Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A simple approach to determining the efficacy of antiperspirants using paper-based colorimetric paper sensors: SweatSENSE†
Rachel A.
Hand
 *a,
Spyridon
Efstathiou
*a,
Spyridon
Efstathiou
 a,
Alan M.
Wemyss
a,
Alan M.
Wemyss
 a,
Maria
Grypioti‡
a,
Gavin
Kirby
a,
Tammie
Barlow
a,
Emmett
Cullen Tinley
b,
Jane
Ford
b,
Andy
Jamieson
b,
Janette
Reynolds
b,
Jean
Miller
b,
Susan
Bates
b,
Ezat
Khoshdel
ab and
David M.
Haddleton
a,
Maria
Grypioti‡
a,
Gavin
Kirby
a,
Tammie
Barlow
a,
Emmett
Cullen Tinley
b,
Jane
Ford
b,
Andy
Jamieson
b,
Janette
Reynolds
b,
Jean
Miller
b,
Susan
Bates
b,
Ezat
Khoshdel
ab and
David M.
Haddleton
 *a
*a
aDepartment of Chemistry, University of Warwick, Coventry, CV4 7AL, UK. E-mail: Rachel.A.Hand@warwick.ac.uk; D.M.Haddleton@warwick.ac.uk
bUnilever Research, Port Sunlight, Bebington CH63 3JW, UK
First published on 5th January 2024
Abstract
Antiperspirants are commonly used daily, or even multiple times a day, by a large percentage of the global population, in order to address the problems associated with sweating. However, there has been no simple way to test and evaluate the effectiveness of antiperspirants in real time. To address this, we have developed a polydiacetylene based chemosensor that undergoes a blue to red colour change when in contact with acids and alcohols found in sweat, but not when exposed to water. The sensor is prepared via inkjet-printing an imidazolium derivative of a diacetylene monomer onto normal paper to give a transparent square followed by UV curing to produce the navy blue 5 × 5 cm “SweatSENSE” patch. A sensor is applied to the underarm for 5 s and the colour changes to red in the presence of sweat and the intensity of the colour change is recorded. From an in vivo trial involving 52 panellists, the sensor was shown to demonstrate a reduction in total sweat area following the application of an antiperspirant, when compared with a deodorant only control. This sensor will allow consumers to test the effectiveness of their antiperspirants during daily life, in real time, without the need for specialised equipment.
Introduction
In humans, the axilla is a unique body site, containing a high density of hair follicles and sebaceous glands, as well as two types of sweat glands: eccrine and apocrine.1,2 These sweat glands differ in their physiology, function and distribution.3,4 Eccrine sweat glands are simple, coiled structures that are present throughout the body, although their density varies with body size. They form part of the body's thermoregulatory system, secreting a sterile, dilute electrolyte solution which is predominantly responsible for wetness (perspiration/sweat). Apocrine sweat glands are unique to the axillae and genital areas and secrete an oily fluid containing proteins, lipids, and steroids. Apocrine sweat is typically produced in response to emotional and/or physical stress. At the skin surface of the axilla, microorganisms thrive on these natural secretions, transforming them into volatile organic compounds.5A consensus has emerged that short and medium chain volatile fatty acids (VFAs), along with thioalcohols, are the primary molecules associated with axillary malodour,5 with a minor contribution from 16-androstene steroids.6 These are by-products of the microbial metabolism of the odourless natural secretions from the eccrine, apocrine and sebaceous glands. In many societies, the detection of underarm wetness and odour brings about feelings of anxiety that affect all aspects of a person's life, from career choices and recreational activities to relationships, emotional well-being, and self-confidence.7 People are conscious of wet patches and staining of clothing in the underarm as well as the potentially negative perception of their body odour.
Antiperspirants and deodorants are a large global business estimated to be worth >$75 billion U.S Dollars in 2019. Many people assume that antiperspirants and deodorants are the same, however, they have different primary functions. The primary aim of a deodorant is to control the concentration of volatile malodorous compounds that are generated as the skin bacteria work on the sweat in the underarm. They contain fragrances and may contain antimicrobial ingredients. Conversely, the first line of defence against underarm wetness is antiperspirants. Antiperspirants are non-invasive, topically applied products worn to reduce the volume of sweat in the axilla. An individual product can have one or both of these functionalities.
Numerous methods exist to test the efficacy of an antiperspirant.8 The most established test is a gravimetric method, whereby the detection and quantification of collected sweat is used to compare the sweat weight reduction (SWR) of an antiperspirant technology versus a control product. Panellists are required to have products applied to the axillae and then sit at elevated temperature in a hot room (for example, at 40 °C and 40% relative humidity) to acclimatise, prior to sweat being collected for a further period on gauze pads applied to the underarms. The pads are weighed before and after application to the underarms which allows the amount of sweat collected to be calculated. This technique provides a means to quantify sweating behaviour under thermal stress conditions but is limited, in that quantification of sweating and measure of sweat control efficacy provided by an antiperspirant is not possible during normal daily activities.
Sensor technology has been driven by advances in materials chemistry combined with the emergence of digital communication technologies and wireless sensor networks. Devices, often quite complex, have been developed for a diverse range of applications, such as forensics, human health, sporting performance, environment and national security.9–14 These advances have led to rapid progress in wearable sensor technologies which offer an opportunity to measure sweat flow rate “in-life”. A number of recent developments employ electrochemical sensors to record sweat volume or the concentration of specific analytes in sweat.15–17 These technologies are more convenient for users than the classic gravimetric methods, however, there can be issues with the response and calibration of such sensors and their use at scale can be cost-prohibitive. Colorimetric sensors use smart materials to induce a response to a specific analyte and can be a simpler, more cost-effective alternative to an electrochemical device.18–20
Polydiacetylenes (PDAs) are a family of conjugated polymers arising from the 1,4 topochemical polymerization of diacetylene monomers.21 The polymerization typically occurs under UV irradiation in the absence of solvents and catalysts, yielding blue π-conjugated polymer networks due to visible light absorption. PDAs exhibit a well-known colorimetric response towards various analytes which is a consequence of polarity changes that disrupt their conjugation. It has been proposed that such disruption causes conformational transitions in the PDA backbone (from all trans to gauche) that eventually lead to colorimetric changes from blue to red due to an increase in the energy required for electronic transitions in the visible region.22,23
It has been reported that PDAs have been used in flexible patch-type hydrochromic sensors for human sweat pore mapping on the hands and fingertips.24,25 PDAs have been formulated into an inkjet-printable micro-emulsion system and applied to paper substrates.26 This simple, low cost approach provides a chemosensor that detects minute amounts of sweat excreted from the sweat glands through a permanent and quantifiable colour change. However, the main issue with this technique for quantifying underarm sweating is that the axilla is a unique and complex body site, hard to access topographically, and with typically high humidity levels (60–99%). In this case, a sensitive hydrochromic sensor is saturated by the water vapour in the underarm area, causing a total colour change of the patch and inhibiting the quantification of sweat.
The work presented herein aims to develop a simple yet effective low cost, chemosensing system that can be used to both demonstrate and quantify antiperspirant efficacy. A “sweatochromic” sensor has been developed that responds to the weak organic acids present in sweat but does not respond to water. The sensor reaction time is tuned to allow complete detection within a 5 seconds skin contact time. This is beneficial as it allows for careful placement and removal of the sensors in the difficult to access axillary vault and enables detection of sweat generated at a single point in time during normal daily life.
Experimental
Materials
10,12-Pentacosadiynoic acid (≥97%), N-hydroxysuccinimide (98%), 1-propanol (≥99.5%, ACS reagent), dichloromethane (anhydrous), propylene glycol methyl ether (PGME), and 3-bromopropionitrile (99%) were purchased from Sigma-Aldrich. Sodium hydroxide (analytical reagent grade), sodium carbonate anhydrous (laboratory reagent grade), potassium hydroxide (analytical reagent grade), potassium carbonate anhydrous (laboratory reagent grade), magnesium sulfate dried (laboratory reagent grade), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC, 98+%) were supplied by Fisher Scientific. Propionic acid (for synthesis) and triethylamine (99%) were sourced from VWR International. All materials were used as received.PCDA-imidazole (PCDA-I+H)
10,12-Pentacosadiynoic acid (PCDA) (5 g, 13.4 mmol) was suspended in anhydrous CH2Cl2 (100 mL) under a N2 atmosphere and cooled to 0 °C in an ice bath with constant stirring, to which was added N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (3.838 g, 20.0 mmol, 1.5 equiv.) and N-hydroxysuccinimide (0.153 g, 1.33 mmol, 0.1 equiv.). Following this, 1-(3-aminopropyl)imidiazole (2.39 mL, 20.0 mmol, 1.5 equiv.) and N,N diisopropylethylamine (2.56 mL, 14.7 mmol, 1.1 equiv.) were added to the reaction mixture. The solution was removed from the ice bath and stirred at 25 °C for 18 h. The CH2Cl2 was removed in vacuo and the oily residue resuspended in ethyl acetate (75 mL) and washed with water (3 × 75 mL). The organic layer was dried over anhydrous magnesium sulfate, filtered, and the solvent removed in vacuo to give PCDA-I as an oily white solid. 1H NMR (CDCl3, 400 MHz) δ (ppm): 7.50 (s, 1H), 7.05 (s, 1H), 6.95 (s, 1H), 5.55 (s, 1H), 4.0 (t, 2H), 3.30 (s, 2H), 2.25 (t, 3H), 2.15 (t, 2H), 2.00 (t, 2H), 1.44 (m, 34H), 1.24 (t, 3H).The PCDA-I product (5 g, 10.4 mmol) was then suspended in acetonitrile (100 mL), to which was added 3-bromopropionitrile (1.1 equiv.) and for 18 h under reflux at 90 °C. The reaction mixture was then allowed to cool before concentration in vacuo to yield a pale yellow solid of PCDA-I+H. 1H NMR (CDCl3, 400 MHz, Fig. S3†) δ (ppm): 14.24 (s, 1H), 9.10 (s, 1H), 7.96 (s, 1H), 7.83 (s, 1H), 7.72 (s, 1H), 4.20 (t, 2H), 3.38 (s, H2O), 3.04 (t, 2H), 2.51 (s, DMSO), 2.27 (t, 2H), 2.08 (t, 2H), 1.94 (t, 2H), 1.44 (m, 6H), 1.24 (m, 26H). For storage, due to the light sensitivity of PCDA-I+H, the solid product was then resuspended at a concentration of 1 M in 1-propanol and stored in the dark.
Ink formulation and ink-jet printing
The ink formulation comprised of PCDA-I+H at 350 mM in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O/1-propanol, with 10% propylene glycol methyl ether (PGME). It was added to the “black” ink tank of an Epson EcoTank inkjet printer, which was then covered with aluminium foil to shield from exposure to light. Images of black rectangles (5 × 5 cm) were then printed on 80 gsm A4 paper, using standard print settings, the sheets were then allowed to dry at room temperature (ca. 5 minutes) before the patches were cured for 12 s at 500
1 H2O/1-propanol, with 10% propylene glycol methyl ether (PGME). It was added to the “black” ink tank of an Epson EcoTank inkjet printer, which was then covered with aluminium foil to shield from exposure to light. Images of black rectangles (5 × 5 cm) were then printed on 80 gsm A4 paper, using standard print settings, the sheets were then allowed to dry at room temperature (ca. 5 minutes) before the patches were cured for 12 s at 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μJ cm−2 in a box UV curer designed for sterilisation (λ = 254 nm).
000 μJ cm−2 in a box UV curer designed for sterilisation (λ = 254 nm).
Sweat study
The performance of the sensors was assessed with a “hot room study” involving 52 subjects, where half were given a topologically applied antiperspirant and the other half a control deodorant product. Subjects applied the products for three consecutive days and on the fourth day they sat in a Hot Room under controlled conditions of temperature and humidity (40 °C/40% relative humidity) for 40 minutes. After this time, the SweatSense™ patches were applied to the subjects’ underarms for 5 seconds using a purpose-built applicator. This applicator allowed for a uniform and consistent force to be applied to each sensor when used in the underarm. Sensors were applied to the centre of the axillary vault. The area of coverage of sweat on the sensor is calculated to determine sweating behaviour at a point in time, i.e., one hour after being in the hot room. This study was performed under approval of the local Unilever Safety and Environmental Assurance Centre (SEAC) following their guidelines.Results and discussion
A common strategy to introduce sensing properties to PDA systems is by covalently attaching polar moieties to diacetylene acid monomers thus enabling polarity changes and colour transitions after exposure to certain analytes.27 A study by Kim et al. demonstrated PCDA bromoacetonitrile imidazolium salts able to sense water by undergoing such transitions.25 However, it was found that smaller chain-length PCDAs showed no response towards water indicating that minor structural changes can affect polarity alternations. Since the goal of this study was to develop PDA sensors with minimal response to water molecules, a comparable approach was envisioned wherein the modification focused on the polar moiety rather than altering the alkyl chain of PCDA.More specifically, a similar imidazolium derivative of PCDA was desired but this time reacting with bromopropionitrile in the final step. The PCDA monomer salt was synthesized in a simple two-step procedure, Fig. 1A. In the first step, a one-pot EDC/NHS coupling was used to generate the neutral imidazolium derivative. The product was then heated under reflux for 18 hours with 1.1 equivalents of bromopropionitrile and was isolated as a pale yellow oily solid.
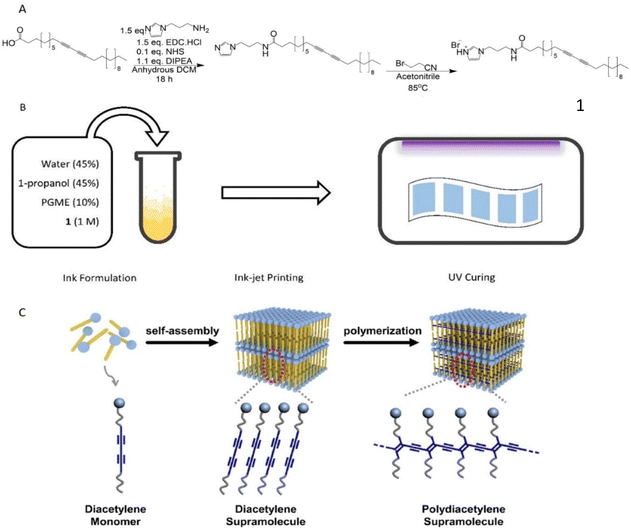 | ||
| Fig. 1 A: Reaction scheme for the synthesis of PCDA-I+H B: schematic illustration of sensor preparation C: cartoon showing ordering and polymerisation adapted from Lee et al. under licence CC BY-NC-ND 3.0.24 | ||
This was quickly redispersed in 1-propanol and protected from light to ensure no polymerisation occurred prior to printing. Surprisingly, the attained final product was not the expected PCDA bromopropionitrile imidazolium salt but instead the protonated PCDA imidazolium salt derivative (PCDA-I+H). This was confirmed by both 1H-NMR and high-resolution mass spectrometry (Fig. S2 and S3†) showing that molecule 1 is the major product of this reaction at the used experimental conditions with the characteristic protonated nitrogen peak (–NH) appearing at δ = 9.20 ppm and an m/z = 496.42 g mol−1. The basic character of the lone pair electrons on nitrogen at position 3 of the imidazole ring likely showed preference to abstract an acidic proton (–CH2–Br) from bromopropionitrile resulting in protonation.
Ink-jet printing is a convenient method for depositing PCDA-I+H onto paper, in a scalable and reproducible manner. However, careful consideration must be given to the ink formulation, which needs to contain appropriate solvents to avoid both particulates forming in the ink and fast evaporation, both of which lead to deposition of solids at the nozzle leading to the printer blocking. This results in streaks forming in the product, Fig. S4.† Ink was produced by dissolving 1 in a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture of water and 1-propanol to form a clear, slightly yellow solution, with added PGME to lower the volatility, Fig. 1B. The ink was transferred into an Epson EcoTank inkjet printer and printed at standard quality on standard 80 gsm A4 paper. The ink was then allowed to fully dry on the paper surface before being irradiated with λ = 254 nm light at 5000 μJ cm−2 for 12 seconds to produce a uniform blue square. Optimizing the time and energy of curing was important to give the highest quality sensors. Three different curing times with two predetermined energy outputs were examined and their performance was assessed in vivo after hot room studies (Fig. S5–S8 and Table S1†). Results indicated that longer irradiation times reduced the sensitivity and quality of the sensor while a 12 seconds curing using 5000 μJ cm−2 was optimal, giving uniform patches with good colour contrast, high droplet clarity and minimal droplet spreading.
50 mixture of water and 1-propanol to form a clear, slightly yellow solution, with added PGME to lower the volatility, Fig. 1B. The ink was transferred into an Epson EcoTank inkjet printer and printed at standard quality on standard 80 gsm A4 paper. The ink was then allowed to fully dry on the paper surface before being irradiated with λ = 254 nm light at 5000 μJ cm−2 for 12 seconds to produce a uniform blue square. Optimizing the time and energy of curing was important to give the highest quality sensors. Three different curing times with two predetermined energy outputs were examined and their performance was assessed in vivo after hot room studies (Fig. S5–S8 and Table S1†). Results indicated that longer irradiation times reduced the sensitivity and quality of the sensor while a 12 seconds curing using 5000 μJ cm−2 was optimal, giving uniform patches with good colour contrast, high droplet clarity and minimal droplet spreading.
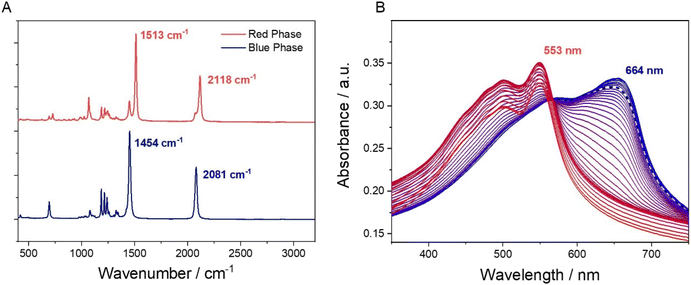
The response of the printed patches to various stimuli was first tested in the laboratory. For the intended application, it is important that the sensor only responds to the acids and alcohols in sweat, and not to water. Fig. 2 demonstrates the colorimetric response to a range of acids, alcohols and tap water. The Raman spectra of a cured PDA patch (blue phase) and the same region after exposure to a drop of propionic acid (red phase) shows indicative changes, with the disappearance of a sharp peak at 1513 cm−1 moving to 1454 cm−1 and 2118 moving to 2081 cm−1, Fig. 3A. It is apparent that the addition of the acid increased the frequency of both the carbon-to-carbon double and triple bond vibrations, indicating that their bond lengths decrease. Upon the addition of water, no visual change was detected (Fig. S9†). This potentially indicated that water molecules did not cause enough polar changes to disrupt the conjugated PDA network thus leading to minor conformational transitions of the backbone.
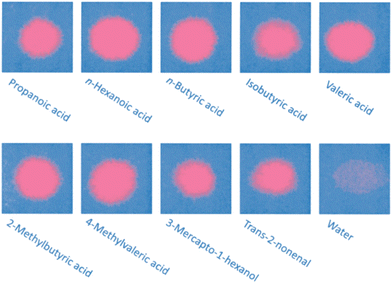 | ||
| Fig. 2 Chromic response of poly(PCDA) printed on paper to a range of odorous sweat components and a tap water control. | ||
Heat can also be used as a stimulus to induce a colour change in these PDAs.28 For underarm sweat analysis, it is necessary that the PDA patches are stable at body temperature. To test this, we taped a sheet of polyethylene film (PE) to paper and printed and cured a PDA patch on to the surface. The blue region on the PE was then clamped in a quartz demountable cuvette and its UV-vis spectra were measured over a temperature range from 25–95 °C (Fig. 3B). With increasing temperature, the absorbance band of the PDA undergoes a significant blue shift, from 664 nm to 553 nm. The increase in the energy of the π* transitions shows the disruption of the large conjugated system of the blue phase PDA and the formation of the red phase, which begins at around 45 °C. The dashed line in Fig. 3B shows that at body temperature the PDA patch is still in the blue phase.
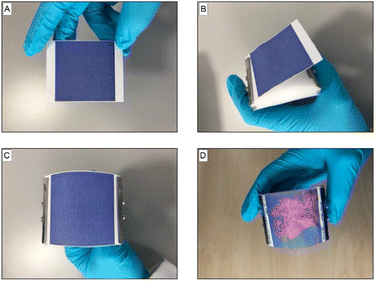
Following the benchtop tests, we proceeded to test the effectiveness of the PDA sensors for detection of sweat from human underarms (Fig. 4A). For this we setup an experiment to test the differences between a deodorant and an antiperspirant product. Antiperspirants typically contain active ingredients that block pores and reduce sweat volume and so we should see a clear difference following the application of these two products. To ensure a controlled environment in the experiment, a hot room study was carried out with 52 panellists. Half were given an antiperspirant to apply for three consecutive days and the other half a deodorant control product. On the fourth day, the panellists sat in a hot room, which was set at 40 °C and 40% relative humidity. After 40 minutes in the hot room, a PDA patch was applied to the underarm of each of the panellists for 5 seconds. For comparison, examples of patches from “untreated” sweat from a hot room study prior to product application are presented in Fig. S10.†
To ensure that an even pressure was applied to the underarms, the PDA patches were secured on an applicator, where the radius of its curvature was designed to maximise surface contact in the underarm (Fig. 4B and C). Following application to the underarm, the area of the patch that was in contact with sweat changed to be red in colour (Fig. 4D).
Images of the patches used in the hot room study were digitized using the Epson EcoTank scanning function. These images were read into MATLAB and processed into binary images with an algorithm based on thresholding of their RBG values. Example images of patches processed from the deodorant control group and the antiperspirant group are given in Fig. 5A, B and C, D respectively. The red area of the patches was determined by calculating the ratio of the number of pixels with a value of 1 to the total number of pixels in the image and finally converting these values to mm2.
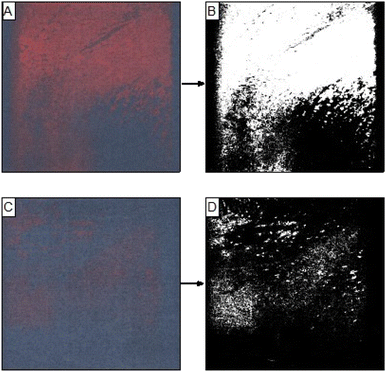 | ||
| Fig. 5 Scanned images of patches after application on subjects with deodorant (A) and antiperspirant (C) and their corresponding processed images (B and D, respectively). | ||
The processed data from all panellists in the hot room study is given in Table 1. It is apparent that when using the patches, a clear difference in the average area of colour change was detected between the groups where deodorant was applied compared with the antiperspirant group, with the latter group showing an area reduction of 46.93%. The difference between the sets of samples was also tested using the Wilcoxon signed-rank test, which showed a significant difference between the two populations (p ≪ 0.05), Fig. 6.
 | ||
| Fig. 6 Comparison of the red area of patches following application on subjects in the antiperspirant and deodorant groups. | ||
| Product | Mean sweat area (mm2) | Std. dev. (σ) | Std. error mean (SEM) | Wilcoxon signed-rank test (p-value) | Sweat area reduction (SAR)a | Number of panellists |
|---|---|---|---|---|---|---|
| a SAR = (1 − mean (Antiperspirant)/mean (Deodorant)) × 100. | ||||||
| Deodorant | 963.37 | 693.26 | 135.96 | 0.0002175 | 46.93% | 26 |
| Antiperspirant | 511.24 | 587.37 | 115.19 | 26 | ||
The capability of the PDA sensor was then further examined by analysing the data collected from three subsets of subjects: light, moderate and heavy “sweaters”, again comparing the area of colour change when using an antiperspirant product or a control, Table S2.† Thus, the patches can be used to differentiate between the subsets when wearing or not wearing an antiperspirant product, Fig. 7.
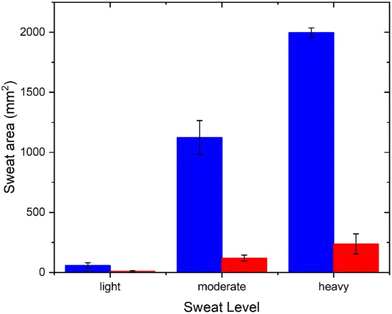 | ||
| Fig. 7 Comparison of the red area of patches following application on subjects in the antiperspirant (red) and deodorant (blue) groups for the data divided into three subsets. | ||
Conclusions
A simple PDA sensor was developed that selectively changes colour from blue to red in response to the acids and alcohols found in sweat, but not to water. Patches were prepared using an inexpensive home/office ink jet printer with refillable ink tanks. The ink, printing method and UV curing time of the sensors were optimised to determine changes in a person's sweat volume in real time and allow for a high data collection rate. This technology is currently being exploited by Unilever.Author contributions
RH and AW contributed to the investigation, supervision and writing. SE contributed to the investigation and writing. MG, GK contributed to the investigation and methodology. TB contributed to the investigation and methodology and supervision. ECT contributed to the investigation and validation. JF contributed to the conceptualization and project administration. JR and JM contributed to the investigation. AJ contributed to the conceptualization. SB, EK and DH were responsible for conceptualization, supervision and funding acquisition.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
RH, SE, AW, MG, GK, and TB would like to thank Unilever for funding. The authors dedicate this paper to the memory of Dr Maria Grypioti. We also thank the Research Technology Platforms (RTP) of the University of Warwick and EPSRC for equipment funded in part by EPSRC EP/V036211/1 and EP/V007688/1.References
- A. Watkinson, R. S. Lee, A. E. Moore, S. E. Paterson, P. Pudney and A. V. Rawlings, Int. J. Cosmet. Sci., 2007, 29(1), 60–60 CrossRef.
- K. Wilke, K. Wick, F. J. Keil, K. P. Wittern, R. Wepf and S. S. Biel, Exp. Dermatol., 2008, 17(1), 73–81 CrossRef PubMed.
- J. Tobin, Chem. Soc. Rev., 2006, 35(1), 52–67 RSC.
- K. Wilke, A. Martin, L. Terstegen and S. S. Biel, Int. J. Cosmet. Sci., 2007, 29(3), 169–179 CrossRef CAS.
- A. G. James, C. J. Austin, D. S. Cox, D. Taylor and R. Calvert, FEMS Microbiol. Ecol., 2013, 83(3), 527–540 CrossRef CAS PubMed.
- C. Austin and J. Ellis, J. Steroid Biochem. Mol. Biol., 2003, 87(1), 105–110 CrossRef CAS PubMed.
- N. Solish, A. Benohanian and W. K. Jonathan, Dermatol. Surg., 2005, 31(4), 405–413 CrossRef CAS.
- H. Murota, Old and new approaches for assessing sweating, in Perspiration Research, Karger Publishers, 2016, vol. 51, pp. 22–29 Search PubMed.
- Y.-R. Kim, S. Jung, H. Ryu, Y.-E. Yoo, S. M. Kim and T.-J. Jeon, Sensors, 2012, 12(7), 9530–9550 CrossRef CAS PubMed.
- D. Grieshaber, R. MacKenzie, J. Vörös and E. Reimhult, Sensors, 2008, 8(3), 1400–1458 CrossRef CAS.
- H. Tao, L. R. Chieffo, M. A. Brenckle, S. M. Siebert, M. Liu, A. C. Strikwerda, K. Fan, D. L. Kaplan, X. Zhang, R. D. Averitt and F. G. Omenetto, Adv. Mater., 2011, 23(28), 3197–3201 CrossRef CAS PubMed.
- S. Kim, C. Young, B. Vidakovic, S. G. A. Gabram-Mendola, C. W. Bayer and B. Mizaikoff, IEEE Sens. J., 2010, 10(1), 145–158 CAS.
- A. J. Bandodkar and J. Wang, Trends Biotechnol., 2014, 32(7), 363–371 CrossRef CAS PubMed.
- D. P. Lobo, A. M. Wemyss, D. J. Smith, A. Straube, K. B. Betteridge, A. H. J. Salmon, R. R. Foster, H. E. Elhegni, S. C. Satchell, H. A. Little, R. Pacheco-Gomez, M. J. Simmons, M. R. Hicks, D. O. Bates, A. Rodger, T. R. Dafforn and K. P. Arkill, Nano Res., 2015, 8(10), 3307–3315 CrossRef PubMed.
- Y. Yang, S. Xing, Z. Fang, R. Li, H. Koo and T. Pan, Lab Chip, 2017, 17(5), 926–935 RSC.
- P. Salvo, F. D. Francesco, D. Costanzo, C. Ferrari, M. G. Trivella and D. D. Rossi, IEEE Sens. J., 2010, 10(10), 1557–1558 Search PubMed.
- W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya, D.-H. Lien, G. A. Brooks, R. W. Davis and A. Javey, Nature, 2016, 529, 509 CrossRef CAS.
- A. Koh, D. Kang, Y. Xue, S. Lee, R. M. Pielak, J. Kim, T. Hwang, S. Min, A. Banks, P. Bastien, M. C. Manco, L. Wang, K. R. Ammann, K.-I. Jang, P. Won, S. Han, R. Ghaffari, U. Paik, M. J. Slepian, G. Balooch, Y. Huang and J. A. Rogers, Sci. Transl. Med., 2016, 8(366), 366ra165 Search PubMed.
- X. Huang, Y. Liu, K. Chen, W.-J. Shin, C.-J. Lu, G.-W. Kong, D. Patnaik, S.-H. Lee, J. F. Cortes and J. A. Rogers, Small, 2014, 10(15), 3083–3090 CrossRef CAS PubMed.
- A. Soni, R. K. Surana and S. K. Jha, Sens. Actuators, B, 2018, 269, 346–353 CrossRef CAS.
- R. Jelinek and M. Ritenberg, Polydiacetylenes – recent molecular advances and applications, 2013 Search PubMed.
- J. Kiji, J. Kaiser, G. Wegner and R. C. Schulz, Polymer, 1973, 14(9), 433–439 CrossRef CAS.
- K. Fahsi, J. Deschamps, K. Chougrani, L. Viau, B. Boury, A. Vioux, A. van der Lee and S. G. Dutremez, CrystEngComm, 2013, 15(21), 4261–4279 RSC.
- J. Lee, M. Pyo, S.-H. Lee, J. Kim, M. Ra, W.-Y. Kim, B. J. Park, C. W. Lee and J.-M. Kim, Nat. Commun., 2014, 5, 3736 CrossRef CAS PubMed.
- M. Seo, D.-H. Park, B. J. Park and J.-M. Kim, J. Appl. Polym. Sci., 2017, 134(6), 44419 CrossRef.
- D.-H. Park, W. Jeong, M. Seo, B. J. Park and J.-M. Kim, Adv. Funct. Mater., 2016, 26(4), 498–506 CrossRef CAS.
- S. Lee, J. Y. Kim, X. Chen and J. Yoon, Chem. Commun., 2016, 52, 9178–9196 RSC.
- B. Yoon, H. Shin, E.-M. Kang, D. W. Cho, K. Shin, H. Chung, C. W. Lee and J.-M. Kim, ACS Appl. Mater. Interfaces, 2013, 5(11), 4527–4535 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3lp00214d |
| ‡ Dr Maria Grypioti died prior to the publication of this work. |
| This journal is © The Royal Society of Chemistry 2024 |