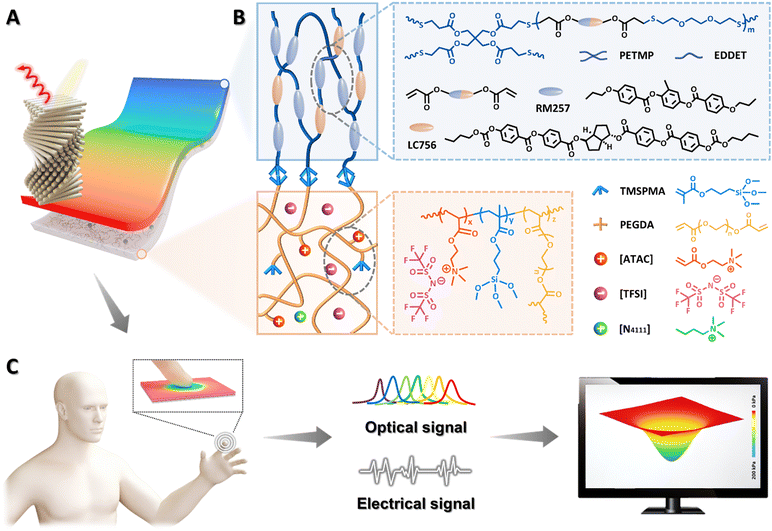
Mechanochromic and ionic conductive cholesteric liquid crystal elastomers for biomechanical monitoring and human–machine interaction†
Jiazhe
Ma‡
a,
Yanzhao
Yang‡
a,
Xuan
Zhang
a,
Pan
Xue
a,
Cristian
Valenzuela
a,
Yuan
Liu
a,
Ling
Wang
 *ab and
Wei
Feng
*ab and
Wei
Feng
 *a
*a
aSchool of Materials Science and Engineering, Tianjin University, Tianjin 300350, P. R. China. E-mail: lwang17@tju.edu.cn; weifeng@tju.edu.cn
bBinhai Industrial Research Institute, Tianjin University, Tianjin 300452, China
First published on 20th October 2023
Abstract
Cholesteric liquid crystal elastomers (CLCEs) that combine rubbery elasticity with structural colour from self-assembled helical nanostructures are of paramount importance for diverse applications such as biomimetic skins, adaptive optics and soft robotics. Despite great advances, it is challenging to integrate electrical sensing and colour-changing characteristics in a single CLCE system. Here, we report the design and synthesis of an ionic conductive cholesteric liquid crystal elastomer (iCLCE) through in situ Michael addition and free-radical photopolymerization of CLCE precursors on silane-functionalized polymer ionic liquid networks, in which robust covalent chemical bonding was formed at the interface. Thanks to superior mechanochromism and ionic conductivity, the resulting iCLCEs exhibit dynamic colour-changing and electrical sensing functions in a wide range upon mechanical stretching, and can be used for biomechanical monitoring during joint bending. Importantly, a capacitive elastomeric sensor can be constructed through facilely stacking iCLCEs, where the optical and electrical dual-signal reporting performance allows intuitive visual localization of pressure intensity and distribution. Moreover, proof-of-concept application of the iCLCEs has been demonstrated with human-interactive systems. The research disclosed herein can provide new insights into the development of bioinspired somatosensory materials for emerging applications in diverse fields such as human–machine interaction, prostheses and intelligent robots.
New conceptsDevelopment of bioinspired colour-changing smart materials with ionic conductivity is currently in the limelight for diverse important applications such as biomimetic skins, adaptive optics and soft robotics. In this work, we report the judicious design and fabrication of ionic conductive cholesteric liquid crystal elastomers (iCLCEs) with excellent mechanochromism and ionic conductivity. The resulting iCLCEs can not only realize optical visualization, but also exhibit electrical sensing properties. It is worth noting that a capacitive elastomeric sensor has been developed through stacking iCLCEs, where the optical and electrical dual-signal reporting performance allows intuitive visual localization of pressure intensity and distribution. Compared with conventional colour-changing materials with electronic conductivity, these ionic conductive photonic materials often show many advantageous features such as low Young's modulus and good biocompatibility, in which sophisticated control of ions as signal carriers similar to that of biological systems could pave the way for the development of biocompatible logic circuits and advanced iontronics toward biomechanical sensing, monitoring, and human–machine interfaces. |
Introduction
Biological skin, as an important window for organisms to directly communicate with the outside world, can perceive environmental stimuli (e.g., pressure, temperature and strain) and transduce them to the nervous system by ion-based electrical signals.1–7 Inspired by the ionic transduction feature of the skins, artificial skin and flexible sensory systems based on ionic conductive materials have been developed, which attract extensive attention in health monitoring,8,9 human–machine interaction,10,11 intelligent robots,12–21 prostheses,22etc. Besides electrical signal response, the skin of some biological organisms can intelligently sense a variety of stimuli and respond to their surrounding environment through colour changes for better survival and reproduction of next-generation individuals.23–28 In particular, chameleons can convert external stimuli into bioelectrical signals to adaptively adjust their skin colours through changing the lattice distance among guanine nanocrystals within the dermal iridophore cells, a characteristic that has long inspired many scientists to develop skin-like advanced colour-changing materials with ionic conductivity.29–35 For instance, silica nanoparticle-based photonic elastomers embedded with lithium salts have been developed as photonic ionic skins capable of outputting synergistic optical and electrical signals under mechanical strain.36 Chromotropic iontronics based on surface-charged 2D bilayered photonic nanostructures and permittivity-switchable hydrogel matrixes have also been demonstrated, which can synchronously realize optical visualization and electrical response to multiple stimuli, including strain, pressure, and temperature.37 Recently, we reported the judicious design and fabrication of mechanochromic chiral nematic nanostructured films with ionic conductivity by infiltrating fluorine-rich ionic liquids into a swollen self-assembled cellulose nanocrystal film with helical nanoarchitectures.38 Compared with colour-changing materials with electronic conductivity that have been extensively investigated,39–44 these ionic conductive photonic materials often show many advantageous features such as low Young's modulus and good biocompatibility, in which sophisticated control of ions as signal carriers similar to that of biological systems could pave the way for the development of biocompatible logic circuits and advanced iontronics toward biomechanical sensing, monitoring, and human–machine interfaces.45 However, the development of bioinspired ionic conductive photonic materials with optical and electrical dual-signal sensing functions is still in a very preliminary stage, therefore, exploring novel colour-changing material systems with ionic conductivity is highly desired for the development of a new generation of intelligent chromotropic sensing platforms.Cholesteric liquid crystal elastomers (CLCEs) that combine elastomeric networks with striking structural colour from self-assembled periodic helical superstructures have been considered as one of the most attractive candidates for advanced colour-changing photonic materials by virtue of their tuneable photonic nanoarchitectures, large elasticity anisotropy, and ultrasensitive stimuli responsiveness as well as the facile and scalable fabrication process.46–51 For example, pneumatically inflating colour-changing materials with broadband spectral modulation ranging from near-infrared to ultraviolet wavelengths have been fabricated based on main-chain CLCEs with large elasticity anisotropy and Poisson's ratio.52 Stretchable and robust CLCE fibres with fast and reversible mechanochromic responses have been achieved and used in smart textiles that can reveal even complex strain patterns.53 Mechanochromic CLCEs have been described that display superior shape programmability and self-healing capabilities by exploiting dynamic covalent chemistry.46 Moreover, a chameleon-inspired camouflage prototype based on thermochromic cholesteric liquid crystals, patterned silver nanowire heaters, and a thermal feedback control logic circuit has been developed to retrieve the local background colour and matches its surface colour in real time.54 Despite extensive efforts that have been devoted to developing and enhancing the colour-changing abilities of CLCEs, conductive CLCEs with sensing functions have not yet been demonstrated.
Ionic liquids (ILs), which are liquid molten salts typically composed of large organic cations and organic or inorganic anions, have witnessed exponential growth of research interest in flexible sensory systems because of their inherent advantageous features, such as replicating the ionic transduction mechanism of biological systems, high ionic conductivity, non-volatility, excellent biocompatibility, low flammability, and good stability.55,56 A recent research study has shown that introducing ILs into a liquid crystal elastomer network with mesogenic anisotropy and polymer elasticity can facilitate significant enhancement of mechanically modulated ionic conductivity due to the formation of fast ion-conducting nanochannels guided by strain-induced smectic mesophases.57 In this context, the much-anticipated integration of ILs and CLCEs could give birth to emerging conductive CLCEs with ion transduction and photonic nanostructures that resemble those of biological skin, allowing promising applications in various fields such as multifunctional stretch and pressure sensing, visualized dynamic displays, and direct intelligent interactions with users. However, considering the easily disturbed self-assembly of the helical superstructure of CLCEs and the poor molecular compatibility between CLCEs and ILs, it remains a formidable challenge to develop conductive CLCEs with dynamic colour changing and electrical sensing capabilities.
In this work, we judiciously design and fabricate ionic conductive cholesteric liquid crystal elastomers (iCLCEs) by in situ thiol-acrylate Michael addition and free-radical photopolymerization of CLCE precursor on polymer ionic liquid networks (PILNs) modified with 3-(trimethoxysilyl)propyl methacrylate (TMSPMA), on the surface of which tough siloxane covalent chemical bonding is formed to ensure high interfacial toughness (Fig. 1). Benefiting from the synergistic effect of mechanochromism and ionic conductivity, the resulting iCLCEs simultaneously exhibit reversible and dynamic colour-changing abilities and electrical sensing functions in a wide range upon mechanical stretching. In particular, a capacitive sensor for pressure visualization was easily constructed by facilely stacking iCLCEs. As a proof-of-concept demonstration, an interactive system was constructed by integrating a customized capacitance acquisition and transmission unit, a display module, and the as-prepared iCLCE sensor, which could be used to control a virtual film and play video games. The strategy disclosed herein can offer new insights into the design and fabrication of bioinspired multifunctional photonic materials for advanced artificial skins, visualized interactive devices, and human–machine interaction.
Results and discussion
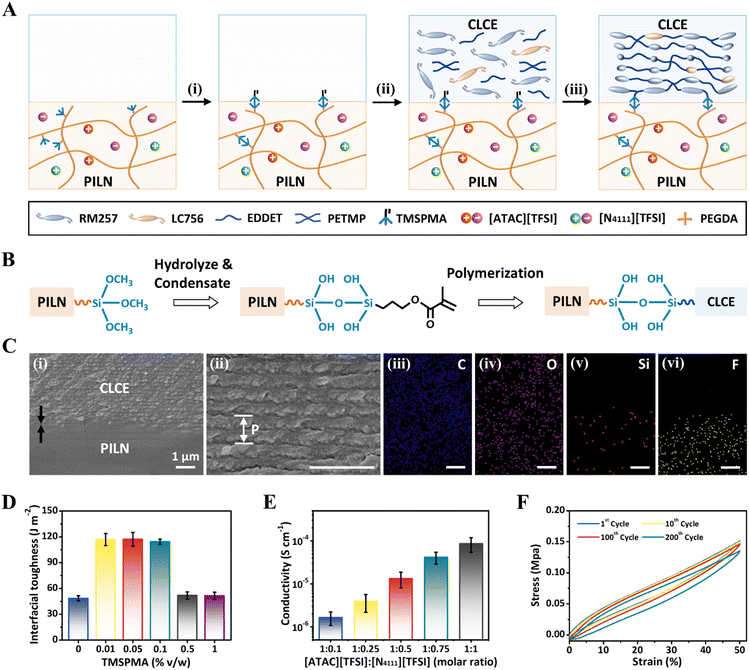
To fabricate ionic conductive CLCEs (iCLCEs), polymer ionic liquid networks (PILNs) were first prepared by one-step photopolymerization of polymerizable ionic liquid monomers ([ATAC][TFSI]), 3-(trimethoxysilyl)propyl methacrylate (TMSPMA) and poly(ethylene glycol)diacrylate (PEGDA) in an IL solvent ([N4111][TFSI]), after which PILNs were chemically functionalized with 3-(trimethoxysilyl)propyl methacrylate (TMSPMA) to introduce polymerizable methacrylate groups on the surface through the hydrolytic condensation reaction of silane (Fig. 2A). Next, the CLCE precursor in toluene solution, including diacrylate reactive mesogen RM257, chiral dopant LC756, chain extender 2,2′-(ethylenedioxy)diethanethiol (EDDET), crosslinker pentaerythritol tetrakis(3-mercaptopropionate) (PETMP), catalyst dipropylamine (DPA) and photoinitiator Irgacure 651, was poured onto the TMSPMA-functionalized PILNs to form cholesteric helical nanostructures by controlled evaporation-induced self-assembly of mesogens (Fig. S1 and S2, ESI†).46 After the Michael addition reaction and photopolymerization, the iCLCEs were finally obtained, in which tough siloxane covalent chemical bonding was formed between the CLCE layer and the PILN layer (Fig. 2B).58–60 The resulting iCLCEs exhibit superior reflection colours due to the well-aligned supramolecular helix of mesogens (Fig. S3, ESI†), which is further demonstrated by polarized optical microscopy (POM) (Fig. S4, ESI†). Moreover, multi-coloured iCLCEs with various patterns can be fabricated by pouring multiple CLCE precursors with different contents of chiral dopants onto TMSPMA-functionalized PILNs (Fig. S5, ESI†). The cross-sectional scanning electron microscopy (SEM) image together with energy-dispersive X-ray spectrometry (EDS) elemental mapping shows the double-layer structure of the iCLCE. A characteristic periodic lamellar nanostructure was observed in the upper part of the iCLCE, confirming the successful formation of the helical nanostructures (Fig. 2C). Particularly, the cross-sectional SEM image indicates a tightly bonded interface without cracks, obviously validating the effectiveness of the siloxane covalent chemical strategy to fabricate iCLCEs.To quantitatively characterize the interfacial toughness of iCLCEs, we carry out a standard 90-degree peeling test with a peeling rate of 50 mm min−1 (Fig. S6, ESI†). The effect of the TMSPMA content in PILNs on the interfacial toughness was first investigated by varying its concentration from 0% to 1% v/w (Table S1, ESI†). As shown in Fig. 2D, the interfacial toughness of the iCLCEs is initially weak without the introduction of TMSPMA in PILNs. When a small amount of TMSPMA (0.01–0.1% v/w) is added to the PILNs, the interfacial toughness of the iCLCEs increases significantly, which is about 2.4 times that of the sample without TMSPMA. This is because the polymerization between the introduced TMSPMA monomer and the CLCE precursor forms tough covalent bonding at the interface, thus improving the interfacial toughness of the iCLCEs. However, when the amount of TMSPMA is too high (more than 0.1% v/w), the cross-linking points of PILNs increase, resulting in the increase of Young's modulus of PILNs (Fig. S7, ESI†) and the reduction of the interfacial toughness of iCLCEs. It is worth noting that besides covalent bonds, there might be also other multiple interactions to enhance the interfacial strength, such as dipole–dipole, ion–dipole, cation–π, and van der Waals interactions (Fig. S8, ESI†).61–63 Additionally, the peeling process of a series of iCLCEs with [ATAC][TFSI]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [N4111][TFSI] molar ratios from 1
[N4111][TFSI] molar ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 1
0.1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was carried out to investigate the effect of the molar ratio of polymerizable ionic liquid monomers and IL solvent on the interfacial toughness (Table S2, ESI†). As shown in Fig. S9 (ESI†), with the molar ratio of [N4111][TFSI] increased from 1
1 was carried out to investigate the effect of the molar ratio of polymerizable ionic liquid monomers and IL solvent on the interfacial toughness (Table S2, ESI†). As shown in Fig. S9 (ESI†), with the molar ratio of [N4111][TFSI] increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 1
0.1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, the interfacial toughness of iCLCEs increases from 86 J m−2 to 198 J m−2, which can be attributed to the increase of the energy dissipation provided by the reversible ion–ion and ion–dipole interactions between [ATAC][TFSI] and [N4111][TFSI] during the peeling process.64 However, with a further increase in the molar ratio of [N4111][TFSI] (from 1
0.25, the interfacial toughness of iCLCEs increases from 86 J m−2 to 198 J m−2, which can be attributed to the increase of the energy dissipation provided by the reversible ion–ion and ion–dipole interactions between [ATAC][TFSI] and [N4111][TFSI] during the peeling process.64 However, with a further increase in the molar ratio of [N4111][TFSI] (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 to 1
0.25 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the interfacial toughness decreases from 198 J m−2 to 29 J m−2 due to the decrease of mechanical strength of PILNs (Fig. S10, ESI†), originated from the reduction of the ionic interaction and the entanglement of polymer chains in poly[ATAC][TFSI], thus forming soft ion domains with a low energy barrier.65 On the other hand, the ionic conductivity of iCLCEs was found to increase from 1.66 × 10−6 to 8.67 × 10−5 S cm−1 as the molar ratio of [N4111][TFSI] increased from 1
1), the interfacial toughness decreases from 198 J m−2 to 29 J m−2 due to the decrease of mechanical strength of PILNs (Fig. S10, ESI†), originated from the reduction of the ionic interaction and the entanglement of polymer chains in poly[ATAC][TFSI], thus forming soft ion domains with a low energy barrier.65 On the other hand, the ionic conductivity of iCLCEs was found to increase from 1.66 × 10−6 to 8.67 × 10−5 S cm−1 as the molar ratio of [N4111][TFSI] increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 1
0.1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2E). Therefore, considering the interfacial toughness and conductivity comprehensively, the [ATAC][TFSI]
1 (Fig. 2E). Therefore, considering the interfacial toughness and conductivity comprehensively, the [ATAC][TFSI]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [N4111][TFSI] molar ratio of 1
[N4111][TFSI] molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 was chosen as the optimal formulation for preparing iCLCEs in the following experiment.
0.75 was chosen as the optimal formulation for preparing iCLCEs in the following experiment.
The mechanical properties of iCLCEs with different thicknesses of PILNs were then investigated via stress–strain measurements (Fig. S11, ESI†). Compared with the pristine CLCE (200 μm), the Young's modulus of iCLCEs decreases with increasing thickness of the introduced PILNs.66 The iCLCE films are denoted as iCLCE-x in the mechanical tests, in which x indicates the thickness of PILNs (in microns). The iCLCE-200 showed better mechanical properties than the iCLCE-400, but the PILN layer of the iCLCEs is relatively uneven and prone to defects because small amounts of PILN precursors are unevenly distributed throughout the Teflon mold. Considering comprehensively, the PILN layer thickness of the iCLCE was fixed at 400 μm in the following experiment. Cyclic loading/unloading curves between 0 and 50% strain for 200 cycles were further obtained (Fig. 2F), revealing the excellent elasticity and mechanical repeatability of iCLCEs. Moreover, iCLCEs exhibit an excellent adhesion ability to various materials such as rubber, polytetrafluoroethylene (PTFE), polyethylene terephthalate (PET), copper, glass, and wood, which results from the synergy of chemical interactions (Fig. S12, ESI†) and energy dissipation during peeling.67 The corresponding adhesive toughness ranges from 14 J m−2 for rubber to a maximum of 209 J m−2 for wood (Fig. S13 and S14, ESI†). Furthermore, the strong adhesion ability is not affected by the distorted curvatures of the substrate (Fig. S15, ESI†), thus enabling the promising applications of iCLCEs as wearable visualized sensors and artificial skins in various scenarios.
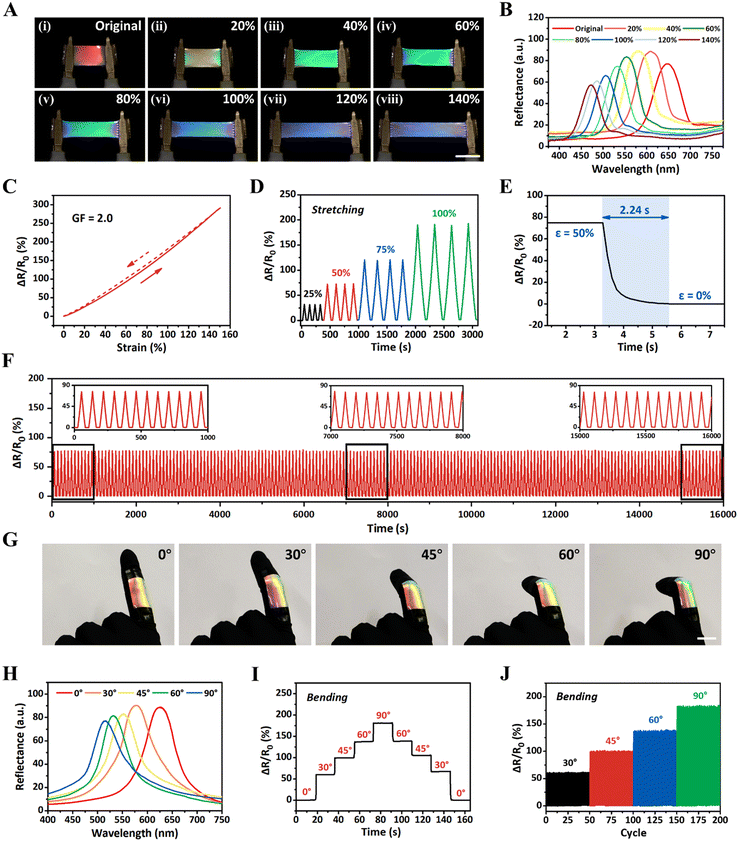
To examine the dynamic sensing performances of iCLCEs, the synchronous optical and electrical response under mechanical stretching was investigated in detail. Taking advantage of tuneable helical nanostructures, the iCLCEs exhibit remarkable dynamic mechanochromic behaviour at room temperature. As shown in Fig. 3A, the iCLCE exhibits sensitive and distinct colour changing within full visible spectral regions from red, green to blue when stretched from the initial state to 140% strain (Movie S1, ESI†). It was also observed that the structural colour of the iCLCE changed immediately when the external force was removed. The entire mechanochromic process is fully reversible with no evident delay between mechanical relaxation and colour recovery. Accordingly, the reflection wavelength continuously shifts from 647 to 471 nm and the mechanochromic sensitivity (Δλ/Δε, where λ is the reflection wavelength and ε is the strain) is 1.26 nm%−1 (Fig. 3B and Fig. S16, ESI†), which results from the shrinkage of thickness along the observed direction perpendicular to the stretched plane (Fig. S17, ESI†). Wide-angle X-ray diffraction (WAXD) was further performed to elucidate the mechanochromic mechanism of the iCLCE. The WAXD pattern transforms from a uniform diffraction ring to a pair of high-intensity arcs along the transverse direction, indicating that the iCLCE is oriented in the stretched state (Fig. S18, ESI†). Meanwhile, the relative resistance (ΔR/R0 = (R − R0)/R0, where R0 is the initial resistance and R is the test resistance) increases gradually and the corresponding gauge factor (GF = (ΔR/R0)/ε, where ε denotes the strain) is 2.0, implying good sensitivity to detect the mechanical strain (Fig. 3C). Repeatable and strain-dependent resistance changes of the iCLCEs under fixed strains of 25, 50, 75, and 100% reveal that the system can recognize mechanical deformation with different amplitudes (Fig. 3D). Besides, the response time of iCLCEs under self-recovery conditions can be observed in Fig. 3E. It is noteworthy that the iCLCEs can output frequency-independent electrical signals for strain at different frequencies (Fig. S19, ESI†). More importantly, the relative resistance change as well as the wavelength shift were measured to remain almost constant during continuous stretching for 200 cycles at a fixed strain of 50%, manifesting the superior durability of the iCLCEs for long-term operation (Fig. 3F and Fig. S20, ESI†). The above results indicate that the as-prepared iCLCEs are capable of sensitively responding to external tensile stimuli via distinct and simultaneous structural colours and ionic resistance changes in a large working range.
On the basis of the aforementioned superior optical and electrical dual-signal sensing properties, the iCLCEs could find important applications in diverse fields such as biomechanical monitoring. As a proof-of-concept demonstration, the iCLCE was attached to the index finger of a prosthetic hand and a gradual transition in structural colours from red to green and the corresponding blue shift in reflection wavelength were observed as the finger joint bending angles increased (Fig. 3G and H). It should be noted that the iCLCE displays excellent and stable reflectance even after repeated and continuous movements of the finger (Fig. S21, ESI†). At the same time, the relative resistance signal presents a stepwise increase, indicating precise recognition of the bending of the finger (Fig. 3I). In particular, the iCLCE provided stable and repeatable relative resistance signal changes when the finger was repeatedly bent at different finger joint bending angles (Fig. 3J). These results indicate that the iCLCEs exhibit interactive colour-changing performance and sensitive ionic conductivity during dynamic activities, and can be used as soft human-motion sensors for real-time optical and electrical dual-signal monitoring.
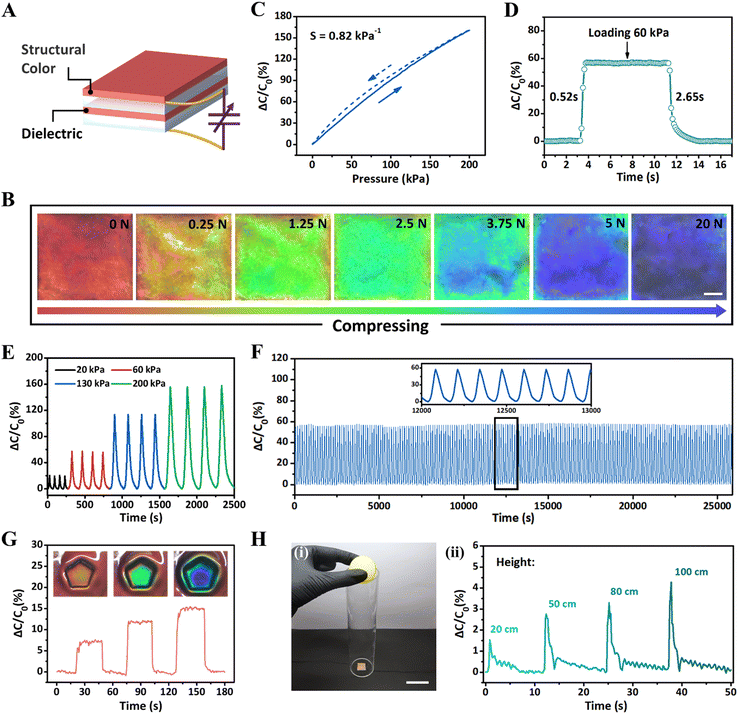
Interestingly, the iCLCEs could function as a visualized capacitive sensor to detect pressure changes via optical and electrical dual signals. As illustrated in Fig. 4A, the capacitive sensor was constructed by directly integrating two pieces of iCLCEs, in which a top layer of CLCE providing the structural colour, an intermediate layer of CLCE acting as the dielectric, and an ionic conductive layer of PILN are connected to electrodes and incorporated into an alternating current circuit.68,69 When a normal force is applied, the reflection wavelength of the capacitive sensor changes from 639 nm (red) to 458 nm (blue) (Fig. 4B and Fig. S22, ESI†). When the pressure is removed, the capacitive sensor quickly returns to its original colour and smooth state due to the inherent elasticity of the cross-linked polymer networks. The pressure-induced colour change of the capacitive sensor is significant and fully reversible within 0 to 800 kPa. The mechanochromic sensitivity (Δλ/ΔP, where λ is the reflection wavelength and P is the pressure) is 0.76 nm kPa−1 for 200 kPa and it changes to 0.05 nm kPa−1 once the pressure reaches 800 kPa, displaying superior colour-pressure responsiveness (Fig. S24 and S25, ESI†). Meanwhile, applying gradually increasing pressure to the capacitive sensor also induces a monotonous increase of relative capacitance change (ΔC/C0 = (C − C0)/C0, where C0 is the initial capacitance and C is the test capacitance) in the pressure range of 0–200 kPa. The capacitance sensitivity is defined as S = (ΔC/C0)/P, which is the slope of the measured capacitance–pressure curve (Fig. 4C). The fabricated capacitive sensor could operate over a wide pressure range with a sensitivity of 0.82 kPa−1. Upon loading pressure of 60 kPa, the response time was 0.52 s, and the recovery time was 2.65 s (Fig. 4D). In addition, a dynamic increase of the applied pressure also produces repeatable and reliable capacitance changes (Fig. 4E). Furthermore, in order to characterize its durability, the capacitive sensor was loaded/unloaded more than 200 cycles under the same pressure; as demonstrated in Fig. 4F, where the highly stable capacitance signals confirm the excellent performance in pressure sensing. As a proof-of-concept illustration, a pentagon-shaped pressure source was used to apply pressure to the capacitive sensor (Fig. 4G). It is observed that the capacitive sensor can sense external pressure through real-time capacitance changes as well as recognize the shape and location of pressure sources via displaying colour information (Fig. S23, ESI†). In addition, the capacitive sensor can also be used to detect the height of a bouncing ball in free-fall motion due to its high electrical sensitivity. As shown in Fig. 4H, dropping a bouncing ball onto the sensor from a fixed height (20, 50, 80, and 100 cm) generated various degrees of impact, which can be fully reflected by the capacitive signals in real time. The simple structure, visual display, and electrical responsiveness of the universal pressure sensor indicate its promising applications in the field of interactive sensing.
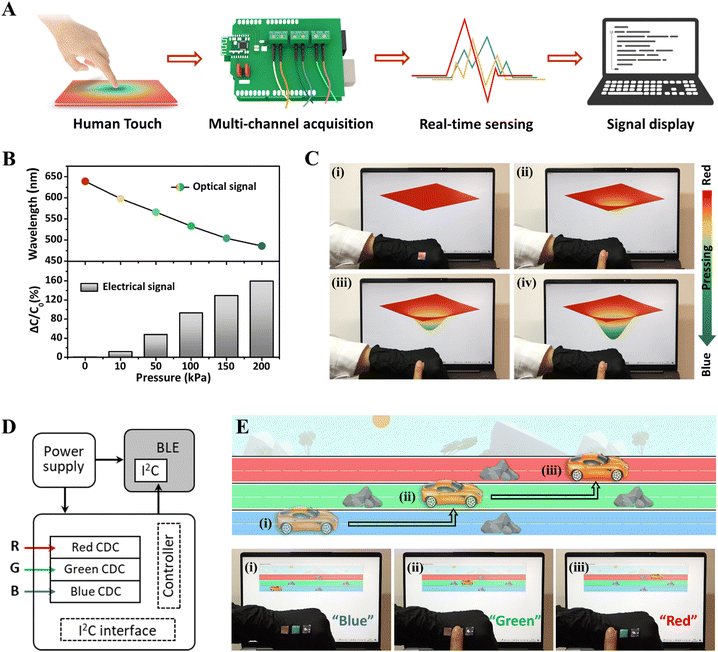
Human–machine interface, as the communication window between the user and the particular equipment or virtual world, is of great importance to achieve intuitive, effective, and seamless operations and execute tasks.70–72 Taking advantage of the real-time optical and electrical dual-signal sensing performance of as-prepared iCLCEs, an interactive system was developed by integrating the iCLCEs, a customized capacitance acquisition and transmission unit (Fig. S26, ESI†), and a display module. As depicted in Fig. 5A, the iCLCEs will generate both optical and capacitive signals when pressed. The capacitive signals can be detected by the multichannel data acquisition system and then transmitted to the host computer through Bluetooth in real time. Fig. 5B shows the reflection wavelength and the corresponding relative capacitance change of the iCLCEs as a function of pressure. It is evident that as the pressure increases, the structural colour gradually shifts from red to blue and the capacitance signal increases simultaneously. Based on this, a virtual film that can alter its colour and shape in response to changes in capacitive signals was developed as a display module for interactive control. As shown in Fig. 5C, the virtual film is initially red, when our finger gently presses the iCLCE, the capacitance signal increases slightly, and the virtual film produces a small deformation and changes from red to yellow. As our finger presses harder, the deformation of the virtual film increases and its colour changes to green and blue in turn. Additionally, the virtual film will retain a certain deformation and colour when our finger keeps pressing the iCLCE at a constant pressure. Once the pressure is removed, the virtual film will return to its original state. This real-time dynamic mechanochromic control of the virtual film is also shown in Movie S2 (ESI†). In addition to manipulating the film in virtual space through finger touch, the iCLCEs can be used to play interactive video games. The sensing system of a skin-like game controller was developed by integrating a RGB (red/green/blue) iCLCE array with a multi-channel data acquisition circuit (Fig. 5D). We attached red, green, and blue iCLCEs to the back of our hand, which correspond to three lanes in the customized obstacle racing game. As displayed in Fig. 5E and Movie S3 (ESI†), the car in the game is initially running in a blue lane and then encounters an obstacle. When our finger touches the green sensor, the car changes to the green lane to avoid obstacles, and similarly, the car changes to the red lane when pressing the red sensor. In this way, we can easily control the movement of the car in this video game on demand. The iCLCEs developed here could play an important role in bridging solid-state electronics and biological systems through sophisticated control of ions as signal carriers, which are of paramount significance in the fields of human–machine interaction, and other intelligent robotic systems.73–75
Conclusions
In summary, we report the judicious design and fabrication of novel optical and electrical dual-signal sensing iCLCEs with excellent mechanochromism and ionic conductivity. The multifunctional iCLCEs were synthesized via in situ thiol-acrylate Michael addition and UV-induced photopolymerization of the CLCE precursor on TMSPMA-functionalized PILNs, on the surface of which robust siloxane covalent chemical bonding was built to ensure high interfacial toughness between CLCEs and PILNs. Thanks to the brilliant structural colour of CLCEs, high ionic conductivity of PILNs, and tough chemical bonding across the interface, the resulting iCLCEs are found to exhibit dynamic colour-changing and electrical sensing functions in a wide range upon mechanical stretching (mechanochromic sensitivity: 1.26 nm%−1; gauge factor: 2.0), which can be used to visually monitor biomechanical movements in real time. It should be noted that a capacitive sensor based on iCLCEs displays optical and electrical dual-signal reporting characteristics for pressure sensing (mechanochromic sensitivity: 0.76 nm kPa−1; pressure sensitivity: 0.82 kPa−1), achieving intuitive visual localization of pressure distribution. As proof-of-concept illustrations, the iCLCEs have been applied to control a virtual film and play video games. The novelty of this work lies in three aspects: (1) a general method is put forward to fabricate iCLCEs that have high ionic conductivity through the integration of CLCEs with polymer ionic conductive networks; (2) the resulting iCLCEs can not only realize optical visualization, but also exhibit electrical sensing properties; (3) the capacitive sensor based on stacking iCLCEs shows excellent optical and electrical dual-signal sensing performances upon pressure, and has been employed in human-interactive systems for the first time. For future study, we can consider using 4D printing technology to develop more sophisticated structures of iCLCEs and achieve their multimodal sensing capabilities, thereby realizing the perception of multiple information such as temperature, humidity, surface texture, touch position, and force at one touch point. The research disclosed herein can provide valuable inspiration for the design and fabrication of bioinspired somatosensory materials and visualized interactive devices toward emerging applications in the areas of wearable electronics, human–machine interaction, and intelligent robotic systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51973155, 52173181, and 52203143), the Key Program of National Natural Science Foundation of China (No. 52130303), the Tianjin Science Fund for Distinguished Young Scholars (22JCJQJC00060), and the National Key R&D Program of China (2023YFB3812800 and 2022YFB3805702).References
- Y. Liu, K. He, G. Chen, W. R. Leow and X. Chen, Chem. Rev., 2017, 117, 12893–12941 CrossRef CAS.
- Y. Dou, Z. P. Wang, W. He, T. Jia, Z. Liu, P. Sun, K. Wen, E. Gao, X. Zhou, X. Hu, J. Li, S. Fang, D. Qian and Z. Liu, Nat. Commun., 2019, 10, 5293 CrossRef PubMed.
- Z. Liu, H. K. Bisoyi, Y. Huang, M. Wang, H. Yang and Q. Li, Angew. Chem., Int. Ed., 2022, 61, e202115755 CrossRef CAS.
- P. Xue, H. K. Bisoyi, Y. Chen, H. Zeng, J. Yang, X. Yang, P. Lv, X. Zhang, A. Priimagi, L. Wang, X. Xu and Q. Li, Angew. Chem., Int. Ed., 2021, 60, 3390–3396 CrossRef CAS.
- K. Yu, X. Ji, T. Yuan, Y. Cheng, J. Li, X. Hu, Z. Liu, X. Zhou and L. Fang, Adv. Mater., 2021, 33, 2104558 CrossRef CAS.
- Y. Hao, J. Gao, Y. Lv and J. Liu, Adv. Funct. Mater., 2022, 32, 2201942 CrossRef CAS.
- K. Sanderson, Nature, 2021, 591, 685–687 CrossRef CAS PubMed.
- S. Chen, H.-Z. Wang, R.-Q. Zhao, W. Rao and J. Liu, Matter, 2020, 2, 1446–1480 CrossRef.
- Y. Lee, J. W. Chung, G. H. Lee, H. Kang, J.-Y. Kim, C. Bae, H. Yoo, S. Jeong, H. Cho, S.-G. Kang, J. Y. Jung, D.-W. Lee, S. Gam, S. G. Hahm, Y. Kuzumoto, S. J. Kim, Z. Bao, Y. Hong, Y. Yun and S. Kim, Sci. Adv., 2021, 7, eabg9180 CrossRef CAS.
- Y. Chang, L. Wang, R. Li, Z. Zhang, Q. Wang, J. Yang, C. F. Guo and T. Pan, Adv. Mater., 2021, 33, 2003464 CrossRef CAS.
- C. C. Kim, H. H. Lee, K. H. Oh and J. Y. Sun, Science, 2016, 353, 682–687 CrossRef CAS.
- J. Hu, Z. Nie, M. Wang, Z. Liu, S. Huang and H. Yang, Angew. Chem., Int. Ed., 2023, 62, e202218227 CrossRef CAS.
- Z. Z. Nie, B. Zuo, M. Wang, S. Huang, X. M. Chen, Z. Y. Liu and H. Yang, Nat. Commun., 2021, 12, 2334 CrossRef CAS PubMed.
- S. Li, R. Zhang, G. Zhang, L. Shuai, W. Chang, X. Hu, M. Zou, X. Zhou, B. An, D. Qian and Z. Liu, Nat. Commun., 2022, 13, 1331 CrossRef CAS PubMed.
- P. Lv, X. Yang, H. K. Bisoyi, H. Zeng, X. Zhang, Y. Chen, P. Xue, S. Shi, A. Priimagi, L. Wang, W. Feng and Q. Li, Mater. Horiz., 2021, 8, 2475–2484 RSC.
- Y. Chen, J. Yang, X. Zhang, Y. Feng, H. Zeng, L. Wang and W. Feng, Mater. Horiz., 2021, 8, 728–757 RSC.
- J. A. Lv, Y. Liu, J. Wei, E. Chen, L. Qin and Y. Yu, Nature, 2016, 537, 179–184 CrossRef CAS PubMed.
- X. Pang, J. A. Lv, C. Zhu, L. Qin and Y. Yu, Adv. Mater., 2019, 31, 1904224 CrossRef CAS.
- X. Yang, Y. Chen, X. Zhang, P. Xue, P. Lv, Y. Yang, L. Wang and W. Feng, Nano Today, 2022, 43, 101419 CrossRef CAS.
- M. Yang, Y. Xu, X. Zhang, H. K. Bisoyi, P. Xue, Y. Yang, X. Yang, C. Valenzuela, Y. Chen, L. Wang, W. Feng and Q. Li, Adv. Funct. Mater., 2022, 32, 2201884 CrossRef CAS.
- X. Yang, C. Valenzuela, X. Zhang, Y. Chen, Y. Yang, L. Wang and W. Feng, Matter, 2023, 6, 1278–1294 CrossRef CAS.
- X. Yu, Z. Xie, Y. Yu, J. Lee, A. Vazquez-Guardado, H. Luan, J. Ruban, X. Ning, A. Akhtar, D. Li, B. Ji, Y. Liu, R. Sun, J. Cao, Q. Huo, Y. Zhong, C. Lee, S. Kim, P. Gutruf, C. Zhang, Y. Xue, Q. Guo, A. Chempakasseril, P. Tian, W. Lu, J. Jeong, Y. Yu, J. Cornman, C. Tan, B. Kim, K. Lee, X. Feng, Y. Huang and J. A. Rogers, Nature, 2019, 575, 473–479 CrossRef CAS.
- C. Xu, G. T. Stiubianu and A. A. Gorodetsky, Science, 2018, 359, 1495–1500 CrossRef CAS PubMed.
- X. Zhang, Y. Yang, P. Xue, C. Valenzuela, Y. Chen, X. Yang, L. Wang and W. Feng, Angew. Chem., Int. Ed., 2022, 61, e202211030 CrossRef CAS.
- X. Zhang, Y. Xu, C. Valenzuela, X. Zhang, L. Wang, W. Feng and Q. Li, Light: Sci. Appl., 2022, 11, 223 CrossRef CAS PubMed.
- P. Xue, Y. Chen, Y. Xu, C. Valenzuela, X. Zhang, H. K. Bisoyi, X. Yang, L. Wang, X. Xu and Q. Li, Nano-Micro Lett., 2022, 15, 1 Search PubMed.
- J. Yang, X. Zhang, X. Zhang, L. Wang, W. Feng and Q. Li, Adv. Mater., 2021, 33, 2004754 CrossRef CAS.
- P. Lv, X. Lu, L. Wang and W. Feng, Adv. Funct. Mater., 2021, 31, 2104991 CrossRef CAS.
- J. Teyssier, S. V. Saenko, D. van der Marel and M. C. Milinkovitch, Nat. Commun., 2015, 6, 6368 CrossRef CAS PubMed.
- Y. Yang, X. Zhang, Y. Chen, X. Yang, J. Ma, J. Wang, L. Wang and W. Feng, ACS Appl. Mater. Interfaces, 2021, 13, 41102–41111 CrossRef CAS.
- P. Xue, C. Valenzuela, S. S. Ma, X. Zhang, J. Z. Ma, Y. H. Chen, X. H. Xu and L. Wang, Adv. Funct. Mater., 2023, 2214867 CrossRef CAS.
- Y. Sun, Y. Wang, Y. Liu, S. Wu, S. Zhang and W. Niu, Adv. Funct. Mater., 2022, 32, 2204467 CrossRef CAS.
- L. Wang, A. M. Urbas and Q. Li, Adv. Mater., 2020, 32, 1801335 CrossRef CAS PubMed.
- G. Isapour and M. Lattuada, Adv. Mater., 2018, 30, 1707069 CrossRef.
- Q. Lyu, M. Li, L. Zhang and J. Zhu, Macromol. Rapid Commun., 2022, 43, 2100867 CrossRef CAS.
- L. Peng, L. Hou and P. Wu, Adv. Mater., 2023, 35, 2211342 CrossRef CAS.
- Y. Wang, X. Cao, J. Cheng, B. Yao, Y. Zhao, S. Wu, B. Ju, S. Zhang, X. He and W. Niu, ACS Nano, 2021, 15, 3509–3521 CrossRef CAS.
- X. Li, Y. Yang, C. Valenzuela, X. Zhang, P. Xue, Y. Liu, C. Liu and L. Wang, ACS Nano, 2023, 17, 12829–12841 CrossRef CAS.
- Y. Lee, J. W. Chung, G. H. Lee, H. Kang, J. Y. Kim, C. Bae, H. Yoo, S. Jeong, H. Cho, S. G. Kang, J. Y. Jung, D. W. Lee, S. Gam, S. G. Hahm, Y. Kuzumoto, S. J. J. C. Yang, J. Mun, S. Y. Kwon, S. Park, Z. Bao and S. Park, Adv. Mater., 2019, 31, 1904765 CrossRef.
- H. H. Chou, A. Nguyen, A. Chortos, J. W. To, C. Lu, J. Mei, T. Kurosawa, W. G. Bae, J. B. Tok and Z. Bao, Nat. Commun., 2015, 6, 8011 CrossRef CAS.
- Y. Wang, Y. Yu, J. Guo, Z. Zhang, X. Zhang and Y. Zhao, Adv. Funct. Mater., 2020, 30, 2000151 CrossRef CAS.
- Z. Zhang, Z. Chen, Y. Wang and Y. Zhao, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 18310–18316 CrossRef CAS PubMed.
- H. Zhang, J. Guo, Y. Wang, L. Sun and Y. Zhao, Adv. Sci., 2021, 8, 2102156 CrossRef CAS.
- G. Lee, G. Y. Bae, J. H. Son, S. Lee, S. W. Kim, D. Kim, S. G. Lee and K. Cho, Adv. Sci., 2020, 7, 2001184 CrossRef CAS.
- C. Yang and Z. Suo, Nat. Rev. Mater., 2018, 3, 125–142 CrossRef CAS.
- J. Ma, Y. Yang, C. Valenzuela, X. Zhang, L. Wang and W. Feng, Angew. Chem., Int. Ed., 2022, 61, e202116219 CrossRef CAS PubMed.
- T. J. White and D. J. Broer, Nat. Mater., 2015, 14, 1087–1098 CrossRef CAS PubMed.
- K. M. Herbert, H. E. Fowler, J. M. McCracken, K. R. Schlafmann, J. A. Koch and T. J. White, Nat. Rev. Mater., 2021, 7, 23–38 CrossRef.
- P. Zhang, L. T. de Haan, M. G. Debije and A. Schenning, Light: Sci. Appl., 2022, 11, 248 CrossRef CAS PubMed.
- C. Valenzuela, Y. Chen, L. Wang and W. Feng, Chem. – Eur. J., 2022, 28, e202201957 CrossRef CAS.
- Z. Guan, L. Wang and J. Bae, Mater. Horiz., 2022, 9, 1825–1849 RSC.
- S. U. Kim, Y. J. Lee, J. Liu, D. S. Kim, H. Wang and S. Yang, Nat. Mater., 2022, 21, 41–46 CrossRef CAS.
- Y. Geng, R. Kizhakidathazhath and J. P. F. Lagerwall, Nat. Mater., 2022, 21, 1441–1447 CrossRef CAS.
- H. Kim, J. Choi, K. K. Kim, P. Won, S. Hong and S. H. Ko, Nat. Commun., 2021, 12, 4658 CrossRef CAS.
- S. Y. Zhang, Q. Zhuang, M. Zhang, H. Wang, Z. Gao, J. K. Sun and J. Yuan, Chem. Soc. Rev., 2020, 49, 1726–1755 RSC.
- M. Wang, P. Zhang, M. Shamsi, J. L. Thelen, W. Qian, V. K. Truong, J. Ma, J. Hu and M. D. Dickey, Nat. Mater., 2022, 21, 359–365 CrossRef CAS PubMed.
- M. Yao, B. Wu, X. Feng, S. Sun and P. Wu, Adv. Mater., 2021, 33, 2103755 CrossRef CAS PubMed.
- Q. Liu, G. Nian, C. Yang, S. Qu and Z. Suo, Nat. Commun., 2018, 9, 846 CrossRef.
- J. Yang, R. Bai, B. Chen and Z. Suo, Adv. Funct. Mater., 2019, 30, 1901693 CrossRef.
- H. Yuk, T. Zhang, S. Lin, G. A. Parada and X. Zhao, Nat. Mater., 2016, 15, 190–196 CrossRef CAS.
- X. Pei, H. Zhang, Y. Zhou, L. Zhou and J. Fu, Mater. Horiz., 2020, 7, 1872–1882 RSC.
- Y. Cao, Y. J. Tan, S. Li, W. W. Lee, H. Guo, Y. Cai, C. Wang and B. C. K. Tee, Nat. Electron., 2019, 2, 75–82 CrossRef.
- H. Fan, J. Wang, Z. Tao, J. Huang, P. Rao, T. Kurokawa and J. P. Gong, Nat. Commun., 2019, 10, 5127 CrossRef.
- Z. Yu and P. Wu, Mater. Horiz., 2021, 8, 2057–2064 RSC.
- Z. Yu and P. Wu, Adv. Mater., 2021, 33, 2008479 CrossRef CAS.
- K. Hisano, S. Kimura, K. Ku, T. Shigeyama, N. Akamatsu, A. Shishido and O. Tsutsumi, Adv. Funct. Mater., 2021, 31, 2104702 CrossRef CAS.
- W. Zhang, B. Wu, S. Sun and P. Wu, Nat. Commun., 2021, 12, 4082 CrossRef CAS.
- Y. Huang, X. Fan, S. C. Chen and N. Zhao, Adv. Funct. Mater., 2019, 29, 1808509 CrossRef.
- J. Y. Sun, C. Keplinger, G. M. Whitesides and Z. Suo, Adv. Mater., 2014, 26, 7608–7614 CrossRef CAS PubMed.
- G. Gao, F. Yang, F. Zhou, J. He, W. Lu, P. Xiao, H. Yan, C. Pan, T. Chen and Z. L. Wang, Adv. Mater., 2020, 32, 2004290 CrossRef CAS.
- M. Zhu, Z. Sun, Z. Zhang, Q. Shi, T. He, H. Liu, T. Chen and C. Lee, Sci. Adv., 2020, 6, eaaz8693 CrossRef CAS PubMed.
- J. Park, D. H. Kang, H. Chae, S. K. Ghosh, C. Jeong, Y. Park, S. Cho, Y. Lee, J. Kim, Y. Ko, J. J. Kim and H. Ko, Sci. Adv., 2022, 8, eabj9220 CrossRef PubMed.
- W. Yang, W. Gong, W. Gu, Z. Liu, C. Hou, Y. Li, Q. Zhang and H. Wang, Adv. Mater., 2021, 33, 2104681 CrossRef CAS PubMed.
- Z. Sun, M. Zhu, X. Shan and C. Lee, Nat. Commun., 2022, 13, 5224 CrossRef CAS.
- B. Hou, L. Yi, C. Li, H. Zhao, R. Zhang, B. Zhou and X. Liu, Nat. Electron., 2022, 5, 682–693 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01386c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |