Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
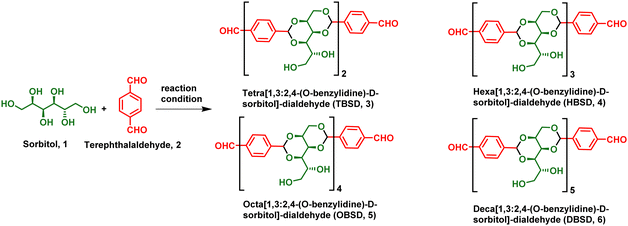
Facile synthesis and self-assembly of pharmaceutically important oligobenzylidene-D-sorbitol dialdehydes: direct encapsulation and stimuli responsive delivery of H2S†
Vara Prasad
Rebaka
,
Yogendra
Kumar
,
Tohira
Banoo
,
Arun Kumar
Rachamalla
and
Subbiah
Nagarajan
 *
*
Assembled Organic & Hybrid Materials Research Lab, Department of Chemistry, National Institute of Technology Warangal, Hanumakonda-506004, Telangana State, India. E-mail: snagarajan@nitw.ac.in
First published on 13th May 2024
Abstract
Over the past two decades, the development of stimuli-responsive drug delivery materials for healthcare applications has drawn significant attention. A detailed investigation of one of the critical endogenously produced gaseous molecules, H2S, revealed its significance in promoting tissue regeneration, angiogenesis, organelle function, stress and antioxidant effect. Even though a continuous delivery of H2S can suppress cancer cell progression, its administration as a therapeutic drug has become highly challenging because of its gaseous nature and reactivity. By considering the limitations of the existing H2S delivery, we have developed a smart, biocompatible drug delivery system for the direct delivery of H2S from bio-based resources. In this report, we have synthesized oligo[1,3:2,4-(O-benzylidene)-D-sorbitol]-dialdehyde (OBSDA) using an FDA-approved GRAS chemical and environmentally benign protocol in good yields. The ability of OBSDA to self-assemble into supramolecular gels was systematically studied in a broad range of solvents. To our fortune, OBSDA displayed gelation in N-methyl pyrrolidone (NMP), one of the FDA-approved solvents displaying a well-established safety profile and anti-inflammatory properties. Molecular level interactions responsible for the formation of the supramolecular gels have been identified using NMR and FTIR analysis. The self-assembly, mechanism, morphology and strength of the gel were systematically studied using XRD, scanning electron microscopy and rheological studies. For the first time, we report the direct in situ encapsulation of H2S into the gel formed by using a pharmaceutical solvent, NMP, by gaining input from the Purisol process. A stimuli-responsive delivery of encapsulated H2S via gel-to-sol transition has been achieved by the addition of acidic buffer. The reported precise delivery will enable future studies of the physiological role of H2S in various biological processes.
Introduction
Supramolecular chemistry refers to “chemistry beyond molecules,” which largely focuses on high-order assemblies and molecular reorganization by means of non-covalent interactions.1 Indeed, supramolecular chemistry involved in biological systems often renders inspirational concepts to the scientists to develop advanced smart materials. In particular, modern and efficient drug delivery systems involving the concept of supramolecular chemistry, such as molecular self-assembly, molecular recognition, and foldamers, receive greater attention and render a finite solution for the treatment of various diseases.2 Generally, these systems deliver active pharmaceutical ingredients like drugs, enzymes, peptides, vaccines and nutrients to a specific target in the body for an extended period of time at a controlled rate. The main advantages of supramolecular delivery systems, generally referred to as nanomedicine, include reduced side effects, controlled dosage, increased bio-availability, improved solubility and efficiency of the drug with broad administration methods.3 In pharmaceutical formulations, various drug delivery systems including liposomal formulations,4 micelles,5 nanoparticles,6 implantable systems,7 biopolymers,8 and low molecular weight gels,9 have been used for treating various diseases. In particular, clinical trials have shown that nanomedicine is pharmacologically and pharmacokinetically more efficient than the drug-alone formulation.10 It is worth mentioning that over 100 nanomedicine-based formulations have been approved by the US Food and Drug Administration (FDA) and the European Medical Agency (EMA). Doxil®, Somavert®, Avinza®, Venofer®, and Abraxane® are the representative examples of commercial lipid, polymer, nanocrystal, inorganic nanoparticle, and protein-based nanomedicine, respectively.11 In order to meet the high pharmaceutical market demand, there is a rapid growth in research and development of nanomedicine-based advancements.H2S, also known as sewer gas or swamp gas with a rotten egg odour, displays corrosiveness, flammability and toxicity. It is recognised as one of the industrial and environmental toxicants.12 In 1996, Abe and Kimura reported that the physiological concentration of H2S selectively enhances the N-methyl-D-aspartate (NMDA) receptor-mediated responses and thus is actively involved in the modulation of synaptic activities regulated by hormones and neurotransmitters.13 Since then, H2S has been widely explored for a vast array of physiological functions of the cardiovascular, gastrointestinal, excretory, nervous and immune systems.14 Recent research reveals the potential of H2S in the treatment of Alzheimer's, arthritis, strokes, Parkinson's and diabetes owing to its anti-inflammatory, antioxidant, pro-angiogenic and anti-apoptotic properties.15–19 Generally, endogenous generation of H2S is effective in carrying out various physiological functions including dietary restriction benefits.20 Direct administration of exogenous sulfide in gaseous or salt form rapidly increases its concentration in the blood, which leads to adverse effects; hence the development of drugs releasing H2S in a controlled way is quite challenging.21 The ideal drug delivery system should release H2S in a controlled manner triggered by a specific response. So many attempts have been made to develop H2S delivery systems using nanomedicine concepts (Table S1, ESI†).22–30 However, the reported methods displayed various limitations, such as tedious synthetic procedures, less loading capacity, biocompatibility and a complex delivery mechanism (Table S1, ESI†).
Among the various nanomedicine-based delivery systems, gels formed by low molecular weight gelators have attracted a broad range of interest because of their 3D-porous network, extensive loading capability, ease of tunability, biocompatibility and stimuli responsiveness.22 Low molecular weight gels are 3D network structures obtained by bottom-up assembly of small molecules using various intermolecular interactions such as H-bonding, π–π, anionic–π, cationic–π, halogen–π interactions and van der Waals forces, which are capable of immobilizing solvents through surface tension and capillary forces.31 Gels derived from small molecules render exciting architectures with unconventional coalesce of properties, which is different from solids, liquids and gases. Because of their fascinating specific physical and chemical properties, gels have applications in cell culture and release, optoelectronics, polymorph control, water purification, drug loading and delivery, and food industries.32 The ready reversibility of gels with respect to the environment enables them to behave like a smart material, where supramolecular manipulations can be triggered by external stimuli.
Matson and co-workers have developed a hydrogel using S-aroylthiooxime conjugated peptides, which can deliver H2S by the decomposition of the S-aroylthiooxime moiety.33–37 Liang et al. have generated an H2S prodrug by grafting 2-aminopyridine-5-thiocarboxamide on partially oxidized alginate, which upon decomposition releases H2S endogenously.38 Recently, Nagarajan and co-workers have developed an enolizable amphiphilic gel from renewable resources and used it for the direct delivery of H2S, wherein the reaction of Na2S and water generated H2S.39 Hyaluronic acid-based hydrogel has been used as a matrix for encapsulating H2S donors, which are released through pH response for repairing wounds on skin.40 In the recent past, various polymer conjugated H2S prodrugs, such as dithiolethiones (DTTS), aryl thioamides, acyl protected perthiols, dialkyl trisulfide, dialkyl tetrasulfide, polysulfides, N-benzoylthiobenzamides N-thiocarboxyanhydrides, Na2S, S-aroylthiooximes (SATOs), Lawesson reagents (phosphonamidoodithiolates), geminal dithiols, thioether ketones, thiocarbamates, tarbanothioates and carbamothioates, have been developed.41 However, controlled release is achieved by a complex chemical/enzymatic process. The main reason for the evolution of the prodrug concept in H2S delivery is because of the poor encapsulation of H2S in solid, liquid and gel phases. A careful analysis of the molecular structure of the H2S prodrug and the release mechanism shows that the overall release of H2S would be very low. In order to achieve the desired concentration of H2S release, a huge amount of H2S prodrug loading is necessitated.
In order to overcome the existing limitations, we have developed an oligo[1,3:2,4-(O-benzylidene)-D-sorbitol]-dialdehyde (OBSDA) based organogelator as a H2S drug delivery system. The idea of developing OBSDA originated from 1,3:2,4-di-(O-benzylidene)-D-sorbitol (DBS), which is one of the well-known chiral amphiphiles prone to form a gel in a broad range of solvents for over a century. In particular, DBS-based gelators have gained attention among researchers because of their bio-compatibility, facile bottom-up assembly in a broad range of solvents, multi-functionality and multi-responsive behaviour.42
For the synthesis of OBSDA, we have used sorbitol as one of the starting materials because of its natural abundance, biodegradability, biocompatibility and eco-friendliness. Sorbitol is a sugar substitute providing dietary energy with an E number of E420 and is also classified as generally recognised as safe (GRAS) by the FDA. Sorbitol is often used in medicine, health care, food and cosmetics.43 Another starting material, terephthalaldehyde, used for the synthesis of OBSDA facilitates π–π stacking interactions. In this report, we have synthesized OBSDA from biologically significant starting materials using a simple protocol in good yield. Bottom-up assembly of OBSDA in polar solvent furnished a 3D network structure, wherein the polar solvents are trapped. To our fortune, OBSDA formed a gel in N-methyl pyrrolidone (NMP), a widely used pharmaceutical solvent displaying bioactive and anti-inflammatory properties. In addition, NMP is a widely preferred solvent for solubilizing many commercial drugs and it has the tendency to enhance the drug penetration in humans as well as animals.44 The famous Purisol process is adopted in petrochemical industries to selectively remove H2S from syn gas using NMP solvent.45 Inspired by the Prusiol process, we have directly encapsulated H2S in the OBSDA gel formed in NMP solvent and demonstrated controlled stimuli-responsive delivery. A plausible self-assembly and pH as a stimuli-responsive H2S release mechanism have been investigated. This is the first report on the direct encapsulation of H2S in a low molecular weight gel system comprising biologically significant materials.
Results and discussion
DBS gels derived from sorbitol display potential applications in pharmaceuticals, food industries, and personal care products. In pharmaceuticals, DBS-based gels act as a stimuli-responsive drug delivery system for the controlled release of naproxen, rosuvastatin, ibuprofen and mesalazine.46–48 In continuation of our ongoing research in the field of fabrication of assembled organic materials and also by considering the potential application of DBS, we envisioned to synthesize an analogue of DBS, i.e. OBSDA, using a simple protocol. The chemistry of hydroxyl functionalization of monosaccharides has been prevalent over 100 years of history and displays a bright future because of displaying high-tech applications such as environmental remediation, tissue engineering, and the fabrication of optical and electronic materials. The chemistry of OBSDA synthesis is in a similar line with DBS, where the formation of acetal is the key step. Traditionally, Lewis acids such as AlCl3, ZnCl2, SnCl2, FeCl3 and BF3, and Brønsted acids such as p-toluenesulfonic acid, phosphoric acid, etc. were typically used for the formation of sugar acetal.49 An extensive literature search revealed that the use of Brønsted acids in excess during the reaction lowers the yield by forming impurities and enhances the purification process. In addition, the use of metal-based Lewis acids allows traces of metal ions in the product because of the strong interaction of sugar acetals with metal ions, which limits its applications on an industrial scale. Even though DBS is commercially available, the reported procedure for the synthesis of DBS did not work well in the case of OBSDA, because of the involvement of simultaneous formation of more than two acetals. In the process of identifying suitable reaction conditions to synthesize OBSDA (3–6) in good yield, we have selected various Lewis and Brønsted acids as a catalyst and a broad range of solvents. Based on the literature, our initial attempts with the use of 0.2 mol% of Lewis acids such as AlCl3, ZnCl2, SnCl2, FeCl3 and BF3.OEt2 as catalysts in MeCN at rt furnished tetrabenzylidene-disorbitol (3) in low to moderate yields (31–67%). In carbohydrate chemistry, it is well known that the use of Brønsted acids can facilitate acetal formation by the protonation of the carbonyl group of the aldehyde followed by the attack of a hydroxyl nucleophile. Among the various Brønsted acids used, p-TSA rendered tetrabenzylidene-disorbitol (3) in moderate yield (55%). To our surprise, the use of heterogeneous catalysts such as Dowex and Amberlyst at room temperature furnished HBSD 4 with 72 and 78% yield, respectively (Table 1). In order to enforce the reaction, the reactivity of all the catalysts was studied at elevated temperature and prolonged reaction time. Unfortunately, the Lewis acids selected for the investigation did not render a satisfactory result. From careful analysis of the reaction mechanism of acetal formation in sorbitol, metal ions in the Lewis acid are entrapped in the benzylidene sorbitol moiety by means of weak van der Waals forces, and hence are not readily available for the further activation of the carbonyl group of pendent aldehyde groups. It is worth mentioning that there is no further progress of the reaction in the case of increasing mol% of Lewis acid. Subsequently, acetal formation in sorbitol using terephthaldehyde in heterogeneous catalysts at 50 °C delivered encouraging results. It is noteworthy to mention that the reaction at elevated temperature furnishes higher analogues of the benzylidene sorbitol product.| Entry | Catalyst | Mol% | Temp. (°C) | Solvent | Time (h) | DBSD | TBSD (3) | HBSD (4) | OBSD (5) | DBSD (6) | Product/reaction nature |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AlCl3 | 0.2 | rt | MeCN | 12 | Traces | 31 | — | — | — | |
| 2 | ZnCl2 | 0.2 | rt | MeCN | 12 | Traces | 45 | — | — | — | |
| 3 | SnCl2 | 0.2 | rt | MeCN | 12 | Traces | 14 | — | — | — | |
| 4 | FeCl3 | 0.2 | rt | MeCN | 12 | Traces | 23 | — | — | — | |
| 5 | BF3.OEt2 | 0.2 | rt | MeCN | 12 | Traces | 62 | ||||
| 6 | pTSA | 0.2 | rt | MeCN | 12 | Traces | 55 | ||||
| 7 | H3PO4 | 0.2 | rt | MeCN | 12 | Traces | Traces | — | |||
| 8 | CH3COOH | 0.2 | rt | MeCN | 12 | Traces | Traces | Sluggish | |||
| 9 | HCl | 0.2 | rt | MeCN | 12 | — | — | Decomposition | |||
| 10 | Dowex | — | rt | MeCN | 12 | Traces | 72 | Traces | |||
| 11 | Amberlyst | — | rt | MeCN | 12 | Traces | 78 | Traces | |||
| 12 | AlCl3 | 0.2 | 50 | MeCN | 12 | Traces | 25 | 12 | — | — | |
| 13 | ZnCl2 | 0.2 | 50 | MeCN | 12 | Traces | 32 | Traces | — | — | |
| 14 | SnCl2 | 0.2 | 50 | MeCN | 12 | Traces | Traces | Traces | — | — | |
| 15 | FeCl3 | 0.2 | 50 | MeCN | 12 | Traces | Traces | Traces | — | — | |
| 16 | BF3.OEt2 | 0.2 | 50 | MeCN | 12 | Traces | 52 | 16 | |||
| 17 | pTSA | 0.2 | 50 | MeCN | 12 | Traces | 38 | 23 | |||
| 18 | H3PO4 | 0.2 | 50 | MeCN | 12 | Traces | Traces | — | |||
| 19 | CH3COOH | 0.2 | 50 | MeCN | 12 | Traces | Traces | Sluggish | |||
| 20 | HCl | 0.2 | 50 | MeCN | 12 | — | — | Decomposition | |||
| 21 | Dowex | — | 50 | MeCN | 12 | Traces | 63 | 17 | |||
| 22 | Amberlyst | — | 50 | MeCN | 12 | Traces | 59 | 15 | |||
| 23 | Dowex | — | 70 | MeCN | 12 | Traces | 45 | 36 | |||
| 24 | Amberlyst | — | 70 | MeCN | 12 | Traces | 39 | 40 | |||
| 25 | Dowex | — | Reflux | MeCN | 12 | Traces | 35 | 42 | Traces | ||
| 26 | Amberlyst | — | Reflux | MeCN | 12 | Traces | 31 | 48 | Traces | ||
| 27 | Dowex | — | Reflux | MeCN | 16 | Traces | 16 | 53 | Traces | ||
| 28 | Amberlyst | — | Reflux | MeCN | 16 | Traces | 24 | 57 | Traces | ||
| 29 | Dowex | — | Reflux | MeCN | 20 | Traces | Traces | 64 | Traces | ||
| 30 | Amberlyst | — | Reflux | MeCN | 20 | Traces | Traces | 67 | Traces | ||
| 31 | Dowex | — | Reflux | MeCN | 24 | Traces | 77 | Traces | Traces | ||
| 32 | Amberlyst | — | Reflux | MeCN | 24 | Traces | 79 | Traces | Traces | ||
| 33 | Dowex | — | Reflux | MeCN | 32 | 65 | Traces | Traces | |||
| 34 | Amberlyst | — | Reflux | MeCN | 32 | 59 | Traces | Traces | |||
| 35 | Dowex | — | Reflux | MeCN | 60 | 18 | 37 | Traces | |||
| 36 | Amberlyst | — | Reflux | MeCN | 60 | 16 | 45 | Traces | |||
| 37 | Dowex | — | Reflux | MeOH | 60 | 18 | 21 | Traces | |||
| 38 | Amberlyst | — | Reflux | MeOH | 60 | 16 | 27 | Traces | |||
| 39 | Dowex | — | Reflux | Cyrene | 60 | 67 | 13 | ||||
| 40 | Amberlyst | — | Reflux | Cyrene | 60 | 72 | 11 | ||||
Having optimized the catalysts, Dowex and amberlyst, the increase in temperature and time render HBSD 4 and OBSD 5. The change of solvents did not provide satisfactory results. Altogether, the use of heterogeneous catalysts such as Dowex and amberlyst at different temperatures and varying times can furnish one of the TBSD, HBSD or OBSD as a major product.
During the progress of the reaction, the acetal product formed is separated as a precipitate or gelatinous substance. After completion of the reaction as identified by TLC, the precipitate is dissolved in a hot cyrene solvent, and the heterogeneous catalyst is separated by filtration. The cyrene solution containing the desired compound is kept under refrigeration for crystallization. The yield denoted in Table 1 is after recrystallization. In order to identify the number of oligomeric units formed during the reaction, 1H-NMR and mass spectral techniques were used. Altogether, we were able to synthesize OBSDA oligomers of 3, 4, 5 and 6 units successfully using Dowex and amberlyst catalysts (Table 1).
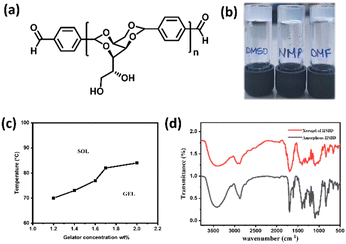
Generally, the reaction of hexose sugar, sorbitol with various aldehydes furnishes the corresponding acetal-based amphiphiles displaying a butterfly-like conformation. The sorbitol moiety is considered as the backbone and the pendant groups attached to it are considered as wings. Bottom-up assembly of butterfly-shaped amphiphiles depends upon the wings, which significantly expand the potential and scope in materials applications. In the current research, the wings were systematically expanded by conjugation with a suitable sorbitol or sorbitol acetals. In an attempt to understand the bottom-up assembly process of the newly synthesized compound HBSD 4, we have performed gelation studies in a broad range of solvents and vegetable oils. The gelation behaviour of various sorbitol acetals bearing varying degrees of H-bonding and π–π stacking units was studied by the stable to inversion method, which involves the dissolution of the gelator in a suitable solvent by heating followed by cooling to room temperature. While cooling, bottom-up assembly of molecules generates the desired architecture with greater precision and error correction capacity by means of H-bonding and π–π interactions (Table S1, ESI†). Interestingly, these compounds display gelation in polar aprotic solvents such as DMSO, DMF and NMP with the critical gelation concentration (CGC) of 0.6–1.5% (wt/v) (Table S2, ESI†). Representative images of the gel formed in DMSO, DMF and NMP are given in Fig. 1.
NMP is one of the well-known pharmaceutical solvents used for commercial drug formulations. Investigation of the gelation behaviour of compounds 3–6 revealed that the tuning of the wing portion by conjugation with benzylidene sorbitol influences the gelation behaviour and assembly pattern. Furthermore, compounds 3–6 were insoluble in most of the non-polar solvents and vegetable oils, whereas a complete or partial dissolution was observed in polar protic solvents. To our fortune, compounds 3–6 displayed excellent gelation in polar aprotic solvents such as DMSO, DMF and NMP. The use of a mixture of solvents such as DMSO, DMF and NMP along with water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for the gelation studies substantially alters the intermolecular interactions, and thereby molecules failed to form a supramolecular architecture by means of a bottom-up assembly process. These results clearly reveal that while forming the gel, the introduction of a proton donor interferes with the molecular aggregation, which results in precipitation. At this point, this class of molecules is prone to form a gel in a specific range of solvents, where it can achieve dissolution as a result of intermolecular interactions and solvent balancing in the bottom-up assembly process. Among the various solvents displaying gelation, NMP is widely used in various industries and pharmaceuticals, and in particular, it is a versatile water-miscible polar aprotic solvent displaying drug solubilization and penetration in humans and animals. The NMP solvent exhibits a well-established safety profile and anti-inflammatory properties.44,50 Hence, our further research focuses on the gel formed in a pharmaceutical solvent, NMP. Purisol is a physical absorption process employed in chemical industries to remove H2S and CO2 from various natural gas streams, crude oil, etc., by using NMP solvent. The high absorption capacity and high boiling point of the NMP solvent allow the removal of acid gases at high pressure and high temperature. A literature review revealed that the Purisol process utilizing NMP is one of the important methods adopted for the purification of syngas at high pressure because of its high selectivity towards H2S. By gaining clues from the literature, we have encapsulated H2S into a gel matrix and studied its release profile.
1 ratio for the gelation studies substantially alters the intermolecular interactions, and thereby molecules failed to form a supramolecular architecture by means of a bottom-up assembly process. These results clearly reveal that while forming the gel, the introduction of a proton donor interferes with the molecular aggregation, which results in precipitation. At this point, this class of molecules is prone to form a gel in a specific range of solvents, where it can achieve dissolution as a result of intermolecular interactions and solvent balancing in the bottom-up assembly process. Among the various solvents displaying gelation, NMP is widely used in various industries and pharmaceuticals, and in particular, it is a versatile water-miscible polar aprotic solvent displaying drug solubilization and penetration in humans and animals. The NMP solvent exhibits a well-established safety profile and anti-inflammatory properties.44,50 Hence, our further research focuses on the gel formed in a pharmaceutical solvent, NMP. Purisol is a physical absorption process employed in chemical industries to remove H2S and CO2 from various natural gas streams, crude oil, etc., by using NMP solvent. The high absorption capacity and high boiling point of the NMP solvent allow the removal of acid gases at high pressure and high temperature. A literature review revealed that the Purisol process utilizing NMP is one of the important methods adopted for the purification of syngas at high pressure because of its high selectivity towards H2S. By gaining clues from the literature, we have encapsulated H2S into a gel matrix and studied its release profile.
It is worth mentioning that the organogel formed by HBSD 4 is stable under heating and cooling cycles from rt to 70 °C. Heating the gel above 120 °C resulted in precipitation, which is attributed to the cleavage of the acetal moiety of HBSD 4. The existence of intermolecular interactions in the process of self-assembly can be studied by using Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and nuclear magnetic resonance (NMR) studies. FTIR spectroscopy provides insights into various molecular interactions that give rise to self-assembled 3D supramolecular architectures. The FTIR spectrum of amorphous HBSD and HBSD xerogels in NMP solvent is shown in Fig. 1. HBSD in an amorphous state displays O–H stretching vibrations at 3428 cm−1 and aldehyde carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching at 1699 cm−1. After undergoing self-assembly in NMP solvent, the HBSD xerogels displayed O–H stretching vibrations at 3410 cm−1, and aldehyde (C
O) stretching at 1699 cm−1. After undergoing self-assembly in NMP solvent, the HBSD xerogels displayed O–H stretching vibrations at 3410 cm−1, and aldehyde (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching at 1695 cm−1, which implies the involvement of hydroxy groups and aldehyde carbonyl groups in the process of H-bonding-mediated self-assembly of molecules into 3D architectures.
O) stretching at 1695 cm−1, which implies the involvement of hydroxy groups and aldehyde carbonyl groups in the process of H-bonding-mediated self-assembly of molecules into 3D architectures.
Further evidence of the self-assembly process is probed from variable temperature 1H-NMR spectral studies. 1H-NMR of HBSD in DMSO at various temperatures is displayed in Fig. 2. Variable temperature 1H-NMR spectral studies revealed that upon increase in temperature, an upfield shift in signals corresponding to the proton of the methylene and free hydroxy groups from δ = 4.84, 4.68, 4.41 ppm to δ = 4.41, 4.30 and 4.05 was observed. In a complex system like carbohydrates, it is very difficult to understand the electronic nature of various groups in free and assembled states. In the assembled state, because of the involvement of –OH groups in intermolecular H-bonding, the electron density decreases and hence the respective proton generates a relatively lower induced magnetic field. Owing to the consequences of the induced magnetic field, the protons of methylene and hydroxyl groups feel a higher magnitude of the applied magnetic field and hence relatively higher frequency is required for the resonance. However, upon disassembly, these protons display an upfield shift because of the disturbance in the intermolecular interaction. Increased electron density in the aggregated π-conjugated systems is fairly known in the literature, which is considered as one of the basic criteria for the development of organic electrons and sensors. However, the influence of aggregation in the σ-system is not known to date, and such investigations are very important to study the structure-function activity of complex biological systems. VT 1H-NMR studies revealed that upon molecular assembly, the CH protons of the carbohydrate moiety resonated at lower frequencies and upon gel-to-sol transition influenced by the heating displayed a downfield shift in protons (Fig. 2). This demonstrates the involvement of hydroxy groups in the self-assembly process by means of H-bonding.
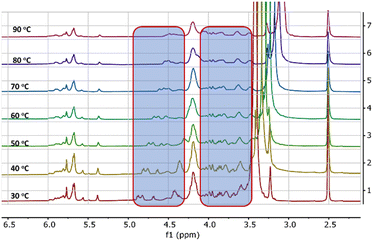 | ||
| Fig. 2 Variable temperature 1H-NMR spectra of the gel formed by HBSD in DMSO-d6. The shift of proton peaks is highlighted in the blue box. | ||
The mechanical characteristics of the HBSD gel formed in NMP were investigated by studying its viscoelastic properties using a rheometer. Gels undergo deformation in response to stress or strain, which depends upon the intermolecular interactions governing the supramolecular architecture. Investigation of the flow characteristics of gel with respect to strain amplitude sweep and angular frequency sweep in terms of storage modulus(G′) and loss modulus(G′′) is very crucial for biomedical applications. In Fig. 3, throughout the frequency sweep measurements of the HBSD gel, it was consistently observed that the storage modulus G′ remained higher than the loss modulus G′′; this indicates that the HBSD gel exhibits stability and resilience towards external forces. The amplitude sweep measurements distinctly demonstrate that the HBSD gels show gel-like behaviour (G′ > G′′) up to a critical strain level of 0.02%. After this point, a decrease in G′ and G′′ indicates the onset of fluid-like behaviour in the gels. After assessing the gel's capacity to endure external forces, we proceeded to evaluate the processability of the gels through continuous temperature ramp-up and ramp-down experiments conducted within the temperature range of 23 °C to 45 °C. This study demonstrates that these gels undergo disassembly upon heating and then reassemble the gel network upon cooling, driven by reversible non-covalent interactions. Typically, the thixotropic nature of the gels provides insight into their capacity to restore the structural integrity that may have been compromised due to the application of different strains to the gel. After applying a constant strain of 100%, the HBSD gel experienced a reduction in strength. However, upon decreasing the strain to 0.1%, the viscosity of the gels returned to the initial state. Through a sequence of three successive cycles of continuous strain ramp-up and ramp-down experiments, we observed a gradual deterioration in gel structural integrity as the strain increased. However, the original state promptly recovered as the strain was eased back to 0.1%.
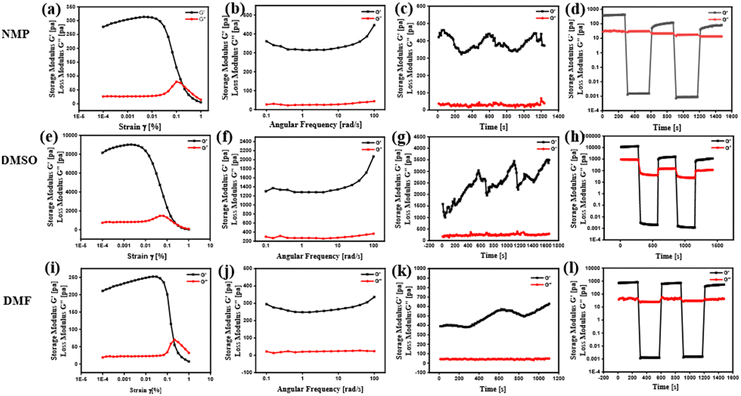 | ||
| Fig. 3 Frequency sweep, amplitude sweep, thixotropy and temperature ramp up studies of the HBSD gel formed in NMP (a)–(d), DMSO (e)–(h) and DMF (i)–(l), respectively. | ||
During the process of self-assembly, the way in which individual molecules are interconnected to create well-ordered 3D architectures such as fibers, tubules, helices, lamellae, twisted fibers, micelles, and vesicles is of utmost significance. These organized patterns contributed distinctive attributes to the resulting soft materials. Scanning electron microscopy is employed to investigate the morphology of the self-assembled gels. Fig. 4 shows that HBSD gels in NMP display fibre like structures in the self-assembled state.
Regardless of its foul-smelling and toxic nature, H2S is an important gasotransmitter in the neurosystem. Initially the biological properties of H2S were investigated by direct administration of NaHS and Na2S, but the main drawback in direct administration is the rapid increase in the concentration of H2S and then it declines rapidly, which causes an inflammatory response. The development of H2S based drugs is quite challenging because of its volatility and rapid metabolism. Hence, a controlled H2S delivery system is necessary. Regulation of H2S delivery has become a prominent area for research since the last few decades. Many types of drug delivery systems from complex macromolecular architectures to nanofiber systems have been used to deliver H2S. Among these drug delivery systems, gels have attracted a wide range of interest because of their 3D-porous network, biodegradability, and stimuli responsive gel–sol transition. In a recent study, Arunan and his colleagues utilized microwave spectroscopy to confirm the existence of hydrogen bonding in H2S,51 which made us curious to establish a new method to encapsulate H2S through an in situ gelation process.
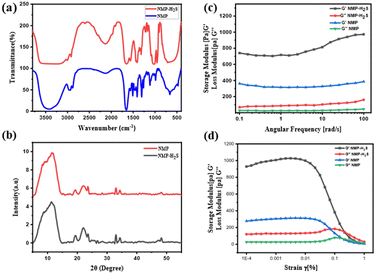
By considering its solubility with H2S gas, we have engineered a drug delivery protocol in which H2S is directly injected into a solution of HBSD dissolved in NMP. This results in a self-assembly process that leads to gel formation, effectively entrapping the H2S gas. Entrapment of H2S is greater in HBSD gel formed in NMP because of dipole–dipole interaction, acid–base interaction, and size and polarizability of H2S with NMP, in addition to the complementary H-bonding extended by the gelator and NMP. Injecting H2S into HBSD solution in NMP has accelerated the self-assembly process and gel formed quickly. The entrapment of H2S can be visually confirmed by the colour change of the HBSD gel from white to yellow. To investigate the self-assembly mechanism after the injection of H2S, we have recorded FTIR spectra for the xerogel of HBSD before and after injection of H2S (Fig. 5a). In the FTIR spectra, the peak observed at 2602 cm−1 represents the S–H stretching frequency, confirming the encapsulation of H2S in the gel matrix, which is absent in the HBSD xerogel. A comparison of O–H stretching vibrations in HBSD and H2S encapsulated xerogels revealed the engagement of hydroxyl groups in H-bonding with H2S. It is worth mentioning that the increase in H-bonding with H2S substantially increases the strength of the gels, which is clearly demonstrated by angular frequency and amplitude sweep experiments (Fig. 5c and d). The storage modulus of the H2S encapsulated gel is higher than the storage modulus of HBSD gel, implying an increase in the strength of the gel upon forming H-bonding with H2S.
Molecular packing of HBSD and H2S encapsulated gels was identified using XRD analysis and the HBSD xerogels displayed peaks at 2θ = 8.28, 10.80, 12.5, 19.29, 22.10, 23.59, 32.97, 34.29, 28.36, 36.48 and 48.19, corresponding to the d-spacing (interplanar spacing) of 10.66, 8.17, 7.15, 4.59, 4.01, 3.76, 2.71, 2.61, 3.14, 2.46 and 1.88 nm, respectively. However, xerogels of HBSD-H2S displayed peaks at 2θ = 8.85, 11.79, 19.37, 22.08, 23.63, 32.97, 34.30, 47.03, 47.51 and 48.24, which correspond to the d-spacing of 9.97, 7.49, 4.57, 4.021, 3.76, 2.71, 2.61, 1.93, 1.91 and 1.88 nm, respectively. The notable variation in the d-spacing of the molecular structures, observed before and after H2S injection, provides strong evidence for the interaction between H2S and HBSD (Fig. 5b).
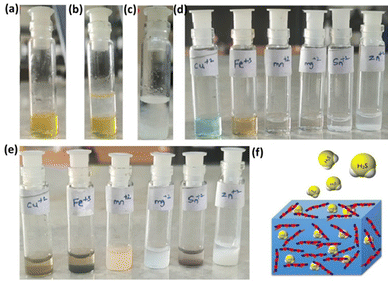
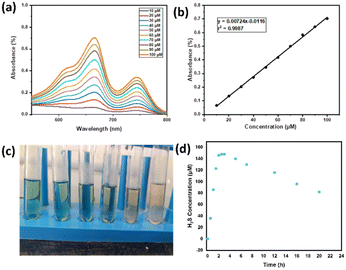
Having established the interactions of H2S in the HBSD gel, we have investigated the stability of these gels at different pH conditions. Initial H2S release studies at neutral pH displayed the stability of the HBSD gel towards distilled water. It is worth mentioning that a little release of H2S is observed because of the exchange of entrapped NMP in the HBSD gel by water molecules rather than a rapid disintegration. It is observed that at pH = 4.0, the gel slowly disintegrates and releases H2S, whereas at neutral and basic pH levels, the gel retains its stability. Having obtained an interesting result, we were curious to propose a plausible mechanism. Visual observation and NMR spectral studies revealed that the release of H2S is due to disruption of H-bonding, visualized by the conversion of the translucent gel into an opaque soft gel and the slow hydrolysis of acetal groups present in the HBSD gelator, which in turn collapses the structural integrity of the self-assembled fibrillar network. During the process of disassembly of the H2S entrapped HBSD gel, H2S gas trapped in the fibrillar network is released. Acetyl-based gels have great potential in controlled delivery of drugs and active pharmaceutical ingredients. As shown in Fig. 6, when a buffer solution of pH 4.0 is added on top of the HBSD-H2S gel, disassembly of the gel occurs, and thereby gradual release of H2S is observed. The deformation of the gel with a significant colour change in buffer solution is physically visible; however, the release of H2S is systematically assessed by converting it into the respective metal sulphides. It is well known that under normal room temperature, the interaction between various metal ions and H2S often gives rise to the formation of the corresponding metal sulfides. Specifically, group II cations exhibited a tendency to form less soluble metal sulfides (with a solubility product constant, Ksp, of less than 10−30) when exposed to lower amounts of H2S. After the addition of buffer solution of pH 4.0, the supernatant liquid is retrieved and further added to a vial containing a solution of different metal ions such as Cu2+, Fe3+, Mn2+, Mg2+, Sn2+ and Zn2+ at 0.1 M concentration. The minimum concentration of H2S present in the aliquots reacting with the respective metal ions in turn generated an insoluble metal sulphide, which was confirmed by the change in colour (Fig. 6). In order to quantify the loaded H2S in the HBSD gel and their release profile, a linear regression analysis is performed by taking the absorbance values obtained from a methylene blue assay (Fig. 7a and b).53 To 1 mL of H2S entrapped HBSD gel, pH = 4 buffer solution is added, and aliquots of 0.5 mL are collected at 30-minute intervals and a methylene blue assay is performed. The interpolation of the obtained UV data with the linear regression plot revealed a slow release of H2S with respect to time and reached a maximum release after 3 h. It is worth mentioning that a slow fall in the H2S concentration is observed because of its volatility. The total amount of H2S loaded in the gel is observed as 148 μm. In contrast to other H2S donors or H2S delivery materials documented in the literature, the reported smart gel offers numerous advantages, such as uncomplicated encapsulation of H2S in the gel without necessitating any covalent bonding, achieved through a self-assembly mechanism. There is no requirement for additional H2S donors. Furthermore, the HBSD gel exhibits pH-triggered H2S delivery.
Experimental
General materials and methods
All reagents and solvents used for the synthesis of diformyl dibenzylidene sorbitol were purchased from commercial suppliers such as Merck, Aldrich, Himedia and Avra chemicals and were used without purification. We have used LR grade solvents for recrystallization and AR grade solvents for gelation studies. The progress of the reactions was monitored by thin-layer chromatography (TLC) using pre-coated Merck silica gel 60 F254 plates and visualized by UV detection or using sulfuric acid spray or molecular iodine. Melting points were recorded on Stuart SMP30 melting point apparatus in capillaries and are uncorrected. 1H- and 13C-NMR spectra were recorded on a Bruker Avance 300 MHz instrument in deuterated solvents such as D2O, DMSO-d6, CDCl3, and CDCl3 with a few drops of DMSO-d6 at room temperature. TMS is used as an internal standard and chemical shifts in NMR spectra (δ) are reported in parts per million (ppm). Multiplicity in the 1H NMR spectra of the synthesized compounds was referred to as singlet (s), doublet (d), triplet (t), quartet (q) and multiplet (m), and coupling constants (J) are given in Hz. Infrared (IR) spectra were recorded using a PerkinElmer spectrum 100 spectrophotometer from 400–4000 cm−1 using KBr. High resolution MS analysis was performed on an Agilent Q-TOF 6230 instrument by dissolving the solid sample in methanol or acetonitrile. To obtain the morphology of the gel, scanning electron microscopy is used with a JEOL JSM-6701F ultrahigh resolution field emission scanning electron microscope. The XRD measurements were taken by keeping a small portion of the xerogel in the X'pert-PRO Diffractometer system.General procedure for the synthesis of HBSD oligomers
To a stirred solution of D-sorbitol (1 mmol) in acetonitrile (10 mL) at rt under N2 atmosphere, terepthaldehyde (2 mmol) and Dowex 50WX8/Amberlyst were added. The reaction mixture was stirred for another 24 h under reflux conditions. After completion of the reaction confirmed by TLC, the reaction mixture was allowed to cool down to room temperature. The formed precipitate is dissolved in hot cyrene and separates the catalyst by filtration. The cyrene solution is refrigerated for crystallization. The catalyst was further reused in other reactions.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) O), 7.95 (d, J = 6.4 Hz, 4H, Ar
O), 7.95 (d, J = 6.4 Hz, 4H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.70 (t, J = 8.6 Hz, 4H, Ar
), 7.70 (t, J = 8.6 Hz, 4H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.57–7.42 (m, 4H, Ar
), 7.57–7.42 (m, 4H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 6.03–5.65 (m, 4H, C
), 6.03–5.65 (m, 4H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (OR)2), 4.97–4.81 (m, 1H), 4.60–4.32 (m, 3H), 4.30–4.10 (m, 6H), 4.09–3.95 (m, 2H), 3.94–3.84 (m, 1H), 3.80 (s, 1H), 3.62 (d, J = 4.9 Hz, 1H), 3.49 (dt, J = 11.9, 5.9 Hz, 1H), 3.67–3.52 (m, 1H), 3.29–3.16 (m, 3H); 13C NMR (101 MHz, DMSO) δ 193.45, 144.66, 137.23, 136.75, 130.48, 129.78, 127.82, 127.36, 126.29, 102.56, 99.54, 98.99, 78.05, 69.81, 69.02, 68.10, 63.03, 48.96, 30.58, 29.48, 17.69. ESI-MS: m/z calculated for C36H38O14 [M + Na]+ is 717.2159: found m/z = 717.2135.
(OR)2), 4.97–4.81 (m, 1H), 4.60–4.32 (m, 3H), 4.30–4.10 (m, 6H), 4.09–3.95 (m, 2H), 3.94–3.84 (m, 1H), 3.80 (s, 1H), 3.62 (d, J = 4.9 Hz, 1H), 3.49 (dt, J = 11.9, 5.9 Hz, 1H), 3.67–3.52 (m, 1H), 3.29–3.16 (m, 3H); 13C NMR (101 MHz, DMSO) δ 193.45, 144.66, 137.23, 136.75, 130.48, 129.78, 127.82, 127.36, 126.29, 102.56, 99.54, 98.99, 78.05, 69.81, 69.02, 68.10, 63.03, 48.96, 30.58, 29.48, 17.69. ESI-MS: m/z calculated for C36H38O14 [M + Na]+ is 717.2159: found m/z = 717.2135.
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) O), 7.94 (d, J = 7.8 Hz, 4H, Ar
O), 7.94 (d, J = 7.8 Hz, 4H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.77–7.65 (m, 4H, Ar
), 7.77–7.65 (m, 4H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.58–7.38 (m, 8H, Ar
), 7.58–7.38 (m, 8H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.99–5.75 (m, 3H, C
), 5.99–5.75 (m, 3H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (OR)2), 5.74–5.63 (3, 3H, C
(OR)2), 5.74–5.63 (3, 3H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (OR)2), 4.59–4.09 (m, 8H), 4.07–3.94 (m, 2H), 3.93–3.82 (m, 2H), 3.82–3.73(m, 2H), 3.72–3.52(m, 4H), 3.52–3.37 (m, 9H), 3.26–3.14(m, 3H); 13C NMR (101 MHz, DMSO) δ: 193.44, 145.00, 136.80, 129.87, 129.79, 127.39, 126.28, 102.81, 99.56, 78.12, 70.55, 69.59, 68.91, 68.14, 63.04. ESI-MS: m/z calculated for C50H54O20 [M + Na]+ is 997.3101: found m/z = 997.3100.
(OR)2), 4.59–4.09 (m, 8H), 4.07–3.94 (m, 2H), 3.93–3.82 (m, 2H), 3.82–3.73(m, 2H), 3.72–3.52(m, 4H), 3.52–3.37 (m, 9H), 3.26–3.14(m, 3H); 13C NMR (101 MHz, DMSO) δ: 193.44, 145.00, 136.80, 129.87, 129.79, 127.39, 126.28, 102.81, 99.56, 78.12, 70.55, 69.59, 68.91, 68.14, 63.04. ESI-MS: m/z calculated for C50H54O20 [M + Na]+ is 997.3101: found m/z = 997.3100.
OBSD(5): white solid; yield: 72%; 1H NMR (400 MHz, DMSO) δ 10.03 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) O), 7.94 (d, J = 7.9 Hz, 4H, Ar
O), 7.94 (d, J = 7.9 Hz, 4H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.70 (q, J = 7.1 Hz, 4H, Ar
), 7.70 (q, J = 7.1 Hz, 4H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.59–7.36(m, 12H, Ar
), 7.59–7.36(m, 12H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 6.00–5.89 (m, 1H, C
), 6.00–5.89 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (OR)2), 5.83 (d, J = 10.9 Hz, 1H, C
(OR)2), 5.83 (d, J = 10.9 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (OR)2), 5.77 (s, 1H, C
(OR)2), 5.77 (s, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (OR)2), 5.69 (q, J = 6.8 Hz, 4H, C
(OR)2), 5.69 (q, J = 6.8 Hz, 4H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (OR)2), 5.60–5.44 (m, 1H, C
(OR)2), 5.60–5.44 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (OR)2), 4.52 (s, 1H), 4.38 (s, 1H), 4.19 (q, J = 11.6 Hz, 10H), 4.05–3.94 (m, 4H), 3.90–3.74 (m, 6H), 3.73–3.65 (m, 2H), 3.59 (m, 6H), 3.45 (m, 9H), 3.17(s, 1H).
(OR)2), 4.52 (s, 1H), 4.38 (s, 1H), 4.19 (q, J = 11.6 Hz, 10H), 4.05–3.94 (m, 4H), 3.90–3.74 (m, 6H), 3.73–3.65 (m, 2H), 3.59 (m, 6H), 3.45 (m, 9H), 3.17(s, 1H).
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) O), 7.94 (d, J = 7.0 Hz, 4H, Ar
O), 7.94 (d, J = 7.0 Hz, 4H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.69 (t, J = 8.3 Hz, 4H, Ar
), 7.69 (t, J = 8.3 Hz, 4H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.60–7.35 (m, 16H, Ar
), 7.60–7.35 (m, 16H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 6.00–5.33 (m, 10H, C
), 6.00–5.33 (m, 10H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (OR)2), 4.94–4.80 (m, 2H), 4.74–4.63 (m, 1H), 4.50–4.22 (m, 6H), 4.27–4.09 (m, 13H), 4.07–3.92 (m, 5H), 3.92–3.73 (m, 6H), 3.69 (d, J = 11.4 Hz, 1H), 3.59 (s, 4H), 3.54–3.46 (m, 6H), 3.23 (s, 6H).
(OR)2), 4.94–4.80 (m, 2H), 4.74–4.63 (m, 1H), 4.50–4.22 (m, 6H), 4.27–4.09 (m, 13H), 4.07–3.92 (m, 5H), 3.92–3.73 (m, 6H), 3.69 (d, J = 11.4 Hz, 1H), 3.59 (s, 4H), 3.54–3.46 (m, 6H), 3.23 (s, 6H).
Conclusions
In summary, we have synthesized oligo[1,3:2,4-(O-benzylidene)-D-sorbitol]-dialdehyde (OBSDA) using an environmentally benign protocol in good yields. Supramolecular gelation of HBSA, one of the OBSDA compounds, displayed gelation in FDA-approved pharmaceutical solvents, N-methyl pyrrolidone (NMP). Molecular level interactions such as H-bonding and π–π stacking responsible for the formation of a supramolecular architecture have been identified using NMR and FTIR analysis. Morphology and mechanical strength analysis clearly establish the suitability of the gel for pharmaceutical applications. Owing to the volatile nature of H2S, to date researchers have utilized additional H2S donors in fabricating drug delivery systems. This report deals with the direct encapsulation of H2S gas into a gel matrix and stimuli responsive delivery via gel-to-sol transition, clearly demonstrating the suitability of NMP gel in evaluating the physiological role of H2S in various biological processes. It also has scope in transdermal drug delivery applications, as NMP has high penetrating capacity through the skin.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the SERB, India (Sanction Order No: CRG/2023/002466) and DST (Sanction Order No: SR/FST/CS-II/2018/65) is gratefully acknowledged. We also thank C Uma Maheswari, SASTRA University, and Vellaisamy Sridharan, Central University of Jammu, for data acquisition help.Notes and references
- F. Huang and E. V. Anslyn, Chem. Rev., 2015, 115, 6999–7000 CrossRef CAS PubMed.
- B. X. Jie Zhou, J. Li and X. Du, Biomaterials, 2017, 129, 1–27 CrossRef PubMed.
- A. Hardenia, N. Maheshwari, S. S. Hardenia, S. K. Dwivedi, R. Maheshwari and R. K. Tekade, Scientific rationale for designing controlled drug delivery systems, Elsevier Inc., 2018, pp. 1–28 Search PubMed.
- D. Yadav, K. Sandeep, D. Pandey and R. K. Dutta, J. Biotechnol. Biomater., 2017, 7, 276 Search PubMed.
- H. S. Kapare and S. R. Metkar, Pharm. Reson., 2020, 2, 21–26 CAS.
- A. Z. Wilczewska, K. Niemirowicz, K. H. Markiewicz and H. Car, Pharmacol Rep., 2012, 64, 1020–1037 CrossRef CAS PubMed.
- E. Chappel, Implantable drug delivery devices, Elsevier Inc., 2021, pp. 129–156 Search PubMed.
- W. B. Liechty, D. R. Kryscio, B. V. Slaughter and N. A. Peppas, Annu. Rev. Chem. Biomol. Eng., 2010, 1, 149–173 CrossRef CAS PubMed.
- Z. Li, J. Cao, H. Li, H. Liu, F. Han, Z. Liu, C. Tong and S. Li, Drug Delivery, 2016, 23, 3168–3178 CrossRef CAS PubMed.
- E. Beltrán-Gracia, A. López-Camacho, I. Higuera-Ciapara, J. B. Velázquez-Fernández and A. A. Vallejo-Cardona, Nanomedicine review: Clinical developments in liposomal applications, Springer Vienna, 2019, vol. 10 Search PubMed.
- R. K. Thapa and J. O. Kim, J. Pharm. Investig., 2023, 53, 19–33 CrossRef CAS PubMed.
- S. H. Roth, Signal Transduction Gasotransm., 2004, 293–313 CAS.
- K. Abe and H. Kimura, J. Neurosci., 1996, 16, 1066–1071 CrossRef CAS PubMed.
- R. Wang, FASEB J., 2002, 16, 1792–1798 CrossRef CAS PubMed.
- A. Katsouda, S.-I. Bibli, A. Pyriochou, C. Szabo and A. Papapetropoulos, Pharmacol. Res., 2016, 113, 175–185 CrossRef CAS PubMed.
- D. Wu, N. Luo, L. Wang, Z. Zhao, H. Bu, G. Xu, Y. Yan, X. Che, Z. Jiao, T. Zhao, J. Chen, A. Ji, Y. Li and G. D. Lee, Sci. Rep., 2017, 7, 455 CrossRef PubMed.
- R. C. O. Zanardo, V. Brancaleone, E. Distrutti, S. Fiorucci, G. Cirino, J. L. Wallace, R. C. O. Zanardo, V. Brancaleone, E. Distrutti, S. Fiorucci, G. Cirino and J. L. Wallace, FASEB J., 2006, 20, 2118–2120 CrossRef CAS PubMed.
- R. Wang, Physiol. Rev., 2012, 92, 791–896 CrossRef CAS PubMed.
- D. J. Lefer, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17907–17908 CrossRef CAS PubMed.
- C. Hine, E. Harputlugil, Y. Zhang, C. Ruckenstuhl, B. C. Lee, L. Brace, A. Longchamp, J. H. Treviño-Villarreal, P. Mejia, C. K. Ozaki, R. Wang, V. N. Gladyshev, F. Madeo, W. B. Mair and J. R. Mitchell, Cell, 2015, 160, 132–144 CrossRef CAS PubMed.
- M. Whiteman and P. G. Winyard, Expert Rev. Clin. Pharmacol., 2011, 4, 13–32 CrossRef CAS PubMed.
- W. Y. Zheng, B. Yu, L. K. De La Cruz, M. R. Choudhury, A. Anifowose and B. Wang, Med. Res. Rev., 2017, 38, 57–100 CrossRef PubMed.
- A. K. Gilbert and M. D. Pluth, J. Am. Chem. Soc., 2022, 144, 17651–17660 CrossRef CAS PubMed.
- C. R. Powell, J. C. Foster, B. Okyere, M. H. Theus and J. B. Matson, J. Am. Chem. Soc., 2016, 138, 13477–13480 CrossRef CAS PubMed.
- W. Wang, X. Sun, H. Zhang, C. Yang, Y. Liu, W. Yang, C. Guo and C. Wang, Int. J. Nanomed., 2016, 11, 3255–3263 CrossRef CAS PubMed.
- M. K. Marwah, H. Shokr, L. Sanchez-Aranguren, R. K. S. Badhan, K. Wang and S. Ahmad, Pharm. Res., 2022, 39, 341–352 CrossRef CAS PubMed.
- S. H. Yu, L. Esser, S. Y. Khor, D. Senyschyn, N. A. Veldhuis, M. R. Whittaker, F. Ercole, T. P. Davis and J. F. Quinn, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 1982–1993 CrossRef CAS.
- H. Zhang, L. Z. Hao, J. A. Pan, Q. Gao, J. F. Zhang, R. K. Kankala, S. Bin Wang, A. Z. Chen and H. L. Zhang, J. Controlled Release, 2021, 329, 286–298 CrossRef CAS PubMed.
- A. Longchamp, K. Kaur, D. Macabrey, C. Dubuis, J. M. Corpataux, S. Déglise, J. B. Matson and F. Allagnat, Acta Biomater., 2019, 97, 374–384 CrossRef CAS PubMed.
- X. Sun, Y. Wang, S. Wen, K. Huang, J. Huang, X. Chu, F. Wang and L. Pang, J. Nanobiotechnol., 2021, 19, 1–16 CrossRef PubMed.
- L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089–6102 RSC.
- E. R. Draper and D. J. Adams, Chem, 2017, 3, 390–410 CAS.
- K. Kaur, Y. Wang and B. M. John, Biomacromolecules, 2020, 21, 1171–1178 CrossRef CAS PubMed.
- Y. Qian, K. Kaur, J. C. Foster and J. B. Matson, Biomacromolecules, 2019, 20, 1077–1086 CrossRef CAS PubMed.
- Y. Wang, K. Kaur, S. J. Scannelli, R. Bitton and J. B. Matson, J. Am. Chem. Soc., 2018, 140, 14945–14951 CrossRef CAS PubMed.
- Y. Qian, A. Altamimi, S. A. Yates, S. Sarkar, M. Cochran, M. Zhou, N. Levi-Polyachenko and J. B. Matson, Biomater. Sci., 2020, 8, 2564–2576 RSC.
- M. Zhou, Y. Qian, Y. Zhu and J. Matson, Chem. Commun., 2020, 56, 1085–1088 RSC.
- W. Liang, J. Chen, L. Li, M. Li, X. Wei, B. Tan, Y. Shang, G. Fan, W. Wang and W. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 14619–14629 CrossRef CAS PubMed.
- A. Thamizhanban, K. Lalitha, G. P. Sarvepalli, C. U. Maheswari, V. Sridharan, J. B. B. Rayappan and S. Nagarajan, J. Mater. Chem. B, 2019, 7, 6238–6246 RSC.
- J. Wu, A. Chen, Y. Zhou, S. Zheng, Y. Yang, Y. An, K. Xu, H. He, J. Kang, J. A. Luckanagul, M. Xian, J. Xiao and Q. Wang, Biomaterials, 2019, 119398 CrossRef CAS PubMed.
- C. R. Powell, K. M. Dillon and J. B. Matson, Biochem. Pharmacol., 2018, 176, 110–123 CrossRef PubMed.
- B. O. Okesola, M. P. Vieira, D. J. Cornwell, N. K. Whitelaw and D. K. Smith, Soft Matter, 2015, 11, 4768–4787 RSC.
- R. C. Deis and M. W. Kearsley, Sweeteners and Sugar Alternatives in Food Technology, 2012, pp. 331–346 Search PubMed.
- A. Jouyban, M. A. A. Fakhree and A. Shayanfar, J. Pharm. Pharm. Sci., 2010, 13, 524–535 CAS.
- R. B. N. L. Kohl, Handbook of Natural Gas Transmission and Processing, 2019, pp. 231–269 Search PubMed.
- E. J. Howe, B. O. Okesola and D. K. Smith, Chem. Commun., 2015, 51, 7451–7454 RSC.
- C. C. Piras, A. K. Patterson and D. K. Smith, Chem. – Eur. J., 2021, 27, 13203–13210 CrossRef CAS PubMed.
- K. Peng, T. Sottmann and C. Stubenrauch, Mol. Phys., 2021, 119, 15–16 Search PubMed.
- B. Springs, C. Xie and J. Xia, US 2006/0079720 A1, 2006.
- M. Roche-Molina, B. Hardwick and C. Sanchez-Ramos, et al. , Sci. Rep., 2020, 10, 11636 CrossRef CAS PubMed.
- A. Das, P. K. Mandal, F. J. Lovas, C. Medcraft, N. R. Walker and E. Arunan, Angew. Chem., Int. Ed., 2018, 57, 15199–15203 CrossRef CAS PubMed.
- J. C. Foster, S. C. Radzinski, X. Zou, C. V. Finkielstein and J. B. Matson, Mol. Pharmaceutics, 2017, 14, 1300–1306 CrossRef CAS PubMed.
- S. Feng, Y. Zhao, M. Xian and Q. Wang, Acta Biomater., 2015, 27, 205–230 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma01110k |
| This journal is © The Royal Society of Chemistry 2024 |