Fabrication of polymeric microspheres for biomedical applications
Xuebing
Li†
ac,
Luohuizi
Li†
a,
Dehui
Wang
 a,
Jun
Zhang
d,
Kangfeng
Yi
d,
Yucai
Su
d,
Jing
Luo
*a,
Xu
Deng
a,
Jun
Zhang
d,
Kangfeng
Yi
d,
Yucai
Su
d,
Jing
Luo
*a,
Xu
Deng
 *ab and
Fei
Deng
*ef
*ab and
Fei
Deng
*ef
aInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, 611731, P. R. China. E-mail: dengxu@uestc.edu.cn; luojing115@mails.ucas.ac.cn
bShenzhen Institute for Advanced Study, University of Electronic Science and Technology of China, Shenzhen, 518110, P. R. China
cState Key Laboratory of Military Stomatology & National Clinical Research Center for Oral Diseases, Department of Oral and Maxillofacial Surgery, School of Stomatology, The Fourth Military Medical University, Xi’an, 710032, P. R. China
dShandong Pharmaceutical Glass Co. Ltd, Zibo, 256100, P. R. China
eDepartment of Nephrology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu 610054, P. R. China
fDepartment of Nephrology, Sichuan Provincial People's Hospital Jinniu Hospital, Chengdu Jinniu District People's Hospital, Chengdu 610054, P. R. China. E-mail: dengfei@med.uestc.edu.cn
First published on 19th March 2024
Abstract
Polymeric microspheres (PMs) have attracted great attention in the field of biomedicine in the last several decades due to their small particle size, special functionalities shown on the surface and high surface-to-volume ratio. However, how to fabricate PMs which can meet the clinical needs and transform laboratory achievements to industrial scale-up still remains a challenge. Therefore, advanced fabrication technologies are pursued. In this review, we summarize the technologies used to fabricate PMs, including emulsion-based methods, microfluidics, spray drying, coacervation, supercritical fluid and superhydrophobic surface-mediated method and their advantages and disadvantages. We also review the different structures, properties and functions of the PMs and their applications in the fields of drug delivery, cell encapsulation and expansion, scaffolds in tissue engineering, transcatheter arterial embolization and artificial cells. Moreover, we discuss existing challenges and future perspectives for advancing fabrication technologies and biomedical applications of PMs.
Wider impactPolymeric microspheres, synthesized from or composed of polymers, are micro-sized particles that possess intriguing properties and functions owing to their small size, high surface-to-volume ratio and tunable characteristics. Consequently, they have found diverse and significant applications in various fields. Particularly in the biomedical field, their distinct structures and properties make them highly appealing. Enhancing the yield and stability of PMs has emerged as an urgent challenge to address. Additionally, scaling up the production of microspheres with specialized structures and functions remains problematic. In this comprehensive review, we present a systematic summary of fabrication technologies for PMs along with different structural variations observed in these microspheres. Furthermore, we highlight typical applications as well as recent advancements in various biomedical areas. We also discuss strategies for designing PMs with specific configurations and physical/chemical properties that meet clinical requirements while summarizing approaches to select and improve fabrication methods accordingly. Lastly, we conclude by outlining future directions towards translating laboratory achievements into industrial-scale production—an essential step for bridging the gap between fundamental science research and clinical applications. The insights provided within this review will enable researchers to gain a comprehensive understanding of recent advances in PM-related areas while facilitating progress towards materials science development. |
1. Introduction
Over the past decades, a large variety of novel materials have been discovered with attractive structures. Among them, materials on the microscale possess fascinating and special functions and properties, like superhydrophobicity,1 tough adhesion,2 and excellent ionic and electronic transport efficiency.3 In recent years, polymeric microspheres (PMs) with sizes in the micron range (1–1000 μm) have attracted great attention due to the characteristics of small particle size, special functionalities shown on the surface and high surface-to-volume ratio, which form a solid–liquid interface with the surrounding liquid, and the interface properties affect the dispersion of PMs. The advantages make them serve as micro-reactors, micro-separators, micro-transporters and microstructural units, which have been widely applied in diverse and important areas such as cosmetics, food industry and biomedicine.4,5There are a variety of PMs. For example, microspheres of larger size (more than tens of microns) are also called microbeads; microspheres capable of loading drugs or cells are also called microcarriers; microspheres which can absorb large amounts of water or solvents are called microgels, and those with core–shell structures are called microcapsules, polymersomes or polymer vesicles. There are a great diversity of materials to fabricate PMs, such as natural materials including carbohydrates (starch,6 chitosan,7 alginate,8 cellulose,9,10etc.) and proteins (gelatin,11,12 collagen (COL),13 albumin,14etc.); synthetic polymers such as degradable polymers (poly(lactide) (PLA),15 poly(lactide-co-glycolide) (PLGA),16etc.), and block copolymers (PLGA-PEG,17 polybutadiene-b-polyethyleneoxide (PBd-b-PEO18)). These materials have good biocompatibility and are attractive in the biomedical field. For example, the degradable PMs are suitable to be applied inside the human body as minimally invasive therapy, which can be excreted by metabolism. The typical and widely applied degradable PMs are PLA and PLGA microspheres which have already been commercially available as drug delivery systems for injection.19
PMs can be fabricated by various strategies in a controllable manner due to the rapid progress of manufacturing technology. The classical methods have long been utilized to fabricate PMs, and it is challenging to produce them with desired properties and uniform size, like emulsion solvent extraction, spray-drying, and coacervation, using these methods. To overcome these manufacturing-related drawbacks, other developing fabrication methods, including microfluidic technology, supercritical fluid, membrane emulsification and superhydrophobic surface-mediated methods, have recently been explored. They allow precise control of the size, composition, configuration and surface properties of the PMs. A variety of PMs with special fascinating structures can be generated, such as hollow, porous, core–shell, Janus microspheres and microspheres with complex structures, which may impart PMs with more advanced functions. In addition, these manufacturing technologies will promote the combination of bioactive functional components within the PMs, such as drugs and cells, which can achieve control over the spatial and temporal distribution of these components that will exhibit unique and special functions.
In this review, we provide a systematic review of fabrication technologies, different structures of PMs and the most recent progress in biomedical applications, as shown in Fig. 1. This review is divided into four sections. In the first section, we give a detailed introduction of the different PM fabrication technologies and their advantages and disadvantages. In the second section, we summarize the different structures and characteristics of the PMs including simple, hollow, porous, core–shell, Janus and complex structures. In the third section, we present the latest applications of PMs in several biomedical fields, including serving as microcarriers for drugs and cells; scaffolds in tissue engineering; transcatheter arterial embolization and artificial cells. In the last section, we give a general conclusion of the review and discuss the present challenges and future opportunities of the PM fabrication technologies and biomedical applications.
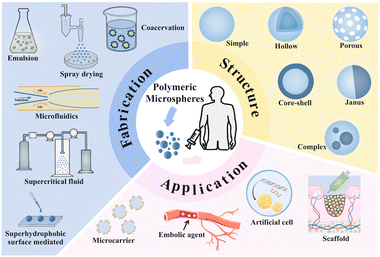 | ||
| Fig. 1 Conceptual image of the fabrication technologies, various structures and biomedical applications of the polymeric microspheres. | ||
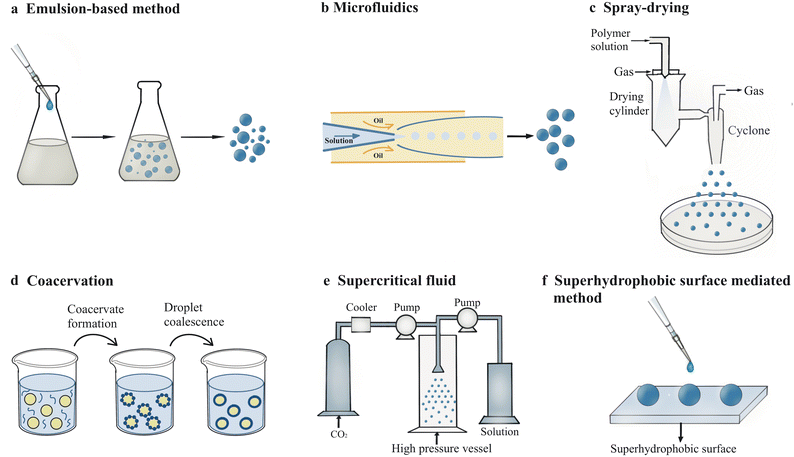
2. Fabrication of microspheres
There are a variety of technologies suitable for fabrication of microspheres with unique properties, including emulsion-based methods, microfluidics, spray-drying, coacervation, supercritical fluid and superhydrophobic surface-mediated method, extrusion, photolithographic methods, etc. Here, six of the above methods are mainly introduced in this review (Fig. 2). The process, mechanism, advantages and disadvantages of these methods will be elucidated in this section and summarized in Table 1.| Methods | Physicochemical principles | Main materials | Advantages | Disadvantages |
|---|---|---|---|---|
| Emulsion-based method | Two immiscible liquids form droplets in the existence of surfactant. The formed droplets go through nucleation, growth, coalescence and agglomeration processes to form particles of the solid phase. | PEG, PS-b-PEO, PLA-b-PEG, PSDVB⊃PAA, SA, EPL, PLA, PLLA | A variety of forms (emulsion solvent evaporation, emulsion interfacial polymerization, other several methods), simple procedures and low costs | Lack of biocompatibility, difficulty in regulation of microsphere morphology, time-consuming |
| Microfluidics | Immiscible fluids form single or double emulsions through injection from a precisely designed microchannel. | Alginate, agarose, gelatin, PLGA, PLA, PEG, PNIPAm | Good polydispersity, improved biocompatibility, low solvent and sample consumption | Increasing cost owing to the exquisite device, limited application at large scale |
| Spray-drying | Liquid feed (e.g., solution, suspension or paste) converting into dry solid phase (e.g., powder) under a hot drying medium in a chamber | Mannitol, pectin, SF, chitosan, propolis, PLGA, PLA, PVP | High production rate, reproducible ability, high encapsulation efficiency and no excessive use of solvents | Large polydispersity in size, high variability in structure, high energy consumption |
| Coacervation | Phase separation process induced by change of media environment | Chitosan, alginate, gelatin, PLGA, PLA, PAA, poly(4-styrenesulfonic acid, sodium salt) (PSS) | Low cost, good reproducibility and high loading efficiency | Easy agglomeration, residual solvents, long process time for removing solvents |
| Supercritical fluid | A substance containing one homogeneous phase over its critical temperature and pressure where the boundary between liquid and vapor vanishes within the SCF area | PLLA, PLA, PCL, PVA, PMMA, poly(glycerol sebacate) (PGS), chitosan | Low organic solvent residue, mild process conditions, enhanced solubility and bioavailability of poorly water soluble drugs | Complex process, limited solvation power, non-uniform mixing |
| Superhydrophobic surface-mediated method | The mixed solution of drugs and monomers is placed on the SHS to maintain multiple spherical droplets. Then microspheres encapsulated with drugs are obtained after the cross-linking of the monomers. | Agarose, alginate, chitosan, PLGA, PLA, | High encapsulation efficiency, high loading of cargo and controlled release rate | Limited production, poor reproducibility, possible contamination of the SHS |
2.1. Emulsion-based method
Emulsion is one of the most common methods which uses two immiscible liquids to form droplets in the existence of a surfactant. The formed droplets go through nucleation, growth, coalescence and agglomeration processes to form particles of the solid phase.20 Depending on which is the dispersed phase and which is the continuous phase, there are two types of emulsions: oil-in-water (O/W) and water-in-oil (W/O) emulsions. Systems with an emulsion as a dispersed phase are called multiple emulsions, such as water-in-oil-in-water (W/O/W) emulsions. With the development of technology, emulsification method has gradually formed a variety of forms, including emulsion solvent evaporation, emulsion interfacial polymerization, reverse phase emulsion, membrane emulsification, Pickering emulsification, emulsion-condensation method, emulsion-crosslinking method and several other methods.21Emulsion droplets generated through simple mechanical interaction are too unstable to agglomerate into droplets with increasing size. To improve the stability of the emulsion, Pickering emulsion utilizing solid particles to prevent coalescence of particles was first reported by Ramsden in 1903.22 With the increasing need to encapsulate different and even incompatible molecules, double emulsions were first discovered in 1925 by William Seifriz23 and later reported to act as a drug carrier in the 1960s due to the versatile and unstable structure.24 By using double emulsions as a template, the emulsion solvent evaporation method was first employed to produce microparticles initially with standard experiment procedure in 1987.25 In order to obtain emulsion droplets with narrow size distribution, membrane emulsification method was reported to produce monodisperse emulsion droplets through a porous glass membrane in 1988.26 Later in 1995, polymer microspheres, microcapsules and microbeads with good monodispersity were fabricated using such technique.27 Recently, emulsion interfacial polymerization emerged as a novel technique for the production of microparticles with anisotropic properties through interfacial instability.28
Although the simple procedure and low cost make the emulsion-based method widely employed for fabrication of microspheres at a large scale, the existing shortcomings cannot be ignored. For example, the lack of biocompatibility due to the oil phase and the difficulty in regulation of microsphere morphology restrict the long-term development of this method. Thus, progress has been made which will be elucidated in the following part.
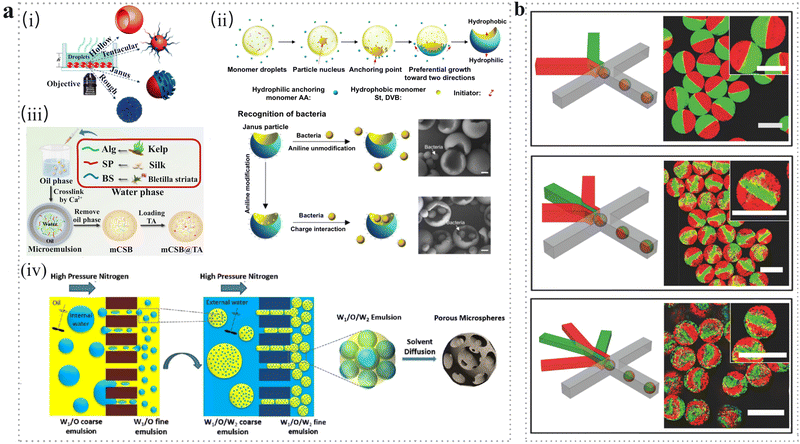 | ||
| Fig. 3 (a) Emulsion-based methods. (i) Microspheres with various morphologies obtained after solvent evaporation.35 Reproduced with permission from ref. 35. Copyright 2019 American Chemical Society. (ii) Schematic illustration of Janus particle preparation. The Janus particles with concave surfaces functionalized by aniline were utilized to selectively recognize and capture live bacteria through electrostatic interactions.28 Scale bar: 500 nm. Reproduced with permission from ref. 28. Copyright 2017 American Association for the Advancement of Science. (iii) Schematic illustration of mCSB@TA microspheres fabricated through reverse phase emulsion method.39 Reproduced with permission from ref. 39. Copyright 2022 Elsevier. (iv) Membrane emulsification method. Schematic illustration of preparation of porous microspheres by two-step premix membrane emulsification, which includes primary emulsification, secondary emulsification and emulsion solidification.46 Reproduced with permission from ref. 46. Copyright 2019 Elsevier. (b) Microfluidic method. Schematic illustration of the microfluidic platforms with different numbers of injection channels used to fabricate multicompartment microgel. Scale bars: 100 μm.64 Reproduced with permission from ref. 64. Copyright 2018 John Wiley and Sons. | ||
Although such direct ME technology exhibits plenty of advantages, it also suffers from limitations such as low emulsification rate and difficulty in producing particles with submicron size, especially for biomedical applications for cellular uptake.44 Thus, an advanced ME technology called premix membrane emulsification was developed to fabricate microspheres with micron size.46 The premix membrane emulsification process contains two steps (Fig. 3a(iv)). First, under high nitrogen pressure, the coarse emulsion with a larger size was extruded through the pores of the porous membrane to obtain uniform droplets. Then, the formed droplets were solidified into porous microspheres with sizes ranging from 100 nm to 5 μm.
2.2. Microfluidics
Microfluidic technologies have attracted extensive attention due to their advantages of good polydispersity, improved biocompatibility, and well-tailored size, structure, morphology as well as multi-functions.47 The exquisite device used in microfluidic technology may increase the cost during large-scale production; therefore, this technology is broadly adopted in laboratory nowadays.Date back to the 1950s, a compartmentalized continuous flow technique was reported for analytical chemistry experiments in fluid pipelines by Skegges and Hochstrasser.48 Droplet microfluidics technology was first proposed by Thorsen et al.49 and then the related research results about microparticles with excellent biocompatibility have been reported by Nie et al. in the medical field.50 After that, the droplet microfluidics technique was widely adopted to encapsulate, incubate, and manipulate individual cells.51 Generally, double emulsions are synthesized in a two-step process. However, it is worth mentioning that in 2005, the microfluidic technique was employed to generate double emulsions using a microcapillary device in one step.52 On this basis, microparticles with Janus structure were prepared in 2009.53
Microfluidics technologies, including droplet microfluidics and flow lithography microfluidics, are employed to achieve precise manipulation of the microflow in microchannels with controlled fabrication of monodisperse microspheres. Among them, the droplet microfluidics is more widely employed to fabricate PMs for biomedical applications. Combining droplet-based microfluidics with droplet-to-particle technology provides a promising method to prepare PMs with narrow size distribution and uniform particle size. Usually, the approaches for making droplets relate to the geometry of the devices, such as commonly used T-junctions,54 co-flowing55 and flow-focusing devices.56–58 In this technology, single or multiple emulsions are produced to act as templates for the formation of microspheres, microcapsules and microgels with tunable sizes. Generally, microfluidic methods for producing single emulsions contain two immiscible liquid phases (the dispersed phase and the continuous phase) through injection from a precisely designed microchannel, followed by the droplets being sheared off at the junction where the immiscible phases meet.59,60
Based on the variance of the device structures, multiple emulsions manipulated by microfluidics technology can function as templates for preparing microspheres with complex structures, such as core–shell microspheres,61 multi-core microcapsules.62 During this process, well-connected microchannels with two different geometries and opposite wettability were employed to produce double emulsions by the formation of an internal droplet in the first geometry and the external droplet in the second.63 For example, Ca-alginate microgels with bi-, tri- and quadri-compartments were fabricated by exploiting microfluidic platforms with different numbers of injection channels (Fig. 3b).64 In addition, the process parameters (such as phase flow rates and fluid properties) have a great impact on the structure, size and morphology of the microspheres. For example, the increasing size was determined by the rising of dispersed phase flow rate, but it is the opposite of the decreased continuous phase flow rate.64 Moreover, the thickness can also be regulated by the flow rate in a double emulsion system. For example, multi-compartment PLGA microcapsules were fabricated through a droplet microfluidic device.65 As the continuous phase flow rate gradually increased, the thicker shell of the microcapsules was obtained.
2.3. Spray-drying
Atomization process involving spray-drying, electrospraying and freeze-drying has been employed to produce PMs with the size ranging from 5 to 1000 μm. Spray-drying is a popular method that has been widely utilized to produce PMs. The process of spray-drying (e.g., solution, suspension or paste) converts a liquid feed into a dry solid phase (e.g., powder) under a hot drying medium in a chamber. The plenty of advantages of the spray-drying method such as high production rate, reproducible ability, high encapsulation efficiency, and no excessive use of solvents have rendered itself attractive for producing simple or core–shell microspheres on an industrial scale. However, the high energy consumption in the conversion process leads to high costs.5 On the other hand, the complicated heat and mass transfer during the spray-drying process can affect the physical and chemical properties of the microspheres.In the 1990s, the spray-drying method was used for microencapsulation of foods, pharmaceuticals, “active” material and other substances.66 The influence of various technological parameters on the structure and release behavior of the microcapsules was systematically investigated after decades, rendering the spray-drying technique as a promising candidate for microcapsule synthesis in drug delivery systems.67 Recently, the nano spray-drying technique has been adopted for manufacturing nanoparticles at the lab scale. On the other hand, the equipment and processing procedure of spray-drying have been improved for high efficiency and high production in manufacturing microparticles.68
The spray-drying method has been applied to produce PMs loaded with various kinds of drugs or bioactive molecules for the treatment of stomach,69 lung,70 and nasal diseases71 and inhibition of cancer.72 Generally, the ratio and interaction of polymers and drugs should be considered because they can affect the morphology, size, and release behaviors. It is reported that inhalable microspheres were prepared based on nafamostat mesylate (NFM), lecithin and mannitol to suppress the penetration of the virus into the cells.73 The higher ratio of mannitol led to improved yield and smaller particle sizes, which was the opposite situation for that of lecithin. In another study, pectin microspheres were produced through spray-drying and the processing parameters were investigated.74 It was demonstrated that the input temperature, aspirator rate, feed flow rate, polymer concentration and polymer feed weight had a great impact on the yield and size of pectin microspheres. For example, a high input temperature at a high feed flow rate led to a high yield. Meanwhile, the pectin microspheres loaded with octreotide acetate possessed rougher surfaces than the control samples. Moreover, the specific process parameters such as inlet air flow, inlet air temperature, pump speed and nozzle size, may affect the heat/mass transfer and other thermodynamic behaviors, and lead to different physicochemical properties of microparticles (Fig. 4a).75
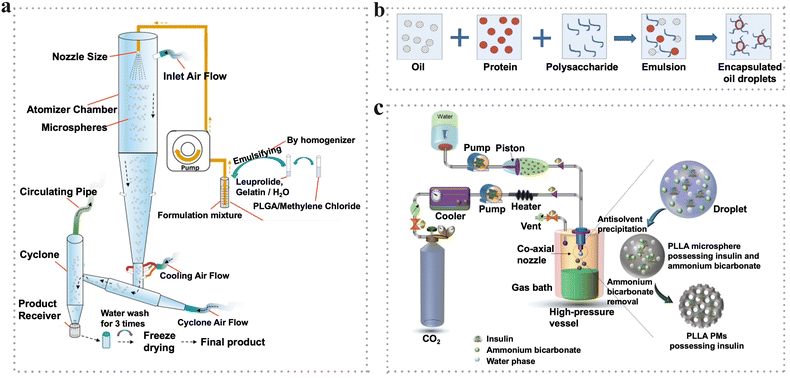 | ||
| Fig. 4 (a) Schematic illustration of spray drying set-up for the preparation of PLGA microspheres.75 Reproduced with permission from ref. 75. Copyright 2020 Elsevier. (b) Flow diagram of microencapsulation by complex coacervation.82 Reproduced with permission from ref. 82. Copyright 2015 Elsevier. (c) Schematic representation of the preparation of porous PLLA PMs by the SCF method.94 Reproduced with permission from ref. 94. Copyright 2018 John Wiley and Sons. | ||
Basically, feedstocks such as single liquids (e.g., emulsions, slurries, melts and pastes) or special compounds (heat-insensitive and heat-sensitive compounds) are applicable to fabricate microspheres with simple structures via spray-drying methods. However, such simple structures cannot fulfil the needs of spatio-temporal release manner and enhanced functionalities. Therefore, combining the spray-drying method with other technologies raises great interest.76 For example, core (poly(lactic acid))-shell (silk fibroin) microspheres were produced via emulsification followed by spray-drying.77 Such core–shell microspheres were incorporated with ciprofloxacin in the shell and ibuprofen in the core, achieving sequential release kinetic which could be applied for the treatment of respiratory diseases.
2.4. Coacervation
The theory of coacervation was discussed earlier in the 1990s78 and was systematically investigated using proteins and anionic polysaccharides as models later in 2004.79Coacervation which contains the coacervate phase and the equilibrium phase is a phase separation process induced by changes in the media environment, such as temperature, pH and solubility. In this process, the coacervate phase is enriched with colloid while the equilibrium phase is devoid of colloid.80 With the basis of the mechanism of the phase separation, coacervation can be classified into simple coacervation and complex coacervation. With the addition of a coacervation agent, usually salt or desolvation liquid, simple coacervation occurs when there is only a single polymer undergoing phase separation owing to dehydration or a “water deficit” process.81 On the other hand, a complex coacervation process contains at least two oppositely charged polymers (such as polysaccharides and proteins) interacting with each other triggered by physical interactions (e.g., electrostatic interaction, hydrogen bonding, polarization-induced attractive interaction or hydrophobic interactions) or chemical agents (e.g., cross-linking agents such as glutaraldehyde or transglutaminase), thus leading to phase separation (Fig. 4b).82 The detailed coacervation process has been elucidated in the reviews.83
Typically, coacervation possesses the advantages of large-scale production rate, low cost, good reproducibility and high loading efficiency. Therefore, plenty of studies have focused on drug delivery systems based on microspheres which were produced by the coacervation method.84 However, after the phase separation process, microspheres tend to agglomerate which is adverse for large-scale industrial production. Recently, coacervation has been effectively employed in the biomedical applications such as encapsulation of drugs,85 and peptides.86 Adopting the coacervation method under mild processing conditions is favorable for protecting the bioactivity of the encapsulated molecules. Meanwhile, the biocompatibility of the microspheres can be assured by utilizing biocompatible polymers, mostly including gelatin, chitosan and other synthetic polymers.85 For example, chitosan-alginate microspheres (CAMPs) containing vancomycin chloride were prepared through a coacervation process.87 The CAMPs showed a highly rough surface, which was proven to support cell adhesion. In addition, microspheres or microcapsules prepared by the coacervation method were broadly used in cancer treatment, antibacterial fabrics, tissue regeneration and anti-adhesive wound dressings due to the customized functionalities and enhanced bioactivity.88–91
2.5. Supercritical fluids
In 1879, supercritical fluids (SCFs) were earlier reported to act as a medium for particle fabrication by Hannay and Hogarth. They demonstrated the phenomenon that a solid could be dissolved in a gas under no measurable gaseous pressure. From the sudden decrease to the increase in the pressure, the solid went through precipitation to redissolution.92 Over a hundred years, the critical points (such as pressure or temperature) of several substances were discovered. Moreover, the physical and chemical properties of the substance were easily affected by the small changes in temperature and/or pressure near the critical point.93Recently, SCF technology has been extensively exploited to generate microspheres due to its advantages of less usage of organic solvents and high efficiency, which is favorable to be used in biomedical applications such as tissue engineering and drug delivery.94,95 The SCF is defined as a substance containing a homogeneous phase over its critical temperature and pressure where the boundary between liquid and vapor vanishes within the SCF area. In general, SCFs possess gas-like properties such as low viscosity and high diffusivity and liquid-like properties such as solvation power and density simultaneously. Recently, among the multiple SCFs used, supercritical carbon dioxide (SC-CO2) has attracted intensive interest for serving as a green candidate with nontoxicity, inertness, low cost and nonflammability. Generally, SC-CO2 can be used as a solvent, antisolvent, solute (porogenic agent) and alternative reagent during the microsphere preparation. The critical temperature (Tc) of 31.4 °C and the medium critical pressure (Pc) of 7.38 MPa can provide protection for thermally unstable materials from degradation and reduce the operational risk for the production process of microparticles.96 The favorable properties of CO2 make it suitable to generate microspheres to be used as pharmaceuticals and biological materials. For example, PLLA porous microspheres encapsulated with insulin were synthesized using ammonium bicarbonate as a porogen based on the SC-CO2 technology (Fig. 4c).94 Under the mild process conditions, these insulin-loaded PLLA PMs exhibited high encapsulation efficiency and excellent aerodynamics performance, thus showing promising potential in the inhalation treatment of diabetes.
Typically, SCF technologies can be classified into supercritical antisolvent process (SAS), rapid expansion of supercritical solutions (RESS), gas antisolvent process (GAS), and solution-enhanced dispersion by supercritical fluids (SEDS).97 Among these, SAS technology has been widely adopted in the microencapsulation owing to the improved solubility and bioavailability of hydrophobic drugs.98 In the SAS process, organic solvent is dispersed in the antisolvent and microcapsules are obtained after the nucleation and precipitation of the solute when the solvent deposited surrounds the solute. Therefore, the affinity between the solvent and antisolvent as well as the mutual solubility between the solute and antisolvent should be considered to achieve high microencapsulation efficiency. In general, SCF technology is suitable for the preparation of microspheres from heat-sensitive drugs and volatile drugs because of its mild process conditions, short microsphere formation time, high encapsulation rate, and low organic solvent residues. In addition, the design of relative equipment is quite mature, making it attractive for the industrial production of microspheres.
2.6. Superhydrophobic surface-mediated method
Wettability indicates the degree of spreading of the liquid on the solid, which is one of the significant characteristics of the solid surface.99 Typically, super-wettability such as superhydrophobicity has attracted considerable attention for the uprising demands of self-cleaning, antiadhesive and antibacterial functions.100–103 In 2010, the superhydrophobic surface (SHS) of polystyrene (PS) was usually adopted for production of natural (such as chitosan and alginate) or synthetic (such as PLGA and PNIPAm) microspheres for drug delivery and encapsulation of cells and proteins.104,105 After five years, SHS made from candle soot was the commonly used template for fabrication of microspheres due to its simplicity and biocompatibility.106,107 Besides, superamphiphobic substrates prepared by etching the surface of aluminum plates and SHS coated by ready-made spray “NeverWet” were also good alternatives for microsphere preparation.108,109Recently, the superhydrophobic surface-mediated (SHSM) method for fabricating microspheres has gained tremendous interest due to its low surface energy and high water contact angles. What's more, through the SHSM method, the desirable morphology, tailored structure and narrow size distribution of the microspheres can be manipulated through the control of the droplets. Applying the SHSM method for microsphere synthesis has lots of benefits such as high encapsulation efficiency, high loading of cargo and controlled release rate, thus showing promising potential for drug delivery and cell encapsulation. In general, the drug-loading process can be described in the following steps. First, the mixed solution of drugs and monomers is placed on the SHS to maintain multiple spherical droplets. Then, microspheres encapsulated with drugs are obtained after the cross-linking of the monomer. The earlier studies adopted the variant SHS (e.g., PS, a fractal-like layer of the candle soot-based SHS110) to fabricate spherical drug-loading hydrogels after going through a crosslinking process.105,111 These hydrogels achieved 100% drug encapsulation and the desired release rate. Meanwhile, the size of the spherical hydrogel can be regulated through the volume of the droplets. However, the size of spherical hydrogels was in millimeter scale, which hindered their application in clinical trials. To overcome this drawback, new strategies combined with the SHS to prepare microspheres have been developed. For example, methacrylamide CS microspheres were obtained on the SHS through spraying followed by photo-crosslinking.112 The obtained microspheres possessed a broad size distribution (80–400 μm), resulting from the droplet fragmentation during spraying. In another example, a superamphiphobic surface with the aid of a microfluidic spinning instrument was employed to fabricate microspheres with various polymers. The diameter of as-prepared microspheres was in an exceptionally wide range (from 1 mm down to 1 μm). Moreover, the morphology of the resultant microspheres can be regulated using coaxial needles. The thickness of the shell was regulated by adjusting the inner and outer diameters of a coaxial needle. For example, core (polyacrylate sodium)-shell (PEO) microspheres and calcium alginate microcapsules incorporated with an oily solvent (glycerin) were obtained based on the above method, providing promising alternatives for drug encapsulation (Fig. 5a).113
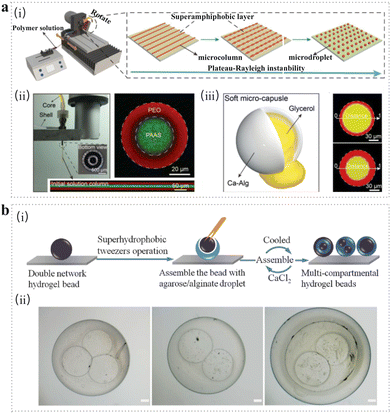 | ||
| Fig. 5 Superhydrophobic surface-mediated method. (a) (i) Schematic illustration of large-scale fabrication of microspheres on superamphiphobic surface with the aid of a microfluidic spin instrument. (ii) Core–shell microspheres fabricated using coaxial needle and loaded with Rhodamine B (red) and FITC (green), respectively. (iii) Schematic of the soft microcapsule and its fluorescence images with different shell thickness.113 Reproduced with permission from ref. 113. Copyright 2021 John Wiley and Sons. (b) (i) Schematic illustration of the fabrication of agarose/alginate microspheres with multicompartmental structure via superhydrophobic surface-mediated droplet formation. (ii) Multicompartmental hydrogel microsphere with core–shell structure.114 Scale bar: 200 μm. Reproduced with permission from ref. 114. Copyright 2014 John Wiley and Sons. | ||
The SHSM method has also been employed to produce microspheres with complex structures.107,114,115 For instance, multi-compartmental agarose/alginate microgels were synthesized on the Cu SHS via a two-step gelation process (Fig. 5b).114 First, agarose/alginate pre-gel solution droplets were placed on the SHS, being cooled at 4 °C to form an agarose microgel and maintain a spherical shape. Then, double microgel network was formed through the addition of a CaCl2 solution. The double-network microgels were used as cores and encapsulated inside the pre-gel solution. At last, the multi-compartmental structure was obtained after the subsequent sol–gel transition. This solvent-free method is suitable for almost 100% encapsulation of biomolecules and living cells under mild conditions without causing toxicity. Besides, the simple process and low cost render the SHS method more effective for preparing microspheres with customized structures and promising functionalities.
Other methods for producing microspheres, such as radical polymerization (including suspension, emulsion, dispersion, and sedimentation polymerization), involve a mixture of monomers and polymerization initiators formed into microspheres in a stabilizing non-miscible phase.116 Meanwhile, electro-spinning, gelation followed by emulsification and ultrasonication are also promising alternatives for microsphere production.117
3. Microsphere structures
With the increasingly complex needs in biomedical fields, simple solid interior microspheres are not enough to realize more sophisticated functions. Therefore, designing different morphologies or more complex structures has aroused intensive interest nowadays. In terms of morphology, the microspheres can be categorized into simple, hollow, porous, core–shell, Janus and complex-structured microspheres, and they are systematically introduced in the following sections (Fig. 6).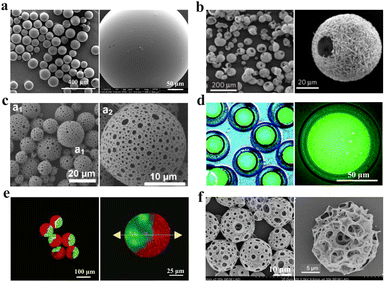 | ||
| Fig. 6 (a) Typical SEM images of simple microspheres.118 Reproduced with permission from ref. 118. Copyright 2015 Frontiers. (b) Typical SEM images of hollow microspheres with nanofibrous surfaces.119 Reproduced with permission from ref. 119. Copyright 2011 Springer Nature. (c) Typical SEM images of porous microspheres.120 Reproduced with permission from ref. 120. Copyright 2014 American Chemical Society. (d) The fluorescence images of core–shell microspheres with the fluorescence-labeled dextran core.121 Reproduced with permission from ref. 121. Copyright 2020 Royal Society of Chemistry. (e) Fluorescence confocal microscopy images of Janus microspheres.122 Reproduced with permission from ref. 122. Copyright 2012 American Chemical Society. (f) Typical SEM images of complex microspheres with both hollow and porous structures.123 Reproduced with permission from ref. 123. Copyright 2019 John Wiley and Sons. | ||
3.1. Simple microspheres
Simple microspheres are fabricated by ordinary methods such as solidification of single emulsion droplets which contain a single kind of chemical component with a solid-interior structure (Fig. 6a).118 According to the types of materials made for microspheres, simple microspheres can be categorized into biodegradable microspheres and non-degradable microspheres, which are prepared utilizing biodegradable polymers (such as COL, gelatin, CS, polycaprolactone (PCL), PLA, poly(glycolic acid) (PGA), and PLGA), or non-degradable polymers (such as poly(methyl methacrylate) (PMMA), poly(tetrafluoroethylene) (PTFE) and poly(ether-urethanes) (PU)).124Generally, such simple microspheres with monodisperse size, customized functionalities and stable mechanical properties demonstrated good potential in tissue engineering and drug delivery. For example, PLGA microspheres loaded with anticancer drugs were prepared through an oil-in-water emulsification process.125 The fabrication parameters such as emulsification speed exert an important effect on the morphology of the PLGA microspheres. Under the relatively high emulsification speed (2000 rpm), the smaller size of microspheres and increased surface area were produced in comparison with the lower emulsification speed (1500 rpm), which led to faster drug release. The PLGA-based microspheres achieved sustained drug release for 50 days in vitro and suppression of the abnormal blood vessel formation in vivo, acting as a promising alternative to antiangiogenic antibody therapy for age-related macular degeneration.
The microfluidic platform has been employed to produce microspheres with precise control and manipulation, favorable for encapsulation of drugs and cells. For instance, COL type I (COL1) microspheres encapsulated with human embryonic stem cells (hESCs) were fabricated by emulsion technology in the microfluidic flow-focusing device.126 The as-produced droplets of COL1 solution mixed with hESCs were released from the oil phase to form the hESCs-laden microspheres which differentiated into hepatocyte-like cells microspheres for subsequent assembly of prevascularized liver tissue, showing a good potential for the regenerative medicine, drug screening, and in vitro liver modeling. Although such simple microspheres have been widely used due to their multiple advantages, the lack of surface area does not satisfy the increasing demands of biomedicine. Therefore, there is an emerging requirement for the design of novel microsphere structures to improve the functionality of customized applications.
3.2. Hollow microspheres
Hollow microspheres possess hierarchical structures with large surface area, narrow size distribution, superior morphological uniformity and desirable biocompatibility, thus making it possible to be used in drug delivery systems, cell encapsulation and immobilization of proteins as well as biomedical imaging (Fig. 6b).119 Synthetic processes such as emulsion polymerization have been adopted to generate hollow structures. In order to obtain hollow microspheres, methods based on templates (e.g., emulsion solvent evaporation and microfluidics) are commonly used because of the simple process and low cost. Typically, the template can be categorized into hard template, soft template and self-template (template-free).127,128For the hard-template method, a myriad of inorganic/organic colloidal materials (e.g., SiO2, calcium bicarbonate, metal oxides and PS) and non-crosslinked polymeric microparticles are generally chosen as ideal templates.129,130 These templates have the advantages of good availability, narrow size distribution, customized size and simple synthesis. In addition, the shape and void size of the hollow microsphere were determined by that of the templates. In this process, the desired components were deposited on the proper templates and then selectively removed by calcination, dissolution or chemical etching. For example, hollow spherical polyaniline microspheres were produced using a PS sphere as a template.130 The resulting sample was finally annealed at high temperature or purified with solvents to remove the PS template. However, the organic solvents or etchants used in the synthesis process are toxic to mammalian cells, which limits the biomedical application of hollow microspheres.
In the soft-template method, thermodynamically metastable liquids (e.g., emulsion droplets, surfactants and other supramolecular micelles, polymer aggregates/vesicles) or gases (e.g., CO2) are employed as sacrificial templates.131 Meanwhile, these soft templates are highly deformable, which can be easily altered via variant parameters, such as temperature, pH value, ionic strength and solvent. What's more, the less synthetic steps and the mild template removal way (e.g., extraction with a mild solvent such as water, ethanol, and acetone, drying in vacuo, gentle evaporation or calcination) render the soft-template method more popular than the hard-template method. In terms of the type of templates, the soft template methods can be categorized into micelle/vesicle templating, emulsion templating,132 electrospraying133 and gas bubbles templating.134 For example, the double-emulsion solvent evaporation method was employed to fabricate hollow PLGA microspheres with coated CS shells.134 The hollow structure of PLGA-CS microspheres was formed by carbon dioxide which was left inside the interior space and hard to diffuse because of the relatively high viscosity. Despite the above advantages of the soft template method, the compromised uniformity and easy aggregation of the PMs cannot be ignored.
In the template-free method, hollow microspheres were prepared without the removal of the core or template. Generally, the migration of the polymer from the center toward the outer shell layer of the hollow microsphere generates the void through polymerization or chemical modification on the seed surface. Then, the core material becomes the principal component of the shell.135 This template-free method has gained popularity due to its high monodispersity, easy processability and large-scale production. Through the mechanism of void expansion, this method can be classified into seeded polymerization,136 one-step polymerization137 and solvent swelling-diffusion for polymers.138 For example, PS hollow microspheres with slightly crosslinked polystyrene–divinylbenzene (PS–DVB) shells were prepared via seeded polymerization.136 In this process, the size enlargement was achieved as the ethanol/water ratio increased, and the underlying reason was the largely decreased surface tension of the emulsion. Meanwhile, the feed time of DVB and reaction conditions were also used to control the size and hollow structure.
3.3. Porous microspheres
Microspheres with interconnective external or internal pores have increased surface area, low density, strong surface penetration ability, and good stability (Fig. 6c),120 thus providing abundant space for incorporation and controlled release of drugs, bioactive molecules and therapeutic cells in terms of the pore size, which can be used for anticancer applications, tissue engineering and regeneration.139 Generally, the method for synthesizing porous microspheres includes emulsion solvent evaporation, emulsion polymerization and spray drying.140Usually, an emulsion/solvent evaporation process and a proper porogen are involved during the preparation of porous microspheres. The commonly used porogens include polymeric materials (e.g., gelatin, alginate, albumin, polyethylene glycol and PVP), gas-forming agents (e.g., ammonium bicarbonate and sodium bicarbonate), osmolytes (e.g., KCl, NaCl and phosphate buffer) and sacrificial microspheres. And the mechanism of porogen-based method is generally identified as solvent casting or particulate leaching. After the porous microspheres are produced, the porogen can be removed using chemical or physical methods. For example, gelatin was used as a porogen to produce poly(L-lactic acid) (PLLA) porous microspheres using a microfluidic device.141 The porous structure was obtained by dissolution of gelatin in a water bath (45 °C). Moreover, the increasing temperature could lead to fewer micropores. In addition, the rising concentration of gelatin also resulted in increased porosity and pore size.142 In another example, ammonium bicarbonate (AB) was adopted as a porogen to fabricate porous microspheres with considerable surface porosity.143 Typically, AB expanded the size of the inner aqueous phase droplets surrounded by the small gas-filled bubble. And the large and small pores were generated because of the inner aqueous phase droplets and small gas-filled bubbles, respectively. Therefore, increasing the concentration of the AB would lead to the uniformity of the pore structure.
However, the porogen-based method suffers from the disadvantages of time-consuming processes, toxic chemicals, deformed morphology and incomplete removal or excessive removal of loaded active substances; therefore, porogen-free methods without using any pores-forming agents attract enormous attention nowadays.144,145 Converting organic solvents into water-soluble organic solvents can give rise to a porous structure. For example, PLGA porous microspheres were produced through the o/w emulsion process.146 In this process, ammonia was added to interact with isopropyl formate to produce water-soluble isopropanol and formamide, which served as antisolvents toward PLGA causing precipitation. Concomitantly, numerous voids and pores were quickly formed through the leaching of the antisolvents into the aqueous continuous phase. In the porogen-free method, the pore size relates to the amount of the formulation of the ingredients or physical characteristics of the microspheres. For example, PCL porous microspheres were fabricated through a one-pot thermally induced phase separation (TIPS) method without using any porogen.145 Increasing concentration of PCL caused the slightly increasing microsphere size but decreasing pore size and porosity. In another example, methacrylated gelatin (GelMA) porous microspheres were synthesized using a microfluidic device.147 It was found that larger pore sizes were relevant to the low elastic modulus of the microspheres.
3.4. Core–shell microspheres
Microspheres or microcapsules with a core–shell structure are typically composed of a liquid or solid surrounded by an outer layer, known as the shell (Fig. 6d).121 The shell enables protection of the valuable encapsulated components from the surrounding environment. The high degree of flexibility in the selection of shell materials allows the production of microspheres with different properties and functions such as controlled release and stimuli-responsiveness.148,149 What's more, the core maintains the stability and viability of the inner biomolecules or cells. Additionally, due to the independence of the core and shell, their properties and functionalities can be designed separately, providing highly tunable platforms for various biomedical applications such as drug delivery, tissue engineering and artificial cells.150–153 There have been various technologies developed to produce the microcapsules, including interfacial polymerization, membrane emulsification, and layer-by-layer assembly. The most frequently used methods are microfluidic technologies and electrospraying, as their production processes are controllable and the products have a narrow size distribution and homogeneous morphology. Besides, superhydrophobic surfaces achieving water contact angles above 150° can be utilized in the fabrication of core–shell structures through cycles of deposition and crosslinking of polymer solutions.106 However, the low yield hinders its further application. Recently, a novel strategy was reported utilizing confinement-free fluid instability to fabricate core–shell microspheres induced by the ultralow surface energy of the superamphiphobic surface. What's more, this strategy was promising for production at an industrial scale with the microfluidic spinning instrument.113 Size, composition, configuration and morphology are important parameters of the core–shell microspheres which should be determined by their applications. For instance, the thickness and molecular weight could affect the degradation and release kinetics of the microcapsules, which could be applied in the controlled release of biomolecules in bone regeneration.154 In another example, a large number of monodisperse PDMS microcapsules were fabricated through a 3D nested capillary microfluidic device. These microcapsules had thin and soft shells which could be applied as physical models for red blood cells. What's more, their size, shell thickness and elasticity could be accurately adjusted by different flow conditions and shell composition, which could help us with exploring haemodynamics and haemorheology in a more effective way.1553.5. Janus microsphere
The name of Janus microspheres stems from the two-faced Roman God Janus. In 1991, P. G. de Gennes raised the concept of Janus particles in his Nobel Prize address, which inspired the preparation and application of various Janus microspheres. Janus microspheres are particles with asymmetry physical or chemical characteristics (e.g., different compositions, polarities, surface modifications, amphiphilic structure (hydrophobic hemisphere and hydrophilic hemisphere) on different sides (Fig. 6e),122 rendering them suitable for many applications, from emulsion stabilization for biomedical imaging and drug delivery vehicles.156 The methods for fabrication of Janus microspheres include emulsion interfacial polymerization, Pickering emulsion and microfluid-based strategies.157Janus microspheres are customized to have two distinct hemispheres with different physical, chemical and biological characteristics, such as dual responsiveness. Such dual-responsive ability can be utilized to lead and release cargo under specific stimuli, while it is difficult to achieve that release behavior for core–shell microspheres. For example, Janus microbeads which were responsive to glucose and pH, respectively, in two compartments were prepared using a UV-assisted centrifugal microfluidic device.158 These hemispheres were composed of glucose-responsive and pH-responsive monomers, respectively, to provide accurate measurement and calibration of glucose concentration, which could be used to monitor the health condition of the patient with diabetes. Moreover, the tunable biological functionalities in different aspects can be achieved by Janus microspheres. For example, Janus microspheres were fabricated by a water-in-oil emulsion solvent evaporation method.159 One part of the microsphere could attach to and orientate on the mucosa which was constructed by ammonioalkyl methacrylate copolymers. The other part could provide a protective sealing from the body fluid which was constructed by hard fat. This Janus microsphere provided a potential enteral drug delivery system for medical applications.
3.6. Microspheres with complex structures
Microspheres with complex structures, such as the combination of hollow and porous structure, hollow microsphere with Janus shell, multicompartment or multicore structure and sphere-in-sphere (capsule) structure have enhanced effects on providing better mechanical and biochemical properties compared to the simple-structured microspheres.Generally, the first kind of microspheres has the dual advantages of hollow and porous structure, such as low density, high bioavailability, high encapsulation efficiency, and good sustained release. Therefore, the hollow microspheres with high porosity are the promising drug carriers. For example, hollow porous microspheres were generated based on poly(ester-thioether)s with the solvent evaporation process without using porogens (Fig. 6f).123 Notably, the hierarchically interconnected microcages were formed because of the partial crosslinking of polymers. The larger pore size generated through the longer alkyl chain length endowed the obtained microspheres with effective encapsulation of hydrophobic and hydrophilic drugs along with faster drug release behavior, indicating great potential for drug delivery.
Recently, microspheres with multicompartment structures were constructed based on microfluidic technologies due to the advantages of controlled structure, designated loading ability and uniform size distribution.160 For example, to co-encapsulate different types of reagents, single- and multi-compartment PLGA microcapsules were produced utilizing the microfluidic method with a liquid-driven compound-fluidic flow-focusing device.65 The number of compartments was determined by the flow rates of the inner aqueous phase and the outer organic phase. When the flow rates of the two inner phases were the same, microdroplets with two compartments were produced. With the decrease in the flow rate ratio of the inner and outer phases, microdroplets with multiple pairs of cores were obtained. The PLGA microspheres with designed compartments had high production rate and encapsulation efficiency, offering a method to prepare multifunctional microcarriers for cell encapsulation, drug delivery and biomedical imaging. The parameters used in microfluidic methods, such as the flow rate of the middle and outer phases, exerted an important role in the morphology and size of the microspheres. For instance, alginate core–shell microspheres with multicores were produced through the combination of coaxial shear microfluidics and coextrusion minifluidics approach using oil-in-water-in-oil (O/W/O) emulsions as templates.161 With the increasing middle flow rate, the size of the core decreased while the thickness of the shell increased. On the other hand, the decreased flow rate of the outer phase resulted in an increasing number of cores and size of the microspheres. In addition, a similar novel structure called polymersomes-in-polymersome has gained broad interest owing to the size homogeneity, monodispersity, and high encapsulation efficiency, which has been used for enzyme cascade bioreaction.162 The sphere-in-sphere structure of microspheres was fabricated using multiple emulsions as templates based on a microfluidic device, showing controlled and responsive release behavior.163 For example, chitosan microspheres with multicores were loaded in the poly(N-isopropylacrylamide) (PNIPAM) microcapsule which was the inner oil core to produce hierarchical sphere-in-sphere microcarriers using quadruple (O1/W2)/O3/W4/O5 emulsions templates generated from the capillary microfluidic device.164 The as-prepared microcarriers showed a burst release of the inner substance along with the CS microspheres due to the volume shrinking of the outer PNIPAM shell triggered by increasing temperature, and then the acid-triggered release of contents and CS caused by the decomposition of an inner CS matrix. Such dual-responsive microcarriers with sphere-in-capsule structures demonstrated promising potential to act as drug delivery systems for co-encapsulation, controlled release and confined microreaction. Similarly, poly(lactic-co-glycolic acid)@CS@capsaicin (CAP@CS@PLGA) dual-core shell microcapsules with enzymatic and pH double responsive ability were fabricated through the composite emulsion method.165 As the degradation of the PLGA shell occurred, CAP@CS and CAP were released under the stimuli of an enzyme, thereby achieving 90% bacterial inhibition.
4. Biomedical applications
The properties of PMs make them attractive for a variety of biomedical applications including bioreactor engineering, bio-separation engineering, pharmaceutical engineering, bio-detection engineering, tissue engineering, etc. In this section, we summarize several important ones, including serving as microcarriers for drugs and cells, as scaffolds for tissue regeneration, agents for transcatheter arterial embolization and constructing artificial cells.4.1. Microcarrier
The small particle size, high surface-to-volume ratio and tunable physical and chemical properties make the PMs ideal carriers for a variety of substances, including drugs, cells, probiotics, enzymes, etc. In this section, we summarize and elucidate two important ones: drugs and cells.4.1.1.1. Natural polymers. PMs can be produced from natural materials including carbohydrates and proteins or from synthetic polymers such as PLA and PLGA, etc. The natural polymers used for the preparation of microspheres are mainly from proteins (e.g., gelatin, albumin and casein) and polysaccharides (e.g., chitosan, starch, sodium hyaluronate, sodium alginate, agarose, pectin, dextran, cellulose and its derivatives).172 Chitosan is a natural cationic polysaccharide obtained from chitin, the second most abundant polysaccharide after cellulose. It is widely used in drug delivery systems due to its biocompatibility, biodegradability, low-toxicity and controlled release ability. Chitosan microspheres have been used as a vehicle for the sustained release of various pharmaceutical agents including anticancer drugs, proteins, antibiotics and antihypertensive drugs. In addition, the abundant amino groups on the surface of chitosan can combine with anionic groups such as sialic acid of the mucosa gel layer, leading to extended residence time of drug absorption at the site of mucus (e.g., buccal, colonic, and nasal mucosa) and subsequently increasing the bioavailability of chitosan microspheres encapsulated drugs. Therefore, chitosan microspheres have great potential for delivering orally or mucosally administered drugs. Chitosan microspheres are usually prepared by crosslinking with multivalent anions (e.g., sulphate, tripolyphosphate, octyl sulphate, lauryl sulphate) or chemicals (e.g., citric acid, glutaraldehyde, formaldehyde, epichlorohydrin, genipin) due to the ample cationic amino groups and hydroxy groups in the polymer. Active pharmaceutic agents could be loaded in the chitosan microspheres before crosslinking and precipitation or incorporated into the pre-formed chitosan microspheres.173,174
Proteins such as albumin and gelatin have been widely used in the construction of PMs for delivering various bioactive molecules. Albumin is the most abundant plasma protein in nature. Due to its good biocompatibility, non-immunogenicity and in vivo stability, albumin has attracted great attention as a carrier for drug delivery. Additionally, its high affinity to secreted protein acidic and rich in cysteine (SPARC) and albondin (gp60) on tumor cells and vascular epithelium offers albumin tumor targetability. Abraxane, an albumin-based nanoparticle loaded with paclitaxel, is now commercially available for the treatment of cancer in many countries. Albumin-based microspheres can be prepared by crosslinking or high-pressure homogenization method. The crosslinking of microspheres may affect the hydrophobicity and subsequently alter the drug release profile. More extensive cross-linking reduces the solubility of albumin, thereby decreasing the drug release rate from microspheres.14,175,176 Gelatin can be attained from degradation or hydrolysis of collagen protein. Due to its excellent biocompatibility, nontoxicity, non-immunogenicity and biodegradability, gelatin is widely accepted as an attractive drug carrier to deliver various pharmaceutic agents. Gelatin microspheres can be prepared by physical or chemical crosslinking. However, physically crosslinked gelatin microspheres are unstable at 37 °C. Therefore, chemical crosslinking is required for preparation of the microspheres. The abundant amine groups on the gelatin chains render the crosslinking agents such as genipin, glutaraldehyde and dimethylaminopropyl-3-ethylcarbodiimide hydrochloride to form covalent bonds and subsequently enhance the stability of microspheres. Gelatin contains cellular adhesive peptide sequences and can be enzymatically degraded in response to matrix metalloproteinases. Drugs loaded in the microspheres are released as the degradation occurs, and therefore, the release behavior can be manipulated by altering the size, surface-to-volume ratio and cross-linking density.12,177–179
4.1.1.2. Synthetic polymers. Due to the advantage of adjustable degradation kinetics and mechanical properties in the stage of synthesis, synthetic biodegradable polymers have more predictable functions and properties when compared with natural polymers.180 PLGA is one of the most widely used synthetic polymers in drug delivery systems. PLGA has been successfully used in clinical with more than 20 approved PLGA-based medical products in the market. PLGA can be prepared by random copolymerization of lactic acid (LA) and glycolic acid (GA) monomers. In addition to its excellent biocompatibility and biodegradability, PLGA is also known to be beneficial for its ability to regulate the degradation properties by varying its molecular weight (Mw) and the ratio of LA/GA.85 After administration, PLGA can be enzymatically degraded into lactic acid and glycolic acid. These two components can enter the tricarboxylic acid cycle and are further metabolized and eliminated as carbon dioxide and water.181 Therefore, PLGA is generally regarded as safe by many regulatory agencies including the FDA (United States Food and Drug Administration), EMA (European Medicines Agency) and NMPA (National Medical Products Administration) and has been officially approved by the FDA as a pharmaceutical excipient.182
PLGA microspheres as a drug carrier. Generally, it is difficult to distinguish a membrane and a core in microspheres due to their homogeneous matrix constitution. Active pharmaceutical ingredient (API) is dispersed in the polymer matrix of microspheres alone or in combination with excipients as molecules or small clusters, with polymers continuously coating around the drug-loaded mixture core. The dosage forms of commercially available PLGA-based microparticles are generally injections. The ideal injectable microspheres should be in the range of 50–100 μm to avoid pain and discomfort during drug administration.183 In clinical practice, patients usually suffer from frequent dosing when medicine is administered by injection. PLGA polymers have been selected as reference materials to create sustainable and controlled drug release manners. Therefore, dosing frequency can be reduced and patient compliance can be improved.
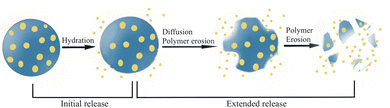
Drug release from PLGA-based microspheres can be regulated by the degradation process of the polymer. The degradation rate of PLGA could be affected by molecular weight, monomer ratio and terminal group functionalization. PLGA with high molecular weight was shown to have a longer degradation time, generally due to the longer polymer chains degrading slower than small polymer chains.19 Monomer composition in the copolymer was reported to have a great impact on the degradation of PLGA. Usually, PLGA copolymers containing a lower glycolic acid content are more hydrophobic and less amorphous, and degrade slower than copolymers containing a higher fraction of glycolic acid. It has been reported that the degradation time of PLGA can be adjusted from months to years by changing the composition of the LA/GA ratio.184 Terminal groups could also affect the degradation kinetics of PLGA copolymers. It has been shown that ester-terminated PLGA exhibited longer degradation time compared with acid-terminated PLGA and are therefore suitable for sustained release applications.185
For drugs encapsulated in PLGA microspheres, diffusion and erosion of the copolymer are the two main release mechanisms (Fig. 7). Therefore, the effects of the encapsulated drug on the release rate should also be taken into account. Water diffusion into the microspheres can be promoted when hydrophilic drugs are incorporated, thus porous polymer network is more likely to be formed and further facilitate the drug release.186 However, water diffusion into the microspheres can be impeded when water-insoluble drugs are encapsulated and further decrease the degradation of the polymer.187 Particle size also potentially influences the drug release rate. For microspheres larger than 200 μm, there is a risk of causing inflammation and immune response, while microspheres with a size less than 10 μm may possibly be phagocytosed by immune cells. The specific surface area of microspheres with large particle sizes is relatively lower than microspheres with smaller diameters, thus water penetration and polymer degradation are relatively slower and drug release is further reduced.182,188
Commercially available PLGA-based microsphere products. PLGA was first approved for preparation of bioresorbable surgical sutures in the 1970s. The first commercialized PLGA-based microsphere (Decapeptyl SR) was approved in Europe in 1986. In Decapeptyl SR, triptorelin as a GnRH agonist was incorporated into the PLGA microsphere for the treatment of prostate cancer. Currently, there are three sustained-release products (1,3 and 6 months) of Decapeptyl SR available on the market. After the approval of Decapeptyl SR, more than 15 PLGA microspheres (MPs) based products have been approved by regulatory agencies and PLGA has become the form of choice for the production of sustained-release delivery systems.183,189
In the past several decades, PLGA MPs have been developed to deliver small-molecule drugs, peptides, proteins and nucleic acids.190 The commercially available PLGA MP-based formulations are described in Table 2 in the order of approval date. From Table 2, we can see that PLGA MP-based formulations delivering the peptide drug, small molecule drug and protein drug administering mainly via injection routes (intra-muscular and subcutaneous injection) have been brought to the market. However, less than 1 new drug depot formulation is approved every year indicating that the development of a PLGA-based microparticulate delivery system is still challenging. Why it is so difficult to develop PLGA MP-based products? First, the complex manufacturing process is one of the main factors that impede the development of PLGA MP products. For example, the only protein-delivering PLGA MP-based formulation in clinical use was Nutropin Depot (somatropin), which was approved in 1999 for the treatment of growth deficiencies, but it was finally withdrawn from the market due to manufacturing difficulties.191 Scale-up from lab, pilot plant to manufacturing plant, the control of foreign particle contamination and the sterilization methods may all be manufacturing difficulties. Additionally, a significant initial burst release is found in many PLGA MPs and almost a quarter of the total drug can be consumed on the first day of injection. Generally, a huge initial burst release with about 100-fold higher drug concentration in comparison with steady-state drug concentration is observed from PLGA MPs formulations. For example, the maximum drug concentration of triptorelin in blood was tested to be about 40 ng mL−1 at 3h after injection of Treslstar® (6-month formulation, 22.5 mg), while the drug concentration at the steady state was less than 1 ng mL−1.192 Lupron depot® is a leuprolide acetate delivered PLGA MPs based formulation developed by Takeda for the treatment of advanced prostate cancer. Patients obtaining a single administration of Lupron depot (4-month formulation, 30 mg) showed a mean leuprolide plasma concentration of 59.3 ng mL−1 at 4 h, and declined to 0.44 ± 0.20 ng mL−1 at the steady state from 3.5 weeks to 16 weeks.193 This indicates > 100 times higher leuprolide concentration of the initial burst release than that of the steady state in blood may still be safe. However, the therapeutic window varies from drug to drug. Drugs with relatively narrow therapeutic windows may easily go from therapeutic to toxic with small changes in drug concentration in the circulation system. Therefore, the huge burst release from PLGA MPs hinders the development of this long-acting formulation. Furthermore, PLGAs may vary from different suppliers even though they have similar LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GA ratios, molecular weights and end groups. Their properties may differ from batches and vendors based on the observation in drug release results. However, currently no thorough characterization methods could clearly elucidate these differences in their properties, making it difficult to duplicate the data and even more difficult to develop long-acting formulations with the desired release behavior.194
GA ratios, molecular weights and end groups. Their properties may differ from batches and vendors based on the observation in drug release results. However, currently no thorough characterization methods could clearly elucidate these differences in their properties, making it difficult to duplicate the data and even more difficult to develop long-acting formulations with the desired release behavior.194
| Commercial name | API | Indications | Administration route | Dose | Company | Year of approval |
|---|---|---|---|---|---|---|
| i.m.-intramuscular; s.c.- subcutaneous. | ||||||
| Decapeptyl® | Triptorelin | Prostate cancer | i.m. | 3.75 mg per month; 11.25 mg per 3 months; 22.5 mg per 6 months | Ipsen | 1986, 2000, 2010 |
| Lupron Depot® | Leuprolide acetate | Prostate cancer/endometrios | i.m. | 7.5 mg per month, 22.5 mg per 3 months, 30 mg per 4 months, and 45 mg per 6 months | Takeda | 1989, 2002, 2003, 2004 |
| Sandostatin® LAR | Octreotide acetate | Acromegaly | i.m. | 10 mg, 20,30 mg per month | Novartis | 1998 |
| Nutropin Depot (discontinued) | Somatropin | Growth deficiencies | s.c. | 13.5 mg, 18 mg, 22.5 mg per month | Genentech/Alkermes | 1999 |
| Trelstar® | Triptorelin pamoate | Prostate cancer | i.m. | 3.75 mg per month; 11.25 mg per 3 months; 22.5 mg per 6 months | Debiopharm | 2000, 2001, 2010 |
| Arestin® | Minocycline Hydrochloride | Periodontitis | Subgingival | 1 mg per 2 weeks | OraPharma | 2001 |
| Risperdal Consta® | Risperidone | Schizophrenia | i.m. | 12.5 mg, 27.5 mg, 37.5 mg, 50 mg per 2 weeks | Janssen | 2003 |
| Vivitrol® | Naltrexone | Opioid use disorder/alcohol use disorder | i.m. | 380 mg per month | Alkermes | 2006 |
| Somatuline® | Lanreotide | Acromegaly | i.m. | 120 mg per month | Ipsen | 2007 |
| Suprecur® MP | Buserelin | Endometriosis, infertility | s.c. | 3.6 mg per month | Sanofi-Aventis | 2006 |
| Lupaneta Pack(discontinued) | Leuprolide acetate | Endometriosis | i.m. | 3.75 mg per month; 11.25 mg per 3 months | Abbvie | 2012 |
| Signifor® LAR | Pasireotide acetate | Cushing's disease/acromegaly | i.m. | 20 mg, 40 mg, 60 mg per month | Novartis | 2014 |
| Zilretta® | Triamcinolone acetonide | Osteoarthritis knee pain | Intra-articular | 32 mg per 3 months | Flexion | 2017 |
| Triptodur | Triptorelin pamoate | Central precocious puberty | i.m. | 22.5 mg per 6 months | Arbor | 2017 |
| Bydureon® | Exenatide | Diabetes | s.c. | 2 mg per week | Astrazeneca AB | 2017 |
| Rykindo® | Risperidone | Atypical antipsychotics | s.c. | 12.5 mg per 2 weeks | Shandong Luye | 2023 |
The release duration of the marketed PLGA MP-based formulations is ranging from 1 week to 6 months. These long-acting PLGA-based microparticle formulations can notably decrease the dose frequency and further lead to improved patient compliance and convenience as well as reduced unwanted side effects and costs. Triptorelin is a synthetic GnRH analog which is more potent than native GnRH for the treatment of endometriosis and advanced prostatic carcinoma.192 Sustained release dosage forms based on PLGA MPs have been developed to improve convenience and treatment adherence. Triptorelin-loaded PLGA MPs were approved in Europe under the name of Decapeptyl®. In 2000, Trelstar® was marketed in the US by the Debiopharm group. Currently the sustained-release dosage forms of triptorelin available in the clinic are 1-month (3.75 mg), 3-month (11.25 mg) and 6-month (22.50 mg). The long-term formulations of triptorelin significantly decrease the number of injections and thus offer better patient comfort and compliance with therapy. Among the marketed PLGA MPs, the duration of Bydureon® is only 2 mg per week, which is much shorter than other long-acting formulations. However, the once-weekly PLGA MP injection is more acceptable compared to the painful and inconvenient twice-daily-administered exenatide, reducing psychological and physical burden on patients with type II diabetes mellitus.191
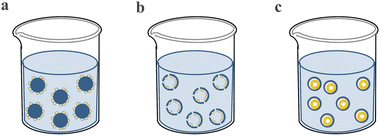
4.1.2.1. Cell expansion. In clinical application, the quantity of cells required for the treatment of disease ranges from millions to billions per patient, while it is far less of the cells isolated from several tissues and is of significant shortage in cell banks.195 For example, NK cells which are a promising strategy for combating cancer due to their innate ability to kill cancer cells make up only 10–15% of lymphocytes in peripheral blood.196 Therefore, it is desperately needed to develop an ideal cell manufacturing method to expand cells of ample quantities. There are a variety of methods for the large expansion of cells, but conventional 2D monolayer culture systems are found to be limited to scaling-up and producing high quality cells.197,198 The PM-based culture systems can solve these problems and have the advantages of increasing cell densities,199 high cell attachment efficiency,200,201 easy to sample and adjust culture parameters and long period continuous subculture by adding empty microspheres.202 Moreover, the microcarrier-assisted cell expansion method can achieve process automation which has great potential in industrial scale-up and replacing laborious and poorly controlled processes.203 According to the configuration of the PMs, there are three typical kinds of microspheres for cell expansion: solid microspheres with cells attached to the outer side (Fig. 8a), which have a simple structure with a high surface-to-volume ratio to increase cell attachment area and expansion efficiency; hollow microspheres with cells attached to the inner side (Fig. 8b), which can provide shelter for the expanded cells; and microcapsules with cells forming colony inside (Fig. 8c), in which the shell can protect cells and the core can promote cell viability and amplification rate. The first kind of PMs is the most widely used which have also been commercially available, such as Cytodex 1™ manufactured by GE Healthcare, Hillex® from Thermo Scientific and SphereCol® from Advanced BioMatrix. The second kind of PMs can provide shelter for the attached cells. For example, a hollow PDMS microsphere was fabricated by photolithography. This special structure could protect cells from shear stress in stirred bioreactors (Fig. 9a).204 The third kind of PMs encapsulates cell colonies in core–shell microspheres which can improve cell viability and expansion efficiency. For example, microcapsules with alginate shells were fabricated through a microfluidic method, which engineered stem cell niche microenvironments inside. These microcarriers significantly improved viability and expansion rates while maintaining pluripotency compared to standard human pluripotent stem cell (hPSC) culture platforms such as 2D cultures, solid microcarriers and aggregates. What's more, the amplification rate was increased to 277-fold in 6.5 days with industrial-scale stirred tank bioreactors which had never been achieved before (Fig. 9b).205
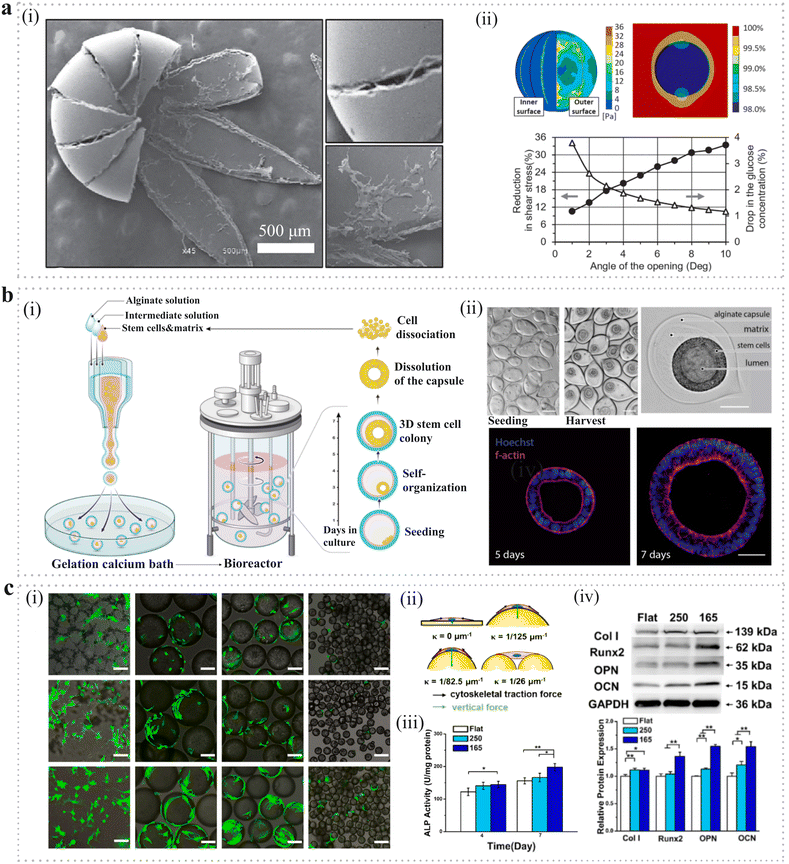 | ||
| Fig. 9 Application of PMs as microcarriers in cell expansion. (a) (i) SEM image of hollow microspheres with cells attached to the inner side. (ii) Numerical analysis on shear stress and glucose diffusion of hollow microspheres.204 Reproduced with permission from ref. 204. Copyright 2018 John Wiley and Sons. (b) (i) Schematic diagram of encapsulation and scale-independent culture of encapsulated 3D hPSC colonies in bioreactors. (ii) Phase contrast and confocal images of the encapsulated hPSCs after seeding. Scale bar: 100 μm.205 Reproduced with permission from ref. 205. Copyright 2023 Elsevier. (c) Microcarriers fabricated with different surface curvature through the microfluidic method. (i) Live/dead staining for BMSCs being cultured on microspheres and membranes. Scale bars: 100 μm. (ii) Schematic diagram of stress analysis of cytoskeleton and nucleus on spherical substrate. (iii) Alkaline phosphatase activity (4 and 7 days) of BMSCs grown onto different surfaces. (iv) Relative expressions of protein markers in relation to osteogenic differentiation after 7 days of culture on different surfaces in osteogenic medium.206 Reproduced with permission from ref. 206. Copyright 2022 Elsevier. | ||
In order to optimize the functions of the microcarriers, there have been a variety of microspheres fabricated with different structures and properties to seek the most effective and ideal cell expansion strategy. For example, a novel GelMA microsphere-based culture system was developed by using a 3D digital light-processing technology. These PMs had a rough surface architecture with many micropoints which had been shown to promote stem cell adhesion, compared with the smooth surface. This system could achieve cell proliferation, passage, harvest, and cryopreservation simultaneously. Fresh microspheres were added to provide more space to achieve cell passage by cell-to-cell transfer without digestion. In a specific example, human adipose-derived stem cells (hADSCs)-loaded microspheres could form macrotissues which were promising in tissue engineering.202 The microspheres could also be optimized with suitable surface curvature to improve the viability and differentiation ability of the expanded cells (Fig. 9c).206 PLGA microsphere curvature was precisely controlled with a microfluidic device and the specific curvature could enhance cytoskeletal organization, nuclear deformation and expression level of osteogenic related genes to regulate bone marrow-derived mesenchymal stem cell (BMSC) differentiation potential. Except for altering surface curvature, highly porous microspheres could offer a larger surface area for they had a structure with interconnected pores to support cell attachment and multidirectional cell–cell interactions.207 What is more, gelatin microspheres fabricated using an emulsion method had an ECM mimicking nanofibrous surface and could support better cell binding, higher proliferation and metabolic activities of stem cells and provide ideal biophysical and biochemical cues for stem cell expansion and differentiation.208 In addition, it is required to harvest cells from the microspheres in some cases. The most frequently used method is proteolytic enzyme treatment, while it sometimes may cause damage to the cells and cannot ensure 100% detachment of cells.209 In recent studies, stimuli-responsive polymers have been developed to detach cells, which ideally reduce the potential damage caused by enzymes. There have been a variety of responsive microspheres fabricated that respond to different stimulations such as temperature and field responsive (magnetic, photo, electronic, ultrasound).209 For example, PNIPAM-based microspheres could allow cell detachment below the lower critical solution temperature (LCST), which holds great promise for the efficient expansion of cells for the industrial-scale culture of therapeutic cells.201 Another magnetic microsphere was developed to obtain relatively pure mesenchymal stem cells (MSCs) in an economical and effective way.210
There have been both primary and adult stem cells expanded on microspheres in laboratory-scale setups including MSCs, hBMSCs, and human umbilical cord mesenchymal stem cells (hUMSCs).206,208,211 For example, BMSCs could be expanded on agarose or gelatin-based microspheres and the osteogenic and chondrogenic differentiation potential was enhanced compared with a 2D monolayer culture system.197,212 Moreover, NK cells were expanded to 387 ± 100-fold in 10 days via a dissolvable polymer-based microsphere platform and maintained ideal cell viability and purity.213 Similarly, HUMSCs were expanded on degradable CS-based microspheres and could increase 4-fold within 5 days’ culture.211 However, it has become obsolete to merely regard the microspheres as an expansion technology. The system can not only achieve big-scale cell culture and expansion but also act as cell delivery vehicles and building blocks for tissue engineering, which is discussed in the next section.214
4.1.2.2. Cell delivery. PMs are ideal carriers for cell delivery in disease therapy and for repairing damaged tissue due to their small size and tunable properties. First, they can deliver cells with the attachment to the surface or infiltrating inside. Second, PMs are able to encapsulate the cells inside which provides a suitable environment to protect them. Both delivering methods enable minimally invasive therapy via injection through very small needles. One of the major advantages of using PMs as cell carriers is that cells are well protected and maintain good viability during the transplanting process. When injected into the target tissue, the relative motion between microspheres brings about limited force transfer, which may improve the viability of the cells even if they are extruded through very small needles. For instance, the enhanced cell protection upon injection could be achieved by crosslinking of hydrogel and a compliant crosslinked alginate hydrogel (G′ = 29.6 Pa) assessed better cell viability than the non-crosslinked alginate solutions.215 Additionally, the physical and chemical properties of PMs are highly tunable, which can improve the viability and loading efficiency of transplanted cells and promote their integration and desired function. Porosity is one of the important properties of microspheres, which can improve the adhesion and loading efficiency of diverse cell types. For example, highly open porous microspheres (OPMs) were fabricated with a natural biopolymer polyhydroxyalkanoate (PHA) through an emulsion method. The OPMs had special structures of suitable surface pores (10–60 μm) and interconnected channels (average 8.8 μm) which enabled improved cell adhesion (93.4%), continuous proliferation (up to 10 days) and improved differentiation of BMSCs. Besides, the interconnected pores enabled the interchange of gases and nutrients which facilitated improved viability and proliferation of the transplanted cells. What's more, the OPMs could improve the viability and migration of cells via protecting them from stress during injection (Fig. 10a).216 Another study demonstrated the optimized pore size (15.5 ± 6.0 μm) in the GelMA microspheres in which the pore sizes were adjusted using the gradient-cooling procedure.217 Except for porosity, the surface topography of PMs can also affect cell shape, orientation, growth and differentiation. For example, hyaluronic acid microspheres with concave surfaces were fabricated through amide crosslinking and gas foaming with an emulsion method. This special surface topography could repress chondrocyte dedifferentiation and promote neocartilage formation via geometric constraints.218 Microspheres with rough surfaces show improved adhesion efficiency on the surface compared to those with smooth surfaces.219,220 Surface chemistry is another important property of microspheres, which is highly tunable for their optimization. For example, to identify candidates that could promote or inhibit the proliferation of fibroblasts, 315 different polymer surfaces were screened and two of them were verified which were pro-proliferative-poly(tetrahydro furfuryl acrylate) (pTHFuA) and anti-proliferative-poly(ethylene glycol phenyl ether acrylate) (pEGPEA). Then, they were synthesized as surfactants on the surface of the PMs. The PMs with pTHFuA modified surface proved to promote wound healing which was novel for harnessing the remodeling potential of the fibroblasts (Fig. 10b).221
 | ||
| Fig. 10 Application of PMs as microcarriers in cell delivery. (a) (i) Schematic diagram and SEM images of PHA highly open porous microspheres (PHA OPMs) and traditional PHA hollow microspheres (PHA HMs). Scale bars: 50 μm. (ii) PHA OPMs could improve the viability of cells via protecting them from stress during injection.216 Reproduced with permission from ref. 216. Copyright 2018 John Wiley and Sons. (b) (i) Discovery of polymers that modulate fibroblast phenotype. Pro-proliferative (pTHFuA) and antiproliferative (pEGPEA) polymers were chosen based on their ability to control fibroblast differentiation and proliferation. (ii)The proliferation and migration ability of fibroblasts was tested by wound healing assay in different groups. Scale bars: 100 μm. (iii) Photographs of wounds healing over time in different groups.221 Reproduced with permission from ref. 221. Copyright 2022 John Wiley and Sons. | ||
In the cell encapsulation method, one of the important factors is how to improve cell viability during the whole delivery process. Initial viability is determined by the fabrication method. For example, electrospraying and gas atomization can achieve an initial cell viability between 90–100% with various materials such as alginate, gelatin and collagen, because the production processes are harmless. For the emulsion method, cell viability is decreased between 70–90%, probably related to the cytotoxicity of emulsifiers and shear force from stirring. As for microfluidic technology, cell viability ranges from 60% to 100%. But recently with the advancement of technology and materials, the viability can be increased to above 90%.222 Before delivery to the target site, the cells need subsequent culture in which the size and porosity of PMs play a crucial role in determining the transportation of nutrients and metabolic wastes. For example, PMs with smaller sizes (diameter <400 μm) enable maintenance of cell viability over a week.223 As for the PMs with diameters over 400 μm, increasing porosity can improve cell viability by providing interconnected channels for the exchange of nutrients and metabolic wastes.224 After reaching the body, the degradability of PMs determines the integration of the transplanted cells and host tissue via cell migration and cell release. A suitable degradation rate that matches the cell release and new tissue formation is important for cell-based therapy. For example, a poly(L-lactic acid)-b-poly(ethylene glycol)-b-poly(N-isopropylacrylamide) copolymer and its self-assembly into nanofibrous gelling microspheres (NF-GMS) were utilized to encapsulate and deliver hESC-derived cardiomyocytes. The PLLA NF microspheres remained intact with about 10% weight loss after 15 weeks which provided sufficient time for myocardium regeneration with vascularization in the infarct zone.225
There have been a variety of cells delivered by PMs.222 The most commonly used are MSCs/BMSCs for bone regeneration. For instance, the GelMA microparticles were generated with the assistance of a microfluidics device, which could carry BMSCs with dispersed fullerol nanocrystals. These BMSC-laden microparticles proved to show enhanced osteogenesis ability in vitro and in vivo and could repair bone defects with robust bone regeneration.226 In another study, chitosan–collagen microspheres were generated as MSCs delivery vehicles using a batch emulsion method. The cells were induced into an osteogenic lineage before implantation into the calvarial defects in vivo. Osteogenically pre-differentiated MSCs encapsulated in the microspheres demonstrated more complete healing of bone defects compared with the undifferentiated ones, indicating that microparticles could be used to deliver cells with regeneration ability that were difficult to transfer with minimally invasive technology.227 PMs have also been demonstrated as carriers for cell delivery in cartilage tissue engineering. Covalently crosslinked assemblies of gelatin microparticles carrying hBMSCs were fabricated via a microfluidic device and the cells could maintain good viability and active migration over a long culture period. These microgels achieved higher-order cartilage tissue formation and rapid bonding with the host tissue mimic.228 Additionally, the microspheres loaded with HUVECs and skin cells obtained from healthy mice could improve wound healing with evenly distributed blood vessels throughout the implants. Moreover, the angiogenic properties of the HUVEC-laden microspheres could be maintained after cryopreservation processes.229 These findings verified the promising potential of functionalized microspheres in cell therapies and regenerative medicine.
Recently, cell-laden microsphere-based biomanufacturing technologies like 3D printing hold tremendous potential in tissue engineering. A promising study reported a new biofabrication approach by utilizing GelMA microparticles carrying MSCs (produced by microfluidics) to assemble osteochondral constructs. These encapsulated MSCs had excellent cell viability and chondrogenic differentiation capacity and could form a robust cartilage tissue construct.230 Another study demonstrated that tyramine-modified hyaluronic acid (HA-TYR) microparticles fabricated using a sizing protocol were excellent bioinks for 3D printing and cartilage maturation, which was promising for cartilage tissue biofabrication.231
4.1.2.3. Tumor models. The lack of high-fidelity tumor models is one of the obstacles to improving the understanding of cancer biology. Thus, it is essential to develop ideal 3D models to mimic the tumor microenvironment and allow a reliable study of tumor features and progression.232 There have been different 3D cancer models developed such as scaffold-free tumor spheroids and scaffold-containing tumor organoids.233 The latter has some advantages compared with the conventional tumor spheroids for the scaffolds could provide an adjustable extracellular microenvironment for cancer cells. PM-based scaffolds offer a variety of biophysical properties, the capability of chemical modification, uniform and controllable size, and homogeneity in structure to ensure convincing results. Therefore, PMs have emerged as 3D biomimetic scaffolds for building 3D tumor models and developing effective therapies for cancer treatment.234 For example, PEG-fibrinogen hydrogel microspheres were generated through both emulsion and microfluidic technology which could encapsulate and maintain different types of cancer cells within this 3D spheroidal scaffold. The tumor microspheres proved to maintain higher uniformity and lower variability in diameter and circularity. The morphology of cancer cells was quite different compared with those in the conventional tumor spheroids with reduced apico-basal polarity and cellular architecture deformation which indicated malignant transformation and tumorigenic progression. These phenomena demonstrated the importance of hydrogel microspheres in the construction of 3D tumor models (Fig. 11a).235 Similarly, honeycomb-like GelMA microspheres fabricated by microfluidic technology were used to construct a 3D model of osteosarcoma, which showed more tumorigenicity and resistance to antitumor drugs compared with 2D cultured osteosarcoma cells (Fig. 11b).236 The special honeycomb-like structure could provide a larger space for tumor cell adhesion and growth. The interconnected pores could promote nutrient exchange and cytokine release, which made the microspheres a novel means for tumor-model construction in vitro. What's more, microspheres could also allow co-culture with other cell types (e.g., endothelial cells, fibroblasts and others) to fabricate a more complex and high-fidelity 3D tumor model.237,238 For example, the core–shell microspheres were fabricated using high-throughput microfluidics technology with a collagen core and an alginate shell of which the physicochemical properties could be modulated. MCF-7 cancer cells were encapsulated in the core to generate microtumors and then assembled with stromal cells (endothelial cells and ADSCs). These microspheres were used as building blocks to fabricate a 3D vascularized human mammary tumor model. Moreover, this complex vascularized tumor model was more resistant to anticancer drugs compared with 2D-cultured cancer cells and avascular microtumors which could be overcome by nanoparticle-mediated drugs, demonstrating its value in cancer study and developing effective therapies for cancer treatment (Fig. 11c).237
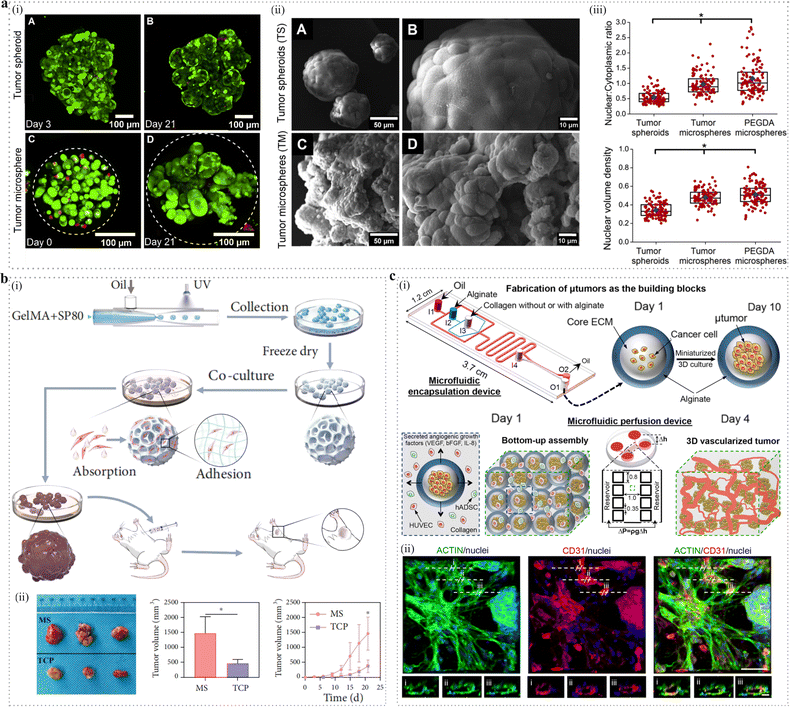 | ||
| Fig. 11 Application of PMs as microcarriers in tumor model construction. (a) (i) Confocal image of tumor spheroids and tumor microspheres stained with Live/Dead viability dyes. (ii) SEM images display ultrastructural differences between tumor spheroids and tumor microspheres. (iii) Enhanced morphometric tumorigenicity of cells in tumor microspheres.235 Reproduced with permission from ref. 235. Copyright 2017 Elsevier. (b) (i) Schematic diagram of fabrication of honeycombed microspheres and its application in tumor model construction. (ii) 3D microsphere cell culture accelerated osteosarcoma formation in mice.236 Reproduced with permission from ref. 236. Copyright 2022 American Association for the Advancement of Science. (c) (i) Schematic diagram of the bottom-up approach for creating a 3D vascularized human tumor. (ii) Confocal images of the vessel structure in micro-tumor. Scale bars: 50 μm.237 Reproduced with permission from ref. 237. Copyright 2017 American Chemical Society. | ||
In another study, pancreatic cancer cells were co-cultured with stromal fibroblasts which contributed to the deposition of extracellular matrix and generation of biomimetic microtumors.238 Except for the solid tumor model, the microspheres could also be utilized to construct a non-solid tumor model. For example, microspheres synthesized with fibronectin and hyaluronic acid allowed non-adherent multiple myeloma cells cultured in both suspension and 3D environment, which could be easily tuned with different functions.239 Thus, these microsphere-based tumor models exhibit great potential in cancer therapeutic screening and drug discovery.
4.1.2.4. Organoids. Organoids are multicellular microtissues formed by the self-organization of stem cells under the conditions of a 3D culture. They can mimic living organs and show similar functions, for example, neural activity (brain organoids), periodic contraction (cardiac organoids) and endocrine secretion (mammary gland organoids), which have great potential in developmental studies, drug screening, disease modelling and regenerative medicine.240 In conventional culture, the formation of organoids relies on the self-assembly of stem cells in a 3D animal-derived matrix like Matrigel. However, this strategy is obscured by the low yield and significant variability of the organoids due to the complex and contents unknown matrix. In comparison with animal-derived matrix, PMs, including natural (e.g., alginate) and synthetic (e.g., PEG) polymers, possess the advantages of proper permeability, uniform morphology and ability of large-scale production. They can also mimic the extracellular matrix microenvironment and possess good control over their physical and chemical properties.241 Therefore, they are recognized as ideal carriers for organoid formation and more and more research is emerging. For example, microcapsules consist of a sodium carboxymethyl cellulose core and sodium alginate shell were fabricated using the microfluidic electrospray method. These microcapsules obtained a uniform size and morphology through regulating key parameters of the microfluidic device. Then, they were applied as 3D carriers and succeeded in the formation of brain organoids from human induced pluripotent stem cells (hiPSCs), which was demonstrated by spontaneous calcium activity observed within the organoids. What's more, these microcapsules containing brain organoids could be co-cultured with endothelial cells and fibroblasts and derived vascular networks integrated with the organoids, indicating more mature of the brain tissues.242 Besides, the size of the microcapsules could be precisely controlled, making them easily administered via a syringe needle as a minimally invasive therapy. Additionally, these microspheres were ideal building blocks for constructing more complex brain organoids which consisted of cortical, thalamic and hippocampal organoids, providing a potential tool to generate artificial human brain models in an efficient and controllable way (Fig. 12a).243
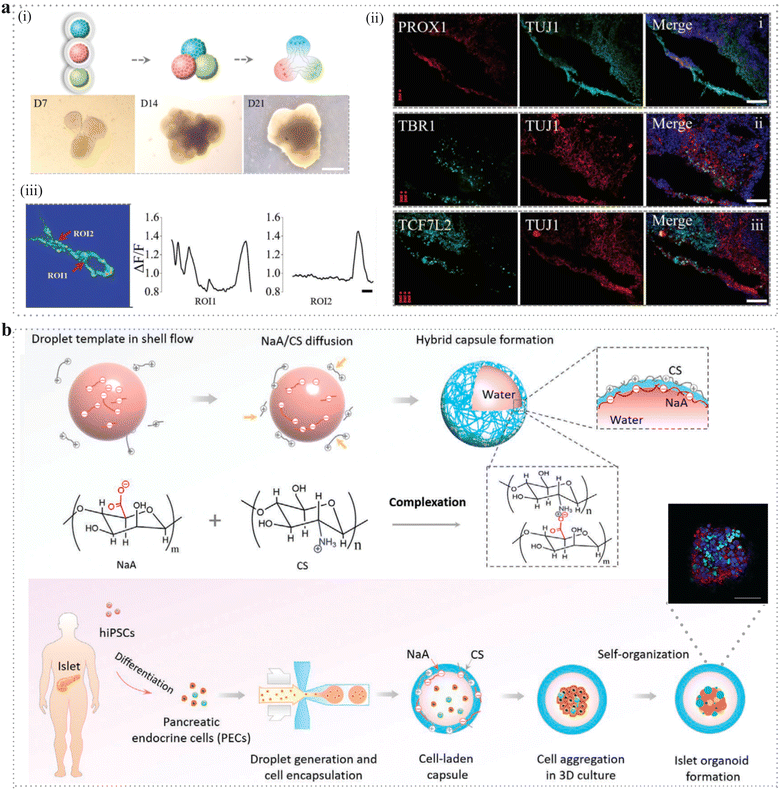 | ||
| Fig. 12 Application of PMs as microcarriers in organoid generation. (a) (i) Schematic digraph and bright-field images of cell spheroid fusion during the formation of cortical–thalamic–hippocampal assembloid. Scale bar: 500 μm. (ii) Immunofluorescence staining of brain assembloids at 25 days for hippocampal (PROX1), cortical (TBR1), and thalamic (TCF7L2) markers and neural cell marker (TUJ1). Scale bar: 100 μm. (iii) Calcium imaging of fused brain organoids at 90 days as measured by changes in fluorescence.243 Reproduced with permission from ref. 243. Copyright 2023 John Wiley and Sons. (b) Schematic diagram of the synthetic principle of hybrid microcapsules in droplet system that enable 3D culture and generation of islet organoids. Scale bar: 50 μm.244 Reproduced with permission from ref. 244. Copyright 2020 John Wiley and Sons. | ||
Additionally, the microcapsules fabricated through the microfluidic method provided a suitable environment for the formation of uniform and functional islet organoids derived from hiPSCs, which had the advantages of high uniformity and stability, and were permeable and scalable for translational applications (Fig. 12b).244 In another typical study, microcapsules with an aqueous core and an alginate shell were generated by using a flow-focusing microfluidic device. An artificial liver organ was formed by the assembly of hepatocytes in the core and fibroblasts in the shell of each microcapsule. It enabled long-period culture of the encapsulated cells for the strong and high permeability of the alginate shell. These microcapsules achieved high-level liver-specific functions of the liver organ model which could be applied for drug screening and disease therapy at an industrial scale.245 Except for large-scale production, the microspheres could also allow better cryopreservation of the organoids. In an interesting study, microcapsules with Matrigel core to support the growth of organoids and alginate shells to protect the organoids in stirred bioreactors were produced using a two-fluidic electrostatic co-spraying method. The recovery efficiency of cell viability was improved to 80% cultured in the microcapsules while it was only 20% in the bulk Matrigel group. These results suggested the microspheres were potential tools for organoid production and cryopreservation which was promising in tissue engineering in clinical application.246
4.2. Scaffold
Tissue engineering is the technology that combines the use of biomaterial scaffolds, cells and bioactive molecules to generate biological replacements for damaged tissues and organs, which is particularly important in modern medicine. Scaffold is one of the key factors in tissue engineering which provides the environment that maintains the viability of cells, guarantee the interaction between cells like adhesion and remodelling and delivering soluble factors like cytokines and growth factors that control cell behavior.247 There are a variety of scaffolds in which PMs have become popular in recent years because they allow direct filling of irregular defects by minimally invasive procedures, which is beneficial in practical clinical applications.According to the different ways of composition, microsphere-based scaffolds can be divided into two types: the first one is that microspheres serve as porogens to generate a porous one; the second one is that microspheres assemble into a bulk one (Fig. 13).
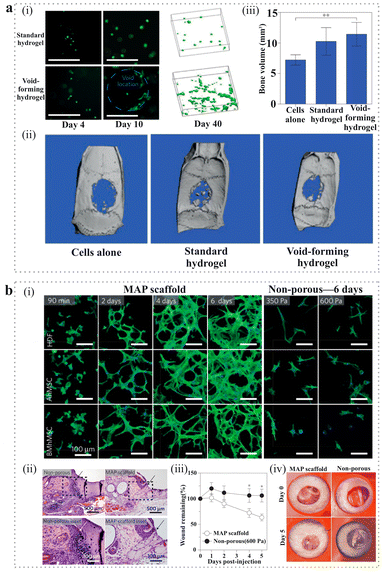 | ||
| Fig. 14 Application of PMs in scaffold construction. (a) (i) Morphology of Calcein-AM-stained mMSC in different groups at days 4 and 10. Right: 3D projections of Calcein-AM-stained cells within different groups after 40 days of in vitro culture. (ii) Representative micro-computed tomographic (μCT) images of regeneration in cranial defects in nude rats within different groups. (iii) Quantitative analysis of the total volume of newly formed bone tissue using μCT.251 Reproduced with permission from ref. 251. Copyright 2015 Springer Nature. (b) (i) Fluorescence images demonstrating the formation of 3D cellular networks during six days of culture in MAP scaffolds in vitro as well as non-porous gels after six days. Scale bars: 100 μm. (ii) Haematoxylin–eosin (HE) staining of tissue sections indicating seamless integration of the injected MAP scaffold as well as the non-porous control 24h post-injection in SKH1-Hrhr mice. (iii) Quantification of wound closure over a five-day period shows statistically significant wound-closure rates for MAP scaffolds when compared with non-porous bilateral controls. (iv) Representative images of wound closure during a five-day in vivo wound-healing model in SKH1-Hrhr mice.258 Reproduced with permission from ref. 258. Copyright 2015 Springer Nature. | ||
One strategy is to fabricate scaffolds through regulating the assembling of microspheres. Compared with a bulk injectable system, a scaffold composed of microspheres can provide sufficient mechanical stability and durability. A significant study was conducted to develop a new kind of injectable scaffold which was termed microporous annealed particle (MAP) scaffold (Fig. 14b).258 This scaffold was fabricated by microgels annealing to each other and could be injected and moulded to any shape. The highlight of MAP scaffolds was that the annealing process was mediated by enzymes via a non-canonical amide linkage between the K and Q peptides under physiological conditions, thus providing a mechanically stable scaffold of interconnected microporous networks for cell growth and precisely matching the rate of material degradation to tissue development. This characteristic was suitable for cell migration and integration with the surrounding tissue that resulting in cutaneous-tissue regeneration in a wounding healing model. Surprisingly, the scaffold not only accelerated re-epithelialization but also regenerated hair follicles and sebaceous glands which was remarkable in real tissue regeneration. This study led to several subsequent applications of MAP scaffolds in tissue engineering such as bone,259–261 cartilage,262 myocardium263 and axons.264 Except for MAP scaffolds, the microspheres could also assemble into bulk scaffolds through different ways such as 3D bioprinting,265–267 selective laser sintering (SLS)268,269 and electrostatic adsorption.264,270,271 Another strategy is to modify the surface topology, physical and chemical compositions of the PMs. There have been studies demonstrated that the chemical and physical signals could interact with receptors in cell membranes and activate intracellular signalling pathways which could regulate cell behaviors and determine cell fate.272 Thus, it is significant to fabricate PMs with proper surface topology and chemical compositions to improve cell microenvironment and facilitate tissue regeneration. For instance, PLGA/PLGA-b-PEG microspheres with controllable fuzzy surface textures were fabricated based on the interfacial instability of an emulsion. However, these microstructures were sensitive to annealing which was conquered by deposition of dopamine. Ultimately these fuzzy microspheres obtained stable microstructures on the surface, which could promote the adhesion of cells and enhance bone regeneration without exogenous cells, proving to be promising scaffolds.273 Chitosan microspheres with an ECM-mimicking nanofibrous structure were fabricated based on a microfluidic technology without introducing any toxic or denaturizing agents. The ECM-mimicking nanofibrous structure could enhance cell attachment and proliferation by providing more effective anchoring sites. These microsphere-based scaffolds had the potential for stem cells to anchor, proliferate, and pre-differentiate and demonstrated promising as modular components for tissue regeneration.261,274 Furthermore the PMs could also be fabricated into electroactive and bioactive ones and facilitate cellular activities such as cell attachment, proliferation and osteogenic differentiation for calvarial defect repair.275
Additionally, the PMs can also be combined with bioactive factors to promote tissue regeneration, such as growth factors whose clinical success is limited by their low stability, short half-life, and rapid diffusion from the delivery site. The PMs enable protection and sustained release of growth factors. The most widely explored application was in the orthopedics field, where microspheres were applied to maintain sustained release of a variety of biomolecules to enhance bone and cartilage regeneration. For example, the bone morphogenetic protein 2 (BMP-2) loaded PLGA-based microspheres have been widely used to provide sustained release of BMP-2 for bone regeneration.200,276 Also the microspheres can be fabricated with a variety of polymers like chitosan, collagen, bacterial cellulose and PLLA for loading growth factors.277–280 Mixed microsphere populations can also be used to optimize the growth factor delivery system and investigate the synergistic effect of multiple growth factors. For example, heparin methacrylamide microspheres fabricated using the W/O emulsion method were designed to load BMP-2 and vascular endothelial growth factor (VEGF) and investigate a tunable delivery system for their sustained release in a composite bone-muscle injury model. These microspheres enabled better control over growth factor delivery and helped to develop an alternative treatment strategy for bone repair.281 In another study, the dual delivery of BMP-2 and MMP-10 using PLGA microspheres promoted bone repair compared to the delivery of either growth factor alone.282 Except for growth factors, the microspheres could also be used to deliver drugs and even minerals and bioactive ions. Microspheres loaded with CaO2 were fabricated with PLGA, PLLA or gelatin to deliver oxygen in the hypoxic host area, which could significantly enhance the osteogenic and angiogenic effects and promote vascularized bone regeneration.283,284 The core–shell microspheres composed of a PLGA-MgO core and an alginate shell were fabricated with a microfluidic device and could precisely control the delivery of Mg2+ at a particular concentration, which enabled effective stimulation of in situ bone regeneration.285 What's more they can also be fabricated as gene carriers because they have disadvantages such as limited protein stability, short half-life, and high cost to deliver protein directly. For instance, chitosan microspheres loading BMP-2 plasmid were generated with microfabrication technology and emulsification method. They could release plasmids in a slow controlled way resulting in sustained secretion of the target protein and thus conducting robust bone regeneration.260
4.3. Embolic agent
Transcatheter arterial embolization (TAE) is a method in which the embolic agent is injected into the artery to selectively block the blood supply of the targeted tissue, which has been used in the treatment of cancers, bleeding, uterine fibroids and other diseases. PMs are the most commonly used embolic agents with the advantages of biocompatibility, chemical stability and easy to obtain and process. The microspheres can be synthesized by non-degradable polymers such as PVA, PEG and degradable polymers such as sodium alginate, gelatin, PLGA, which are commercially available.The non-degradable microspheres are utilized for permanent embolization so that the blockage of blood vessels is complete and non-transient, which can decrease the size of the tumor and reduce the intraoperative bleeding. The parameters of the microspheres are crucial for their clinical application, such as their size, shape, uniformity and mechanical properties.286 For example, larger microspheres tend to be retained proximally to the site of administration and thus block larger caliber vessels, whereas smaller microspheres cause catheter penetration from the distal end within the artery and ultimately block smaller caliber vessels. In addition, irregularly shaped microspheres are more likely to cluster together, so the degree of occlusion is less predictable. The elasticity determines the ability of the microspheres to pass through the delivery catheter. For example, CalliSpheres®, the first drug-loaded embolic microspheres developed in China, has excellent elasticity and can be compressed to a much smaller size, which enables it to pass through the microcatheter easily and recover to the initial size quickly without blocking the microcatheter.287
Biodegradable microspheres can cause transient embolization of blood vessels from minutes to months. The microspheres used for embolization that only lasts within 1 hour are suitable for patients with decreased organ functions and not tolerable for permanent embolization because they can protect the organ from ischaemia following TAE. For instance, Spherex® and EmboCept® are starch microspheres which can be degraded by serum amylase. The vessels can be recanalized within one hour. On the other hand, the embolic microspheres which can last from weeks to months are mainly applied to patients who need repeated embolization treatment to avoid tumor recurrence caused by vascular regeneration or incomplete blockage of tributary blood vessels. For example, Occlusin 500® is the most representative long-term temporary embolic microspheres consisting of PLGA coated with bovine collagen.288 Following injection into the target vasculature, the collagen binds circulating platelets resulting in a platelet-rich clot which occludes the target vasculature. The microspheres can slowly degrade and are removed from the body over 4–6 months.
Over the last decades, the functions of embolic microspheres are increasing, such as drug and radioisotope delivery289–291 and imageability.292,293 The most common function of embolic microspheres is drug loading and has already been commercially available, such as DC Bead®, LifePearl® and Hepasphere®.294 Current research is focused on enabling the microspheres to be loaded with more bioactive molecules to achieve more functions. For example, the embolic microspheres can also be loaded with biomolecules to improve the hypoxia microenvironment caused by embolization and in synergy with chemotherapy to achieve enhanced cancer treatment. In an interesting study, a multifunctional PVA/HA-based microsphere was fabricated through the emulsion cross-linking method, which was also a carrier of both doxorubicin and an effective hypoxia-inducible factors 2α (HIF-2α) inhibitor. This inhibitor could effectively suppress tumors through inhibiting the expression level of HIF-2α in hypoxic cancer cells and VEGF expression in combination with doxorubicin. Thus this multifunctional microsphere was a promising embolic agent for enhanced cancer treatment via the synergy of embolization, chemotherapy and hypoxia microenvironment improvement.295 Additionally, the microsphere could also be functionalized with thrombin to promote blood clotting and enhance the strength of the blood clots, thus were potential for effective hemostasis and embolization (Fig. 15a).296 What's more, researchers also functionalized the embolic microspheres with other substances such as viruses, polydopamine, and CaCO3 to achieve a combination of TAE to enhance the antitumor efficacy in cancer treatment.297–299
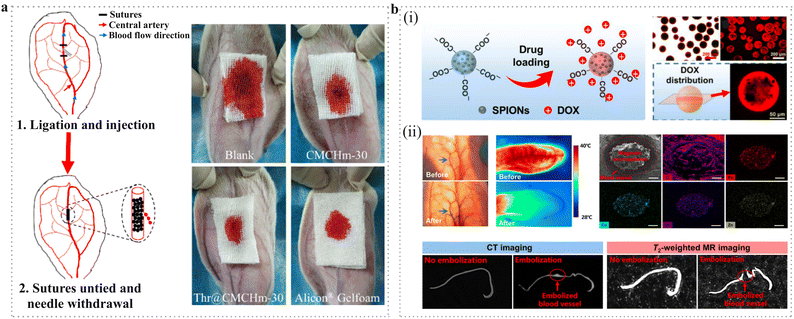 | ||
| Fig. 15 Application of PMs as an embolic agent in transcatheter arterial embolization. (a) Thrombin-functionalized carboxymethyl chitin microspheres for effective hemostasis and endovascular embolization.296 Reproduced with permission from ref. 296. Copyright 2022 Elsevier. (b) (i) Drug loading capacity of magnetic microspheres. (ii) In vivo embolization study and CT/MR imaging capacity of magnetic microspheres.302 Reproduced with permission from ref. 302. Copyright 2022 Elsevier. | ||
Another function of embolic microspheres is imaging capability. In the early times, the clinical requirement promoted the development of X-ray imageable microspheres by introducing radiopaque moieties into the microspheres.300 For instance, X-SphereTM and LC Bead LUMTM, two radiopaque microspheres have been authorized for TAE.288 Recently, studies have focused on fabricating microspheres capable of multi-modal imaging such as X-ray, computed tomography (CT), magnetic resonance imaging (MRI) and surface-enhanced Raman spectroscopy, which can realize real-time visualization and allow accurate tracking of the embolic agents.301 In recent years, researchers have attempted to develop multifunctional embolic microspheres which integrate the functions of drug loading, CT/MR dual-modality imaging, and photothermal together (Fig. 15b).302 For example, magnetic liquid metal nanoparticles were encapsulated into calcium alginate microspheres to realize the multi-functions mentioned above.303
4.4. Artificial cells
Artificial cells are micrometer-sized systems that can mimic the structure, function and behavior of living cells.304 In 1964, Chang of McGill University put forward the concept of artificial cells for the first time, who constructed a biocompatible semi-permeable membrane for encapsulating cells.305 As a simplified model, artificial cells enable a better understanding of the cellular functions and the origin of life, which can accelerate the development of biomedical applications such as disease therapy and tissue regeneration.306 According to their composition and structure, they have been classified into lipid vesicles,307 polymer vesicles,308–310 hybrid vesicles,311–313 and vesicles made of cell membrane-derived native materials (e.g., proteo-liposomes with extracted membrane proteins).314,315 In this part, we focus on artificial cells constructed by giant vesicles which are made of polymers, whose size is in the micrometer-scale and is comparable to natural cells.Lipid vesicles are the first to be studied. In recent years, more researchers have tended to use polymer or lipid–polymer hybrid vesicles to assemble artificial cells because they have higher physical and chemical stability. These polymer vesicles are mainly composed of amphiphilic block copolymers (e.g., PEO-based block copolymers, PNIPAM) which have a hydrophilic end covalently connected to the hydrophobic end.316,317 What's more, their chemical and physical properties (e.g., charge, stimulus responsiveness, membrane permeability, fluidity and intermembrane dynamics) can be easily controlled by adjusting the total molecular weight, the molecular weight distribution, the fraction of blocks and the chemical and structure of each block.18,315 For instance, the membrane permeability can either be increased or decreased by mixing with lower molecular weight analogs or utilizing high f value polymers.
In recent years, the development of stimulus-responsive artificial cells has been rapidly expanding for the purpose of sensing environmental signals or possessing more features.318 The stimuli responsiveness can be achieved by inserting molecules containing a hydrophobic domain within the polymeric membrane. For example, thermo-responsive maltotriose-b-poly(N-n-propylglycine) was utilized to fabricate solute-permeable polymer vesicles, which exhibited an LCST-like behavior as hydrophobic segments. Micrometer-scale vesicles could be prepared using the film hydration method. It was also demonstrated that electrical charge was the most important factor in determining the solute permeability.319 In another interesting study, micro-sized polymer vesicles were fabricated to mimic artificial cells through a modified double emulsion method using the block copolymer poly(butadiene)-block-poly(ethylene oxide) (PB22-b-PEO12). To conquer the low permeability of the polymeric membranes, a photo-transducer consisting of a photo-responsive spiropyran (SP) with a hydrophobic C16 tail (SP-C16) was designed to be integrated into the membranes. Only small hydrophilic molecules could pass through the polymer membrane when the vesicles were exposed to a UV light pulse. What's more, these polymer vesicles could also compartmentalize water-soluble molecules to act as light-activated microreactors and an ideal platform for the light-activated formation of internal coacervate subcompartments (Fig. 16a).309 The polymer vesicles can also be modified with protein, DNA or RNA integrated into the membrane or encapsulated inside the cavity for their specific functionality and to mimic the natural cells better. In a significant study, cytochrome bo3 ubiquinol oxidase, which was a transmembrane protein and proton pump, was integrated in the PDMS-g-PEO membranes of the polymer vesicles using a fusion/electroformation approach. This technology enabled more functions for artificial cells like membrane rearrangement, enhanced stability and fluidity, while keeping the compartments proton-tight.320 For the purpose of practical commercialization and convenience for scientific research, the polymer-constructed artificial cell membrane can also extend its stability. Recently, the Brain Science Institute of the Korea Institute of Science and Technology (KIST) reported that they had developed an artificial cell membrane that could remain stable on a silicon substrate for more than 50 days. The membrane was fabricated using an electric-field-assisted self-assembly technology with a 3-dimensional block copolymer (BCP) in a controllable and scalable way. BCPs are macromolecules composed of two or more blocks that can be repeatedly arranged into a long row of blocks with counteracting properties that mimic the hydrophilicity and hydrophobicity of human cell membranes. Additionally, they could be generated in a diversity of morphology on a microscale, such as a unilamellar spherical structure, a cilia-like elongated structure or diverse shapes with different curvatures, which was modulated by the concentration of the block copolymer and the frequency and amplitude of the electric field. This is a new achievement in the field of artificial cells which has the longest stability reported until now (Fig. 16b).18 The polymer microspheres can also be fabricated with multicompartment to resemble the multicompartmental composition of eukaryotic cells. In an interesting study, mangosteen-like microspheres with chitosan shells and multicompartmental alginate cores were generated through an oil-free gas-shearing approach. These microspheres were suitable for multiple cascade reactions to mimic the physiological functions of natural cells. This study also developed an example of artificial islet β cells which could sense high concentrations of glucose and release insulin.321
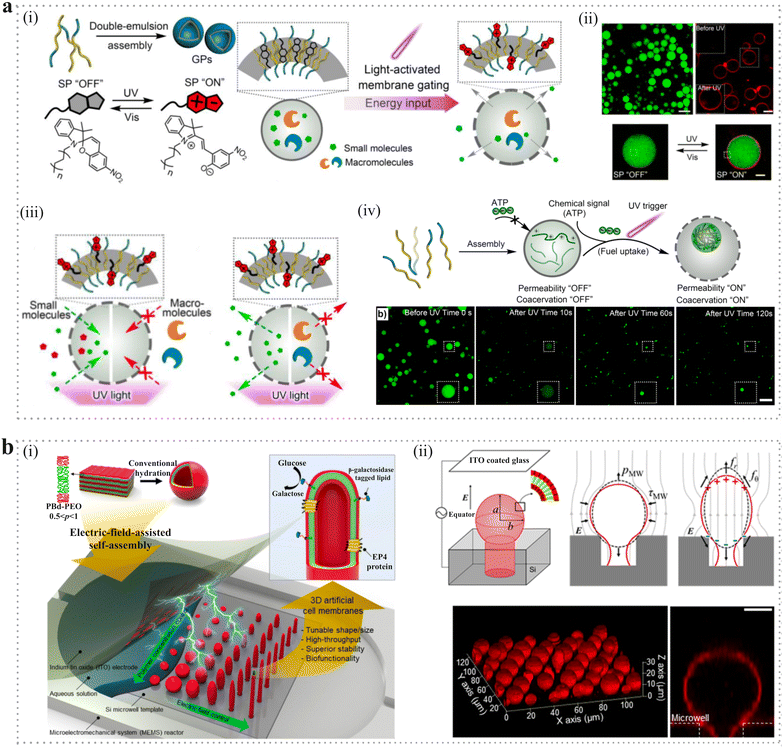 | ||
| Fig. 16 Application of PMs in artificial cells. (a) (i) Schematic diagram of design and construction of the photo-activated giant polymersomes. (ii) Reversible fluorescence “SP ON” and “SP OFF” of spiropyran-embedded polymersomes (SP-GPs) membrane upon UV and Vis light irradiation, scale bar: 20 μm. (iii) Schematic diagram of light-induced permeabilization of SP-GP and size-selective release of internal molecules and uptake of external compounds. (iv) Light-triggered formation of sub-compartments inside SP-GPs.309 Reproduced with permission from ref. 309. Copyright 2022 John Wiley and Sons. (b) (i) Schematic diagram of electric-field-assisted self-assembly method for the scalable fabrication of 3-dimensional block copolymer artificial cell membranes (3DBCPMs) with diverse shapes/sizes, high stability, and biofunctionality on silicon (Si) microwell template. (ii) Mechanism for the morphological transformation of a 3DBCPM. (iii) Confocal fluorescence microscopy image of spherical 3DBCPM array (left) and its magnified cross-sectional view (right). Scale bar: 5 μm.18 Reproduced with permission from ref. 18. Copyright 2022 Springer Nature. | ||
5. Conclusions and perspectives
In summary, this article mainly reviews from three aspects. In the first section, we introduced different PM fabrication technologies including emulsion-based methods, microfluidics, spray-drying, coacervation, supercritical fluids and superhydrophobic surface-mediated methods. We summarized the fabrication mechanism, some processing parameters, equipment and the materials used to generate the PMs. In the second section, we summed up the different structures and characteristics of the PMs including simple, hollow, porous, core–shell, Janus, complex structures and the technologies to fabricate them. Besides, we also discussed the advantages and drawbacks of each structure in biomedical applications. In the third section, we presented the latest applications of PMs in several biomedical fields, including serving as microcarriers for drugs and cells; scaffolds in tissue engineering; embolic agents and artificial cells.In terms of materials for fabricating PMs, a diverse range of natural and synthetic polymers have been employed. Natural polymers are derived from nature and are abundant from renewable sources, exhibiting high organization that renders them highly biocompatible and biodegradable. Their possession of cell-binding sites also positions them as ideal candidates for cell-related studies. However, certain limitations still impede their further applications. First, some natural polymers have limited availability and exhibit batch-to-batch variation depending on the polymer extraction process. Second, the composition of certain components remains unclear, resulting in unpredictable properties when fabricating PMs from natural polymers. Third, processing these materials can be complex at times. On the other hand, synthetic polymers offer readily available alternatives that can be prepared in a controlled manner with well-defined compositions and predictable properties such as solubility and degradability. Moreover, their physical and chemical properties can be easily tuned to meet the requirements of biomedical applications. Nevertheless, there exist drawbacks hindering their further application as well. For instance, several fabrication conditions impede their use in cell encapsulation due to the utilization of organic solvents or non-physiological pH levels along with high temperatures. Additionally, they lack inherent cell binding sites necessitating chemical modifications to enhance cell adhesion.
Regarding PM fabrication, microspheres with uniform size are critical for their application. Therefore, innovation and development of fabrication technologies that can generate PMs with more uniform size at an industrial scale are at the cutting edge of research. For example, there are many methods to generate uniform PMs from monomers. Emulsion/soap-free emulsion polymerization and dispersion/precipitation polymerization can generate uniform microspheres. However, these methods are difficult to generate larger or smaller microspheres and embed functional substances. Besides, it is difficult to regulate the morphology of the PMs and most of them are not biocompatible, which hinders them from being used as drug carriers in the human body. PMs generated from natural polymers such as polysaccharides and proteins and biodegradable polymers such as polyester have good biocompatibility which can be used in the human body. However, these methods cannot fabricate PMs with uniform sizes. Special methods should be developed to control the particle size according to the characteristics of the material. The novel membrane emulsification technology has been emerging to produce homogeneous microspheres ranging from submicrons to hundreds of micrometers in one step. Besides, it is suitable for producing microspheres from monomer systems and biodegradable polymer systems, which has become a universal chemical process. Additionally, this technology makes the droplets more stable in the process of solidification which can significantly improve the encapsulation efficiency. The commercially available equipment has enabled large-scale production and the production rate can be up to 60 L h−1 or even higher.322 However, there are still many challenges that need to be overcome in the future.
First, the chemical modification of PMs will determine their safety in vivo. Thus, it is very urgent to fabricate PMs with novel configurations through these innovative manufacturing methods which can give them more functionality, such as hollow, porous, Janus and biomimetic microspheres. Second, it is quite important to reduce the use of organic solvents in the process of fabrication and improve manufacturing efficiency, for example, using an aqueous two-phase system to prepare PMs with uniform size. Third, it is very challenging to enable the production of PMs on an industrial scale. How to make the production process in a clean, controlled setting and reduce the manufacturing cost is also a difficulty we need to overcome. For example, the industry of microcarriers for cell culture and drug delivery is thriving. It is critical to reduce the cost which is beneficial for their commercial industry to develop.
Microfluidics is also an innovative and advanced method to generate PMs. The fabrication process is controllable which enables generation of PMs with narrow size distribution and special configurations such as porous, Janus or multicompartmental PMs. However, this method is easy for laboratory production of PMs but not feasible to employ them for industry applications because the devices are not robust enough and the cost is too much. For example, PDMS or other polymer-based devices with complex geometries need to be fabricated using lithographic technologies, which is a big payout. Another obstacle is mass production for industry applications. PMs are generated droplet-by-droplet in a microfluidic device and the production rate ranges from 0.1–10 mL h−1, which is far below the traditional emulsion methods.323 While the industry scale production requires more than 1 L h−1. Therefore, the challenge remains in large-scale production at low cost to realize industrial applications.
In terms of biomedical applications, PMs used as microcarriers for drugs and cells are of great significance for modern medicine which needs to be industrially produced. However, there are still many issues that need to be addressed. As for cells, they are vulnerable to the culture conditions. Thus, the future research direction is to design the microcarriers to achieve high cell density, good cell viability and high yield of target products. For example, the surface pattern of microcarriers has to be well controlled to improve the attachment and proliferation of the cells. The mechanical properties of microcarriers should be characterized and regulated for improved cell functions because the stiffness of the microcarriers will determine cell fate. As for drugs, there have been a great number of excellent papers published. The challenge is how to develop advanced fabrication technologies and promote laboratory achievements to industrialization. PM formulations are much more complicated compared to normal injectable solutions. Manufacturing challenges such as scale up production and sterilization lengthen the duration of PM products development and decrease the chances of success. For scale-up from lab to manufacturing plant, scalable equipment plays a key role in the manufacturing process. In addition, as parenteral formulations, PMs must be produced with high purity, quality and sterility according to the pharmacopeia. For manufacturing standard parenteral solutions, sterile filtration or terminal sterilization is preferred to obtain the sterilized final product. However, these sterilization strategies cannot be used for PM products. Nevertheless, the complex and expensive manufacturing process of PM products makes it difficult for generic drug-makers to copy these products. For successful and time-saving manufacturing of PM products, close collaboration between formulation scientists, pharmaceutical equipment designers and production engineers is required at an early stage of development. Moreover, novel feasible terminal sterilization methods such as γ-irradiation should be developed for terminal sterilization of PM drug products. In conclusion, we firmly believe that addressing the aforementioned challenges necessitates interdisciplinary collaboration among researchers to discover solutions. This not only advances the development of polymeric microspheres but also unlocks exciting possibilities for their extensive utilization in the biomedical field.
Author contributions
Conceptualization and supervision: X. Deng; writing – original draft: X. B. Li, L. H. Z. Li; writing – review & editing: D. H. Wang, J. Luo, J. Zhang, K. F. Yi, Y. C. Su, F. Deng.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (22325201, 52103136, 22275028, U22A20153 and 22205033), the Sichuan Outstanding Young Scholars Foundation (2021JDJQ0013) and the Sichuan Science and Technology Program (2022JDRC0128, 2023JDRC0081); “Oncology Medical Engineering Innovation Foundation” project of University of Electronic Science and Technology of China and Sichuan Cancer Hospital (ZYGX2021YGCX009); “Medical and Industrial Cross Foundation” of University of Electronic Science and Technology of China and Sichuan Provincial People's Hospital (ZYGX2021YGLH207), Shandong Key R&D grant (2022CXGC010509).Notes and references
- D. Wang, Q. Sun, M. J. Hokkanen, C. Zhang, F. Y. Lin, Q. Liu, S. P. Zhu, T. Zhou, Q. Chang, B. He, Q. Zhou, L. Chen, Z. Wang, R. H. A. Ras and X. Deng, Nature, 2020, 582, 55–59 CrossRef CAS PubMed
.
- X. Zhang, G. Chen, Y. Yu, L. Sun and Y. Zhao, Research, 2020, 2020, 3672120 CAS
.
- K. J. Griffith, K. M. Wiaderek, G. Cibin, L. E. Marbella and C. P. Grey, Nature, 2018, 559, 556–563 CrossRef CAS PubMed
.
- E. Campos, J. Branquinho, A. S. Carreira, A. Carvalho, P. Coimbra, P. Ferreira and M. H. Gil, Eur. Polym. J., 2013, 49, 2005–2021 CrossRef CAS
.
- H. El Itawi, S. Fadlallah, F. Allais and P. Perré, Green Chem., 2022, 24, 4237–4269 RSC
.
- D. Tesfay, S. Abrha, Z. Yilma, G. Woldu and F. Molla, Biomed Res. Int., 2020, 2020, 2147971 Search PubMed
.
- M. Cirri, F. Maestrelli, S. Scuota, V. Bazzucchi and P. Mura, Int. J. Pharm., 2021, 598, 120375 CrossRef CAS PubMed
.
- L. M. Caballero Aguilar, S. Duchi, C. Onofrillo, C. D. O’Connell, C. Di Bella and S. E. Moulton, J. Colloid Interface Sci., 2021, 587, 240–251 CrossRef CAS PubMed
.
- T. Mohan, U. Ajdnik, C. Nagaraj, F. Lackner, A. Dobaj Štiglic, T. Palani, L. Amornkitbamrung, L. Gradišnik, U. Maver, R. Kargl and K. Stana Kleinschek, ACS Appl. Mater. Interfaces, 2022, 14, 3726–3739 CrossRef CAS PubMed
.
- W. Shi, Y. C. Ching and C. H. Chuah, Int. J. Biol. Macromol., 2021, 170, 751–767 CrossRef CAS PubMed
.
- R. T. Annamalai, P. A. Turner, W. F. Carson, B. Levi, S. Kunkel and J. P. Stegemann, Biomaterials, 2018, 161, 216–227 CrossRef CAS PubMed
.
- Z. Dong, X. Meng, W. Yang, J. Zhang, P. Sun, H. Zhang, X. Fang, D. A. Wang and C. Fan, Mater. Sci. Eng., C, 2021, 122, 111949 CrossRef CAS PubMed
.
- C. Snider, M. Bellrichard, A. Meyer, R. Kannan, D. Grant and S. Grant, J. Biomed. Mater. Res., Part B, 2020, 108, 2789–2798 CrossRef CAS PubMed
.
- F. Yan, B. Li, F. Shen and Q. Fu, Drug Delivery, 2015, 22, 862–868 CrossRef CAS PubMed
.
- A. Butreddy, R. P. Gaddam, N. Kommineni, N. Dudhipala and C. Voshavar, Int. J. Mol. Sci., 2021, 22, 8884 CrossRef CAS PubMed
.
- Y. Su, B. Zhang, R. Sun, W. Liu, Q. Zhu, X. Zhang, R. Wang and C. Chen, Drug Delivery, 2021, 28, 1397–1418 CrossRef CAS PubMed
.
- S. M. Jusu, J. D. Obayemi, A. A. Salifu, C. C. Nwazojie, V. Uzonwanne, O. S. Odusanya and W. O. Soboyejo, Sci. Rep., 2020, 10, 14188 CrossRef CAS PubMed
.
- D. H. Kang, W. B. Han, H. Il Ryu, N. H. Kim, T. Y. Kim, N. Choi, J. Y. Kang, Y. G. Yu and T. S. Kim, Nat. Commun., 2022, 13, 1261 CrossRef CAS PubMed
.
- J. M. Anderson and M. S. Shive, Adv. Drug Delivery Rev., 2012, 64, 72–82 CrossRef
.
- S. Chen, R. Guo, C. Xie, Q. Liang and X. Xiao, Mater. Sci. Eng., C, 2020, 110, 110655 CrossRef CAS PubMed
.
- J. Zhang, Y. Wang, Q. Qu, T. Lu, F. Li, J. Wang, A. Yang, Y. Zou and C. Huang, Macromol. Mater. Eng., 2021, 306, 2000593 CrossRef CAS
.
- W. Ramsden, Proc. R. Soc. London, 1903, 72, 156–164 CAS
.
- W. Seifriz, J. Phys. Chem., 1925, 29, 738–749 CrossRef
.
- R. H. Engel, S. J. Riggi and M. J. Fahrenbach, Nature, 1968, 219, 856–857 CrossRef CAS PubMed
.
- O. Yasuaki, Y. Masaki, H. Okada, O. Hiroaki, T. Yashiki and S. Tsugio, Chem. Pharm. Bull., 1988, 36, 1095–1103 CrossRef PubMed
.
- T. Nakashima and M. Shimizu, Pharm. Technol., Jpn., 1988, 4, 75–82 CrossRef
.
- N. Muramatsu and T. Kondo, J. Microencapsulation, 1995, 12, 129–136 CrossRef CAS PubMed
.
- J.-B. Fan, Y. Song, H. Liu, Z. Lu, F. Zhang, H. Liu, J. Meng, L. Gu, S. Wang and L. Jiang, Sci. Adv., 2017, 3, e1603203 CrossRef PubMed
.
- D. Y. Zhu, M. Z. Rong and M. Q. Zhang, Prog. Polym. Sci., 2015, 49–50, 175–220 CrossRef CAS
.
- M. N. Freitas and J. M. Marchetti, Int. J. Pharm., 2005, 295, 201–211 CrossRef CAS PubMed
.
- I. D. Rosca, F. Watari and M. Uo, J. Controlled Release, 2004, 99, 271–280 CrossRef CAS PubMed
.
- H. Raza, S. Javeria and Z. Rashid, Mater. Res. Express, 2020, 7, 015343 CrossRef CAS
.
- J. Zhu and R. C. Hayward, Angew. Chem., 2008, 120, 2143–2146 CrossRef
.
- J. Zhu and R. C. Hayward, J. Colloid Interface Sci., 2012, 365, 275–279 CrossRef CAS PubMed
.
- M. Hussain, J. Xie, K. Wang, H. Wang, Z. Tan, Q. Liu, Z. Geng, K. Shezad, L. Noureen, H. Jiang, J. Xu, L. Zhang and J. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 42734–42743 CrossRef CAS PubMed
.
- F. Zhang, J. Fan and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 21840–21856 CrossRef CAS PubMed
.
- Y. Song, J. Fan, X. Li, X. Liang and S. Wang, Adv. Mater., 2019, 31, 1900391 CrossRef PubMed
.
- K. Ishihara, Y. Narita, Y. Teramura and K. Fukazawa, ACS Biomater. Sci. Eng., 2021, 7, 5107–5117 CrossRef CAS PubMed
.
- N. Wang, X. Tian, B. Cheng, S. Guang and H. Xu, Int. J. Biol. Macromol., 2022, 220, 1329–1344 CrossRef CAS PubMed
.
- I. Capek, Adv. Colloid Interface Sci., 2010, 156, 35–61 CrossRef CAS PubMed
.
- X. Ouyang, L. Zhao, F. Jiang, J. Ling, L.-Y. Yang and N. Wang, Carbohydr. Polym., 2022, 293, 119688 CrossRef CAS PubMed
.
- Y. Chen, Z. Lu and Q. Liu, J. Membr. Sci., 2019, 592, 117384 CrossRef CAS
.
- G. Ma, J. Controlled Release, 2014, 193, 324–340 CrossRef CAS PubMed
.
- L. Wang, T. Yang and G. Ma, Curr. Pharm. Des., 2015, 21, 2563–2598 CrossRef CAS PubMed
.
- Y. Wei, Y. Wu, K. Wen, N. Bazybek and G. Ma, J. Mater. Chem. B, 2020, 8, 6322–6332 RSC
.
- X. Na, W. Zhou, T. Li, D. Hong, J. Li and G. Ma, Particuology, 2019, 44, 22–27 CrossRef CAS
.
- M. Zhang, W. Wang, R. Xie, X. Ju, Z. Liu, L. Jiang, Q. Chen and L. Chu, Particuology, 2016, 24, 18–31 CrossRef
.
- L. T. Skeggs and H. Hochstrasser, Clin. Chem., 1964, 10, 918–936 CrossRef CAS
.
- T. Thorsen, R. W. Roberts, F. H. Arnold and S. R. Quake, Phys. Rev. Lett., 2001, 86, 4163–4166 CrossRef CAS PubMed
.
- Z. Nie, S. Xu, M. Seo, P. C. Lewis and E. Kumacheva, J. Am. Chem. Soc., 2005, 127, 8058–8063 CrossRef CAS PubMed
.
- S. Da Ling, Y. Geng, A. Chen, Y. Du and J. Xu, Biomicrofluidics, 2020, 14, 061508 CrossRef PubMed
.
- A. S. Utada, E. Lorenceau, D. R. Link, P. D. Kaplan, H. A. Stone and D. A. Weitz, Science, 2005, 308, 537–541 CrossRef CAS PubMed
.
- C.-H. Chen, R. K. Shah, A. R. Abate and D. A. Weitz, Langmuir, 2009, 25, 4320–4323 CrossRef CAS PubMed
.
- W. Zeng, Z. Tong, X. Shan, H. Fu and T. Yang, Chem. Eng. Sci., 2021, 243, 116799 CrossRef CAS
.
- J. Lian, J. Wu, S. Wu, W. Yu, P. Wang, L. Liu and Q. Zuo, Colloids Surf., A, 2021, 629, 127468 CrossRef CAS
.
- Z. Yin, Z. Huang, X. Lin, X. Gao and F. Bao, Micromachines, 2020, 11, 743 CrossRef PubMed
.
- S. G. Sontti and A. Atta, Ind. Eng. Chem. Res., 2020, 59, 3702–3716 CrossRef CAS
.
- W. Zeng and H. Fu, Chem. Eng. Res. Des., 2020, 160, 321–325 CrossRef CAS
.
- C. X. Zhao, Adv. Drug Delivery Rev., 2013, 65, 1420–1446 CrossRef CAS PubMed
.
- R. K. Shah, H. C. Shum, A. C. Rowat, D. Lee, J. J. Agresti, A. S. Utada, L. Y. Chu, J. W. Kim, A. Fernandez-Nieves, C. J. Martinez and D. A. Weitz, Mater. Today, 2008, 11, 18–27 CrossRef CAS
.
- J. Shi, P. Zhu, J. Liu, R. Shen, H. Xia, H. Jiang, S. Xu and F. Zhao, Ind. Eng. Chem. Res., 2022, 61, 17593–17606 CrossRef CAS
.
- N. Leister, G. T. Vladisavljević and H. P. Karbstein, J. Colloid Interface Sci., 2022, 611, 451–461 CrossRef CAS PubMed
.
- S. Ding, C. A. Serra, T. F. Vandamme, W. Yu and N. Anton, J. Controlled Release, 2019, 295, 31–49 CrossRef CAS PubMed
.
- L. Zhang, K. Chen, H. Zhang, B. Pang, C. H. Choi, A. S. Mao, H. Liao, S. Utech, D. J. Mooney, H. Wang and D. A. Weitz, Small, 2018, 14, 1702955 CrossRef PubMed
.
- Z. Zhu, Q. Wu, S. Han, W. Xu, F. Zhong, S. Yuan, P. Dwivedi, T. Si and R. X. Xu, Sens. Actuators, B, 2018, 275, 190–198 CrossRef CAS
.
- M. I. Ré, Drying Technol., 1998, 16, 1195–1236 CrossRef
.
- E. Esposito, R. Roncarati, R. Cortesi, F. Cervellati and C. Nastruzzi, Pharm. Dev. Technol., 2000, 5, 267–278 CrossRef CAS PubMed
.
- D. Strojewski and A. Krupa, Polim. Med., 2022, 52, 101–111 CrossRef PubMed
.
- D. W. Ko, J. H. Cho and H. G. Choi, Pharm. Dev. Technol., 2021, 26, 701–708 CrossRef CAS PubMed
.
- E. Fernández-Paz, L. Feijoo-Siota, M. M. Gaspar, N. Csaba and C. Remuñán-López, Pharmaceutics, 2021, 13, 1377 CrossRef PubMed
.
- C. Bartos, P. Varga, P. Szabó-Révész and R. Ambrus, Pharmaceutics, 2021, 13, 608 CrossRef CAS PubMed
.
- H. Wei, W. Li, H. Chen, X. Wen, J. He and J. Li, Carbohydr. Polym., 2020, 241, 116351 CrossRef CAS PubMed
.
- J.-H. Kang, Y.-J. Kim, M.-S. Yang, D. H. Shin, D.-W. Kim, I. Y. Park and C.-W. Park, Pharmaceutics, 2021, 13, 1519 CrossRef CAS PubMed
.
- T. Li, B. Wan, R. Jog, A. Costa and D. J. Burgess, Int. J. Pharm., 2022, 613, 121384 CrossRef CAS PubMed
.
- N.-Q. Shi, J. Zhou, J. Walker, L. Li, J. K. Y. Hong, K. F. Olsen, J. Tang, R. Ackermann, Y. Wang, B. Qin, A. Schwendeman and S. P. Schwendeman, J. Controlled Release, 2020, 321, 756–772 CrossRef CAS PubMed
.
- R. Zhong, Q. Zhong, M. Huo, B. Yang and H. Li, Int. J. Biol. Macromol., 2020, 146, 939–945 CrossRef CAS PubMed
.
- D. M. Silva, L. G. dos Reis, M. J. Tobin, J. Vongsvivut, D. Traini and V. Sencadas, Mater. Sci. Eng., C, 2021, 122, 111831 CrossRef CAS PubMed
.
- D. Burgess, J. Colloid Interface Sci., 1990, 140, 227–238 CrossRef CAS
.
- C. G. de Kruif, F. Weinbreck and R. de Vries, Curr. Opin. Colloid Interface Sci., 2004, 9, 340–349 CrossRef CAS
.
- G. Ozkan, P. Franco, I. De Marco, J. Xiao and E. Capanoglu, Food Chem., 2019, 272, 494–506 CrossRef CAS PubMed
.
- A. M. M. Costa, L. K. Moretti, G. Simões, K. A. Silva, V. Calado, R. V. Tonon and A. G. Torres, Lwt, 2020, 131, 109519 CrossRef CAS
.
- P. Kaushik, K. Dowling, C. J. Barrow and B. Adhikari, J. Funct. Foods, 2015, 19, 868–881 CrossRef CAS
.
- C. E. Sing and S. L. Perry, Soft Matter, 2020, 16, 2885–2914 RSC
.
- Z. Yang, H. Peng, W. Wang and T. Liu, J. Appl. Polym. Sci., 2010, 116, 2658–2667 CrossRef CAS
.
- R. Wang, Q. Bao, A. G. Clark, Y. Wang, S. Zhang and D. J. Burgess, Int. J. Pharm., 2022, 628, 122292 CrossRef CAS PubMed
.
- R. Yilmaz-Ozturk, H. Calik, S. Yaman, E. Ustun-Karatop and R. Cakir-Koc, Comp. Immunol., Microbiol. Infect. Dis., 2023, 92, 101927 CrossRef CAS PubMed
.
- J. M. Unagolla and A. C. Jayasuriya, Eur. J. Pharm. Sci., 2018, 114, 199–209 CrossRef CAS PubMed
.
- N. Yaman Turan, E. Turker and Ö. Insaatci, Cellulose, 2021, 28, 4469–4483 CrossRef CAS
.
- C. Phan, T. T. C. Nguyen, T. V. T. Do and G. Tang, Rev. Mater., 2023, 28, 20230015 Search PubMed
.
- D. B. Lima, M. A. A. de Souza, G. G. de Lima, E. P. Ferreira Souto, H. M. L. Oliveira, M. V. L. Fook and M. J. C. de Sá, Carbohydr. Polym., 2020, 245, 116575 CrossRef CAS PubMed
.
- S. Ito, A. Nishiguchi, H. Ichimaru, K. Nagasaka, H. Hirade and T. Taguchi, Acta Biomater., 2022, 149, 139–149 CrossRef CAS PubMed
.
- J. B. Hannay and J. Hogarth, Proc. R. Soc. London, 1879, 30, 178–188 Search PubMed
.
- J. L. Tveekrem, S. C. Greer and D. T. Jacobs, Macromolecules, 1988, 21, 147–153 CrossRef CAS
.
- X. F. Lin, R. K. Kankala, N. Tang, P. Y. Xu, L. Z. Hao, D. Y. Yang, S. Bin Wang, Y. S. Zhang and A. Z. Chen, Adv. Healthcare Mater., 2019, 8, 1800910 CrossRef PubMed
.
- V. Prosapio, I. De Marco and E. Reverchon, Chem. Eng. J., 2016, 292, 264–275 CrossRef CAS
.
- C. A. García-González, A. Concheiro and C. Alvarez-Lorenzo, Bioconjugate Chem., 2015, 26, 1159–1171 CrossRef PubMed
.
- S. H. Soh and L. Y. Lee, Pharmaceutics, 2019, 11, 21 CrossRef CAS PubMed
.
- S. M. Abuzar, S. M. Hyun, J. H. Kim, H. J. Park, M. S. Kim, J. S. Park and S. J. Hwang, Int. J. Pharm., 2018, 538, 1–13 CrossRef CAS PubMed
.
- J. Chi, X. Zhang, Y. Wang, C. Shao, L. Shang and Y. Zhao, Mater. Horiz., 2021, 8, 124–144 RSC
.
- W. Song, A. C. Lima and J. F. Mano, Soft Matter, 2010, 6, 5868–5871 RSC
.
- E. Kobina Sam, D. Kobina Sam, X. Lv, B. Liu, X. Xiao, S. Gong, W. Yu, J. Chen and J. Liu, Chem. Eng. J., 2019, 373, 531–546 CrossRef CAS
.
- M. Zaman Khan, J. Militky, M. Petru, B. Tomková, A. Ali, E. Tören and S. Perveen, Eur. Polym. J., 2022, 178, 111481 CrossRef CAS
.
- D. Jia, Y. Lin, Y. Zou, Y. Zhang and Q. Yu, Macromol. Biosci., 2023, 23, 2300191 CrossRef CAS PubMed
.
- M. B. Oliveira, W. Song, L. Martín, S. M. Oliveira, S. G. Caridade, M. Alonso, J. C. Rodríguez-Cabello and J. F. Mano, Soft Matter, 2011, 7, 6426 RSC
.
- A. C. Lima, W. Song, B. Blanco-Fernandez, C. Alvarez-Lorenzo and J. F. Mano, Pharm. Res., 2011, 28, 1294–1305 CrossRef CAS PubMed
.
- A. M. S. Costa and J. F. Mano, J. Am. Chem. Soc., 2017, 139, 1057–1060 CrossRef CAS PubMed
.
- A. M. S. Costa, M. Alatorre-Meda, C. Alvarez-Lorenzo and J. F. Mano, Small, 2015, 11, 3648–3652 CrossRef CAS PubMed
.
- C. Yousry, M. M. Amin, A. H. Elshafeey and O. N. El Gazayerly, Drug Delivery, 2018, 25, 1448–1460 CrossRef CAS PubMed
.
- C. Yousry, I. S. Ahmed, M. M. Amin and O. N. El Gazayerly, Pharmaceutics, 2019, 11, 257 CrossRef CAS PubMed
.
- Y. Fan, D. H. Wang, J. L. Yang, J. N. Song, X. M. Li, C. L. Zhang, D. S. Wang, L. Q. Chen, J. X. Cui and X. Deng, Chinese J. Polym. Sci., 2020, 38, 1286–1293 CrossRef CAS
.
- C. Schlaich, Y. Fan, P. Dey, J. Cui, Q. Wei, R. Haag and X. Deng, Adv. Mater. Interfaces, 2018, 5, 1701536 CrossRef
.
- A. M. S. Costa, M. Alatorre-Meda, N. M. Oliveira and J. F. Mano, Langmuir, 2014, 30, 4535–4539 CrossRef CAS PubMed
.
- J. Song, W. Zhang, D. Wang, Y. Fan, C. Zhang, D. Wang, L. Chen, B. Miao, J. Cui and X. Deng, Adv. Mater., 2021, 33, 2007154 CrossRef CAS PubMed
.
- R. Luo, Y. Cao, P. Shi and C. H. Chen, Small, 2014, 10, 4886–4894 CrossRef CAS PubMed
.
- A. C. Lima, C. A. Custõdio, C. Alvarez-Lorenzo and J. F. Mano, Small, 2013, 9, 2487–2492 CrossRef CAS PubMed
.
- K. Saralidze, L. H. Koole and M. L. W. Knetsch, Materials, 2010, 3, 3537–3564 CrossRef CAS
.
- K. M. Z. Hossain, U. Patel and I. Ahmed, Prog. Biomater., 2015, 4, 1–19 CrossRef CAS PubMed
.
- C. Jiang, L. Kuang, M. P. Merkel, F. Yue, M. A. Cano-Vega, N. Narayanan, S. Kuang and M. Deng, Front. Endocrinol., 2015, 6, 169 Search PubMed
.
- X. Liu, X. Jin and P. X. Ma, Nat. Mater., 2011, 10, 398–406 CrossRef CAS PubMed
.
- Z. Li, H. Liu, L. Zeng, H. Liu, S. Yang and Y. Wang, Langmuir, 2014, 30, 12154–12163 CrossRef CAS PubMed
.
- N. D. Dinh, M. Kukumberg, A. T. Nguyen, H. Keramati, S. Guo, D. T. Phan, N. B. Ja’afar, E. Birgersson, H. L. Leo, R. Y. J. Huang, T. Kofidis, A. J. Rufaihah and C. H. Chen, Lab Chip, 2020, 20, 2756–2764 RSC
.
- M. Marquis, D. Renard and B. Cathala, Biomacromolecules, 2012, 13, 1197–1203 CrossRef CAS PubMed
.
- F. Cheng, T. Su, Y. Pu, W. Gao and B. He, Macromol. Biosci., 2019, 19, 1900171 CrossRef CAS PubMed
.
- M. S. B. Reddy, D. Ponnamma, R. Choudhary and K. K. Sadasivuni, Polymers, 2021, 13, 1105 CrossRef CAS PubMed
.
- E. S. Kim, M. S. Lee, H. Jeong, S. Y. Lim, D. Kim, D. Kim, J. Jung, S. Lyu, H. J. Cho, D. M. Kim, W. Suh and J. H. Jeong, Pharmaceutics, 2021, 13, 1548 CrossRef CAS PubMed
.
- S. Deng, X. Zhao, Y. Zhu, N. Tang, R. Wang, X. Zhang, F. Qu, Y.-P. Ho, W. Y.-W. Lee, J. Chen, M. Li, Y. Tao and H. F. Chan, Biofabrication, 2023, 15, 015016 CrossRef CAS PubMed
.
- H. Zou and K. Shang, Mater. Chem. Front., 2021, 5, 3765–3787 RSC
.
- W. Wichaita, D. Polpanich and P. Tangboriboonrat, Ind. Eng. Chem. Res., 2019, 58, 20880–20901 CrossRef CAS
.
- J. Yu, T. R. Huang, Z. H. Lim, R. Luo, R. R. Pasula, L. De Liao, S. Lim and C. H. Chen, Adv. Healthcare Mater., 2016, 5, 2983–2992 CrossRef CAS PubMed
.
- Y. Wu, H. Chang, J. Peng, Y. Liu, B. Sun, Z. Yang, S. Gao and F. Liu, Polym. Bull., 2023, 80, 3675–3688 CrossRef CAS
.
- J. Wang, Z. Wang, D. Mao and D. Wang, Sci. China: Chem., 2022, 65, 7–19 CrossRef CAS
.
- S. Meng, S. Wang and M. G. Piao, J. Drug Delivery Sci. Technol., 2022, 70, 103235 CrossRef CAS
.
- C. Xie, Q. Xiong, Y. Wei, X. Li, J. Hu, M. He, S. Wei, J. Yu, S. Cheng, M. Ahmad, Y. Liu, S. Luo, X. Zeng, J. Yu and H. Luo, Mater. Today Bio, 2023, 20, 100628 CrossRef CAS PubMed
.
- J. Wu, X. Wang, H. Li, M. Qu, W. Sun, X. Yan, Z. Zhao and B. Li, J. Drug Delivery Sci. Technol., 2022, 73, 103482 CrossRef CAS
.
- Y. Si, M. Chen and L. Wu, Chem. Soc. Rev., 2016, 45, 690–714 RSC
.
- L. Tan and B. Tan, Polym. Chem., 2021, 12, 2689–2694 RSC
.
- T. Omura, T. Suzuki and H. Minami, Langmuir, 2021, 37, 9371–9377 CrossRef CAS PubMed
.
- T. Omura, T. Suzuki and H. Minami, Langmuir, 2020, 36, 14076–14082 CrossRef CAS PubMed
.
- N. R. Visaveliya, C. W. Leishman, K. Ng, N. Yehya, N. Tobar, D. M. Eisele and J. M. Köhler, Adv. Mater. Interfaces, 2017, 4, 1700929 CrossRef
.
- D. Ghosh Dastidar, S. Saha and M. Chowdhury, Int. J. Pharm., 2018, 548, 34–48 CrossRef CAS PubMed
.
- Y. Xu, Y. Gu, F. Cai, K. Xi, T. Xin, J. Tang, L. Wu, Z. Wang, F. Wang, L. Deng, C. L. Pereira, B. Sarmento, W. Cui and L. Chen, Adv. Funct. Mater., 2020, 30, 2006333 CrossRef CAS
.
- C. Zhang, Z. Zhou, W. Liu, T. Huang, Y. Zhao, P. Chen, Z. Zhou, D. Wang, M. Yi and J. Fang, J. Macromol. Sci., Part B: Phys., 2021, 60, 313–323 CrossRef CAS
.
- B. Xiong, Y. Chen, Y. Liu, X. Hu, H. Han and Q. Li, Colloids Surf., B, 2021, 206, 111937 CrossRef CAS PubMed
.
- F. Leng, F. Chen and X. Jiang, Carbohydr. Polym., 2021, 270, 118348 CrossRef CAS PubMed
.
- Q. Yao, Y. Liu, Y. Pan, J. M. Miszuk and H. Sun, J. Biomed. Mater. Res., Part B, 2020, 108, 2699–2710 CrossRef CAS PubMed
.
- S. Kim and H. Sah, J. Biomater. Sci., Polym. Ed., 2019, 30, 1725–1743 CrossRef CAS PubMed
.
- Z. Chen, Z. Lv, Y. Zhuang, Q. Saiding, W. Yang, W. Xiong, Z. Zhang, H. Chen, W. Cui and Y. Zhang, Adv. Mater., 2023, 35, 2300180 CrossRef CAS PubMed
.
- X. Wang, C. Mao, Q. Li and R. Wang, J. Polym. Res., 2022, 29, 314 CrossRef CAS
.
- S. Wang, H. Liu, D. Wu and X. Wang, J. Colloid Interface Sci., 2021, 583, 470–486 CrossRef CAS PubMed
.
- J. Huang, D. Fu, X. Wu, Y. Li, B. Zheng, Z. Liu, Y. Zhou, Y. Gan, Y. Miao and Z. Hu, Biofabrication, 2023, 15, 025007 CrossRef PubMed
.
- Q. Meng, S. Zhong, J. Wang, Y. Gao and X. Cui, Carbohydr. Polym., 2023, 300, 120265 CrossRef CAS PubMed
.
- L. Yang, Y. Liu, L. Sun, C. Zhao, G. Chen and Y. Zhao, Nano-Micro Lett., 2022, 14, 4 CrossRef CAS PubMed
.
- W. Jiang, Z. Wu, Z. Gao, M. Wan, M. Zhou, C. Mao and J. Shen, ACS Nano, 2022, 16, 15705–15733 CrossRef CAS PubMed
.
- Y. Wang, L. Zhao, L. Zhou, C. Chen and G. Chen, Int. J. Biol. Macromol., 2023, 232, 123330 CrossRef CAS PubMed
.
- Q. Chen, N. Singh, K. Schirrmann, Q. Zhou, I. L. Chernyavsky and A. Juel, Soft Matter, 2023, 19, 5249–5261 RSC
.
- Z. Liu, D. J. McClements, A. Shi, L. Zhi, Y. Tian, B. Jiao, H. Liu and Q. Wang, Crit. Rev. Food Sci. Nutr., 2022, 10093–10104 Search PubMed
.
- E. Poggi and J. F. Gohy, Colloid Polym. Sci., 2017, 295, 2083–2108 CrossRef CAS
.
- M. Ando, M. Tsuchiya, S. Itai, T. Murayama, Y. Kurashina, Y. J. Heo and H. Onoe, Sensors, 2021, 21, 4829 CrossRef CAS PubMed
.
- A. Matsumoto, C. Watanabe and M. Murakami, Drug Discoveries Ther., 2019, 13, 343–353 CrossRef CAS PubMed
.
- W. Wang, B. Y. Li, M. J. Zhang, Y. Y. Su, D. W. Pan, Z. Liu, X. J. Ju, R. Xie, Y. Faraj and L. Y. Chu, Chem. Eng. J., 2023, 452, 139277 CrossRef CAS
.
- L. Huang, K. Wu, R. Zhang and H. Ji, Ind. Eng. Chem. Res., 2019, 58, 17017–17026 CrossRef CAS
.
- R. J. R. W. Peters, M. Marguet, S. Marais, M. W. Fraaije, J. C. M. Van Hest and S. Lecommandoux, Angew. Chem., Int. Ed., 2014, 53, 146–150 CrossRef CAS PubMed
.
- J. W. Kim, S. H. Han, Y. H. Choi, W. M. Hamonangan, Y. Oh and S. H. Kim, Lab Chip, 2022, 22, 2259–2291 RSC
.
- C. L. Mou, W. Wang, X. J. Ju, R. Xie, Z. Liu and L. Y. Chu, J. Taiwan Inst. Chem. Eng., 2019, 98, 63–69 CrossRef CAS
.
- Y. Guo, H. Feng, W. Li, W. Wang, M. Yu and S. Chen, Biochem. Eng. J., 2023, 196, 108956 CrossRef CAS
.
- J. A. Floyd, A. Galperin and B. D. Ratner, Adv. Drug Delivery Rev., 2015, 91, 23–37 CrossRef CAS PubMed
.
- Z. Zhu, Q. Yu, H. Li, F. Han, Q. Guo, H. Sun, H. Zhao, Z. Tu, Z. Liu, C. Zhu and B. Li, Bioact. Mater., 2023, 28, 167–182 CAS
.
- A. McMillan, M. K. Nguyen, C. T. Huynh, S. M. Sarett, P. Ge, M. Chetverikova, K. Nguyen, D. Grosh, C. L. Duvall and E. Alsberg, Acta Biomater., 2021, 124, 315–326 CrossRef CAS PubMed
.
- W. Li, S. Chen, L. Zhang, Y. Zhang, X. Yang, B. Xie, J. Guo, Y. He and C. Wang, Colloids Surf., B, 2020, 196, 111350 CrossRef CAS PubMed
.
- J. Gan, L. Sun, G. Chen, W. Ma, Y. Zhao and L. Sun, Adv. Healthcare Mater., 2022, 11, 2201105 CrossRef CAS PubMed
.
- S. E. Bae, J. S. Son, K. Park and D. K. Han, J. Controlled Release, 2009, 133, 37–43 CrossRef CAS PubMed
.
- M. Lengyel, N. Kállai-Szabó, V. Antal, A. J. Laki and I. Antal, Sci. Pharm., 2019, 87, 20 CrossRef CAS
.
- J. Guo, X. Sun, H. Yin, T. Wang, Y. Li, C. Zhou, H. Zhou, S. He and H. Cong, Front. Cell. Infect. Microbiol., 2018, 8, 163 CrossRef PubMed
.
- V. R. Sinha, A. K. Singla, S. Wadhawan, R. Kaushik, R. Kumria, K. Bansal and S. Dhawan, Int. J. Pharm., 2004, 274, 1–33 CrossRef CAS PubMed
.
- W. Khan, R. Kumar, S. Singh, S. K. Arora and N. Kumar, Drug Test. Anal., 2013, 5, 468–473 CrossRef CAS PubMed
.
- Y. Xu, Y. Liu, Q. Liu, S. Lu, X. Chen, W. Xu and F. Shi, J. Controlled Release, 2021, 338, 705–718 CrossRef CAS PubMed
.
- Y. Jin, I. Y. Kim, I. D. Kim, H. K. Lee, J. Y. Park, P. L. Han, K. K. Kim, H. Choi and J. K. Lee, Acta Biomater., 2014, 10, 3126–3135 CrossRef CAS PubMed
.
- Y. Gu, G. Wen, H. Zhao, H. Qi, Y. Yang and T. Hu, Mol. Med. Rep., 2023, 28, 137 CrossRef CAS PubMed
.
- A. K. Kudva, A. D. Dikina, F. P. Luyten, E. Alsberg and J. Patterson, Acta Biomater., 2019, 90, 287–299 CrossRef CAS PubMed
.
- S. S. Panchal and D. V. Vasava, ACS Omega, 2020, 5, 4370–4379 CrossRef CAS PubMed
.
- H. K. Makadia and S. J. Siegel, Polymers, 2011, 3, 1377–1397 CrossRef CAS PubMed
.
- F. Y. Han, K. J. Thurecht, A. K. Whittaker and M. T. Smith, Front. Pharmacol., 2016, 7, 185 Search PubMed
.
- N. Y. Abu-Thabit and A. S. H. Makhlouf, Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, Polymers, 2018, 1, 3–41 CAS
.
- D. Essa, P. P. D. Kondiah, Y. E. Choonara and V. Pillay, Front. Bioeng. Biotechnol., 2020, 8, 48 CrossRef PubMed
.
- P. Gentile, V. Chiono, I. Carmagnola and P. V. Hatton, Int. J. Mol. Sci., 2014, 15, 3640–3659 CrossRef CAS PubMed
.
- S. Feng, F. Lu, Y. Wang and J. Suo, J. Appl. Polym. Sci., 2015, 132, 41943 CrossRef
.
- D. Klose, F. Siepmann, K. Elkharraz and J. Siepmann, Int. J. Pharm., 2008, 354, 95–103 CrossRef CAS PubMed
.
- G. J. S. Dawes, L. E. Fratila-Apachitei, K. Mulia, I. Apachitei, G. J. Witkamp and J. Duszczyk, J. Mater. Sci.: Mater. Med., 2009, 20, 1089–1094 CrossRef CAS PubMed
.
- A. Vlachopoulos, G. Karlioti, E. Balla, V. Daniilidis, T. Kalamas, M. Stefanidou, N. D. Bikiaris, E. Christodoulou, I. Koumentakou, E. Karavas and D. N. Bikiaris, Pharmaceutics, 2022, 14, 359 CrossRef CAS PubMed
.
- F. Wan and M. Yang, Int. J. Pharm., 2016, 498, 82–95 CrossRef CAS PubMed
.
- C. I. Nkanga, A. Fisch, M. Rad-Malekshahi, M. D. Romic, B. Kittel, T. Ullrich, J. Wang, R. W. M. Krause, S. Adler, T. Lammers, W. E. Hennink and F. Ramazani, Adv. Drug Delivery Rev., 2020, 167, 19–46 CrossRef CAS PubMed
.
- E. A. Lundström, R. K. Rencken, J. H. van Wyk, L. J. E. Coetzee, J. C. M. Bahlmann, S. Reif, E. A. Strasheim, M. C. Bigalke, A. R. Pontin, L. Goedhals, D. G. Steyn, C. F. Heyns, L. A. Aldera, T. M. Mackenzie, D. Purcea, P. Y. Grosgurin and H. C. Porchet, Clin. Drug Investig., 2009, 29, 757–765 CrossRef PubMed
.
- Label of LUPRON DEPOT (leuprolide acetate for depot suspension). Reference ID: 4398579, FDA.
- K. Park, S. Skidmore, J. Hadar, J. Garner, H. Park, A. Otte, B. K. Soh, G. Yoon, D. Yu, Y. Yun, B. K. Lee, X. Jiang and Y. Wang, J. Controlled Release, 2019, 304, 125–134 CrossRef CAS PubMed
.
- V. Bunpetch, H. Wu, S. Zhang and H. Ouyang, Stem Cells Dev., 2017, 26, 1662–1673 CrossRef PubMed
.
- E. O. Ojo, A. A. Sharma, R. Liu, S. Moreton, M. A. Checkley-Luttge, K. Gupta, G. Lee, D. A. Lee, F. Otegbeye, R. P. Sekaly, M. de Lima and D. N. Wald, Sci. Rep., 2019, 9, 14916 CrossRef PubMed
.
- S. Sulaiman, S. R. Chowdhury, M. B. Fauzi, R. A. Rani, N. H. Mohamadyahaya, Y. Tabata, Y. Hiraoka, R. B. H. Idrus and N. M. Hwei, Int. J. Mol. Sci., 2020, 21, 2688 CrossRef CAS PubMed
.
- W. Kim, Y. Gwon, S. Park, H. Kim and J. Kim, Bioact. Mater., 2023, 19, 50–74 CAS
.
- W. J. Seeto, Y. Tian, S. Pradhan, P. Kerscher and E. A. Lipke, Small, 2019, 15, 1902058 CrossRef CAS PubMed
.
- D. Wei, R. Qiao, J. Dao, J. Su, C. Jiang, X. Wang, M. Gao and J. Zhong, Small, 2018, 14, 1800063 CrossRef PubMed
.
- H. F. Darge, Y. H. Lin, T. Hsieh-Chih, S. Y. Lin and M. C. Yang, Biomater. Adv., 2022, 139, 213008 CrossRef CAS PubMed
.
- Q. He, Y. Liao, J. Zhang, X. Yao, W. Zhou, Y. Hong and H. Ouyang, Small, 2020, 16, 1906539 CrossRef CAS PubMed
.
- M. D. Neto, M. B. Oliveira and J. F. Mano, Trends Biotechnol., 2019, 37, 1011–1028 CrossRef CAS PubMed
.
- A. YekrangSafakar, A. Acun, J. W. Choi, E. Song, P. Zorlutuna and K. Park, Biotechnol. Bioeng., 2018, 115, 1717–1728 CrossRef CAS PubMed
.
- P. J. R. Cohen, E. Luquet, J. Pletenka, A. Leonard, E. Warter, B. Gurchenkov, J. Carrere, C. Rieu, J. Hardouin, F. Moncaubeig, M. Lanero, E. Quelennec, H. Wurtz, E. Jamet, M. Demarco, C. Banal, P. Van Liedekerke, P. Nassoy, M. Feyeux, N. Lefort and K. Alessandri, Biomaterials, 2023, 295, 122033 CrossRef CAS PubMed
.
- Z. Jin, Y. Zhai, Y. Zhou, P. Guo, M. Chai, W. Tan, Y. Zhou and L. Cen, Chem. Eng. J., 2022, 448, 137739 CrossRef CAS
.
- Z. Zhou, W. Wu, J. Fang and J. Yin, Int. Mater. Rev., 2021, 66, 77–113 CrossRef CAS
.
- N. Sarviya, S. M. Basu, R. Mani, M. Chauhan, P. Kingshott and J. Giri, Biomater. Adv., 2022, 139, 212981 CrossRef CAS PubMed
.
- I. Mawji, E. L. Roberts, T. Dang, B. Abraham and M. S. Kallos, Biotechnol. Bioeng., 2022, 119, 3062–3078 CrossRef CAS PubMed
.
- S. Wu, Z. Wang, Y. Wang, M. Guo, M. Zhou, L. Wang, J. Ma and P. Zhang, Front. Bioeng. Biotechnol., 2022, 10, 1–16 Search PubMed
.
- S. Zhang, B. Ma, S. Wang, J. Duan, J. Qiu, D. Li, Y. Sang, S. Ge and H. Liu, Chem. Eng. J., 2018, 331, 675–684 CrossRef CAS
.
- H. Tanimowo Aiyelabegan, M. Ebadi, G. Ali Kardar, N. Lotfibakhshaiesh, F. Abedin Dorkoosh, S. Ebrahimi_Barough and E. Sadroddiny, Int. J. Polym. Mater. Polym.
Biomater., 2020, 69, 373–380 CrossRef CAS
.
- C. D. L. Johnson, N. E. Zale, E. D. Frary and J. A. Lomakin, Front. Immunol., 2022, 13, 13–16 Search PubMed
.
- S. L. Ding, X. Liu, X. Y. Zhao, K. T. Wang, W. Xiong, Z. L. Gao, C. Y. Sun, M. X. Jia, C. Li, Q. Gu and M. Z. Zhang, Bioact. Mater., 2022, 17, 81–108 CAS
.
- B. A. Aguado, W. Mulyasasmita, J. Su, K. J. Lampe and S. C. Heilshorn, Tissue Eng., Part A, 2012, 18, 806–815 CrossRef CAS PubMed
.
- D. X. Wei, J. W. Dao and G. Q. Chen, Adv. Mater., 2018, 30, 1802273 CrossRef PubMed
.
- Z. Yuan, X. Yuan, Y. Zhao, Q. Cai, Y. Wang, R. Luo, S. Yu, Y. Wang, J. Han, L. Ge, J. Huang and C. Xiong, Small, 2021, 17, 2006596 CrossRef CAS PubMed
.
- S. Ding, X. Zhao, W. Xiong, L. Ji, M. Jia, Y. Liu, H. Guo, F. Qu, W. Cui, Q. Gu and M. Zhang, Adv. Mater., 2023, 35, 2212114 CrossRef CAS PubMed
.
- Y. Zheng, Z. Wu, Y. Hou, N. Li, Q. Zhang and J. M. Lin, ACS Appl. Mater. Interfaces, 2023, 15, 7833–7840 CrossRef CAS PubMed
.
- M. H. Amer, M. Alvarez-Paino, J. McLaren, F. Pappalardo, S. Trujillo, J. Q. Wong, S. Shrestha, S. Abdelrazig, L. A. Stevens, J. B. Lee, D.-H. Kim, C. González-García, D. Needham, M. Salmerón-Sánchez, K. M. Shakesheff, M. R. Alexander, C. Alexander and F. R. Rose, Biomaterials, 2021, 266, 120450 CrossRef CAS PubMed
.
- A. Latif, L. E. Fisher, A. A. Dundas, V. Cuzzucoli Crucitti, Z. Imir, K. Lawler, F. Pappalardo, B. W. Muir, R. Wildman, D. J. Irvine, M. R. Alexander and A. M. Ghaemmaghami, Adv. Mater., 2022, 2208364, 1–13 Search PubMed
.
- Q. He, J. Zhang, Y. Liao, E. V. Alakpa, V. Bunpetch, J. Zhang and H. Ouyang, Biotechnol. Adv., 2020, 39, 107459 CrossRef CAS PubMed
.
- J. Li, A. T.-L. Lam, J. P. W. Toh, S. Reuveny, S. K.-W. Oh and W. R. Birch, Langmuir, 2017, 33, 3068–3079 CrossRef CAS PubMed
.
- D. Wu, Y. Yu, C. Zhao, X. Shou, Y. Piao, X. Zhao, Y. Zhao and S. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 33716–33724 CrossRef CAS PubMed
.
- C. Zhao, S. Tian, Q. Liu, K. Xiu, I. Lei, Z. Wang and P. X. Ma, Adv. Funct. Mater., 2020, 30, 2000776 CrossRef CAS PubMed
.
- J. Yang, J. Liang, Y. Zhu, M. Hu, L. Deng, W. Cui and X. Xu, Bioact. Mater., 2021, 6, 4801–4815 CAS
.
- R. T. Annamalai, X. Hong, N. G. Schott, G. Tiruchinapally, B. Levi and J. P. Stegemann, Biomaterials, 2019, 208, 32–44 CrossRef CAS PubMed
.
- F. Li, V. X. Truong, P. Fisch, C. Levinson, V. Glattauer, M. Zenobi-Wong, H. Thissen, J. S. Forsythe and J. E. Frith, Acta Biomater., 2018, 77, 48–62 CrossRef CAS PubMed
.
- H. Zhao, Z. Wang, S. Jiang, J. Wang, Z. Hu, P. E. Lobie and S. Ma, Cell Rep. Phys. Sci., 2020, 1, 100047 CrossRef
.
- X. Cui, C. R. Alcala-Orozco, K. Baer, J. Li, C. A. Murphy, M. Durham, G. Lindberg, G. J. Hooper, K. S. Lim and T. B. F. Woodfield, Biofabrication, 2022, 14, 034101 CrossRef CAS PubMed
.
- K. Flégeau, A. Puiggali-Jou and M. Zenobi-Wong, Biofabrication, 2022, 14, 034105 CrossRef PubMed
.
- Y. Li and E. Kumacheva, Sci. Adv., 2018, 4, eaas8998 CrossRef PubMed
.
- A. K. Badekila, S. Kini and A. K. Jaiswal, J. Cell. Physiol., 2021, 236, 741–762 CrossRef CAS PubMed
.
- K. Alessandri, B. R. Sarangi, V. V. Gurchenkov, B. Sinha, T. R. Kießling, L. Fetler, F. Rico, S. Scheuring, C. Lamaze, A. Simon, S. Geraldo, D. Vignjević, H. Doméjean, L. Rolland, A. Funfak, J. Bibette, N. Bremond and P. Nassoy, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 14843–14848 CrossRef CAS PubMed
.
- S. Pradhan, J. M. Clary, D. Seliktar and E. A. Lipke, Biomaterials, 2017, 115, 141–154 CrossRef CAS PubMed
.
- J. He, C. Chen, L. Chen, R. Cheng, J. Sun, X. Liu, L. Wang, C. Zhu, S. Hu, Y. Xue, J. Lu, H. Yang, W. Cui and Q. Shi, Research, 2022, 2022, 9809763 CAS
.
- P. Agarwal, H. Wang, M. Sun, J. Xu, S. Zhao, Z. Liu, K. J. Gooch, Y. Zhao, X. Lu and X. He, ACS Nano, 2017, 11, 6691–6702 CrossRef CAS PubMed
.
- V. Brancato, V. Comunanza, G. Imparato, D. Corà, F. Urciuolo, A. Noghero, F. Bussolino and P. A. Netti, Acta Biomater., 2017, 49, 152–166 CrossRef CAS PubMed
.
- S. Clara-Trujillo, L. Tolosa, L. Cordón, A. Sempere, G. Gallego Ferrer and J. L. Gómez Ribelles, Biomater. Adv., 2022, 135, 212749 CrossRef CAS PubMed
.
- G. Fang, Y. C. Chen, H. Lu and D. Jin, Adv. Funct. Mater., 2023, 33, 2215043 CrossRef CAS
.
- Z. Gan, X. Qin, H. Liu, J. Liu and J. Qin, Bioact. Mater., 2023, 28, 386–401 CAS
.
- Y. Zhu, L. Sun, X. Fu, J. Liu, Z. Liang, H. Tan, W. Li and Y. Zhao, Chem. Eng. J., 2021, 424, 130427 CrossRef CAS
.
- Y. Zhu, X. Zhang, L. Sun, Y. Wang and Y. Zhao, Adv. Mater., 2023, 35, 2210083 CrossRef CAS PubMed
.
- H. Liu, Y. Wang, H. Wang, M. Zhao, T. Tao, X. Zhang and J. Qin, Adv. Sci., 2020, 7, 1903739 CrossRef CAS PubMed
.
- Q. Chen, S. Utech, D. Chen, R. Prodanovic, J. M. Lin and D. A. Weitz, Lab Chip, 2016, 16, 1346–1349 RSC
.
- Y. C. Lu, D. J. Fu, D. An, A. Chiu, R. Schwartz, A. Y. Nikitin and M. Ma, Adv. Biosyst., 2017, 1, 1700165 CrossRef PubMed
.
- A. K. Gaharwar, I. Singh and A. Khademhosseini, Nat. Rev. Mater., 2020, 5, 686–705 CrossRef CAS
.
- C. Fan and D. A. Wang, Tissue Eng., Part B, 2017, 23, 451–461 CrossRef CAS PubMed
.
- S. J. Hollister, Nat. Mater., 2005, 4, 518–524 CrossRef CAS PubMed
.
- G. Lutzweiler, A. N. Halili and N. E. Vrana, Pharmaceutics, 2020, 12, 602 CrossRef CAS PubMed
.
- N. Huebsch, E. Lippens, K. Lee, M. Mehta, S. T. Koshy, M. C. Darnell, R. M. Desai, C. M. Madl, M. Xu, X. Zhao, O. Chaudhuri, C. Verbeke, W. S. Kim, K. Alim, A. Mammoto, D. E. Ingber, G. N. Duda and D. J. Mooney, Nat. Mater., 2015, 14, 1269–1277 CrossRef CAS PubMed
.
- S. Khan, M. Ul-Islam, M. Ikram, S. U. Islam, M. W. Ullah, M. Israr, J. H. Jang, S. Yoon and J. K. Park, Int. J. Biol. Macromol., 2018, 117, 1200–1210 CrossRef CAS PubMed
.
- J. Fu, C. Wiraja, H. B. Muhammad, C. Xu and D. A. Wang, Acta Biomater., 2017, 58, 225–237 CrossRef CAS PubMed
.
- I. Eugenis, D. Wu, C. Hu, G. Chiang, N. F. Huang and T. A. Rando, Biomaterials, 2022, 290, 121818 CrossRef CAS PubMed
.
- L. Shao, Q. Gao, C. Xie, J. Fu, M. Xiang, Z. Liu, L. Xiang and Y. He, Bio-Des. Manuf., 2020, 3, 30–39 CrossRef CAS
.
- T. Lu, S. Feng, F. He and J. Ye, J. Biomed. Mater. Res., Part A, 2020, 108, 645–653 CrossRef CAS PubMed
.
- I. Lukin, I. Erezuma, L. Maeso, J. Zarate, M. F. Desimone, T. H. Al-Tel, A. Dolatshahi-Pirouz and G. Orive, Pharmaceutics, 2022, 14, 1177 CrossRef CAS PubMed
.
- D. R. Griffin, W. M. Weaver, P. O. Scumpia, D. Di Carlo and T. Segura, Nat. Mater., 2015, 14, 737–744 CrossRef CAS PubMed
.
- P. Wang, X. Meng, R. Wang, W. Yang, L. Yang, J. Wang, D. Wang and C. Fan, Adv. Healthcare Mater., 2022, 11, 2102818 CrossRef CAS PubMed
.
- R. Ding, Y. Liu, D. Cheng, G. Yang, W. Wu, H. Du, X. Jin, Y. Chen, Y. Wang, B. C. Heng, Q. Yang and J. Xu, Nano Res., 2022, 15, 6348–6360 CrossRef CAS
.
- H. Yang, S. Wang, H. Bian, X. Xing, J. Yu, X. Wu, L. Zhang, X. Liang, A. Lu and C. Huang, Carbohydr. Polym., 2022, 292, 119693 CrossRef CAS PubMed
.
- A. C. Daly, B. N. Sathy and D. J. Kelly, J. Tissue Eng., 2018, 9, 1–12 CAS
.
- J. Fang, J. Koh, Q. Fang, H. Qiu, M. M. Archang, M. M. Hasani-Sadrabadi, H. Miwa, X. Zhong, R. Sievers, D. W. Gao, R. Lee, D. Di Carlo and S. Li, Adv. Funct. Mater., 2020, 30, 2004307 CrossRef CAS PubMed
.
- R. Hsu, P. Chen, J. Fang, Y. Chen, C. Chang, Y. Lu and S. Hu, Adv. Sci., 2019, 6, 1900520 CrossRef PubMed
.
- R. Zhang, J. Guo, X. Yang, X. Jiang, L. Zhang, J. Zhou, X. Cao and B. Duan, ACS Appl. Mater. Interfaces, 2023, 15, 15917–15927 CrossRef CAS PubMed
.
- M. Xie, Y. Shi, C. Zhang, M. Ge, J. Zhang, Z. Chen, J. Fu, Z. Xie and Y. He, Nat. Commun., 2022, 13, 3597 CrossRef CAS PubMed
.
- A. J. Seymour, S. Shin and S. C. Heilshorn, Adv. Healthcare Mater., 2021, 10, 2100644 CrossRef CAS PubMed
.
- X. Gu, Y. Zha, Y. Li, J. Chen, S. Liu, Y. Du, S. Zhang and J. Wang, Acta Biomater., 2022, 141, 190–197 CrossRef CAS PubMed
.
- Z. Sun, F. Wu, H. Gao, K. Cui, M. Xian, J. Zhong, Y. Tian, S. Fan and G. Wu, ACS Appl. Bio Mater., 2020, 3, 8739–8747 CrossRef CAS PubMed
.
- X. Luo, L. Zhang, Y. Luo, Z. Cai, H. Zeng, T. Wang, Z. Liu, Y. Chen, X. Sheng, A. E. D. G. Mandlate, Z. Zhou, F. Chen and L. Zheng, Adv. Funct. Mater., 2023, 33, 2214036 CrossRef CAS
.
- A. Salerno, A. Palladino, C. Pizzoleo, C. Attanasio and P. A. Netti, Biofabrication, 2022, 14, 045002 CrossRef CAS PubMed
.
- A. L. Kersey, T. U. Nguyen, B. Nayak, I. Singh and A. K. Gaharwar, Mater. Today, 2023, 64, 98–120 CrossRef
.
- D. Zhang, H. Zheng, K. Geng, J. Shen, X. Feng, P. Xu, Y. Duan, Y. Li, R. Wu, Z. Gou and C. Gao, Biomaterials, 2021, 272, 120783 CrossRef CAS PubMed
.
- Y. Zhou, H. L. Gao, L. L. Shen, Z. Pan, L. B. Mao, T. Wu, J. C. He, D. H. Zou, Z. Y. Zhang and S. H. Yu, Nanoscale, 2016, 8, 309–317 RSC
.
- H. Yan, Z. Wang, L. Li, X. Shi, E. Jia, Q. Ji, Y. Wang, Y. Ito, Y. Wei and P. Zhang, Chem. Eng. J., 2021, 416, 129129 CrossRef CAS
.
- H. J. Kim, S. J. Hong, S. Lee, J. M. Park, J. I. Park, J. S. Park, S. H. Shim and K. H. Park, Adv. Ther., 2021, 4, 2000245 CrossRef CAS
.
- J. Mao, P. Wei, Z. Yuan, W. Jing, J. Cao, G. Li, J. Guo, H. Wang, D. Chen and Q. Cai, J. Biomater. Sci., Polym. Ed., 2021, 32, 229–247 CrossRef CAS PubMed
.
- W. Zhang, X. Chuan Wang, X. Yue Li, L. Le Zhang and F. Jiang, Carbohydr. Polym., 2020, 236, 116043 CrossRef CAS PubMed
.
- B. Cai, Q. Zou, Y. Zuo, Q. Mei, J. Ma, L. Lin, L. Chen and Y. Li, ACS Appl. Mater. Interfaces, 2018, 10, 25099–25112 CrossRef CAS PubMed
.
- M. Whitely, G. Rodriguez-Rivera, C. Waldron, S. Mohiuddin, S. Cereceres, N. Sears, N. Ray and E. Cosgriff-Hernandez, Acta Biomater., 2019, 93, 169–179 CrossRef CAS PubMed
.
- R. Subbiah, A. Cheng, M. A. Ruehle, M. H. Hettiaratchi, L. E. Bertassoni and R. E. Guldberg, Acta Biomater., 2020, 114, 63–75 CrossRef CAS PubMed
.
- R. Reyes, J. A. Rodríguez, J. Orbe, M. R. Arnau, C. Évora and A. Delgado, Drug Delivery, 2018, 25, 750–756 CrossRef CAS PubMed
.
- E. Mohseni-Vadeghani, R. Karimi-Soflou, S. Khorshidi and A. Karkhaneh, Colloids Surf., B, 2021, 197, 111376 CrossRef CAS PubMed
.
- C. Wang, H. Xu, C. Liu, Z. Peng, R. Min, Z. Zhang, J. Li, Y. Jin, Y. Wang, Z. Li, J. Guo and L. Zhu, Biomater. Sci., 2021, 9, 3005–3018 RSC
.
- Z. Lin, J. Wu, W. Qiao, Y. Zhao, K. H. M. Wong, P. K. Chu, L. Bian, S. Wu, Y. Zheng, K. M. C. Cheung, F. Leung and K. W. K. Yeung, Biomaterials, 2018, 174, 1–16 CrossRef CAS PubMed
.
- S. H. Yang, X. J. Ju, C. F. Deng, Q. W. Cai, X. Y. Tian, R. Xie, W. Wang, Z. Liu, D. W. Pan and L. Y. Chu, Ind. Eng. Chem. Res., 2023, 62, 2636–2648 CrossRef CAS
.
- E. Lu, G. Shao, J. Ma, Y. He, Y. Gong, Z. Yan and X. Sha, Pharmaceutics, 2021, 13, 799 CrossRef CAS PubMed
.
- A. Pérez-López, C. Martín-Sabroso, L. Gómez-Lázaro, A. I. Torres-Suárez and J. Aparicio-Blanco, Acta Biomater., 2022, 149, 1–15 CrossRef PubMed
.
- T. C. Ho, J.-S. Lim, S.-J. Kim, S.-Y. Kim and B.-S. Chun, Mar. Drugs, 2023, 21, 287 CrossRef CAS PubMed
.
- M. M. Welling, N. Duszenko, M. P. van Meerbeek, T. J. M. Molenaar, T. Buckle, F. W. B. van Leeuwen and D. D. D. Rietbergen, J. Clin. Med., 2023, 12, 918 CrossRef CAS PubMed
.
- F. Pang, Y. Li, W. Zhang, C. Xia, Q. He, Z. Li, L. Xiao, S. Song, P. Dong, H. Zhou, T. Shao, H. Cai and L. Li, Adv. Healthcare Mater., 2020, 9, 2000028 CrossRef CAS PubMed
.
- Q. Xiao, P. Chen, M. Chen, Y. Zhou, J. Li, Y. Lun, Q. Li and G. Ye, Drug Delivery Transl. Res., 2023, 13, 2664–2676 CrossRef CAS PubMed
.
- K. Vogt, L. Aryan, S. Stealey, A. Hall, K. Pereira and S. P. Zustiak, J. Biomed. Mater. Res., Part A, 2022, 110, 131–142 CrossRef CAS PubMed
.
- A. S. Mikhail, A. H. Negussie, M. Mauda-Havakuk, J. W. Owen, W. F. Pritchard, A. L. Lewis and B. J. Wood, Expert Opin. Drug Delivery, 2021, 18, 383–398 CrossRef CAS PubMed
.
- M. Chen, G. Shu, X. Lv, X. Xu, C. Lu, E. Qiao, S. Fang, L. Shen, N. Zhang, J. Wang, C. Chen, J. Song, Z. Liu, Y. Du and J. Ji, Biomaterials, 2022, 284, 121512 CrossRef CAS PubMed
.
- F. Leng, S. Lei, B. Luo, S. Lv, L. Huang and X. Jiang, Carbohydr. Polym., 2022, 286, 119274 CrossRef CAS PubMed
.
- P. Lv, H. Chen, H. Cheng, X. Liu, C. Liu, Y. Zeng, L. Jiang, X. Wang, J. Mao and G. Liu, Adv. Ther., 2023, 6, 2200174 CrossRef CAS
.
- X. Yang, S. Wang, X. Zhang, C. Ye, S. Wang and X. An, Biomater. Adv., 2022, 135, 112677 CrossRef PubMed
.
- A. Zhang, Z. Xiao, Q. Liu, P. Li, F. Xu, J. Liu, H. Tao, L. Feng, S. Song, Z. Liu and G. Huang, Adv. Healthcare Mater., 2021, 10, 2100748 CrossRef CAS PubMed
.
- K. V. Sharma, Z. Bascal, H. Kilpatrick, K. Ashrafi, S. L. Willis, M. R. Dreher and A. L. Lewis, Biomaterials, 2016, 103, 293–304 CrossRef CAS PubMed
.
- Y. He, Y. Zhang, Y. Gong, Z. Zhang, T. Xu, L. Tian, T. Pan, H. Yang, H. Pan, Q. Kou, H. Wang and G. Shao, Front. Bioeng. Biotechnol., 2022, 10, 1024174 CrossRef PubMed
.
- C. Wei, C. Wu, X. Jin, P. Yin, X. Yu, C. Wang and W. Zhang, Acta Biomater., 2022, 153, 453–464 CrossRef CAS PubMed
.
- D. Wang, Q. Wu, R. Guo, C. Lu, M. Niu and W. Rao, Nanoscale, 2021, 13, 8817–8836 RSC
.
- A. J. Dzieciol and S. Mann, Chem. Soc. Rev., 2012, 41, 79–85 RSC
.
- T. M. S. Chang, Science, 1964, 146, 524–525 CrossRef CAS PubMed
.
- X. Cai, M. Jian, S. Zhou, Z. Wang, K. Wang and J. Liu, Prog. Chem., 2022, 34, 2462–2475 CAS
.
- E. S. Köksal, S. Liese, I. Kantarci, R. Olsson, A. Carlson and I. Gözen, ACS Nano, 2019, 13, 6867–6878 CrossRef PubMed
.
- J. J. Green and J. H. Elisseeff, Nature, 2016, 540, 386–394 CrossRef CAS PubMed
.
- S. Cao, L. C. da Silva and K. Landfester, Angew. Chem., Int. Ed., 2022, 61, e202205266 CrossRef CAS PubMed
.
- C. G. Palivan, R. Goers, A. Najer, X. Zhang, A. Car and W. Meier, Chem. Soc. Rev., 2016, 45, 377–411 RSC
.
- J. F. Le Meins, C. Schatz, S. Lecommandoux and O. Sandre, Mater. Today, 2013, 16, 397–402 CrossRef CAS
.
- S. Khan, M. Li, S. P. Muench, L. J. C. Jeuken and P. A. Beales, Chem. Commun., 2016, 52, 11020–11023 RSC
.
- X. Huang, D. Hürlimann, H. T. Spanke, D. Wu, M. Skowicki, I. A. Dinu, E. R. Dufresne and C. G. Palivan, Adv. Healthcare Mater., 2022, 11, 2202100 CrossRef CAS PubMed
.
- W. Liu, M. Zou, S. Qin, Y. Cheng, Y. Ma, Y. Sun and X. Zhang, Adv. Funct. Mater., 2020, 30, 2003559 CrossRef CAS
.
- Y. Lu, G. Allegri and J. Huskens, Mater. Horiz., 2022, 9, 892–907 RSC
.
- C. E. Meyer, S. L. Abram, I. Craciun and C. G. Palivan, Phys. Chem. Chem. Phys., 2020, 22, 11197–11218 RSC
.
- D. Gaur, N. C. Dubey and B. P. Tripathi, Adv. Colloid Interface Sci., 2022, 299, 102566 CrossRef CAS PubMed
.
- M. A. Boyd and N. P. Kamat, Trends Biotechnol., 2021, 39, 927–939 CrossRef CAS PubMed
.
- Y. Okuno, T. Nishimura, Y. Sasaki and K. Akiyoshi, Biomacromolecules, 2021, 22, 3099–3106 CrossRef CAS PubMed
.
- N. Marušič, L. Otrin, Z. Zhao, R. B. Lira, F. L. Kyrilis, F. Hamdi, P. L. Kastritis, T. Vidakovic-Koch, I. Ivanov, K. Sundmacher and R. Dimova, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 15006–15017 CrossRef PubMed
.
- Q. Qu, X. Zhang, H. Ravanbakhsh, G. Tang, J. Zhang, Y. Deng, K. Braeckmans, S. C. De Smedt, R. Xiong and C. Huang, Chem. Eng. J., 2022, 428, 132607 CrossRef CAS
.
- Z. Chen, Z. Lv, Y. Zhuang, Q. Saiding, W. Yang, W. Xiong, Z. Zhang, H. Chen, W. Cui and Y. Zhang, Adv. Mater., 2023, 13, 14–27 Search PubMed
.
- W. Li, L. Zhang, X. Ge, B. Xu, W. Zhang, L. Qu, C. H. Choi, J. Xu, A. Zhang, H. Lee and D. A. Weitz, Chem. Soc. Rev., 2018, 47, 5646–5683 RSC
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |