Open Access Article
Open Access ArticleSynthesis of N-methylol polyamide 4: characterization, properties, and biodegradability†
Norioki
Kawasaki
 *,
Naoko
Yamano
and
Atsuyoshi
Nakayama
*,
Naoko
Yamano
and
Atsuyoshi
Nakayama
National Institute of Advanced Industrial Science and Technology (AIST), 1-8-31 Midorigaoka, Ikeda, Osaka 563-8577, Japan. E-mail: n-kawasaki@aist.go.jp
First published on 5th December 2023
Abstract
Polyamide 4 has attractive features: easy biodegradability in the natural environment and in vivo, potential production of monomers from biomass, excellent thermal and mechanical properties, and facile molecular design of various polymer structures utilizing a polymerization mechanism. With the aim of varying the physical properties of biodegradable polyamide 4, a series of N-methylol polyamide 4 with various degrees of methylolation were synthesized by using paraformaldehyde as a reagent. By varying the quantity of paraformaldehyde, it was possible to control the degree of methylolation. The purified products were hard or elastic solids that were white, translucent, or transparent. Depending on the degree of methylolation (mol%), the melting point of N-methylol polyamide 4 varied from 251.2 °C (4.5 mol%) to 164.1 °C (22.5 mol%) and finally disappeared (36.5 mol%). The heat of fusion decreased from 60.6 J g−1 (4.5 mol%) to 2.7 J g−1 (22.5 mol%) and eventually was not observed (36.5 mol%). There was no significant difference in thermal decomposition points, whereas polyamide 4 with a higher degree of methylolation exhibited a slightly higher thermal decomposition point. The tensile strength also depended on the degree of methylolation as in 29.6 MPa (4.5 mol%), 3.0 MPa (22.5 mol%), or 1.3 MPa (36.5 mol%). The elongation at break increased remarkably to 332% even at a low degree of methylolation such as 4.5 mol%, whereas there was no clear proportional relationship with the degree of methylolation. The presence of the methylol group on polyamide 4 main chains had a suppression effect on the biodegradation of polyamide 4, which was dependent on the degree of methylolation. The methylolation could control the high biodegradability of polyamide 4.
Introduction
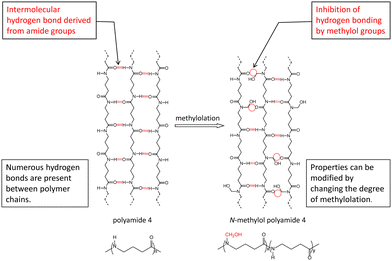
Polyamide 4 was first synthesized in 1953 by William O. Ney et al.1 by ring-opening polymerization of 2-pyrrolidone with a basic catalyst and acyl compounds. Since then, the synthesis of polyamide 4 by polymerization of 2-pyrrolidone has been studied intermittently.2–4 While polyamide 4 can be made into a polymer solution to form a cast film and electrospinning nonwoven fabrics,5,6 the practical application of polyamide 4 has not progressed because of the difficulty in melt molding due to the proximity of its melting point and thermal decomposition temperature.7–10 On the other hand, polyamide 4 is an attractive material with the following features: easy biodegradability in the natural environment and in vivo; efficient production of monomers from biomass through bio- and chemical processes (syrup → glutamic acid → γ-aminobutyric acid → 2-pyrrolidone); excellent thermal and mechanical properties owing to hydrogen bonding between amide groups in the polymer chains; and facile molecular design of various polymer structures utilizing a polymerization mechanism with a variety of initiators. The specific results on the above features of polyamide 4 were described in previous papers. In terms of biodegradability, there have been reports on biodegradation in soil,11 and activated sludge,12 on the isolation of biodegrading bacteria from activated sludge13 or soil14 and its biodegradation mechanism,13 on biodegradation in sea water for laboratory or field studies15,16 and isolation of biodegrading bacteria,16 on biodegradation control by the introduction of a fatty acid group,17 on biodegradation switching by utilizing a titanium dioxide composite as a photooxidative antibacterial material,18 and on bioabsorption and low inflammation reactions in the backs of rats.19 In terms of bioproduction, bioprocess conversion from L-glutamic acid to 2-pyrrolidone via γ-aminobutyric acid has been reported.20 In terms of the design of polymer structures, there have been reports on the synthesis of three-star polyamide 4 and their properties,12 on the synthesis of polyamide 4 with long-chain fatty acid and their biodegradability,21 and on the synthesis of a polyamide 4 containing azo group and its initiation ability for vinyl polymerization and subsequent synthesis of polyamide 4-block-poly(vinyl acetate).22,23Polyamide 4 is a material with useful characteristics as described above. Therefore, it is important to modify its properties in order to develop a wide range of applications. One method of modifying the properties is to introduce other constitutional units into the polymer chain by copolymerization with another monomer during synthesis. With regard to this method, our previous work on modifying the properties of polyamide 4 focused on the synthesis, properties, and biodegradation of copolyamide (4/6) which consists of a polyamide 4 constitutional unit (2-pyrrolidone: PRN) and a polyamide 6 constitutional unit (ε-caprolactam: CLM).24 It was found that the properties and the biodegradability of copolyamide (4/6) can be controlled according to polymer composition (PRN/CLM). In particular, copolyamide (4/6) having low content of the CLM unit maintained a high melting point, strong tensile strength, and easy biodegradability similar to polyamide 4, and additionally had large elongation. Another method of modifying the properties is to substitute hydrogen of the amide group in the polymer chain for other groups through a polymer reaction. For example, when formaldehyde is used as a reagent, amide hydrogen undergoes a substitution reaction to be methylolated or alkoxymethylated. There have been several studies on methylolation or alkoxymethylation of polyamides and their properties and applications. Although the methylolation or alkoxymethylation has been performed on protein,25 polyamide 66,26 polyamide 610,26 polyamide 3,27 and polyamide 6,28,29 little is known about methylolation of polyamide 4. Since polyamide 4 has physical properties derived from hydrogen bonds between polymer chains,30 methylolation of the amide groups in polyamide 4 chains inhibits intermolecular hydrogen bonding and is expected to modify physical properties (Fig. 1). The purpose of the present study is to investigate the synthesis of N-methylol polyamide 4 by a methylolation reaction, and its properties: melting point, heat of fusion, glass transition point, thermal decomposition point, tensile strength, elongation at break, elastic modulus, and also biodegradability.
Experimental
Materials
Polyamide 4 was prepared by ring-opening polymerization of 2-pyrrolidone according to our previous paper.12 Among several types of polyamide 4 structures, three-star polyamide 4 (center: Benzene-1,3,5-tricarbonyl structure), which has better tensile strength than the linear type, was used for the methylolation reaction. Formic acid (Fujifilm Wako Pure Chemical Corp., Osaka, Japan), paraformaldehyde (Fujifilm Wako Pure Chemical), sodium hydroxide (Fujifilm Wako Pure Chemical), methanol (Fujifilm Wako Pure Chemical), acetone (Fujifilm Wako Pure Chemical), and 2,2,2-trifluoroethanol (Kishida Chemical Co. Ltd, Osaka, Japan) were used as received.Synthesis
Three-star polyamide 4 (4.26 g; constitutional unit equivalent 50.1 mmol) was dissolved in formic acid (31.5 mL) at room temperature to prepare a homogeneous polymer solution. Separately, paraformaldehyde (1.07 g; 35.6 mmol) was mixed with methanol (5.4 mL) and sodium hydroxide-methanol solution (concentration 0.05 g mL−1; 50 mL) was added to make a homogeneous solution. This prepared paraformaldehyde-methanol solution was added to the previous polymer solution, and the mixture was heated and stirred at 60 °C for 10 minutes under slightly reduced pressure. The mixture was then diluted by adding methanol (5.4 mL) to reduce the viscosity, and the polymer reaction was carried out by heating and stirring at 60 °C for about 2 hours under slightly reduced pressure. After the reaction, the reactants were concentrated by removing the solvent at reduced pressure using a vacuum pump. The concentrated reactants were purified by precipitation and washing with acetone and dried under reduced pressure at 40 °C in a hot desiccator. The obtained polymeric product was a white solid with elasticity. Yield 5.05 g; 94.7% (basis of polyamide 4 + paraformaldehyde)Methods
The molecular weight (Mn, Mw) and the distribution (Mw/Mn) of N-methylol polyamide 4 were determined by gel permeation chromatography (GPC: HLC-8420 GPC system, Tosoh Corp., Tokyo, Japan; sample columns: TSK gel Super HM-N with two connections, Tosoh; reference columns with two connections: TSK gel Super H-RC, Tosoh). Poly(methyl methacrylate) standards (Shodex Standard M-75, Showa Denko K.K., Tokyo, Japan) and hexafluoroisopropyl alcohol (HFIP, Central Glass Co. Ltd, Tokyo, Japan) as the eluent (flow rate 0.2 mL min−1) were used and maintained at 40 °C.The structure and composition of the N-methylol polyamide 4 were determined by 1H nuclear magnetic resonance (NMR) spectroscopy (JNM-ECA-500 NMR spectrometer 500 MHz, JEOL Ltd, Tokyo, Japan) using deuterated formic acid DCOOD as the solvent. The concentration of the specimen was about 25 mg mL−1.
The melting points (Tm) and the enthalpy of fusion (ΔHm) were measured by differential scanning calorimetry (DSC: DSC3100S Calorimeter, Bruker AXS K.K., Yokohama, Japan). Samples (4–6 mg) were analyzed at a heating rate of 10 °C min−1 using the heating program 30–150–30–350 °C under a nitrogen atmosphere. The glass transition points (Tg) were measured by the heating program −30–120 °C through the use of the same apparatus. Tg were estimated from the midpoint of the distance between the tangent lines of the low and high temperature baselines at the step change. The thermal decomposition points (Td) were measured with thermogravimetry (TG: TG-DTA2000SA, Bruker AXS) by heating from 50 to 500 °C at a rate of 10 °C min−1 under a nitrogen atmosphere. The Td values were determined from the intersection of both tangent lines for the horizontal part and decreasing part on TG curves. From the measured numerical data, 5% weight loss temperature Td5 and 10% weight loss temperature Td10 were also determined.
The tensile strength (ultimate strength) and elongation at break of the N-methylol polyamide 4 film specimens were measured by using a universal tensile testing machine (Auto Com/AC-50C, T.S.E Co. Ltd, Yokohama, Japan) performed with a crosshead speed of 10 mm min−1 at room temperature. All the films were prepared by means of a solvent casting method. Specifically, the N-methylol polyamide 4 were dissolved in 2,2,2-trifluoroethanol (8–10%, w/v), and subsequently dried on a Petri dish at room temperature for a few days. The films were further dried at 40 °C under reduced pressure and then placed in the atmosphere for several hours to reach hygroscopic equilibrium. The average thickness of each film ranged from 110–200 μm. Only the sample with 36.5 mol% methylolation had a film thickness of about 80 μm. The films were cut into rectangles (5 mm × 30 mm) and both edges of the specimens were fixed by square cardboard (25 mm × 25 mm) taking a gauge distance of 10 mm. Most of the specimens were measured 15 times and anomalous data were excluded. The mechanical properties reported here correspond to the average of the measured values, excluding unreasonable data. To indicate the extent of variation in the data, statistical processing was performed, and the standard deviation values are shown after the symbol ±.
The percent biodegradation of the N-methylol polyamide 4 with standard activated sludge (Chemicals Evaluation and Research Institute, Japan) metabolism was evaluated using a closed manometric respirometer system (BOD TESTER 200F and COOLNIT CL-150R system, Taitec Co. Ltd, Koshigaya, Japan). N-Methylol polyamide 4 sample (30 mg) was dispersed in an inorganic culture medium (200 mL) as described in ISO 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851, and standard activated sludge (20 mL; dry weight 20 mg) was added to the medium. The biodegradation test was carried out for about one month at 27 °C with a thermostatic water tank. The percent biodegradation was corrected by subtracting the experimental values on the samples from the experimental values on the activated sludge.
851, and standard activated sludge (20 mL; dry weight 20 mg) was added to the medium. The biodegradation test was carried out for about one month at 27 °C with a thermostatic water tank. The percent biodegradation was corrected by subtracting the experimental values on the samples from the experimental values on the activated sludge.
Results and discussion
N-Methylol polyamide 4 was prepared by substituting hydrogen on an amide bond with a hydroxymethyl group (Scheme 1). The purified products were hard or elastic solids that were white, translucent, or transparent.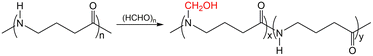 | ||
| Scheme 1 Synthesis of N-methylol polyamide 4 by methylolation reaction using paraformaldehyde as a reagent. | ||
The examples of 1H NMR spectra of polyamide 4 and N-methylol polyamide 4 are shown in Fig. 2. The simple peaks a (2.50–2.51 ppm), b (1.96–1.97 ppm), and c (3.41–3.42 ppm) are due to methylene protons in the polyamide 4 constitutional unit. The complex peaks a′ (2.46–2.94 ppm) and c′ (3.71–3.85 ppm) in the vicinity of peak a and peak c appear to be originated from methylene protons in the polyamide 4 constitutional unit in which the amide hydrogen is substituted with a methylol group. This is supported by the fact that each peak area of a + a′, b, and c + c′ is of equal integration intensity (methylene protons, 2H). Peak d (5.12 ppm) is due to methylene protons in the methylol group. The amide proton peaks were observed at 8.39–8.70 ppm for methanol-d4 + LiCl as a solvent in previous studies, whereas the amide proton peak was vaguely observed for formic acid-d2 as a solvent in this work. The faint peak around 7.88 ppm seems to come from the amide proton. The peak at 8.31 ppm comes from formic acid used as the NMR solvent. Incidentally, when only formic acid was used as a reaction solvent, the same 1H NMR spectrum was obtained in the case where the mixed solvent of formic acid and methanol were used for the reaction. Therefore, it was confirmed that the formation of methoxymethyl groups did not occur in this reaction. The degree of methylolation was calculated by the ratio of peak areas, with integrated intensity d/integrated intensity b. When calculated for b (methylene protons 2H: 2.00), the following is obtained.
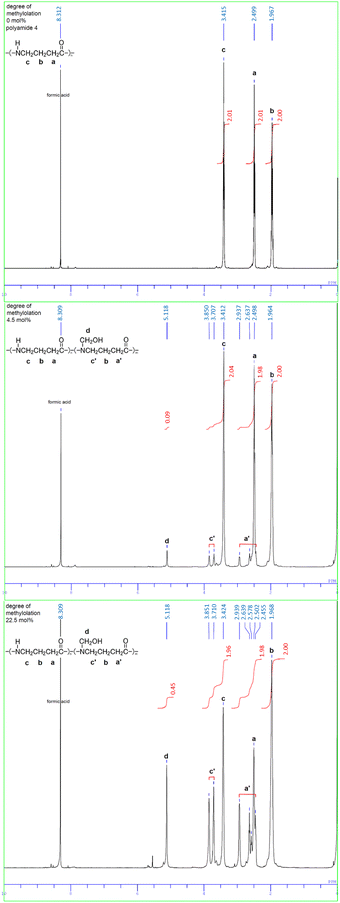 | ||
| Fig. 2 1H NMR spectra of polyamide 4 and N-methylol polyamide 4 with different degrees of methylolation (4.5 mol%, 22.5 mol%), (solvent: deuterated formic acid DCOOD). | ||
integrated intensity d/integrated intensity b = 0.09/2.00 = 0.045; 4.5 mol%
integrated intensity d/integrated intensity b = 0.45/2.00 = 0.225; 22.5 mol%
By varying the quantity of paraformaldehyde, although there was a little variation, and it was possible to control the degree of methylolation.
e.g.
the quantity of paraformaldehyde 0.27 g
→ the degree of methylolation 4.5 mol%
the quantity of paraformaldehyde 1.07 g
→ the degree of methylolation 22.5 mol%
The data of molecular weight, thermal and mechanical properties of the obtained N-methylol polyamide 4 with various degrees of methylolation are listed in Table 1. The molecular weight of N-methylol polyamide 4 was almost the same as that of the raw material polyamide 4. This suggests that the proportion of crosslinks and microgels formed by the further reaction of methylol groups with the amide groups in polyamide 4 is very low.
| Degree of methylolation (mol%)a | M n × 10−3 (g mol−1)b | M w × 10−3 (g mol−1)b | M w/Mn | T m (°C) | ΔHm (J g−1) | T g (°C) | T d (°C) | T d5 (°C) | T d10 (°C) | Tensile strength (MPa) | Tensile strain at break (%) | Elastic modulus (GPa)c | Noted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Calculated by the ratio of the 1H NMR peak areas. b GPC solvent: 1,1,1,3,3,3-hexafluoro-2-propanol, standard: poly(methyl methacrylate). c Measured after several hours keeping in the air, parenthesis dry: measured immediately after vacuum drying at 100 °C. d Yield: wt% = (weight of N-methylol polyamide 4)/(weight of polyamide 4 + weight of reagent) × 100, reagent: paraformaldehyde. e Amorphous. | |||||||||||||
| 0 | 32.2 | 94.9 | 2.95 | 267.9 | 83.4 | 30.7 | 278.9 | 255.0 | 278.9 | 49.5 ± 4.0 | 20.1 ± 2.5 | 0.426 | Raw material |
| 39.0 ± 2.6 | 66.7 ± 6.4 | 0.370 | Poly | ||||||||||
| 36.9 ± 3.5 | 65.6 ± 6.3 | 0.365 | Amide 4 | ||||||||||
| 51.0 ± 3.5 | 19.4 ± 3.0 | 0.412 | |||||||||||
| av. 44.1 | av. 43.0 | av. 0.393 (dry 1.685) | |||||||||||
| 4.5 | 38.0 | 116.7 | 3.07 | 251.2 | 60.6 | 32.6 | 276.0 | 238.4 | 274.1 | 25.6 ± 1.2 | 330.3 ± 32.0 | 0.149 | Yield |
| 30.5 ± 1.3 | 295.1 ± 36.0 | 0.176 | 92.9% | ||||||||||
| 32.7 ± 1.8 | 370.8 ± 32.9 | 0.157 | Reagent | ||||||||||
| av. 29.6 | av. 332.1 | av. 0.161 (dry 0.890) | 0.27 g | ||||||||||
| 6.5 | 34.7 | 111.2 | 3.21 | 245.8 | 47.0 | 33.7 | 276.3 | 247.0 | 275.9 | 25.4 ± 1.9 | 375.9 ± 57.5 | 0.144 | Yield |
| 28.9 ± 1.1 | 276.0 ± 33.4 | 0.173 | 91.9% | ||||||||||
| 25.0 ± 1.0 | 326.7 ± 28.8 | 0.146 | Reagent | ||||||||||
| av. 26.4 | av. 326.2 | av. 0.154 (dry 1.695) | 0.53 g | ||||||||||
| 9.5 | 31.6 | 81.1 | 2.56 | 227.8 | 30.9 | 34.8 | 277.3 | 269.3 | 282.3 | 13.6 ± 0.6 | 233.7 ± 30.6 | 0.087 | Yield |
| 164.8 | 2.1 | 22.1 ± 1.4 | 265.6 ± 25.8 | 0.130 | 96.4% | ||||||||
| 14.4 ± 0.5 | 190.1 ± 16.8 | 0.089 | Reagent | ||||||||||
| av. 16.7 | av. 229.8 | av. 0.102 (dry 1.359) | 0.80 g | ||||||||||
| 13.5 | 34.3 | 82.8 | 2.41 | 214.9 | 16.1 | 36.4 | 280.7 | 262.4 | 284.8 | 10.5 ± 1.0 | 265.2 ± 33.3 | 0.067 | Yield |
| 161.3 | 1.4 | 11.7 ± 1.0 | 237.6 ± 27.9 | 0.063 | 91.1% | ||||||||
| 13.8 ± 1.4 | 286.2 ± 36.0 | 0.071 | Reagent | ||||||||||
| av. 12.0 | av. 263.0 | av. 0.067 (dry 1.392) | 0.67 g | ||||||||||
| 22.5 | 34.0 | 95.5 | 2.81 | 164.1 | 2.7 | 38.7 | 281.9 | 275.9 | 288.2 | 2.5 ± 0.4 | 275.2 ± 37.5 | 0.015 | Yield |
| 3.1 ± 0.2 | 286.8 ± 32.2 | 0.013 | 94.7% | ||||||||||
| 3.3 ± 0.2 | 221.0 ± 34.2. | 0.025 | Reagent | ||||||||||
| av. 3.0 | av. 261.0 | av. 0.018 (dry 0.897) | 1.07 g | ||||||||||
| 36.5 | 24.2 | 70.4 | 2.90 | —e | —e | 38.2 | 282.7 | 242.6 | 286.8 | 1.3 ± 0.2 | 613.6 ± 57.6 | 0.0007 | Yield |
| 65.6% | |||||||||||||
| Reagent | |||||||||||||
| 4.26 g | |||||||||||||
The melting point and heat of fusion of N-methylol polyamide 4 were measured by DSC. DSC thermograms of various N-methylol polyamide 4 and polyamide 4 are shown in Fig. 3. The major melting peaks based on the crystalline region of polyamide 4 shifted to the low temperature side with increasing degree of methylolation from 0 mol% to 13.5 mol%. At the same time, the major melting peaks became smaller and broader, resulting in a decrease in the heat of fusion. The slight melting peaks were also observed in N-methylol polyamide 4 with a degree of methylolation of 9.5 mol% to 22.5 mol%. The melting peaks disappeared when the degree of methylolation increased to 36.5 mol%. It is thought that random methylolation of polyamide 4 chains increased the irregularity of chain architecture, inhibited hydrogen bonding of amide groups in the polymer chains, limited crystallinity and lowered the major melting peaks. This is supported by the fact that the heat of fusion is reduced at the same time. The dependence of melting point Tm of N-methylol polyamide 4 on the degree of methylolation is shown in Fig. 4. The major melting points are plotted as black filled circles. The slight melting points on the low temperature side are plotted as white open circles. The major melting points were proportional to the degree of methylolation. By changing the degree of methylolation, it is possible to control the melting point of N-methylol polyamide 4. The dependence of heat of fusion of N-methylol polyamide 4 on the degree of methylolation is shown in Fig. 5. The black filled circle and the white open circle correspond to major melting peaks and slight melting peaks, respectively. The heat of fusion decreased with increasing the degree of methylolation as well. These observations suggest that the methylolation of polyamide 4 reduces the crystallinity and becomes amorphous.
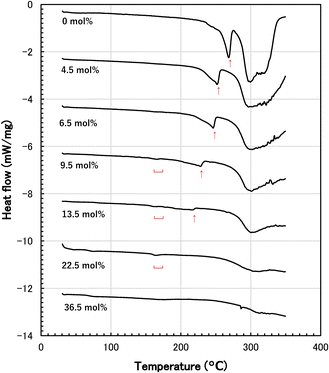 | ||
| Fig. 3 DSC thermograms of polyamide 4 and N-methylol polyamide 4 with different degrees of methylolation (arrow: major melting point, underbar: low melting point). | ||
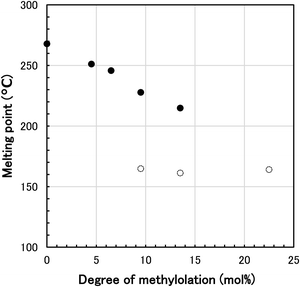 | ||
| Fig. 4 Relationship between melting point and degree of methylolation for N-methylol polyamide 4 (black filled circle: major melting point, white open circle: slight melting point). | ||
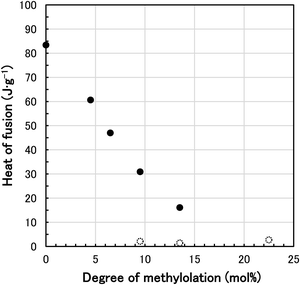 | ||
| Fig. 5 Relationship between heat of fusion and degree of methylolation for N-methylol polyamide 4 (black filled circle: major heat of fusion, white open circle: slight heat of fusion). | ||
The glass transition point Tg were measured by DSC. DSC thermograms of polyamide 4 and various N-methylol polyamide 4 are shown in Fig. S1 (ESI†). The shape was a simple stepwise pattern up to a degree of methylolation of 6.5 mol%, whereas the shape change as a degree of methylolation increased to 9.5 mol%. Tg increased gradually with increasing the degree of methylolation. The Tg of polyamide 4 was 30.7 °C, and those of N-methylol polyamide 4 were 32.6–38.7 °C. Polymers with bulky or polar functional groups in the side chain generally have a higher Tg. For example, the reported data for the vinyl polymer series are summarized in the Table S1 (ESI†).31 Thus, in analogy with vinyl polymers, the Tg of polyamide 4 modified with a methylol group would have changed to higher values. The modification of the amide groups of polyamide 4 with methylol groups, which made the side chains bulky and polar, is thought to have restricted the micro-Brownian motion of the polymer chains and slightly increased Tg.
The thermogravimetric analysis curves of various N-methylol polyamide 4 and polyamide 4 are shown in Fig. 6. Thermal decomposition point Td was estimated from the intersection of the tangent line for the previous flat stage and the tangent line for the weight loss stage. Additionally, 5% weight loss temperature Td5 and 10% weight loss temperature Td10 were also determined. These data, Td, Td5, and Td10, are listed in Table 1. The values of Td were approximately close to the values of Td10. There was no clear trend for Td5, whereas the polyamide 4 with higher methylolation showed a slightly higher thermal decomposition point for Td10. In the gradual weight loss stage up to Td, the weight loss curves of all samples were similar. On the other hand, in the rapid weight loss stage beyond Td, the slope of the weight loss curve of polyamide 4 with higher methylolation tended to be slower. It is known that methylol groups react with amide groups when heated.29 Therefore, this crosslinking reaction between methylol groups and amide groups may provide the possibility of suppressing thermal decomposition. There is also a possibility that methylolation of amide groups suppresses the formation of 2-pyrrolidone in cyclization through backbiting, namely, the depolymerization of polyamide 4 chains. Due to the crosslinking reaction during the heating process, N-methylol polyamide 4 above 6.5 mol% degree of methylolation remained as char and increased the residual weight at 500 °C. From the comparison between melting point and thermal decomposition temperature, methylolation lowered the melting point, whereas the thermal decomposition temperature (Td, Td10) was about the same or slightly higher.
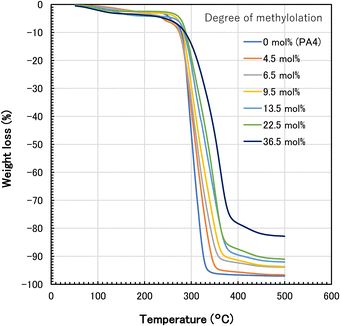 | ||
| Fig. 6 Thermogravimetric analysis curves of polyamide 4 and N-methylol polyamide 4 with different degrees of methylolation. | ||
In the case of polyamide 4 with lower methylolation (4.5 mol%, 6.5 mol%), the melting point remained relatively high, and the difference between the melting point and the thermal decomposition temperature (Td, Td10) widened. As it turned out, the melting point and thermal decomposition temperature become separated wider, N-methylol polyamide 4 is expected to be easier for processing by melt molding than polyamide 4.
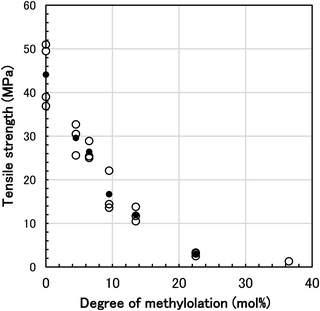
The mechanical properties, tensile strength (ultimate strength), elongation at break, and elastic modulus of N-methylol polyamide 4 and polyamide 4 were measured to observe the influence of methylolation. The values in Table 1 are averages of multiple measurements up to 15 times for each specimen. Typical tensile stress–strain curves for polyamide 4 and N-methylol polyamide 4 (degree of methylolation 4.5 mol%, 22.5 mol%) are shown in Fig. 7. These typical specimens lots are the lot (49.5 ± 4.0 MPa, 20.1 ± 2.5%) for polyamide 4, the lot (30.5 ± 1.3 MPa, 295.1 ± 36.0%) for 4.5 mol% N-methylol polyamide 4, and the lot (3.1 ± 0.2 MPa, 286.8 ± 32.2%) for 22.5 mol% N-methylol polyamide 4 in Table 1. Each fitted stress–strain curve of the lot is plotted from the average of the data for polyamide 4 (repetition 15 times) and N-methylol polyamide 4 (repetition 12 times) respectively. The tensile stress–strain curves showed that polyamide 4 was strong and brittle, whereas N-methylol polyamide 4 changed to ductile (4.5 mol%) or elastomeric (22.5 mol%) thermoplastic material as the degree of methylolation increased. The relationship between tensile strength and degree of methylolation for N-methylol polyamide 4 is shown in Fig. 8. Since the tensile properties of each specimen tend to vary due to the influence of film shape, stochastic defects in the film, and the measurement environment, many measurements were performed to obtain an average value. Each lot of specimens was measured 15 times, and anomalous data were excluded, and then the averaged values are plotted as white open circles. The black filled circles refer to the average value of the lots. While some variation in the tensile strength of each lot appeared, it showed a clear relationship between lower tensile strength with increasing the degree of methylolation.
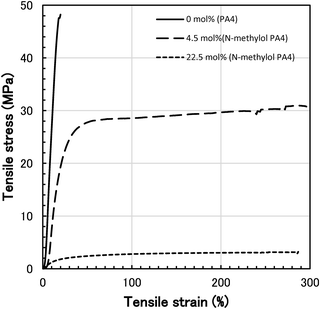 | ||
| Fig. 7 Typical tensile stress–strain curves for polyamide 4 and N-methylol polyamide 4 (degree of methylolation 4.5 mol%, 22.5 mol%). | ||
The elongation at break of the specimens remarkably increased from a low degree of methylolation, whereas there was no clear proportional relationship with the degree of methylolation (Fig. S2, ESI†). Although there are differences and dispersion in data for elongation at break, the methylolation of the amide group in polyamide 4 played an important role in increasing the elongation at break. For most N-methylol polyamide 4, the elongation at break was in the range of 190–380%. When the degree of methylolation is 36.5 mol%, it showed an exceptionally large elongation at break. For this specimen only, the data is the average of four measurements. The reason is that the specimen absorbed moisture from the air during handling and became slightly sticky, making it difficult to perform many measurements.
For obtaining information on the moisture absorption of polyamide 4 and N-methylol polyamide 4, hygroscopicity of each sample with a different degree of methylolation was evaluated from the weight loss as the temperature increased from 30–150 °C using thermogravimetry. To achieve hygroscopic equilibrium, each sample was kept overnight in the air. The moisture contents of the representative samples were 5.41% for polyamide 4, 6.47% and 10.07% for N-methylol polyamide 4 (degree of methylolation 4.5 mol% and 22.5 mol%). The weight percent of moisture content increased with increasing the degree of methylolation (Fig. S3, ESI†).
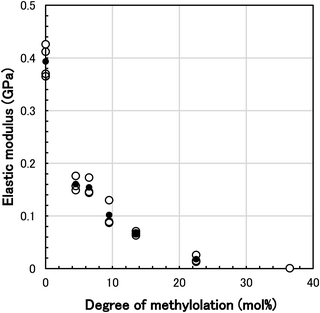
The values of elastic modulus for each specimen lot at hygroscopic equilibrium and at dry state are listed in Table 1. The conditions for measurement at hygroscopic equilibrium were as follows; temperature: 23 °C to 19 °C, humidity: 49% to 66%. Specimens in the dry state were dried at 100 °C under reduced pressure and measured immediately afterwards. The elastic modulus of specimens in the dry state were 0.890–1.695 GPa. These values in the dry state were the same order of magnitude of polyamide 6 and were reasonable.32 On the other hand, the elastic modulus at hygroscopic equilibrium in the atmosphere became lower and decreased with increasing the degree of methylolation. The elastic modulus at hygroscopic equilibrium for polyamide 4 was 0.393 GPa, while those for N-methylol polyamide 4 were 0.161–0.0007 GPa. The elastic modulus of specimens at hygroscopic equilibrium with the degree of methylolation is plotted in Fig. 9. The trend of the degree of methylolation on the elastic modulus was analogous to that on the tensile strength. By methylolating polyamide 4, the mechanical properties at hygroscopic equilibrium changed from strong and brittle → ductile → elastomeric. These properties are reasonable based on the change in elastic modulus at hygroscopic equilibrium. On the other hand, compared to the Tg for polyamide 4 (30.7 °C), a slight increase in Tg for N-methylol polyamide 4 (32.6–38.7 °C) is observed, however, both are close to ambient temperature, so the effect of the change in Tg is thought to be small.
To elucidate the relationship between biodegradability and degree of methylolation, the biodegradation test for various N-methylol polyamide 4 was carried out. The oxygen consumption of only activated sludge without polymer sample was measured as a blank. The blank measurement was in 2.1–7.4 mL range during the biodegradation test. These values were well below the tested values for biodegradable polymer samples. The oxygen consumption of the sample by biodegradation was corrected by subtraction of the oxygen consumption of the blank. The percent biodegradation was calculated from the corrected oxygen consumption caused by the respiration of microbial metabolism for the polymer sample. The time course of biodegradation for various N-methylol polyamide 4 by standard activated sludge is shown in Fig. 10. As the degree of methylolation increased from 0 mol% to 22.5 mol%, the percent biodegradation for 35 days decreased from 52.0% to 20.9%, and at 36.5 mol% methylolation, the percent biodegradation was almost 0%. The results showed that the higher the degree of methylolation of the polyamide 4 main chains, the more inhibited the biodegradation. To clarify the relationship between percent biodegradation and degree of methylolation, a plot of the percent biodegradation against the degree of methylolation for 35 days was made as shown in Fig. 11. The effect of methylolation of polyamide 4 on biodegradability was clearly proportional to the degree of methylolation with a negative correlation. It became evident that modification of polyamide 4 chains with methylol groups reduced the biodegradability of polyamide 4. It is likely that the presence of side chains, such as methylol groups, on the polyamide 4 main chains inhibited the susceptibility to the microbe's enzyme. While polyamide 4 is easily biodegradable, the biodegradability can be controlled to suit the usage environment by adjusting the degree of methylolation.
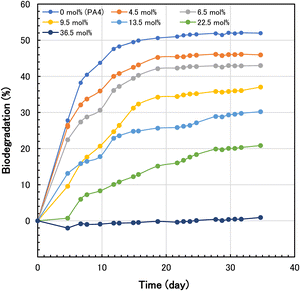 | ||
| Fig. 10 Time course of biodegradation for various N-methylol polyamide 4 using standard activated sludge. | ||
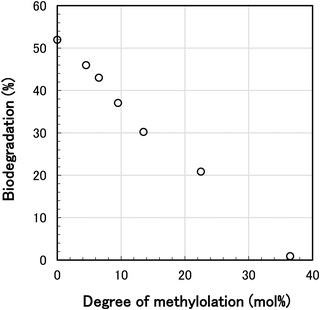 | ||
| Fig. 11 Relationship between percent biodegradation of N-methylol polyamide 4 and degree of methylolation for 35 days. | ||
Conclusions
Through substituting hydrogen on an amide linkage for the hydroxymethyl group, a series of N-methylol polyamide 4 with various degrees of methylolation were synthesized by using paraformaldehyde as a reagent. The degree of methylolation could be adjusted by varying the amount of paraformaldehyde. The melting point and the heat of fusion for N-methylol polyamide 4 decreased upon increasing the degree of methylolation. The glass transition point was slightly increased by modifying the amide groups of polyamide 4 with methylol groups, with the side chains becoming more bulky and polar. The thermal decomposition temperature of N-methylol polyamide 4 remained almost the same as that of polyamide 4 and was slightly higher for a higher degree of methylolation. The methylolation of polyamide 4 was effective in separating the melting points and the thermal decomposition temperature. The tensile strength and the elastic modulus at hygroscopic equilibrium decreased upon increasing the degree of methylolation. The elongation at break increased remarkably even at a low degree of methylolation, whereas there was no clear proportional relationship between them. The biodegradability of N-methylol polyamide 4 was suppressed as the degree of methylolation increased. These results made it evident that physical properties and biodegradability could be controlled by methylolating polyamide 4.Conflicts of interest
There are no conflicts to declare.Notes and references
- W. O. Ney, US Pat., 2638463, 1953 Search PubMed.
- S. Barzakay, M. Levy and D. Vofsi, J. Polym. Sci. A1 Polym. Chem., 1966, 4, 2211 CrossRef.
- J. Roda, J. Kralicek and Z. Bouskova, Eur. Polym. J., 1977, 13, 119 CrossRef.
- G. Costa, M. Nencioni and S. Russo, Makromol. Chem., 1981, 182, 1399 CrossRef.
- L. Blazkova, L. Malinova, V. Benesova, J. Roda and J. Brozek, J. Polym. Sci. A Polym. Chem., 2017, 55, 2203 CrossRef.
- J. Gu, S. Yagi, J. Meng, Y. Dong, C. Qian, D. Zhao, A. Kumar, T. Xu, A. Lucchetti and H. Xu, J. Membr. Sci., 2022, 654, 120571 CrossRef.
- H. Tani and T. Konomi, J. Polym. Sci. A1 Polym. Chem., 1968, 6, 2281 CrossRef.
- H. Sekiguchi, P. Tsourkas, F. Carriere and R. Audebert, Eur. Polym. J., 1974, 10, 1185 CrossRef.
- S. K. Kakar, Text. Res. J., 1976, 46, 776 CrossRef.
- R. Bacskai, Polym. Bull., 1984, 11, 229 CrossRef.
- K. Hashimoto, T. Hamano and M. Okada, J. Appl. Polym. Sci., 1994, 54, 1579 CrossRef.
- N. Kawasaki, A. Nakayama, N. Yamano, S. Takeda, Y. Kawata, N. Yamamoto and S. Aiba, Polymer, 2005, 46, 9987 CrossRef.
- N. Yamano, A. Nakayama, N. Kawasaki, N. Yamamoto and S. Aiba, J. Polym. Environ., 2008, 16, 141 CrossRef.
- K. Tachibana, K. Hashimoto, M. Yoshikawa and H. Okawa, Polym. Degrad. Stab., 2010, 95, 912 CrossRef.
- K. Tachibana, Y. Urano and K. Numata, Polym. Degrad. Stab., 2013, 98, 1847 CrossRef CAS.
- N. Yamano, N. Kawasaki, S. Ida and A. Nakayama, Polym. Degrad. Stab., 2019, 166, 230 CrossRef CAS.
- N. Yamano, N. Kawasaki, M. Oshima and A. Nakayama, Polym. Degrad. Stab., 2014, 108, 116 CrossRef CAS.
- A. Masui, S. Iwata, N. Kawasaki, N. Yamano and A. Nakayama, Polym. Degrad. Stab., 2019, 167, 44 CrossRef CAS.
- N. Yamano, N. Kawasaki, S. Ida, Y. Nakayama and A. Nakayama, Polym. Degrad. Stab., 2017, 137, 281 CrossRef CAS.
- N. Yamano, N. Kawasaki, S. Takeda and A. Nakayama, J. Polym. Environ., 2013, 21, 528 CrossRef CAS.
- N. Yamano, N. Kawasaki, M. Oshima and A. Nakayama, Polym. Degrad. Stab., 2014, 108, 116 CrossRef CAS.
- N. Kawasaki, N. Yamano, S. Takeda, H. Ando and A. Nakayama, J. Appl. Polym. Sci., 2012, 126, E425 CrossRef CAS.
- N. Kawasaki, N. Yamano and A. Nakayama, J. Appl. Polym. Sci., 2015, 132, 42466 CrossRef.
- N. Kawasaki, N. Yamano and A. Nakayama, J. Appl. Polym. Sci., 2020, 137, 49165 CrossRef CAS.
- H. F. Conrat, M. Cooper and H. S. Olcott, J. Am. Chem. Soc., 1945, 67, 950 CrossRef.
- T. L. Cairns, H. D. Foster, A. W. Larchar, A. K. Schneider and R. S. Schreiber, J. Am. Chem. Soc., 1949, 71, 651 CrossRef CAS.
- F. Suzuki, H. Kimura, K. Onozato and S. Kuroda, J. Appl. Polym. Sci., 1986, 32, 4573 CrossRef CAS.
- T. Arakawa, F. Nagatoshi and N. Arai, J.Polym.Sci. A2 Polym. Phys., 1969, 7, 1461 CrossRef CAS.
- J. Shieh and R. Y. M. Huang, J. Appl. Polym. Sci., 1997, 64, 855 CrossRef CAS.
- M. A. Bellinger, A. J. Waddon, E. D. T. Atkins and W. J. MacKnight, Macromolecules, 1994, 27, 2130 CrossRef CAS.
- R. J. Andrews and E. A. Grulke, in Polymer Handbook, ed. J. Brandrup, E. H. Immergut and E. A. Grulke, John Wiley & Sons, Ltd, New York, USA, 4th edn, Section VI, 1999, p. 193 Search PubMed.
- R. H. Mehta, in Polymer Handbook, ed. J. Brandrup, E. H. Immergut and E. A. Grulke, John Wiley & Sons, Ltd, New York, USA, 4th edn, Section V, 1999, p. 121 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma00047h |
| This journal is © The Royal Society of Chemistry 2024 |