Droplet manipulation on an adjustable closed-open digital microfluidic system utilizing asymmetric EWOD†
Jingsong
Xu
,
Xingcheng
Wang
 ,
Qingyuan
Huang
and
Xiaodong
He
,
Qingyuan
Huang
and
Xiaodong
He
 *
*
School of Information Science and Engineering, Lanzhou University, No. 222 Tianshui South Road, Lanzhou 730000, China. E-mail: xdhe@lzu.edu.cn
First published on 20th November 2023
Abstract
The closed-open digital microfluidic (DMF) system offers a versatile and powerful platform for various applications by combining the advantages of both closed and open structures. The current closed-open DMF system faces challenges in scaling up due to electrode structural differences between closed and open regions. Here we developed an adjustable closed-open DMF platform by utilizing the modified slippery liquid-infused porous surfaces (SLIPS) with asymmetric electrowetting on dielectric (AEWOD) as a hydrophobic dielectric layer. The consistent electrode structures of the bottom printed circuit board (PCB) electrode array on both the closed and open regions, and the utilization of a transparent acrylic with floating potential as the top plate allow a low-cost and easily scalable closed-open DMF system to be achieved. The impacts of applied voltage, parallel plate spacing, electrode switching interval, and electrode driving strategies on various droplet manipulations were investigated. The results show that the optimal plate spacings range from 340–510 μm within the closed region. Meanwhile, we also studied the influence of the thickness, geometry, and position of the top plate on the droplet movement at the closed-open boundary. Through force analysis and experimentation, it is found that a thin top plate and a bevel of ∼4° can effectively facilitate the movement of droplets at the boundary. Finally, we successfully achieved protein staining experiments on this platform and developed a customized smartphone application for the accurate detection of protein concentration. This innovative closed-open DMF system provides new possibilities for future applications in real-time biological sample processing and detection.
1. Introduction
Digital microfluidic (DMF) technology has attracted significant attention for its capability to conduct bioassays and chemical analyses using minimal samples and reagents.1–5 As a part of the broader microfluidic technology, DMF aims to develop miniaturized devices that can perform the key functions of an analytical laboratory. Within the DMF system, droplet manipulation occurs on a plate without the need for micropumps, microvalves, microtubes, or other complex mechanical configurations.6–8 This eliminates contamination risks caused by the production, assembly, and cross-use of intricate components, making it a promising technology for various applications, such as point-of-care diagnostics, chemical and biological manipulations, and detection.9,10A diverse range of methods have been used to precisely manipulate droplets in DMF systems, such as acoustic waves,11 optical actuation,12 and electrical actuation.13,14 One particularly attractive method is electrowetting on dielectric (EWOD),15–17 which offers simplicity, flexibility, parallel performance, and vertical addressing capability. Additionally, programmable droplet control enhances the automation level of the DMF system.18,19 EWOD-based DMF devices can have either an open structure with a single plate or a closed structure with double plates. The open structure is simple and easily accessible, allowing efficient mixing of droplets.20 Meanwhile, the spherical droplet shape facilitates more effective fluorescence focusing, thereby improving detection sensitivity for fluorescence-based applications.21 Additionally, the open structure allows for straightforward integration with various liquid handling and manipulation tools as well as surface analytical equipment.22,23 However, precise dispensing and splitting of droplets can be challenging in an open structure, limiting its applications. Furthermore, the droplet manipulation typically requires a configuration where both the ground and driving electrodes are located on a single bottom plate. The closed structure enables droplet dispensing, transporting, splitting, and mixing manipulations, and provides reliable volume control. However, it needs to remove the top plate for offline processing, such as purification,24 biological culture,25 or mass spectrometry evaluation26 in most EWOD biological experiments, which may lead to undesired volume changes or contamination. By integrating the advantages of both structures onto the same chip, droplets in microliters/nanoliters can be dispensed and controlled independently in the closed region for sample pretreatment and then driven to the open region for sample post-processing and detection. If necessary, the droplets can also be transported back to the closed region for further processes. Several research works have been reported closed-open DMF systems. Chang et al. designed a curved boundary structure of the top plate using hydrophobically modified polydimethylsiloxane (PDMS), enabling contactless manipulation of droplets from a closed region to an open region.27 This design reduces resistance, allowing more flexible integration of EWOD systems. Wang et al. studied water and oil droplet transport at the closed-open boundary.28 The beveled edge can facilitate droplet movement and detachment at the boundary of the closed-open structure, and an oleophobic surface on the top plate also allows free transportation of oil droplets at the boundary. However, in the current closed-open DMF system, fabricating electrode chips always needs high cost, lengthy production, and a complex photolithography process on indium tin oxide (ITO) glass.29 The wiring challenges for single-layer ITO glass result in significant difficulties in designing large-scale electrode arrays, thereby limiting high-throughput droplet manipulation. Furthermore, for the current closed-open DMF system relying on EWOD, it is necessary to ground the top plate. This requirement for a conductive layer restricts the choice of materials for the top plate. Moreover, the electrode structure design on both sides has significant differences, hindering the expansion of large-scale array electrodes and limiting the ability to adjust the open and closed regions.
To address the above problems, one approach involves creating a DMF system using mature multi-layer printed circuit board (PCB) technology, which has been widely used for large-scale DMF chip preparation due to its low-cost and multilayer wiring method.30 To avoid the differences in electrode structure in both regions, a potential solution is to use asymmetric electrowetting on dielectric (AEWOD) for droplet manipulation. AEWOD exhibits an asymmetric contact angle variation with respect to voltage polarity, and the θV curve (contact angle–applied voltage) is asymmetric along the V = 0 axis by sessile drop and coplanar electrode experiments.31–33 Therefore, the droplet can be driven directionally between any two adjacent coplanar electrodes on the bottom substrate in both open and closed regions. Meanwhile, the droplet is driven by the bottom electrodes, and the top plate of the closed structure would become a floating potential as it does not require grounding. Our previous works found that the modified slippery liquid-infused porous surfaces (SLIPS) yielded an opposite AEWOD phenomenon, namely the droplet tends to move from the positively biased single electrode to the negatively biased single electrode.34 Furthermore, the SLIPS film also shows the ability to overcome the obstacle of the gap between PCB electrodes.35,36 Therefore, the combination of modified SLIPS and PCB technologies shows a promising strategy for designing a large-scale, simplified wiring and low-cost closed-open DMF system.
In this paper, we proposed an adjustable closed-open DMF platform by utilizing the modified SLIPS with AEWOD. Using PCB electrodes as the bottom substrate and transparent acrylic with floating potential as the top plate, along with a digitally controllable boost circuit, enables us to achieve a vertical addressing, cost-effective, scalable, and high-integration digital microfluidic system that combines the advantages of both closed and open structures. The impacts of a series of physical parameters on droplet manipulation were investigated. Meanwhile, a user-friendly graphical user interface (GUI) is designed using Qt to enhance the accessibility of the DMF platform. Finally, protein staining experiments are conducted on this platform, and a customized smartphone application is also developed for accurate offline detection of stained protein concentrations. This method provides new possibilities for future applications of biological detection on DMF platforms and expands the potential for real-time detection scenarios.
2. Materials and methods
2.1. Materials and reagents
The electrode board and controller board of the DMF device were manufactured using standard FR-4 substrates, a common choice in the PCB industry. These substrates have specifications, including a copper thickness of 35 μm and a substrate thickness of 1.6 mm. The design of the PCB components was conducted using Altium Designer software, while the physical boards were produced by a PCB manufacturing company (Shenzhen Jialichuang Technology Development Co.). For the assembly process, electronic components were soldered and assembled within our laboratory. To prepare SLIPS, anhydrous ethanol, polytetrafluoroethylene, and 1H,1H,2H,2H-perfluorooctyltrichlorosilane (PFOTS) were purchased from Shanghai Aladdin Biochemical Technology Co, Ltd. The acrylic sheets are cut to dimensions using a laser cutter.Bovine serum albumin (BSA, Sigma-Aldrich (Shanghai) Trading Co, Ltd) was stained with Coomassie Brilliant Blue G-250 (CBB, Hefei Qiansheng Biotechnology Company, China) to determine its concentration. The CBB G-250 solution configuration requires a 95% alcohol solution (Beijing Innochem Science & Technology Co, Ltd), an 85% phosphoric acid solution (H3PO4, Guangdong HP Chemical Reagent Company, China), and distilled water. For the BSA solution, dilution was performed using a 0.15 mol L−1 NaCl solution.
2.2. Droplet manipulation by AEWOD
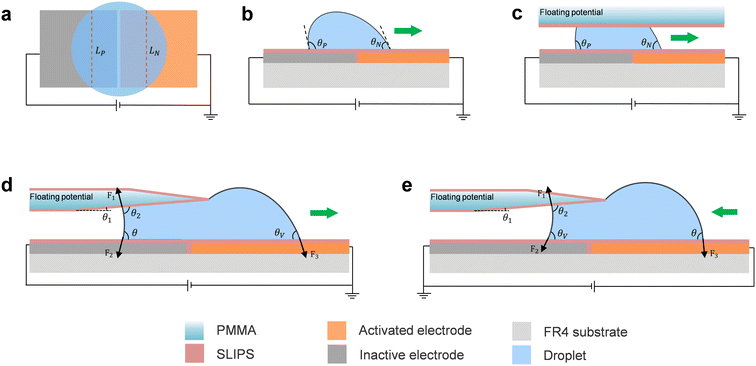
The AEWOD effect is related to the polarity of the applied voltage which has been found on the coplanar electrode experiment.32,37 When applying a DC voltage on the coplanar electrodes, the droplet would be pumped directionally. The AEWOD enables the controlled movement of a droplet on an open surface between two coplanar electrodes coated with a dielectric layer and a hydrophobic layer. The above research works found that the droplet exhibits a more pronounced change in contact angle on the positively biased electrode compared to the negatively biased one. In contrast, our earlier research involving modified SLIPS yielded an opposite AEWOD phenomenon on the coplanar electrodes.34 The droplet tends to move from the positively biased electrode to the negatively biased electrode. As illustrated in Fig. 1a, a droplet is positioned in the middle of two coplanar electrodes. The left electrode is applied a positive bias voltage, while the right electrode remains grounded. LP and LN are the effective width (chord length) of the three-phase contact line (TCL) on the positively biased electrode and the grounded electrode, respectively. When LP and LN are equal to L, the electrowetting force F applied on the droplet due to the surface tension can be expressed as detailed as follows.32,34,36,38F = γLG × L(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θN − cos θN − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θP) θP) | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θN is much higher than cos
θN is much higher than cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θP, the droplet will be transported toward the grounded electrode under the force of AEWOD. Fig. 1b demonstrates the movement of the droplet on the open surface. Similarly, using the AWEOD mechanism for directional manipulation of droplets in closed structures is considered. As shown in Fig. 1c, as the droplet is driven by the bottom electrodes, the top plate of the closed structure remains at a floating potential, thus it does not need to be grounded. Different from the previous configuration of the top plate grounding in a closed structure, the top plate of AEWOD only serves to limit droplets.
θP, the droplet will be transported toward the grounded electrode under the force of AEWOD. Fig. 1b demonstrates the movement of the droplet on the open surface. Similarly, using the AWEOD mechanism for directional manipulation of droplets in closed structures is considered. As shown in Fig. 1c, as the droplet is driven by the bottom electrodes, the top plate of the closed structure remains at a floating potential, thus it does not need to be grounded. Different from the previous configuration of the top plate grounding in a closed structure, the top plate of AEWOD only serves to limit droplets.
For the closed-open DMF system, the droplet is easy to transport from the open region to the closed region due to the capillary force from the closed region. However, challenges emerge when attempting to transport the droplet from the closed region to the open region, prior to the treatment of the edge of the top plate. As shown in Fig. S1 (see the ESI†), it is difficult to move the droplet from the closed region to the open region due to the large adhesion force from the edge of the top plate and the capillary force generated by the narrow spacing of the closed structure. To reduce the adhesion force and the capillary force, we treated the edge of the top plate by introducing a tilt angle. Through a comprehensive analysis of the forces acting on the droplet at the closed-open boundary, we aim to determine the optimal tilt angle parameter that will promote droplet transportation at this boundary. The interfacial forces exerted on the droplet at the boundary are illustrated in Fig. 1d. When the edge of the top plate is thin enough, there is no contact between the upper portion of the droplet and the edge of the top plate, so the side resistance F4 of the top plate can be neglected, as shown in Fig. S1b.† As a result, when a droplet moves from the closed region to the open region, the total surface tension FC–O exerted on the droplet is as follows:28
FC–O = F3|cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θV| − F1|cos θV| − F1|cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ2| − F2|cos θ2| − F2|cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ| θ| | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ2|, that is to increase θ1. As seen in Fig. S2a (see the ESI†), θ ≈ 85.76° when the droplet moves from the closed region to the open region. Consequently, when θ1 varies between 0° and 8.48°, it results in an increased FC–O. By adjusting the θ2 closer to 90° (θ1 ≈ 4.24°), FC–O is allowed to have a maximum value.
θ2|, that is to increase θ1. As seen in Fig. S2a (see the ESI†), θ ≈ 85.76° when the droplet moves from the closed region to the open region. Consequently, when θ1 varies between 0° and 8.48°, it results in an increased FC–O. By adjusting the θ2 closer to 90° (θ1 ≈ 4.24°), FC–O is allowed to have a maximum value.
Similar conclusions were also reported in previous research works. Wang et al.28 reported that sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ1 < 0.15 (that is θ1 < 8.63°) for the oil droplet transporting at the boundary. Additionally, Chang et al.27 designed a 3D curvature surface structure of the top plate based on the theory of droplet diffusion resistance and the marginal effect proposed by Oliver in 1977.39 The boundary angle of the specific house shape proposed is 171.70° (equivalent to θ1 ≈ 8.3°), which reduces the impact of friction and allows the droplet to move smoothly from a closed region to an open region. Thereby, a smaller tilt angle of the top plate can promote droplet transportation between the two regions.
θ1 < 0.15 (that is θ1 < 8.63°) for the oil droplet transporting at the boundary. Additionally, Chang et al.27 designed a 3D curvature surface structure of the top plate based on the theory of droplet diffusion resistance and the marginal effect proposed by Oliver in 1977.39 The boundary angle of the specific house shape proposed is 171.70° (equivalent to θ1 ≈ 8.3°), which reduces the impact of friction and allows the droplet to move smoothly from a closed region to an open region. Thereby, a smaller tilt angle of the top plate can promote droplet transportation between the two regions.
Fig. 1e depicts the side view and force analysis of a droplet moving back to the closed region from the open region (snapshots are shown in Fig. S2a and b in the ESI†). Here, the total force becomes:
FO–C = F1|cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ2| + F2|cos θ2| + F2|cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θV| − F3|cos θV| − F3|cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ| θ| | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ| is pretty small because θ very close to 90°, and the condition of FO–C > 0 can be easily achieved by applying a small voltage, making it easy for the droplet to enter the closed region from the open region.
θ| is pretty small because θ very close to 90°, and the condition of FO–C > 0 can be easily achieved by applying a small voltage, making it easy for the droplet to enter the closed region from the open region.
2.3. Fabrication of DMF system components
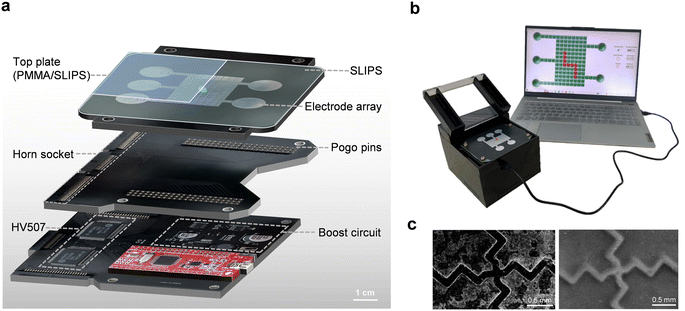
The DMF system comprises four primary components: the control circuit board, the electrical connection board, the DMF chip, and the personal computer (PC) interactive interface for DMF platform operation.(1) The control circuit board integrates three modules: a microcontroller, a boost converter, and two high-voltage switching chips (HV507), as shown in Fig. 2a. The chosen microcontroller STC12C5A60S2 controls the state of electrodes to facilitate continuous movement of droplets. The DC–DC boost circuit converts the microcontroller's 5 V voltage into an adjustable 50–300 V output, using the MAX1771 boost chip, MCP41050 digital potentiometer, and other components. Notably, the digital potentiometer programmatically sets the actuation voltage without the need for an external mechanical adjustment. The HV507 chip, connected to the output of the boost circuit, serves as a low-voltage serial to high-voltage parallel converter with 64 push–pull outputs. This cascading capability allows the connection of two HV507 chips in the DMF device, delivering 128 parallel level signals. This arrangement enables vertical addressing of the electrode array, i.e. each driving electrode is individually addressable, enhancing droplet control accuracy.
(2) The electrical connection board, depicted in Fig. 2a, serves as the intermediary between the control circuit board and the DMF chip. It's equipped with three horn sockets, each featuring 50, 50, and 30 pins, respectively, along with 132 pogo pin connectors securely affixed to the board. This modular design offers flexibility, allowing optimization and upgrades as practical application needs evolve.
(3) The DMF chip is composed of distinct components: an electrode array board, a hydrophobic dielectric membrane, a spacer, and an acrylic top plate. The electrode array consists of 127 available electrodes (122 actuating electrodes of 3 mm × 3 mm and 5 circular reservoirs with a diameter of 13.5 mm), which were made on a standard double-layer PCB substrate. The inter-electrode gap is kept at ∼100 μm. The thickness of spacers is 170 μm unless otherwise specified. A 1 mm thick transparent acrylic plate coating with SLIPS serves as the top plate, which helps improve the smoothness of the top plate and promotes the movement of droplets under AEWOD.
(4) A user-friendly graphical user interface (GUI) is designed using Qt to enhance the accessibility of the DMF platform for non-programmers, as shown in Fig. 2b. The GUI communicates with the microcontroller through the UART protocol, enabling users to effortlessly adjust the electrode voltage, and define droplet movement paths and speed according to their preferences, as shown in Video S1.†
2.4. Fabrication of the dielectric layer
In EWOD devices, the dielectric layer is an important part of determining the performance of the device. However, it is worth noting that biofouling and high voltage breakdown caused by conventional dielectric layers are still the main obstacles preventing the scaling of DMF devices to various applications. The SLIPS as an alternative dielectric layer offers numerous advantages, including small contact angle hysteresis, high breakdown voltage, self-cleaning, self-repairing, anti-freezing, and anti-biological contamination. Moreover, the uniform-thickness lubricant layer can also overcome the obstacle of the deep gap between PCB electrodes, facilitating the movement of droplets between adjacent electrodes.The preparation process of SLIPS modified with 1H,1H,2H,2H-perfluorooctyltrichlorosilane (PFOTS) is similar to a previous work.33 Firstly, the polytetrafluoroethylene (PTFE) membrane (composed of nanofiber network, ∼25 μm thick, average pore size 100 nm, Beijing Fuliu Material Technology Co. Ltd.) is cut according to the size of the electrode array board and immersed in 0.03 wt% PFOTS ethanol solution for 1 h to graft the self-assembled monolayer of perfluorosilane molecules onto the PTFE membrane. Next, the modified membrane is carefully coated onto the clean electrode array board, avoiding contact with the surface and being free from contamination. The side edge of the SLIPS was moderately stretched to achieve a flattened surface and eliminate any bubbles that may exist between the SLIPS and the electrodes. Finally, after ethanol evaporated, H201 methyl silicone oil with a viscosity of 10 cSt is injected into the modified PTFE nanoporous membrane by capillarity to form a transparent SLIPS. To remove any excess silicone oil, the SLIPS-covered electrode array board was placed vertically for 1 h at room temperature, followed by a horizontal placement for an additional 1 h to achieve a uniform thickness of the lubricant layer. After these processes, a hydrophobic modified SLIPS membrane was prepared.
As illustrated in Fig. 2c, the left SEM picture represents the electrode array without SLIPS covering. It is evident that there is a large gap between adjacent electrodes, and the surface of the electrodes appears noticeably rough. The gap depth measures ∼40 μm, rendering conventional spin coating techniques insufficient to bridge such a height. In contrast, the right picture shows the electrode array with SLIPS covering. It is apparent from the image that the surface smoothness of the SLIPS-covered electrode array is significantly improved, and the gaps between the electrodes are effectively filled. Consequently, a smoother droplet-driving effect can be achieved.
3. Results and discussion
Droplet manipulation depends on the parallel plate spacing, droplet volume, and applied voltage. In addition, the uniformity of the generated daughter droplets is closely related to the electrode driving strategies. Exploring their impacts is especially vital to droplet dispensing and splitting. To ensure the control rationality and accuracy of the platform, the physical parameters for droplet manipulation on the platform were verified first.3.1. Parameter validation of droplet manipulation
The spacing between parallel plates plays a key role in both the dispensing and splitting of droplets. Fig. 3a (see Video S2†) shows the droplet dispensing at different spacings between the parallel plates on the fourth square electrode. It is found that the small plate spacing causes a trailing effect, which leads to droplet residue accumulation behind the electrodes when the plate spacing is ≤170 μm. Meanwhile, the larger plate spacing (≥680 μm) results in the daughter droplets being hard to generate from the reservoirs. In this DMF system, the optimal droplet manipulation occurs at the plate spacings of 340 μm to 510 μm. At these distances, dispensed daughter droplets measure approximately from 2.7 μL to 4.5 μL. Fig. 3b shows the snapshot of droplet's successful movement in the open region and closed region, respectively. Fig. 3c shows the variation of droplet velocity with applied voltage in the open and closed regions, and it is found that the velocity of the droplet is proportional to the applied voltage in both regions. However, due to the combined influence of top plate resistance and capillary forces generated by the narrow spacing between parallel plates, the velocity of droplets in the closed region is smaller than that in the open region.Fig. 3d and e demonstrate that the transport velocity of droplets varies with the droplet volume under three different applied voltages. The droplet velocity is calculated by dividing the electrode size by the time of the frame image taken by a high-speed camera. The transport velocity increases with the droplet volume when the volume is small. This trend is attributed to a short effective three-phase contact line between the droplet and the activating electrode. This leads to a weak driving force, resulting in slow droplet movement. However, with the effective three-phase contact line increasing to around 3 mm (approximately the width of the electrode), the driving force on the droplet reaches its maximum value. Beyond this point, further increasing the droplet volume causes a decrease in the droplet velocity, as the driving force does not increase, while the adhesion force and resistance force increase with the droplet size. It's worth noting that the maximum velocity and the corresponding volume of the droplet are also different under different voltages. With the decrease of applied voltage, both the maximum velocity and the corresponding volume of the droplet decrease. In the open region, the velocity of droplets reaches the maximum value of 9.18 mm s−1 for the volume of 15 μL under the applied voltage of 274 V, 4.67 mm s−1 for the volume of 10 μL under the applied voltage of 201 V, and 1.65 mm s−1 for the volume of 9 μL under the applied voltage of 103 V, respectively. The minimum volume threshold for a droplet moving continuously over a long distance is ∼5 μL. In the closed structure, the droplet velocity reaches the maximum value of 4.5 mm s−1 for the volume of 2.8 μL under the applied voltage of 274 V, 2.71 mm s−1 for the volume of 2.6 μL under the applied voltage of 201 V, and 1.1 mm s−1 for the volume of 2.4 μL under the applied voltage of 103 V, respectively. The minimum volume threshold for a droplet moving continuously over a long distance is ∼2 μL.
The speed of droplet transport is determined by the switching time between adjacent electrodes. Moreover, different switching times correspond to a minimum threshold voltage that enables the continuous driving of droplets. Therefore, the relationship between the electrode switching interval and the minimum applied voltage for achieving continuous droplet driving is investigated in both the closed and open regions. Fig. 4a and b show the minimum applied voltage that enables the droplets to move continuously in both the open and closed regions under different electrode switching times. As expected, the minimum driving voltage increases as the electrode switching interval decreases. The driving voltage for an 8 μL droplet is ∼105 V when the electrode switching interval is set to 3000 ms, while it requires ∼225 V for a 500 ms interval. This phenomenon can be attributed to the longer duration of the electrowetting force exerted on the droplets as the electrode switching time increases. As the surface pinning forces remain constant, the cumulative electrowetting force still can drive the droplet to move to the activated electrode even under a smaller voltage as the duration increases. Interestingly, it has been observed that droplets with larger volumes exhibit lower threshold-driven voltages in both regions. This phenomenon can be attributed to the fact that larger droplets initially have a greater effective width of TCL with the active electrode. Consequently, the greater EWOD force of a large volume droplet makes it easier to be driven.
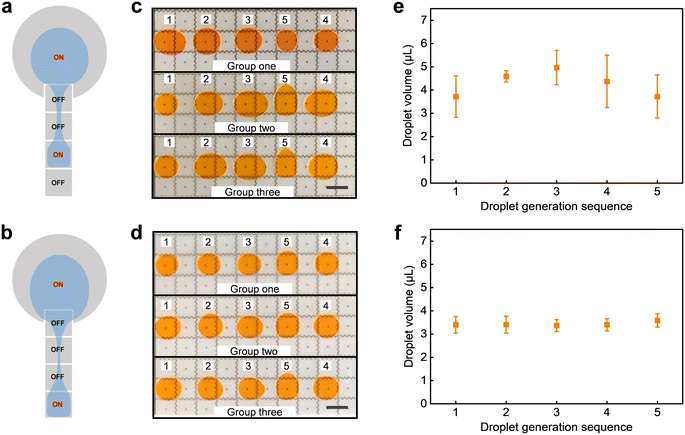
Moreover, evaluating the efficacy of a DMF system depends on the consistent dispensation of stable droplets from its reservoir. For this reason, we examined the uniformity in droplet volume dispensed from the reservoir. Fig. 5a and b illustrate the schematic representation of droplet dispensing under two different driving strategies. It is found that the small liquid column is easier to form when the activated electrode is farther from the reservoir, as shown in Fig. 5b. Fig. 5c and d (see Video S3†) show three sets of repeated experiments, respectively, where droplets are generated at diverse positions within the reservoir. Each set comprises five droplets, all droplets were dispensed from the same reservoir, the numbers above the droplets represent the sequence of daughter droplet generation, and their generation positions are consistent with those depicted in Fig. 5a and b, respectively. The droplets generated on the third square electrode exhibit inadequate volume uniformity, displaying inconsistencies not only among different groups but also within the same group of five droplets. In contrast, the droplets generated on the fourth square electrode demonstrate a significant enhancement in uniformity. Fig. 5e and f show the average volume of daughter droplets. The values and error bar more clearly illustrate the volume differences of daughter droplets generated at different positions. This can be attributed to the fact that the small liquid column formed between the farther electrode and the reservoir is more susceptible to splitting.
3.2. Droplet manipulation on the DMF platform
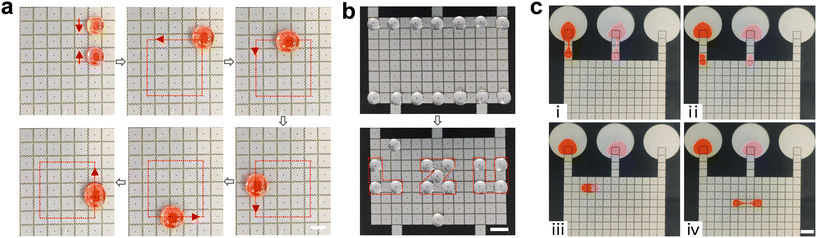
Based on the above optimal parameters, the droplet manipulations were investigated next. The spacing between parallel plates is 340 μm and the driving voltage is 274 V. Fig. 6a (see Video S4†) shows a sequence of video frames for two ∼15 μL droplets transported and mixed smoothly on the open surface. Furthermore, the system demonstrates the capability to transport multiple droplets, as seen in Fig. 6b (see Video S4†). Multiple droplets were smoothly transported to the specified position according to the preset program to display the letters “LZU”. They can be transported independently and would not merge, even at a small distance. Accordingly, this DMF platform allows efficient, large-scale, and high-throughput droplet reactions with its stability in droplet manipulation. Fig. 6c (see Video S5†) shows snapshots of four basic manipulations of droplets in the closed region: dispensing, transporting, mixing, and splitting. Two ∼3 μL daughter droplets with different colors were dispensed from the two reservoirs with 15 μL droplets, respectively. Then, the daughter droplets were transported to mix. Finally, the ∼6 μL droplet was split into two ∼3 μL droplets. Our results indicate that the closed structure can be rapidly and thoroughly mixed while maintaining relatively uniform volumes after splitting. This observation confirms the complete feasibility of manipulating droplets within a closed structure using AEWOD.Fig. 7a (see Video S6†) illustrates snapshots of droplet manipulation and back-and-forth transport on the closed-open DMF chip. Firstly, two daughter droplets were generated from different reservoirs and thoroughly mixed, and then waited for another group of droplets to merge with them and be transported to the open structure. Subsequently, the experiment involves moving droplets from the open region back to the closed region, completing the bidirectional transportation process. In addition, it is found that the smaller droplets are pulled out of the closed structure more smoothly than the larger droplets, as shown in Video S6.† This observation indicates that the resistance caused by the narrow spacing cannot be ignored. Conversely, droplets are smoothly transported from the open region to the closed region, regardless of the volume of the droplets. Therefore, it is crucial to set an appropriate volume to ensure smooth back-and-forth transportation of droplets at the boundary. Typically, droplets with a size equivalent to 2–4 electrode units are chosen on this DMF platform. Certainly, the successful execution of these experiments effectively demonstrates the feasibility and flexibility of droplet manipulation within this integrated closed-open system.
Note that the top plate does not require grounding in this platform, allowing for the utilization of a more cost-effective acrylic material as a substitute for ITO glass. Moreover, the acrylic plate material allows the formation of a tilt angle by polishing directly. After conducting experiments on multiple acrylic plates with varying tilt angles, we have achieved a relatively ideal droplet transport at a tilt angle of ∼4°. In our experiments, a large tilt angle (∼24°) caused a failure of droplet transport; the droplet was trapped at the junction of the parallel plate and the bevel. The experiments are consistent with our previous theoretical analysis.
Fig. 7b (see Video S7†) investigated the droplet transport across the closed-open boundary at three different horizontal positions of the top plate. The plate spacing is 340 μm for the three experiments. The results show that the 5 μL droplets can be successfully transported from the closed region to the open region, regardless of the horizontal positions of the top plate. Meanwhile, the droplet transport at the boundary under the parallel plate spacings of 510 μm was also investigated, as shown in Fig. S3 (see the ESI†). Our results show the smooth transportation of droplets from the closed region to the open region was achieved at both plate spacings. To ensure continuous transportation upon entering the open area, the droplet volume was set to 5 μL and 8 μL at the spacing of 340 μm and 510 μm, respectively. Significantly, the closed-open boundary can be adjusted above any square electrodes as all the square electrodes have the same structure and driven method. Moreover, our results found that the horizontal positions of the top plate have little effect on droplet transportation. The minimum threshold voltage for effecting droplet transport from the closed region to the open region was found to be approximately 80 V at a spacing of 340 μm, and about 67 V at a spacing of 510 μm. This dynamic flexibility in adjusting the position of the top plate further expands the potential applications of the DMF system.
To verify the stability of droplet transportation at the boundary, a ∼10 μL droplet was transported back and forth circularly between the closed and open regions at a plate spacing of 340 μm. As shown in Video S8,† when the edge of the top plate is placed exactly above the center of the gap between the two electrodes, the droplet was successfully driven across the closed-open boundary 30 times in the initial 31 rounds of transportation, with only one instance of failure. Beyond the 31st round, the droplet volume reduced to approximately 7.5 μL due to evaporation during transportation. This gradual decrease in volume made it challenging for the droplets to establish contact with the next electrode during transportation from a closed region to an open region. Therefore, the failure beyond the 31st round is attributed to droplet evaporation. If we only consider the first 31 transports, the success rate of droplet transportation back and forth at the boundary exceeded 96.5%. This result demonstrates the system's stability and further validates the efficacy of droplet transportation at the open-closed boundary.
3.3. CBB G-250 solution and BSA solution preparation
There are four primary methods for protein staining: organic reagent staining, silver staining, fluorescent staining, and isotopic chromatography. One prominent representative of organic reagent staining is CBB G-250. The underlying principle of the CBB G-250 determination of protein concentration rests on the dye-binding technique. Under acidic environments, the CBB G-250 dye binds to the hydrophobic regions of proteins, resulting in a peak absorption shift from 465 nm to 595 nm. This binding transformation triggers a change in color from brown to blue, with the intensity of the blue directly proportional to the protein concentration. The interaction between CBB G-250 and proteins attains equilibrium in approximately 2 minutes, and the formed conjugate remains stable for up to an hour at room temperature. Furthermore, CBB G-250 boasts remarkable detection sensitivity, allowing for the quantification of microgram-level protein content. As a consequence, this method is commonly used for the rapid determination of trace proteins. For evaluating the DMF platform based on SLIPS in terms of anti-biological contamination and its compatibility with high-voltage driven processes, a staining procedure involving BSA was performed on the platform.The reagents required for protein staining are prepared as follows. Firstly, dissolve 50 mg CBB G-250 in 25 mL ethanol solution with a concentration of 95%, then add 85% phosphoric acid (H3PO4), dilute to 500 mL with distilled water, filter the solution into a brown bottle with filter paper and set aside. Then 4.3875 g NaCl was dissolved in 500 mL distilled water to prepare a 0.15 mol L−1 NaCl solution. Add 10 mg BSA to 100 mL of 0.15 mol L−1 NaCl solution to prepare 0.1 g L−1 standard protein solution. In addition, the standard protein solution of 0.1 g L−1 was prepared into a gradient concentration protein solution according to Table 1. Fig. S4a (see the ESI†) illustrates the mixing of the diluted BSA gradient solution and CBB G-250 reagent. It demonstrates that as the protein concentration increases, the blue color of the solution progressively becomes darker.
| Tube number | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 g L−1 BSA (mL) | 0.0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 |
| 0.15 mol L−1 NaCl (mL) | 1 | 0.9 | 0.8 | 0.7 | 0.6 | 0.5 | 0.4 | 0.3 | 0.2 |
| Diluted BSA concentration (g L−1) | 0 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.06 | 0.07 | 0.08 |
| CBB G-250 (mL) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
3.4. Establishment of a standard curve of protein concentration–relative image intensity
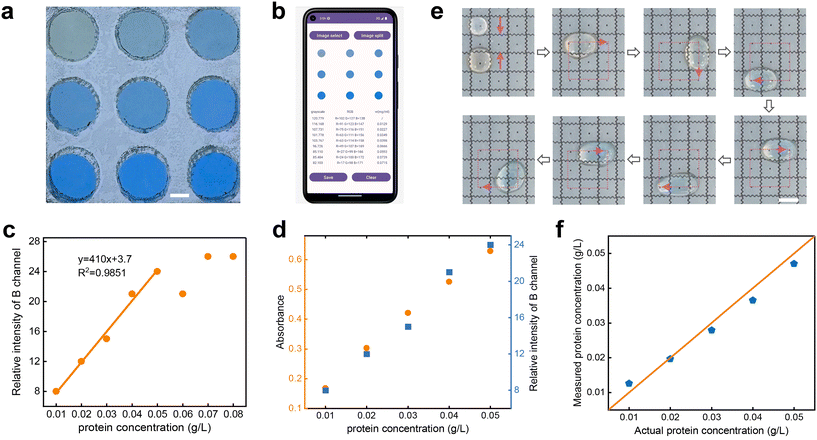
The conventional method for determining protein concentration using CBB G-250 involves colorimetric analysis with a spectrophotometer. However, this method requires a large number of samples for wavelength measurement (at least ∼1000 μL of sample solution per measurement), and integrating bulky spectrophotometers with DMF systems is not feasible, limiting its application in real-time detection and similar scenarios. Consequently, we developed an Android app capable of performing colorimetric analysis. By measuring the grayscale or relative intensity of captured protein-stained images, we established a correlation between these values and protein concentration. This allows us to construct a standard curve, enabling further determination of the concentration of unknown samples.Before constructing the standard curve, it is necessary to measure the RGB value of the image to establish the relationship between its color information and protein concentration. In the colorimetric analysis application, bitmap segmentation was employed, extracting RGB values of multiple droplets from one image. Fig. 8a and b illustrate the segmentation of a 9-hole colorimetric cell image. The hole is fabricated by cutting an acrylic plate with a laser cutter. The center of each hole served as the center of an individual image segment, encompassing a circular region with a 100-pixel radius. The resultant RGB value represents the average of all pixel RGB values within the segmented region. To counter irregular or intense light exposure on samples, an airtight box crafted from white acrylic material was employed on the platform. This enclosure effectively eliminated external light interference. A white LED light source beneath the box, coupled with a white translucent acrylic plate functioning as a light diffusion plate, ensured even light distribution within the box. This strategy enhanced sample illumination consistency, and thus elevated protein concentration detection accuracy. This approach not only heightened the analysis efficiency but also reduced the impact of external light variations.
The colorimetric application, created using Android Studio, has revealed a linear correlation between protein concentration and the B channel value in RGB. This relationship suggests that higher protein concentrations are associated with larger B values, which is consistent with our visual analysis. Here, by calculating the difference values between the B channel of the protein concentration group and the blank control group, the relationship of the relative B channel with protein concentration was obtained, as shown in Fig. 8c. Note that the colorimetric app exhibited a threshold with the increase of concentrations. The relative intensity of the B channel demonstrated a robust linear correlation with protein concentration within the 0.01–0.05 g L−1 concentration range. However, the relative intensity of the B channel exhibited irregular fluctuations when the protein concentrations were higher than 0.05 g L−1. Accordingly, the relative intensity of the B channel within 0.01–0.05 g L−1 concentration was employed to construct a standard curve for protein concentration determination.
3.5. Protein staining on the DMF platform
Before detecting the protein concentration on the DMF platform, a comparison study was performed between smartphone colorimetry and the commonly utilized spectrophotometric method for protein concentration detection. As shown in Fig. 8d, the spectrophotometric approach depicted a positive correlation between absorbance at 595 nm and concentration. As expected, the relative intensity of the B channel is also almost linearly positively correlated with protein concentration. Furthermore, Fig. S4b† indicates that the Pearson correlation coefficient between the two methods reached 0.99. It further verifies the rationality of this method for protein detection.For offline concentration determination, 6 μL of 0.01 g L−1 BSA solution and 12 μL of CBB G-250 reagent were added to the DMF platform. Then, they were mixed thoroughly for 2 minutes along a predefined path. Subsequently, the mixed reagent was transferred to a colorimetric cell that was situated within an airtight acrylic box. This process was replicated for protein solutions ranging from 0.01 to 0.05 g L−1, each mixed sequentially on the DMF platform. The relative intensity of the B channel was measured three times at each concentration to derive an average value. Notably, to minimize error, a distinct control group was established for each protein concentration measurement using smartphone colorimetry.
Fig. 8e depicts the process of a 6 μL droplet of 0.03 g L−1 BSA mixing with a 12 μL CBB G-250 droplet on the platform. As CBB G-250 binds to hydrophobic protein areas during staining, their thorough combination leads to decreased hydrophobicity in the mixed droplet. Consequently, the droplet eventually halts on the electrode within 2 minutes, indicating full integration. After a 2 minute wait, the droplets are retrieved for offline colorimetric detection. Fig. 8f shows the comparison between the concentration measured by the app and the actual concentration after fully mixing protein solutions of different concentrations and CBB G-250 solution. The orange line indicates that the actual concentration is equal to the measured concentration. The small disparity between the detected and actual concentrations after platform-based mixing (variance approximately 0.0057) reaffirms the feasibility of protein concentration detection on this DMF platform. In addition, to explore the highest protein concentration limit of this AEWOD system, the droplets driven for five different solutions with concentrations of 0.01 g L−1, 0.1 g L−1, 1 g L−1, 10 g L−1, and 100 g L−1 were performed. As shown in Fig. S5 (see the ESI†), although the contact angle of the droplet decreases gradually with an increase in the concentration of the protein solution, the transport speed of the droplets on the SLIPS surface slightly decreases with increasing protein concentration. Additionally, no residue was observed on the SLIPS surface after transporting droplets of varying concentrations.
Through the staining experiment, the DMF platform based on SLIPS showed a very small error between the detected BSA concentration and the actual concentration. This result substantiates that the platform is bio-friendly toward proteins, opening avenues for potential biological experiments such as DNA extraction and even PCR amplification on this platform in the future.
4. Conclusions
In summary, we proposed a novel adjustable closed-open DMF platform by utilizing the modified SLIPS with AEWOD. The various droplet manipulations, including dispensing, transporting, mixing, and splitting, were successfully achieved in this DMF platform. The consistent electrode structure design on both regions allows the proportion of closed and open regions to be adjusted optionally. Meanwhile, the utilization of the PCB electrodes as the bottom substrate, a transparent acrylic with floating potential as the top plate, and a digitally controllable boost circuit allows a highly integrated, vertical addressing, flexible, and easily scalable closed-open EWOD-based DMF platform to be achieved. The protein staining is successfully conducted on the digital microfluidic platform, demonstrating the potential in biological sample processing of closed-open DMF. This closed-open DMF system provides new possibilities for developing chemical engineering, bioengineering, and biomedical engineering point-of-care test devices.Author contributions
Xiaodong He: conceptualization, methodology, supervision, writing – review & editing, funding acquisition. Jingsong Xu: investigation, writing – original draft, writing – review & editing, software. Xingcheng Wang: software. Qingyuan Huang: writing – original draft.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 62061025, No. 61804071), Fundamental Research Funds for the Central Universities (lzujbky-2022-31), and the Science and Technology support program of Gansu Province (No. 22JR5RA485).References
- A. H. C. Ng, K. Choi, R. P. Luoma, J. M. Robinson and A. R. Wheeler, Anal. Chem., 2012, 84, 8805–8812 CrossRef CAS.
- J. Shen, L. Zhang, J. Yuan, Y. Zhu, H. Cheng, Y. Zeng, J. Wang, X. You, C. Yang, X. Qu and H. Chen, Anal. Chem., 2021, 93, 15033–15041 CrossRef CAS.
- A. Das, C. Weise, M. Polack, R. D. Urban, B. Krafft, S. Hasan, H. Westphal, R. Warias, S. Schmidt, T. Gulder and D. Belder, J. Am. Chem. Soc., 2022, 144, 10353–10360 CrossRef CAS PubMed.
- M. Abdelgawad and A. R. Wheeler, Adv. Mater., 2009, 21, 920–925 CrossRef CAS.
- M. D. M. Dryden, D. D. G. Rackus, M. H. Shamsi and A. R. Wheeler, Anal. Chem., 2013, 85, 8809–8816 CrossRef CAS PubMed.
- W. Qiu and S. Nagl, ACS Sens., 2021, 6, 1147–1156 CrossRef CAS PubMed.
- H. Wang, L. Chen and L. Sun, Front. Mech. Eng., 2017, 12, 510–525 CrossRef.
- Z. Gu, M.-L. Wu, B.-Y. Yan, H.-F. Wang and C. Kong, ACS Omega, 2020, 5, 11196–11201 CrossRef CAS.
- B. Coelho, B. Veigas, H. Águas, E. Fortunato, R. Martins, P. Baptista and R. Igreja, Sensors, 2017, 17, 2616 CrossRef PubMed.
- N. Grant, B. Geiss, S. Field, A. Demann and T. W. Chen, Micromachines, 2021, 12, 1065 CrossRef PubMed.
- H. Wu, Z. Tang, R. You, S. Pan, W. Liu, H. Zhang, T. Li, Y. Yang, C. Sun, W. Pang and X. Duan, Nanotechnol. Precis. Eng., 2022, 5, 023001 CrossRef CAS.
- S. K. Thio, S. Bae and S.-Y. Park, Sens. Actuators, B, 2020, 308, 127704 CrossRef CAS.
- W. Wang, X. Rui, W. Sheng, Q. Wang, Q. Wang, K. Zhang, A. Riaud and J. Zhou, Sens. Actuators, B, 2020, 324, 128763 CrossRef CAS.
- J.-H. Chang and J. J. Pak, Sens. Actuators, B, 2011, 160, 1581–1585 CrossRef CAS.
- X. Min, C. Bao and W. S. Kim, ACS Sens., 2019, 4, 918–923 CrossRef CAS PubMed.
- M. Torabinia, U. S. Dakarapu, P. Asgari, J. Jeon and H. Moon, Sens. Actuators, B, 2021, 330, 129252 CrossRef CAS.
- S. von der Ecken, A. A. Sklavounos and A. R. Wheeler, Adv. Mater. Technol., 2021, 7, 2101251 CrossRef.
- Z. Luo, J. Xu, Z. Pan, H. Yin, L. Cao, G. Zhou and S. Liu, IEEE Access, 2022, 10, 30573–30582 Search PubMed.
- C. Li, K. Zhang, X. Wang, J. Zhang, H. Liu and J. Zhou, Sens. Actuators, B, 2018, 255, 3616–3622 CrossRef CAS.
- A. N. Banerjee, S. Qian and S. W. Joo, J. Colloid Interface Sci., 2011, 362, 567–574 CrossRef CAS PubMed.
- X. Zeng, K. Zhang, J. Pan, G. Chen, A. Q. Liu, S. K. Fan and J. Zhou, Lab Chip, 2013, 13, 2714–2720 RSC.
- P. Y. Paik, V. K. Pamula and K. Chakrabarty, IEEE Trans. Very Large Scale Integr. VLSI Syst., 2008, 16, 432–443 Search PubMed.
- M. Abdelgawad, P. Park and A. R. Wheeler, J. Appl. Phys., 2009, 105, 094506 CrossRef.
- H. Ding, S. Sadeghi, G. J. Shah, S. Chen, P. Y. Keng, C. J. Kim and R. M. van Dam, Lab Chip, 2012, 12, 3331–3340 RSC.
- X. Xu, L. Cai, S. Liang, Q. Zhang, S. Lin, M. Li, Q. Yang, C. Li, Z. Han and C. Yang, Lab Chip, 2023, 23, 1169–1191 RSC.
- V. N. Luk and A. R. Wheeler, Anal. Chem., 2009, 81, 4524–4530 CrossRef CAS PubMed.
- Y. N. Chang and D. J. Yao, Micromachines, 2022, 13, 898 CrossRef.
- W. Wang and T. B. Jones, Lab Chip, 2015, 15, 2201–2212 RSC.
- Y. Xing, Y. Liu, R. Chen, Y. Li, C. Zhang, Y. Jiang, Y. Lu, B. Lin, P. Chen, R. Tian, X. Liu and X. Cheng, Lab Chip, 2021, 21, 1886–1896 RSC.
- D. Li, X. Liu, Y. Chai, J. Shan, Y. Xie, Y. Liang, S. Huang, W. Zheng and Z. Li, Lab Chip, 2022, 22, 709–716 RSC.
- E. Seyrat and R. A. Hayes, J. Appl. Phys., 2001, 90, 1383–1386 CrossRef CAS.
- S. K. Fan, H. Yang, T. T. Wang and W. Hsu, Lab Chip, 2007, 7, 1330–1335 RSC.
- X. He, W. Qiang, C. Du, Q. Shao, X. Zhang and Y. Deng, J. Mater. Chem. A, 2017, 5, 19159–19167 RSC.
- X. He, J. Zhang, X. Zhang and Y. Deng, Soft Matter, 2019, 15, 5211–5219 RSC.
- Z. Yi, H. Feng, X. Zhou and L. Shui, Front. Phys., 2020, 8, 193 CrossRef.
- X. He, J. Xu, B. Yang and F. Yang, Results Phys., 2023, 48, 106401 CrossRef.
- T. T. Wang, P. W. Huang and S. K. Fan, Presented in part at the 19th IEEE International Conference on Micro Electro Mechanical Systems, Istanbul, Turkey, January, 2006 Search PubMed.
- J. Berthier, P. Dubois, P. Clementz, P. Claustre, C. Peponnet and Y. Fouillet, Sens. Actuators, A, 2007, 134, 471–479 CrossRef CAS.
- J. F. Oliver, C. Huh and S. G. Mason, J. Colloid Interface Sci., 1977, 59, 568–581 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3lc00856h |
| This journal is © The Royal Society of Chemistry 2024 |