Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Manganese dioxide (MnO2) and biomass-derived carbon-based electroactive composite materials for supercapacitor applications
Pranoti H.
Patil
 and
Sushilkumar A.
Jadhav
and
Sushilkumar A.
Jadhav
 *
*
School of Nanoscience and Technology, Shivaji University Kolhapur, Vidyanagar, Kolhapur 416004, Maharashtra, India. E-mail: sushil.unige@gmail.com
First published on 3rd May 2024
Abstract
Manganese dioxide (MnO2) is the most promising electrode material for supercapacitors (SCs) due to its low cost, non-toxic nature, high theoretical capacitance, and wide potential window. Meanwhile, biomass-derived carbon has also become a prominent electrode material in recent years due to its cost-effectiveness, eco-friendliness, and availability of biomass in abundance. Carbon can be synthesized from biomass precursors such as plants, animals, and microorganisms via various synthesis and activation techniques. MnO2 is combined with carbon to obtain composite materials with improved electrochemical properties and structural stability. Sustainable porosity in MnO2-biomass-derived carbon composites increases the conductivity and electrochemical performance of the electrode material. Hence, MnO2 and biomass-derived composite materials have received great attention regarding their potential use as electrode materials in SCs. Recently, significant new developments in the synthesis and testing of such composite materials have been made. In this review, recent reports about such composite materials are listed and analyzed with numerous examples providing the authors with important collective information. Here, we place a strong emphasis on carbons obtained from a variety of biomass and different types of MnO2 and composites made from them for SC application. The current challenges and prospects in this field of research are also highlighted.
1. Introduction
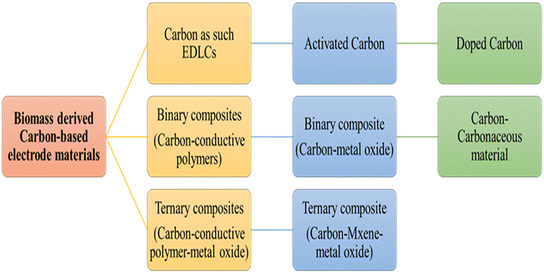
Supercapacitors (SCs) are energy storage devices with unique properties and applications.1 They are categorized into three types: electric double-layer capacitors (EDLCs), pseudo capacitors, and hybrid-based devices, each with a different energy storage mechanism.2 EDLCs use carbon and non-faradaic reactions to store charge. Faradaic reactions are used in pseudocapacitive materials such as metal oxides (MOs), metal sulfides, metal hydroxides, and polymers. Hybrid devices combine the advantages of EDLCs and pseudocapacitive materials to enhance energy density.3 However, compared to batteries (which have an energy density of 30–200 W h kg−1), SCs have a lower energy density (20 W h kg−1).4 They need to have a high energy density to be compact. To overcome this problem, a lot of research is being conducted regarding the synthesis and development of novel electrode materials. To enhance the electrochemical properties of SCs, the choice of electrode materials and their fabrication are crucial. SC electrodes must have chemical stability, surface wettability, high thermal stability, high specific surface area, corrosion resistance, and high electrical conductivity. Additionally, they must be affordable and environmentally sustainable.5–7 In actuality, electrochemical factors are not only influenced by the surface area but also by other crucial parameters such as morphology, including distributions of pore sizes and availability of active sites for the electrolyte.8–10 The smaller the pore size, the greater the capacitance and the higher the energy density, but it suffers from lower power density. Therefore, the selection of electrode material is critical. Different electrode materials are being investigated for SCs such as MOs or layered double hydroxides (LDHs), MXene, covalent organic frameworks (COFs), etc.11,12 The most extensively investigated strategies are the development of new electrodes from biomass-derived carbon-based materials and nanostructured materials such as hard carbons, dichalcogenides, bio-mass engineered MnxOy nano-scaled oxides like Mn3O4, and metal–organic frameworks (MOFs) as high-capacity electrodes with higher surface area, porosity, capacitance, energy density, and power density.13–15 Biomass-derived carbon-based materials are extensively used as electrode materials for SCs due to the presence of non-metal elements and various functional groups; also their low cost, easy availability, and environmentally friendly nature make them efficient electrode materials for SCs. Various biomass-derived carbon-based electrode materials are shown in Fig. 1. As a precursor, biomass with naturally hierarchical structures could be a viable option for SCs. Recently, heteroatom-doped carbon materials have attracted much attention as another potential electrode material for a variety of electrochemical applications.16 The synthesis of heteroatom-doped carbon is reasonably simple as merely changing the synthesis conditions can yield it. But every material has its strengths and weaknesses; Mos suffer from poor cyclic stability, while carbon-based materials suffer from low electrical conductivity. Therefore, there is a need to combine both materials to achieve better electrochemical performances.In comparison to bulk MO materials which have a lower specific area and capacitance, the emerging trend of designing and fabricating innovative porous nanostructure composites with carbon materials is beneficial to increase the specific surface area and achieve good electrochemical properties. MOs demonstrate many oxidation states and participate in faradaic redox reactions that provide high capacitance. On the other hand, activated carbon (AC) exhibits good conductivity with a large surface area and porosity. MO/AC composites have a wide range of applications in energy storage devices.17
Nowadays, bimetallic and trimetallic oxides are often utilized as electrode materials due to their high capacitance and electrical conductivity. Trimetallic oxides, compared to single and bimetallic oxides, showed good electrochemical properties. In comparison to single MOs including NiO, MnO2, SiO2, Co3O4, V2O5, CuO, and ZrO2, bi- or tri-MOs such as NiCo2O4 delivered high electrical conductivity. Moreover, the doping of Ni enhances the electrical conductivity from 3.1 × 10−5 of (CO3O4) to 0.1 to 0.3 S cm−1 of (NixCO3−xO4). Novel heterostructure and nanostructured (OD), (1D) and (3D) mixed MOs are typically porous and can offer more active sites for charge transfer and full contact between the active materials and electrolyte results in the enhancement of the electrochemical characteristics compared to bulk MO materials. Recently, plant-based AC has attracted much attention due to its natural 3D porous structure, which plays a vital role in the storage and rapid migration of electrolyte ions and can compensate for the underdeveloped pore structure of conventional AC.18–25 AC made from biomass is abundant in functional groups and non-metal elements like N, S, and O that can be used in faradaic processes to create specific types of pseudo capacitors. When compared to conventional AC, biomass-derived AC is renewable, affordable, and environmentally benign. It is possible to predict that biomass-derived AC will eventually replace conventional AC as the dominant trend in the development of AC. An abundance of porous structures makes AC a good material to make a composite with MnO2.
The importance of MnO2 and activated carbon materials has been pointed out separately and the electrochemical and charge storage properties of these materials were investigated in several studies. The main aim of this review is to point out the importance of MnO2 and biomass-derived carbon-based electroactive composite materials for energy storage application. Herein, we have initially presented the basic and important information about MnO2 and its various types and their role in energy storage devices. Then the information about biomass-derived carbon is presented by highlighting the characteristic properties of carbons and importance of activation. This section also highlights different biomass sources to produce the carbon. Meanwhile, the main section about the MnO2-biomass-derived carbon-based composite materials contains discussion of several recent examples of such composites for SC application. The electrochemical properties shown by such composites are correlated to the type, structure, morphology, and shape of MnO2 and the source of biomass from which the carbon is obtained as well as the main properties of the carbon such as N-doping and porosity. The review is concluded by highlighting the main findings and possible future directions in this field of research.
2. MnO2: a famous supercapacitive material
Among all transition MOs, MnO2 is the most promising electrode material because of its various advantages, such as excellent faradaic properties, superior electrochemical performance for pseudo capacitors, high theoretical capacitance (1370 F g−1), and a wide potential window.26–29 It has higher theoretical capacity than a typical graphite anode and low conductivity which limits the capacitance of thick MnO2 electrodes.30 The Mn ion exists in different oxidation states such as +2, +3, +4, +6 and +7. Nanostructured MnO2 can be synthesized by different methods such as hydrothermal synthesis, solvothermal synthesis, precipitation, thermal decomposition, and sol–gel. MnO2 has excellent electrochemical properties in neutral electrolytes which guarantees less chemical corrosion of the collector.31 MnO2 may get easily dissolved in the electrolyte and this harms its cycling performance. The material's poor electron-transporting capacity also restricts its capacitive efficiency and makes it incapable of exhibiting high-power performance, which limits its potential for use in SCs. MnO2 has been reported in a variety of nanostructures, including nanosheets, nanorods, nanowires, nanoflakes, nanoflowers, and nanoplates. MnO2 exhibits a variety of crystalline phases by joining the MnO6 octahedron unit in various ways, α-, β-, γ-, ε-, δ- and λ-MnO2 which are shown in Fig. 2.32 The specific capacitance of these crystalline phases decreases in the order of α > δ > γ > λ > β. It can be divided into three types, including a 1D tunnel structure, 2D layered structure, and 3D mesh structure.33 The performance of MnO2 electrode materials is significantly influenced by their polymorphs. Due to the several polymorphs, it exhibits hierarchical electrochemical characteristics. The shape, surface area, and tunnel size of MnO2 might vary depending on the conditions and synthesis process used. The atom configurations inside these diverse crystallographic phases result in various sorts of pores, which have an effect on the electrolytic ion moment or electron transfer process in the charge storage mechanism.34 Types of MnO2 are discussed in detail in the following section.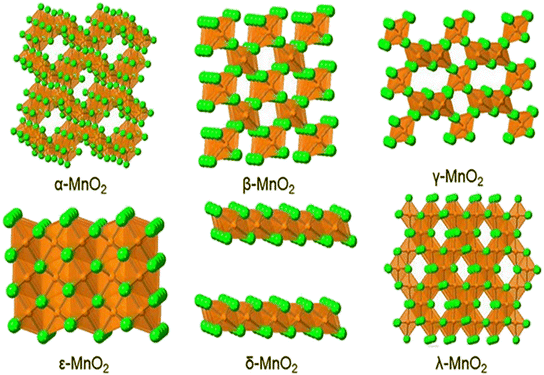 | ||
| Fig. 2 Schematic diagram of the crystal structure of α-, β-, γ-, ε-, δ- and λ-MnO2. Reproduced from ref. 32 with permission from RSC, copyright 2022. | ||
Types of MnO2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The MnO2@Zn-MOF composite electrode material exhibited a notably better performance due to its unique morphology with enhanced reactive sites and redox-active MnO2. The asymmetric SC displayed a specific capacitance of 160 F g−1 at 3 A g−1 with an energy density of 40.68 W h kg−1 at a power density of 2024.98 W kg−1. The device also showed a capacitance retention of 90% after 10
000 cycles. The MnO2@Zn-MOF composite electrode material exhibited a notably better performance due to its unique morphology with enhanced reactive sites and redox-active MnO2. The asymmetric SC displayed a specific capacitance of 160 F g−1 at 3 A g−1 with an energy density of 40.68 W h kg−1 at a power density of 2024.98 W kg−1. The device also showed a capacitance retention of 90% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.43 Furthermore, Patil et al. prepared α-MnO2 coated with polyaniline (PANI) and reduced graphene oxide (rGO) for SC application. The specific capacitance of the α-MnO2/PANI composite material was increased by using rGO. The α-MnO2/PANI/rGO composite displayed a specific capacitance of 261 F g−1 at a scan rate of 5 mV s−1 with a 25% loss of capacitance retention after 2000 cycles at a current density of 5 A g−1. The composite material exhibited an energy density of 11 W h kg−1 at a power density of 1250 W kg−1.44
000 cycles.43 Furthermore, Patil et al. prepared α-MnO2 coated with polyaniline (PANI) and reduced graphene oxide (rGO) for SC application. The specific capacitance of the α-MnO2/PANI composite material was increased by using rGO. The α-MnO2/PANI/rGO composite displayed a specific capacitance of 261 F g−1 at a scan rate of 5 mV s−1 with a 25% loss of capacitance retention after 2000 cycles at a current density of 5 A g−1. The composite material exhibited an energy density of 11 W h kg−1 at a power density of 1250 W kg−1.44
Discussed below are some representative examples of the use of MnO2-based materials for SC application.
Jayachandran et al. produced α-MnO2 nanorods as an electrode material for high-quality SCs. In three distinct aqueous electrolytes, including 1 M Na2SO4, 0.5 M KOH, and 1 M Na2SO4 + 0.5 M KOH, they examined the electrochemical performance of MnO2 nanorods. In this study, 1 M Na2SO4 + 0.5 M KOH showed outstanding electrochemical performance. It produced a 570 F g−1 specific capacitance at 1 A g−1 current density and 20% capacitance retention loss over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.50 Moniruzzaman et al. synthesized a cobalt doped@MnO2 nanosheet composite on highly conductive nickel foam for SC application. The composite material delivered a specific capacitance of 337.8 F g−1 in 2 M KOH at 0.5 A g−1 current density. This material exhibited a huge loss of capacitance retention i.e. 13% for 3000 charge–discharge cycles.51 Deng et al. fabricated α-MnO2 electrodes for high-performance asymmetric sodium (Na) ion SCs. In this work, they introduced a pre-activation strategy into the α-MnO2 nanosheet electrode to enhance the Na-storage performance. Based on α-MnO2 positive and m-WO3 negative, they developed a novel aqueous ASSC in aqueous Na2SO4 electrolyte. The electrode material showed a volumetric capacitance of 377 F cm−3 with an excellent capacitance retention of 123% after 10
000 cycles.50 Moniruzzaman et al. synthesized a cobalt doped@MnO2 nanosheet composite on highly conductive nickel foam for SC application. The composite material delivered a specific capacitance of 337.8 F g−1 in 2 M KOH at 0.5 A g−1 current density. This material exhibited a huge loss of capacitance retention i.e. 13% for 3000 charge–discharge cycles.51 Deng et al. fabricated α-MnO2 electrodes for high-performance asymmetric sodium (Na) ion SCs. In this work, they introduced a pre-activation strategy into the α-MnO2 nanosheet electrode to enhance the Na-storage performance. Based on α-MnO2 positive and m-WO3 negative, they developed a novel aqueous ASSC in aqueous Na2SO4 electrolyte. The electrode material showed a volumetric capacitance of 377 F cm−3 with an excellent capacitance retention of 123% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. It delivered an energy and power density of 0.965 W h cm−3 and 65.2 W cm−3 respectively.52 Dai et al. reported the effect of current density on the electrochemical performance of MnO2 electrodes of SCs and lithium-ion batteries. In SCs, the electrode material showed a specific capacitance of 415.4 F g−1 at the current density of 1 A g−1 while in a lithium-ion battery, it delivered 818. 1 mA h g−1 at 1 A g−1 after 200 cycles. The MnO2 electrode exhibited an energy density of 1000 mA g−1 in a lithium-ion battery.53 For high-performance aqueous SCs, Wu et al. prepared zinc (Zn)-doped MnO2 ultrathin nanosheets with defects. The Zn ion doped in MnO2 results in an increase of conductivity of the electrode material. The prepared material showed a specific capacitance of 392 F g−1 with a good capacitance retention of 95.2% after 10
000 cycles. It delivered an energy and power density of 0.965 W h cm−3 and 65.2 W cm−3 respectively.52 Dai et al. reported the effect of current density on the electrochemical performance of MnO2 electrodes of SCs and lithium-ion batteries. In SCs, the electrode material showed a specific capacitance of 415.4 F g−1 at the current density of 1 A g−1 while in a lithium-ion battery, it delivered 818. 1 mA h g−1 at 1 A g−1 after 200 cycles. The MnO2 electrode exhibited an energy density of 1000 mA g−1 in a lithium-ion battery.53 For high-performance aqueous SCs, Wu et al. prepared zinc (Zn)-doped MnO2 ultrathin nanosheets with defects. The Zn ion doped in MnO2 results in an increase of conductivity of the electrode material. The prepared material showed a specific capacitance of 392 F g−1 with a good capacitance retention of 95.2% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. It delivered an energy density of 55.28 W h kg−1 at a power density of 555.6 W kg−1.54 Saini et al. fabricated an asymmetric device using ZnCo2O4@MnO2 with a porous nanosphere decorated flower-shaped structure. The asymmetric device delivered a specific capacitance of 791.11 F g−1 at a 5 A g−1 scan rate with an energy density of 247.22 W h kg−1 at a power density of 1250 W kg−1. ZnCo2O@MnO2 showed a significant specific capacitance of 4223 F g−1 in 6 M KOH in 1 A g−1. It exhibited only a 3% loss of capacitance retention after 5000 cycles.55
000 cycles. It delivered an energy density of 55.28 W h kg−1 at a power density of 555.6 W kg−1.54 Saini et al. fabricated an asymmetric device using ZnCo2O4@MnO2 with a porous nanosphere decorated flower-shaped structure. The asymmetric device delivered a specific capacitance of 791.11 F g−1 at a 5 A g−1 scan rate with an energy density of 247.22 W h kg−1 at a power density of 1250 W kg−1. ZnCo2O@MnO2 showed a significant specific capacitance of 4223 F g−1 in 6 M KOH in 1 A g−1. It exhibited only a 3% loss of capacitance retention after 5000 cycles.55
3. Biomass-derived carbon
Carbon plays an important role in energy storage devices. Coal, natural gas, and biomass are all sources of carbon-based materials but biomass is a renewable and natural source of carbon. The surface area and tunable porous architecture of biomass-derived carbon make it an efficient electrode material in the field of energy storage devices. Additionally, due to its natural abundance, low cost, non-toxicity, environmental friendliness, and biocompatibility, it is frequently used by researchers as a promising electrode material for SCs.56 It is synthesized from naturally present biomass precursors such as wood, agricultural waste, and other organic materials. Additionally, plant-based biomaterials (such as sugar cane bagasse, orange peel, soybean, aquatic weeds, banana peel, peas, and maize cob) as well as animals and microorganism-based biomass (such as fungal [mushroom] and bacterial cellulose) have been established. While choosing a carbon source from biomass, the precursor should have a significant number of cross-linked molecules, a low elemental oxygen content, a high molecular weight, and thermally stable molecules. It exists in different morphologies, including 0D spherical, 1D fibrous, 2D sheet, and 3D porous structure.57 The carbon compounds prepared from biomass can be used in various reactions, including electrocatalytic, photocatalytic, and organic transformations.58–60 Biomass-derived carbon materials are synthesized by processes like pyrolysis and thermal carbonization. Pyrolysis is a dry-carbonization reaction that takes place at high temperatures (between 300 °C and 900 °C) in an inert atmosphere or low-oxygen environment. In pyrolysis, the performance of carbon is influenced by the catalyst, temperature, and time. On the other hand, thermal carbonization is a chemical process that transforms biomass into carbonaceous compounds and takes place in an aqueous environment at a higher temperature (300 °C) and high self-generated pressure. In this process, the water-to-biomass ratio, temperature, pressure, and duration are crucial. In comparison to pyrolysis, thermal carbonization produces more biochar yield.61 It can also be obtained by physical and chemical activation methods. Physical activation is carried out in the presence of air, steam, or gas. At low temperatures (<500 °C), air activation is carried out. Steam is the most favorable activating agent that is conducted at 800–1200 °C. The activation of CO2 is conducted at a higher degree of graphitization. Chemical activation is carried out at 450–900 °C in the presence of KOH, NaOH, ZnCl2, FeCl3 or H3PO4.62Biomass-derived carbon has several notable features such as high carbon content, porosity, and stability. Compared to conventional carbon materials, it has a larger charge storage capacity, faster charge/discharge rates, and longer cycle life due to its wide surface area. It can resist high temperatures without degrading because of its relatively good thermal stability.63 Pore size distribution, connectivity, and wettability of porous carbon are significant characteristics that have an impact on the ion diffusion and energy density of active materials. The entry of ions into pore systems and the transport of electrons are significantly influenced by the wettability of the pores. There are many different kinds of porous carbon materials, including microporous, mesoporous, and microporous, each of which has a specific function in the ion storage and transport kinetics of the material.64 Macro pores serve as ion reservoirs. They can store a large quantity of ions and act as a buffer or storage area. It keeps the ion concentration stable within the material. Furthermore, mesoporous materials are small to medium-sized porous carbon materials that offer several channels for ions to permeate through a substance. They provide ion transport by promoting ion movement within the porous carbon. This helps to maintain even ion distribution throughout the material. Materials with microporosity can present a challenge for ions due to their size. Even though some micropore regions might not directly absorb ions, they still have an impact on the material's overall charge condition. The overall charge storage capability of the porous carbon material may be impacted by the selective ion diffusion. For the structure of porous carbon, interconnected pores of various sizes are essential. The creation of 3D interconnected microporous and mesoporous structures is still challenging. To achieve acceptable wettability, surface modification of porous carbon materials is progressively becoming an issue. The majority of biomass contains heteroatoms, which increase the number of active sites and decrease the hydrophobicity of porous carbon. These components include boron, nitrogen, sulfur, and other trace elements.61 Hence, biomass-derived carbon materials are considered as the most promising electrode materials for energy storage devices. Recently reported examples of biomass-derived carbon as electrode materials for energy storage devices such as batteries and SCs are discussed below.
Mariappan et al. constructed a MnFe layer double hydroxide on carbon derived from orange peel for electrocatalytic water splitting. The MnFe-biomass-derived carbon catalyst was synthesized by pyrolysis followed by the hydrothermal method. The electrocatalyst showed better hydrogen evolution reaction and oxygen evolution reaction activities in 1 M KOH. Due to the synergic effect between MnFe-LDH and carbon, it achieved noteworthy electrolytic performance.65 Sui et al. synthesized biomass-derived carbon-coated SiO2 nanotubes for lithium-ion batteries. They used SiO2 nanotubes (SNTs) as an effective anode material due to their excellent properties and lignin furfural resin (C-PDLF) derived carbon as a cathode material for lithium-ion batteries. SNTs@C-PDLF exhibited 661 mA h g−1 specific capacity at 100 mA g−1. Compared with pristine SNTs, SNTs@C-lignin showed a better life span.66 For hydrogen fuel cells, Ding et al. prepared carbon derived from brinjal peel immobilized ultrafine Pt nanoparticles as a highly efficient catalyst. The prepared catalyst exhibited a power density of 1.246 V cm−2 at 2000 mA cm−2 in an H2 air fuel cell.67 Jin et al. constructed biomass carbon quantum dots from a spent coffee ground modified TiO2 photocatalyst for methylene blue degradation. The TiO2 catalyst showed high photocatalytic activity as well as chemical stability. N-doped carbon quantum dots (NCQDs) were used to decorate TiO2. As compared to pristine TiO2, 3-NCQDs/TiO2 showed a faster degradation rate for methylene blue. It also showed an adequate photocatalytic degradation rate of 93.1% within 60 min.68 Wang et al. synthesized N, P co-doped hard carbon derived from tea tormenta material for Na-ion batteries. The material showed excellent electrochemical performance due to the active sites in N and P groups.69 Zhang et al. synthesized a Cu-doped bamboo/polypyrrole-derived porous carbon material for SCs. Moreover, they used citric acid and copper sulfate as catalysts. Due to the synergic effects of catalysts and heteroatom doping the synthesized composite material exhibited good electrochemical performance. It showed the lowest energy density of 17.42 W h kg−1 at the highest power density of 5000 W kg−1 in Na2SO4 aqueous electrolyte.70 For high-performance SCs, Wang et al. synthesized hierarchical cornstalk-derived porous carbon (CSPC) with decorated Ag-nanoparticles. The materials were characterized using SEM and TEM as shown in Fig. 3. The CSPC-Ag electrode has a higher specific capacitance because it uses electron transport channels and active sites of Ag NPs to facilitate electrolyte ion diffusion.71
 | ||
| Fig. 3 SEM images of CSPC-0.5 (a), CSPC-1 (b), CSPC-1.5 (c), CSPC-2 (d) and CSPC-1-5% Ag (e); (f) TEM image of CSPC-1-5% Ag; (g) EDS element mapping analyses and spectrum of CSPC-1-5% Ag. Reproduced from ref. 71 with permission from Elsevier, copyright 2022. | ||
Yao et al. synthesized cheese-like hierarchical porous red dates derived activated carbon (RDAC) materials for high-performance SCs. Furthermore, Fig. 4 illustrates the SEM images of RDAC-0 and RDAC-4 samples. The material exhibited a large specific surface area (SSA) of 1115.7 m2 g−1. Due to the large SSA and porous structure, the material showed good electrochemical properties.72
 | ||
| Fig. 4 SEM images of the samples: (a and b) RDAC-0-800; (c and d) RDKC-4-800. Reproduced from ref. 72 with permission from Elsevier, copyright 2023. | ||
Agrawal et al. fabricated a symmetric SC using waste biomass Phyllanthus emblica leaves (PELC) derived high-performance AC (PELAC). The surface characterizations are depicted in Fig. 5. The PELAC electrode exhibited good cyclic stability i.e. 98.7% capacitance retention after 8000 cycles at 10 A g−1 in KOH electrolyte. The symmetric SC showed an energy density of 29 W h kg−1 at a remarkable power density of 6750 W kg−1 in PVA/KOH.73
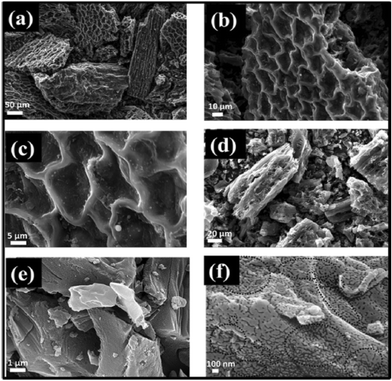 | ||
| Fig. 5 (a–c) SEM images of PELC-800, (d) SEM image of PELAC, and (e and f) FESEM images at different magnifications (inset dotted circles indicate many tiny pores/surface defects existing inside the material at the 100 nm scale). Reproduced from ref. 73 with permission from Elsevier, copyright 2023. | ||
Mehdi et al. prepared biomass-derived carbon from date seeds for SC application. The date seed biochar (DSBC) material was synthesized by slow pyrolysis at 600 °C (DSBC-600) and chemical activation at 700 °C (DSAC-700), 800 °C (DSAC-800) and 900 °C (DSAC-900) by H2SO4. Fig. 6 shows SEM images at different temperatures. Due to 3D porous structure, DSAC-700 showed good specific capacitance of 487.5 F g−1 at 1 A g−1.74
 | ||
| Fig. 6 (a) SEM micrographs for biochar produced at 600, (b) shows a micrograph of DSAC700, (c) shows a micrograph of DSAC800, and (d) shows a micrograph of DSAC900. Reproduced from ref. 74 with permission from Elsevier, copyright 2023. | ||
For a high mass loading SC, Tian et al. synthesized porous carbon from Linum usitatissimum L. root by a facile in situ activation method. The material was carbonized at 800 °C for 1 h in a N2 atmosphere. The synthesized material enhanced surface utilization and ion diffusion due to active sites as shown in Fig. 7. The material showed gravimetric/volumetric/areal capacitances of 421 F g−1 /316 F cm−3 /8.0 F cm−2 at 1 A g−1. The symmetric SC delivered an energy density of 8.16 W h kg−1.75
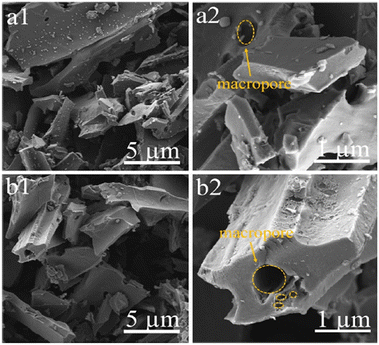 | ||
| Fig. 7 SEM images of LRC-0 (a1 and a2) and LRC-1 (b1 and b2). Reproduced from ref. 75 with permission from Elsevier, copyright 2023. | ||
Li et al. prepared N, P co-doped hierarchical porous carbon nanosheets derived from pomelo peel for SC application. The co-doping improved the conductivity, morphology, and specific surface area of the material. Fig. 8 shows the SEM images of the synthesized material. At 750 °C temperature, the N, P co-doped material exhibited an energy density of 36 ± 1.5 W h kg−1 and power density of 1000 W kg−1. It also showed outstanding capacitance retention of 99% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.76
000 cycles.76
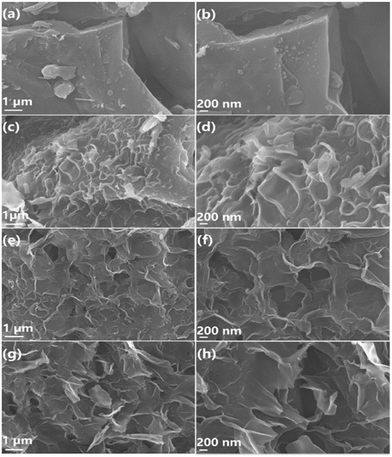 | ||
| Fig. 8 SEM images of (a and b) BC, (c and d) NPCNs-650, (e and f) NPCNs-750, and (g and h) NPCNs-850. Reproduced from ref. 76 with permission from Elsevier, copyright 2022. | ||
Tarimo et al. fabricated a symmetric SC device from a waste chicken bone-derived carbon electrode material. The SEM and TEM images at different magnifications are shown in Fig. 9. The material was carbonized at 700 °C for 2 h under an Ar atmosphere. Due to carbonization, the material improved pore size and specific surface area (2235.8 m2 g−1).77
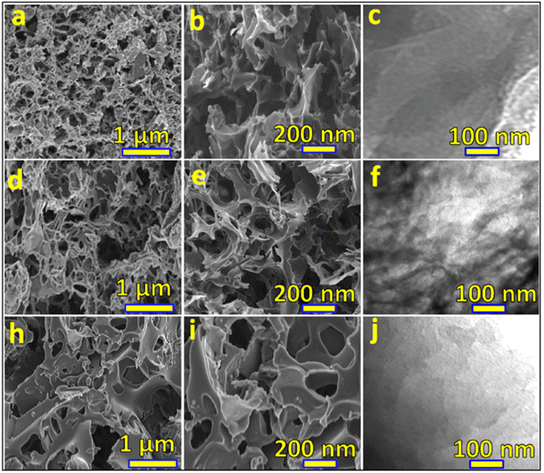 | ||
| Fig. 9 (a and b), (d and e) and (h and i) are SEM images of CCBW-0.5, CCBW-1, and CCBW-2 at low and high magnifications, respectively. (c), (f) and (j) are TEM images of CCBW-0.5, CCBW-1, and CCBW-2, respectively. Reproduced from ref. 77 with permission from Elsevier, copyright 2022. | ||
Overall, the synthesis of biomass-derived carbon materials as electrodes is a subject of intense research. Recently used biomasses obtained from various biomass-derived carbon precursors, their synthesis methods, and conditions with dimensions, porosity, and surface area are listed in Table 1.
| Sr. no. | Biomass source | Condition of carbonization/pyrolysis under N2 or in air | Carbon dimensions/pore size/surface area (m2 g−1) | Ref. |
|---|---|---|---|---|
| 1. | Agaric | Pyrolyzed at 750 °C for 2 h under N2 atmosphere | — | 78 |
| Microporous 2225.5 | ||||
| 2. | Lignin | Carbonization at 1000 °C for 1 h under N2 atmosphere | 1D | 79 |
| Mesopores 674 | ||||
| 3. | Bamboo | Carbonization at 800 °C for 2 h under N2 atmosphere | 1D | 80 |
| Micropores 1987.76 | ||||
| 4. | Lotus seedpod | Carbonization at 800 °C for 3 h under Ar atmosphere | — | 81 |
| Micropores 908.9 | ||||
| 5. | Marine biowaste | Carbonization at 1000 °C for 1 h under N2 atmosphere | — | 82 |
| Micropores 1526 | ||||
| 6. | Orange peel | Carbonization at 400 °C for 8 h in air | 3D | 83 |
| — | ||||
| — | ||||
| 7. | Agave sisalana | Carbonization at 550 °C for 60 min under N2 atmosphere | — | 84 |
| Micropores and mesopores 1464 | ||||
| 8. | Oil palm empty fruit bunch | Carbonization at 800 °C for 2 h under N2 atmosphere | — | 85 |
| Mesopores 640.61 | ||||
| 9. | Palmyra palm flower | Carbonization at 400 °C for 1 h under N2 atmosphere | — | 86 |
| Micropores 950 | ||||
| 10. | Eggfruit | Carbonization at 500 °C for 4 h under N2 atmosphere | 3D | 87 |
| Mesoporous 87.88 | ||||
| 11. | Acacia wood | Carbonization at 800 °C for 2 h under N2 atmosphere | 3D | 88 |
| Nanoporous 1563.43 | ||||
| 12. | Cassia fistula dry fruits | Carbonization at 800 °C for 3 h under N2 atmosphere | — | 89 |
| Mesoporous 625 | ||||
| 13. | Wood/sticks | Carbonization at 800 °C for 4 h under N2 atmosphere | 3D | 90 |
| Microporous and mesoporous 973 | ||||
| 14. | Caesalpinia sappan | Pyrolysis at 800 °C for 1 h under N2 atmosphere | — | 91 |
| Super microporous 675 | ||||
| 15. | Bamboo | Carbonization at 600 °C for 2 h under N2 atmosphere | — | 92 |
| Micro-mesoporous 1985 | ||||
| 16. | Acacia auriculiformis bark | Carbonization at 800 °C for 2 h under Ar atmosphere | 3D | 93 |
| Microporous and mesoporous 1147 | ||||
| 17. | Sugar beet pulp | Pyrolyzed at 700 °C for 150 min in an inert atmosphere | — | 94 |
| Micro/mesoporous 950.31 | ||||
| 18. | Eucalyptus oil | Carbonization at 700 °C for 1 h under N2 atmosphere | — | 95 |
| Microporous 329.66 | ||||
| 19. | Lemongrass | Pyrolyzed at 600 °C under N2 atmosphere | — | 96 |
| Micro-mesoporous 1694 | ||||
| 20. | Chicken feet (collagen) | Carbonization at 800 °C for 30 min under Ar atmosphere | 3D | 97 |
| Microporous 1087 | ||||
| 21. | Soybean pods | Pyrolyzed at 600 °C for 90 min under Ar atmosphere | 3D | 98 |
| Mesoporous 1807.56 | ||||
| 22. | Tobacco waste | Carbonization at 850 °C for 30 min under Ar atmosphere | — | 99 |
| Microporous 1079.2 | ||||
| 23. | Bamboo fiber | Pyrolyzed at 800 °C for 2 h under Ar atmosphere | — | 100 |
| Microporous 1900 | ||||
| 24. | Vegetable sponge | Carbonization at 600 °C for 2 h under N2 atmosphere | — | 101 |
| Mesoporous 1619.3 | ||||
| 25. | Pisum sativum | Carbonization at 800 °C for 2 h under N2 atmosphere | — | 102 |
| Microporous 240 |
4. MnO2-Biomass-derived carbon-based composites
In comparison to pure MOs, the combination of MO with carbon-based nanostructures has demonstrated superior performance in galvanostatic cycling investigations.103 Recently, MO–carbon composites have attracted considerable attention because of their chemical, physical, electrical, and optical properties as well as many potential applications.104,105 The composite combines the energy characteristics of pseudocapacitive materials (via fast redox reversible reaction) with cyclic stability and power characteristics of carbon materials (electrostatic fast ion adsorption and desorption at electrode–electrolyte interfaces) and boost cycle life. The composite materials have various benefits over conventional electrode materials. Due to more active sites for transporting electrolyte ions and a wider potential window, the composite results in high specific capacitance, good stability, and high energy and power densities.106 MnO2 is a low-cost, non-toxic and environmentally friendly MO. Additionally, it has strong electrochemical functionality for energy storage systems. Its high specific capacity but its poor conductivity and stability limits its application as an SC.107 Biomass-derived carbon can improve the electrical conductivity of MnO2 by forming a conductive network or coating on the surface of MnO2. This can increase the charge transfer rate and reduce the internal resistance of the electrode.108 A comparatively large surface area, high stability, superior electrical conductivity, and cost-effectiveness all are the features of porous carbon's structure. Porous carbon has been widely employed in batteries and SCs because of its tremendous benefits.109 MnO2 easily collapses therefore, carbon combines with it to give structural stability. MnO2 and biomass-derived carbon composites have high energy densities, good rate capabilities, and extended cycle life, making them suitable electrode materials for SCs.110 However, there are still some challenges and opportunities for further improvements, such as optimizing the synthesis methods, enhancing the conductivity and stability of the composites, exploring new biomass sources, and designing novel architectures.MnO2 and N-doped biomass-derived carbon
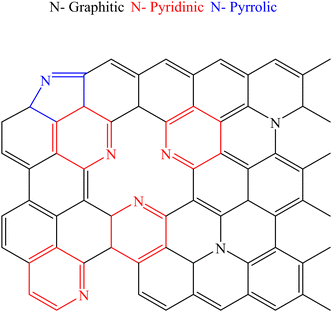
N-doping improves carbon materials' capacitance and conductivity by introducing pseudo-capacitance and active sites.111–113 In N-doped carbons, the nitrogen functional groups are typically found in the molecular configurations (chemical states) (Fig. 10): quaternary nitrogen is referred to as “graphitic nitrogen” or “substituted nitrogen”, in which nitrogen atoms are integrated into the carbon network. Pyridinic nitrogen refers to nitrogen atoms at the edges of graphite planes, each of which is bonded to two carbon atoms and donates one π electron to the aromatic system; pyrrolic N refers to nitrogen atoms that are bonded to two carbon atoms and contributes two π electrons to the system.114MnO2/N-doped biomass-derived carbon composites have been synthesized using various techniques, including solution growth, hydrothermal treatment, vapor deposition, etc. Compared to pure MnO2 or carbon materials, the composites have better chemical performance and stability for SCs.115–117 For examples, one study reported that MnO2/N-doped porous carbon spheres prepared from Na alginate showed a specific capacitance of 419.3 F g−1 at 1 A g−1 and an ultrafast charge–discharge rate of 2.5 V s−1.118
Li et al. fabricated a quasi-solid asymmetric SC using MnO2-coated and N-doped pine cone porous carbon (N-PC). MnO2-coated and N-doped porous carbon was prepared by a carbonization and hydrothermal process. Due to carbonization, the material enhanced the contact area and improved the electron transmission. The asymmetric SC showed a specific capacitance of 167.8 F g−1 at 1 A g−1 current density with a 15.5% loss of capacitance retention after 3000 cycles in PVA/Na2SO4 quasi-solid electrolyte. It also displayed an energy density and power density of 20.42 W h kg−1 and 799.9 W kg−1 respectively.119
Sui et al. prepared an asymmetric SC using polypyrrole, MnO2, and N-doped carbon nanowires (PCNW). The PCNW porous structure made of polypyrrole nanowires serves as a supportive substrate for the deposition of MnO2via a redox reaction between carbon and KMnO4. The asymmetric SC displayed a specific capacitance of 55.7 F g−1 at 0.5 A g−1 with an energy density of 23.7 W h kg−1 at a good power density of 2000 W kg−1. It exhibited capacitance retention of 87.6% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.120
000 cycles.120
For flexible solid-state asymmetric SC, Sun et al. synthesized a MnO2@N-doped AC (NAC) composite. The N-doped carbon was activated by KOH while the MnO2@NAC composite was obtained by a simple hydrothermal method. The NAC has significant interactions with MnO2 due to interface modification, allowing for fast charge transfer. The electrode material showed a specific capacitance of 408.5 F g−1 at 0.5 A g−1. The flexible solid-state asymmetric SC fabricated with NAC as the anode and MnO2@NAC as the cathode in PVA-KOH electrolyte displayed a specific capacitance of 75 F g−1 at 1 A g−1 current density with 89.5% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. It also delivered an energy density of 26.7 W h kg−1 at a power density of 400 W kg−1.121
000 cycles. It also delivered an energy density of 26.7 W h kg−1 at a power density of 400 W kg−1.121
Kongthong et al. prepared coin- and pouch-cell SCs using N-doped pineapple leaf fiber-derived activated carbon (PALF-NAC) with MnO2. The α-MnO2/PALF-NAC electrode material showed a specific capacitance of 195 F g−1 at 0.1 A g−1 in 1 M H2SO4. This electrode material showed good electrochemical performance in coin- and pouch-cell SCs. The specific capacitance exhibited 133 F g−1 and 120 F g−1 in coin- and pouch-cell SC respectively. In the coin-cell SC, the energy density is 27 W h kg−1 at 148 W kg−1 power density with a capacitance retention of 84% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Similarly in the pouch-cell SC, this electrode material delivered an energy density of 26.8 W h kg−1 at a power density of 120 W kg−1.122
000 cycles. Similarly in the pouch-cell SC, this electrode material delivered an energy density of 26.8 W h kg−1 at a power density of 120 W kg−1.122
Li et al. fabricated an asymmetric SC using MnO2 nanosheets and N-doped agaric-derived porous carbon (N-APC). The three-dimensional pore structure of N-APC enhanced the electron transmission. The electrode material exhibited a specific capacitance of 330 F g−1 at 1 A g−1. An asymmetric SC developed with N-APC as a negative electrode and MnO2@N-APC as a positive electrode in 1 M Na2SO4 showed an energy density and power density of 28 W h kg−1 and 560 W kg−1 respectively.123
Wang et al. reported the multicore–shell MnO2@Ppy@N-doped porous carbon nanofiber ternary composite electrode material for a high-performance SC. The composite material showed a specific capacitance of 595.77 F g−1 with a 4% loss of capacitance retention after 1000 cycles. The asymmetric SC delivered an energy density of 9.36 W h kg−1 at 1000 W kg−1 power density.124
Synergic effects
The MO present in the carbon matrix provides electrical conductivity to the electrons produced during the redox reaction from the MO. It is believed that the device will have outstanding electrochemical performance due to the faradaic redox reaction in MO and the huge specific surface area of AC.125 The electrical conductivity, specific capacitance, rate capability, and cyclic stability of the electrodes can all be enhanced by the synthesis of a composite of MnO2 and carbon produced from biomass. The synthesis techniques, shape, and crystal structure of the composite all affect the synergistic effects of MnO2 and carbon produced from biomass.126 Layered MnO2 can limit volume expansion and improve the utilization of MnO2 such as α-, β-, γ-, ε-, δ-, and λ which have distinct electrochemical features and affinity with carbon materials.127 Porous carbon can provide more active sites and channels for ion transport inside the film. Therefore, it is essential to optimize the synthesis and production of MnO2 and carbon composites obtained from biomass to produce high-performance SCs. MnO2 can be synthesized in different morphologies/shapes such as nanosheets, nanorods, nanoflowers, nano wrinkles, and nanoparticles. There are several reports available regarding MnO2 nanosheets like morphology and biomass-derived carbon for SC applications. All the experiments except MnO2/egg yolk by Feng et al. were carried out using a three-electrode cell configuration in Na2SO4 electrolyte. Some examples are explained below.Nanosheets
He et al. fabricated flax textile-derived carbon fiber cloth coated with MnO2 nanosheets for SC application. Although flax textile has good stability it suffers from low specific capacitance; therefore to achieve good specific capacitance it is coated with MnO2 nanosheets. The composite material delivered the highest specific capacitance of 683.73 F g−1 at a current density of 2 A g−1 and 94% capacitance retention after 1000 cycles. The electrode material showed an energy density of 46.54 W h kg−1 at the highest power density of 45.50 kW kg−1.128Li et al. prepared a 3D hollow carbon skeleton from lotus pollen and hexagonal MnO2 nanosheet composite for a high-performance SC. Due to chemical activation in the presence of KMnO4, the MnO2/C material improved the morphology and enhanced the electrochemical performance. The composite exhibited a high specific capacitance of 257 F g−1 at a current density of 0.5 A g−1. It showed an energy density and power density of 51.5 W h kg−1 and 303 W kg−1 respectively. The composite displayed very high capacitance loss i.e. 12% for 2000 cycles.129
Mao et al. synthesized the fungal conidium-derived carbon/MnO2 composite for SC application. Sulfur element from conidium enhanced electron transit and created readily available active sites for MnO2 nanosheet binding. The specific capacitance of 263.5 F g−1 was reported at 1 A g−1. The composite material revealed an energy density of 542 W h kg−1 at an outstanding power density of 1000 W kg−1 but 17% capacitance loss was observed for only 2000 cycles.130
The MnO2/allium-giganteum-like biocarbon (KWB) derived from sugarcane bagasse composite was fabricated by Chen et al. KWBM was created by binding MnO2 nanosheets on the surface of biocarbon. The composite material showed a specific capacitance of 402 F g−1 at 1 A g−1 in a three-electrode assembly. KWBM-4 was used as the positive electrode and KWB as the negative electrode to create an asymmetric two-electrode setup which exhibited an energy density of 25.9 W h kg−1 at a superior power density of 750 W kg−1. The composite material displayed excellent cyclic stability with 94.2% capacitance retention after 2000 cycles.131
Chen et al. synthesized carbon derived from soybean stalk with MnO2. They reported that nanostructured MnO2 with a highly porous and conductive matrix had improved pseudocapacitive kinetics. The electrode material exhibited a specific capacitance of 384.9 F g−1 at low current density. It revealed an energy density and good power density of 32.3 W h kg−1 and 9.58 kW kg−1 respectively. The material showed 90.7% capacitance retention after 5000 cycles.132
For a high-performance SC, Kong et al. fabricated carbon derived from wheat bran which was further decorated with MnO2 nanosheets. Due to hierarchical porous carbon, the material enhances ion or electron transmission. MnO2@PAC as the positive electrode and PAC as the negative electrode were used for the asymmetric SC. The electrode material displayed a specific capacitance of 258 F g−1 at 1 A g−1. Also, it exhibited an energy density of 32.6 W h kg−1 at a power density of 450 W kg−1. The capacitance retention of 93.6% was achieved after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles.133
000 charge–discharge cycles.133
Feng et al. reported biomass-derived carbon with incorporation of MnO2 for SC application. In this work, P, N, and O-tri-doped egg yolk-derived carbon were used. The heteroatom-doped biomass-derived carbon improved electrochemical properties. The electrode material delivered a specific capacitance of 341 F g−1 at a current density of 1 A g−1. The electrode material exhibited a power density of 0.2 W kg−1 with 38.4 W h kg−1 energy density and low capacitance loss was observed for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In this report, the material showed good specific capacitance and energy density but very low power density than other reports.134
000 cycles. In this report, the material showed good specific capacitance and energy density but very low power density than other reports.134
Li et al. prepared 3D porous MnO2@carbon nanosheets synthesized from rambutan peel. They fabricated an asymmetric SC of MnO2@R//NR-800 by using the MnO2@R composite material as a positive electrode and NR-800 as a negative electrode. The results showed a low specific capacitance of 139.6 F g−1 at very low current density. It showed cycling life i.e., 92% capacitance retention after 5000 charge–discharge cycles with an energy density of 9.2 W h kg−1 and good power density of 1283.7 W kg−1.135
Li et al. reported MnO2 nanosheets derived from agaric grown on biomass-derived N-doped carbon for an asymmetric SC. The composite material showed good electrochemical performance due to the 3D pore structure and large specific surface area. It exhibited a specific capacitance of 330 F g−1 at 1 A g−1 and very low cyclic stability i.e., 75% capacitance retention after 1000 cycles. Also, the electrode showed a low energy density of 28 W h kg−1 at a power density of 560 W kg−1.136
Li et al. fabricated a MnOx nanosheet anchored on lotus seed pod-derived carbon composite for a high-performance SC. The prepared MnOx@LSCF hybrid electrode material comprised a 3D conductive network and ultrathin MnOx nanosheets with oxygen vacancies, which can boost intrinsic electron and ion transmission and allow for adequate surface faradaic redox reactions. The electrode material showed a specific capacitance of 406 F g−1 with a capacitance retention of 91% after 5000 cycles. The material delivered low energy as well as power density.137
Yu et al. fabricated an electrode material for an SC from a MnO2 nanosheet composite with bamboo leaf (MnO2@BL) carbon. The SEM and TEM images of BL and MnO2@BL are depicted in Fig. 11 along with the EDS map. A specific capacitance of 76 F g−1 was achieved at a current density of 0.5 A g−1. It showed a 14% loss for 5000 cycles.138
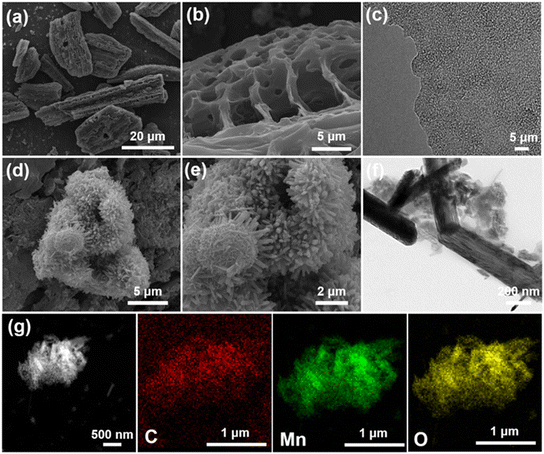 | ||
| Fig. 11 (a and b) SEM images of BL-3, (c) TEM image of BL-3, (d and e) SEM images of MnO2@BL-3 h, (f) TEM image of MnO2@BL, and (g) MnO2@BL-3-6 h EDS map. Reproduced from ref. 138 with permission from ACS, copyright 2023. | ||
Nanowires
Yang et al. fabricated a porous 3D structure of MnO2 nanowires and hemp stem-derived AC (HC) as an electrode material for SC application. It exhibited a specific capacitance of 340 F g−1 at 1 A g−1 current density with 12% capacitance loss after 3000 cycles. The electrochemical performance was measured in a three-electrode assembly using 1 m Na2SO4 as an electrolyte. For the asymmetrical SC, 3D HC as the anode and 3D MnO2/HC as the cathode were used. The electrode material exhibited a specific energy of 33.3 W h kg−1 at a specific power density of 14.8 W kg−1.139The work done by Mohammed et al. demonstrated a hybrid SC device. The MnO2 nanowires were decorated on the Faidherbia Albida fruit shell (FAFSC) carbon sphere. They fabricated a symmetric device using MnO2/FAFSC as a negative and positive electrode while MnO2/FAFSC as a negative electrode for an asymmetric SC. The composite electrode material showed electrochemical performance in two different electrolytes such as 3 M KOH and 1 M Na2SO4. It revealed specific capacitances of 426 F g−1 and 202.5 F g−1 in 3 M KOH and 1 M Na2SO4 respectively. The electrode material delivered a good cycle life of 97% capacitance retention after 1000 cycles. Also, it exhibited 32 W h kg−1 energy density at 400 W kg−1 power density.140
Zang et al. synthesized a MnO2/biomass-derived carbon composite using silkworm excrement. The thin and flower-like MnO2 nanowires were decorated on biomass-derived carbon. The hybrid electrode material displayed a specific capacitance of 238 F g−1 at 0.5 A g−1 current density of 1 M Na2SO4. It exhibited an energy density of 38.6 W h kg−1 at a power density of 698 W kg−1 with 7% capacitance loss for 2000 cycles.141
Nanorods
Shen et al. fabricated a δ-MnO2/soybean stalk carbon composite electrode material which delivered a superior specific capacitance of 530 F g−1 at 0.2 A g−1. It showed better cycling stability with 91% capacitance retention after 6000 consecutive charge–discharge cycles with an energy density of 5607 W h kg−1 at a power density of 90 W kg−1.142For a high-energy asymmetric SC, Nirmaladevi et al. synthesized MnO2 nanorods with carbon derived from Acacia leucophloea wood sawdust. Electrochemical characteristics were determined using three and two electrode set ups. The composite material revealed a specific capacitance of 290 F g−1 in the two-electrode cell configuration and 512 F g−1 in the three-electrode assembly at a current density of 0.5 A g−1 and 7% capacitance loss for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The electrode materials showed an energy density and power density of 74.3 W h kg−1 and 1996.4 W kg−1 respectively.143
000 cycles. The electrode materials showed an energy density and power density of 74.3 W h kg−1 and 1996.4 W kg−1 respectively.143
Nanoflakes
Yuan et al. successfully synthesized a hierarchically porous MnO2 nanoflakes/rice husk-derived carbon composite as an electrode material for an SC. The MnO2 film was composed of well-dispersed nanoflakes that anchored on the surface of rice husks for adequate structural stability. The composite material showed electrochemical properties in 0.5 M Na2SO4 electrolyte. The material exhibited a specific capacitance of 197.6 F g−1 at 5 mV s−1 scan rate and 210.3 F g−1 at 0.5 A g−1. The composite displayed a 20% capacitance loss for 5000 charge–discharge cycles.144Hu et al. synthesized MnO2/poly(3,4-ethylene dioxythiophene) core–shell nanoflakes on ramie-derived fiber. The electrochemical performances shown in Fig. 12 were measured using a two-electrode assembly in PVA/KCl gel solid-state electrolyte. The hybrid electrode material revealed a high specific capacitance of 922 F g−1 at 1 A g−1. The fabricated flexible solid-state symmetric device showed a specific capacitance of 138 F g−1 at a current density of 1 A g−1 and 17% capacitance loss after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. It showed an energy density of 19.17 W h kg−1 at a power density of 500 W kg−1.145
000 cycles. It showed an energy density of 19.17 W h kg−1 at a power density of 500 W kg−1.145
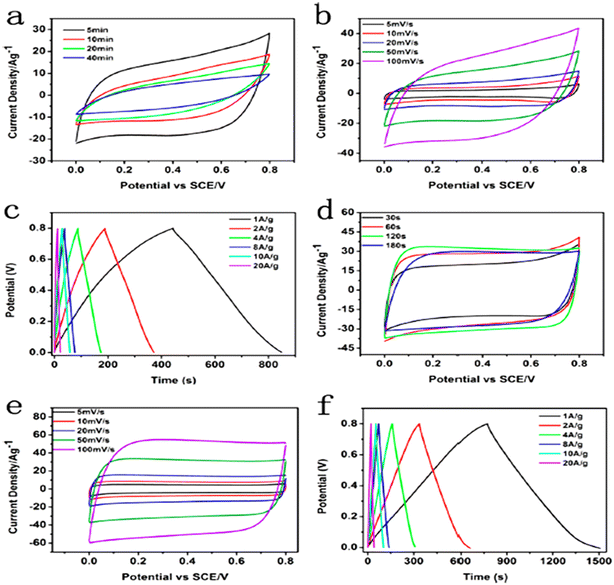 | ||
| Fig. 12 (a) CV curves of the RCFs/MnO2 electrodes with different electrodeposition times at 50 mV s−1. (b) CV curves at varied potential scan rates of the RCFs/MnO2 electrode with MnO2 deposited for 5 min. (c) Charge–discharge curves at different current densities of the RCFs/MnO2 electrode with MnO2 deposited for 5 min. (d) CV curves of the RCFs/MnO2/PEDOT electrodes with different polymerization times at 50 mV s−1. (e) CV curves at varied potential scan rates of the RCFs/MnO2/PEDOT electrode with PEDOT deposited for 120 s. (f) Charge–discharge curves at different current densities of the RCFs/MnO2/PEDOT electrode with PEDOT deposited for 120 s. Reproduced from ref. 145 with permission from ACS, copyright 2016. | ||
Nanoneedles
Biowaste-derived carbon from human hair (Acs) was synthesized by Deng et al., and decorated with a needle-like morphology of MnO2. The ACs and MnO2 were synthesized in different weight ratios i.e., ACs/KMnO4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10
1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 12
1, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 14
1, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 16
1 and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, MnO2/ACs (1
1. Then, MnO2/ACs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) delivered a specific capacitance of 300 F g−1 after 500 cycles in 1.0 M H2SO4. The MnO2/ACS (1
12) delivered a specific capacitance of 300 F g−1 after 500 cycles in 1.0 M H2SO4. The MnO2/ACS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) electrode material showed capacitance values of 410 F g−1, 345 F g−1, and 251 F g−1 in 1 M H2SO4, 1 M KOH, and 1 M Na2SO4 aqueous electrolyte respectively. Therefore, the electrode material exhibited the highest capacitance value in 1 M H2SO4 electrolyte solution.146
12) electrode material showed capacitance values of 410 F g−1, 345 F g−1, and 251 F g−1 in 1 M H2SO4, 1 M KOH, and 1 M Na2SO4 aqueous electrolyte respectively. Therefore, the electrode material exhibited the highest capacitance value in 1 M H2SO4 electrolyte solution.146
Nano wrinkles
Yuan et al. fabricated a prototype asymmetric SC device using chicken bones as a 3D hierarchical porous carbon scaffold (HPCS) and MnO2 composite. They reported that MnO2 showed a nano wrinkle morphology when it was deposited on the 3D-HPCS template. Electrochemical performance was measured using two and three-electrode cell configurations in 1 M Na2SO4 aqueous electrolytic solution. The composite electrode material revealed a specific capacitance of 476.4 F g−1 at a current density of 1 A g−1 and approximately 84.9% capacitance retention for 4000 cycles. The electrode material exhibited a specific energy of 60.8 W h kg−1 at a power density of 20.7 W kg−1.147Nanoplates
For a high-performance asymmetric SC, Li et al. fabricated MnO2 nanoplates on biomass-derived carbon nanosheets from Willow Catkin. Interconnected thin carbon nanosheets behave as 3D conductive networks which is useful for rapid electron transmission. The electrode material tests were carried out using three electrode assemblies in 1 M Na2SO4. The composite electrode material exhibited a gravimetric capacitance of 188 F g−1 at 5 mV s−1. It showed superior cycling performance with only 1.4% capacitance loss after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles with 23.6 W h kg−1 energy density at 188.8 W kg−1 power density.148
000 charge–discharge cycles with 23.6 W h kg−1 energy density at 188.8 W kg−1 power density.148
Nanowalls
In the work done by Wang et al., they synthesized δ-MnO2/ carbon sheets from biomass-derived cornstalk for an SC. Due to the synergic effects of δ-MnO2 nanowalls and biomass-derived carbon nanosheets, the hybrid electrode material showed a significant specific capacitance of 520 F g−1 at 1 mV s−1 scan rate in Na2SO4, but it suffered from poor cyclic stability. A 20% capacitance loss was observed after 5000 charge–discharge cycles.149Others
Lu et al. synthesized a biomass-derived carbon from activated Ganoderma lucidum spore and pompon-like MnO2 composite. In this case, the morphology of MnO2 was rare and unique. O and N heteroatoms were used to enhance the electrochemical properties such as surface polarity, reaction activity, wettability, and electronic conductivity. The electrochemical performances are depicted in Fig. 13. The composite material delivered a remarkable specific capacitance of 1324 F g−1 at 1 A g−1 current density using an electrode system in a 6 M KOH aqueous electrolyte. Although the electrode material showed a significant energy density of 43.75 W h kg−1 it suffers from low power density i.e., 250 W kg−1. It showed 92.3% capacitance retention after 5000 charge–discharge cycles.150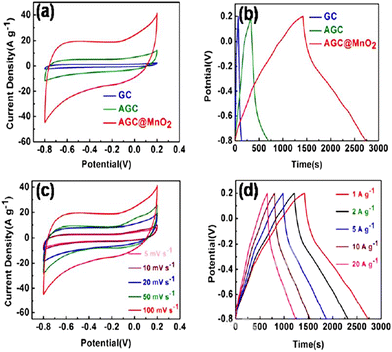 | ||
| Fig. 13 (a) CV curves of GC, AGC, and AGC@MnO2 at 100 mV s−1. (b) GCD curves of GC, AGC, and AGC@MnO2 at 1 A g−1. (c) CV curves of AGC@MnO2 at different scan rates. (d) GCD curves of AGC@MnO2 at 1, 2, 5, 10, and 20 A g−1. Reproduced from ref. 150 with permission from Elsevier, copyright 2020. | ||
Tan et al. synthesized the composite of MnO2 with sisal hemp active carbon (SHAC) and used it as an electrode material which showed a specific capacitance of 397.9 F g−1. An asymmetric SC was assembled by employing flower-like MnO2/SHAC as the positive electrode and layered SHAC as the negative electrode. It exhibited capacitance retention of 80.4% after 5000 cycles. The electrode material revealed an energy density and superior power density of 46.2 W h kg−1 and 3679.5 W kg−1 respectively.151
Li et al. prepared an asymmetric all solid-state SC from biomass waste-derived carbon from corncob and MnO2 composite. The MnO2@corncob composite material delivered an excellent electrochemical performance in PVA/LiCl gel electrolyte. The composite electrode material revealed an areal specific capacitance of 3455 mF cm−2 at 1 mA cm−2 and only 1% loss of capacitance was observed after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. It exhibited a very low energy and power density of 1.56 mW h cm−2 and 900 mW cm−2 respectively.152
000 cycles. It exhibited a very low energy and power density of 1.56 mW h cm−2 and 900 mW cm−2 respectively.152
Yu et al. synthesized Typha orientalis leaves biomass-derived P-doped porous carbon and MnO2 hybrid electrodes for high-performance SCs. The electrode material delivered a specific capacitance of 384 F g−1 in 1 M KOH at 1 A g−1 current density. The fabricated asymmetric SC device displayed an energy density of 46 W h kg−1 at a high-power density of 1000 W kg−1. However, it showed a huge capacitance loss i.e. 17% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.153
000 cycles.153
Konnerth et al. synthesized MO-doped AC from biomass-derived materials such as bakery waste and coffee grounds. They used MOs such as Fe2O3, Fe3O4, and MnO2, to fabricate composites with bakery waste and coffee grounds. The electrochemical performance tests were carried out in 0.5 M H2SO4 aqueous electrolyte. The better electrochemical results were obtained for the AC spent coffee ground-MnO2 electrode material. An 875 F g−1 specific capacitance achieved at 5 mV s−1 scan rate and 40.3 F g−1 was observed for bakery waste containing Fe2O3 doping.154
The work done by Palem et al. synthesized carboxymethyl cellulose with a CuO@MnO2 hybrid material. The material showed good electrochemical performance due to its unique nanocrystalline morphology. The electrochemical properties were measured in 3 M KOH as an electrolyte. This composite material showed a specific capacitance of 414 F g−1 at 0.5 A g−1 current density with 96.2% capacitance retention after 5000 cycles.155
The experiment performed by Wen et al. used disposable bamboo chopsticks and converted them into carbon fibers. MnO2 was grown on carbon fiber to obtain a composite material. The MnO2 nanoparticles were observed on the surface of carbon fibers which are clearly shown in Fig. 14. The self-supported and binder-free composite electrode material showed better electrochemical performance in aqueous 1 M Na2SO4 solution. The composite material exhibited a specific capacitance of 375 F g−1 at 1 A g−1 and outstanding capacitance retention i.e., 110.3% was achieved after 5000 cycles. The symmetric SC consists of carbon fiber and MnO2 electrodes delivering an energy density of 11 W h kg−1 at 17.4 kW kg−1 power density.156
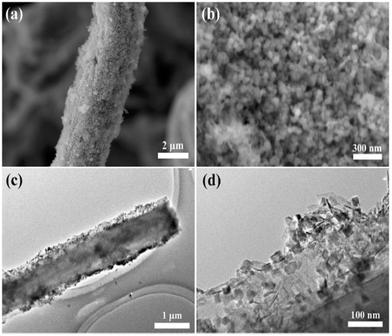 | ||
| Fig. 14 (a) SEM images and (b) corresponding enlarged view of CFS/MnO2. (c) TEM image and (d) corresponding enlarged view of CFS/MnO2. Reproduced from ref. 156 with permission from Elsevier, copyright 2017. | ||
A three-dimensional hybrid kenaf stem-derived porous carbon and MnO2 composite was prepared by Wang et al., for a high-performance SC. The three-dimensional porous structure of carbon (as shown in Fig. 15) delivered a conductive network to enhance the charge transport and mass transfer in the electrochemical process and improve large MnO2 mass loading capacity. The specific capacitance achieved by the composite material was 416 F g−1 at a scan rate of 1 mV s−1 with the lowest energy density as well as power density of 17.3 W h kg−1 and 198 W kg−1 respectively. Moreover, it also suffers from low cyclic stability i.e., 86% capacitance retention after 1000 cycles.157
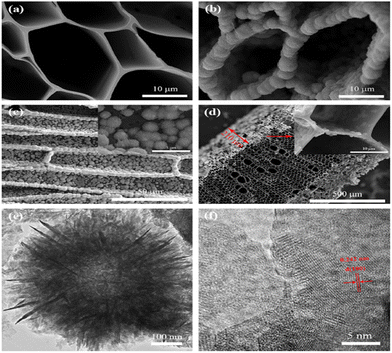 | ||
| Fig. 15 (a) SEM image of 3D-PC. (b–d) SEM images of MnO2/3D-PC after a 200 min deposition. (b) Top view. (c) Side view. (d) Cross-section. TEM (e) and HRTEM (f) images of MnO2/3D-PC. Reproduced from ref. 157 with permission from Elsevier, copyright 2014. | ||
Yang and Park synthesized MnO2/biomass-derived 3D porous carbon from banana peel by a hydrothermal method. The pore images of the BPC composite are shown in Fig. 16. Due to the 3D porous structure, the material showed good electrochemical performance. The electrochemical tests were carried out using three-electrode cell configurations in 1 M Na2SO4 aqueous electrolyte. The composite material exhibited a gravimetric capacitance of 139.6 F g−1 at 300 mA g−1. Also, it delivered a good cyclic life of 92.3% capacitance retention after 1000 cycles.158
 | ||
| Fig. 16 SEM images of (a and b) BPC at different magnifications, (c) MnO2 particles, and (d–f) MnO2/BPC composites at different magnifications. Reproduced from ref. 158 with permission from Elsevier, copyright 2018. | ||
Wang et al. fabricated a device using MnO2/orange peel as the biomass-derived material for a solid-state carbon-based SC. The schematic illustration of the synthesized material and characterization techniques such as XRD, XPS, SEM, TEM, and HRTEM are shown in Fig. 17. The electrochemical performance of the electrode material was measured using a three-electrode assembly in 1 M Na2SO4 while PVA gel electrolyte was used for the device. The composite material displayed 186 F g−1 gravimetric capacitance and areal capacitance of 3987 mF cm−1. The material showed huge capacitance loss i.e. 16%.159
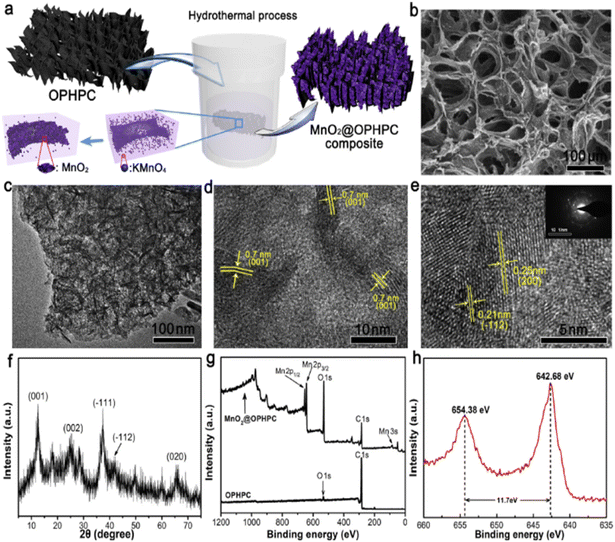 | ||
| Fig. 17 (a) The schematic illustration of the fabrication of the MnO2@OPHPC composite. (b) SEM image, (c) TEM image, and (d and e) HRTEM images of MnO2@OPHPC. (f) XRD of MnO2@OPHPC, (g) survey XPS spectra of OPHPC and MnO2@OPHPC, and (h) Mn 2p core-level XPS spectrum of MnO2@OPHPC. Reproduced from ref. 159 with permission from Elsevier, copyright 2018. | ||
For a high-performance SC, Liu et al. deposited MnO2 nanostructures on graphene-like porous carbon nanosheets (GPCN-SS). The SEM and TEM images of porous carbon and MnO2/GPCN-SS composites are shown in Fig. 18. Due to the synergistic effect of GPCN-SS and MnO2 nanostructures, the composite material showed a gravimetric specific capacitance of 438 F g−1 at a current density of 0.5 A g−1. The asymmetric SC was prepared with the MnO2/GPCN-SS composite as the cathode and GPCN-SS as the anode. Moreover, the MnO2/GPCN-SS electrode material displayed an energy density of 50.2 W h kg−1 at 516 W kg−1 power density. However, 22% of capacitance loss was observed for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.160
000 cycles.160
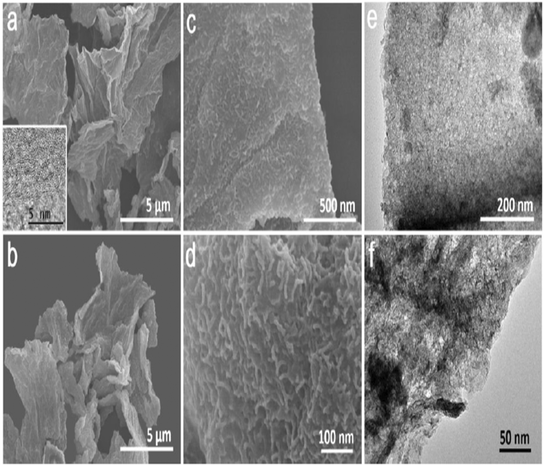 | ||
| Fig. 18 SEM images of GPCN-SS (a) and MnO2/GPCN-SS (b–d) at different magnifications; the inset of (a) shows the HRTEM image of GPCN-SS. (e and f) TEM images of MnO2/GPCN-SS at different magnifications. Reproduced from ref. 160 with permission from ACS, copyright 2019. | ||
Ping et al., in their work, incorporated MnO2 with biomass-derived porous carbon from natural Juncus effusus. The electrochemical performances were measured in a neutral 1 M Na2SO4 electrolyte. The composite material delivered a low specific capacitance of 238 F g−1 at 1 A g−1. The energy density and power density for the symmetric device were 19.13 W h kg−1 and 225 W kg−1. However, it suffered from poor cyclic stability and very huge capacitance loss i.e. 14% after 5000 cycles.161 Yumak et al. successfully synthesized biomass-derived AC from Kanlow switchgrass and decorated it with MnO2. The specific surface area of the material increased by direct KOH and H3PO4 activation. Fig. 19 shows the SEM images of materials. However, a notable effect was observed for the KOH-activated biochar. The hybrid composite material showed low specific capacitance of 110 F g−1 for 1000 cycles in a 6 M KOH aqueous electrolyte.162
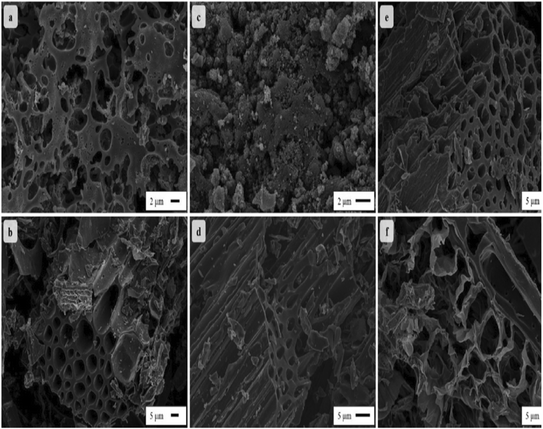 | ||
| Fig. 19 SEM microstructures of the activated carbons: a) KOH-K, b) KOH-KB, c) H3PO4-K, d) H3PO4-KB, e) MnO2/KOH-KB, and f) MnO2/H3PO4-KB. Reproduced from ref. 162 with permission from Elsevier, copyright 2020. | ||
For a high-performance SC, Du et al. synthesized an electrode material from a wheat flour-derived matrix decorated with MnO2 nanoparticles. The synthesized materials were characterized by SEM, TEM, HRTEM, and elemental mapping along with the SAED pattern as shown in Fig. 20. The electrode material exhibited a specific capacitance of 197 F g−1 at 1 A g−1 current density with a remarkable capacitance retention of 100% after 5000 cycles. The symmetric SC was prepared with WFC@MnO2 as positive and negative electrodes which resulted in an energy density of 17.1 W h kg−1 at an excellent power density of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 and only 5% loss of capacitance retention after 5000 charge–discharge cycles.163
000 W kg−1 and only 5% loss of capacitance retention after 5000 charge–discharge cycles.163
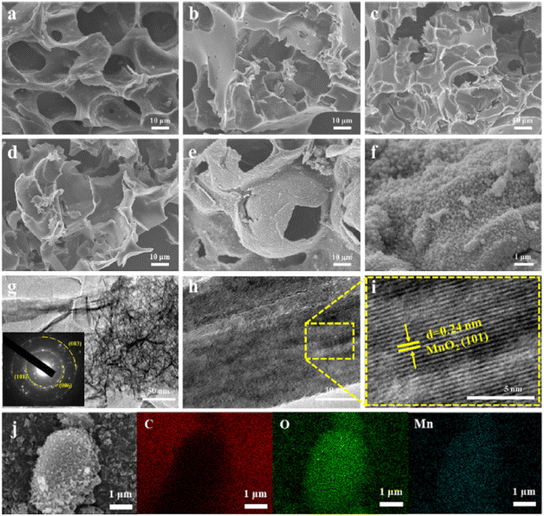 | ||
| Fig. 20 SEM images of (a) WFC-0, (b) WFC-2, (c) WFC-4, (d) WFC-6, (e and f) WFC-4@MnO2, (g and h) TEM images of WFC-4@MnO2, the inset shows the corresponding SAED pattern from the edge of MnO2, and (i) HRTEM image of WFC-4@MnO2. (j) Element mapping images of C, O, and Mn. Reproduced from ref. 163 with permission from Elsevier, copyright 2023. | ||
Table 2 highlights the latest reports on MnO2 and biomass-derived carbon composites for SC applications. Notably, these studies emphasize on the morphology of MnO2 and electrochemical studies of composite materials.
| Sr. no. | Morphology of metal oxide | Biomass-derived carbon | Specific capacitance (F g−1) | Scan rate/current density | Energy density (W h kg−1) | Power density (W kg−1) | Cyclic stability (capacitance retention %, cycles) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1. | MnO2 nanorods | Sweet potato | 718 | 10 mV s−1 | 66.4 | 1980 | 89%, 5000 | 1 M Na2SO4 | 164 |
| 2. | Two-dimensional layered δ-MnO2 | Bamboo fibre | 364.4 (2E) | 0.5 A g−1 | 12.6 | 164 | 99.7%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6 M KOH | 165 |
| 3. | MnO2/CuO/rGO | Lemon peel | 177 | 2 A g−1 | 79.60 | 2430 | 90%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 M Na2SO4 | 166 |
| 4. | MnO2 | Eggplant | 652 | 0.5 A g−1 | — | — | 79.2%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 M Na2SO4 | 167 |
| 5. | MnO2 | Wood | 87 | 1 A g−1 | 12.2 | 22.3 | 75.2%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 168 |
| 6. | MnO2 | Datura stramonium seedpod | 700 | 5 mV s−1 | — | — | 86%, 3000 | 0.5 M Na2SO4 | 169 |
| 7. | MnO2 nanofibers | Pineapple leaf | 195 | 0.1 A g−1 | 26.8 | 120.5 | 84.5%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 M H2SO4 | 170 |
| 8. | δ-MnO2 nanosheets | Lignin | 198 | — | 3.82 | 125 | — | — | 171 |
| 9. | MnO2 nanoflowers | Bacterial cellulose | 170 | 1 A g−1 | 9.8 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
76%, 5000 | 1 M Na2SO4 | 172 |
| 10. | MnO2 | Luffa sponge | 586 | 1 A g−1 | 31.4 | 400 | 95%, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.5 M Na2SO4 | 173 |
| 11. | δ-MnO2 | Reed residue | 128.75 | 0.5 A g−1 | 22.7 | 15.1k | 98.7%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 M Na2SO4 | 174 |
| 12. | MnO2 | Wood tracheids | 13.55 F cm−2 | 10 mA cm−2 | 0.875 mW cm−2 | 9 mW cm−2 | 93.21%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 M Na2SO4 | 175 |
Recent reports on MnO2 and several carbon-based composite electrode materials for SCs obtained from different biomasses are shown in Table 2. It illustrates the specific capacitance, power density, current density, scan rate, cyclic stability, and various electrolytes used in electrochemical reactions. Different shapes of MnO2 such as nanorods, nanofibers, nanosheets, and nanoflowers were mentioned. Most of the experiments were conducted using plant-derived carbon. In the studies, the electrolytes Na2SO4, KOH, and H2SO4 were widely used. The table elucidates that the highest specific capacitance reported in MnO2 nanorod and carbon derived from sweet potato material is 718 F g−1 with 10 mV s−1 scan rate. Although it has a low energy density and power density, the two-dimensional layered δ-MnO2 and biomass-generated carbon from bamboo fiber exhibits exceptional cyclic stability, with 99.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The outstanding power density of 15.1 kW kg−1 with good capacitance retention of 98.7% after 10
000 cycles. The outstanding power density of 15.1 kW kg−1 with good capacitance retention of 98.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles is revealed by δ-MnO2 and carbon generated from reed residue waste. MnO2 and carbon made from bacterial cellulose have good power densities of 10
000 cycles is revealed by δ-MnO2 and carbon generated from reed residue waste. MnO2 and carbon made from bacterial cellulose have good power densities of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 W kg−1 but have low specific energies and energy densities of 170 F g−1 and 9.8 W h kg−1, respectively. With a specific capacitance of 177 F g−1 at 2 A g−1 current density and an energy density of 79.60 W h kg−1 at a power density of 2430 W kg−1, the MnO2/CuO/r-GO composite with carbon generated from lemon peel was employed to synthesize an electrode material. The lowest specific capacitance, energy density, and power density were found in the MnO2 and carbon produced from wood tracheids, with values of 13.55 F cm−2, 0.875 mW h cm−2, and 9 mW cm−2, respectively. MnO2 and carbon produced from wood have poor cycle stability. In most of the reports, electrochemical testing was done using a three-electrode assembly.
500 W kg−1 but have low specific energies and energy densities of 170 F g−1 and 9.8 W h kg−1, respectively. With a specific capacitance of 177 F g−1 at 2 A g−1 current density and an energy density of 79.60 W h kg−1 at a power density of 2430 W kg−1, the MnO2/CuO/r-GO composite with carbon generated from lemon peel was employed to synthesize an electrode material. The lowest specific capacitance, energy density, and power density were found in the MnO2 and carbon produced from wood tracheids, with values of 13.55 F cm−2, 0.875 mW h cm−2, and 9 mW cm−2, respectively. MnO2 and carbon produced from wood have poor cycle stability. In most of the reports, electrochemical testing was done using a three-electrode assembly.
5. Conclusions and future perspective
This review offered information about recent advancements in MnO2 and biomass-derived carbon-based composites for SC application. The electrochemical properties of SCs are affected by electrode materials, preparation process, and electrolytes. Thus, the selection of electrode material plays an important role in achieving good electrochemical properties. As the analyzed literature reports suggest, MnO2 is a famous supercapacitive electrode material among all TMOs due to its various advantages. The types and morphologies of MnO2 play an important role in the final electrochemical performance of the material. However, MnO2 suffers from low electrical conductivity and poor stability and it was found that this weakness can be overcome by preparing composites of MnO2 with different materials. Like MnO2, biomass-derived carbon materials have also received increased attention in the field of energy storage devices. Here, we collectively reported various biomass precursors to obtain the carbon materials and the properties of carbon such as structural features, surface area, and porosity. It was observed that biomass-derived carbon is synthesized from a variety of materials such as crops, plants, animals, and waste biomass. Due to high porosity, the carbon provides several active sites for the electrolyte ions resulting in enhanced stability and specific capacitance. The carbon can be combined with MnO2 to achieve better electrochemical performances. In fact, all the examples of MnO2-biomass-derived carbon-based materials discussed above proved this point. The future research in this area will be continued along following main directions.The first main approach involves development of newer and more efficient synthesis techniques both for MnO2 and carbon. Although most of the synthesis methods are well-optimized to obtain MnO2 with desired features there is still considerable scope for the development of in situ methods that will directly form the film of material on the desired substrate to be used as an electrode. The new developments in thin film technologies have come up with several efficient methods but they need to be adapted or suitably modified to obtain complex shapes (such as core–shell or porous) of the desired materials. Layer by layer assembly of the materials is also a requirement in some applications. Meanwhile, almost all reports about biomass-derived carbon have used pyrolysis and carbonization methods at high temperatures in an inert atmosphere to obtain the carbon. As these methods require high energy, a one-step preparation process that yields the highest quality carbon at the lowest possible temperatures should be invented under the advocacy of energy saving and emission reduction. So far, in the real application of SCs, the flexibility of electrodes and cyclic stability of the electrode materials are highly valued. So, there is a need to develop efficient synthesis methods that will yield stable electroactive materials sustainable under various conditions.
As far as electrochemical testing is concerned, the most used electrolytes are still Na2SO4, KOH, H2SO4, etc. So, there is a need to develop and test new electrolytes that are more stable as well as less corroding in nature and can offer wide potential windows. Polymer or gel-based electrolytes that can be used in all solid-state devices need to be tested and compared. The use of non-aqueous or solid electrolytes sounds more practical towards device fabrication but it will need detailed studies about the electrode–electrolyte (solid–solid or solid–gel) interfaces that will reveal the mechanisms of energy storage and conduction. The interface barriers (resistance) should be minimized by using suitable surface modification techniques for the electrodes or by inventing electrolytes compatible with different electrode materials. For this purpose, functional polymer-based electrolytes are being investigated with great interest.
Another important consideration is the film forming property and processability of various materials developed. The thin films of MnO2 and carbon-based active electrodes influence the faradaic processes at the electrode–electrolyte interface. Not all materials or composites give stable films. Hence there is a need to develop composite materials that are adherent with excellent film-forming properties on various substrates such as nickel foam, graphite, carbon cloth etc. to give the best electrochemical performance. To summarise, significant efforts should be made to develop new and efficient composite electrode materials to be included in flexible and compressible SC devices.
Conflicts of interest
There are no conflicts of interest to declare.References
- P. H. Patil, V. V. Kulkarni and S. A. Jadhav, J. Compos. Sci., 2022, 6, 363 CrossRef CAS.
- P. H. Patil, V. V. Kulkarni, A. K. Mane, T. D. Dongale and S. A. Jadhav, Polym. Adv. Technol., 2024, 35, e6362 CrossRef.
- V. S. Patil, S. S. Thoravat, S. S. Kundale, T. D. Dongale, P. S. Patil and S. A. Jadhav, Chem. Phys. Lett., 2023, 814, 140334 CrossRef CAS.
- G. Smdani, M. R. Islam, A. N. Ahmad Yahaya and S. I. Bin Safie, Energy Environ., 2023, 34, 1094–1141 CrossRef.
- L. Lai, H. Yang, L. Wang, B. K. Teh, J. Zhong, H. Chou, L. Chen, W. Chen, Z. Shen, R. S. Ruoff and J. Lin, ACS Nano, 2012, 6, 5941–5951 CrossRef CAS PubMed.
- L. Xie, G. Sun, F. Su, X. Guo, Q. Kong, X. Li, X. Huang, L. Wan, W. Song, K. Li, C. Lv and C.-M. Chen, J. Mater. Chem. A, 2016, 4, 1637–1646 RSC.
- E. Frackowiak and F. Béguin, Carbon, 2001, 39, 937–950 CrossRef CAS.
- V. V. Kulkarni, P. H. Patil, T. D. Dongale, A. V. Takaloo and S. A. Jadhav, Chem. Phys. Lett., 2024, 841, 141173 CrossRef CAS.
- B. K. Kim, S. Sy, A. Yu and J. Zhang, in Handbook of Clean Energy Systems, ed. J. Yan, Wiley, 1st edn, 2015, pp. 1–25 Search PubMed.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250–1280 RSC.
- P. Forouzandeh, V. Kumaravel and S. C. Pillai, Catalysts, 2020, 10, 969 CrossRef CAS.
- S. Rajagopal, R. Pulapparambil Vallikkattil, M. Mohamed Ibrahim and D. G. Velev, Condens. Matter, 2022, 7, 6 CrossRef CAS.
- A. Diallo, N. Tandjigora, S. Ndiaye, T. Jan, I. Ahmad and M. Maaza, SN Appl. Sci., 2021, 3, 562 CrossRef CAS.
- S. A. Jadhav, V. V. Kulkarni, P. H. Patil and I. U. Shah, J. Appl. Electrochem., 2023, 53, 1911–1926 CrossRef CAS.
- M. Vangari, T. Pryor and L. Jiang, J. Energy Eng., 2013, 139, 72–79 CrossRef.
- J. Hong, H. Kim, J. E. Lee, Y. N. Ko, K. T. Park, Y. E. Kim, M. H. Youn, S. K. Jeong, J. Park and W. Lee, Korean J. Chem. Eng., 2020, 37, 1218–1225 CrossRef CAS.
- V. V. Kulkarni, P. H. Patil and S. A. Jadhav, Period. Polytech., Chem. Eng., 2024, 68, 1–15 CrossRef.
- B. T. Sone, X. G. Fuku and M. Maaza, Int. J. Electrochem. Sci., 2016, 11, 8204–8220 CrossRef CAS.
- B. T. Vetrikarasan, A. R. Nair, T. Karthick, S. K. Shinde, D. Y. Kim, S. N. Sawant and A. D. Jagdale, J. Energy Storage, 2023, 72, 108403 CrossRef.
- J. Wei, F. Hu, X. Shen, B. Chen, L. Chen, Z. Wang, C. Lv and Q. Ouyang, J. Colloid Interface Sci., 2023, 649, 665–674 CrossRef CAS PubMed.
- A. C. Nwanya, M. M. Ndipingwi, C. O. Ikpo, R. M. Obodo, S. C. Nwanya, S. Botha, F. I. Ezema, E. I. Iwuoha and M. Maaza, J. Alloys Compd., 2020, 822, 153581 CrossRef CAS.
- J. B. K. Kana, J. M. Ndjaka, P. O. Ateba, B. D. Ngom, N. Manyala, O. Nemraoui, A. C. Beye and M. Maaza, Appl. Surf. Sci., 2008, 254, 3959–3963 CrossRef CAS.
- B. Padmadevi and T. Kalaivani, Ceram. Int., 2022, 48, 36101–36109 CrossRef CAS.
- J. Sackey, A. C. Nwanya, A. K. H. Bashir, N. Matinise, J. B. Ngilirabanga, A. E. Ameh, E. Coetsee and M. Maaza, Mater. Chem. Phys., 2020, 244, 122714 CrossRef CAS.
- A. Diallo, K. Kaviyarasu, S. Ndiaye, B. M. Mothudi, A. Ishaq, V. Rajendran and M. Maaza, Green Chem. Lett. Rev., 2018, 11, 166–175 CrossRef CAS.
- S. A. Jadhav, S. D. Dhas, K. T. Patil, A. V. Moholkar and P. S. Patil, Chem. Phys. Lett., 2021, 778, 138764 CrossRef CAS.
- T. S. Bhat, S. A. Jadhav, S. A. Beknalkar, S. S. Patil and P. S. Patil, Inorg. Chem. Commun., 2022, 141, 109493 CrossRef CAS.
- D. Wu, X. Xie, Y. Zhang, D. Zhang, W. Du, X. Zhang and B. Wang, Front. Mater., 2020, 7, 2 CrossRef.
- R. Liang, Y. Du, P. Xiao, J. Cheng, S. Yuan, Y. Chen, J. Yuan and J. Chen, Nanomaterials, 2021, 11, 1248 CrossRef CAS PubMed.
- A. Abdollahi, A. Abnavi, F. Ghasemi, S. Ghasemi, Z. Sanaee and S. Mohajerzadeh, Electrochim. Acta, 2021, 390, 138826 CrossRef CAS.
- S. Azmi, A. Klimek and E. Frackowiak, Mater. Today, 2023, S1369702123002092 Search PubMed.
- J. Liu, J. Bao, X. Zhang, Y. Gao, Y. Zhang, L. Liu and Z. Cao, RSC Adv., 2022, 12, 35556–35578 RSC.
- Y.-F. Li, S.-C. Zhu and Z.-P. Liu, J. Am. Chem. Soc., 2016, 138, 5371–5379 CrossRef CAS PubMed.
- R. Devi, V. Kumar, S. Kumar, M. Bulla, S. Sharma and A. Sharma, Appl. Sci., 2023, 13, 7907 CrossRef CAS.
- S. Sivakumar and L. Nelson Prabu, Mater. Today: Proc., 2021, 47, 52–55 CAS.
- J. Liu, J. Bao, X. Zhang, Y. Gao, Y. Zhang, L. Liu and Z. Cao, RSC Adv., 2022, 12, 35556–35578 RSC.
- P. Tagsin, P. Suksangrat, P. Klangtakai, P. Srepusharawoot, C. Ruttanapun, P. Kumnorkaew, S. Pimanpang and V. Amornkitbamrung, Appl. Surf. Sci., 2021, 570, 151056 CrossRef CAS.
- D. Kiymaz, A. Kiymaz, S. Tekoglu, F. Mayr, H. Dincalp and C. Zafer, Thin Solid Films, 2022, 762, 139535 CrossRef CAS.
- M. He, L. Cao, W. Li, X. Chang and Z. Ren, J. Alloys Compd., 2021, 865, 158934 CrossRef CAS.
- R. A. Davoglio, G. Cabello, J. F. Marco and S. R. Biaggio, Electrochim. Acta, 2018, 261, 428–435 CrossRef CAS.
- Y. Li, H. Xie, J. Wang and L. Chen, Mater. Lett., 2011, 65, 403–405 CrossRef CAS.
- S. Rong, P. Zhang, F. Liu and Y. Yang, ACS Catal., 2018, 8, 3435–3446 CrossRef CAS.
- B. Chettiannan, A. K. Srinivasan, G. Arumugam, S. Shajahan, M. AbuHaija and R. Rajendran, ACS Omega, 2023, 8, 6982–6993 CrossRef CAS PubMed.
- P. H. Patil, V. V. Kulkarni, T. D. Dongale and S. A. Jadhav, J. Compos. Sci., 2023, 7, 167 CrossRef CAS.
- R. Yang, Y. Fan, R. Ye, Y. Tang, X. Cao, Z. Yin and Z. Zeng, Adv. Mater., 2021, 33, 2004862 CrossRef CAS PubMed.
- X. Liao, C. Pana, Y. Pana and C. Yin, J. Alloys Compd., 2021, 888, 161619 CrossRef CAS.
- S. Pundir, S. Upadhyay, R. Priya, N. Kumar, S. Chetana, I. Hossain, N. C. Joshi and O. P. Pandey, J. Solid State Electrochem., 2023, 27, 531–538 CrossRef CAS.
- Y. Zhu, H. Xu, P. Chen, Y. Bao, X. Jiang and Y. Chen, Electrochim. Acta, 2022, 413, 140146 CrossRef CAS.
- J. M. O. Cremonezzi, D. Y. Tiba and S. H. Domingues, SN Appl. Sci., 2020, 2, 1689 CrossRef CAS.
- M. Jayachandran, A. Rose, T. Maiyalagan, N. Poongodi and T. Vijayakumar, Electrochim. Acta, 2021, 366, 137412 CrossRef CAS.
- M. Moniruzzaman, Y. Anil Kumar, M. R. Pallavolu, H. M. Arbi, S. Alzahmi and I. M. Obaidat, Nanomaterials, 2022, 12, 3187 CrossRef CAS PubMed.
- C. Deng, Z. He, J. Huang, L. Liu, Y. Liu, T. Wang, G. Chen, Y. Yi and K. Du, Appl. Surf. Sci., 2023, 627, 157299 CrossRef CAS.
- X. Dai, M. Zhang, T. Li, X. Cui, Y. Shi, X. Zhu, P. Wangyang, D. Yang and J. Li, Vacuum, 2022, 195, 110692 CrossRef CAS.
- J. Wu, W. Raza, P. Wang, A. Hussain, Y. Ding, J. Yu, Y. Wu and J. Zhao, Electrochim. Acta, 2022, 418, 140339 CrossRef CAS.
- S. Saini, P. Chand and A. Joshi, J. Energy Storage, 2023, 71, 108209 CrossRef.
- Z. Zhai, L. Zhang, T. Du, B. Ren, Y. Xu, S. Wang, J. Miao and Z. Liu, Mater. Des., 2022, 221, 111017 CrossRef CAS.
- J. Zhou, S. Zhang, Y. N. Zhou, W. Tang, J. Yang, C. Peng and Z. Guo, Electrochem. Energy Rev., 2021, 4, 219–248 CrossRef CAS.
- Y. Chen, X. Guo, A. Liu, H. Zhu and T. Ma, Sustainable Energy Fuels, 2021, 5, 3017–3038 RSC.
- A. Shetty, V. Molahalli, A. Sharma and G. Hegde, Catalysts, 2022, 13, 20 CrossRef.
- Y. Liu, J. Chen, B. Cui, P. Yin and C. Zhang, C, 2018, 4, 53 CAS.
- H. Yang, S. Ye, J. Zhou and T. Liang, Front. Chem., 2019, 7, 274 CrossRef CAS PubMed.
- H. Lu and X. S. Zhao, Sustainable Energy Fuels, 2017, 1, 1265–1281 RSC.
- J. Wang, X. Zhang, Z. Li, Y. Ma and L. Ma, J. Power Sources, 2020, 451, 227794 CrossRef CAS.
- Z. Pan, S. Yu, L. Wang, C. Li, F. Meng, N. Wang, S. Zhou, Y. Xiong, Z. Wang, Y. Wu, X. Liu, B. Fang and Y. Zhang, Nanomaterials, 2023, 13, 1744 CrossRef CAS PubMed.
- A. Mariappan, R. K. Dharman, T. H. Oh, S. Prabu and K.-Y. Chiang, Mater. Chem. Phys., 2023, 309, 128321 CrossRef CAS.
- D. Sui, M. Yao, L. Si, K. Yan, J. Shi, J. Wang, C. C. Xu and Y. Zhang, Carbon, 2023, 205, 510–518 CrossRef CAS.
- Q. Ding, M. Zhang and H. Huang, Mater. Lett., 2023, 333, 133675 CrossRef CAS.
- Y. Jin, W. Tang, J. Wang, F. Ren, Z. Chen, Z. Sun and P.-G. Ren, J. Alloys Compd., 2023, 932, 167627 CrossRef CAS.
- H. Wang, H. Chen, C. Chen, M. Li, Y. Xie, X. Zhang, X. Wu, Q. Zhang and C. Lu, Chin. Chem. Lett., 2023, 34, 107465 CrossRef CAS.
- Z. Zhang, S. Lu, Y. Li, J. Song, E. Han, H. Wang and Y. He, Ind. Crops Prod., 2023, 203, 117155 CrossRef CAS.
- R. Wang, X. Li, Z. Nie, Q. Jing, Y. Zhao, H. Song and H. Wang, J. Energy Storage, 2022, 51, 104364 CrossRef.
- Y. Yao, T. Xie, P. Li, W. Du, J. Jiang, H. Ding, T. Zhao, G. Xu and L. Zhang, Diamond Relat. Mater., 2023, 138, 110169 CrossRef CAS.
- A. Agrawal, A. Gaur and A. Kumar, J. Energy Storage, 2023, 66, 107395 CrossRef.
- R. Mehdi, S. R. Naqvi, A. H. Khoja and R. Hussain, Fuel, 2023, 348, 128529 CrossRef CAS.
- W. Tian, P. Ren, J. Wang, X. Hou, A. Sun, Y. Jin and Z. Chen, J. Energy Storage, 2023, 63, 107039 CrossRef.
- G. Li, Y. Li, X. Chen, X. Hou, H. Lin and L. Jia, J. Colloid Interface Sci., 2022, 605, 71–81 CrossRef CAS PubMed.
- D. J. Tarimo, K. O. Oyedotun, N. F. Sylla, A. A. Mirghni, N. M. Ndiaye and N. Manyala, J. Energy Storage, 2022, 51, 104378 CrossRef.
- D. Li, Y. Huang, C. Yu, C. Tang and J. Lin, Diamond Relat. Mater., 2023, 136, 109956 CrossRef CAS.
- M. Zhou, P. Wang, Y. Yu, W. Ma, Z. Cai, F. Ko, M. Li and Q. Wang, Energy, 2023, 278, 127705 CrossRef CAS.
- Z. Zhang, S. Lu, Y. Li, J. Song, E. Han, H. Wang and Y. He, Ind. Crops Prod., 2023, 203, 117155 CrossRef CAS.
- S. Cheng, X. Wang, K. Du, Y. Mao, Y. Han, L. Li, X. Liu and G. Wen, Molecules, 2023, 28, 5020 CrossRef CAS PubMed.
- A. T. S. C. Brandão, S. State, R. Costa, P. Potorac, J. A. Vázquez, J. Valcarcel, A. F. Silva, L. Anicai, M. Enachescu and C. M. Pereira, ACS Omega, 2023, 8, 18782–18798 CrossRef PubMed.
- A. Singh and A. K. Ojha, J. Mater. Sci.: Mater. Electron., 2023, 34, 1003 CrossRef CAS.
- T. E. Kibona, D. J. Mahushi and G. N. Shao, Biofuels, Bioprod. Biorefin., 2023, bbb.2523 Search PubMed.
- H. Rustamaji, T. Prakoso, H. Devianto, P. Widiatmoko and W. H. Saputera, J. Energy Storage, 2022, 52, 104724 CrossRef.
- V. Raghavan and S. J. Rajasekaran, J. Electrochem. Sci. Eng., 2022, 12, 545–556 Search PubMed.
- R. Zou, B. Wang, L. Zhu, L. Yan, F. Shi, Y. Sun, B. Shao, S. Zhang and W. Sun, Diamond Relat. Mater., 2022, 126, 109060 CrossRef CAS.
- H. A. Hamouda, H. I. Abdu, Q. Hu, M. A. Abubaker, H. Lei, S. Cui, A. I. Alduma, H. Peng, G. Ma and Z. Lei, Front. Chem., 2022, 10, 1024047 CrossRef CAS PubMed.
- E. Elanthamilan, S. J. Jennifer, S.-F. Wang and J. P. Merlin, Mater. Chem. Phys., 2022, 286, 126188 CrossRef CAS.
- A. R. Selvaraj, D. Chinnadurai, I. Cho, J.-S. Bak and K. Prabakar, J. Energy Storage, 2022, 52, 104928 CrossRef.
- V. S. Bhat, A. Toghan, G. Hegde and R. S. Varma, J. Energy Storage, 2022, 52, 104776 CrossRef.
- G. Qiu, Z. Miao, Y. Guo, J. Xu, W. Jia, Y. Zhang, F. Guo and J. Wu, Colloids Surf., A, 2022, 650, 129575 CrossRef CAS.
- M. Jalalah, S. Rudra, B. Aljafari, M. Irfan, S. S. Almasabi, T. Alsuwian, M. I. Khazi, A. K. Nayak and F. A. Harraz, Electrochim. Acta, 2022, 414, 140205 CrossRef CAS.
- E. Gür, T. G. Semerci and F. Semerci, J. Energy Storage, 2022, 51, 104363 CrossRef.
- K. Li, J. Luo, M. Wei, X. Yao, Q. Feng, X. Ma and Z. Liu, Diamond Relat. Mater., 2022, 127, 109196 CrossRef CAS.
- E. Taer, N. Y. Effendi, R. Taslim and A. Apriwandi, J. Mater. Res. Technol., 2022, 19, 4721–4732 CrossRef CAS.
- K. Subhani, X. Jin, N. Hameed, A. K. Lau, J. A. M. Ramshaw, V. Glattauer and N. V. Salim, Mater. Today Sustain., 2022, 18, 100152 CrossRef.
- F. Wu, X. Ren, F. Tian, G. Han, J. Sheng, Y. Yu, Y. Liu and W. Yang, New J. Chem., 2022, 46, 19667–19674 RSC.
- Y. Liu, X. Cheng and S. Zhang, Carbon Lett., 2022, 32, 251–263 CrossRef.
- W. Hu, B. Wang, Y. Yu, N. Wang and X. Wu, J. Alloys Compd., 2021, 884, 161149 CrossRef CAS.
- Y. Yin, S. Yan, Z. Ni, C. Jin and L. Zhao, Biomass Convers. Biorefin., 2023, 13, 12115–12124 CrossRef CAS.
- P. H. Patil, S. B. Ravan, S. S. Thoravat, T. D. Dongale and S. A. Jadhav, Korean J. Chem. Eng., 2023, 40, 2087–2090 CrossRef CAS.
- J. R. Choudhuri, S. P. Singh, A. K. Agarwal, K. Kumar and S. K. Srivastav, Metal oxide-carbon nanocomposites for electrochemical storage, Springer Nature Singapore, 2022, pp. 49–67 Search PubMed.
- S. S. Thoravat, V. S. Patil, S. S. Kundale, T. D. Dongale, P. S. Patil and S. A. Jadhav, Synth. Met., 2023, 294, 117312 CrossRef CAS.
- M. Bilal, Z. U. Rehman, J. Hou, S. Ali, S. Ullah and J. Ahmad, Metal oxide carbon composite: Synthesis and properties by using conventional enabling technologies, Elsevier, 2022, pp. 25–60 Search PubMed.
- J. R. Choi, J. W. Lee, G. Yang, Y.-J. Heo and S.-J. Park, Catalysts, 2020, 10, 256 CrossRef CAS.
- D. Ma, X. Mu, G. Zhao, X. Qin and M. Qi, Coatings, 2023, 13, 707 CrossRef CAS.
- T. Huang, C. Zhao, L. Wu, X. Lang, K. Liu and Z. Hu, Ceram. Int., 2017, 43, 1968–1974 CrossRef CAS.
- T. Bi, H. Chen, J. Li, X. Zhang and Q. Lin, Electrochim. Acta, 2022, 433, 141266 CrossRef CAS.
- S. Saini, P. Chand and A. Joshi, J. Energy Storage, 2021, 39, 102646 CrossRef.
- J. Zhang, H. Chen, J. Bai, M. Xu, C. Luo, L. Yang, L. Bai, D. Wei, W. Wang and H. Yang, J. Alloys Compd., 2021, 854, 157207 CrossRef CAS.
- G. Lin, R. Ma, Y. Zhou, Q. Liu, X. Dong and J. Wang, Electrochim. Acta, 2018, 261, 49–57 CrossRef CAS.
- J. Li, Y. Zou, C. Xiang, F. Xu, L. Sun, B. Li and J. Zhang, J. Energy Storage, 2021, 42, 103017 CrossRef.
- K. Cong, M. Radtke, S. Stumpf, B. Schröter, D. G. G. McMillan, M. Rettenmayr and A. Ignaszak, Mater. Renew. Sustain. Energy, 2015, 4, 5 CrossRef.
- S. Gao, Y. Chen, H. Fan, X. Wei, C. Hu, H. Luo and L. Qu, J. Mater. Chem. A, 2014, 2, 3317 RSC.
- A. J. Christina Mary, C. I. Sathish, P. S. Murphin Kumar, A. Vinu and A. C. Bose, Electrochim. Acta, 2020, 342, 136062 CrossRef CAS.
- D. Bejjanki, P. Banothu, V. B. Kumar and P. S. Kumar, C, 2023, 9, 24 CAS.
- J. Bu, Z. Zhou, X. Wu, C. Zhang and Z. Li, J. Mater. Sci., 2021, 56, 17694–17708 CrossRef CAS.
- Z. Li, R. Wang, R. Zhong, A. Zhou, X. Wang and Z. Yang, J. Mater. Sci.: Mater. Electron., 2022, 33, 1899–1909 CrossRef CAS.
- Z. Sui, Z. Chang, X. Xu, Y. Li, X. Zhu, C. Zhao and Q. Chen, Diamond Relat. Mater., 2020, 108, 107988 CrossRef CAS.
- B. Sun, X. Zhang, X. Fan, R. Wang, H. Bai and X. Wei, Energy, 2022, 249, 123659 CrossRef CAS.
- T. Kongthong, C. Poochai, C. Sriprachuabwong, A. Tuantranont, S. Nanan, N. Meethong, P. Pakawatpanurut, T. Amornsakchai and J. Sodtipinta, J. Sci.: Adv. Mater. Devices, 2022, 7, 100434 CAS.
- D. Li, J. Lin, Y. Lu, Y. Huang, X. He, C. Yu, J. Zhang and C. Tang, J. Alloys Compd., 2020, 815, 152344 CrossRef CAS.
- Y. Wang, J. Wang, D. Wei and L. Xu, J. Colloid Interface Sci., 2023, 648, 925–939 CrossRef CAS PubMed.
- Handbook of Nanocomposite Supercapacitor Materials II: Performance, ed. K. K. Kar, Springer International Publishing, Cham, 2020, vol. 302 Search PubMed.
- J.-W. Wang, Y. Chen and B.-Z. Chen, J. Electrochem. Soc., 2015, 162, A1654–A1661 CrossRef CAS.
- Y. Liu, S. Zuo, B. Shen, Y. Wang and H. Xia, Int. J. Electrochem. Sci., 2020, 15, 7646–7662 CrossRef.
- S. He and W. Chen, J. Power Sources, 2015, 294, 150–158 CrossRef CAS.
- H. Li, B. Wang, X. He, J. Xiao, H. Zhang, Q. Liu, J. Liu, J. Wang, L. Liu and P. Wang, J. Mater. Chem. A, 2015, 3, 9754–9762 RSC.
- C. Mao, S. Liu, L. Pang, Q. Sun, Y. Liu, M. Xu and Z. Lu, RSC Adv., 2016, 6, 5184–5191 RSC.
- J. Chen, J. Qiu, B. Wang, H. Feng, Y. Yu and E. Sakai, J. Electroanal. Chem., 2017, 791, 159–166 CrossRef CAS.
- Q. Chen, J. Chen, Y. Zhou, C. Song, Q. Tian, J. Xu and C.-P. Wong, Appl. Surf. Sci., 2018, 440, 1027–1036 CrossRef CAS.
- S. Kong, B. Jin, X. Quan, G. Zhang, X. Guo, Q. Zhu, F. Yang, K. Cheng, G. Wang and D. Cao, J. Electroanal. Chem., 2019, 850, 113412 CrossRef CAS.
- C. Feng, D. Chen, Y. Tang, S. Chen, Y. Liu, C. Zhu, W. Xie, L. Ye, Q. Zhang, P. Qian, X. Feng, X. Dong and H. Jin, Int. J. Electrochem. Sci., 2019, 14, 8284–8295 CrossRef CAS.
- M. Li, J. Yu, X. Wang and Z. Yang, Appl. Surf. Sci., 2020, 530, 147230 CrossRef CAS.
- D. Li, J. Lin, Y. Lu, Y. Huang, X. He, C. Yu, J. Zhang and C. Tang, J. Alloys Compd., 2020, 815, 152344 CrossRef CAS.
- C. Li, X. Dong, Y. Zhang, J. Hu, W. Liu, X. Cui and A. Hao, Appl. Surf. Sci., 2020, 527, 146842 CrossRef CAS.
- J. Yu, M. Li, X. Wang and Z. Yang, ACS Omega, 2020, 5, 16299–16306 CrossRef CAS PubMed.
- M. Yang, D. S. Kim, S. B. Hong, J.-W. Sim, J. Kim, S.-S. Kim and B. G. Choi, Langmuir, 2017, 33, 5140–5147 CrossRef CAS PubMed.
- A. A. Mohammed, C. Chen and Z. Zhu, J. Power Sources, 2019, 417, 1–13 CrossRef CAS.
- P. Zhang, Y. Wu, H. Sun, J. Zhao, Z. Cheng and X. Kang, Int. J. Miner., Metall. Mater., 2021, 28, 1735–1744 CrossRef CAS.
- H. Shen, X. Kong, P. Zhang, X. Song, H. Wang and Y. Zhang, J. Alloys Compd., 2021, 853, 157357 CrossRef CAS.
- S. Nirmaladevi, R. Boopathiraja, S. K. Kandasamy, S. Sathishkumar and M. Parthibavarman, Surf. Interfaces, 2021, 27, 101548 CrossRef CAS.
- C. Yuan, H. Lin, H. Lu, E. Xing, Y. Zhang and B. Xie, Appl. Energy, 2016, 178, 260–268 CrossRef CAS.
- X. Hu, W. Xiong, W. Wang, S. Qin, H. Cheng, Y. Zeng, B. Wang and Z. Zhu, ACS Sustainable Chem. Eng., 2016, 4, 1201–1211 CrossRef CAS.
- D. Deng, B.-S. Kim, M. Gopiraman and I. S. Kim, RSC Adv., 2015, 5, 81492–81498 RSC.
- X. Yuan, Y. Zhang, Y. Yan, B. Wei, K. Qiao, B. Zhu, X. Cai and T.-W. Chou, Chem. Eng. J., 2020, 393, 121214 CrossRef CAS.
- Y. Li, N. Yu, P. Yan, Y. Li, X. Zhou, S. Chen, G. Wang, T. Wei and Z. Fan, J. Power Sources, 2015, 300, 309–317 CrossRef CAS.
- J. Wang, H. Yang, Q. Sun, C. Zhou, X. Zhang, L. Ge and X. Ma, Mater. Lett., 2021, 285, 129116 CrossRef CAS.
- Q. Lu, S. Zhou, M. Cheng, B. Li, H. Wei, L. Zhang, Y. Zhang, J. Zhao, J. Zhang and Q. Liu, Electrochim. Acta, 2020, 363, 137240 CrossRef CAS.
- Y. Tan, C. Yang, W. Qian and C. Teng, J. Alloys Compd., 2020, 826, 154133 CrossRef CAS.
- X.-S. Li, M.-M. Xu, Y. Yang, Q.-B. Huang, X.-Y. Wang, J.-L. Ren and X.-H. Wang, Materials, 2019, 12, 2379 CrossRef CAS PubMed.
- T. Yu, F. Wang, X. Zhang, G. Lv, H. Lv, J. Wang, Y. Zhai and M. Li, Diamond Relat. Mater., 2021, 116, 108450 CrossRef CAS.
- P. Konnerth, D. Jung, J. W. Straten, K. Raffelt and A. Kruse, Mater. Sci. Energy Technol., 2021, 4, 69–80 CAS.
- R. R. Palem, S. Ramesh, C. Bathula, V. Kakani, G. D. Saratale, H. M. Yadav, J.-H. Kim, H. S. Kim and S.-H. Lee, Ceram. Int., 2021, 47, 26738–26747 CrossRef CAS.
- Y. Wen, T. Qin, Z. Wang, X. Jiang, S. Peng, J. Zhang, J. Hou, F. Huang, D. He and G. Cao, J. Alloys Compd., 2017, 699, 126–135 CrossRef CAS.
- L. Wang, Y. Zheng, S. Chen, Y. Ye, F. Xu, H. Tan, Z. Li, H. Hou and Y. Song, Electrochim. Acta, 2014, 135, 380–387 CrossRef CAS.
- G. Yang and S.-J. Park, J. Alloys Compd., 2018, 741, 360–367 CrossRef CAS.
- C. Wang, Y. Xiong, H. Wang and Q. Sun, J. Colloid Interface Sci., 2018, 528, 349–359 CrossRef CAS PubMed.
- B. Liu, Y. Liu, H. Chen, M. Yang and H. Li, ACS Sustainable Chem. Eng., 2019, 7, 3101–3110 CrossRef CAS.
- Y. Ping, Z. Liu, J. Li, J. Han, Y. Yang, B. Xiong, P. Fang and C. He, J. Alloys Compd., 2019, 805, 822–830 CrossRef CAS.
- T. Yumak, G. A. Yakaboylu, O. Oginni, K. Singh, E. Ciftyurek and E. M. Sabolsky, Colloids Surf., A, 2020, 586, 124150 CrossRef CAS.
- X. Du, C. Hou, H. Kimura, X. Xie, H. Jiang, X. Sun, X. Yang, Y. Zhang and W. Du, J. Energy Storage, 2023, 72, 108355 CrossRef.
- A. Ganesh, T. Sivakumar and G. Sankar, J. Mater. Sci.: Mater. Electron., 2022, 33, 14772–14783 CrossRef CAS.
- Z. Wang, S. Wen, J. Ma, Z. Li, J. Wang and X. Liu, Diamond Relat. Mater., 2023, 110451 CrossRef CAS.
- P. Sengodan, R. Govindan, G. Arumugam, B. Chettiannan, M. Navaneethan, M. R. Pallavolu, M. Hussien, M. Selvaraj and R. Rajendran, J. Energy Storage, 2022, 50, 104625 CrossRef.
- X. Wang, J. Chu, H. J. Yan and H. K. Zhang, Heliyon, 2022, 8, e10631 CrossRef CAS PubMed.
- L. Chen, F. Wang, Z. Tian, H. Guo, C. Cai, Q. Wu, H. Du, K. Liu, Z. Hao, S. He, G. Duan and S. Jiang, Small, 2022, 18, 2201307 CrossRef CAS PubMed.
- S. Dhinesh, M. Priyadharshini, T. Pazhanivel and R. Gobi, Mater. Technol., 2022, 37, 1837–1845 CrossRef CAS.
- T. Kongthong, C. Poochai, C. Sriprachuabwong, A. Tuantranont, S. Nanan, N. Meethong, P. Pakawatpanurut, T. Amornsakchai and J. Sodtipinta, J. Sci.: Adv. Mater. Devices, 2022, 7, 100434 CAS.
- T. Niu, R. Lou, Q. Cao, Y. Zhang, Y. Zhang, G. Wei and Z. Wang, Mater. Chem. Phys., 2023, 305, 127941 CrossRef CAS.
- J. Zhang, X. Du, H. Kimura, C. Hou, X. Sun, X. Yang, Y. Zhang, X. Xie and W. Du, Appl. Surf. Sci., 2023, 623, 157095 CrossRef CAS.
- V. Babaahmadi, S. E. M. Pourhosseini, O. Norouzi and H. R. Naderi, Nanomaterials, 2023, 13, 1866 CrossRef CAS PubMed.
- P. Li, J. Wu, L. Tang, H. Liu, Y. Xu and D. Zhang, Ionics, 2023, 29, 3629–3639 CrossRef CAS.
- L. Wang, X. Wang, J. Ouyang, Y. Guo, W. Xiong, L. Zhao, M. Li, Z. Hua, Z. Li, K. Du, C. Zhou and Y. Luo, Appl. Surf. Sci., 2023, 612, 155821 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |