Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Exploring the Mpemba effect: a universal ice pressing enables porous ceramics
Xiaodan
Yang
ab,
Yao
Shan
ab,
Ying
Hong
ab,
Zhuomin
Zhang
ab,
Shiyuan
Liu
ab,
Xiaodong
Yan
ab,
Xuetian
Gong
c,
Guangzu
Zhang
c and
Zhengbao
Yang
*a
aDepartment of Mechanical and Aerospace Engineering, Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, China. E-mail: zbyang@ust.hk
bDepartment of Mechanical Engineering, City University of Hong Kong, Hong Kong, China
cSchool of Optical and Electronic Information, Engineering Research Center for Functional Ceramic MOE and Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, Wuhan 430074, China
First published on 20th March 2024
Abstract
Correction for ‘Exploring the Mpemba effect: a universal ice pressing enables porous ceramics’ by Xiaodan Yang et al., Mater. Horiz., 2024, DOI: https://doi.org/10.1039/d3mh01869e.
The authors regret some errors in the published article, as outlined below. These corrections do not affect any of the conclusions of the article.
Ref. 1–3 included in the published article were incorrect, and should be as included here.
In addition, in Section 2.2, in the 3rd paragraph a citation to ref. 21 should be replaced with a citation to ref. 2, as follows: “Research conducted by J.D. Brownridge's group suggests that convection likely played a role in facilitating the faster freezing of water at higher temperatures.2”
In the Introduction, the sentence “During frost weathering,7 when water fills the gaps in rocks and freezes in place, the resulting ice growth can exert pressures of up to 207 MPa inside cracks in the rock, assuming a temperature of −22 °C.8–10” should read as follows: “During frost weathering, when water fills the gaps in rocks and freezes, the underlying intermolecular forces associated with unfrozen water can exert enormous pressures.7 The bulk equilibrium pressure of approximately 207 MPa inside cracks in the rock, assuming a temperature of −22 °C, is also substantial if circumstances allow it to be reached.8–10”
In Section 2.2, the word “exponentially” should be removed from two sentences in paragraph 3. The corrected sentences are as follows:
“According to the results of in situ temperature mappings (Movie S1, ESI†) and temperature curves (Fig. 2c & inset), hot water shows a faster cooling rate to reach the relative equilibrium than that in the cool case, which takes less time (about 15 seconds) to freeze.”
and
“Therefore, the temperature of hot water cools rapidly at the beginning and then slows down.”
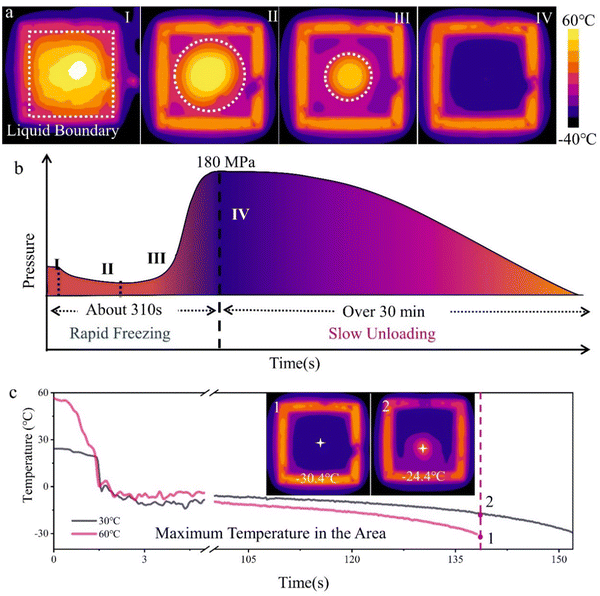
In Fig. 2c, the temperature curves were labelled incorrectly, in addition the caption of Fig. 2c stated an incorrect temperature of 20 °C, which should be replaced with 30 °C. The corrected image and caption are as shown here.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- E. B. Mpemba and D. G. Osborne, Phys. Educ., 1969, 4, 172 CrossRef.
- J. D. Brownridge, Am. J. Phys., 2011, 79, 78–84 CrossRef CAS.
- M. Vynnycky and S. L. Mitchell, Heat Mass Transfer, 2010, 46, 881–890 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |