Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cyanation of aryl halides using potassium hexacyanoferrate(II) via direct mechanocatalysis†
Suhmi
Hwang
,
Phil M.
Preuß
,
Wilm
Pickhardt
,
Sven
Grätz
 and
Lars
Borchardt
and
Lars
Borchardt
 *
*
Inorganic Chemistry I, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany. E-mail: lars.borchardt@rub.de
First published on 27th August 2024
Abstract
A cyanation reaction was performed inside a ball mill system utilizing catalytically active milling balls, while avoiding the use of solvents and ligands. Additionally, replacing the highly toxic cyanide source with potassium hexacyanoferrate(II) leads to a safer reaction environment. Yields of up to 90% were achieved in as little as 4 hours at room temperature. The oxidative addition and transmetalation step could be observed via X-ray photoelectron spectroscopy (XPS) and powder X-ray diffraction (PXRD) analysis, respectively, giving a first indication of the mechanism of this mechanochemical reaction.
Introduction
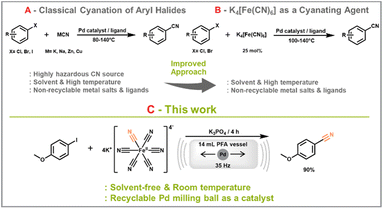
Cyano-containing compounds are useful building blocks that are applied in a wide range of syntheses, for example, pharmaceuticals, agrochemicals, and dyes.1,2 Due to its dominant electron-withdrawing properties, the cyano group can be used as a versatile functional group to modify the reactivity of molecules. Despite its great importance in organic synthesis, the classic cyanide reactions have the critical drawback of inevitably using highly toxic cyanide sources. For example, the Sandmeyer reaction, which is a typical method for the cyanation of aryl groups, involves the use of copper(I) cyanide.3Several publications have since emerged aiming to replace the hazardous cyanide agent with the significantly safer alternative potassium hexacyanoferrate(II) (Scheme 1) that is used as a food additive.4 After pioneering work by Beller's group in 2004, Frech research group employed a well-known cross-coupling Pd catalyst, dichloro[bis{1-(dicyclohexylphosphanyl)piperidine}]palladium, to convert versatile aryl bromides into aromatic nitriles.5,6 Nakao and Hiyama's research group introduced a carbocyanation reaction that yields a diverse range of acrylonitriles by introducing acetonitrile as the cyanide source.7 In another approach Cabanetos and co-workers employed a mild μ-wave synthesis approach to modify dibromo benzothioxanthene imide derivatives, resulting in the formation of two regioisomeric nitrile products.8
Beyond the success achieved in developing less harmful pathways for cyanation reactions, our group found that there remains room for further improvement. For instance, the use of solvents that contribute to the main waste stream and the incorporation of catalytic metal salts along with their supporting ligands, which are neither cost-effective nor recyclable, present notable challenges. Mechanochemical ball milling holds great promise as an approach to tackle these challenges primarily due to the solvent-free system, or significantly reduced solvent uses in certain instances.9 In 2018, the Bolm group successfully utilized the redox mediator properties of K3[Fe(CN)6] and K4[Fe(CN)6]·3H2O via a Strecker-type reaction inside a ball mill, generating α-aminonitriles based on different milling conditions in a solvent-free environment.10 Recently, a cyanation reaction using potassium hexacyanoferrate(II) was reported using an electromagnetic mill.11 This approach allowed successful conversion of 4-bromoacetophenone under liquid assisted grinding (LAG) conditions as well as gram scale reactions.11 Despite sustainable aspects in those previous findings, the above reports still involve the use of salt catalysts or ligands such as Pd(OAc)2 or Ni(cod)2, and xantphos.
In ongoing efforts to maximize the utility of the ball mill system, our group is actively exploring the utilization of catalytically active milling equipment (e.g., Pd vessel or Pd balls).12,13 This effort allows us to conveniently and efficiently recycle the catalyst as well as avoiding the need for expensive ligands. This concept is referred to as “direct mechanocatalysis (DM)”.14 In this study, we apply this concept to the advancement of a sustainable and efficient synthetic route for the cyanation reaction.
Results and discussion
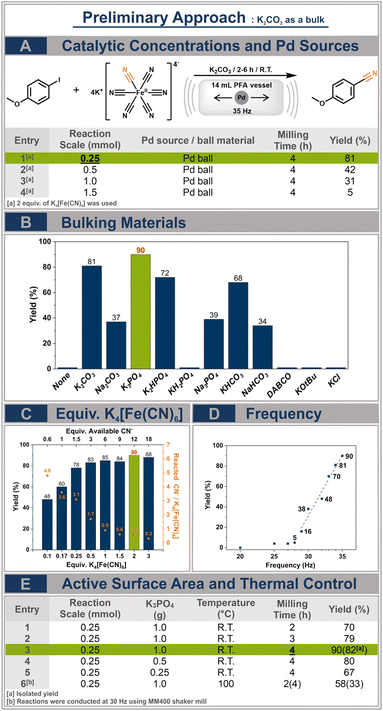
We selected 4-iodoanisole as a coupling partner of potassium hexacyanoferrate(II) due to its low toxicity and electron-donating properties, making it a suitable model reactant. Potassium carbonate was initially chosen as a bulking material because it has been frequently used as a base in previously reported approaches for cyanation reactions using potassium hexacyanoferrate(II).15 K2CO3 (1 g), 4-iodoanisole (0.5 mmol), potassium hexacyanoferrate(II) (1 mmol), and a Pd ball were placed inside a 14 mL perfluoroalkoxy alkane (PFA) vessel. After 4 h of milling at 35 Hz in an MM500 vario mixer mill (Retsch GmbH), the entire reaction mixture was subjected to liquid–liquid extraction using water and dichloromethane (DCM). The yield was analysed via1H nuclear magnetic resonance (NMR) spectroscopy using dibromomethane as an internal standard. The quantitative 1H NMR analysis showed a yield of 40%, confirming the feasibility of the cyanation reaction with potassium hexacyanoferrate(II) under solvent-free ball milling conditions.Building upon these promising findings, we conducted in-depth investigations into the chemical reaction parameters to optimize the yield. We initiated our investigations with reaction scale control experiments (Fig. 1A). Given the nature of DM, where reactions occur on the surface of the catalytically active milling ball,16 this parameter not only influences the catalyst concentration but also the energy dissipation and transfer within the reaction medium.17 Within the range of 0.25 mmol to 1.5 mmol, 0.25 mmol was determined to be the most effective condition, resulting in a yield of 59%, while the yield decreased with increasing reaction scale, consistent with the lower apparent catalyst concentration.
Subsequently, the effect of bulking material was investigated (Fig. 1B). In the absence of the bulking material, no reaction took place; however, using 0.25 g of bulking material resulted in 67% yield, which suggests that absence of basicity during the reaction attributed to no product being formed rather than the amount of bulking material used. Among the choice of bulking materials, K3PO4 provided moderately higher yield compared to the initial approach of using K2CO3. A general trend was observed, wherein potassium-based inorganic bases exhibited better yields compared to sodium-based salts, consistent with the HSAB (hard and soft acids and bases) theory. Simultaneously, the level of basicity also appeared to play an important role in this system. Potassium phosphate mono-, di-, and tri-basic compounds followed the pattern of higher basicity leading to better yields. Conversely, KOtBu and KCl demonstrated that excessively high or absent basicity did not contribute to the formation of the product. Overall, it was deduced that potassium-containing bases with pKa values greater than 7 are required to achieve good yields.
The next investigation was focused on determining the optimal number of equivalents of potassium hexacyanoferrate(II) (Fig. 1C). Using the right amount of potassium hexacyanoferrate(II) is particularly significant in preventing the formation of catalytically inactive Pd(II)-cyano complexes, as observed in previously reported classical solvent-based approaches.5 Interestingly, the yield already reached nearly 80% upon using only 0.25 equiv. of potassium hexacyanoferrate(II). In contrast to the solution-based approach, cyanation in ball milling could achieve a high degree of utilization of the CN-ligands from potassium hexacyanoferrate(II) without the need for elevated temperatures. Despite the decrease in atom economy with the introduction of a larger number of equivalents of potassium salt, we chose to further investigate with 2 equiv. as we prioritized yield at this point.
After investigating the chemical parameters, we turned our attention to the technical parameters to further optimize the protocol (Fig. 1D and E). Initially, we conducted a small screening to establish the required reaction time (Fig. 1E, entries 1–3). While 2 hours resulted in a 70% yield of the product, 4 hours of milling time was necessary to achieve a satisfactory yield of 90%.
Furthermore, we investigated reactions at various milling frequencies, as they are dominant factors in providing mechanical energy input and a higher number of cycles to refresh the surface of the Pd ball, thereby accelerating the reaction rate (Fig. 1D). These results showed that 29 Hz is the apparent threshold milling frequency for this reaction, as only negligible yields were observed below that point. Subsequently, the yield increases linearly with the milling frequency until it reaches 90% at 35 Hz. This linear increase correlates nicely with the number of impacts between the wall and the ball, assuming optimal movement of the milling ball.
Reactions with decreased amounts of bulk material (Fig. 1E, entries 3–5) were conducted to further investigate the impact of mechanical energy. A higher amount of bulk material leads to a more extensive distribution of the mechanical energy applied to reactants, while a lower amount of bulk material is expected to provide higher energy input to reactants during the given milling time. However, as the amount of bulk material is reduced, the yield also decreases. Combined with the results of reaction scale control experiments (Fig. 1A, entries 1–4), it can be deduced that factors possibly hindering the reaction are not related to the introduction of mechanical energy. This is likely due to the limited catalytic surface area that the Pd ball provides during milling.
Subsequently, elevated temperatures were applied to the system, as thermal energy is necessary to activate the stable potassium hexacyanoferrate(II) to release cyanide, in the case of solvent-based systems. We utilized a custom-made heating jacket on the MM400 mixer mill (Retsch GmbH) to conduct thermal control milling experiments at a frequency of 30 Hz (Fig. 1E, entry 6). To investigate the necessity and efficiency of introducing heat compared to reactions at room temperature, 2 h and 4 h of milling time were applied. While the yield is slightly superior to that in room temperature experiments after 2 h at 30 Hz (58% vs. 38% for 4 h at R.T.), the yield starts decreasing with prolonged milling time, hinting at thermal decomposition or side reactions occurring under these conditions. Contrary to the conventional solvent-based approach, rather mild chemical and mechanical parameters are sufficient to activate potassium hexacyanoferrate(II), resulting in excellent yields, and applying excess thermal energy, along with the associated extra energy consumption, is not necessary. In addition to these discoveries, we conducted a screening of several LAG (liquid assisted grinding) additives; however, introducing solvent into this system proved to be ineffective (Table S1†).
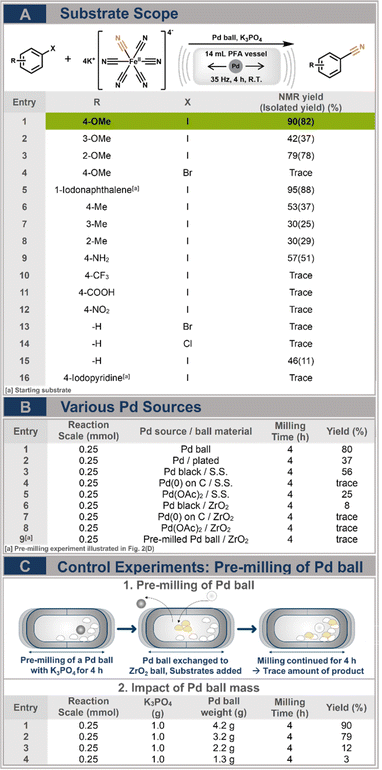
We further studied the reactivities of diverse aryl halides under the optimized conditions (Fig. 2A). Overall, this cyanation system was found to be more applicable to substrates with less perturbing properties to the aromatic system of aryl halides. Electron-donating functional groups at ortho positions and the 1-naphthalene moiety showed moderate to excellent yields (entries 1–4 and 5–8). Interestingly, the substrates containing electron withdrawing groups (entries 10 and 11) did not lead to the formation of the product, despite their known ability to enhance the electrophilicity of aryl halides. Additionally, only iodides yielded a product under our conditions, while bromides and chlorides (entries 12–14) did not undergo any reaction. In case of entry 15, the isolated yield of benzonitrile significantly decreased compared to the NMR yield due to its volatile nature.
Additionally, we examined the role of the catalyst in the reaction. To do so, we conducted several control experiments to further investigate the direct mechanocatalytic performance of the Pd ball. Firstly, two types of Pd balls were employed (Fig. 2B, entries 1 and 2): a pure Pd ball and galvanostatically coated Pd ball.18 Despite the cost-saving advantages of using a cheaper inert stainless-steel ball as the coating body for the Pd layer, the coating's durability did not endure throughout the milling process, resulting in a yield of only 37% (Fig. S1†). Abrasion was also observed for the pure Pd ball (3–5 mg per reaction, Fig. S2†), necessitating an assessment of the reactivity of potential Pd particles in the reaction (Fig. 2C).16 As a reference experiment, the Pd ball was pre-milled with 1 g of K3PO4 for 4 hours to induce abrasion similar to a standard reaction. Subsequently, reactants were added for further milling with an inert 10 mm ZrO2 ball (approximately 3.3 g), which replaced the Pd ball to provide mechanical energy to the reaction. Four hours of continued milling in the presence of abraded Pd powder did not result in the desired product, indicating that abraded Pd particles did not catalyse the reaction. To rule out differences in weight between the Pd and ZrO2 balls as a potential source of these differences, we utilized Pd balls of varying mass and surface area (Fig. 2C, entries 1–4). Utilizing a 3.2 g Pd ball led to a 79% yield, comparable to the standard condition (4.2 g), while masses of 2.2 g and 1.3 g showed negligible yields. Moreover, the inclusion of common Pd catalysts (Fig. 2B, entries 3–8) in a ball milling system with inert milling balls failed to produce significant yields. Only Pd black in combination with a stainless-steel ball exhibited a moderate yield of 56%.
Furthermore, the reaction mixtures, including those in pre-milling experiments, were analyzed via XPS measurements. When the Pd ball was milled with potassium phosphate to induce abrasion, this mixture only contained Pd(0) (335.6 eV) (Fig. S3†), which was inactive in our reference experiments. On the other hand, milling the Pd ball in the presence of iodoanisole and potassium phosphate led to the formation of a Pd(II) species (338 and 338.9 eV), confirming the formation of the oxidative addition product (Fig. S4†), but also indicating the leaching of Pd from the ball into the reaction mixture. This was also confirmed for the reaction mixture of the optimized protocol (Fig. 1E, entry 3), which contained both Pd(0) (355.6 eV) and Pd(II) species (338 and 338.9 eV) (Fig. 3A), indicating that abraded Pd(0) powder could be converted into active Pd(II) during the reaction, which is also supported by the experiments with Pd black (Fig. S5†).
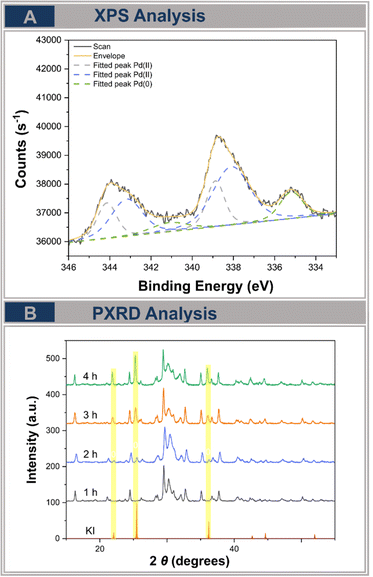 | ||
| Fig. 3 (a) XPS analysis of the reaction mixture under the optimized reaction conditions (Fig. 1(E), entry 3). (b) Time dependent PXRD analysis of the optimized reaction conditions (Fig. 1(E), entry 3). | ||
To study the formation of inactive Pd(II) species, we added two different quantities of potassium hexacyanoferrate(II) to a mixture containing the aryl-Pd(II) (Fig. S6†). In both cases of 1 equiv. and 2 equiv., two different Pd(II) species were found and no signal of Pd(0) was detected. However, using 1 equiv. of potassium hexacyanoferrate(II) resulted in a 72% yield, while 2 equiv. of potassium ferrocyanide yielded no product. This indicates the possibility of excess hexacyanoferrate(II) consuming the reactive Pd(II) in the mixture to form an inactive Pd(II) species such as Pd(CN)2. From these results, we can deduce that the abraded Pd(0) species becomes active when milled with iodoanisole in the absence of a CN source, promoting the formation of the oxidative addition product. On the other hand, in the presence of excess potassium ferrocyanide, the inactive Pd(CN)2 complex is formed, resulting in the quenching of the catalytic cycle.
After investigating the oxidative addition, PXRD analysis was conducted to elucidate the transmetallation step of the cyanation reaction (Fig. 3B). We observed the formation of potassium iodide starting after about 2 hours of milling, with the intensity of the reflections increasing with extended reaction time. This observation supports the ligand exchange between the arylpalladium intermediate and potassium hexacyanoferrate(II).
Conclusions
In conclusion, we present a mechanochemical ball mill system that offers a promising and sustainable method for cyanation reactions, eliminating the need for solvents and toxic cyanide sources while providing high yields of up to 90% in 4 hours. By employing potassium hexacyanoferrate(II) as a safer alternative and utilizing a catalytically active Pd ball, this approach not only enhances the environmental friendliness of the process but also ensures a safer reaction environment. We studied the influence of several chemical and process parameters on the reaction and established that with as little as 0.25 equiv. of potassium hexacyanoferrate(II), yields of up to 80% are possible. While high temperatures are usually employed in solution, the mechanochemical reaction proceeds at room temperature. Furthermore, we studied several other catalysts, but none could exceed the performance of the utilized Pd balls. However, compared to conventional synthetic approaches, this system exhibited a more limited range of substrates capable of achieving moderate to high yields. Analyzing the reaction mixture with XPS confirmed the occurrence of the oxidative addition step. Additionally, the transmetalation step could be observed by PXRD, further contributing to the mechanistic understanding of the mechanochemical reaction.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Suhmi Hwang: preparation of the manuscript, mechanochemical reactions and sample purifications, XPS sample preparation, 1H-NMR sample preparation and analysis, GC-MS measurements, PXRD sample preparation. Phil Marvin Preuß: mechanochemical reactions and sample purifications, 1H-NMR sample preparation and analysis, GC-MS measurements. Wilm Pickhardt: project supervision, design of reference experiments. Sven Grätz: preparation of the manuscript, project coordination and supervision. Lars Borchardt: preparation of the manuscript, project coordination and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the support of the European Research Council (ERC) and Deutscher Akademischer Austauschdienst (DAAD). Furthermore, we thank Miriam Sander for PXRD measurements of reaction mixtures and Maximilian Wohlgemuth for the XPS measurements and analysis of milling mixtures as well as the surface of milling balls. Lastly, we acknowledge Stefanie Hutsch for recording the SEM image of the coated milling balls.References
- M. Sundermeier, A. Zapf and M. Beller, Angew. Chem., Int. Ed., 2003, 42, 1661–1664 CrossRef CAS PubMed.
- U. S. Kanchana, T. V. Mathew and G. Anilkumar, J. Organomet. Chem., 2020, 920, 121337 CrossRef CAS.
- T. Sandmeyer and B. Dtsch, Chem. Ges., 1884, 17, 2650–2653 CrossRef.
- M. Younes, P. Aggett, F. Aguilar, R. Crebelli, B. Dusemund, M. Filipič, M. J. Frutos, P. Galtier, D. Gott, U. Gundert-Remy, G. G. Kuhnle, C. Lambré, J.-C. Leblanc, I. T. Lillegaard, P. Moldeus, A. Mortensen, A. Oskarsson, I. Stankovic, I. Waalkens-Berendsen, M. Wright, A. Di Domenico, H. van Loveren, A. Giarola, Z. Horvath, F. Lodi and R. A. Woutersen, EFSA J., 2018, 16, e05374 Search PubMed.
- T. Schareina, A. Zapf and M. Beller, J. Organomet. Chem., 2004, 689, 4576–4583 CrossRef CAS.
- R. Gerber, M. Oberholzer and C. M. Frech, Chem. Eur. J., 2012, 18, 2978–2986 CrossRef CAS PubMed.
- A. Yada, T. Yukawa, H. Idei, Y. Nakao and T. Hiyama, Bull. Chem. Soc. Jpn., 2010, 83, 619–634 CrossRef CAS.
- P. Simón Marqués, J. M. Andrés Castán, L. A. Galan, M. Allain, O. Maury, T. Le Bahers, P. Blanchard and C. Cabanetos, J. Org. Chem., 2021, 86, 5901–5907 CrossRef PubMed.
- T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed.
- C. Bolm, R. Mocci, C. Schumacher, M. Turberg, F. Puccetti and J. G. Hernández, Angew. Chem., Int. Ed., 2018, 57, 2423–2426 CrossRef CAS PubMed.
- Y. Hou, H. Wang, J. Xi, R. Jiang, L. Zhang, X. Li, F. Sun, Q. Liu, Z. Zhao and H. Liu, Green Chem., 2023, 25, 2279–2286 RSC.
- C. G. Vogt, M. Oltermann, W. Pickhardt, S. Grätz and L. Borchardt, Adv. Energy Sustainability Res., 2021, 2, 2100011 CrossRef CAS.
- W. Pickhardt, E. Siegfried, S. Fabig, M. F. Rappen, M. Etter, M. Wohlgemuth, S. Grätz and L. Borchardt, Angew. Chem., Int. Ed., 2023, 62, e202301490 CrossRef CAS PubMed.
- W. Pickhardt, S. Grätz and L. Borchardt, Chem. Eur. J., 2020, 26, 12903–12911 CrossRef CAS PubMed.
- J. Zhang, X. Chen, T. Hu, Y. Zhang, K. Xu, Y. Yu and J. Huang, Cat. Lett., 2010, 139, 56–60 CrossRef CAS.
- W. Pickhardt, C. Beaković, M. Mayer, M. Wohlgemuth, F. J. L. Kraus, M. Etter, S. Grätz and L. Borchardt, Angew. Chem., Int. Ed., 2022, 61, e202205003 CrossRef CAS PubMed.
- S. Hwang, S. Grätz and L. Borchardt, A guide to direct mechanocatalysis, Chem. Commun., 2022, 58, 1661–1671 RSC.
- M. Wohlgemuth, M. Mayer, M. Rappen, F. Schmidt, R. Saure, S. Grätz and L. Borchardt, Angew. Chem., Int. Ed., 2022, 61, e202212694 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mr00054d |
| This journal is © The Royal Society of Chemistry 2024 |