Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
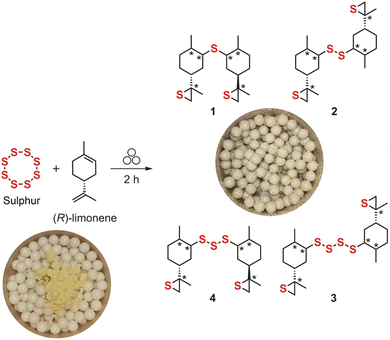
Iron-free mechanochemical limonene inverse vulcanization†
Rima
Tedjini‡
ab,
Raquel
Viveiros
b,
Teresa
Casimiro
 b and
Vasco D. B.
Bonifácio
b and
Vasco D. B.
Bonifácio
 *cd
*cd
aLaboratory of Applied Organic Chemistry, Faculty of Chemistry, University of Science and Technology Houari Boumediene, BP 32, Alia Bab-Ezzouar, 16111 Algiers, Algeria
bLAQV-REQUIMTE, Chemistry Department, NOVA School of Science & Technology|FCT NOVA, NOVA University of Lisbon, Caparica, 2829-516 Caparica, Portugal
ciBB-Institute for Bioengineering and Biosciences, i4HB-Institute for Health and Bioeconomy, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049-001 Lisboa, Portugal. E-mail: vasco.bonifacio@tecnico.ulisboa.pt
dBioengineering Department, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049-001 Lisboa, Portugal
First published on 29th January 2024
Abstract
An iron-free mechanochemical-assisted limonene inverse vulcanization is reported. The process makes use of only limonene and sulphur, industrial waste by-products, under mild conditions (ca. 40 °C) and short time (2 h) using a zirconium oxide reactor and a planetary ball mil. The obtained high value products are light yellow solids, readily soluble in chloroform, optically active oligosulfides, which are different from polysulfides reported under conventional conditions (ca. 185 °C), as confirmed by NMR spectroscopy and mass spectrometry. A general reaction mechanism is proposed, initiated by homolytic sulphur ring opening triggered by mechanical stress, and involving thiirane intermediates, via an addition–elimination reaction of sulphur to the limonene double bonds.
The valorisation of industrial waste by-products is of enormous importance since impacts on both sustainability and circular economy. From this perspective, the development of new products from residues, especially with high added value, is not only challenging but economically attractive. Sulphur has been known since antiquity and is an unwanted by-product of the petrochemical industry, with a worldwide production estimated in 78 million tonnes in 2020.1 Similarly, limonene is a well-known cyclic monoterpene that is obtained as a by-product from the citrus juice industry, by cold pressing or distillation from orange and lemon fruit peel, reaching a production around 60 thousand tonnes per year.2 The preparation of polysulfides by inverse vulcanization, a process were elemental sulphur (S8) is a comonomer and reaction medium, has been explored in the last decades.3 Applications of these polysulfides have been focused on the development of repairable materials, energy generation and storage, optical devices, and environmental remediation.3,4 Recently, advances in catalytic inverse vulcanization reactions enabled the use of a wide range of crosslinkers, thus reducing reaction time and temperature, also preventing harmful H2S production.5 Very recently, a mechanochemical synthesis of inverse vulcanized polymers was reported using a stainless steel vibratory mill, where different co-polymers where prepared, starting from synthetic and renewable monomers, and aromatic and aliphatic crosslinkers.6
Herein, we show the potential of mechanochemistry to perform limonene inverse vulcanization under iron-free conditions at very “low temperatures”. In this work (R)-limonene was studied as a model olefin for the preparation of high value oligosulfides. The reaction proceeds without heating (ca. 40 °C, temperature reached inside the zirconium oxide reactor during the milling process using a planetary ball mil) under solventless conditions. We studied the progress of the reaction over time, at 500 rpm, up to 2 hours. In this time frame, we found that (R)-limonene is fully consumed. To find the optimal milling conditions the progress of the reaction was followed by 1H-NMR over time, and information about (R)-limonene consumption was achieved by stopping the milling in fixed time intervals. After milling, the obtained crude mixture (light yellow paste) was removed from the reactor (and balls) by washings with chloroform and filtered. The chloroform fraction, after evaporation, led to a yellow solid. The remaining solid obtained in the filtration (chloroform insoluble fraction) was found to be highly soluble in carbon disulfide (CS2). Attempts to trace a 1H-NMR spectrum using a CDCl3/CS2 mixture failed since the sample precipitated even with a small amount of CDCl3 (10% v/v), thus precluding full characterization, nevertheless the 1H-NMR using CDCl3/CS2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 did not show any signal. Fig. 1 shows the 1H-NMR spectrum of the chloroform fraction acquired in CDCl3/CS2 9
1 did not show any signal. Fig. 1 shows the 1H-NMR spectrum of the chloroform fraction acquired in CDCl3/CS2 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The addition of a small amount of CS2 (10% v/v) allowed the full solubilization of the solid in CDCl3, in which, unexpectedly, the oligosulfides are insoluble. CS2 was chosen as the NMR co-solvent since does not affect chemical shifts7 and is a good solvent for S8, which allowed purity control.
1. The addition of a small amount of CS2 (10% v/v) allowed the full solubilization of the solid in CDCl3, in which, unexpectedly, the oligosulfides are insoluble. CS2 was chosen as the NMR co-solvent since does not affect chemical shifts7 and is a good solvent for S8, which allowed purity control.
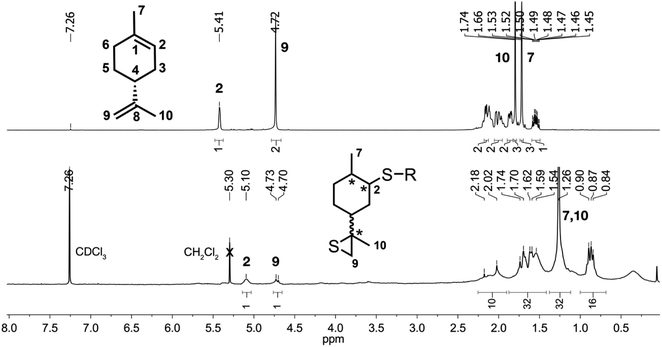 | ||
Fig. 1 Limonene mechanochemical inverse vulcanization. 1H-NMR spectrum of limonene in CDCl3 (top) and limonene oligosulfides in CDCl3/CS2 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (bottom). 1 (bottom). | ||
The 1H-NMR spectrum does not show the characteristic signals of (R)-limonene protons from endocyclic (1H, 5.41 ppm) and exocyclic (2H, 4.72 ppm) double bonds. In this region, the presence of three residual signals may be attributed to (R)-limonene thiirane (5.10 ppm, endocyclic proton, H2) and a (R)-limonene dimer (4.73 and 4.70 ppm, exocyclic protons, H9), both identified in the mass spectra. The endocyclic proton of (R)-limonene thiirane is slightly downshifted (ΔH2 = 0.27 ppm) to the reported racemate value, the only reference available for these family of compounds (5.37 ppm,8 8,9-epithio-1-p-menth-1-ene). In analogy, different H2 chemical shifts were found for (R)-limonene oxide pure stereoisomers (5.36 (ref. 9) or 5.40 (ref. 10) ppm for (4R,8S)-8,9-epoxy-p-menth-1-ene; 5.38 (ref. 9 and 10) ppm for (4R,8R)-8,9-epoxy-p-menth-1-ene) and the racemic mixture (5.37 (ref. 11) ppm for 8,9-epoxy-p-menth-1-ene), which could explain the observed chemical shift.
The reaction products were also identified by mass spectrometry, following an optimized protocol using silver(I) infused samples.12 The mass spectra were acquired in the positive mode, as reported, which allowed us to identify the products formed in the mechanochemical inverse vulcanization (Scheme 1).
We also acquired the spectra in the negative mode, but only the peaks from AgNO3 clusters were observed, with the same pattern of the control injection (AgNO3 and solvents mixture). The spectra acquired in the negative mode, showing the peaks corresponding to AgNO3 (m/z = 230.9, 100.0%; 232.9, 94.5%, duplet), and the corresponding dimer (m/z = 399.7, 55.1%; 401.6, 100%; 403.6, 47.5%, triplet) and trimer (m/z = 568.4, 34.6%; 570.4, 100%; 572.3, 92.9%; 574.3, 30.5%, quartet) clusters were useful in the interpretation of the species observed in the positive mode spectra (see Fig. S2–S4 in the ESI†). Multiple Ag+ ion complexation with sulphur atoms leads to characteristic mass patterns, correlated with the silver isotopic abundance. As in the case of inverse vulcanization by a conventional route (solventless heating),13 only low molecular weight limonene oligosulfides were found. However, mechanochemical inverse vulcanization leads to the formation of different oligosulfides.
One interesting and rather unusual feature of our methodology using a zirconium oxide reactor and a planetary ball mill, is that, beyond the expected oligosulfides, thiiranes are also formed. The reaction must occur by a catalyst-free sulphur insertion into the limonene double bonds. The homo-ROP of S8 has been explored in the preparation of different sulphur copolymers,14 and is based on the formation of sulphur diradicals upon melting. For the preparation of stable copolymers, avoiding reversible depolymerization, reactions are performed at high temperatures (ca. 185 °C), and a yellow to red (or even black) colour change is usually observed. This colour changes have been thoroughly investigated, and associated with the monomer ability to form terminal structures by chain transfer, especially the ones containing aromatic or aliphatic terminal olefins, where aliphatic internal olefins (as in the case of limonene) are less prone to the formation of coloured by-products.15
In the previously reported6 mechanochemical synthesis of inverse vulcanized polymers, the use of a zirconium oxide reactor was also investigated in control experiments using monomers 1,3-diisopropenylbenzene (DIB) and styrene. In this case lighter coloured products are obtained, with no remaining sulphur, but found to be insoluble in THF and chloroform, precluding full characterization. However, as we demonstrate in this work, an iron catalysis may be discarded, being mechanical energy a key driving force in this type of reactions.
More recently, a “low temperature” inverse vulcanization (110 °C, below the sulphur melting point) was achieved by the addition of sodium diethyldithiocarbamate trihydrate as a catalyst.16 Nevertheless, under these harsh conditions, and depending on the co-monomers, side reactions are always observed. In the case of limonene, the aromatization to p-cymene is observed.13 Herein, we used a zirconium oxide reactor and a short reaction time (2 h), being the reaction temperature lower than 30 °C. Under these mild conditions, a decolourization of the initial yellow mixture is observed, and no aromatic signals are detected in the NMR spectra, thus precluding further limonene oxidation.
Thiiranes are important heterocycles, and many synthetic methods have been developed in the last decades.17,18 To the best of our knowledge, this is the first report of thiiranes mechanosynthesis, under mild catalyst-free conditions and using elemental sulphur as the sulphur donor. Only a few examples of thiirane synthesis are reported using elemental sulphur as the sulphur source, some proceeding with high stereoselectivity,19,20 not investigated in this work.
The obtained oligosulfides are optically active, with [α]20D = −64.4 (c = 1.0, CHCl3), a value that is close to the one found for polysulfides obtained by a conventional route, [α]20D = −27.3 (c = 1.0, CHCl3).
We believe that the reaction may follow a radical mechanism, involving an addition–elimination reaction of sulphur to the limonene double bonds (Scheme 2). The homolytic S8 ring opening may be triggered by mechanical stress, as in the case of ultrasound induced disulfide metathesis.21
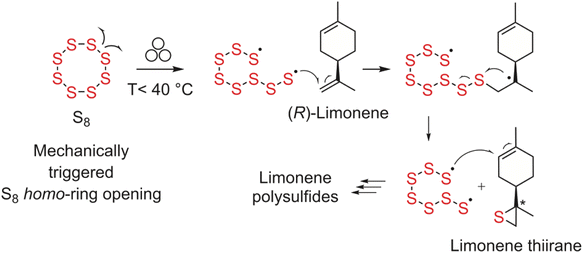 | ||
| Scheme 2 Proposed mechanism for the mechanosynthesis of limonene polysulfides using elemental sulphur as the sulphur donor. | ||
In the mass spectrum we can also observe a region (m/z = 200–400) with a cluster of peaks corresponding to molecules or fragments without sulphur incorporation (absence of the Ag+ isotopic signature). The peak with the highest intensity, with m/z = 274.34, was attributed to a limonene dimer (m/z = 274.27), formed via a C2–C2′ coupling. The dimerization of limonene is reported to occur in high yield and short reaction time under acid catalytic conditions in refluxing conditions (85%, 2 h, Nafion SAC-13).22
Limonene thiiranes and oligosulfides were identified by the [M + Agn]+ peaks found in the mass spectra obtained using the positive mode. In the case of polysulfides the silver ions were found to form adducts with acetonitrile (Fig. 2). These adducts were also present in the injection control using the positive mode (see Fig. S2 in the ESI†).
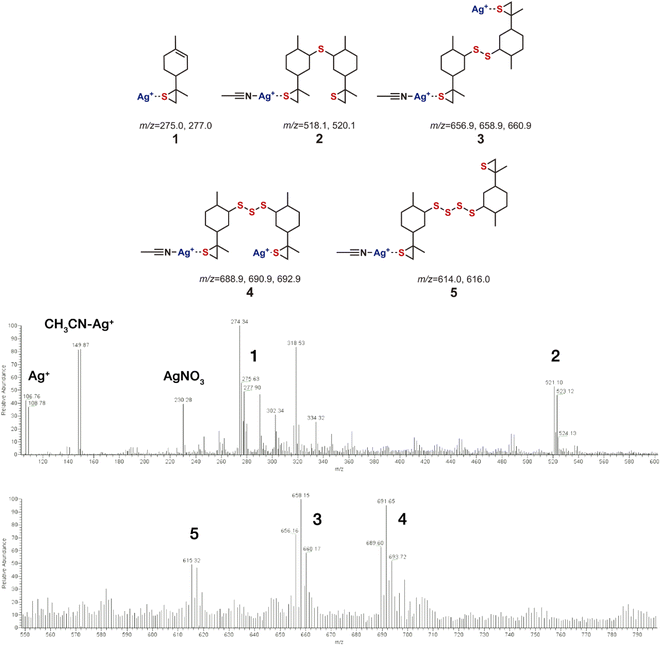 | ||
| Fig. 2 Identification of limonene inverse vulcanization products obtained by mechanosynthesis. The assignment of [M + Agn]+ adducts is shown in the mass spectra. | ||
In summary, we demonstrate that mechanosynthesis using zirconium oxide reactors may be explored as a valuable methodology to produce high value products from abundant raw materials such as sulphur and limonene. Also, the milder mechanically triggered reactions originate new products, which are not described by mechanochemical inverse vulcanization using stainless steel reactors or detected under conventional high temperature reactions. Our data highlight the importance of the reactor material, as a key factor to enlarge the scope of mechanically driven synthesis.
Conflicts of interest
There are no conflits to declare.Acknowledgements
This research was funded by Fundação para a Ciência e a Tecnologia, Ministério da Ciência, Tecnologia e Ensino Superior (FCT/MCTES Portugal), through projects PTDC/EQU-EQU/32473/2017 and PTDC/MECONC/29327/2017. The Associate Laboratory Research Unit for Green Chemistry – Clean Technologies and Processes – LAQV is financed by national funds from FCT/MCTES (UIDB/QUI/50006/2020) and co-funded by the ERDF under the PT2020 Partnership Agreement (POCI-01-0145-FEDER-007265). We thank Dr Conceição Oliveira (Mass Spectrometry Facility at CQE, node IST/RNEM campus Alameda) for performing the mass spectrometry experiments.References
- U.S. Geological Survey, Mineral Commodity Summaries 2021, U.S. Geological Survey, 2021, DOI:10.3133/mcs2021.
- Global Limonene Market Size, Manufacturers, Supply Chain, Sales Channel and Clients, 2020–2026, https://www.360marketupdates.com, accessed November 2023.
- M. J. H. Worthington, R. L. Kuceraa and J. M. Chalker, Green Chem., 2017, 19, 2748–2761 RSC; J. M. Chalker, M. J. H. Worthington, N. A. Lundquist and L. J. Esdaile, Top. Curr. Chem., 2019, 377, 16 CrossRef CAS PubMed.
- K. W. Park and E. M. Leitao, Chem. Commun., 2021, 57, 3190–3202 RSC.
- X. Wu, J. A. Smith, S. Petcher, B. Zhang, D. J. Parker, J. M. Griffin and T. Hasell, Nat. Commun., 2019, 10, 647 CrossRef PubMed.
- P. Yan, W. Zhao, F. McBride, D. Cai, J. Dale, V. Hanna and T. Hasel, Nat. Commun., 2022, 13, 4824 CrossRef CAS PubMed.
- D. B. Walters, Anal. Chim. Acta, 1972, 60, 421–425 CrossRef CAS.
- E. Demole, P. Enggist and G. Ohloff, Helv. Chim. Acta, 1982, 65, 1785–1794 CrossRef CAS.
- R. M. Carman, J. J. De Voss and K. L. Greenjield, Aust. J. Chem., 1986, 39, 441–446 CrossRef CAS.
- A. Kergomard and H. Veschambre, Tetrahedron, 1977, 33, 2215–2224 CrossRef CAS.
- R. Carlson, N. Behn and C. Cowles, J. Org. Chem., 1971, 36(24), 3832–3833 CrossRef CAS.
- K. Wang, M. Groom, R. Sheridan, S. Zhang and E. Block, J. Sulfur Chem., 2013, 34, 55–66 CrossRef CAS.
- M. P. Crockett, A. M. Evans, M. J. H. Worthington, I. S. Albuquerque, A. D. Slattery, C. T. Gibson, J. A. Campbell, D. A. Lewis, G. J. L. Bernardes and J. M. Chalker, Angew. Chem., Int. Ed., 2016, 55, 1714–1718 CrossRef CAS PubMed.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- Y. Onose, Y. Ito, J. Kuwabara and T. Kanbara, Polym. Chem., 2022, 13, 5486–5493 RSC.
- B. Zhang, H. Gao, P. Yan, S. Petcher and T. Hasell, Mater. Chem. Front., 2020, 4, 669–675 RSC.
- M. Sander, Chem. Rev., 1966, 66, 297–339 CrossRef.
- W. Chew and D. N. Harpp, Sulfur Rep., 1993, 15, 1–39 CrossRef CAS.
- W. Adam and R. M. Bargon, Chem. Rev., 2004, 104, 251–261 CrossRef CAS PubMed.
- T. B. Nguyen, Adv. Synth. Catal., 2017, 359, 1066–1130 CrossRef CAS.
- U. F. Fritze and M. von Delius, Chem. Commun., 2016, 52, 6363–6366 RSC.
- H. A. Meylemans, R. L. Quintana and B. G. Harvey, Fuel, 2012, 97, 560–568 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mr00002h |
| ‡ Present address: Centro de Química Estrutural, Instituto Superior Técnico, Av. Rovisco Pais, 1049-001, Lisboa, Portugal. |
| This journal is © The Royal Society of Chemistry 2024 |