intensification.
Also from the MPICI, Manuel Garcia Ricardo presented his work that leverages an automated glycan assembly (AGA) platform to merge “…Solid-Phase Peptide and Glycan Synthesis to Prepare Biomolecular Chimeras”.15 AGA enabled the fully automated preparation of diverse glycopeptide chimeras. The robust AGA protocol involves a single purification step, and opens avenues for the automated generation of glycan-based nanomaterials.
Eric Sletten gave us a glimpse of “AGA from a Chemist's Perspective”. He and his co-workers are synthesizing sulfated16 and phosphorylated oligosaccharides17 with unprecedented length and control. Their on-resin approach utilizing AGA involves regioselective sulfation and protecting-group hydrolysis, enabling the preparation of diverse, biologically relevant sulfated and phosphorylated glycans. He concluded with some preliminary results for producing even longer structures with AGA.
Sandhya Mardhekar also showed how she is capitalizing on the added value of AGA and rapidly expanding the scope of her previous PhD work, synthesizing “Biomimetic Analogs of Cell Surface Proteoglycans”. AGA continues to showcase how enabling technologies can impact synthesis by providing a highly efficient, reproducible, and automated workflow for constructing molecules, such as glycans, that have a diverse portfolio of complex structures.18
José Angel Danglad-Flores and Sebastián Pinzón-López shared their insights on AGA from the chemical engineering perspective. In particular, José shared his experiences in “Redesigning an Automated Glycan Assembly Platform”. In his opinion, breaking the old version was the best way to understand the necessary technical improvements to solve the synthetic challenges at hand. He showed how cost and time analyses played a role in prioritizing his efforts, ultimately resulting in a completely revamped platform that was faster, more efficient, and more compact, thus saving lab space.
Sebastián's approach was similarly analytical, breaking down all the operations to conduct the synthesis into a pie chart to identify the most time-consuming steps.19 He showed us his results for determining the average mixing times that were essential for optimizing the efficiency of multi-step automated oligosaccharide syntheses. Their work underscores the critical need for interdisciplinary teams to advance enabling technologies in synthesis.
A physicist from the MPICI, Sebastian Ronneberger, introduced us to “NanoFDM – A Nanolayer Printing Technique for Polymers with Additives”. His work in the Löffler group is pushing the limits of our ability to precisely control the transfer of matter with applications not only in femtoliter-scale chemistry,20 but also the patterning of high-resolution information labels.21,22
Wei-hsin Hsu also shared her preliminary work on overcoming problems related to combining biphasic reactions and automation, setting up an “Automated Flow Platform for the Optimization of Photochemical Liquid–Gas Reactions”. Her platform takes advantage of the many benefits that flow chemistry has to offer biphasic reactions involving liquids and gases in photochemical reactions.23 What became increasingly evident during her presentation was the large amount of work that goes into planning, setting up, and validating a robust system for screening reaction conditions. We look forward to what results from the work she put into automating reaction sampling, analysis, data storage and processing. Collectively, these researchers’ advancement of our physical chemical and technical knowledge was an excellent segue into one of the conference's and the CTC's thematic areas – Automation and Standardization.
IV. Automation in chemical research
Automation has yet to fully take off in chemical R&D in the same way that it has in biotech, where it has streamlined processes, reduced human error, accelerated experimentation and standardised analysis. Automation has the potential to enable chemistry researchers to precisely control reactions, perform high-throughput screening, and collect data at an unprecedented scale, relieving highly experienced chemists from mind-numbingly repetitive tasks. Automation can thereby empower chemists to focus on creative problem-solving and innovation, ultimately transforming chemical research into a more efficient, data-driven, and knowledge-rich discipline.Michael Schneider's insights into “Accelerating, Standardizing, Digitalizing the Transformation of Chemical Industry” further emphasized the role that technology will play. His team at Chemspeed is continuously improving the toolbox of automated workstations/workflows for sample preparation, synthesis, process development, formulation, screening and testing. Their platforms have found applications in powder dispensing for pharmaceutical research,24 sample and thin-layer preparation for perovskite materials,25 and the discovery of organic lasers,26 among others.
Pascal Miéville and his team at the EPFL have integrated some of Chemspeed's equipment into their data-driven automated laboratory for homogeneous catalyst discovery and optimization.27 His presentation on “Storms and Drones in a Laboratory” showcased their progress in handling solids on a microgram scale using their stochastic robotized micro-sampling system, as well as their approach for transferring samples between instruments using an open-source 2D drone-swarm. Pascal's and SwissCat+'s efforts are bringing the field progressively closer to autonomous labs.28
Antonio LaPorte of the Burke lab discussed their contributions to developing “Chemistry that machines can do”. Their work with MIDA- and TIDA-boronates29 for Suzuki–Miyaura cross-couplings30 in closed-loop workflows31 provides a unique approach for stitching together complex molecules that is comparable with classical iterative solid-support methods that have shaped automated oligonucleotide, oligopeptide, and oligosaccharide synthesis.
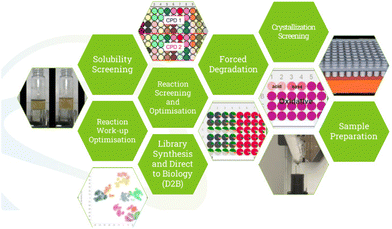
Synthesis is, however, only part of the innovation pipeline. Paola Ferrini gave us an overview of “Automation at GSK…” in particular with applications in drug development. She explained that we as a community can automate just about anything we put our minds to, but emphasised that we should ask why or when we should automate – questions the flow chemistry community would have been good to ask. Material saving and recovery, quick turnarounds, saving time and thereby money, the generation of large data sets with standardized processes ensuring data integrity and above all worker safety, are considerations that are made at GSK when deciding when to automate. The GSK team is pushing the company's capacities in the areas of solubility screening, reaction condition and work-up screening and optimisation, library synthesis, forced degradation, crystallisation screening, and standardised sample preparation (Fig. 4). Paola pointed to advances in analytics playing a key role in the next generation of automation in chemical R&D, posing the question: while we can now set up 384- or even 1536-well plates, how do we actually analyse that number of samples and translate that data into information at a comparable rate?
V. Analytics in translators for digital tools and collaboration
Open-source projects offer an agile, versatile and community-driven approach for collaboration. Tools like GitHub and Jupyter Notebook have played a significant role in modern software development and collaborative projects. Distributed version control, web-based platforms with integrated features and built-in analytical tools have expanded collaborative efforts by providing a central hub for code hosting, issue tracking, and pulling requests. The tools have become essential for fostering transparency, code and knowledge sharing, and collaboration among developers worldwide. There is a growing demand for chemical R&D to reach the level of remote collaboration and efficiency of software developers. Automation has the ability to effectively capture data, i.e., experimentation parameters, and yet there are still gaps in our ability to translate chemistry or molecules into digital information. This process is essential for communicating chemical inputs and experimental results digitally.Christian Haas presented his perspective for “Transforming Chemistry: Analytical Chemistry as a Catalyst for Change” and pointed to the role that analytics will play in “digital translators decoding chemical reactions…”. Decentralized, automated, comprehensive tracking and analysis of raw data will be necessary to facilitate data-based decision-making for geographically separated working groups. He emphasized that not only technical advancements will need to be made, but also a paradigm shift in the groups of people trying to solve chemistry's riddles – chemists, engineers, and data scientists will all need to contribute to modernizing chemistry.
Jakob Wolf's presentation on “Canonization of thioglycoside reactivity” tied in numerous aspects of how this might look in practice. His work on automating the screening of continuous reaction parameters like temperature, equivalents and reaction time, and coupling it with multi-dimensional analysis (HPLC/UV-Vis/ELSD/MS) is revealing new insights into glycosylation reactions and forming the foundation of a more efficient, systematic data-driven approach to glycan assembly. Jakob's work interfaces Python scripts with database software to facilitate data capture and underscores how systematically generating vast datasets through automated processes better equips researchers to harness the power of multi-dimensional analysis and extract chemical knowledge. This synergy between equipment interfaced with Python scripts and models to analyse data is shaping the future of chemistry into what could be an exciting acceleration of scientific discovery.
At times, ideas like the remote operation of self-driving labs, which communicate with researchers sitting at home, does seem to be far in the future. However, early progress is already showing how there is a significantly reduced need for researchers to be physically present in the lab, or at least allowing them to be present in only one lab. For example, Felix Strieth-Kalthoff presented his work on “A Delocalized Self-Driving Lab for Organic Materials Discovery”.32 His work in the Aspuru-Guzik lab, together with the Adachi, Burke, Cronin, Grzybowski and Hein labs, takes a cloud-based strategy that enables delocalized and asynchronous design–make–test–analyse cycles in the exploration of new materials for organic solid-state lasers (OSLs) (Fig. 5).33 Illustrating the seamless coordination among five globally distributed laboratories, their workflow serves as a model for decentralizing scientific discovery. It establishes, arguably, a benchmark for international collaboration and the incorporation of cutting-edge technologies for discovery.
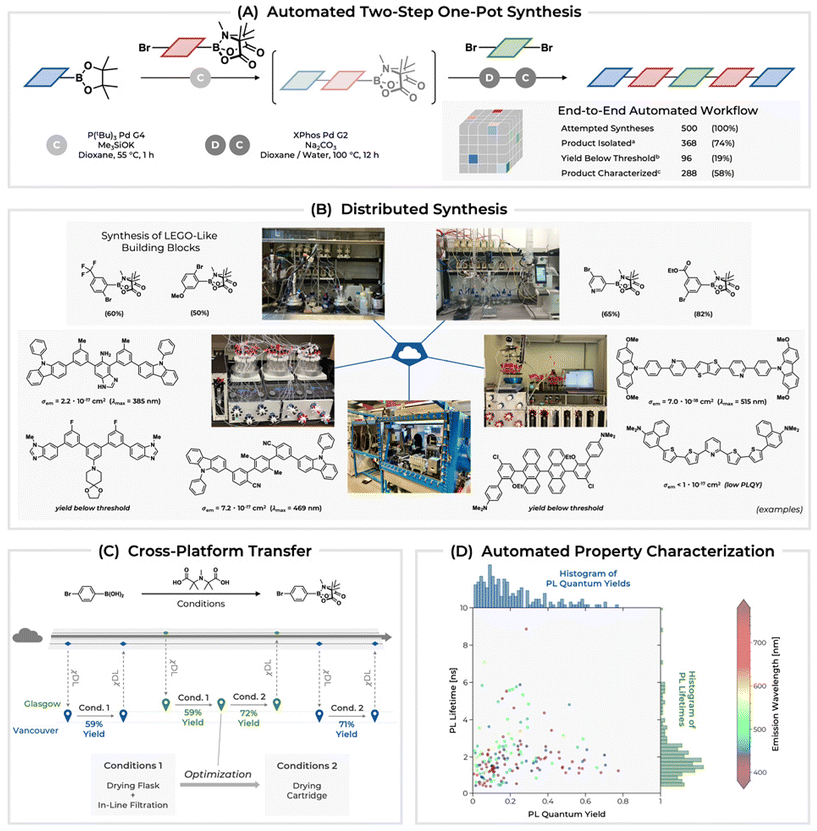 | ||
| Fig. 5 Automated synthesis and characterization of OSLs: (A) End-to-end automated workflow. (B) Examples of gram-scale LEGO-like building blocks and small-scale OSL synthesis. (C) Cross-platform optimization via standardized χDL protocols. (D) Scatter plot and histograms of photoluminescence quantum yields and lifetimes for a seed dataset of 500 OSL candidate compounds. Reused from Strieth-Kalthoff et al.33 under CC BY NC ND 4.0. | ||
The analytics in their material discovery campaign, using multiple automated synthesis and characterization modules across different laboratories and time zones with a central, cloud-based AI optimizer, enabled data-based decision-making and digital communication of results, and is an exciting new direction for international collaboration in chemical R&D.
VI. Data- and computer-driven approaches
In this ever-evolving landscape of digitalizing R&D, analytics appears to be the bridge between chemistry and the digital age, facilitating efficient, geographically separated collaboration. Data-driven approaches and machine learning represent a new frontier, where the analytical data not only facilitate communication but also have the potential to generate knowledge and progress like never before. Several presentations at the Ringberg Conference shed light on different aspects of how computer-driven approaches are transforming the landscape, paving the way for more efficient, systematic, and data-driven scientific exploration.Marwin Segler talked about his work with Microsoft Research using “Machine Learning for Organic Synthesis and Molecular Discovery”. Organic chemistry, known for its intricate nature, is frequently distilled through general models, including Lewis formulae, arrow-pushing diagrams, trends, heuristics, and empirical rules. These tools, drawn from careful observation of experimental data, facilitate the comprehension, explanation, and prediction of chemical reactions.34 It is, however, often to varying degrees, inefficient to predict what will happen. In order to grapple with this complexity, he described AI's potential role at each step of the typical Design–Make–Test cycle of R&D, where classification and regression models aid in testing, generative models can enable better design, and machine learning can enhance chemists’ ability to make molecules. While these machine learning methods are extremely powerful, they are simply statistical models of the data that we provide, making data acquisition, curation and translation crucial for action.
Heng Ji's presentation on “Knowledge-Empowered Scientific Language Modelling”, described one approach to gathering data that scientists have produced in the form of scientific literature. Her research at the University of Illinois Urbana-Champaign stands out as a compelling application of AI, addressing the challenge in the natural sciences, where the sheer volume of papers has surpassed human capacity for comprehension. In 2022 alone, over 5.1 million academic articles were published.35 While GPT-4 serves as a valuable tool, it falls short of contributing to scientific discovery. The specific challenge lies in extracting actionable knowledge from the text to inform decision-making. Her group is working on developing Domain-Specific Knowledge Enhanced Large Language Models to use the vast amount of literature data to generate actionable knowledge. This includes not only text-based molecular and procedural descriptors, but also 2D images of molecules, representing the underlying molecules involved in the reactions or studies.36
Interpretation of chemistry in the literature is complex, in particular organic structures, because they are communicated in numerous forms: as IUPAC names, condensed formulae, arbitrary abbreviations and 2-D drawings, such as bond-line or simply line structures. Carl Edwards presented his work on “Language-Guided Scientific Discovery for Chemistry” towards structural information extraction from the literature. He strives to overcome the complexities of integrating diverse modalities like text and molecular drawings, all while mitigating alignment loss, which refers to lost information due to the discrepancy between the two different modes of representation. With his Text2Mol task, he showed how he was able to construct a paired dataset of molecules and their corresponding text descriptions, which learns an aligned common semantic embedding space for retrieval.37 He also showed how MolT5-based models are able to generate outputs, both molecules and captions.38 He admitted the need for improvements to deal with inconsistencies in the literature as well as to generate knowledge with missing negative data. The latter point is a recurring topic among scientists, not only at this conference but also in the community.39 How can we predict what will work without being able to predict what will not?
John Bradshaw's insights into “Reaction Discovery: ML methods for predicting what will happen and what has happened!” presented data-driven approaches and machine learning in chemistry, in particular in the discovery of unknown structures.40 During the course of reaction discovery and optimization, and especially mechanism elucidation, chemists are confronted with molecules of unknown identity. Mass spectrometry is a quick, easy way to compare an observed spectrum with a library of previously collected spectra to identify the molecule. While this is a popular approach, it often fails, necessitating the development of new techniques to identify compounds based on their mass.41 Computational methods to predict the spectra in silico remain difficult. His work with Samuel Goldman in the Coley group is working on an approach called SCARF, which utilizes prefix tree data structures to efficiently decode mass spectra in order to identify molecules’ identities. John and the Coley group are tackling methodological challenges that have previously hindered autonomous discovery in the chemical sciences. One crucial aspect for overcoming R&D hurdles is not only developing tools to explain what happened, but also the invention of robust predictive tools. Many have speculated that to expand our capabilities to predict, improvements in the reporting and curation of reaction data must be made first.42
One way that scientists will be able to overcome R&D hurdles like professional, structured data reporting and curation will be via academic–industry collaborations. Companies like BASF have unmatched experience in chemistry, and yet, they themselves are facing challenges in digitalization. Hergen Schultze's presentation “Towards autonomous research machines” gave us insights into the motivation for digitalization related to materials and process development at BASF. He sees scientific modelling, statistics and machine learning playing an important role in closing the gap between what we actually want and what we can actually control. That means defining a product function and tracing that function back to the parameters that we can control, i.e., reagents and reaction parameters such as temperature, concentration, mixing, etc. There are, however, numerous potential stumbling blocks related to capturing, processing, contextualizing and interpreting data. Volume, velocity, variety and veracity of data are all issues. System integration and a researcher's willingness to adapt to new practises are also issues, especially on the implementation side of things. In addition, the high diversity of lab equipment – vendor, age, operating system, etc. – constitutes a hurdle to the execution of a data management plan that can deliver said potential. Setting those issues aside, Hergen pointed to the more exciting downstream challenges that we will then be able to work on, such as the development of material and composite representations to create complex yet generic models, dealing with conflicting goals as well as consistently applying models to discover new materials (Fig. 6).
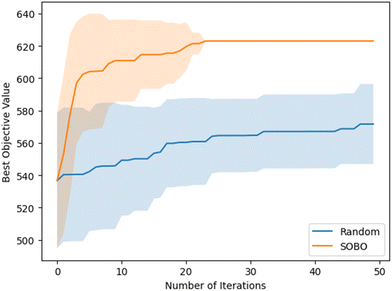 | ||
| Fig. 6 Sample plot from an example notebook for a Bayesian optimisation on a molecular dataset using a Tanimoto fingerprint kernel and the photoswitch dataset.43 Part of the Bayesian Optimization Framework Intended for Real Experiments (BoFire), a pre-competitive open source project between Evonik, BASF, and Boehringer.44 | ||
Computer-driven approaches currently pose a strategic competitive advantage in R&D. However, in the future, they may simply become every-day digital tools, for example in the planning of waste utilization towards the production of valuable new materials or chemicals. Tracing all possible syntheses, selecting feasible paths and imposing sustainability criteria would arguably be too complex or too time-consuming for humans alone, but is a challenge well-suited for computer-driven approaches. Sara Szymkuć presented the role that Allchemy played in the “Computer-designed revalorization and controlled-degradation of chemical wastes”.45 Allchemy's platform utilizes broad synthetic knowledge and computational algorithms to generate extensive synthetic networks. Out of around 200 waste chemicals, the Allchemy platform identified tens of thousands of routes leading to approximately 300 crucial drugs and agrochemicals, algorithmically ranked based on sustainability metrics (Fig. 7). This predictive capability was validated in the lab by On Demand Pharmaceuticals, and showcases the potential algorithms have to efficiently identify viable routes for the productive reuse of chemical waste, thereby accelerating sustainable circular chemistry initiatives.
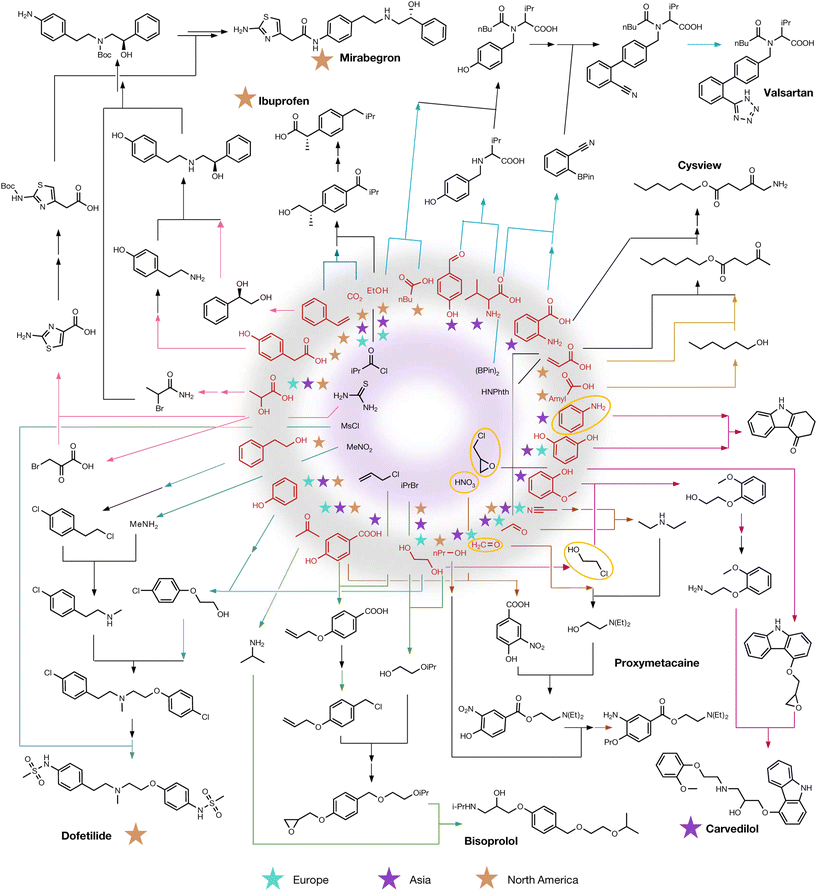 | ||
| Fig. 7 Selected intermediates for various synthesis routes from waste substrates. Auxiliary substrates are shown in the inner circle, while ‘waste’ substrates are highlighted in red in the outer circle. Hazardous substances are marked with yellow ellipses. Geographical locations of substrate-producing companies are denoted by stars. Notably, drugs and agrochemicals with stars can be synthesized from substrates available at the same location. Different colours represent distinct synthetic pathways, with coloured arrows indicating literature-reported steps. Taken from Wołos et al.45 | ||
Computer-driven approaches play a crucial role in predicting and planning syntheses, yet there is limited exploration differentiating the feasible from best for application. Bartosz Grzybowski's talk on “Algorithms and gigascale reaction networks for the chemical industry” addressed how computers can help chemists answer questions related to what is the most reasonable path forward in the context of a desired cost or scale. Since the emergence of the computer program called Chematica (a.k.a. Synthia), which was shown to autonomously plan multistep syntheses of complex natural products,46 there have been a number of improvements.47 While these algorithms produce a plethora of plausible syntheses, they do not weight cost and scale. By selecting and scoring the most economical and diverse synthetic pathways from large graphs of synthetically viable solutions, his team has provided an approach that can interface with catalogues of starting materials and their corresponding prices to incorporate cost into synthesis planning.48 Moreover, he also gave us a glimpse of his teams work, delving into patent literature and incorporating reaction scale into planning. This work would be vital for discovery pipelines that require larger scales for proof of principle or process-oriented studies. Without reliable predictions about cost-effectively scaling our discoveries, it will be difficult to bring innovative, sustainable solutions to the market.
VII. Innovations in sustainable chemistry
Automation and data sciences have the potential to change molecular design, synthesis planning and property optimization – vital components of the discovery pipeline. This shift towards data- or computer-driven methodologies and machine learning heralds a new era in chemistry. To create impactful tools, it is essential to tailor them to the needs of the users, specifically the scientists addressing fundamental challenges in chemistry. Ringberg 2023 featured several participants who exemplified this user group, sharing innovative perspectives on sustainable processes, polymers, and materials.In the area of repurposing waste streams, Juliana Cárdenas showed us her work on “A modified heteropolyacid as a phase-transfer catalyst for used cooking oil epoxidation with hydrogen peroxide”. Exploitation of waste lipids in the production of higher value-added and green chemicals is one approach to enhancing circularity in the oleochemical industry.49 Her research is helping to advance transformations of renewable waste feedstocks, i.e., cooking oil waste, into valuable resources.
Michael Meier's work on “New cellulose chemistry from a sustainability perspective” gave us insights into how his group uses Environmental-factors (E-factors = kg waste per kg product)50 as a simple metric for quickly assessing sustainability to guide their progress. For example, a significant hurdle in handling cellulose, the planet's most abundant organic material, lies in the solubilisation of cellulose.51 The Meier group was able to optimise a switchable solvent system, DBU/CO2, for efficient cellulose solubilization and derivatization, achieving rapid cellulose transformation under mild conditions. Full life cycle analyses (LCAs) for each of their processes would prove time-consuming; however, a quick calculation of the E-factors helped them identify four factors to consider in developing a new cellulose modification protocol: (1) solvent selection; (2) derivatization reagent; (3) functionalization method (catalysed, stoichiometric activation, etc.); (4) toxicity, biodegradability and stability of the modified cellulose.52 Michael also showed how E-factors guided his group while developing a more sustainable method for producing isocyanides.53
Clara Scheelje, of the Meier group, presented her work on “Improving the sustainability of polyurethanes”. She and her colleagues are pursuing alternate routes, such as trans-urethanisation or activation of cyclic carbonates, as well as utilising sustainable diols and carbonates for producing polyurethanes with the same properties when compared with commercial polyurethanes.
Manuel Häußler outlined his vision for “Closing the Carbon Loop for Plastics”, highlighting the urgency of shifting from fossil fuels as the primary source for chemicals and materials. In 2020, 84% of plastics originated from fossil fuels, while bio-based, recycled, and CO2-based sources constituted less than 10%, 5%, and 0.5%, respectively. Addressing the plastic problem gains significance as the demand for carbon-containing materials is expected to double.54 In this way, Manuel emphasized the urgency for the next generation of sustainable plastics, citing long-chain polyesters and polycarbonates as promising platform chemicals.55 He showcased a simple solvolysis method for the chemical recycling of these long-chain polycarbonates and polyesters, strategically targeting the polyethylene-like chains at the sparsely incorporated functional groups that act as breakpoints. This innovative approach achieved an impressive recovery rate surpassing 96%, all while maintaining the crystalline polyethylene structure and preserving the high-performance properties of the materials. He also gave us a glimpse of his start-up, which is working on synthesizing aliphatic dicarboxylic acids from polyethylene waste, paving a potential way to transform current waste streams into materials with higher potential for circularity.56
Sustainable CO2 loops would be ground-breaking achievements. Katharina Eisenhardt presented her efforts on “Using Carbon Dioxide to Make Plastics”, highlighting catalysis’ role in developing the next generation of sustainable materials.57 Her work in the Williams group on ring-opening copolymerization (ROCOP) of propylene oxide (PO) and CO2 represents an eco-friendly approach to utilize CO2 waste gas (Fig. 8). Despite recent progress, challenges persist in understanding the polymerization mechanism and developing more efficient catalysts. She introduced a novel Co(III)K(I) catalyst that enables low-pressure ROCOP (<5 bar of CO2), while still exhibiting unprecedented activity and selectivity.58 Katharina's valuable insights into the polymerization mechanism are already leading to the exploration of new ligand systems and could aid future catalyst design for large-scale, low-pressure industrial production of CO2-based polycarbonates and block polymers.
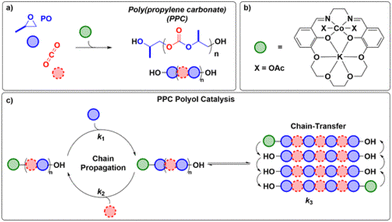 | ||
| Fig. 8 (a) PO/CO2 ROCOP to make poly(propylene carbonate) (PPC) polyols. (b) Illustration of one of the heterodinuclear catalysts. (c) Catalytic cycle for PO/CO2 ROCOP, illustrating both propagation and chain transfer reactions. Taken from Deacy et al.58 and reused under CC BY 4.0. | ||
VIII. Moving forward together
In navigating the highly complex interconnected chemical industry, the chemistry community is regularly confronted with the growing necessity to communicate what are chemical industry's most pressing issues. This environment leads to a challenge wherein academics form distinct groups, all vying for the same resources to support their efforts to tackle their problems. The funding agencies essentially then have to answer: how do we begin to prioritize which challenges are more important?Tyler McQuade from On Demand Pharmaceuticals (ODP) shared his strategy for developing a business model that not only serves ODP's objectives but also aligns with customer goals. Central to Tyler's approach is a deep understanding of customers’ underlying needs, enabling the alignment of incentives to effectively address those needs. This customer-centric focus enhances engagement, aligns incentives with customer satisfaction, and facilitates the delivery of solutions that add significant value. ODP's subscriber model illustrates this strategy by aligning company objectives with sustained customer satisfaction, as demonstrated in the case where ODP addresses shortages of acute medicines by working closely with clinicians. Tyler's strategy at ODP resonated with the CTC team, who look to align incentives within the chemical community.
Divya Varadharajan presented a similar “Consumer-driven optimization in chemistry”. She highlighted that the most vocal preferences expressed by certain consumers do not consistently align with the factors influencing the majority of consumers’ purchasing decisions. While purchasing decisions in general are very strongly driven by rationale, there is a large mistrust of anything synthetic and surprisingly, ethical reasons tend to play a less important role.59 Purchases are heavily driven by lowest cost or the perception of added value for the money. Sustainability is, however, playing an ever-growing role in brand choices. Divya argued that even academics need to consider how consumers drive decisions in the chemical industry. She explained her view on how cooperation between companies and academics can help meet consumer demands and generate a positive public image, while navigating demographic changes, labour shortages for digitalisation and scepticism of new enabling technologies, factors that will influence the community as it attempts to drive sustainable innovations in chemistry.
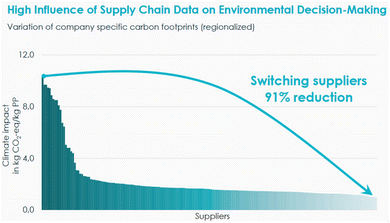
Leif Rohrbach, from the Cologne-based company Carbon Minds, highlighted their efforts at the boundary between science and business, with his presentation on “Product Carbon Footprints – From Science to Business Implementation”.60 Their use of third-party certified data to conduct LCAs and estimate product carbon footprints (PCFs) provides a basis for business strategies related not only to corporate image, but also risk mitigation, cost reduction and revenue optimization. Their work demonstrates how incentives can be aligned to provide companies with viable economic solutions while simultaneously contributing to progress in a more sustainable, environmentally friendly industry. Their comprehensive database is not only able to estimate production volumes and technologies by location, but also provides insights into international material flows and the impact of regional consumers on global footprints (Fig. 9). In this way, their interdisciplinary team of chemists, engineers, data scientists and sustainability experts are producing data that is useful to increase understanding, and thereby form the basis for effective action.
The challenges we are facing are increasingly multi-disciplined, and also require the expertise and resources of diverse groups of stakeholders. Tobias Klement is concentrated on “Shaping the structural change towards a sustainable bioeconomy”, emphasizing the engagement of CLIB (Cluster industrielle Biotechnologie). The cluster facilitates connections among enterprises of varying sizes, including large, small, and medium-sized, along with start-ups, research institutions, and universities. This initiative to facilitate collaborative efforts aims to synergize expertise for the development of sustainable bio-based strategies. Tobias’ non-profit organisation, based in the Rheinisches Revier in North Rhine-Westphalia, is looking to play a pivotal role in the regional structural change as Germany aims to exit the coal industry by 2030. Similar to the Mitteldeutsches Revier where the CTC is situated (Fig. 10), the Rheinisches Revier is significantly shaped by not only the coal industry, but also the chemical sector. Consequently, both regions encounter analogous challenges as they endeavour to shift toward more stable and sustainable industry models. The similarities in goals between CLIB’s partners and the CTC network provides an opportunity for a much larger synergistic strategy to help both regions generate chemical innovations in the areas of the bio-economy and move towards a more circular economy.
IX. Conclusion
The CTC Kick-off conference convened a diverse community of scientists, ranging from PhD students to seasoned professionals, across a spectrum of disciplines including chemical synthesis, catalysis, automation, AI, and natural language processing. Over three and a half days, attendees engaged in stimulating discussions that transcended specific scientific focuses, fostering a rich exchange of ideas and insights. Their enthusiastic participation underscores the role the CTC hopes to play in driving innovation and collaboration across diverse scientific domains, particularly in advancing sustainable practices and enabling technologies within chemistry. As we reflect on the success of this conference, we look forward to building upon this momentum and shaping the future of chemistry's global endeavours towards sustainability and resilience.Author contributions
M. B. P: funding acquisition, conceptualization, writing – original draft, writing – review & editing. P. H. S. funding acquisition, review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the Max Planck Society, the German Federal Ministry of Education and Research for funding the CTC (Funding Reference: 03WSP1570), and the highly talented Ringberg Castle team headed by Jochen Essl. We are also grateful to all participants for their time, presentations and engaging discussions. (Participants in alphabetical order according to their surname: John Bradshaw, Juliana Cárdenas, José Angel Danglad-Flores, Joachim Dickhaut, Dimitrije Djukanovic, Carl Edwards, Katharina Eisenhardt, Paola Ferrini, Manuel Garcia Ricardo, Bartosz Grzybowski, Christian Haas, Manuel Häußler, Wei-Hsin Hsu, Heng Ji, Tobias Klement, Hannah Kortman, Klara Krämer-Klement, Antonio LaPorte, Sandhya Mardhekar, Lewis Mcghie, D. Tyler McQuade, Pascal Miéville, Michael Meier, Sebastian Pinzon Lopez, Leif Rohrbach, Sebastian Ronneberger, Clara Scheelje, Michael Schneider, Hergen Schultze, Rebecca Schweier, Marwin Segler, Eric Sletten, Felix Strieth-Kalthoff, Sara Szymkuć, Arne Thomas, Divya Varadharajan, Siegfried Waldvogel, and Jakob Wolf).References
- Richtlinie zur Förderung von Vorhaben im Rahmen der Initiative “Wissen schafft Perspektiven für die Region!”, https://www.bmbf.de/bmbf/shareddocs/bekanntmachungen/de/2021/01/<?pdb_no 3295?>3295<?pdb END?>_bekanntmachung.html.
- A lunar landing program, often referred to as a “moon-shot”, is a highly ambitious and audacious initiative or project that aims to achieve a ground-breaking or revolutionary goal within a specific timeframe. The term “moon-shot” originates from the Apollo 11 mission, where the United States successfully landed the first humans on the moon in 1969.
- UniSysCat Berlin, https://www.unisyscat.de/, (accessed November 16, 2023).
- M. Traxler, S. Gisbertz, P. Pachfule, J. Schmidt, J. Roeser, S. Reischauer, J. Rabeah, B. Pieber and A. Thomas, Angew. Chem., Int. Ed., 2022, 61, e202117738 CrossRef CAS PubMed.
- C. Li, S. Cao, J. Lutzki, J. Yang, T. Konegger, F. Kleitz and A. Thomas, J. Am. Chem. Soc., 2022, 144, 3083–3090 CrossRef CAS PubMed.
- M. Rüscher, A. Herzog, J. Timoshenko, H. S. Jeon, W. Frandsen, S. Kühl and B. Roldan Cuenya, Catal. Sci. Technol., 2022, 12, 3028–3043 RSC.
- J. Klein and S. R. Waldvogel, ChemSusChem, 2023, 16, e202202300 CrossRef CAS PubMed.
- T. Ashraf, A. P. Rodriguez, B. T. Mei and G. Mul, Faraday Discuss., 2023, 247, 252–269 RSC.
- X. Dong, J. L. Roeckl, S. R. Waldvogel and B. Morandi, Science, 2021, 371, 507–514 CrossRef CAS PubMed.
- M. Zirbes, L. L. Quadri, M. Breiner, A. Stenglein, A. Bomm, W. Schade and S. R. Waldvogel, ACS Sustainable Chem. Eng., 2020, 8, 7300–7307 CrossRef CAS.
- M. M. Hielscher, M. Dörr, J. Schneider and S. R. Waldvogel, Chem. – Asian J., 2023, 18, e202300380 CrossRef CAS PubMed.
- D. Djukanovic, M. A. Ganiek, K. Nishi, K. Karaghiosoff, K. Mashima and P. Knochel, Angew. Chem., Int. Ed., 2022, 61, e202205440 CrossRef CAS PubMed.
- D. Djukanovic, B. Heinz, F. Mandrelli, S. Mostarda, P. Filipponi, B. Martin and P. Knochel, Chem. – Eur. J., 2021, 27, 13977–13981 CrossRef CAS PubMed.
- B. Heinz, D. Djukanovic, P. Filipponi, B. Martin, K. Karaghiosoff and P. Knochel, Chem. Sci., 2021, 12, 6143–6147 RSC.
- M. G. Ricardo and P. H. Seeberger, Chem. – Eur. J., 2023, 29, e202301678 CrossRef CAS PubMed.
- T. Tyrikos-Ergas, E. T. Sletten, J.-Y. Huang, P. H. Seeberger and M. Delbianco, Chem. Sci., 2022, 13, 2115–2120 RSC.
- E. T. Sletten, J. Danglad-Flores, S. Leichnitz, A. Abragam Joseph and P. H. Seeberger, Carbohydr. Res., 2022, 511, 108489 CrossRef CAS PubMed.
- M.-H. Lin, J. B. Wolf, E. T. Sletten, D. Cambié, J. Danglad-Flores and P. H. Seeberger, ChemBioChem, 2023, 24, e202200607 CrossRef CAS PubMed.
- S. Pinzón-López, M. Kraume, J. Danglad-Flores and P. H. Seeberger, React. Chem. Eng., 2023, 8, 2951 RSC.
- J. Zhang, Y. Liu, S. Ronneberger, N. V. Tarakina, N. Merbouh and F. F. Loeffler, Adv. Mater., 2022, 34, 2108493 CrossRef CAS PubMed.
- S. Ronneberger, J. Zhang, Y. Liu and F. F. Loeffler, Adv. Funct. Mater., 2023, 33, 2210116 CrossRef CAS.
- J. Zhang, Y. Liu, C. Njel, S. Ronneberger, N. V. Tarakina and F. F. Loeffler, Nat. Nanotechnol., 2023, 18, 1027–1035 CrossRef CAS PubMed.
- M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796–11893 CrossRef CAS PubMed.
- M. N. Bahr, M. A. Morris, N. P. Tu and A. Nandkeolyar, Org. Process Res. Dev., 2020, 24, 2752–2761 CrossRef CAS.
- E. Reinhardt, A. M. Salaheldin, M. Distaso, D. Segets and W. Peukert, ACS Comb. Sci., 2020, 22, 6–17 CrossRef CAS PubMed.
- T. C. Wu, A. Aguilar-Granda, K. Hotta, S. A. Yazdani, R. Pollice, J. Vestfrid, H. Hao, C. Lavigne, M. Seifrid, N. Angello, F. Bencheikh, J. E. Hein, M. Burke, C. Adachi and A. Aspuru-Guzik, Adv. Mater., 2023, 35, 2207070 CrossRef CAS PubMed.
- P. Laveille, P. Miéville, S. Chatterjee, E. Clerc, J.-C. Cousty, F. de Nanteuil, E. Lam, E. Mariano, A. Ramirez, U. Randrianarisoa, K. Villat, C. Copéret and N. Cramer, Chimia, 2023, 77, 154 CrossRef CAS PubMed.
- SwissCat+, https://swisscatplus.ch/.
- A. J. LaPorte, J. E. Feldner, J. C. Spies, T. J. Maher and M. D. Burke, Angew. Chem., Int. Ed., 2023, 62, e202309566 CrossRef CAS PubMed.
- W. Wang, N. Angello, D. Blair, K. Medine, T. Tyrikos-Ergas, A. LaPorte and M. Burke, ChemRxiv, 2023 DOI:10.26434/chemrxiv-2023-qpf2x.
- N. H. Angello, V. Rathore, W. Beker, A. Wołos, E. R. Jira, R. Roszak, T. C. Wu, C. M. Schroeder, A. Aspuru-Guzik, B. A. Grzybowski and M. D. Burke, Science, 2022, 378, 399–405 CrossRef CAS PubMed.
- F. Strieth-Kalthoff, H. Hao, V. Rathore, J. Derasp, T. Gaudin, N. H. Angello, M. Seifrid, E. Trushina, M. Guy, J. LiuX. Tang, M. Mamada, W. Wang, T. Tsagaantsooj, C. Lavigne, R. Pollice, T. C. Wu, K. Hotta, L. Bodo, S. Li, M. Haddadnia, A. Wolos, R. Roszak, C.-T. Ser, C. Bozal-Ginesta, R. J. Hickman, J. Vestfrid, A. Aguilar-Gránda, E. L. Klimareva, R. C. Sigerson, W. Hou, D. Gahler, S. Lach, A. Warzybok, O. Borodin, S. Rohrbach, B. Sanchez-Lengeling, C. Adachi, B. A. Grzybowski, L. Cronin, J. E. Hein, M. D. Burke and A. Aspuru-Guzik, ChemRxiv, 2023. DOI:10.26434/chemrxiv-2023-wqp0d.
- F. Strieth-Kalthoff, H. Hao, V. Rathore, J. Derasp, T. Gaudin, N. H. Angello, M. Seifrid, E. Trushina, M. Guy, J. Liu, X. Tang, M. Mamada, W. Wang, T. Tsagaantsooj, C. Lavigne, R. Pollice, T. C. Wu, K. Hotta, L. Bodo, S. Li, M. Haddadnia, A. Wolos, R. Roszak, C.-T. Ser, C. Bozal-Ginesta, R. J. Hickman, J. Vestfrid, A. Aguilar-Gránda, E. L. Klimareva, R. C. Sigerson, W. Hou, D. Gahler, S. Lach, A. Warzybok, O. Borodin, S. Rohrbach, B. Sanchez-Lengeling, C. Adachi, B. A. Grzybowski, L. Cronin, J. E. Hein, M. D. Burke and A. Aspuru-Guzik, ChemRxiv, 2023. DOI:10.26434/chemrxiv-2023-wqp0d.
- F. Strieth-Kalthoff, F. Sandfort, M. H. S. Segler and F. Glorius, Chem. Soc. Rev., 2020, 49, 6154–6168 RSC.
- D. Curcic, Number of Academic Papers Published Per Year, https://wordsrated.com/number-of-academic-papers-published-per-year/, (accessed 16.11.2023, 2023).
- H. Wang, W. Li, X. Jin, K. Cho, H. Ji, J. Han and M. D. Burke, arXiv, 2021, preprint, arXiv:2109.09888. DOI:10.48550/arXiv.2109.09888.
- C. Edwards, C. Zhai and H. Ji, Online and Punta Cana, Dominican Republic, 2021 Search PubMed.
- C. Edwards, T. Lai, K. Ros, G. Honke, K. Cho and H. Ji, Translation between Molecules and Natural Language, Abu Dhabi, United Arab Emirates, 2022 Search PubMed.
- M. P. Maloney, C. W. Coley, S. Genheden, N. Carson, P. Helquist, P.-O. Norrby and O. Wiest, J. Org. Chem., 2023, 88, 5239–5241 CrossRef CAS PubMed.
- S. Goldman, J. Bradshaw, J. Xin and C. Coley, presented in part at the Thirty-seventh Conference on Neural Information Processing Systems, 2023.
- J. N. Wei, D. Belanger, R. P. Adams and D. Sculley, ACS Cent. Sci., 2019, 5, 700–708 CrossRef CAS PubMed.
- R. Mercado, S. M. Kearnes and C. W. Coley, J. Chem. Inf. Model., 2023, 63, 4253–4265 CrossRef CAS PubMed.
- R.-R. Griffiths, J. L. Greenfield, A. R. Thawani, A. R. Jamasb, H. B. Moss, A. Bourached, P. Jones, W. McCorkindale, A. A. Aldrick, M. J. Fuchter and A. A. Lee, Chem. Sci., 2022, 13, 13541–13551 RSC.
- Evonik-dev, https://github.com/experimental-design/bofire.git, (accessed December 18, 2023).
- A. Wołos, D. Koszelewski, R. Roszak, S. Szymkuć, M. Moskal, R. Ostaszewski, B. T. Herrera, J. M. Maier, G. Brezicki, J. Samuel, J. A. M. Lummiss, D. T. McQuade, L. Rogers and B. A. Grzybowski, Nature, 2022, 604, 668–676 CrossRef PubMed.
- B. A. Grzybowski, S. Szymkuć, E. P. Gajewska, K. Molga, P. Dittwald, A. Wołoś and T. Klucznik, Chem, 2018, 4, 390–398 CAS.
- B. A. Grzybowski, T. Badowski, K. Molga and S. Szymkuć, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2023, 13, e1630 Search PubMed.
- T. Badowski, K. Molga and B. A. Grzybowski, Chem. Sci., 2019, 10, 4640–4651 RSC.
- J. Cárdenas, A. Orjuela, D. L. Sánchez, P. C. Narváez, B. Katryniok and J. Clark, J. Cleaner Prod., 2021, 289, 125129 CrossRef.
- R. A. Sheldon, Chem. Commun., 2008, 3352–3365 RSC.
- K. N. Onwukamike, S. Grelier, E. Grau, H. Cramail and M. A. R. Meier, ACS Sustainable Chem. Eng., 2019, 7, 1826–1840 CrossRef CAS.
- K. N. Onwukamike, T. Tassaing, S. Grelier, E. Grau, H. Cramail and M. A. R. Meier, ACS Sustainable Chem. Eng., 2018, 6, 1496–1503 CrossRef CAS.
- K. A. Waibel, R. Nickisch, N. Möhl, R. Seim and M. A. R. Meier, Green Chem., 2020, 22, 933–941 RSC.
- M. Carus, F. Kähler and O. Porc, Global Carbon Demand for Chemicals and Derived Materials, https://renewable-carbon.eu/publications/product/global-carbon-demand-for-chemicals-and-derived-materials-png/, 92 KB.
- M. Eck, S. T. Schwab, T. F. Nelson, K. Wurst, S. Iberl, D. Schleheck, C. Link, G. Battagliarin and S. Mecking, Angew. Chem., Int. Ed., 2023, 62, e202213438 CrossRef CAS PubMed.
- M. Häussler and L. Odenwald, Germany Pat, DE102021133861A1, 2023 Search PubMed.
- K. C. Poon, G. L. Gregory, G. S. Sulley, F. Vidal and C. K. Williams, Adv. Mater., 2023, 35, 2302825 CrossRef CAS PubMed.
- A. C. Deacy, E. Moreby, A. Phanopoulos and C. K. Williams, J. Am. Chem. Soc., 2020, 142, 19150–19160 CrossRef CAS PubMed.
- E. Bazzoni, M. Mijer, O. Gediehn, M. Jacob, S. Welchering, S. Land,J. Moulton and D. Spillecke, European Consumer Sentiment Survey: How current events in Europe are shaping consumer behavior – Insights from Germany, McKinsey & Company, 2022 Search PubMed.
- R. Meys, A. Kätelhön, M. Bachmann, B. Winter, C. Zibunas, S. Suh and A. Bardow, Science, 2021, 374, 71–76 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |