The afterglow of carbon dots shining in inorganic matrices
Xiaoyan
He†
a,
Yihao
Zheng†
ab,
Chaofan
Hu
 a,
Bingfu
Lei
a,
Bingfu
Lei
 a,
Xingcai
Zhang
*c,
Yingliang
Liu
a,
Xingcai
Zhang
*c,
Yingliang
Liu
 *a and
Jianle
Zhuang
*a and
Jianle
Zhuang
 *a
*a
aKey Laboratory for Biobased Materials and Energy of Ministry of Education/Guangdong Provincial Engineering Technology Research Center for Optical Agriculture, College of Materials and Energy, South China Agricultural University, Guangzhou 510642, China. E-mail: tliuyl@scau.edu.cn; zhuangjl@scau.edu.cn
bJoint Key Laboratory of the Ministry of Education, Institute of Applied Physics and Materials Engineering, University of Macau, Avenida da Universidade, Taipa, Macau SAR 999078, China
cSchool of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA. E-mail: xingcai@seas.harvard.edu
First published on 5th October 2023
Abstract
Carbon dots (CDs) are a new type of quasi-spherical and zero-dimension carbon nanomaterial with a diameter less than 10 nm. They exhibit a broad absorption spanning from the ultraviolet (UV) to visible light regions and inspire growing interests due to their excellent performance. In recent years, it was identified that the CDs embedded in various inorganic matrices (IMs) can effectively activate afterglow emission by suppressing the nonradiative transitions of molecules and protecting the triplet excitons of CDs, which hold broad application prospects. Herein, recent advances in CDs@IMs are reviewed in detail, and the interaction and luminescence mechanisms between CDs and IMs are also summarized. We highlight the synthetic strategies of constructing composites and the roles of IMs in facilitating the applications of CDs in diverse areas. Finally, some directions and challenges of future research in this field are proposed.
Wider impactCarbon dot (CD)-based afterglow materials have become an attractive star after organic phosphorescent materials in the optical field. In order to realize the afterglow of CDs, two key issues need to be focused on. One is to promote intersystem crossing from the singlet to triplet state to generate triplet excitons, and the other is to protect triplet excitons from quenching by molecular motions and surrounding oxygen to enable radiative transition. With continuous efforts, various strategies have been developed to solve these issues. In particular, the method of introducing CDs into inorganic matrices (IMs) has been widely proven effective, where the number of articles accounts for a considerable proportion of CD-based afterglow materials. Therefore, a comprehensive understanding of the afterglow emission behaviors, formation mechanisms, and inherent interactions of various CDs@IMs systems is indispensable. In this review, the key research progress of representative CDs@IMs systems is firstly summarized, and then the functions of IMs to CDs on confinement effect, stabilization effect and electron/energy transfer are analyzed. The multifunctional applications of CDs@IMs in various fields are introduced, and the challenges and perspectives of future research are proposed. This review also provides guidance for extending the afterglow lifetime and emission wavelength from some new perspectives. |
1. Introduction
Carbon dots (CDs) have swiftly occupied a place in the field of new luminescent materials due to their advantages of facile preparation, excellent optical properties, low toxicity, good biocompatibility, etc.1–4 In 2004, Xu et al. first detected fluorescent carbon particles serendipitously in the process of purifying single-walled carbon nanotubes from arc-discharge soot.5 In 2006, surface-passivated nanoscale carbon particles were called “carbon dots”, and luminescent CDs with strong photoluminescence and good water solubility were discovered.6 The CDs family has been expanded during the research process, including carbon nanodots (CNDs), carbon quantum dots (CQDs), graphene quantum dots (GQDs), and carbonized polymer dots (CPDs).7 They mainly consist of sp2/sp3-hybridized carbon cores and abundant surface functional groups (e.g., –OH, –COOH, and –NH2).8 So far, the luminescence mechanisms of CDs have been explained by several theories, including molecular states, defect states, quantum confinement effects, crosslink-enhanced emission effects, etc.7,8 Accordingly, the luminescent properties of CDs can be regulated by changing the size of carbon cores, modifying the surface functional groups, and doping other heteroatoms.9,10 To date, CDs have widespread applications in sensing,11 catalysis,12–14 security,15 bioimaging,16 theranostics,4,17 optoelectronics,18,19 energy conversion and storage,20etc.Luminescent materials refer to the materials that can generate luminescence under the excitation of ultraviolet (UV), visible, infrared light, or X-ray, etc.15,21–23 Both fluorescent and afterglow materials in the field of photoluminescence have triggered a research boom since their discovery.24–28 The afterglow materials can eliminate the interference of background fluorescence and light scattering, and have greater advantage over fluorescence in some areas such as bio-imaging and anti-counterfeiting.8,29 To date, traditional afterglow materials still mainly include metal–organic complexes,30,31 pure organic compounds,32,33 and inorganic phosphors.34–36 However, the high cytotoxicity, strict crystal growth conditions, complex preparation process and short afterglow lifetime have greatly hindered their further development and application. Consequently, it is vital to design and prepare new metal-free inorganic afterglow materials that with low toxicity, excellent water solubility, ultralong lifetimes, and high quantum efficiency. In recent years, scientists have continuously been discovering long-lived afterglow emission phenomena in CDs, specifically room temperature phosphorescence (RTP), which absorbs energy at room temperature and exhibits long-lived emission after turning off the radiation. Obviously, the CD-based afterglow materials have greater advantages over traditional afterglow materials and have swiftly become a new star in the field of afterglow research.37–41 RTP is normally harder to obtain than fluorescence. It principally consists of two key processes: (1) the radiative transition from the lowest singlet state (S1) to the triplet state (T1) through the intersystem crossing (ISC) process; (2) the radiative transition from the lowest triplet state (T1) to the ground state (S0). Furthermore, delayed fluorescence (DF) will occur in the radiative transition from S1 to S0via the reverse intersystem crossing (RISC) process (Fig. 1).42,43 The processes mentioned above are generally influenced by the molecular vibration/rotation and surrounding dissolved oxygen.38,44 Hence, the promotion of ISC/RISC processes and inhibition of nonradiative transitions are vital for the production of afterglow effectively.45 To date, several strategies for afterglow enhancement have been proposed, such as reinforcing spin–orbit coupling (SOC) by introducing hetero atoms,46 heavy halogens,47 or transition metals,48 which are profitable for the more effective production of triplet excitons. Additionally, nonradiative transitions can be inhibited by crystalline inducement,39 crosslink-enhanced,49,50 or matrix-assisted processes.38
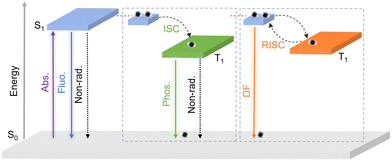 | ||
| Fig. 1 A schematic diagram of the emission process of fluorescence, phosphorescence, and delayed fluorescence. | ||
The matrix-assisted method principally embedded the CDs in the various rigid matrices, such as inorganic salts,39 boric acid (BA),51,52 silica (SiO2),53,54 urea/biuret,55 zeolites,37,56 NaCNO,44,57,58 polyacrylamide (PAM),59 cyanuric acid,60 melamine,61 polyvinyl alcohol (PVA), etc.62–64 From many previous studies, it is clear that nano-sized CDs can be easily embedded into matrix materials and then the abundant functional groups on the surface of CDs can form strong interactions such as covalent bonds, hydrogen bonds, and ionic bonds with the matrix to construct a stable structure and achieve afterglow effectively.65,66 In 2013, Zhao et al. first observed CD-based long lifetime RTP (up to 380 ms) in the PVA matrix, principally due to the strong SOC of aromatic carbonyls.67 This historic discovery brings a new breakthrough to the development of CD-based afterglow materials. Similar to the crosslinking enhancement process of CDs and the matrix-assisted process of organic polymers, inorganic matrices (IMs) can also provide a strong rigid structure and serve as an ideal host for CDs (Fig. 2). In 2015, Zhang et al. used the potash alum as the matrix for CDs in their experiments and showed that the composite materials could provide remarkable RTP with an average lifetime of 655 ms.68 Undoubtedly, they discovered a new continent where the inorganics can serve as ideal matrices to induce an efficient afterglow of CDs. Many studies have demonstrated that afterglow emissions are closely related to the triplet excitons of CDs and different long lifetime afterglow emissions can be obtained by choosing different precursors and matrices. In addition, researchers are similarly devoted to optimizing other properties of CDs@IMs and have made great progress in related research, such as water solubility, quantum yield (QY), and emission wavelength. In 2017, Lin et al. for the first time reported afterglow materials (m-CDs@nSiO2) with a long lifetime in an aqueous solution.69 In 2020, Shan and his group prepared water-soluble phosphorescent nanomaterials with a lifetime of 1.86 s and a phosphorescent QY of 11.6%.53 In 2021, Li et al. designed a novel material with a phosphorescent quantum efficiency of 48% via a grinding-induced amorphous to crystallization transformation.70 Qiu et al. prepared CDs@IMs with full-color ultralong afterglow, in which the RTP emission ranges from blue to red.71 Due to the excellent phosphorescent properties of CDs@IMs, they have important scientific significance and application prospects in bioimaging,53,72 fingerprint recognition,52,73 information encryption,71,73,74 time delay lighting,44 and many other fields.
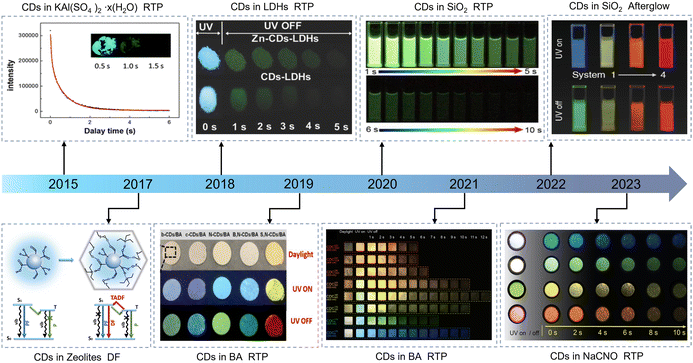 | ||
| Fig. 2 The development timeline of CDs in IMs. Reproduced with permission from ref. 37, 44, 48, 51, 53, 68, 71 and 75 Copyright 2015, Royal Society of Chemistry. Copyright 2017, American Association for the Advancement of Science. Copyright 2018, Wiley-VCH. Copyright 2019, Wiley-VCH. Copyright 2020, Elsevier Ltd. Copyright 2021, Wiley-VCH. Copyright 2022, Wiley-VCH. Copyright 2023, Wiley-VCH. | ||
In this review, the research progress of CDs@IMs in recent years will be introduced from the following aspects. Based on the different synthesis strategies and luminescence properties of composites, the function of IMs to CDs on structural confinement effect, structure stability, electron/energy transfer, and their applications in information encryption, anti-counterfeiting, sensing, fingerprint recognition, and biological imaging were analyzed. This is the first review to systematically summarize the work focused on the afterglow of CDs in IMs. We also outline the current challenges and development perspectives in this field for the future.
2. CDs in IMs: synthesis and properties
To date, a variety of IMs such as silica (SiO2), boric acid (BA), zeolites, layered double hydroxides (LDHs), molten salts (MS) and so on, have been used to activate the afterglow of CDs. We summarized some of the latest research results in recent years. Table 1 shows that the afterglow emissions of CDs in different IMs have different lifetimes (from milliseconds to seconds) and emission wavelengths (from 300 to 800 nm). The state of CDs can be solid or dispersed in a solvent. In addition, different properties provide CDs with versatile applications. The synthesis strategies and luminescent properties of CDs@IMs are illustrated below.| Matrices | Material Name | State | Afterglow Properties | Application | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Color | Ex (nm) | Em (nm) | Lifetime (s) | QY (%) | |||||
| NA: not available; Ex: excitation; Em: emission; QY: quantum yield. | |||||||||
| SiO2 | m-CDs@nSiO2 | Solution | Blue | 365 | 470 | 0.703 | NA | Information protection | 201769 |
| CDs/silica gel | Solid | Green | 380 | 525 | 1.8 | NA | NA | 201776 | |
| CDs@SiO2 | Solution | Green | 356 | 520 | 1.64 | NA | Tissue/cell imaging | 201972 | |
| CPDs/SiO2 | Solid | yellow green | 365 | 520 | 1.26 | NA | NA | 201977 | |
| WSP-CNDs@silica | Solution | Green | 350 | 520 | 1.86 | 11.6 | Bioimaging | 202053 | |
| CDs@SiO2 | Solid | Green | 330 | 480 | 1.62 | NA | Anti-counterfeiting, information encryption | 202078 | |
| CDs@SiO2-600 | Solid | Blue | 260 | 464 | 5.72 | 26.36 | Time-resolved anti-counterfeiting | 202040 | |
| CDs-SiO2 | Solid | Green | 340 | 517 | 1.76 | NA | Temperature sensing | 202079 | |
| CD@SiO2 | Solid | Blue | 370 | 440 | 0.030 | NA | Lifetime thermal sensing | 202080 | |
| CNDs-RhB@silica | Solution | Red | 350 | 600 | 0.91 | 3.56 | In vivo afterglow imaging | 202154 | |
| CNDs@silica | Solution | Green | 350 | 520 | 1.57 | 12.6 | Time division duplexing | 202181 | |
| F,NCDs@SiO2 | Solution | Green | 460 | 500 | 0.48 | NA | Information encryption, cellular imaging | 202182 | |
| CDs/silica | Solid | Deep-blue | 365 | 430 | 0.81 | NA | NA | 202183 | |
| PCDs-SNSs | Solution | Green | 360 | 505 | 2.19 | 7.4 | Information encryption, bioimaging | 202284 | |
| MSNs-CPDs | Solution | Blue | 365 | 430 | 0.176 | 6 | Information security, ion detection | 202285 | |
| CDs@SiO2-550 | Solid | Green | 360 | 508 | 0.451 | NA | Anti-counterfeiting | 202286 | |
| GCDs@SiO2-OCDs | Solution | Orange | 360 | 580 | 0.376 | 11.6 | Information security, fingerprint recognition | 202287 | |
| System 1–4 | Solution | Green-red | 355 | 510–610 | 0.99–1.61 | NA | Information encryption, in vivo/in vitro bioimaging | 202275 | |
| PAT-CDs@SiO2 | Solution | Green | 360 | 430,480 | 1.78 | NA | Multi-channel detections | 202288 | |
| SiO2/CDs | Solid | Green | 320 | 488 | 1.3 | 11.22 | Anti-counterfeiting | 202289 | |
| (B,G,Y,R)-CD@SiO2 | Solid | Bule-red | 260–360 | 465–680 | 0.25–2.11 | 3.3–36.68 | 3D information storage, encryption system | 202290 | |
| B,N,P-CDs280@SiO2 | Solution | Green | 345 | 502 | 1.97 | 3.15 | Afterglow imaging | 202391 | |
| BA (B2O3) | a-CDs/BA | Solid | yellow green | 350 | 530 | 1.6 | 8.7 | Information encryption | 201951 |
| G-CDs/B2O3 | Solid | Blue | 420 | 480 | 0.477 | NA | Information encryption, photo writing | 202092 | |
| BD50 | Solid | Yello green | 365 | 512 | 1.17 | 23.5 | Data encryption | 202147 | |
| CDs-I/B2O3 | Solid | Blue, green | 440 | 475, 555 | 0.423, 0.445 | 17.61 | Information encryption, fingerprint recognition | 202193 | |
| B-CD | Solid | Full-color | 260–420 | 466–638 | 0.113–0.581 | 0.42–13.7 | Anti-counterfeiting, 3D encryption | 202171 | |
| 7HOCA0.01%/BA | Solid | Cyan | 365 | 495 | 1.74 | 66.13 | Anti-counterfeiting, information security | 202194 | |
| CD@BA | Solid | Yellow green | 365 | 520 | 1.76 | 30 | Single-phase white-light-emitting diodes (WLEDs) | 202195 | |
| g-t-CD@BA | Solid | Green | 365 | 530 | 1.67 | 48 | NA | 202270 | |
| B-CDs | Solid | Yello green | 365 | 530 | 0.487 | 14.5 | Information encryption | 202274 | |
| P-CDs@B2O3 | Solid | Blue | 365 | 440 | 2.15 | NA | Information encryption/decryption | 202273 | |
| BCDs-0.1N | Solid | Blue | 273 | 424 | 2.02 | 24.1 | Bioimaging, information security and encryption | 202396 | |
| (G,Y,R,NIR)-CDs@BA | Solid | Green-NIR | 365–500 | 530–750 | 0.0004–1.66 | 0.51–38.2 | Dynamic information encryption | 202397 | |
| CB-I | Solid | Cyan | 392 | 438 | 0.193 | 35.07 | Information anticounterfeiting and encryption | 202398 | |
| Zeolite | CNDs-2@LEV-p | Solid | Green | 365 | 510 | 0.273 | 18.3 | Information encryption | 201799 |
| CDs@2D-AlPO | Solid | Blue | 370 | 440 | 0.197 | 52.14 | Security protection | 201737 | |
| CDs@MnAPO-CJ50 | Solid | Red | 420 | 620 | 0.010 | 9.6 | Anticounterfeiting, light emitting diodes (LEDs) | 201941 | |
| CDs@SBT-1 | Solid | Green | 370 | 525 | 0.574 | NA | Security protection | 2019100 | |
| CDs@Mn-LEV | Solid | Red | 420 | 620 | 0.001 | 5.7 | NA | 2019101 | |
| CD@MgAPO-5 | Solid | Blue-green | 360 | 425–515 | 0.271–0.859 | 20.86–42 | Time-dependent security protection | 2020102 | |
| CDs@AlPO-5 | Solid | Green | 400 | 530 | 2.1 | 24.4 | Information encryption, LED supplementary lighting | 2020103 | |
| CDs@zeolite | Solid | Green | 360 | 516–520 | 0.38–2.1 | NA | Optical multiplexing | 2021104 | |
| CDs@EuAPO-5 | Solid | Green | 360 | 516 | 1.4 | NA | Anti-counterfeiting | 202156 | |
| NA | Solid | Blue-red | 365 | 463–614 | 0.232–1.5 | NA | Time-space division information multiplexing | 2023105 | |
| LDHs | CDs-MgAl-LDHs | Solid | Green | 365 | ca. 525 | 0.319 | NA | Oxygen sensors | 2017106 |
| Zn-CDs-LDH | Solid | Green | 365 | 490 | 0.734 | 9.44 | NA | 201848 | |
| GQDs-LDHs | Solid | Green | 365 | 525 | 0.434 | 4.81 | Anti-counterfeiting | 2018107 | |
| Zn-CDs-LDHs | Solid | Green | 365 | 490 | 0.719 | 9.58 | Information security | 2019108 | |
| CDs-LDHs | Solid | Green | 300–500 | NA | 0.170 | 1.48 | Information encryption | 2022109 | |
| MS | CDs@MS | Solid | Green | 420 | 566 | 0.886 | NA | High-level information anti-counterfeiting | 2019110 |
| CDs@MP | Solution | Yello | 350 | 506 | 1.28 | 26.4 | pH/temperature detection | 2020111 | |
| CD71 | Solution | Yello | 340 | 520 | 0.616 | 22.5 | In vivo phosphorescence bioimaging | 2022112 | |
| ZnAl2O4 | CZAO700 | Solid | Red | 455 | 650 | NA | NA | NA | 2018113 |
| CDs@ZnAl2O4 | Solid | Yello-red | 290 | 400–570 | 0.41–1.05 | NA | Data encryption/decryption | 2021114 | |
| Potash Alum | CDs-APS1 | Solid | NA | 360 | 500 | 0.655 | NA | NA | 201568 |
| Al2(SO4)3 | NA | Solid | Green | 320 | 500 | 0.876 | NA | NA | 2017115 |
| NaCl | CDs-NaCl | Solid | Green | 365 | 519 | 0.314 | NA | NA | 2017116 |
| PDDA-CDs@NaCl | Solid | Green | 505 | NA | NA | 23–35 | Solution-based sensor | 2021117 | |
| Na0.5Mg0.25Cl | CDs/Na0.5Mg0.25Cl | Solid | Yello to green | 360 | 560 | 0.464 | 2.49 | Dynamic information encryption | 2023118 |
| (Ca,Sr,Ba)CO3 | F-CND/(Ca,Sr,Ba)CO3 | Solid | Green | 365 | NA | 0.072–0.127 | 0.3–1.3 | NA | 201939 |
| BNO | GQD@BNO | Solid | NA | 325 | 467 | 0.783 | NA | Time-resolved information security | 2020119 |
| Y(OH)xF3−x | CDs@YOHF | Solid | Green-orange | 360–380 | 494–602 | 0.37–0.53 | NA | Anti-counterfeiting, fingerprint detection | 2022120 |
| YF3 | CDs@YF3:Yb,Tm | Solid | Green | 380 | 538 | 0.82 | NA | Anti-counterfeiting, information encryption | 2023121 |
| NaCNO | CNDs-2@NaCNO | Solid | UV | 310 | 348 | 0.01582 | 16.2 | Antibacterial | 202257 |
| (UV,B,G,Y,R,NIR)-CNDs | Solid | UV-VIS-NIR | UV-VIS | 300–800 | 0.015–0.818 | NA | Time delay lighting | 202344 | |
| uCDs@SiO2/NaOH | Solid | Red | 365 | 600 | 0.1096 | NA | Anti-counterfeiting | 202358 | |
| NaOH | (b,g,r)-R-CDs | Solid | Blue-red | 290–320 | 483–635 | 0.058–0.389 | 0.63–9.05 | Information encryption | 2022122 |
| Al2O3 | g-CDs@Al2O3 | Solid | Green | 360 | 510 | 0.0007 | 42.6 | information encryption, fingerprint detection | 2023123 |
| Clay | CDs@clay | Solid | Blue | 254 | 450 | 1.05 | 1.08 | Anti-counterfeiting, sensing | 2019124 |
2.1. CDs in silica
SiO2 is a widely used inorganic non-metallic material, which has excellent characteristics of non-toxic, pollution-free and facile preparation. When SiO2 is used as the matrix to stabilize the triplet states of CDs, the common construction strategies of CDs@IMs composite materials include a one-step method and a two-step method (sol–gel method). In the one-step hydrothermal process, the formation of CDs is accompanied by the hydrolysis and condensation reactions of silane coupling agent under high temperature and pressure.77,83 Subsequently, the CDs are in situ embedded in the SiO2 matrix and they are connected by stable Si–C/Si–O–C covalent bonds. The traditional Stöber synthesis route is normally used in the field of sol–gel materials.125 Tetraethyl orthosilicate (TEOS) will undergo hydrolysis and condensation reactions in the presence of water and ammonia. The ammonia as a catalyst has an important effect on the reaction rate of the whole system.126 In the process of TEOS transforming into the gel, it can be considered that it has experienced monomer polymerization nucleation, particle growth, and particle link in three stages, then ultimately formed tiny and dispersed colloidal particles.127 These colloidal particles are linked by van der Waals forces, hydrogen bonds, or other chemical bonds to form a frame structure with open space. The CDs can be easily embedded in the SiO2 network (Fig. 3a).53 The functional groups on SiO2 are combined with those on the surface of CDs to form stable Si–O–C chemical bonds. Meanwhile, the dense network formed by Si–O–Si bonds provides a rigid structure for CDs. Yang et al. prepared CPD-embedded SiO2 microspheres using TEOS and ethylenediamine as precursors via a one-step hydrolysis-assisted crosslinking carbonization method (Fig. 3b).77 The rigid environment provided by the Si–O–Si network can protect triplet excitons and give composites with excellent optical performance and an RTP lifetime up to 1.26 s. This strategy provided a new idea for preparing long-lifetime afterglow materials. They then prepared CDs@IMs with deep-blue emission and a lifetime of 0.81 s through a one-step hydrothermal method.83 In this reaction system, they used only 3-aminopropyltriethoxylsilane (APTES) as a precursor to provide both the luminescent groups and rigid environment (Fig. 3c), and the potential chromophores on the CDs surface were linked to SiO2 microparticles by Si–C covalent bonds. Moreover, the rigid structure of matrices can effectively limit the motion of the groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N) and reduce the energy gap. This will spur researchers to design more blue or dark-blue CDs@IMs using a simple method.
O and C–N) and reduce the energy gap. This will spur researchers to design more blue or dark-blue CDs@IMs using a simple method.
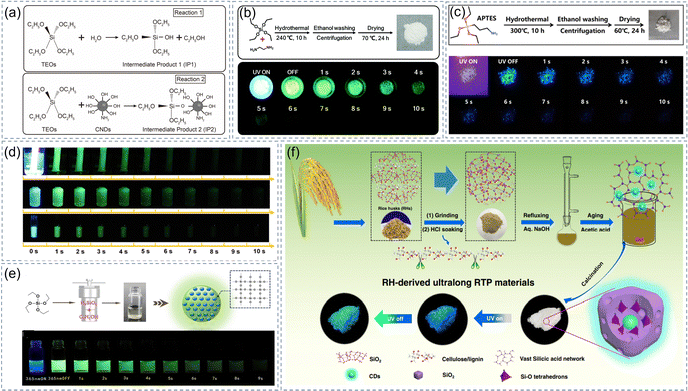 | ||
| Fig. 3 SiO2 as a matrix of CD-based afterglow materials. (a) Hydrolysis and condensation process of TEOS. Reproduced with permission.53 Copyright 2020, Elsevier Ltd. (b) Schematic representation of preparing CPDs-embedded SiO2 microspheres and their luminescence properties. Reproduced with permission.77 Copyright 2019, Royal Society of Chemistry. (c) Schematic diagram of preparing RTP materials and their luminescence properties. Reproduced with permission.83 Copyright 2021, American Chemical Society. (d) Photographs of the CDs@SiO2 under UV on or off. Reproduced with permission.72 Copyright 2019, American Chemical Society. (e) Schematic diagram of the synthesis process and photographs of the sample under 365 nm on or off. Reproduced with permission.84 Copyright 2021, Elsevier Ltd. (f) Specific synthesis steps from rice husks raw material to the CDs@SiO2 sample. Reproduced with permission.40 Copyright 2020, Springer Nature. | ||
Compared with short-lifetime fluorescent probes, afterglow probes have the advantage of a high signal-to-noise ratio, which helps to eliminate the interference from autofluorescence. Nevertheless, achieving long-lifetime afterglow in an aqueous solution remains a challenge as the transition of excitons from T1 to S1 is prohibited. It's very gratifying to see that researchers have made many attempts and also made some breakthroughs continually over the past few years. Our research group prepared composite materials (CDs@SiO2) by embedding CDs into colloidal silica and TEOS as the raw materials, which accomplished green afterglow in air-saturated aqueous solution, and its ultralong RTP lifetime was 1.64 s (Fig. 3d).72 The analysis indicated that CDs acted as a luminescence center while SiO2 provided a rigid environment and they are connected by the Si–O–C bonds. The resulting CDs@SiO2 had excellent hydrophilicity due to the abundant Si–OH groups on its surface, which enabled the application of CDs@SiO2 for practical imaging of biological samples. Subsequently, Shan et al. made another breakthrough in this area by embedding CNDs in the silica layer to limit the rotation/vibration of the bonds and isolate it from oxygen molecules.53 The RTP lifetime and phosphorescent QY of the CNDs reached 1.86 s and 11.6% in aqueous solution. They could be used as an afterglow agent in the study of in vivo/in vitro bioimaging because of their excellent phosphorescent properties, small size, and good water solubility. Feng et al. selected TEOS as the precursor and synthesized polymer carbon dot-silica nanosphere composites with a lifetime of 2.19 s using a one-step hydrothermal method (Fig. 3e).84 The analysis shows that the interaction of the strong covalent/hydrogen bonds between CDs and SiO2 are the main reasons for the RTP and ultralong lifetimes. In summary, the main reasons for obtaining effective afterglow are as follows: (1) the effect of the hydrogen bonds can promote triplet states emission; (2) the strong covalent bonds are profitable for prolonging the time of afterglow emission and realizing the state of afterglow from solid to water-soluble compared with the common hydrogen/halogen bonds; (3) the rigid environment protects the luminescent centers from the surrounding dissolved oxygen and prohibits the nonradiative transition of triplet excitons.
In addition to exploring how to extend the afterglow lifetimes and gain water-soluble CDs@IMs, researchers are also looking for their other properties such as their high stability and multicolor afterglow. Our group designed a material with a multi-confinement structure of the highly rigid network that has excellent stability in strong oxidants, acids, bases, and polar solvents.40 The multiple constraints imposed by the tetrahedral interstice are different from the Si–OH network by TEOS hydrolysis, which is unique in that CDs are embedded in situ in the huge SiO2 network composed of Si–O tetrahedron (Fig. 3f). The stable covalent bonds and three-dimensional nano-space constraints can effectively suppress the vibration of molecules and successfully protect the triplet states of CDs. Lu et al. successfully obtained long-lived multicolor (blue to red) phosphorescent CD-based composites by embedding multicolor CDs in silica via hydrolysis of TEOS.90 This is mainly attributed to the construction of rigid Si–C bonds after calcination.
2.2. CDs in boric acid (BA)
Boron atom with a vacant p orbit can attract π electrons to form the p–π conjugate system when CDs are dispersed in BA.92 It can effectively reduce the lowest unoccupied molecular orbital (LUMO) energy level of the system and promote the ISC between S1 and T1. Our group reported for the first time that BA were used as the matrix of CDs to activate the multi-color RTP for CDs by one-step heat treatment of CDs and BA (Fig. 4a).51 To verify the universality of this strategy, we used heteroatom-free CDs as the luminescence centers to activate their RTP. This type of CDs containing C, H, and O atoms find it more difficult to produce RTP and they have a larger energy gap between S1 and T1 compared with those of heteroatom doping. Amazingly, some heteroatom-free CDs embedded in the BA matrix also exhibit long lifetime RTP. Subsequently, we carried out a series of experimental studies to further demonstrate that BA can be used as a universal matrix. Based on Dexter's energy transfer (ET) theory, the ET rate constant depends on the pre-design of the appropriate energy donors and acceptors, and the overlap between the excitation spectrum of energy acceptors and the emission spectrum of energy donors reveals the possibility of effective ET. Inspired by this theory, we have constructed an efficient method for strengthening RTP by embedding CDs in the BA matrix and the RTP emission intensity and lifetime of CDs are markedly prolonged in this design system via ET (Fig. 4b).73 In order to address the issue that CDs@IMs can be only excited by UV or visible light, we recombine CDs@IMs with rare earth upconversion materials to achieve near-infrared-excited multicolor afterglow.52 It has great significance for the long-wavelength excitation of CDs afterglow and their application in biological imaging.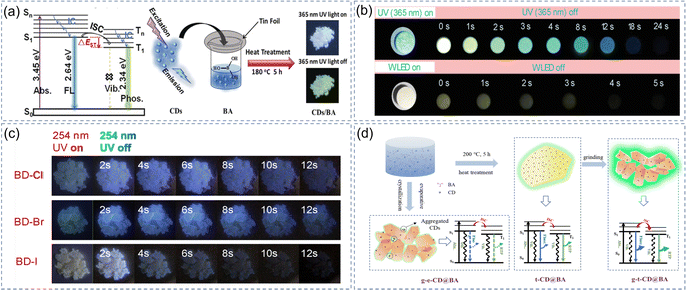 | ||
| Fig. 4 BA as a matrix of CD-based afterglow materials. (a) Schematic diagram of the luminescence mechanism of phosphorescence and representation of the recombination process of CDs and BA. Reproduced with permission.51 Copyright 2019, Wiley-VCH. (b) Photographs of the P-CDs@B2O3 under UV or white light-emitting diodes on or off. Reproduced with permission.73 Copyright 2021, Wiley-VCH. (c) Fluorescence and afterglow of BD-Cl, BD-Br, and BD-I composites excited at 254 nm. Reproduced with permission.47 Copyright 2020, Elsevier B.V. (d) Formation process of the crystalline complex and phosphorescent emission mechanism. Reproduced with permission.70 Copyright 2021, Wiley-VCH. | ||
In recent years, many researchers have constructed a series of CDs@IMs using different methods after BA was found to be a universal matrix for CDs. Although these methods considerably promote the phosphorescent emission of CDs, the QY of existing CDs is still inferior to some organic phosphorescent materials. In order to solve this problem, Zheng et al. introduced halogens (Cl, Br, and I) into the precursor compounds and prepared a series of color-tunable RTP materials (Fig. 4c). The materials have an ultralong-lifetime (up to 2.61 s) and high phosphorescent QY (up to 34.4%) because of the heavy-atom effect.47 Recently, Li and his group notably prepared a phosphorescent composite material (CD@BA) with a phosphorescent QY of up to 48%.70 It is found that the high quantum efficiency of CD@BA is closely related to the mechanical grinding process (Fig. 4d). The grinding process prompts the amorphous complexes to transform into a crystalline phase, and the rigid crystalline structure can effectively protect the triplet excitons. Chen et al. prepared an RTP material using a one-step method that had an ultra-long lifetime and high QY up to 1.74 s and 66.13% respectively.94 This is primarily attributed to the rigid structure of the matrices and the interaction of the B–C covalent/hydrogen bonds between BA and CDs, which can effectively inhibit the motion of CDs and thus successfully protect the triplet states of CDs. Although the quantum efficiency of afterglow obtained in different systems cannot be compared together, it can be seen from the summary of some research achievements in recent years that researchers have made a lot of efforts in this area and put forward some effective strategies (such as heavy atom effect and mechanical grinding) for future reference.
2.3. CDs in zeolite
Zeolite is a type of inorganic crystal microporous material with ordered distribution and its pore size is less than 2 nm, which has high thermal and chemical stability. Pyrolysis is a simple method of preparing this type of CDs@IMs: (1) inorganic ions in zeolite pores are exchanged with organic carbon sources and then calcined at a certain temperature; (2) uniform CDs can be directly generated by pyrolysis of organo-templated zeolites or an in situ solvent-free thermal crystallization method.99,128–130 Meanwhile, the particle size of CDs is greatly larger than the micropore size of the zeolite, so the zeolite framework can be used as a suitable matrix to disperse CDs, and effectively avoid the aggregation and phosphorescent quenching of CDs. Since 2008, CDs with a uniform size have been dispersed/supported into the zeolite matrix to achieve stable solid-state photoluminescence by thermal oxidation of the zeolite host.128 The molecular framework of zeolite can effectively isolate the oxygen around CDs and stabilize the triplet states. So far, the composite materials that contain zeolites as the matrix have achieved thermally activated delayed fluorescence (TADF) and RTP. In 2017, Yu et al. prepared a series of new CD-based TADF materials (CNDs@zeolite) with a high QY up to 52.14% by pyrolysis of template-containing organic zeolite.37 The organic structure-directing agents similarly play an indispensable role in the efficient TADF emission of composite materials in addition to the confined space effect of zeolites. The different RTP composite materials with lifetimes of 273–312 ms were mainly obtained by adjusting the zeolite matrix (host) and the organic template (guest) (Fig. 5a), which was proved to be an effective strategy for designing CDs@IMs.99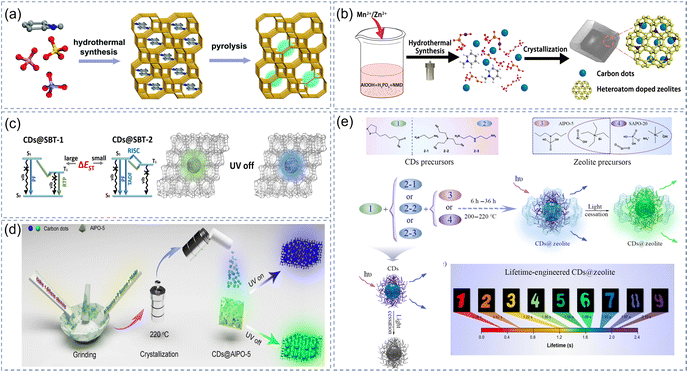 | ||
| Fig. 5 Zeolite as a matrix of CD-based afterglow materials. (a) Hydrothermal synthesis and pyrolysis of the CNDs@zeolite. Reproduced with permission.99 Copyright 2017, Royal Society of Chemistry. (b) Hydrothermal synthesis and crystallization of the CD-based afterglow materials. Reproduced with permission.101 Copyright 2019, American Chemical Society. (c) Phosphorescence/delay fluorescence mechanisms of the CNDs@zeolite. Reproduced with permission.100 Copyright 2019, American Chemical Society. (d) The preparation process and luminescence properties of CDs@AIPO-5. Reproduced with permission.103 Copyright 2020, Chinese Chemical Society publishing. (e) The synthesis process of CNDs@zeolite and the lifetime engineered. Reproduced with permission.104 Copyright 2021, Chinese Chemical Society publishing. | ||
The formation of CDs is commonly accompanied by the crystallization of zeolites in the process of preparing afterglow materials using the solvothermal method. To be more precise, CDs are embedded and restricted in the zeolite matrix in the growth process of crystals. In this way, the vibration and rotation of the CDs themselves can be suppressed, and the triplet states can be stabilized. Two necessary conditions are as follows for ET to effectively reduce the energy gaps in the donor–acceptor systems: (1) selecting a suitable carbon source so that the absorption spectrum of the donor overlaps the emission spectrum of the acceptor; (2) by introducing transition metal atoms into the zeolite matrices, the zeolite matrices with doped heteroatoms can act as an acceptor to promote the ET process. Based on this strategy, CDs (N-Methylpiperidine) were used as the energy donor, and the aluminophosphate zeolite frameworks with doped heteroatoms (Mn2+) were used as an energy acceptor (Fig. 5b).101 The emission spectra of CDs and absorption spectra of aluminumphosphate had a large overlap, which resulted in effective ET and reduced the energy gaps. The red RTP composite material was prepared by doping heteroatoms in the zeolite, whose lifetime and QY were 1.814 ms and 5.7% respectively. Significantly, the clever combination of CDs prepared by different precursors and zeolite matrices with variable constraint space facilitated the preparation of CDs@IMs with some new luminescence properties and applications. For example, when 4-(2-aminoethyl)-morpholine was used as a precursor, the composites emitted green RTP with a lifetime of 574 ms (Fig. 5c).100
It is worth noting whether small organic molecules can also synthesize CDs in the zeolite frameworks in situ under solvent-free conditions to achieve efficient ultralong afterglow emission. First of all, it is essential to understand some principles in the type selection of CDs precursors and zeolite matrices in the process of synthesizing CDs@zeolite composites. For example, the precursors of CDs rich in O, N, or P atoms and the zeolite matrices that can provide strong chemical bonds will be the best option to obtain long-lifetime composite materials. Yu and colleagues used organic amines and acids as the precursors to prepare the composites (CDs@AIPO-5) using a solvent-free thermal crystallization method. The CDs@AIPO-5 had ultralong TADF (1.7 s) and RTP (2.1 s) dual emission, and the phosphorescent QY was up to 24.4% (Fig. 5d).103 The diversity of CDs precursors and the tunability of the zeolite matrices enabled the afterglow lifetime of the composites to be tuned in a wide range. Yu et al. selected α-lipoic acid and three kinds of amine analogs with different structures as CDs precursors and combined the different CDs precursors with AlPO-5 and SAPO-20 zeolite matrices in the synthesis of CDs@zeolite composites. Different amounts of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N–H bonds introduced by CDs precursors and different host–guest interactions generated by the two zeolite matrices are the key factors for the tunability of the afterglow lifetime of the composites. Thus, CDs@zeolite composites with afterglow lifetimes ranging from 0.38 to 2.1 s can be fabricated in solvent-free conditions by means of rational design.104 At the same time, the regulation mechanism of various reaction factors such as the intersystem cross rate (KISC) and nonradiative decay rate (Knr) on RTP lifetime was further clarified (Fig. 5e).
N and N–H bonds introduced by CDs precursors and different host–guest interactions generated by the two zeolite matrices are the key factors for the tunability of the afterglow lifetime of the composites. Thus, CDs@zeolite composites with afterglow lifetimes ranging from 0.38 to 2.1 s can be fabricated in solvent-free conditions by means of rational design.104 At the same time, the regulation mechanism of various reaction factors such as the intersystem cross rate (KISC) and nonradiative decay rate (Knr) on RTP lifetime was further clarified (Fig. 5e).
2.4. CDs in layered double hydroxides (LDHs)
LDHs are composed of several positively charged layers and anions of balanced charge.131 The commonly used preparation method is co-precipitation,132 a sol–gel method,133 and hydrothermal synthesis.134 Its chemical formula can be expressed as [M1−x2+Mx3+(OH)2]x+(An−)x/n·mH2O, where M2+ and M3+ represent non-noble metal cations (e.g., Mg, Fe, Cu, Al, Ga, etc.), the value of x is a ratio of M3+/(M2+ + M3+); An− is an anion of balanced charge layers; m is the space-occupying amount of water molecules in the interlayer region in the absence of anions.131,135 The special structure of LDHs provides a rigidly confined space for some functional molecules to enhance their optical,136 drug delivery,137 and catalytic properties.138The chemical composition and spatial structure of LDHs are highly tunable, and the anions in the interlamellar can be easily replaced by other ions. Hence, Shi et al. used LDHs as the rigid matrices for CDs to activate the afterglow by the synergistic action of ordered non-noble metals and dual structural confinements.106 In this system, the non-noble metal Zn with a small atomic number was arranged in order on LDHs, which strengthened the SOC of electrons and reduced the energy gap of the singlet–triplet state. In addition, the nonradiative relaxation process of CDs is inhibited by the dual structural confinements of the matrices. CDs and LDHs are connected by some chemical bonds (e.g., hydrogen bonds, coordination bonds, covalent bonds), and there is also electrostatic interaction. CDs-LDHs materials were calcined at different temperatures (from 200 °C to 400 °C) to study the effect of dual structural confinements on luminescence properties. Its phosphorescence intensity increased first and then decreased with the increase in temperature and the optimum phosphorescence intensity occurred at 300 °C. The structure of LDHs collapsed at 350 °C, resulting in weakening of the structural confinement and a decrease in the CDs-LDHs phosphorescence intensity. They carried out further research since the LDHs have ordered non-noble metal arrangement, dual structural confinements and tunability of different metal types and proportions (Fig. 6a).48 Based on the tunneling-related mechanism, they combined the theory of electron spin resonance, thermoluminescence, and positron annihilation lifetime spectroscopy to study how the LDH defects affect the exciton transfer process. The results demonstrated that the presence of Zn increased the defect depth and was more conducive to the stability of excitons and the prolongation of lifetime. After a series of experiments, they proposed three design principles to activate the afterglow and prolong its lifetime based on the design idea of the synergistic effect: structural confinement effect, heavy atom effect, and chemical bonds (Fig. 6b),108 which opens up a new path for the construction of organic–inorganic luminescent materials with ultralong afterglow.
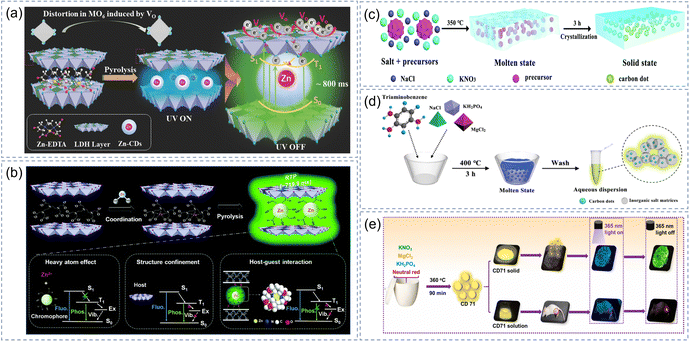 | ||
| Fig. 6 LDHs and MS as the matrices of CD-based afterglow materials. (a) Structure diagram of the Zn-CDs and LDH Layer after forming a composite, and their luminescence properties. Reproduced with permission.48 Copyright 2018, Wiley-VCH. (b) The luminescence properties of Zn-CDs-LDH. Reproduced with permission.108 Copyright 2019, Royal Society of Chemistry. (c) The synthesis process of the CDs/MS complex. Reproduced with permission.110 Copyright 2019, Royal Society of Chemistry. (d) Preparation process of water-soluble CDs@MP. Reproduced with permission.111 Copyright 2020, American Chemical Society. (e) Preparation of solid and water-soluble CD71 and its application. Reproduced with permission.112 Copyright 2021, Elsevier B.V. | ||
2.5. CDs in Molten salt
Molten salts (MS) are solid at room temperature and can be converted to liquid at high temperatures. The molten salt method (MSM) is a new method to synthesize materials using MS as a reaction medium.139,140 Researchers have prepared a number of inorganic crystals and carbon nanomaterials containing different ions by selecting different types of salt (NaCl, KNO3, MgCl2, etc.) and regulating the melting temperature (operating range between 100 °C and 1000 °C). MSM is suitable for the large-scale synthesis of nanomaterials due to its advantages of facile preparation, rapid ion diffusion, and adjustable temperature. So far, Fe2O3 nanocrystals, semiconductors, graphene and carbon nanomaterials have been prepared via MSM.141 In recent years, the MSM has been used in the preparation of CDs composites and some relevant research results have been reported in the literature. Song et al. developed an MSM for the in situ preparation of CDs@IMs with a long lifetime and long-wavelength emission.110 CDs were embedded in the special crystal structure during the melting and recrystallization of inorganic salts (Fig. 6c), which inhibited nonradiative relaxation by stabilizing triplet excitons and preventing intermolecular collisions. The resulting CDs/MS nanocomposites showed excitation dependence at different wavelengths of excitation and color-tunable RTP (green to yellow).For many years, how to achieve stable emission of afterglow in an aqueous solution has puzzled many scientists. They have tried many different matrices and found that the MS is another possible IM for realizing water-soluble afterglow in addition to hydrophilic silica. CDs precursors and inorganic salt can be calcined directly through MSM to prepare water-soluble CDs@IMs. Based on previous studies, Song et al. chose phosphate salts and magnesium with a high-charge-density as doping salts for the synthesis of CDs. CDs were produced and embedded into the crystalline salt matrix during melting and recrystallization (Fig. 6d).111 Since magnesium phosphate is insoluble in water, it mainly exists in possible forms of magnesium hydrogen phosphate, magnesium hydrogen phosphate trihydrate, and magnesium oxide to provide rigid protection for CDs. Therefore, the CDs composite (CDs@MP) synthesized using this method had bright RTP, and its afterglow QY and lifetime were 26.4% and 1.28 s, respectively (about 6 s in an aqueous solution by the naked eye). More importantly, their good water solubility resulted from the synergistic effect of crystal confinement and aggregation-induced phosphorescence (AIP). They dissolved CDs@MP in HCl solutions and regulated the degree of aggregation by adjusting the volume fraction of ethanol (fe) to test the properties of AIP. The CDs@MP aggregates would self-assemble to form a network when the fe reached 70%, which reinforced the rigid structure of the crystalline salt matrix and stabilized the triplet states. In addition, the good crystal structure of the matrix is closely related to the addition of inorganic salts, and the high-charge-density of metal ions helps to reduce the energy gap and achieve the ISC process. Subsequently, Zheng et al. chose neutral red, KNO3, MgCl2, and KH2PO4 as the raw materials to synthesize the CD71 composite material, which showed water-soluble yellow RTP and were successfully applied in fingerprint recognition and in vivo biological imaging (Fig. 6e).112 In another research study, they used BA as the matrix for CDs and obtained solid phosphorescent (2.61 s) by the heavy-atom effect, but there was no afterglow in the aqueous solution.47 Consequently, the rigid protection and solubility of the matrices in solution are crucial for the realization of water-soluble afterglow.
2.6. CDs in other matrices
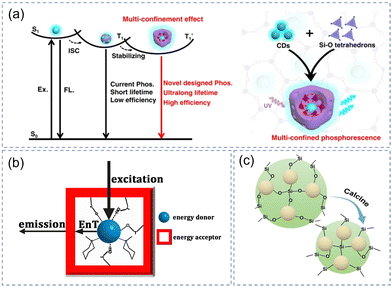
Carbonate/sulfate, zinc aluminate, hydroxy fluorides, and NaCNO crystal can also be used as excellent matrices for CDs besides SiO2, BA, zeolite, LDHS, and MS mentioned above. The afterglow emission of CDs@IMs is predominantly in the green region in these existing studies, while blue, yellow and orange are comparatively uncommon, not to mention UV, red and NIR. Several strategies have been proposed to resolve this dilemma, such as interfering with the electronic transitions, increasing the deep levels/defects, and forming the multi-confinement effect of the matrices to regulate the energy gap and protect the triple states.The physical properties and luminescence properties of various nanocomposites were studied when F-CND were embedded in crystalline particles of carbonates, sulfates, and oxalates by Meldrum et al.39 It was discovered that the phosphorescence QY and lifetimes were related to the atomic number of cations. At the same time, the synergy of the matrices and heavy atoms brings the cations close to the surface of F-CNDs and affects the electronic transitions between the active carbonyl group and the surface groups of F-CNDs. In addition, the long lifetimes and radiative transition of excited states can be systematically regulated by changing the deep levels of the matrix. Hirata et al. synthesized CDs in situ in the zinc aluminate (CZAO) matrix.113 They used a simple and convenient method to obtain extremely long-lived green/yellow and orange/red RTP composite materials, and the RTP lasted for 5 and 15 minutes, respectively, which were 2–3 orders of magnitude longer than previously reported. The color change was largely adjusted by the annealing temperature of the CZAO sample, because the oxidation degree of the sample increased with the annealing temperature, resulting in a red-shift of fluorescence emission and an increase in the energy gap from 1.2 eV to 3.2 eV. Moreover, the generation of deep levels causes ET from one trap level to another and then prolongs the phosphorescent lifetime of the emission center. Based on this strategy, an ultra-wideband RTP composite (CDs@ZnAl2O4) with UV and visible excitation was prepared by in situ calcination in our group.114 The multi-color RTP emission from yellow to red is primarily due to matrix-assisted action and different triplet states generated via ISC. Recently, our group achieved multi-color long-wavelength RTP emission by embedding CDs in the hydroxy fluorides (Y(OH)xF3−x) matrix.120 Due to its multiple confinements (space confinement, hydrogen bonds, C–F bonds) effect, the RTP of CDs was activated. The strong protection of the rigid matrix also ensured the stability of the material. Recently, Shan's group obtained UV to NIR (300–800 nm) phosphorescence of CDs@IMs under rigid NaCNO matrix constraints by improving the strength of SOC and the rate of ISC of CNDs by introducing hetero atoms (N, O) with a lone pair of electrons.44 This facile strategy provides a new way to generate afterglow materials with specific performances.
3. Roles of IMs in CD-based afterglow materials
3.1. Structural confinement effect on CDs
The rigid structure of the matrix occupies an important position in the afterglow emission of CDs (Fig. 7a).40 The porous nanostructure/dense network of the IMs can not only disperse CDs but also limit the growth of CDs, resulting in good dispersion and uniform size distribution of CDs. Joseph et al. used aluminum sulfate as a matrix for CDs, which effectively limits the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and inhibited the nonradiative transition of triplet excitons, enabling the composite to emit distinct green RTP with a lifetime of 876 ms.115 Some IMs cannot produce afterglow by themselves, but can provide a rigid environment for CDs to assist in inducing the afterglow of CDs. Molten salts KNO3, MgCl2, and KH2PO4 can be used to embed CDs during melting and recrystallization, which made CDs have excellent dispersion and stability in the aqueous solution and realized water-soluble yellow phosphorescent emission.112 Shan et al. designed a crystal engineering process for in situ confining citric acid-derived CDs and achieved full-color RTP by suppressing nonradiative transition of triplet excitons through rigid crystal NaOH.122 In addition, SiO2 with a dense network and three-dimensional space constraints, zeolite with a regular porous structure, LDHs with a nano-interlayer structure, and aluminate with a spinel structure are likewise considered as outstanding matrices to activate the afterglow of CDs by a structural confinement effect.
N and inhibited the nonradiative transition of triplet excitons, enabling the composite to emit distinct green RTP with a lifetime of 876 ms.115 Some IMs cannot produce afterglow by themselves, but can provide a rigid environment for CDs to assist in inducing the afterglow of CDs. Molten salts KNO3, MgCl2, and KH2PO4 can be used to embed CDs during melting and recrystallization, which made CDs have excellent dispersion and stability in the aqueous solution and realized water-soluble yellow phosphorescent emission.112 Shan et al. designed a crystal engineering process for in situ confining citric acid-derived CDs and achieved full-color RTP by suppressing nonradiative transition of triplet excitons through rigid crystal NaOH.122 In addition, SiO2 with a dense network and three-dimensional space constraints, zeolite with a regular porous structure, LDHs with a nano-interlayer structure, and aluminate with a spinel structure are likewise considered as outstanding matrices to activate the afterglow of CDs by a structural confinement effect.
 | ||
| Fig. 7 Roles of IMs in CD-based composites. (a) Three-dimensional nano-space confinement effect of the SiO2 matrix. Reproduced with permission.40 Copyright 2020, Springer Nature. (b) Energy transfer between donor and receptor. Reproduced with permission.101 Copyright 2019, American Chemical Society. (c) Some chemical bonds in SiO2 microspheres. Reproduced with permission.90 Copyright 2022, Wiley-VCH. | ||
3.2. Energy/electron transfer in host–guest systems
The ET in host–guest systems is an effective way to improve the afterglow performance (Fig. 7b),101 which can predominantly be used to prolong the lifetime and tune the emission wavelength of the afterglow. ET normally includes the following forms: (1) the effective ET occurs between matrices (contains metal ions or defects) and CDs. Lu et al. reported a mechanism that utilized traps to capture and release excitons.48 In the design of this hybrid system, Zn2+ was selected as a co-dopant to induce trap generation on the LDH matrices. The traps can be used as an exciton capture center to produce a defect-related ET process, stabilize triplet excitons and promote the RTP emission of Zn-CDs-LDHs. The afterglow lifetime and emission wavelength of the composite can be cleverly adjusted via efficient ET when the CDs are embedded in the Mn-matrix.41 The emission wavelength of the afterglow is closely related to the energy gap, traps, and defect states. Consequently, the ET mechanism of CDs@IMs remains to be elucidated and further study is required. Our group investigated the RTP mechanism of CDs by combining the effects of intrinsic emission and surface group emission.114 The yellow to red RTP of CDs@ZnAl2O4 composites can be achieved by efficient energy transfer between the inorganic defects and the triplet excited states of CDs. (2) The CDs and fluorescent dyes are embedded in the IMs, and the effective ET occurs between different CDs and CDs/fluorescent dyes. The absorption band of the acceptor and the emission band of the donor have a large spectral overlap integral, which provides a possibility for effective ET. Yu's group first proposed ET between two different CDs in the zeolite matrix based on their previous in-depth studies of ET in the CDs-in-Zeolite system.102 Liang et al. designed and synthesized a red afterglow imaging agent (CNDs-RhB@SiO2) composed of Rhodamine B and CDs.54 In this composite system, CDs with long-lifetime green RTP acted as the energy donor, and Rhodamine B dye with red fluorescence acted as the energy acceptor, both of which were enclosed within the hydrophilic SiO2. The distance between donor and receptor is less than 10nm, so that an effective Förster resonance energy transfer (FRET) process can take place in abundant SiO2 microspheres with the transfer efficiency reaching 99.2%. Consequently, a good host–guest energy/electron transfer system design can enhance unprecedented performance in many luminescence applications. Recently, our group utilized the same strategy to design the cascade resonance ET systems to achieve multi-color long afterglow.75 We demonstrated the reliability of FRET in regulating afterglow emission wavelengths by studying the changes in the overlap integral between the emission spectra of energy donors and acceptors in one-step, two-step, and three-step FRET processes. (3) The effective ET occurs between CDs@IMs and CDs/fluorescence quantum dots. Wang et al. designed a strategy that enables energy-transfer-induced delayed fluorescence in aqueous solutions,87 in which the silica nanoparticles (GCDs@SiO2) with green phosphorescent emission are used as energy donors and the CDs (OCDs) with orange fluorescence emission are used as energy acceptors. Yu et al. achieved tunable afterglow emission through resonant energy transfer by integrating CDs@zeolite composites and multicolor fluorescent quantum dots into a small container.1053.3. Stabilization effect on CDs
Due to the abundant functional groups (hydroxyl, amino, and carboxyl) on the surface of CDs (Fig. 7c),90 strong rigid bonds (covalent bonds, hydrogen bonds, ionic bonds) can be formed between CDs and the matrix, thus stabilizing the triplet states of CDs. Joseph et al. synthesized CDs with green RTP (lifetime is about 1.8 s) by using malonic acid and ethylenediamine rich in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functional groups as raw materials. This is due to the rigid bonds between the SiO2 and CDs that forbid intermolecular vibration and protect the radiative transition from T1 to S0.76 In our previous studies, we found for the first time that the glassy states were the new forms to stabilize triplet excitons in addition to hydrogen bonds, covalent bonds, and crystalline states and demonstrated the formation of the B–C bonds was the main cause of activation of RTP when BA was used as the matrix.51 Some porous materials such as zeolites can also form hydrogen bonds or even covalent bonds with the surface functional groups of CDs, which considerably limits the vibration/rotation of bonds and reduces the nonradiative transition, resulting in efficient afterglow emission and lifetime up to 2.1 s.104 Compared with the covalent and hydrogen bonds mentioned above, Shan et al. recently found that the high-density ionic bonds between cations (Na+) and CDs had stronger bond energy to suppress the molecular-vibration-induced nonradiative transitions when NaCNO and NaOH served as the protective matrix of CDs.44,57,58,122
N functional groups as raw materials. This is due to the rigid bonds between the SiO2 and CDs that forbid intermolecular vibration and protect the radiative transition from T1 to S0.76 In our previous studies, we found for the first time that the glassy states were the new forms to stabilize triplet excitons in addition to hydrogen bonds, covalent bonds, and crystalline states and demonstrated the formation of the B–C bonds was the main cause of activation of RTP when BA was used as the matrix.51 Some porous materials such as zeolites can also form hydrogen bonds or even covalent bonds with the surface functional groups of CDs, which considerably limits the vibration/rotation of bonds and reduces the nonradiative transition, resulting in efficient afterglow emission and lifetime up to 2.1 s.104 Compared with the covalent and hydrogen bonds mentioned above, Shan et al. recently found that the high-density ionic bonds between cations (Na+) and CDs had stronger bond energy to suppress the molecular-vibration-induced nonradiative transitions when NaCNO and NaOH served as the protective matrix of CDs.44,57,58,122
4. Applications of CDs@IMs
CDs@IMs have the advantages of multicolor luminescence, long lifetime, low toxicity, good biocompatibility, and eliminating interference from background fluorescence and light scatter, which have broad application prospects in information encryption, anti-counterfeiting, sensing, biological imaging and fingerprint identification. In this section, we will show some reported representative applications in these fields and briefly introduce the mechanism.4.1. Information encryption
Due to the unique fluorescence and afterglow properties of the CDs@IMs, they have great potential in the application of information encryption.142 The problem of the spatio-temporal overlapping information was solved and the advanced information encryption of the time division duplexing technology was accomplished utilizing the difference in the lifetimes of two materials.81 As shown in Fig. 8a, the encrypted information in channel 1 (CNDs) and channel 2 (CNDs@SiO2) cannot be read due to the spatio-temporal overlapping. The nano-second channel and the second channel can be obtained after the introduction of water molecules so that the correct information can be recognized. Tunable full-color long lifetime RTP composites were prepared by thermal decomposition of citric acid and BA raw materials with different mass ratios under different reaction conditions.71 The emission wavelengths of the composites were continuously adjustable from 466–638 nm. As displayed in Fig. 8b, multiple barcodes are obtained by permutation and combination of eight composite materials with different RTP colors. In different combinations, the RTP color varies with the excitation wavelength and time, so the information can be stored and encrypted by changing the permutation mode or excitation wavelength. As shown in Fig. 8c, a 3D multichannel information encryption system is demonstrated utilizing the unique afterglow properties of CD/SiO2 composites. The CD/SiO2 composite materials were placed in pre-prepared five grooves to encrypt and store the 3D information by turning on and off the UV lamps (254, 365, and 395 nm) and adding or removing filters.90 Advanced encryption can be carried out while storing a large amount of information due to the thermally enhanced and time/temperature-dependent emission nature of the uCDs@SiO2/NaOH materials (Fig. 8d).58 The code “520” shows the wrong information when the 365 nm UV lamp is on and off. The correct code information “52” can only be obtained when the CDs sample is heated. The color of the pattern “ZZU” shows the change from orange to bright red as the temperature increases from 298 K to 373 K. Intriguingly, the characteristics of uCDs@SiO2/NaOH samples have been used to design an information encryption/decryption model based on the principle of Morse codes.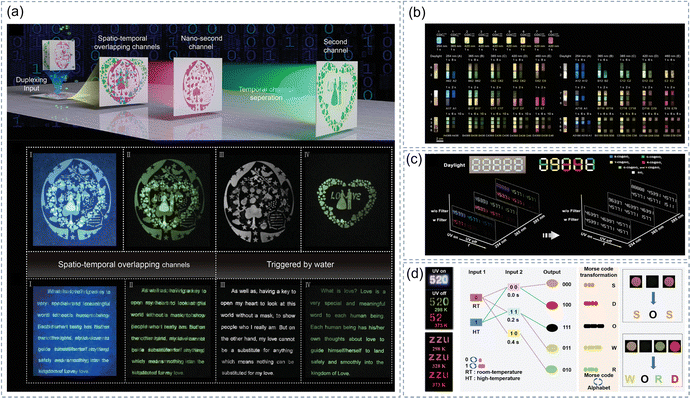 | ||
| Fig. 8 (a) Schematic illustration of the time division duplexing technology based on the CNDs and CNDs@silica. Reproduced with permission.81 Copyright 2021, Wiley-VCH. (b) Multiple barcodes made with eight composite materials with different RTP colors. Reproduced with permission.71 Copyright 2021, Wiley-VCH. (c) A 3D multichannel information encryption system. Reproduced with permission.90 Copyright 2022, Wiley-VCH. (d) Demonstration of anti-counterfeiting, information protection, and encrypted information processing. Reproduced with permission.58 Copyright 2023, Wiley-VCH. | ||
4.2. Anti-counterfeiting
Progressively abundant commodities make anti-counterfeiting more and more crucial in food, medicine, clothing, electronic products, and other fields. Nonetheless, the existing anti-counterfeiting technologies for fluorescent information identification cannot meet the current production needs, so it is urgent to develop anti-counterfeiting technologies with excellent anti-counterfeiting performance and higher encryption levels. Luminescence anti-counterfeiting results from the color change of luminescent materials under various external stimuli143,144 (such as laser, solvent, heating, and pressure, etc.). In this regard, some progress has been made in the application of afterglow composite materials based on CDs. As shown in Fig. 9a, the composite materials with TADF properties and the traditional photoluminescent materials are used to create a security pattern of roses.37 The flower part shows a blue afterglow and the stems/leaves disappear when the UV light is turned off. Similarly, flower patterns made with CDs@MnAPO-CJ50 powder display three distinct colors when exposed to sunlight and UV light on/off (Fig. 9b).41 Our group have found an interesting material that can readily convert RTP and TADF in response to temperature changes.78 When 360 nm UV lamp excitation stops, the anti-counterfeiting patterns prepared by the CDs@SiO2 material exhibit green, cyan, and blue afterglow at room temperature, 120 °C, and 200 °C respectively (Fig. 9c).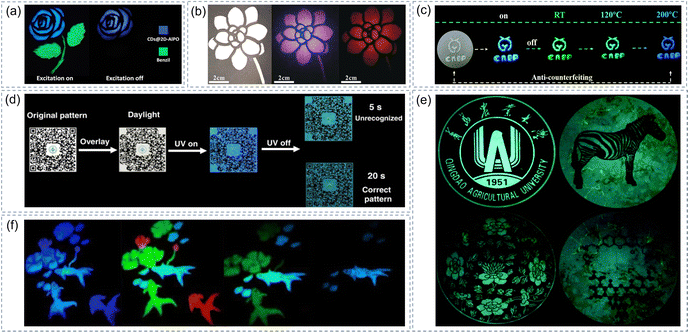 | ||
| Fig. 9 (a) The security pattern of a rose. Reproduced with permission.37 Copyright 2017, American Association for the Advancement of Science (b) Time-resolved security features for triple-mode color switching encryption. Reproduced with permission.41 Copyright 2019, Wiley-VCH. (c) Photographs of CDs@SiO2 with temperature-responsive afterglow in anti-counterfeiting applications. Reproduced with permission.78 Copyright 2020, Royal Society of Chemistry. (d) The anti-counterfeit QR code fabricated using composite materials. Reproduced with permission.40 Copyright 2020 Springer Nature. (e) Digital photos of afterglow projections on the G-CDs/B2O3 composite after removing the irradiation. Reproduced with permission.92 Copyright 2020, Royal Society of Chemistry. (f) A four-level anti-counterfeiting coding model. Reproduced with permission.94 Copyright 2021, Elsevier B.V. | ||
CDs@SiO2 composite materials with a multi-confined effect have an ultralong RTP lifetime (5.72 s) and extraordinary stability (stability exists in oxidants, acids, and bases), which can be applied in the field of graph anti-counterfeiting in some special environments.40 As illustrated in Fig. 9d, the anti-counterfeit QR code was fabricated using composite materials with different afterglow lifetimes. The pattern could not be identified and provided useful messages under the illumination of sunlight or UV light because there were three parts covered by CDs@SiO2 powder. Interestingly, the incomplete pattern was still unrecognizable after the UV light was shut off, but the three corners would slowly appear after 20 s, and the correct blue-green pattern could be recognized by the scanner. As shown in Fig. 9e, the pattern of the badge, zebra, flowers, and structural formula are projected on a piece of glass and applied in graphic anti-counterfeiting according to the afterglow properties of G-CDs/B2O3 materials.92Fig. 9f shows a four-level anti-counterfeiting coding model based on the tunable phosphorescence properties of the three materials.94 Using the materials to draw anti-counterfeiting labels, the bright blue fluorescence can be observed at the excitation of 365 nm and then the blue, green and orange RTP can be seen when the UV lamp is turned off. At the same time, the RTP lifetimes of the anti-counterfeiting pattern composed of composite materials changes with different times, which has the potential application for advanced anti-counterfeiting technology.
4.3. Sensing
The afterglow of some composites responds to changes in the surrounding environment (gas concentration, temperature, humidity) and thus can be used in sensors. We all understand that oxygen is one of the most important components in nature and has great significance to the growth of plants and animals. Consequently, oxygen concentrations need to be monitored over a wide range of concentrations in various environments. Some existing oxygen sensors have been widely used in life, scientific research, industrial production and medical fields. Optical sensors are reliable tools for measuring oxygen content due to their high sensitivity, simplicity, and wide adjustable range. To demonstrate the potential application of CDs-MgAl-LDHs as an oxygen sensor, the variation trend in phosphorescent emission intensity with increasing oxygen concentration was monitored in real-time.106Fig. 10a shows the graph fitted by the Stern–Volmer quenching equation. By using the basic principle of optical oxygen quenching, the full-range optical sensing detection of oxygen concentration from 0% to 100% was achieved.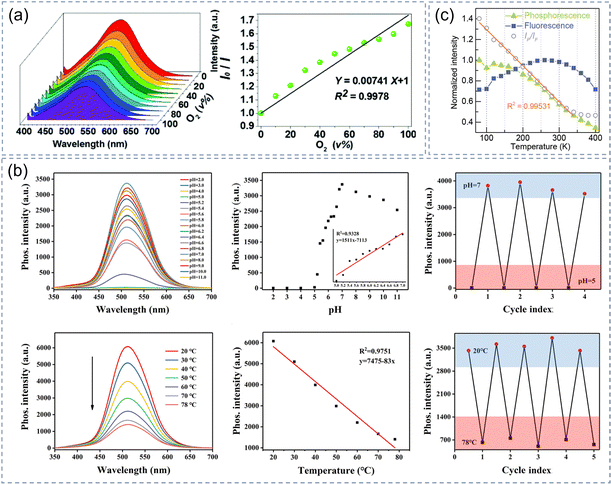 | ||
| Fig. 10 (a) Phosphorescence and I0/I intensity as a function of oxygen content. Reproduced with permission.106 Copyright 2017, Royal Society of Chemistry. (b) The RTP measurement probe based on temperature and pH value. Reproduced with permission.111 Copyright 2020, American Chemical Society. (c) The intensity of phosphorescence, fluorescence, and their ratio changes with temperature. Reproduced with permission.79 Copyright 2020, Wiley-VCH. | ||
Temperature and pH value are two basic physical parameters of a chemical process and cell activity in a biological microenvironment. The afterglow probe has the advantages of a long lifetime, high signal-to-noise ratio and eliminating self-fluorescence interference, thus providing a reliable path for biological analysis. CDs@MP composites can be used as an intelligent probe due to its long RTP lifetime, good water dispersibility, and the phosphorescence intensity affected by the pH value and temperature of the solution.111 As shown in Fig. 10b, there is no phosphorescence when CDs@MP composites are dispersed in a buffer solution with pH values of 2.0 to 5.0. However, when the pH value of the buffer solution was increased from 5.0 to 7.5, the phosphorescence intensity was significantly enhanced because of its aggregation network construction, indicating that CDs@MP had a sensitive pH response. Similarly, it can also be used as a temperature sensor by monitoring the change of phosphorescence intensity with temperature in the range of 20–78 °C. Our group likewise utilized a similar principle to demonstrate that CDs-SiO2 could be applied as temperature sensors.79 The phosphorescent/fluorescence intensity ratio had a good correlation with temperature within the 80–340 K and the correlation coefficient was as high as 0.995 (Fig. 10c).
4.4. Biological imaging
Some CDs@IMs have the potential to be used for in vivo/in vitro imaging of plants/animals due to their low toxicity and good biocompatibility. As shown in Fig. 11a, our group processed mung bean sprouts and onion bulb epidermal tissue with CDs@SiO2 aqueous solution and then observed the intracellular fluorescence and phosphorescence signal with an inverted fluorescence microscope.72 Some RTP images were continuously garnered at different time intervals after closing the excitation light source and the time-resolved RTP images were obtained eventually. The images indicate that CDs@SiO2 are mainly located in the external cell walls and in vascular bundles. Furthermore, CDs@SiO2 solution can also be used for phosphorescent imaging of animal cells. In our other work, HeLa cancer cells were initially cultured with CDS-SiO2 solution and then the bright green phosphorescence images of the cells were observed in the cytoplasmic region under a 405 nm confocal laser.79 The application of CDs@SiO2 aqueous solution in biological samples opens a new chapter in afterglow bioimaging for CDs@IMs and is of great significance to the development of this field.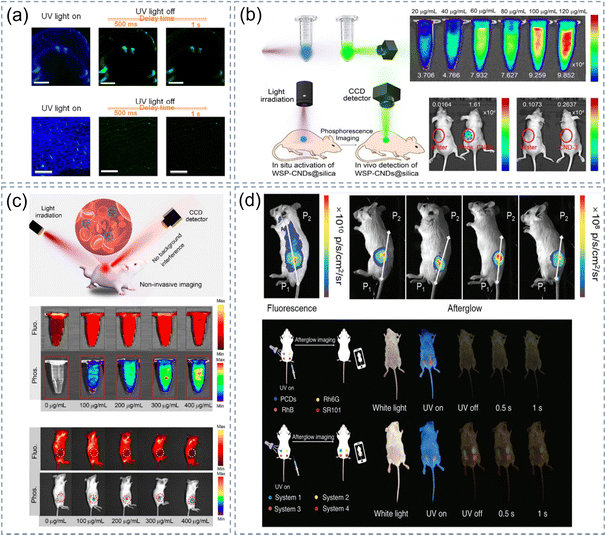 | ||
| Fig. 11 (a) Images of CDs@SiO2 aqueous solution in the stem segment of mung bean sprout. Reproduced with permission.72 Copyright 2019, American Chemical Society. (b) Schematic illustration of phosphorescence detection and imaging of the WSP-CNDs@silica. Reproduced with permission.53 Copyright 2020, Elsevier Ltd. (c) In vivo imaging of a red afterglow imaging agent in mice. Reproduced with permission.54 Copyright 2021, American Chemical Society. (d) Photographs of mice injected subcutaneously with commercial fluorescent dyes and system 1–4 under UV lamps turned on and off, respectively. Reproduced with permission.75 Copyright 2022, Wiley-VCH. | ||
Some other reports have further investigated the application of CDs@IMs for in vivo imaging. Shan et al. detected cytotoxicity and studied the bioimaging of WSP-CNDs@SiO2 solution in cells. The green RTP images were observed distinctly without background fluorescence after the 405 nm excitation source was turned off.53 They applied WSP-CNDs@SiO2 solution in vivo afterglow imaging after collecting mouse organs to further evaluate its safety in clinical studies (Fig. 11b), greatly increasing the possibility that CDs@IMs could be used in the biomedical field. The water-soluble red afterglow material has deeper tissue penetration capabilities. Therefore, they performed in vivo fluorescence and phosphorescence imaging of mice using CNDs-RhB@SiO2 nanocomposites with red afterglow and evaluated the afterglow imaging utilizing the in vivo imaging system (IVIS).54 As shown in Fig. 11c, the phosphorescence phenomenon was most obvious in the marked place in the mouse body when the solution concentration was 400 μg mL−1. Our group used systems 1–4 to perform fluorescence/afterglow imaging in the living mouse by subcutaneous injection and compared them with the commercial fluorescent dyes. It was found thatsystems 1–4 exhibited low background noise and a high signal-to-noise ratio, which made it easy to obtain afterglow images using a mobile phone camera (Fig. 11d).75
4.5. Fingerprint identification
Fingerprints have become one of the most important pieces of information in modern identity authentication and they are widely used in crime forensics, bank encryption and security systems in addition to the familiar mobile phone fingerprint unlocking system. Nevertheless, the details of fingerprints are normally hard to see using our naked eyes in daily life, and thus require some visualization technology for detection and display. The afterglow imaging of CDs@IMs has a low background fluorescence and a high signal-to-noise ratio, making it more suitable for fingerprint detection than fluorescence. The composite material can be simply applied to fingerprint identification by means of mechanical adhesion. As shown in Fig. 12a–c, whether the finger was pressed on the glass or the back of the mobile phone shell, a complete fingerprint afterglow image could be seen after the UV lamp was shut off, and clean ridges and edges could be observed by the naked eye.73,87,112 Recently, our group prepared CDs@YOHF composite materials with multicolor long-wavelength RTP emission which can not only be used for information encryption but also fingerprint recognition. As displayed in Fig. 12d, the fingerprint photograph can be presented through the afterglow of the CDs@YOHF. Some unique texture details (core, bifurcation, termination, pore and island) can be well observed after zooming in on certain areas of the fingerprint photograph.120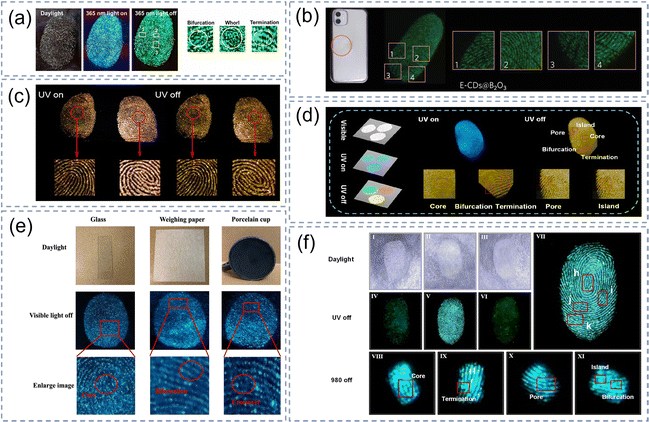 | ||
| Fig. 12 (a) Fluorescence and phosphorescence photographs of latent fingerprints on tin foil and on a copper plate. Reproduced with permission.112 Copyright 2021, Elsevier B.V. (b) The application of E-CDs@B2O3 powder in fingerprint identification. Reproduced with permission.73 Copyright 2021, Wiley-VCH. (c) The images of GCDs@SiO2-OCDs-stained fingerprints on the slide with UV light on and off. Reproduced with permission.87 Copyright 2022, American Chemical Society. (d) Images of fingerprint recognition application of CDs@YOHF. Reproduced with permission.120 Copyright 2022, American Chemical Society. (e) The pictures of CDs-I/B2O3 composites applied to latent fingerprints. Reproduced with permission.93 Copyright 2021, American Chemical Society. (f) The fingerprint pattern under daylight on and UV/980 nm off. Reproduced with permission.52 Copyright 2021, Wiley-VCH. | ||
CDs@IMs have exhibited great potential for practical applications in the visualization and detection of rapid fingerprint identification. The reported fingerprint afterglow imaging can be obtained not only by UV light excitation but also by visible light and near-infrared light.52,93 As shown in Fig. 12e, the fingerprints on glass, weighing paper and ceramic cups are unrecognizable under daylight but can be successfully detected after visible light excitation is stopped, and even some details can be observed in some magnified areas. Subsequently, our group prepared CDs@IMs that can be excited by near-infrared light and successfully applied it to fingerprint recognition, and the results were consistent with the UV-excited afterglow image of the corresponding region (Fig. 12f).52 The details of the fingerprint image excited by near-infrared light such as the core, termination, pore, island, and bifurcation were clear and had high contrast.
4.6 Other applications
In addition to the common applications mentioned above, CDs@IMs in some new applications have also been realized such as light-emitting diodes (LEDs), antibacterial and laser writing. The related research of Yu's group promoted the practical application of afterglow materials in LEDs. The LEDs showed CIE coordinates of (0.32, 0.33) and CRI of 92.8 by mixing afterglow materials and gels evenly and then coating them on diode chips.41 Subsequently, they also realized the first application of the afterglow material CDs@AlPO-5 in the supplementary lighting of alternating-current light-emitting diode (AC LED) devices.103 Furthermore, the CD@BA powder prepared by Li et al. was successfully applied to single-component white light-emitting diodes (WLEDs), and the WLEDs had a broad spectral region from 400 to 750 nm.95 It had the advantages of no phase separation and good stability compared with multi-component white phosphors. Intriguingly, the white light excited-afterglow CNDs were assembled with wood to make the CND-based functional wood lampshades.44 This will provide a buffer time for the user's eyes when the LEDs switch suddenly between light and dark indoors.Although the afterglow emissions of CDs@IMs have been extended from blue light to red light, UV afterglow is very uncommon. Shan et al. prepared the UV phosphorescent CNDs and showed their application in novel antibacterial activities by emitting high-energy photons for a long time. The antibacterial efficiency was even as high as 99.9% for some bacteria.57 In addition, the advantage of the long persistent afterglow of CDs@IMs can be applied to advanced optical anti-counterfeiting photo writing.52,92
5. Summary and outlook
The excellent luminescence properties of CDs@IMs are undoubtedly noticeable and have also been widely investigated in recent years. In this paper, we focus on the construction, mechanism and application of the CDs embedded in IMs. This is the first review on the afterglow of CDs from the perspective of IMs. The interaction and luminescence mechanism of CDs with SiO2, BA, zeolite and other IMs are summarized, and the applications of CDs@IMs in anti-counterfeiting, information encryption, biological imaging, and fingerprint recognition are introduced. The special structure of the IMs not only provides a rigid environment for CDs but also combines with CDs through covalent bonds/hydrogen bonds/ionic bonds, promoting CDs@IMs to obtain afterglow in both solid-state and aqueous solutions. Although CDs@IMs have made great progress in a short period, there are still some problems and challenges in the development process:(1) The afterglow emission of CDs@IMs is mostly green, but with less long-wavelength emission, while UV and infrared emissions still need to be further expanded.
(2) Although some existing studies have obtained the long-wavelength emission of afterglow through high-temperature calcination, heteroatomic doping, and ET, the relevant mechanism is not thoroughly studied. Further research is required on the structure–activity relationship between CDs and IMs so that we can carry out functional design according to needs.
(3) Most of the reported afterglow phenomena in aqueous solutions are found in CDs@SiO2. For other types of CDs@IMs, they often do not have good water solubility. Especially, many CD@IMs have a large size and thus are not suitable for biological applications. Therefore, it is necessary to improve the preparation process and explore new matrices to develop uniformly nanoscale water-soluble CDs@IMs.
(4) Various precursors and construction methods will lead to differences in luminescence mechanisms and properties. Different crystal phases and morphologies of the IMs may also have a modulating effect on CDs afterglow. Consequently, the selection of precursors and reasonable design of the structure of new CD-based afterglow functional materials are particularly important, and analyzing the internal relationship between them is of great significance for designable synthesis and further development.
(5) Advanced high-resolution structural characterizations can be employed to help us gain a deeper insight into the structure–property relationships of CDs@IMs. In addition, in situ characterization techniques such as in situ X-ray diffraction, in situ Fourier transform infrared spectroscopy, and in situ transmission electron microscopy are needed to monitor the process of matrix growth and in situ generation/embedding of CDs, as well as the changes of CDs during this process, etc. This may increase the understanding of the interactions between CDs and matrices, and provide guidelines for theoretical designs and experiments.
(6) For the synthesis of CDs and CDs@IMs, we may be able to assist or even fully automate the synthesis of carbon nanomaterials with the help of artificial intelligence and machine learning in the future. Compared with the traditional experimental operation, it can predict material properties and guide material synthesis through simulation analysis. Especially in the selection of matrices and CDs, it is necessary to design a set of effective screening procedures based on the luminescent mechanism of CDs and the theory of inorganic crystal structure and with the help of high-throughput calculations. This can help us match different CDs and matrices in a more targeted manner to obtain CD-based afterglow materials with higher quantum efficiency and longer lifetime.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52172142, 12174119), The Guangdong Basic and Applied Basic Research Foundation (2022A1515011958, 2020A1515011210), and the Science and Technology Planning Project of Guangzhou City (202102080288, 202007020005).References
- J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S.-T. Lee, J. Zhong and Z. Kang, Science, 2015, 347, 970–974 CrossRef CAS PubMed.
- F. Yuan, Y.-K. Wang, G. Sharma, Y. Dong, X. Zheng, P. Li, A. Johnston, G. Bappi, J. Z. Fan, H. Kung, B. Chen, M. I. Saidaminov, K. Singh, O. Voznyy, O. M. Bakr, Z.-H. Lu and E. H. Sargent, Nat. Photonics, 2019, 14, 171–176 CrossRef.
- N. Gong, X. Ma, X. Ye, Q. Zhou, X. Chen, X. Tan, S. Yao, S. Huo, T. Zhang, S. Chen, X. Teng, X. Hu, J. Yu, Y. Gan, H. Jiang, J. Li and X. J. Liang, Nat. Nanotechnol., 2019, 14, 379–387 CrossRef CAS.
- S. Li, W. Su, H. Wu, T. Yuan, C. Yuan, J. Liu, G. Deng, X. Gao, Z. Chen, Y. Bao, F. Yuan, S. Zhou, H. Tan, Y. Li, X. Li, L. Fan, J. Zhu, A. T. Chen, F. Liu, Y. Zhou, M. Li, X. Zhai and J. Zhou, Nat. Biomed. Eng., 2020, 4, 704–716 CrossRef CAS PubMed.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff and X. Wang, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- L. Ethordevic, F. Arcudi, M. Cacioppo and M. Prato, Nat. Nanotechnol., 2022, 17, 112–130 CrossRef PubMed.
- B. Wang and S. Lu, Matter, 2022, 5, 110–149 CrossRef CAS.
- M. Zhang, X. Zhai, M. Sun, T. Ma, Y. Huang, B. Huang, Y. Du and C. Yan, Chem. Soc. Rev., 2020, 49, 9220–9248 RSC.
- L. Dordević, F. Arcudi and M. Prato, Nat. Protoc., 2019, 14, 2931–2953 CrossRef PubMed.
- K. Nekoueian, M. Amiri, M. Sillanpaa, F. Marken, R. Boukherroub and S. Szunerits, Chem. Soc. Rev., 2019, 48, 4281–4316 RSC.
- Y. Wang, X. Liu, X. Han, R. Godin, J. Chen, W. Zhou, C. Jiang, J. F. Thompson, K. B. Mustafa, S. A. Shevlin, J. R. Durrant, Z. Guo and J. Tang, Nat. Commun., 2020, 11, 2531 CrossRef CAS PubMed.
- Q. Wu, J. Cao, X. Wang, Y. Liu, Y. Zhao, H. Wang, Y. Liu, H. Huang, F. Liao, M. Shao and Z. Kang, Nat. Commun., 2021, 12, 483 CrossRef CAS.
- C. Xia, Y. Qiu, Y. Xia, P. Zhu, G. King, X. Zhang, Z. Wu, J. Y. Kim, D. A. Cullen, D. Zheng, P. Li, M. Shakouri, E. Heredia, P. Cui, H. N. Alshareef, Y. Hu and H. Wang, Nat. Chem., 2021, 13, 887–894 CrossRef CAS PubMed.
- H. Yang, Y. Liu, Z. Guo, B. Lei, J. Zhuang, X. Zhang, Z. Liu and C. Hu, Nat. Commun., 2019, 10, 1789 CrossRef.
- Q. Zhang, R. Wang, B. Feng, X. Zhong and K. K. Ostrikov, Nat. Commun., 2021, 12, 6856 CrossRef CAS PubMed.
- L. Qin, J. Gan, D. Niu, Y. Cao, X. Duan, X. Qin, H. Zhang, Z. Jiang, Y. Jiang, S. Dai, Y. Li and J. Shi, Nat. Commun., 2022, 13, 91 CrossRef CAS PubMed.
- H. Choi, S.-J. Ko, Y. Choi, P. Joo, T. Kim, B. R. Lee, J.-W. Jung, H. J. Choi, M. Cha, J.-R. Jeong, I.-W. Hwang, M. H. Song, B.-S. Kim and J. Y. Kim, Nat. Photonics, 2013, 7, 732–738 CrossRef CAS.
- B. Zhao, Z. Wang and Z. A. Tan, Nat. Photonics, 2020, 14, 130–131 CrossRef CAS.
- C. Hu, M. Li, J. Qiu and Y. P. Sun, Chem. Soc. Rev., 2019, 48, 2315–2337 RSC.
- K. Jinnai, R. Kabe, Z. Lin and C. Adachi, Nat. Mater., 2022, 21, 338–344 CrossRef CAS.
- B. Zhou, B. Shi, D. Jin and X. Liu, Nat. Nanotechnol., 2015, 10, 924–936 CrossRef CAS.
- P. Pei, Y. Chen, C. Sun, Y. Fan, Y. Yang, X. Liu, L. Lu, M. Zhao, H. Zhang, D. Zhao, X. Liu and F. Zhang, Nat. Nanotechnol., 2021, 16, 1011–1018 CrossRef CAS PubMed.
- X. Wang, J. Cao, Z. Lu, A. Cohen, H. Kitadai, T. Li, Q. Tan, M. Wilson, C. H. Lui, D. Smirnov, S. Sharifzadeh and X. Ling, Nat. Mater., 2021, 20, 964–970 CrossRef CAS PubMed.
- R. Kabe and C. Adachi, Nature, 2017, 550, 384–387 CrossRef CAS PubMed.
- Q. Miao, C. Xie, X. Zhen, Y. Lyu, H. Duan, X. Liu, J. V. Jokerst and K. Pu, Nat. Biotechnol., 2017, 35, 1102–1110 CrossRef CAS PubMed.
- M. S. Kwon, Y. Yu, C. Coburn, A. W. Phillips, K. Chung, A. Shanker, J. Jung, G. Kim, K. Pipe, S. R. Forrest, J. H. Youk, J. Gierschner and J. Kim, Nat. Commun., 2015, 6, 8947 CrossRef PubMed.
- D. Li, F. Lu, J. Wang, W. Hu, X.-M. Cao, X. Ma and H. Tian, J. Am. Chem. Soc., 2018, 140, 1916–1923 CrossRef CAS PubMed.
- Z. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu and W. Huang, Nat. Mater., 2015, 14, 685–690 CrossRef CAS PubMed.
- J. Lee, H. F. Chen, T. Batagoda, C. Coburn, P. I. Djurovich, M. E. Thompson and S. R. Forrest, Nat. Mater., 2016, 15, 92–98 CrossRef CAS PubMed.
- K. H. Kim, S. Lee, C. K. Moon, S. Y. Kim, Y. S. Park, J. H. Lee, J. Woo Lee, J. Huh, Y. You and J. J. Kim, Nat. Commun., 2014, 5, 4769 CrossRef CAS PubMed.
- O. Bolton, K. Lee, H. J. Kim, K. Y. Lin and J. Kim, Nat. Chem., 2011, 3, 205–210 CrossRef CAS.
- J. Yang, X. Zhen, B. Wang, X. Gao, Z. Ren, J. Wang, Y. Xie, J. Li, Q. Peng, K. Pu and Z. Li, Nat. Commun., 2018, 9, 840 CrossRef PubMed.
- Y. Zhuo, A. Mansouri Tehrani, A. O. Oliynyk, A. C. Duke and J. Brgoch, Nat. Commun., 2018, 9, 4377 CrossRef.
- X. Ou, X. Qin, B. Huang, J. Zan, Q. Wu, Z. Hong, L. Xie, H. Bian, Z. Yi, X. Chen, Y. Wu, X. Song, J. Li, Q. Chen, H. Yang and X. Liu, Nature, 2021, 590, 410–415 CrossRef CAS PubMed.
- L. Liang, J. Chen, K. Shao, X. Qin, Z. Pan and X. Liu, Nat. Mater., 2023, 22, 289–304 CrossRef CAS.
- J. Liu, N. Wang, Y. Yu, Y. Yan, H. Zhang, J. Li and J. Yu, Sci. Adv., 2017, 3, e1603171 CrossRef PubMed.
- Q. Li, M. Zhou, M. Yang, Q. Yang, Z. Zhang and J. Shi, Nat. Commun., 2018, 9, 734 CrossRef PubMed.
- D. C. Green, M. A. Holden, M. A. Levenstein, S. Zhang, B. R. G. Johnson, J. Gala de Pablo, A. Ward, S. W. Botchway and F. C. Meldrum, Nat. Commun., 2019, 10, 206 CrossRef PubMed.
- Y. Sun, S. Liu, L. Sun, S. Wu, G. Hu, X. Pang, A. T. Smith, C. Hu, S. Zeng, W. Wang, Y. Liu and M. Zheng, Nat. Commun., 2020, 11, 5591 CrossRef CAS PubMed.
- B. Wang, Y. Yu, H. Zhang, Y. Xuan, G. Chen, W. Ma, J. Li and J. Yu, Angew. Chem., Int. Ed., 2019, 58, 18443–18448 CrossRef CAS PubMed.
- H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259–3302 RSC.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS.
- S. Song, K. Liu, X. Mao, Q. Cao, N. Li, W. Zhao, Y. Wang, Y. Liang, J. Zang, X. Li, Q. Lou, L. Dong and C. Shan, Adv. Mater., 2023, 35, 2212286 CrossRef CAS.
- J. Li, Y. Wu and X. Gong, Chem. Sci., 2023, 14, 3705–3729 RSC.
- K. Jiang, Y. Wang, X. Gao, C. Cai and H. Lin, Angew. Chem., Int. Ed., 2018, 57, 6216–6220 CrossRef CAS.
- Q. Feng, Z. Xie and M. Zheng, Chem. Eng. J., 2021, 420, 127647 CrossRef CAS.
- W. Shi, J. Yao, L. Bai and C. Lu, Adv. Funct. Mater., 2018, 28, 1804961 CrossRef.
- S. Tao, S. Lu, Y. Geng, S. Zhu, S. A. T. Redfern, Y. Song, T. Feng, W. Xu and B. Yang, Angew. Chem., Int. Ed., 2018, 57, 2393–2398 CrossRef CAS.
- K. Jiang, Y. Wang, C. Cai and H. Lin, Adv. Mater., 2018, 30, 1800783 CrossRef PubMed.
- W. Li, W. Zhou, Z. Zhou, H. Zhang, X. Zhang, J. Zhuang, Y. Liu, B. Lei and C. Hu, Angew. Chem., Int. Ed., 2019, 58, 7278–7283 CrossRef CAS PubMed.
- Y. Zheng, H. Wei, P. Liang, X. Xu, X. Zhang, H. Li, C. Zhang, C. Hu, X. Zhang, B. Lei, W. Y. Wong, Y. Liu and J. Zhuang, Angew. Chem., Int. Ed., 2021, 60, 22253–22259 CrossRef CAS.
- Y. Liang, S. Gou, K. Liu, W. Wu, C. Guo, S. Lu, J. Zang, X. Wu, Q. Lou, L. Dong, Y. Gao and C. Shan, Nano Today, 2020, 34, 100900 CrossRef CAS.
- Y. Liang, Q. Cao, K. Liu, X. Peng, L. Sui, S. Wang, S. Song, X. Wu, W. Zhao, Y. Deng, Q. Lou, L. Dong and C. Shan, ACS Nano, 2021, 15, 16242–16254 CrossRef CAS PubMed.
- Q. Li, M. Zhou, Q. Yang, Q. Wu, J. Shi, A. Gong and M. Yang, Chem. Mater., 2016, 28, 8221–8227 CrossRef CAS.
- X. Yu, K. Liu, H. Zhang, B. Wang, W. Ma, J. Li and J. Yu, Small, 2021, 17, 2103374 CrossRef CAS PubMed.
- S. Song, K. Liu, Q. Cao, X. Mao, W. Zhao, Y. Wang, Y. Liang, J. Zang, Q. Lou, L. Dong and C. Shan, Light: Sci. Appl., 2022, 11, 146 CrossRef CAS PubMed.
- Q. Lou, N. Chen, J. Zhu, K. Liu, C. Li, Y. Zhu, W. Xu, X. Chen, Z. Song, C. Liang, C. X. Shan and J. Hu, Adv. Mater., 2023, 35, 2211858 CrossRef CAS PubMed.
- H. Wang, L. Zhou, H. Yu, X. Tang, C. Xing, G. Nie, H. Akafzade, S. Wang and W. Chen, Adv. Opt. Mater., 2022, 10, 2200678 CrossRef CAS.
- Y. Zheng, Q. Zhou, Y. Yang, X. Chen, C. Wang, X. Zheng, L. Gao and C. Yang, Small, 2022, 18, 2201223 CrossRef CAS PubMed.
- Y. Zheng, Z. Wang, J. Liu, Y. Zhang, L. Gao, C. Wang, X. Zheng, Q. Zhou, Y. Yang, Y. Li, H. Tang, L. Qu, Y. Zhao and C. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 15706–15715 CrossRef CAS PubMed.
- K. Jiang, L. Zhang, J. Lu, C. Xu, C. Cai and H. Lin, Angew. Chem., Int. Ed., 2016, 55, 7231–7235 CrossRef CAS PubMed.
- Z. Tian, D. Li, E. V. Ushakova, V. G. Maslov, D. Zhou, P. Jing, D. Shen, S. Qu and A. L. Rogach, Adv. Sci., 2018, 5, 1800795 CrossRef.
- Q. Cao, K. Liu, Y. Liang, S. Song, Y. Deng, X. Mao, Y. Wang, W. Zhao, Q. Lou and C. Shan, Nano Lett., 2022, 22, 4097–4105 CrossRef CAS.
- H. Shi, Y. Wu, J. Xu, H. Shi and Z. An, Small, 2023, 19, 2207104 CrossRef CAS.
- K. Wang, L. Qu and C. Yang, Small, 2023, 19, 2206429 CrossRef CAS PubMed.
- Y. Deng, D. Zhao, X. Chen, F. Wang, H. Song and D. Shen, Chem. Commun., 2013, 49, 5751–5753 RSC.
- X. Dong, L. Wei, Y. Su, Z. Li, H. Geng, C. Yang and Y. Zhang, J. Mater. Chem. C, 2015, 3, 2798–2801 RSC.
- K. Jiang, Y. Wang, C. Cai and H. Lin, Chem. Mater., 2017, 29, 4866–4873 CrossRef CAS.
- Q. Li, Z. Zhao, S. Meng, Y. Li, Y. Zhao, B. Zhang, Z. Tang, J. Tan and S. Qu, SmartMat, 2022, 3, 260–268 CrossRef CAS.
- Y. Ding, X. Wang, M. Tang and H. Qiu, Adv. Sci., 2021, 9, 2103833 CrossRef PubMed.
- W. Li, S. Wu, X. Xu, J. Zhuang, H. Zhang, X. Zhang, C. Hu, B. Lei, C. F. Kaminski and Y. Liu, Chem. Mater., 2019, 31, 9887–9894 CrossRef CAS.
- Z. Zhou, Z. Song, J. Liu, B. Lei, J. Zhuang, X. Zhang, Y. Liu and C. Hu, Adv. Opt. Mater., 2022, 10, 2100704 CrossRef CAS.
- R. Liang, L. Huo, A. Yu, J. Wang, C. Jia and J. Li, Chin. Chem. Lett., 2022, 33, 243–246 CrossRef CAS.
- L. Mo, H. Liu, Z. Liu, X. Xu, B. Lei, J. Zhuang, Y. Liu and C. Hu, Adv. Opt. Mater., 2022, 10, 2102666 CrossRef CAS.
- J. Joseph and A. A. Anappara, Phys. Chem. Chem. Phys., 2017, 19, 15137–15144 RSC.
- G. Tang, K. Zhang, T. Feng, S. Tao, M. Han, R. Li, C. Wang, Y. Wang and B. Yang, J. Mater. Chem. C, 2019, 7, 8680–8687 RSC.
- Y. Sun, J. Liu, X. Pang, X. Zhang, J. Zhuang, H. Zhang, C. Hu, M. Zheng, B. Lei and Y. Liu, J. Mater. Chem. C, 2020, 8, 5744–5751 RSC.
- J. He, Y. Chen, Y. He, X. Xu, B. Lei, H. Zhang, J. Zhuang, C. Hu and Y. Liu, Small, 2020, 16, 2005228 CrossRef CAS.
- J. Liu, X. Kang, H. Zhang, Y. Liu, C. Wang, X. Gao and Y. Li, Dyes Pigm., 2020, 181, 108576 CrossRef CAS.
- Y. Liang, K. Liu, X. Wu, Q. Lou, L. Sui, L. Dong, K. Yuan and C. Shan, Adv. Sci., 2021, 8, 2003433 CrossRef CAS PubMed.
- L. Mo, X. Xu, Z. Liu, H. Liu, B. Lei, J. Zhuang, Z. Guo, Y. Liu and C. Hu, Chem. Eng. J., 2021, 426, 130728 CrossRef CAS.
- G. Tang, C. Wang, K. Zhang, Y. Wang and B. Yang, Langmuir, 2021, 37, 13187–13193 CrossRef CAS PubMed.
- C. Hao, Y. Bai, Z. Chen, F. Geng, J. Qin, T. Zhou and F. Feng, Dyes Pigm., 2022, 197, 109890 CrossRef CAS.
- C. Zheng, S. Tao, Y. Liu, C. Kang and B. Yang, Chin. Chem. Lett., 2022, 33, 4213–4218 CrossRef CAS.
- Y. Liu, X. Kang, Y. Xu, Y. Li, S. Wang, C. Wang, W. Hu, R. Wang and J. Liu, ACS Appl. Mater. Interfaces, 2022, 14, 22363–22371 CrossRef CAS PubMed.
- X. Teng, X. Sun, W. Pan, Z. Song and J. Wang, ACS Appl. Nano Mater., 2022, 5, 5168–5175 CrossRef CAS.
- J. Wang, X. Sun, W. Pan and J. Wang, Microchem. J., 2022, 178, 107408 CrossRef CAS.
- T. Chao, J. Wang, X. Dong, J. Ren, H. Zhang, R. Song and Z. Xie, J. Phys. Chem. Lett., 2022, 13, 9558–9563 CrossRef CAS PubMed.
- Y. Zhang, M. Li and S. Lu, Small, 2023, 19, 2206080 CrossRef CAS PubMed.
- L. Chen, S. Zhao, Y. Wang, S. Yu, Y. Yang and X. Liu, Sens. Actuators, B, 2023, 390, 133946 CrossRef CAS.
- Z. Xu, X. Sun, P. Ma, Y. Chen, W. Pan and J. Wang, J. Mater. Chem. C, 2020, 8, 4557–4563 RSC.
- W. He, X. Sun and X. Cao, ACS Sustainable Chem. Eng., 2021, 9, 4477–4486 CrossRef CAS.
- S. Cui, B. Wang, Y. Zan, Z. Shen, S. Liu, W. Fang, X. Yan, Y. Li and L. Chen, Chem. Eng. J., 2021, 431, 133373 CrossRef.
- Q. Li, Y. Li, S. Meng, J. Yang, Y. Qin, J. Tan and S. Qu, J. Mater. Chem. C, 2021, 9, 6796–6801 RSC.
- Y. Jie, D. Wang, R. Chen, J. Zhang, W. Li, J. Huang, P. Dai, Y. Gao, F. Li and J. Fang, Nanoscale, 2023, 15, 3337–3344 RSC.
- Q. Li, D. Cheng, H. Gu, D. Yang, Y. Li, S. Meng, Y. Zhao, Z. Tang, Y. Zhang, J. Tan and S. Qu, Chem. Eng. J., 2023, 462, 142339 CrossRef CAS.
- J. Gao, X. Wu, X. Jiang, M. Li, R. He and W. Shen, Carbon, 2023, 208, 365–373 CrossRef CAS.
- Y. Mu, H. Shi, Y. Wang, H. Ding and J. Li, J. Mater. Chem. C, 2017, 5, 10894–10899 RSC.
- J. Liu, H. Zhang, N. Wang, Y. Yu, Y. Cui, J. Li and J. Yu, ACS Mater. Lett., 2019, 1, 58–63 CrossRef CAS.
- B. Wang, Y. Mu, H. Zhang, H. Shi, G. Chen, Y. Yu, Z. Yang, J. Li and J. Yu, ACS Cent. Sci., 2019, 5, 349–356 CrossRef CAS PubMed.
- H. Zhang, J. Liu, B. Wang, K. Liu, G. Chen, X. Yu, J. Li and J. Yu, Mater. Chem. Front., 2020, 4, 1404–1410 RSC.
- H. Zhang, K. Liu, J. Liu, B. Wang, C. Li, W. Song, J. Li, L. Huang and J. Yu, CCS Chem., 2020, 2, 118–127 CrossRef CAS.
- X. Yu, K. Liu, H. Zhang, B. Wang, G. Yang, J. Li and J. Yu, CCS Chem., 2021, 3, 252–264 CrossRef CAS.
- X. Yu, K. Liu, B. Wang, H. Zhang, Y. Qi and J. Yu, Adv. Mater., 2023, 35, 2208735 CrossRef CAS PubMed.
- L. Q. Bai, N. Xue, X. R. Wang, W. Y. Shi and C. Lu, Nanoscale, 2017, 9, 6658–6664 RSC.
- L. Bai, N. Xue, Y. Zhao, X. Wang, C. Lu and W. Shi, Nano Res., 2018, 11, 2034–2045 CrossRef CAS.
- X. Kong, X. Wang, H. Cheng, Y. Zhao and W. Shi, J. Mater. Chem. C, 2019, 7, 230–236 RSC.
- R. Wang, Y. Zhu, Z. Xia, K. Liang, L. Kong, J. Liu, W. Shi and C. Lu, J. Mater. Chem. C, 2022, 10, 17182–17189 RSC.
- C. Wang, Y. Chen, T. Hu, Y. Chang, G. Ran, M. Wang and Q. Song, Nanoscale, 2019, 11, 11967–11974 RSC.
- C. Wang, Y. Chen, Y. Xu, G. Ran, Y. He and Q. Song, ACS Appl. Mater. Interfaces, 2020, 12, 10791–10800 CrossRef CAS.
- Q. Feng, Z. Xie and M. Zheng, Sens. Actuators, B, 2022, 351, 130976 CrossRef CAS.
- L. A. Diaz-Torres, C. Gomez-Solis, J. Oliva, C. R. García, A. I. Oliva, C. Angeles-Chavez and G. A. Hirata, J. Phys. D: Appl. Phys., 2018, 51, 415104 CrossRef.
- Z. Song, Y. Liu, X. Lin, Z. Zhou, X. Zhang, J. Zhuang, B. Lei and C. Hu, ACS Appl. Mater. Interfaces, 2021, 13, 34705–34713 CrossRef CAS PubMed.
- J. Joseph and A. A. Anappara, ChemistrySelect, 2017, 2, 4058–4062 CrossRef CAS.
- H. Liu, F. Wang, Y. Wang, J. Mei and D. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 18248–18253 CrossRef CAS.
- M. Madhu and W. L. Tseng, Anal. Methods, 2021, 13, 4949–4954 RSC.
- W. Shi, R. Wang, J. Liu, F. Peng, R. Tian and C. Lu, Angew. Chem., Int. Ed., 2023, 62, e202303063 CrossRef CAS PubMed.
- M. Park, H. S. Kim, H. Yoon, J. Kim, S. Lee, S. Yoo and S. Jeon, Adv. Mater., 2020, 32, 2000936 CrossRef CAS PubMed.
- P. Liang, Y. Zheng, X. Zhang, H. Wei, X. Xu, X. Yang, H. Lin, C. Hu, X. Zhang, B. Lei, W. Y. Wong, Y. Liu and J. Zhuang, Nano Lett., 2022, 22, 5127–5136 CrossRef CAS PubMed.
- P. Liang, Y. Zheng, F. Liu, H. Shao, C. Hu, B. Lei, X. Zhang, Y. Liu, J. Zhuang and X. Zhang, JACS Au, 2023, 3, 2291–2298 CrossRef CAS PubMed.
- Z. Ding, C. Shen, J. Han, G. Zheng, Q. Ni, R. Song, K. Liu, J. Zang, L. Dong, Q. Lou and C. Shan, Small, 2023, 19, 2205916 CrossRef CAS PubMed.
- D. Lu, K. Lu, H. T. Wen, Z. Wei, A. Bianco, G. G. Wang and H. Y. Zhang, Small, 2023, 19, 2207046 CrossRef CAS PubMed.
- Y. Deng, P. Li, H. Jiang, X. Ji and H. Li, J. Mater. Chem. C, 2019, 7, 13640–13646 RSC.
- R. Ciriminna, A. Fidalgo, V. Pandarus, F. Beland, L. M. Ilharco and M. Pagliaro, Chem. Rev., 2013, 113, 6592–6620 CrossRef CAS PubMed.
- J. E. Lofgreen and G. A. Ozin, Chem. Soc. Rev., 2014, 43, 911–933 RSC.
- P. P. Ghimire and M. Jaroniec, J. Colloid Interface Sci., 2021, 584, 838–865 CrossRef CAS PubMed.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, V. Georgakilas and E. P. Giannelis, Chem. Mater., 2008, 20, 4539–4541 CrossRef CAS.
- Y. Wang, Y. Li, Y. Yan, J. Xu, B. Guan, Q. Wang, J. Li and J. Yu, Chem. Commun., 2013, 49, 9006–9008 RSC.
- H. G. Baldovi, S. Valencia, M. Alvaro, A. M. Asiri and H. Garcia, Nanoscale, 2015, 7, 1744–1752 RSC.
- T. Hibino, Eur. J. Inorg. Chem., 2018, 722–730 CrossRef CAS.
- Z. Meng, Y. Zhang, Q. Zhang, X. Chen, L. Liu, S. Komarneni and F. Lv, Appl. Surf. Sci., 2017, 396, 799–803 CrossRef CAS.
- P. Saikia, N. g B. Allou, A. Borah and R. L. Goswamee, Mater. Chem. Phys., 2017, 186, 52–60 CrossRef CAS.
- S. N. Golovin, M. N. Yapryntsev, I. G. Ryltsova, A. A. Veligzhanin and O. E. Lebedeva, Chem. Zvesti, 2019, 74, 367–370 CrossRef.
- M. V. Bukhtiyarova, J. Solid State Chem., 2019, 269, 494–506 CrossRef CAS.
- J. C. Munyemana, J. Chen, Y. Han, S. Zhang and H. Qiu, Mikrochim. Acta, 2021, 188, 80 CrossRef CAS PubMed.
- V. Shirin, R. Sankar, A. P. Johnson, H. V. Gangadharappa and K. Pramod, J. Controlled Release, 2021, 330, 398–426 CrossRef PubMed.
- Y. Song, S. K. Beaumont, X. Zhang, K. Wilson and A. F. Lee, Curr. Opin. Green Sustain. Chem., 2020, 22, 29–38 CrossRef.
- L. Huang, Z. Hu, H. Jin, J. Wu, K. Liu, Z. Xu, J. Wan, H. Zhou, J. Duan and B. Hu, Adv. Funct. Mater., 2020, 30, 1908486 CrossRef CAS.
- S. Li, J. Song, Y. Che, S. Jiao, J. He and B. Yang, Energy Environ. Mater., 2021, 6, e12339 CrossRef.
- L. Luo, S. Wang, H. Wang, C. Tian and B. Jiang, Energy Technol., 2021, 9, 2000945 CrossRef CAS.
- C. Peng, X. Chen, M. Chen, S. Lu, Y. Wang, S. Wu, X. Liu and W. Huang, Research, 2021, 2021, 1–27 CrossRef.
- J. Yang, M. Fang and Z. Li, Acc. Mater. Res., 2021, 2, 644–654 CrossRef CAS.
- X. Yu, H. Zhang and J. Yu, Aggregate, 2021, 2, 20–34 CrossRef CAS.
Footnote |
| † X. H. and Y. Z. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |





