Isotype heterojunction: tuning the heptazine/triazine phase of crystalline nitrogen-rich C3N5 towards multifunctional photocatalytic applications†
Sue-Faye
Ng
ab,
Joel Jie
Foo
ab and
Wee-Jun
Ong
 *abcde
*abcde
aSchool of Energy and Chemical Engineering, Xiamen University Malaysia, Selangor Darul Ehsan 43900, Malaysia. E-mail: weejun.ong@xmu.edu.my
bCenter of Excellence for NaNo Energy & Catalysis Technology (CONNECT), Xiamen University Malaysia, Selangor Darul Ehsan 43900, Malaysia
cState Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
dGulei Innovation Institute, Xiamen University, Zhangzhou 363200, China
eShenzhen Research Institute of Xiamen University, Shenzhen 518057, China
First published on 21st September 2023
Abstract
Photocatalytic technology has been well studied as a means to achieve sustainable energy generation through water splitting or chemical synthesis. Recently, a low C/N atomic ratio carbon nitride allotrope, C3N5, has been found to be highly prospective due to its excellent electronic properties and ample N-active sites compared to g-C3N4. Tangentially, crystalline g-C3N4 has also been a prospective candidate due to its improved electron transport and extended π-conjugated system. For the first time, our group successfully employed a one-step molten salt calcination method to prepare novel N-rich crystalline C3N5 and elucidate the effect of calcination temperature on the heptazine/triazine phase. Calcination temperatures of 500 °C (CC3N5-500) and 550 °C (CC3N5-550) lead to crystalline carbon nitride with both heptazine and triazine phases, forming an intimate isotype heterojunction for robust interfacial charge separation. An excellent photocatalytic hydrogen evolution rate (359.97 μmol h−1; apparent quantum efficiency (AQE): 12.86% at 420 nm) was achieved using CC3N5-500, which was 15-fold higher than that of pristine C3N5. Furthermore, CC3N5-500 exhibited improved activity for simultaneous benzyl alcohol oxidation and hydrogen production, as well as H2O2 production (AQE: 9.49% at 420 nm), signifying its multitudinous photoredox capabilities. Moreover, the recyclability tests of the optimal CC3N5-500 on a 3D-printed substrate also showed a 92% performance retention after 4 cycles (16 h). This highlights that crystalline C3N5 significantly augmented the reaction performance for diverse multifunctional solar-driven applications. As such, these results serve as a guide toward the structural tuning of 2D metal-free carbon nanomaterials with tunable crystallinity toward achieving boosted photocatalysis.
New conceptsStructural engineering of 2D nanomaterials is an appealing strategy for boosting electron transport along the in-plane direction attributed to their extended π-conjugated system and delocalized π-electrons. Since 2009, g-C3N4 has been widely studied as a low-cost and chemically stable photocatalyst. However, in recent two years, the N-rich C3N5 allotrope has gained momentum owing to its ample active sites and improved visible-light harvesting ability. For the first time, this study explores a novel approach for the C3N5 allotrope through a facile one-pot molten salt synthesis to engineer 2D porous crystalline C3N5 with a heptazine/triazine isotype heterojunction. Notably, the calcination temperature strongly affects the heptazine/triazine phase. At 500 and 550 °C, both heptazine and triazine peaks were observed in the X-ray diffraction pattern, indicating the presence of a robust isotype heterojunction that facilitates interfacial electron transfer. Furthermore, crystalline C3N5 exhibits markedly improved activity for the half-reaction of the hydrogen evolution reaction (HER), simultaneous photoredox HER and benzyl alcohol oxidation, and H2O2 production, ascertaining its multifunctional photocatalytic performances, outperforming various carbon nitride catalysts. Fascinatingly, the recyclability of crystalline C3N5 has been tested on a 3D-printed substrate over 16 h with 92% performance retention after 4 cycles. Hence, this is a testament to the feasibility of its application in multifarious catalytic as well as potential scale-up reactions. |
Introduction
The conversion of solar energy to renewable hydrogen energy has been an important topic due to ongoing global warming and climate concerns.1,2 Traditional non-renewable fossil fuel reserves are also rapidly depleting, which has led to a global search for green power generation to reduce our reliance on fossil fuels.3–9 Currently, hydrogen production relies majorly on fossil fuels, thereby contributing to around 830 million tonnes of CO2 emissions per annum.10 Therefore, global support for hydrogen energy research is on the rise due to the declining cost of renewable energy.As non-metallic semiconductor photocatalysts, 2D carbon nitrides have attracted attention due to their superior stability, high surface-area-to-volume ratio and tunability of surface engineering.11–14 Discovered in 2009 by Wang et al.,15 graphitic carbon nitride (g-C3N4) has been highly regarded for its high thermal and chemical stability, non-toxicity, and affordability. Therefore, it has been a popular choice for solar-driven water splitting,16–19 CO2 reduction,20–24 and other environmental applications.25–28 Despite its unprecedented characteristics, pristine 2D g-C3N4 suffers from much of the same pitfalls of other photocatalysts, that is a narrow visible light absorption region (with an onset wavelength of ca. 440–450 nm),29 which indicates limited light-harvesting abilities in a broad extended visible light region. Furthermore, other reasons for low activity of bare g-C3N4 include insufficient reactant adsorption and reactive sites as well as a rapid electron–hole recombination rate.30
In light of that, a low C/N atomic ratio carbon nitride allotrope, C3N5, has emerged as a promising successor to overcome the drawbacks of g-C3N4. This 2D nitrogen-rich C3N5 possesses excellent electronic properties and ample N-active sites, and induces more electrons in the π–π conjugation compared to g-C3N4, which is attributed to the electron contribution of the sp2 hybridized N on the triazole moiety.31 As such, the emergence of a carbon nitride allotrope represents a new paradigm of nanoengineering through chemical composition tuning. C3N5 has shown a remarkable performance in photo- and electrocatalytic CO2 reduction,32,33 hydrogen evolution,31,34 and other environmental applications.35,36
The typical bulk condensation of N-rich precursors (i.e. urea,37 thiourea,38,39 cyanamide,40 dicyandiamide,41 and melamine42) leads to their incomplete polymerization with large amounts of non-condensed surface amino groups that result in sluggish charge carrier transport.18,43 The thermal shock during high-temperature calcination has also been linked to agglomeration and a disordered arrangement of homo-triazine units.44
Therefore, there has been increasing interest in the synthesis of fully condensed crystalline carbon nitride, which has an improved electron transport along the in-plane direction ascribed to the extended π-conjugated system and delocalized π-electrons and reduced recombination centers for charge carriers.45 In addition, its high crystallinity has been known to improve its charge transfer kinetics and enhance its light harvesting efficiency.46 As such, molten-salt synthesis is a green and cost-effective method for structural modification. Molten-salts act as the reaction medium for synthesis and flux for crystal growth. The molten-salt synthesis method is also more facile than a soft/hard templating approach, which requires toxic ammonium bifluoride and hydrofluoric acid to remove the silicon. In contrast, salt can be easily removed with warm water.47
Unfortunately, purely poly(triazine imide) (PTI)-based carbon nitride displayed poorer photocatalytic performance compared to heptazine-based carbon nitrides with an extended and fully condensed conjugation structure.48 Thus, Wang et al. have combined different carbon nitride phases in g-C3N4 with the incorporation of 2,4-diamino-6-hydroxypyrimidine as an effective strategy towards regulating its band structure and improving its photocatalytic hydrogen evolution activity (569.5 μmol g−1 h−1).49 Thus, achieving an isotype heterojunction through heptazine/triazine phase tuning can effectively realize an intimate interface contact with a low resistance interface due to the interfacial electric field.50
Because the C3N5 allotrope has been reported more recently, to the best of our knowledge, there has been a lack of research on crystalline C3N5 phase tuning. Therefore, a one-step molten salt method was used in this study to prepare crystalline C3N5 nanosheets with a heptazine/triazine isotype heterojunction (Fig. 1). Alkali salts LiCl and KCl were chosen as they favor the formation of triazine and heptazine structures, respectively.48 The units of heptazine form a π electron system, which can enhance photocatalytic stability and enhance the transfer of charge.51 Meanwhile, the incorporation of the triazine phase with the heptazine phase leads to an isotype heterojunction that synergistically improves the photogenerated electron–hole separation.49 An increase in calcination temperature and the salt/carbon precursor ratio results in the transition of the heptazine phase to the PTI phase, allowing for tunable phase control with a moderate calcination temperature and salt ratio.52,53 The effects of temperature on the properties of crystalline C3N5 as well as its versatility in photocatalytic reactions will be analyzed. This includes multifunctional photocatalytic applications toward H2 production and H2O2 production as well as benzyl alcohol oxidation.
Results and discussion
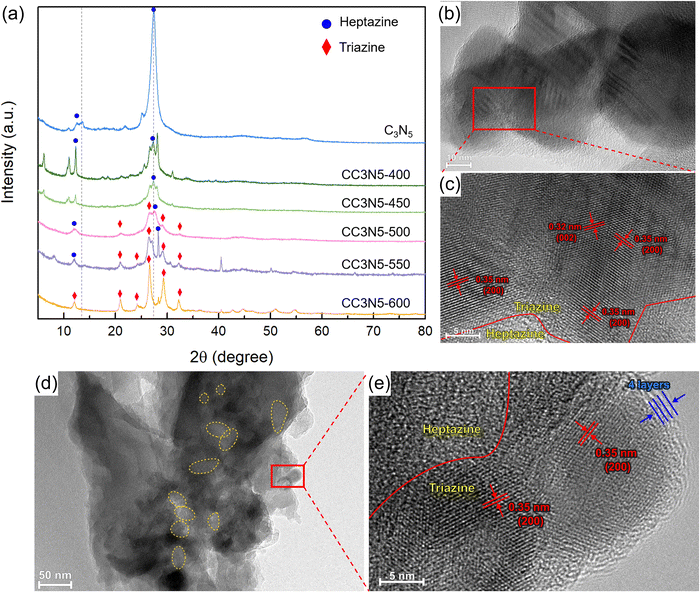
Crystalline C3N5 was synthesized through thermal calcination of 3-amino-1,2,4-triazole with LiCl/KCl molten salts (Fig. S1, ESI†). As seen in the X-ray diffraction (XRD) patterns (Fig. 2a), pristine C3N5 only has two main peaks at 2θ = 12.6° and 27.53°, which were ascribed to the in-plane repeating unit (100) and the interlayer stacking unit (002), respectively. Notably, the increase of synthetic temperature (CC3N5-600) leads to the complete change of heptazine-based carbon nitrides to PTI with higher crystallinity. This is attributed to the higher thermal stability of PTI compared to the heptazine phase at higher temperature.At a moderate synthesis temperature (CC3N5-500), the co-existence of triazine-based carbon nitride can be seen through the diffraction peaks at 21.04°, 26.63°, 29.15°, and 32.41°, which can be assigned to the (110), (200), (102) and (210) planes of PTI, while the peaks at 12.04° and 27.64° correspond to the (100) and (002) planes of the heptazine phase CN, respectively. At 500 °C, the heptazine and triazine phases are calculated to be 55 and 45%, respectively. The triazine phase increases to 74% and 93% for the CC3N5-550 and CC3N5-600 samples, respectively. Additionally, the full width at half-maximum (FWHM) values of the (002) peak of the CC3N5-500 (0.65°) and CC3N5-550 (0.15°) samples were narrowed compared to that of the pristine C3N5 sample (1.05°), which indicates their improved crystallinity over bulk C3N5. This is consistent with the Jin et al.'s observations on crystalline g-C3N4.52
Furthermore, the high-resolution transmission electron microscopy (HRTEM) image (Fig. 2b and c) demonstrates the coexistence of heptazine- and triazine-based crystalline carbon nitrides in CC3N5-500, whereby the lattice fringe with d = 0.32 nm corresponds to the (002) plane of heptazine-based carbon nitride and another lattice fringe of 0.35 nm corresponds to the (200) plane of triazine-based carbon nitride.54 The presence of pores (ca. 15–20 nm) can also be clearly seen in Fig. 2d, which is an indication of a high surface area. It can also be seen in the HRTEM image in Fig. 2e that CC3N5-500 is around 4 layers thick.
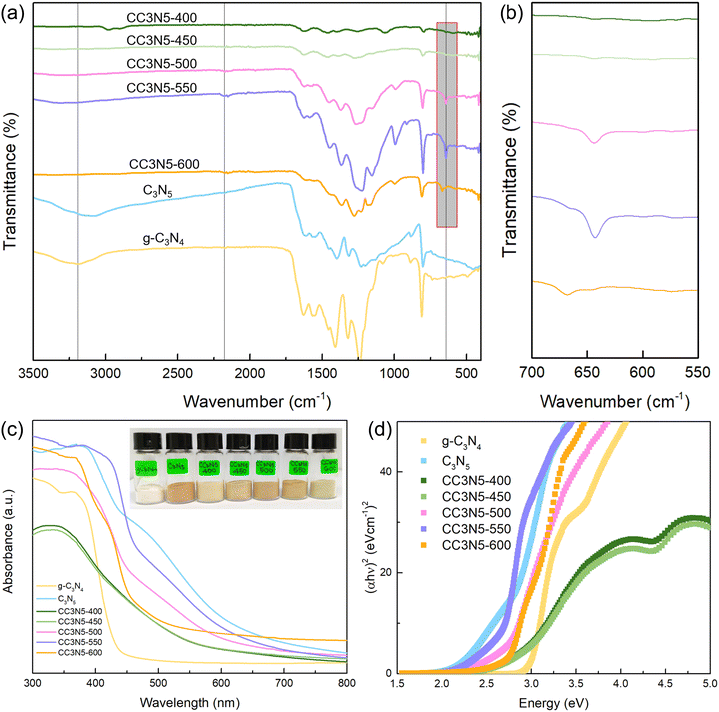
The chemical structure of the as-prepared crystalline carbon nitride samples was investigated using Fourier transform infrared (FTIR) measurements (Fig. 3a). The pristine g-C3N4 and C3N5 samples have a broad peak in the range of ∼3000–3500 cm−1, which is attributed to the N–H stretching vibrations of the terminal amine group or the bridging amine group between s-triazine units. The pristine carbon nitrides also possess typical stretching modes of aromatic CN heterocycles in the region of 1100–1700 cm−1. A peak at ∼850 cm−1 is due to the heptazine ring-bending vibrations.53 As for the crystalline C3N5 samples, a new weak peak emerged at ∼2100 cm−1 attributed to the asymmetric contraction vibrations of the cyano group (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), due to the loss of ammonia.47 A reduced broad peak at ∼3000–3500 cm−1 for the crystalline C3N5 samples indicates a reduced concentration of the terminal amino group (N–H) and adsorbed H2O. This can be correlated with the molten-salt induced deamination in the formation of a tri-s-triazine-based crystalline structure.55 A band at ∼640 cm−1 for CC3N5-500, CC3N5-550 and CC3N5-600 is attributed to the stretching vibrations of C–Cl formed during the molten salt process (Fig. 3b) and confirms the successful intercalation of chloride into carbon nitrides.47,54 As such, all these observations resulted from the one-pot molten-salt synthesis for varying the as-synthesized heptazine/triazine phases of crystalline C3N5 with temperature control.
N), due to the loss of ammonia.47 A reduced broad peak at ∼3000–3500 cm−1 for the crystalline C3N5 samples indicates a reduced concentration of the terminal amino group (N–H) and adsorbed H2O. This can be correlated with the molten-salt induced deamination in the formation of a tri-s-triazine-based crystalline structure.55 A band at ∼640 cm−1 for CC3N5-500, CC3N5-550 and CC3N5-600 is attributed to the stretching vibrations of C–Cl formed during the molten salt process (Fig. 3b) and confirms the successful intercalation of chloride into carbon nitrides.47,54 As such, all these observations resulted from the one-pot molten-salt synthesis for varying the as-synthesized heptazine/triazine phases of crystalline C3N5 with temperature control.
The UV-Vis spectra of all the samples are displayed in Fig. 3c. g-C3N4 shows a characteristic absorption peak between 300 and 400 nm,56 while C3N5 has a broad absorption band due to the π → π* transition in the conjugated network and is red-shifted with a band tail up to 650 nm. This difference is due to the increased azo-bridge (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) between the heptazine units, which extends the π conjugated network due to the overlap between N 2p orbitals of bridging azo moieties and N 2p in the heptazine π conjugated system.57 The π → π* transition also facilitates the absorption in the visible light spectrum and results in the orange color of the C3N5 sample.58
N–) between the heptazine units, which extends the π conjugated network due to the overlap between N 2p orbitals of bridging azo moieties and N 2p in the heptazine π conjugated system.57 The π → π* transition also facilitates the absorption in the visible light spectrum and results in the orange color of the C3N5 sample.58
As for the crystalline C3N5 systems, CC3N5-400 and CC3N5-450 show the lowest absorption intensity in the range of 300–350 nm and an absorption edge of ∼520 nm, while CC3N5-550 and CC3N5-500 have an absorption edge at 613 and 595 nm, respectively. Furthermore, the absorption edge of CC3N5-600 is at 462 nm. The optical bandgaps were determined with a Tauc plot, exemplifying that the band gap of C3N5 (2.67 eV) is narrowed compared to that of g-C3N4 (2.98 eV), which facilitates an enhanced light utilization and harvesting. Meanwhile, the crystalline C3N5 samples have band gaps of 2.78, 2.79, 2.65, 2.64 and 2.72 eV for CC3N5-400, CC3N5-450, CC3N5-500, CC3N5-550 and CC3N5-600, respectively. Notably, the triazine phase of crystalline C3N5 samples has downshifted band positions compared to the heptazine phase (Fig. S4, ESI†), which enables tuning the band structure through controlling the calcination temperature in the salt medium. As such, the isotype heterojunction formed between the heptazine/triazine units with interleaved band gaps aids in the interfacial charge transfer.59
X-ray photoelectron spectroscopy (XPS) analysis was conducted on CC3N5-500 to further confirm its surface compositions and elemental chemical state (Fig. 4). The C 1s spectra have four peaks at 284.8, 286.5, 288.4, and 289.8 eV, which are attributed to the sp2 C–C bond, the C–O bond, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and the N–C
N and the N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N species, respectively. The N 1s spectra also show a peak assigned to C
N species, respectively. The N 1s spectra also show a peak assigned to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N for CC3N5-500 (400.2 eV), along with the existence of the C
N for CC3N5-500 (400.2 eV), along with the existence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C peak (398.8 eV),60 which is also in line with the FTIR results. The valence state of K was analyzed through the K 2p XPS, which shows two binding energy peaks at 293.6 and 295.5 eV assigned to the K+ ion, which are different from that of metallic K (294.7 eV).61 The presence of Cl− ions can also be observed through XPS analysis (0.63%), as evidenced by the Cl 2p1/2 and Cl 2p3/2 peaks at 200.3 and 199.1 eV, respectively.62 The elemental ratios calculated from XPS are shown in Table S1 (ESI†). Trace Li, Cl and K elements were estimated to be less than 10% according to the XPS results. As for CC3N5-600 (Fig. S5a–d, ESI†), the O 1s spectra have two peaks located at 529 and 531 eV, corresponding to C–O and the surface adsorbed H2O, respectively. The C
N–C peak (398.8 eV),60 which is also in line with the FTIR results. The valence state of K was analyzed through the K 2p XPS, which shows two binding energy peaks at 293.6 and 295.5 eV assigned to the K+ ion, which are different from that of metallic K (294.7 eV).61 The presence of Cl− ions can also be observed through XPS analysis (0.63%), as evidenced by the Cl 2p1/2 and Cl 2p3/2 peaks at 200.3 and 199.1 eV, respectively.62 The elemental ratios calculated from XPS are shown in Table S1 (ESI†). Trace Li, Cl and K elements were estimated to be less than 10% according to the XPS results. As for CC3N5-600 (Fig. S5a–d, ESI†), the O 1s spectra have two peaks located at 529 and 531 eV, corresponding to C–O and the surface adsorbed H2O, respectively. The C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N peak was also shifted towards a slightly higher binding energy (400.71 eV) in the N 1s spectra of CC3N5-600. For pristine C3N5 (Fig. S5e–h, ESI†), the C 1s peak is deconvoluted into three peaks with binding energies of 284.8, 287.9 and 288.2 eV, corresponding to the sp2 C–C bond, the C
N peak was also shifted towards a slightly higher binding energy (400.71 eV) in the N 1s spectra of CC3N5-600. For pristine C3N5 (Fig. S5e–h, ESI†), the C 1s peak is deconvoluted into three peaks with binding energies of 284.8, 287.9 and 288.2 eV, corresponding to the sp2 C–C bond, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and the N–C
N and the N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N species, respectively. Notably, the at% of C
N species, respectively. Notably, the at% of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N species in the N 1s spectra of pristine C3N5 (44%) was lower than those of CC3N5-500 (87%) and CC3N5-600 (78%). There are no Li, Cl or K peaks for pristine C3N5 according to the survey spectra.
N species in the N 1s spectra of pristine C3N5 (44%) was lower than those of CC3N5-500 (87%) and CC3N5-600 (78%). There are no Li, Cl or K peaks for pristine C3N5 according to the survey spectra.
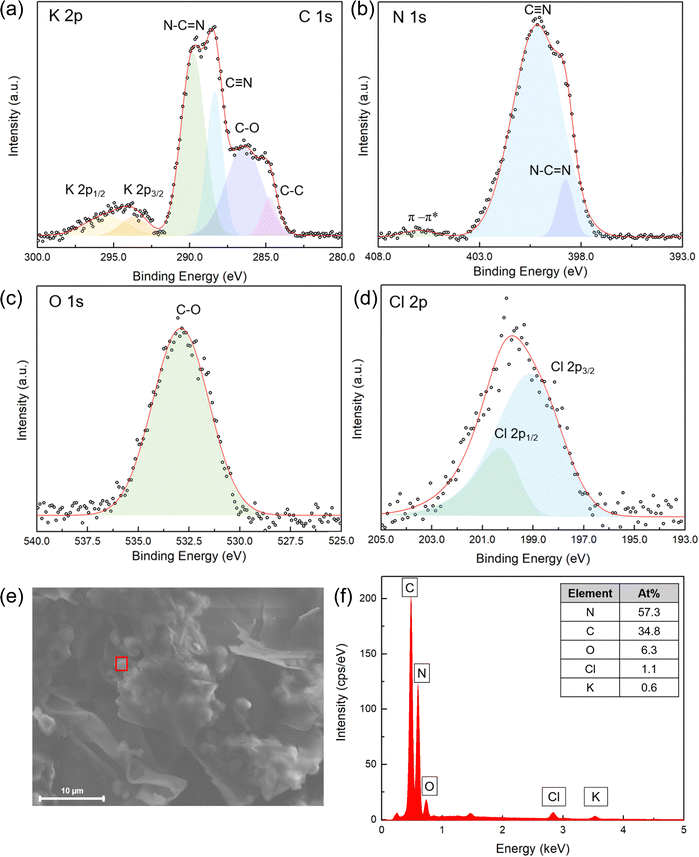 | ||
| Fig. 4 High-resolution XPS spectra of (a) C 1s and K 2p, (b) N 1s, (c) O 1s, and (d) Cl 2p of CC3N5-500. (e) SEM and (f) EDX analysis of the CC3N5-500 sample. | ||
Scanning electron microscopy (SEM) combined with energy-dispersive X-ray (EDX) analysis was also performed for CC3N5-500 (Fig. 4e and f). The EDX spectrum demonstrates the presence of corresponding C, N, O, Cl, and K elements in the material. The C/N ratio calculated from SEM-EDX analysis (C:N = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.93) is also similar to that calculated from XPS (C:N = 3
4.93) is also similar to that calculated from XPS (C:N = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.51) (Table S1, ESI†).
5.51) (Table S1, ESI†).
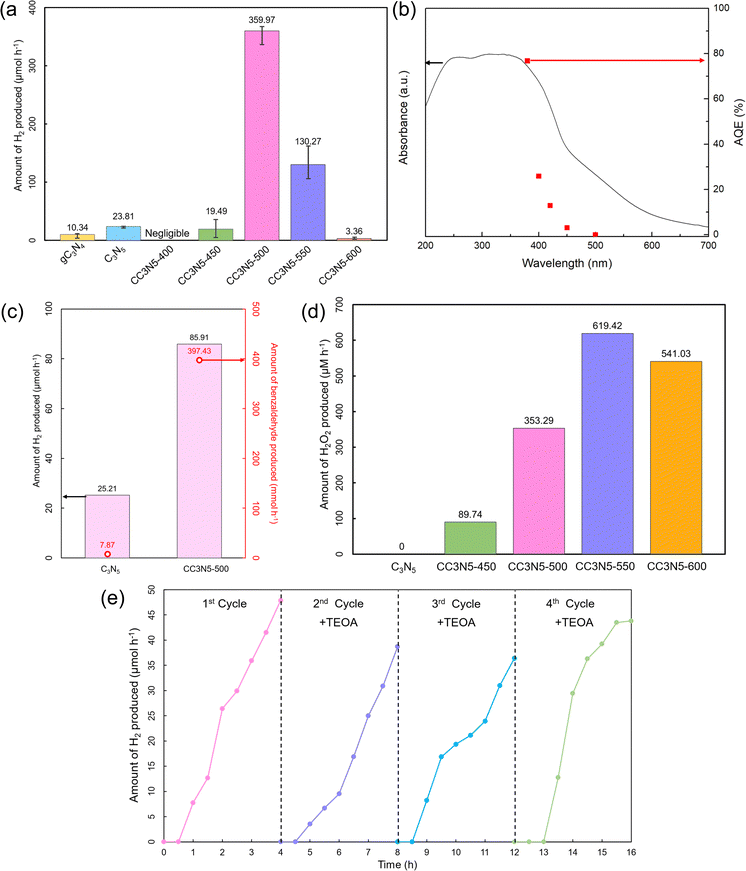
The photocatalytic activities of the various carbon nitride catalysts for hydrogen production are shown in Fig. 5a. The hydrogen evolution rate of pristine C3N5 is 2.3-fold higher than that of g-C3N4, in the presence of a sacrificial agent (TEOA), which can be attributed to the higher C/N atomic ratio that facilitates more catalytically active sites. As for the crystalline C3N5 samples, the CC3N5-400 and CC3N5-600 have negligible HER activity, while the CC3N5-500 catalyst has the highest HER rate of ∼360 μmol h−1 (20 mg), which outperforms those of other carbon nitride catalysts (Table S2, ESI†). This stems from the poor light absorption and excess content of PTI for CC3N5-600 (93%) and incomplete polymerization of CC3N5-400.49 This highlights that the heptazine/PTI ratio resulting from the calcination temperature plays a predominant role in its photocatalytic performance. This is based on the XRD results that show CC3N5-500 possesses a heptazine/triazine ratio of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45. Such a trend is similar to that of studies done on crystalline g-C3N4, whereby the temperature range of 500 to 550 °C contributes to the highest photocatalytic performance.54
45. Such a trend is similar to that of studies done on crystalline g-C3N4, whereby the temperature range of 500 to 550 °C contributes to the highest photocatalytic performance.54
The solar-to-hydrogen (STH) efficiency of CC3N5-500 measured using an AM1.5 light filter was calculated to be 1.31%. The apparent quantum efficiency (AQE) was measured at various wavelengths (380, 400, 420, 450, and 500 nm) to better evaluate its photocatalytic activity (Fig. 5b and Table S3, ESI†). At 420 nm, CC3N5-500 had an AQE of 12.86%, which is higher than that of other single materials or doped carbon nitride photocatalysts reported to date.63–66 The AQE action spectrum of the CC3N5-500 sample indicates that it is able to generate H2 in the visible light range up to 450 nm, consistent with the UV-Vis spectrum.
Apart from the HER half-reaction, the crystalline carbon nitride semiconductor also demonstrates a significant improvement toward simultaneous photocatalytic HER and benzyl alcohol oxidation. As shown in Fig. 5c, the CC3N5-500 sample produced 3 times more hydrogen and 50 times more benzaldehyde than pristine C3N5 when the sacrificial agent (TEOA) was switched to benzyl alcohol (3 vol%). Furthermore, the benzaldehyde consumption rate of CC3N5-500 (223.37 μmol h−1) is also higher than that of pristine C3N5 (161.54 μmol h−1). Therefore, it can be deduced that the improved crystallinity of CC3N5-500 leads to better utilization of both electrons and holes in order to partake in simultaneous HER and organic synthesis. This is an indication of its versatility towards photoredox chemical synthesis. Additionally, these results are also better than those of similar carbon nitride research works published in recent years (Table S4, ESI†), ascertaining the vital role of interfacial electron–hole transfer in phase tuning.
The application of crystalline C3N5 can also be extended toward H2O2 production (Fig. 5d). The most optimal sample was identified to be CC3N5-550 with a H2O2 evolution rate of 619.42 μmol L−1 h−1, whereas pristine C3N5 displayed negligible H2O2 production (Table S5, ESI†). This is followed by CC3N5-600, CC3N5-500, and CC3N5-450. There exists an optimal trend in the photocatalytic performance, which indicates that the presence of both heptazine and triazine phases aids in the oxygen reduction reaction towards H2O2 due to the interfacial electron transfer across the junction. Additionally, as in the control experiments, the photocatalytic H2O2 production activity of CC3N5-550 was studied in purified air and N2 atmospheres to ensure the O2 dependency of the reaction (Fig. S6c, ESI†). Compared to the experiment conducted with O2 purging, only 364.9 and 71.35 μmol L−1 h−1 H2O2 was produced with purified air and N2 purging, respectively, demonstrating that O2 is the contributing factor in the reaction.67
The reusability of a photocatalyst is a crucial measure for its practical applications. The CC3N5-500 catalyst was then tested for its recyclability in conjunction with 3D printing technology (see Methodology, Video in the ESI†). It was found that the catalyst retained 92% of photocatalytic H2 production even after 16 h (4 cycles) (Fig. 5e), thus proving its potential to be applied in scaled-up HER setups such as in a continuous flow reactor. From Fig. S7 (ESI†), it can be seen that the catalyst has largely been retained on the 3D substrate after each cycle. Thus, this demonstrates the importance of integrating additive manufacturing processes such as 3D printing to narrow the gap between lab-scale and commercial-scale photocatalytic green fuel and chemical production.
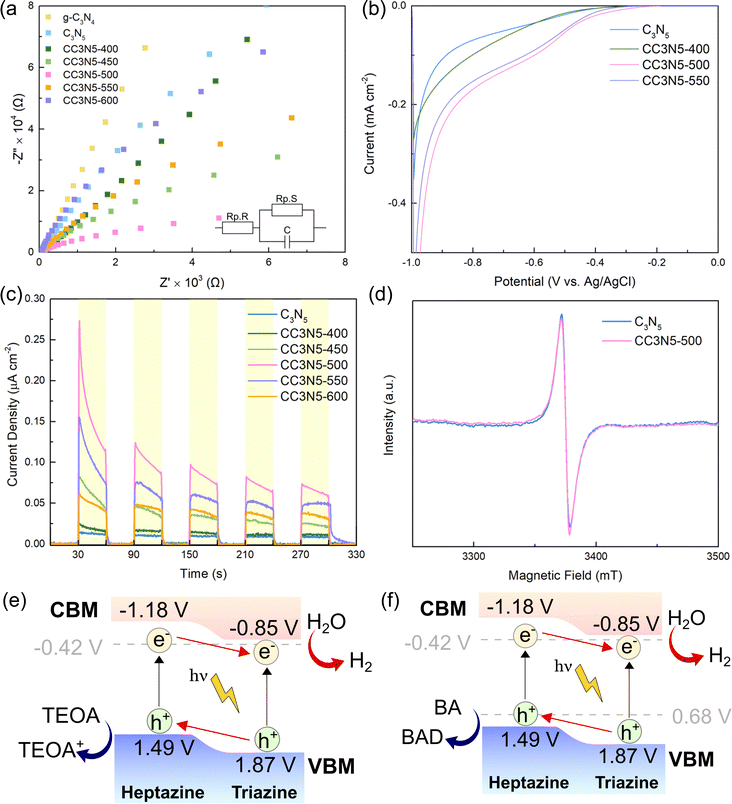
Electrochemical analysis has also been carried out to further unveil the underlying electronic properties. Electrochemical impedance spectroscopy (EIS) was conducted to uncover the charge carrier recombination processes of the different carbon nitride catalysts. From the Nyquist plot in Fig. 6a, the arc radius of CC3N5-500 is smaller than that of the other crystalline C3N5 samples and pristine C3N5. This decreased radius demonstrates that the photogenerated carrier separation and fast surface interfacial charge transfer in CC3N5-500 are crucial factors for its high HER performance. The charge transfer resistances (Rp.R) for the different samples are shown in Table S6 (ESI†), whereby CC3N5-500 has the lowest Rp.R. This result is in line with the photocatalytic HER results and supports the interfacial electron–hole transfer hypothesis.
The photocatalyst samples were also studied through linear sweep voltammetry (LSV) (Fig. 6b). Pristine C3N5 only generates a low current density of −0.069 mA cm−2 at an applied potential of −0.8 V (vs. Ag/AgCl), while the CC3N5-500 photoelectrode exhibits a current density of −0.8 mA cm−2 at the same applied potential. The ameliorated current density is due to the well separation of electron–hole pairs in the CC3N5-500 sample.68 The charge transfer ability was also confirmed through transient photocurrent responses (Fig. 6c). The order of photocurrent response intensities is CC3N5-500 > CC3N5-550 > CC3N5-600 > CC3N5-450 > CC3N5-600 > CC3N5-400 > C3N5. This is further evidence of the high separation efficiency of charge carriers in crystalline carbon nitride,69 particularly CC3N5-500, which enables its superb photocatalytic performance due to the heptazine/triazine phase junction.
Electron paramagnetic resonance (EPR) analysis was conducted for g-C3N4, C3N5 and CC3N5-500 samples (Fig. 6d). All three samples show an obvious EPR signal at a g-value of 2.0034, which corresponds to the defects in the carbon nitride framework.70 The EPR intensity of C3N5 is higher than that of CC3N5-500, signifying that the molten salt calcination treatment leads to a lower defect concentration due to the improved polymerization degree of CC3N5-500.71 Hence, this could lead to a reduced charge recombination of crystalline C3N5 and excellent photocatalytic results.
The active radical species involved in photocatalytic H2 evolution were further investigated using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) spin-trapped EPR spectroscopy to detect the hydroxyl (˙OH−) and the superoxide radicals (˙O2−). In Fig. S8a (ESI†), no obvious DMPO-˙OH signal can be found for pristine C3N5, which implies that no ˙OH radicals were generated. In comparison, 4 characteristic peaks of the DMPO-˙OH adducts can be found for the CC3N5-500 sample, indicating that these radicals are generated during photocatalytic reactions.72 The signals for active radicals for ˙O2− were also observed for CC3N5-500 (Fig. S8b, ESI†), demonstrating that they are part of the photocatalytic mechanism. As such, the EPR results revealed that crystalline C3N5 can produce more ˙OH− and ˙O2− radicals under visible-light irradiation, which could be due to the reduced electron–hole recombination rate of crystalline C3N5 compared to its pristine form.73
The Mott–Schottky curves for all samples are consistent with that of a typical n-type semiconductor (Fig. S9a–g, ESI†). In addition, the Mott–Schottky curves were extrapolated to identify the flat band potential and subsequently their conduction band (CB). The CB potential of an n-type semiconductor is ∼0.1 V more negative than its flat band potential.74 Therefore, the CB potentials of all catalysts are listed in Table S7 (ESI†), and their band positions were calculated with the Eg data obtained from UV-Vis diffuse reflectance spectroscopy (DRS) analysis. Notably, the CB positions of all crystalline C3N5 samples were more positive than that of pristine C3N5. By combining the CB position obtained through the Mott–Schottky curves and the band gap positions obtained through UV-Vis analysis, the band positions of the heptazine phase and triazine phase were proposed.
Based on the aforementioned various characterization analyses and the superior photocatalytic activity, the possible photocatalytic mechanism was proposed and shown in Fig. 6e and f. Under light irradiation, electrons are excited from the VB to the CB of the catalyst. Due to the presence of the isotype heterojunction, the electrons flow from the CB of the heptazine to the triazine phase, while the holes migrate from the VB of the triazine to the heptazine phase.75 This is in line with previous studies that indicate the phase junctions between heptazine-based carbon nitride and triazine-based carbon nitride belong to the type-II heterojunction.54,76 The electrons on the triazine-based carbon nitride then react with H+ to produce H2, whereas the holes oxidize TEOA to TEOA+. For the simultaneous HER and benzyl alcohol oxidation, the holes will oxidize benzyl alcohol to benzaldehyde at an oxidation potential of 0.68 V (vs. the normal hydrogen electrode, NHE).77 The H2O2 production mechanism shown in Fig. S9h (ESI†) is also similar to the HER half-reaction mechanism, whereas the ORR reaction takes place with a reduction potential of 0.68 V (vs. NHE). As a whole, this proposed photocatalytic mechanism for the isotype heterojunction crystalline C3N5 facilitates charge carrier separation, which markedly accelerates the photocatalytic redox performance.
Conclusion
In this research work, we have successfully synthesized crystalline C3N5 with a heptazine/triazine isotype heterojunction with a molten salt synthesis route. Notably, temperature has a great influence on the phase engineering of the carbon nitride, with 500 and 550 °C displaying both heptazine and triazine phases. Notably, a low or high synthesis temperature leads to a more dominant heptazine or triazine phase, respectively, which leads to good interfacial charge transfer. The temperature–performance relationship has a strong influence on the physicochemical properties of the crystalline carbon nitrides, which in turn affects the photocatalytic performance. CC3N5-500 has a heptazine/triazine ratio of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45, and the triazine phase increases to 74% and 93% for CC3N5-550 and CC3N5-600, respectively. From the UV-Vis results, the light absorption of C3N5 improved in the visible light region, with a red-shifted absorption band edge at 603 nm compared to g-C3N4 (427 nm). XPS analysis also revealed that the C:N ratio of CC3N5-500 is 3
45, and the triazine phase increases to 74% and 93% for CC3N5-550 and CC3N5-600, respectively. From the UV-Vis results, the light absorption of C3N5 improved in the visible light region, with a red-shifted absorption band edge at 603 nm compared to g-C3N4 (427 nm). XPS analysis also revealed that the C:N ratio of CC3N5-500 is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5, compared to that of CC3N5-600 (3
5.5, compared to that of CC3N5-600 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.1). Furthermore, the CC3N5-500 sample exhibited the highest HER performance of 359.97 μmol h−1 and an AQE of 12.86% at 420 nm, which were 15-fold higher than those of pristine C3N5. The recyclability tests on a 3D printed substrate also exemplified a 92% performance retention after 4 cycles (16 h), which indicates its promising potential for its use in large-scale photocatalytic reactions. The crystalline C3N5 sample also outperformed pristine C3N5 in benzyl alcohol oxidation. Meanwhile, CC3N5-550 exhibited the highest performance in photocatalytic H2O2 production with an AQE of 9.49% at 420 nm. This indicates that crystalline C3N5 improves the reaction kinetics for multitudinous solar-driven photoredox reactions due to the boosted charge transfer kinetics as evidenced by the electrochemistry results, resulting in enhanced interfacial electron–hole transfer in the crystalline heptazine/triazine carbon nitride heterojunction compared to the pure heptazine or triazine phase. In short, these results provide guidance for the structural and phase tuning of carbon nitride to aid future studies on its crystallinity for its photocatalyic applications.
6.1). Furthermore, the CC3N5-500 sample exhibited the highest HER performance of 359.97 μmol h−1 and an AQE of 12.86% at 420 nm, which were 15-fold higher than those of pristine C3N5. The recyclability tests on a 3D printed substrate also exemplified a 92% performance retention after 4 cycles (16 h), which indicates its promising potential for its use in large-scale photocatalytic reactions. The crystalline C3N5 sample also outperformed pristine C3N5 in benzyl alcohol oxidation. Meanwhile, CC3N5-550 exhibited the highest performance in photocatalytic H2O2 production with an AQE of 9.49% at 420 nm. This indicates that crystalline C3N5 improves the reaction kinetics for multitudinous solar-driven photoredox reactions due to the boosted charge transfer kinetics as evidenced by the electrochemistry results, resulting in enhanced interfacial electron–hole transfer in the crystalline heptazine/triazine carbon nitride heterojunction compared to the pure heptazine or triazine phase. In short, these results provide guidance for the structural and phase tuning of carbon nitride to aid future studies on its crystallinity for its photocatalyic applications.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to acknowledge the financial support provided by the Ministry of Higher Education (MOHE) Malaysia under the Fundamental Research Grant Scheme (FRGS) (Ref no: FRGS/1/2020/TK0/XMU/02/1). The authors would like to thank the Ministry of Science, Technology and Innovation (MOSTI) Malaysia under the Strategic Research Fund (SRF) (S.22015). This research was supported by the National Natural Science Foundation of China (Ref no: 22202168) and Guangdong Basic and Applied Basic Research Foundation (Ref no: 2021A1515111019). We would also like to acknowledge the financial support from the State Key Laboratory of Physical Chemistry of Solid Surfaces, Xiamen University (ref. no.: 2023X11). This work was also funded by Xiamen University Malaysia Investigatorship Grant (Grant no: IENG/0038), Xiamen University Malaysia Research Fund (ICOE/0001, XMUMRF/2021-C8/IENG/0041 and XMUMRF/2019-C3/IENG/0013) and Hengyuan International Sdn. Bhd. (Grant no: EENG/0003).References
- Assessing the Global Climate in 2021, https://www.ncei.noaa.gov/news/global-climate-202112).
- L. Ai, S.-F. Ng and W.-J. Ong, ChemSusChem, 2022, 15, e202200857 CrossRef CAS PubMed.
- H. Mai, T. C. Le, D. Chen, D. A. Winkler and R. A. Caruso, Chem. Rev., 2022, 122, 13478–13515 CrossRef CAS PubMed.
- A. K. Singh, K. Mathew, H. L. Zhuang and R. G. Hennig, J. Phys. Chem. Lett., 2015, 6, 1087–1098 CrossRef CAS PubMed.
- V. B.-Y. Oh, S.-F. Ng and W.-J. Ong, EcoMat, 2022, 4, e12204 CrossRef CAS.
- J. J. Foo, S.-F. Ng and W.-J. Ong, Nano Res., 2023, 16, 4310–4363 CrossRef CAS.
- C. Yang, R. Zhao, H. Xiang, J. Wu, W. Zhong, W. Li, Q. Zhang, N. Yang and X. Li, Adv. Energy Mater., 2020, 10, 2002260 CrossRef CAS.
- C.-F. Fu, X. Wu and J. Yang, Adv. Mater., 2018, 30, 1802106 CrossRef PubMed.
- G. Z. S. Ling, S.-F. Ng and W.-J. Ong, Adv. Funct. Mater., 2022, 32, 2111875 CrossRef CAS.
- The Future of Hydrogen, https://www.iea.org/reports/the-future-of-hydrogen).
- W.-J. Ong, L.-L. Tan, Y. H. Ng, S.-T. Yong and S.-P. Chai, Chem. Rev., 2016, 116, 7159–7329 CrossRef CAS PubMed.
- A. Naseri, M. Samadi, A. Pourjavadi, A. Z. Moshfegh and S. Ramakrishna, J. Mater. Chem. A, 2017, 5, 23406–23433 RSC.
- B. Huang, Y. Wu, B. Chen, Y. Qian, N. Zhou and N. Li, Chin. J. Catal., 2021, 42, 1160–1167 CrossRef CAS.
- G. Z. S. Ling, S. H. W. Kok, P. Zhang, J. S. Tan, C. Y. Haw, L.-L. Tan, B. H. Chen and W.-J. Ong, J. Mater. Chem. A, 2023 10.1039/D3TA04204A.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2008, 8, 76–80 CrossRef PubMed.
- C. Wu, S. Xue, Z. Qin, M. Nazari, G. Yang, S. Yue, T. Tong, H. Ghasemi, F. C. R. Hernandez, S. Xue, D. Zhang, H. Wang, Z. M. Wang, S. Pu and J. Bao, Appl. Catal., B, 2021, 282, 119557 CrossRef CAS.
- X. Liu, Y. Deng, L. Zheng, M. R. Kesama, C. Tang and Y. Zhu, ACS Catal., 2022, 12, 5517–5526 CrossRef CAS.
- L. Lin, Z. Yu and X. Wang, Angew. Chem., Int. Ed., 2019, 58, 6164–6175 CrossRef CAS PubMed.
- M. Volokh, G. Peng, J. Barrio and M. Shalom, Angew. Chem., Int. Ed., 2019, 58, 6138–6151 CrossRef CAS PubMed.
- R. Kuriki, K. Sekizawa, O. Ishitani and K. Maeda, Angew. Chem., Int. Ed., 2015, 54, 2406–2409 CrossRef CAS PubMed.
- J. Liang, Z. Jiang, P. K. Wong and C.-S. Lee, Solar RRL, 2021, 5, 2000478 CrossRef CAS.
- Y. Li, B. Li, D. Zhang, L. Cheng and Q. Xiang, ACS Nano, 2020, 14, 10552–10561 CrossRef CAS PubMed.
- L. Cheng, P. Zhang, Q. Wen, J. Fan and Q. Xiang, Chin. J. Catal., 2022, 43, 451–460 CrossRef CAS.
- M. Sayed, B. Zhu, P. Kuang, X. Liu, B. Cheng, A. A. A. Ghamdi, S. Wageh, L. Zhang and J. Yu, Adv. Sustainable Syst., 2022, 6, 2100264 CrossRef CAS.
- A. Yuan, H. Lei, Z. Wang and X. Dong, J. Colloid Interface Sci., 2020, 560, 40–49 CrossRef CAS PubMed.
- T. Uekert, H. Kasap and E. Reisner, J. Am. Chem. Soc., 2019, 141, 15201–15210 CrossRef CAS PubMed.
- T. He, C. Zhang, L. Zhang and A. Du, Nano Res., 2019, 12, 1817–1823 CrossRef CAS.
- X.-Q. Tan, S.-F. Ng, A. R. Mohamed and W.-J. Ong, Carbon Energy, 2022, 4, 665–730 CrossRef CAS.
- J. Fu, J. Yu, C. Jiang and B. Cheng, Adv. Energy Mater., 2018, 8, 1701503 CrossRef.
- X. Yu, S.-F. Ng, L. K. Putri, L.-L. Tan, A. R. Mohamed and W.-J. Ong, Small, 2021, 17, 2006851 CrossRef CAS PubMed.
- S.-F. Ng, X. Chen, J. J. Foo, M. Xiong and W.-J. Ong, Chin. J. Catal., 2023, 47, 150–160 CrossRef CAS.
- Y. Wang, T. Ngoc Pham, Y. Tian, Y. Morikawa and L. Yan, J. Colloid Interface Sci., 2021, 585, 740–749 CrossRef CAS PubMed.
- J. Zhang, Z. Li, J. He, H. Tao, M. Chen, Y. Zhou and M. Zhu, ACS Catal., 2023, 13, 785–795 CrossRef CAS.
- C. Peng, L. Han, J. Huang, S. Wang, X. Zhang and H. Chen, Chin. J. Catal., 2022, 43, 410–420 CrossRef CAS.
- Q. Li, S. Song, Z. Mo, L. Zhang, Y. Qian and C. Ge, Appl. Surf. Sci., 2022, 579, 152006 CrossRef CAS.
- C. Hu, Y.-H. Lin, M. Yoshida and S. Ashimura, ACS Appl. Mater. Interfaces, 2021, 13, 24907–24915 CrossRef CAS PubMed.
- Y. Yang, L. Geng, Y. Guo, J. Meng and Y. Guo, Appl. Surf. Sci., 2017, 425, 535–546 CrossRef CAS.
- H. Wang, Z. Sun, Q. Li, Q. Tang and Z. Wu, J. CO2 Utiliz., 2016, 14, 143–151 CrossRef CAS.
- F. Dong, Y. Sun, L. Wu, M. Fu and Z. Wu, Catal. Sci. Technol., 2012, 2, 1332–1335 RSC.
- J. Liu, J. Yan, H. Ji, Y. Xu, L. Huang, Y. Li, Y. Song, Q. Zhang, H. Xu and H. Li, Mater. Sci. Semicond. Process., 2016, 46, 59–68 CrossRef CAS.
- L. Yang, X. Liu, Z. Liu, C. Wang, G. Liu, Q. Li and X. Feng, Ceram. Int., 2018, 44, 20613–20619 CrossRef CAS.
- N. Tian, Y. Zhang, X. Li, K. Xiao, X. Du, F. Dong, G. I. N. Waterhouse, T. Zhang and H. Huang, Nano Energy, 2017, 38, 72–81 CrossRef CAS.
- S.-F. Ng, J. J. Foo and W.-J. Ong, InfoMat, 2022, 4, e12279 CrossRef CAS.
- Y. Shao, X. Hao, S. Lu and Z. Jin, Chem. Eng. J., 2023, 454, 140123 CrossRef CAS.
- H. Li, B. Zhu, S. Cao and J. Yu, Chem. Commun., 2020, 56, 5641–5644 RSC.
- G. Chen, F. Wei, Z. Zhou, B. Su, C. Yang, X. F. Lu, S. Wang and X. Wang, Sustainable Energy Fuels, 2023, 7, 381–388 RSC.
- Y. Zhou, C. Liao, Y. Fan, S. Ma, M. Su, Z. Zhou, T.-S. Chan, Y.-R. Lu and K. Shih, Environ. Sci.: Nano, 2019, 6, 3324–3335 RSC.
- Y. Li, B. Wang, Q.-J. Xiang, Q. Zhang and G. Chen, Dalton Trans., 2022, 51, 16527–16535 RSC.
- W. Wang, X. Kou, T. Li, R. Zhao and Y. Su, J. Photochem. Photobiol., A, 2023, 435, 114308 CrossRef CAS.
- C. Cheng, L. Mao, X. Kang, C.-L. Dong, Y.-C. Huang, S. Shen, J. Shi and L. Guo, Appl. Catal., B, 2023, 331, 122733 CrossRef CAS.
- J. Liu, W. Fu, Y. Liao, J. Fan and Q. Xiang, J. Mater. Sci. Technol., 2021, 91, 224–240 CrossRef CAS.
- A. Jin, Y. Jia, C. Chen, X. Liu, J. Jiang, X. Chen and F. Zhang, J. Phys. Chem. C, 2017, 121, 21497–21509 CrossRef CAS.
- L. Lin, W. Ren, C. Wang, A. M. Asiri, J. Zhang and X. Wang, Appl. Catal., B, 2018, 231, 234–241 CrossRef CAS.
- Z. Zhao, Z. Shu, J. Zhou, T. Li, K. Wang and J. Song, J. Alloys Compd., 2023, 938, 168484 CrossRef CAS.
- W. Wang, Z. Shu, J. Zhou, D. Meng, Z. Zhao and T. Li, J. Mater. Chem. A, 2020, 8, 6785–6794 RSC.
- Y. Zhou, L. Zhang, W. Huang, Q. Kong, X. Fan, M. Wang and J. Shi, Carbon, 2016, 99, 111–117 CrossRef CAS.
- K. M. Alam, C. E. Jensen, P. Kumar, R. W. Hooper, G. M. Bernard, A. Patidar, A. P. Manuel, N. Amer, A. Palmgren, D. N. Purschke, N. Chaulagain, J. Garcia, P. S. Kirwin, L. C. T. Shoute, K. Cui, S. Gusarov, A. E. Kobryn, V. K. Michaelis, F. A. Hegmann and K. Shankar, ACS Appl. Mater. Interfaces, 2021, 13, 47418–47439 CrossRef CAS PubMed.
- P. Kumar, E. Vahidzadeh, U. K. Thakur, P. Kar, K. M. Alam, A. Goswami, N. Mahdi, K. Cui, G. M. Bernard, V. K. Michaelis and K. Shankar, J. Am. Chem. Soc., 2019, 141, 5415–5436 CrossRef CAS PubMed.
- W. Shi, L. Cao, Y. Shi, W. Zhong, Z. Chen, Y. Wei, F. Guo, L. Chen and X. Du, J. Mol. Struct., 2023, 1280, 135076 CrossRef CAS.
- P. Xia, M. Antonietti, B. Zhu, T. Heil, J. Yu and S. Cao, Adv. Funct. Mater., 2019, 29, 1900093 CrossRef.
- G. Zhang, Y. Xu, C. He, P. Zhang and H. Mi, Appl. Catal., B, 2021, 283, 119636 CrossRef CAS.
- G. Zhang, Y. Xu, M. Rauf, J. Zhu, Y. Li, C. He, X. Ren, P. Zhang and H. Mi, Adv. Sci., 2022, 9, 2201677 CrossRef CAS PubMed.
- S. Mondal, G. Mark, L. Abisdris, J. Li, T. Shmila, J. Tzadikov, M. Volokh, L. Xing and M. Shalom, Mater. Horiz., 2023, 10, 1363–1372 RSC.
- W. Dai, R. Wang, Z. Chen, S. Deng, C. Huang, W. Luo and H. Chen, J. Mater. Chem. A, 2023, 11, 7584–7595 RSC.
- L. Cao, Y. Shi, Z. Chen, Y. Tian, L. Chen, F. Guo and W. Shi, Mater. Today Commun., 2022, 33, 104354 CrossRef CAS.
- X. Wang, K. Wu, W. Cao, K. Rui, W. Wang, R. Zhu, J. Zhu and Z. Yan, Adv. Mater. Interfaces, 2023, 10, 2201627 CrossRef CAS.
- B. P. Mishra, L. Acharya and K. Parida, Catal. Sci. Technol., 2023, 13, 1448–1458 RSC.
- C. Hegde, T. Rosental, J. M. R. Tan, S. Magdassi and L. H. Wong, Mater. Horiz., 2023, 10, 1806–1815 RSC.
- B. Wu, T. Sun, N. Liu, L. Lu, R. Zhang, W. Shi and P. Cheng, ACS Appl. Mater. Interfaces, 2022, 14, 26742–26751 CrossRef CAS PubMed.
- G. Chen, Z. Zhang, W. Zhang, L. Xia, X. Nie, W. Huang, X. Wang, L. Wang, C. Hong, Z. Zhang and Y. You, Mater. Horiz., 2021, 8, 2018–2024 RSC.
- Y. Lin, X. Wang, X. Fu and W. Su, J. Mater. Chem. A, 2022, 10, 8252–8257 RSC.
- X. Luan, Z. Yu, J. Zi, F. Gao and Z. Lian, Adv. Funct. Mater., 2023, 2304259 CrossRef CAS.
- X. Zhang, Z. Li, N. Ta and H. Han, ACS Sustainable Chem. Eng., 2022, 10, 4059–4064 CrossRef CAS.
- S. Kaowphong, W. Choklap, A. Chachvalvutikul and N. Chandet, Colloids Surf., A, 2019, 579, 123639 CrossRef CAS.
- F. Li, X. Yue, Y. Liao, L. Qiao, K. Lv and Q. Xiang, Nat. Commun., 2023, 14, 3901 CrossRef CAS PubMed.
- G. Zhang, Y. Xu, J. Zhu, Y. Li, C. He, X. Ren, P. Zhang and H. Mi, Appl. Catal., B, 2023, 338, 123049 CrossRef CAS.
- X. Xiang, B. Zhu, J. Zhang, C. Jiang, T. Chen, H. Yu, J. Yu and L. Wang, Appl. Catal., B, 2023, 324, 122301 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01115a |
| This journal is © The Royal Society of Chemistry 2024 |