Open Access Article
Open Access ArticleFacile development of copper ferrite nanospheres for UV light-driven photocatalytic degradation of cloxacillin sodium
Muhammad
Naeem
 ab,
Faheem
Haider
a,
Adnan
Ashraf
ab,
Faheem
Haider
a,
Adnan
Ashraf
 *a,
Saeed
Ahmed
*a,
Saeed
Ahmed
 *c,
Khalid Mujasam
Batoo
*c,
Khalid Mujasam
Batoo
 d,
Waseeq Ahmad
Siddiqui
d,
Waseeq Ahmad
Siddiqui
 e,
Muhammad
Imran
b,
Muhammad Asam
Raza
e,
Muhammad
Imran
b,
Muhammad Asam
Raza
 f,
Muhammad
Pervaiz
g and
Sajjad
Hussain
h
f,
Muhammad
Pervaiz
g and
Sajjad
Hussain
h
aDepartment of Chemistry, The University of Lahore, Lahore, Pakistan. E-mail: adnanashraf7772@gmail.com
bCentre for Inorganic Chemistry, School of Chemistry, University of the Punjab, Lahore, Pakistan
cDepartment of Chemistry, University of Chakwal, Chakwal-48800, Pakistan. E-mail: saeedahmedlaghari@chemist.com
dCollege of Science, King Saud University, P.O. Box-2455, Riyadh-11451, Saudi Arabia
eInstitute of Chemistry, University of Sargodha, Sargodha-40100, Pakistan
fDepartment of Chemistry, The University of Gujrat, Gujrat, Pakistan
gDepartment of Chemistry, Govt. College University, Lahore, Pakistan
hGraphene Research Institute and Institute of Nano and Advanced Materials Engineering, Sejong University, Seoul, 143-747, Republic of Korea
First published on 23rd November 2023
Abstract
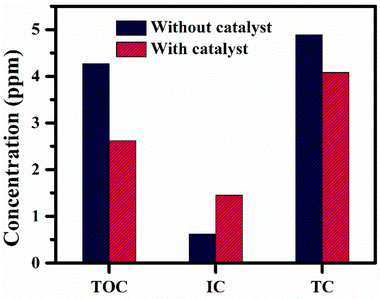
Copper ferrite nanoparticles (CuFe2O4 NPs) have multiple applications in various fields. Herein, a co-precipitation method under mild basic conditions has been used to develop CuFe2O4 NPs. The obtained precipitates were annealed at 500 °C for 4 hours to form copper ferrite. The synthesis of CuFe2O4 NPs was initially confirmed by UV-visible spectroscopy. The presence of specific functional groups like Fe–O was found at 593 cm−1 using Fourier transform infrared spectroscopy. The tetrahedral crystal structure for the CuFe2O4 NPs has been confirmed by X-ray diffraction analysis. The nanosphere-like morphology was confirmed by field emission scanning electron microscopy and the average calculated size of the nanoparticles is ∼45 nm and the pHPZC for the CuFe2O4 NPs is 6.67. The synthesized CuFe2O4 NPs were used for UV light-mediated degradation of cloxacillin sodium (CLX). The degradation of CLX was optimized under different sets of conditions including times of exposure of the drug with the catalyst under UV light, pH values, concentrations, and dosages of the catalyst. The total organic carbon (TOC) confirms 39% degradation of the drug, which is converted into inorganic carbon (IC) and in line with other experimental results. This study provides new insight into the use of copper ferrite to overcome pharmaceutical pollution of water bodies.
Introduction
Water pollution has been a major global issue.1 Different types of waste from various sources, including industrial, agricultural, medical, domestic, and home waste, can cause water pollution.2 In many developing countries, there is no proper system to monitor the discharged water from industries to make it safe for the environment.3 The contaminated water from industries drains into the open channels of canals, rivers, and oceans. The dissolving of waste materials in water bodies is very dangerous and toxic for human health.4 Many pollutants, including primary and secondary, can cause water pollution.5 Industrial waste is now dumped into water faster because of urbanization. New hazards to the environment and human health are various kinds of effluents. The characteristics of organic contaminants, including high toxicity and lower solubility, are serious concerns to human health.6 In particular, in many developing countries, wastes from the pharmaceutical industry have become an annoyance with adverse consequences for the environment and human health. In this regard, a major factor of water pollution is caused by pharmaceutical drugs. Inappropriate disposal of hospital and pharmaceutical industry waste seriously threatens communities.7 Researchers have been paying considerable attention to the excessive use of drugs in recent years because of their hazardous impact. These have been regarded as the main organic contaminants frequently found in wastewater.2It has been well established that antibiotic drugs have several hazardous effects on the environment that can affect both animals and humans.8 Harmful effects of drugs in the environment are usually observed even at very low concentrations.9 CLX is a member of the penicillin family of antibiotic drugs, extensively used to treat infections caused by bacteria in animals and humans.10 Conventionally applied biological wastewater treatment procedures cannot remove CLX from water bodies. Previously, several conventional methods such as electrodialysis,11 filtration,12 adsorption,13 electrodeionization,14 electrochemical reduction,15 and biological treatments have been used for the degradation of all these kinds of organic pollutants present in waste water, which are not appropriate to remove drug contents from wastewater.16 Physical methods like filtration or sedimentation are considered safe ways, but they produce sludge, which produces further secondary pollution.17
Furthermore, these methods are complicated and costly, and by transferring pollutants among fluids, various wastes and by-products are produced, making wastewater treatment difficult using conventional methods.18 The most effective and efficient method is the advanced oxidation process with the aid of photocatalysts.19,20 The use of photocatalysts under UV-visible light is very fruitful for the treatment of wastewater21,22 with drug-based pollutants.23
One powerful approach for reducing pollutants in the environment is the development of nanoparticles as catalysts.24–26 The basic properties like charge transport, band gap, quantum effect and surface states of the material change as its size decreases from bulk to nanoscale.27 The photocatalysis technique has garnered significant attention in the environmental refining industry because of its unique characteristics, which include low cost, easy operating environment, and energy efficiency. When light irradiation activates the semiconductor behaviors of photocatalysts, the electrons and holes are produced. Spinel ferrites are effective agents for the photocatalysis and photodegradation of dyes and drugs, because of their catalytic activity, lower solubility, stable crystalline structure, and exceptional magnetic activity. These characteristics can also make it simple to recover nanoparticles like ZnFe2O4,28 CoFe2O4,29 MgFe2O4,30 MnFe2O4,31 CuFe2O4 and nickel ferrite (NiFe2O4) from solutions using an external magnetic field.32,33 Due to their ferrimagnetic activity, ferrites are primarily complex oxides containing ferric ions that are categorized as magnetic materials. The interactions between metallic ions at specific locations with oxygen ions in the oxide crystal structure give rise to the magnetic properties of the ferrites. Snoek and associates at the Philips Research Laboratories in the Netherlands enhanced the range of ferrites between 1945 and 1993.34
Ferrites can immediately eliminate contaminants using easily available visible light, making them the most effective light-sensitive photocatalysts. One of the most important ways to reduce water contamination is to use photocatalysts for wastewater retreatment. It was previously found that CuFe2O4 NPs were a 75% efficient photocatalyst for rhodamine B dye.35 Since the physical properties of CuFe2O4 nanoparticles such as phase transitions, electrical switching, semi-conductivity, magnetic properties, and chemical stability can alter in response to varying environmental circumstances, they are the ideal spinel ferrite.36 CuFe2O4 nanoparticles have been employed in the production of lithium-ion rechargeable batteries, magnetic resonance imaging materials, photocatalysts for hydrogen evolution using visible light, energy storage materials, coupling reaction catalysts, CO2 reduction catalysts, photoanodes for solar water oxidation, supports for enzyme immobilization, and catalysts in nanomedicine for the treatment of breast cancer.37
Copper ferrite is widely used in numerous fields, such as photocatalysis, high-density magnetic storage media, high-performance electromagnetic devices, and heterogeneous catalysis. Its use has also expanded to include antibacterial properties, magnetic resonance imaging, magnetic separation of cancer cells, biomedicine, and medication delivery.38 With a band gap of less than 2.5 eV, ferrites with remarkable magnetic properties have enhanced photocatalytic activities and absorb significant visible light.39 The possible strategies for producing ferrite nanoparticles must have the maximum rate of recombination properties as spinel ferrite nanoparticles like CuFe2O4 may degrade organic pollutants.40 Several applications of copper ferrite as a photocatalyst have recently been studied, including H2 production, and degradation of rhodamine B and methylene blue.41 Furthermore, CuFe2O4 nanoparticles show potential applications for lithium batteries in high-frequency devices, magnetic memory, drug delivery, sensors, and anode materials.42 CuFe2O4 is found in a tetragonal shape and has an inverse spinel structure. Each unit cell encloses eight Cu-II ions in octahedral sites and sixteen ferric (Fe-II and Fe-III) ions equally distributed among tetrahedral and octahedral sites.43 The magnetic properties of the NPs adapt due to the Cu-II ions as the magnetic moments of Fe-II and Fe-III cancel each other.44 This phenomenon of charge distribution plays an important role in their potential applications as sensors, for electrical applications, and as active photocatalysts.45
Sol–gel, co-precipitation, hydrothermal, and solid-state methods are commonly used to synthesize CuFe2O4 NPs.46 However, these synthetic procedures have some limitations, such as toxicity, high bioaccumulation, and not being eco-friendly.47 Compared to other conventional methods of synthesis, chemical co-precipitation to synthesize nanoparticles of CuFe2O4 has more advantages due to its simplicity, productivity, high purity, stability, uniformity, low cost, and controllable particle size, shape, and composition.48
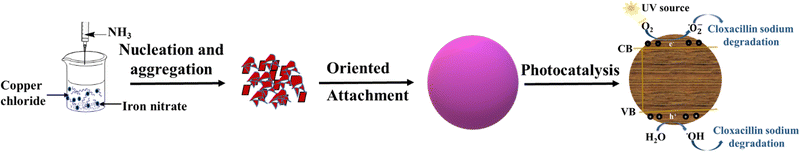
The CuFe2O4 NPs were successfully prepared using the co-precipitation method in this research work. The novelty of these prepared nanoparticles includes their good photocatalytic efficiency for the degradation of the least explored cloxacillin sodium (CLX) drug, easy preparation, good recyclability, high structural and thermal stability, and easy separation. The prepared CuFe2O4 nanoparticle photocatalyst was characterized for structural, elemental, and morphological properties. Furthermore, the photocatalytic activity of the prepared sample was evaluated for the degradation of CLX, and various process parameters for the reaction were also optimized. Scheme 1 shows the formation of copper ferrite and its photocatalytic degradation of pharmaceuticals.
Results and discussion
Structure of copper ferrite
FT-IR spectroscopy of the synthesized nanoparticles after calcination was employed to investigate the various functional groups. Fig. 1 displays the FT-IR spectrum of the CuFe2O4 NPs. The high-frequency band 593 cm−1 indicates the deformation of Fe–O in tetrahedral and octahedral sites. In comparison, the low-frequency band at ∼456 cm−1 is ascribed to the deformation of Fe–O in the octahedral hematite site.49 The OFe–OH bond was observed at 1091 cm−1. The two bands at 1536 cm−1 and 1644 cm−1 correspond to metal hydroxyl group bending vibration.50The XRD pattern of the CuFe2O4 NPs is shown in Fig. 2. As can be seen, the X-ray diffraction for the prepared sample has six major strong peaks at 2θ = 33.62°, 36.06°, 49.92°, 54.49°, 64.35°, and 66.63° (JCPDS card # 34-0425), which describe the tetragonal shape of copper ferrite.51 Moreover, two peaks are seen at 2θ = 39.31° and 58.1° corresponding to the monoclinic CuO phase plane (JCPDS card # 80-1916).52 The peaks of Fe2O3 were still found at 2θ = 41.34° and 62.77°, which corresponded to the well-accepted JCPDS card (33-0664).53 Based on X-ray diffraction data, the Debye–Scherrer equation was used to calculate the crystallite size of copper ferrite based on eqn (1).
 | (1) |
Morphology of copper ferrite
The surface morphology of the nanoparticles was investigated using SEM analysis. Fig. 3(A) and (B) show SEM images of the CuFe2O4 NPs. The magnetic properties of the CuFe2O4 NPs indicated aggregation.54 The morphology of the NPs was recognized as spherical and cubic shapes because over half of the particles were prepared when the reaction was mixed under a magnetic field. In contrast, other NPs did not have the chance to grow up in a heating oven through a hydrothermal reaction. As shown in Fig. 3B, the CuFe2O4 NPs have an average diameter size of about 45 nm. Further, the elemental composition of the synthesized CuFe2O4 was confirmed by EDX analysis as shown in Fig. 4, which is well in agreement with the literature data.PL spectroscopy
The separation–recombination process of the photoinduced electron–hole (e−/h+) pair was monitored by photoluminescence spectroscopy. The PL spectrum of the CuFe2O4 NPs presented in Fig. 5 has sharp bands, which are designated to Fe3+ charge transfer and O2− ions surrounding the octahedral sites. The PL spectrum exhibits four PL transitions at 455, 539, 576, and 659 nm. The transitions at 455 nm might be assisted by the intra-band gap defects and oxygen vacancies. These defects are responsible for affording donor levels near the conduction band edge of the metal oxide. The peaks observed at 539 and 576 nm are relatively small intensity peaks that indicate the exciton separation efficiency and are designated to transition between the conduction band and defect sites on the surface of the catalyst. The peak found at 659 nm indicates the least charge carrier separation. The obtained results are in good agreement with the literature data.55Surface area
The surface area analysis of the CuFe2O4 nanocomposite was conducted through a comprehensive examination using nitrogen adsorption and desorption analyses. The adsorption isotherm, presented in Fig. 6, is a key indicator of the material's porosity and structure. This isotherm's classification as type IV, according to the IUPAC criteria,56 confirms that CuFe2O4 can be categorized as a mesoporous material. It can be seen that the CuFe2O4 nanomaterial synthesized via the co-precipitation method exhibits a large specific surface area of 65.24 m2 g−1.57Determination of point of zero charge (pHPZC)
The pH of the point of zero charge is a crucial factor that directly indicates the acidity and basicity of the adsorbent along with the net charge on the catalyst. The equal concentration of positive and negative charges on the surface of the adsorbent can be determined by pHPZC.58 The experimental results are presented in Fig. 7, which indicates that the pHPZC of copper ferrite is 6.67. The presence of positive charge on the surface of copper ferrite is expected below this pH, while negative charges may be available above this pH.Photocatalytic degradation of CLX
| S. no. | Synthetic methods | Morphology | Targeted pollutant | Degradation efficiency (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Hydrothermal | Cubic | Methylene blue | 95.9 | 61,62 |
| 2 | Co-precipitation | Irregular blocks | Lignin | 92.21 | 63 |
| 3 | co-precipitation | Irregular blocks | Lignin | 58.77 | |
| 4 | Solid reaction process | Irregular blocks | Lignin | 71.24 | |
| 5 | Ethylene glycol-assisted sol–gel process | Irregular blocks | Lignin | 49.73 | |
| 6 | Hydrothermal process | Irregular blocks | Lignin | 58.34 | |
| 7 | Green genesis approach | Spherical shaped | Methylene blue dye | 88 | 64 |
| 8 | Co-precipitation | Sphere-shaped | 2,4-Dichloro phenoxy acetic acid | 88.9 | 65 |
| 9 | Hydrothermal | Cubic | Methylene blue dye | 95.9 | 66 |
| 10 | Via a one-step hydrothermal | Spherical and cubic shapes | Nitroanilines | — | 67 |
| 11 | Sol–gel method | Spherical | Atenolol | 90 | 68 |
| 12 | Sol–gel | Cubic-to-tetragonal | Gallic acid | Up to 98 | 69 |
| 13 | Facile co-precipitation | Spherical and cubic | CLX | 40 | This work |
The degradation of CLX using different catalysts is presented in Table 2. The complete degradation of CLX required a photo-Fenton process at various pH values or more time is required under UV irradiation. The CuFe2O4 NPs as photocatalysts require less time for the degradation of CLX.
| S. no. | Catalyst | Degradation time | pH | Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| a Not mentioned. | |||||
| 1 | UV/ZnO | 300 min | 11 | 100 | 60 |
| 2 | UV/TiO2 | 300 min | 5 | —a | 71 |
| UV/H2O2/TiO2 | 30 min | 100 | |||
| 3 | UV/TiO2 | 480 min | — | 45 | 72 |
| UV/H2O2/TiO2 | 240 min | 15 | |||
| 4 | UV/TiO2/H2O2 | 24 h | 5 | 100 | 73 |
| 5 | UV/ZnS | 120 min | 4.5 | 82–100 | 74 |
| 6 | H2O2/Fe2+ | 2 min | 3 | 100 | 75 |
| 6 | CuFe2O4 | 5 min | 8 | 40 | This work |
Reusability
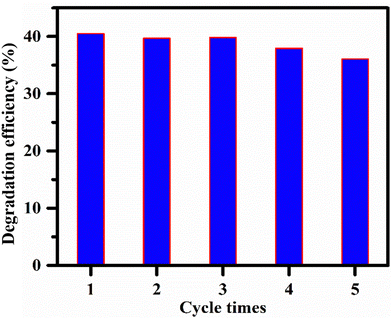
The photocatalytic stability and reusability of the copper ferrite were investigated by performing the previously indicated photocatalytic activity measurements by treating the aqueous solution of CLX (5 ppm) with the catalyst five times. The used photocatalyst was extracted from the treated CLX solution after each cycle, cleaned with deionized water, dried in an oven for an hour at 80 degrees Celsius, and then put back to use in the next photodegradation cycle. Furthermore, photocatalytic stability is significant in large-scale procedures. Hence, to investigate the stability of the photocatalyst, the recycling of copper ferrite for the photocatalytic degradation of CLX under UV irradiation was carried out. The efficiency of the degradation of CLX reduced from 40% to 36% after five cycles (Fig. 13), which shows that it is stable for multiple cycles. These results show the effective and stable nature of the developed catalyst.Experimental
Materials
All the reagents and chemicals used were of analytical grade and used without further purification. Copper chloride (99%), iron nitrate (98%), and ammonium hydroxide (30–33%) were purchased from Sigma Aldrich. Sodium hydroxide (97%) and hydrochloric acid (37%) were of analytical grade and were purchased from Sigma Aldrich.Synthesis of copper ferrite nanoparticles
2 M copper chloride solution and 2 M iron nitrate solutions are mixed followed by the addition of 1 M solution of ammonia NH3 as a precipitating agent. After adding ammonia drop by drop, precipitates are formed in the solution. The formed precipitates were separated using filter paper. The precipitates were washed several times with distilled water and dried in an oven at 100 °C to get a final precursor powder. The final powder was calcined in a furnace at 600 °C for 4 h to get the final copper ferrite.Characterization of copper ferrite
The FTIR (Fourier transformed infrared) spectra were obtained using a Thermos-Scientific infrared spectrophotometer with ZnSe ATR in the range from 4000 cm−1 to 400 cm−1. UV-visible (UV-vis) spectroscopy (UV-8000) was utilized in the range from 200 nm to 800 nm for photocatalytic activities. XRD analysis of the synthesized catalyst was performed using a Bruker D2 Phaser, and diffractometer with Cu Kα radiation. SEM with EDS analysis was performed for the elemental composition surface morphology of the photocatalyst, which was observed using scanning electron microscopy (SEM, FEI NOVA nano SEM 450). PL spectroscopy of the copper ferrite catalyst was studied using a spectrofluorometric FS5 (Edinburgh Instruments, United Kingdom) system. BET of the CuFe2O4 NPs was studied using a Quantachrome Nova 2200e system, Kingsville, TX. TOC analysis was performed using a Sievers 860 laboratory TOC analyzer.Photocatalytic degradation of cloxacillin sodium
The mechanism of the photocatalytic process under UV irradiation is based on the excitation of electrons of the catalyst by absorbing energy equal to or greater than its band gap. In this case, electrons from the valence band progress to the conduction band energy level to form electron–hole pairs.76,77 The photocatalytic activity of CuFe2O4 nanoparticles for the degradation of CLX was determined by using a UV lamp of a longer wavelength with an emission intensity of 365 nm and a power of 10 watts. The experiment was performed in a volumetric flask inside a wooden box. The visible lamp was 20 cm above the solution. In each experiment, 100 mL of aqueous solution of CLX with a specified concentration was dispersed into the photoreactor. Subsequently, a certain amount of photocatalyst CuFe2O4 was introduced into the flask, providing continuous stirring. The experiments were performed under different conditions depending on the pH value, the concentration of the drug, the dosage of the catalyst, and the time of exposure to find the best photocatalytic activity of the CuFe2O4 NPs. After a specified interval of time, 5 mL of the sample was collected, and finally, the sample was analyzed using a UV-visible spectrophotometer by scanning the samples from 200 nm to 800 nm. The maximum absorption for the CLX was found at 217 nm.Effect of time
The time-dependent photocatalytic efficiency of the copper ferrite NPs was tested against a 50 ppm aqueous solution of CLX under UV irradiation. For this purpose, 20 mg of catalyst was added to 100 mL solution in a reaction vessel with continuous stirring under UV light irradiation. After time intervals of 5, 10, 15, 30, 60, 120, 180, 240, and 300 min, aliquots (5 mL) were collected and analyzed using a UV-visible spectrophotometer. The drug solution was stirred for 30 min in the dark before it was exposed to light. The rate of degradation of CLX solution was assessed by eqn 2.| Degradation rate (%) = Co − Ct/Co | (2) |
Here, Co is the initial solution's absorption intensity, and Ct is the CLX's absorption intensity after time t.
Effect of cloxacillin sodium concentration
The concentration of a drug is a key parameter in optimizing the photocatalytic degradation process. A study was performed with CLX concentrations of 5, 10, 15, 20, and 25 ppm with five different dosages of copper ferrite catalyst for the photocatalytic degradation process with a constant time of 60 min at room temperature.Effect of solution pH
The maximum adsorption was analyzed at different pH values (4–9) of drug solution by adding 20 mg copper ferrite NPs as a photocatalyst, 5 ppm constant concentration of CLX, and 120 min for irradiation at room temperature. A phosphate buffer of pH 8 was used for sample preparation, and different pH values were adjusted using NaOH and HCl.Effect of catalyst dose
The dosage-dependent study was carried out at 0.05, 0.1, 0.15, and 0.2 mg mL−1 of drug solution with different concentrations of 5, 10, 15, 20, and 25 ppm with continuous stirring under UV light for 60 min at room temperature.Conclusions
Copper ferrite was successfully synthesized using a co-precipitation method under mild conditions. Various analytical techniques confirmed the synthesized material, including UV-visible, FT-IT, XRD, and FESEM with EDX and BET analysis. The copper ferrite has a particle size of 45 nm and a spherical shape. The average surface area for the CuFe2O4 NPs is found to be 65.24 m2 g−1. The calculated pHPZC for the catalyst is 6.64. CLX was degraded at different pH values, concentrations, times, and catalyst dosages. The TOC analysis also confirmed that the same degradation percentage as maximum degradation of CLX was observed using CuFe2O4 NPs as the photocatalyst under UV light irradiation. After 5 min, maximum degradation of CLX was observed, while pH-based studies confirmed the maximum degradation at pH 8 and 0.05 and 0.1 mg mL−1 doses are more effective for the degradation of the drug CLX. This study provides insight into the use of metal ferrites for the reduction of emerging pollutants.Author contributions
Writing – original draft: M. N., F. H., methodology: S. A., formal analysis, A. A., S. A., and M. A. R., supervision: A. A., Conceptualization: W. A. S., writing – review and editing: M. I., K. M. B., visualization: S. H., software: M. P., validation: S. H.Conflicts of interest
All the authors declare no conflicts of interest.Acknowledgements
The authors are thankful to the Researchers Supporting Project No. (RSP2023R148) at King Saud University, Riyadh, Saudi Arabia for financial support.Notes and references
- M. Mariana, H. P. S. Abdul Khalil, E. B. Yahya, N. G. Olaiya, T. Alfatah, A. B. Suriani and A. Mohamed, J. Water Process Eng., 2022, 45, 102481 CrossRef
.
- G. Gupta, S. K. Kansal, A. Umar and S. Akbar, Chemosphere, 2023, 314, 137611 CrossRef PubMed
.
- A. Mostafa, J. Biodivers. Environ. Sci., 2015, 7, 501–525 Search PubMed
.
- U. Riaz, L. Shahzad, A. Aslam, M. A. Qazi, M. Nasim, W. Anum, R. Ullah, M. M. Khan and I. Ahmad, Abasyn J. Life Sci., 2022, 5, 56–71 Search PubMed
.
- U. Shareef, M. H. D. Othman, A. F. Ismail and A. Jilani, J. Aust. Ceram. Soc., 2020, 56, 29–39 CrossRef
.
- Y. Wang, X. Cui, P. Zhang, Y. Wang and W. Lu, Environ. Technol. Innovation, 2022, 29, 102972 CrossRef
.
- E. S. Okeke, T. P. C. Ezeorba, C. O. Okoye, Y. Chen, G. Mao, W. Feng and X. Wu, Sustainable Chem. Pharm., 2022, 30, 100865 CrossRef
.
- C. Bouki, D. Venieri and E. Diamadopoulos, Ecotoxicol. Environ. Saf., 2013, 91, 1–9 CrossRef
.
- I. Michael, L. Rizzo, C. S. McArdell, C. M. Manaia, C. Merlin, T. Schwartz, C. Dagot and D. Fatta-Kassinos, Water Res., 2013, 47, 957–995 CrossRef PubMed
.
- Y. Ito, Y. Ikai, H. Oka, H. Matsumoto, Y. Miyazaki, K. Takeba and H. Nagase, J. Chromatogr. A, 2001, 911, 217–223 CrossRef
.
- A. Zularisam, A. Ismail and R. Salim, Desalination, 2006, 194, 211–231 CrossRef
.
- M. A. Zazouli and L. R. Kalankesh, J. Environ. Health Sci. Eng., 2017, 15, 1–10 CrossRef PubMed
.
- A. Azimi, A. Azari, M. Rezakazemi and M. Ansarpour, ChemBioEng Rev., 2017, 4, 37–59 CrossRef
.
- E. Mousset and K. Doudrick, Curr. Opin. Electrochem., 2020, 22, 221–227 CrossRef CAS
.
- M. T. Yagub, T. K. Sen and S. Afroze, Science, 2014, 209, 172–184 CAS
.
- A. Majumder, B. Gupta and A. Gupta, Environ. Res., 2019, 176, 108542 CrossRef CAS PubMed
.
- A. G. Akerdi and S. H. J. Bahrami, J. Environ. Chem. Eng., 2019, 7, 103283 CrossRef CAS
.
- Ö. Arar, Ü. Yüksel, N. Kabay and M. Yüksel, Desalination, 2014, 342, 16–22 CrossRef
.
- Y. Liu, Z. Hu and J. C. Yu, Chemosphere, 2021, 278, 130376 CrossRef CAS
.
- P. Chen, P. Zhang, Y. Cui, X. Fu and Y. Wang, Mater. Today Sustainability, 2023, 21, 100276 CrossRef
.
- A. Hassani, S. Krishnan, J. Scaria, P. Eghbali and P. Nidheesh, Curr. Opin. Solid State Mater. Sci., 2021, 25, 100941 CrossRef CAS
.
- A. Hassani, P. Eghbali, F. Mahdipour, S. Wacławek, K.-Y. A. Lin and F. Ghanbari, Chem. Eng. J., 2023, 453, 139556 CrossRef CAS
.
- E. S. Elmolla and M. Chaudhuri, Desalination, 2010, 256, 43–47 CrossRef CAS
.
- N. Baig, I. Kammakakam and W. Falath, Mater. Adv., 2021, 2, 1821–1871 RSC
.
- Y. Zhang, D. Pan, Y. Tao, H. Shang, D. Zhang, G. Li and H. Li, Adv. Funct. Mater., 2022, 32, 2109600 CrossRef CAS
.
- A. Sudhaik, P. Raizada, S. Rangabhashiyam, A. Singh, V. H. Nguyen, Q. Van Le, A. A. P. Khan, C. Hu, C. W. Huang and T. Ahamad, Surf. Interfaces, 2022, 33, 102182 CrossRef CAS
.
- P. Anushkkaran, M. A. Mahadik, W.-S. Chae, H. H. Lee, S. H. Choi and J. S. Jang, Chem. Eng. J., 2023, 472, 144998 CrossRef CAS
.
- J. Zhu, Y. Zhu, Z. Chen, S. Wu, X. Fang and Y. Yao, Int. J. Environ. Res. Public Health, 2022, 19, 10710 CrossRef CAS PubMed
.
- A. Miri, M. Sarani, A. Najafidoust, M. Mehrabani, F. A. Zadeh and R. S. Varma, Mater. Res. Bull., 2022, 149, 111706 CrossRef CAS
.
- M. Kaur and K. Jeet, J. Photochem. Photobiol., A, 2022, 425, 113717 CrossRef
.
- M. Abdo, R. Al-Wafi and M. Al Hammad, Ceram. Int., 2023, 49, 29245–29258 CrossRef CAS
.
- A. Manohar, V. Vijayakanth, S. P. Vattikuti and K. H. Kim, Phys. Mater. Chem., 2022, 286, 126117 CrossRef CAS
.
- A. Hassani, P. Eghbali and Ö. Metin, Environ. Sci. Pollut. Res., 2018, 25, 32140–32155 CrossRef CAS PubMed
.
- P. A. Udhaya, A. Ahmad, M. Meena, M. A. J. Queen, M. Aravind, P. Velusamy, T. M. Almutairi, A. A. Mohammed and S. Ali, J. Mol. Struct., 2023, 1277, 134807 CrossRef CAS
.
- A. Q. Malik, H. Singh, A. Kumar, R. Aepuru, D. Kumar, Q. Ul Ain, A. A. Bhat and A. Mubayi, Energy Environ. Monitoring Assess., 2022, 19, 744 Search PubMed
.
- S. M. Rathod, A. R. Chavan, S. S. Jadhav, K. M. Batoo, M. Hadi and E. H. Raslan, Chem. Phys. Lett., 2021, 765, 138308 CrossRef CAS
.
- S. Anandan, T. Selvamani, G. G. Prasad, A. Asiri and J. Wu, J. Magn. Magn. Mater., 2017, 432, 437–443 CrossRef CAS
.
- S. Anandan, T. Selvamani, G. G. Prasad, A. M. Asiri and J. J. Wu, J. Magn. Magn. Mater., 2017, 432, 437–443 CrossRef CAS
.
- A. Anantharaman, B. A. Josephine, V. M. Teresita, T. Ajeesha and M. George, J. Nanosci. Nanotechnol., 2019, 19, 5116–5129 CrossRef CAS PubMed
.
- M. Ismael, Mater. Res. Bull., 2022, 151, 111803 CrossRef CAS
.
- M. Israr, J. Iqbal, A. Arshad, A. Sadaf, M. Rani, M. Rani and S. Jabeen, J. Phys. D: Appl. Phys., 2021, 54, 395501 CrossRef CAS
.
- M. Kılıç, N. D. Kahya, B. S. Mısırlıoğlu, Ö. Çakır and Z. G. Özdemir, Ferroelectrics, 2021, 571, 183–199 CrossRef
.
- E. Agouriane, B. Rabi, A. Essoumhi, A. Razouk, M. Sahlaoui, B. Costa and M. Sajieddine, J. Mater. Environ. Sci., 2016, 7, 4116–4120 CAS
.
- P. Sarkar, D. Roy, B. Bera, S. De and S. Neogi, Chem. Eng. J., 2022, 430, 132834 CrossRef CAS
.
- A. Mohseni-Bandpei, S. M. Ghasemi, A. Eslami, M. Rafiee, M. Sadani and F. P. Ghanbari, J. Photochem. Photobiol., A, 2021, 418, 113425 CrossRef CAS
.
- A. Subha, M. Shalini, B. Sahu, S. Rout and S. C. Sahoo, Mater. Chem. Phys., 2022, 286, 126212 CrossRef CAS
.
- J. Singh, T. Dutta, K.-H. Kim, M. Rawat, P. Samddar and P. Kumar, J. Nanobiotechnol., 2018, 16, 1–24 CrossRef
.
- A. El Khanchaoui, M. Sajieddine, M. Ounacer, A. Fnidiki, F. Richomme, J. Juraszek, M. Mansori, M. Dib and A. Essoumhi, Appl. Phys. A: Mater. Sci. Process., 2022, 128, 406 CrossRef CAS
.
- T. Kaur, B. Kaur, B. H. Bhat, S. Kumar and A. Srivastava, Phys. B: Condens. Matter, 2015, 456, 206–212 CrossRef CAS
.
- M. Ghaani and J. Saffari, J. Nanostructures, 2016, 6, 172–178 CAS
.
- X. Guo, K. Wang and Y. Xu, Mater. Sci. Eng. B, 2019, 245, 75–84 CrossRef CAS
.
- H. Yan, Y. Zhang, Y. Wang, J. Liu, X. Li, Y. Zhang and P. Dong, Ceram. Int., 2019, 45, 20796–20802 CrossRef CAS
.
- L. Plyasova, T. Y. Kardash, D. Svintsitskiy, E. Paukshtis, N. Shtertser and T. Minyukova, Mater. Res. Bull., 2019, 118, 110481 CrossRef CAS
.
- K. Atacan, B. Topaloğlu and M. Özacar, Appl. Catal., A, 2018, 564, 33–42 CrossRef CAS
.
- P. Paramasivan and P. Venkatesh, Phys. E, 2016, 84, 258–262 CrossRef CAS
.
- Z. A. ALOthman, Materials, 2012, 5, 2874–2902 CrossRef CAS
.
- N. Masunga, B. B. Mamba, Y. W. Getahun, A. A. El-Gendy and K. K. Kefeni, Mater. Sci. Eng. B, 2021, 272, 115368 CrossRef CAS
.
- H. A. Kiwaan, T. M. Atwee, E. A. Azab and A. A. El-Bindary, J. Chin. Chem. Soc., 2019, 66, 89–98 CrossRef CAS
.
- A. Akyol, H. Yatmaz and M. Bayramoglu, Appl. Catal., B, 2004, 54, 19–24 CrossRef CAS
.
- E. S. Elmolla and M. Chaudhuri, J. Hazard. Mater., 2010, 173, 445–449 CrossRef CAS PubMed
.
- M. Rashad, R. Mohamed, M. Ibrahim, L. Ismail and E. Abdel-Aal, Adv. Powder Technol., 2012, 23, 315–323 CrossRef CAS
.
- F. Sinfrônio, J. Rodrigues, F. Silva, R. Fonseca, A. De Menezes, R. Mouta, S. Sharma and M. Castro Jr, J. Electron. Mater., 2018, 47, 6821–6832 CrossRef
.
- Z. Ye, Z. Deng, L. Zhang, J. Chen, G. Wang and Z. Wu, Mater. Res. Express, 2020, 7, 035007 CrossRef CAS
.
- R. Priya, S. Stanly, R. Anuradha and S. Sagadevan, Mater. Res. Express, 2019, 6, 095014 CrossRef CAS
.
- N. Jaafarzadeh, F. Ghanbari and M. Ahmadi, Chem. Eng. J., 2017, 320, 436–447 CrossRef CAS
.
- M. Rashad, R. Mohamed, M. Ibrahim, L. Ismail and E. Abdel-Aal, Adv. Powder Technol., 2012, 23, 315–323 CrossRef CAS
.
- S. Naghash-Hamed, N. Arsalani and S. B. Mousavi, ChemistryOpen, 2022, 11, e202200156 CrossRef CAS PubMed
.
- A. Mohseni-Bandpei, S. M. Ghasemi, A. Eslami, M. Rafiee, M. Sadani and F. Ghanbari, J. Photochem. Photobiol., A, 2021, 418, 113425 CrossRef CAS
.
- M. V. López-Ramón, M. A. Álvarez, C. Moreno-Castilla, M. A. Fontecha-Cámara, Á. Yebra-Rodríguez and E. Bailón-García, J. Colloid Interface Sci., 2018, 511, 193–202 CrossRef PubMed
.
- P. Y. Motlagh, A. Khataee, A. Hassani and Y. Orooji, J. Ind. Eng. Chem., 2023, 126, 465–479 CrossRef
.
- E. S. Elmolla and M. Chaudhuri, Desalination, 2010, 252, 46–52 CrossRef CAS
.
- E. A. Serna-Galvis, A. L. Giraldo-Aguirre, J. Silva-Agredo, O. A. Flórez-Acosta and R. A. Torres-Palma, Environ. Sci. Pollut. Res., 2017, 24, 6339–6352 CrossRef CAS PubMed
.
- E. S. Elmolla and M. Chaudhuri, Desalination, 2010, 252, 46–52 CrossRef CAS
.
- H. R. Pouretedal and M. A. Hasanali, Desalin. Water Treat., 2013, 51, 2617–2623 CrossRef CAS
.
- E. S. Elmolla and M. Chaudhuri, J. Hazard. Mater., 2009, 172, 1476–1481 CrossRef CAS PubMed
.
- U. I. Gaya and A. Abdullah, J. Photochem. Photobiol., C, 2008, 9, 1–12 CrossRef CAS
.
- M. Mariana, H. P. S. Abdul Khalil, E. B. Yahya, N. Olaiya, T. Alfatah, A. Suriani and A. Mohamed, J. Water Process Eng., 2022, 45, 102481 CrossRef
.
| This journal is © The Royal Society of Chemistry 2024 |