Open Access Article
Open Access ArticleSingle particle ICP-MS: a tool for the characterization of gold nanoparticles in nanotheranostics applications†
Meritxell
Cabré
 ab,
Gabriel
Fernández
ab,
Gabriel
Fernández
 a,
Esther
González
a,
Esther
González
 a,
Jordi
Abellà
a,
Jordi
Abellà
 a and
Ariadna
Verdaguer
a and
Ariadna
Verdaguer
 *a
*a
aAnalytical and Applied Chemistry Department, IQS School of Engineering, Ramon Llull University, Via Augusta 390, 08017 Barcelona, Spain. E-mail: ariadna.verdaguer@iqs.url.edu
bPerkinElmer Scientific Spain, S.L., Ronda de Poniente 19, 28760 Tres Cantos, Spain
First published on 13th August 2024
Abstract
Nanotheranostics aims to perform a premature and non-invasive diagnosis combined with therapy focused on the specific place where the disease is by using nanomaterials. To evaluate the ability to penetrate and retain the inorganic nanoparticles (NPs) in the cells, analytical techniques such as Single-Particle ICP-MS (SP-ICP-MS) are required to characterize these NPs. SP-ICP-MS provides not only the size distribution and concentration of NPs but also the concentration of the dissolved elements. In recent years, direct alkaline dilution of blood, serum, and urine is performed in clinical laboratories for routine analysis. This alkaline diluent is named clinical diluent and it is a mixture of ammonia, EDTA, 2-propanol, Triton X100, and purified water. In this work, a methodology to characterize AuNPs in blood and urine samples using SP-ICP-MS has been developed. Samples were directly diluted with clinical diluent before multi-quadrupole ICP-MS analysis. The effect of this clinical diluent on the behaviour and stability of AuNPs has been studied. Good stability of AuNPs was observed for both the particle size and particle concentration (<17% difference in 10 days). Moreover, analytical parameters of this method such as linearity, detection limit, accuracy, and precision in blood and urine samples were studied for both the particle size and particle concentration. Linearity was evaluated for particle size (from 10 to 100 nm) and particle concentration (from 5 × 103 to 1 × 104 NP per mL). Furthermore, recoveries between 88% and 103% for the NP concentration and between 100% and 110% for the nanoparticle size were obtained. Dissolved and gold nanoparticle detection limits have also been estimated.
1. Introduction
The term nanomedicine emerged in the 1990s.1 This branch of science aims to harness advances in nanotechnology to address complex challenges in disease detection, drug delivery, and therapeutic efficacy. To achieve these goals, nanomaterials such as liposomes or nanoparticles have been used. These materials have physicochemical properties that allow them to interact with biological systems at the cellular and molecular levels. Nanotheranostics is a specialised branch of nanomedicine that focuses on the development of nanoplatforms that integrate therapy and diagnosis into a single system. There are different nanoplatforms such as micelles, carbon nanotubes, liposomes, dendrimers, and inorganic nanoparticles, among others.2Particularly, inorganic nanoparticles (NPs) have unique mechanical, optical, magnetic, electric, and structural properties due to their size (from 1 to 100 nm) and surface/volume ratio.3–5 Furthermore, at the nanoscale, the particles are more bioactive and more chemically active, allowing easy entry into the cells and organs. Therefore, NPs hold great potential for use in nanotheranostics as they allow real-time monitoring of how the drug gets delivered and distributed.
Nanotheranostics studies are performed using different nanoparticle compositions and size distribution depending on the target disease. Some of the most commonly used nanoparticles are gold, silver, titanium IV oxide, zinc II oxide, cerium IV oxide, and iron oxides.6 Due to their optical properties, gold nanoparticles (AuNPs) are used as contrast agents in diagnostics. In addition, their plasmonic properties allow AuNPs to be used as diagnostic and therapeutic agents. Furthermore, their photothermal properties can be applied in therapeutic applications and their biocompatibility as drug delivery systems.2–9 Thus, gold nanoparticles can be widely used in nanotheranostics applications. Since these NPs will be introduced into the body, it is important to have analytical techniques for the characterization of these nanoparticles and their interaction with cells. The characterization of these NPs will provide information on their bioavailability, their transport in the body, their excretion, and whether these NPs transform when inside the organism.
Currently, these nanoparticles can be characterized by several techniques. These include Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), Dynamic Light Scattering (DLS), Nanoparticle Tracking Analysis (NTA), Single Particle ICP-MS (SP-ICP-MS) and Asymmetric Flow Field-Flow Fractionation coupled to ICP-MS (AF4-ICP-MS), among others.6,10–15 Each of these techniques provides relevant information about the nanoparticles, but each has its own limitations. For example, TEM and SEM supply details about the structure, shape, size, and composition of the nanoparticles. However, preparing the sample is time-consuming and only allows a small portion of the sample to be examined. They also do not give information about the concentration of NPs. In contrast, DLS and NTA offer information about nanoparticle hydrodynamic diameter and concentration but also have some limitations such as lack of selectivity, low sensitivity, and polydispersity. Thus, more techniques are needed to obtain information regarding both the nanoparticle size and concentration.
In 2003, Degueldre et al. first described the basic principles of SP-ICP-MS.16 Since then the ICP-MS equipment has undergone improvements. Today's detectors analyse faster, allowing dwell time values to be reduced to 10 μs. Furthermore, this new generation of instruments offers increased sensitivity, facilitating the characterization of smaller nanoparticles. With its high selectivity and specificity, this technique holds great promise for nanoparticle characterization. Compared to other techniques, SP-ICP-MS provides not only the size distribution and the concentration of NPs but also the concentration of the dissolved element in the suspension.13–15,17–24 This technique can therefore reveal relevant information on inorganic nanoparticle behaviour, which will help to understand how NPs are transported within the body and how they are excreted. However, there are only a few studies where this technique has been used to obtain such information.6,12,25,26
The metal content of biological fluids is routinely measured in clinical analytical laboratories. Due to the complex nature of the matrix, the clinical diluent is widely used. This is an alkaline solution that offers several advantages in the analysis of biological samples such as blood and urine. Firstly, the clinical diluent facilitates erythrocyte lysis, a crucial step in the sample preparation that ensures efficient extraction of target analytes. In addition, alkaline solutions prevent precipitation, reducing the risk of nebuliser clogging during analysis. They also help to mitigate memory effects and minimise spectral and matrix interferences, enhancing the accuracy and reliability of subsequent measurements. In summary, the use of alkaline solutions simplifies sample preparation, improves analytical performance and increases the robustness of analytical methods.25
The aim of this work is to provide new methodologies to characterize nanoparticles in the blood and urine of living organisms involved in nanotheranostics applications by SP-ICP-MS. To achieve this, we propose to investigate the feasibility of sample preparation by direct dilution of blood and urine using clinical diluent. All experiments were performed in accordance with the Guidelines of Spanish Biomedical Research Law 14/2007, and experiments were approved by the ethics committee at Ramon Llull University (approved ethical protocols CER_URL_2019_2020_016 and 200039). Samples were obtained from healthy volunteers who gave their informed consent.
2. Materials and methods
2.1. Standards and reagents
Clinical diluent was prepared with 0.5 g of EDTA, 10 mL isopropyl alcohol, 10 mL Ammonia solution, 0.5 g Triton-X100 and filled up to 1 L Milli-Q water. This clinical diluent was used to dilute all standard solutions. Ethylenediaminetetraacetic acid (EDTA for analysis, ref. 131026.1210) was supplied by Panreac. Isopropyl alcohol (HPLC grade, ref. P/7507/17) was purchased from Fisher Scientific. Ammonia solution (25%, ref. 1.05428) was supplied by Merck. Triton-X100 was supplied by Sigma Aldrich.Gold standard solution (1000 mg L−1, ref. N9303759) was supplied by PerkinElmer. Gold nanoparticle standard solutions with nominal sizes of 10 nm (9.1 ± 0.8), 20 nm (20.8 ± 1), 30 nm (27.1 ± 2.2), 50 nm (49.6 ± 4.2) and 100 nm (99.4 ± 6) were purchased from NanoComposix, Inc (San Diego, USA).
2.2. Instrumentation
A PerkinElmer NexION® 5000 (PerkinElmer, Waltham, USA) was used for this study. This ICP-MS is equipped with four quadrupoles to guide the ions generated in the plasma and to suppress interferences. The sample introduction system consists of a PFA nebulizer and a quartz cyclonic spray chamber. The sample flow rate was determined daily as the difference in weight after 4 minutes of uptake. A 50 nm gold nanoparticle suspension (104 NPs per mL) was prepared in clinical diluent to determine the transport efficiency (TE%). The mean of two measurements was used for sample analysis.Homogenisation of the nanoparticle suspension prior to dilution was performed in an ultrasonic bath (J. P. Selecta, S.A.).
2.3. Sample preparation
3. Results and discussion
3.1. Instrumental method optimization
The use of single quad (Q3 only: 197 m/z) and tandem quad (MS/MS: 197/197 m/z) was evaluated for gold nanoparticle characterization. A 2 μg L−1 dissolved gold solution and 50 nm gold standard nanoparticle solutions prepared in clinical diluent were analysed under both conditions (ESI Table S1†). For both solutions, higher mean intensities were obtained in Q3 only mode compared to MS/MS (266 and 59 mean AuNP counts, respectively). Nevertheless, no differences in nanoparticle size and concentration were observed. Thus, MS/MS is sensitive enough to characterize gold nanoparticles with a nominal size of 50 nm.Once the acquisition mode is optimized the dwell time was evaluated as described in other studies.18,27 In this case it was carried out with a suspension of gold nanoparticles of 50 nm. The dwell time values studied were: 20, 50, 100, 500 and 1000 μs obtaining transient signals (ESI Fig. S1†). As indicated in the literature, longer dwell times result in fewer events defining the peak. Therefore, a dwell time of 50 μs was chosen. This ensures a well-defined peak, minimising double nanoparticle events and providing sufficient sensitivity for this study. The optimised conditions for the characterization of gold nanoparticles are summarised in Table 1.
| Sample flow rate (mL min−1) | 0.40 ± 0.03 |
| Transport efficiency (%) | 8.4 ± 1.0 |
| Spray chamber | Quartz – cyclonic |
| Nebulizer | PFA |
| Cones | Pt |
| Plasma gas (L min−1) | 16 |
| Nebulizer gas (L min−1) | 0.99 |
| Auxiliary gas (L min−1) | 1.2 |
| Isotope | 197Au |
| Dwell time (μs) | 50 |
| Acquisition time (s) | 60 |
| Replicates per sample | 3 |
| Scan mode | MS/MS |
| Gas profile | Standard |
3.2. Analytical parameters evaluation
The analytical parameters including linearity and detection limit for dissolved gold, and both nanoparticle size and concentration were evaluated.Good linearity was observed for the dissolved gold within the concentration range of 1 to 5 μg L−1, with a coefficient of determination higher than 0.995. Furthermore, a detection limit of 0.012 μg L−1 was determined, calculated as the 3-fold standard deviation of 10 blank measurements.
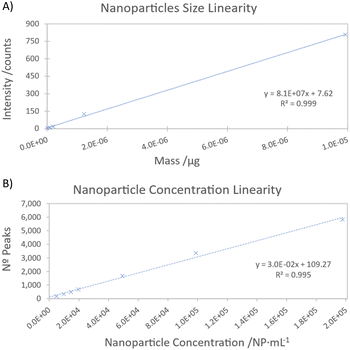
To assess the linearity of the nanoparticle size, suspensions of different nanoparticle sizes (10, 20, 30, 50 and 100 nm) were analysed as samples. A coefficient of determination greater than 0.999 was obtained (Fig. 1A). To further confirm this linearity, a residual error study was conducted. Residual errors were found to be below 7% for standards between 20 and 100 nm, while the 10 nm standard exhibited a residual error of 56%. This higher error can be attributed to the proximity to the detection limit. Under these instrumental conditions, the Syngistix™ Nano Application software module provides a LODsize value of 13 nm by using a 3-sigma approach. This LODsize is consistent with the values reported in the literature, typically ranging from 10 to 20 nm.6,18,22,28
In this study, we focused on AuNPs ranging from 10–100 nm, sizes commonly used in various medical applications. The ICP-MS equipment enables precise characterization of these nanoparticle sizes. This capability allows for the detection of nanoparticles, such as those preferred for cancer diagnostics due to their enhanced tumor penetration capabilities and utilization of the enhanced permeation and retention (EPR) effect.29 Furthermore, the versatility of nanoparticle sizes enables strategic targeting in cancer therapy, where a combination of sizes can address both peripheral accumulation and deep tissue penetration within tumors. Thus, our developed method holds promise for adapting to a wider range of nanoparticle sizes, enhancing its clinical relevance.
Numerous dilutions were performed to establish the linearity range for the concentration of gold nanoparticles. A satisfactory linearity was observed within the range of 5000 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 NP per mL (Fig. 1B). The limit of detection for nanoparticle concentration was determined according to the guidelines of ISO 19590:2017, resulting in a LODNP of 147 NP per mL. This result is in agreement with the results reported by Zhang et al.,28 who also documented an LODNP of 135 NP per mL, which indicates the consistency of the results obtained.
000 NP per mL (Fig. 1B). The limit of detection for nanoparticle concentration was determined according to the guidelines of ISO 19590:2017, resulting in a LODNP of 147 NP per mL. This result is in agreement with the results reported by Zhang et al.,28 who also documented an LODNP of 135 NP per mL, which indicates the consistency of the results obtained.
3.3. AuNP stability in clinical diluent
Three stability tests were performed at different concentrations (104, 5 × 104 and 105 NP per mL) to investigate the behaviour of AuNPs when exposed to the clinical diluent. Freshly prepared samples (t0h) and samples stored for 6 hours (t6h), 1 (t1d), 2 (t2d), 6 (t6d) and 10 days (t10d) were analysed. The results for AuNP size and concentration are shown in Fig. 2. These results were compared with those obtained at t0h to assess the variations in the nanoparticle size and concentration over the specified period.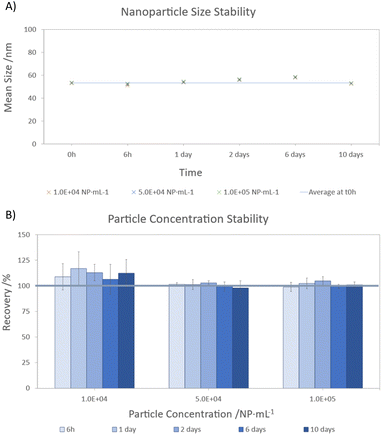 | ||
| Fig. 2 Gold nanoparticle stability in clinical diluent. (A) Nanoparticles size (mean ± s); (B) nanoparticles concentration (mean ± s). | ||
During the stability test, it was observed that the nanoparticle size showed consistent behaviour across all three concentration levels (Fig. 2A). However, slight variations in size were observed during storage, with deviations less than 10%. The largest difference from the initial measurement (<10%) was observed after 6 days of storage. Interestingly, despite this initial increase, the difference decreased to 1% after 10 days. This suggests that the observed variation may be due to sample introduction or insufficient stirring rather than environmental factors or interactions with the clinical diluent. For the gold nanoparticle concentration (Fig. 2B), recoveries between 95–117% were achieved. In particular, the most diluted suspensions (104 NP per mL) showed higher recoveries (106–117%) over time, whereas minimal differences were observed for higher AuNPs concentrations (below 5%). Despite these differences, no apparent trend of increasing or decreasing AuNPs concentration was observed over the storage period.
Therefore, these results suggest that dilution with clinical diluent and storage at 5 °C for 10 days does not adversely affect the size or concentration of these specific gold nanoparticles. Instead, the differences observed are likely to be inherent to the analytical methodology (sample preparation and instrumental technique). This highlights the feasibility of storing these nanoparticles under these conditions for subsequent analysis within this timeframe.
3.4. Method suitability for AuNP characterization in blood and urine
The suitability of the developed methodology for gold nanoparticles characterization in blood and urine using SP-ICP-MS was evaluated. As described in Section 2.3, gold nanoparticle standards (50 nm) were added to blood and urine at three different concentrations as a model analyte.Sizes between 53.5 and 54.0 nm were obtained when characterizing the size of gold nanoparticles in the blood (Table 2). Compared to the certified values (49.6 ± 4.2 nm), a maximum difference of 8.9% was observed. A t-student test was performed, and no significant difference was observed (p > 0.05). However, a slight increase in the signal of the gold nanoparticles in blood cannot be ruled out, which may lead to a bias towards larger sizes. Despite a lower concentration of gold nanoparticles detected in blood compared to the added concentration, recoveries between 88–93% were achieved (t-student test: p > 0.05 for all cases), indicating a uniform recovery efficiency independent of the nanoparticle concentration.
| Sample ID | Nanoparticles concentration | Nanoparticles size | ||||
|---|---|---|---|---|---|---|
| AuNP addition (NP per mL) | Nanoparticles conc. (NP per mL) | % Recovery (NP conc.) | Mean size ± 2 s (nm) | Size certified (nm) | % Error (mean size) | |
| Blood addition 1 | 9890 | 8947 | 91 | 53.5 ± 0.5 | 49.6 ± 4.2 | 7.9 |
| Blood addition 2 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 124 |
93 | 53.9 ± 0.4 | 49.6 ± 4.2 | 8.6 |
| Blood addition 3 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 141 141 |
88 | 54.0 ± 0.2 | 49.6 ± 4.2 | 8.9 |
Similarly, the same procedure was followed to assess the suitability of the method for gold nanoparticle characterization in urine (Table 3). In this case, the nanoparticle size values ranged from 49.2 to 50.0 nm, practically matching the certified values (49.6 ± 4.2 nm). Good recoveries were obtained for the gold nanoparticle concentration (between 90 and 95%), suggesting no significant matrix effects (t-student test: p > 0.05 in all cases). To summarize, the results demonstrate the reliability and consistency of the characterization methodology used in this study, highlighting its suitability for characterizing gold nanoparticles in both blood and urine matrices.
| Sample ID | Nanoparticles concentration | Nanoparticles size | ||||
|---|---|---|---|---|---|---|
| AuNP addition (NP per mL) | Nanoparticles conc. (NP per mL) | % Recovery (NP conc.) | Mean size ± 2 s (nm) | Size certified (nm) | % Error (mean size) | |
| Urine addition 1 | 9890 | 9441 | 96 | 49.2 ± 0.3 | 49.6 ± 4.2 | −0.7 |
| Urine addition 2 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411 |
102 | 49.6 ± 0.2 | 49.6 ± 4.2 | 0.1 |
| Urine addition 3 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 359 359 |
103 | 50.0 ± 0.3 | 49.6 ± 4.2 | 0.9 |
4. Conclusions
This study evaluated the suitability of the clinical diluent for the characterization of gold nanoparticles in blood and urine using SP-ICP-MS. Several analytical parameters were investigated, including linearity, where coefficients of determination higher than 0.995 were obtained for both the dissolved gold and nanoparticles (size and concentration). In addition, detection limits were determined, resulting in values of 0.012 μg L−1 for dissolved gold, 13 nm for the nanoparticle size and 147 NP per mL for nanoparticle concentration.An assessment of whether the clinical diluent could affect the gold nanoparticles was also made. It was observed that after 10 days of storage at 5 °C, both AuNP size and concentration were unaffected, with differences of less than 10% and recoveries between 95–117%.
The behaviour of gold nanoparticles was also investigated when spiked into blood and urine and characterized by direct dilution with clinical diluent. Notably, differences of less than 9% were observed for nanoparticle size with respect to the certified value, along with recoveries of 88–103% for total nanoparticle concentration in both matrices.
Based on these results, it can be concluded that clinical diluent is a suitable solution for characterizing gold nanoparticles in blood and urine under these conditions. This underlines the usefulness of clinical diluent as a matrix diluent for analysing nanoparticles in biological samples.
List of abbreviations
| AF4-ICP-MS | Asymmetric Flow Field-Flow Fractionation coupled to Induction Coupled Plasma-Mass Spectrometry |
| AuNPs | Gold nanoparticles |
| DLS | Dynamic Light Scattering |
| EDTA | Ethylenediaminetetraacetic acid |
| ICP-MS | Inductively Coupled Plasma – Mass Spectrometry |
| LODNP | Detection limit for nanoparticle concentration |
| LODsize | Detection limit for nanoparticle size |
| NPs | Nanoparticles |
| NTA | Nanoparticle Tracking Analysis |
| SEM | Scanning Electron Microscopy |
| SP-ICP-MS | Single Particle-Inductively Coupled Plasma-Mass Spectrometry |
| TEM | Transmission Electron Microscopy |
Data availability
Data are available at https://dataverse.csuc.cat/dataverse/URL.Author contributions
Meritxell Cabré: investigation, methodology, validation, writing-original draft and writing–review and editing. Gabriel Fernández: investigation, methodology, validation, writing-original draft. Esther González: resources, visualization, and writing – review and editing. Jordi Abellà: conceptualization, funding acquisition, project administration, supervision, visualization, and writing – review and editing. Ariadna Verdaguer: conceptualization, funding acquisition, methodology, project administration, resources, supervision, visualization, and writing – review and editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to express their gratitude for the financial support received from the Industrial Doctorate's Plan of Research and Universities Department of the Generalitat de Catalunya, 2021_DI_109. PerkinElmer Scientific Spain is also acknowledged for its support in the development of the project.References
- L. Leon, E. J. Chung and C. Rinaldi, in Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications, Elsevier Inc., 2020, pp. 1–4 Search PubMed.
- C. Enrico, in Design of Nanostructures for Theranostics Applications, William Andrew Publishing, 2018, pp. 41–68 Search PubMed.
- X. Y. Wong, A. Sena-Torralba, R. Álvarez-Diduk, K. Muthoosamy and A. Merkoçi, ACS Nano, 2020, 14, 2585–2627 CrossRef.
- M. Nikzamir, A. Akbarzadeh and Y. Panahi, J. Drug Delivery Sci. Technol., 2023, 61, 102316 CrossRef.
- N. Sarfraz and I. Khan, Chem.–Asian J., 2021, 16, 720–742 CrossRef.
- S. Fernandez Trujillo, M. Jimenez Moreno, A. Ríos and R. D. C. Rodríguez Martín-Doimeadios, J. Anal. At. Spectrom., 2021, 36, 528–534 RSC.
- S. J. Soenen, W. J. Parak, J. Rejman and B. Manshian, Chem. Rev., 2015, 115, 2109–2135 CrossRef CAS.
- C. Soica, I. Pinzaru, C. Trandafirescu, F. Andrica, C. Danciu, M. Mioc, D. Coricovac, C. Sitaru and C. Dehelean, in Design of Nanostructures for Theranostics Applications, Elsevier, 2018, pp. 161–242 Search PubMed.
- M. G. Soliman, P. del Pino, W. J. Parak and B. Pelaz, inReference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2017 Search PubMed.
- O. A. Sadik, N. Du, V. Kariuki, V. Okello and V. Bushlyar, in ACS Sustainable Chemistry and Engineering, American Chemical Society, 2014, vol. 2, pp. 1707–1716 Search PubMed.
- F. Barbero, C. Mayall, D. Drobne, J. Saiz-Poseu, N. G. Bastús and V. Puntes, Sci. Total Environ., 2021, 768, 144792 CrossRef CAS.
- M. Logozzi, D. Mizzoni, B. Bocca, R. Di Raimo, F. Petrucci, S. Caimi, A. Alimonti, M. Falchi, F. Cappello, C. Campanella, C. C. Bavisotto, S. David, F. Bucchieri, D. F. Angelini, L. Battistini and S. Fais, Eur. J. Pharm. Biopharm., 2019, 137, 23–36 CrossRef CAS PubMed.
- R. S. Amais, G. L. Donati and M. A. Zezzi Arruda, Trends Anal. Chem., 2020, 133, 116094 CrossRef CAS.
- R. M. Galazzi, K. Chacón-Madrid, D. C. Freitas, L. F. da Costa and M. A. Z. Arruda, Rapid Commun. Mass Spectrom., 2020, 34, 1–14 CrossRef.
- W. Liu, H. Shi, K. Liu, X. Liu, E. Sahle-Demessie and C. Stephan, J. Agric. Food Chem., 2021, 69, 1115–1122 CrossRef CAS PubMed.
- C. Degueldre and P. Y. Favarger, in Colloids and Surfaces A: Physicochemical and Engineering Aspects, Elsevier, 2003, vol. 217, pp. 137–142 Search PubMed.
- O. V. Kuznetsova, G. M. D. M. Rubio, B. K. Keppler, J. M. Chin, M. R. Reithofer and A. R. Timerbaev, Anal. Biochem., 2020, 611, 114003 CrossRef CAS.
- J. Liu, K. E. Murphy, R. I. Maccuspie and M. R. Winchester, Anal. Chem., 2014, 86, 3405–3414 CrossRef CAS.
- H. E. Pace, N. J. Rogers, C. Jarolimek, V. A. Coleman, C. P. Higgins and J. F. Ranville, Anal. Chem., 2011, 83, 9361–9369 CrossRef CAS.
- C. Degueldre, P. Y. Favarger and S. Wold, Anal. Chim. Acta, 2006, 555, 263–268 CrossRef CAS.
- J. Campbell, S. Burkitt, N. Dong and C. Zavaleta, in Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications, Elsevier Inc., 2020, pp. 129–144 Search PubMed.
- F. Laborda, E. Bolea and J. Jiménez-Lamana, Anal. Chem., 2013, 86, 2270–2278 CrossRef PubMed.
- H. Goenaga-Infante and D. Bartczak, in Characterization of Nanoparticles: Measurement Processes for Nanoparticles, Elsevier, 2019, pp. 65–77 Search PubMed.
- E. Bolea-Fernandez, D. Leite, A. Rua-Ibarz, T. Liu, G. Woods, M. Aramendia, M. Resano and F. Vanhaecke, Anal. Chim. Acta, 2019, 1077, 95–106 CrossRef PubMed.
- Y. Lu, M. Kippler, F. Harari, M. Grandér, B. Palm, H. Nordqvist and M. Vahter, Clin. Biochem., 2015, 48, 140–147 CrossRef PubMed.
- F. Laborda, A. C. Gimenez-Ingalaturre, E. Bolea and J. R. Castillo, Spectrochim. Acta, Part B, 2020, 169, 105883 CrossRef.
- F. Laborda, J. Jiménez-Lamana, E. Bolea and J. R. Castillo, J. Anal. At. Spectrom., 2013, 28, 1220–1232 RSC.
- X. He, H. Zhang, H. Shi, W. Liu and E. Sahle-Demessie, J. Am. Soc. Mass Spectrom., 2020, 31, 2180–2190 CrossRef CAS.
- E. Casals, G. Casals, V. Puntes and J. M. Rosenholm, in Theranostic Bionanomaterials, Elsevier, 2019, pp. 3–26 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ja00141a |
| This journal is © The Royal Society of Chemistry 2024 |