Stability of iron single atoms in graphene structures from X-ray absorption spectroscopy data†
Anna
Krot
 ,
Serguei
Savilov
,
Ekatherina
Arkhipova
and
Stepan
Kalmykov
*
,
Serguei
Savilov
,
Ekatherina
Arkhipova
and
Stepan
Kalmykov
*
Lomonosov Moscow State University, Leninskie Gory 1-3, 119991, Russia. E-mail: anna.d.krot@gmail.com
First published on 21st November 2023
Abstract
We investigate iron oxidation states and its local environment in graphene nanoflakes (GNFs) as well as those treated with nitric acid using X-ray absorption spectroscopy (XAS). Ppb-concentrated single-atom Fe was found to be intercalated into the structure of GNFs during the synthesis and is highly stabilized between graphene layers.
The chemistry of carbon is extremely interesting due to the multitude of its allotropic modifications (graphite, graphene, diamond, lonsdaleite, fullerene, carbon nanotubes, etc.),1 which demonstrate various, sometimes opposite, characteristics. Despite this, most of them have already found their practical use in modern industry. To enhance their application spheres, it is important to understand the structural features at levels higher than atomic but lower than micron. Special interest from this viewpoint is turned to self-organizing carbon structures. This process takes place due to interactions of ionogenic groups at the surface and local doping of the material by metallic nanoparticles, which provides opportunities for application in many fields of modern industry.2–5
After the Nobel Prize for the discovery of graphene was awarded to A. Geim and K. Novoselov,6 a lot has been written about its properties, applications and production techniques. However, practically, it is almost impossible to obtain single-layer graphene in significant quantities. Therefore, graphene nanoflakes (GNFs) which are few-layer graphenes or, as is more correct, few-layer graphites can be in the sphere of interest. Depending on the number of layers and particle size, their planarity, associated with the number of carbon atoms with uncompensated valences and functional groups, can vary. GNF fragments are characterized by a continuous, extended structure, consisting of 1–10 carbon layers.7
Normally, the method of GNF synthesis implies the pyrolysis of hydrocarbons over a MgO or CaO template8 in quartz reactors, followed by its elimination with non-oxidizing acids. From the commercial viewpoint the template purity normally is limited to 99%. The residual amount of Fe impurities there is less than 0.1% and can be detected on the background level by the X-ray Fluorescence (XRF) technique. Nevertheless, it was observed that Fe originating from the template material can intercalate into the structure of carbon nanomaterials during synthesis. Metal atoms introduced in this way are present in the resulting material at concentrations as low as ppb in different forms, including single atoms.9 Recently it was shown that carbon materials doped with single atom Fe demonstrate high catalytic activity in the oxygen reduction reaction (ORR)10,11 and benzene oxidation.12 A remarkable property of the encapsulated single-atom Fe is its stability under oxidation treatment with nitric acid, that is widely used for surface modification of nanoflakes, making them a promising material for extraction of low-concentrated metals, catalytic, magnetic, and electronic applications and as a component of composites.
As a result of the synthesis of GNFs using a MgO template (containing up to 0.1% of Fe impurities) iron in a single-atom form was found to be intercalated into the structure of the carbon material. The residual amount of Fe impurities was less than 0.1% which was possible to detect on the background level by X-ray Fluorescence (XRF) and Mössbauer spectroscopy. According to ICP-MS measurements, the total amount of Fe in samples does not exceed 1.3 ppm. The bulk properties of the pristine carbon material and that oxidized with HNO3 were investigated previously using Raman spectroscopy, XPS and TEM techniques.13 In brief, according to TEM data, GNFs are of 15–50 nm size and contain up to 7 graphene layers. Upon oxidation, their size is reduced, but the number of layers is preserved. Although TEM measurements proved to be a powerful technique for determination of metal single sites in carbon-based materials,14,15 the extremely low concentrations of Fe make it difficult to apply in our investigation. Raman data indicates that the bulk structure of the material is preserved after oxidation, and XPS revealed that the surface of the oxidized material is functionalized by carboxylic groups. Again, none of these methods was capable of probing iron chemistry due to its low concentration and more sensitive synchrotron-based techniques are required.
This study became possible due to the development of ultra-sensitive detectors and electronics at the large-scale facilities – synchrotrons – adjusted for iron in X-ray absorption beamlines and setups. X-ray absorption spectroscopy is well known as one of the most powerful tools for the determination of an element's valence state and local environment even when it is present at extremely low (up to ppm) concentrations. We show here that even ppb concentrations are possible to study now at the Rossendorf Beamline (BM20) of the European Synchrotron (France).16 In the present study we focus on the chemical state and stability of intercalated Fe atoms provided by XAS data on the Fe K-edge.
Synthesized according to previous studies13,17 GNFs with intercalated Fe were heated to 80 °C with nitric acid to study transformations of iron under oxidation treatment. For XAS investigation of the Fe local environment, 4 samples corresponding to different reaction timesteps were sealed in special holders and measured: pristine (initial material before oxidation), 15 min, 1 h and 4 h. Details of XAS data collection and treatment are given in the Electronic Supporting Information (ESI).†
The XANES (X-ray Absorption Near Edge Structure) regions of XAS spectra recorded at the Fe K-edge of Fe-incorporated GNFs at different stages of the reaction with nitric acid are shown in Fig. 1a.
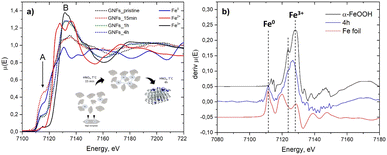 | ||
| Fig. 1 (a) XANES spectra of the GNF samples recorded at the Fe K-edge with reference spectra of Fe foil (Fe0), FeCO3 (Fe2+),20 and FeOOH (Fe3+);21 (b) the 1st derivative μ(E) of the GNFs_4 h oxidized sample, compared to the goethite (α-FeOOH)21 and Fe foil references. | ||
The position of the absorption edge in spectra of untreated GNFs (marked as “GNFs_pristine”) and GNFs after oxidation with nitric acid for 15 minutes (marked as “GNFs_15 min”) indicates that during the first 15 min of the reaction iron remain in the unoxidized Fe0 state. The intensity of pre-edge feature A is higher in the 15 min sample compared with others. The pre-edge structure of the Fe K-edge XANES is due to the quadrupole allowed 1s–3d (or a mixture of the dipole allowed and quadrupole allowed transitions).18,19 The pre-edge intensity increases when the distortion of the metal's surroundings occurs. The main XANES transitions (feature B) at the Fe K-edge of 3d metals correspond to the 1s to 4p transitions. The position of the absorption edge coincides in the GNFs_1 h and 4 h samples, and the GNFs_4 h sample represents mainly Fe3+ along with a fraction of Fe0, which is justified by the presence of a pre-edge maximum similar to the Fe0 reference on the derivative plot (Fig. 1b). Hence the major amount of Fe oxidizes in the 15 min to 1 hour interval of heating with nitric acid. The XANES spectral shapes of samples exposed for 1 h and 4 h to nitric acid are very similar, which indicates that significant changes in the Fe oxidation state and local environment do not occur during the corresponding timestep. However, as could be seen from the derivative plot (Fig. 1b), a part of Fe atoms remains as Fe0 even after 4 h of oxidation treatment.
According to the XANES spectra analysis, several significant stages of the oxidation process can be distinguished: (i) strong disordering of the initial structure during the first 15 minutes of oxidation, Fe atoms remain unoxidized; (2) partial oxidation to Fe3+ in the interval of 15 min to 1 h accompanied by a reverse ordering of the new structure; (iii) the final stage, reached after 1 h of oxidation. At the final step iron is mostly oxidized to Fe3+, but partially preserved in graphene nanoflakes as Fe0. It is important to note that Fe is not fully oxidized even after 4 h of the reaction with nitric acid, which is stressed in Fig. 1b: features attributed to Fe foil are present in the deriv. μ(E) spectrum of the 4 h oxidized sample.
A more precise investigation of Fe speciation in GNFs at different stages of the oxidation by nitric acid can be done by analysis of the oscillated structure of XAS spectra that corresponds to EXAFS spectroscopy. An important question is whether incorporated iron is present in the form of single-atoms, clusters or metallic particles. Based on the EXAFS spectra, it is possible to establish the local atomic environment of Fe in the samples – type of atoms in the nearest coordination spheres, interatomic distances and their coordination numbers. From this information the predominant type of Fe coordination in the carbon material at different stages of the reaction could be better understood. Up to now it has been impossible to obtain such information on ultra-low ppb concentrations of elements in complex materials. In Fig. S1† Fe K-edge k2-weighted EXAFS spectra and their Fourier transforms (FTs) along with fitting curves are shown. Spectra were recorded on GNFs with incorporation of Fe at the ppb level at different timesteps of heating with nitric acid. EXAFS fitting results are listed in Table S1.†
FT of the spectrum recorded on untreated GNFs (marked as GNFs_pristine) shows a single peak corresponding to split coordination sphere of C atoms at distances of 2.02 Å and 2.39 Å. Coordination numbers obtained through EXAFS analysis are somewhat arbitrary due to the strong correlation of CN with the Debye–Waller factor and may qualitatively indicate the presence of only graphene nanoplanes in the local surroundings of the Fe atom. There is no significant contribution of any coordination spheres at distances of 3–5 Å. Specifically, note the absence of the neighboring Fe coordination shell in the untreated sample indicating that initially Fe0 is represented by single atoms only. An attempt to add Fe–Fe scattering paths, assuming clustering, has resulted in low-quality fit and physically unrealistic parameters. The same conclusion is evident from the analysis of wavelet transforms of the GNFs_pristine spectrum and reference spectrum of Fe foil (Fig. S2†). The absence of peaks at ∼5.2 and ∼8.2 Å−1 on the k-axis (horizontal) and at 3.8–5 Å on the R axis (vertical) in GNFs_pristine compared to Fe foil points to the formation of single-atom Fe intercalates.
Therefore, EXAFS fitting results indicate the presence of Fe single atoms intercalated between graphene layers in the untreated material. The same result was previously obtained for multi-walled carbon nanotubes during their study by Mössbauer spectroscopy.8,9 A schematic representation of possible Fe local surroundings in the GNFs_pristine sample according to the obtained results is shown in Scheme 1.
As was established based on the position of the absorption edge in the XANES spectra, during the first 15 minutes of the reaction, oxidation of Fe atoms does not occur. Only the disordering of the local structure takes place due to the oxidation of the edges of carbon sheets that is described well using other analytical techniques.22 The first stage of the reaction is illustrated in Fig. S3,† showing a comparison of the Fourier transforms of the EXAFS spectra of GNFs_pristine and GNFs_15 min samples. In the GNFs_15 min sample, the first peak broadened and shifted to higher R compared to the GNFs_ pristine, and a more pronounced contribution of coordination spheres at 3–5 Å is observed. According to the EXAFS fitting results, the distance to carbon shells increases by c.a. 0.4 Å in GNFs_15 min. At the same time, Fe coordination shell appears at the distance of 4.29 Å with CN 5.1, which indicates the compression of Fe in the GNF structure at the first stage: atoms came closer to each other in the GNF structure possibly due to the oxidation and destruction of graphene sheets. However, according to XANES, Fe is still predominantly present as Fe0. This fact is additionally confirmed by the absence of Fe–O contribution at distances around 2 Å in the EXAFS region as is common for Fe3+/Fe2+ oxides. The coordination numbers obtained during fitting of the GNFs_15 min spectrum, 8.4 and 14.3, seem to be overestimated. Taking into account the high uncertainty of CN (±25–30%) obtained from EXAFS and strong correlation of the CN and Debye–Waller factor, these values could not be analyzed quantitatively and only indicate that local coordination of Fe includes only Fe–C/O paths (not Fe–Fe), and the interatomic distance (>2.45 Å) is not typical for Fe2+/3+ oxides or ions coordinated to carboxylic groups.
The quality of the EXAFS spectrum of the GNFs_1 h sample does not allow the fitting procedure to be accurately performed. However, from the shape and position of the absorption edge in the XANES spectrum, it can be concluded that after 1 hour Fe is already mostly oxidized to Fe3+ and the local surroundings are very similar to those of the GNFs_4 h sample. Therefore, the local environment of Fe at the final stage of the reaction is formed after 1 hour and subsequently changes slightly.
At the initial (GNFs_ pristine) and intermediate (GNFs_15 min) states of the reaction, Fe0 single-atoms were clearly observed, and at the final stage they are still present along with Fe3+ coordinated by the oxygen-containing functional groups of graphene oxide (Fig. 1b). A possible explanation of the enhanced stability of single-atom Fe0 is the highly hindered diffusion of nitric acid to Fe atoms through graphene planes. Thus, the nano-scale structure of the carbon material protects the intercalated metal from oxidation. After 4 h of oxidation treatment nitric acid reaches the majority of Fe atoms. In the GNFs_4 h sample, the nearest coordination sphere of O atoms appears at the distance of 1.95 Å with fixed CN = 6, which is typical for Fe3+ interaction with O-donating ligands. The CN for this coordination sphere was fixed at 6, assuming the octahedral coordination of Fe3+. This assumption is based on two points. Firstly, it is supported by the similarity of the shape of the absorption edge in the GNFs_4 h sample and goethite (Fig. S4†). Secondly, it is known that the position, shape and intensity of the pre-edge feature in the Fe K-edge is sensitive to the oxidation state and coordination environment. Fitting it with a combination of Gaussian and Lorentzian functions (PsdVoigt 2) gave the best fit by two components with centroids at 7113.3 and 7116.5 eV (Fig. S5†), which is in agreement with reported data for model compounds of Fe3+ in octahedral coordination.23 The coordination spheres of C/O atoms at the distances of 2.45 and 2.90 Å may reflect the contribution of graphene layers, O-containing functional groups of oxidized graphene edges, NO3− ions of nitric acid, etc. More detailed assignment of C/O coordination spheres at 2–4 Å would be speculative due to a high disorder of the sample and poor quality of the data. However, especially important is the presence of Fe contribution at 4.32 Å that appeared during oxidation as a result of compaction and partial destruction of graphene layers. It is also interesting to notice that the distance to the Fe coordination sphere and CN (with the same fixed Debye–Waller factor) are similar, which points to the preservation of Fe-GNF agglomerates during oxidation.
Conclusions
During the synthesis of few-layer graphene fragments, a very small amount of iron impurities from the MgO template intercalates into the structure in the atomic state and stabilizes between the layers. During the interaction with nitric acid, Fe in the structure of GNFs also undergoes oxidation together with C atoms at the edges. However, after 4 hours of the reaction intercalated Fe is not completely oxidized, some of the Fe atoms remain in a stabilized single-atom Fe0 form due to the steric inaccessibility of iron atoms surrounded by graphene planes.At the initial stage of the reaction, the distance between graphene planes increases due to the repulsion of ionogenic groups. At the same time, the arrangement of Fe atoms in the structure compresses which is proven by the appearance of a closed Fe-neighbouring coordination shell at ∼4.3 Å in GNFs_15 min and GNFs_4 h samples. The oxidation state of Fe remains unchanged at the first stage of the reaction and only distortion of the local structure occurs during the initial interval of 0 to 15 min. Oxidation of intercalated Fe atoms begins only after prolonged treatment with nitric acid followed by the substantial restructuring of the GNFs. At the end of the reaction, according to spectral data, Fe3+ is predominantly coordinated to oxygen-containing functional groups at the edges and defects of graphene planes, while a small fraction of Fe remains stabilized in Fe0 form inside of graphene nanoaggregates. The obtained results reveal that the system of graphene layers in the GNF structure possesses an extremely high affinity to iron single-atoms and enables their «extraction» from the template with further encapsulation in the material. Moreover, the single-atom Fe form is highly stable in the few-layered graphene structure and cannot be fully oxidized even by long acid treatment that causes the partial decay of the GNF structure. These effects can reveal the features of graphene and carbon nanotube behaviour when they are applied as components of modern devices.
This study demonstrates new opportunities that have opened up thanks to the development of new 4th generation synchrotron light sources, one of which is ESRF-EBS, and implementation of advanced electronics and detectors at the X-ray absorption beamline. Now it has become possible to investigate systems with ultra-low ppb metal concentrations, which will inevitably boost the research in fields of catalysis and electronics.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
XAS data collection and analysis were carried out under financial support of the Russian Science Foundation grant no. 23-73-30006, sample preparation was supported by the Federal Assignment of MSU “Physical chemistry of surface, adsorption and catalysis” (No. АААА-А21-121011990019-4). We acknowledge HZDR for granting a beamtime under proposal A20-1-836. We thank Dr D. Prieur and PhD E. Bazarkina for their help in data collection. The authors are especially grateful to Dr K. Kvashnina for the help with XAS data collection and valuable discussions.Notes and references
- A. Hirsch, The era of carbon allotropes, Nat. Mater., 2010, 9(11), 868–871 CrossRef CAS PubMed.
- I. Píš, S. Nappini and F. Bondino, et al., Fe intercalation under graphene and hexagonal boron nitride in-plane heterostructure on Pt(111), Carbon, 2018, 134, 274–282 CrossRef.
- M. Cattelan, G. W. Peng and E. Cavaliere, et al., The nature of the Fe-graphene interface at the nanometer level, Nanoscale, 2015, 7(6), 2450–2460 RSC.
- R. Arrigo, T. Sasaki, J. Callison, D. Gianolio and M. E. Schuster, Monitoring dynamics of defects and single Fe atoms in N-functionalized few-layer graphene by in situ temperature programmed scanning transmission electron microscopy, J. Energy Chem., 2021, 64, 520–530 CrossRef.
- V. Sessi, S. Stepanow and A. N. Rudenko, et al., Single 3d transition metal atoms on multi-layer graphene systems: Electronic configurations, bonding mechanisms and role of the substrate, New J. Phys., 2014, 16, 062001 CrossRef CAS.
- K. S. Novoselov, et al., Electric Field Effect in Atomically Thin Carbon Films, Science, 2004, 306(5696), 666–669 CrossRef CAS PubMed.
- F. Chen, J. Yang, T. Bai, B. Long and X. Zhou, Facile synthesis of few-layer graphene from biomass waste and its application in lithium ion batteries, J. Electroanal. Chem., 2016, 768, 18–26 CrossRef CAS.
- A. V. Sobolev, S. V. Savilov, N. B. Cherkasov, I. A. Presniakov and A. S. Ivanov, Magnetic iron-containing nanoparticles on the surface of multiwalled carbon nanotubes, Hyperfine Interact., 2012, 207(1–3), 29–32 CrossRef CAS.
- A. V. Sobolev, N. B. Cherkasov, I. A. Presniakov and S. V. Savilov, Iron-containing microphases in multiwalled carbon nanotubes, Hyperfine Interact., 2012, 207(1–3), 25–28 CrossRef CAS.
- J. Zhou, P. N. Duchesne and Y. Hu, et al., Fe-N bonding in a carbon nanotube-graphene complex for oxygen reduction: An XAS study, Phys. Chem. Chem. Phys., 2014, 16(30), 15787–15791 RSC.
- Y. Cheng, S. He and S. Lu, et al., Iron Single Atoms on Graphene as Nonprecious Metal Catalysts for High-Temperature Polymer Electrolyte Membrane Fuel Cells, Advanced Science, 2019, 6, 1802066 CrossRef PubMed.
- D. Deng, X. Chen and L. Yu, et al., Chemistry: A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature, Sci. Adv., 2015, 1, e1500462 CrossRef PubMed.
- S. A. Chernyak, A. M. Podgornova and E. A. Arkhipova, et al., Jellyfish-like few-layer graphene nanoflakes: Synthesis, oxidation, and hydrothermal N-doping, Appl. Surf. Sci., 2018, 439, 371–373 CrossRef CAS.
- J. Li, C. Wu and C. Yuan, et al., Single-Atom Iron Anchored on 2-D Graphene Carbon to Realize Bridge-Adsorption of O-O as Biomimetic Enzyme for Remarkably Sensitive Electrochemical Detection of H2O2, Anal. Chem., 2022, 94(41), 14109–14117 CrossRef CAS PubMed.
- Z. Chen, Y. Ye and T. Peng, et al., Iron-Single Sites Confined by Graphene Lattice for Ammonia Synthesis under Mild Conditions, ACS Catal., 2023, 13, 14385–14394 CrossRef CAS.
- A. C. Scheinost, J. Claussner and J. Exner, et al., ROBL-II at ESRF: A synchrotron toolbox for actinide research, J. Synchrotron Radiat., 2021, 28, 333–349 CrossRef CAS PubMed.
- S. A. Chernyak, A. S. Ivanov and A. M. Podgornova, et al., Kinetics of the defunctionalization of oxidized few-layer graphene nanoflakes, Phys. Chem. Chem. Phys., 2018, 20(37), 24117–24122 RSC.
- G. A. Waychunas, M. J. Apted and G. E. Brown, X-ray K-edge absorption spectra of Fe minerals and model compounds: Near-edge structure, Phys. Chem. Miner., 1983, 10(1), 1–9 CrossRef CAS.
- G. S. Henderson, F. M. F. De Groot and B. J. A. Moulton, X-ray Absorption Near-Edge Structure (XANES) Spectroscopy, Rev. Mineral. Geochem., 2014, 78, 75–138 CrossRef CAS.
- D. Testemale and C. Sanchez-Valle, Fe K Edge XAS Transmission of Natural Siderite FeCO3 at Ambient Conditions, SSHADE/FAME (OSUG Data Center). Dataset/Spectral Data, Published online 2016 Search PubMed.
- D. Testemale and C. Sanchez-Valle, Fe K Edge XAS Transmission of Natural Goethite FeO(OH) at Ambient Conditions, SSHADE/FAME (OSUG Data Center). Dataset/Spectral Data, Published online 2016 Search PubMed.
- E. A. Arkhipova, A. S. Ivanov, K. I. Maslakov, A. V. Egorov, S. V. Savilov and V. V. Lunin, Mesoporous graphene nanoflakes for high performance supercapacitors with ionic liquid electrolyte, Microporous Mesoporous Mater., 2020, 294, 109851 CrossRef CAS.
- M. Wilke, F. Farges, P. E. Petit, G. E. Brown and F. Martin, Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study, Am. Mineral., 2001, 86(5–6), 714–730 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ja00357d |
| This journal is © The Royal Society of Chemistry 2024 |