Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Modulating mediation medium for few layered dichalcogenides enhances inhibition of common pathogens†
Ashamoni
Neog
a,
Rajib
Biswas
 *a,
Muzamil Ahmad
Rather
b,
Pritam
Bardhan
b,
Manabendra
Mandal
b and
Nirmal
Mazumder
*a,
Muzamil Ahmad
Rather
b,
Pritam
Bardhan
b,
Manabendra
Mandal
b and
Nirmal
Mazumder
 *c
*c
aDepartment Of Physics, Applied Optics and Photonics Lab, Tezpur University, Tezpur, Assam, India. E-mail: rajib@tezu.ernet.in
bDepartment Of Molecular Biology and Biotechnology, Applied Microbiology and Biotechnology, Tezpur University, Tezpur, Assam, India
cDepartment of Biophysics, Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, India. E-mail: nirmal.mazumder@manipal.edu
First published on 20th February 2024
Abstract
As mandated by the United Nations Ad hoc Interagency Coordination Group, there is a looming prospect of acute health crises and poverty by 2030 in the absence of action against microbial resistance. Nanomaterials possess the capability to disrupt pathogenic cell membranes or induce cell death through the production of reactive oxygen species and free radicals. Hence, nanomaterials have emerged as promising agents to combat the impending crises. While research on nanomaterial-based approaches for drug-resistant infections has commenced, it is imperative to conduct parallel investigations to ascertain the maximal effectiveness of nanomaterials against common pathogens. Transition metal dichalcogenides represent the next generation of antibiotics to counter common and multidrug-resistant infections. However, existing studies predominantly focus on a limited spectrum of microorganisms or pathogens, with minimal reports on their efficacy against pathogens such as Pseudomonas aeruginosa and Candida albicans. Notably, many studies have explored the functionalization, doping, or composite formation of these nanostructures to enhance their antipathogenic activity, overlooking the intrinsic antibiotic potential of the materials in their original form. Consequently, this study investigates the antipathogenic activity of non-functionalized few-layer WS2 and MoS2 nanosheets against a range of pathogens, including Mycobacterium smegmatis, Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, Yersinia pestis, Escherichia coli and Candida albicans, in lysogeny broth (LB) and potato dextrose broth (PDB) media. Remarkably, few-layer MoS2 and WS2 exhibit significant antipathogenic activity against all tested pathogens, surpassing standard antibiotics in the case of Pseudomonas aeruginosa and Candida albicans.
1. Introduction
Transition metal dichalcogenides (TMDCs) have made significant strides in various applications, including photosensing,1–3 bio and chemical sensing,4–7 future electronic and valleytronic devices,8–11 catalysis,5,12,13 wastewater treatment, and toxic gas adsorption and removal,14 among others. Despite these advancements, research on TMDCs for biomedical applications is still nascent. Only in the past two decades have researchers begun exploring the cytotoxicity of these materials.15–17 Notably, inorganic fullerene-type and few-layer structures of WS2 and MoS2 have garnered attention due to their low cytotoxicity and genotoxicity, as assessed by various biocompatibility tests.18These findings have spurred investigations into the antipathogenic activities of WS2 and MoS2. Although limited, several studies have yielded promising results. WS2 nanosheets synthesized via the hydrothermal method have exhibited significant bactericidal activity, with a mortality rate of up to 99.97% against Staphylococcus epidermidis.19 Additionally, WS2 nanosheets have shown efficacy against Escherichia coli, Salmonella typhimurium, and Bacillus subtilis at a concentration of 250 μg mL−1, as assessed using the colony counting method.19 Furthermore, the antibacterial activity of WS2 against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus was evaluated through colony-forming unit studies, resulting in nearly 0% bacterial viability at a concentration of 200 μg mL−1.20 The activity of WS2 and the WS2/ZnO nanohybrid against Candida albicans was investigated using the disc diffusion method, inhibiting fungal growth by up to 74% and 91%, respectively, at a concentration of 300 μg mL−1.21
MoS2 nanosheets synthesized via Li-intercalation exhibited a reduction in Escherichia coli viability of 91.8% ± 1.4% at a concentration of 80 μg mL−1.22 The superior performance of MoS2 nanosheets compared to bulk counterparts underscores the role of their high specific surface area and conductivity in bacterial cell destruction.22 Moreover, Li-intercalated and ligand-functionalized MoS2 nanosheets were effective against Staphylococcus aureus and Pseudomonas aeruginosa, with positively charged exfoliated MoS2 demonstrating enhanced bactericidal effects.23 Similarly, MoS2 nanosheets exfoliated through solvo-sonication displayed antibacterial activity against Salmonella and wild-type Salmonella typhimurium at a concentration of 20 μg mL−1.24
It is intriguing to note that the antipathogenic activity of transition metal dichalcogenides (TMDCs) is phase-dependent. Both the 1T and 2H phases exhibit promising antibacterial properties. The 1T phase demonstrates significant enhancement through surface functionalization, rendering it valuable for antibacterial applications. Conversely, the 2H phase of TMDCs, the semiconducting variant, plays a pivotal role in inducing oxidative stress by generating reactive oxygen species (ROS), thereby augmenting antibacterial activity. The membrane depolarization associated with 2H-MoS2 based antipathogenic activity is attributed to functionalized ligands, resulting in heightened antibacterial effectiveness. In contrast to the 1T phase, the 2H phase of TMDCs displays superior antibacterial activity against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa. This enhanced activity is ascribed to its semiconducting nature and the synergistic effect of functionalized ligands. Furthermore, the 2H phase of TMDCs, exemplified by MoS2, exhibits enhanced antibacterial activity compared to the metallic 1T phase when functionalized with thiolated ligands, showing potential antibacterial effects against both Gram-positive and Gram-negative bacterial strains. It is also reported that in comparison to commonly used antibiotics and other nanomaterial-based antibacterial agents, positively charged 2H-MoS2 demonstrates higher antibacterial efficacy at lower dosages. The synergistic effect of the 2H phase and functionalized ligands contributes to this enhanced antibacterial activity. While the 1T phase of TMDCs does exhibit antibacterial activity, it is less potent compared to the 2H phase as it is observed that positively charged 1T-MoS2 did not exhibit effective antibacterial activity against Pseudomonas aeruginosa.25
In the study on ligand-mediated exfoliation and antibacterial activity of 2H transition-metal dichalcogenides, the researchers found that the relative antibacterial activity of the functionalized 2H TMDCs tested against Gram-positive MRSA and Gram-negative Pseudomonas aeruginosa was as follows:
| MoS2 > WS2 > MoSe2 > WSe2 |
The observed differences in antibacterial activity among the TMDCs can be attributed to the generation of intracellular reactive oxygen species (ROS). This study indicated that the core material of the functionalized TMDCs plays a crucial role in ROS generation. Semiconducting TMDCs can generate ROS through processes involving hole and excited electrons, leading to the production of reactive oxygen species such as hydroxyl radicals and superoxide radicals. Furthermore, the band gap energy of the TMDCs was highlighted as a factor influencing ROS generation and antibacterial activity. TMDs with higher band gap energies, such as MoS2 and WS2, exhibited increased ROS generation compared to MoSe2 and WSe2, which have lower band gap energies. This difference in band gap energies contributed to the varying extent of antibacterial activities observed among the functionalized TMDs. Therefore, the differential antibacterial activity of the TMDs can be attributed to the core material's ability to generate intracellular ROS, influenced by factors such as band gap energy and material composition.26
While semiconducting 2H transition metal dichalcogenides (2H-TMDCs) exhibit significant antibacterial activity owing to their intrinsic material characteristics, their full potential remains largely unexplored in this regard. Again, most TMDC-based antibiotic studies have targeted Staphylococcus aureus and Escherichia coli, neglecting common pathogens such as Mycobacterium smegmatis, Bacillus cereus, and Yersinia pestis. Furthermore, research on TMDC nanostructure-based antibiotics against pathogens like Pseudomonas aeruginosa and Candida albicans is scarce.
In light of these gaps in research, an experiment was conducted to address the dearth of information regarding nanomaterial-based antibiotics. The antipathogenic activity of 2H-WS2 and 2H-MoS2 nanosheets against six pathogens, including five bacterial cultures and one fungus, was evaluated, with results compared between the two materials. Four different synthesis methods of WS2 and MoS2 were employed to elucidate key factors influencing antipathogenic activity. Antipathogenic activity was assessed using the agar well diffusion method, with all analyses performed on liquid-dispersed specimens.
2. Experimental details
2.1. Agar well diffusion assay
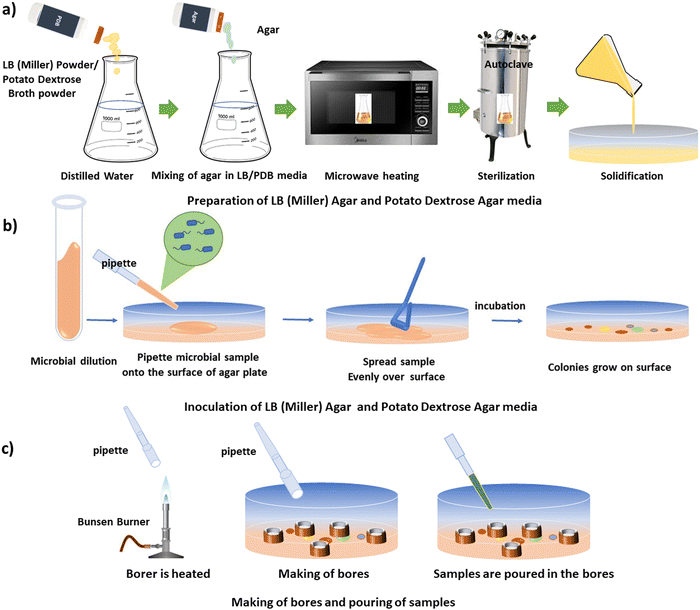
The agar well diffusion method is a commonly used antibacterial assay. The steps of this assay are discussed below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. For the preparation of LB agar media, bacteriological agar (HiMedia) was added to LB broth to achieve a final concentration of 1.8% (w/v). The mixture was then heated in a microwave for 1–2 minutes to dissolve the agar, followed by sterilization of the culture media in an autoclave at 15 psi and 121 °C for 20 minutes.
2. For the preparation of LB agar media, bacteriological agar (HiMedia) was added to LB broth to achieve a final concentration of 1.8% (w/v). The mixture was then heated in a microwave for 1–2 minutes to dissolve the agar, followed by sterilization of the culture media in an autoclave at 15 psi and 121 °C for 20 minutes.
To prepare PDB media, 12 g of potato dextrose broth powder (granulated, HiMedia) were dissolved in 500 mL of distilled water. For the preparation of potato dextrose agar media, bacteriological agar was added to PDB to achieve a final concentration of 2% (w/v). The mixture was heated in a microwave for 1–2 minutes to dissolve the agar, followed by sterilization of the culture media in an autoclave at 15 psi and 121 °C for 20 minutes.
Following autoclaving, 25 mL of the agar media were poured into each Petri dish under sterile conditions, within a laminar airflow hood, and allowed to solidify. Subsequently, the Petri dishes containing solidified agar media were utilized for the antimicrobial assay. The schematic illustration of the preparation of LB (Miller) and PDB media is depicted in Fig. 1(a).
| S. No. | Name | MTCC No. |
|---|---|---|
| 1 | Mycobacterium smegmatis (MS) | MTCC 14468 |
| 2 | Staphylococcus aureus (SA) | MTCC 3160 |
| 3 | Bacillus cereus (BC) | MTCC 430 |
| 4 | Pseudomonas aeruginosa (PA) | MTCC 2297 |
| 5 | Yersinia pestis (YP) | NA |
| 6 | Candida albicans (CA) | MTCC 3017 |
| 7 | Escherichia coli (EC) | MTCC 40 |
2.2. Preparation of WS2 and MoS2 nanostructures (Method 1)
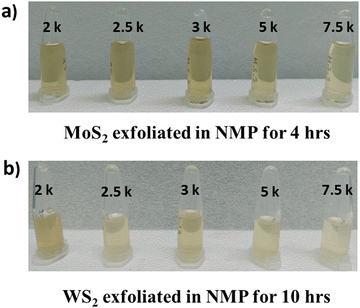
WS2 and MoS2 flakes (1.6 mg each) were dispersed in 1 mL of N-methyl-2-pyrrolidone (NMP, obtained from Merck®) in separate beakers and subjected to ultrasonication in a bath sonicator (Jain Scientific Glass Works) operating at an output power of 100 W and an output frequency of 50 Hz. The temperature of the system was carefully maintained below 30 °C throughout the process. Sonication of MoS2 was conducted for 4 hours, while sonication of WS2 was carried out for 10 hours, followed by a resting period of 24 hours. After this resting period, the TMDC specimens underwent centrifugation at 1k revolution per minute (rpm) for 2 hours at 25 °C.Following centrifugation, the supernatant was carefully separated from the pellet. Subsequently, 2 mL of the supernatant were retained for characterization, while another portion of the sample underwent centrifugation for 2 hours at 25 °C at 1.5 krpm. The supernatant obtained after this centrifugation was again separated from the pellets, as previously described. A fresh portion of the supernatant (2 mL) was retained for characterization, while the remaining portion underwent further centrifugation for 2 hours at 25 °C at higher rpms, including 2, 2.5, 3, 5, and 7.5 krpm.
The process of repeated centrifugation of the supernatant of the specimens at progressively higher rpms is known as liquid cascade centrifugation. This methodology for synthesizing a monolayer-enriched dispersion of TMDC nanosheets was adapted from Backes et al.29 The specimens obtained after each centrifugation step are depicted in Fig. 2. To ascertain the concentration of dispersed transition metal dichalcogenide (TMDC) nanosheets within the material system, the quantity of pellets obtained subsequent to each centrifugation iteration was subtracted from the initial amount of TMDC flakes utilized prior to exfoliation. The concentration of dispersed MoS2 nanosheets obtained after 2 krpm and 7.5 krpm of centrifugation was ∼198 μg mL−1 and ∼180 μg mL−1, respectively, whereas the concentration of dispersed WS2 nanosheets obtained after 2 krpm and 7.5 krpm of centrifugation was ∼610 μg mL−1 and ∼590 μg mL−1, respectively. These specimens obtained after 2 krpm and 7.5 krpm of centrifugation are used for the antimicrobial assay. In this method, the concentration of the dispersed material and yield of mono/few-layer nanosheets after exfoliation and centrifugation process are found to be the same.
3. Results (Method 1)
3.1. Characterization of the synthesized nanostructures
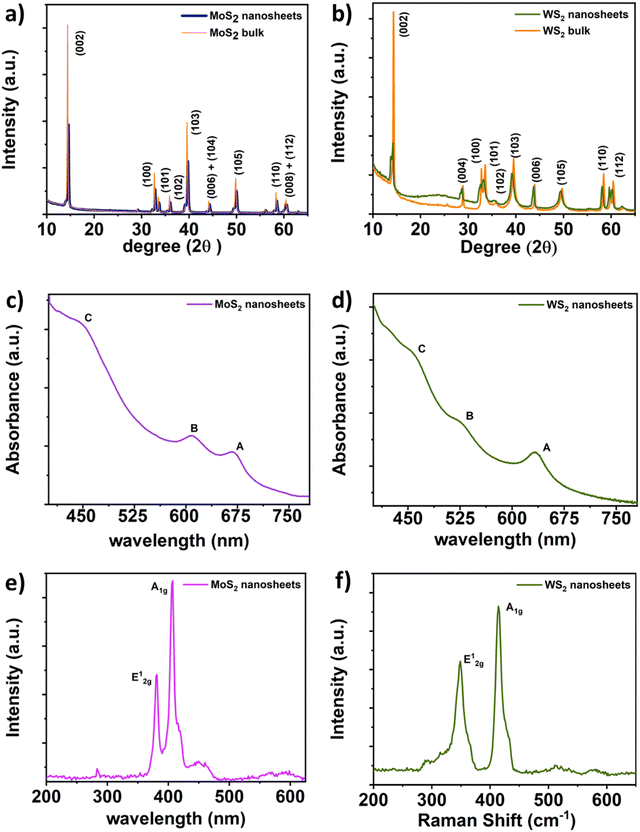
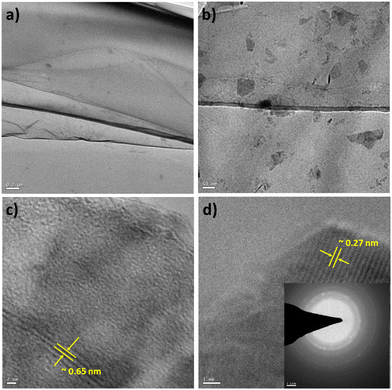
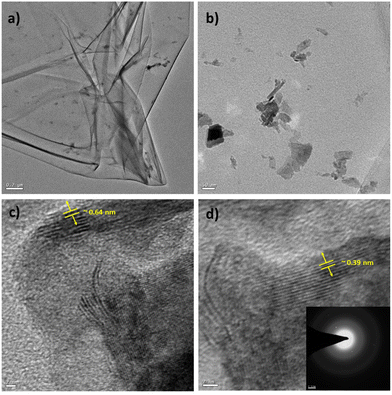
Similarly, TEM characterization of the WS2 nanosheets was conducted. Fig. 5(a) illustrates the sheet-like structure of the as-synthesized WS2, with a scale bar of 0.2 μm. Fig. 5(b) presents a magnified view of the WS2 nanostructures, with a scale bar of 50 nm. A highly resolved micrograph of the WS2 nanostructures, as shown in Fig. 5(c) with a scale bar of 2 nm, was analyzed to determine the interlayer distance to be approximately 0.64 nm, corresponding to the (002) plane. Furthermore, Fig. 5(d) displays a high-resolution micrograph of WS2 nanosheets, with a scale bar of 2 nm, along with the SAED pattern in the inset. Analysis revealed that the thickness of the WS2 nanosheets was approximately 8 nm (as presented in Fig. S4 and Table TS6 in the ESI†).
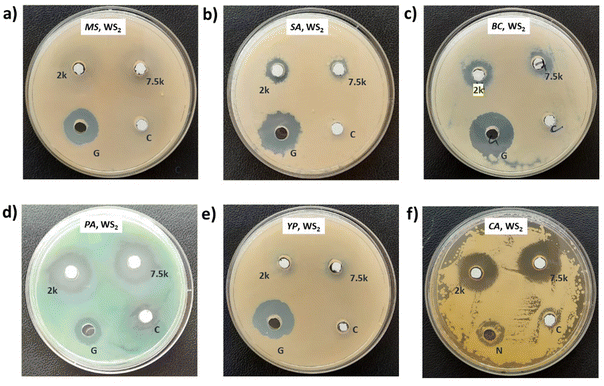
3.2. Screening for antimicrobial activity
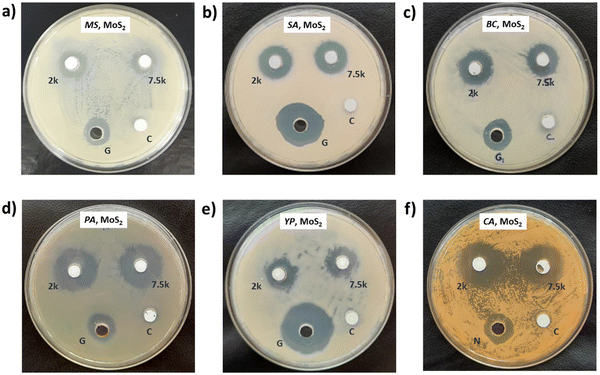
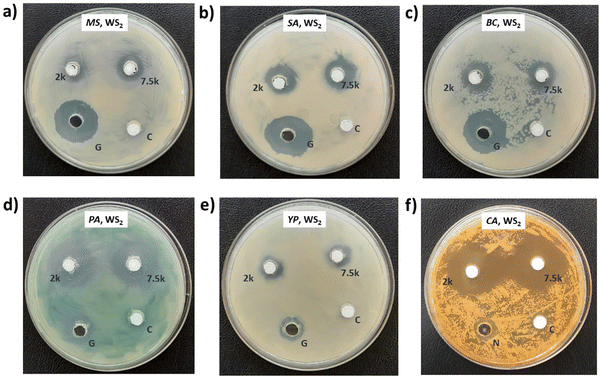
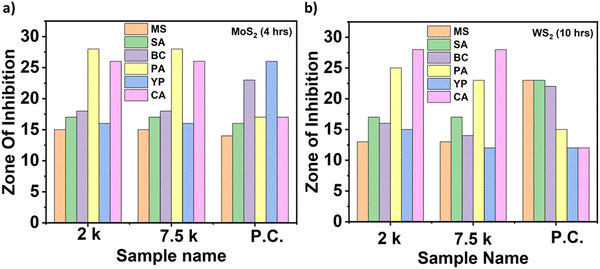
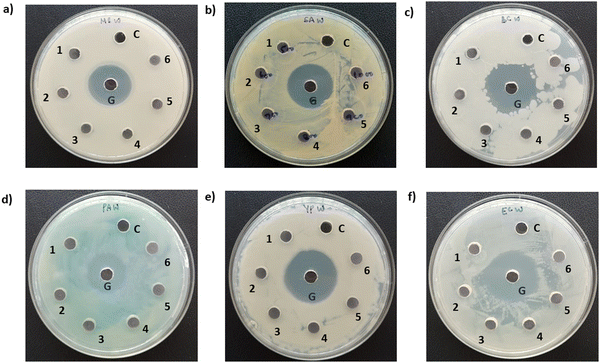
Photographs depicting bacterial and fungal cultures after 24 hours of incubation with MoS2 and WS2 nanosheets exfoliated in NMP for varying durations are presented in Fig. 6 and 7, respectively. Gentamicin (G) served as the positive control for bacterial cultures, namely MS, SA, BC, PA, and YP, while Nystatin (N) functioned as the positive control for fungal culture CA. WS2 and MoS2 nanosheets centrifuged at 2 krpm and 7.5 krpm were denoted as 2 k and 7.5 k, respectively. The solvent NMP utilized for specimen dispersion was regarded as the carrier control and labeled as C.Fig. 8 illustrates the zone of inhibition (ZOI) of MoS2 and WS2 nanosheets against bacterial and fungal cultures. It indicates that MoS2 and WS2 outperformed the positive controls for PA and CA. Conversely, the positive controls exhibited superior performance over the specimens for BC. The susceptibility pattern of pathogens towards the TMDC specimens was analyzed based on the categorization provided in Table 2,31 with Table 3 summarizing the susceptibility pattern of pathogens towards the TMDC nanostructures. Few-layer MoS2 nanostructures demonstrated susceptibility across all pathogens, while for WS2, susceptibility was observed in all pathogens except MS, which exhibited intermediate susceptibility towards WS2 specimens. Notably, few-layer MoS2 nanostructures exhibited superior antipathogenic efficacy compared to few-layer WS2 nanostructures, even at concentrations three times lower (Table 3). Additionally, the preparation time for few-layer WS2 nanostructures was at least 6 hours longer than that for MoS2 nanostructures. Consequently, it may be concluded that non-functionalized MoS2 is more effective as an antipathogenic agent than WS2.
| ≥15 mm | Susceptible (S) |
| 11–14 mm | Intermediate (I) |
| ≤10 mm | Resistant (R) |
| 0 mm | No zone (NZ) |
| Nanostructures | Concentration (μg mL−1) | ZOI for MS | ZOI for SA | ZOI for BC | ZOI for PA | ZOI for YP | ZOI for CA |
|---|---|---|---|---|---|---|---|
| Key: Intermediate = [I], Susceptible = [S]. | |||||||
| Few-layer MoS2 | ∼198 | S | S | S | S | S | S |
| Few-layer WS2 | ∼610 | I | S | S | S | S | S |
In addition to the aforementioned synthesis method, several other methodologies were employed to determine key parameters affecting the antimicrobial activity of MoS2 and WS2. WS2 and MoS2 were exfoliated, and their antimicrobial activities were investigated by altering the sonication time, solvent, and concentration of WS2 and MoS2 flakes. Subsequently, some of these methods and their antibacterial performances are discussed in the subsequent sections.
4. Experimental details and antimicrobial assessment (Method 2)
4.1. Preparation of WS2 and MoS2 specimens
Using the same process described in method 1, exfoliation of WS2 was performed. However, this time, sonication was performed for 13 h. The concentration of the dispersed WS2 nanosheets in the material system was determined by the same method mentioned in Section 2.2. The concentration of the dispersed WS2 nanosheets obtained after 2 krpm and 7.5 krpm of centrifugation was found to be ∼270 μg mL−1 and 258 μg mL−1 respectively. In this method, the concentration of the dispersed material and yield of mono/few-layer nanosheets after exfoliation and centrifugation were found to be the same. The specimens obtained after 2 krpm and 7.5 krpm of centrifugation were used for the antimicrobial assay.4.2. Screening for antimicrobial activity
The pathogen viability when treated with exfoliated WS2 is shown in Fig. 9. It is observed that for MS and YP, the activity is negligible. PA and CA are susceptible to these WS2 nanostructures. However, SA and BC showed intermediate susceptibility.5. Experimental details and antimicrobial assessment (Method 3)
5.1. Preparation of WS2 and MoS2 specimens
A solution comprising isopropanol (IPA) (obtained from Merck®) and double-distilled (DD) water was prepared in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, guided by the investigation conducted by Sajedi-Moghaddam et al.32 Subsequently, 1.6 mg of WS2 and MoS2 were individually combined with 1 mL of the solution in separate beakers and subjected to ultrasonication for durations of 6, 7, 8, 9, and 10 hours in a bath sonicator (Jain Scientific Glass Works) with an output power of 100 W and an output frequency of 50 Hz. The system temperature was carefully maintained below 30 °C throughout the process. To prevent any aggregation of WS2 and MoS2 flakes at the bottom of the container, the beakers were periodically shaken every 10 minutes during the initial hour of sonication. Following ultrasonication periods of 6, 7, 8, 9, and 10 hours, the solutions comprising TMDC specimens were collected and subsequently centrifuged for 1 hour at 2.5 krpm, after which the supernatants were extracted for antimicrobial analysis. The concentration of the dispersed WS2 and MoS2 nanosheets was measured as discussed in Section 2.2. After 10 h of ultrasonication and subsequent centrifugation, the concentrations of the dispersed WS2 and MoS2 nanosheets were found to be ∼80 μg mL−1 and ∼50 μg mL−1, respectively. In this method, the concentration of the dispersed material and yield of mono/few-layer nanosheets after exfoliation processes are found to be the same.
4, guided by the investigation conducted by Sajedi-Moghaddam et al.32 Subsequently, 1.6 mg of WS2 and MoS2 were individually combined with 1 mL of the solution in separate beakers and subjected to ultrasonication for durations of 6, 7, 8, 9, and 10 hours in a bath sonicator (Jain Scientific Glass Works) with an output power of 100 W and an output frequency of 50 Hz. The system temperature was carefully maintained below 30 °C throughout the process. To prevent any aggregation of WS2 and MoS2 flakes at the bottom of the container, the beakers were periodically shaken every 10 minutes during the initial hour of sonication. Following ultrasonication periods of 6, 7, 8, 9, and 10 hours, the solutions comprising TMDC specimens were collected and subsequently centrifuged for 1 hour at 2.5 krpm, after which the supernatants were extracted for antimicrobial analysis. The concentration of the dispersed WS2 and MoS2 nanosheets was measured as discussed in Section 2.2. After 10 h of ultrasonication and subsequent centrifugation, the concentrations of the dispersed WS2 and MoS2 nanosheets were found to be ∼80 μg mL−1 and ∼50 μg mL−1, respectively. In this method, the concentration of the dispersed material and yield of mono/few-layer nanosheets after exfoliation processes are found to be the same.
5.2. Screening for antimicrobial activity
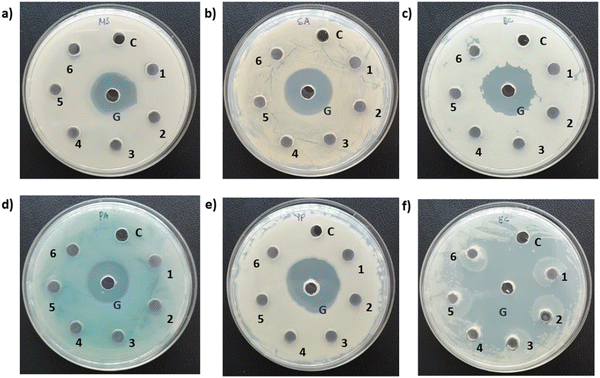
Photographs depicting bacterial and fungal cultures after 24 hours of incubation with MoS2 and WS2 specimens exfoliated in IPA-H2O mixtures for durations of 6, 7, 8, 9, and 10 hours are presented in Fig. 10 and 11, respectively. The specimens are denoted as 6, 7, 8, 9, and 10 corresponding to the duration of sonication. The IPA-H2O solution serves as the carrier control and is labelled as C. Fig. 10 and 11 illustrate that these specimens exhibited no antibacterial activity, as evidenced by the absence of a zone of inhibition (ZOI) for all specimens.6. Experimental details and antimicrobial assessment (Method 4)
6.1. Preparation of WS2 and MoS2 specimens
A solution consisting of IPA and double-distilled (DD) water was prepared in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, as described in method 3. Subsequently, various quantities of WS2 and MoS2 flakes were introduced into the prepared mixture to achieve concentrations of 500, 600, 700, 800, 900, and 1000 μg mL−1. The mixtures were then subjected to ultrasonication for a duration of 6 hours. After the 6-hour sonication period, the specimens were utilized directly for antimicrobial assessment without undergoing any further treatment. The specimens obtained are depicted in Fig. 12. In this instance, the yield of mono/few-layer nanosheets was determined to be negligible and regarded as zero as the exfoliated TMDCs were multilayered.
4, as described in method 3. Subsequently, various quantities of WS2 and MoS2 flakes were introduced into the prepared mixture to achieve concentrations of 500, 600, 700, 800, 900, and 1000 μg mL−1. The mixtures were then subjected to ultrasonication for a duration of 6 hours. After the 6-hour sonication period, the specimens were utilized directly for antimicrobial assessment without undergoing any further treatment. The specimens obtained are depicted in Fig. 12. In this instance, the yield of mono/few-layer nanosheets was determined to be negligible and regarded as zero as the exfoliated TMDCs were multilayered.
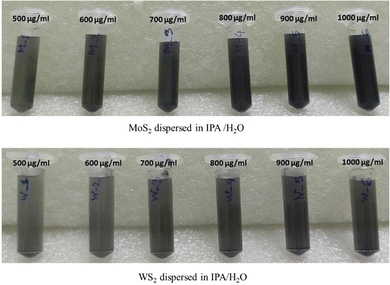 | ||
| Fig. 12 Different concentrations of exfoliated MoS2 and WS2 in IPA-H2O solvent after 6 h of exfoliation. | ||
6.2. Screening for antimicrobial activity
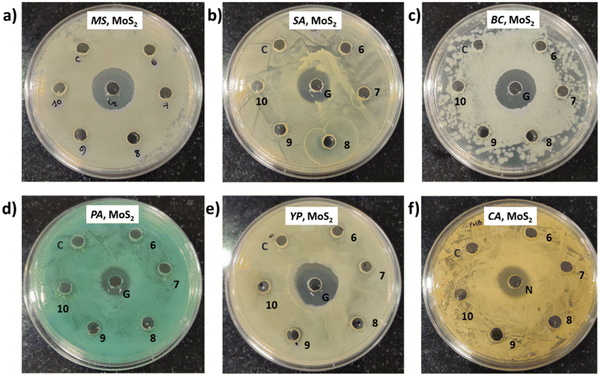
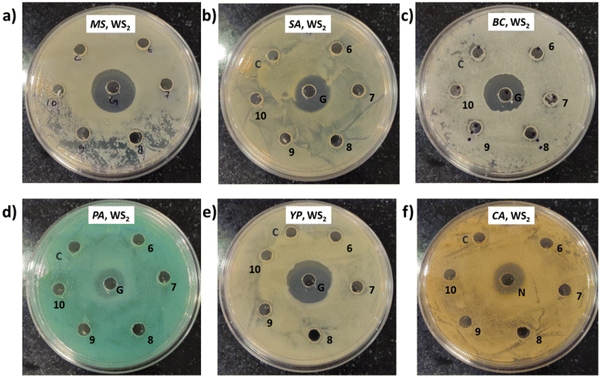
In this instance, antibacterial analysis was conducted against MS, SA, BC, PA, YP, and EC. Photographs depicting bacterial and fungal cultures after 24 hours of incubation with MoS2 and WS2 specimens are presented in Fig. 13 and 14, respectively. Gentamicin (G) served as the positive control for antibacterial assessment involving MS, SA, BC, PA, YP, and EC. Interactions of bacterial cultures with WS2 and MoS2 specimens at different concentrations, namely 500, 600, 700, 800, 900, and 1000 μg mL−1, are denoted as 1, 2, 3, 4, 5, and 6, respectively, while the IPA-H2O solution, serving as the carrier control, is labelled as C. Fig. 13 and Fig. 14 demonstrate that these WS2 and MoS2 specimens do not exhibit antimicrobial activity, as evidenced by the absence of a zone of inhibition (ZOI) for all specimens.7. Discussions
7.1. Comparison of antimicrobial activities of WS2 and MoS2 specimens exfoliated through different methods.
Table 4 presents a comparative analysis of the antimicrobial activities of WS2 and MoS2 specimens exfoliated through different methodologies. WS2 and MoS2 were exfoliated in NMP and IPA-H2O varying sonication times and initial concentrations of TMDC bulk flakes. In Method 1, ultrasonication of WS2 was conducted for 10 hours, while in Method 2, it was extended to 13 hours. However, the results were only moderately improved for the latter, indicating that an increase in sonication time may not necessarily enhance antimicrobial activity. Furthermore, Method 3, involving exfoliation of WS2 and MoS2 in an IPA-H2O mixture followed by varying sonication durations, resulted in the complete absence of a zone of inhibition (ZOI), reinforcing the notion that antimicrobial properties are not solely dependent on sonication time.| Method | Concentration of WS2 and MoS2 (μg mL−1) | ZOI (MS) | ZOI (SA) | ZOI (BC) | ZOI (PA) | ZOI (YP) | ZOI (CA) | ZOI (EC) | |
|---|---|---|---|---|---|---|---|---|---|
| Key: NZ = no zone, Intermediate = [I], Susceptible = [S], Not applicable [NA]. | |||||||||
| 1 | MoS2 | ∼198 | S | S | S | S | S | S | NA |
| WS2 | ∼610 | I | S | S | S | S | S | NA | |
| 2 | MoS2 | NA | NA | NA | NA | NA | NA | NA | NA |
| WS2 | ∼270 | NZ | I | I | S | NZ | S | NA | |
| 3 | MoS2 | ∼50 | NZ | NZ | NZ | NZ | NZ | NZ | NA |
| WS2 | ∼80 | NZ | NZ | NZ | NZ | NZ | NZ | NA | |
| 4 | MoS2 | 500,600,700,800, 900 & 1000 | NZ | NZ | NZ | NZ | NZ | NZ | NZ |
| WS2 | 500,600,700,800, 900 & 1000 | NZ | NZ | NZ | NZ | NZ | NZ | NZ | |
Additionally, the antimicrobial properties of highly poly-dispersed specimens (Method 4) were evaluated, wherein high concentrations of WS2 and MoS2 multi-layered nanostructures were employed. However, the results were found to be insignificant, suggesting that merely increasing the concentrations of WS2 and MoS2 specimens does not correlate with enhanced antimicrobial activity. Consequently, from the discourse, it can be inferred that the antimicrobial activity of WS2 and MoS2 nanosheets is not directly influenced by sonication time or the concentration of material particles within the system.
7.2. Parameters responsible for the antimicrobial activities of WS2 and MoS2 specimens
Yang et al.22 conducted an investigation into the antimicrobial properties of chemically exfoliated (CE) MoS2 nanosheets, comparing monolayer specimens with a thickness of 1 nm and a size of approximately 200 nm, to aggregated CE-MoS2 nanosheets with a thickness of about 10 nm and a size ranging from 1 to 2 μm, as well as bulk nanosheets. Their findings indicated a dependence of antimicrobial efficacy on the morphology, specifically the shape and specific surface area, of the material. Navale et al.19 synthesized few-layered WS2 nanosheets with thicknesses ranging from 1 to 5 nm and lengths from 1 to 3 μm, observing an increase in antibacterial activity with higher concentrations of nanosheets and longer incubation times. Pandit et al.23 investigated the antibacterial properties of single-layer MoS2, while Liu et al.20 synthesized monolayer WS2 using a surfactant exfoliation method, both concluding that antibacterial activity correlated positively with concentration and incubation time.These studies collectively suggest that WS2 and MoS2 nanosheets exhibit antimicrobial activity when the material comprises a sufficient proportion of monolayer and few-layer nanostructures. In our study, TEM analysis revealed predominantly mono/few-layer nanostructures in the exfoliated sheets obtained via Method 1, consistent with the specimen photographs (Fig. 2) and micrographs (Fig. 4) presented. Conversely, exfoliated sheets derived from Method 4 exhibited a multilayer composition. Comparative analysis (Table 4) indicated that despite higher concentrations of WS2 and MoS2 in Method 4, no Zone of Inhibition (ZOI) was observed. This observation suggests a positive correlation between antibacterial activity and the concentration of monolayer/few-layer transition metal dichalcogenides.
7.3. Mechanism of antipathogenic activity
Limited investigations have provided comprehensive insights into the specific anti-pathogenic mechanisms of MoS2 and WS2 against distinct pathogens. Notably, for MoS2 nanosheets, a complex sequence of events unfolds. Initially, nanosheets adhere to the cell membrane due to electrostatic attractions, subsequently penetrating the cell body through van der Waals forces with phospholipids. This infiltration process often leads to the extraction of phospholipid molecules.33 In certain instances, the simultaneous embedding of nanosheets onto bacterial cells and phospholipid extraction induces rapid depolarization, disrupting membrane permeability and normal respiratory functions, ultimately impeding bacterial metabolism. Concomitantly, oxidative stress intensifies, hastening bacterial demise.22–24,34 Conversely, the antimicrobial action of WS2 nanosheets predominantly involves membrane disruption.19,20In our observations, it is notable that the MoS2 and WS2 nanosheets employed for antimicrobial applications display nearly neutral characteristics, as evidenced by the measured zeta potentials. Specifically, the zeta potentials for WS2 and MoS2 were determined to be −3.3 mV and −2.12 mV, respectively (refer to Fig. S5 and S6, ESI†). Again, one of the explanations for the antimicrobial activity of semiconducting TMDCs is the generation of intracellular reactive oxygen species (ROS) through a mechanism involving (e–h) pairs.26 Karunakaran et al. reported an increasing order of intracellular ROS generation as follows: MoS2 > WS2. Despite the absence of functionalization in our study, both MoS2 and WS2 demonstrate antimicrobial efficacy. Therefore, the generation of intercellular ROS presents a plausible explanation for the consistent trend observed. Consequently, the antimicrobial mechanism in our context is explicated thus: upon interaction, nanomaterials adhere to microbial cells via van der Waals forces with phospholipids. Remarkably thinner than microbial cells by approximately 102–103 times, nanomaterials potentially induce physical membrane disruption, disrupting essential cellular components. The production of reactive oxygen species further accelerates the demise of the pathogen.
A comparative analysis in Table 5 delineates various antimicrobial studies on WS2 and MoS2 nanosheets and their composites.
| Material | Concentration | Method of analysis | Pathogens | Incubation time | Ref |
|---|---|---|---|---|---|
| a E. coli (EC). b S. typhimurium (ST). c B. subtilis (BS). d S. epidermidis (SE). e S. aureus (SA). f C. albicans (CA). g E. coli DH5α (EC DH5α). h P. aeruginosa (PA). i Alternaria alternata (AA). j Klebsiella pneumoniae (KP). k F. oxysporum (FO). l P. diminuta, & M. smegmatis (MS). m B. cereus (BC). n Y. pestis (YP). | |||||
| WS2 | 250 μg mL−1 | Colony counting method | EC , STb, BSc, SEd | 6 h | 19 |
| r-GO-WS2 | 250 μg mL−1 | ||||
| WS2 nanosheets | 200 μg mL−1 | Colony forming unit | EC , SAe | 2 h | 20 |
| WS2/ZnO | 300 μg mL−1 | Disc diffusion | CA | NA | 21 |
| MoS2 nanosheets | 20 μg mL− | Colony counting method | EC DH5α | 6 h | 22 |
| MoS2 nanosheets Functionalized with thiol ligands | 25.12 μg mL−1 | Microbroth dilution method | SA , PAh | 72 h | 23 |
| MoS2 nanosheets | Colony counting method | Salmonella wild-type Salmonella | 24 h | 24 | |
| MoS2 nanosheets | 1000 μg mL−1 | metabolomics | EC | 12 h | 35 |
| Iron doped MoS2 coated on titanium | 100 μg mL−1 | Agar diffusion assay | EC , SAe | 24 h | 36 |
| MoS2 nanostructures | NA | Agar method | AA | NA | 37 |
| MoS2 -modified curcumin nanostructures | 50 μg mL−1 | Confocal analysis | KP | 18 h | 38 |
| Chitosan/Ag/MoS2 | NA | Colony counting method | SA , ECa | 20 min | 39 |
| O, N co-doped MoS2 nanoflowers | 2 mg mL−1 | Triphenyl tetrazolium chloride assay | AA , FOk | 24 h | 40 |
| MoS2 | ∼ 198 μg mL−1 (MoS2) | Agar well diffusion assay | SA , CAf, PAh, PDl, MSl, BCm, YPn | 24 h | Present |
| WS2 | ∼610 μg mL−1 (WS2) | ||||
8. Conclusion
The present study investigates the antimicrobial properties of WS2 and MoS2 nanosheets exfoliated using varied techniques. Antipathogenic assessment was conducted via the agar well diffusion assay. MoS2 flakes, exfoliated in N-methyl-2-pyrrolidone (NMP) for 4 hours followed by liquid cascading centrifugation, exhibited antimicrobial efficacy against all examined microbes, including M. smegmatis, S. aureus, B. cereus, P. aeruginosa, Y. pestis, and C. albicans. Notably, all pathogens demonstrated susceptibility to this exfoliated MoS2 specimen, wherein the concentration of few layers approximated 198 μg mL−1. Similarly, WS2 flakes, exfoliated in NMP for 10 hours followed by liquid cascading centrifugation, displayed antimicrobial activity against all pathogens. M. smegmatis exhibited intermediate susceptibility to this sample, while all other pathogens were fully susceptible. The concentration of few layers in this sample was approximately 610 μg mL−1.It was elucidated that these materials exhibit antimicrobial efficacy only when they contain a specific concentration of mono or few-layer nanostructures within the material matrix. Notably, alterations in exfoliation parameters impact antimicrobial activity solely if they augment the number of monolayers or few layers within the system. From the findings, it can be inferred that MoS2 demonstrates superior effectiveness as an antipathogenic agent compared to WS2 few-layer nanostructures. This conclusion is substantiated by the fact that MoS2 synthesis is time-efficient and even lesser quantities of MoS2 nanosheets exhibit enhanced antimicrobial activity relative to WS2 nanosheets.
Author contributions
A. N. designed the experiment, conducted the synthesis, characterization, and analysis of TMDCs, and authored the manuscript. M. A. R. and P. B. conducted the Agar well diffusion assay, with M. A. R. and P. B also contributing to the corresponding manuscript section. R. B. and M. M., along with N. M., oversaw the entire investigation and finalized the editing process.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors are thankful to the Department of Physics, Tezpur University, Tezpur for providing the UV-Vis spectrophotometer to carry out the spectroscopic investigations, and to SAIC, Tezpur University, for providing the facilities of XRD, Raman spectrometer and TEM. Author A.N. would like to acknowledge Prof. Dambarudhar Mohanta and late Prof. Ashok Kumar, Department Of Physics, Tezpur University, Tezpur for allowing access to their labs. A.N. acknowledges the funding from Research and Innovation Grant, Tezpur University and Tezpur University for the institutional fellowship. A.N. would like to extend heartful thanks to Upama Das, Bhupali Deka, Stuti Tamuli, Kaushik Nath, Research Scholars, Department of Physics, Tezpur University, Tezpur for their cooperation while carrying out the synthesis processes and characterization.References
- A. Splendiani, L. Sun, Y. Zhang, T. Li, J. Kim, C. Y. Chim, G. Galli and F. Wang, Emerging photoluminescence in monolayer MoS2, Nano Lett., 2010, 10(4), 1271–1275 CrossRef CAS PubMed.
- W. Choi, M. Y. Cho, A. Konar, J. H. Lee, G. B. Cha, S. C. Hong, S. Kim, J. Kim, D. Jena, J. Joo and S. Kim, High-detectivity multilayer MoS2 phototransistors with spectral response from ultraviolet to infrared, Adv. Mater., 2012, 24(43), 5832–5836 CrossRef CAS PubMed.
- A. Neog and R. Biswas, Evidence of Laser-Induced Amplification of Random Noise in WS2 Nanosheets Based Resistive System, Phys. Status Solidi RRL, 2022, 16(8), 2200142 CrossRef CAS.
- Y. Xu, C. Y. Hsieh, L. Wu and L. K. Ang, Two-dimensional transition metal dichalcogenides mediated long range surface plasmon resonance biosensors, J. Phys. D: Appl. Phys., 2018, 52(6), 065101 CrossRef.
- D. Monga, S. Sharma, N. P. Shetti, S. Basu, K. R. Reddy and T. M. Aminabhavi, Advances in transition metal dichalcogenide-based two-dimensional nanomaterials, Mater. Today Chem., 2021, 19, 100399 CrossRef CAS.
- A. Neog and R. Biswas, WS2 nanosheets as a potential candidate towards sensing heavy metal ions: A new dimension of 2D materials, Mater. Res. Bull., 2021, 144, 111471 CrossRef CAS.
- A. Neog and R. Biswas, A novel route for sensing heavy metal ion in aqueous solution, Europhys. Lett., 2022, 46002 CrossRef CAS.
- W. Liao, S. Zhao, F. Li, C. Wang, Y. Ge, H. Wang, S. Wang and H. Zhang, Interface engineering of two-dimensional transition metal dichalcogenides towards next-generation electronic devices: recent advances and challenges, Nanoscale Horiz., 2020, 5(5), 787–807 RSC.
- H. Zeng, J. Dai, W. Yao, D. Xiao and X. Cui, Valley polarization in MoS2 monolayers by optical pumping, Nat. Nanotechnol., 2012, 7(8), 490–493 CrossRef CAS PubMed.
- T. Cao, G. Wang, W. Han, H. Ye, C. Zhu, J. Shi, Q. Niu, P. Tan, E. Wang, B. Liu and J. Feng, Valley-selective circular dichroism of monolayer molybdenum disulphide, Nat. Commun., 2012, 3(1), 1–5 Search PubMed.
- A. Ramasubramaniam, D. Naveh and E. Towe, Tunable band gaps in bilayer transition-metal dichalcogenides, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84(20), 205325 CrossRef.
- Y. Zhao, J. Liu, X. Zhang, C. Wang, X. Zhao, J. Li and H. Jin, Convenient Synthesis of WS2–MoS2 Heterostructures with Enhanced Photocatalytic Performance, J. Phys. Chem. C, 2019, 123(45), 27363–27368 CrossRef CAS.
- J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis, Nat. Mater., 2012, 11(11), 963–969 CrossRef CAS PubMed.
- X. Zhang, S. Y. Teng, A. C. M. Loy, B. S. How, W. D. Leong and X. Tao, Transition metal dichalcogenides for the application of pollution reduction: A review, Nanomaterials, 2020, 10(6), 1012 CrossRef CAS PubMed.
- M. Redlich, A. Katz, L. Rapoport, H. D. Wagner, Y. Feldman and R. Tenne, Improved orthodontic stainless-steel wires coated with inorganic fullerene-like nanoparticles of WS2 impregnated in electroless nickel–phosphorous film, Dent. Mater., 2008, 24(12), 1640–1646 CrossRef CAS PubMed.
- H. Wu, R. Yang, B. Song, Q. Han, J. Li, Y. Zhang, Y. Fang, R. Tenne and C. Wang, Biocompatible inorganic fullerene-like molybdenum disulfide nanoparticles produced by pulsed laser ablation in water, ACS Nano, 2011, 5(2), 1276–1281 CrossRef CAS PubMed.
- W. Z. Teo, E. L. K. Chng, Z. Sofer and M. Pumera, Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues, Chem. - Eur. J., 2014, 20(31), 9627–9632 CrossRef CAS PubMed.
- J. H. Appel, D. O. Li, J. D. Podlevsky, A. Debnath, A. A. Green, Q. H. Wang and J. Chae, Low cytotoxicity and genotoxicity of two-dimensional MoS2 and WS2, ACS Biomater. Sci. Eng., 2016, 2(3), 361–367 CrossRef CAS PubMed.
- G. R. Navale, C. S. Rout, K. N. Gohil, M. S. Dharne, D. J. Late and S. S. Shinde, Oxidative and membrane stress-mediated antibacterial activity of WS2 and rGO-WS2 nanosheets, RSC Adv., 2015, 5(91), 74726–74733 RSC.
- X. Liu, G. Duan, W. Li, Z. Zhou and R. Zhou, Membrane destruction-mediated antibacterial activity of tungsten disulfide (WS2), RSC Adv., 2017, 7(60), 37873–37880 RSC.
- V. K. Bhatt, M. Patel, P. M. Pataniya, B. D. Iyer, C. K. Sumesh and D. J. Late, Enhanced antifungal activity of WS2/ZnO nanohybrid against Candida albicans, ACS Biomater. Sci. Eng., 2020, 6(11), 6069–6075 CrossRef CAS PubMed.
- X. Yang, J. Li, T. Liang, C. Ma, Y. Zhang, H. Chen, N. Hanagata, H. Su and M. Xu, Antibacterial activity of two-dimensional MoS2 sheets, Nanoscale, 2014, 6(17), 10126–10133 RSC.
- S. Pandit, S. Karunakaran, S. K. Boda, B. Basu and M. De, High antibacterial activity of functionalized chemically exfoliated MoS2, ACS Appl. Mater. Interfaces, 2016, 8(46), 31567–31573 CrossRef CAS PubMed.
- J. Kaur, M. Singh, C. Dell‘Aversana, R. Benedetti, P. Giardina, M. Rossi, M. Valadan, A. Vergara, A. Cutarelli, A. M. I. Montone and L. Altucci, Biological interactions of biocompatible and water-dispersed MoS2 nanosheets with bacteria and human cells, Sci. Rep., 2018, 8(1), 1–15 CAS.
- S. Karunakaran, S. Pandit, B. Basu and M. De, Simultaneous exfoliation and functionalization of 2H-MoS2 by thiolated surfactants: applications in enhanced antibacterial activity, J. Am. Chem. Soc., 2018, 140(39), 12634–12644 CrossRef CAS PubMed.
- S. Karunakaran, S. Sahoo, J. Sahoo and M. De, Ligand-Mediated Exfoliation and Antibacterial Activity of 2H Transition-Metal Dichalcogenides, ACS Appl. Bio. Mater., 2022, 6(1), 126–133 CrossRef PubMed.
- C. Backes, B. M. Szydłowska, A. Harvey, S. Yuan, V. Vega-Mayoral, B. R. Davies, P. L. Zhao, D. Hanlon, E. J. Santos, M. I. Katsnelson and W. J. Blau, Production of highly monolayer enriched dispersions of liquid-exfoliated nanosheets by liquid cascade centrifugation, ACS Nano, 2016, 10(1), 1589–1601 CrossRef CAS PubMed.
- A. Neog, S. Deb and R. Biswas, Atypical electrical behavior of few layered WS2 nanosheets based platform subject to heavy metal ion treatment, Mater. Lett., 2020, 268, 127597 CrossRef CAS.
- C. Gamazo, S. Prior, M. Concepción Lecároz, A. I. Vitas, M. A. Campanero, G. Pérez, D. Gonzalez and M. J. Blanco-Prieto, Biodegradable gentamicin delivery systems for parenteral use for the treatment of intracellular bacterial infections, Expert Opin. Drug Delivery, 2007, 4(6), 677–688 CrossRef CAS PubMed.
- N. H. Park and M. K. Kang, Antifungal and antiviral agents, Pharmacology and therapeutics for dentistry, Mosby Co, New Delhi, 5th edn, 2004, p.660 Search PubMed.
- A. Hayat and F. Munnawar, Antibacterial effectiveness of commercially available hand sanitizers, Int. J. Biol. Biotechnol., 2016, 13(3), 427–431 CAS.
- A. Sajedi-Moghaddam and E. Saievar-Iranizad, High-yield exfoliation of tungsten disulphide nanosheets by rational mixing of low-boiling-point solvents, Mater. Res. Exp., 2018, 5(1), 015045 CrossRef.
- Z. Gu, W. Li, L. Hong and R. Zhou, Exploring biological effects of MoS2 nanosheets on native structures of α-helical peptides, J. Chem. Phys., 2016, 144(17), 175103 CrossRef PubMed.
- S. Roy, A. Mondal, V. Yadav, A. Sarkar, R. Banerjee, P. Sanpui and A. Jaiswal, Mechanistic insight into the antibacterial activity of chitosan exfoliated MoS2 nanosheets: membrane damage, metabolic inactivation, and oxidative stress, ACS Appl. Bio. Mater., 2019, 2(7), 2738–2755 CrossRef CAS PubMed.
- N. Wu, Y. Yu, T. Li, X. Ji, L. Jiang, J. Zong and H. Huang, Investigating the influence of MoS2 nanosheets on E. coli from metabolomics level, PLoS One, 2016, 11(12), e0167245 CrossRef PubMed.
- K. Tang, L. Wang, H. Geng, J. Qiu, H. Cao and X. Liu, Molybdenum disulfide (MoS2) nanosheets vertically coated on titanium for disinfection in the dark, Arabian J. Chem., 2020, 13(1), 1612–1623 CrossRef CAS.
- P. Basu, J. Chakraborty, N. Ganguli, K. Mukherjee, K. Acharya, B. Satpati, S. Khamrui, S. Mandal, D. Banerjee, D. Goswami and P. M. Nambissan, Defect-engineered MoS2 nanostructures for reactive oxygen species generation in the dark: antipollutant and antifungal performances, ACS Appl. Mater. Interfaces, 2019, 11(51), 48179–48191 CrossRef CAS PubMed.
- A. K. Singh, H. Mishra, Z. Firdaus, S. Yadav, P. Aditi, N. Nandy, K. Sharma, P. Bose, A. K. Pandey, B. S. Chauhan and K. Neogi, MoS2-modified curcumin nanostructures: the novel theranostic hybrid having potent antibacterial and antibiofilm activities against multidrug-resistant hypervirulent klebsiella pneumoniae, Chem. Res. Toxicol., 2019, 32(8), 1599–1618 Search PubMed.
- M. Zhu, X. Liu, L. Tan, Z. Cui, Y. Liang, Z. Li, K. W. K. Yeung and S. Wu, Photo-responsive chitosan/Ag/MoS2 for rapid bacteria-killing, J. Hazard. Mater., 2020, 383, 121122 CrossRef CAS PubMed.
- P. Basu, K. Mukherjee, S. Khamrui, S. Mukherjee, M. Ahmed, K. Acharya, D. Banerjee, P. M. Nambissan and K. Chatterjee, Oxygen, nitrogen co-doped molybdenum disulphide nanoflowers for an excellent antifungal activity, Mater. Adv., 2020, 1(6), 1726–1738 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma01128c |
| This journal is © The Royal Society of Chemistry 2024 |