3D printing of polyvinyl alcohol hydrogels enabled by aqueous two-phase system†
Rahul
Karyappa
ac,
Nidhi
Nagaraju
ab,
Kento
Yamagishi
a,
Xue Qi
Koh
c,
Qiang
Zhu
 cde and
Michinao
Hashimoto
cde and
Michinao
Hashimoto
 *ab
*ab
aDigital Manufacturing and Design Centre, Singapore University of Technology and Design, 8, Somapah Road, Singapore 487372, Republic of Singapore. E-mail: hashimoto@sutd.edu.sg
bPillar of Engineering Product Development, Singapore University of Technology and Design, 8, Somapah Road, Singapore 487372, Republic of Singapore
cInstitute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Singapore 138634, Republic of Singapore
dSchool of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore 637371, Republic of Singapore
eInstitute of Sustainability for Chemicals, Energy and Environment (ISCE2), Agency for Science, Technology and Research (A*STAR), 1 Pesek Road, Jurong Island, Singapore 627833, Republic of Singapore
First published on 14th February 2024
Abstract
The synthesis of PVA hydrogels (PVA-Hy) requires a highly basic environment (e.g., an aqueous solution of sodium hydroxide, NaOH, 14% w/w, 4.2 M), but the rapid crosslinking of PVA due to high pH makes it challenging to perform layer-by-layer three-dimensional (3D) printing of PVA-Hy. This work demonstrated 3D printing of PVA-Hy in moderate alkaline conditions (e.g., NaOH, 1% w/w, 0.3 M) assisted by aqueous two-phase system (ATPS). Salting out of PVA to form ATPS allowed temporal shape retention of a 3D-printed PVA structure while it was physically crosslinked in moderate alkaline conditions. Crucially, the layer-to-layer adhesion of PVA was facilitated by delayed crosslinking of PVA that required additional reaction time and overlapping between the layers. To verify this principle, we studied the feasibility of direct ink write (DIW) 3D printing of PVA inks (5–25% w/w, μ = 0.1–20 Pa s, and MW = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 74
000 and 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800) in aqueous embedding media offering three distinct chemical environments: (1) salts for salting out (e.g., Na2SO4), (2) alkali hydroxides for physical crosslinking (e.g., NaOH), and (3) a mixture of salt and alkali hydroxide. Our study suggested the feasibility of 3D-printed PVA-Hy using the mixture of salt and alkali hydroxide, demonstrating a unique concept of embedded 3D printing enabled by ATPS for temporary stabilization of the printed structures to facilitate 3D fabrication.
800) in aqueous embedding media offering three distinct chemical environments: (1) salts for salting out (e.g., Na2SO4), (2) alkali hydroxides for physical crosslinking (e.g., NaOH), and (3) a mixture of salt and alkali hydroxide. Our study suggested the feasibility of 3D-printed PVA-Hy using the mixture of salt and alkali hydroxide, demonstrating a unique concept of embedded 3D printing enabled by ATPS for temporary stabilization of the printed structures to facilitate 3D fabrication.
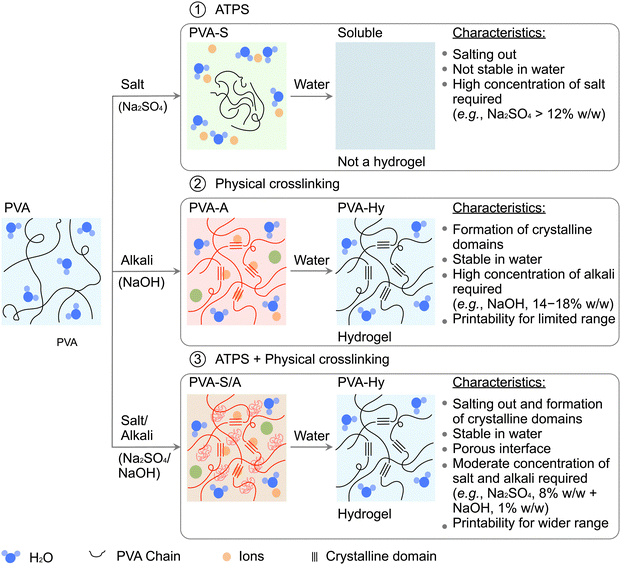
New conceptsPolyvinyl alcohol (PVA) forms a strong, stretchable, durable hydrogel suitable for biomedical applications. Fabrication of PVA hydrogel (PVA-Hy) from an aqueous PVA ink requires rapid physical crosslinking of PVA in high-concentration alkali solution (e.g., NaOH, 4.2 M). However, 3D printing of PVA-Hy is challenging due to rapid crosslinking that impedes layer-to-layer adhesion. To overcome this challenge, we developed a unique method to fabricate 3D structures of PVA-Hy by direct ink writing (DIW). Our approach involved the use of an embedding media containing salt and base. In situ salting out with the salt provided temporal stability to the printed structures by the formation of the aqueous two-phase system (ATPS), while the formed ATPS was physically crosslinked by the alkaline solution (e.g., NaOH, 0.3 M). Crucially, such a moderately basic condition allowed layer-to-layer overlap of the printed inks, which enabled the 3D printing of PVA-Hy. The mechanical properties of the 3D-printed PVA-Hy were readily controlled by various factors, such as the polymer concentration in the ink and alkali and salt concentrations in the embedding media. This work is the first demonstration of 3D printing of PVA hydrogels via physical crosslinking mediated by ATPS, which should find various applications in biomedical engineering, including soft robotics, microfluidics, and tissue engineering. |
1. Introduction
This paper describes a unique approach to 3D-print polyvinyl alcohol hydrogels (PVA-Hy) via direct ink writing (DIW) 3D printing in embedded liquid media via slow physical crosslinking mediated by aqueous two-phase systems (ATPS). 3D printing of PVA-Hy has been demonstrated only in highly basic conditions (i.e., sodium hydroxide (NaOH), 14% w/w, 4.2 M). Rapid physical crosslinking of PVA solution in such conditions, however, makes it challenging to ensure the adhesion between filaments in 3D printing. In this research, we demonstrated an alternative approach to 3D-print physically crosslinked PVA-Hy by employing salt-induced precipitation (or salting out) of aqueous PVA in alkali conditions. The printed PVA solution was stabilized as ATPS while crosslinking in moderate alkali conditions (i.e., NaOH, 1% w/w, 0.3 M). Crucially, slow physical crosslinking of salt-induced ATPS facilitated layer-to-layer adhesion between the overlapping filaments, which ensured the fidelity of 3D printing. The developed method allowed the fabrication of 3D models of physically crosslinked PVA-Hy with varying flexibility and stretchability, which should find applications in soft robotics, tissue engineering, implantable electronics, and drug delivery.1.1. PVA and PVA hydrogels
PVA is a semi-crystalline, biodegradable, biocompatible, and non-toxic synthetic polymer that shows characteristics of film orientation and adhesive properties.1,2 PVA contains polar hydroxyl groups, and the solubility of PVA in water depends on the degree of hydrolysis. PVA is synthesized by two-step polymerization of vinyl acetate. Different grades of PVA can be synthesized by controlling the hydrolysis that would affect its solubility, crystallinity, and chemical properties.3–5 For a broad range of biomedical applications, PVA must be crosslinked into hydrogels. Physical crosslinking (e.g., freezing–thawing cycle,6 hydrogen bonding7), chemical crosslinking (e.g., glutaraldehyde,8 boric acid9), and radiation crosslinking (e.g., g-ray,10 electron beam11) have been demonstrated to prepare the PVA hydrogels. Amongst these methods, the physical crosslinking, especially the freezing–thawing cycle of PVA, is preferred to tune the mechanical properties of the hydrogels. The repeated freezing and thawing cycles induce the growth of the polymer crystallites that act as points of physical crosslinking between the chains of PVA to produce PVA hydrogels (PVA-Hy).6,12,13 Applications in tissue engineering require the fabrication of a porous scaffold mimicking the extracellular matrix for cell attachment and proliferation.8,14–16 The conventional fabrication techniques of porous scaffolds of PVA hydrogels include solvent casting,17 gas foaming,18 freeze drying,4 sol–gel method/thermal annealing,19 and electrospinning.20,21 These conventional techniques have inherent drawbacks in the required time and steps to complete syntheses.22 The energy consumption is high (e.g., freeze drying22). Moreover, removing the residual organic solvents from the fabricated scaffolds is difficult, which may render the hydrogel cytotoxic.221.2. 3D printing of PVA hydrogels
Recent advancements in additive manufacturing have enabled 3D printing of freeform and complex structures of different materials for industrial use (e.g., automotive, construction, sensors, actuators, and foods), medical use (e.g., tissue engineering, drug delivery, and medical devices), and sociocultural use (e.g., education, art, and jewelry).23 3D printing of biocompatible hydrogels has become attractive and promising for pharmaceutical and biomedical applications. 3D printing of hydrogels has enabled overcoming wastage and allowed higher accuracy and greater complexity in shape than conventional fabrication methods. Multiple methods of 3D printing, such as stereolithography (SLA), digital light processing (DLP), and direct ink writing (DIW), have been demonstrated for the 3D printing of hydrogels.15,16 DIW 3D printing has attracted much attention owing to its low cost, simplicity, and ability to combine more than one material in a single processing step.24 DIW 3D printing prints liquid precursors through a deposition nozzle in a layer-by-layer approach to form 3D objects. DIW is one of the most popular techniques demonstrated to print PVA and PVA-based hydrogels.25–30 DIW 3D printing of PVA hydrogels has been performed in three major approaches: (1) alteration of the rheological characteristics of the PVA solution by adding rheology modifiers (e.g., κ-carrageenan (κ-CA); prominent interactions between PVA and κ-CA are induced due to –OH and –SO3− groups, hydrogen bonds, and van der Waals forces),25,26,30 (2) alteration of the rheological characteristics of the PVA solution by incorporating filler materials (e.g., hydroxyapatite, nanomaterials; PVA acts as a binder to hold the filler materials to form a paste),29,31,32 and (3) phase change of the printed solution (e.g., in situ freezing).27,28,33 The yield stress characteristics of the PVA inks allowed them to retain the printed shape and ensured the fidelity of printing. The main disadvantage of the existing methods is the requirement for the structural or chemical modification of pristine PVA, which does not allow retaining its original properties. Moreover, in many cases, postprocessing (such as freezing-thawing cycles) was required for the 3D-printed objects to complete the crosslinking and tune the mechanical properties of the printed hydrogels. Overall, despite these successful demonstrations, the fabrication of 3D structures of PVA hydrogels using pristine PVA ink (without altering the rheological characteristics) is not demonstrated.1.3. 3D printing in embedding media
Successful DIW 3D printing of liquid inks requires arresting the shape of the printed materials in situ by solidification or gelation. To this end, the use of liquid embedding media has been widely explored, collectively termed embedded 3D printing.34–38 We previously demonstrated a novel method of embedded 3D printing (i.e., immersion precipitation 3D printing, ip3DP, and freeform polymer precipitation, FPP) based on the polymer–solvent–nonsolvent ternary system in which the dissolved polymers were dispensed directly in a nonsolvent.34,39,40 In those works, we showcased the fabrication of 3D structures of PVA polymer dissolved in water (solvent) and printed in acetone (nonsolvent). The printed structure of PVA remained a thermoplastic polymer; it did not have the characteristics of a hydrogel (e.g., elastic, flexible, water-insoluble). Microparticulate embedding media allowed freeform fabrication of thermoplastic polymers,39,40 and curable resins.41–43PVA can be converted to a hydrogel by two simple routes with embedding media: (1) with a salt solution (e.g., sodium sulfate, sodium chloride),44–46 and (2) with an alkali hydroxide solution (e.g., sodium hydroxide, potassium hydroxide).47 When two aqueous solutions containing appropriate concentrations of specific polymers and salts are mixed, they separate into two immiscible polymer-rich phases and a salt-rich phase with water as a solvent in both phases. Such a system is well-known as ATPS.48 The presence of salts in the embedding media affects the interactions between PVA and water molecules to form hydrogen bonding, resulting in the precipitation of PVA.44,49,50 In aqueous solutions of salts, salt ions are surrounded by hydration shells. For the ions of salts (e.g., potassium chloride, ammonium chloride, sodium carbonate), the number of water molecules bound to the ion only in the first hydration layer ranges from five to nine.51,52 When the concentration of water molecules is sufficiently small, the ions of the salts borrow water molecules from the hydration shells of PVA chains to maintain their hydration shells. Therefore, hydrogen bonds between PVA and water molecules are compromised, leading to the packing of PVA chains into crystalline structures (or the formation of crystalline domains called microcrystallites) stabilized by the hydrogen bonds between the hydroxyl group of PVA.44,53,54 The timescale of the polymer precipitation (also called salting out) and the size and density of the microcrystallite are dependent on the concentration of the salt.53,55 Similarly, the alkali hydroxides dissolved in water when contacted with PVA cause in situ physical crosslinking of PVA chains by rapidly inducing crystallinity (i.e., forming microcrystallite domains). The mechanical properties of PVA-Hy can be tuned by the salt and alkali concentrations.47,53 Besides, PVA hydrogels exhibit shape memory properties and are cytocompatible, hemocompatible, and biocompatible.47,55 Despite the simplicity of methods to fabricate PVA-Hy, digital fabrication of PVA-Hy via embedded 3D printing using inks prepared with pristine PVA has not been demonstrated.
To bridge this gap, we present the first demonstration of direct writing of liquid PVA inks continuously printed in liquid media to form 3D structures with controlled dimensions. Pristine PVA ink facilitates easy extrusion through the nozzles, and arresting the printed shape is the requirement for 3D printing. We took three different approaches for embedded 3D printing. The embedding media consisted of (1) salt solution, (2) alkali hydroxide solution, and (3) both salt and alkali hydroxide solution (Fig. 1). These three approaches enabled arresting the printed structure of the aqueous PVA solutions by (1) salting out, (2) alkali physical crosslinking, or (3) a combination of both processes. Providing such chemical environments for the formation of hydrogels cannot be achieved without liquid embedding media. In this research, we studied the effect of critical parameters (such as concentration and molecular weight of PVA in the printing ink as well as types and concentrations of salts and alkali hydroxide in the embedding media) on the printability of PVA and the resulting properties of the PVA hydrogels. Notably, the 3D printing of PVA hydrogels by physical crosslinking was facilitated by salting out in ATPS. This is the first report to combine these two processes in embedded 3D printing. The proposed method of layer-by-layer 3D printing offered unique opportunities to print various formulations designed from a breadth of synthetic and natural hydrophilic polymers. We highlighted the capability of our approach by fabricating 3D structures of functionalized PVA hydrogels by mixing PVA with synthetic polymers such as polyvinylpyrrolidone (PVP), and natural polymers such as sodium alginate and gelatin. The concepts and methods presented here will expand the toolkit in the digital fabrication of PVA hydrogels to fabricate functional structures for tissue engineering, soft robotics, and biomedical applications such as wound dressing, vascular grafts, and artificial meniscus.
2. Results and discussions
2.1. Experimental design
This work aims to develop a novel method of 3D printing of hydrogels mediated by ATPS. The initial part of this study explored salting out of ATPS to achieve this goal. Proteins in aqueous solutions can be precipitated using different salts, called ion-specific effect or Hofmeister effect.56 The ability of the salts to precipitate proteins depends on cationic and anionic effects, represented in the Hofmeister series (Fig. S1, ESI†). In the Hofmeister series, the ions are divided based on their ability to order water molecules beyond their immediate solvation shells: (1) kosmotropes (structure makers) and (2) chaotropes (structure breakers). The effect of salting out is generally led by the strongly hydrated kosmotropes that remove water from the polymer effectively.56 On the other hand, weakly hydrated chaotropes do not possess this ability. The effect of interactions among the ions, the water molecule, and synthetic polymer chains on solubility and swelling of the polymers and mechanical properties of the hydrogels are well studied.45,57–60 We hypothesized that 3D printing would be facilitated with Hofmeister effect.We selected PVA as a model polymer. PVA has a simple molecular structure with a hydrophobic (CH2–CH2) backbone and hydrophilic (–OH) side-groups. PVA in an aqueous solution of salts forms ATPS, and the effect of different salts on salting out of PVA has been well-studied.44–46,53,55,61 Depending on the type of ions, three types of interactions among the ions, the PVA chains and the hydration of water molecules have been reported: (1) polarization of the hydration by anions leading to destabilization of the hydrogen bonds between PVA and the water molecules, (2) restriction of hydrophobic hydration of PVA chains by ions, and (3) binding of anions and addition of extra charges to PVA chains, which leads to increase in solubility of PVA (salting in).45 To this end, three types of salts with different concentrations were investigated in the current study: sodium sulfate (Na2SO4), tripotassium phosphate (K3PO4), and ammonium sulfate ((NH4)2SO4). These salts were selected to understand the ion-specific effects on in situ salting out of PVA inks.
Apart from salts, we used alkali hydroxides such as sodium hydroxide (NaOH) as a physical crosslinker in the embedding media. We chose NaOH at different concentrations in the embedding media, as it can rapidly induce crystallinity in PVA polymer chains. Successful formation of PVA-Hy using PVA of high molecular weight (molecular weight, MW = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2
000–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) has been reported.47 The stability and swelling of the formed PVA-Hy depend on the molecular weight (MW) of PVA.61 For PVA with MW less than 31
000) has been reported.47 The stability and swelling of the formed PVA-Hy depend on the molecular weight (MW) of PVA.61 For PVA with MW less than 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the physical crosslinking to form PVA-Hy was ineffective; PVA-Hy prepared with high MW PVA was more stable than the low MW. Based on these studies, we selected PVA of high MW (PVAh) and low MW (PVAl) to understand its effect on physical crosslinking. With the selected inks and embedding media, DIW 3D printing was performed to fabricate complex structures in a layer-by-layer manner (Supporting Movie 1, ESI†). At moderate concentrations of NaOH in the embedding media, the printed layer of PVA ink remained in a sol–gel state when the next layer was printed, which facilitated interfacial bonding between the layers to form 3D structures. However, the increased concentration of NaOH resulted in the increased rate of crosslinking of PVA in the media, thus limiting the duration of time available for interfacial bonding. Due to these constraints, practically it is preferred to perform crosslinking of PVA at a reduced concentration of NaOH. This requirement motivated us to explore the crosslinking of PVA stabilized by salts while crosslinking at low concentrations of NaOH. To this end, we examined the printability of the PVA inks to fabricate 3D structures of PVA-Hy under the different operating parameters and materials.
000, the physical crosslinking to form PVA-Hy was ineffective; PVA-Hy prepared with high MW PVA was more stable than the low MW. Based on these studies, we selected PVA of high MW (PVAh) and low MW (PVAl) to understand its effect on physical crosslinking. With the selected inks and embedding media, DIW 3D printing was performed to fabricate complex structures in a layer-by-layer manner (Supporting Movie 1, ESI†). At moderate concentrations of NaOH in the embedding media, the printed layer of PVA ink remained in a sol–gel state when the next layer was printed, which facilitated interfacial bonding between the layers to form 3D structures. However, the increased concentration of NaOH resulted in the increased rate of crosslinking of PVA in the media, thus limiting the duration of time available for interfacial bonding. Due to these constraints, practically it is preferred to perform crosslinking of PVA at a reduced concentration of NaOH. This requirement motivated us to explore the crosslinking of PVA stabilized by salts while crosslinking at low concentrations of NaOH. To this end, we examined the printability of the PVA inks to fabricate 3D structures of PVA-Hy under the different operating parameters and materials.
2.2. PVA inks
First, we prepared aqueous PVA inks for 3D printing. We prepared PVA inks using a low MW PVA (PVAl, MW = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and a high MW PVA (PVAh, MW = 74
000) and a high MW PVA (PVAh, MW = 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800) in concentrations of 5% w/w to 25% w/w. We studied the process-related viscosity (μ) of the printing inks as a function of the applied shear rate (
800) in concentrations of 5% w/w to 25% w/w. We studied the process-related viscosity (μ) of the printing inks as a function of the applied shear rate (![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) for four concentrations of PVAh and PVAl (Fig. S2, ESI†). Throughout this paper, we denoted the solution of 25% w/w of PVAh as PVAh(25). The same nomenclature applies to PVAh and PVAl with varying concentrations. Over the investigated ranges of the shear rates, the viscosity of PVA inks displayed no change over the increasing shear rate (which is a characteristic of a Newtonian fluid). The apparent viscosities of PVA inks tested in this work were calculated to be 0.1–20 Pa s.
) for four concentrations of PVAh and PVAl (Fig. S2, ESI†). Throughout this paper, we denoted the solution of 25% w/w of PVAh as PVAh(25). The same nomenclature applies to PVAh and PVAl with varying concentrations. Over the investigated ranges of the shear rates, the viscosity of PVA inks displayed no change over the increasing shear rate (which is a characteristic of a Newtonian fluid). The apparent viscosities of PVA inks tested in this work were calculated to be 0.1–20 Pa s.
2.3. Preparation of embedding media
We used three types of embedding aqueous media to print the PVA inks: (1) salt solution, (2) alkali hydroxide solution, and (3) salt and alkali hydroxide solution. We studied three salts: sodium sulfate (Na2SO4), tripotassium phosphate (K3PO4), and ammonium sulfate ((NH4)2SO4)) based on their distinct effects on the salting out in the Hofmeister series (Fig. S1, ESI†). In the presence of salts, PVA experiences salting out at a rate that depends on the concentration of salts.55,61 We varied the concentration of the salts in the embedding media from 2% w/w to 14% w/w. Throughout this paper, we denoted the solution of 2% w/w of Na2SO4 as Na2SO4(2). The same nomenclature applies to other concentrations of Na2SO4 and other salts. In general, aggregation of free PVA chains leads to the formation of large particles (Fig. 1(a)). As time progresses, the dimensions of the aggregated particles increase due to the continuous addition of the free PVA chains in the solution. Finally, the coalescence of the large particles leads to precipitation or salting-out of PVA. The formation of hydrogen bonds mainly stabilizes the aggregates of PVA.55 We attempted to use this mechanism to 3D-print aqueous PVA solutions (Fig. 1(a)).Next, we studied the physical crosslinking of PVA in the base solution. It has been reported that PVA is readily crosslinked when the solution is rendered basic.47 We performed 3D printing of an aqueous PVA solution by directly printing it in the solution of concentrated alkali hydroxides (Fig. 1(b)). The alkali hydroxides rapidly induce crystallinity into the dispensed dense PVA solutions, causing in situ physical crosslinking. We selected NaOH to study in situ physical crosslinking of PVA chains. We varied the concentrations of all the salts in the embedding media from 4% w/w to 20% w/w. Following the same notation, the solution of 4% w/w NaOH is denoted as NaOH(4), and similar nomenclature applies to the samples described in this paper. Lastly, we studied the combination of salting out and physical crosslinking. We hypothesized that the required concentration of NaOH can be reduced in the presence of salts facilitating the formation of ATPS (Fig. 1(c)). To test the mixture of salts and NaOH as embedding media, we varied the concentration of salts (2–8% w/w) and NaOH (1–4% w/w), respectively.
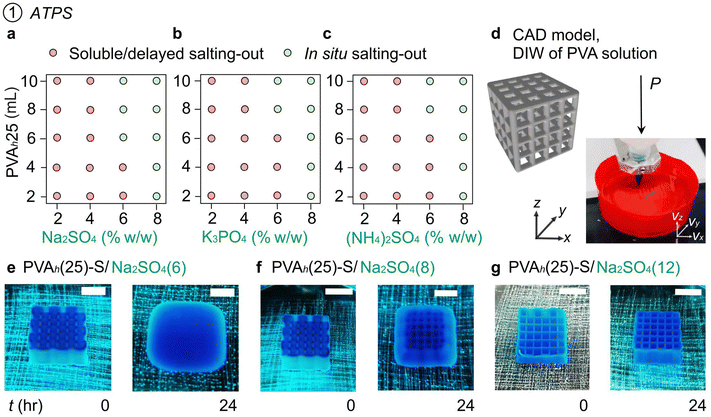
2.4. 3D printing of PVA solutions by salting out (PVA-S)
First, we studied the salting out of PVA from the aqueous PVA ink with three types of salts (Na2SO4, K3PO4, and (NH4)2SO4, 2–8% w/w). We filled 30 mL of salt solutions in the glass vials and mixed PVAh(25) in different amounts (2–10 mL). The 2D plot indicates the effect of the concentration of salts and the volume of PVAh(25) ink on the salting out of PVA (Fig. 2(a)–(c) and Fig. S3, ESI†). Although the salting out (or aggregation of PVA) was observed at all salt concentrations we investigated, the solutions with 6–8% w/w of salts exhibited rapid in situ precipitation resulting from the fast aggregation of PVA.To demonstrate 3D printing of PVA using salt solutions as liquid media, rapid, in situ phase separation of PVA solution is essential. Continuous formation of the printed filament is necessary for layer-by-layer deposition in 3D modeling.34 Printing PVA inks in the air formed a droplet due to the capillary effect, which was unsuitable for 3D printing (Supporting Movie S2, ESI†). In contrast, all the PVA inks formed continuous filaments in the liquid embedding media with any of the three salts we studied (Supporting Movie S3, ESI†). Based on these observations, we performed 3D printing of PVA inks in the embedding liquid media containing 6–8% w/w of salts (Fig. 2(d)). To enhance the color contrast of PVA ink while printing and to check the diffusion of the molecules from the ink to the surrounding aqueous media, we mixed a water-soluble blue dye in PVA ink. PVAh(25) printed in Na2SO4(6) resulted in situ salting-out of PVA with good fidelity of printing (Fig. 2(e)). The blue dye in the printed ink diffused into the surrounding liquid media during and after printing. To understand the stability of the printed object after printing, we kept the printed model in the embedding media for 24 h. The spreading of the printed ink was observed over time in Na2SO4(6) (Fig. 2(e)). Similarly, the spreading of the object printed in Na2SO4(8) was observed after 24 h of printing (Fig. 2(f)). In the combination of the ink and embedded media we investigated, different intermolecular interactions can potentially influence the stability of the printed objects; interactions may occur (1) between ions and water molecules, (2) between ions and PVA chains, (3) between PVA chains and water molecules, and (4) among PVA chains. Although the in situ gelation of PVA occurred to maintain the printed shape of the 3D objects just after printing, the dissociation of hydrogen bonds among PVA chains could have caused the instability of the printed object, followed by spreading over time. A further investigation would warrant an understanding of the stability of the printed object in varying salt concentrations in the embedding media.
Despite the spreading after 24 h, the degree of spreading was less in Na2SO4(8) than in Na2SO4(6) (Fig. 2(e) and (f)). Crucially, a further increase in the concentration of Na2SO4 to 12% w/w resulted in no spreading or sagging of printed PVAh(25) after 24 h (Fig. 2(g)). Interestingly, the object remained the same shape for 120 h in the embedding media without spreading or sagging. A similar effect of salt concentration on the stability of the printed object was observed when K3PO4 was used in the embedding media. In contrast, the use of (NH4)2SO4(12) as embedding media resulted in the spreading or sagging of the printed PVA after 24 h of printing. This observation could be attributed to the ability of NH4+ and SO4− ions to form hydrogen bonds with OH groups of PVA that prevented the crystallization.54 The printed objects underwent plastic deformations when detached from the substrate (Fig. S4 and Supporting Movie S4, ESI†). Overall, we demonstrated a method of DIW 3D printing of PVA-salt ATPS via in situ salting out in an embedding media. The concentration of salts in the embedding media affected the rate of salting out of PVA. The relatively fast salting out of PVA in embedding media with a concentration of salts ≥6% w/w allowed the printing of PVA inks, but the spreading of the printed object was observed at extended residence times (>24 h). For the successful DIW of PVA solutions by salting out with long-term stability, a salt concentration of ≥12% w/w was necessary for Na2SO4 and K3PO4. However, the same salt concentration for (NH4)2SO4 did not ensure the stability of the printed object after 24 h.
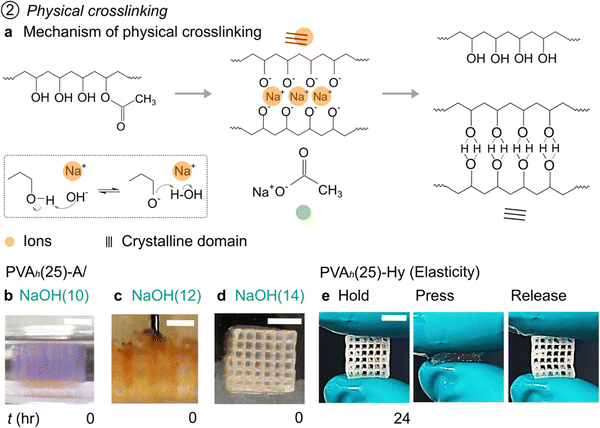
2.5. 3D Printing of PVA inks by in situ physical crosslinking (PVA-A)
PVA in contact with alkali hydroxides, such as NaOH, has been shown to form in situ crystallinity.47 In this process, OH− ions in the alkali hydroxide solutions deprotonate hydroxyl groups of PVA (Fig. 3(a), inset). Subsequently, a complex is formed between O− groups and free Na+. The formation of complex facilitates the stretching and alignment of PVA chains and leads to the formation of crystalline domains. The reaction with the OH− ions also leads to the hydrolysis of the ester groups of the acetate moieties from the backbone of PVA (Fig. 3(a), reaction). PVA solution in contact with alkali solution is termed PVA-A; once crosslinked, the PVA construct is placed in DI water to form PVA-Hy. The density of crosslinked PVA-Hy was reported to be dependent on the concentration of the alkali hydroxides.47 Fabrication of the 3D object of PVA-Hy has been showcased by combining 2D printing and assembly. The assembled object was then brought into contact with NaOH to form PVA-Hy by physical crosslinking.47 Despite all these studies, embedded 3D printing of PVA solutions in a strong alkali solution to fabricate 3D objects of PVA-Hy has not been performed.First, we tested the gelation of PVA by physical crosslinking from the aqueous PVA ink with an alkali (NaOH). We filled 2 mL of NaOH solutions in a six-well microplate and mixed PVAh(25) in different amounts (0.1–0.7 mL). The 2D plot illustrates the effect of the concentration of NaOH and the volume of PVAh(25) ink on the gelation of PVA (Fig. S5, ESI†). Although the gelation by physical crosslinking was observed at all NaOH concentrations we investigated, the solutions with 8–10% w/w of NaOH exhibited the formation of strong and thick membranes of PVA resulting from complete crosslinking of PVA. At these concentrations, swelling of PVA films was observed. It is plausible that the concentration of NaOH higher than 10% w/w would result in the formation of strong and thick membranes of crosslinked PVA as well.
When PVAh(25) was printed in a low concentration of NaOH (2–8% w/w), the printed ink spread in the embedding media before crosslinking; presumably because the rate of physical crosslinking was not sufficiently fast to hold the printed layers together. A stable printed object was obtained with NaOH(10) and NaOH(12) as embedding media (Fig. 3(b) and (c)). However, the swelling of the printed object was observed while printing PVAh(25). Crucially, with NaOH(12) as embedding media, the motion of the nozzle was disrupted by the previously printed layers (Fig. 3(c) and Supporting Movie S5, ESI†). The vertical swelling of the previously printed layer disrupted the motion of the nozzle. With NaOH(14) as the embedded media, the swelling of the printed object was not observed, and the printability of the 3D object was demonstrated (Fig. 3(d)). The swelling of the printed ink observed in NaOH(10) and NaOH(12) was presumably due to the lower density of crosslinking than in NaOH(14). The embedding media was then replaced with water; the printed object was left for 24 h to remove Na+ ions and to stabilize the formed crystalline domains permanently in the printed object. The fabricated 3D object of PVA-Hy exhibited flexibility and elasticity (Fig. 3(e) and Supporting Movie S6, ESI†).
The increase in the concentration of NaOH in the embedding media increased the rate of crosslinking, which was necessary for the 3D printing of PVA-A. However, it, unfortunately, entailed some drawbacks: (1) detachment and (2) warping of the printed object (Figure S6(a) and (b), ESI†), which are inherent problems when highly-concentrated NaOH solutions were used. We overcame these problems by decreasing the distance between the nozzle and the substrate (zo). Printing the first layer close to the substrate ensured the attachment of the first layer to the substrate until the printing was complete (Fig. S6(c), ESI†). It is to be noted that the color of the aqueous dye mixed with PVA ink changed from blue to violet while printing, presumably due to the reaction of the dye with NaOH (Fig. S6(a) and (b), ESI†). The gradual change in the color of the dye indicated the progress of the physical crosslinking of PVA. The changed color faded when the object was kept in water for the removal of Na+ to form a stable PVA-Hy (Fig. S6c, ESI†). Overall, we demonstrated 3D printing of PVA-A by in situ physical crosslinking that can be converted to PVA-Hy by the removal of Na+. NaOH(12) (and lower concentration of NaOH solutions) were unsuitable embedding media for PVA solutions we tested due to slow physical crosslinking that resulted in the spreading or swelling of the printed object. Adequate print fidelity was achieved with NaOH(14). However, it is worth noting that such rapid crosslinking can be a drawback to 3D printing; layer-by-layer adhesion can be achieved in DIW 3D printing only when the previous layer remains partially crosslinked. As such, we investigated the printability of a PVA solution at a low-to-moderate concentration of an alkaline bath that also contained salts for additional stabilization.
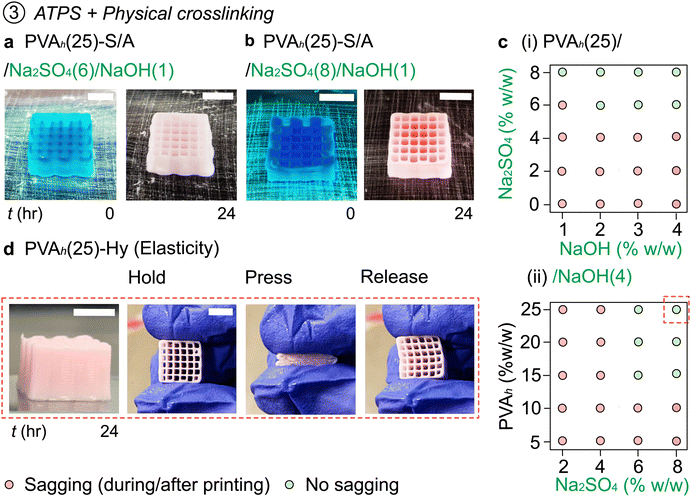
2.6. 3D printing of PVA inks by combining salting out and in situ physical crosslinking (PVA-S/A)
Our experiments thus far suggested that DIW 3D printing of PVA inks was achieved with (1) Na2SO4 concentration ≥12% w/w, and (2) NaOH concentration of ≥14% w/w. Based on these observations, we hypothesized that in situ salting out and physical crosslinking of PVA may be simultaneously performed to enhance the printability of the same ink, preferably with reduced concentrations of Na2SO4 and NaOH (Fig. 4).We varied the Na2SO4 concentration (0–8% w/w) and NaOH concentration (0–4% w/w) in the embedding media to understand their effects on the printability of the PVA solutions. At the concentration of Na2SO4 less than 4% w/w, the ink was not printable due to spreading. With Na2SO4(6) as an embedding media (without NaOH), the printed object exhibited spreading after 24 h from the printing (Fig. 2(e)). As such, we increased the concentration of NaOH in the embedded media. The embedding media of Na2SO4(6)/NaOH(1) enhanced the stability of the printed object (Fig. 4(a)); although minor sagging was observed of the printed object, the sagging of the printed object was noticeably less prominent than the object printed without NaOH. The printability was further increased when the same ink was printed in Na2SO4(8)/NaOH(1) (Fig. 4(b)). We summarized the printability of PVAh(25) in the embedding media containing Na2SO4 (0–8% w/w) and NaOH (0–4% w/w) (Fig. 4(c-i)). The ink did not maintain the printed structures in Na2SO4(4) (or less concentration of Na2SO4) regardless of the concentration of NaOH. Under these conditions, the spreading of the ink was observed upon printing or within 24 h of printing. The stability of the printed object was enhanced with increasing concentrations of either Na2SO4 or NaOH. A good fidelity of printing of PVAh(25) was observed when the concentration of Na2SO4 and NaOH was at ≥6% w/w and ≥2% w/w, respectively, in the embedding media. This observation suggested that both salting out and physical crosslinking can enhance the fidelity of printing. Crucially, these results are in critical contrast with the observations discussed in the previous sections: DIW of PVAh(25) inks was not readily printable via either salting out (Na2SO4, <10% w/w) or physical crosslinking (NaOH, <10% w/w). Only when in situ salting out and physical crosslinking simultaneously occurred was the printability attained at reduced concentrations of Na2SO4 and NaOH. The printed PVA models (PVA-S/A) can be readily converted to PVA-Hy by immersing them in DI water to remove Na+. We note that the fabricated 3D object of PVAh(25)-Hy was elastic, which exhibited similar mechanical properties to those crosslinked only with NaOH(14) (Fig. 4(d) and Supporting Movie S7, ESI†).
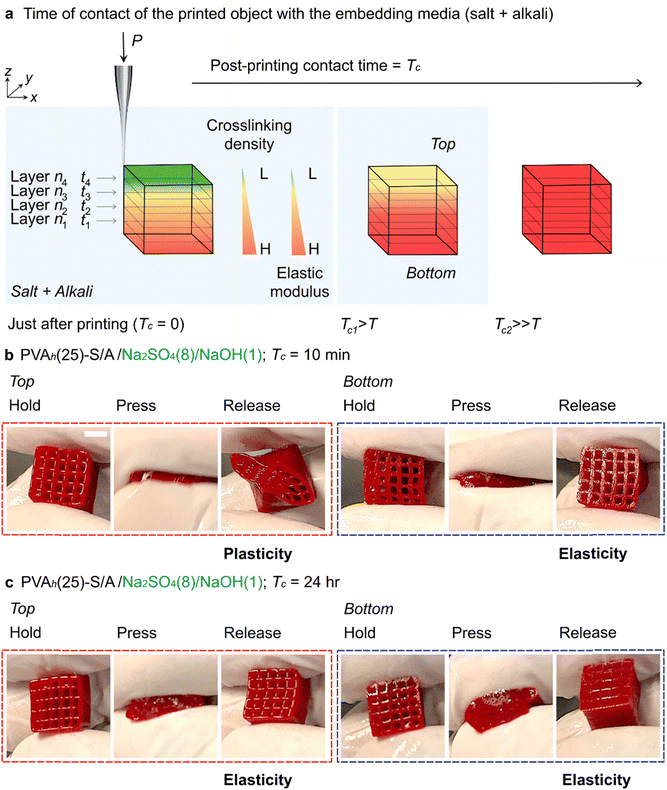
To describe the mechanism of stabilization of PVA, PVAh ink was printed in Na2SO4(8) (Fig. 2(f)). In this case, the structure began sagging and spreading after leaving the printed structure in the embedding media for some time (Fig. S7(a)); the spreading was obvious when the printed object was left in the bath for 120 min. The addition of NaOH(1) to Na2SO4(8) prevented the sagging of the printed object and facilitated the formation of PVAh-Hy. When the first layer of the PVAh ink was printed in Na2SO4(8)/NaOH(1) (i.e., layer n1 at time t1), it was stabilized by in situ salting out and by the formation of crystalline domains that acted as crosslinking points (Fig. 5(a)). As the subsequent layers (i.e., layers n2, n3, and n4 at t2, t3, and t4, respectively) were printed, the degree of crosslinking in the bottom layers was enhanced due to the extended contact time in the media (i.e., the first layer was left in the media for the longest time; t1 > t2 > t3 > t4) (Fig. 5(b), (c) and Supporting Movie S8, ESI†).
When the printing was just completed (post-printing contact time, Tc = 0), the degree of crosslinking was spatially varied within the printed structure; the bottom layer (n1) was exposed to the embedding medium longer than the top layer (n4). To highlight the effect of Tc on the degree of crosslinking within the printed object, we printed PVAh(20) in Na2SO4(8)/NaOH(1) and Na2SO4(8)/NaOH(4) (Fig. S7(b), ESI†). After printing, the printed object was left in the embedding media (Tc = 10 min, 30 min, and 24 h). After each Tc, the printed object was placed in DI water for dialysis to remove Na+ for 24 h to form PVAh(20)-Hy. The 3D-printed objects with Tc = 10 min and 30 min appeared to be more swollen than the objects with Tc = 24 h (which did not swell). This observation highlighted the effect of Tc on the crosslinking of PVAh(20)-Hy (Fig. S7(b), ESI†). Moreover, for a fixed Tc, the degree of swelling was less for the sample printed in Na2SO4(8)/NaOH(4) than the sample printed in Na2SO4(8)/NaOH(1) (Fig. S7(b), ESI†). This observation highlighted the effect of NaOH concentration on the crosslinking. To ensure uniform crosslinking within the printed object, we selected Tc = 24 h for all subsequent experiments. Overall, the simultaneous use of salt and alkali in the embedding media (such as Na2SO4(8)/NaOH(1)) allowed forming PVAh-Hy at reduced concentrations of each additive, suggesting potential synergistic stabilization effects between the salt and alkali. Further study is warranted to understand the full molecular mechanism of the formation of stable PVAh-Hy.
2.7. Concentration of PVA on embedded 3D printing
The variation in concentration of PVA in ink can affect printability due to changes in the rate of salting out and physical crosslinking. For example, a reduced concentration of PVA can lower the rate of salting out and physical crosslinking due to an increase in water content in the ink. To understand the effect of the concentration of PVA solution (5–25% w/w) on their printability in embedding media (Na2SO4 (2–8% w/w) and NaOH (4% w/w)), the printability of the ink was summarized in a 2D plot (Fig. 4(c-ii)). PVAh(5) and PVAh(10) were not printable in any embedding media; the threading of the ink due to the attachment of the ink to the nozzle tip was also observed. However, PVAh(15), PVAh(20) and PVAh(25) were printable in Na2SO4(6)/NaOH(4) and Na2SO4(8)/NaOH(4). Overall, these experiments suggested the requirement for the concentration of PVA in the printed ink in embedded media with moderate concentrations of Na2SO4 and NaOH. PVA concentration serves as one of the major parameters to control the rate of salting out and physical crosslinking of PVA.2.8. Molecular weight of PVA on embedded 3D printing
Physical crosslinking of PVA was successfully demonstrated for high MW (>31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) polymers.47 We studied the printability of inks containing low MW PVA (PVAl, MW = 22
000) polymers.47 We studied the printability of inks containing low MW PVA (PVAl, MW = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000); the concentration of PVAl (5–25% w/w) was printed in the embedding media containing Na2SO4 (2–8% w/w) and NaOH (4% w/w). We hypothesized that the molecular weight of PVA affected the tendency of aggregation upon contact with ions and alkali. We observed that any PVAl inks (5–25% w/w) were unsuitable for embedded 3D printing (Fig. S8, ESI†). The printing was unsuccessful due to either (1) threading of the ink at the nozzle tip or (2) spreading of the ink after printing. The threading of the inks was mainly due to the low viscosity of PVAl inks, and the spreading of the ink was due to the slow precipitation of PVA. We increased the concentration of PVAl to 50% w/w. In this condition, the viscosity of the ink was increased and the precipitation of PVAl was accelerated. As such, PVAl(50) was successfully printed in Na2SO4(8)/NaOH(4) (Fig. S9(a), ESI†). The printed object was elastic and flexible (Fig. S9(a) and Supporting Movie S9, ESI†). The printed object was then kept in water for 24 h to remove Na+ ions. After 24 h, the printed structure became brittle (Fig. S9(b) and Supporting Movie S10, ESI†). These experiments suggested that DIW of PVA-Hy was possible for PVAl (MW = 22
000); the concentration of PVAl (5–25% w/w) was printed in the embedding media containing Na2SO4 (2–8% w/w) and NaOH (4% w/w). We hypothesized that the molecular weight of PVA affected the tendency of aggregation upon contact with ions and alkali. We observed that any PVAl inks (5–25% w/w) were unsuitable for embedded 3D printing (Fig. S8, ESI†). The printing was unsuccessful due to either (1) threading of the ink at the nozzle tip or (2) spreading of the ink after printing. The threading of the inks was mainly due to the low viscosity of PVAl inks, and the spreading of the ink was due to the slow precipitation of PVA. We increased the concentration of PVAl to 50% w/w. In this condition, the viscosity of the ink was increased and the precipitation of PVAl was accelerated. As such, PVAl(50) was successfully printed in Na2SO4(8)/NaOH(4) (Fig. S9(a), ESI†). The printed object was elastic and flexible (Fig. S9(a) and Supporting Movie S9, ESI†). The printed object was then kept in water for 24 h to remove Na+ ions. After 24 h, the printed structure became brittle (Fig. S9(b) and Supporting Movie S10, ESI†). These experiments suggested that DIW of PVA-Hy was possible for PVAl (MW = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) only at the high concentration of PVAl (50 w/w%); the precipitation of PVAl was ineffective at low concentration.47,62 Despite the printability, however, the mechanical properties of PVA-Hy (after the removal of Na+) were dependent on the molecular weight of PVA. Further investigation will justify the effect of the molecular weight of PVA on the property of PVA-Hy, which is crucial to determining the application of 3D-printed objects.
000) only at the high concentration of PVAl (50 w/w%); the precipitation of PVAl was ineffective at low concentration.47,62 Despite the printability, however, the mechanical properties of PVA-Hy (after the removal of Na+) were dependent on the molecular weight of PVA. Further investigation will justify the effect of the molecular weight of PVA on the property of PVA-Hy, which is crucial to determining the application of 3D-printed objects.
2.9. Stability of 3D-printed structures of PVA
The long-term stability of the PVA constructs is often required for their applicability in cell encapsulation, tissue engineering, and drug delivery. The dissociation of hydrogels may affect their properties due to swelling, shrinkage, and changes in the hardness and elasticity of the hydrogels.63 As such, we investigated the stability of the 3D-printed PVA constructs by immersing them in DI water and PBS buffer at different temperatures (25, 40, 50, 60, 70, and 80 °C).We tested the stability of the 3D-printed PVA in various states: (1) pristine PVA, (2) PVA-S (stabilized by salting out), (3) PVA-A (stabilized by alkali solution, NaOH), (4) PVA-S/A (stabilized by salting out and NaOH), and (5) PVA-Hy (Na+ removed from PVA-A or PVA-S/A by immersing them in DI water at least 24 h). We previously reported a method to 3D-print pristine PVA by immersion precipitation.34 This 3D-printed pristine PVA was kept in DI water, and it was completely dissolved after 24 h (Fig. S10(a)). PVA-S fabricated by in situ salting out with Na2SO4(12) was entirely dissolved in DI water after 48 h (Fig. S10(b), ESI†). In critical contrast, PVA-S/A was stable in DI water (Fig. S10(c), ESI†). Physically crosslinked PVA hydrogel was observed to be stable in DI water; the long residence time in DI water removed Na+, and the printed PVA became PVA-Hy. Over six months, no evidence of degradation or solubility of PVA-Hy was observed. We performed ten cycles of swelling and drying of the printed PVA-Hy; over the repeated cycles, no changes were observed for PVA-Hy (Fig. S11 and Supporting Movie S11, ESI†). Similar observations were made when 3D-printed PVA-Hy were maintained in a PBS buffer. In contrast, once PVAh-Hy was left in the ambient condition, noticeable volume shrinkage was observed. For example, PVAh(20)-Hy printed in Na2SO4(8)/NaOH(1–4) exhibited a volume shrinkage of ∼75–80% after five days (Fig. S12, ESI†).
We observed that the printed PVA-S eventually dissolved in DI water over time, suggesting that the partially hydrolyzed PVA (88% hydrolyzed) used in this study did not form microcrystalline domains by salting out. In critical contrast, previous studies on PVA (>99% hydrolyzed) suggested the formation of microcrystalline domains only by salting out, exhibiting improved stability in water. Indeed, a previous study revealed that the time scale of dissolution of the fabricated PVA-S depended on the degree of hydrolysis of PVA.64 Thin strips of PVA-S (13 μm wide, 13 μm high, and 220 μm long) were prepared by mixing two types of PVA (>99% and 86.7–88.7% hydrolyzed) at different ratios (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 8
0, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 6
2, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 4
4, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 2
6, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, and 0
8, and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). When those PVA samples (that are stabilized only by salting out) were exposed to DI water, they exhibited different time scales of dissolution. PVA-S prepared with PVA with >99% degree of hydrolysis (10
10). When those PVA samples (that are stabilized only by salting out) were exposed to DI water, they exhibited different time scales of dissolution. PVA-S prepared with PVA with >99% degree of hydrolysis (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) remained stable without dissolution for more than two weeks, although a few structures underwent swelling and deformation.64 Multiple studies reported that the salting out of PVA with >99% degree of hydrolysis introduced crystallinity in the fabricated structures.45,64–66 In critical contrast, when increasing the ratio of partially hydrolyzed PVA to 2
0) remained stable without dissolution for more than two weeks, although a few structures underwent swelling and deformation.64 Multiple studies reported that the salting out of PVA with >99% degree of hydrolysis introduced crystallinity in the fabricated structures.45,64–66 In critical contrast, when increasing the ratio of partially hydrolyzed PVA to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 or 0
8 or 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, complete dissolution of PVA-S occurred within several hours. These observations are in agreement with our experiments where PVA-S samples dissolved in DI water over time, also suggesting the absence of microcrystalline domains in the partially hydrolyzed PVA (88%) stabilized only by the salts.
10, complete dissolution of PVA-S occurred within several hours. These observations are in agreement with our experiments where PVA-S samples dissolved in DI water over time, also suggesting the absence of microcrystalline domains in the partially hydrolyzed PVA (88%) stabilized only by the salts.
To investigate the stability of PVA-Hy against temperature changes, we immersed them in DI water at 40, 50, 60, 70, and 80 °C for 2 h. After 2 h, PVA-Hy remained stable below 60 °C; no change in the morphology of the printed object was observed (Fig. S13, ESI†). For temperatures above 60 °C, the printed object appeared to be swelled, and the degree of swelling increased as the temperature increased (Fig. S13, ESI†). The heating of PVA-Hy presumably led to the dissociation of microcrystallites and increased absorption of water.47 This experiment suggested that the temperature of the surrounding media altered the stability of the PVA-Hy in DI water and PBS buffer. The same study suggested that swelling of PVA-Hy can be tuned by varying the concentration of NaOH. Our study also confirmed that temperature offered another way to control the swelling of PVA-Hy. These studies can help design the applications of PVA-Hy where long-term stability is essential.
2.10. Effect of embedding media on the crystallinity of PVA constructs
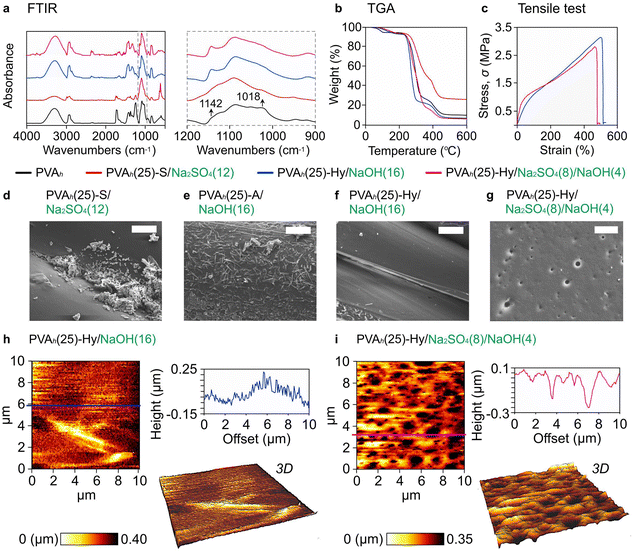
Lastly, to understand the effect of embedding media on the crystallinity of the printed object, we characterized the fabricated 3D objects with Fourier transform infrared (FTIR) spectrometry, thermogravimetric analysis (TGA), tensile testing, and scanning electron microscopy (SEM). We characterized four states of PVA: (1) pristine PVA, (2) PVA-S, (3) PVA-Hy synthesized from PVA-A, (4) PVA-Hy synthesized from PVA-S/A.First, FTIR spectra of four samples were studied: (a) pristine PVAh, (b) PVAh(25)-S/Na2SO4(12), (c) PVAh(25)-Hy/NaOH(16) and (d) PVAh(25)-Hy/Na2SO4(8)/NaOH(4) (Fig. 6(a)). For all 3D-printed samples (i.e., samples (b), (c), and (d)), the fingerprint peak for the acetate group at 1750–1735 cm−1 disappeared (Fig. 6(a)). This peak is the signature of the stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the acetate group.47 A sharp peak at 1142 cm−1 appeared for PVAh-Hy (i.e., samples (c) and (d)); this peak is related to the crystallinity of PVA47,67 (Fig. 6(a), inset). The same peak also confirmed the reorganization of the PVA chains after its reaction with NaOH. In contrast, the peak at 1142 cm−1 was not observed for PVA stabilized by salt (i.e., (b) PVAh(25)-S), suggesting the absence of crystallinity in the ATPS of PVA. The salting out did not cause the formation of microcrystallites in PVA that supported the observation of complete dissolution of the printed object in DI water (Fig. S10(b), ESI†). The absence of crystallinity in the printed PVAh(25)-S justifies further investigation; the increase in crystallinity of PVA was highlighted after the addition of salts in PVA solutions54 while the degree of crystallinity also depends on the degree of hydrolysis of PVA.64 Additionally, in PVAh(25)-Hy, the decrease in the peak at 1018 cm−1 was observed. It was attributed to the CH2 wagging and twisting that suggested the confinement of PVA chains due to the prevention of free deformation of C–H bonds of the CH2 groups of the backbone mainly owing to the van der Waals interactions (Fig. 6(a), inset).
O in the acetate group.47 A sharp peak at 1142 cm−1 appeared for PVAh-Hy (i.e., samples (c) and (d)); this peak is related to the crystallinity of PVA47,67 (Fig. 6(a), inset). The same peak also confirmed the reorganization of the PVA chains after its reaction with NaOH. In contrast, the peak at 1142 cm−1 was not observed for PVA stabilized by salt (i.e., (b) PVAh(25)-S), suggesting the absence of crystallinity in the ATPS of PVA. The salting out did not cause the formation of microcrystallites in PVA that supported the observation of complete dissolution of the printed object in DI water (Fig. S10(b), ESI†). The absence of crystallinity in the printed PVAh(25)-S justifies further investigation; the increase in crystallinity of PVA was highlighted after the addition of salts in PVA solutions54 while the degree of crystallinity also depends on the degree of hydrolysis of PVA.64 Additionally, in PVAh(25)-Hy, the decrease in the peak at 1018 cm−1 was observed. It was attributed to the CH2 wagging and twisting that suggested the confinement of PVA chains due to the prevention of free deformation of C–H bonds of the CH2 groups of the backbone mainly owing to the van der Waals interactions (Fig. 6(a), inset).
Next, TGA was performed to understand the thermal properties of the same samples (Fig. 6(b)). Around 100 °C, the evaporation of water trapped in PVA was observed. The evaporation of water was slower for PVAh(25)-Hy than pristine PVAh. The delayed evaporation (as indicative of the change of the slope) was due to the presence of the crystalline structure that decreased the diffusion of water molecules through the crystalline region of PVA chains.47,68 As the temperature increased, the hydrogen bonds between the hydroxyl groups of PVA chains broke, followed by the degradation of the backbone of PVA chains. PVAh(25)-S exhibited higher stability than PVAh(25)-Hy presumably due to its amorphous nature. It is known that crystallites degrade faster than the amorphous chains that are entangled. The entanglement of the polymer chains in the amorphous polymers inhibited their free movement and hence required high energy for the degradation of the material.47,69
It was highlighted that the concentration of NaOH affected the degree of crosslinking and crystallinity in PVA.47 The effect can be seen on the mechanical properties of PVA-Hy created in different concentrations of NaOH: (c) PVAh(25)-Hy/NaOH(16) and (d) PVAh(25)-Hy/Na2SO4(8)/NaOH(4) (Fig. 6(c)). PVA printed in NaOH(16) was more elastic (E = 10.0 ± 2.1 MPa) than in Na2SO4(8)/NaOH(4) (E = 37.3 ± 4.6 MPa), which can be attributed to a higher degree of crosslinking. The high elasticity of PVA printed in NaOH(16) than in Na2SO4(8)/NaOH(4) can be further supported by the presence of pores on the surface of the printed object in Na2SO4(8)/NaOH(4) (Fig. 6(f) and (g)). The salting out led to the formation of pores in the printed object. The porous microstructure of PVAh(25)-Hy printed in Na2SO4(8)/NaOH(4) was also confirmed by atomic force microscopy (AFM) topographic images (Fig. 6(h) and (i)). The surface of PVAh(25)-Hy printed in Na2SO4(8)/NaOH(4) was more rough than in NaOH(16). Although both the samples exhibited elastic behaviors when stretched to 100% strain, the maximum elongation was higher for PVA-Hy printed in NaOH(16) (524.1 ± 102.3%) than in Na2SO4(8)/NaOH(4) (376.2 ± 91.3%). Moreover, PVA concentration in the ink affected the mechanical properties of PVA-Hy when PVA was printed in the same embedding media. For example, PVAh(25) printed in NaOH(16) was more elastic (E = 10.0 ± 2.1 MPa) and higher maximum elongation (524.1 ± 102.3%) than PVAh(15) printed in NaOH(16) (E = 37.3 ± 4.6 MPa and maximum elongation = 292 ± 10.6%) (Fig. S14, ESI†). Similarly, PVAh(25) printed in Na2SO4(8)/NaOH(4) was more elastic (E = 37.3 ± 4.6 MPa) and higher maximum elongation (376.2 ± 91.3%) than PVAh(15) printed in Na2SO4(8)/NaOH(4) (E = 45.2 ± 3.8 MPa and maximum elongation = 239 ± 7.2%).
As discussed in the early section (Fig. 5 and Fig. S7, ESI†), the 3D-printed samples in the embedding medium may have anisotropic mechanical properties when the crosslinking is prematurely terminated. Typically, the fabrication of a 3D mesh cube (1 cm × 1 cm × 1 cm) in the embedding media with printing parameters (applied pressure, P = 90 kPa; print speed, v = 1 mm s−1; nozzle size, di = 420 μm; layer-to-layer distance, Δz = 2 mm) required 35 min to complete the printing. Therefore, immediately after completing the 3D printing (Tc = 0), the first printed layer has already been in contact with the embedding medium for ∼35 min. We hypothesized that the first layer (or the bottom part of the printed object) had a higher crosslinking density than the last layer (or the top part of the printed object) immediately after printing (Tc = 0). Interestingly, for the sample with Tc = 10 min, PVAh(20)-Hy underwent plastic deformation when squeezed (Fig. S7(b), ESI†). For Tc = 30 min, the printed samples exhibited flexibility and elasticity (Fig. S7(b), ESI†). In order to ensure the consistency of the degree of crosslinking within the object, we used Tc = 24 h in this study unless otherwise stated. Regardless, it should be noted that Tc affected the spatial variation in the crosslinking density and hence the elastic modulus (E) of the printed object. Such 3D-printed objects with a considerable difference in mechanical properties from top to bottom provide an interesting platform to understand the behavior of the biological cells and tissues when are in contact.70 The engineered mechanical properties of these structures can be helpful for the selective promotion or repulsion of biological tissues via cell integration and attachment to the surface.
Overall, these studies highlighted that embedded 3D printing of PVA solutions in salt solutions (PVA-S) did not induce crystallinity in the printed objects; as such, the structure merely stabilized by salting out did not offer a physically stable structure in water. Further postprocessing such as cycles of freezing and thawing of the printed objects is required to induce crystallinity in PVA-S.45 Crucially, we revealed that adding NaOH in the embedding media is a simple way to induce crystallinity in the printed objects to form PVA hydrogel after the removal of Na+ ions. To this end, the PVA stabilized by salting out (PVA-S) can be crosslinked with a moderate concentration of NaOH (4% w/w, 1.2 M) to form PVA-Hy, which exhibited similar properties (albeit less elastic) to those crosslinked with a high concentration of NaOH (16% w/w, 4.8 M).
2.11. Fabrication of 3D models of PVA-Hy
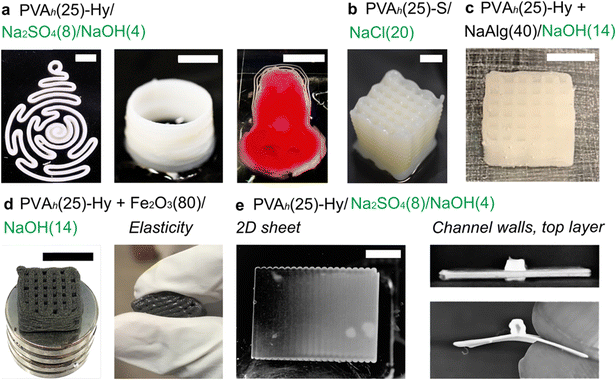
Finally, we highlighted the capability to fabricate 3D models with overhang structures of PVA-Hy (Fig. 7). We fabricated various 3D structures of PVA-Hy using PVAh(25) printed in Na2SO4(8)/NaOH(4) (Fig. 7(a)). We also demonstrated the applicability of sodium chloride (NaCl) in the embedding medium for 3D printing of PVA inks (Fig. 7(b)). A high concentration of NaCl (20% w/w) allowed the stabilization of the printed object by salting out. Similarly to Na2SO4 discussed earlier, the fabricated structure does not confer crystallinity and long-term physical stability.One of the advantages of using liquid inks for 3D printing is the ease of tailoring its contents by mixing additional solid and/or liquid components. PVA inks used in embedded 3D printing can be blended with other polymers such as sodium alginate (NaAlg) (Fig. 7(c)). The mechanical properties of the fabricated PVA-Hy can be tuned by varying the concentration of sodium alginate in the ink.71 Besides, the reinforcement of PVA with different types of solid composites has been studied to enhance their mechanical, electrical, and magnetic properties.47,72,73 Magnetically active PVA by the addition of ferric oxide nanoparticles for anticancer drug delivery applications has been demonstrated.74 To this end, we demonstrated the functionalization of PVAh(25) by adding ferric oxide microparticles (80% w/w of the PVAh ink) to impart magnetic properties to PVAh-Hy (Fig. 7(d)). The printed object also exhibited elasticity and flexibility (Fig. 7(d)).
Lastly, we demonstrated that PVAh-Hy can be printed overhang structures (i.e., structures without physical support underneath). Microfluidic channels require such overhang structures for covering layers for microchannels. A previously demonstrated method to fabricate a microfluidic device consisting of PVAh-Hy required manual layer-by-layer assembly.47 Our method enabled the fabrication of entirely 3D-printed microfluidic devices by direct writing of PVA inks in an aqueous medium containing salt and alkali. To this end, we first printed a 2D sheet of PVAh-S/A in Na2SO4(8)/NaOH(4), followed by printing channel walls and the top layer on the 2D sheet (Fig. 7(e)). The fabricated microchannel was observed as the cross-section to ensure the formation of the channel (Fig. 7(e)). The channel size can be adjusted by tuning the design of the overhang structure or nozzle size. Overall, the developed method allows facile functionalization of PVAh inks and the fabrication of complex 3D structures using biocompatible and elastic PVAh-Hy, which should find diverse applications in microfluidics and soft robotics.
3. Conclusions
In conclusion, we presented an unprecedented method of embedded 3D printing of PVA hydrogels by uniquely combining salting out of ATPS and alkali-based physical crosslinking. The method allowed for fabricating 3D structures of PVA hydrogels using pristine PVA without any rheological modifiers. Simultaneous salting out and physical crosslinking allowed the use of a reduced concentration of strong base (i.e., NaOH, 4% w/w), which offered less corrosive and more suitable conditions to perform additive manufacturing by embedded 3D printing. The use of a low-concentration base permitted slow precipitation of the printed inks, which permitted layer-by-layer overlap, leading to the adhesion between printed materials necessary for extrusion-based 3D printing. To date, DIW 3D printing of PVA inks has been demonstrated only with (1) PVA inks conferred with yield stress properties or (2) embedding media with stringent conditions such as highly basic conditions (14% w/w, NaOH). To our knowledge, this is the first study to demonstrate the reproducible 3D printing of physically crosslinked PVA hydrogels with low viscosity (μ ∼ 0.1 to 20 Pa s) in moderately basic conditions (NaOH, 4% w/w).This study highlighted the unique conceptual merit of using an ATPS to enable embedded 3D printing. ATPS by salting out offers a physical reaction to stabilize the printed aqueous ink in the embedding aqueous media. Importantly, this reaction is reversible; the ion present in the system (i.e., Na+) can be readily removed after additional reaction (i.e., physical crosslinking by strong base) is performed. This mechanism is crucial to achieve layer-by-layer 3D printing with the adhesion of the filaments, which is crucial for the fabrication of PVA hydrogels. Due to the liquid nature of the printing material, DIW 3D printing offers potential in fabricating structures consisting of PVA hydrogels with composite additives. The degradation of the fabricated PVA hydrogels at higher temperatures (T > 60 °C) allows recycling of the polymer making the proposed method sustainable.
Some characteristics of the developed method and PVA-Hy can be highlighted as follows: (1) the molecular weight of PVA is crucial to ensure the print fidelity of the printed PVA-Hy; (2) the concentration of PVAh (15–25% w/w) was used in the developed method, which is higher than typical concentration reported for the formation of PVA hydrogels; (3) the post-printing time of 24 h in the bath is required to ensure uniform mechanical properties of the printed object. These characteristics should motivate further research to improve the method and attainable properties of PVA-Hy. Nevertheless, the concept demonstrated in this study should apply to the 3D printing of elastic, flexible, and biocompatible PVA hydrogels for various applications, including microfluidics, wearable devices, and scaffolds for tissue engineering.
4. Experimental section
4.1. Preparation of PVA inks
Two commercially available PVA powders were used for this study: PVAh (1788L, MW = 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, 88% hydrolyzed, Eastchem Lab, China) and PVAl (PVA500, MW 22
800, 88% hydrolyzed, Eastchem Lab, China) and PVAl (PVA500, MW 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 86.5–89% hydrolyzed, Kanto Chemical Co., Inc., Japan). The polymers were used without further purification. The deionized water was first heated at 90 °C, and the desired amount of PVA was then added under continuous stirring. The solution was continuously stirred until it became homogeneous and kept overnight at ambient conditions for the removal of air bubbles. The degassed solution was then used immediately for 3D printing. PVA-based inks were prepared by adding sodium alginate (Sigma-Aldrich, Singapore) into the PVA solution under continuous stirring at 90 °C. The mixtures were stirred continuously to be homogeneous and free of bubbles. The functionalized PVA inks were prepared by mixing PVA solution with ferric oxide microparticles (Sigma-Aldrich, Singapore) under continuous stirring. The mixtures were stirred continuously to be homogeneous and free of bubbles. PVA-based composite inks were then placed immediately into the dispensing syringes for 3D printing.
000, 86.5–89% hydrolyzed, Kanto Chemical Co., Inc., Japan). The polymers were used without further purification. The deionized water was first heated at 90 °C, and the desired amount of PVA was then added under continuous stirring. The solution was continuously stirred until it became homogeneous and kept overnight at ambient conditions for the removal of air bubbles. The degassed solution was then used immediately for 3D printing. PVA-based inks were prepared by adding sodium alginate (Sigma-Aldrich, Singapore) into the PVA solution under continuous stirring at 90 °C. The mixtures were stirred continuously to be homogeneous and free of bubbles. The functionalized PVA inks were prepared by mixing PVA solution with ferric oxide microparticles (Sigma-Aldrich, Singapore) under continuous stirring. The mixtures were stirred continuously to be homogeneous and free of bubbles. PVA-based composite inks were then placed immediately into the dispensing syringes for 3D printing.
4.2. Preparation of embedding liquid media
The salt solution was prepared by dissolving the desired concentration of sodium sulfate (Na2SO4, Sigma-Aldrich, Singapore), tripotassium phosphate (K3PO4, Sigma-Aldrich, Singapore), ammonium sulfate ((NH4)2SO4, Sigma-Aldrich, Singapore), and sodium chloride (NaCl, Sigma-Aldrich, Singapore) in deionized water under continuous stirring until a homogeneous mixture was obtained at ambient conditions. Similarly, the alkali solution was prepared by dissolving the desired concentration of sodium hydroxide (NaOH, Sigma-Aldrich, Singapore) and potassium hydroxide (KOH, Sigma-Aldrich, Singapore) in deionized water under continuous stirring until a homogeneous mixture was obtained at ambient conditions. All the chemicals were used without further purification. The solutions were freshly prepared and used as embedding media before every 3D printing experiment.4.3. Characterization of PVA inks
The steady-state shear viscosity of the inks was characterized using a rheometer (Discovery HR-2, TA Instruments, USA) with a 20-mm parallel plate. The shear rate was ramped stepwise from 0.001 s−1 to 1000 s−1. The gap between the plate and the stationary flatbed was 500 μm in all the rheological experiments. A spatula was used to deposit the ink carefully on the bottom plate. The top plate was lowered to the set gap of 500 μm. The excess sample was squeezed out between the plates, which was removed neatly to avoid the edge effects. A similar loading procedure was followed for all the measurements. All experiments were performed at room temperature and under ambient pressure.4.4. DIW 3D printer
MuCAD V software (Musashi Engineering Inc., Japan) was used to generate the design and print using a DIW 3D printer using a commercial 3D printing robot and a liquid dispenser (SHOTmini 200 Sx and IMAGE MASTER 350 PC Smart, Musashi Engineering Inc., Japan). For the designs of CAD, STL data was generated using a commercial CAD program and sliced using Slic3r software75 into 100 μm to 200 μm thick layers to generate the G-code instructions. The G-code was then converted to the format readable by MuCAD V using a house-made Python script.4.5. Protocol for 3D printing
Before each printing, the nozzle was attached to the cylindrical syringe and placed into its respective position in the liquid dispenser. For every nozzle attached, calibrations in the horizontal (x and y) and the vertical (z) direction (distance between the nozzle tip and the substrate) were performed. A Petri dish was filled with the embedding liquid media, and the printing was performed. The inks were extruded through nozzles of different internal diameters (di = 160–680 μm) with varying applied pressure (P = 10–600 kPa). After completion of the printing, the printed structure was kept in the embedding media for 24 h to ensure complete salting out or ionic crosslinking of PVA ink in the embedding media. The physically crosslinked objects were then kept in deionized water for 24 h for the removal of Na+. All experiments were performed at room temperature.4.6. Thermogravimetric analysis (TGA)
Pyrolysis tests were performed in a differential thermogravimetric analyzer (Q50, TA Instruments, USA) with a precision of temperature measurement of ±0.1 °C and weight measurement of ±0.01%. The sample weight loss and the rate of weight loss were recorded continuously as a function of time and temperature from 30 °C to 1000 °C. The experiments were performed at atmospheric pressure, under a nitrogen atmosphere, with a flow rate of 30 mL min−1 at various linear heating rates of 5,10, 20, and 30 °C min−1.4.7. Fourier transform infrared spectroscopy (FTIR) analysis
FTIR measurements with an attenuated total reflection (ATR) were performed to identify the functional groups present in the prepared samples. FTIR spectrophotometer (FTIR610, Jasco International Co. Ltd., Japan) with absorbance mode was used, and the interferograms were recorded in the spectral range of 4000–400 cm−1 with 128 scans per pixel at 4 cm−1 spectral resolution and aperture of 5 mm.4.8. Tensile testing of PVA hydrogel sheets
First, PVA solution was poured onto a glass slide, and a film of 200–300 μm size was formed by spin coating at 500–1000 rpm for 10–20 s. The glass slide with the PVA film was immediately embedded into the liquid media for physical crosslinking for 24 h. The crosslinked PVA hydrogel films were then kept in deionized water for the removal of Na+. The specimen was cut into a rectangular shape with a width of 20 mm and a length of 15 mm, of which top and bottom edges were supported by an adhesive tape to be held by the clamps of the instrument for tensile testing. Tensile tests were carried out with a universal testing machine (Instron 5943, Illinois Tool Works Inc., USA). The tensile rate was 2% strain per min.4.9. Atomic force microscopy (AFM)
Surface topography of the hydrogels was measured in water using JPK NanoWizard 3 AFM (Bruker Singapore Pte. Ltd., Singapore) quantitative imaging (QI) mode with QP-cont probes (NANOSENSORS™, Switzerland) with a spring constant of 0.08–0.51 N m−1. The probes were calibrated in water using Sader method.4.10. Imaging
Photographs were taken using a Nikon D5600 camera (Nikon, Japan) under white-light illumination. All image processing was done using ImageJ (National Institute of Health, USA). The microscopic morphologies of the 3D printed objects were observed using a field emission scanning electron microscope (JSM-7600F, JEOL, Japan) at 5–15 kV. The membranous structures were sampled in liquid nitrogen and then sputtered with gold for 30–60 s at 20 mA using an auto fine coater (JFC-1600, JEOL, Japan) before imaging.Author contributions
R. K. and M. H. planned the study; R. K. designed and performed the experiments and analyzed the data; N. N. performed FTIR measurements and analyzed the data; K. Y. performed tensile testing measurements and analyzed the data; K. X. perform AFM and analyzed the data. R. K., Z. Q. and M. H. wrote the paper.Data availability statement
The raw data and the processed data required to reproduce these findings are available from the corresponding author on reasonable request.Conflicts of interest
The authors declare no competing interests.Acknowledgements
M. H. thanks to Agency for Science, Technology and Research (A*STAR) (A1983c0039 and M22K2c0085) and Digital Manufacturing and Design (DManD) Centre at Singapore University of Technology and Design (RGDM1620503) for the project support.References
- J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1880 CrossRef CAS PubMed.
- J. Chana, B. Forbes and S. A. Jones, J. Nanosci. Nanotechnol., 2008, 8, 5739–5747 CrossRef CAS PubMed.
- S. Jiang, S. Liu and W. Feng, J. Mech. Behav. Biomed. Mater., 2011, 4, 1228–1233 CrossRef CAS PubMed.
- Y. Liu, L. M. Geever, J. E. Kennedy, C. L. Higginbotham, P. A. Cahill and G. B. McGuinness, J. Mech. Behav. Biomed. Mater., 2010, 3, 203–209 CrossRef PubMed.
- S. R. Stauffer and N. A. Peppas, Polymer, 1992, 33, 3932–3936 CrossRef CAS.
- Y.-N. Chen, L. Peng, T. Liu, Y. Wang, S. Shi and H. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 27199–27206 CrossRef CAS PubMed.
- R. Rudra, V. Kumar and P. P. Kundu, RSC Adv., 2015, 5, 83436–83447 RSC.
- T. Miyazaki, Y. Takeda, S. Akane, T. Itou, A. Hoshiko and K. En, Polymer, 2010, 51, 5539–5549 CrossRef CAS.
- A. Danno, J. Phys. Soc. Jpn., 1958, 13, 722–727 CrossRef CAS.
- N. A. Peppas and E. W. Merrill, J. Appl. Polym. Sci., 1977, 21, 1763–1770 CrossRef CAS.
- T. H. Kim, D. B. An, S. H. Oh, M. K. Kang, H. H. Song and J. H. Lee, Biomaterials, 2015, 40, 51–60 CrossRef CAS PubMed.
- S. Lin, X. Liu, J. Liu, H. Yuk, H.-C. Loh, G. A. Parada, C. Settens, J. Song, A. Masic, G. H. McKinley and X. Zhao, Sci. Adv., 2019, 5, eaau8528 CrossRef PubMed.
- M. Irimia-Vladu, Chem. Soc. Rev., 2014, 43, 588–610 RSC.
- J. Lia, C. Wu, P. K. Chu and M. Gelinsky, Mater. Sci. Eng., R: Rep., 2020, 140, 100543 CrossRef.
- C. Xu, G. Dai and Y. Hong, Acta Biomater., 2019, 95, 50–59 CrossRef CAS PubMed.
- J. M. Ino, P. Chevallier, D. Letourneur, D. Mantovani and C. Le Visage, Biomatter, 2013, 3, e25414 CrossRef PubMed.
- W. Luo, S. Zhang, P. Li, R. Xu, Y. Zhang, L. Liang, C. D. Wood, Q. Lu and B. Tan, Polymer, 2015, 61, 183–191 CrossRef CAS.
- R. Bryaskova, N. Georgieva, T. Andreeva and R. Tzoneva, Surf. Coat. Technol., 2013, 235, 186–191 CrossRef CAS.
- S. A. Stone, P. iGosavi, T. J. Athauda and R. R. Ozer, Mater. Lett., 2013, 112, 32–35 CrossRef CAS.
- M. A. Teixeira, M. Teresa, P. Amorim and H. P. Felgueiras, Polymers, 2020, 12, 7 CrossRef CAS PubMed.
- E. Sachlos and J. T. Czernuszka, Eur. Cell Mater., 2003, 5, 29–39 CrossRef CAS PubMed.
- C. M. Thakar, S. S. Parkhe, A. Jain, K. Phasinam, G. Murugesan and R. J. M. Ventayen, Mat. Today: Proc, 2022, 51, 842–849 Search PubMed.
- M. A. S. R. Saadi, A. Maguire, N. T. Pottackal, M. S. H. Thakur, M. M. Ikram, A. J. Hart, P. M. Ajayan and M. M. Rahman, Adv. Mater., 2022, 34, 2108855 CrossRef CAS PubMed.
- P. Jiang, C. Yan, Y. Guo, X. Zhang, M. Cai, X. Jia, X. Wang and F. Zhou, Biomater. Sci., 2019, 7, 1805–1814 RSC.
- A. Li, Y. Si, X. Wang, X. Jia, X. Guo and Y. Xu, ACS Appl. Nano Mater., 2019, 2, 707–715 CrossRef CAS.
- Y. Meng, J. Cao, Y. Chen, Y. Yu and L. Ye, J. Mater. Chem. B, 2019, 8, 677–690 RSC.
- Z. Tan, C. Parisi, L. Di Silvio, D. Dini and A. E. Forte, Sci. Rep., 2017, 7, 16293 CrossRef PubMed.
- N. M. Ergul, S. Unal, I. Kartal, C. Kalkandelen, N. Ekren, O. Kilic, L. Chi-Chang and O. Gunduz, Polym. Test., 2019, 79, 106006 CrossRef CAS.
- F. Liu, W. Li, H. Liu, T. Yuan, Y. Yang, W. Zhou, Y. Hu and Z. Yang, Macromol. Biosci., 2021, 2000398 CrossRef CAS PubMed.
- M. Angjellari, E. Tamburri, L. Montaina, M. Natali, D. Passeri, M. Rossi and M. L. Terranova, Mater. Des., 2017, 119, 12–21 CrossRef CAS.
- J. Li, X. Li, S. Long, H. Li and D. Huang, Acta Mater. Compos. Sin., 2016, 33, 2412–2418 Search PubMed.
- H. Kim, G. H. Yang, C. H. Choi, Y. S. Cho and G. H. Kim, Int. J. Biol. Macromol., 2018, 120, 119–127 CrossRef CAS PubMed.
- R. Karyappa, A. Ohno and M. Hashimoto, Mater. Horiz., 2019, 6, 1834–1844 RSC.
- R. Karyappa, T. Ching and M. Hashimoto, ACS Appl. Mater. Interfaces, 2020, 12, 23565–23575 CrossRef CAS PubMed.
- F. Zhu, L. Cheng, J. Yin, Z. L. Wu, J. Qian, J. Fu and Q. Zheng, ACS Appl. Mater. Interfaces, 2016, 8, 31304–31310 CrossRef CAS PubMed.
- G. M. Gratson, M. Xu and J. Lewis, Nature, 2004, 428, 386 CrossRef CAS PubMed.
- A. McCormack, C. B. Highley, N. R. Leslie and F. P. W. Melchels, Trends Biotechnol., 2020, 38, 584–593 CrossRef CAS PubMed.
- R. Karyappa and M. Hashimoto, ACS Appl. Polym. Mater., 2021, 3, 908–919 CrossRef CAS.
- R. Karyappa, H. Liu, Q. Zhu and M. Hashimoto, ACS Appl. Mater. Interfaces, 2023, 15, 21575–21584 CrossRef CAS PubMed.
- T. Bhattacharjee, S. M. Zehnder, K. G. Rowe, S. Jain, R. M. Nixon, W. G. Sawyer and T. E. Angelini, Sci. Adv., 2015, 1, e1500655 CrossRef PubMed.
- T. J. Hinton, A. Hudson, K. Pusch, A. Lee and A. W. Feinberg, ACS Biomater. Sci. Eng., 2016, 2, 1781–1786 CrossRef CAS PubMed.
- M. Stang, J. Tashman, D. Shiwarski, H. Yang, L. Yao and A. Feinberg, Adv. Mater. Technol., 2023, 8, 2200984 CrossRef CAS PubMed.
- B. Filova, L. Musilova, A. Mracek, M. L. Ramos, L. M. P. Veríssimo, A. J. M. Valente and A. C. F. Ribeiro, J. Mol. Liq., 2020, 304, 112728 CrossRef CAS.
- S. Wu, M. Hua, Y. Alsaid, Y. Du, Y. Ma, Y. Zhao, C.-Y. Lo, C. Wang, D. Wu, B. Yao, J. Strzalka, H. Zhou, X. Zhu and X. He, Adv. Mater., 2021, 33, 2007829 CrossRef CAS PubMed.
- W. Cui, Y. Zheng, R. Zhu, Q. Mu, X. Wang, Z. Wang, S. Liu, M. Li and R. Ran, Adv. Funct. Mater., 2022, 2204823 CrossRef CAS.
- M. A. Darabi, A. Khosrozadeh, Y. Wang, N. Ashammakhi, H. Alem, A. Erdem, Q. Chang, K. Xu, Y. Liu, G. Luo, A. Khademhosseini and M. Xing, Adv. Mater., 2020, 7, 1902740 CAS.
- A. L. Grilo, M. R. Aires-Barros and A. M. Azevedo, Sep. Purif. Rev., 2016, 45, 68–80 CrossRef.
- A. Bhattacharya and P. Ray, J. Appl. Polym. Sci., 2004, 93, 122–130 CrossRef CAS.
- B. Briscoe, P. Luckham and S. Zhu, Polymer, 2000, 41, 3851–3860 CrossRef CAS.
- A. Bankura, V. Carnevale and M. L. Klein, J. Chem. Phys., 2013, 138, 014501 CrossRef PubMed.
- B. Hribar, N. T. Southall, V. Vlachy and K. A. Dill, J. Am. Chem. Soc., 2002, 124, 12302–12311 CrossRef CAS PubMed.
- S. Komiya, E. Otsuka, Y. Horashima and A. Suzuki, Prog. Nat. Sci.: Mater. Int., 2011, 21, 375–379 CrossRef.
- O. N. Tretinnikov and S. A. Zagorskaya, J. Appl. Spectrosc., 2012, 78, 904–908 CrossRef CAS.
- M. Perfetti, N. Gallucci, I. R. Krauss, A. Radulescu, S. Pasini, O. Holderer, G. D’Errico, G. Vitiello, G. O. Bianchetti and L. Paduano, Macromolecules, 2020, 53, 852–861 CrossRef CAS.
- P. Jungwirth and P. S. Cremer, Nat. Chem., 2014, 6, 261–263 CrossRef CAS PubMed.
- Y. Zhang and P. S. Cremer, Curr. Opin. Chem. Biol., 2006, 10, 658–663 CrossRef CAS PubMed.
- Y. Zhang, S. Furyk, D. E. Bergbreiter and P. S. Cremer, J. Am. Chem. Soc., 2005, 127, 14505–14510 CrossRef CAS PubMed.
- R. S. Carnegie, C. L. D. Gibb and B. C. Gibb, Angew. Chem., Int. Ed., 2014, 53, 11498–11500 CrossRef CAS PubMed.
- Q. He, Y. Huang and S. Wang, Adv. Funct. Mater., 2018, 28, 1705069 CrossRef.
- B. E. B. Jensen, A. A. A. Smith, B. Fejerskov, A. Postma, P. Senn, E. Reimhult, M. Pla-Roca, L. Isa, D. S. Sutherland, B. Stadler and A. N. Zelikin, Langmuir, 2011, 27, 10216–10223 CrossRef CAS PubMed.
- F. Auriemma, C. De Rosa and R. Triolo, Macromolecules, 2006, 39, 9429–9434 CrossRef CAS.
- B. Semmling, S. Nagel, K. Sternberg, W. Weitschies and A. Seidlitz, J. Pharm. Technol. Drug Res., 2013, 2, 19 CrossRef.
- R. Sanchis-Gual, H. Ye, T. Ueno, F. C. Landers, L. Hertle, S. Deng, A. Veciana, Y. Xia, C. Franco, H. Choi, J. Puigmartí-Luis, B. J. Nelson, X. Z. Chen and S. Pané, Adv. Funct. Mater., 2023, 33, 2212952 CrossRef CAS.
- S. Wu, T.-W. Wang, Y. Du, B. Yao, S. Duan, Y. Yan, M. Hua, Y. Alsaid, X. Zhu and X. He, NPG Asia Mater., 2022, 14, 65 CrossRef CAS.
- X. Fu, H. Tong, X. Zhang, K. Zhang, L. Douadji, S. Kang, J. Luo, Z. Pan and W. Lu, ACS Appl. Polym. Mater., 2023, 5, 9876–9887 CrossRef CAS.
- O. N. Tretinnikov and S. A. Zagorskaya, J. Appl. Spectrosc., 2012, 79, 521–526 CrossRef CAS.
- M. Hedenqvist and U. W. Gedde, Prog. Polym. Sci., 1996, 21, 299–333 CrossRef CAS.
- Y. K. Godovsky, Thermophysical Properties of Polymers, Springer, Berlin, 2012 Search PubMed.
- M. Hakim Khalili, V. Panchal, A. Dulebo, S. Hawi, R. Zhang, S. Wilson, E. Dossi, S. Goel, S. A. Impey and A. I. Aria, ACS Appl. Polym. Mater., 2023, 5, 3034–3042 CrossRef CAS PubMed.
- M. Bahadoran, A. Shamloo and Y. D. Nokoorani, Sci. Rep., 2020, 10, 7342 CrossRef CAS PubMed.
- A. A. Novakova, V. Y. Lanchinskaya, A. V. Volkov, T. S. Gendler, T. Y. Kiseleva, M. A. Moskvina and S. B. Zezin, J. Magn. Magn. Mater., 2003, 258–259, 354–357 CrossRef CAS.
- Y. Pan, L. Fu, Q. Zhou, Z. Wen, C.-T. Lin, J. Yu, W. Wang and H. Zhao, Polym. Compos., 2020, 41, 210–218 CrossRef CAS.
- A. K. Bajpai and R. Gupta, J. Mater. Sci.: Mater. Med., 2011, 22, 357–369 CrossRef CAS PubMed.
- A. Ranellucci, Slic3r, https://slic3r.org/ Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01714a |
| This journal is © The Royal Society of Chemistry 2024 |