Open Access Article
Open Access ArticlePhase transformation between CsPbBr3 and Cs4PbBr6 nanocrystals by a cationic oligomeric ligand and water, and their water resistance†
Norio
Saito
 *ab,
Akihiro
Urayama
b,
Takahiro
Takei
*ab,
Akihiro
Urayama
b,
Takahiro
Takei
 a and
Nobuhiro
Kumada
a and
Nobuhiro
Kumada
 a
a
aCenter for Crystal Science and Technology, University of Yamanashi, 7-32 Miyamae, Kofu, Yamanashi 400-8511, Japan. E-mail: n-saito@yamanashi.ac.jp
bDepartment of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku, Tokyo 162-8601, Japan
First published on 24th January 2024
Abstract
This article explores the origin of the significant water resistance of CsPbBr3 nanocrystals passivated by a cationic oligomeric ligand. Ligand exchange and X-ray photoemission spectroscopies indicate that a phase transformation between CsPbBr3 and Cs4PbBr6 is induced in the presence of the ligand and water, prolonging the duration of the nanocrystals.
Lead halide perovskite nanocrystals (LHP NCs) and related compounds have been intensively investigated for the last decade. Many articles have reported on their attractive photophysical properties, including a very narrow emission window,1 near-unity photoluminescence quantum yield,2 and photocatalytic properties,3 as well as their potential application to optoelectronic devices.4 Because of the strong ionicity of LHP NCs, they can easily form surface defects when their surface ionic species dissociate to the solvent phase. However, these surface defects greatly deteriorate the electronic and optical properties of LHP NCs.5 Hence, the surface defects must be sufficiently passivated to exploit their optoelectronic properties. Surface passivation of LHP NCs with aliphatic surfactants (ligands) is a primary method of improving their colloidal stability and optoelectronic properties. Various ligand molecules such as pairs of aliphatic acids and bases,6 quaternary alkylammonium halides,7 phosphonic acids,8 and zwitterionic ligands9 have been investigated so far. Recent studies10 have indicated that some surface-passivating ligands can mediate the crystalline phase transformation from three-dimensional (3D) CsPbX3 NCs to zero-dimensional (0D) Cs4PbBr6 or two-dimensional CsPb2Br5, accompanied by considerable changes in their morphology, a blue-shift of absorption, and improved luminescence properties.
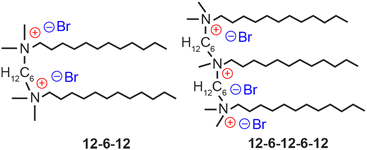
We herein explore a novel surface-passivating ligand for LHP NCs and their crystallization processes. Some of the co-authors have reported the surface passivation of CsPbBr3 NCs using a cationic dimeric (gemini) ligand.11 These NCs exhibited excellent luminescence performance and prominent stability in water compared to those passivated using conventional ligands such as a pair of oleic acid and oleylamine (OA–OAm) and didodecyldimethylammonium bromide (DDAB).12 Although gemini ligands containing long methylene spacers are strongly bound to the surfaces of CsPbBr3 NCs via two-point adsorption,11 no clear evidence has been presented so far to account adequately for their remarkable water resistance. This article explores the origin of the water resistance of CsPbBr3 NCs passivated using cationic oligomeric ligands, such as gemini and trimeric ligands (Fig. 1), from the perspectives of ligand engineering and crystal chemistry. We demonstrate repeatable phase transformation between 3D CsPbBr3 and 0D Cs4PbBr6, mediated by water and an oligomeric ligand, and discuss the mechanism by which the phase transformation contributes to their chemical stability.
The cationic gemini ligand 1,6-bis(dimethyldodecylammonio)hexane dibromide, referred to as 12-6-12 from now onwards,13 and trimeric ligand methyldodecylbis[6-(dimethyldodecylammonio)hexane]ammonium tribromide, denoted as 12-6-12-6-12,13 were synthesized according to the methods described in the literature (for details, see the ESI†). CsPbBr3 NCs were synthesized by the hot injection method14 using OA and OAm, followed by purification by conducting centrifugation (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 10 min at least three times. Then, the synthesized CsPbBr3 NCs were used in the ligand-exchange procedure.12 CsPbBr3 NCs (0.7 μM) were agitated in 2.0 mL of toluene containing 0.2 mL of OA and 0.4 mL of DDAB (50 mM), or dichloromethane containing 0.4 mL of 12-6-12 (25 mM) or 12-6-12-6-12 (17 mM). These ligand concentrations are optimal for good NC production; otherwise, the production yield will decrease significantly. After adding 4 mL of ethyl acetate, the precipitate was collected by conducting centrifugation (13
000 rpm) for 10 min at least three times. Then, the synthesized CsPbBr3 NCs were used in the ligand-exchange procedure.12 CsPbBr3 NCs (0.7 μM) were agitated in 2.0 mL of toluene containing 0.2 mL of OA and 0.4 mL of DDAB (50 mM), or dichloromethane containing 0.4 mL of 12-6-12 (25 mM) or 12-6-12-6-12 (17 mM). These ligand concentrations are optimal for good NC production; otherwise, the production yield will decrease significantly. After adding 4 mL of ethyl acetate, the precipitate was collected by conducting centrifugation (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 10 min and then redispersing it in 3 mL of toluene. Hereafter, the synthesized CsPbBr3 NCs are denoted as CsPbBr3 NCs/“ligand” (e.g., CsPbBr3 NCs/12-6-12).
000 rpm) for 10 min and then redispersing it in 3 mL of toluene. Hereafter, the synthesized CsPbBr3 NCs are denoted as CsPbBr3 NCs/“ligand” (e.g., CsPbBr3 NCs/12-6-12).
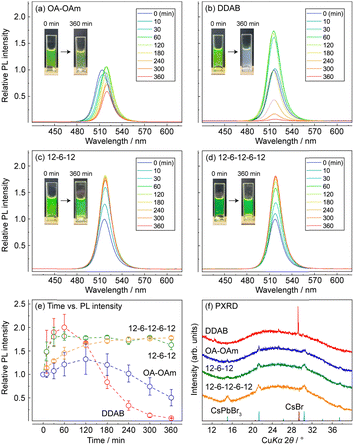
First, the water resistance of the synthesized CsPbBr3 NCs was tested using the experimental setup reported in our previous article (see details in the ESI†). In this test, 0.5 mL of pure water was added to 2.0 mL of CsPbBr3 NCs, and the mixture was then vigorously stirred. This process accelerated the degradation of CsPbBr3. The yellowish-green solution of CsPbBr3 NCs with an absorbance of 0.5 at 500 nm became white opaque following water exposure for 360 min, when DDAB was used as the passivating ligand (Fig. 2b). However, CsPbBr3 NCs/12-6-12 and 12-6-12-6-12 maintained their green colours (Fig. 2c and d). Powder X-ray diffraction (PXRD) studies on the supernatant obtained after 360 min exposure to water showed that the diffraction patterns of the CsPbBr3 NCs/DDAB exhibited only a strong peak at 2θ = 29.20° corresponding to the 110 diffraction of CsBr, whereas those of CsPbBr3 NCs/12-6-12 and 12-6-12-6-12 exhibited the diffraction of CsPbBr3 (Fig. 2f). Changes in the photoluminescence intensities during the water-resistance test (Fig. 2e) reveal that the relative luminescence intensity of every CsPbBr3 NC except OA–OAm increased by approximately 1.7–1.8 times during the first 120 min due to water insertion into the lattice of CsPbBr3.15 The luminescence intensities of the CsPbBr3 NCs/12-6-12 and 12-6-12-6-12 were maintained during water exposure for 360 min, in contrast to critical luminescence degradation of CsPbBr3 NCs/OA–OAm and DDAB. These results indicate that the surface passivation using the oligomeric ligands obviously improved the stability of CsPbBr3 NCs toward water exposure.
We also evaluated the moisture resistance of dried CsPbBr3 NCs by observing changes in the PXRD patterns of them deposited on a glass XRD holder stored in a 97% relative humidity at 30 °C for several days. The PXRD patterns of CsPbBr3 NCs/OA–OAm (Fig. S1a, ESI†) indicate that the intensities of the diffraction peaks at 2θ = 11.44°, 29.18°, and 33.14° sharply increased during exposure to moisture. These peaks are matched with the diffraction pattern of CsPb2Br5, indicating the degradation of CsPbBr3 to CsPb2Br5. The PXRD patterns of the ligand-exchanged samples (Fig. S1b–d, ESI†) show that they prevented the degradation to CsPb2Br5. However, the diffraction peaks corresponding to CsPbBr3 were split and sharpened by exposure to moisture. This indicates an expansion of the lattice volume of CsPbBr3 NCs and their aggregation. Compared to the colloidal NCs, dried ones do not contain free ligand molecules. They are thus likely to aggregate each other. It is speculated that adsorption of water on the surfaces of CsPbBr3 NCs would lead to detachment of the passivating ligands and then cause aggregation of ligand-detached NCs.
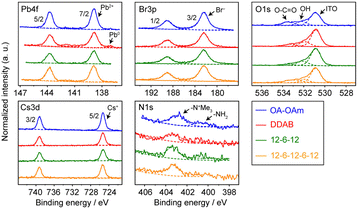
The chemical compositions of the synthesized CsPbBr3 NCs were evaluated using a combination of hard X-ray photoemission spectroscopy (HAXPES, hν = 7.939 keV) and angle-resolved XPS (ARXPS). As the escape depth of the photoelectrons in HAXPES is a few tens of nanometers long, HAXPES is sensitive to the bulk states of the samples. Conversely, ARXPS, which can control the photoelectron escape depth by its take-off angle (TOA, θ), is sensitive to the surface state. The combination of HAXPES and ARXPS can thus clarify the chemical composition of both the surface and bulk states of the samples. Fig. 3 shows the high-resolution HAXPES narrow scans of the Cs 3d, Pb 4f, Br 3p, N 1s, and O 1s regions. As shown in Table S1 (ESI†) listing the binding energies and peak areas of the HAXPES narrow scans determined by peak fitting calculations, the spectral shapes and binding energies of the HAXPES spectra were not significantly varied by the passivating ligand. The atomic concentration of the sample was evaluated from the peak areas of the narrow scans weighted with sensitivity factors.16 The results of the HAXPES elemental analysis showed that the concentration of Cs:Pb:Br was modified among the passivating ligands (Fig. 4 and Table S1, ESI†). Interestingly, 12-6-12 and 12-6-12-6-12 exhibited a higher concentration of Br and a lower concentration of Pb than that expected by stoichiometry (the stoichiometry of Br and Pb is 60% and 20%, respectively). Thus, the atomic ratio of Br to Pb was higher in these samples. The ARXPS spectra and results of their peak fitting calculations (Fig. S2 and Table S2, respectively, ESI†) show that the concentration of Br increased with lower θ. As the photoelectron escape depth in ARXPS is proportional to sinθ,17 the abundant Br was present on the surfaces of the CsPbBr3 NCs. On the contrary, the concentration of Cs decreased with lower θ while that of Br and Pb was close to or higher than stoichiometry, suggesting dissociation of the Cs atoms from the NC surface and ligand passivation of the Cs defects.
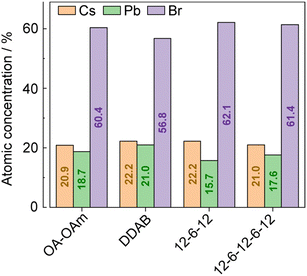 | ||
| Fig. 4 Atomic concentrations of CsPbBr3 NCs estimated from the peak areas of the HAXPES narrow scans weighted with sensitivity factors. | ||
Fig. S3 (ESI†) shows a Cs–Pb–Br ternary phase diagram that plots the atomic concentrations of CsPbBr3 NCs evaluated by HAXPES. The figure also displays the concentrations for crystallization of ternary compounds estimated from previous literature.10 These plots demonstrate that the atomic concentrations evaluated by HAXPES are situated on a boundary where a mixture of CsPbBr3 and Cs4PbBr6 would crystallize. Considering that the ligand exchange process induced the dissolution of CsPbBr3 NCs and modification of their atomic concentrations, the ligands would influence the crystallization of CsPbBr3, Cs4PbBr6, and/or other competitive phase(s).
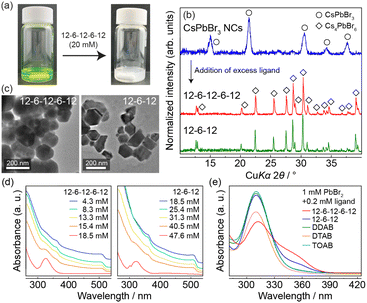
To examine how the oligomeric ligands influence the crystallization behaviour of the CsPbBr3 NCs, we added excess ligands to the CsPbBr3 NC solution during ligand exchange. The clear green solution became a white opaque suspension with no luminescence (Fig. 5a) when either 12-6-12 or 12-6-12-6-12 was added to the solution until its total concentration exceeded 50 and 20 mM, respectively. The PXRD study of the precipitate obtained by the centrifugation of the suspension indicated that it matched well with the diffraction pattern of Cs4PbBr6 (Fig. 5b). The transmission electron microscopy (TEM) images of the obtained Cs4PbBr6 show 50–300-nm quasi-spherical or hexagonal particles (Fig. 5c). As its particle size was significantly greater than that of the CsPbBr3 NCs (11–13 nm),11 the latter were first dissolved and then relatively large Cs4PbBr6 was recrystallized. Ultraviolet-visible (UV-vis) spectra of the CsPbBr3 NC solution containing the oligomeric ligand (Fig. 5d) showed that the absorption peaks characteristic of the CsPbBr3 NCs at 505 nm corresponding to the interband transitions gradually weakened with an increase in the concentration of the oligomeric ligand. Moreover, an absorption peak at 325 nm, corresponding to the localized 6S1/2–6P1/2 transitions within the individual [PbBr6]4− unit in Cs4PbBr6, simultaneously appeared.10 Hence, the UV-vis spectra suggest that the oligomeric ligands induce the dissolution of CsPbBr3 NCs and crystallization of Cs4PbBr6. Fig. 5d shows that 18.5 mM of 12-6-12-6-12 transformed almost all CsPbBr3 NCs into Cs4PbBr6, which is approximately 0.4 times less than that of 12-6-12 (i.e., 47.6 mM). This result indicates that the trimeric ligand is likely to facilitate the phase transformation.
Notably, the transformation of CsPbBr3 into Cs4PbBr6 was not possible with monocationic ligands such as DDAB, dodecyltrimethylammonium bromide (DTAB), and tetraoctylammonium bromide (TOAB). This is because these ligands are not involved in the formation of the multivalent plumbic bromide complex, an essential intermediate in the crystallization of Cs4PbBr6. The UV-vis spectra of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of toluene and dimethylformamide (DMF) containing 1-mM PbBr2 and 0.2-mM ligand (Fig. 5e) suggest a different behaviour of the plumbic bromide complex among the ligands. The solutions containing either DDAB, DTAB, or TOAB show an absorption peak at 310 nm only, corresponding to the monovalent [PbBr3]− complex.18 In contrast, those containing 12-6-12 or 12-6-12-6-12 exhibited a shoulder-like broad peak at approximately 355 nm corresponding to the divalent [PbBr4]2− complex.18 These results suggest that oligomeric ligands, in the presence of Pb2+ and Br− ions, facilitate the formation of plumbate complexes richer in Br than the tribromide ones, which preferentially crystallize in Cs4PbBr6 by electrostatic packing with the Cs+ ions. Although OAm and thiol ligands have been reported to transform CsPbBr3 into Cs4PbBr6 because of their coordinating effect on the Pb2+ ion,10 the transformation mechanism for the oligomeric ligands should be different because they repel the metal cation.
1 solution of toluene and dimethylformamide (DMF) containing 1-mM PbBr2 and 0.2-mM ligand (Fig. 5e) suggest a different behaviour of the plumbic bromide complex among the ligands. The solutions containing either DDAB, DTAB, or TOAB show an absorption peak at 310 nm only, corresponding to the monovalent [PbBr3]− complex.18 In contrast, those containing 12-6-12 or 12-6-12-6-12 exhibited a shoulder-like broad peak at approximately 355 nm corresponding to the divalent [PbBr4]2− complex.18 These results suggest that oligomeric ligands, in the presence of Pb2+ and Br− ions, facilitate the formation of plumbate complexes richer in Br than the tribromide ones, which preferentially crystallize in Cs4PbBr6 by electrostatic packing with the Cs+ ions. Although OAm and thiol ligands have been reported to transform CsPbBr3 into Cs4PbBr6 because of their coordinating effect on the Pb2+ ion,10 the transformation mechanism for the oligomeric ligands should be different because they repel the metal cation.
Hereafter, we discuss the water resistance of the CsPbBr3 NCs passivated using oligomeric ligands. With respect to water-induced perovskite degradation, Nakamura et al. recently clarified that (CH3NH3)PbI3 thin films degrade to CH3NH3I and PbI2 under high humidity.19 Lin et al. reported that the structures of the perovskite crystals can be tuned from CsPbBr3 to CsPb2Br5 by controlling the water-to-dimethylsulfoxide ratios.20 Accordingly, the possible degradation reactions of CsPbBr3 NCs in contact with water can be assumed as follows:
| CsPbBr3 → PbBr2 + CsBr | (1) |
| 2CsPbBr3 → CsPb2Br5 + CsBr. | (2) |
Eqn (1) and (2) suggest that the surfaces of CsPbBr3 NCs were exfoliated as either PbBr2 or CsPb2Br5 when in contact with water because of the dissociation of highly ionic CsBr into the aqueous phase. The resulting plumbic compounds decomposed into plumbic bromide complexes according to the following reactions:
| PbBr2 + (Ligand)Brn + mH2O → (Ligand)[PbBr2+n·mH2O] | (3) |
| CsPb2Br5 + 2(Ligand)Brn + 2mH2O → 2(Ligand)[PbBr2+n·mH2O] + CsBr, | (4) |
Cs4PbBr6 obtained by using excess oligomeric ligand can be changed into an orange solid when water is added to the system. The orange solid was dispersible in toluene and dichloromethane. It exhibited green emission at ∼522 nm under UV light (λ = 365 nm), where the wavelength was slightly longer than the as-synthesized samples (Fig. S4, ESI†) due to the larger particle size of the re-formed NCs. The PXRD pattern of the solid (Fig. S5, ESI†) showed the diffraction patterns of the concomitant phases of Cs4PbBr6, CsPbBr3, and CsBr, suggesting that Cs4PbBr6 partially transformed into CsPbBr3. As discussed in the literature,22 Cs4PbBr6 decomposes into CsPbBr3 by the dissociation of CsBr into the aqueous phase, as follows:
| Cs4PbBr6 → CsPbBr3 + 3CsBr. | (5) |
Interestingly, the addition of oligomeric ligands to the CsPbBr3/Cs4PbBr6-dispersed solution resulted in the re-transformation of Cs4PbBr6 (Fig. S5, ESI†). This result suggests that repeatable phase transformation between CsPbBr3 and Cs4PbBr6 is possible with an oligomeric ligand and water (Fig. 6). Note that the luminescence intensity is not fully maintained during the phase transformation due to the dissociation of CsBr into the aqueous phase. However, the oligomeric ligand plays a role not only in protecting the surfaces of CsPbBr3 NCs, but also in recovering their luminescence properties from their degraded states. Hence, it can be concluded that these characteristics contribute to the high chemical stability of oligomeric ligand-passivating perovskite NCs.
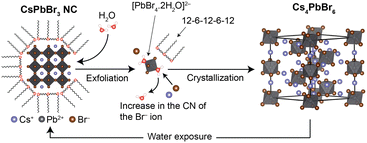 | ||
| Fig. 6 Schematic illustration of crystalline phase transformation between CsPbBr3 and Cs4PbBr6, mediated by 12-6-12-6-12 and water. | ||
In summary, we demonstrated a repeatable transformation between CsPbBr3 and Cs4PbBr6 mediated by oligomeric ligands and water. This phenomenon is characteristic of oligomeric ligands because of their affinity for multivalent anions. The oligomeric ligands investigated in this study can be used as efficient passivation agents to improve the chemical stability of perovskite NCs and thin films that can be applied to photovoltaic solar cells and electronic luminescent devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by JSPS KAKENHI (grant numbers 21K14706 and 23K04888), a research grant from the Murata Science Foundation (grant number AN20030), and research grants from the Iketani Science, Technology Foundation (grant number 0341150-A) and the Nippon Sheet Glass Foundation. The HAXPES measurements were performed at the Japanese synchrotron radiation facility, SPring-8, with non-proprietary approval from the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal numbers 2021B1852/BL46XU and 2022A1758/BL46XU).References
- F. Zhang, H. Zhong, C. Chen, X.-G. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. Dong, ACS Nano, 2015, 9, 4533 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692 CrossRef CAS PubMed; H. Huang, F. Zhao, L. Liu, F. Zhang, X.-G. Wu, L. Shi, B. Zou, Q. Pei and H. Zhong, ACS Appl. Mater. Interfaces, 2015, 7, 28128 CrossRef PubMed.
- H. Huang, B. Pradhan, J. Hofkens, M. B. J. Roeffaers and J. A. Steele, ACS Energy Lett., 2020, 5, 1107 CrossRef CAS; J. Yuan, H. Liu, S. Wang and X. Li, Nanoscale, 2021, 13, 10281 RSC.
- J. Liu, Y. Xue, Z. Wang, Z.-Q. Xu, C. Zheng, B. Weber, J. Song, Y. Wang, Y. Lu, Y. Zhang and Q. Bao, ACS Nano, 2016, 10, 3536 CrossRef CAS PubMed; T. Chiba, S. Ishikawa, J. Sato, Y. Takahashi, H. Ebe, S. Ohisa and J. Kido, Adv. Opt. Maters., 2020, 8, 2000289 CrossRef; Y.-H. Kim, C. Wolf, Y.-T. Kim, H. Cho, W. Kwon, S. Do, A. Sadhanala, C. G. Park, S.-W. Rhee, S. H. Im, R. H. Friend and T.-W. Lee, ACS Nano, 2017, 11, 6586 CrossRef PubMed.
- D. Yang, X. Li and H. Zeng, Adv. Mater. Interfaces, 2018, 5, 1701662 CrossRef CAS; D. P. Nenon, K. Pressler, J. Kang, B. A. Koscher, J. H. Olshansky, W. T. Osowiecki, M. A. Koc, L.-W. Wang and A. P. Alivisatos, J. Am. Chem. Soc., 2018, 140, 17760 CrossRef PubMed.
- A. Pan, B. He, X. Fan, Z. Liu, J. J. Urban, A. P. Alivisatos, L. He and Y. Liu, ACS Nano, 2016, 10, 7943 CrossRef CAS PubMed; Z. Liang, S. Zhao, Z. Xu, B. Qiao, P. Song, D. Gao and X. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 28824 CrossRef PubMed.
- J. H. Park, A.-Y. Lee, J. C. Yu, Y. S. Nam, Y. Choi, J. Park and M. H. Song, ACS Appl. Mater. Interfaces, 2019, 11, 8428 CrossRef CAS PubMed.
- B. Zhang, L. Goldoni, J. Zito, Z. Dang, G. Almeida, F. Zaccaria, J. W. I. Infante, L. D. Trizio and L. Manna, Chem. Mater., 2019, 31, 9140 CrossRef CAS.
- F. Krieg, S. T. Ochsenbein, S. Yakunin, S. Brinck, P. Aellen, A. Süess, B. Clerc, D. Guggisberg, O. Nazarenko, Y. Shynkarenko, S. Kumar, C.-J. Shih, I. Infante and M. V. Kovalenko, ACS Energy Lett., 2018, 3, 641 CrossRef CAS PubMed.
- Q. Jing, Y. Xu, Y. Su, X. Xing and Z. Lu, Nanoscale, 2019, 11, 1784 RSC; Y. Li, H. Huang, Y. Xiong, S. V. Kershaw and A. L. Rogach, CrystEngComm, 2018, 20, 4900 RSC; S. K. Balakrishnan and P. V. Kamat, Chem. Mater., 2018, 30, 74 CrossRef CAS; F. Palazon, G. Almeida, Q. A. Akkerman, L. D. Trizio, Z. Dang, M. Prato and L. Manna, Chem. Mater., 2017, 29, 4167 CrossRef PubMed; Z. Liu, Y. Bekenstein, X. Ye, S. C. Nguyen, J. Swabeck, D. Zhang, S.-T. Lee, P. Yang, W. Ma and A. P. Alivisatos, J. Am. Chem. Soc., 2017, 139, 5309 CrossRef PubMed.
- N. Saito, A. Urayama, M. Ikezawa and Y. Kondo, Adv. Mater. Interfaces, 2022, 9, 2101836 CrossRef CAS.
- J. Pan, L. N. Quan, Y. Zhao, W. Peng, B. Murali, S. P. Sarmah, M. Yuan, L. Sinatra, N. M. Alyami, J. Liu, E. Yassitepe, Z. Yang, O. Voznyy, R. Comin, M. N. Hedhili, O. F. Mohammed, Z. Hong Lu, D. H. Kim, E. H. Sargent and O. M. Bakr, Adv. Mater., 2016, 28, 8718 CrossRef CAS PubMed.
- E. Alami, G. Beinert, P. Marie and R. Zana, Langmuir, 1993, 9, 1465 CrossRef CAS; R. Zana, J. Colloid Interface Sci., 2002, 248, 203 CrossRef PubMed; R. Zana, H. Levy, D. Papoutsi and G. Beinert, Langmuir, 1995, 11, 3694 CrossRef; M. In and V. Bec, Langmuir, 2000, 16, 141 CrossRef.
- M. I. Bodnarchuk, S. C. Boehme, S. Brinck, C. Bernasconi, Y. Shynkarenko, F. Krieg, R. Widmer, B. Aeschlimann, D. Günther, M. V. Kovalenko and I. Infante, ACS Energy Lett., 2019, 4, 63 CrossRef CAS PubMed; C. K. Ng, C. Wang and J. J. Jasieniak, Langmuir, 2019, 35, 11609 CrossRef PubMed.
- M. Kim, J. H. Kim, M. Kim, C. S. Kim, J. W. Choi, K. Choi, J. H. Lee, J. Park, Y.-C. Kang, S.-H. Jin and M. Song, J. Ind. Eng. Chem., 2020, 88, 84 CrossRef CAS.
- J. J. Yeh and I. Lindau, At. Data Nucl. Data Tables, 1985, 32, 1 CrossRef CAS.
- T. Schneider, K. Artyushkova, J. E. Fulghum, L. Broadwater, A. Smith and O. D. Lavrentovich, Langmuir, 2005, 21, 2300 CrossRef CAS PubMed.
- S. J. Yoon, K. G. Stamplecoskie and P. V. Kamat, J. Phys. Chem. Lett., 2016, 7, 1368 CrossRef CAS PubMed; M. Liu, J. Zhao, Z. Luo, Z. Sun, N. Pan, H. Ding and X. Wang, Chem. Mater., 2018, 30, 5846 CrossRef.
- Y. Nakamura, N. Shibayama, K. Fujiwara, T. Koganezawa and T. Miyasaka, ACS Mater. Lett., 2022, 4, 2409 CrossRef CAS.
- Q. Lin, S. Bernardi, B. Shabbir, Q. Ou, M. Wang, W. Yin, S. Liu, A. S. R. Chesman, S. O. Fürer, G. Si, N. Medhekar, J. Jasieniak, A. Widmer-Cooper, W. Mao and U. Bach, Adv. Funct. Maters., 2022, 32, 2109442 CrossRef CAS.
- M. C. Weidman, M. Seitz, S. D. Stranks and W. A. Tisdale, ACS Nano, 2016, 10, 7830 CrossRef CAS PubMed.
- L. Wu, H. Hu, Y. Xu, S. Jiang, M. Chen, Q. Zhong, D. Yang, Q. Liu, Y. Zhao, B. Sun, Q. Zhang and Y. Yin, Nano Lett., 2017, 17, 5799 CrossRef CAS PubMed; D. Lu, A. Urayama and N. Saito, Colloids Surf., A, 2022, 648, 129345 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma00597f |
| This journal is © The Royal Society of Chemistry 2024 |