Open Access Article
Open Access ArticleEDTA-functionalized hierarchical porous microspheres for effective cobalt ion recovery from water†
Mao-Hsuan
Peng
and
Chia-Chen
Li
 *
*
Department of Materials Science and Engineering, National Tsing Hua University, Hsinchu 30013, Taiwan. E-mail: cc.li@mx.nthu.edu.tw
First published on 10th September 2024
Abstract
The new adsorbent EDTA@PSV, which is a hierarchical porous microsphere modified with ethylenediaminetetraacetic acid, has demonstrated its effectiveness in extracting valuable metals. With a high surface area of 665 m2 g−1 and a large porosity of 90%, it exhibits significant potential for adsorbing Co2+, as supported by density functional theory simulations and experimental results.
Introduction
The sustainable utilization of valuable metals in industry is increasingly important due to metal resource scarcity and higher pollution levels. In order to promote a circular economy,1 which emphasizes resource recovery and recycling rather than wasteful consumption, it is crucial to extract indestructible metal elements from waste. This way, we can innovate and create new materials that align with sustainable industrial practices. Several methods have been developed for removing or recovering valuable metal ions from wastewater. These include chemical precipitation,2 chemical oxidation–reduction,3 electrochemical treatment,4 and adsorption.5,6 Among these methods, adsorption is the most effective, as it generates less sludge and is more cost-effective than other techniques.In the field of adsorption techniques, a wide range of adsorbents have been proposed, from activated carbon to metal–organic frameworks and polymers.7,8 However, many of these materials struggle to achieve satisfactory adsorption capacities and rates. To address this issue, surface modification of adsorbents with ligands such as ethylene diamine tetraacetic acid (EDTA) has become increasingly popular.9,10 EDTA possesses noteworthy chelating properties, thanks to its four carboxylic acids and two amine groups, which allow it to form stable water-soluble complexes with various transition metal ions, even at neutral pH levels. EDTA, which is a promising candidate for improving adsorption performance, has been widely used in industry and laboratory applications due to its high sorption efficiency, selectivity, and sensitivity.11,12
Cobalt is a valuable metal that is highly important in various industrial and military applications.13 It is classified as a strategic metal asset by several countries and is included in the European Union's list of 34 critical raw materials.14 This highlights its scarcity as it only makes up 0.001% of the Earth's crust. Cobalt is usually obtained as a by-product of copper and nickel refining, but its importance goes beyond traditional uses. For example, it plays a crucial role in aerospace technology where it is used to create superalloys that are essential for aircraft turbine engines.15 Moreover, it is a key component in cathode materials such as lithium cobalt oxide (LiCoO2)16 and lithium nickel manganese cobalt oxides (LiNixMnyCo1−x−yO2),17 which are used in lithium-ion batteries (LIBs) that power a wide range of electronic devices such as computers and communication equipment. Due to cobalt‘s criticality and its higher required amount (5–10% w/w) in battery applications compared to its natural availability, an efficient recovery method from waste is essential. Recycling cobalt from industrial waste streams can reduce the environmental impact of mining, conserve resources, and ensure a stable supply for the future. Furthermore, it is possible to utilize recycled metal-adsorbent complexes to develop novel materials, such as catalysts for organic pollution degradation18 or electromagnetic wave absorption,19,20 thereby adding value to the recycling process and contributing to environmental sustainability.
It has been suggested in the literature that chitosan beads and silica gel can be used as adsorbents to recover cobalt ions (Co2+ or Co3+).21,22 To improve their adsorption capability, it has been recommended that these adsorbents be modified with ligands like EDTA. Nevertheless, the adsorption efficiency of the ligand-based adsorbent still remains insufficient and poses a challenge in the field.23,24 To address the issue, this study proposes using a highly porous microsphere with a hierarchical pore structure as the adsorbent. The porous microsphere has a substantial specific surface area and the presence of numerous surface functional groups. It will be functionalized with EDTA to enhance its ability to adsorb Co2+. In this preliminary exploration, the adsorption efficiency of the EDTA-modified porous microsphere will be evaluated. Based on the analyses including adsorption isotherms, X-ray photoelectron spectroscopy (XPS), kinetic studies, and simulations utilizing density functional theory (DFT), the adsorption behavior will be explained.
Experimental
The synthesis of poly(styrene-co-vinyl benzyl chloride) (PSV) and the subsequent production of PSV porous microspheres, namely @PSV, have been described in detail in our prior research.25 The @PSV was cross-linked by immersing it in hot concentrated sulfuric acid (95%, Honeywell, Germany) at 120 °C for 1 h. This acid-treated product, called ac@PSV, was rinsed with deionized water multiple times and then dried. The amination process involved adding 0.1 g of ac@PSV to a mixture of 30 mL ethylenediamine (EDA; 99%, Thermo Scientific, Germany) and methanol (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 ratio) and allowing the reaction to proceed under reflux at 80 °C for 7 h.26,27 The resulting precipitate, called en@PSV, was washed with methanol before drying in a vacuum oven at 30 °C for at least 24 h. For grafting with ethylenediaminetetraacetic acid (EDTA), 0.1 g of en@PSV was mixed with 0.5 g of ethylenediaminetetraacetic dianhydride (EDTA-dianhydride; ≥98.0%, Aladdin, China) in 120 mL N,N-dimethylacetamide (DMAc; 99.5%, Echo, Taiwan) and subjected to a reaction under reflux at 75 °C for 20 h.28 After the reaction, the resulting product, EDTA@PSV, was washed with DMAc, a saturated aqueous solution of sodium bicarbonate (99.5%, Showa, Japan), and several rounds of deionized water before final drying in a vacuum oven at 55 °C.
15 ratio) and allowing the reaction to proceed under reflux at 80 °C for 7 h.26,27 The resulting precipitate, called en@PSV, was washed with methanol before drying in a vacuum oven at 30 °C for at least 24 h. For grafting with ethylenediaminetetraacetic acid (EDTA), 0.1 g of en@PSV was mixed with 0.5 g of ethylenediaminetetraacetic dianhydride (EDTA-dianhydride; ≥98.0%, Aladdin, China) in 120 mL N,N-dimethylacetamide (DMAc; 99.5%, Echo, Taiwan) and subjected to a reaction under reflux at 75 °C for 20 h.28 After the reaction, the resulting product, EDTA@PSV, was washed with DMAc, a saturated aqueous solution of sodium bicarbonate (99.5%, Showa, Japan), and several rounds of deionized water before final drying in a vacuum oven at 55 °C.
In the adsorption experiment, the aqueous solution of cobalt sulfate heptahydrate (CoSO4·7H2O; 99%, Showa, Japan) was used to model the Co2+ in wastewater. Firstly, CoSO4·7H2O was dissolved in water to create standard solutions with various Co2+ concentrations ([Co2+]) of 200, 300, 600, 800, 1000, and 1200 mg L−1, and the pH of the aqueous solution was fixed at 6. A calibration curve was created by measuring the absorbance peak intensity of the aqueous solution in the visible light range (wavelength: 350–800 nm) using a UV-Vis spectrometer (U-3010, Hitachi, Japan). In the isothermal adsorption test, various 10 mL aqueous solutions with different [Co2+] were prepared at a fixed pH of 6. Then, 20 mg of EDTA@PSV was added, and the mixture was stirred at 25 °C for 24 h. After that, EDTA@PSV was separated, and the concentration of non-adsorbed Co2+ in the filtrate was determined using UV-Vis. The amount of Co2+ adsorbed was calculated based on mass balance. In an adsorption kinetic test, a 10 mL aqueous solution with a fixed [Co2+] of 1000 mg L−1 was prepared, with pH fixed at 6. 20 mg of EDTA@PSV was added, and the mixture was kept at 25 °C for different time durations. The EDTA@PSV was then separated, and the non-adsorbed [Co2+] in the filtrate and the adsorbed [Co2+] onto EDTA@PSV was determined via UV-Vis adsorption measurements.
The morphology and composition of the @PSV were analyzed using field-emission scanning electron microscopy (FE-SEM; SU8010, Hitachi, Japan) and an energy-dispersive X-ray spectrometer (EDS; EMAX, Horiba, Japan). Pore size was measured by a mercury porosimeter (Autopore IV 9520, Micromeritics Instrument Co., USA). The surface area was measured using the gas adsorption method (ASAP 2020, Micrometrics Instrument Co., USA). Surface chemistry of various porous microspheres was characterized using Fourier-transform infrared spectroscopy (FT-IR; Vertex 80v, Bruker, Germany) and XPS (PHI 5000 Versaprobe II, ULVAC-PHI, Japan). Notably, all samples were stored in a nitrogen desiccator prior to analysis, and the FT-IR spectrometer was continuously purged with N2 gas during measurements. Zeta potential was assessed using the electroacoustic method (ZetaProbe, Colloidal Dynamics Inc., North Attleborough, MA, USA), and a contact angle test (FTA-1000B, First Ten Angstroms, USA) was also conducted. To understand how EDTA@PSV interacts with Co2+, we carried out a simulation based on the DFT method using the DMol3 module within the Materials Studio 2020 software package. The specific parameters for the numerical calculations are listed in Table S1 (ESI†).
Results and discussion
The image displayed in Fig. 1a illustrates the morphology of the synthesized @PSV, with particle size varying from 5 to 20 μm. Each microsphere has evenly distributed pores throughout its entire volume, as seen in the magnified image in Fig. 1b. The pores are present in different sizes, as shown in Fig. 1c, with an average large pore size of 3 μm, medium pore size of 300 nm, and small pore size of 3 nm. The @PSV has a high porosity of 90%25 and a high surface area of 665 m2 g−1 (Fig. S1, ESI†). These characteristics, along with the hierarchical pores, make it a potential adsorbent for metal ions. However, the @PSV is hydrophobic and poorly wetted by water, as revealed in Fig. 1d, where the contact angle is greater than 129°. This hydrophobic feature may limit its practical use in aqueous systems. To be an effective adsorbent for recycling valuable metals or removing harmful heavy metals from industrial wastewater, the @PSV should undergo surface modification with the potential ligand EDTA to enhance its capability of attracting adsorbate.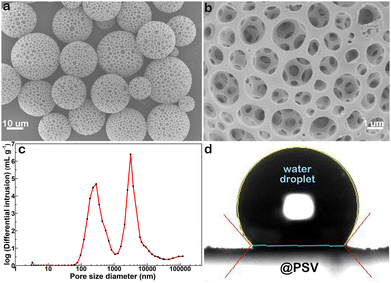 | ||
| Fig. 1 (a) and (b) SEM images of @PSV with small (a) and large (b) magnifications. (c) Size distribution of pores in @PSV. (d) Contact angle measurement for @PSV. | ||
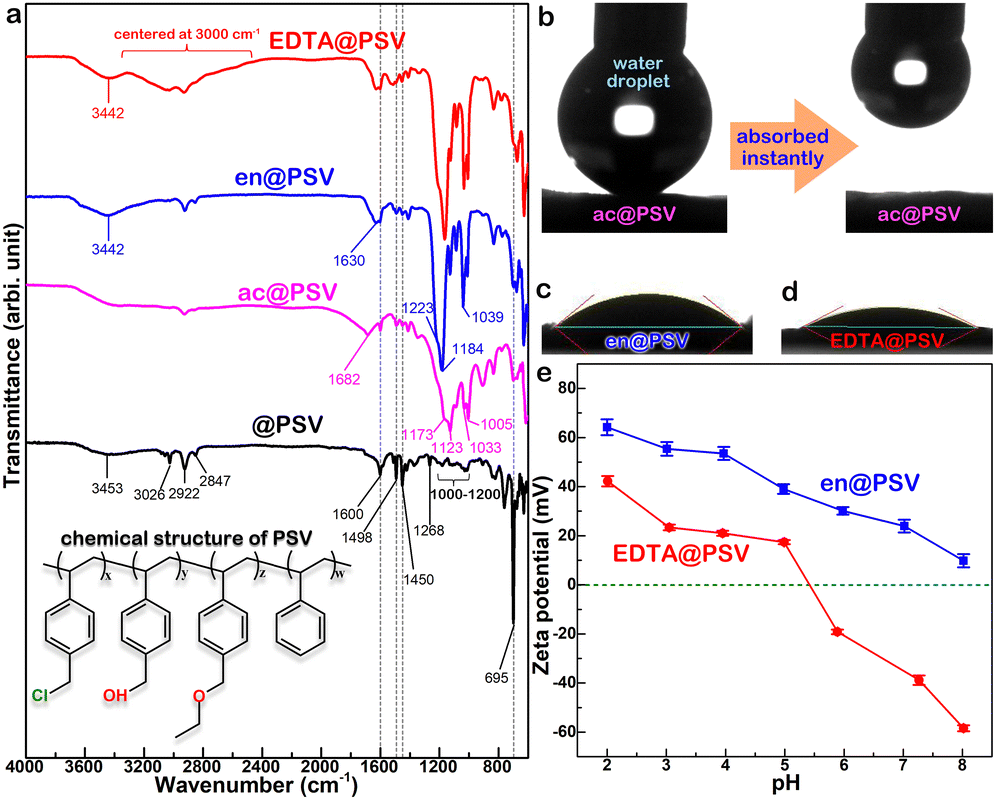
Prior to modification with EDTA, crosslinking converted @PSV to ac@PSV, resulting in improved structural and thermal stabilities (Fig. S2, ESI†).25,29,30 Next, the reaction of the ac@PSV with amine yielded en@PSV,26,27 which contains –NH2 groups and is capable of reacting with EDTA-dianhydride to form EDTA@PSV.26,28 The FT-IR spectra of @PSV, ac@PSV, en@PSV, and EDTA@PSV are shown in Fig. 2a, with the chemical structure of PSV displayed in the inset. For @PSV, the absorption band centered at 3453 cm−1 is usually associated with O–H stretching, which corresponds to the hydroxyl group. The benzene ring of @PSV shows vibrations that correspond to the aromatic C–H stretch at 3026 cm−1 and the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretches at 1600, 1498, and 1450 cm−1.31 The absorptions at 2922 and 2847 cm−1 are assigned to the C–H stretches. The peaks at 1000–1200 cm−1 are assigned to the C–O stretching and may belong to the –CH2OH or –CH2OC2H5 groups of @PSV. The peak at 1268 cm−1 could correspond to the –CH2Cl wagging, and that at 695 cm−1 could be related to the C–Cl stretch, both of which can correlate with the benzyl chloride of @PSV.31,32 In the IR absorptions of ac@PSV, there is a reduction in the intensity of the peaks related to benzyl chloride, particularly the one at 695 cm−1. This suggests that the crosslinking reaction may have taken place via the –CH2Cl group.30 Additionally, there is an emergence of peaks that correspond to the asymmetric S
C stretches at 1600, 1498, and 1450 cm−1.31 The absorptions at 2922 and 2847 cm−1 are assigned to the C–H stretches. The peaks at 1000–1200 cm−1 are assigned to the C–O stretching and may belong to the –CH2OH or –CH2OC2H5 groups of @PSV. The peak at 1268 cm−1 could correspond to the –CH2Cl wagging, and that at 695 cm−1 could be related to the C–Cl stretch, both of which can correlate with the benzyl chloride of @PSV.31,32 In the IR absorptions of ac@PSV, there is a reduction in the intensity of the peaks related to benzyl chloride, particularly the one at 695 cm−1. This suggests that the crosslinking reaction may have taken place via the –CH2Cl group.30 Additionally, there is an emergence of peaks that correspond to the asymmetric S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the SO3 group at 1173 and 1123 cm−1 and the symmetric SO3 vibrations at 1033 and 1005 cm−1, indicating the sulfonation of @PSV.31,33 Moreover, the appearance of a new peak at 1682 cm−1 related to the carbonyl (C
O stretching of the SO3 group at 1173 and 1123 cm−1 and the symmetric SO3 vibrations at 1033 and 1005 cm−1, indicating the sulfonation of @PSV.31,33 Moreover, the appearance of a new peak at 1682 cm−1 related to the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibration of conjugated ketones or aldehydes confirms the oxidation of some –OH groups of @PSV.
O) vibration of conjugated ketones or aldehydes confirms the oxidation of some –OH groups of @PSV.
 | ||
| Fig. 2 (a) FT-IR spectra of various porous microspheres. Contact angle measurements for (b) ac@PSV, (c) en@PSV, and (d) EDTA@PSV. (e) Zeta potential curves of en@PSV and EDTA@PSV. | ||
The FT-IR spectrum of en@PSV displays some similarities with the spectra of @PSV and ac@PSV. However, a new peak centered at 1630 cm−1 appears, which should be associated with the N–H bending of a primary amine or the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of an imine.34 The broad band centered at 3442 cm−1 is due to the overlapping stretches of O–H and N–H. The multiple absorption peaks located between 1039 cm−1 and 1223 cm−1 correspond to the C–N vibrations. All these observations confirm a successful amination process. After reacting with EDTA-dianhydride, the resulting EDTA@PSV shows IR absorptions similar to en@PSV. This suggests that there might be some amine groups that remained unreacted on EDTA@PSV.
N of an imine.34 The broad band centered at 3442 cm−1 is due to the overlapping stretches of O–H and N–H. The multiple absorption peaks located between 1039 cm−1 and 1223 cm−1 correspond to the C–N vibrations. All these observations confirm a successful amination process. After reacting with EDTA-dianhydride, the resulting EDTA@PSV shows IR absorptions similar to en@PSV. This suggests that there might be some amine groups that remained unreacted on EDTA@PSV.
Additionally, a broad band centered around 3000 cm−1 is observed on top of the usual C–H absorption. This band is typically attributed to the O–H stretch of a carboxylic acid group.31 This finding indicates that the –NH groups from en@PSV had reacted with the EDTA-dianhydride. Based on the FT-IR characterization results, we proposed the potential reactions that took place during the syntheses of ac@PSV, en@PSV, and EDTA@PSV as outlined in Scheme 1. In Scheme 1a, the reaction with concentrated H2SO4 not only caused PSV to crosslink but should have resulted in the sulfonation of PSV and the oxidation of the carried –OH. Additionally, apart from the amination, the ketone and aldehyde groups of ac@PSV may have undergone condensation with ethylenediamine.34,35
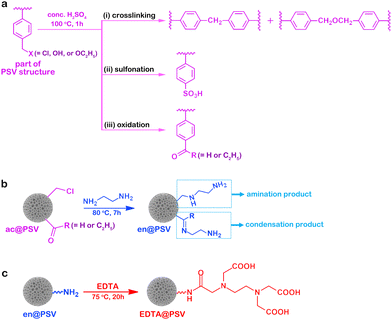 | ||
| Scheme 1 Potential reactions occurring during the syntheses of (a) ac@PSV, (b) en@PSV, and (c) EDTA@PSV. | ||
During the contact angle measurement, it was observed that the water-repelling nature of @PSV (Fig. 1d) changed after it was converted to ac@PSV. As demonstrated in Fig. 2b, the ac@PSV absorbs the dripping water upon contact, revealing its higher hydrophilicity with a contact angle of nearly 0°. After amination, en@PSV shows a different level of hydrophilicity, with an increased contact angle of 40°, as shown in Fig. 2c. EDTA@PSV shows a slight improvement in hydrophilicity compared to en@PSV, with a reduced contact angle of 33°, as shown in Fig. 2d. Although the contact angles of en@PSV and EDTA@PSV are similar, zeta potential measurements demonstrate that they have distinct surface chemistries, as depicted in Fig. 2e. At pH <8, en@PSV exhibits positive zeta potentials, and there is no presence of an isoelectric point (IEP). The IEP is the pH at which the positive and negative charge densities on a particle surface are equal, resulting in zero net charge. On the other hand, EDTA@PSV has an IEP of pH 5–6, meaning that its surface is negatively charged at a pH greater than 5–6 and positively charged at other pH values. Besides, EDTA@PSV has a more negative zeta potential compared to en@PSV, making it an effective electron donor and chelator. When the pH of the solution is above 5, the negative zeta potentials of EDTA@PSV should facilitate the attraction for metal ion adsorption.
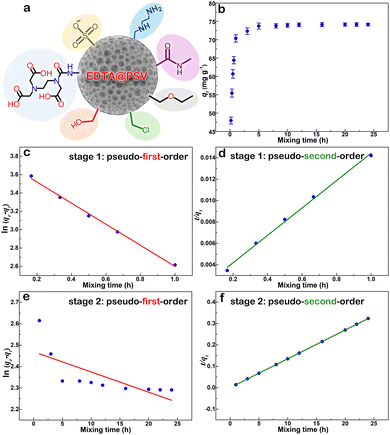
It has been previously identified through FT-IR analysis that EDTA@PSV contains several functional groups such as –CH2Cl, –CH2OH, –CH2OC2H5, –SO3, –NH2, –N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–, and the –COOH and –NH– of EDTA, as depicted in Fig. 3a. EDTA@PSV works as an adsorbent and the amount of Co2+ adsorbed has been estimated using UV-Vis spectroscopy (Fig. S3, ESI†). Fig. 3b presents the adsorption kinetics of Co2+ onto EDTA@PSV at pH = 6 by measuring the adsorption amount (qt) as a function of mixing time. It is noteworthy that Co2+ remains soluble in aqueous solutions up to pH 7 (Fig. S4, ESI†). A more alkaline solution will cause precipitation with OH−, which can disrupt the adsorption experiment. In Fig. 3b, an initial concentration of Co2+ at 1000 mg L−1 was used as an example, and it can be observed that the adsorption process occurs in two stages. During the first stage, the adsorption amount increases to over 70 mg g−1 within an hour. In the second stage, the adsorption continues to progress, but at a slower rate until the amount reaches an equilibrium at a plateau. Both stages were analyzed using the rate equations of pseudo-first-order (Lagergren model as in eqn (1)) and pseudo-second-order (Ho and Mckay model as in eqn (2)) models.26,36 The pseudo-first-order kinetic model assumes that physisorption limits the adsorption rate, while the pseudo-second-order model considers chemisorption as the rate-limiting mechanism.
O)–, and the –COOH and –NH– of EDTA, as depicted in Fig. 3a. EDTA@PSV works as an adsorbent and the amount of Co2+ adsorbed has been estimated using UV-Vis spectroscopy (Fig. S3, ESI†). Fig. 3b presents the adsorption kinetics of Co2+ onto EDTA@PSV at pH = 6 by measuring the adsorption amount (qt) as a function of mixing time. It is noteworthy that Co2+ remains soluble in aqueous solutions up to pH 7 (Fig. S4, ESI†). A more alkaline solution will cause precipitation with OH−, which can disrupt the adsorption experiment. In Fig. 3b, an initial concentration of Co2+ at 1000 mg L−1 was used as an example, and it can be observed that the adsorption process occurs in two stages. During the first stage, the adsorption amount increases to over 70 mg g−1 within an hour. In the second stage, the adsorption continues to progress, but at a slower rate until the amount reaches an equilibrium at a plateau. Both stages were analyzed using the rate equations of pseudo-first-order (Lagergren model as in eqn (1)) and pseudo-second-order (Ho and Mckay model as in eqn (2)) models.26,36 The pseudo-first-order kinetic model assumes that physisorption limits the adsorption rate, while the pseudo-second-order model considers chemisorption as the rate-limiting mechanism.
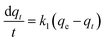 | (1) |
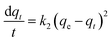 | (2) |
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t | (3) |
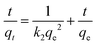 | (4) |
During the analysis for stage 1, we generated plots of ln(qe − qt) vs. t and t/qtvs. t, which are depicted in Fig. 3c and d, respectively. By utilizing the slope and intercept derived from these plots, we are able to calculate the adsorption parameters, including qe, k1, and k2. We repeated this process for stage 2 analysis and displayed the results in Fig. 3e and f. We have compiled the adsorption parameters obtained from both analyses in Table 1 for comparison. Our findings indicated that the pseudo-first-order and pseudo-second-order models were both well-suited to the adsorption process in stage 1, as demonstrated by the high R2 values. However, only the pseudo-second-order model was capable of predicting a qe value that corresponds with the observation (∼75 mg g−1) in Fig. 3b, indicating that this kinetic model is more appropriate. Furthermore, the adsorption in stage 2 was exclusively fitted by the pseudo-second-order model, which led to a reasonable qe prediction. Thus, based on the above analysis, it is evident that the adsorption of Co2+ onto EDTA@PSV obeys the chemically pseudo-second-order kinetics.
| Stage | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| q e (mg g−1) | k 1 (min−1) | R 2 | q e (mg g−1) | k 2 (g mg−1 min−1) | R 2 | |
| 1 | 42.49 | 0.053 | 0.996 | 78.13 | 0.101 | 0.995 |
| 2 | 11.81 | 0.034 | 0. 488 | 74.35 | 0.229 | 0.999 |
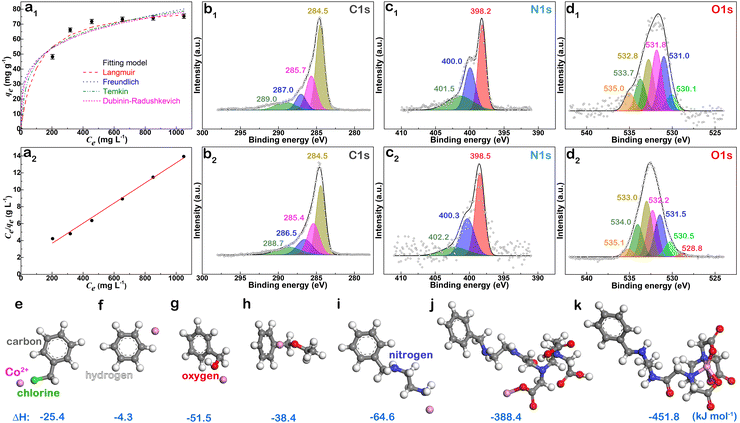
Furthermore, the adsorption isotherm of Co2+ on EDTA@PSV was measured to determine the qe of Co2+ at different equilibrium concentrations (Ce) in the solution. The results are displayed in Fig. 4a1. Initially, increasing Ce leads to an increase in qe, and then the increase gradually levels off. This suggests that the adsorption could be either monolayer adsorption on homogeneous sites or multilayer adsorption on heterogeneous sites. To confirm this, the adsorption data was analyzed using the adsorption isotherm models of Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich, which are described in eqn (5)–(8),26,37 respectively.
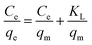 | (5) |
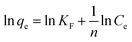 | (6) |
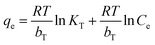 | (7) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qm − KDRε2 qm − KDRε2 | (8) |
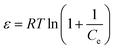 | (9) |
To investigate how EDTA@PSV interacts with Co2+, the XPS spectra of EDTA@PSV were measured before and after Co2+ adsorption (Fig. S6, ESI†). The chemical state spectra of C 1s, N 1s, and O 1s are shown in Fig. 4b1, b2, c1, c2, and d1, d2, respectively. Prior to Co2+ adsorption, the C 1s spectrum reveals four peaks at 284.5, 285.7, 287.0, and 289.0 eV for C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, C–O/C–Cl/O
C, C–N, C–O/C–Cl/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N, and O
C–N, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, respectively, in Fig. 4b1. After Co2+ adsorption in Fig. 4b2, the peak corresponding to C–C/C
C–O, respectively, in Fig. 4b1. After Co2+ adsorption in Fig. 4b2, the peak corresponding to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C remains almost unchanged at 284.5 eV, while those corresponding to C–N, C–O/C–Cl/O
C remains almost unchanged at 284.5 eV, while those corresponding to C–N, C–O/C–Cl/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N, and O
C–N, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O shift to lower binding energies at 285.4, 286.5, and 288.7 eV, respectively. These changes indicate that complexes between EDTA@PSV and Co2+ have been formed. This suggests that the N and O atoms share their electrons with Co2+, leading to an increase in electron density at their adjacent C atoms.38 As a result, the binding energy of the carbons is reduced. In Fig. 4c1 of the N 1s spectrum, the EDTA@PSV displays three peaks at 398.2, 400.0, and 401.5 eV. These peaks correspond to the C–N, –NH2, and O
C–O shift to lower binding energies at 285.4, 286.5, and 288.7 eV, respectively. These changes indicate that complexes between EDTA@PSV and Co2+ have been formed. This suggests that the N and O atoms share their electrons with Co2+, leading to an increase in electron density at their adjacent C atoms.38 As a result, the binding energy of the carbons is reduced. In Fig. 4c1 of the N 1s spectrum, the EDTA@PSV displays three peaks at 398.2, 400.0, and 401.5 eV. These peaks correspond to the C–N, –NH2, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N(H)– species, respectively. After Co2+ adsorption, all three peaks shift towards higher binding energies and are now observed at 398.5, 400.3, and 402.2 eV, as shown in Fig. 4c2. This indicates that these N-containing groups act as electron donors in complex formation with Co2+.
C–N(H)– species, respectively. After Co2+ adsorption, all three peaks shift towards higher binding energies and are now observed at 398.5, 400.3, and 402.2 eV, as shown in Fig. 4c2. This indicates that these N-containing groups act as electron donors in complex formation with Co2+.
Prior to Co2+ adsorption, Fig. 4d1 shows the O 1s spectrum of EDTA@PSV with multiple binding energies at 530.1, 531.0, 531.8, 532.8, and 533.7 eV. These peaks represent the O atoms in SO3−, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/O
C/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, C–OH, C–O–C, and O–S, respectively.33,39 The peak at 535.0 eV is more related to the sodium Auger peak (Na KLL). After the adsorption of Co2+, the binding energies of almost all O atoms have shifted to higher values, indicating that these O atoms share their lone pair electrons with Co2+. This results in a decrease in electron density, leading to an increase in the binding energy. Additionally, a new peak appears at 528.8 eV, corresponding to the binding energy of O–Co.40 This indicates that a chemical bond is formed between Co2+ and the –OH of the hydroxyl or carboxylic acid group. We also observed a simultaneous decrease in the relative peak intensities of O
S, C–OH, C–O–C, and O–S, respectively.33,39 The peak at 535.0 eV is more related to the sodium Auger peak (Na KLL). After the adsorption of Co2+, the binding energies of almost all O atoms have shifted to higher values, indicating that these O atoms share their lone pair electrons with Co2+. This results in a decrease in electron density, leading to an increase in the binding energy. Additionally, a new peak appears at 528.8 eV, corresponding to the binding energy of O–Co.40 This indicates that a chemical bond is formed between Co2+ and the –OH of the hydroxyl or carboxylic acid group. We also observed a simultaneous decrease in the relative peak intensities of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/O
C/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and C–OH to SO3, suggesting that the functional group that forms a chemical bond with Co2+ is the carboxylic acid rather than the hydroxyl group. In other words, the –COOH of the EDTA unit not only forms a complex but also chemically bonds with Co2+.
S and C–OH to SO3, suggesting that the functional group that forms a chemical bond with Co2+ is the carboxylic acid rather than the hydroxyl group. In other words, the –COOH of the EDTA unit not only forms a complex but also chemically bonds with Co2+.
The XPS analysis revealed that the N- and O-containing groups have a tendency to interact with Co2+, but we need to note that not all of these groups are part of the EDTA unit. That is, other functional groups aside from EDTA may also exhibit interactions with Co2+. To confirm this, we conducted simulation calculations using DFT to determine the energy variations (ΔEads) of these species before and after Co2+ interaction. We simplified the calculations by modeling the functional groups present on EDTA@PSV with those shown in Fig. 4e–k. With the exception of the benzene ring (Fig. 4f) and the EDTA-grafted unit (Fig. 4j and k), other species exhibit ΔEads in the range of −25 to −65 kJ mol−1, which is similar to the heat energy (−40 to −50 kJ mol−1) released from physical adsorptions.41 The small value of ΔEads in Fig. 4f indicates that there is negligible interaction between the π electrons of the benzene ring and Co2+. The high values of ΔEads obtained in Fig. 4j (−388.4 kJ mol−1) (Fig. S7, ESI†) and Fig. 4k (−451.8 kJ mol−1) suggest that the EDTA unit forms a complex with Co2+ and uses its –COOH to bond with it. These values are comparable to the amount of energy released during chemical bond formation. Therefore, they are a primary mechanism responsible for the chemisorption of Co2+ onto EDTA@PSV as determined by the earlier adsorption analyses. Additionally, the DFT results suggest that other functional groups, aside from EDTA, also contribute to the chemical affinity of EDTA@PSV towards Co2+ adsorption.
Conclusions
A new adsorbent material named EDTA@PSV has been created by modifying a hierarchical porous microsphere with an EDTA functional unit. This material demonstrates excellent adsorption properties for Co2+ ions when used in an aqueous environment. The original porous microsphere is hydrophobic, but after the introduction of EDTA, it became hydrophilic with a small contact angle of 33°. This change in surface properties makes it an ideal material for extracting valuable metal ions from an aqueous system. Furthermore, the EDTA@PSV carries a negative charge when the pH is greater than 5, which can be advantageous for attracting cationic metal ions. Most importantly, the EDTA@PSV contains various functional groups that involve N and O, such as –OH, –NH2, –N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–. These polar functional groups are thermodynamically favorable to interact with Co2+ according to the DFT simulation results. In XPS characterizations, it was discovered that EDTA@PSV exhibits an electron-donation behavior during Co2+ adsorption. This means that the polar groups in EDTA@PSV increase their chemical affinity for Co2+ adsorption. Kinetic adsorption analyses showed that Co2+ adsorbed onto EDTA@PSV through a pseudo-second-order model, indicating a major chemisorption behavior. This conclusion was further confirmed by the adsorption isotherm measurement, which demonstrated that the Co2+ adsorption on EDTA@PSV was better described by the chemisorption-based monolayer Langmuir model, rather than the Freundlich, Temkin, or Dubinin–Radushkevich model. It is worth noting that the use of EDTA@PSV resulted in a high adsorption capacity of 84 mg g−1 for Co2+. This remarkable adsorption capability can be attributed to the unique features of EDTA@PSV, such as its large surface area, porous structure, negative surface charge, and good affinity of the multiple functional groups for Co2+. Given its excellent adsorption capacity, further investigations will be conducted to study its adsorption potentials and selectivity for other metal ions in the future.
O)–. These polar functional groups are thermodynamically favorable to interact with Co2+ according to the DFT simulation results. In XPS characterizations, it was discovered that EDTA@PSV exhibits an electron-donation behavior during Co2+ adsorption. This means that the polar groups in EDTA@PSV increase their chemical affinity for Co2+ adsorption. Kinetic adsorption analyses showed that Co2+ adsorbed onto EDTA@PSV through a pseudo-second-order model, indicating a major chemisorption behavior. This conclusion was further confirmed by the adsorption isotherm measurement, which demonstrated that the Co2+ adsorption on EDTA@PSV was better described by the chemisorption-based monolayer Langmuir model, rather than the Freundlich, Temkin, or Dubinin–Radushkevich model. It is worth noting that the use of EDTA@PSV resulted in a high adsorption capacity of 84 mg g−1 for Co2+. This remarkable adsorption capability can be attributed to the unique features of EDTA@PSV, such as its large surface area, porous structure, negative surface charge, and good affinity of the multiple functional groups for Co2+. Given its excellent adsorption capacity, further investigations will be conducted to study its adsorption potentials and selectivity for other metal ions in the future.
Author contributions
Chia-Chen Li: conceptualization, writing – original draft, writing – review and editing, supervision, project administration. Mao-Hsuan Peng: investigation, methodology, validation.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the Industrial Technology Research Institute under the grant of 112A7042J4.Notes and references
- X. X. Peng, Y. S. Jiang, Z. G. Chen, A. I. Osman, M. Farghali, D. W. Rooney and P.-S. Yap, Environ. Chem., 2023, 21, 765–801 CAS.
- W. Y. Zhang, L. Y. Zhao, M. G. Xue, X. H. Duan, C. H. Feng and J. P. Zhu, J. Cleaner Prod., 2023, 396, 136455 CrossRef CAS.
- X. C. Peng, Y. Su, H. J. Guo and J. Guo, Fuel, 2024, 367, 131202 CrossRef CAS.
- H. A. Petersen, T. H. T. Myren, S. J. O’Sullivan and O. R. Luca, Mater. Adv., 2021, 2, 1113–1138 RSC.
- T. Dutta, T. Kim, K. Vellingiri, D. C. W. Tsang, J. R. Shon, K. H. Kim and S. Kumar, Chem. Eng. J., 2019, 364, 514–529 CrossRef CAS.
- A. Islam, S. H. Teo, Y. H. Taufiq-Yap, C. H. Ng, D. V. N. Vo, M. L. Ibrahim, M. M. Hasan, M. A. R. Khan, A. S. Nur and M. R. Awual, Resour., Conserv. Recycl., 2021, 175, 105849 CrossRef CAS.
- Y. L. Wu, D. Lan, J. W. Ren and S. J. Zhang, Mater. Today Phys., 2023, 36, 101178 CrossRef CAS.
- F. Amalina, A. S. A. Razak, S. Krishnan, A. W. Zularisam and M. Nasrullah, Cleaner Waste Syst., 2022, 3, 100051 CrossRef.
- K. Zhang, Z. W. Dai, W. L. Zhang, Q. Gao, Y. Dai, F. Xia and X. Zhang, Coord. Chem. Rev., 2021, 434, 213809 CrossRef CAS.
- L. Q. Liang, J. H. Wang and Y. X. Zhang, Microporous Mesoporous Mater., 2023, 347, 112344 CrossRef CAS.
- X. C. Chen, C. C. Yao, A. Wang, Z. D. Zhang, L. Z. Chen, J. Y. Zhang, X.-H. Liu and H.-B. Li, J. Hazard. Mater., 2023, 444, 130416 CrossRef CAS.
- X. D. Cui, J. K. Zhang, Y. W. Sun, F. B. Yan, J. F. Zhao, D. D. He, Y. S. Pan, L. Yuan, Y. J. Zhai and G. Z. Hu, Poult. Sci., 2023, 102, 102346 CrossRef CAS PubMed.
- C. A. Emond, V. B. Vergara, E. D. Lombardini, S. R. Mog and J. F. Kalinich, Int. J. Toxicol., 2015, 34, 44–54 CrossRef CAS PubMed.
- S. E. Zhang, J. E. Bourdeau, G. T. Nwaila and Y. Ghorbani, Resour. Policy, 2023, 86, 104247 CrossRef.
- G. Prashar, K. Thakur, S. Singh, P. Singh and V. K. Srivastava, Superalloys for high-temperature applications: An overview, in AIP Conference Proceedings, 2024, 2986, AIP Publishing.
- L. L. Wang, B. B. Chen, J. Ma, G. L. Cui and L. Q. Chen, Chem. Soc. Rev., 2018, 47, 6505–6602 RSC.
- B. B. Chu, Y. J. Guo, J. L. Shi, Y. X. Yin, T. Huang, H. Su, A. Yu, Y. G. Guo and Y. Li, J. Power Sources, 2022, 544, 231873 CrossRef CAS.
- S. Bo, J. Luo, Q. An, X. Zhao, Z. Xiao, S. Zhai and Z. Li, J. Cleaner Prod., 2019, 236, 117630 CrossRef CAS.
- J. Luo, S. Bo, Y. Qin, Q. An, Z. Xiao and S. Zhai,, Int. J. Biol. Macromol., 2021, 166, 1513–1525 CrossRef CAS PubMed.
- Y. H. Wang and C. C. Li, Appl. Mater. Today, 2024, 36, 102041 CrossRef.
- S. Zhuang, K. Zhu and J. Wang, J. Cleaner Prod., 2021, 285, 124911 CrossRef CAS.
- E. Repo, L. Malinen, R. Koivula, R. Harjula and M. Sillanpää, J. Hazard. Mater., 2011, 187, 122–132 CrossRef CAS.
- F. H. Wang, P. Wu, L. Shu, D. Huang and H. H. Liu, Environ. Sci. Pollut. Res., 2022, 29, 1–11 CrossRef PubMed.
- E. Repo, J. K. Warchol, T. A. Kurniawan and M. E. T. Sillanpää, Chem. Eng. J., 2010, 161, 73–82 CrossRef CAS.
- C. C. Li, S. Yang, Y. J. Tsou, J. T. Lee and C. J. Hsieh, Chem. Mater., 2016, 28, 6089–6095 CrossRef CAS.
- O. R. U. López, H. S. Ortega, R. E. Navarro, J. L. Valenzuela-García, L. Machi and J. M. Quiroz-Castillo, Results Mater., 2023, 17, 100369 CrossRef.
- W. I. Harris, D. A. Keeley, D. J. Gisch, M. H. Tegen, J. A. Jagodzinski and D. C. McDonald, U.S. Pat., 8273799, U.S. Patent and Trademark Office, Washington, DC, 2012 Search PubMed.
- A. Jalil, M. H. Asim, Z. B. Akkus, M. Schoenthaler, B. Matuszczak and A. Bernkop-Schnürch, J. Mol. Liq., 2019, 295, 111649 CrossRef CAS.
- T. M’Hiri, C. Catusse, R. Catusse and J. L. J. Dubry, React. Kinet. Catal. Lett., 1983, 22, 425–428 CrossRef.
- M. S. M. D. Silva, C. L. D. Costa, M. D. M. Pinto and E. R. Lachter, React. Polym., 1995, 25, 55–61 CrossRef.
- K. Nakamoto, Infrared and Raman Characteristic Frequencies of Organic Molecules, 1999.
- M. Wilson, R. Kore, A. W. Ritchie, R. C. Fraser, S. K. Beaumont, R. Srivastava and J. P. S. Badyal, Colloids Surf., A, 2018, 545, 78–85 CrossRef CAS.
- N. Joseph, K. K. M. Illam, R. V. Bhat, N. E. Veetil, A. Kaipamangalath, M. R. Varma and S. Thomas, J. Phys. Chem. Solids, 2020, 145, 109527 CrossRef CAS.
- S. H. Lee, S. R. Shin and D. S. Lee, Mater. Des., 2019, 172, 107774 CrossRef CAS.
- E. Kolobova1, P. Mäki-Arvela, A. Pestryakov, E. Pakrieva, L. Pascual, A. Smeds, J. Rahkila, T. Sandberg, J. Peltonen and D. Y. Murzin, Catal. Lett., 2019, 149, 3432–3446 CrossRef.
- N. F. Al-Harby, E. F. Albahly and N. A. Mohamed, Polymers, 2021, 13, 4446 CrossRef CAS.
- T. Wang, Y. Y. An, J. X. Sun, H. X. Yang, Y. Y. Huang and H. L. Zheng, Chem. Eng. J., 2023, 465, 142950 CrossRef CAS.
- Y. Li, Y. Q. Liang, X. M. Mao and H. Li, Chem. Eng. J., 2022, 438, 135531 CrossRef CAS.
- Y. Gao, L. F. Yao, S. Z. Zhang, Q. Y. Yue and W. Y. Yin, Environ. Pollut., 2023, 316, 120622 CrossRef CAS.
- N. S. McIntyre, D. D. Johnston, L. L. Coatsworth, R. D. Davidson and J. R. Brown, Surf. Interface Anal., 1990, 15, 265–272 CrossRef CAS.
- J. G. Dash, Films on solid surfaces: the physics and chemistry of physical adsorption, Elsevier, 2012 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma00601a |
| This journal is © The Royal Society of Chemistry 2024 |