Open Access Article
Open Access ArticleModification of chitosan-coated magnetic material with glycidyltrimethylammonium chloride and its application as heterogeneous base catalyst for levulinic acid esterification†
Feri
Mukhayani
 a,
Yuichi
Kamiya
a,
Yuichi
Kamiya
 *b,
Ryoichi
Otomo
*b,
Ryoichi
Otomo
 b,
Eko Sri
Kunarti
b,
Eko Sri
Kunarti
 a and
Nuryono
Nuryono
a and
Nuryono
Nuryono
 *a
*a
aDepartment of Chemistry, Universitas Gadjah Mada, 55281, Yogyakarta, Indonesia. E-mail: nuryono_mipa@ugm.ac.id
bFaculty of Environmental Earth Science, Hokkaido University, 060-0810, Sapporo, Japan. E-mail: kamiya@ees.hokudai.ac.jp
First published on 18th March 2024
Abstract
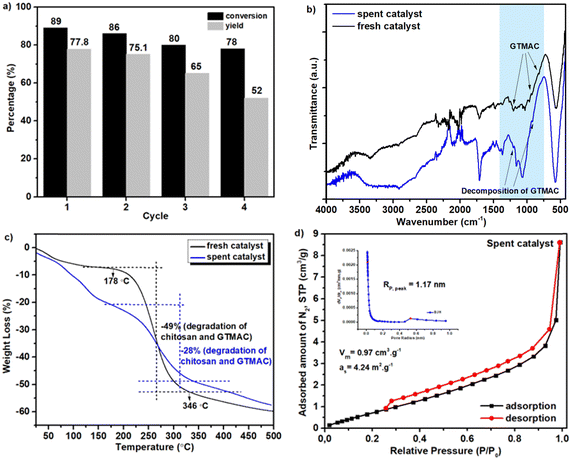
To promote more widespread use of biodiesel, it is essential to add additives to improve its fuel properties, and one such additive is ethyl levulinate (EL). EL is often produced by acid-catalyzed esterification of levulinic acid (LA) with ethanol, but acid catalysis has a critical problem of low catalytic activity. This problem can be solved by using a base catalyst for this reaction. In the present study, we developed a novel composite material that was composed of natural magnetic material (MM), which was obtained from iron sand, with chitosan (Chi) and glycidyltrimethylammonium chloride (GTMAC), and applied it as a base catalyst for esterification of LA to EL. In the first step, the surface of MM was modified with chitosan in 1% acetic acid, and then it was further modified with GTMAC at room temperature. The obtained composite (MM/Chi/GTMAC) was comprehensively characterized by various physical and chemical methods to verify that it had a core (MM)–shell (Chi/GTMAC) structure as expected. In addition, TPD measurements demonstrated that MM/Chi/GTMAC had base sites owing to chloride ions in GTMAC. MM/Chi/GTMAC exhibited high catalytic performance for esterification of LA to EL and 77% of EL yield, and 89% of LA conversion were achieved at 80 °C for 6 h, while MM and MM/Chi showed only little catalytic activity for EL formation. Additionally, the catalyst was reusable, while the performance was gradually decreased with each repeated use for the reaction.
1. Introduction
Essential points that should be considered for developing and using biofuel are the amount of produced energy, reduced greenhouse gas emissions, and, of course, its production cost.1 Biodiesel is one of the most popular biofuels that can replace fossil fuels in the transport and fuel sector. Compared to conventional diesel oil produced from petroleum, the sulfur content in biodiesel is low. Thus, its combustion produces less SO2 emission, avoiding acid rain and photochemical smog, which is currently caused by the combustion of fossil fuels.2 Thus, expansion of biodiesel utilization can reduce the negative environmental impact caused by fuel combustion. Although biodiesel has many advantages over conventional diesel oil, it has the same problem of generating soot during combustion as conventional diesel oil. To avoid soot generation, additives that have high oxygen content must be added to biodiesel. One biodiesel additive with sufficient oxygen content is ethyl levulinate (EL),3 whose oxygen content is 33 wt%. In addition to reducing the soot generation, advantages of EL as a biodiesel additive include improvement in lubricity, flash point stability, reduction of sulfur content,4,5 and decrease of viscosity of biodiesel.6The development of practical methods and processes for the production of EL is still keenly desired. Levulinic acid (LA) is one of the platform chemicals produced from biomass7 and is utilized as a raw material for the production of EL. EL is usually synthesized by esterification of LA with ethanol (EtOH) in the presence of a catalyst,8 and homogeneous and heterogeneous acid catalysts promote this reaction. Commonly used homogeneous catalysts are mineral acids such as sulfonic acid, sulfuric acid, and hydrochloric acid, but they have disadvantages of not being reusable, difficulty in separation from the solution,9 and hazardous and high cost.2 Therefore, many researchers tried to develop and apply heterogeneous acid catalysts, namely solid acid catalysts, including sulfonated lignin-based carbon catalysts,10 sulfonic acid-functionalized silica,11,12 and Amberlyst-1513 for the reaction. Indeed, solid acid catalysts promoted the reaction with high selectivity to EL, even under mild reaction conditions.14 Nevertheless, the acid catalysts still have a critical problem of low catalytic activity, regardless of homogeneous or heterogeneous form.15
Therefore, researchers have investigated base catalysts for the reaction to overcome the problems with acid catalysts, while the research is only a little compared to the acid ones. Heterogeneous base catalysts such as CaO,16 MgO–CaO,17 and SrO18 were applied for transesterification reactions. Therefore, we were interested in developing heterogeneous base catalysts that are active for the esterification of LA with EtOH.
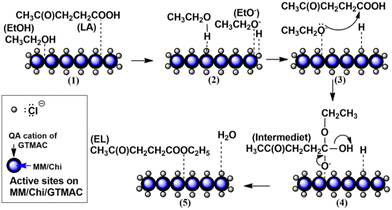
Previous research demonstrated that chloride ion (Cl−)-intercalated MgAl-layered double hydroxide acted as a solid catalyst for the hydrolysis of carbonyl sulfide.19 A report showed that adding chloride ions to Li/SnO2 improved the catalytic performance for the conversion reaction of chloromethane to ethylene.20 Those previous studies suggest that chloride ion gives a base site in the materials. Based on this speculation, we focused on glycidyltrimethylammonium chloride (GTMAC) as an active component for a base function to support materials. GTMAC is a compound with an epoxide group at one end and a quaternary ammonium at the other. Unique points of this compound are that chloride ions at the quaternary ammonium cation are known to act as a Lewis base site, and an epoxide group is usable to fix this compound tightly to support materials through a covalent bond. As a support material for GTMAC, magnetite/silica,21 chitosan,22 and polyvinyl alcohol (PVA) membranes23 have been investigated. Among them, chitosan is one of the most attractive materials because of its high biocompatibility, biodegradability, and environmentally friendliness.24,25 In addition to a base function, easy separation is also essential in a solid base catalyst for practical applications. Environmentally benign natural magnetic materials help to provide magnetic properties to solid catalysts, so the catalysts are separated simply by applying an external magnetic field.
In the present study, we synthesized a magnetically separable heterogeneous base catalyst in which natural magnetic material was modified sequentially with chitosan and GTMAC, and the synthesized catalysts were thoroughly characterized by physical and chemical methods. Furthermore, the catalysts were applied for the esterification of LA with EtOH to EL, and a kinetic study was done for the reaction over the catalyst.
2. Experimental section
2.1 Chemicals
Natural magnetic material (MM) was isolated from iron sand collected from Meliwis Beach, Kebumen, Central Java, Indonesia, containing iron oxide as a main component (70 wt%) and was treated as previously reported26 as follows. The iron sand (25 g, 200 mesh) was heated in 50 mL of 1 M HCl solution at 60 °C for 5 h, and then 1 M of NaOH solution was added to the mixture until pH 10. The precipitate was collected by filtration and washed with distilled water until the filtrate became neutral. After that, it was dried at 90 °C for 10 h, and MM was obtained.The following chemicals were used for the synthesis of the materials, which included chitosan powder (75–85% deacetylated, Merck), GTMAC (Sigma Aldrich), acetic acid (Merck), and sodium hydroxide (Merck). The chemicals used for the catalytic reaction (LA, EtOH, EL, and o-xylene) were purchased from Wako Pure Chemical Co. All the reagents were used as received.
2.2 Synthesis of magnetically separable heterogeneous base catalyst (MM/Chi/GTMAC)
Chitosan powder (0.5 g) was dissolved in 50 mL of 1 mass% acetic acid solution, and the mixture was stirred for 15 min until chitosan power was completely dissolved.27 To the solution, 3 mL of 1 M HCl solution containing 1.5 g of MM was poured, and the resulting suspension was treated with ultrasonication for 10 min. Then, the mixture was stirred at 60 °C for 4 h. After that, 1 M of aqueous NaOH solution was added until the pH of the mixture was 10, and a precipitate of chitosan-modified MM, which is named MM/Chi, was formed. The precipitate was collected by filtration and washed with distilled water until the filtrate became pH 7.The modification of MM/Chi with GTMAC was performed as follows: 1.5 mL of GTMAC was added to 1.75 g of MM/Chi, and the mixture was stirred at room temperature for 1 h. Afterward, the resulting solid was separated by an external magnet, washed with distilled water two times (50 mL × 2), and dried in an oven at 90 °C for 10 h. The obtained material is named MM/Chi/GTMAC.
2.3 Characterization
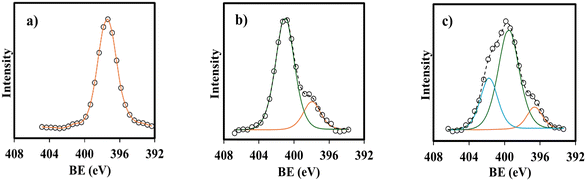
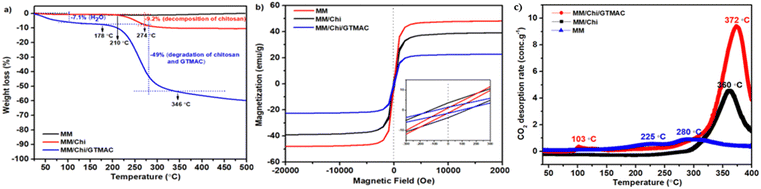
The synthesized materials were thoroughly characterized using FTIR-ATR, XPS, XRD, TGA, FE-SEM, and TEM. FTIR spectra of the materials were taken on a Fourier transform infrared-attenuated total reflection (FTIR-ATR) spectrometer (NICOLET, Is10 Thermi Gisher Scientific) in the 400–4000 cm−1 range. The resolution and number of scans were 0.5 cm−1 and 16, respectively. The electronic states of elements in the materials were investigated using XPS spectroscopy. Before measurement, all samples were coated with Pt–Pd to impart conductivity. The XPS spectra were obtained with a JEOL JPS_9200 spectrometer using an aluminum anode, and the scanning range was 0–1000 eV. XRD patterns of the materials were taken on an X-ray diffractometer (Bruker, D2 PHASER 2nd Generation) with Cu Kα radiation operating at 30 kV and 10 mA in the range of 2θ = 5–90° at a scanning rate of 0.02° min−1. Thermogravimetric analysis (TGA) of the materials was performed using a Pyris 1 TGA thermal analyzer (PerkinElmer). The temperature was increased from room temperature to 500 °C at 10 °C min−1 in air flow (50 mL min−1). The morphologies and EDX mapping of the materials were observed using a JEOL JSM-6510LA SEM microscope operating at 5 kV and 30 mA. To afford measurements, the material was coated with Au. The core–shell structure of the material was identified using transmission electron microscopy (TEM, EM Philips EM 208S). The TEM specimens were prepared by dispersing the sample powder in ethanol and placing a drop of the dispersion liquid onto a carbon-coated copper grid.The materials were also characterized using a surface area and pore size analyzer, zeta-sizer, VSM, CO2-TPD, and ion-chromatograph. Surface area, pore volume, and pore size distribution were estimated from N2 adsorption–desorption isotherm taken at −196 °C on a Micrometrics ASAP analyzer. Before measurement, the samples were pretreated at 200 °C in a vacuum. The Brunauer–Emmett–Teller theory was applied to determine the specific surface area of the materials. For zeta-sizer measurement, 5 mg of material was dispersed in 10 mL of citric acid solution (1%), and the suspension was treated under sonication for 15 min. The analysis was performed by transferring 1.5 mL of the sample solution into a disposable folding capillary cuvette. Zeta potential and particle size were measured simultaneously using Malvern zeta-sizer Pro-Red MAL1266214 with a 293 nm laser. The refractive index and the detection angle were set at 1.493–1.509 and 173°, respectively. The measurement was performed three times at room temperature, and the average was taken. The magnetic properties of the materials were evaluated using a superconducting quantum interference device (SQUID) MPMS-5 (Quantum Design) magnetometer at room temperature. The VSM hysteresis curve was scanned with the range of magnetic field between −20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe (−2 T) and 20000 Oe (2 T) at a scanning speed of 0.1 T min−1. CO2-TPD was performed to analyze the basicity of the materials, and a BelCAT instrument (Bel Japan) was used for the measurement. About 0.1 g of the sample was placed in a quartz reactor and pretreated in a He flow (25 mL min−1) at 200 °C for 48 min. The sample was then cooled to 35 °C in CO2 flow (25 mL min−1), followed by saturation of the sample with CO2 at 40 °C for 35 min. Desorption of CO2 was carried out by heating the sample up to 400 °C at a heating rate of 10 °C min−1. The concentration of chloride ions in the materials was determined using an ion chromatograph (TOSOH, IC-8100) equipped with an electrical conductivity detector. The solid sample (0.05 g) was added to 2 mmol L−1 of NaBr solution to release Cl− from the sample, and then the filtrate was analyzed by the ion chromatograph to determine the concentration of Cl− in the solution.
000 Oe (−2 T) and 20000 Oe (2 T) at a scanning speed of 0.1 T min−1. CO2-TPD was performed to analyze the basicity of the materials, and a BelCAT instrument (Bel Japan) was used for the measurement. About 0.1 g of the sample was placed in a quartz reactor and pretreated in a He flow (25 mL min−1) at 200 °C for 48 min. The sample was then cooled to 35 °C in CO2 flow (25 mL min−1), followed by saturation of the sample with CO2 at 40 °C for 35 min. Desorption of CO2 was carried out by heating the sample up to 400 °C at a heating rate of 10 °C min−1. The concentration of chloride ions in the materials was determined using an ion chromatograph (TOSOH, IC-8100) equipped with an electrical conductivity detector. The solid sample (0.05 g) was added to 2 mmol L−1 of NaBr solution to release Cl− from the sample, and then the filtrate was analyzed by the ion chromatograph to determine the concentration of Cl− in the solution.
2.4 Evaluation of catalytic performance
Esterification of LA with EtOH in the presence of MM/Chi/GTMAC was performed in a test tube heated in an aluminum block heater. Typically, 0.567 g (5 mmol) of LA and 3.945 g (86 mmol) of EtOH were charged in a test tube with the catalyst. The reaction was performed with different catalyst masses (15 and 30 mg) and reaction time (1, 2, 3, 6, 8, 9, 10, 20, and 24 h) to investigate their effects on the reaction. After the reaction, the catalyst was separated with an external magnet, and the obtained reaction solution was analyzed by a gas-chromatograph (Shimadzu, GC-2014) equipped with a flame ionization detector (FID) and a capillary column (Restek, SH-Rtx-Wax). Quantifications of the unreacted LA and produced EL were carried out with an internal standard method using o-xylene as an internal standard. The conversion of LA and yield of EL were calculated with eqn (1) and (2), respectively.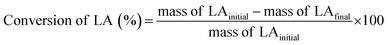 | (1) |
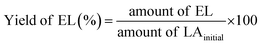 | (2) |
2.5 Recycling test
After a reaction, the catalyst was separated with an external magnet and washed three times with 5 mL of ethanol. After drying in an oven at 60 °C for 10 h, the catalyst was used for the second. The recycling test was performed up to 4 times, namely, first-time use and three times reuse.2.6 Kinetic model for the esterification of LA
The esterification of LA with EtOH is a reversible reaction, as shown in eqn (3), and the whole reaction rate equation is expressed in eqn (4),| LA + EtOH ⇄ EL + H2O | (3) |
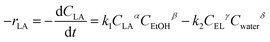 | (4) |
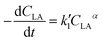 | (5) |
Eqn (5) can be integrated according to the value of α as eqn (6)–(8), having α = 0, 1, and 2, respectively,
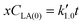 | (6) |
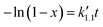 | (7) |
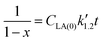 | (8) |
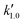 ,
, 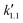 , and
, and 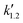 are pseudo-zero, pseudo-first, and pseudo-second-order rate constants, respectively. These three equations have been used in previous studies for kinetic analysis,10,11,28,29 and the reaction order was determined by fitting the experimental data using those reaction equations.
are pseudo-zero, pseudo-first, and pseudo-second-order rate constants, respectively. These three equations have been used in previous studies for kinetic analysis,10,11,28,29 and the reaction order was determined by fitting the experimental data using those reaction equations.
3. Results and discussion
3.1 Characterization of the synthesized materials
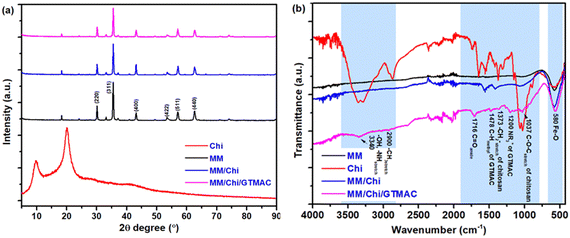
Pour et al. reported that GTMAC showed IR bands of the epoxide group, stretching of NR4+ complexes, and symmetrical bending of –CH3 on the quaternary ammonium substituent appeared at 880–980, 600–1000 and 1475 cm−1, respectively.34 These absorption bands were observed for MM/Chi/GTMAC, but the band of the epoxide group disappeared, indicating the opening of the epoxide ring. Therefore, GTMAC was fixed by the reaction of the epoxide, presumably with –NH2 in chitosan, to make a covalent N–C bond.
| Material | Zeta potential (mV) | Particle size (nm) |
|---|---|---|
| MM | −11.7 | 1575 |
| MM/Chi | 34.1 | 651 |
| MM/Chi/GTMAC | 36.2 | 1428 |
| Chitosan | 39.4 | 743 |
| GTMAC | 5.5 | 768 |
The particle size of MM was 1575 nm. It is known that magnetite particles like MM tend to aggregate very easily owing to strong hydrogen bonding,37 causing attraction between the particles.38 The modification of MM with chitosan decreased the particle size, which was 651 nm. Considering pKa, almost all amino groups in chitosan are present as amino cations (–NH3+) in solution at pH 4.39 Since the modification of MM with chitosan was performed in 1 M hydrochloric acid, the amino group of chitosan in MM/Chi must exist as –NH3+, causing electrostatic repulsion between the particles. In addition, covering the surface of MM further weakened the interaction due to hydrogen bonding that acted by the surface hydroxyl groups of MM. At higher pH levels, the number of –NH3+ and the net of the interchain repulsive electrostatic forces are reduced. Hydrogen bonding and hydrophobic interactions between chains would be favored.40,41 This can promote the formation of a more compact structure, so the particle size of the material becomes smaller. By those effects, the aggregation of the MM particles was eliminated with the chitosan modification, decreasing the particle size of the material (MM/Chi). However, the particle size was significantly increased by the modification of MM/Chi with GTMAC. This is because GTMAC covered the surface of MM/Chi, thereby weakening the effect brought about by chitosan.
A schematic image of the structures of the materials in each preparation step speculated based on zeta potential is proposed in Fig. 3. As Table 1 shows, MM had a zeta potential of −11.7 mV, indicating that a lot of hydroxyl groups were present on the surface. On the other hand, the zeta potential of chitosan was 39.4 mV, meaning that the surface of chitosan was positively charged.
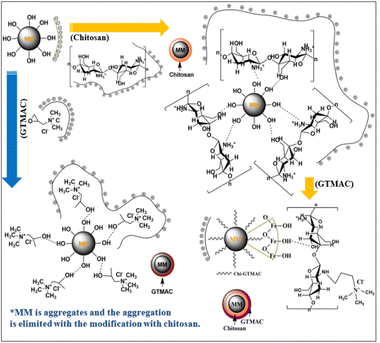 | ||
| Fig. 3 Images of structures and surface charges of MM, chitosan, GTMAC, MM/Chi, MM/GTMAC, and MM/Chi/GTMAC. | ||
Chitosan is a positively charged polymer with polar functional groups such as –NH2 and –OH.38 In an acidic solution like 1% citric acid, those polar functional groups of chitosan undergo protonation to give a positive charge on the surface. Chitosan is a polycation, and its charge density changes depending on the degree of acetylation and pH of the solution. Large amounts of protonated –NH2 groups, namely –NH3+, on the chitosan structure account for its solubility in acid-aqueous media since its pKa is approximately 6.5. Chitosan becomes soluble when around 50% of the amino groups are protonated.42
GTMAC also had positive zeta potential, being 5.5 mV, and it was due to the presence of quaternary ammonium cations (–N(CH3)3+). The modification of MM with chitosan and GTMAC changed the zeta potential of the materials. MM/Chi had a zeta potential of 34.1 mV, meaning the surface was positively charged in an acidic solution. The drastic change in the zeta potential with the modification of MM with chitosan indicated that the surface of MM was successfully covered with chitosan. It should be noted that MM/Chi/GTMAC had zeta potential that was more positive than those of MM/Chi and MM/GTMAC. This indicates that the positive charges of chitosan and GTMAC contributed together to produce a more significant positive charge on the surface since a large amount of positively charged organic compounds was present on the surface of MM/Chi/GTMAC.
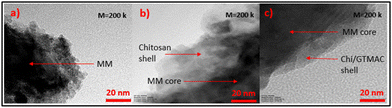
Fig. 5 displays the detailed morphologies of MM, MM/Chi, and MM/Chi/GTMAC observed by TEM. In Fig. 5(b), it was clearly observed that the MM core was covered with the chitosan shell. The thickness of the shell became thinner after the modification with GTMAC (Fig. 5(c)). This is because GTMAC was strongly bound to chitosan on the MM surface, and the structure became very dense.
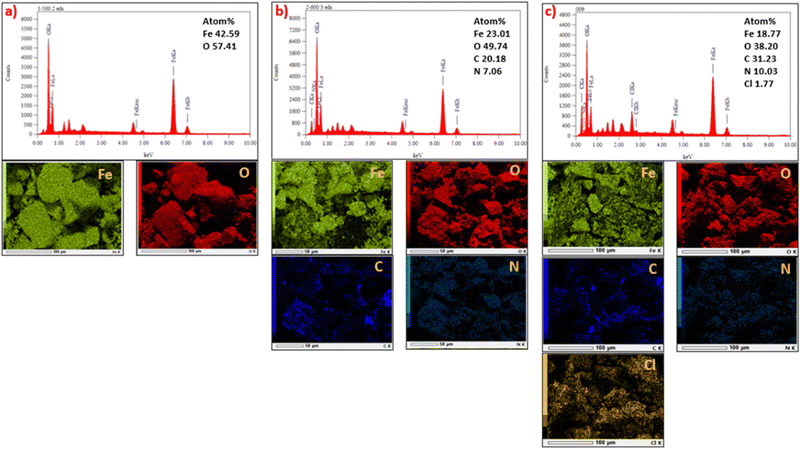
The EDX spectra and elemental EDX mapping of MM, MM/Chi, and MM/Chi/GTMAC are given in Fig. 6. It is obvious that MM contained Fe and O, while C and N were present in MM/Chi in addition to Fe and O. Moreover, the EDX spectrum for MM/Chi/GTMAC demonstrated the presence of more amounts of C and N in MM/Chi/GTMAC. It is noted that Cl was detected only for MM/Chi/GTMAC, demonstrating the successful modification with GTMAC. These sequentially decrease content in the Fe composition (MM > MM/Chi > MM/Chi/GTMAC) strongly supported the formation of ideal core (MM)–shell (Chi/GTMAC) structure.
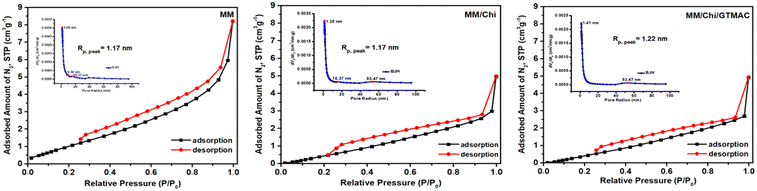
| Material | S BET (m2 g−1) | V BET (mm3 g−1) | R p (nm) |
|---|---|---|---|
| MM | 5.20 | 12.0 | 1.17 |
| MM/Chi | 4.02 | 6.4 | 1.17 |
| MM/Chi/GTMAC | 3.95 | 6.2 | 1.22 |
3.2 Catalytic performance of MM/Chi/GTMAC for esterification of levulinic acid with ethanol
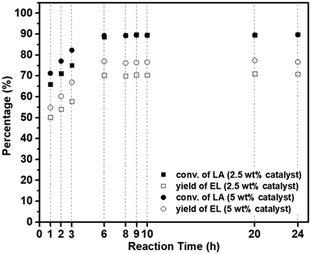 | ||
| Fig. 10 Catalytic performance of MM/Chi/GTMAC for esterification of LA with EtOH to EL. Reaction conditions: the temperature of 80 °C and EtOH/LA = 17. | ||
Here, we compared the catalytic performance of our catalyst with previously reported ones which are summarized in Table 3 with the given reaction condition. Unfortunately, since few base-catalyzed esterifications of LA have been reported, we compared our results with those obtained using acid catalysts. Compared to the catalytic performances in previous reports, our catalyst (MM/Chi/GTMAC) shows comparable performance, so it can be concluded that our result is at least as good as theirs. In addition, we want to emphasize that quaternary ammonium salt (GTMAC) is environmentally friendly and prevents the environment from contamination.22 Introducing a magnetic property in the base catalyst offers another benefit: the catalyst is easily separated from the system with an external magnet after the reaction.
| Catalyst | Condition | Conv. (%) | Yield (%) |
|---|---|---|---|
| Porous sulfonated carbon47 | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); 8 h; 80° 5); 8 h; 80° |
94 | NA |
| Carbon cryogel28 | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12); 3 h; 78 °C 12); 3 h; 78 °C |
90 | 86.5 |
| Amberlite IR12048 | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15); 4 h; 150 °C 15); 4 h; 150 °C |
86.5 | 85 |
| 1-Ethyl-3-mehylimidazolium tosylate ([EMim][OTS])49 | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10); 5.25 h; 90 °C 10); 5.25 h; 90 °C |
84 | 78 |
| ZSM-12 nanolayers50 | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 24 h; 100 °C 1); 24 h; 100 °C |
78.5 | NA |
| SO42−/SnO251 | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15); 7 h; 70 °C 15); 7 h; 70 °C |
77 | NA |
| SiO2–SO3H11 | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10); 4 h; 70 °C 10); 4 h; 70 °C |
70.6 | NA |
| MM/Chi/GTMAC (this work) | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17); 6 h; 80 °C 17); 6 h; 80 °C |
89 | 77 |
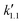 , was estimated to be 2.42 × 10−2 min−1. This value is comparable to the esterification of LA with EtOH over previously reported solid acid catalyst, which is SiO2–SO3H modified with methyl group (5.40 × 10−3 min−1).11
, was estimated to be 2.42 × 10−2 min−1. This value is comparable to the esterification of LA with EtOH over previously reported solid acid catalyst, which is SiO2–SO3H modified with methyl group (5.40 × 10−3 min−1).11
| Reaction order | Linear regression | R 2 value |
|---|---|---|
| Pseudo-zeroth | y = 0.0478x + 0.6737 | 0.9623 |
| Pseudo-first | y = 0.0557x − 2.7784 | 0.9833 |
| Pseudo-second | y = 1.6342x + 1.4374 | 0.9504 |
4. Conclusions
In this study, a novel composite material (MM/Chi/GTMAC) has been successfully synthesized by modifying MM with chitosan and GTMAC in sequence. The structure and properties of MM/Chi/GTMAC were comprehensively characterized with various physical and chemical methods, and it was found that base sites were successfully introduced to the composite material by modification with GTMAC. This composite material acted as a heterogeneous base catalyst and effectively promoted the esterification of LA with EtOH to produce EL due to the basicity of chloride ions. This heterogeneous base catalyst was magnetically separable and reusable for the esterification, though the catalytic performance decreased gradually with each repeated use.Author contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript. Feri Mukhayani: methodology, investigation, data visualization, writing – original draft; Nuryono, Yuichi Kamiya, Ryoichi Otomo, and Eko Sri Kunarti: conceptualization, resources, formal analysis, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
All authors thank Indonesia's Ministry of Education, Culture, Research, and Technology for the financial support through the Enhancing International Program (EIP-PMDSU) within contract no. 2192/UN1/DITLIT/Dit-Lit/PT.01.03/2023, the Scientific Publication Quality Improvement Program with contract no. 165.23/E4.4/KU/2023, and Promotor Collaboration Improvement Program with contract no. 224/E4.4/KU/2023. A part of this work was conducted at the Laboratory of XPS and SQUID-VSM analysis, Joint-use facilities, Hokkaido University.References
- S. Heger, K. Bluhm, J. Brendt, P. Mayer, N. Anders, A. Schaffer, T. B. Seiler and H. Hollert, Microscale in Vitro Assays for the Investigation of Neutral Red Retention and Ethoxyresorufin-O-Deethylase of Biofuels and Fossil Fuels, PLoS One, 2016, 11, 1–24 Search PubMed.
- S. K. Hoekman, A. Broch, C. Robbins, E. Ceniceros and M. Natarajan, Review of biodiesel composition, properties, and specifications, Renewable Sustainable Energy Rev., 2012, 16, 143–169 CrossRef CAS.
- B. C. Windom, T. M. Lovestead, M. Mascal, E. B. Nikitin and T. J. Bruno, Advanced Distillation Curve Analysis on Ethyl Levulinate as A Diesel Fuel Oxygenate and A Hybrid Biodiesel Fuel, Energy Fuels, 2011, 25, 1878–1890 CrossRef CAS.
- C. C. L. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman and J. C. Peters, T. F. Jaramillo, Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed.
- W. Ying, Z. Longbao and W. Hewu, Diesel Emission Improvements by the Use of Oxygenated DME/Diesel Blend Fuels, Atmos. Environ., 2006, 40, 2313–2320 CrossRef.
- L. M. Schmidt, L. D. Mthembu, P. Reddy, N. Deenadayalu, M. Kaltschmitt and I. Smirnova, Levulinic Acid Production Integrated into A Sugarcane Bagasse Based Biorefinery Using Thermal-Enzymatic Pretreatment, Ind. Crops Prod., 2017, 99, 172–178 CrossRef CAS.
- B. Girisuta and H. J. Heeres, Levulinic Acid from Biomass: Synthesis and Applications, in Production of Platform Chemicals from Sustainable Resources, ed. Z. Fang, R. L. Smith and X. Qi, Biofuel and Biorefineries, Springer, Singapore, 2017, pp. 143–169 DOI:10.1007/978-981-10-4172-3_5.
- G. D. Yadav and A. R. Yadav, Synthesis of Ethyl Levulinate as Fuel Additives Using Heterogeneous Solid Superacidic Catalysts: Efficacy and Kinetic Modeling, Chem. Eng. J., 2014, 243, 556–563 CrossRef CAS.
- F. Link, N. Ahad and A. De Klerk, Low-Pressure Hydrocracking of Wax over Pt/SiO2–Al2O3 to Produce Kerosene for Synthetic Jet Fuel, ACS Symp. Ser., 2021, 1379, 311–352 CrossRef CAS.
- A. H. Hassan, M. M. Zainol, M. A. Samion, M. A. Azlan, M. Asmadi, A. R. Mohamad Daud, I. Saad and N. A. N. M. Nor Azman, Synthesis of Ethyl Levulinate Over Sulfonated Lignin-Based Carbon Catalyst as A Fuel Additive to Biodiesel-Diesel Blends Towards Engine Emissions, J. Cleaner Prod., 2023, 418, 138101 CrossRef CAS.
- D. D. Ristiana, S. Suyanta and N. Nuryono, Sulfonic Acid-Functionalized Silica with Controlled Hydrophobicity as an Effective Catalyst for Esterification of Levulinic Acid, Mater. Today Commun., 2022, 32, 103953 CrossRef CAS.
- D. D. Ristiana, S. Suyanta and N. Nuryono, Simple One-Pot Synthesis of Sulfonic-Acid-Functionalized Silica for Effective Catalytic Esterification of Levulinic Acid, Indones. J. Chem., 2021, 22, 157–170 CrossRef.
- D. T. Melfi, M. K. Lenzi, L. P. Ramos and M. L. Corazza, Kinetic Modeling of ScCO2-Assisted Levulinic Acid Esterification with Ethanol Using Amberlyst-15 as a Catalyst in a Batch Reactor, Energy Fuels, 2021, 35, 14770–14779 CrossRef CAS.
- A. H. Hassan, M. M. Zainol, K. R. Zainuddin, H. A. Rosmadi, M. Asmadi, N. A. Rahman and N. A. S. Amin, A Review on Alkyl Levulinates Synthesis from Renewable Levulinic Acid using Various Modified Carbon-Based Catalysts, Malays. J. Chem., 2022, 24, 264–282 Search PubMed.
- D. W. Lee, Y. M. Park and K. Y. Lee, Heterogeneous Base Catalysts for Transesterification in Biodiesel Synthesis, Catal. Surv. from Asia, 2009, 13, 63–77 CrossRef CAS.
- M. Jayakumar, N. Karmegam, M. P. Gundupalli, K. Bizuneh Gebeyehu, B. Tessema Asfaw, S. W. Chang, B. Ravindran and M. K. Awasthi, Heterogeneous Base Catalysts: Synthesis and Application for Biodiesel Production – A Review, Bioresour. Technol., 2021, 331, 125054 CrossRef CAS PubMed.
- A. G. Margellou, A. A. Koutsouki, D. E. Petrakis, M. G. Kontominas and P. J. Pomonis, Catalysis and Inhibition of Transesterification of Rapeseed Oil over MgO–CaO, BioEnergy Res., 2023, 16, 528–538 CrossRef CAS.
- A. Mukhtar, S. Saqib, H. Lin, M. U. Hassan Shah, S. Ullah, M. Younas, M. Rezakazemi, M. Ibrahim, A. Mahmood, S. Asif and A. Bokhari, Current Status and Challenges in the Heterogeneous Catalysis for Biodiesel Production, Renewable Sustainable Energy Rev., 2022, 157, 112012 CrossRef CAS.
- C. Li, S. Zhao, X. Yao, L. He, S. Xu, X. Shen and Z. Yao, The Catalytic Mechanism of Intercalated Chlorine Anions as Active Basic Sites in Mgal-Layered Double Hydroxide for Carbonyl Sulfide Hydrolysis, Environ. Sci. Pollut. Res., 2022, 29, 10605–10616 CrossRef CAS PubMed.
- F. Cheng, J. Yang, L. Yan, J. Zhao, H. Zhao, H. Song and L. Chou, Impact of Chloride Ions on the Oxidative Coupling of Methane Over Li/Sno2 Catalyst, React. Kinet., Mech. Catal., 2018, 125, 675–688 CrossRef CAS.
- R. B. Istiningrum, S. J. Santosa and N. Nuryono, Preparation of Magnetic/silica/quarternary-chitosan by Sol-Gel Method and Its Stability in Various pH Medium, Rasayan J. Chem., 2021, 14, 2767–2775 CrossRef CAS.
- S. P. Rwei, Y. M. Chen, W. Lin and W. Y. Chiang, Synthesis and Rheological Characterization of Water-Soluble Glycidyltrimethylammonium-Chitosan, Mar. Drugs, 2014, 5547–5562 CrossRef CAS PubMed.
- A. M. Sajjan, B. K. Jeevan Kumar, A. A. Kittur and M. Y. Kariduraganavar, Development of Novel Grafted Hybrid PVA Membranes Using Glycidyltrimethylammonium Chloride For Pervaporation Separation of Water–Isopropanol Mixtures, J. Ind. Eng. Chem., 2013, 19, 427–437 CrossRef CAS.
- K. Wang, F. Zhang, K. Xu, Y. Che, M. Qi and C. Song, Modified Magnetic Chitosan Materials for Heavy Metal Adsorption: A Review, RSC Adv., 2023, 13, 6713–6736 RSC.
- T. M. Budnyak, I. V. Pylypchuk, V. A. Tertykh, E. S. Yanovska and D. Kolodynska, Synthesis and Adsorption Properties of Chitosan-Silica Nanocomposite Prepared by Sol-Gel Method, Nanoscale Res. Lett., 2015, 10, 87 CrossRef PubMed.
- N. Nuryono, D. Miswanda, S. C. W. Sakti, B. Rusdiarso, P. A. Krisbiantoro, N. Utami, R. Otomo and Y. Kamiya, Chitosan-Functionalized Natural Magnetic Particle@Silica Modified with (3-Chloropropyl)Trimethoxysilane as A Highly Stable Magnetic Adsorbent for Gold(III) Ion, Mater. Chem. Phys., 2020, 255, 123507 CrossRef CAS.
- F. W. Mahatmanti and N. Nuryono, Physical Characteristics of Chitosan Based Film Modified with Silica and Polyethylene Glycol, Indones. J. Chem., 2014, 131–137 CrossRef CAS.
- M. M. Zainol, N. A. S. Amin and M. Asmadi, Kinetics and Thermodynamic Analysis of Levulinic Acid Esterification Using Lignin-Furfural Carbon Cryogel Catalyst, Renew, Energy, 2019, 130, 547–557 CAS.
- T. A. Degfie, T. T. Mamo and Y. S. Mekonnen, Optimized Biodiesel Production from Waste Cooking Oil (WCO) using Calcium Oxide (CaO) Nano-catalyst, Sci. Rep., 2019, 9, 18982 CrossRef CAS PubMed.
- S. Lotfi, F. Ghaderi, A. Bahari and S. Mahjoub, Preparation and Characterization of Magnetite–Chitosan Nanoparticles and Evaluation of Their Cytotoxicity Effects on MCF7 and Fibroblast Cells, J. Supercond. Novel Magn., 2017, 30, 3431–3438 CrossRef CAS.
- F. Shi, Y. Li, Q. Zhang and H. Wang, Synthesis of Fe3O4/C/TiO2 Magnetic Photocatalyst via Vapor Phase Hydrolysis, Int. J. Photoenergy, 2012, 365401 Search PubMed.
- N. E. A. El-Naggar, A. M. Shiha, H. Mahrous and A. B. A. Mohammed, Green Synthesis of Chitosan Nanoparticles, Optimization, Characterization and Antibacterial Efficacy Against Multi-Drug Resistant Biofilm-Forming Acinetobacter Baumannii, Sci. Rep., 2022, 12, 1–19 CrossRef PubMed.
- M. F. Queiroz, K. R. T. Melo, D. A. Sabry, G. L. Sassaki and H. A. O. Rocha, Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation?, Mar. Drugs, 2015, 13, 141–158 CrossRef PubMed.
- Z. Sekhavat Pour, P. Makvandi and M. Ghaemy, Performance Properties and Antibacterial Activity of Crosslinked Films of Quaternary Ammonium Modified Starch and Poly(Vinyl Alcohol), Int. J. Biol. Macromol., 2015, 80, 596–604 CrossRef CAS PubMed.
- L. Y. Huang, W. Li, N. Du, H. Q. Lu, L. D. Meng, K. Y. Huang and K. Li, Preparation of Quaternary Ammonium Magnetic Chitosan Microspheres and Their Application for Congo Red Adsorption, Carbohydr. Polym., 2022, 297, 119995 CrossRef CAS PubMed.
- T. A. Dickstein, E. Zhou, K. K. Hershberger, A. K. Haskell, D. G. Morgan, M. Pink, B. D. Stein, L. Z. Nikoshvili, V. G. Matveeva and L. M. Bronstein, Chitosan as Capping Agent in A Robust One-Pot Procedure for A Magnetic Catalyst Synthesis, Carbohydr. Polym., 2021, 269, 118267 CrossRef CAS PubMed.
- D. Wilson and M. A. Langell, MA, XPS Analysis of Oleylamine/Oleic Acid Capped Fe3O4 Nanoparticles as a Function of Temperature, Appl. Surf. Sci., 2014, 303, 6–13 CrossRef CAS.
- S. Favela-Camacho, E. Samaniego, A. Godínez-García, L. Aviles-Arellano and J. F. Pérez-Robles, How to Decrease the Agglomeration of Magnetite Nanoparticles and Increase Their Stability Using Surface Properties, Colloids Surf., A, 2019, 1, 574 Search PubMed.
- F. P. Ramanery, A. A. P. Mansur and H. S. Mansur, One-step Colloidal Synthesis of Biocompatible Water-Soluble ZnS Quantum Dot/Chitosan Nanoconjugates, Nanoscale Res. Lett., 2013, 8, 1–13 CrossRef PubMed.
- A. Chenite, M. Buschmann, D. Wang, C. Chaput and N. Kandani, Rheological Characterisation of Thermogelling Chitosan/Glycerol-Phosphate Solutions, Carbohydr. Polym., 2000, 46, 39–47 CrossRef.
- T. Puangkaew, N. Booranabunyat, S. Kiatkamjornwong, P. Thanyasrisuung and V. P. Hoven, Amphiphilic Quaternized Chitosan: Synthesis, Characterization, and Anti-Cariogenic Biofilm Property, Carbohydr. Polym., 2022, 277, 118882 CrossRef PubMed.
- I. Aranaz, A. R. Alcántara, M. C. Civera, C. Arias, B. Elorza, A. Heras Caballero and N. Acosta, Chitosan: An Overview of Its Properties and Applications, Polymers, 2021, 13, 3256 CrossRef CAS PubMed.
- V. M. Lenart, R. de Fátima Turchiello, M. P. Calatayud, G. F. Goy and S. L. Gómez, Synthesis of Magnetite Nanoparticles of Different Size and Shape by Interplay of Two Different Surfactants, Braz. J. Phys., 2019, 49, 829–835 CrossRef CAS.
- D. Zhang, L. Shang, J. Shen, Z. Shi, L. Wu, C. Tung and T. Zhang, A Mild One-Step Solvothermal Route to Truncated Octahedral Magnetite Crystals, Particuology, 2014, 15, 51–55 CrossRef CAS.
- J. Guo, Z. Zheng, C. Chen, X. Lu, Y. Zhang and B. Zheng, Enhanced Production of κ-Carrageenase and κ-Carrageenan Oligosaccharides through Immobilization of Thalassospira sp. Fjfst-332 with Magnetic Fe3O4-Chitosan Microspheres, J. Agric. Food Chem., 2017, 65, 7934–7943 CrossRef CAS PubMed.
- J. Stejskal, I. Sapurina, J. Vilčáková, M. Jurča, M. Trchová, Z. Kolská, J. Prokes and I. Krivka, One-Pot Preparation of Conducting Melamine/Polypyrrole/Magnetite Ferrosponge, ACS Appl. Polym. Mater., 2021, 3, 1107–1115 CrossRef CAS.
- N. Li, Q. Wang, S. Ullah, X. C. Zheng, Z. K. Peng and G. P. Zheng, Esterification of Levulinic Acid in the Production of Fuel Additives Catalyzed by Porous Sulfonated Carbon Derived from Pine Needle, Catal. Commun., 2019, 129, 105755 CrossRef CAS.
- V. Russo, R. Tesser, C. Rossano, T. Cogliano, R. Vitiello, S. Leveneur and M. Di Serio, Kinetic Study of Amberlite IR120 Catalyzed Acid Esterification of Levulinic Acid with Ethanol: from Batch to Continuous Operation, Chem. Eng. J., 2020, 401, 126126 CrossRef CAS.
- L. D. Mthembu, D. Lokhat and N. Deenadayalu, Esterification of levulinic Acid to Ethyl Levulinate: Optimization of Process Conditions Using Commercial Levulinic Acid and Extension to The Use of Levulinic Acid Derived from Depithed Sugarcane Bagasse, Biomass Convers. Biorefin., 2023, 13, 3113–3122 CrossRef CAS.
- P. Dugkhuntod, T. Imyen, W. Wannapakdee, T. Yutthalekha, S. Salakhum and C. Wattanakit, Synthesis of Hierarchical ZSM-12 Nanolayers for Levulinic Acid Esterification with Ethanol to Ethyl Levulinate, RSC Adv., 2019, 9, 18087–18097 RSC.
- M. Popova, P. Shestakova, H. Lazarova, M. Dimitrov, D. Kovacheva, A. Szegedi, G. Mali, V. Dasireddy and B. Likozar, Efficient Solid Acid Catalysts Based on Sulfated Tin Oxides for Liquid Phase Esterification of Levulinic Acid with Ethanol, Appl. Catal., A, 2018, 560, 119–131 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma00181h |
| This journal is © The Royal Society of Chemistry 2024 |