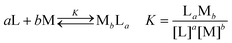Open Access Article
Open Access ArticlePentatomic carbon ring conjugated nitrogen-doped nanographene†
Jinku
Bai
a,
Xiao
Xu
b,
Xin-Yue
Wang
a,
Xin
Sun
a,
Jiaqi
Liang
a,
Tongling
Liang
 c and
Han-Yuan
Gong
c and
Han-Yuan
Gong
 *a
*a
aCollege of Chemistry, Beijing Normal University, No. 19, XinJieKouWai St, HaiDian District, Beijing 100875, P. R. China. E-mail: hanyuangong@bnu.edu.cn
bGoods and Material Department of CASIC Tertiary Research Institute, Building 30, No. 1 Xili, Beiqu, Fengtai District, Beijing, P. R. China
cInstitute of Chemistry, Chinese Academy of Science, Beijing 100190, P. R. China
First published on 12th January 2024
Abstract
A π extended N-doped nanographene molecule with pentatomic carbon ring conjugation was synthesized. The molecule, 1,14-dichloro-6,9-dimethoxyquinolino[2′,3′,4:3,4]indeno[2,1,7-ghi] phenanthridine (1), exhibited potential as a metal cation and proton receptor with a UV-vis and fluorescence response to selected metal cations (e.g., Zn2+, Zr4+, Ag+, Cu2+, or Cd2+) or protons.
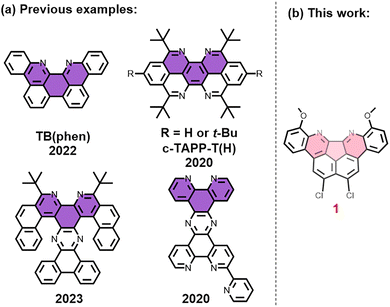
Over the past two decades, there has been growing interest in N-doped carbon-rich materials.1–17 These materials exhibit excellent characteristics and a wide range of applications, including ion recognition, optical materials, electronic devices, and catalysts.18–23 A crucial strategy for generating these materials with precise structures is bottom-up organic synthesis.24 A potential candidate of the smallest N-doped nanographene fragments is 2,2′-bipyridine. It has shown an impressive ability to coordinate with different metal cations and also displays effective catalytic properties.25 Nanographene molecules containing 1,10-phenanthroline were synthesized, demonstrating limited examples of hexatomic carbon ring conjugation involving the 2,2′-bipyridinyl moiety (Fig. 1a).23,26–28 Meanwhile, non-hexatomic carbon rings in conjugated carbon structures lead to the formation of nanographene with unique topological features and distinct properties.29,30 However, it remains difficult to examine the effect of conjugation and substituents on non-hexatomic carbon rings (such as a pentatomic carbon ring) in N-doped nanographene.
Herein, we present the compound 1,14-dichloro-6,9-dimethoxyquinolino[2′,3′,4′:3,4]indeno[2,1,7-ghi]phenanthridine (1), a N-doped nanographene derivative consisting of a pentatomic carbon ring with conjugation (Fig. 1b). When compared to the previously reported hexatomic carbon ring conjugated N-doped nanographene (e.g., tetrabenzo [b,de,gh,j] [1,10]phenanthroline, TB(phen)), 1 has smaller ε values at 450 nm (about a quarter of TB(phen)'s) and larger Stokes shift (55 nm vs. 35 nm, respectively). Furthermore, as a result of the pentatomic carbon ring conjugation and the influence of substituents, 1 also showed various binding selectivities and/or modes with tested metal cations or protons. The results indicated that non-hexatomic ring extension and/or substituents can be employed to control carbon-rich materials.
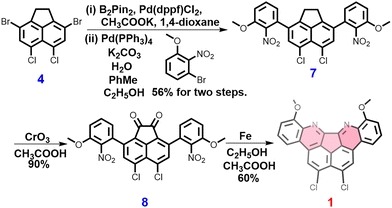
Initially, there was an attempt to synthesize a specific hydrogen-carbon molecule, matching to 1, without the presence of methoxy and chloride groups. However, the final product displayed significant insolubility in all solvents, rendering exact purification and further characterisation impracticable. The synthesis of 1 is presented in Scheme 1. The incorporation of chlorine functional groups modifies the localization effect of subsequent bromination reaction. After undergoing boron esterification and a subsequent Suzuki coupling reaction, the compound 3,8-dibromo-5,6-dichloro-1,2-dihydroacenaphthylene (4)31 was transformed into the coupling product 5,6-dichloro-3,8-bis(3-methoxy-2-nitrophenyl)-1,2-dihydro-acenaphthylene (7) with an overall yield of 56%. 7 was oxidized using chromium trioxide in glacial acetic acid to produce 5,6-dichloro-3,8-bis(3-methoxy-2-nitrophenyl)acenaphthylene-1,2-dione (8) with a yield of 90%. Ultimately, the reduction of nitro groups on 8 and carbonyl-amine condensation in one pot generated 1 with a remarkable yield of 60% using iron powder in CH3COOH/C2H5OH (Scheme 1).
1 was characterized with high resolution mass spectrometry (HRMS) ([M + H]+ calcd 457.0511; found: 457.0502), as well as 1H and 13C NMR spectroscopic study in CDCl3 (Fig. S9, S10 and S14, ESI†). The structure of 1 was further confirmed using a single crystal X-ray diffraction analysis (Fig. S15, ESI†). The single crystal sample of 1·C2H5OH was obtained by slowly evaporating a solution mixture consisting of 1 (5 mg) and 6 mL CH2Cl2/C2H5OH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
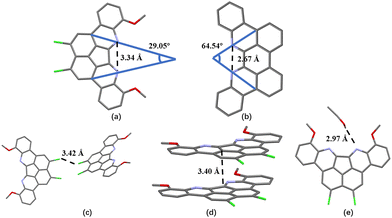
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The interatomic distance between two nitrogen atoms on 1 is 3.34 Å, representing a 25% increase compared to the distance on TB(phen) (2.67 Å). The angle formed with the intersection of the lines connecting the N atom and the carbon atom on the 4-site of the pyridinyl group is utilized to determine the angle of the nitrogen electron pair. The angle for 1 or TB(phen) is 29.05° and 64.54°, respectively (Fig. 2a and b). A neutral halogen bonding interaction between two adjacent 1 molecules occurred, with an intermolecular atomic distance of 3.42 Å (Fig. 2c). Additionally, 1 can bind to another neighbouring 1via π–π donor acceptor interaction, with the intermolecular C–C atomic distance as 3.40 Å (Fig. 2d). The distance between the nitrogen atom on 1 and the oxygen atom on the C2H5OH is 2.97 Å. This distance means that each 1 can form a hydrogen bonding contact with an ethanol molecule (Fig. 2e).
1, v/v). The interatomic distance between two nitrogen atoms on 1 is 3.34 Å, representing a 25% increase compared to the distance on TB(phen) (2.67 Å). The angle formed with the intersection of the lines connecting the N atom and the carbon atom on the 4-site of the pyridinyl group is utilized to determine the angle of the nitrogen electron pair. The angle for 1 or TB(phen) is 29.05° and 64.54°, respectively (Fig. 2a and b). A neutral halogen bonding interaction between two adjacent 1 molecules occurred, with an intermolecular atomic distance of 3.42 Å (Fig. 2c). Additionally, 1 can bind to another neighbouring 1via π–π donor acceptor interaction, with the intermolecular C–C atomic distance as 3.40 Å (Fig. 2d). The distance between the nitrogen atom on 1 and the oxygen atom on the C2H5OH is 2.97 Å. This distance means that each 1 can form a hydrogen bonding contact with an ethanol molecule (Fig. 2e).
Although 1 is not soluble in tetrahydrofuran (THF) or carbon disulfide (CS2), it is soluble in CH2Cl2. In the following study, a solvent system of CH2Cl2/CH3OH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
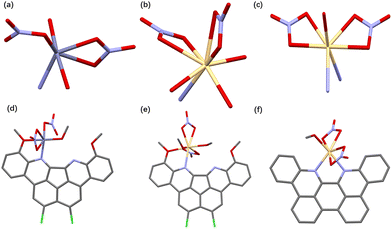
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was utilized to dissolve both the receptor (1) and substrates (e.g., metal salts), respectively. 1 displays three distinct UV-vis absorption peaks at wavelengths of 400 nm, 425 nm, and 450 nm. These peaks correspond to molar absorptivity (ε) values of 1.12 × 104 M−1 cm−1, 8.54 × 103 M−1 cm−1 and 8.16 × 103 M−1 cm−1, respectively. The maximum ε values of TB(phen) are found under the same conditions at longer wavelengths, specifically as 1.50 × 104 M−1 cm−1 (398 nm), 2.36 × 104 M−1 cm−1 (427 nm) and 2.92 × 104 M−1 cm−1 (451 nm). Excitation at a wavelength of 400 nm in the same solvent generates a single emission peak at 505 nm (Fig. 3). The fluorescence lifetime (τ) of 1 is 3.44 ns, and the fluorescence quantum yield (ΦF) is 0.20. The fluorescence of TB(phen) was measured under identical conditions, with ΦF as 0.49, τ as 3.51 ns, and a maximum value at 515 nm (Fig. S50, ESI†).
1, v/v) was utilized to dissolve both the receptor (1) and substrates (e.g., metal salts), respectively. 1 displays three distinct UV-vis absorption peaks at wavelengths of 400 nm, 425 nm, and 450 nm. These peaks correspond to molar absorptivity (ε) values of 1.12 × 104 M−1 cm−1, 8.54 × 103 M−1 cm−1 and 8.16 × 103 M−1 cm−1, respectively. The maximum ε values of TB(phen) are found under the same conditions at longer wavelengths, specifically as 1.50 × 104 M−1 cm−1 (398 nm), 2.36 × 104 M−1 cm−1 (427 nm) and 2.92 × 104 M−1 cm−1 (451 nm). Excitation at a wavelength of 400 nm in the same solvent generates a single emission peak at 505 nm (Fig. 3). The fluorescence lifetime (τ) of 1 is 3.44 ns, and the fluorescence quantum yield (ΦF) is 0.20. The fluorescence of TB(phen) was measured under identical conditions, with ΦF as 0.49, τ as 3.51 ns, and a maximum value at 515 nm (Fig. S50, ESI†).
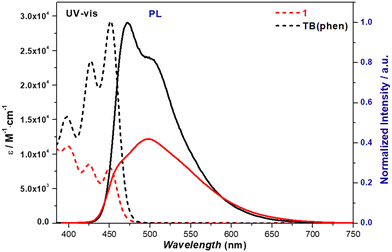 | ||
Fig. 3 UV-vis (dashed lines) and fluorescence emission spectra (solid lines) corresponding to 1 (red) and TB(phen) (black) (each concentration as 0.020 mM, λex = 400 nm) in CH2Cl2/CH3OH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1, v/v). | ||
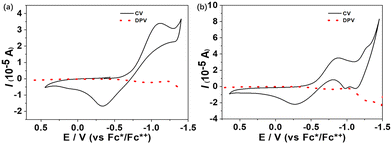
The electrochemical properties of 1 and TB(phen) in CH2Cl2/CH3OH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) were evaluated using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Multistage redox behaviors were observed (Fig. 4). 1 exhibited a quasi-reversible reduction process with an E1/2 value of −0.73 V. TB(phen) displayed two quasi-reversible reduction processes (Ere11/2 = −0.57 V, Ere21/2 = −1.20 V).
1, v/v) were evaluated using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Multistage redox behaviors were observed (Fig. 4). 1 exhibited a quasi-reversible reduction process with an E1/2 value of −0.73 V. TB(phen) displayed two quasi-reversible reduction processes (Ere11/2 = −0.57 V, Ere21/2 = −1.20 V).
In order to gain a deeper understanding of the spectra and electrochemical differences between 1 and TB(phen), density functional theory (DFT) calculations were carried out at the B3LYP/6-31G(d) level. The results suggest that the absorption peak observed at a wavelength of 450 nm can be attributed to the transition from the ground state (S0) to the first excited state (S1), which corresponds to the transition from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). The respective oscillator strength (f) values were determined to be 0.3643 and 0.6236. The incorporation of a pentatomic carbon ring conjugation and the substituents led to similar energy gap values between the HOMO and LUMO of 1 and TB(phen) (Fig. S71 and S72, ESI†). This is consistent with the observation that the wavelengths of their longest absorption peaks were comparable.
The UV-vis test demonstrated that compound 1 exhibits selective response towards Co2+, Ni2+, Cu2+, Zn2+, Ag+, Zr4+ and Cd2+ out of the tested 32 metal cations. These metal cations include alkali, alkaline earth, transition, and lanthanide cations. All the metal cations were explored in the form of their NO3− salts, except for AgBF4, ZrCl4 and NiCl2 (Fig. S20–S34, ESI†). In order to examine the specific modes of the complexation between 1 and metal cations, an initial investigation was carried out using Cd2+. The spectroscopic Job plot revealed a maximum value at a molar fraction of 0.66 ([M]/[M] + [L]) (where L represents 1 and M represents Cd2+). It suggested that the most stable interaction between 1 and Cd2+ is best described with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (L/M) complexation (Fig. S33, ESI†). In the UV-vis titration, the absorbance of 1 decreased at wavelengths less than 460 nm (known as the isosbestic point) as the concentration of Cd2+ grew. However, a shoulder peak increased at wavelengths above 460 nm (see Fig. 5a). The association constant (log
2 (L/M) complexation (Fig. S33, ESI†). In the UV-vis titration, the absorbance of 1 decreased at wavelengths less than 460 nm (known as the isosbestic point) as the concentration of Cd2+ grew. However, a shoulder peak increased at wavelengths above 460 nm (see Fig. 5a). The association constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka) was calculated via nonlinear fitting analysis as 6.1. The fluorescence intensity of 1 increased with the addition of Cd2+ in the range of 0–66 molar equiv., and then decreased in the range of 66–135 molar equiv. (Fig. 5b).
Ka) was calculated via nonlinear fitting analysis as 6.1. The fluorescence intensity of 1 increased with the addition of Cd2+ in the range of 0–66 molar equiv., and then decreased in the range of 66–135 molar equiv. (Fig. 5b).
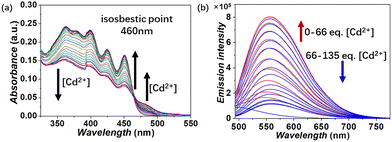 | ||
Fig. 5 UV-vis (a) and fluorescence (b) titration of 1 (2.00 × 10−5 M) with increasing Cd2+ in CH2Cl2/CH3OH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1, v/v). | ||
The interactions between 1 and the other tested metal cations, including Zr4+, Co2+, Ni2+, Cu2+, Zn2+ or Ag+, were examined using comparable methods. Table 1 provided a comparison of the binding stoichiometries and association constants (expressed as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka) for the metal cations evaluated with 1 or TB(phen). When examining the complexation of Cd2+ as an example, it was shown that its interaction with 1 or TB(phen) showed significant differences in their values (6.1 vs. 12.3). In addition, the equilibrium ratios of 1 and TB(phen) with the identical metal exhibited disparity. The equations corresponding to the equilibria, which have been calculated using the most accurate non-curve fitting method, are provided below:
Ka) for the metal cations evaluated with 1 or TB(phen). When examining the complexation of Cd2+ as an example, it was shown that its interaction with 1 or TB(phen) showed significant differences in their values (6.1 vs. 12.3). In addition, the equilibrium ratios of 1 and TB(phen) with the identical metal exhibited disparity. The equations corresponding to the equilibria, which have been calculated using the most accurate non-curve fitting method, are provided below:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v)
1, v/v)
| Metal cations |
1 [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [L] [L] |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka UV-vis Ka UV-vis |
TB(phen) [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [L] [L] |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka UV-vis Ka UV-vis |
Metal cations |
1 [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [L] [L] |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka UV-vis Ka UV-vis |
TB(phen) [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [L] [L] |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka UV-vis Ka UV-vis |
|---|---|---|---|---|---|---|---|---|---|
Only the association constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka) of the most thermostable complex of 1 was calculated due to the weak and complicated interaction (including possible solvent competition and/or the other complexations, etc.). Ka) of the most thermostable complex of 1 was calculated due to the weak and complicated interaction (including possible solvent competition and/or the other complexations, etc.). |
|||||||||
| Co2+ | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.5(3) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.3(3) | Zn2+ | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
23.6(4) | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.3(6) | Zr4+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4.1(2) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.7(5) | |||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
23.9(2) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
16.5(5) | ||||||
| Ni2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.7(2) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.8(3) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16.9(8) | |||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
23.5(2) | Ag+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.6(2) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.8(6) | |||
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.5(3) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
12.6(3) | ||||||
| Cu2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.3(2) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8.9(3) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
24.6(3) | |||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
16.2(2) | Cd2+ | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.1(3) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.3(5) | |||
| Zn2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.2(2) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.3(5) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.3(2) | |||
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.9(2) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
24.9(4) | ||||||
The interaction between 1 and metal cations was verified using electrospray ionization high resolution mass spectrometric (ESI-HRMS) analysis (Fig. S59–S63, ESI†). This indicates that the binding is also chemically stable in the gas phase.
To gain deeper insight into the coordination modes between 1 and metal cations, single crystal X-ray diffraction analysis of [1·Zn2+]·(NO3−)2·CH3OH and [1·Cd2+]·(NO3−)2·2CH3OH was carried out (Fig. 6d and e). After dissolving 1 (2 mg) and 25 molar equiv. of Zn(NO3)2·6H2O or 20 molar equiv. of Cd(NO3)2·4H2O in a mixed solvent of 4 mL ClCH2CH2Cl/CH3OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), red needle-shaped single crystal samples of [1·Zn2+]·(NO3−)2·CH3OH or [1·Cd2+]·(NO3−)2·2CH3OH were grown through gradual evaporation. Zinc and cadmium, although they are in the same group, have different coordination numbers of 6 and 8, respectively, in 1 complexation. This discrepancy emerges due to their distinct atomic sizes (Fig. 6a and b). Both complexes demonstrated metal coordination with a single nitrogen atom and an adjacent oxygen atom on 1. In order to investigate the various binding modes between 1 or TB(phen) and Cd2+, the two complex structures were compared. The Cd2+ ion in the crystal formed a 1
1, v/v), red needle-shaped single crystal samples of [1·Zn2+]·(NO3−)2·CH3OH or [1·Cd2+]·(NO3−)2·2CH3OH were grown through gradual evaporation. Zinc and cadmium, although they are in the same group, have different coordination numbers of 6 and 8, respectively, in 1 complexation. This discrepancy emerges due to their distinct atomic sizes (Fig. 6a and b). Both complexes demonstrated metal coordination with a single nitrogen atom and an adjacent oxygen atom on 1. In order to investigate the various binding modes between 1 or TB(phen) and Cd2+, the two complex structures were compared. The Cd2+ ion in the crystal formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 combination with 1. In addition, the Cd2+ ion displayed octacoordination, with two methanol molecules, two nitrate anions, and one nitrogen and one neighboring oxygen atom on 1 surrounding it. The crystal structure of [TB(phen)·Cd2+·(NO3−)2]·CH3OH, as previously described, revealed a coordination number of 7 for Cd2+. In this case, Cd2+ formed a complex with four oxygen atoms from nitrate anions, two nitrogen atoms from TB(phen), and one oxygen atom from methanol (Fig. 6c and f). Unlike the TB(phen) complex, the 1 complex has only one nitrogen atom that can coordinate with a single Cd2+ ion. The results may be due to the relationship between the longer N–N distance, the lower electron pair angle, and the substituent effect.
1 combination with 1. In addition, the Cd2+ ion displayed octacoordination, with two methanol molecules, two nitrate anions, and one nitrogen and one neighboring oxygen atom on 1 surrounding it. The crystal structure of [TB(phen)·Cd2+·(NO3−)2]·CH3OH, as previously described, revealed a coordination number of 7 for Cd2+. In this case, Cd2+ formed a complex with four oxygen atoms from nitrate anions, two nitrogen atoms from TB(phen), and one oxygen atom from methanol (Fig. 6c and f). Unlike the TB(phen) complex, the 1 complex has only one nitrogen atom that can coordinate with a single Cd2+ ion. The results may be due to the relationship between the longer N–N distance, the lower electron pair angle, and the substituent effect.
The investigation focused on the complexation process between 1 or TB(phen) and Cd2+ ions, characterized by the pseudoequilibrium (Scheme 2). Theoretical calculations were performed separately using either the MM+ force field or the PM7 method, in either a vacuum or aqueous environment. The findings were in agreement with the experimental results. More precisely, when the ratio of L to M is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the TB(phen) complex shows a stronger interaction compared to 1's. When the ratio of L to M is 2
1, the TB(phen) complex shows a stronger interaction compared to 1's. When the ratio of L to M is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, they display similar powerful interactions. When the ratio of L to M is 1
1, they display similar powerful interactions. When the ratio of L to M is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, it is observed that only the complex of 1 can exist in a stable manner (Table 2).
2, it is observed that only the complex of 1 can exist in a stable manner (Table 2).
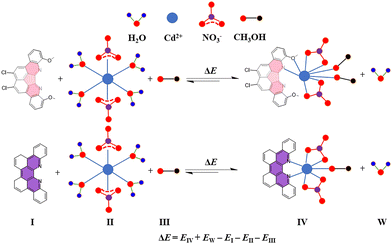 | ||
Scheme 2 Scheme presentation of the complexation equilibriums between Cd(NO3)2·4H2O and 1 or TB(phen) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. 1 ratio. | ||
| Methoda | Ligand | ΔE (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cd2+) Cd2+) |
||
|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||
| a Molecular mechanics (MM+) under vacuum and PM7 in water. Solvation is accounted for by applying a 1.65 nm × 1.65 nm × 1.65 nm periodic box. b The complex exhibits excessive instability and the structure optimization is unsuccessful. | ||||
| MM+ | 1 | 2.26 | 4.81 | −12.92 |
| TB(phen) | −7.53 | 14.03 | ||
| PM7 | 1 | −187.19 | −113.35 | −233.68 |
| TB(phen) | −274.52 | −216.18 | ||
The proton sensitivity of 1 was further investigated via carefully monitoring the changes in UV-vis or fluorescence spectra as the trifluoromethanesulfonic acid (TfOH) concentration increased (Fig. S79 and S80, ESI†). Specifically, the UV-vis spectra of compound 1 (2.00 × 10−5 M in CH2Cl2/CH3OH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v)) exhibited a drop in intensity with wavelength shorter than 462 nm (i.e., the isosbestic point). Additionally, a new broad absorption band with two distinct peaks at 515 and 550 nm was seen. 1 has an emission peak at 630 nm with the excitation wavelength at 540 nm. ΦF of the mixture containing 1 and 20 molar equiv. of TfOH was 5.8%, and τ was 1.78 ns.
1, v/v)) exhibited a drop in intensity with wavelength shorter than 462 nm (i.e., the isosbestic point). Additionally, a new broad absorption band with two distinct peaks at 515 and 550 nm was seen. 1 has an emission peak at 630 nm with the excitation wavelength at 540 nm. ΦF of the mixture containing 1 and 20 molar equiv. of TfOH was 5.8%, and τ was 1.78 ns.
In conclusion, we have described a N-doped nanographene derivative 1 as a π extended 2,2′-bipyridine, featuring a conjugation of a pentatomic carbon ring. Experimental and theoretical calculations indicated that including a pentatomic carbon ring with a conjugated structure, combined with the influence of substituents, led to substantial modifications in the overall skeleton structure. The modifications include the distribution of nitrogen atoms and the angle between electron pairs. Subsequently, the molecule can display distinctive characteristics when it interacts with metal cations or protons, as opposed to the hexatomic carbon ring conjugated TB(phen). This implies that involving a conjugated system with a non-hexatomic carbon ring can effectively regulate the structure and properties of N-doped nanographene molecules.
Prof. H.-Y. Gong is grateful to the National Natural Science Foundation of China (92156009 and 21971022), the Fundamental Research Funds for the Central Universities, the Beijing Municipal Commission of Education, and Beijing Normal University for financial support. Dr J. Bai is grateful to Dr S. Wang for the theoretical calculation.
Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Zhang, S. H. Pun and Q. Miao, Chem. Rev., 2022, 122, 14554 CrossRef CAS PubMed.
- S. Yang, M. Chu and Q. Miao, J. Mater. Chem. C, 2018, 6, 3651 RSC.
- Y.-M. Liu, Y.-Q. Huang, S.-H. Liu, D. Chen, C. Tang, Z.-L. Qiu, J. Zhu and Y.-Z. Tan, Angew. Chem., Int. Ed., 2019, 58, 13276 CrossRef CAS PubMed.
- R. Gao, Z. Liu, Z. Liu, T. Liang, J. Su and L. Gan, Angew. Chem., Int. Ed., 2023, 62, e202300151 CrossRef CAS PubMed.
- S. Sun, Z. Liu, F. Colombo, R. Gao, Y. Yu, Y. Qiu, J. Su and L. Gan, Angew. Chem., Int. Ed., 2022, 61, e202212090 CrossRef CAS PubMed.
- M. Wang, H. Shi, P. Zhang, Z. Liao, M. Wang, H. Zhong, F. Schwotzer, A. S. Nia, E. Zschech, S. Zhou, S. Kaskel, R. Dong and X. Feng, Adv. Funct. Mater., 2020, 30, 2002664 CrossRef CAS.
- G. Wang, N. Chandrasekhar, B. P. Biswal, D. Becker, S. Paasch, E. Brunner, M. Addicoat, M. Yu, R. Berger and X. Feng, Adv. Mater., 2019, 31, 1901478 CrossRef PubMed.
- Y. Fu, H. Yang, Y. Gao, L. Huang, R. Berger, J. Liu, H. Lu, Z. Cheng, S. Du, H.-J. Gao and X. Feng, Angew. Chem., Int. Ed., 2020, 59, 8873 CrossRef CAS PubMed.
- F. C. Parks, E. G. Sheetz, S. R. Stutsman, A. Lutolli, S. Debnath, K. Raghavachari and A. H. Flood, J. Am. Chem. Soc., 2022, 144, 1274 CrossRef CAS PubMed.
- W. Zhao, J. Tropp, B. Qiao, M. Pink, J. D. Azoulay and A. H. Flood, J. Am. Chem. Soc., 2020, 142, 2579 CrossRef CAS PubMed.
- G. Li, T. Matsuno, Y. Han, H. Phan, S. Wu, Q. Jiang, Y. Zou, H. Isobe and J. Wu, Angew. Chem., Int. Ed., 2020, 59, 9727 CrossRef CAS PubMed.
- J. Zhu, S. Wu, X. Hou and J. Wu, Angew. Chem., Int. Ed., 2021, 60, 25323 CrossRef CAS PubMed.
- W. Zhou, T. Sarma, L. Yang, C. Lei and J. L. Sessler, Chem. Sci., 2022, 13, 7276 RSC.
- X. Guo, Z. Yuan, Y. Zhu, Z. Li, R. Huang, Z. Xia, W. Zhang, Y. Li and J. Wang, Angew. Chem., Int. Ed., 2019, 58, 16966 CrossRef CAS PubMed.
- W.-W. Zhang, Q. Wang, S.-Z. Zhang, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2023, 62, e202214460 CrossRef CAS PubMed.
- Q. Wang, W.-W. Zhang, C. Zheng, Q. Gu and S.-L. You, J. Am. Chem. Soc., 2021, 143, 114 CrossRef CAS PubMed.
- Y. Wang, X.-S. Ke, S. Lee, S. Kang, V. M. Lynch, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2022, 144, 9212 CrossRef CAS PubMed.
- X.-Y. Wang, X. Yao, A. Narita and K. Müllen, Acc. Chem. Res., 2019, 52, 2491 CrossRef CAS PubMed.
- Y.-M. Liu, H. Hou, Y.-Z. Zhou, X.-J. Zhao, C. Tang, Y.-Z. Tan and K. Müllen, Nat. Commun., 2018, 9, 1901 CrossRef PubMed.
- A. Narita, X.-Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616 RSC.
- D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361 CrossRef CAS PubMed.
- H. Yin, C. Zhang, F. Liu and Y. Hou, Adv. Funct. Mater., 2014, 24, 2930 CrossRef CAS.
- X. Xu, T. Xia, X.-L. Chen, X. Hao, T. Liang, H.-R. Li and H.-Y. Gong, New J. Chem., 2022, 46, 11835 RSC.
- L. Zhou, B. Wu, Y. Chen, J. Gong, J. Wang, G. Dai, C. Chi and Q. Wang, Org. Lett., 2021, 23, 8640 CrossRef CAS PubMed.
- S. Banerjee, B. Senthilkumar and N. T. Patil, Org. Lett., 2019, 21, 180 CrossRef CAS PubMed.
- W. Yuan, J. Cheng, X. Li, M. Wu, Y. Han, C. Yan, G. Zou, K. Müllen and Y. Chen, Angew. Chem., Int. Ed., 2020, 59, 9940 CrossRef CAS PubMed.
- Z. Yu, G. Shi, K.-P. Wang, L.-Z. Xu, S. Chen and Z.-Q. Hu, Tetrahedron, 2023, 130, 133178 CrossRef CAS.
- R. Zibaseresht, Synth. Commun., 2020, 50, 904 CrossRef CAS.
- C. Wang, Z. Deng, D. L. Phillips and J. Liu, Angew. Chem., Int. Ed., 2023, 62, e202306890 CrossRef CAS PubMed.
- H. Luo and J. Liu, Angew. Chem., Int. Ed., 2023, 62, e202302761 CrossRef CAS PubMed.
- Y. Fei, Y. Fu, X. Bai, L. Du, Z. Li, H. Komber, K.-H. Low, S. Zhou, D. L. Phillips, X. Feng and J. Liu, J. Am. Chem. Soc., 2021, 143, 2353 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2305337, 2305338 and 2305344. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ma01082a |
| This journal is © The Royal Society of Chemistry 2024 |