Cold-resistant, highly stretchable ionic conductive hydrogels for intelligent motion recognition in winter sports†
Tongda
Lei
a,
Jiajun
Pan
a,
Ning
Wang
a,
Zhaopeng
Xia
*a,
Qingsong
Zhang
*b,
Jie
Fan
*a,
Lei
Tao
 c,
Wan
Shou
d and
Yu
Gao
b
c,
Wan
Shou
d and
Yu
Gao
b
aSchool of Textile Science and Engineering, Tiangong University, Tianjin 300387, China. E-mail: xia_zhaopeng@163.com; fanjie@tiangong.edu.cn
bSchool of Material Science and Engineering, Tiangong University, Tianjin 300387, China. E-mail: zhangqingsong@tiangong.edu.cn
cThe Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, P. R. China
dDepartment of Mechanical Engineering, University of Arkansas, Fayetteville, AR 72701, USA
First published on 22nd December 2023
Abstract
Conductive hydrogels have attracted much attention for their wide application in the field of flexible wearable sensors due to their outstanding flexibility, conductivity and sensing properties. However, the weak mechanical properties, lack of frost resistance and susceptibility to microbial contamination of traditional conductive hydrogels greatly limit their practical application. In this work, multifunctional polyvinyl alcohol (PVA)/carboxymethyl cellulose (CMC)/poly(acrylamide-co-1-vinyl-3-butylimidazolium bromide) (P(AAm-co-VBIMBr)) (PCPAV) ionic conductive hydrogels with high strength and good conductive, transparent, anti-freezing and antibacterial properties were constructed by introducing a network of chemically crosslinked AAm and VBIMBr copolymers into the base material of PVA and CMC by in situ free radical polymerization. Owing to the multiple interactions between the polymers, including covalent crosslinking, multiple hydrogen bonding interactions, and electrostatic interactions, the obtained ionic conductive hydrogels exhibit a high tensile strength (360.6 kPa), a large elongation at break (810.6%), good toughness, and fatigue resistance properties. The introduction of VBIMBr endows the PCPAV hydrogels with excellent transparency (∼92%), a high ionic conductivity (15.2 mS cm−1), antimicrobial activity and good flexibility and conductivity at sub-zero temperatures. Notably, the PCPAV hydrogels exhibit a wide strain range (0–800%), high strain sensitivity (GF = 3.75), fast response, long-term stability, and fantastic durability, which enable them to detect both large joint movements and minute muscle movements. Based on these advantages, it is believed that the PCPAV-based hydrogel sensors would have potential applications in health monitoring, human motion detection, soft robotics, ionic skins, human–machine interfaces, and other flexible electronic devices.
New conceptsConductive hydrogels exhibit potential for a wide range of applications including artificial intelligence devices, human–computer interfaces, electronic skins, and health and motion detecting devices. However, electrically conductive hydrogels based on conductive polymers affect wearing comfort due to their inherent rigidity. In addition, their opaqueness and incompatibility with conductive fillers limit their application in wearable sensors. In contrast, ionic conductive hydrogels are some of the candidates for application in flexible wearable sensors due to their high conductivity, high stretchability, high flexibility and high transparency. However, there are still some outstanding issues to overcome. Here, multifunctional PVA/CMC/P(AAm-co-VBIMBr) (PCPAV) ionic conductive hydrogels were constructed using ionic liquids. The obtained ionic conductive hydrogels exhibited excellent tensile properties (810.6%), outstanding tensile stress (360.6 kPa), good toughness and anti-fatigue properties due to their multiple crosslinked network structure with covalent crosslinked networks, multiple hydrogen bonding interactions, and electrostatic interactions. The introduction of VBIMBr endows PCPAV hydrogels with high ionic conductivity (15.2 mS cm−1), excellent transparency (∼92%), and outstanding frost resistance (−45.5 °C). In addition, the good resistance to low temperature, volatilization and microbial contamination of VBIMBr facilitate the PCPAV hydrogels to exhibit excellent anti-freezing, moisturizing and antimicrobial properties. A flexible sensor assembled from this hydrogel exhibited a high strain sensitivity (GF = 3.75), fast response, long-term stability and durability, and it can not only monitor various large-scale human joint motions, but can also sense tiny muscle movements. In addition, it can realize human–computer interactions, and has a broad application prospect in flexible wearable electronic products. |
1. Introduction
In recent years, flexible and wearable sensor devices are attracting considerable attention because of their potential applications in health monitoring, human motion detection, electronic skins, human–machine interfaces, soft robotics, and implantable biosensors.1–7 However, traditional sensing devices based on metals or semiconductors typically only perceive minor deformations with small strains and are usually accompanied by an inherent rigidity which affects wearing comfort.8,9 In addition, the requirements for stretchability, transparency, mechanical durability, and biocompatibility of wearable sensor devices have increased for their practical applications. Therefore, there is an urgent need to develop various strategies to produce flexible wearable sensors with high sensitivity, high stretchability and excellent mechanical properties.Hydrogels, as classical soft materials, consist of a three-dimensional elastic crosslinked polymer network and a large amount of water.10–12 Due to their good hydrophilicity, high flexibility, high stretchability, tunable mechanical properties, good biocompatibility and structural similarity to natural soft tissues, hydrogels have become promising candidate materials for the fabrication of flexible wearable sensors.13,14 It is possible to create conductive hydrogels by incorporating conductive materials into the hydrogel matrix endowing them with excellent electrical properties, which can be used directly as sensing materials for wearable sensors.15 So far, conductive hydrogels can be divided into electronically conductive hydrogels and ionically conductive hydrogels depending on the transmission medium. By integrating conductive fillers such as carbon-based nanomaterials,16–18 metal nanomaterials,19,20 and conductive polymers21,22 into hydrogel matrices, a variety of electronically conductive hydrogels with excellent electronic conductivity and unique biomechanical properties have been developed. For example, Chen et al. reported an electronically conductive hydrogel composed of acrylamide (AAm), cellulose nanofibers (CNFs), and carbon nanotubes (CNTs) synthesised via in situ polymerization, and appropriately increasing the content of CNTs (from 0.5 wt% to 1 wt%) could increase the electronic conductivity of PAAm/CNTs-based electrically conductive hydrogels from 0.74 to 0.85 mS cm−1, as well as increase the tensile strength of the hydrogels from 0.28 to 0.32 MPa.23 However, due to the poor interfacial interaction between the embedded materials and the hydrogel matrix, the hydrogels not only exhibit low sensitivity, but also low stretchability and poor mechanical properties.13,15 Furthermore, electronically conductive hydrogels are usually opaque, which limits their further application in wearable sensors that require visualization.24
Compared to electrically conductive hydrogels based on conductive fillers, ionic conductive hydrogels are conducted through directional transport of free ions, which greatly enhances the ionic conductivity of the hydrogels. In addition, ionic conductive hydrogels usually present inherent transparency, tunable mechanical properties, a greater sensing range, and a more straightforward fabrication process. More importantly, signals in biological systems are typically transmitted via ions, which gives ionic conductive hydrogels flexibility similar to that of biological systems, and thus they have emerged as promising candidates for flexible wearable sensors.25,26 Double network ionic conductive hydrogels prepared using K+ ionic crosslinked κ-carrageenan (κ-CG-K+) as the first network and hydrogen bonded crosslinked poly(N-hydroxyethyl acrylamide) as the second network exhibited a high tensile strength (2.02 MPa), excellent tensile strain (1550%), good optical transparency, and a good electrical conductivity (2.9 mS cm−1), and could be used in strain sensors for monitoring human motion.27 Nevertheless, there are still some key scientific issues to be overcome. For example, traditional ionic conductive hydrogels with water as the ionic conduction medium usually lose their original flexibility, conductivity and optical transparency at sub-zero temperatures due to the freezing of water.28 Inspired by the fact that lipids in plant cell membranes can inhibit freezing of water, researchers introduced binary solvent systems (such as glycerol/water, ethylene glycol (EG)/water and dimethyl sulfoxide/water) as the dispersion media of hydrogels to prepare organic hydrogels with antifreeze properties.29,30 A polyvinyl alcohol (PVA)/EG conductive hydrogel constructed by replacing the single water solvent with an EG/water mixed solvent exhibited excellent anti-freezing and moisturizing properties, and showed excellent flexibility and stretchability at both −20 °C and 25 °C.31 However, the introduction of organic solvents not only increases the crosslink density of the hydrogel network, but decreases the degree of ion dissociation, which inevitably hinders ion mobility and thus reduces ionic conductivity.29,32 In addition, the volatility and toxicity of organic solvents limit their use in skin contact utilization. Introducing soluble metal ionic salts (such as Na+, K+, Li+, Ca2+, Fe3+ and Al3+) into a hydrogel matrix is another method widely used to produce ionic conductive hydrogels with excellent anti-freezing properties.33 For instance, Zhang et al. constructed polyacrylamide/sodium alginate/LiCl ionic conductive hydrogels by free radical polymerization, and the introduction of LiCl enhanced the freeze resistance of the hydrogels.34 The hydrogels exhibited excellent ionic conductivity (60.61 mS cm−1) at −30 °C and maintained good stretchability ranging from −30 °C to 25 °C. Zhang et al. reported an ionic conductive composite hydrogel prepared using LiCl and glycerol.35 Due to the ionic hydration of LiCl and the hydrogen bonding interactions between water molecules and glycerol, the hydrogel exhibited excellent anti-freezing properties and could work at low temperatures as low as −30 °C. Tian et al. reported a double-network cellulose/polyacrylic acid (Cel/PAA) composite ionic hydrogel based on the dissolution of cellulose in an aqueous system of AlCl3/ZnCl2.36 AlCl3/ZnCl2 endowed the Cel/PAA composite hydrogel with excellent anti-freezing properties (−45 °C). However, the high concentration of salt ions disrupts the network structure and the interactions between polymers in the hydrogel, and the mechanical characteristics of the hydrogels were restricted.37,38
Ionic liquids (ILs) that consist of organic cations and inorganic/organic anions are molten salts in the liquid state at temperatures below 100 °C.39 Recently, ILs have attracted great interest in the field of electronic devices due to their high ionic conductivity, wide electrochemical window, easy to fine-tune physicochemical properties and excellent electrochemical stability.40,41 In addition, the inherent low freezing point, high boiling point and nonvolatility of ILs endow IL-based ionic conductive hydrogels with excellent freezing resistance and water retention for their application in flexible wearable sensors, especially in extreme environments.42,43 For example, Wang et al. reported a novel ionic liquid hydrogel wound dressing with anti-freezing properties, in which the introduction of ionic liquids endowed it with good tensile mechanics even after being placed at −20 °C for one day.44 Chen et al. developed a series of double crosslinked ionic conductive hydrogels based on polyacrylamide/Ca-alginate and adjusted the electrical and mechanical properties of the hydrogels by introducing imidazolium ionic liquid monomers with various chain lengths.45 But the highest tensile strength of the pAMAL-IMC6-Ca hydrogel was only 8.25 kPa. Many ionic liquid hydrogels, especially imidazolium-based ionic liquids hydrogels, exhibit weak mechanical properties due to insufficient non-covalent bonding interactions between imidazolium chain segments and other chain segments in the hydrogels, thus limiting their application in the field of flexible sensors.46–48 Therefore, it is particularly important to develop robust IL-based ionic conductive hydrogels to meet the demands of high-performance flexible wearable sensors.
Currently, researchers have tried to enhance the mechanical strength of ionic liquid-based hydrogels by establishing chemical cross-linking and physical interactions between the molecules of the hydrogels.49,50 Chemical cross-linking can effectively improve the mechanical strength of hydrogels, and physical interactions can not only recover and rebuild after destruction, but also confer a high degree of deformation tolerance and excellent fatigue resistance to ionic liquid-based hydrogels.51 Therefore, constructing semi-interpenetrating networks by combining the chemical cross-linking and physical interactions between the components of the hydrogels is an effective strategy to improve the mechanical properties of ionic liquid-based hydrogels. For example, Wang et al. prepared a semi-interpenetrating ionic conductive hydrogel by means of ILs and acrylic acid in an aqueous solution of poly(ethylene oxide).52 The hydrogel consisted of a covalently cross-linked network and a dense hydrogen-bonded network, which enabled it to show superelasticity, large reversible tensile/compressive properties and excellent fatigue resistance. PVA is a common water-soluble polymer and is a popular elastic matrix for hydrogels due to its good biocompatibility, biodegradability, mechanical properties and environmental friendliness.53,54 In addition, PVA molecular chains contain a large number of hydroxyl groups (–OH), which can form hydrogen bonds with other water-soluble components to build multifunctional hydrogels. For example, Tavakolizadeh et al. reported a salep/PVA double network hydrogel for wound dressing.55 The dynamic hydrogen bonding interactions formed between –OH in the PVA network enhanced the mechanical properties and self-healing ability of the hydrogel. Carboxymethyl cellulose (CMC), as a non-toxic cellulose derivative, contains abundant carboxyl groups (–COOH) in the molecular chains, which can form strong interactions with PVA through hydrogen bonds and subsequent crosslinking to result a hydrogel with excellent mechanical properties and good hygroscopicity.56 For example, a study by Yang et al. showed that PVA/CMC composite hydrogels have higher tensile strength than pure PVA hydrogels.57 In practical applications, the moist surface of the PVA/CMC hydrogels may provide a suitable environment for the growth of bacteria, leading to allergies of human skin and contamination of the hydrogels. Fortunately, ILs also show excellent antibacterial activity due to their ability to cross bacterial membranes, enter the cytosol and alter membrane characteristics of the bacterial cell wall, severely affecting membrane function.58 Thus, ILs are promising doping materials for the preparation of flexible wearable hydrogel sensors with simultaneously high electrical conductivity, good antifreeze properties, water retention, and excellent antimicrobial activity.
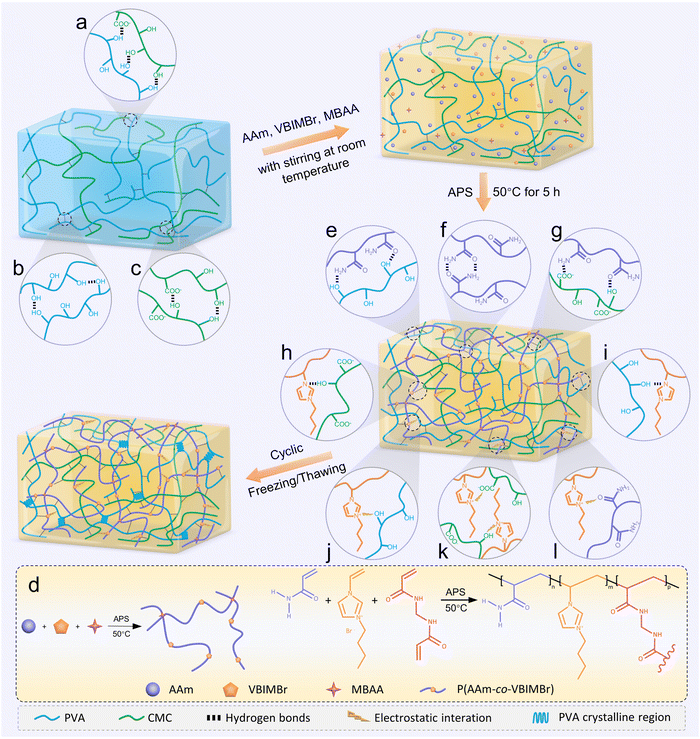
Herein, a simple two-step process of free radical polymerization and subsequent freeze–thaw cycling is proposed to prepare multifunctional ionic conductive hydrogels with high tensile strength, good conductivity, and high transparent, anti-freezing and antibacterial properties for the construction of high-performance flexible wearable sensors. PVA and CMC were used as the backbone structure of the ionic conductive hydrogela, and a copolymer network of chemically crosslinked AAm and 1-vinyl-3-butylimidazolium bromide (VBIMBr) is introduced to construct a semi-interpenetrating network structure. Finally, PVA/CMC/P(AAm-co-VBIMBr) (PCPAV) ionic conductive hydrogels were obtained after freeze–thaw cycles. The three-dimensional network structure of the PCPAV hydrogels was formed by the synergistic interaction of covalent interactions, multiple hydrogen bonding interactions and electrostatic interactions. The crystalline domains formed by the PVA chains after freeze–thaw cycles and the semi-interpenetrating network endow the hydrogels with good mechanical properties. In addition, the physical cross-linking network consisting of hydrogen bonding and electrostatic interactions also enhances the self-recovery and fatigue resistance properties of the hydrogels. The introduction of VBIMBr increased the ionic conductivity of the hydrogels up to 15.2 mS cm−1. In addition to ensuring that the hydrogel presents high ionic conductivity, it also endows the ionic conductive hydrogel containing an ionic liquid with excellent mechanical properties. At the same time, without adding any external biocides and inorganic salts, excellent resistance to low temperature, volatilization and microbial contamination of VBIMBr endow the PCPAV ionic conductive hydrogels with excellent anti-freezing, moisture retention and antibacterial properties. In addition, without the addition of any inorganic salts and bactericides, excellent resistance to low temperature, volatilization, and microbial contamination of VBIMBr endow the PCPAV hydrogels with excellent anti-freezing, moisture retention, and antimicrobial properties, which enable the hydrogels to work over a wide temperature range and enhance their long-term storage capacity. Furthermore, excellent electromechanical properties enable the PCPAV ionic conductive hydrogels to be used in flexible strain sensors that can be used to monitor a variety of large-scale human joint movements and sense small muscle movements. In summary, this work developed multifunctional PCPAV ionic conductive hydrogels that combine high electrical conductivity, tunable mechanical properties, excellent anti-freezing properties, and superior antimicrobial activity, providing a new perspective on next-generation flexible sensors and broadening their application prospects in wearable sensing devices, soft robotics, and human–computer interactions.
2. Results and discussion
2.1. Crosslinking mechanism and structure analyses of PCPAV ionic conductive hydrogels
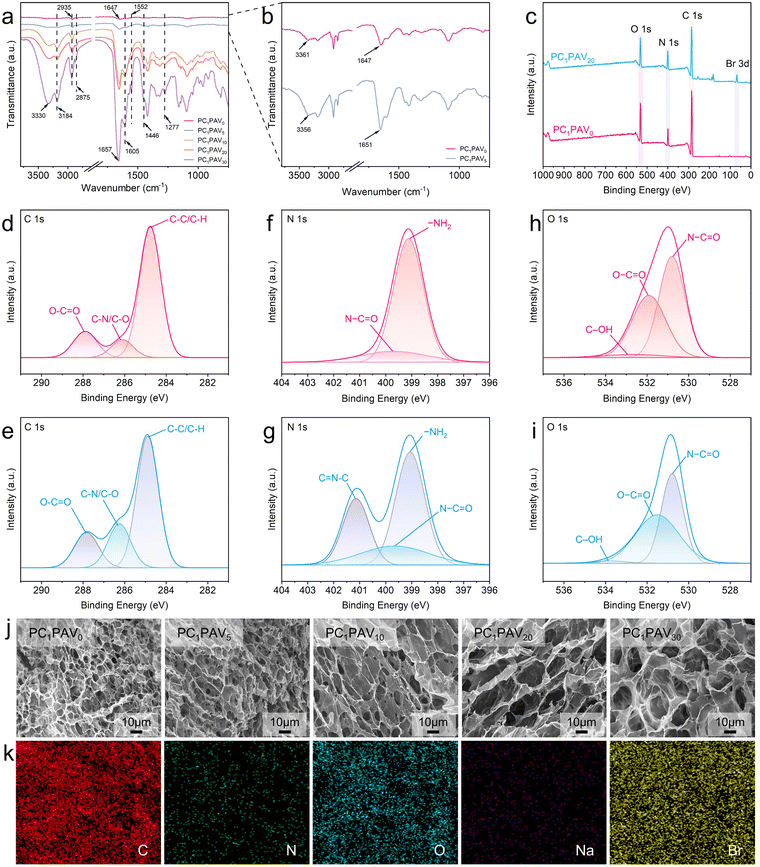
In this work, multifunctional ionic conductive hydrogels (PCPAV) were synthesised by a facile two-step process of thermally induced free radical polymerization and subsequent freeze–thaw cycling as shown in Fig. 1. Depending on the detailed compositional proportions, the obtained PCPAV is labelled PCxPAVy, where x and y represent the mass ratio of CMC and VBIMBr, respectively, to the PVA solution. For example, PC1PAV20 indicates that the mass of CMC is 1% to the PVA solution and the mass of VBIMBr is 20% to the PVA solution. PVA provides a large number of –OH in the main chain, and the CMC chain provides –OH along with a large number of –COOH. Thus, PVA chains and CMC chains are intertwined with each other via intramolecular and intermolecular hydrogen bonds (Fig. 1a–c) to form the basic skeleton of the hydrogels with excellent mechanical properties and elasticity.59 In addition, AAm and VBIMBr monomers were polymerized to form poly(AAm-co-VBIMBr) (PAV chains) by radical initiation under heating conditions by using MBAA as the chemical crosslinker, APS as the thermal initiator, and TEMED as the promoter (Fig. 1d). The covalently crosslinked PAV chains provide strong chemical bonding networks for the hydrogels. After incorporating the PAV chains into the PVA/CMC skeleton, the dynamic crosslinking between PVA/CMC and PAV is initiated, and a more complex network structure is obtained via hydrogen bonding and electrostatic interactions between the molecular chains. The hydrogen bonds not only exist between the amide groups of the PAV chains and the –OH of the PVA chains (Fig. 1e), but also present between the amide groups of the PAV chains (Fig. 1f), the PAV chains and the –COOH/–OH groups of the CMC chains (Fig. 1g), the uncharged nitrogen atoms on the imidazole ring of VBIMBr and –OH of PVA/CMC (Fig. 1h and i). Meanwhile, electrostatic interactions were formed between the positively charged nitrogen atoms on the imidazole ring in VBIMBr and the –OH of PVA/CMC (Fig. 1j), the negatively charged –COOH of CMC (Fig. 1k), as well as the amide groups in the PAV chains (Fig. 1l). After cyclic freeze–thaw treatment, crystalline domains were generated from the PVA polymer chains in the hydrogel, which enhanced the mechanical properties of the PCPAV ionic conductive hydrogel. Moreover, the multiple hydrogen bonds and electrostatic interactions endow the hydrogel with a self-healing performance. VBIMBr and CMC provide a high number of freely moving ions such as imidazole cations, Br−, and Na+, which increase the ionic conductivity of the hydrogel. And the superior resistance to low temperature and microbial contamination of VBIMBr endow the PCPAV ionic conductive hydrogels with excellent frost resistance and antibacterial properties.The structural and physical interactions of the PCPAV ionic conductive hydrogels were investigated by FTIR as shown in Fig. 2a and b. The absorption band of O–H in the spectrum of the PC1PAV0 hydrogel was at 3361 cm−1, which shifted to 3356 cm−1 in the spectrum of the PC1PAV5 hydrogel (Fig. 2b), implying that more strong hydrogen bonds formed in the polymer network of the PC1PAV5 hydrogel.60 Compared with the spectrum of PC1PAV5, the intensity of O–H at 3330 cm−1 in the spectrum of PC1PAV30 was greatly increased indicating the formation of a large number of hydrogen bonds between the –OH groups. Meanwhile the peak at 3184 cm−1 was attributed to N–H stretching vibrations. It could be seen that the intensity of the peak increased with increasing content of VBIMBr, indicating the formation of a large number of hydrogen bonds. The peaks at 2935 and 2875 cm−1 can be assigned to –CH3 and –CH2 in-plane stretching of alkyl groups,61 and the intensity of the double peaks increased with increasing VBIMBr content. The absorption peak at around 1647 cm−1 was attributed to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching vibration of the acrylamide unit, and the location of the peak gradually shifts to 1657 cm−1 in the spectrum of the PC1PAV30 hydrogel with increasing addition of VBIMBr. This result indicates that the amide groups of the PAV chains created significant electrostatic interactions with the VBIMBr of the PAV chains, and the electrostatic interactions were strengthened as the VBIMBr concentration increased. Furthermore, the amide groups of the PAV chains also formed strong hydrogen bonding interactions with –OH and –COOH of the PVA/CMC chains. Moreover, the peak at 1605 cm−1 is attributed to the asymmetric stretching vibrations of –COOH in the CMC chain, and the intensity of the peak increased with the introduction of VBIMBr, indicating that electrostatic interactions are formed between the imidazole ring cation and –COO−. The significant characteristic absorption peaks at 1552 cm−1, 1446 cm−1, and 1277 cm−1 are attributed to the C
O asymmetric stretching vibration of the acrylamide unit, and the location of the peak gradually shifts to 1657 cm−1 in the spectrum of the PC1PAV30 hydrogel with increasing addition of VBIMBr. This result indicates that the amide groups of the PAV chains created significant electrostatic interactions with the VBIMBr of the PAV chains, and the electrostatic interactions were strengthened as the VBIMBr concentration increased. Furthermore, the amide groups of the PAV chains also formed strong hydrogen bonding interactions with –OH and –COOH of the PVA/CMC chains. Moreover, the peak at 1605 cm−1 is attributed to the asymmetric stretching vibrations of –COOH in the CMC chain, and the intensity of the peak increased with the introduction of VBIMBr, indicating that electrostatic interactions are formed between the imidazole ring cation and –COO−. The significant characteristic absorption peaks at 1552 cm−1, 1446 cm−1, and 1277 cm−1 are attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–N stretching vibrations within the imidazole ring, respectively. And these peaks were all intensified with increasing VBIMBr content.
C and C–N stretching vibrations within the imidazole ring, respectively. And these peaks were all intensified with increasing VBIMBr content.
The chemical composition and elements present in the PC1PAV0 and PC1PAV20 hydrogels were explored using XPS, and the results are shown in Fig. 2c. It could be seen that the characteristic peaks of C 1s, N 1s and O 1s were recorded at the binding energies of 285, 400 and 532 eV for the two hydrogels in the broad scan XPS spectra, respectively. The Br 3d signal was detected at 68 eV for the PC1PAV20 hydrogel, indicating the integration of VBIMBr into the PCPVA hydrogel. The high-resolution XPS spectra were further analyzed. As shown in Fig. 2d, the PC1PAV0 hydrogel displayed three characteristic peaks at 284.8, 286.2 and 287.9 eV in the C 1s spectrum, corresponding to C–C/C–H, C–N/C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.62,63 The characteristic C 1s peaks of PC1PAV20 hydrogels were at 284.8, 286.3 and 287.8 eV for the C–C/C–H, C–N/C–O and O–C
O, respectively.62,63 The characteristic C 1s peaks of PC1PAV20 hydrogels were at 284.8, 286.3 and 287.8 eV for the C–C/C–H, C–N/C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively (Fig. 2e). Compared with the PC1PAV0 hydrogels, the increased C–N/C–O binding energy and the decreased O–C
O, respectively (Fig. 2e). Compared with the PC1PAV0 hydrogels, the increased C–N/C–O binding energy and the decreased O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O binding energy of the PC1PAV20 hydrogels were attributed to the hydrogen bonding between the uncharged N atom and –OH, and the electrostatic interaction between the imidazole cation in VBIMBr and –COO−.64 For the N 1s spectrum, as shown in Fig. 2f, the PC1PAV0 hydrogel had two characteristic peaks at 399.1 and 399.7 eV, corresponding to –NH2 and N–C
O binding energy of the PC1PAV20 hydrogels were attributed to the hydrogen bonding between the uncharged N atom and –OH, and the electrostatic interaction between the imidazole cation in VBIMBr and –COO−.64 For the N 1s spectrum, as shown in Fig. 2f, the PC1PAV0 hydrogel had two characteristic peaks at 399.1 and 399.7 eV, corresponding to –NH2 and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. In contrast to the PC1PAV0 hydrogel, the N 1s spectrum of the PC1PAV20 hydrogel showed a new characteristic peak at 401.1 eV (Fig. 2g), which was attributed to C
O, respectively. In contrast to the PC1PAV0 hydrogel, the N 1s spectrum of the PC1PAV20 hydrogel showed a new characteristic peak at 401.1 eV (Fig. 2g), which was attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C in the imidazole ring. In the O 1s spectrum shown in Fig. 2h, the PC1PAV0 hydrogel showed three characteristic peaks located at 530.9 eV for N–C
N–C in the imidazole ring. In the O 1s spectrum shown in Fig. 2h, the PC1PAV0 hydrogel showed three characteristic peaks located at 530.9 eV for N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 532.1 eV for O–C
O, 532.1 eV for O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and 532.6 eV for C–OH. Compared to the PC1PAV0 hydrogel, as shown in Fig. 2i, the lower binding energies of N–C
O and 532.6 eV for C–OH. Compared to the PC1PAV0 hydrogel, as shown in Fig. 2i, the lower binding energies of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (530.8 eV) and O–C
O (530.8 eV) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (531.9 eV) of the PC1PAV20 hydrogels indicated the electrostatic interactions between VBIMBr of PAV chains and amide groups of PAV chains as well as –COOH of CMC in the hydrogels.
O (531.9 eV) of the PC1PAV20 hydrogels indicated the electrostatic interactions between VBIMBr of PAV chains and amide groups of PAV chains as well as –COOH of CMC in the hydrogels.
The inside morphology of the PCPAV ionic conductive hydrogels is shown in Fig. 2j. It can be seen that the PCPAV hydrogels exhibit a three-dimensional porous structure, and the pore size of the PCPAV hydrogels gradually increased from 5.52 ± 1.87 μm of the PC1PAV0 hydrogel to 17.06 ± 6.37 μm of the PC1PAV30 hydrogel with increasing VBIMBr content (Fig. S1, ESI†). It can be mainly attributed to the decreased crystallinity of PVA in PCPAV hydrogels caused by the introduction of the VBIMBr component. Furthermore, EDS studies of the PC1PAV30 ionic conducting hydrogels showed that the C, N, O, Na and Br elements were uniformly distributed in the three-dimensional network of the hydrogels (Fig. 2k).
2.2. Mechanical properties, light transmittance and conductivity of PCPAV ionic conductive hydrogels
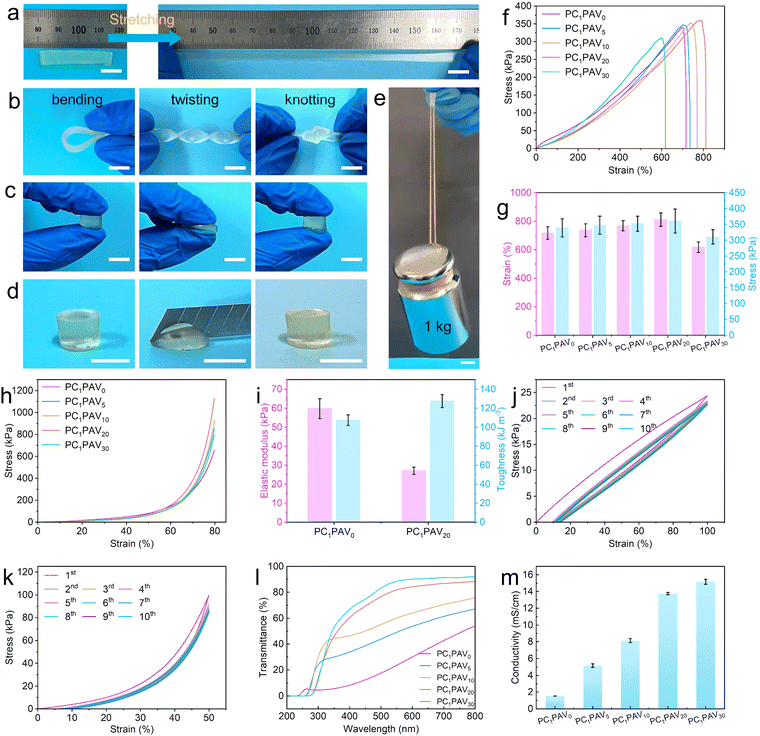
It is of interest that the obtained PC1PAV20 hydrogels maintain their original shape integrity without fracture after being subjected to stretching, bending, twisting, knotting, compression and cutting, as shown in Fig. 3a–d. It was also worth mentioning that the hydrogel strip (30 mm × 8 mm × 2 mm, about 0.25 g) could lift a weight of 1 kg, which was about 4000 times of its own weight without breaking (Fig. 3e and Video S1, ESI†). As is known to all, the cross-linking degree or the CMC content plays a key role in the conductivity, mechanical properties and transmittance of the hydrogels. Accordingly, a comprehensive analysis and discussion were performed, the results of which could be found in Fig. S2 and S3 in the ESI.† Ultimately, the crosslinking degree of PCPAV hydrogels was set to be 0.1% and the CMC addition was set to be 1%.Besides above, VBIMBr is also involved in the formation of covalent crosslinks, multiple hydrogen bonds and electrostatic interactions. Therefore, the tensile stress–strain curves of the PCPAV hydrogels with various VBIMBr content are shown in Fig. 3f. It can be found that the tensile stress of the hydrogel increased from 339.1 kPa to 360.6 kPa and the elongation at break increased from 716.8% to 810.6% with increasing VBIMBr content (Fig. 3g). That is because the electrostatic interactions were formed between the positively charged imidazole cations in VBIMBr, the –OH in PVA/CMC and the negatively charged –COOH in CMC.65 These dynamic physical interactions were gradually disrupted when the PCPAV hydrogel was stretched. However, during the slow stretching of the hydrogels, electrostatic interactions will again form between the imidazole cationic groups located in the other PAV chains and the –OH and –COOH. These new interactions could continue to maintain the integrity of the PCPAV hydrogels during the stretching process, thus endowing the hydrogels with good tensile properties.65 This situation was more pronounced in PCPAV hydrogels added with higher amounts of VBIMBr. However, excessive VBIMBr addition would inversely reduce the tensile properties (Fig. 3g) because the structural homogeneity of the PCPAV hydrogels will be disrupted by increasing covalent crosslinking of VBIMBr and AAm with excessive VBIMBr.44 More importantly, the PCPAV hydrogels exhibit excellent compressive capabilities as well. As shown in Fig. 3h, the PC1PAV20 hydrogel exhibited the highest compressive strength of 1131.8 kPa.
Given the excellent tensile and compressive abilities of the PC1PAV20 hydrogel, we further compared the elastic modulus and toughness of PC1PAV0 and PC1PAV20 hydrogels as shown in Fig. 3i. Compared with the PC1PAV0 hydrogel, the PC1PAV20 hydrogel shows a lower elastic modulus of 27 kPa and a higher toughness of 127.3 kJ m−3. The lower elastic modulus of the PC1PAV20 hydrogel means improved flexibility of the hydrogel due to the decreased crystallinity of PVA chains by addition of VBIMBr.66 Meanwhile, VBIMBr provided rich electrostatic interactions for the network structure of the PC1PAV20 hydrogel, and acted as an energy dissipator to take up part of the stress during the tensile process, leading to a better toughness of the PC1PAV20 hydrogel than that of the PC1PAV0 hydrogel.
For hyEdrogel strain sensors, the self-recovery and anti-fatigue properties of the hydrogels are essential to provide a reliable and stable output signal. The physical cross-linking network in the PC1PAV20 hydrogel consisting of hydrogen bonding and electrostatic interactions is expected to improve the self-recovery and anti-fatigue properties of the hydrogel. Therefore, the self-recovery and anti-fatigue capabilities of the PC1PAV20 hydrogel were evaluated by a 10-cycle loading–unloading test with 100% elongation. As shown in Fig. 3j, the first loading–unloading cycle formed a large closed curve with an obvious hysteresis loop, indicating that the breakage and remodeling of a large number of hydrogen bonds and electrostatic interactions effectively dissipated the energy. As shown in Fig. S4a (ESI†), the dissipation energy of the first cycle of stretching was as high as 3.15 kJ m−3, which was due to the continuous stretching that prevented the hydrogen bonding and electrostatic interactions from reconfiguring rapidly. Interestingly, the next 9 cycles of hysteresis loops were relatively smaller (Fig. 3j), and the dissipation energy was about 1.30 kJ m−3 and kept almost unchanged at 1.20 kJ m−3 (Fig. S4a, ESI†). Moreover, the compression curve of the 10-times cycled PC1PAV20 hydrogel is shown in Fig. 3k. Similar to the loading–unloading tensile curve, a significant hysteresis loop was observed at the first test cycle with a compression strength of 22.57 kPa and a dissipated energy of 22.16 kJ m−3 at 50% compression (Fig. S4b, ESI†). For the other 9 cycles, the hydrogel showed small hysteresis loops (Fig. 3k) and the dissipation energy almost maintained at 5.7 kJ m−3 (Fig. S4b, ESI†). These results demonstrated that the PC1PAV20 hydrogel presents good self-recovery and fatigue resistance, showing potential for flexible strain sensor applications.
Optical transparency is another key parameter of a hydrogel to be used in wearable electronic devices for detecting the state of human skin in real time. As shown in Fig. 3l and Fig. S5a and b (ESI†), the light transmission of PCPAV hydrogels gradually increased with increasing VBIMBr content. The PC1PAV0 hydrogel only presents about 54% light transmission at 800 nm, while the PC1PAV30 hydrogel exhibits a high light transmission of about 92%. That is because the introduction of VBIMBr could make the PVA crystals inside the hydrogels slightly dissociated, and the high steric effect endowed the hydrogel with high light transmittance.66 As shown in Fig. S6 (ESI†), the XRD patterns of different PCPAV hydrogels all exhibited two blunt peaks near 2θ = 27° and 2θ = 40°, indicating that the polymer chains in the hydrogel matrix were in the semi-crystalline state. As the VBIMBr content gradually increased, the height of the diffraction peak near 2θ = 27° gradually decreased. According to the calculated results, the crystallinity of the PC1PAV30 hydrogel has decreased to 3.62% from 14.34% of the PC1PAV0 hydrogel (Table S1, ESI†). The PCPAV hydrogel with high light transmittance has great potential for application in the field of human–machine interfaces.
Electrical conductivity is the core element of a hydrogel for wearable sensor applications. The conductivity of the PCPAV hydrogels is shown in Fig. 3m. It can be seen that the ionic conductivity of the hydrogels increased with increasing VBIMBr content. The conductivity of the PC1PAV30 hydrogel increased to 15.2 mS cm−1 from 1.5 mS cm−1 of the PC1PAV0 hydrogel, which is much higher than those of many previously reported ionic conductive hydrogels (Table S2, ESI†). It was mainly attributed to the strong electrostatic interactions between the positively charged imidazole cations of VBIMBr and the negatively charged –COOH of CMC, which constructed a denser conductive network with strong ion transport capacity in the hydrogel.65 Furthermore, VBIMBr also serves as an ionic source for the conductive hydrogel to promote the carrier concentration in the electrolyte hydrogel. Therefore, considering the balanced mechanical properties, light transmission and ionic conductivity, PC1PAV20 hydrogels were selected for further evaluation unless otherwise stated.
2.3. Anti-freezing and water retention of PCPAV ionic conductive hydrogels
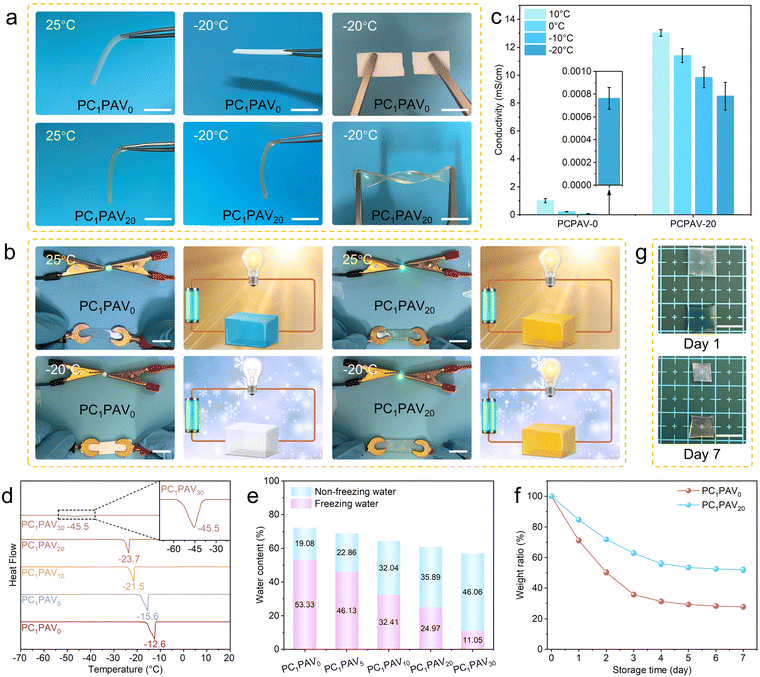
Traditional hydrogels are usually frozen in cold environments (below 0 °C) and their original functions, such as flexibility, transparency, electrical conductivity, are lost correspondingly. Therefore, it is necessary to develop anti-freeze hydrogels to facilitate low temperature applications.Fig. 4a demonstrates the mechanical properties of PC1PAV0 and PC1PAV20 hydrogels in different temperature environments. It can be seen that both hydrogels are flexible at 25 °C, while the PC1PAV20 hydrogel shows superior flexibility than the PC1PAV0 hydrogel. However, the mechanical properties of the two hydrogels were significantly different at −20 °C. The PC1PAV0 hydrogel turned to be white and easy to be snapped, while the PC1PAV20 hydrogel still maintained good transparency and flexibility, and could still withstand twisting at such a low temperature. The conductivity of the hydrogel at different temperatures was also evaluated. As is shown in Fig. 4b, both hydrogels could light a bulb at 25 °C. However, unlike the PC1PAV0 hydrogel, the PC1PAV20 hydrogel could light up the bulb normally in a cold environment. The conductivity of the two hydrogels at different temperatures (10 °C, 0 °C, −10 °C and −20 °C) was subsequently tested, and the results are shown in Fig. 4c. The conductivity of the PC1PAV0 hydrogel was 1.01, 0.21, 0.06, and 7.63 × 10−4 mS cm−1, respectively, at 10 °C, 0 °C, −10 °C and −20 °C. While the PC1PAV20 hydrogel exhibited a higher conductivity of 13.03, 11.39, 9.84, and 8.49 mS cm−1, respectively, at 10 °C, 0 °C, −10 °C and −20 °C. It indicates that the PC1PAV0 hydrogel can be considered as an insulator at low temperature, while the PC1PAV20 hydrogel still had an acceptable conductivity to serve as an effective conductor in such an environment.
Given the excellent anti-freezing performance of the PC1PAV20 hydrogel, the quantitative relationship between the VBIMBr content and anti-freezing capacity was further investigated by DSC. DSC curves of the PCPAV hydrogels (Fig. 4d) suggested that the freezing point of the PCPAV hydrogels gradually decreased with increasing VBIMBr content. The freezing points of the PCPAV hydrogels gradually decreased from −12.6 °C for the PC1PAV0 hydrogel to −45.5 °C for the PC1PAV30 hydrogel, which is lower than those of many existing anti-freezing conductive hydrogels (Table S3, ESI†). The improved anti-freezing properties of the PCPAV hydrogel were due to the strong interaction between VBIMBr and H2O molecules that inhibits the formation of ice crystals at low temperature.67
As we know that the water in the hydrogel can be divided into three groups: free water, freezable bound water and non-freezable bound water, among which the free water and freezable bound water can be frozen at low temperature.68 The heat absorption peak in Fig. 4d is related to the melting of frozen water (free water and freezable bound water) in the hydrogel. As shown in Fig. 4e, the content of non-freezable bound water in the hydrogel gradually increased with increasing VBIMBr content due to the strong interaction between VBIMBr and H2O. As a result, the freezing point of the PC1PAV30 hydrogel has lowered to −45.5 °C. This result indicates that the anti-freezing properties of the PCPAV hydrogels could be adjusted by changing the state of water caused by the addition of VBIMBr.
Long-term water retention is another key property of a hydrogel for its practical application in a stable sensor. As seen in Fig. 4f, the PC1PAV20 hydrogel retained more than 50% of its original weight after one week. In addition, it remained moist and retained its original shape with only a slight decrease in volume (Fig. 4g). In contrast, the PC1PAV0 hydrogel lost about 50% of its original weight after the first two days and weighed only 28% of its original weight after 7 days with severe deformation. This result indicates that the PC1PAV20 hydrogel exhibited better water retention properties than the PC1PAV0 hydrogel due to the inhibition of water evaporation by the large number of hydrogen bonds formed between VBIMBr and water molecules.69 It can be seen that after one week storage, the tensile strength of the PC1PAV20 hydrogel improved, while the elongation at break decreased (Fig. S7a, ESI†), which can be attributed to the evaporation of water inside the hydrogel. In addition, the conductivity of the hydrogel decreased to 8.313 mS cm−1 (Fig. S7b, ESI†).
The above results indicate that the PC1PAV20 hydrogel showed good anti-freezing and water retention capabilities, and could be a potential candidate for flexible sensor applications under extreme conditions.
2.4. Self-healing properties of PCPAV ionic conductive hydrogels
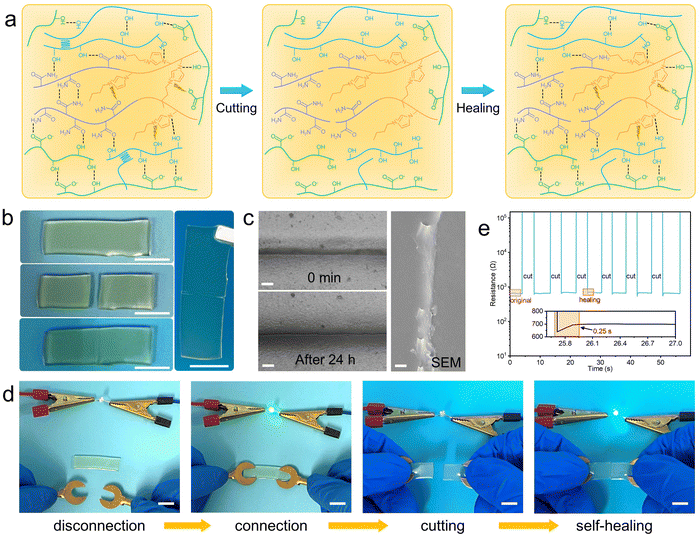
In practical applications, damage caused by external forces may attenuate the mechanical and electrical properties of hydrogels. Therefore, the self-healing properties are necessary for hydrogels. The self-healing mechanism of the PC1PAV20 hydrogel is shown in Fig. 5a. When the PC1PAV20 hydrogel was broken, the hydrogen bonds and electrostatic interactions of the hydrogel were disrupted. Meanwhile, a large number of donors and acceptors on the new surfaces are capable of forming reversible non-covalent bonds facilitating the self-healing properties of the hydrogel. When the fractured surfaces were in contact, they could quickly become one by the reconstructed hydrogen bonds, the electrostatic interactions between VBIMBr and the –COOH of CMC, the –OH of PVA/CMC, and the amide groups in the PAV chains between the two surfaces. As is shown in Fig. 5b, the two pieces of the PC1PAV20 hydrogel weld with each other at 25 °C, and the incision gradually disappeared and eventually a new cross-linked network was formed (Fig. 5c). As shown in Fig. S8 (ESI†), the tensile strength of the PC1PAV20 hydrogel after heating at 25 °C for 24 h was only 48.55% of the original hydrogel. This is due to the low recovery efficiency of the covalent crosslinks in the hydrogel after disruption. In addition, the presence of PVA crystalline domains also reduces the self-healing ability of hydrogels.70 Interestingly, the self-healing efficiency of the hydrogel at −20 °C for 24 h can reach 43.83%, which is very close to the self-healing efficiency of the hydrogel at 25 °C (48.55%). As shown in Fig. 5d, when the hydrogel in the circuit was cut into half, the LED lamp extinguished immediately. Then, after the two pieces of PC1PAV20 hydrogels were brought into contact for a certain time, the LED lamp was lit again, indicating that the repaired hydrogel still maintained good electrical conductivity as the original hydrogel. In addition, the real-time resistance changes of the hydrogels during the cutting-recovery process at 25 °C were recorded (Fig. 5e) with a digital source meter. It can be seen that the hydrogel showed an instantaneous great increase in the resistance after the cut. And the resistance value of the PC1PAV20 hydrogel immediately decreased to the original value within 0.25 seconds when the cut was re-contacted and maintained stable in the repeated cutting-recovery process due to the rapid self-healing properties of the hydrogel. These results indicated that the PC1PAV20 hydrogel still shows a stable electrical conductivity after self-healing.2.5. Antibacterial properties of PCPAV hydrogels
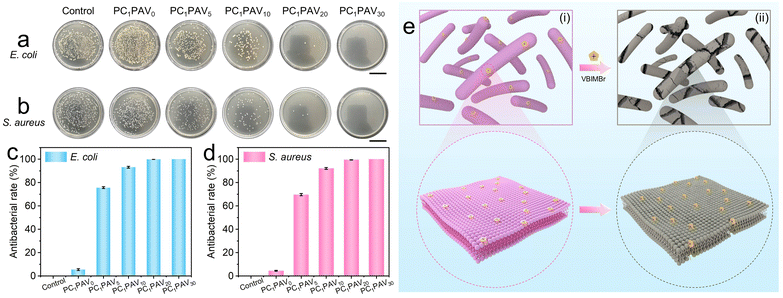
In practical applications, the moist surface of a hydrogel provides a suitable environment for bacterial growth, which might cause allergies during wear. To avoid this problem, the hydrogel should be replaced frequently. Therefore, the antibacterial capability of wearable hydrogel flexible sensors is a necessary property to be considered. In this work, the antibacterial activity of PCPAV hydrogels against Gram-negative E. coli and Gram-positive S. aureus bacteria was evaluated using the colony formation counting assay.As shown in Fig. 6a and b, the antibacterial performance of the PCPAV hydrogels against both E. coli and S. aureus was significantly enhanced with increasing VBIMBr content. Compared to the blank control group and the hydrogel with a lower VBIMBr content, there was no colony on the PC1PAV30 hydrogel after 24 hour culture. In addition, the antibacterial rate of PCPAV hydrogels was calculated by counting the number of colonies on the plates (Fig. 6c and d). It could be clearly seen that the antibacterial rates of the PC1PAV0 hydrogel against E. coli and S. aureus were only 5.43% and 4.48%, respectively, while that of the PC1PAV30 hydrogel was as high as 100% for both bacteria. This excellent antibacterial effect of the PC1PAV30 hydrogel can be attributed to two aspects. On the one hand, the electrostatic interaction between the positive charge on the imidazole ring of VBIMBr and the negative phosphate group of the bacterial cell membrane makes VBIMBr preferentially adsorbed on the cell membrane to disrupt the cytoplasmic membrane of bacteria. And cytoplasmic contents (such as potassium ions, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA)) were then released (Fig. 6e(i)).71 On the other hand, the terminal alkyl chains of VBIMBr could insert into the lipophilic region of the cell membrane, causing perforation of the cell membrane and irreversible physical damage to cell, which eventually results in cell death (Fig. 6e(ii)).58,67,72
2.6. Sensitivity of PC1PAV20 ionic conductive hydrogels
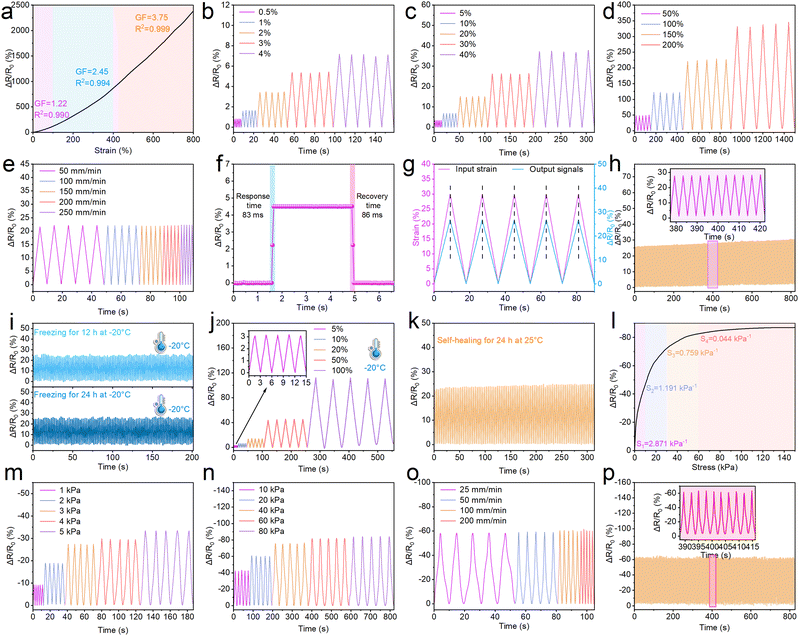
Sensitivity is an indispensable property of the PC1PAV20 hydrogel for its potential application in piezoresistive hydrogel strain sensors for flexible wearable electronics. As is shown in Fig. S9a (ESI†), elongation on a hydrogel strip (20 mm × 5 mm) or pressure on a hydrogel cylinder (8 mm high, 10 mm diameter) that is connected to the circuit will change the brightness of the LED light. The LED light turned to be a little bit dimmer when the hydrogel strip was stretched because the conductive pathway in the hydrogel became narrower and longer. In contrast, it can be seen in Fig. S9b (ESI†) that the LED light became brighter when the hydrogel cylinder was compressed due to the reduction of the conductive pathway.The strain sensitivity of hydrogel sensors is usually characterized by the gauge factor (GF), which is defined as the change in relative resistance to the applied strain.73Fig. 7a shows the relative resistance change of the PC1PAV20 hydrogel sensor at different tensile strain loads. It can be seen that the hydrogel sensor exhibits a strain-dependent sensitivity in the elongation range of 0–800%. With increasing strain, the GF of the PC1PAV20 hydrogel could be roughly further divided into three linear regions with different slopes. In the low strain regime (0–100%), the GF increases from 0 to 1.22 and then to 2.45 as the strain increases to 400%. Finally, the GF reaches 3.75 in the high strain regime (400–800%). These results demonstrate the excellent sensitivity of the PC1PAV20 hydrogel strain sensors in a broad sensing range. Notably, the GF was as high as 3.75 when the strain reached 800%, and the value was higher than the corresponding GF of many previously reported conductive hydrogel-based sensors (Fig. S10, ESI†). This means that PC1PAV20 hydrogels as flexible wearable sensors could greatly improve the accuracy and reliability of the output signal in practical applications. Then, the dynamic electromechanical properties of the hydrogels were investigated through real-time relative resistance change tests. As shown in Fig. S7b–d (ESI†), the hydrogel sensors exhibited stable relative resistance changes during repeated stretching at small (0.5–4%), medium (5–40%) and large (50–200%) elongation, which indicated that they have good sensing reliability. Notably, the hydrogel sensor exhibited an ultra-low detection limit of 0.5% elongation (Fig. 7b), implying that the PC1PAV20 hydrogel can be used to detect subtle human body movements. In addition, as shown in Fig. 7e, the stable signal tested by the hydrogen will not be affected by the stretching rate variation (50 to 250 mm min−1) for a certain elongation. And the significant density at different stretching rates can be detected implying that the hydrogel has an excellent frequency sensitivity, which was important for accurate monitoring.
The response time of hydrogel sensors is another key performance indicator, as a fast response ensures a real-time response. As shown in Fig. 7f, the hydrogel sensor exhibited negligible hysteresis when elongation is 5% on the hydrogel and maintained for 3 seconds. The response time and recovery time were 83 ms and 86 ms, which were faster than that of the human skin (100 ms).74 They are also faster than those of the reported CPDA-P (400 ms response time and 600 ms recovery time),75 PACF (498 ms response time and 501 ms recovery time),76 and PVA-NaCl-GL-AMY (97 ms response time and 100 ms recovery time)77 hydrogels. As a result, the hydrogel exhibits excellent signal synchronization transmission. As shown in Fig. 7g, the output relative resistance change of the hydrogel sensor is fully synchronized with the input strain during the deformation cycles (with 30% elongation). The hydrogel sensor also showed excellent sensing stability and reversibility during the 200-times load–unload cycles with 30% constant strain (Fig. 7h), indicating that the PC1PAV20 hydrogel sensor presented a fantastic durability. The slight upward drift of the ΔR/R0 baseline was mainly due to the permanent fatigue hysteresis of the hydrogel during repeated stretch-unload cycles, which is a common phenomenon for the other hydrogel strain sensors.78–80 It is worth mentioning that since the PC1PAV20 hydrogel possesses excellent anti-freezing properties, the strain sensor assembled from it can still output stable electrical signals at −20 °C after freezing for 12 and 24 h (Fig. 7i). In addition, the strain sensor can accurately and reliably output electrical signals at different tensile strains in a −20 °C environment as it works at 25 °C (Fig. 7j). Moreover, the healing hydrogel after self-healing also exhibited stable and reliable sensing properties (Fig. 7k). These results clearly demonstrated that the PC1PAV20 hydrogel has great potential for application in flexible wearable strain sensors with good performance even at low temperature for human motion detection and soft robotics.
Pressure sensing performance of the PC1PAV20 hydrogel was also evaluated. As shown in Fig. 7l, the pressure sensitivity of the PC1PAV20 hydrogel gradually decreased with increasing pressure, and the pressure sensitivity curve can be divided into 4 regions over the stress range from 0 to 150 kPa. Specifically, the pressure sensitivity value was 2.871, 1.191, 0.759 and 0.044 kPa−1 in the pressure range of 0–10, 10–30, 30–60 and 60–150 kPa, respectively. In addition, the hydrogel exhibited a stable and repeatable pressure signal over a large pressure range (1–80 kPa, Fig. 7m and n) and a large deformation speed range (25–200 mm min−1, Fig. 7o). In addition, the hydrogel sensor could output a stable electrical signal during the 300 compression-recovery cycle (Fig. 7p). The above results suggested that the PC1PAV20 hydrogel exhibited good stability, repeatability and durability for application in the field of flexible pressure sensors.
2.7. PC1PAV20 ionic conductive hydrogel for wearable sensing devices
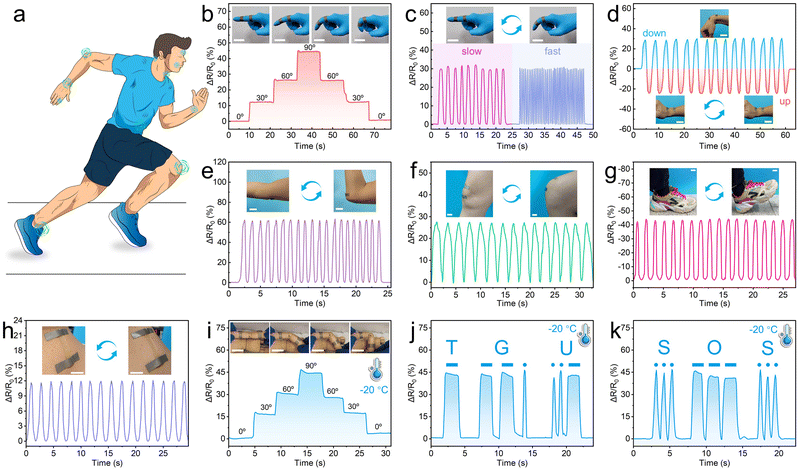
The PC1PAV20 hydrogel sensor is a promising strain and pressure sensor for application as a wearable sensing device or soft electronics for the detection of human motion and other biosignals due to its skin-like malleability and elasticity, excellent strain sensitivity, superior fatigue resistance, remarkable electrical properties, and excellent antibacterial activity. It can be used by simply attaching to different parts of the body, including fingers, wrists, elbows, knees, and ankles (Fig. 8a). Fig. 8b shows the relative resistance of the hydrogel sensor stacked on a finger when the finger was at different bending angles. Elongation of the hydrogel caused by the bending movement from 0°, 30°, 60° to 90° synchronously led to the variation of ΔR/R0 from 0% to 45%, and the increased relative resistance was then decreased to 0 accompanied by the bending recovery of the finger. Meanwhile, the ΔR/R0 value corresponding to each bending angle of the finger was stable, indicating that the wearable hydrogel sensor had a high electrical signal stability.When the finger bending movement repeated, a stable and repeatable resistance pattern could be accurately recorded (Fig. 8c). Meanwhile, the bending frequency could be accurately identified according to the number of response peaks within a certain time. Besides, the hydrogel sensor also exhibits satisfactory performance in detecting the wrist bending, elbow flexion, and knee, ankle and neck movement of a volunteer (Fig. 8d–h). It is worth mentioning that the PC1PAV20 hydrogel strain sensor exhibited good signal transmission performance even at −20 °C due to its good anti-freezing properties. For example, different bending angles could be accurately recognized (Fig. 8i). Moreover, by combining with Morse code, it could output the letter combinations of “TGU” (Fig. 8j) and “SOS” (Fig. 8k) for signal transmission and distress in cold environments. In addition, excellent water retention gives the hydrogel good signal transmission after one week storage at 25 °C. As shown in Fig. S11 (ESI†), the PC1PAV20 hydrogel as a strain sensor was still able to detect the bending of the finger and output a stable and reliable electrical signal (Fig. S11a, ESI†). Furthermore, it could also output “HELP”, through the bending of the finger (Fig. S11b, ESI†), further verifying the reliability and stability of its sensing performance.
The above results well demonstrate that the hydrogel sensor based on PC1PAV20 possessed good repeatability, stability and responsiveness to detect large scale movements of various joints, as well as exhibited good signal output even in low-temperature environments.
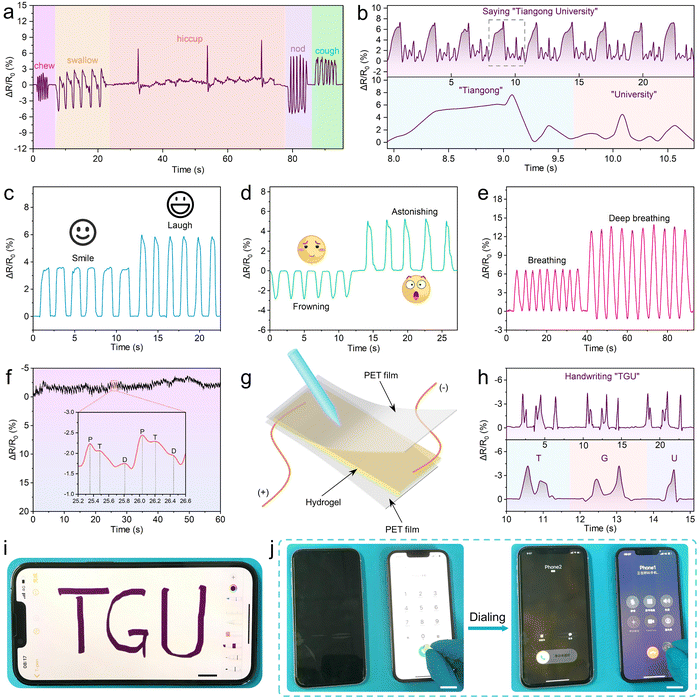
As shown in Fig. 9a, different movements of the throat (such as chewing, swallowing, hiccupping, nodding and coughing) resulted in different deformations of the hydrogel, which generated different electrical signals. It was noticeable that the relative resistance and its variation caused by each kind of weak motion on the throat are stable and repeatable, indicating that the hydrogel sensor has a potential application in the intensive care of the critically ill patients. In addition, the hydrogel attached to the throat can also sense the signals generated by the weak vibrations of human vocal cords. As shown in Fig. 9b and Fig. S12 (ESI†), the pronunciation of different words such as “Tiangong University”, “Great”, “Hydrogel” and “Sensor” could be accurately distinguished by recognition of the special signals corresponding to the vocal fold vibration of different words. As a result, the PCPAV hydrogels could be used to design speech recognition devices. Besides, the hydrogel sensors can be used to differentiate facial expressions, such as smile and laugh (Fig. 9c), frown or surprised (Fig. 9d) and the breathing state (Fig. 9e). The hydrogel sensor fixed at the radial artery on the wrist can also serve as a pulse monitor by recording the frequency of the resistance signal. Moreover, the shape of the signal curve can reflect the information on the complex blood flow and heartbeat (Fig. 9f). When the rectangular region pattern was fitted by a low-pass filter, two distinctive diacritical peaks and a late systolic augmentation shoulder caused by the blood pressure from left ventricle shrinkage and the repercussive waveform can be observed for healthy individuals.81 These results proved that the PCPAV hydrogel sensors are promising as wearable devices for real-time monitoring the human health and body movement.
The PCPAV hydrogel sensors are expected to be suitable for advanced applications such as a flexible writing pad made by the hydrogel between two PET films (Fig. 9g). As shown in Fig. 9h, a unique signal for a specific letter written could be detected, and the letter can be accurately recognized. In addition, because of the handwriting of each individual is different in strength, movement speed and writing sequence, the obtained relative resistance variation curves of the same word written by different people are unique, as well (Fig. S13, ESI†). As a result, the hydrogel sensor could be used for anti-counterfeiting applications.
Moreover, based on their excellent flexibility and electrical conductivity, the PCPAV hydrogels can be used as touch screen pens (T-pen). As shown in Fig. 9i and Fig. S14a (ESI†), a T-pen could write the letters “TGU” and “Sensor” clearly and coherently on a smartphone screen, as well as draw various graphics such as pentagrams and the sun (Fig. S14b, ESI†). Similarly, the T-pen could press the screen of a smartphone to make a phone call (Fig. 9j) as well as tap a calculator (Fig. S14c, ESI†). These above applications demonstrate the great potential of the PCPAV hydrogels as electro-sensing hydrogels for application in electronic skins, implantable electronics, artificial intelligence devices, wearable electronics and other fields.
3. Conclusions
In this work, multifunctional PCPAV ionic conductive hydrogels were successfully prepared by a simple two-step process of free radical polymerization and subsequent freeze–thaw cycling. The hydrogels possessed improved mechanical properties, which were achieved through well-designed network structures and interactions. The semi-interpenetrating network structure formed by PVA chains, CMC chains and PAV chains was the basis for the improved mechanical properties of the hydrogels. In addition, the multiple crosslinked network structures, including covalent crosslinking, multiple hydrogen bonding interactions, and electrostatic interactions also contribute to the mechanical properties of the hydrogels. Furthermore, the physical cross-linking network consisting of hydrogen bonds and electrostatic interactions in the hydrogels imparts good self-recovery and anti-fatigue properties to the hydrogels. The introduction of VBIMBr is the key for the PCPAV hydrogels exhibiting multifunctionality, including excellent transparency (∼92%), a high ionic conductivity (up to 15.2 mS cm−1), good antimicrobial activity, and good flexibility and conductivity at temperatures as low as −20 °C. Moreover, the PCPAV hydrogels present a wide strain range (0–800%), high strain sensitivity (GF = 3.75), fast response, long-term stability, and fantastic durability. The above excellent properties of the PCPAV hydrogels make them promising for application in flexible hydrogel sensors to be used in health monitoring, human motion detection, soft robotics, ionic skins, human–machine interfaces, and other wearable electronic devices.4. Experimental
All the information about the materials and methods is provided in the ESI.†Author contributions
Tongda Lei: methodology, investigation, formal analysis, visualization, investigation, and writing – original draft. Jiajun Pan: visualization and investigation. Ning Wang: data curation and formal analysis. Zhaopeng Xia: supervision. Qingsong Zhang: methodology, supervision, data curation, writing – review and editing, and funding acquisition. Jie Fan: conceptualization, investigation, supervision, methodology, and writing – review and editing. Lei Tao: conceptualization, investigation, and supervision. Wan Shou: conceptualization, investigation, supervision, and writing – review and editing. Yu Gao: methodology and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (52173060), the Natural Science Foundation of Shandong Province of China (ZR2022ME095), the 2023 Nanshan Holdings Research Project (Project No. 2023-5-3), and Tianjin Research Innovation Project for Postgraduate Students (2022SKY133). We would also like to thank the Shiyanjia Lab (www.shiyanjia.com) for the XRD, FTIR, XPS, SEM and DSC work.References
- S. Mirjalali, S. Peng, Z. Fang, C.-H. Wang and S. Wu, Adv. Mater. Technol., 2022, 7, 2100545 CrossRef CAS PubMed.
- H. Zhang, D. Zhang, J. Guan, D. Wang, M. Tang, Y. Ma and H. Xia, J. Mater. Chem. C, 2022, 10, 15554–15564 RSC.
- M. Wang, H. Zhou, X. Jin, H. Liu, A. Ma, H. Yan, L. Chen and W. Chen, J. Mater. Chem. C, 2021, 9, 1822–1828 RSC.
- R. Yin, D. Wang, S. Zhao, Z. Lou and G. Shen, Adv. Funct. Mater., 2021, 31, 2008936 CrossRef CAS.
- D. Jiao, Q. L. Zhu, C. Y. Li, Q. Zheng and Z. L. Wu, Acc. Chem. Res., 2022, 55, 1533–1545 CrossRef CAS PubMed.
- G. Liu, Z. Lv, S. Batool, M.-Z. Li, P. Zhao, L. Guo, Y. Wang, Y. Zhou and S.-T. Han, Small, 2023, 19, 2207879 CrossRef CAS PubMed.
- A. Qureshi and J. H. Niazi, Mater. Horiz., 2023, 10, 1580–1607 RSC.
- L. Fan, J. Xie, Y. Zheng, D. Wei, D. Yao, J. Zhang and T. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 22225–22236 CrossRef CAS PubMed.
- L. Tang, S. Wu, J. Qu, L. Gong and J. Tang, Materials, 2020, 13, 3947–3963 CrossRef CAS PubMed.
- G. Ge, Y. Lu, X. Qu, W. Zhao, Y. Ren, W. Wang, Q. Wang, W. Huang and X. Dong, ACS Nano, 2020, 14, 218–228 CrossRef CAS PubMed.
- L. Lao, H. Bai and J. Fan, Adv. Fiber Mater., 2023, 5, 1076–1087 CrossRef CAS.
- X. Li, M. Jiang, Y. Du, X. Ding, C. Xiao, Y. Wang, Y. Yang, Y. Zhuo, K. Zheng, X. Liu, L. Chen, Y. Gong, X. Tian and X. Zhang, Mater. Horiz., 2023, 10, 2945–2957 RSC.
- L. Wang, T. Xu and X. Zhang, TrAC-Trend Anal. Chem., 2021, 134, 116130 CrossRef CAS.
- R. Liu, L. Cui, H. Wang, Q. Chen, Y. Guan and Y. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 42052–42062 CrossRef CAS PubMed.
- G. Li, C. Li, G. Li, D. Yu, Z. Song, H. Wang, X. Liu, H. Liu and W. Liu, Small, 2022, 18, 2101518 CrossRef CAS PubMed.
- L.-Y. Hsiao, L. Jing, K. Li, H. Yang, Y. Li and P.-Y. Chen, Carbon, 2020, 161, 784–793 CrossRef CAS.
- X. Sun, Z. Qin, L. Ye, H. Zhang, Q. Yu, X. Wu, J. Li and F. Yao, Chem. Eng. J., 2020, 382, 122832 CrossRef CAS.
- L. Wu, M. Fan, M. Qu, S. Yang, J. Nie, P. Tang, L. Pan, H. Wang and Y. Bin, J. Mater. Chem. B, 2021, 9, 3088–3096 RSC.
- X. Wang, Z. Wang, X. Wang, L. Shi and R. Ran, Carbohydr. Polym., 2021, 257, 117665 CrossRef CAS PubMed.
- K. S. Kumar, L. Zhang, M. S. Kalairaj, H. Banerjee, X. Xiao, C. C. Jiayi, H. Huang, C. M. Lim, J. Ouyang and H. Ren, ACS Appl. Mater. Interfaces, 2021, 13, 37816–37829 CrossRef CAS PubMed.
- T. Yang, C. Xu, C. Liu, Y. Ye, Z. Sun, B. Wang and Z. Luo, Chem. Eng. J., 2022, 429, 132430 CrossRef CAS.
- Y. Li, X. Zhou, B. Sarkar, N. Gagnon-Lafrenais and F. Cicoira, Adv. Mater., 2022, 34, 2108932 CrossRef CAS PubMed.
- C. Chen, Y. Wang, T. Meng, Q. Wu, L. Fang, D. Zhao, Y. Zhang and D. Li, Cellulose, 2019, 26, 8843–8851 CrossRef CAS.
- Z. Qin, S. Liu, J. Bai, J. Yin, N. Li and T. Jiao, Int. J. Biol. Macromol., 2022, 220, 90–96 CrossRef CAS PubMed.
- Z. Chen, Y. Chen, M. S. Hedenqvist, C. Chen, C. Cai, H. Li, H. Liu and J. Fu, J. Mater. Chem. B, 2021, 9, 2561–2583 RSC.
- Z. Wang, Y. Cong and J. Fu, J. Mater. Chem. B, 2020, 8, 3437–3459 RSC.
- J. Xu, Z. Guo, Y. Chen, Y. Luo, S. Xie, Y. Zhang, H. Tan, L. Xu and J. Zheng, Polymer, 2021, 236, 124321 CrossRef CAS.
- H. Wang, Z. Li, M. Zuo, X. Zeng, X. Tang, Y. Sun and L. Lin, Carbohydr. Polym., 2022, 280, 119018 CrossRef CAS PubMed.
- Y. Ye, Y. Zhang, Y. Chen, X. Han and F. Jiang, Adv. Funct. Mater., 2020, 30, 2003430 CrossRef CAS.
- Z. Wu, X. Yang and J. Wu, ACS Appl. Mater. Interfaces, 2021, 13, 2128–2144 CrossRef CAS PubMed.
- Z. Li, F. Yin, W. He, T. Hang, Z. Li, J. Zheng, X. Li, S. Jiang and Y. Chen, Int. J. Biol. Macromol., 2023, 230, 123117 CrossRef CAS PubMed.
- L. Wang, Y. Chen, L. Lin, H. Wang, X. Huang, H. Xue and J. Gao, Chem. Eng. J., 2019, 362, 89–98 CrossRef CAS.
- C. Zhou, T. Wu, X. Xie, G. Song, X. Ma, Q. Mu, Z. Huang, X. Liu, C. Sun and W. Xu, Eur. Polym. J., 2022, 177, 111454 CrossRef CAS.
- C. Zhang, J. Wang, S. Li, X. Zou, H. Yin, Y. Huang, F. Dong, P. Li and Y. Song, Eur. Polym. J., 2023, 186, 111827 CrossRef CAS.
- J. Zhang, W. Xue, Y. Dai, C. Wu, B. Li, X. Guo, B. Liao and W. Zeng, Composites, Part A, 2023, 171, 107572 CrossRef CAS.
- Y. Tian, L. Zhang, X. Li, M. Yan, Y. Wang, J. Ma and Z. Wang, Int. J. Biol. Macromol., 2023, 253, 126550 CrossRef CAS PubMed.
- Y. Yang, Y. Yang, Y. Cao, X. Wang, Y. Chen, H. Liu, Y. Gao, J. Wang, C. Liu, W. Wang, J.-K. Yu and D. Wu, Chem. Eng. J., 2021, 403, 126431 CrossRef CAS.
- G. Mu, W. He, J. He, Y. Muhammad, Z. Shi, B. Zhang, L. Zhou, Z. Zhao and Z. Zhao, Int. J. Biol. Macromol., 2023, 236, 123936 CrossRef CAS PubMed.
- G. Kaur, H. Kumar and M. Singla, J. Mol. Liq., 2022, 351, 118556 CrossRef CAS.
- K. Liu, Z. Wang, L. Shi, S. Jungsuttiwong and S. Yuan, J. Energy Chem., 2021, 59, 320–333 CrossRef CAS.
- Y. Pei, Y. Zhang, J. Ma, M. Fan, S. Zhang and J. Wang, Mater. Today Nano, 2022, 17, 100159 CrossRef CAS.
- Y. Zhang, J. Xu and H. Wang, RSC Adv., 2021, 11, 37661–37666 RSC.
- Y. Ding, J. Zhang, L. Chang, X. Zhang, H. Liu and L. Jiang, Adv. Mater., 2017, 29, 1704253 CrossRef PubMed.
- K. Wang, J. Wang, L. Li, L. Xu, N. Feng, Y. Wang, X. Fei, J. Tian and Y. Li, Chem. Eng. J., 2019, 372, 216–225 CrossRef CAS.
- C.-K. Chen, P.-W. Chen, H.-J. Wang and M.-Y. Yeh, Gels, 2021, 7, 164 CrossRef CAS PubMed.
- M. Amjadi, A. Pichitpajongkit, S. Lee, S. Ryu and I. Park, ACS Nano, 2014, 8, 5154–5163 CrossRef CAS PubMed.
- Z. Cao, H. Liu and L. Jiang, Mater. Horiz., 2020, 7, 912–918 RSC.
- Y. Cao, T. G. Morrissey, E. Acome, S. I. Allec, B. M. Wong, C. Keplinger and C. Wang, Adv. Mater., 2017, 29, 1605099 CrossRef PubMed.
- H. Zhang, W. Niu and S. Zhang, Chem. Eng. J., 2020, 387, 124105 CrossRef CAS.
- Z. Guo, H. Gu, Y. He, Y. Zhang, W. Xu, J. Zhang, Y. Liu, L. Xiong, A. Chen and Y. Feng, Chem. Eng. J., 2020, 388, 124282 CrossRef CAS.
- M. X. Wang, Y. M. Chen, Y. Gao, C. Hu, J. Hu, L. Tan and Z. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 26610–26617 CrossRef CAS PubMed.
- A. Wang, Y. Wang, B. Zhang, K. Wan, J. Zhu, J. Xu, C. Zhang and T. Liu, Chem. Eng. J., 2021, 411, 128506 CrossRef CAS.
- Q. Liu, J. Qiu, C. Yang, L. Zang, G. Zhang and E. Sakai, Adv. Mater. Technol., 2021, 6, 2000919 CrossRef CAS.
- H. Wei, D. Kong, T. Li, Q. Xue, S. Wang, D. Cui, Y. Huang, L. Wang, S. Hu, T. Wan and G. Yang, ACS Sens., 2021, 6, 2938–2951 CrossRef CAS PubMed.
- M. Tavakolizadeh, A. Pourjavadi, M. Ansari, H. Tebyanian, S. J. Seyyed Tabaei, M. Atarod, N. Rabiee, M. Bagherzadeh and R. S. Varma, Green Chem., 2021, 23, 1312–1329 RSC.
- H. Nargesi Khoramabadi, M. Arefian, M. Hojjati, I. Tajzad, A. Mokhtarzade, M. Mazhar and A. Jamavari, J. Compos. Compound, 2020, 2, 69–76 Search PubMed.
- Y. Li, C. Zhu, D. Fan, R. Fu, P. Ma, Z. Duan, X. Li, H. Lei and L. Chi, Int. J. Polym. Mater. Polym. Biomater., 2020, 69, 505–515 CrossRef CAS.
- N. Nikfarjam, M. Ghomi, T. Agarwal, M. Hassanpour, E. Sharifi, D. Khorsandi, M. Ali Khan, F. Rossi, A. Rossetti, E. Nazarzadeh Zare, N. Rabiee, D. Afshar, M. Vosough, T. Kumar Maiti, V. Mattoli, E. Lichtfouse, F. R. Tay and P. Makvandi, Adv. Funct. Mater., 2021, 31, 2104148 CrossRef CAS.
- Y. Wang, Z. Qu, W. Wang and D. Yu, Colloids Surf., B, 2021, 208, 112088 CrossRef CAS PubMed.
- S.-N. Li, X.-F. He, Z.-F. Zeng, B. Jiang, Q. Wu, L.-X. Gong, Y. Li, J. Bae, S. Wang and L.-C. Tang, Nano Energy, 2022, 103, 107789 CrossRef CAS.
- A. Porfarzollah, M. Bagheri and R. Mohammad-Rezaei, Polym. Adv. Technol., 2019, 30, 1767–1776 CrossRef CAS.
- J. Ren, Y. Liu, Z. Wang, S. Chen, Y. Ma, H. Wei and S. Lü, Adv. Funct. Mater., 2022, 32, 2107404 CrossRef CAS.
- K. Li, Y. Liu, Y. Ge, H. Cao, S. Zhuang, X. Yang, Y. Zhao and X. Gu, J. Mater. Chem. C, 2023, 11, 1908–1918 RSC.
- Y. Liu, Q. Liu, L. Zhong, C. Chen and Z. Xu, Chem. Eng. J., 2023, 452, 139314 CrossRef CAS.
- Y. Zhou, X. Fei, J. Tian, L. Xu and Y. Li, J. Colloid Interface Sci., 2022, 606, 192–203 CrossRef CAS PubMed.
- B. Liu, R. Fu, Z. Duan, C. Zhu, J. Deng and D. Fan, Composites, Part B, 2022, 236, 109804 CrossRef CAS.
- Y.-R. Gao, J.-F. Cao, Y. Shu and J.-H. Wang, Green Chem. Eng., 2021, 2, 368–383 CrossRef.
- W. Ge, S. Cao, Y. Yang, O. J. Rojas and X. Wang, Chem. Eng. J., 2021, 408, 127306 CrossRef CAS.
- Y. Jian, S. Handschuh-Wang, J. Zhang, W. Lu, X. Zhou and T. Chen, Mater. Horiz., 2020, 7, 3339 RSC.
- W. J. Zheng, Y. Gao, X. X. Fan, X. F. Cui, W. Zou, D. Zheng, J. Yan and B. Li, Macromol. Mater. Eng., 2018, 303, 1800104 CrossRef.
- A. M. Curreri, S. Mitragotri and E. E. L. Tanner, Adv. Sci., 2021, 8, 2004819 CrossRef CAS PubMed.
- Y. Yu, Z. Yang, S. Ren, Y. Gao and L. Zheng, J. Mol. Liq., 2020, 299, 112185 CrossRef CAS.
- Y. Jiao, Y. Lu, K. Lu, Y. Yue, X. Xu, H. Xiao, J. Li and J. Han, J. Colloid Interface Sci., 2021, 597, 171–181 CrossRef CAS PubMed.
- H. Ding, Z. Wu, H. Wang, Z. Zhou, Y. Wei, K. Tao, X. Xie and J. Wu, Mater. Horiz., 2022, 9, 1935–1946 RSC.
- X. Li, L. Jiang, M. Yan, H. Bi and Q. Wang, Int. J. Biol. Macromol., 2023, 242, 124780 CrossRef CAS PubMed.
- L. Zhong, Y. Zhang, F. Liu, L. Wang, Q. Feng, C. Chen and Z. Xu, Int. J. Biol. Macromol., 2023, 248, 125973 CrossRef CAS PubMed.
- Y. Gao, Y. Gao, Z. Zhang, Y. Wang, X. Ren, F. Jia and G. Gao, J. Mater. Chem. C, 2022, 10, 12873–12882 RSC.
- F. Ding, W. Tang, L. Fu, R. Zhang, G. Wang, Z. Han, R. Wu, Y. Dong and X. Zou, Polymer, 2022, 238, 124387 CrossRef CAS.
- H. Zheng, M. Chen, Y. Sun and B. Zuo, Chem. Eng. J., 2022, 446, 136931 CrossRef CAS.
- H. Chen, J. Huang, J. Liu, J. Gu, J. Zhu, B. Huang, J. Bai, J. Guo, X. Yang and L. Guan, J. Mater. Chem. A, 2021, 9, 23243–23255 RSC.
- H. Wu, Q. Zhao, Y. Liang, L. Ren and L. Ren, ACS Sustainable Chem. Eng., 2022, 10, 4425–4437 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh02013d |
| This journal is © The Royal Society of Chemistry 2024 |