Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oil-in-oil droplet stability dependence on dimensions of 2D Pickering particles of controlled size†
Simon D.
Dale
a,
James
Beament
b,
Andrew P.
Dove
 *a and
Rachel K.
O'Reilly
*a and
Rachel K.
O'Reilly
 *a
*a
aSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. E-mail: a.dove@bham.ac.uk; r.oreilly@bham.ac.uk
bInfineum UK Ltd., Milton Hill, Abingdon, OX13 6BB, UK
First published on 22nd July 2024
Abstract
Non-aqueous emulsions are employed for a host of important applications; however, their long-term stability often limits their use. 2D particles have been reported to provide greater emulsion stability compared to surfactants and isotropic particles as a result of their greater interfacial area interaction. Here, control over the particle size resulted in control over the droplet diameter and increased stability. Non-aqueous emulsions are widely employed; therefore, characterising the effect of the dimensions of 2D particles on their stability is key to making oil-in-oil (o/o) emulsions with enhanced properties. This study investigates the self-assembly of uniform 2D particles of a controlled size, and their application as Pickering particles in o/o emulsions. The correlation between 2D particle dimensions and emulsion characteristics was investigated, a comparison that has not been reported for o/o emulsions prior to this study.
Introduction
Emulsions that do not contain an aqueous phase have seen an increase in interest in recent years due to their unique properties and applications.1 The absence of an aqueous phase has been pivotal in the study of water-sensitive functionalities that otherwise would not be accessible in emulsion technology. Oil-in-oil (o/o) emulsions have a wide range of applications, from polymer synthesis to pharmaceutical development.2 For example, polymeric particles, of a well-defined size, can be synthesised using water-sensitive catalysts and avoiding water-initiated degradation to undesired side products.3–5 In the drive for achieving new and improved pharmaceuticals, o/o emulsions have enabled the study of new drugs that were either water sensitive or poorly soluble. This has aided their administration and, in some cases, their residence time.6–8 For example, the encapsulation of ibuprofen was demonstrated to be greater in an o/o emulsion compared to a water containing emulsion.9 With these ever expanding, and important, roles of o/o emulsions, they are seen as an exciting field for new developments.Emulsifier choice plays a key role in the properties of the resulting emulsion, including stability or the droplet size. Control over such properties has previously proven difficult compared to water containing emulsions, due to the much lower interfacial tension between two oil phases.1,2 Pickering particles have demonstrated many benefits over block copolymers (BCPs) and small molecule surfactants as emulsifiers. The solid particles require much higher energies to remove them from a liquid–liquid interface, whilst providing greater steric resistance to droplet coalescence.10,11 Among this class of emulsifying agents, there are particular benefits in the use of 2 dimensional (2D) particles as a consequence of their high aspect ratio. This large surface area, and their expected orientation to lay with their edge parallel to the liquid–liquid interface, makes them even less likely to desorb compared to spherical or cylindrical particles.12 The most common 2D materials that have been studied as Pickering particles are clay platelets13–16 and graphene oxide (GO) nanosheets.8,17,18 However, the preparation of these particles lacks any significant control over the dimensions of the resulting 2D emulsifier, which may be limiting their ability to control aspects of the emulsion such as stability or the droplet size. Therefore, to date, there has been no investigation into the role that the particle size plays in the properties of oil-in-oil (o/o) emulsions. Previously, our group demonstrated the important role 2D particle dimensions play in the droplet size and stability of water-in-water (w/w) emulsions.19 Uniform PLLA-based diamond platelets with a larger surface area provided greater stability and a smaller droplet size compared to smaller platelets of the same chemistry and width.
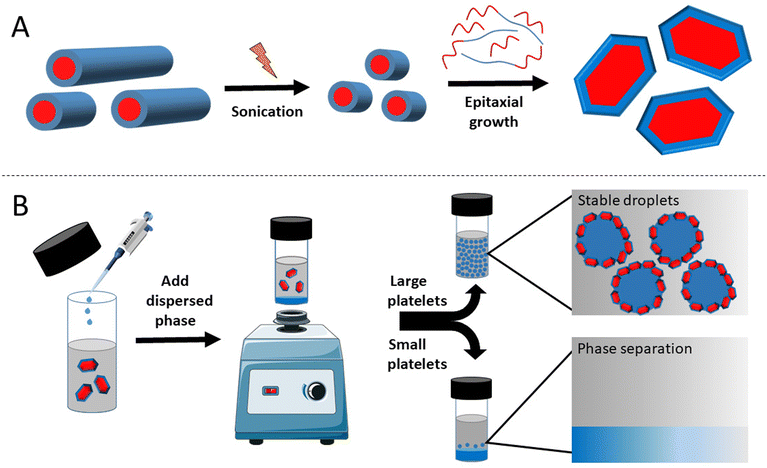
BCP self-assembly is a well-studied method of nanoparticle synthesis. Of particular note, crystallisation-driven self-assembly (CDSA) has been reliably demonstrated to assemble anisotropic particles, including 2D platelets.20 Control over the platelet size can be achieved by a process termed living CDSA: the epitaxial growth of a BCP unimer and, in some cases, a crystalline homopolymer to uniform “seed” particles created from the sonication of particles with high size dispersity.21 The size of the resulting platelets can be controlled through the mass ratio between the seed particles and the added unimer and semi-crystalline homopolymer. This process was first reported by Eisenberg and co-workers, where the addition of the poly(ε-caprolactone) (PCL) homopolymer to the assembly of PCL–PEO drove a morphological ripening of 1D cylinders to 2D platelet particles.22 This has recently been improved upon as a method of obtaining PCL-based platelets of a uniform size by a living growth mechanism, demonstrating robust control over the particle dimensions.23
Given the importance of o/o emulsions, the development of emulsifiers that can be tuned to provide emulsions with defined properties would be a significant achievement. Considering the previously observed dependency of droplet stability on particle size for w/w emulsions, we postulated that a similar dependence would be observed in o/o emulsions. We therefore investigated a living CDSA methodology of 2D particle synthesis in nonpolar solvents to generate platelets of different sizes with low size dispersity. These platelets were used as emulsifiers to investigate the dependency of droplet properties on the dimensions. To directly compare the effects of particle dimension control with previous reports, we replicated the same systems that employed GO sheets of high size dispersity as emulsifiers: a dispersed phase of N,N-dimethylformamide (DMF) within a continuous phase of octane in a 1/5 ratio.17 As octane is the continuous phase, particle formation would be conducted in this solvent prior to the addition of DMF. Particles of two sizes were investigated as emulsifiers at varying platelet concentrations. The droplet size was observed to differ significantly from the comparable study, and a droplet stability dependency on the platelet size was observed at a certain concentration.
Results and discussion
PCL has been widely studied as a core forming block for CDSA, with reports demonstrating its ability to form 2D particles of a controlled size, mostly taking place in water.23 As a biocompatible and biodegradable aliphatic polyester, PCL has been of great interest for pharmaceutical and biological applications.24,25 Hence, utilising PCL as the base of these Pickering particles, compared to GO, increases the potential applications in the biological field. The biodegradability of the PCL core may also offer opportunities to control degradation and hence release from emulsions, thus highlighting the potential advantages of using PCL-containing particles as Pickering stabilizers.Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct solubility calculations normalised to the surface area (log
Poct solubility calculations normalised to the surface area (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/SA) demonstrated a significant difference in solubility parameters between PCL (log
Poct/SA) demonstrated a significant difference in solubility parameters between PCL (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/SA = 0.010) and octane (log
Poct/SA = 0.010) and octane (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/SA = 0.022), suggesting this would be a suitable core forming block for self-assembly in octane. As methacrylates with linear alkyl sidechains are reported as steric stabilising blocks for a range of n-alkane soluble nanoparticles, this type of polymer was investigated as a soluble stabilising block.26–28 From log
Poct/SA = 0.022), suggesting this would be a suitable core forming block for self-assembly in octane. As methacrylates with linear alkyl sidechains are reported as steric stabilising blocks for a range of n-alkane soluble nanoparticles, this type of polymer was investigated as a soluble stabilising block.26–28 From log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct calculations, poly(n-decyl methacrylate) (PnDMA) possessed a closely matching hydrophobicity to that of octane (log
Poct calculations, poly(n-decyl methacrylate) (PnDMA) possessed a closely matching hydrophobicity to that of octane (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/SA = 0.023); therefore this was selected as the stabilising block for this study.
Poct/SA = 0.023); therefore this was selected as the stabilising block for this study.
A PCL50 macro-CTA was targeted using a bifunctional ring-opening polymerisation (ROP) initiator and a reversible-addition fragmentation chain transfer (RAFT) agent as previously reported (Fig. S1 & S2†).29 A degree of polymerisation (DP) of 50 was achieved after 6 h with a narrow dispersity (ĐM = 1.07) (Scheme S1, Fig. S3 & S4†). RAFT polymerisation of nDMA using the PCL50 macro chain transfer agent (CTA) provided the amphiphilic BCP PCL50-b-PnDMA155 after 18 h as determined by 1H NMR spectroscopy (Scheme S1, Fig. S5 & S6†).
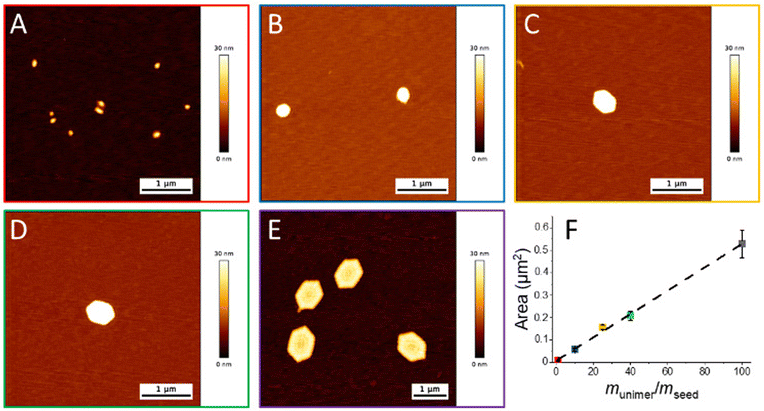
Size exclusion chromatography (SEC) analysis indicated good control over the polymerisation, with a narrow dispersity of molecular weight (ĐM = 1.16) observed (Fig. S7†). Good agreement of the refractive index (RI) and UV (λ = 309 nm) indicated retention of the trithiocarbonate end group in the purified product. The semi-crystallinity of the PCL block was maintained in the di-block as observed through thermal analysis by differential scanning calorimetry (DSC), providing a melting temperature (Tm) of 49.5 °C, a crystallisation temperature (Tc) of −54.6 °C, and a glass transition temperature (Tg) of −64.9 °C (Fig. S8†). The retention of the semi-crystallinity of the PCL block, along with the hydrophobic nature of the methacrylate block, indicates that this BCP should be suitable to undergo CDSA in octane. Transmission electron microscopy (TEM) imaging of a solution of the polymer after self-assembly provided evidence that cylindrical particles had formed as targeted (Fig. S9A†). As controlled crystallisation into anisotropic nanoparticles had been demonstrated, we moved to the next stages of creating 2D particles of a controlled size via a living CDSA mechanism (Scheme 1A). Sonication of the cylindrical micelle solution resulted in the formation of seed particles with an average length (Lave) of 54 ± 15 nm (Fig. S9B & C†). These seed particles were subsequently used to form platelets through the addition of a blend of the dissolved PCL50-b-PnDMA155 unimer and the PCL50 homopolymer. The mass ratio of the unimer to PCL50 was set at 1 (munimer/mPCL50 = 1) for all living growth experiments, whilst the mass ratio of seeds and the unimer (munimer/mseed) was varied from 1, 10, 25, 40 to 100. Atomic force microscopy (AFM) analysis provided images of platelets at each of these munimer/mseed ratios, with the size of the platelet increasing as munimer/mseed increased (Fig. 1A–E & S10†). The linear relationship between munimer/mseed and the average platelet area demonstrates the controlled nature of the platelet size increase, meaning that the aim of achieving low dispersity platelets of defined dimensions was achieved (Fig. 1F). Control over the lateral dimensions was achieved; however, the width of the platelets remained constant. Therefore, the shape factor of the resulting platelets changes with increasing epitaxial growth. Alterations of the platelet width can be made through altering the BCP composition;23 however, this was not considered for this study. The thickness of 2D Pickering stabilisers can have an effect on the emulsion, for example, increasing the density of clay platelets leads to destabilisation and, therefore, a less stable emulsion.30 We therefore only altered the lateral dimensions of the platelets used in this study.
Given the fact that the control over the 2D particle size had been achieved, the ability of these particles to act as emulsifiers was investigated. Two platelet sizes were selected for this: munimer/mseed = 25 (P25, platelet area = 0.15 ± 0.01 μm, Fig. 1C) and 100 (P100, platelet area = 0.53 ± 0.06 μm, Fig. 1E). These were chosen as they possessed significantly different areas; therefore any changes in the resulting emulsions would likely be as a result of the difference in the platelet area. We selected DMF, an aprotic polar solvent with a boiling point >100 °C, as the dispersed phase. As described earlier, the ability to use emulsions where both phases are stable at temperatures >100 °C unlocks the possibility to use emulsion technology for applications that would not be possible when one of the phases is water. After the addition of DMF (octane/DMF = 5/1 v/v), the solutions were vortexed for 30 seconds (Scheme 1B). The concentration of platelets in octane was varied as the platelet concentration has been demonstrated to play a role in droplet stability, and it was therefore of great interest to determine the effect of altering this variable on droplet properties.
After vortexing the solutions containing 1% w/w platelets (Fig. S11†), both sizes of platelets provided stable emulsions with a consistent droplet size over 4 weeks (Fig. S12–S14, and Table S1†). Interestingly, the droplet size did not appear to be dependent on the platelet dimensions, possibly as a consequence of the higher particle concentration compared to other systems.17 Interestingly, the droplet size observed for these controlled emulsifier systems was significantly smaller than that using GO sheets using the same solvent system.17 The droplet size has a direct influence on a range of emulsion properties including optics,31 rheology,32 pharmaceutical activity,33 and stability.34 Smaller droplets also benefit from longer stability times as a consequence of being more resistant to gravitational forces, leading to greater resilience against sedimentation or creaming.34 The ability to produce smaller droplets can benefit these applications, as well as provide longer term stability in storage so that the properties remain consistent over time.35
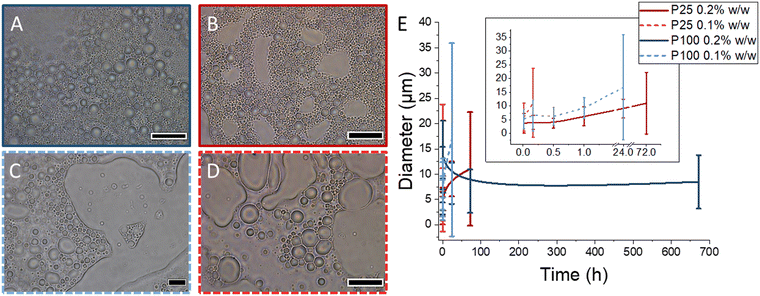
Subsequently, the concentration of platelets of both sizes was reduced to 0.2% w/w in octane. As in the case when the concentration was 1% w/w, droplets with a relatively low size dispersity were observed, with the droplet diameter <6 μm on average (Fig. 2, Fig. S15 & S16, Table S2†). Analysis of the emulsion stabilised by P25 indicated good stability over 1 h. However, ripening was evident after this as the average droplet diameter steadily increased over the 3 day aging period (Fig. 2E, Fig. S15†). Long term instability of these emulsions was observed after 4 weeks of aging, with phase separation observed in the microscopy analysis (Fig. 2B). However, when imaging the emulsion of the same concentration of P100, no phase separation was observed after the same 4 week aging period (Fig. 2A & Fig. S16†). The droplet diameter remained relatively stable over this period, indicating that ripening was not taking place (Fig. 2E, Table S2†). These results demonstrate that the area of the platelet employed as the Pickering particle does indeed play an important role in droplet stability.
After observing the long-term stability of the larger platelets at 0.2% w/w, a further concentration reduction to 0.1% w/w was tested using P100. The droplet size steadily increased over the first 1 h with some phase separation clearly observed when imaging after 24 h (Fig. 2C and E, Fig. S17†). Significantly larger areas of free solvent were present after 3 days, as phase separation continued over the longer aging period. However, in comparison, P25 only provided droplet stability for 1 minute at 0.1% w/w, as large ill-defined droplets caused by ripening were observed after 10 minutes and clear phase separation was observed after 30 minutes (Fig. 2D and E, Fig. S18†). This again evidenced the increased stability afforded by the larger platelet dimensions at the same concentration of the emulsifier.
These observations were further investigated by analysing how the presence of the particles affected the surface tension (ST) between the two solvents through drop shape analysis of DMF in the platelet solutions at 0.1 and 0.2% w/w (Fig. 3, Fig. S19†). As anticipated, a droplet of DMF in solutions that contained P25 reduced the surface tension to a lower extent than those in solutions that contained P100 (Fig. 3). Inherently, a lower surface tension reduces the surface free energy (ΔG) of an emulsified system, as demonstrated in the following equation:36
| ΔG = σΔA |
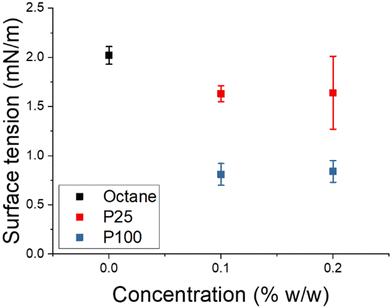 | ||
| Fig. 3 Surface tension of a droplet of DMF in octane and solutions of platelets in octane at 0.2 and 0.1% w/w. | ||
Additionally, the morphological dependency was further studied through changing the shape of the emulsifier. Short cylindrical particles of PCL50-b-PnDMA155 were substituted for platelet particles as Pickering particles (Fig. S20†). Using the same emulsification strategy as above, at 0.2% w/w, emulsion droplets were observed after 1 minute; however phase separation was prevalent after only 2 minutes. Increasing the cylinder concentration up to even 4% w/w did not lead to stable emulsions over a long time period, as phase separation was observed after only 5 minutes. This demonstrates that the higher surface area results in platelets having a greater ability to sit at the interface and stabilise droplets compared to 1D particles.
To further expand the applicability of these emulsifiers, we investigated P100 as a stabiliser for a second polar non-aqueous solvent, acetonitrile (CH3CN), in octane. Drop shape analysis of CH3CN in solutions of P100 at 0.1 and 0.2% w/w demonstrated that the particles reduced ST from 1.35 ± 0.02 mN m−1 without particles to a similar level at both concentrations: 0.73 ± 0.38 and 0.53 ± 0.06 mN m−1 respectively (Fig. S21†). These reductions in ST resulted in emulsion droplets forming in the presence of P100 at 0.1 and 0.2% w/w following the same conditions as when DMF was the dispersed phase (Fig. S22†). These droplets were stable after 24 h, as no phase separation was observed at this timepoint. This result demonstrates the utility of these particles of a controlled size to act as stabilisers for multiple o/o systems, enhancing their applicability in the field of o/o emulsions.
Conclusions
It was demonstrated that the dimensions of 2D Pickering particles play a significant role in emulsion droplet stability. The controlled assembly of 2D platelets with a low size dispersity using PCL-based BCPs was achieved through matching of the solubility of an alkyl methacrylate corona block with the selected solvent by log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/SA computational calculations. The epitaxial growth of the unimer and the semi-crystalline PCL50 homopolymer to seed particles was observed to provide 2D platelet particles of controlled dimensions. This is the first report of PCL-based BCPs assembling into 2D platelets of a controlled size in non-polar organic solvents, unlocking these materials to be implemented in many applications with the potential to be degradable post-use. Droplets of DMF in octane were stabilised over 4 weeks by platelets of two different sizes at a relatively high weight percentage; however, the droplet size was significantly smaller than that of a comparable study using GO sheets. This demonstrates the importance of the size of particles in how they act as Pickering stabilisers in non-aqueous emulsions. A relationship between the platelet size and droplet stability was indicated, as the platelets with a larger size stabilised droplets to a greater extent compared to their smaller counterparts with the same chemistry. This highlights the potential advantages of controlling the dimensions of 2D particles as stabilisers for o/o emulsions, and this is an important consideration to make in any future formulations. We envisage that emulsion stability can be tailored to an application through simply altering the dimensions of the stabilising platelet. This system could therefore be utilised for timed release of the cargo, or to ensure that a desired reaction time can be achieved within the dispersed phase. Particles of this type are expected to find further use in emulsions for biological or environmental applications, as a consequence of the biologically compatible, and biodegradable, PCL core.
Poct/SA computational calculations. The epitaxial growth of the unimer and the semi-crystalline PCL50 homopolymer to seed particles was observed to provide 2D platelet particles of controlled dimensions. This is the first report of PCL-based BCPs assembling into 2D platelets of a controlled size in non-polar organic solvents, unlocking these materials to be implemented in many applications with the potential to be degradable post-use. Droplets of DMF in octane were stabilised over 4 weeks by platelets of two different sizes at a relatively high weight percentage; however, the droplet size was significantly smaller than that of a comparable study using GO sheets. This demonstrates the importance of the size of particles in how they act as Pickering stabilisers in non-aqueous emulsions. A relationship between the platelet size and droplet stability was indicated, as the platelets with a larger size stabilised droplets to a greater extent compared to their smaller counterparts with the same chemistry. This highlights the potential advantages of controlling the dimensions of 2D particles as stabilisers for o/o emulsions, and this is an important consideration to make in any future formulations. We envisage that emulsion stability can be tailored to an application through simply altering the dimensions of the stabilising platelet. This system could therefore be utilised for timed release of the cargo, or to ensure that a desired reaction time can be achieved within the dispersed phase. Particles of this type are expected to find further use in emulsions for biological or environmental applications, as a consequence of the biologically compatible, and biodegradable, PCL core.
Author contributions
Conceptualisation: S. D. D., J. B., A. P. D., and R. K. O.; experimental data collection: S. D. D.; data analysis: S. D. D.; writing: S. D. D.; review and editing: S. D. D. and A. P. D.; supervision: A. P. D. and R. K. O.; funding acquisition: J. B.Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge funding from Infineum UK Ltd.References
- A. Zia, E. Pentzer, S. Thickett and K. Kempe, ACS Appl. Mater. Interfaces, 2020, 12, 38845–38861 CrossRef CAS.
- D. Crespy and K. Landfester, Soft Matter, 2011, 7, 11054–11064 RSC.
- R. Dorresteijn, R. Haschick, M. Klapper and K. Müllen, Macromol. Chem. Phys., 2012, 213, 1996–2002 CrossRef CAS.
- C. Herrmann, D. Crespy and K. Landfester, Colloid Polym. Sci., 2011, 289, 1111–1117 CrossRef CAS.
- M. Klapper, S. Nenov, R. Haschick, K. Müller and K. Müllen, Acc. Chem. Res., 2008, 41, 1190–1201 CrossRef CAS PubMed.
- L. I. Atanase and G. Riess, Int. J. Pharm., 2013, 448, 339–345 CrossRef CAS.
- O. Suitthimeathegorn, J. A. Turton, H. Mizuuchi and A. T. Florence, Int. J. Pharm., 2007, 331, 204–210 CrossRef CAS PubMed.
- B. J. Rodier, A. de Leon, C. Hemmingsen and E. Pentzer, Polym. Chem., 2018, 9, 1547–1550 RSC.
- Z. Mana, Y. Pellequer and A. Lamprecht, Int. J. Pharm., 2007, 338, 231–237 CrossRef CAS PubMed.
- R. Aveyard, B. P. Binks and J. H. Clint, Adv. Colloid Interface Sci., 2003, 100–102, 503–546 CrossRef.
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21–41 CrossRef.
- P. Wei, Q. Luo, K. J. Edgehouse, C. M. Hemmingsen, B. J. Rodier and E. B. Pentzer, ACS Appl. Mater. Interfaces, 2018, 10, 21765–21781 CrossRef PubMed.
- N. P. Ashby and B. P. Binks, Phys. Chem. Chem. Phys., 2000, 2, 5640–5646 RSC.
- B. Brunier, N. Sheibat-Othman, Y. Chevalier and E. Bourgeat-Lami, Langmuir, 2016, 32, 112–124 CrossRef PubMed.
- J. Dong, A. J. Worthen, L. M. Foster, Y. Chen, K. A. Cornell, S. L. Bryant, T. M. Truskett, C. W. Bielawski and K. P. Johnston, ACS Appl. Mater. Interfaces, 2014, 6, 11502–11513 CrossRef.
- X. Lu, J. S. Katz, A. K. Schmitt and J. S. Moore, J. Am. Chem. Soc., 2018, 140, 3619–3625 CrossRef.
- B. Rodier, A. de Leon, C. Hemmingsen and E. Pentzer, ACS Macro Lett., 2017, 6, 1201–1206 CrossRef CAS PubMed.
- Y. He, F. Wu, X. Sun, R. Li, Y. Guo, C. Li, L. Zhang, F. Xing, W. Wang and J. Gao, ACS Appl. Mater. Interfaces, 2013, 5, 4843–4855 CrossRef CAS PubMed.
- M. Inam, J. R. Jones, M. M. Pérez-Madrigal, M. C. Arno, A. P. Dove and R. K. O'Reilly, ACS Cent. Sci., 2018, 4, 63–70 CrossRef CAS.
- J. C. Foster, S. Varlas, B. Couturaud, Z. Coe and R. K. O'Reilly, J. Am. Chem. Soc., 2019, 141, 2742–2753 CrossRef CAS.
- L. MacFarlane, C. Zhao, J. Cai, H. Qiu and I. Manners, Chem. Sci., 2021, 12, 4661–4682 RSC.
- G. Rizis, T. G. M. van de Ven and A. Eisenberg, Angew. Chem., Int. Ed., 2014, 53, 9000–9003 CrossRef CAS PubMed.
- Z. Tong, Y. Xie, M. C. Arno, Y. Zhang, I. Manners, R. K. O'Reilly and A. P. Dove, Nat. Chem., 2023, 15, 824–831 CrossRef CAS PubMed.
- L. S. Nair and C. T. Laurencin, Prog. Polym. Sci., 2007, 32, 762–798 CrossRef CAS.
- E. Chiellini and R. Solaro, Adv. Mater., 1996, 8, 305–313 CrossRef CAS.
- B. T. Seyrnour, R. K. E. Wright, A. C. Parrott, H. Y. Gao, A. Martini, J. Qu, S. Dai and B. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 25038–25048 CrossRef.
- L. A. Fielding, M. J. Derry, V. Ladmiral, J. Rosselgong, A. M. Rodrigues, L. P. D. Ratcliffe, S. Sugihara and S. P. Armes, Chem. Sci., 2013, 4, 2081–2087 RSC.
- M. J. Derry, O. O. Mykhaylyk, A. J. Ryan and S. P. Armes, Chem. Sci., 2018, 9, 4071–4082 RSC.
- Y. Su, Y. Jiang, L. Liu, Y. Xie, S. Chen, Y. Wang, R. K. O'Reilly and Z. Tong, Macromolecules, 2022, 55, 1067–1076 CrossRef CAS.
- M. Vis, J. Opdam, I. S. J. van ‘t Oor, G. Soligno, R. van Roij, R. H. Tromp and B. H. Erné, ACS Macro Lett., 2015, 4, 965–968 CrossRef CAS PubMed.
- W. Chantrapornchai, F. Clydesdale and D. J. McClements, Colloids Surf., A, 1999, 155, 373–382 CrossRef CAS.
- R. Pal, AIChE J., 1996, 42, 3181–3190 CrossRef CAS.
- M. Jaiswal, R. Dudhe and P. K. Sharma, 3 Biotech, 2015, 5, 123–127 CrossRef.
- T. Tadros, P. Izquierdo, J. Esquena and C. Solans, Adv. Colloid Interface Sci., 2004, 108–109, 303–318 CrossRef CAS.
- S. Kumar, J. Ali and S. Baboota, Nanotechnology, 2016, 27, 435101 CrossRef PubMed.
- K. Schroën, J. de Ruiter and C. Berton-Carabin, ChemEngineering, 2020, 4, 63 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Polymer synthesis, self-assembly, characterisation of nano-objects, emulsion methods and analysis. See DOI: https://doi.org/10.1039/d4lp00091a |
| This journal is © The Royal Society of Chemistry 2024 |