Open Access Article
Open Access ArticleLiquid-assisted mechanochemical synthesis of thioamide building blocks with the Lawesson reagent: ex situ monitoring and detection of intermediate polymorphs†
Carlos
Naranjo-Castañeda
 a,
Marco A.
Leyva-Ramírez
a,
Marco A.
Leyva-Ramírez
 a and
Eusebio
Juaristi
a and
Eusebio
Juaristi
 *ab
*ab
aDepartment of Chemistry, Centro de Investigación y de Estudios Avanzados, Avenida IPN # 2508, Col. San Pedro Zacatenco, 07360 Ciudad de México, Mexico. E-mail: ejuarist@cinvestav.mx
bEl Colegio Nacional, Donceles # 104, Centro Histórico, 06000 Ciudad de México, Mexico
First published on 10th September 2024
Abstract
Thioamidation of various classes of carboxamide substrates with Lawesson's reagent under liquid-assisted mechanical activation for the synthesis of relevant building blocks including aromatic thioamides, thiopeptides, thiolactams, and thioenones is described. A thorough analysis of the effect of the specific material of milling jars and milling balls was carried out. The effect of different additives for liquid-assisted grinding (LAG) and the potential of the synthetic protocol for scale-up were explored. The simple and mild reaction conditions involved in this solvent-minimized mechanochemical protocol proved rather effective with a wide variety of substrates. Comparison with the corresponding reactions in solution shows comparable or better yields under mechanochemical activation. Ex situ powder X-ray diffraction (PXRD) monitoring with analysis at multiple points was performed in order to compare the diffraction patterns of reagents and products, to detect potential morphological changes of the reagents induced by milling prior to the reaction, and to perceive the occurrence of phase transitions during the mechanochemical reaction.
Introduction
Organic compounds containing the thiocarbonyl function, especially thioamides and thiopeptides, are encountered in numerous molecules of pharmaceutical interest, as well as in agrochemicals, cosmetics, adhesives, lubricants and corrosion inhibitors, among others.1–3 Importantly, in the pharmaceutical industry various thiocarbonyl-containing compounds have been found to be effective in the treatment of diseases such as hypothyroidism4 and tuberculosis.5 Thus, not surprisingly, the interesting pharmacological properties achieved upon substitution of oxygen by sulfur in carbonyl-containing compounds have aroused special interest in medicinal chemistry.6,7 On the other hand, the notable efficiency of chiral thiopeptides as organocatalysts in asymmetric synthesis has been recently demonstrated.8–11In this context, it is known that the carbonyl group in cyclic and acyclic amides is usually more readily transformed into the corresponding thiocarbonyl group relative to other common carbonyl derivatives such as ketones, esters and anhydrides.12 Consequently, several synthetic procedures have been developed for the preparation of thioamides from amides, using various reagents under appropriate reaction conditions. More generally, thioamides are prepared by two synthetic strategies, namely redox and non-redox transformations. Redox transformations with thioacylating reagents include the classical Willgerodt–Kindler reaction13 and its variations.14,15 In addition, iodine-catalyzed oxidative coupling16 or reactions involving dimethyl sulfoxide (DMSO) as an oxidizing agent and sulfur as a catalyst17 have been documented. Non-redox transformations include thionation (either direct or following prior electrophilic activation) of carboxamides with sulfurizing agents such as hydrogen sulfide (H2S),18 phosphorus pentasulfide (P2S5),19 bis(trimethylsilyl)sulfide,20 rhodanine21 or Lawesson's reagent [2,4-bis(4-methoxyphenyl)-1,3-dithia-2,4-phosphetane-2,4-disulphide].22,23
Of special interest in the present work, Lawesson's reagent has been employed in numerous thionation reactions under gentle reaction conditions.22–25 Relevantly, Lawesson's reagent is notably effective with amide substrates, requiring short reaction times and affording high yields of thiocarbonyl derivatives.25,26 Lawesson's reagent is usually applied in solution with organic solvents such as benzene, toluene, xylene or pyridine, methylene chloride, tetrahydrofuran, acetonitrile or 1,4-dioxane, all of which are notoriously toxic.27 Alternative techniques making use of microwave activation have been successfully developed, either in solution28,29 or under neat conditions.30,31 In this context, activation by means of sonication has also been employed to carry out thioamidation reactions.32 Nevertheless, there is still a need to set forth synthetic alternatives that could have lower environmental impact.
Mechanochemical activation33–47 would seem to be an attractive alternative to carry out sulfuration reactions, as this technique has proved sustainable and particularly friendly to the environment as it eliminates or at least greatly reduces the use of solvent. Furthermore, mechanochemical reactions usually take place at shorter reaction times and with reduced energy consumption. Furthermore, mechanochemical activation enables the manipulation of insoluble reactants as well as air- and water-sensitive reagents.
In the context of the present report, very recently Bolm's group48 reported the use of mechanochemistry to obtain α-ketothioamides from acetophenone derivatives employing elemental sulfur under metal and solvent-free mechanochemical activation in a ball mill (Fig. 1).
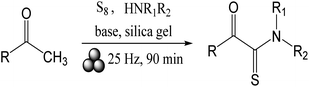 | ||
| Fig. 1 Mechanochemical approach for the preparation of α-ketothioamides with elemental sulfur.48 | ||
Also central in the field of mechanochemical sulfidation protocols is the work of Kotyk and co-workers, who prepared several thiolactams and thioamides by means of Lawesson's reagent under mechanical activation.49
Nevertheless, leading players in the field of mechanochemistry have stressed the urgent need for a less empirical, more rational development of the technique.50–64
In particular, in the year 2013, Boldyreva emphasized the importance of understanding the effects of treatment using various mechanical devices (vibrational or planetary mills, twin screw extruders, etc.) on solid-state properties and reactivity.55 In this regard, mechanical action can cause damage to and induce phase transitions in solid substrates, altering their morphology and crystallinity upon milling.54–58
On the other hand, Friščić, Emmerling, and Hernández and their co-workers pointed out the fact that little attention has been paid to determine the mechanistic aspects of mechanochemical reactions, that are not necessarily similar to solution chemistry.52,59–61
In this regard, since mechanochemical reactions are carried out in the solid-state, it would be informative to know if polymorphic changes occur upon milling. Therefore, monitoring of mechanochemical processes is important for the identification of short-lived intermediates.62–64 Importantly, Emmerling and co-workers established that the stability of polymorphic species depends strongly on the material of the milling vessel.50,52
As it was pointed out by Friščić, Halasz and co-workers, assigning the mechanism of mechanochemical reactions involves detecting a sequence of crystalline or amorphous phases leading from reactants to products, which can be accomplished by ex situ and in situ monitoring at multiple points.53,59
In the present paper, we describe a simple mechanochemical approach for the conversion of different substrates containing the carboxamide group into the corresponding thiocarboxamide groups by means of Lawesson's reagent. A thorough examination of the milling parameters such as milling jars and ball milling material, assistance by liquid additives, the effect of milling frequency, reaction time, and scalability was determined. Furthermore, it is worth noting that no special experimental setups are required, thus conventional laboratory equipment is used in the thionation procedure described in this paper.
Importantly, we report the results of ex situ powder X-ray diffraction (PXRD) monitoring of the mechanochemical process, which revealed an interesting polymorphic behaviour of the organic reagents during the solid-state transformation. Additionally, the present study demonstrated that the nature of the grinding materials affects the morphology of the solid phases of the substrates and products induced by milling. Consequently, the efficiency of the solid-state reaction is influenced by the nature of the milling materials.
Results and discussion
Amide precursors of the corresponding thioamides of interest were synthesized with excellent yields (85–95%; see the ESI†) according to previous reports in the literature.65–68In initial exploratory experiments we performed the mechanochemical thionation reaction employing grinding vessels and balls made of different milling materials. The reaction was carried out with benzamide (1.23 mmol) as the model substrate with 0.61 mmol of Lawesson's reagent (0.5 equiv.) in 1 mL of freshly distilled THF for liquid-assisted grinding (see below). Milling jars (5–8 mL capacity) of acrylic material, Teflon, stainless steel, and agate were employed, as well as milling balls (10–15 mm of diameter) of copper, Teflon, stainless steel or agate. The mechanochemical reactions were carried out for 90 minutes at 25 Hz frequency in a Retsch200 or Retsch400 ball mill (vibrational ball-milling). The desired products were obtained with 81–96% yield (see Table 1). Satisfactory results were achieved with acrylic components, but higher yields were achieved employing a vibrational Retsch400 mill and a Teflon milling jar with one Teflon ball. Thus, it was decided to employ Teflon grinding vessels and Teflon milling balls in further experiments.
| Entrya | Milling jarb | Number and material of ballsc | Yieldd (%) |
|---|---|---|---|
| a Reaction conditions, benzamide (150 mg 1.23 mmol), LR (250 mg, 0.61 mmol), freshly distilled THF (1.0 mL). b Acrylic (7.5 mL capacity), Teflon (8 mL capacity), stainless steel (7 mL capacity), and agate (5 mL capacity). c Milling balls of copper (11 mm, 5.6 g), Teflon (10 mm, 1.7 g), stainless steel (12 mm, 6.8 g) and agate (15 mm, 4.8 g) were tested. The reaction was performed for 90 minutes at a frequency of 25 Hz. d Average isolated yield. | |||
| 1 | Acrylic | 1, copper | 88.2 |
| 2 | Acrylic | 1, steel | 93.4 |
| 3 | Teflon | 1, Teflon | 95.6 ± 1.4 |
| 4 | Teflon | 2, Teflon | 96.1 ± 0.8 |
| 5 | Teflon | 1, copper | 84.35 ± 3.04 |
| 6 | Teflon | 1, steel | 81.45 ± 3.8 |
| 7 | Teflon | 1, agate | 89.25 ± 1.3 |
| 8 | Stainless steel | 1, copper | 85.4 ± 2.1 |
| 9 | Stainless steel | 1, steel | 86.2 ± 6.4 |
| 10 | Agate | 1, agate | 89.40 ± 4.1 |
Grinding conditions were optimized by variation of the nature and number of milling balls. Thus, copper balls (11 mm diameter, 5.6 g weight), Teflon (10 mm diameter, 1.7 g weight), stainless steel (12 mm diameter, 6.8 g weight) and agate (15 mm diameter, 4.8 g weight) milling balls were examined using a Retsch400 vibrational mill. As it turned out, the number of balls (one or two Teflon balls) did not induce a significant difference in the reaction yield (Table 1, entries 3 and 4), so for subsequent experiments it was decided to work with one Teflon ball. Stainless steel milling jars and milling balls afforded less satisfactory results in terms of yield (Table 1, entries 5 and 6), probably as a consequence of the partial chemical reaction between the metal and the thioamide being formed.69 This leaching process resulted in blackish aggregates on the metal surface.
The use of a liquid additive to facilitate the grinding process (liquid assisted grinding, LAG) was then investigated since the presence of solvent can have a drastic influence on the outcome of a mechanical treatment.70 The solvents that were examined include acetonitrile (dielectric constant = 36.64), methylene chloride (dielectric constant = 10.42), or tetrahydrofuran (dielectric constant = 7.52), which are commonly used in thionation reactions with Lawesson's reagent.23 The results show that the reaction proceeds best in the least polar liquid additive THF, which afforded 95–96% yield of the desired product in 90 minutes of reaction in a Retsch400 mill (see entries 2–4 in Table 2). Additionally, undistilled THF was examined as a liquid additive and it was found that the yield of thiobenzamide product decreased by more than 30% (see entry 3 in Table 2); thus, it is recommended that the THF employed as the liquid additive is dry and freshly distilled.
| Entrya | Liquid additiveb | η | Yieldd (%) |
|---|---|---|---|
| a Reaction conditions: benzamide (150 mg, 1.23 mmol), LR (250 mg, 0.61 mmol), reactions were carried out in duplicate, Teflon milling jars with 1 Teflon ball. b Additives (1 or 0.5 mL), freshly distilled methylene chloride, tetrahydrofuran, or acetonitrile. c (η = μL mg−1) measures the ratio of the solvent additive in μL over the weight of the sample in mg. The reaction was performed for 90 minutes (at a frequency of 25 Hz). d Average isolated yield. | |||
| 1 | CH2Cl2 (1 mL) | 2.5 (slurry) | 31.2 ± 4.1 |
| 2 | THF (1 mL) | 2.5 (slurry) | 96.0 ± 0.6 |
| 3 | Undistilled THF (1 mL) | 2.5 (slurry) | 65.7 ± 5.3 |
| 4 | THF (0.5 mL) | 1.25 (slurry) | 94.9 ± 1.9 |
| 5 | THF (0.25 mL) | 0.62 (LAG) | 67.1 ± 3.4 |
| 6 | 0 | 0 (neat grinding) | 19.9 ± 3.8 |
| 7 | CH3CN (1 mL) | 2.5 (slurry) | 88.4 ± 2.1 |
In order to optimize the amount of the liquid additive, that is to determine the most convenient value of the parameter η, which measures the ratio of solvent in μL relative to the weight of the sample in mg,70,71 four different working amounts of solvent were handled: 1000 μL, 500 μL, 250 μL and 0 μL (Table 2). As it turned out, 500 μL of THF (η = 1.25, which satisfies the criterion for a slurry protocol70) was most convenient, resulting in 95% reaction yield (see entry 4 in Table 2). Reactivity of the reagents seems to depend on their capacity to get in close contact with each other. In the present mechanochemically activated system, the feasibility of the chemical reaction in the mill is determined by the amount of liquid that is present, impacting the performance and showing that the assisting solvent can play a crucial role in the development of the reaction.70 In this regard, the reaction yields were notably lower when the amount of the liquid additive was decreased (Table 2, entries 5 and 6).
The effect of vibration frequencies was examined since it influences the number of collisions within the milling jar, including productive collisions between molecules to afford the product. The optimum input energy for the mechanochemical process was then determined by adjusting the vibration frequencies to 6, 15 and 25 Hz in a Retsch400. It was found that at a frequency of 25 Hz the desired product is obtained with an excellent yield of 94–95% (entry 5 in Table 3).
| Entrya | Frequencyb | Timec (min) | Yieldd (%) |
|---|---|---|---|
| a Reaction conditions: benzamide (150 mg 1.23 mmol), LR (250 mg, 0.61 mmol), THF (0.5 mL) freshly distilled, a Teflon milling jar with 1 Teflon ball. b The reaction was performed at different working frequencies (6, 15, and 25 Hz). c Times (90, 60, 40, and 20 min). d Average isolated yield. | |||
| 1 | 6 | 90 | 25.4 ± 1.2 |
| 3 | 15 | 90 | 72.5 ± 4.4 |
| 5 | 25 | 90 | 94.7 ± 1.0 |
| 7 | 25 | 60 | 95.9 ± 0.4 |
| 9 | 25 | 40 | 86.6 ± 3.0 |
| 11 | 25 | 20 | 52.8 ± 3.6 |
The time required for the reaction to be complete was then determined. The process was carried out in Retsch200 and Retsch400 ball mills, finding that the thionation reaction is complete after 60 min, affording the desired product with 95–96% yield (see entry 7 in Table 3).
One of the most challenging objectives in mechanochemical procedures is the scaling up of the chemical process, especially with the aim to eventually transport the procedures of interest to the chemical industry. In the present study, the optimal mechanochemical reaction conditions were employed with two and four times the initial quantity of the substrates, that is, 300 and 600 mg of benzamide. In the event, a rather small decrease in yield, which is nevertheless higher than 90.0% yield, was observed (Table 4). In summary, it was established that the thionation reaction of model substrate benzamide with half equivalent of Lawesson's reagent in the presence of 500 μL of freshly distilled THF as the liquid additive under mechanochemical activation (Teflon milling jar and one Teflon ball with 10 mm of diameter and 1.7 g of weight) afforded the desired product 1 with an excellent yield of 95–96% in 60 minutes of milling time.
| Entrya | Substrateb (mg) | Yieldc (%) |
|---|---|---|
| a The reaction was performed for 60 min at a frequency of 25 Hz. b Scale tested: 300 mg benzamide + 500 mg LR, freshly distilled THF (1 mL); 600 mg + 1 g LR, and 2 mL of freshly distilled THF. A Teflon milling jar with 1 Teflon ball (10 mm diameter, 1.7 g weight). c Isolated yield. | ||
| 1 | 300 | 93.3 |
| 2 | 300 | 90.1 |
| 3 | 600 | 91.6 |
| 4 | 600 | 90.0 |
Ex situ monitoring of the mechanochemical thionation reaction: effect of the material of milling jars and balls
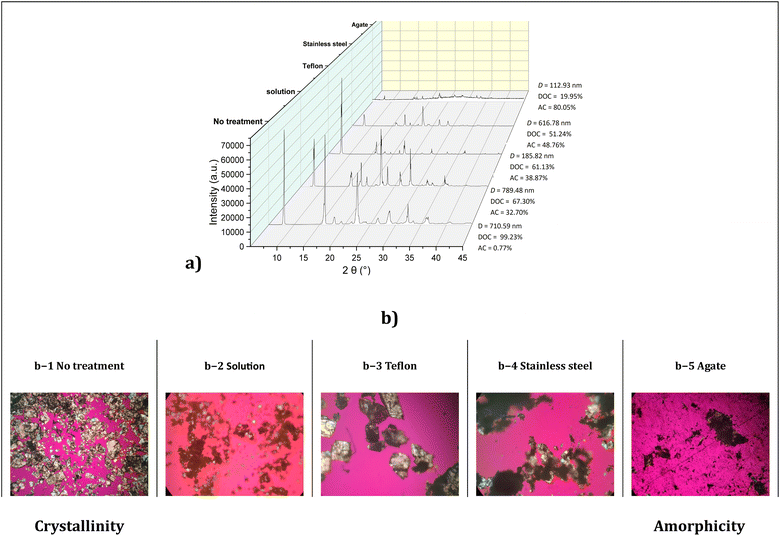
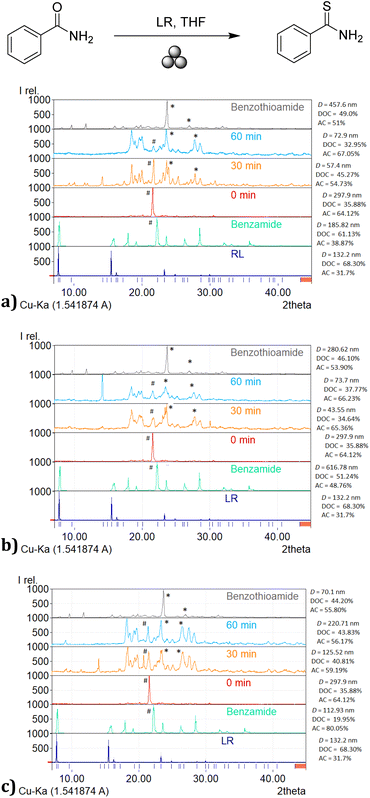
In order to determine the potential influence of the material of milling jars upon milling50,52 of benzamide prior to the addition of Lawesson's reagent, the milling action was carried out using Teflon, stainless steel, and agate milling jars and in the presence of 500 μL of freshly distilled THF as the liquid additive. After 60 min of milling, THF was removed under vacuum and the resulting residues were analyzed by powder X-ray diffraction (PXRD) (Fig. 2). Analysis showed that the material of the milling jars has indeed a distinct effect on the crystallinity of benzamide, revealing the presence of two component solid phases in the milled sample, one crystalline and the other amorphous. The diffraction patterns of these components differed from the diffraction pattern obtained from a commercial sample (Sigma-Aldrich®) of benzamide, as well as from a reference sample of benzamide that had been dissolved in THF solution (0.1 M) before concentration and recovery. The sample that showed the highest degree of crystallinity corresponded to the one processed in the Teflon milling jar, presenting 66.13% crystallinity and a crystallite size of 185.82 nm. By contrast, the sample recovered from the stainless steel milling jar presented 51.24% crystallinity and a crystallite size of 616.78 nm. Finally, the milled sample recovered from the agate milling jar presented 19.95% crystallinity and a crystallite size equal to 112.93 nm. The above observations confirm the damaging effect suffered by the solid benzamide substrate upon impact on the milling vessel walls.As it can be appreciated in Fig. 2, distinct phase transitions are induced by milling using the different milling materials. This means that the recovered solid was obtained in a modified state relative to the original one. In this regard, it is anticipated that the solid substrate recovered after milling will exhibit a certain degree of amorphicity since, for the mechanochemical reaction to take place, amorphicity gives rise to a greater available surface area on the solid substrate that enables the reaction.58,72
To verify whether the observed new polymorphic states of milled benzamide are a consequence of the choice of the milling vessel and milling ball material, we compared the diffractograms obtained from ex situ PXRD experiments after addition of Lawesson's reagent at different milling times (0, 30 and 60 min). The three experiments afforded different results depending on the material of the grinding jars (Fig. 3). The formation of thiobenzamide was observed after 30 min of milling, with a concomitant decrease in the amount of remaining starting benzamide. This finding is better appreciated in Fig. 3a, where the reaction in Teflon shows greater efficiency since starting benzamide is present in lower abundance relative to thiobenzamide. This difference in the benzamide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thiobenzamide ratio was greater in Teflon milling jars when compared with reactions carried out in stainless steel and agate vessels.
thiobenzamide ratio was greater in Teflon milling jars when compared with reactions carried out in stainless steel and agate vessels.
As anticipated, PXRD analysis at time zero of the three mechanochemical thionation reactions enabled using Lawesson's reagent and employing Teflon, stainless steel or agate milling jars showed only physical mixtures of the reactants. By contrast, a decrease in crystallite size of the benzamide substrate was observed after 30 min of milling, particularly in the case of Teflon vessels. Indeed, crystals that initially measured 185.82 nm decreased in size to 57–72 nm after 30 min of milling, which is in line with the experimentally observed higher efficiency of the reactions carried out with the Teflon milling material (cf. Table 1). When stainless steel and agate milling jars were used, the decrease in crystallite size was not uniform.
The above observations are in line with the anticipation that smaller crystals of the substrate should induce greater reactivity since a greater surface area is available for reactivity in the thionation reaction.58,72
Most relevantly, the PXRD analysis of the crystallinity and morphological characteristics of the solid-state components in the mechanochemical processes reported herein confirm Emmerling's finding that the stability of polymorphic species depends strongly on the material of the milling vessel.50,52
Aiming to expand the scope of the process, other carboxamides were converted into the corresponding thioamides under the optimized reaction conditions (Fig. 4). Thus, we initially carried out the reaction with benzamide-like amides, which upon reaction with Lawesson's reagent generated the desired thioamide products 2–5 with yields varying between 89 and 93% (Fig. 4).
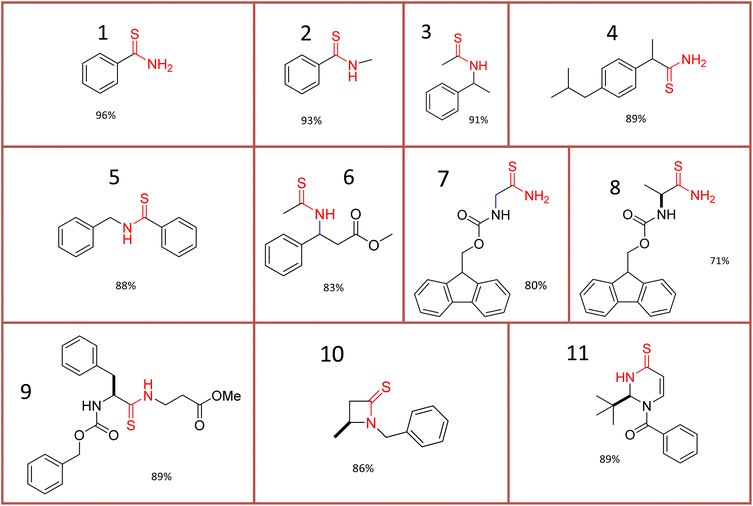 | ||
| Fig. 4 Thiocarbonyl-containing compounds obtained by treatment of the corresponding carbonyl-containing substrates with Lawesson's reagent under solvent-assisted mechanochemical activation. | ||
Discrimination between the carbonyl groups present in esters vis-a-vis amides was evidenced when thioamide formation took place exclusively in a good yield of 83% (see product 6 in Fig. 2). This result is in line with recorded precedent that amides are generally more reactive than esters.69 It should be noted that by comparison of the thionation reaction in refluxing THF solution and occupying 1 equiv. of Lawesson's reagent, the mechanochemical methodology can afford higher yields. So, for instance, N-benzyl-benzothioamide 5 was obtained in 88% yield in the present mechanochemical procedure (Fig. 4), whereas Huang and Xu achieved 74% yield under flow conditions in MeCN.73
Subsequently, amino amides derived from N-Fmoc protected (Fmoc = fluorenylmethoxycarbonyl) proteinogenic α-amino acids such as Gly, Ala, Val, Phe and Pro were used as substrates. The thionation reaction proceeded especially well with the less hindered Gly and Ala derivatives to deliver the anticipated products 7 and 8 (Fig. 4) with yields of 80% and 70%, respectively. On the other hand, thioamide derivatives 6 and 11, which are of interest in the area of enantioselective synthesis of β-amino acids,74,75 were obtained in 83% and 89% yield, respectively (Fig. 4).
In this context, the ability of mechanochemical ball milling to reduce or eliminate epimerization during peptide preparation and handling has been established.76,77 Gratifyingly, the present liquid-assisted mechanochemical thionation methodology proceeded efficiently to afford thiodipeptide 9 with a yield of 89% and without racemization. However, loss of stereochemical integrity (racemization) was observed with chiral N-Fmoc-protected alanine as the substrate (see 8 in Fig. 4).
Other types of substrates that were examined include one β-lactam and one enone. To our satisfaction, the target products 10 and 11 were isolated with high 86% and 89% yields, respectively (Fig. 4).
Finally, all starting materials and products were examined by PXRD. Significant differences in powder morphology (degree of crystallinity) were observed (see the ESI†).
Conclusions
Herein, an efficient method for the synthesis of thioamides by sulphidation of amide precursors with Lawesson's reagent under solvent-assisted mechanical activation is described. The mechanochemical technique was thoroughly optimized and a series of structurally diverse derivatives containing the thioamide moiety were subsequently synthesized in good yields. Ex situ PXRD monitoring revealed that the formation and interconversion of polymorphs during milling depends strongly on the choice of grinding materials – the softer milling jars and milling balls made out of Teflon induced the formation of amorphous phases at the interphase with the crystallite lattice of the benzamide substrate, which translates into higher reactivity.78,79 Importantly, the method proved efficient for the thionation of a model N-Cbz-protected peptide, which proceeded in good yield and preserved the stereochemical identity of the chiral substrate. On the other hand, the thionation of N-Fmoc-protected amino acid amides was problematic, as it provided the desired product in only moderate yields and with racemization of the center of chirality.Experimental
Most reagents were commercially available from Sigma-Aldrich Chemical Co. and the rest were prepared as described in the literature. The high-speed ball grinding reactions were carried out in a Retsch MM200 or MM400 mixing mill. The 1H and 13C NMR spectra were recorded on a 500 MHz Bruker spectrometer in deuterated chloroform (CDCl3) or deuterated dimethyl sulfoxide (DMSO-d6). NMR data are presented in the following order: chemical displacement in ppm, multiplicities as s (singlet), d (doublet), t (triplet), m (multiplet), etc. The NMR signals of carboxamides are in agreement with those reported in the literature.65,66,80,81 X-ray powder diffraction was carried out in Bragg–Brentano mode on a BRUKER D8-ADVANCE eco diffractometer equipped with a LynxEye detector (λCu-Kα1+2 = 1.541874 Å. Data were collected at room temperature in the range of 2θ = 5–45° (step of 0.02 and step time 0.5 s).Procedure of the solvent-assisted mechanochemical synthesis of benzothioamide (1)
In a Teflon milling jar (7.5 mL capacity) with a Teflon grinding ball (10 mm diameter and 1.7 g weight), carboxamide (1 equiv., 1.23 mmol) and Lawesson's reagent (LR, 0.5 equiv., 0.61 mmol) were placed before the addition of 500 μL of freshly distilled THF. The jar was closed and the reaction was milled for 60 minutes at 25 Hz frequency using the Retsch MM400 mixing mill. The desired thioamide product was purified by silica gel flash chromatography with hexane/EtOAc (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) as the eluent. Compound 1 was obtained as a yellow solid, mp 116.2–116.9 (lit.21 mp 114–116 °C) in 96% yield (161 mg). 1H NMR (500 MHz, DMSO-d6): δ 9.87 (br. s, 1H), 9.5 (br. s, 1H), 7.88–7.90 (m, 2H), 7.54–7.47 (m, 1H), 7.45–7.38 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 200.20, 139.49, 131.12, 127.91, 127.25. Calculated m/z for C7H7NS +1, 138.03, found 138.1.
20) as the eluent. Compound 1 was obtained as a yellow solid, mp 116.2–116.9 (lit.21 mp 114–116 °C) in 96% yield (161 mg). 1H NMR (500 MHz, DMSO-d6): δ 9.87 (br. s, 1H), 9.5 (br. s, 1H), 7.88–7.90 (m, 2H), 7.54–7.47 (m, 1H), 7.45–7.38 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 200.20, 139.49, 131.12, 127.91, 127.25. Calculated m/z for C7H7NS +1, 138.03, found 138.1.
Data availability
NMR spectra, HPLC chromatograms, and powder X-ray diffraction data are provided in the ESI† file.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are indebted to SEP-CINVESTAV and CONAHCYT, Mexico, for financial support via grant A1-S-44097. C. N.-C. is grateful to CONAHCYT, Mexico, for doctoral scholarship 770118. We thank Dr Luis D. Miranda, Instituto de Química, UNAM, for his support in obtaining several high-resolution mass spectra.References
- R. N. Hurd and G. Delamater, Chem. Rev., 1961, 61, 45–86 CrossRef CAS
.
- R. A. Begum, D. Powell and K. Bowman-James, Inorg. Chem., 2006, 45, 964–966 CrossRef CAS PubMed
.
- T. Kanbara, K. Okada, T. Yamamoto, H. Ogawa and T. Inoue, J. Organomet. Chem., 2004, 689, 1860–1864 CrossRef CAS
.
- A. Fumarola, A. Di Fiore, M. Dainelli, G. Grani and A. Calvanese, Exp. Clin. Endocrinol. Diabetes, 2010, 118, 678–684 CrossRef CAS PubMed
.
- F. Wang, R. Langley, G. Gulten, L. G. Dover, G. S. Besra, W. R. Jacobs and J. C. Sacchettini, J. Exp. Med., 2007, 204, 73–78 CrossRef CAS PubMed
.
- K. S. Gayen and N. Chatterjee, Asian J. Org. Chem., 2020, 9, 508–528 CrossRef CAS
.
- T. N. Hansen and C. A. Olsen, Chem.–Eur. J., 2023, 2023, e202303770 Search PubMed
.
- J. G. Hernández, V. García-López and E. Juaristi, Tetrahedron, 2012, 68, 92–97 CrossRef
.
- N. Kumagai and M. Shibasaki, Isr. J. Chem., 2012, 52, 604–612 CrossRef CAS
.
- N. Majumdar, A. Saito, L. Yin, N. Kumagai and M. Shibasaki, Org. Lett., 2015, 17, 3362–3365 CrossRef CAS PubMed
.
- A. Quintavalla, D. Carboni and M. Lombardo, Molecules, 2023, 28, 2234 CrossRef CAS PubMed
.
- S. Banala and R. D. Süssmuth, ChemBioChem, 2010, 11, 1335–1337 CrossRef CAS PubMed
.
- M. Carmack, J. Heterocycl. Chem., 1989, 26, 1319–1323 CrossRef CAS
.
- D. L. Priebbenow and C. Bolm, Chem. Soc. Rev., 2013, 42, 7870–7880 RSC
.
- Q. Zhang, L. Soulère and Y. Queneau, Molecules, 2023, 28, 3527 CrossRef CAS PubMed
.
- J. Chen, L. Mei, J. Liu, C. Zhong, B. Yuan and Q. Li, RSC Adv., 2019, 9, 28576–28580 RSC
.
- T. B. Nguyen, L. P. A. Nguyen and T. T. T. Nguyen, Adv. Synth. Catal., 2019, 361, 1787–1791 CrossRef CAS
.
- T. T. Nguyen, T. N. Le, P. E. Hansen and F. Duus, Tetrahedron Lett., 2006, 47, 8433–8435 CrossRef CAS
.
- T. J. Curphey, J. Org. Chem., 2002, 67, 6461–6473 CrossRef CAS PubMed
.
- A. Degl'Innocenti, A. Capperucci, A. Mordini, G. Reginato, A. Ricci and F. Cerreta, Tetrahedron Lett., 1993, 34, 873–876 CrossRef
.
- S. Ray, A. Bhaumik, A. Dutta, R. J. Butcher and C. Mukhopadhyay, Tetrahedron Lett., 2013, 54, 2164–2170 CrossRef CAS
.
- B. S. Pedersen, S. Scheibye, N. H. Nilsson and S.-O. Lawesson, Bull. Soc. Chim. Belg., 1978, 87, 223–228 CrossRef CAS
.
- T. Ozturk, E. Ertas and O. Mert, Chem. Rev., 2007, 107, 5210–5278 CrossRef CAS PubMed
.
- L. A. Kayukova, K. D. Praliyev, V. G. Gut'Yar and G. P. Baitursynova, Russ. J. Org. Chem., 2015, 51, 148–160 CrossRef CAS
.
- M. P. Cava and M. I. Levinson, Tetrahedron, 1985, 41, 5061–5087 CrossRef CAS
.
- H. Khatoon and E. Abdulmalek, Molecules, 2021, 26, 6937 CrossRef CAS PubMed
.
- M. Jesberger, T. P. Davis and L. Barner, Synthesis, 2003, 1929–1958 CrossRef CAS
.
- S. V. Giofrè, R. Mancuso, F. Araniti, R. Romeo, D. Iannazzo, M. R. Abenavoli and B. Gabriele, ChemPlusChem, 2019, 84, 942–950 CrossRef PubMed
.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed
.
- A. Pawełczyk and L. Zaprutko, Flavour Fragrance J., 2011, 26, 101–106 CrossRef
.
- R. S. Varma and D. Kumar, Org. Lett., 1999, 1, 697–700 CrossRef CAS PubMed
.
- N. P. T. Le, T. H. T. Nguyen, T. K. Nguyen, P. K. B. Tran and P. H. Tran, J. Chem. Technol. Biotechnol., 2023, 98, 2823–2829 CrossRef CAS
.
- T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed
.
- F. Cuccu, L. De Luca, F. Delogu, E. Colacino, N. Solin, R. Mocci and A. Porcheddu, ChemSusChem, 2022, 15, e202200362 CrossRef CAS PubMed
.
- K. J. Ardila-Fierro and J. G. Hernández, ChemSusChem, 2021, 14, 2145–2162 CrossRef CAS PubMed
.
- O. Bento, F. Luttringer, T. M. El Dine, N. Pétry, X. Bantreil and F. Lamaty, Eur. J. Org Chem., 2022, 2022, e202101516 CrossRef CAS
.
- K. Kubota and H. Ito, Trends Chem., 2020, 2, 1066 CrossRef CAS
.
- Q. Cao, D. E. Crawford, C. Shi and S. L. James, Angew. Chem., Int. Ed., 2020, 59, 4478–4483 CrossRef CAS PubMed
.
- J. Arciszewski and K. Auclair, ChemSusChem, 2022, 15, e202102084 CrossRef CAS PubMed
.
- M. Wohlgemuth, M. Mayer, M. Rappen, F. Schmidt, R. Saure, S. Grätz and L. Borchardt, Angew. Chem., Int. Ed., 2022, 61, e202212694 CrossRef CAS PubMed
.
- M. Pérez-Venegas and E. Juaristi, ACS Sustainable Chem. Eng., 2020, 8, 8881–8893 CrossRef
.
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC
.
- M. Rautenberg, B. Bhattacharya, J. Witt, M. Jain and F. Emmerling, CrystEngComm, 2022, 24, 6747–6750 RSC
.
- I. d'Anciaes, A. Silva, E. Bartalucci, C. Bolm and T. Wiegand, Adv. Mater., 2023, 2304092 Search PubMed
.
- E. Juaristi and C. G. Avila-Ortiz, Synthesis, 2023, 55, 2439–2459 CrossRef CAS
.
- D. Margetić, Pure Appl. Chem., 2023, 95, 315–328 CrossRef
.
- I. D′Anciães Almeida Silva, E. Bartalucci, C. Bolm and T. Wiegand, Adv. Mater., 2023, 35, 202304092 Search PubMed
.
- C. C. Sahu, S. Biswas, R. Hommelsheim and C. Bolm, RSC Mechanochem., 2024, 1, 38–42 RSC
.
- M. D. Goodwin, M. Q. Costa, J. R. Robinson and C. M. Kotyk, Results Chem., 2022, 4, 100528 CrossRef CAS
.
- H. Kulla, C. Becker, A. A. L. Michalchuk, K. Linberg, B. Paulus and F. Emmerling, Cryst. Growth Des., 2019, 19, 7271–7279 CrossRef CAS
.
- L. S. Germann, M. Arhangelskis, M. Etter, R. E. Dinnebier and T. Friščić, Chem. Sci., 2020, 11, 10092–10100 RSC
.
- K. Linberg, F. Emmerling and A. A. L. Michalchuk, Cryst. Growth Des., 2023, 23, 19–23 CrossRef CAS
.
- L. Stipe, L. S. Germann, T. Friščić and I. Halasz, Acc. Chem. Res., 2022, 55, 1262–1277 CrossRef PubMed
.
- K. J. Ardila-Fierro, M. Rubčić and J. G. Hernández, Chem.–Eur. J., 2022, 28, e202200737 CrossRef CAS PubMed
.
- E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719–7738 RSC
.
- A. A. L. Michalchuk, K. S. Hope, S. R. Kennedy, M. V. Blanco, E. V. Boldyreva and C. R. Pulham, Chem. Commun., 2018, 54, 4033–4036 RSC
.
- Y. Zheng, J. Jiang, M. Jin, D. Miura, F. X. Lu, K. Kubota, T. Nakajima, S. Maeda, H. Ito and J. P. Gong, J. Am. Chem. Soc., 2023, 145, 7376–7389 CrossRef CAS PubMed
.
- C. Bolm and J. G. Hernández, Angew. Chem., Int. Ed., 2019, 58, 3285–3299 CrossRef CAS PubMed
.
- P. A. Julien and T. Friščić, Cryst. Growth Des., 2022, 22, 5726–5754 CrossRef CAS
.
- K. J. Ardila-Fierro and J. G. Hernández, Angew. Chem., Int. Ed., 2024, 63, e202317638 CrossRef CAS PubMed
.
- A. Cortés-Lobo and J. G. Hernández, ChemPlusChem, 2024, e202400257 CrossRef PubMed
.
- H. Kulla, S. Greiser, S. Benemann, K. Rademann and F. Emmerling, Cryst. Growth Des., 2017, 17, 1190–1196 CrossRef CAS
.
- J. L. Howard, M. C. Brand and D. L. Browne, Angew. Chem., Int. Ed., 2018, 57, 16104–16108 CrossRef CAS PubMed
.
- O. V. Lapshin, E. V. Boldyreva and V. V. Boldyrev, Russ. J. Inorg. Chem., 2021, 66, 433–453 CrossRef CAS
.
- P. B. W. Ten Kortenaar, B. G. Van Dijk, J. M. Peeters, B. J. Raaben, P. J. H. M. Adams and G. I. Tesser, Int. J. Pept. Protein Res., 1986, 27, 398–400 CrossRef CAS
.
- T. Ezawa, Y. Kawashima, T. Noguchi, S. Jung and N. Imai, Tetrahedron: Asymmetry, 2017, 28, 1690–1699 CrossRef CAS
.
- H. S. Lalithamba, K. Uma, T. S. Gowthami and G. Nagendra, Org. Prep. Proced. Int., 2020, 52, 181–191 CrossRef CAS
.
- S. Wang, X. Zhao, D. Zhang-Negrerie and Y. Du, Org. Chem. Front., 2019, 6, 347–351 RSC
.
-
T. Murai, Chemistry of Thioamides, Springer, New York, NY, 2019 Search PubMed
.
- T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, CrystEngComm, 2009, 11, 418–426 RSC
.
- P. Ying, J. Yu and W. Su, Adv. Synth. Catal., 2021, 363, 1246–1271 CrossRef CAS
.
- F. Cao, L. Wang, R. Zheng, L. Guo, Y. Chen and X. Qian, RSC Adv., 2022, 12, 31564–31576 RSC
.
- C. Huang and H. C. Xu, Sci. China: Chem., 2019, 62, 1501–1503 CrossRef CAS
.
-
E. Juaristi, 1-Benzoyl-2(S)-tert-butyl-3-methyl-perhydro-pyrimidin-4-one, in Handbook of Reagents for Organic Synthesis. Chiral Reagents for Asymmetric Synthesis, ed. L. A. Paquette, Wiley, Chichester, 2003, p. 53–56 Search PubMed
.
- M. Pérez-Venegas, G. Reyes-Rangel, A. Neri, J. Escalante and E. Juaristi, Beilstein J. Org. Chem., 2017, 13, 1728–1734 CrossRef PubMed
.
- J. G. Hernández and E. Juaristi, J. Org. Chem., 2010, 75, 7107–7111 CrossRef PubMed
.
- T. M. El-Dine, M. Lavayssiere, H. Adihou, G. Subra, T.-X. Métro, O. Ludemann-Hombourger and F. Lamaty, Chem.–Eur. J., 2014, 1, e202400007 Search PubMed
.
- G. D. Semchenko, I. N. Opryshko, L. A. Angolenko, K. P. Vernigora and V. V. Kalin, Refract. Ind. Ceram., 2004, 45, 34–38 Search PubMed
.
- A. A. L. Michalchuk, E. V. Boldyreva, A. M. Belenguer, F. Emmerling and V. V. Boldyrev, Front. Chem., 2021, 9, 685789 CrossRef CAS PubMed
.
- L. Legnani, L. Toma, P. Caramella, M. A. Chiacchio, S. Giofrè, I. Delso, T. Tejero and P. Merino, J. Org. Chem., 2016, 81, 7733–7740 CrossRef CAS PubMed
.
- H. S. Lalithamba, K. Uma, T. S. Gowthami and G. Nagendra, Org. Prep. Proced. Int., 2020, 52, 181–191 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mr00013g |
| This journal is © The Royal Society of Chemistry 2024 |