Open Access Article
Open Access ArticleThe introduction of gallium ions into V2O5 interlayers for highly reversible Zn ion batteries
Ming
Zhao
a,
Shilong
Li
a,
Xiang
Wu
 *a and
Abdukayum
Abdukader
*a and
Abdukayum
Abdukader
 *b
*b
aSchool of Materials Science and Engineering, Shenyang University of Technology, Shenyang 110870, P. R. China. E-mail: wuxiang05@sut.edu.cn
bXinjiang Key Laboratory of Novel Functional Materials Chemistry, College of Chemistry and Environmental Sciences, Kashi University, Kashi 844000, P. R. China. E-mail: abdukadera@sina.com
First published on 19th March 2024
Abstract
It is very important to construct energy storage systems with high safety and excellent electrochemical performance. In particular, aqueous Zn ion batteries (AZIBs) possess the characteristics of low-cost and environmental benignity. However, there are few cathode materials that match well with the zinc anode. Herein, Ga3+ pre-intercalation into V2O5 layers promotes the insertion/extraction kinetics of zinc ions. The assembled Zn/V2O5–0.1Ga battery with 3 M Zn(CF3SO3)2 electrolyte shows a specific capacity of 512.07 mA h g−1 at a current density of 0.1 A g−1. It delivers an energy density of 281.64 W h kg−1 at a power density of 55 W kg−1. It can also provide a reversible capacity of 110 mA h g−1 at 10 A g−1 with a retention rate of 91.43% after 5000 cycles, revealing its potential applications in future energy storage devices.
1. Introduction
In the past few years, the excessive depletion of fossil fuels has caused a serious energy crisis and environmental pollution. It is necessary to design and develop many sustainable energy sources.1–4 Among them, Li-ion batteries (LIBs) have been utilized in smartphones, electric vehicles and laptops. Nevertheless, their future development is still restricted considering the high cost and toxic organic electrolyte.5,6 Thus, it is crucial to seek an alternative to LIBs, such as rechargeable aqueous multivalent ion batteries (Na, K, Ca, Zn, and Mg).7–10 Aqueous zinc ion batteries (AZIBs) attract wide attention owing to their high theoretical capacity (820 mA h g−1), and the abundant resources and low redox potential of Zn2+/Zn (−0.76 V vs. SHE).11–13 However, it is still an urgent task to explore suitable cathode materials, such as Prussian blue analogues, and Mn-based and vanadium-based materials.14,15 Prussian blue analogues show low capacity and short cycle life.16 Manganese-based materials still need to be improved owing to low rate performance and unstable structures.17,18Recently, various V2O5 structures have been widely investigated as cathode materials for AZIBs,19 owing to their diverse oxidation states (V2+, V3+, V4+, and V5+) and unique crystal features.20,21 The crystal structure of V2O5 connects the distorted VO5 square cones through the shared edges and corners to form a 2D layered structure with weak van der Waals interactions between the layers. For instance, Chen et al. prepared V2O5 samples supported on carbon cloth by a template route. The fabricated cells delivered a capacity of 370 mA h g−1 at 0.2 A g−1.22 The Zn/V2O5@CNT devices presented a capacity of 485.8 at 0.1 A g−1.23 However, the capacity of the reported V2O5 electrode materials is far from their theoretical capacity (589 mA h g−1). The strong electrostatic repulsion between the zinc ions and the host structure results in a slow electrochemical kinetic behavior. Therefore, some strategies have been proposed to modify the cathode material of AZIBs, such as metal ion pre-embedding, defect engineering and coating of other materials.
Metal ion pre-embedding is thought to be an efficient strategy to enhance the electrochemical performance of AZIBs. Many metal cations have been introduced into target materials, such as Zn, K, and Ca. Zhang et al. introduced La3+ into a layered V2O5 structure. The assembled device shows a capacity of 405 mA h g−1 at 0.1 A g−1 and energy density of 227.5 W h kg−1 at 55 W kg−1.24 The Zn/V2O5 cells deliver a discharge capacity of 350 mA h g−1 at 0.1 A g−1 by the intercalation of PANI.25 Liu and coworkers used K+ ions as a structure pillar for introduction into V2O5 interlayers. The resulting material possessed a capacity of 479.8 mA h g−1 at 0.2 A g−1 and maintained 91.3% of its initial capacity at 10 A g−1 after 3000 cycles.26
In this work, we prepare Ga3+-intercalated V2O5 nanobelts by a simple hydrothermal route. Gallium possesses natural advantages, such as its strong electronegativity forming stable chemical bonds. It has a small radius compared to other cations, making it easier to approach the target sample. The introduction of metal ions promotes the transfer of Zn ions, and maintains structural stability of the electrode materials. A series of zinc ion batteries are assembled using the obtained V2O5–0.1Ga samples as cathodes. The cells deliver a discharge capacity of 512.07 mA h g−1 at 0.1 A g−1. They maintain 91.43% of the original capacity at 10 A g−1 after 5000 cycles. The batteries present an energy density of 281.64 W h kg−1 at a power density of 55 W kg−1. This work provides new ideas in designing cathode materials that match well with Zn anodes.
2. Experimental section
All the purchased chemicals were used without any purification. Typically, 4 mmol V2O5 (Alfa Aesar) powder was dissolved into 50 mL of de-ionized water and stirred for 30 min at 50 °C. Then, 1 mL of 30% H2O2 was added into the above solution. After that, a certain amount of HCl (Codow) solution is uniformly dispersed into the above mixture to adjust the pH to 1. Subsequently, 0.1 mmol Ga(NO3)3·xH2O (Macklin) was added into the prepared solution with stirring for half an hour. Then, the solution was placed into an 80 mL Teflon-lined stainless-steel autoclave and maintained at 180 °C for 48 h. After cooling to room temperature, the solution was washed with alcohol and deionized water several times and dried under vacuum at 60 °C for 12 h. For comparison, different amounts of Ga(NO3)3·xH2O (0, 0.05, and 0.2 mmol) were also added to similar solutions and the prepared samples were labeled as V2O5, V2O5–0.05Ga, and V2O5–0.2Ga, respectively.2.1. Structural characterization
The crystallographic information of the as-prepared samples was characterized by powder X-ray diffraction (XRD, 7000, Shimadzu, Cu Kα radiation, λ = 0.1541 nm, 40 kV), scanning electron microscopy (SEM, Gemini 300-71-31), and X-ray photoelectron spectroscopy (XPS, Thermo Kalpha). The specific surface area was studied by the Brunauer–Emmett–Teller (BET, Micromeritics ASAP, JW-TB200) method.2.2. Electrochemical measurements
The cathode materials consist of the active materials, carbon black (Power Origin Limited) and polyvinylidene fluoride (Power Origin Limited) with a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then a certain amount of N-methyl-L-2-pyrrolidone (Damao) was added to form a slurry, which was evenly pressed onto graphite paper. A piece of zinc foil was used as the anode with a thickness of 0.2 mm. The coin cells (CR2032) were assembled by using 3 M Zn(CF3SO3)2 (Bidepharm) as the electrolyte. The average loading mass of the cathode is about 1.5 mg. The galvanostatic intermittent titration technique (GITT), galvanic charge–discharge (GCD) analysis and cycling stability tests were performed at a voltage range of 0.4–1.5 V using a Neware battery tester (CT-4008T-5V6A-164). The CV curves and Nyquist plots of the cells were measured by using an electrochemical workstation (Shanghai Chenhua, CHI660E).
1. Then a certain amount of N-methyl-L-2-pyrrolidone (Damao) was added to form a slurry, which was evenly pressed onto graphite paper. A piece of zinc foil was used as the anode with a thickness of 0.2 mm. The coin cells (CR2032) were assembled by using 3 M Zn(CF3SO3)2 (Bidepharm) as the electrolyte. The average loading mass of the cathode is about 1.5 mg. The galvanostatic intermittent titration technique (GITT), galvanic charge–discharge (GCD) analysis and cycling stability tests were performed at a voltage range of 0.4–1.5 V using a Neware battery tester (CT-4008T-5V6A-164). The CV curves and Nyquist plots of the cells were measured by using an electrochemical workstation (Shanghai Chenhua, CHI660E).
3. Results and discussion
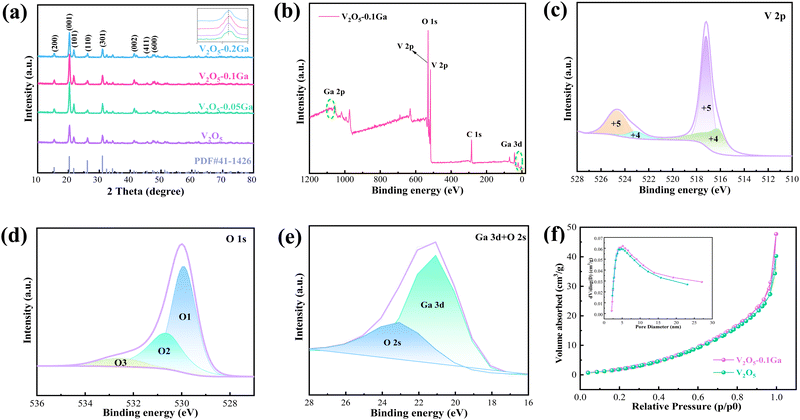
First, the crystal structures of the samples are investigated by XRD, as shown in Fig. 1a. It can be observed that the diffraction peaks match well with the V2O5 phase (PDF# 41-1426). The V2O5–0.1Ga sample presents sharp and strong diffraction peaks. The main peaks at 15.30, 20.32, 21.68, 26.16, 31.04, and 41.36° belong to the (200), (001), (101), (110), (301) and (002) crystal planes, respectively. By enlarging the XRD pattern, it can be observed that the (101) crystal plane shifts to the right. This demonstrates the successful doping of Ga ions into the V2O5 host structure. The lattice parameters a = 11.516 Å, b = 3.566 Å, and c = 4.373 Å. Then XPS is used to investigate the valence states and elemental composition of the electrode materials. Fig. 1b presents the full spectrum of the V2O5–0.1Ga sample, revealing the existence of the V, O, Ga and C elements. From Fig. 1c, the binding energies at 516.28/523.08 eV and 517.18/524.78 eV are in accordance with V4+ and V5+.27 According to previous reports, V2O5 samples with mixed valences (V4+ and V5+) possess fast reaction kinetics and low polarization intensity.28 The O 1s spectrum can be fitted into three peaks, as shown in Fig. 1d. The peaks at 529.98, 530.68 and 532.68 eV can be indexed to oxygens in the metal oxide,29 V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds,30 and O–H from surface-absorbed water,31 respectively. The Ga 3d spectrum in Fig. 1e shows the signal peak at 20.8 eV, which confirms the presence of Ga3+ in the V2O5 samples.32Fig. 1f shows nitrogen adsorption and desorption isotherms of the V2O5 and V2O5–0.1Ga samples. The specific surface area of V2O5–0.1Ga is 27.42 m2 g−1, which is larger than that of the V2O5 product (24.09 m2 g−1). In addition, the total pore volumes of the V2O5–0.1Ga and V2O5 samples are 0.061 and 0.052 cm3 g−1, respectively. The results demonstrate that the addition of Ga element can increase the specific surface area and the active sites.
O bonds,30 and O–H from surface-absorbed water,31 respectively. The Ga 3d spectrum in Fig. 1e shows the signal peak at 20.8 eV, which confirms the presence of Ga3+ in the V2O5 samples.32Fig. 1f shows nitrogen adsorption and desorption isotherms of the V2O5 and V2O5–0.1Ga samples. The specific surface area of V2O5–0.1Ga is 27.42 m2 g−1, which is larger than that of the V2O5 product (24.09 m2 g−1). In addition, the total pore volumes of the V2O5–0.1Ga and V2O5 samples are 0.061 and 0.052 cm3 g−1, respectively. The results demonstrate that the addition of Ga element can increase the specific surface area and the active sites.
SEM is then utilized to observe the morphologies of the samples. As described in Fig. 2a–d, the growth process of the V2O5 samples is investigated by controlling the content of Ga3+. In Fig. 2b, the V2O5–0.1Ga electrode possesses a belt-like shape. In contrast, the other three samples show uneven microcrystals. Using V2O5–0.1Ga samples as a cathode, the exposed surface is expected to provide abundant active sites to obtain superior rate performance. As seen in Fig. 2e, the elemental mapping confirms a uniform distribution of three elements of V, O and Ga along the surface of the nanobelts, which demonstrates that Ga3+ is doped into the V2O5 host structure.
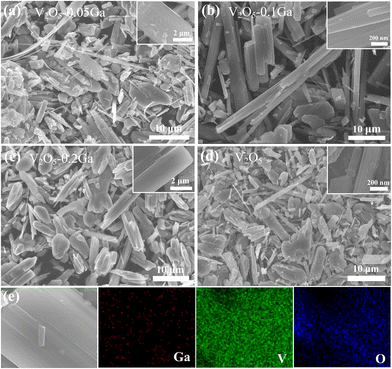 | ||
| Fig. 2 SEM images of (a) V2O5–0.05Ga, (b) V2O5–0.1Ga, (c) V2O5–0.2Ga and (d) V2O5 samples. (e) The corresponding elemental mapping of the V2O5–0.1Ga samples. | ||
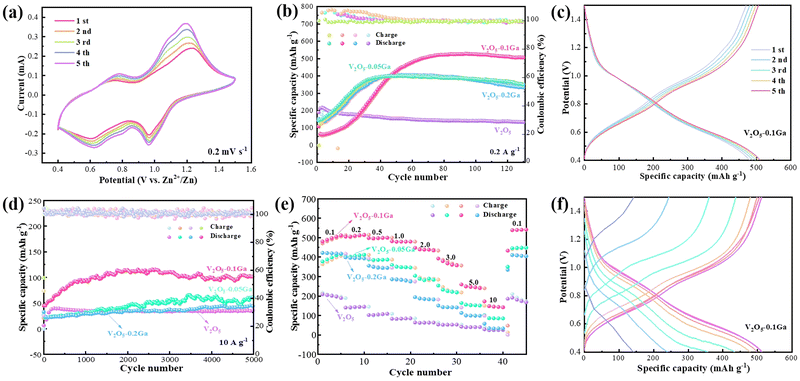
To study the electrochemical performance of the samples, some CR2032 coin cells are assembled with 3 M Zn(CF3SO3)2 as the electrolyte. Fig. 3a shows the first five cycle CV curves of the V2O5–0.1Ga sample at 0.2 mV s−1. The curves maintain the same shapes well, indicating their high reversibility of the redox reactions. Furthermore, there are two pairs of redox peaks located at 1.19/0.96 V and 0.77/0.62 V, which are related to the multi-step intercalation/de-intercalation of Zn2+. Fig. 3b presents the cycling stability of the samples. After many cycles of activation, the discharge capacity of the V2O5–0.1Ga sample delivers a specific capacity of 526.8 mA h g−1 at 0.2 A g−1 and maintains a retention rate of 95.8% after 130 cycles. The GCD curves of the V2O5–0.1Ga products show several charge–discharge platforms (Fig. 3c), which are in accordance with the corresponding CV curves shown in Fig. 3a.
The cycling stability of the cells is also evaluated at 10 A g−1, as depicted in Fig. 3d. The capacity of the V2O5–0.1Ga sample reaches 110 mA h g−1 with a retention rate of 91.43% after 5000 cycles. Fig. 3e shows the corresponding rate performance of the batteries. The discharge specific capacities of the V2O5–0.1Ga electrodes are 512.07, 514.03, 499.67, 479.18, 437.59, 360.66, 244.64, are 148.40 mA h g−1 at current densities from 0.1 to 10 A g−1. When it returns to 0.1 A g−1, the battery still obtains a capacity of 540.57 mA h g−1. This demonstrates that the moderate amount of Ga3+ is conducive to the enhancement of the specific capacity and rate capability. On the contrary, excessive Ga3+ incorporation may lead to the disruption of the crystal structure and volume expansion of the V2O5 samples, thus producing adverse effects. Furthermore, the GCD curves (Fig. 3f) of the Zn/V2O5–0.1Ga batteries show that the capacity decreases with increasing current density. Table 1 lists the zinc-ion storage performance of several electrode materials.33–41 It demonstrates the excellent electrochemical performance of Zn/V2O5–0.1Ga cells.
| Materials | Morphology | Current density (A g−1) | Discharge capacity (mA h g−1) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| VO2@PPy | Hollow spheres | 0.1 | 440 | 3 M Zn(CF3SO3)2 | 33 |
| V2O5 | Nanofibers | 0.02 | 319 | 3 M Zn(CF3SO3)2 | 34 |
| Mn-doped-VO2 | Nanobelts | 0.1 | 209.6 | 3 M Zn(CF3SO3)2 | 35 |
| Ni0.25V2O5·nH2O | Nanoribbons | 0.2 | 389 | 3 M ZnSO4 | 36 |
| VO2·0.2H2O | Nanocuboids | 0.25 | 423 | 2 M ZnSO4 | 37 |
| V2O5x/PANI | Nanosheets | 0.1 | 400 | 2 M ZnSO4 | 38 |
| K1.15V5O13·1.3H2O | Nanobelts | 0.2 | 461 | 3 M Zn(OTF)2 | 39 |
| Ba1.2V6O16·3H2O | Nanobelts | 0.1 | 321 | 2 M ZnSO4 | 40 |
| V2O5 | Hollow spheres | 0.2 | 280 | 3.65 M ZnSO4 | 41 |
| V2O5–0.1Ga | Nanobelts | 0.1 | 512.07 | 3 M Zn(CF3SO3)2 | This work |
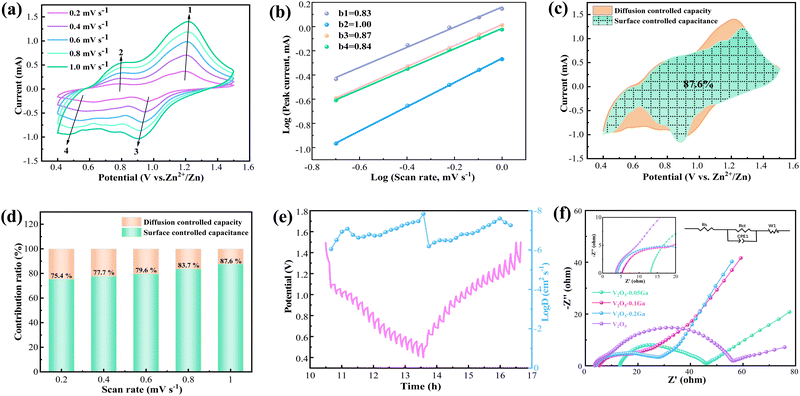
Fig. 4a presents the CV curves of the Zn/V2O5–0.1Ga cells at various scan rates (0.2–1.0 mV s−1). With the increasing of the sweep speed, the positive and negative peak currents move to high and low potentials, respectively. The shape of the curves remains almost unchanged, showing their excellent reversibility. For the CV curves, the relationship between peak current (i) and sweep speed (v) can be obtained through the equation as follows:
| i = avb | (1) |
In addition, the following equation can be utilized to calculate the pseudocapacitive and diffusion contributions:
| i(V) = k1v + k2v1/2 | (2) |
The energy density and power density can be calculated based on eqn (3) and (4):
| E = QU/2m | (3) |
| P = iU/2m | (4) |
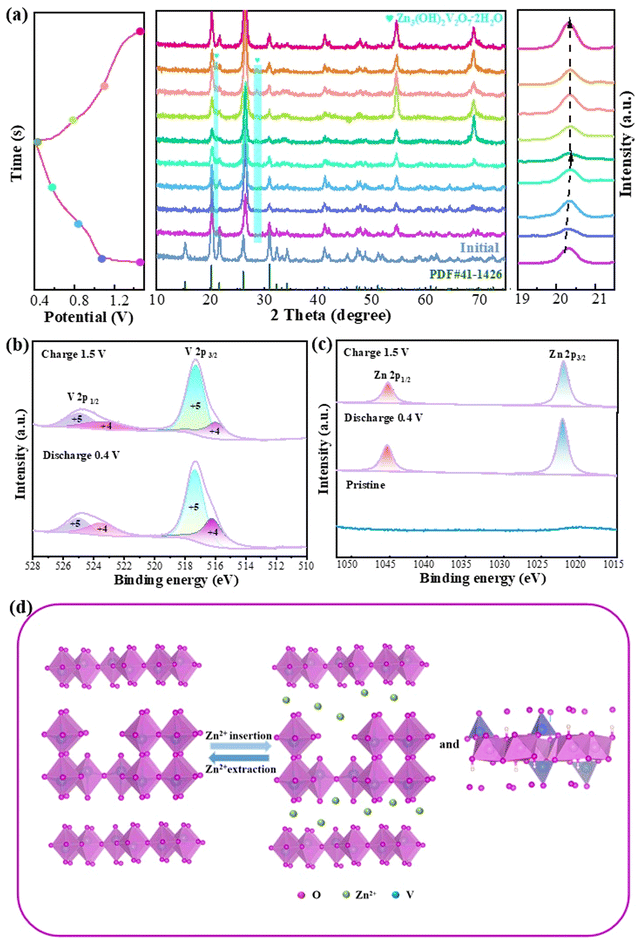
Ex situ XRD was performed to study the Zn2+ storage mechanism of the Zn/V2O5–0.1Ga batteries at different stages (Fig. 5a). The (001) crystal plane shifts to a high angle when the voltage drops from 1.5 V to 0.4 V. This is due to the strong electrostatic adsorption between the intercalated Zn2+ and V2O5 materials, which reduces the interplanar spacing of the (001) plane.42 After full charging, the plane returns to its original state, which is attributed to the reversible removal of Zn2+ in the host material. Besides, a new phase Zn3(OH)2V2O7·2H2O appears during the reaction due to the insertion of Zn2+, demonstrating that Zn2+ is embedded successfully into the V2O5–0.1Ga samples. The generation of a new phase is related to H+ in the electrolyte. The electrochemical reaction process can be represented as below:
| V5+ + 4H2O → VO2(OH)− + 6H+ | (5) |
| Zn → Zn2+ + 2e− | (6) |
| 2VO2(OH)− + 3Zn2+ + 3H2O → Zn3(OH)2V2O7·2H2O + 4H+ | (7) |
Finally, XPS spectra are again obtained to study the composition and chemical valence of the V2O5–0.1Ga electrode during the charge and discharge states. In Fig. 5b, the V 2p1/2 and V 2p3/2 diffraction peaks can be attributed to V4+ (523.68/516.28 eV) and V5+ (524.98/517.38 eV), respectively.43 In the charge state, the signal of the V4+ peaks decreases, while that of V5+ increases. This is caused by reversible insertion/extraction of Zn2+ in the redox reactions. As seen in Fig. 5c, there are no Zn2+ signals detected in the pristine state. When discharged to 0.4 V, the peaks at 1022.18 eV and 1045.38 eV belong to Zn 2p3/2 and Zn 2p1/2. The peak signal is lower during charging than discharging. It is worth noting that the intensity of the Zn signal peak does not disappear completely after charging, which is due to the irreversible de-embedding of some Zn2+. Fig. 5d illustrates an electrochemical reaction schematic of the reversible insertion/extraction of Zn2+.
4. Conclusions
In summary, we have prepared several Ga-ion pre-embedded V2O5 nanobelts by a hydrothermal strategy. The obtained samples show large specific surface areas, which is beneficial to increasing the reaction active sites. The pre-embedding of Ga3+ effectively maintains the stability of the electrode material during repeated charging and discharging. The assembled Zn/V2O5–0.1Ga possesses an outstanding specific capacity and long cycle life after 5000 cycles and ultrafast Zn2+ diffusion capability. Moreover, they also achieve high energy density and power density. The superior performance V2O5–0.1Ga cathode materials show great potential for advanced and safe aqueous zinc ion batteries.Author contributions
Ming Zhao: conceptualization, software, data curation, writing – original draft preparation, and methodology, Shilong Li: visualization, and Xiang Wu and Abdukayum Abdukader: supervision, writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project is supported by the Natural Science Foundation of China (no. 52172218) and the Tianshan Innovation Team Plan of Xinjiang Uygur Autonomous Region (2023D14002).References
- Y. Liu, Y. Liu and X. Wu, Defect engineering of vanadium-based electrode materials for zinc ion battery, Chin. Chem. Lett., 2023, 34, 107839 CrossRef CAS
.
- S. Zhong, Y. S. Yu, Y. Yang, Y. Yao, L. F. Wang, S. N. He, Y. X. Yang, L. Liu, W. P. Sun, Y. Z. Feng, H. G. Pan, X. H. Rui and Y. Yu, Molecular engineering on solvation structure of carbonate electrolyte toward durable sodium metal battery at −40 °C, Angew. Chem., Int. Ed., 2023, 62, e2023011693 Search PubMed
.
- N. M. Tri, S. Preston, P. Andrea, F. Michael G, H. Xiao, G. Ilja and S. Ullrich, Polymer-templated mesoporous lithium titanate microspheres for high-performance lithium batteries, Mater. Adv., 2022, 3, 362–372 RSC
.
- Q. Xia, T. Xia and X. Wu, PPy decorated α-Fe2O3 nanosheets as flexible supercapacitor electrode, Rare Met., 2022, 41, 1195–1201 CrossRef CAS
.
- S. Stefan, L. Andreas, Z. Veronika, R. Joseph, G. Steffen, R. Daniel and F. Jurgen, Li+/H+ exchange of Li7La3Zr2O12 single and polycrystals investigated by quantitative LIBS depth profiling, Mater. Adv., 2022, 3, 8760–8770 RSC
.
- J. X. Chen, P. Alessandro, Y. Shinhee, C. D. Valbjorn, E. Vincenzo and P. Nini, Mater. Adv., 2023, 4, 6638–6644 RSC
.
- X. M. Xia, S. T. Xu, F. Tang, Y. Yao, L. F. Wang, L. Liu, S. N. He, Y. X. Yang, W. P. Sun, C. Xu, Y. Z. Feng, H. G. Pan, X. H. Rui and Y. Yu, A multifunctional interphase layer enabling superior sodium-metal batteries under ambient temperature and −40 °C, Adv. Mater., 2023, 35, 2209511 CrossRef CAS PubMed
.
- J. J. Zhang and X. Wu, Dual-ion carrier storage through Mg2+ addition for high-energy and long-life zinc-ion hybrid capacitor, Int. J. Miner., Metall. Mater., 2024, 31, 179–185 CrossRef CAS
.
- J. Xie and Q. C. Zhang, Recent progress in multivalent metal (Mg, Zn, Ca, and Al) and metal-ion rechargeable batteries with organic materials as promising electrodes, Small, 2019, 15, 1805061 CrossRef PubMed
.
- X. Lv, F. Tang, Y. Yao, C. Xu, D. Chen, L. Liu, Y. Z. Feng, X. H. Rui and Y. Yu, Sodium gallium alloy layer for fast and reversible sodium deposition, SusMat, 2022, 2, 699–707 CrossRef CAS
.
- M. Zhao, S. L. Li and X. Wu, (NH4)2V10O25.8H2O nanowire materials for stable zinc ion storage, Mater. Today Chem., 2023, 33, 101686 CrossRef CAS
.
- Y. X. Zeng, X. Y. Zhang, R. F. Qin, X. Q. Liu, P. P. Fang, D. Z. Zheng, Y. X. Tong and X. H. Lu, Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries, Adv. Mater., 2019, 31, 1903675 CrossRef PubMed
.
- Y. Liu, Y. Liu, X. Wu and Y. R. Cho, General carbon modiffcation avenue to construct highly stable V2O5 electrodes for aqueous zinc-ion batteries, ACS Sustainable Chem. Eng., 2023, 11, 13298–13305 CrossRef CAS
.
- S. Q. Zhao, Y. Liu and X. Wu, Rose-shaped VS2 nanosheets as cathode materials for rechargeable zinc ion batteries, CrystEngComm, 2023, 25, 1986–1992 RSC
.
- Y. Liu and X. Wu, High durable aqueous zinc ion batteries by synergistic effect of V6O13/VO2 electrode materials, J. Energy Chem., 2023, 87, 334–341 CrossRef CAS
.
- Y. X. Zeng, X. F. Lu, S. L. Zhang, D. Y. Luan, S. Li and X. W. Lou, Construction of Co-Mn prussian blue analog hollow spheres for efficient aqueous Zn-ion batteries, Angew. Chem., Int. Ed., 2021, 60, 22189–22194 CrossRef CAS PubMed
.
- K. X. Li, Y. Liu and X. Wu, Mn2+ Intercalation into hydrated vanadium pentoxide nanosheets for highly durable zinc ion batteries, ACS Appl. Nano Mater., 2023, 6, 12439–12446 CrossRef CAS
.
- F. X. Shi, C. C. Mang, H. W. Liu and Y. F. Dong, Flexible and high-energy-density Zn/MnO2 batteries enabled by electrochemically exfoliated graphene nanosheets, New J. Chem., 2020, 44, 653–657 RSC
.
- Y. Liu, Y. Liu, Y. Yamauchi, Z. A. Alothman, Y. V. Kaneti and X. Wu, Enhanced zinc ion storage capability of V2O5 electrode materials with hollow interior cavities, Batteries Supercaps, 2021, 4, 1867–1873 CrossRef CAS
.
- Y. Liu and X. Wu, Hydrogen and sodium ions co-intercalated vanadium dioxide electrode materials with enhanced zinc ion storage capacity, Nano Energy, 2021, 86, 106124 CrossRef CAS
.
- F. Wan and Z. Q. Niu, Design strategies for vanadium-based aqueous zinc-ion batteries, Angew. Chem., Int. Ed., 2019, 58, 16358–16367 CrossRef CAS PubMed
.
- X. Zhang, Y. C. Tang, P. He, Z. Zhang and T. F. Chen, Edge-rich vertical graphene nanosheets templating V2O5 for highly durable zinc ion battery, Carbon, 2021, 172, 207–213 CrossRef CAS
.
- X. Wang, Y. M. Wang, J. N. Hao, Y. Liu, H. H. Xiao, Y. Ma, L. M. Chen, Y. Y. Huang and G. H. Yuan, Pseudocapacitive zinc cation intercalation with superior kinetics enabled by atomically thin V2O5 nanobelts for quasi-solid-state microbatteries, Energy Storage Mater., 2022, 50, 454–463 CrossRef
.
- D. D. Zhang, J. Cao, Y. L. Yue, T. Pakornchote, T. Bovornratanaraks, J. Han, X. Y. Zhang, J. Q. Qin and Y. H. Huang, Two birds with one stone: boosting zinc-ion insertion/extraction kinetics and suppressing vanadium dissolution of V2O5 via La3+ incorporation enable advanced zinc-ion batteries, ACS Appl. Mater. Interface, 2021, 13, 38416–38424 CrossRef CAS PubMed
.
- R. Li, F. Xing, T. Y. Li, H. M. Zhang, J. W. Yan, Q. Zheng and X. F. Li, Intercalated polyaniline in V2O5 as a unique vanadium oxide bronze cathode for highly stable aqueous zinc ion battery, Energy Storage Mater., 2021, 38, 590–598 CrossRef
.
- Y. Liu, Y. Liu, X. Wu and Y. R. Cho, High performance aqueous zinc battery enabled by potassium ion stabilization, J. Colloid Interface Sci., 2022, 628, 33–40 CrossRef CAS PubMed
.
- G. A. Sawatzky and D. Post, X-ray photoelectron and auger spectroscopy study of some vanadium oxides, Phys. Rev. B: Condens. Matter Mater. Phys., 1979, 20, 1546–1555 CrossRef CAS
.
- F. Liu, Z. X. Chen, G. Z. Fang, Z. Q. Wang, Y. S. Cai, B. Y. Tang, J. Zhou and S. Q. Liang, V2O5 nanospheres with mixed vanadium valences as high electrochemically active aqueous zinc-ion battery cathode, Nano-Micro Lett., 2019, 11, 25 CrossRef CAS PubMed
.
- C. M. Ghimbeu, E. Raymundo-Pinero, P. Fioux, F. Beguin and C. Vix-Guterl, Vanadium nitride/carbon nanotube nanocomposites as electrodes for supercapacitors, J. Mater. Chem. A, 2011, 21, 13268 RSC
.
- C. X. Liu, R. Li, W. J. Liu, G. Z. Shen and D. Chen, Chitosan-assisted fabrication of a network C@V2O5 cathode for high-performance Zn-ion batteries, ACS Appl. Mater. Interfaces, 2021, 13, 37194–37200 CrossRef CAS PubMed
.
- Y. X. Zeng, Z. Z. Lai, Y. H. Han, H. Z. Zhang, S. L. Xie and X. H. Lu, Oxygen-vacancy and surface modulation of ultrathin nickel cobaltite nanosheets as a high-energy cathode for advanced Zn-ion batteries, Adv. Mater., 2018, 33, 1802396 CrossRef PubMed
.
- J. Zhang, C. Q. Dong, Z. B. Wang, H. Gao, J. Z. Niu, Z. Q. Peng and Z. H. Zhang, A new defect-rich CoGa layered double hydroxide as efficient and stable oxygen evolution electrocatalyst, Small Methods, 2019, 3, 1800286 CrossRef
.
- Y. Liu, P. Hu, H. Liu, X. Wu and C. Zhi, Tetragonal VO2 hollow nanospheres as robust cathode material for aqueous zinc ion batteries, Mater. Today Energy, 2020, 17, 100431 CrossRef
.
- X. Y. Chen, L. B. Wang, H. Li, F. Y. Cheng and J. Chen, Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries, J. Energy Chem., 2019, 38, 20–25 CrossRef
.
- S. Y. Deng, H. Li, B. H. Chen, Z. J. Xu, Y. Jiang, C. H. Li, W. Xiao and X. M. Yan, High performance of Mn-doped VO2 cathode for aqueous zinc-ion batteries: an insight into Zn2+ storage mechanism, Chem. Eng. J., 2023, 452, 139115 CrossRef CAS
.
- J. W. Li, K. McColl, X. K. Lu, S. Sathasivam, H. B. Dong, L. Q. Kang, Z. N. Li, S. Y. Zhao, A. G. Kafizas, R. Wang, D. J. L. Brett, P. R. Shearing, F. Cora, G. J. He, C. J. Carmalt and I. P. Parkin, Multi-Scale investigations of δ-Ni0.25V2O5·nH2O cathode materials in aqueous zinc-ion batteries, Adv. Energy Mater., 2020, 10, 2000058 CrossRef CAS
.
- D. D. Jia, K. Zheng, M. Song, H. Tan, A. Zhang, L. H. Wang, L. J. Yue, D. Li, C. W. Li and J. Q. Liu, VO2·0.2H2O nanocuboids anchored onto graphene sheets as the cathode material for ultrahigh capacity aqueous zinc ion batteries, Nano Res., 2020, 13, 215–224 CrossRef CAS
.
- W. J. Li, C. Han, Q. F. Gu, S. L. Chou, J. Z. Wang, H. K. Liu and S. X. Dou, Electron delocalization and dissolution-restraint in vanadium oxide superlattices to boost electrochemical performance of aqueous zinc-ion batteries, Adv. Energy Mater., 2020, 10, 2001852 CrossRef CAS
.
- N. Qiu, Z. M. Yang, R. Xue, Y. Wang, Y. M. Zhu and W. Liu, Toward a high-performance aqueous zinc ion battery: potassium vanadate nanobelts and carbon enhanced zinc foil, Nano Lett., 2021, 21, 2738–2744 CrossRef CAS PubMed
.
- X. Wang, B. J. Xi, X. J. Ma, Z. Y. Feng, Y. X. Jia, J. K. Feng, Y. T. Qian and S. L. Xiong, Boosting zinc-ion storage capability by effectively suppressing vanadium dissolution based on robust layered barium vanadate, Nano Lett., 2020, 20, 2899–2906 CrossRef CAS PubMed
.
- H. G. Qin, L. L. Chen, L. M. Wang, X. Chen and Z. H. Yang, V2O5 hollow spheres as high rate and long life cathode for aqueous rechargeable zinc ion batteries, Electrochim. Acta, 2019, 306, 307–316 CrossRef CAS
.
- H. F. Liang, Z. Cao, F. W. Ming, W. L. Zhang, D. H. Anjum, Y. Cui, L. Cavallo and H. N. Alshareef, Aqueous zinc-ion storage in MoS2 by tuning the intercalation energy, Nano Lett., 2019, 19, 3199–3206 CrossRef CAS PubMed
.
- Z. Y. Feng, Y. F. Zhang, J. J. Sun, Y. Y. Liu, H. M. Jiang, M. Cui, T. Hu and C. G. Meng, Dual ions enable vanadium oxide hydration with superior Zn2+ storage for aqueous zinc-ion batteries, Chem. Eng. J., 2022, 433, 133795 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2024 |