Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One step synthesis of nitrogen-rich green primary explosives from secondary explosives: synthesis, characterization, and performance study†
Parasar
Kumar
 a,
Vikas D.
Ghule
a,
Vikas D.
Ghule
 b and
Srinivas
Dharavath
b and
Srinivas
Dharavath
 *a
*a
aEnergetic Materials Laboratory, Department of Chemistry, Indian Institute of Technology Kanpur, Kanpur-208016, Uttar Pradesh, India. E-mail: srinivasd@iitk.ac.in
bDepartment of Chemistry, National Institute of Technology Kurukshetra, Kurukshetra-136119, Haryana, India
First published on 28th June 2024
Abstract
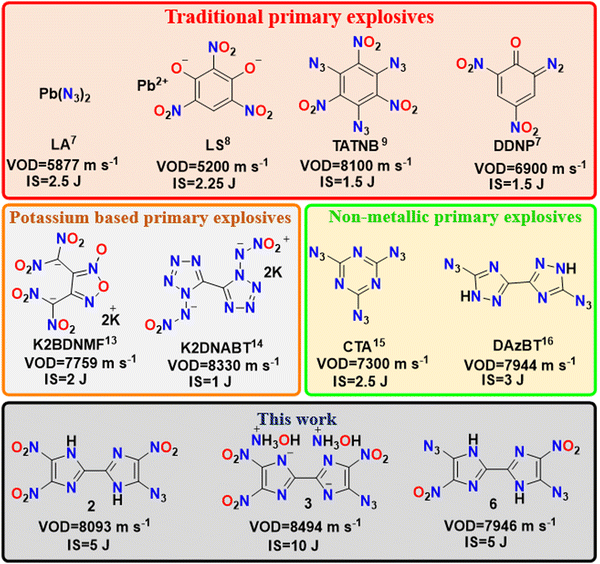
Primary explosives are essential for initiating combustion or detonation in propellants and secondary explosives. Hence, there is a critical need to find a green and safe alternative primary explosive to replace the extremely toxic metal-based compounds. We have synthesized metal-free primary explosives and characterized them using various analytical techniques such as multi-nuclear magnetic resonance (NMR; 1H, 13C, 15N), infrared spectroscopy (IR), elemental analysis (EA), high-resolution mass spectrometry (HRMS) and differential scanning calorimetry (DSC). The structure of 5,5′-diazido-4,4′-dinitro-1H,1′H-2,2′-biimidazole (6) was further supported by single crystal X-ray data. 5-Azido-4,4′,5′-trinitro-1H,1′H-2,2′-biimidazole (2) and dihydroxylammonium-5-azido-4,4′,5′-trinitro-[2,2′-biimidazole]-1,1′-diide (3) show excellent thermal stabilities (252 and 245 °C) and detonation velocities (8093 and 8494 m s−1) compared to diazodinitrophenol (DDNP, 6900 m s−1) and Pb(N3)2 (5877 m s−1). Both compounds 2 and 3 are more insensitive to friction (>240 N) and impact sensitivity (5 to 10 J) than the benchmark materials DDNP (1 J, 24.7 N) and Pb(N3)2 (2.5 J, 0.1 N). Considering the overall fine-tuned performance, these newly synthesized compounds have significant potential to serve as primary explosives.
Introduction
Primary explosives are an essential class of energetic materials and refer to highly sensitive mixtures/compounds crucial in initiating explosive reactions.1,2 Primary explosives can be quickly initiated by small external stimuli or a simple initiating impulse (such as heat, a flame, friction, impact, or an electric spark).3 They are primarily applied in detonators to induce shock for initiating less-sensitive secondary explosives.4 Another vital application of them is as sensitizers in priming mixtures. Primary explosives play a crucial role in the reliable ignition of these priming compositions, making them sensitive to external stimuli enough to be easily ignited.5,6 Lead styphnate, lead azide, mercury fulminate, silver azide, dinol, and tetrazene are classic examples of primary explosives, as shown in Fig. 1.7–9 Notably, concerns over environmental hazards, lead and mercury toxicity, and health threats of the combustion products have led to a search for heavy metal-free compositions that would be less harmful yet have performance properties and other essential characteristics comparable to those of their lead-containing predecessors.10,11 In recently reported works, promising replacements for lead-free primary explosives were obtained using nitrogen-rich materials and their complexes with nontoxic sodium, ammonium, and iron cations.12 Subsequent attempts to develop alternative primary explosives with potassium metal, such as potassium bis(dinitromethanide) furoxan (K2BDNMF),13 potassium dinitramino bistetrazole (K2DNABT),14 and other neutral nitrogen rich heterocyclic compounds like cyanuric acid triazide (CTA)15 and diazidobistriazole (DAzBT),16 have encountered challenges such as insufficient detonation properties and very high sensitivity, which could lead to casualties in the event of premature or accidental explosions. There is a continuous search for effective strategies for the design and synthesis of energetic materials by incorporating explosophores such as –NO2, –NHNO2, –NH2, –N3, driven by diverse objectives.17 One noteworthy approach involves the azidation of heterocyclic backbones, proving to be effective in creating primary explosives.18 Additionally, the incorporation of both azido and nitro groups has been investigated for the ability to create a balance of detonation properties.19 These materials, due to their susceptibility to impact initiation, have been extensively investigated as lead-free primary explosives.20–25 In light of these advancements, imidazoles stand out as a distinctive class of compounds, offering a higher number of functional sites.26 Backbones featuring two or more nitro groups, in conjunction with azido groups, are anticipated to serve as promising energetic ingredients for primary explosive formulations.27–29 The incorporation of a nitro-containing imidazole backbone presents numerous advantages, including a substantial nitrogen and oxygen content, good thermal stability, chemical robustness, and versatile structural functionality.30–32 Introduction of multiple nitro groups in these energetic compounds results in elevated density and favorable oxygen balance.33 To obtain a better understanding of balanced properties, we report the synthesis of 5-azido-4,4′,5′-trinitro-1H,1′H-2,2′-biimidazole (2), and 5,5′-diazido-4,4′-dinitro-1H,1′H-2,2′-biimidazole (6) using the Lewis acid, zinc chloride. Later, compound 2 was treated with various nitrogen-rich bases to obtain energetic salts. Recently, Tang et al. reported the synthesis of compound 6 from the reaction of the diazonium salt of 4,4′-diamino-5,5′-dinitro-1H,1′H-2,2′-biimidazole with a mixture of hydrazine monohydrate and sodium nitrite in acidic conditions or sodium azide.34Synthesis
4,4′,5,5′-Tetranitro-1H,1′H-2,2′-biimidazole (1) was synthesized according to the procedure reported in the literature.35,36 It was treated with different concentrations of sodium azide and zinc chloride in water at 80 °C for 12 hours, followed by acidification to pH = 2 using conc. HCl, which led to the formation of new precipitates, di and mono azido-substituted compounds 5-azido-4,4′,5-trinitro-1H,1′H-2,2′-biimidazole (2) and 5,5′-diazido-4,4′-dinitro-1H,1′H-2,2′-biimidazole (6) in good yield rather than the expected furoxan and tetra azido substituted compounds 4H,4′H-[5,5′-biimidazo[4,5-c][1,2,5]oxadiazole] 1,1′-dioxide (6a) and 4,4′,5,5′-tetraazido-1H,1′H-2,2′-biimidazole (6b). Even after using an excess amount of sodium azide, only compound 6 was produced and attempts to obtain the poly-substituted azide (6b) failed. The main reason behind the failure of this attempt is the addition-elimination mechanism which is not favored after the substitution of two azide. Moreover, to improve the yields of compounds 2 and 6, various solvent screenings were performed as shown in Table S1 (ESI†). The additive ZnCl2 in water was found to be the most suitable combination. Compounds 2 and 6 were then treated with various nitrogen-rich bases such as hydroxyl amine monohydrate, hydrazine monohydrate and 3,5-diaminotriazole in methanol at room temperature to form their energetic salts. We failed to get the energetic salts from compound 6 but compound 2 afforded dihydroxylammonium-5-azido-4,4′,5′-trinitro-[2,2′-biimidazole]-1,1′-diide (3), dihydrazinium-5-azido-4,4′,5′-trinitro-[2,2′-biimidazole]-1,1′-diide (4), and bis-3,5-diamino-4H-1,2,4-triazol-1-ium-5-azido-4,4′,5′-trinitro-[2,2′-biimidazole]-1,1′-diide (5) in quantitative yields as shown in Scheme 1.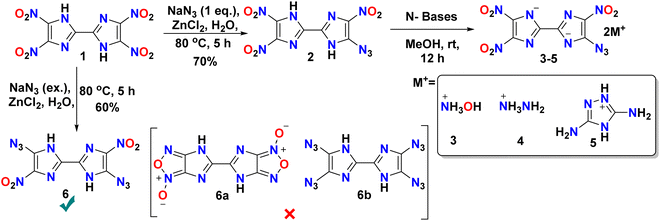 | ||
| Scheme 1 Synthesis of mono and di-azido substituted bis-imidazoles (2 and 6) and energetic salts of 2 (3–5). | ||
15N NMR
Proton decoupled 15N measurements were carried out for compound 3 and are shown in the ESI† (Fig. S9). The nitro group nitrogen signals appeared at δ (ppm) = −25.91, and −35.40; imidazole moiety signals appeared at δ (ppm) −105.21, −117.28, −123.02, and −129.06; azide group signals appeared at δ (ppm) = −157.03, −187.09, −231.50; while the hydroxylammonium peak appeared at −276.70 ppm.Crystal structure
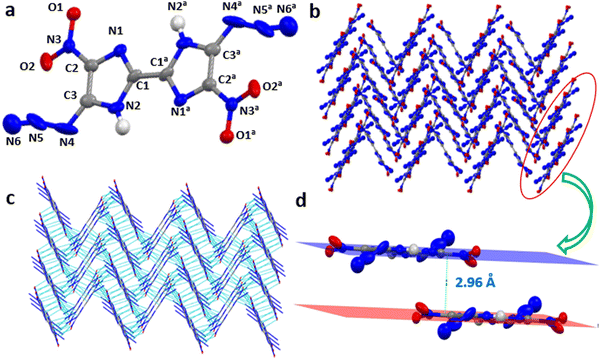
A suitable crystal of 5,5′-diazido-4,4′-dinitro-1H,1′H-2,2′-biimidazole (6) was obtained by slow evaporation of water having monoclinic space group Pc with two molecular units per unit cell and a crystal density of 1.69 g cm−3 at 100 K. Compound 6 has low crystal density as it crystallized with six water molecules. The bond length of C2–N3 is 1.426(4) Å, while that of C1–C1a is 1.453(7) Å and of C2–C3 is 1.365(4) Å, which indicates their partial single and double bond character, respectively. The nitrogen bond lengths in azide are N4–N5= 1.253(7) Å and N5–N6= 1.008(7) Å. The two rings are coplanar with the torsional angle of N1–C1–C1a–N1a = −180.0°. The substituent on C2 (nitro) is in plane with the ring having the bond angle N1–C2–N3 = 120.3°, while the substituent on C3 (azido) is slightly tilted C2–C3–N4 = 139.1°. The interlayer distance between two planes is 2.96 Å. The 3D crystal arrangement shows cross-linked crystal packing with intense non-covalent interactions (NCI) (Fig. 2a–d).Physicochemical properties
Thermal stability is a crucial parameter to assess the resistance of energetic materials to detonation or decomposition when subjected to heat. A higher value of thermal decomposition (Td) indicates better thermal stability and the compound becomes less prone to accidental detonation. The thermal stabilities of newly synthesized poly nitro- and azido-containing imidazole compounds were determined using differential scanning calorimetry (DSC) measurements at a heating rate of 5 °C min−1. As shown in Table 1, the thermal decomposition temperatures (Td) of compounds 2–6 range between 112 and 252 °C. The diazido-substituted compound 6 has a decomposition temperature of 112 °C while its monoazido-substituted bis imidazole derivative 2 has a decomposition temperature of 252 °C. Di-cationic salts of compound 2, such as compounds 3, 4, and 5, possess decomposition temperatures of 245, 131, and 120 °C, respectively. Compounds 2 (252 °C) and 3 (245 °C) have the highest thermal decomposition temperatures, better than those of diazodinitrophenol (DDNP; 157 °C). The experimental densities of all the synthesized compounds (2–6) were measured using a gas pycnometer under helium environment at ambient temperature. The measured densities range between 1.65 and 1.77 g cm−3. The high densities of these molecules ensure better packing, which in turn results in high energy per unit volume. All these compounds possess high nitrogen and oxygen content (>75%). The Gaussian 09 suite of programs was employed to compute the heats of formation (HOF) of synthesized imidazole derivatives.37 Compounds incorporating polyazides exhibit elevated heats of formation, a trend attributed to increased energy content that correlates with the increased number of energetic azido groups within the molecule. The azido group is well known for its high energy content and is frequently employed to enhance HOF by approximately +300 kJ mol−1, an outcome associated with an increase in nitrogen content. Both compounds 2 and 6 demonstrate highly positive HOF values, 458 and 755 kJ mol−1, respectively. This high energy content is attributed to the presence of azido groups and a high number of C–N and N–N bonds within these compounds. The detonation velocity (VOD) and detonation pressure (DP) for all the synthesized compounds were evaluated using Explo-5 (version 7.01.01).38 This assessment utilized measured densities and calculated heats of formation (HOFs). Neutral compounds 2 and 6 possess good VOD (8093, 7946 m s−1) and DP (26.7, 24.9 GPa) properties. Energetic salts of compound 2 possess good detonation values such as compounds 3 (8494 m s−1, 30 GPa), 4 (8072 m s−1, 25.1 GPa), and 5 (7593 m s−1, 21.3 GPa), which are better than those of classical primary explosives, DDNP (6900 m s−1, 24.2 GPa) and lead azide (5877 m s−1, 33.4 GPa). To ensure safe handling, storage, and transportation of energetic materials, the sensitivities of compounds 2–6 to mechanical shock (impact) and friction were measured. The data for the impact and friction tests are summarized in Table 1. The impact and friction sensitivities of these compounds fall in the ranges 5–10 J and 240–360 N, respectively. Among them, neutral compounds 2 and 6 are comparatively more susceptible to impact (IS = 5 J) than its salts (IS = 7.5–10 J), but are better than DDNP (IS = 1 J) and lead azide (IS = 2.5 J). However, the friction-sensitivity tests show that all the compounds are insensitive towards friction (FS = 240–360 N).| Compd | T d [°C] | ρ [g cm−3] | HOFc [kJ mol−1] | VODd [m s−1] | DPe [GPa] | N + Of [%] | ISg [J] | FSh [N] |
|---|---|---|---|---|---|---|---|---|
| a Onset decomposition temperature under nitrogen gas (DSC, 5 °C min−1). b Density measured by Anton parr Ultrapyc 5000 micro gas pycnometer (25 °C). c Computed heat of formation. d Detonation velocity. e Detonation pressure. f Nitrogen and oxygen percentage. g Impact sensitivity. h Friction sensitivity. i Ref. 7. | ||||||||
| 2 | 252 | 1.77 | 458 | 8093 | 26.7 | 76.11 | 5 | 240 |
| 3 | 245 | 1.77 | 466 | 8494 | 30.0 | 78.70 | 10 | 360 |
| 4 | 131 | 1.65 | 684 | 8072 | 25.1 | 78.05 | 7.5 | 360 |
| 5 | 120 | 1.69 | 819 | 7593 | 21.3 | 73.99 | 10 | 360 |
| 6 | 112 | 1.76 | 755 | 7946 | 24.9 | 75.80 | 5 | 240 |
| DDNP | 157 | 1.72 | 321 | 6900 | 24.2 | 64.74 | 1 | 24.7 |
| Pb(N3)2 | 315 | 4.8 | 450.1 | 5877 | 33.4 | 28.86 | 2.5 | 0.1 |
Conclusion
In summary, we have synthesized 5-azido-4,4′,5′-trinitro-1H,1′H-2,2′-biimidazole (2), its energetic salts (3–5), and 5,5′-diazido-4,4′-dinitro-1H,1′H-2,2′-biimidazole (6) in good yields. Compounds 2, 3, and 6 showed good densities (>1.76 g cm−3). As a metal-free primary explosive, compound 3 revealed good detonation velocity and detonation pressure of 8494 m s−1 and 30.00 GPa, respectively. Compounds 2 and 4 also have good detonation velocities of 8093 and 8072 m s−1 and detonation pressures of 26.7 and 25.1 GPa, respectively. Compounds 2 and 3 show high thermal stabilities of 252 and 245 °C, respectively. All the synthesized compounds are sensitive to impact (5–10 J) and less sensitive to friction (240–360 N). The high nitrogen and oxygen percentages (>75%) in their molecular backbones also ensure that fewer toxic gases are released after explosion. Considering all the aspects, these molecules can be used as a potential metal-free primary explosive in the defence field.Author contributions
Parasar Kumar: conceptualization; methodology; investigation, validation; formal analysis; writing – original draft. Vikas D. Ghule: investigation, validation, software, formal analysis, review & editing. Srinivas Dharavath: conceptualization; methodology; validation, supervision; funding acquisition; software, project administration; writing – original draft; writing – review & editing. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
The data underlying this study are available in the published article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
PK thanks IIT Kanpur for the fellowship and infrastructure. SD is grateful for the financial support from the core research grant (No. CRG/2023/000007), Science and Engineering Research Board, Department of Science and Technology, Government of India.References
- H. Gao and J. M. Shreeve, Azole-Based Energetic Salts, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS.
- S. Banik, A. K. Yadav, P. Kumar, V. D. Ghule and S. Dharavath, Unfolding the Chemistry of FOX-7: Unique Energetic Material and Precursor with Numerous Possibilities, Chem. Eng. J., 2022, 431, 133378 CrossRef CAS.
- W. Huang, Y. Tang, G. H. Imler, D. A. Parrish and J. M. Shreeve, Nitrogen-Rich Tetrazolo[1,5-b]Pyridazine: Promising Building Block for Advanced Energetic Materials, J. Am. Chem. Soc., 2020, 142, 3652–3657 CrossRef CAS.
- M. Benz, T. M. Klapotke, J. Stierstorfer and M. Voggenreiter, Synthesis and Characterization of Binary, Highly Endothermic, and Extremely Sensitive 2,2′-Azobis5-Azidotetrazole, J. Am. Chem. Soc., 2022, 144, 6143–6147 CrossRef CAS.
- Y. Du, J. Zhang, P. Peng, H. Su, S. Li and S. Pang, Synthesis and characterization of three pyrazolate inner diazonium salts: green, powerful and stable primary explosives, New J. Chem., 2017, 41, 9244 RSC.
- Y. Huang, Y. Zhang and J. M. Shreeve, Nitrogen-Rich Salts Based on Energetic Nitroaminodiazido[1,3,5]Triazine and Guanazine, Chem. – Eur. J, 2011, 17, 1538–1546 CrossRef CAS PubMed.
- P. Kumar, N. Kumar, V. D. Ghule and S. Dharavath, Zwitterionic fused pyrazolo-triazole based high performing energetic materials, Chem. Commun., 2024, 60, 1646–1649 RSC.
- R. Matyáš and J. Pachman, Prim. Explos., 2012, 9783642284366, 1–338 Search PubMed.
- D. Adam, K. Karaghiosoff, T. M. Klapötke, G. Holl and M. Kaiser, Triazidotrinitro Benzene: 1,3,5-(N3)3-2,4,6-(NO2)3C6, Propellants, Explos. Pyrotech., 2002, 27, 7–11 CrossRef CAS.
- J. Zhang, Y. Feng, Y. Bo, A. K. Chinnam, J. Singh, R. J. Staples, X. He, K. Wang, J. Zhang and J. M. Shreeve, Synthesis of a high-energy-density material through rapid replacement of crystal water of hydrates, Chem, 2022, 8, 2678–2687 CAS.
- J. W. Fronabarger, M. D. Williams, W. B. Sanborn, J. G. Bragg, D. A. Parrish and M. Bichay, DBX-1 – A Lead Free Replacement for Lead Azide, Propellants, Explos., Pyrotech., 2011, 36, 541–550 CrossRef CAS.
- M. H. V. Huynh, M. A. Hiskey, T. J. Meyer and M. Wetzler, Green Primaries: Environmentally Friendly Energetic Complexes, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 5409–5412 CrossRef CAS PubMed.
- J. Ma, J. Tang, H. Yang, Z. Yi, G. Wu, S. Zhu, W. Zhang, Y. Li and G. Cheng, Polynitro-Functionalized Triazolylfurazanate Triaminoguanidine: Novel Green Primary Explosive with Insensitive Nature, ACS Appl. Mater. Interfaces, 2019, 11, 26053–26059 CrossRef CAS.
- A. K. Yadav, V. D. Ghule and S. Dharavath, Promising Thermally Stable Energetic Materials with the Combination of Pyrazole–1,3,4-Oxadiazole and Pyrazole–1,2,4-Triazole Backbones: Facile Synthesis and Energetic Performance, ACS Appl. Mater. Interfaces, 2022, 14(44), 49898–49908 CrossRef CAS.
- L. M. Foroughi, R. A. Wiscons, D. R. Du Bois and A. J. Matzger, Improving Stability of the Metal-Free Primary Energetic Cyanuric Triazide (CTA) through Cocrystallization, Chem. Commun., 2020, 56, 2111 RSC.
- A. A. Dippold and T. M. Klapötke, Nitrogen-Rich Bis-1,2,4-Triazoles—A Comparative Study of Structural and Energetic Properties, Chem. – Eur. J., 2012, 18, 16742–16753 CrossRef CAS PubMed.
- A. K. Yadav, V. D. Ghule and S. Dharavath, Facile fabrication of functionalized pyrimidine derivatives: constructing a new family of high performance and less sensitive energetic compounds, J. Mater. Chem. A, 2022, 10, 12702–12712 RSC.
- C. Ye, H. Gao, J. A. Boatz, G. W. Drake, B. Twamley and J. M. Shreeve, Polyazidopyrimidines: High-Energy Compounds and Precursors to Carbon Nanotubes, Angew. Chem., Int. Ed., 2006, 45, 7262–7265 CrossRef CAS.
- M. Benz, T. M. Klapötke and J. Stierstorfer, 1-Nitrimino-5-Azidotetrazole: Extending Energetic Tetrazole Chemistry, ChemPlusChem, 2022, 87, e202200186 CrossRef CAS PubMed.
- M. Deng, Y. Feng, W. Zhang, X. Qi and Q. Zhang, A green metal-free fused-ring initiating substance, Nat. Commun., 2019, 10, 1339 CrossRef.
- P. Kumar, V. D. Ghule and S. Dharavath, Facile Synthesis of Thermally Stable Tetrazolo[1,5-b][1,2,4]Triazine Substituted Energetic Materials: Synthesis and Characterization, Dalt. Trans., 2023, 52, 747–753 RSC.
- P. Kumar, V. D. Ghule and S. Dharavath, 1,3,5-Tris[(2 H-Tetrazol-5-Yl)Methyl]Isocyanurate and Its Tricationic Salts as Thermostable and Insensitive Energetic Materials, Org. Lett., 2022, 24, 3555–3559 CrossRef CAS.
- S. Manzoor, Q. U. Nisa Tariq, X. Yin and J. G. Zhang, Nitro-Tetrazole Based High Performing Explosives: Recent Overview of Synthesis and Energetic Properties, Def. Technol, 2021, 17, 1995–2010 CrossRef.
- P. Yin, Q. Zhang and J. M. Shreeve, Dancing with Energetic Nitrogen Atoms: Versatile N-Functionalization Strategies for N-Heterocyclic Frameworks in High Energy Density Materials, Acc. Chem. Res., 2016, 49, 4–16 CrossRef CAS.
- O. T. O’Sullivan and M. J. Zdilla, Properties and Promise of Catenated Nitrogen Systems As High-Energy-Density Materials, Chem. Rev., 2020, 120, 5682–5744 CrossRef PubMed.
- C. Xie, L. Pei, J. Cai, P. Yin and S. Pang, Imidazole-Based Energetic Materials: A Promising Family of N-Heterocyclic Framework, Chem. – Asian J., 2022, 17, e202200829 CrossRef CAS PubMed.
- S. Feng, B. Zhang, C. Luo, Y. Liu, S. Zhu, R. Gou, S. Zhang, P. Yin and S. Pang, Challenging the Limitations of Tetranitro Biimidazole through Introducing a Gem-Dinitromethyl Scaffold, Org. Lett., 2023, 25, 1290–1294 CrossRef CAS.
- Y. F. Li, Z. Y. Wang, X. H. Ju and X. W. Fan, Computational Study of Substituted 2,2 0-Bi-1H-Imidazole as High Energetic Materials, J. Mol. Struct. Theochem., 2009, 907, 29–34 CrossRef CAS.
- L. Liu, P. Sun, Y. Zhang and A. Pang, Energetic Double Salts Based on 4, 4′,5, 5′-Tetranitro-2, 2′-Biimidazolate, J. Mol. Struct., 2020, 1208, 127861 CrossRef CAS.
- Y. Zhang, H. Gao, Y. H. Joo and J. M. Shreeve, Ionic Liquids as Hypergolic Fuels, Angew. Chem., Int. Ed., 2011, 50, 9554–9562 CrossRef CAS PubMed.
- Y. Xu, C. Shen, Q. Lin, P. Wang, C. Jiang and M. Lu, 1-Nitro-2-Trinitromethyl Substituted Imidazoles: A New Family of High Performance Energetic Materials, J. Mater. Chem. A, 2016, 4, 17791–17800 RSC.
- T. M. Klapötke, P. C. Schmid and S. Schnell, Thermal Stabilization of Energetic Materials by the Aromatic Nitrogen-Rich 4, 4′,5, 5′-Tetraamino-3, 3′-Bi-1,2,4-Triazolium Cation, J. Mater. Chem. A, 2015, 3, 2658–2668 RSC.
- S. Banik, P. Kumar, V. D. Ghule, S. Khanna, D. Allimuthu and S. Dharavath, Facile Synthesis of Nitroamino-1,3,4-Oxadiazole with Azo Linkage: A New Family of High-Performance and Biosafe Energetic Materials, J. Mater. Chem. A, 2022, 10, 22803–22811 RSC.
- Y. Lai, Y. Liu, W. Huang, Z. Zeng, H. Yang and Y. Tang, Synthesis and characterization of pyrazole- and imidazole- derived energetic compounds featuring ortho azido/nitro groups, FirePhysChem, 2022, 2, 140–146 CrossRef.
- T. M. Klapötke, A. Preimesser and J. Stierstorfer, Energetic Derivatives of 4, 4′,5, 5′-Tetranitro-2, 2′-Bisimidazole (TNBI), Z. Anorg. Allg. Chem., 2012, 638, 1278–1286 CrossRef.
- S. G. Cho, J. R. Cho, E. M. Goh, J. K. Kim, R. Damavarapu and R. Surapaneni, Synthesis and Characterization of 4,4′,5,5′-Tetranitro-2,2′-Bi-1H-Imidazole (TNBI), Propellants, Explos., Pyrotech., 2005, 30, 445–449 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision E.01, Gaussian, Inc, Wallingford, CT, 2013 Search PubMed.
- M. Suceska, EXPLO5; Brodarski Institute: Zagreb, Croatia, 2013.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterization data, computational details, crystal refinements, X-ray crystallographic file in CIF format for 6. This material is available free of charge. CCDC 2312111. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ma00430b |
| This journal is © The Royal Society of Chemistry 2024 |