DOI:
10.1039/D4MA00632A
(Paper)
Mater. Adv., 2024,
5, 7387-7400
Gas sensing properties of a Cu-doped PANI nanocomposite towards ammonia
Received
18th June 2024
, Accepted 18th August 2024
First published on 20th August 2024
Abstract
This study exhibits the use of nanocomposite films containing copper nanoparticles at various concentrations to selectively detect ammonia gas. Using the chemical oxidative polymerization method, the samples were submerged in a PANI matrix and then subjected to varying concentrations of NH3 at room temperature to investigate their gas-sensing capabilities. For 100 ppm of NH3 gas, a gas sensing response of 51% was achieved for PANI loaded with 0.2 g of Cu nanoparticles (PANI@Cu1). Combining PANI@Cu with nanocomposite porosity augmentation improves the resistance, which was already robust before NH3 deprotonation. An extraordinarily high level of selectivity towards NH3 gas at ambient temperature was seen in films with a 2 g loading of Cu embedded in a PANI matrix (PANI@Cu5), among the many compositions that were tested. With a recovery time of less than 20 seconds, the resultant sensor responded with 62% accuracy at 300 ppm. Aside from NH3 gas, the designed sensor is less selective for ethanol, CO, CO2, and H2S.
1. Introduction
Ammonia (NH3) is a major polluting gas that has substantial consequences for human health. It exists in industries such as the chemical industry, agriculture, coal, and manufacturing, and its concentration requires precise monitoring.1 Recently, ammonia has been identified as a biomarker in exhaled gas. Exhaled gas with a high proportion of NH3 is thought to be an indicator of kidney illness.2,3 The Occupational Safety and Health Administration of the United States has issued several ammonia-related warnings. Eight hours at 25 ppm and fifteen minutes at 35 ppm are considered safe levels of ammonia for humans to be exposed to. Serious health concerns, and even death, can result from prolonged exposure and inhalation of it at concentrations greater than 5000 ppm.4 Therefore, it is extremely desired and crucial to build a low concentration ammonia monitoring system in addition to preventing ammonia leakage. Several metal oxide semiconductors, including Cu2O–MoS2, SnO2, In2O3 and so on, have been utilised by researchers as ammonia sensors thus far.5–7 High temperatures, usually between 200 and 500 °C, are optimal for the operation of sensors based on metal oxide semiconductors. Power consumption would be significant due to a high operating temperature, which would limit their usefulness in many areas. Since then, there has been a flurry of activity among scientists working to perfect gas sensors that can detect gases at ambient temperature. Polyaniline (PANI) was singled out as a promising choice among conductive polymers due to its many advantageous characteristics, including its inexpensive cost, ease of production, distinctive electrical and redox characteristics, rapid response time, and ability to operate at room temperature.8,9 Doping and de-doping are two common methods for controlling PANI resistance, while varying the degree of protonation with certain additives and target gases alters the material electrical resistivity. It has been demonstrated that the sensing characteristics of PANI composites could be significantly enhanced by the addition of metal.10
Our present focus is on developing a sensor by combining a PANI matrix with copper (Cu), an inorganic material. The decision to choose copper as a material was influenced by multiple considerations such as good biocompatibility, lack of toxicity, excellent chemical stability, high electron transfer capability, excellent mechanical strength, and direct wide band gap (Eg = 1.0–1.9 eV).11,12 The incorporation of copper within the PANI matrix significantly enhances the composite properties, rendering it a promising material for various advanced applications. Copper's excellent electrical conductivity, coupled with PANI's intrinsic conductivity, results in an improvement in the overall conductivity of the composite. Furthermore, the presence of copper imparts superior catalytic activity, making the PANI–Cu composite an attractive candidate for applications in sensors, energy storage devices, and catalysis. The enhanced stability and durability of the composite, attributed to the strong interaction between copper and the PANI matrix, further underscores its potential for practical application. Several studies have examined employing PANI or a PANI–metal nanoparticle combination to improve electrical and optical aspects. For example, Shivam Kumar et al.13 developed a highly sensitive Cu–ethylenediamine/PANI composite film for gas sensing, by integrating I and Cu functionalities into the Zr-UiO-67 material, Khalid Mujasam Batoo et al.14 demonstrated not only enhanced CO uptake capacity but also a significant increase in CO conversion efficiency, as measured by evolution rate, and presented a MOF-based design strategy for achieving high selectivity and productivity in the photocatalytic reduction of CO to MeOH. A novel MXene/TiO2/C-NFs heterojunction-based sensory component for NH3 detection was created by Sagar Sardana et al.,15 with cellulose nanofibers (C-NFs) serving as the substrate. This sensor is very sensitive to NH3, has a high selectivity, and is very reproducible. For self-powered breathing and environmental monitoring, Sagar Sardana et al.16 investigated edge-site-enriched Ti3C2Tx MXene/MoS2 nanosheet heterostructures. In addition, Zhang et al.17 developed a polyaniline/silver nanocomposite for the determination of formaldehyde, and Cui et al.18 fabricated a polyaniline/titanium dioxide nanocomposite film by layer-by-layer self-assembly, but a lot still remains to be explored. These considerations motivated the chemical polymerization synthesis of copper in a PANI matrix, which formed the basis of a room temperature ammonia sensor. In this study, PANI and its composites were made on a Si substrate using the spin-coating procedure with five different concentrations of copper sources. The nanocomposites were studied with XRD, FTIR, EDX, FE-SEM, UV-Vis absorption spectroscopy and TGA techniques, respectively. The gas-sensing capabilities of the samples were tested by exposing them to varying amounts of NH3 at room temperature. Furthermore, the mechanisms and impacts of copper on sensor behaviour for ammonia detection were thoroughly examined.
2. Experimental details
2.1 Materials and reagents
Ammonium persulfate (APS) (M.W. 228.20 g mol−1, Sigma-Aldrich), copper acetate Cu(OAc2) (M.W. 181.63 g mol−1, Sigma-Aldrich), aniline monomer (M.W. 93.13, AR), and hydrochloric acid (M.W. 36.46 g mol−1, AR) were procured. No additional purification was performed on the other reagents prior use. All of the solutions used in the experiment were prepared using double distilled water.
2.2 Synthesis of polyaniline (PANI)
PANI was synthesized using a method involving the mixing of solutions. Scheme 1 shows the use of ammonium persulfate as an oxidant/initiator in conjunction with aniline polymerization in concentrated hydrochloric acid to produce an acidic medium. A standard procedure was followed in preparing an aqueous solution of aniline (1 M) and HCl (0.1 M) in a beaker. After being stirred, the reaction mixture was cooled to a temperature between 0–5 °C for approximately 15 minutes. An ammonium persulfate solution (0.1 g in 5 mL distilled water) acting as an oxidant was gradually added to the aforementioned reaction mixture while being continuously stirred. The reaction mixture was stirred for 2 hours until a solid precipitate formed and then it was left overnight to set, under static conditions for 12 h at 0–5 °C. The polymer (PANI) drops to the bottom due to its high molecular weight, while unreacted aniline and oligomer remain in the supernatant and are removed. After separation, 10 mL distilled water was added and incubated for 2 hours. To remove aniline and oligomers, the process was repeated twice and the supernatant was discarded. And then distilled water was used to wash the precipitate after it had been obtained by filtering. The sample was obtained by drying it in an oven set at 60 °C for 12 hours.19
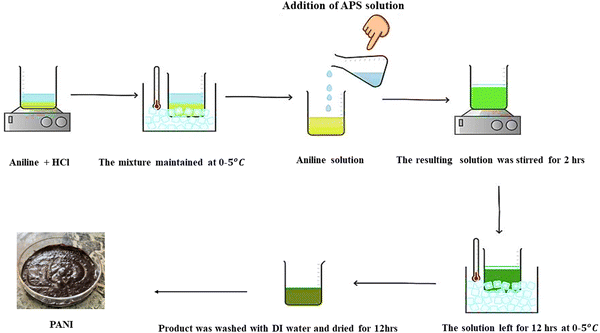 |
| | Scheme 1 Aniline and ammonium persulfate are used in the synthesis of PANI in acidic medium. | |
2.3 Synthesis of a PANI@Cu nanocomposite
The aniline hydrochloride solution was prepared by dissolving 1 M aniline monomer in 0.1 M HCl to produce an acidic medium. Another beaker was taken and 0.015 M of copper acetate was mixed with distilled water to form a solution and here, copper ions will be produced. Immediately, the copper acetate solution was added to the aniline solution while stirring with a magnetic stirrer operated at 600 rpm. After gently adding copper ion-containing aniline to the solution, an oxidant solution of ammonium persulfate (0.1 g in 5 mL distilled water) was added to the reaction mixture. Simultaneously, this started the polymerization of aniline and the reduction of copper ions, and over time, the solution becomes darkened as a result of the formation of the PANI@Cu nanocomposite. The reaction was allowed to continue for a particular duration, typically hours, in order to allow full polymerization and reduction of copper ions. Once the reaction was finished, distilled water was used to wash the precipitate after it had been obtained by filtering. The sample was obtained by drying it in an oven set at 60 °C. A PANI@Cu nanocomposite may be synthesized according to the steps shown in Fig. 1. PANI@Cu nanocomposites were prepared with different concentrations of copper acetate (0.015, 0.03, 0.06, 0.09 and 0.12 M), hereafter called PANI@Cu1, PANI@Cu2, PANI@Cu3, PANI@Cu4 and PANI@Cu5, respectively, as shown in Table 1.
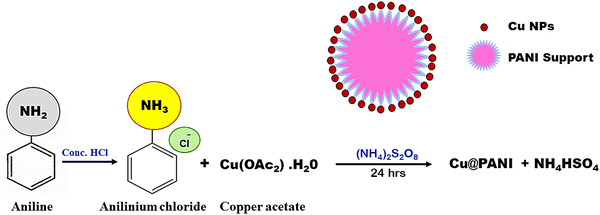 |
| | Fig. 1 Methods for the preparation of the PANI@Cu nanocomposite. | |
Table 1 PANI with various concentrations of copper acetate
| Sample |
PANI (mL) |
Cu (g) |
| PANI |
10 |
0 |
| PANI@Cu1 |
10 |
0.2 |
| PANI@Cu2 |
10 |
0.5 |
| PANI@Cu3 |
10 |
1 |
| PANI@Cu4 |
10 |
1.5 |
| PANI@Cu5 |
10 |
2 |
2.4 Preparation of gas concentrations, sensing response and sensitivity
The gas response was measured using a 900 cm3 gas chamber that had been specifically prepared. We used a static mode to detect gas levels. In the gas chamber, the artificial sensor was placed. In Fig. 2 we can see a simplified representation of a gas sensing device. Using a syringe, the specified amount of test gas was delivered into the chamber until the gas concentration (in parts per million) was achieved. A scientific programmable digital sourcemeter was used to test the electrical resistance and connected to a computer for data collection. After reaching the steady state, the sensor was exposed to fresh air to record its recovery. The chamber's input and outflow valves, labelled V1 and V2, were opened simultaneously to achieve this. The hoover pump was turned on simultaneously and the gas may leave the chamber through the outlet valve (V2) and fresh air might enter through the inlet valve (V1). The degassing process was expedited by the vacuum pump and everything was done at room temperature for measuring gas sensitivity. The required concentration of the gas is prepared by the static gas distribution method given by the formula20,21| | 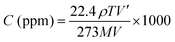 | (1) |
where C (ppm) is the desired target gas concentration, ρ is (g mL−1) the density of the liquid (gas), V′ is the volume of gas, T is the temperature in Kelvin, M is the molecular weight of gas (g mol−1), and V is the volume of the chamber (L). The humidity present inside the chamber and temperature reading on the day of the experiment was 40% and ∼26 °C, respectively. The performance of the sensor response was evaluated using:22,23| | 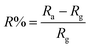 | (2) |
In this case, Ra represents the air standby resistance and Rg the resistance after being exposed to a reducing gas. The sensor is able to respond to NH3 gas by modulating the response/recovery time between different concentrations of metal doped and various amounts of NH3 gas. According to the IUPAC, sensitivity is defined as the slope of the calibration curve (sensing response versus target gas concentration):here ΔR and ΔC are the change in sensor response and concentration.
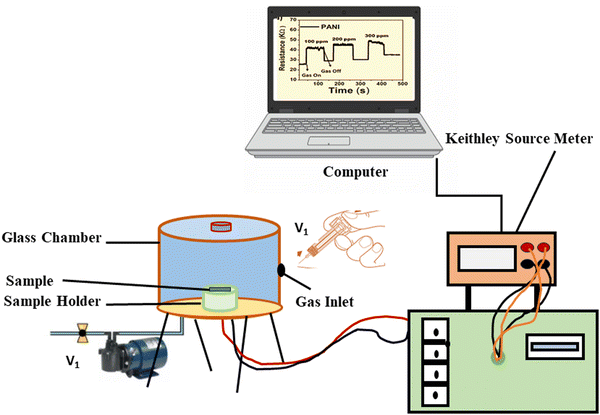 |
| | Fig. 2 Schematic diagram of a gas sensing experimental setup. | |
3. Results and discussion
3.1 X-Ray diffraction
In addition to providing information on the size of the particles, XRD spectra also offer insights into the crystallinity, lattice constant, and d-spacing of the compounds, among other structural characteristics. Fig. 3a and b illustrate the XRD spectra of pure PANI and PANI@Cu. The diffraction patterns of PANI reveal the presence of both crystalline and amorphous components, as evidenced by the peaks at 2θ of approximately 11.66°, 18.38°, 20.43°, and 25.55°. Similar to the findings of Bhagwat et al.,24 the crystalline PANI is attributed to the planar character of the benzenoid and quinoid functional groups, as well as the nanofiber structure. The polymer chain parallel periodicity correlates to the peak at 20.43°, whereas the monoclinic periodicity can be related to the peak at 26.36°. In the XRD pattern of the PANI@Cu nanocomposite, in addition to the PANI broad peak, three distinct diffraction peaks can be seen with maximum intensity at approximately 43°, 50° and 74°. These peaks correspond to Bragg reflections from the (111), (200), and (220) planes of Cu nanoparticles, and their intensities align with the values found in the literature (JCPDS no. 04-0836),25 indicating that the PANI@Cu nanocomposites contain face-cantered cubic lattice Cu. In the Williamson–Hall plot as shown Fig. 3c and d in order to ascertain the strain as well as particle size, the intercept point on the Y-axis and the slope, respectively, are calculated by fitting the given plot. Despite the model's inability to take into consideration the fact that crystals display spatial variation, the current study used a Williamson–Hall analysis of nanocomposite Cu in a PANI matrix assuming uniform deformation. Fig. 3b depicts our model for Cu nanoparticle system uniform displacement. In nanocomposites the size of the Cu phase crystals is determined by applying Scherrer's formula, which corresponds to a peak at 2θ = 43° with D = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where λ is the wavelength of the X-rays and β is the full width at half maximum (FWHM).26,27 The average size range for Cu phase crystallites is 14 nm.28Table 2 indicates that increasing copper concentration results in an increase in particle size and d-spacing, reflecting the intricate interplay between nucleation, growth, and crystal structure in the material system being studied.
θ, where λ is the wavelength of the X-rays and β is the full width at half maximum (FWHM).26,27 The average size range for Cu phase crystallites is 14 nm.28Table 2 indicates that increasing copper concentration results in an increase in particle size and d-spacing, reflecting the intricate interplay between nucleation, growth, and crystal structure in the material system being studied.
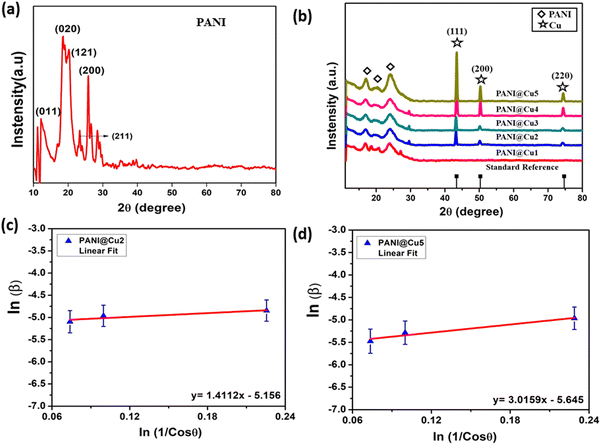 |
| | Fig. 3 XRD spectra of (a) PANI and (b) the PANI@Cu nanocomposite, and (c) and (d) Williamson–Hall plot of the nanocrystalline copper sample. | |
Table 2 The crystallite size of the PANI@Cu nanocomposite
| Conc. (g) |
2θ of the intense peak (degree) |
hkl
|
FWHM of intense peak (β) radians |
Size of the particle (D) nm |
d-Spacing nm |
Lattice parameter (a) Å |
Macrostrain (ε × 10−3) |
Dislocation density (δ × 10−3/nm2) |
| 0.5 |
43.50 |
111 |
0.0061 |
12.2 |
2.07 |
3.60 |
3.82 |
1.67 |
| 1 |
43.31 |
111 |
0.0055 |
13.3 |
2.08 |
3.51 |
3.51 |
1.48 |
| 1.5 |
43.41 |
111 |
0.0045 |
16.4 |
2.08 |
3.60 |
2.85 |
0.92 |
| 2 |
43.42 |
111 |
0.0041 |
17.8 |
2.08 |
3.60 |
2.63 |
0.78 |
3.2 FT-IR study
FTIR was then used to explore the interaction between Cu and PANI, as shown in Fig. 4. The peak at 1460 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of a quinoid,29 while the peaks at 1303, 1137, and 810 cm−1 correspond to the C
C stretching vibration of a quinoid,29 while the peaks at 1303, 1137, and 810 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C extension vibration mode of benzenoid rings, the stretching mode of N
C extension vibration mode of benzenoid rings, the stretching mode of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (Q belongs to the quinoid ring), and the C–H bonding mode of aromatic rings, respectively.30 All samples show substantial absorption bands in the 720–1595 cm−1 range, indicating PANI features. The absorbance peak at around 750 cm−1 corresponds to the C–H out-of-plane bending vibration of the 1,4-distributed benzene ring.31 In the PANI, a strong absorption intensity band at ∼868 cm−1 is assigned to the C–H out of plane bending vibration of the benzene ring deformation32. The benzenoid C–N33 is responsible for the 1303 cm−1 band, while N–H stretching of secondary amines34 causes the peak at 3446 cm−1. These results confirmed that the Cu@PANI nanocomposite could be successfully obtained. Compared to PANI, the peak at 1561 cm−1 is somewhat red-shifted, while the peaks at 1302 and 1458 cm−1 have weakened. This is due to the π–π interaction between the Cu nanoparticles in the PANI matrix. Table 3 displays the appropriate peak positions from the infrared spectra of the PANI and PANI@Cu nanocomposites.
N (Q belongs to the quinoid ring), and the C–H bonding mode of aromatic rings, respectively.30 All samples show substantial absorption bands in the 720–1595 cm−1 range, indicating PANI features. The absorbance peak at around 750 cm−1 corresponds to the C–H out-of-plane bending vibration of the 1,4-distributed benzene ring.31 In the PANI, a strong absorption intensity band at ∼868 cm−1 is assigned to the C–H out of plane bending vibration of the benzene ring deformation32. The benzenoid C–N33 is responsible for the 1303 cm−1 band, while N–H stretching of secondary amines34 causes the peak at 3446 cm−1. These results confirmed that the Cu@PANI nanocomposite could be successfully obtained. Compared to PANI, the peak at 1561 cm−1 is somewhat red-shifted, while the peaks at 1302 and 1458 cm−1 have weakened. This is due to the π–π interaction between the Cu nanoparticles in the PANI matrix. Table 3 displays the appropriate peak positions from the infrared spectra of the PANI and PANI@Cu nanocomposites.
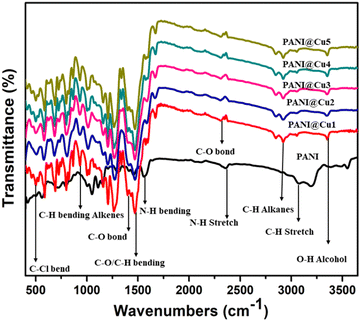 |
| | Fig. 4 FT-IR spectra of PANI and the PANI@Cu nanocomposite. | |
Table 3 The FTIR analysis of the PANI and PANI@Cu nanocomposite
| Assignment |
Band position (cm−1) |
| PANI |
PANI@Cu |
| Stretching vibrations of –OH groups |
3547 |
3553 |
| C–H stretching vibrations (alkanes) |
3074 |
2922 |
| C–O bond |
— |
2350 |
| N–H bending vibrations of amino groups |
1561 |
1575 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of quinoid ring C stretching vibrations of quinoid ring |
1458 |
— |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration of benzenoid rings C vibration of benzenoid rings |
1302 |
1303 |
| C–O primary/secondary alcohol |
— |
1207 |
| C–H bending alkenes |
1157 |
1160 |
| C–O stretching vibrations/alkyl ammine |
1047 |
1027 |
| Chloride group incorporation in the polymer chain |
573 |
501 |
3.3 Optical analysis and band gap values
One significant component of expanding the applications of PANI and PANI@Cu nanocomposites is the investigation of their optical characteristics. In the UV-vis spectra of PANI (Fig. 5a), the π–π* transition and the polaron band transitions are indicated by two absorption peaks at 255 and 294 nm. It seems that an excitonic transition in the quinoid ring causes another strong absorption shoulder at approximately 412 nm.35 On the other hand, the PANI@Cu1 to PANI@Cu5 (Fig. 5b) nanocomposites all exhibited three absorption peaks at different wavelengths: 233, 316 and 434 nm; 230, 315 and 433 nm; 218, 314 and 429 nm; 227, 317 and 430 nm; and 221, 318, and 431 nm, respectively, in their spectra. At approximately 430 nm, an electron and polaron transition from the benzenoid ring to the quinonoid ring is attributed to the absorption peak. The PANI@Cu nanocomposite exhibited a considerably greater capacity for light absorption than pure PANI nanoparticles.20 Crucially as previously described by C. Burda et al.36 and S. Linic et al.37 the PANI@Cu1 nanocomposites exhibited a peak at 434 nm, which corresponds to the surface plasmon resonance of the implanted copper nanoparticles in the polymer matrix. The band in the visible region is also defined, which means that the absorbance in the visible region is higher as a result of the Cu nanoparticle behaviours. According to the Tauc equation α = (h − Eg)2/hv, the optical band gap of powder samples can be approximated using the direct allowed transition type, where Eg is the optical band gap, h is the Planck constant, and v is the reciprocal of the wavelength with α as the absorption coefficient.38,39 The spectrum analysis yielded transition bandgaps (Eg) of approximately 2.21 eV for pure PANI, 2.46 eV for PANI@Cu1, 2.47 eV for PANI@Cu2, 2.48 eV for PANI@Cu3, 2.49 eV for PANI@Cu4 and 2.49 eV for the PANI@Cu5 nanocomposite, as shown in Fig. 5c. Charge neutrality, the introduction of dopant energy levels, coulombic interactions and quantum confinement effects are all variables that alter the material electronic structure, which causes the band gap to widen as the copper concentration increases in the PANI matrix.
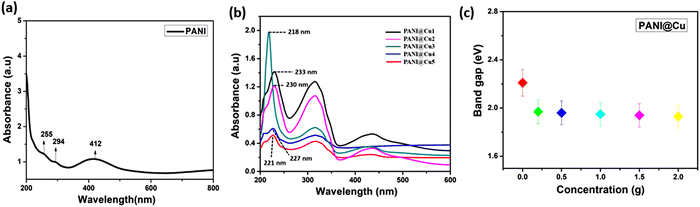 |
| | Fig. 5 UV spectrum of (a) pure PANI and (b) the PANI@Cu nanocomposite, (c) the band gap of PANI and PANI@Cu1–5 with various concentrations. | |
3.4 Morphological analysis
Images from field emission scanning electron microscopy (FESEM) of PANI, and the PANI@Cu1 and PANI@Cu5 nanocomposites are presented in Fig. 6, in that sequence. Fig. 6a and d show that PANI is characterized by nanofibrous agglomeration of inhomogeneous-shaped particles with widths of around 100 nm and lengths varying from hundreds of nanometres to several micrometres. Clusters of unevenly sized particles in the nanofibers are formed when aniline oligomer self-assembly occurs due to π–π contact, hydrogen bonding, and van der Waals forces, according to the research of Sheng Du et al.40 When comparing the PANI@Cu (Fig. 6b and c) nanocomposite samples, it is clear that the former exhibits heterogonous nucleation-induced aggregation in the form of huge globules. The fact that there are no holes in the structure proves that the particles truly do interact with one other. The FE-SEM micrographs of the composite samples show that the nano-scaled particles are evenly distributed throughout the polymer matrix. This could be because the grass particles are chemically bound to the polymer and the polymer is preventing the undesirable grass particles from obstructing the narrower particle dispersion.41 High surface area to volume ratio Cu nanoparticles improve their gas detecting properties, therefore enabling more effective sensor designs and improved sensitivity and reactivity. Furthermore, the nonporous nanofibers have a much higher surface area; so, the homogeneous coating of Cu nanoparticles with PANI chains reduces as the Cu content in the composite rises. Particles might group or come together to create more complex forms. More metal atoms from higher metal concentrations help to increase particle–particle interactions, hence promoting aggregation. On the other hand, very high concentrations could cause particle crowding, therefore restricting their capacity for aggregation and hence producing lower individual particle sizes. Many times, nanofibers and other materials feature related vacant areas or channels. These pathways allow gas molecules to permeate through the material, facilitating quick and efficient gas interaction with the sensor devices.42,43 Because of this, it can respond faster and identify gases more accurately.
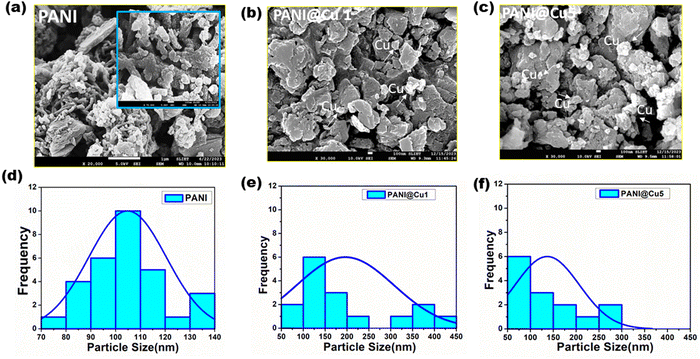 |
| | Fig. 6 FE-SEM of (a) PANI and (b) and (c) the PANI@Cu nanocomposites, and (d)–(f) particle size analysis of the samples. | |
3.5 Dispersive X-ray spectroscopy (EDX)
Sample elements are revealed by EDS. The sample elemental composition is ascertained by analysing its characteristic dispersive X-rays. As seen in Fig. 6, the EDS analysis returns the following results. The presence of carbon, nitrogen, and oxygen would be most prominently exhibited in an EDS analysis of pure polyaniline. These components make up the backbone of the polymer, as illustrated in Fig. 7a. It is clear from the pictures that copper ions can get through the PANI matrix (see Fig. 7b). Visual inspection confirmed the presence of Cu, N, and C in the PANI@Cu5 blend. Since the H signal has a lower energy, it is not present.44 More elements, namely Cl and O, came into being as a result of the addition of HCl and ammonium persulfate solution. Thus, the surface plays a pivotal role in gas sensing, and the characteristics of the fibre samples are highly advantageous for these applications.
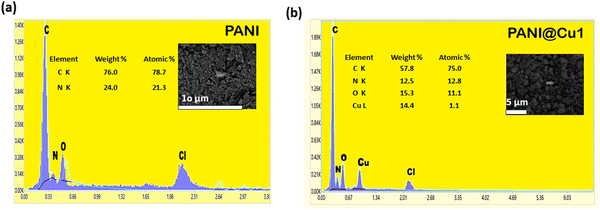 |
| | Fig. 7 (a) EDX spectra of PANI and (b) the PANI@Cu nanocomposite. | |
3.6 Thermogravimetric (TG) and differential thermal analysis (DTA)
The thermal stability of the synthesized PANI and typical PANI@Cu1 composites was investigated using a 100 °C min−1 heating rate and a dynamic nitrogen flow, in conjunction with TGA and DTA studies. Fig. 8a and b show pure PANI as a result of the TGA/DTA trace and adsorbed water molecules desorb at 100 °C, with the sample losing 5.53% of its weight between room temperature and that temperature. An endothermic peak at 156 °C was observed on the DTA trace, indicating this desorption. There is no noticeable effect on weight loss between 100 °C and 203 °C. Here, we can see that the material can resist quite high temperatures. The DTA trace then showed a big endothermic peak at 299 °C and a constant loss of mass from 203 °C to 638 °C. A staggering 42.46% was lost. This mass loss occurs when the polymer burns. Chemically oxidized PANI is also compatible with it, according to the temperature data from this study.45 When PANI samples are produced by chemical oxidation pathways, they exhibit an uneven loss of weight and do not contain a distinct dehydration step. The interfacial polymer method includes a clear dehydration step and a steady weight loss for breakdown, making it a potential candidate for synthesising substituted PANI for targeted uses. Degradation of the PANI@Cu2 nanocomposites is two-stage as shown Fig. 8c and d by the TGA-DTA results: (a) the first weight loss of 4.23% was likely associated with planar water loss; (b) the subsequent weight loss of 46.6% was caused by polymeric chain degradation and the polyaniline benzene ring-opening breakdown.46 At the temperatures used to measure thermal conductivity, the PANI@Cu2 nanocomposite showed remarkable stability, according to the TGA data.
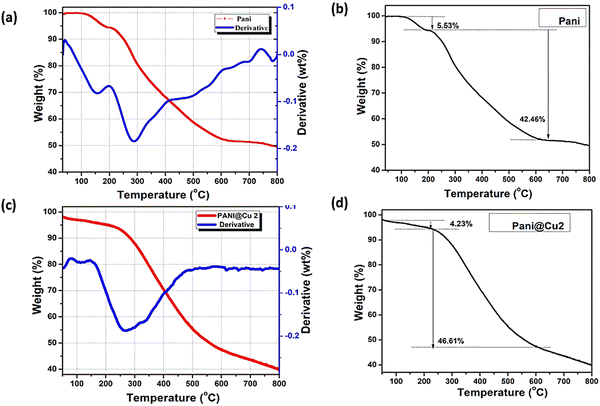 |
| | Fig. 8 TG/DTA curves of (a) and (b) traces of pure PANI and (c) and (d) the PANI@Cu nanocomposite. | |
3.7 Gas sensing studies
3.7.1 The preparation of PANI film.
A viscous solution was prepared by dissolving the pure polyaniline in N-methyl pyrrolidone (NMP). This solution was used to deposit the PANI layer on a silicon substrate using the spin coating method. The process was carried out at 1500 rpm for 20 seconds. Additionally, silver paste was used to create electrical connections on the PANI film. The width of the track and the gaps between two successive tracks were 1 mm each.
3.7.2 The preparation of the PANI@Cu nanocomposite film.
The prepared sample of the PANI@Cu nanocomposite film (2 mg) was dissolved in 0.1 M polyvinyl pyrrolidone to make a solution. The spin coating process was employed to deposit a layer of PANI@Cu nanocomposite on a silicon substrate using this solution. For 10 seconds, the procedure was run at 1500 rpm. In addition, the manufactured nanocomposite film was electrically connected using silver paste.
3.7.3 Gas response.
Fig. 8 shows the results of the study, which included monitoring the fluctuation in resistance over time at room temperature. According to the data, films that are exposed to NH3 gas have an increased resistance, which diminishes as the gas is closed.47Fig. 9a illustrates the detection range for ammonia, which is between 100 and 300 ppm, as a function of the sensor resistance. In spite of being subjected to varying amounts of NH3 gas, the resistance value of the pure PANI-based sensor does not change significantly. Simultaneously, the resistance values of sensors built of PANI@Cu nanocomposite films with different concentrations of doped metal experienced a significant decrease in the resistance compared to PANI. The resistance values revert to their approximate baseline levels after each reaction and recovery time, which occurs when the test chamber is filled with air. To be more specific, the resistance of the PANI@Cu nanocomposite sensors ranges from 16 kΩ to 48.5 kΩ for the PANI@Cu1 nanocomposite film, from 15.7 kΩ to 46.1 kΩ for the PANI@Cu2 nanocomposite film, from 18.7 kΩ to 43.1 kΩ for the PANI@Cu3 nanocomposite film, from 18.7 kΩ to 48.1 kΩ for the PANI@Cu4 nanocomposite film, 19.3 kΩ to 49.4 kΩ for the PANI@Cu5 nanocomposite film, and from 25.2 kΩ to 47.3 kΩ for the pure PANI sensor, as shown in Fig. 9a. The responsiveness of the composite conducting polymer sensor to NH3 vapours is depicted in Fig. 9b. For the purpose of illustrating how the gas sensor reacts to different concentrations, we developed a graph visual. At a concentration of 300 ppm, the bar chart makes it abundantly evident that the PANI film reacts 50% and PANI@Cu5 reacts 62% towards NH3 gas. This demonstrates that the PANI@Cu nanocomposite film sensor is suited for real-time gas sensor design for the detection of NH3 gas under ambient conditions due to the fact that it has an outstanding selectivity for this gas. Using the data in Fig. 9b and the response values displayed in Table 4, we can see how the sensors assembled for this work react to different amounts of ammonia gas. For example, at a concentration of 100 ppm of NH3 gas, sensors based on PANI@Cu1, PANI@Cu2, PANI@Cu3, PANI@Cu4, and PANI@Cu5 nanocomposite films exhibit 51%, 53%, 55%, 54%, and 59% improvement over sensors based on pure PANI, respectively. There are potential synergistic effects that improve the sensing performance when Cu and PANI are combined. Cu has the ability to modify PANI's electronic structure, which in turn enhances the mobility of charge carriers and their interaction with gas molecules. A more sensitive and responsive sensor may be the outcome of the synergy between Cu and PANI. In comparison to a pure PANI sensor, the PANI@Cu nanocomposite film exhibits a significantly more favourable response to NH3 gas, according to these results. Over the course of seven days, we monitored the sensor reactions after exposure to a 100 ppm NH3 gas alternative in order to assess the formed gas sensor initial long-term stability. Fig. 9c displays the measured sensor response (ΔR/Rg) to NH3 exposure for up to seven days for the PANI and PANI@Cu1 to PANI@Cu5 nanocomposite film gas sensors. After 7 days, the results show that the prepared gas sensor maintains a consistent sensing performance with just a slight decrease in response. Sensors based on PANI can be made more stable and long-lasting by using copper nanoparticles. The longevity of the sensor makes it resistant to repeated gas exposure without major degradation, and the enhanced stability makes sure that its performance remains consistent over time. In addition, we have examined the impact of humidity on the prepared PANI and PANI@Cu1–5 nanocomposite film gas sensors when exposed to 100 ppm of NH3 gas in various humid environments. In Fig. 9d, we can observe the sensor reaction to varying humidity levels. Increased humidity causes the sensor to respond more strongly. At a relative humidity (RH%) of 40%, the sensor demonstrates its highest sensing response. Thereafter, a reduction in sensor response with increasing humidity is observed. Consequently, as discussed in Section 3.8,36–42 the conductivity changes when PANI and copper nanoparticles interact with humidity (H2O) because of the complex proton exchange mechanism.
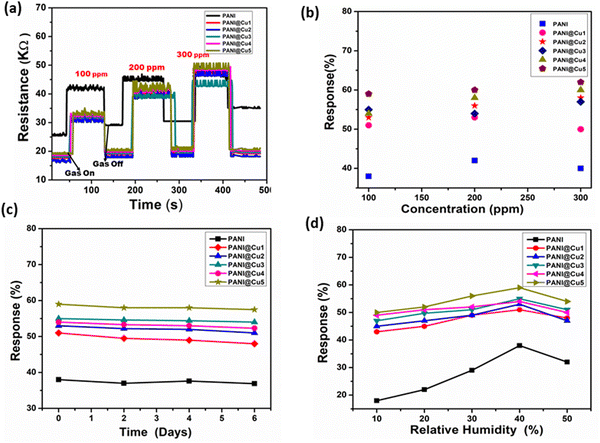 |
| | Fig. 9 (a) The shift in resistance for the pure PANI film and different concentrations of Cu nanoparticles (PANI@Cu1 to PANI@Cu5 nanocomposite) relative to time when exposed to NH3 gas at room temperature, (b) the sensing response of PANI and PANI@Cu1 to the PANI@Cu5 nanocomposite film with varying NH3 concentrations, (c) stability of the gas sensor based on PANI and the PANI@Cu1 to PANI@Cu5 nanocomposite film toward 100 ppm ammonia and (d) effect of humidity on the prepared sensor when exposed to 100 ppm ammonia. | |
Table 4 The response values of sensors prepared in this work regarding NH3 gas
| NH3 gas concentration response (%) |
100 (ppm) |
200 (ppm) |
300 (ppm) |
| PANI |
38 |
42 |
40 |
| PANI@Cu1 |
51 |
53 |
50 |
| PANI@Cu2 |
53 |
56 |
58 |
| PANI@Cu3 |
55 |
54 |
57 |
| PANI@Cu4 |
54 |
58 |
60 |
| PANI@Cu5 |
59 |
60 |
62 |
3.7.4 Response and recovery time.
The subsequent crucial elements that should be taken into consideration are the evaluation of a sensor reaction and the recovery periods. The amount of time that has passed after the intake of gas and the occurrence of a discernible change in the parameter is referred to as the response time. On the other hand, the amount of time that is necessary for the parameter to return to its initial state following the removal of gas is referred to as the recovery time. Two methods are available for determining them: first, the amount of time it takes for the sensor to reach 90% saturation of Rg from Ra during gas intake, and second, the value of Ra at 10% during gas withdrawal. Both of these methods are able to be measured. Using different quantities of NH3 gas, Fig. 10 shows the reaction and recovery period of the pure PANI film (Fig. 10a) and PANI@Cu nanocomposite film (Fig. 10b–f). As the NH3 concentration increases from 100 to 300 ppm, the response time for pure PANI film increases from 27 to 29 s and the recovery time decreases from 22 to 20 s. In contrast, for the Cu-doped PANI (PANI@Cu1) nanocomposite film, the response time decreases from 18 to 17 s and the recovery time increases from 17 to 20 s. Table 5 presents the response times (T1) and recovery times (T2) of PANI and the PANI@Cu nanocomposite sensors after they were exposed to NH3 gas at a concentration of 100 ppm. According to V. G. Bairi et al.48 reduced response times are the consequence of surface roughness enhancement and beneficial dedoping brought about by the formation of a restricted polaron band when metal is present. Due to the presence of Cu ions in the PANI chain, the recovery time for the PANI@Cu3 nanocomposite film appears to be rapid. Previous tests that have been published have demonstrated response times that are significantly greater than the minimum response time of the PANI@Cu nanocomposite film, which is approximately 20 seconds. Fast oxidation of NH3 gas could be a reason for the PANI@Cu sensor's quick response to the gas. Sensing measures in real-world contexts often necessitate a fast reaction time; yet, recovery time is little impacted even if it is crucial for the sample to return to its original state.49 It is evident that PANI@Cu nanocomposite sensors often have lower response times than pure PANI sensors. Incorporating copper into the PANI matrix can enable the nanocomposite sensitive film to have a quicker response and recovery time. One possible explanation for the longer reaction and recovery durations is that the PANI nanofibers are coated and deposited on the copper during chemical polymerization, leading to a more uniform distribution and a larger surface area. Part 3.4 details how to assess this by integrating morphology and microstructure studies of the PANI@Cu nanocomposite film. A faster response and recovery rate with less recovery time is achieved by providing more active sites for the adsorption of NH3 gas molecules. As a result, the PANI@Cu nanocomposite film sensor is ideal for practical applications that require rapid and reliable ammonia detection under room temperature conditions. Table 6 provides a summary of the NH3 gas-sensitive properties of the PANI@Cu nanocomposite sensor that was used in this study. These qualities are compared to those of some others that have been reported in recent times.
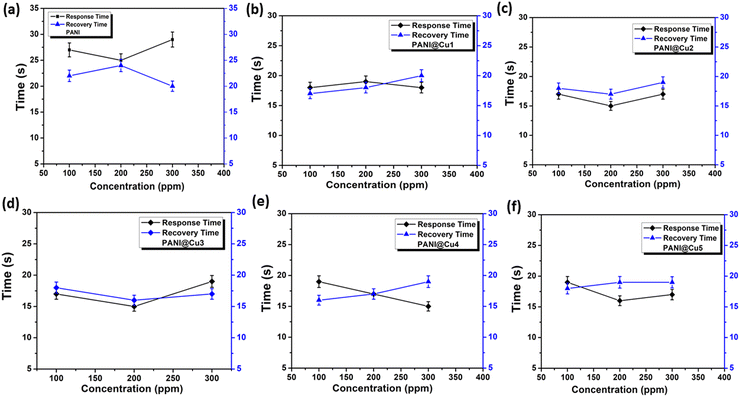 |
| | Fig. 10 Response and recovery time plot for (a) pure PANI film and (b)–(f) the PANI@Cu nanocomposite film when exposed to various concentrations of NH3 gas at room temperature. | |
Table 5 Response times (T1) and recovery times (T2) of the sensors under 100 ppm NH3 gas
| Sensitive films |
Pure PANI |
PANI@Cu1 |
PANI@Cu2 |
PANI@Cu3 |
PANI@Cu4 |
PANI@Cu5 |
| Response time (T1) |
27 |
18 |
17 |
18 |
19 |
19 |
| Recovery time (T2) |
22 |
17 |
18 |
18 |
16 |
18 |
Table 6 Comparison of the PANI@Cu nanocomposite film-based gas sensor and those reported in the literature
| Material |
Substrate |
Temp. |
Gas |
ppm |
Response (%) |
T
1 (s) |
T
2 (s) |
Ref. |
|
(Rg − Ra)/Ra.
(Ia − Ig)/Ia.
|
| PAN1 |
PET |
RT |
NH3 |
100 |
26 |
33 |
— |
50
|
| PANI |
Glass |
RT |
NH3 |
10 |
22 |
— |
— |
51
|
| PANI/graphene |
|
25 °C |
|
20 |
3.65 |
50 |
23 |
52
|
| PANI@CuO |
Glass |
RT |
NH3 |
100 |
— |
80 |
496 |
41
|
| PANI/graphene |
|
RT |
|
20 |
0.6 |
— |
— |
53
|
| Cu-en@PANI |
Glass |
RT |
NH3 |
100 |
3.8a |
100 |
— |
13
|
| PANI–CeO2 |
PET |
RT |
NH3 |
16 ppb |
2.4a |
6 min |
|
54
|
| Pd/PANI |
IDE |
RT |
NH3 |
100 |
∼10 |
— |
|
55
|
| Au/PANI |
— |
RT |
NH3 |
100 |
∼3 |
5 |
7 |
56
|
| CuO/PANI |
PET |
RT |
NH3 |
100 |
0.98a |
5.7 |
— |
57
|
| Ag/PANI |
PET |
Rt |
NH3 |
10 |
9.1 |
|
|
58
|
| Pd/PANI |
— |
RT |
NH3 |
500 |
21.9 |
— |
|
55
|
| Cu@PANI |
Glass |
RT |
NH3 |
50 |
0.55b |
7 |
— |
59
|
| PANI |
Si |
RT |
NH3 |
100 |
38 |
27 |
22 |
This work |
| 200 |
42 |
25 |
24 |
| 300 |
40 |
29 |
20 |
| PANI@Cu1 |
Si |
RT |
NH3 |
100 |
51 |
18 |
17 |
This work |
| 200 |
53 |
19 |
18 |
| 300 |
50 |
17 |
20 |
| PANI@Cu2 |
Si |
RT |
NH3 |
100 |
53 |
17 |
18 |
This work |
| 200 |
56 |
15 |
17 |
| 300 |
58 |
16 |
19 |
| PANI@Cu3 |
Si |
RT |
NH3 |
100 |
55 |
17 |
18 |
This work |
| 200 |
54 |
15 |
16 |
| 300 |
57 |
19 |
17 |
| PANI@Cu4 |
Si |
RT |
NH3 |
100 |
54 |
19 |
16 |
This work |
| 200 |
58 |
17 |
17 |
| 300 |
60 |
15 |
19 |
| PANI@Cu5 |
Si |
RT |
NH3 |
100 |
59 |
19 |
18 |
This work |
| 200 |
60 |
16 |
19 |
| 300 |
62 |
17 |
19 |
3.7.5 Selectivity.
In this work, selectivity tests were carried out for the PANI film and PANI@Cu nanocomposite films. Fig. 11b shows the sensor response of the PANI@Cu1 nanocomposite sensor to 100 ppm concentrations of NH3 gas, ethanol (C2H5OH), carbon monoxide (CO), hydrogen sulphide (H2S) and carbon dioxide (CO2), respectively. It is clear that the response of the PANI@Cu sensor toward NH3 gas is significantly higher than that of the other gases measured. By combining PANI with copper nanoparticles, a synergistic effect can be achieved, where the combined qualities of both components improve the nanocomposite performance. A composite can be made more stable and conductive with the inclusion of PANI and ammonia can be adsorbed more effectively with the help of copper nanoparticles. The chemical affinity, high surface area, presence of copper nanoparticles, conductivity changes upon exposure to ammonia, and selective detection mechanisms of the PANI@Cu1 nanocomposite contribute to its good selectivity towards ammonia gas. The nanocomposite selectivity for ammonia gas detection and sensing is due, in part, to these components. There is a clear difference between ammonia and other gases in terms of its sensor response. Being highly polar and capable of hydrogen bonding, it has the potential to interact strongly with the composite film in comparison to other gases.
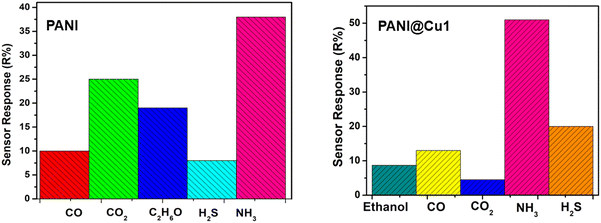 |
| | Fig. 11 Selectivity of (a) PANI and (b) the PANI@Cu1 nanocomposite film gas sensor. | |
3.8 Sensing mechanism
A decrease in free charge carriers, which are partially consumed during complex formation as a result of an interaction between the PANI film and NH3, an acceptor and a donor, respectively, are responsible for the rise in film resistance that occurs when the amount of NH3 that is consumed is increased.44 In order to counteract the rising concentration of NH3 gas, the system must move in the direction that is prescribed by the Le Chatelier primary equilibrium. The elimination of ammonia, on the other hand, results in a different direction of balance within the system. When it comes to NH3 gas, the electronegative charge of nitrogen is dominant over that of hydrogen. In the presence of PANI-based molecules, ammonia (NH3) is able to create a partial polar bond with hydrogen atoms. As a result, the final product will have a greater degree of interchain separation in the PANI film. This results in a decrease in the likelihood of electron hopping, which is the fundamental conduction mechanism in PANI systems, occurring between neighbouring chains, which ultimately leads to an increase in the film resistance. It is possible for the PANI emeraldine salt to be transformed into the PANI emeraldine base form when the concentration of ammonia vapour is at its greatest.60 Changing the emeraldine base back into emeraldine salt once the ammonia vapour has been removed is all that is required to bring the sensor functionality back to its original state. In order to perform the sensing mechanism, ammonia molecules make chemical contact with the surface of the PANI@Cu nanocomposite film. There is a possibility that, between the ammonia molecules and the copper nanoparticles, hydrogen bonds, dipole–dipole interactions, or weak chemical bonds could result. Because of these interactions, the composite material electrical properties, such as its conductivity and other quantifiable characteristics, are susceptible to change. Fig. 12 provides a schematic representation of the PANI film and PANI@Cu nanocomposite film for ammonia detection.
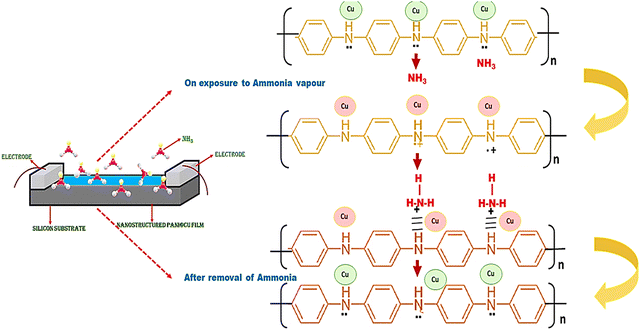 |
| | Fig. 12 Schematic of the ammonia sensing mechanism. | |
4. Conclusions
We have synthesized polyaniline and a polyaniline–copper (PANI@Cu) nanocomposite by a chemical oxidative polymerization method. The formation of copper nanoparticles and their presence in the prepared nanocomposite were confirmed by XRD, FTIR, FE-SEM, UV-Vis spectroscopy, TGA and EDS. The XRD patterns indicated that the crystalline phase of Cu nanoparticles is cubic with a crystallite size of 14 nm. FE-SEM analysis showed uniform dispersion of copper nanoparticle clusters in the PANI matrix. The optical studies indicated that the absorption mechanism is due to direct allowed transition and the optical band gap of polyaniline is less than that of the PANI@Cu nanocomposite. Comparing the nanocomposite to the naked PANI film, the TGA and DTG curves reveal that the latter is relatively unstable. Copper nanoparticle intercalated PANI nanocomposite films were tested at room temperature for their gas sensing capabilities with respect to NH3, CO, CO2, H2S, and ethanol. At ambient temperature, the nanocomposite films showed improved response kinetics and a highly selective response toward NH3. For instance, among all of the materials evaluated, the sensor film with 2 g of Cu nanoparticles (PANI@Cu5) had the best sensor response (62%), with response and recovery times of 19 and 18 s, respectively, towards 300 ppm of NH3. The greater response might be because the copper nanoparticle intercalation in the PANI matrix increased the surface area and improved the charge transfer. Therefore, nanocomposite film sensors show promise in the gas-sensor industry due to their rapid reaction time, reasonable selectivity towards ammonia, and somewhat stable operation at room temperature. In conclusion, we assert that the addition of copper to PANI has resulted in a much-enhanced ammonia sensor.
Data availability
The data used to support the Findings of this study are available from the corresponding author upon request.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We acknowledge Dr Pawan Kulriya from JNU Delhi and NPL, Delhi for sensor measurements and analysis. We are also thankful to Dr Monika for helping us in the analysis of results, validation and editing of the manuscript.
References
- M. J. Palys,
et al.
, Curr. Opin. Chem. Eng., 2021, 31, 100667 CrossRef.
- G. Wu,
et al.
, Sens. Actuators, B, 2024, 418, 136328 CrossRef.
- A. Verma and T. Kumar, PANI@Ag nanocomposites gas sensors for rapid detection of ammonia, Polyhedron, 2024, 116982 CrossRef CAS.
- B. Timmer, W. Olthuis and A. V. Berg, Ammonia sensors and their applications-a review, Sens. Actuators, B, 2005, 107(2), 666–677 CrossRef CAS.
- Y. J. Zhang, W. Zeng and Y. Q. Li, Porous MoS2 microspheres decorated with Cu2O nanoparticles for ammonia sensing property, Mater. Lett., 2019, 241, 223–226 CrossRef CAS.
- B. C. Omur, Humidity effect on adsorption kinetics of ammonia onto electrospun SnO2 nanofiber, Mater. Res. Express, 2019, 6(4) DOI:10.1088/2053-1591/aafa27.
- S. K. Bhardwaj, N. Bhardwaj, M. Kukkar, A. L. Sharma, K.-H. Kim and A. Deep, Formation of high-purity indium oxide nanoparticles and their application to sensitive detection of ammonia, Sensors, 2015, 15(12), 31930–31938 CrossRef CAS PubMed.
- M. Das and D. Sarkar, One-pot synthesis of zinc oxide–polyaniline nanocomposite for fabrication of efficient room temperature ammonia gas sensor, Ceram. Int., 2017, 43(14), 11123–11131 CrossRef CAS.
- A. Verma,
et al., Review—Recent Advances and Challenges of Conducting Polymer–Metal Nanocomposites for the Detection of Industrial Waste Gases, ECS J. Solid State Sci. Technol., 2023, 12, 047002 CrossRef.
- E. Detsri and J. Popanyasak, Fabrication of silver nanoparticles/polyaniline composite thin films using layer-by-layer self-assembly technique for ammonia sensing, Colloids Surf., A, 2015, 467, 57–65 CrossRef CAS.
- V. Pentyala,
et al., Carbon dioxide gas detection by open metal site metal organic frameworks and surface functionalized metal organic frameworks, Sens. Actuators, B, 2016, 225, 363–368 CrossRef CAS.
- A. A. Sagade and R. Sharma, Copper sulphide (CuxS) as an ammonia gas sensor working at room temperature, Sens. Actuators, B, 2008, 133, 135–143 CrossRef CAS.
- S. Kumar Gautam and S. Panda, Highly sensitive Cu ethylenediamine/PANI composite sensor for NH3 detection at room temperature, Talanta, 2023, 258, 124418 CrossRef PubMed.
- K. M. Batoo, E. Ali, B. A. Hussein, A. Al-khalidi, U. S. Altimari, S. Hussain, S. Hameed Kareem, M. Kadhem Abid, A. Alawadi and A. Ihsan, The cooperative performance of iodo and copper in a Zr-based UiO-67 metal-organic framework for highly selective photocatalytic CO2 reduction to methanol, J. Mol. Struct., 2024, 1307, 137927 CrossRef CAS.
- S. Sardana, H. Kaur, B. Arora, D. Kumar Aswal and A. Mahajan, ACS Sens., 2022, 7(1), 312–321 CrossRef CAS PubMed.
- C. N. Zhu, Z. J. Wang, X. P. Li, S. Y. Chen, D. Y. Zheng, C. Liu, X. J. Liu, D. B. Cheng and Z. Y. Qiao, Site-specific activation of mitochondria-targeting peptide nanomaterials for treatment of drug-resistant tumors, ACS Appl. Nano Mater., 2023, 6, 15641–15650 CrossRef CAS.
- J. Zhang, P. Guan, W. Li, Z. Shi and H. Zhai, Synthesis and characterization of a polyaniline/silver nanocomposite for the determination of formaldehyde, Instrum. Sci. Technol., 2016, 44(3), 249–258 CrossRef CAS.
- S. Bai, Y. Tian and M. Cui,
et al., Polyaniline@SnO2 heterojunction loading on flexible PET thin film for detection of NH3 at room temperature, Sens. Actuators, B, 2016, 226, 540–547 CrossRef CAS.
- R. G. Bavane, M. D. Shirsat and A. M. Mahajan, Sens. Transducers J., 2010, 113(2), 63 CAS.
- S. T. Navale, C. Liu, P. S. Gaiker, V. B. Patil, R. U. R. Sagar, B. Du, R. S. Mane and F. J. Stadler, Sens. Actuators, B, 2017, 245, 524–532 CrossRef CAS.
- P. Dipak, D. C. Tiwari and A. Samadhiya,
et al., Synthesis of polyaniline (printable nanoink) gas sensor for the detection of ammonia gas, J. Mater. Sci.: Mater. Electron., 2020, 31, 22512–22521, DOI:10.1007/s10854-020-04760-2.
- D. C. Tiwari, P. Atri and R. Sharma, Sensitive detection of ammonia by reduced graphene oxide/polypyrrole nanocomposites, Synth. Met., 2015, 203, 228–234 CrossRef CAS.
- L. M. García Rojas, C. A. Huerta-Aguilar, E. Navarrete, E. Llobet and P. Thangarasu, Enhancement of the CO2 Sensing/Capture through High Cationic Charge in M–ZrO2 (Li+, Mg2+, or Co3+): Experimental and Theoretical Studies, ACS Appl. Mater. Interfaces, 2023, 15, 25952–25965 CrossRef PubMed.
- A. D. Bhagwat, S. S. Sawant and C. M. Mahajan, Facile rapid synthesis of polyaniline (PANI) nanofibers, J. Nano-Electron. Phys., 2016, 8(1), 8–10 Search PubMed.
- H. Liu, T. Wang and H. Zeng, Part. Part. Syst. Charact., 2015, 32, 869–873 CrossRef CAS.
- Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report).
- A. E. A. Said, M. M. E-Wahab, S. A. Soliman and M. N. Goda, Nanosci. Nanoeng., 2014, 2, 17–28 CAS.
- B. N. Meethal, R. Ramanarayanan and S. Swaminathan, Appl. Nanosci., 2018, 8, 1545–1555 CrossRef.
- X. Wang, S. Feng, W. Zhao, D. Zhao and S. Chen, New J. Chem., 2017, 41, 9354–9360 RSC.
- B. K. Sharma, A. K. Gupta, N. Khare, S. K. Dhawan and H. C. Gupta, Synth. Met., 2009, 159, 391–395 CrossRef CAS.
- M. Trchova and J. Stejskal, Undoped Appl. Chem., 2011, 83, 1803–1817 CrossRef CAS.
- S. Khasim, S. C. Raghavendra, M. Revanasiddappa and M. V. N. Ambika Prasad, Ferroelectrics, 2005, 325, 111–119 CrossRef CAS.
- J. Li, T. Peng, Y. Zhang, C. Zhou and A. Zhu, Sep. Purif. Technol., 2018, 201, 120–129 CrossRef CAS.
- M. R. Nabid, M. Golbabaee, A. B. Moghaddam, R. Dinarvand and R. Sedghi, Int. J. Electrochem. Sci., 2008, 3, 1117–1126 CrossRef CAS.
- J. Bhadra, N. K. Madi, N. J. Al-Thani and M. A. Al-Maadeed, Polyaniline/polyvinyl alcohol blends: Effect of sulfonic acid dopants on microstructural, optical, thermal and electrical properties, Synth. Met., 2014, 191, 126–134 CrossRef CAS.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 36, 1025–1102 CrossRef PubMed.
- S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911–921 CrossRef CAS PubMed.
- P. Chrysicopoulou, D. Davazoglou, C. Trapalis and G. Kordas, Optical properties of very thin (<100 nm) sol–gel TiO2 films, Thin Solid Films, 1998, 323, 188 CrossRef CAS.
- C. A. Huerta-Aguilar, Y. S. G. Gutiérrez and P. Thangarasu, Crystal plane directed interaction of TiO2 [1 0 1] with AgNPs [1 1 1] silver nanoparticles enhancing solar light induced photo-catalytic oxidation of ciprofloxacin: Experimental and theoretical studies, Chem. Eng. J., 2020, 394, 124286 CrossRef CAS.
- X.-S. Du, C.-F. Zhou, G.-T. Wang and Y.-W. Mai, Novel Solid-State and Template-Free Synthesis of Branched Polyaniline Nanofibers, Chem. Mater., 2008, 20, 3806–3808 CrossRef CAS.
- S. K. Singh, R. K. Shukla, R. Kumar, U. K. Tripathi and S. K. Mishra, Investigation on PANI/CuO nanocomposites for ammonia gas, Mater. Lett., 2022, 309, 131325 CrossRef CAS . sensing applications.
- X. Liu, W. Zheng, R. Kumar, M. Kumar and J. Zhang, Conducting polymer-based nanostructures for gas sensors, Coord. Chem. Rev., 2022, 462(1), 214517 CrossRef CAS.
- J. Song, X. Lin and L. Y. Ee,
et al., A Review on Electrospinning as Versatile Supports for Diverse Nanofibers and Their Applications in Environmental Sensing, Adv. Fiber Mater., 2023, 5, 429–460 CrossRef CAS.
- K. Crowley, A. Morrin, A. Hernandez, E. O’Malley, P. G. Whitten, G. G. Wallace, M. R. Smyth and A. J. Killard, Talanta, 2008, 77, 710–717 CrossRef CAS.
- P. K. Khanna, V. V. V. S. Subbarao, N. Singh, R. Gokhale and U. P. Mulik, Synthesis and characterization of Ag/PVA nanocomposite by chemical reduction method, Mater. Chem. Phys., 2005, 93, 117 CrossRef CAS.
- V. Tauc and A. Menth, States in the gap, J. Non-Cryst. Solids, 1972, 8–10, 569–585 CrossRef.
- S. Konwer, A. K. Guha and S. K. Dolui, Graphene oxide-filled conducting polyaniline composites as methanol-sensing materials, J. Mater. Sci., 2013, 48(4), 1729–1739 CrossRef CAS.
- V. G. Bairi, S. E. Bourdo, N. Sacre, D. Nair, B. C. Berry, A. S. Biris and T. Viswanathan, Ammonia gas sensing behavior of tanninsulfonic acid doped polyaniline–TiO2 composite, Sensors, 2015, 15, 26415–26429 CrossRef CAS PubMed.
- C. Drake, A. Amalu, J. Bernard and S. Seal, Enhancing the low temperature hydrogen sensitivity of nanocrystalline SnO2 as a function of trivalent dopants, J. Appl. Phys., 2007, 101(10), 104307 CrossRef.
- D. K. Bandgar, S. T. Navale, S. R. Nalage, R. S. Mane, F. J. Stadler, D. K. Aswal, S. K. Gupta and V. B. Patil, Simple and low-temperature polyaniline-based flexible ammonia sensor: a step towards laboratory synthesis to economical device design, J. Mater. Chem., 2015, 3, 9461–9468 RSC.
- Q. X. Nie, Z. Y. Pang, D. W. Li, H. M. Zhou, F. L. Huang and Y. B. Cai,
et al., Facile fabrication of flexible SiO2/PANI nanofibers for ammonia gas sensing at room temperature, Colloids Surf., A, 2018, 537, 523–539, DOI:10.1016/j.colsurfa.2017.10.065.
- Z. Wu, X. Chen and S. Zhu,
et al., Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite, Sens. Actuators, B, 2013, 178, 485–493 CrossRef CAS.
- M.-S. Kim, S. Kim and H. J. Kong,
et al., Tunable electrical-Sensing performance of random-alternating layered graphene/polyaniline Nanoarchitectures, J. Phys. Chem. C, 2016, 120, 18289–18295 CrossRef CAS.
- C. Liu, H. Tai, P. Zhang, Z. Yuan, X. Du, G. Xie and Y. Jiang, A high-performance flexible gas sensor based on self-assembled PANI–CeO2 nanocomposite thin film for trace-level NH3 detection at room temperature, Sens. Actuators, B, 2018, 261, 587–597 CrossRef CAS.
- H. T. Hien, H. T. Giang and N. V. Hieu,
et al., Elaboration of Pd-nanoparticle decorated polyaniline films for room temperature NH3 gas sensors, Sens. Actuators, B, 2017, 249, 348–356 CrossRef CAS.
- U. V. Patil, N. S. Ramgir and N. Karmakar,
et al., Room temperature ammonia sensor based on copper nanoparticle intercalated polyaniline nanocomposite thin films, Appl. Surf. Sci., 2015, 339, 69–74 CrossRef CAS.
- B. Sakthivel, L. Manjakkal and G. Nammalvar, High performance CuO nanorectanglesbased room temperature flexible NH3 sensor, IEEE Sens. J., 2017, 17, 6529–6536 CAS.
- S. Bai, C. Sun and P. Wan,
et al., Transparent conducting films of hierarchically nanostructured polyaniline networks on flexible substrates for high-performance gas sensors, Small, 2015, 11, 306–310 CrossRef CAS PubMed.
- U. V. Patil, N. S. Ramgir, N. Karmakar, A. Bhogale, A. K. Debnath, D. K. Aswal, S. K. Gupta and D. C. Kothari, Room temperature ammonia sensor based on copper nanoparticle intercalated polyaniline nanocomposite thin films, Appl. Surf. Sci., 2015, 339, 69–74 CrossRef CAS.
- Z. Pang, Z. Yang, Y. Chen, J. Zhang, Q. Wang, F. Huang and Q. Wei, Colloids Surf., A, 2016, 494, 248–255 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*
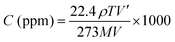
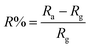
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where λ is the wavelength of the X-rays and β is the full width at half maximum (FWHM).26,27 The average size range for Cu phase crystallites is 14 nm.28Table 2 indicates that increasing copper concentration results in an increase in particle size and d-spacing, reflecting the intricate interplay between nucleation, growth, and crystal structure in the material system being studied.
θ, where λ is the wavelength of the X-rays and β is the full width at half maximum (FWHM).26,27 The average size range for Cu phase crystallites is 14 nm.28Table 2 indicates that increasing copper concentration results in an increase in particle size and d-spacing, reflecting the intricate interplay between nucleation, growth, and crystal structure in the material system being studied.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of a quinoid,29 while the peaks at 1303, 1137, and 810 cm−1 correspond to the C
C stretching vibration of a quinoid,29 while the peaks at 1303, 1137, and 810 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C extension vibration mode of benzenoid rings, the stretching mode of N
C extension vibration mode of benzenoid rings, the stretching mode of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (Q belongs to the quinoid ring), and the C–H bonding mode of aromatic rings, respectively.30 All samples show substantial absorption bands in the 720–1595 cm−1 range, indicating PANI features. The absorbance peak at around 750 cm−1 corresponds to the C–H out-of-plane bending vibration of the 1,4-distributed benzene ring.31 In the PANI, a strong absorption intensity band at ∼868 cm−1 is assigned to the C–H out of plane bending vibration of the benzene ring deformation32. The benzenoid C–N33 is responsible for the 1303 cm−1 band, while N–H stretching of secondary amines34 causes the peak at 3446 cm−1. These results confirmed that the Cu@PANI nanocomposite could be successfully obtained. Compared to PANI, the peak at 1561 cm−1 is somewhat red-shifted, while the peaks at 1302 and 1458 cm−1 have weakened. This is due to the π–π interaction between the Cu nanoparticles in the PANI matrix. Table 3 displays the appropriate peak positions from the infrared spectra of the PANI and PANI@Cu nanocomposites.
N (Q belongs to the quinoid ring), and the C–H bonding mode of aromatic rings, respectively.30 All samples show substantial absorption bands in the 720–1595 cm−1 range, indicating PANI features. The absorbance peak at around 750 cm−1 corresponds to the C–H out-of-plane bending vibration of the 1,4-distributed benzene ring.31 In the PANI, a strong absorption intensity band at ∼868 cm−1 is assigned to the C–H out of plane bending vibration of the benzene ring deformation32. The benzenoid C–N33 is responsible for the 1303 cm−1 band, while N–H stretching of secondary amines34 causes the peak at 3446 cm−1. These results confirmed that the Cu@PANI nanocomposite could be successfully obtained. Compared to PANI, the peak at 1561 cm−1 is somewhat red-shifted, while the peaks at 1302 and 1458 cm−1 have weakened. This is due to the π–π interaction between the Cu nanoparticles in the PANI matrix. Table 3 displays the appropriate peak positions from the infrared spectra of the PANI and PANI@Cu nanocomposites.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of quinoid ring
C stretching vibrations of quinoid ring![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration of benzenoid rings
C vibration of benzenoid rings











