Open Access Article
Open Access ArticleDirect arylation of alkyl fluorides using in situ mechanochemically generated calcium-based heavy Grignard reagents†
Pan
Gao
a,
Julong
Jiang
b,
Yamato
Fukuzawa
a,
Satoshi
Maeda
 bc,
Koji
Kubota
bc,
Koji
Kubota
 *ac and
Hajime
Ito
*ac and
Hajime
Ito
 *ac
*ac
aDivision of Applied Chemistry, Faculty of Engineering, Hokkaido University, Sapporo, Hokkaido, Japan. E-mail: kbt@eng.hokudai.ac.jp; hajito@eng.hokudai.ac.jp
bDepartment of Chemistry, Faculty of Science, Hokkaido University, Sapporo, Hokkaido, Japan
cInstitute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Sapporo, Hokkaido, Japan
First published on 13th August 2024
Abstract
Here, we report the reaction of calcium-based heavy Grignard reagents, which are easily generated by a mechanochemical method, with unactivated alkyl fluorides in the absence of transition metal catalysts to produce the corresponding arylated products in moderate to good yields. This is the first example of the nucleophilic substitution of an inert C(sp3)–F bond by an organocalcium species. Preliminary mechanistic studies based on theoretical calculations indicate that tetrameric aryl calcium species facilitate the unprecedented C(sp3)–F bond arylation.
Organocalcium reagents have demonstrated remarkable synthetic potential as carbon nucleophiles because they frequently exhibit distinct reactivity owing to the large electronegativity difference between calcium and carbon.1–5 However, in contrast to the extensive research on traditional organometallic carbon nucleophiles, such as Grignard reagents, studies on synthetic applications involving calcium-based nucleophiles are scarce.6 This is due to a lack of operationally simple protocols for obtaining these nucleophiles from commercially available starting materials, which may impede comprehensive investigations into the reactivity profile of calcium-based carbon nucleophiles; this in turn can result in basic reactions being overlooked.1–5
We recently developed a simple mechanochemical protocol using ball milling to generate calcium-based heavy Grignard reagents (R-CaX)7–13 from commercially available, unactivated calcium metal.14 Conventionally, such direct synthesis necessitates the production of activated calcium metal (Rieke calcium) under harsh conditions using alkali metals.10–12 However, in our mechanochemical approach, the strong mechanical impact during ball milling may provide sufficient energy to overcome the high atomization energy of calcium metal, enabling the direct generation of calcium-based nucleophiles from commercial calcium metal without the need for laborious pre-activation processes.14,15 Furthermore, all synthetic operations can be carried out under ambient conditions. We discovered a new reactivity of aryl calcium nucleophiles using this operationally simple protocol to rapidly generate organocalcium nucleophiles; they reacted smoothly with alkyl iodides or bromides, yielding the corresponding arylated products in good-to-high yields.14
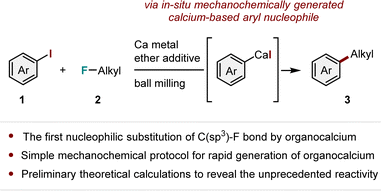
In this study, we discovered that direct arylation of alkyl fluorides using in situ mechanochemically generated aryl calcium nucleophiles is highly efficient even in the absence of transition metal catalysts (Scheme 1).16,17 This is the first example of the nucleophilic substitution of an inert C(sp3)–F bond by an organocalcium species.18,19 Although direct C(sp3)–F bond arylation is also feasible using Grignard reagents, as discovered by Matsubara et al.,16a the present study sheds light on the previously overlooked reactivity of calcium-based heavy Grignard reagents. The developed organocalcium-mediated arylation exhibited a significantly shorter reaction time (1 h) than that under Matsubara's conditions using Grignard reagents (>12 h),16a which is likely owing to the higher nucleophilicity of the organocalcium species. Density functional theory (DFT) calculations were used to propose the mechanism of the C(sp3)–F bond arylation. This study demonstrates the effectiveness of a simple mechanochemical protocol to explore the novel reactivity of organocalcium species more easily.
 | ||
| Scheme 1 Arylation of alkyl fluorides using mechanochemically generated calcium-based heavy Grignard reagents. | ||
All the reactions were conducted in a Retsch MM400 mill (stainless-steel milling jar; 30 Hz; stainless-steel balls). Based on our previous study,14 the reaction between iodobenzene (1a, 3.0 equiv.) and 1-fluorododecane (2a, 1.0 equiv.) was performed in the presence of commercially available calcium metal (2.0 equiv.) and tetrahydropyran (THP) (4.0 equiv.) in a 1.5 mL stainless steel jar with two stainless steel balls (diameter: 7 mm) in air. Following 60 min of ball milling (30 Hz) using a heat gun as a temperature controller20 to maintain an internal temperature of 80 °C (for details, refer to the ESI†), the C(sp3)–F bond arylation product 3aa was successfully obtained in good yield (86%, Table 1, entry 1). When the reaction was performed at room temperature without the use of a heat gun, the yield of 3aa decreased significantly (28%; Table 1, entry 2). Ether additives are thought to coordinate with the calcium(II) center to form thermodynamically stable species,5 facilitating the formation of aryl calcium nucleophiles under mechanochemical conditions.14 The reaction did not proceed without the addition of THP (<5%; Table 1, entry 3). The use of tetrahydrofuran (THF) resulted in a moderate yield (51%; Table 1, entry 4). The reaction was completely suppressed when dimethoxyethane (DME) was used (<5%; Table 1, entry 5). The reaction with two equivalents of THP gave a slightly lower yield of 3aa (71%, Table 1, entry 6). Lowering the vibration frequency of ball milling resulted in a significant decrease in yield, indicating that mechanical impact played an important role in this transformation (62%; Table 1, entry 7). Lower yields were obtained when the amounts of 1a and calcium metal were reduced (61 and 41%, respectively; Table 1, entries 8 and 9). When the reaction was performed in a 10 mL stainless-steel jar with two stainless-steel balls (diameter: 10 mm), a moderate yield of 3aa was obtained (66%; Table 1, entry 10). Given the possibility of cross-electrophile coupling catalyzed by transition metals derived from stainless-steel milling jars and/or balls,17 we performed the reaction in a commercially available 10 mL zirconium oxide jar with two zirconium oxide balls (diameter: 10 mm), yielding the desired arylation product 3aa (43%; Table 1, entry 11). This result suggests that the C(sp3)–F bond arylation process is likely to proceed only by organocalcium species without catalysis of transition metal contamination.17 We believe that the lower mechanical impact of zirconium oxide balls, which are much lighter than stainless-steel balls, is a primary factor contributing to the observed decrease in yield. The mechanical activation of calcium metal is crucial for the formation of organocalcium compounds, and the reduced impact with zirconium oxide balls likely hampers this activation process. While Matsubara et al. reported that phenyl Grignard reagent reacted with alkyl fluorides in THF solution,16a the use of magnesium instead of calcium did not result in the formation of arylation product 3aa under mechanochemical conditions (<5%; Table 1, entry 12).21 To verify the role of the ball-milling technique, we performed the reaction in THP solution at 80 °C (Table 1, entry 13). We discovered that 3aa was not detected, and almost no conversion of 1a was observed (3%), suggesting that the mechanical impact of ball milling is required for the generation of the aryl calcium nucleophile, which subsequently reacts with alkyl fluoride 2a. Because it is difficult to generate the corresponding calcium nucleophiles, using bromo- or chlorobenzene instead of 1a resulted in no product formation (Table 1, entries 14 and 15).
| Entry | Deviation from “standard conditions” | Yield (%) of 3aab |
|---|---|---|
| a Conditions: 1a (1.5 mmol), 2a (0.5 mmol), Ca (0.75 mmol), and THP (2.0 mmol) in a stainless-steel ball-milling jar (1.5 mL) with two stainless-steel balls (diameter: 7 mm). b Yields determined by 1H NMR spectroscopy using dibromo methane as an internal standard. The isolated yields are shown in parentheses. | ||
| 1 | None | 86 (74) |
| 2 | Room temperature | 28 |
| 3 | Without THP | <5 |
| 4 | THF instead of THP | 51 |
| 5 | DME instead of THP | <5 |
| 6 | 2.0 equiv. of THP | 71 |
| 7 | Frequency: 15 Hz | 62 |
| 8 | 2.0 equiv. of 1a | 61 |
| 9 | 1.0 equiv. of Ca metal | 41 |
| 10 | 10 mL jar with a 10 mm ball × 2 (stainless-steel) | 66 |
| 11 | 10 mL jar with 10 mm ball × 2 (zirconium oxide) | 43 |
| 12 | Mg metal instead of Ca metal | <5 |
| 13 | In THP solution (0.2 M) using a test tube | <5 |
| 14 | Bromobenzene instead of iodobenzene (1a) | <5 |
| 15 | Chlorobenzene instead of iodobenzene (1a) | <5 |
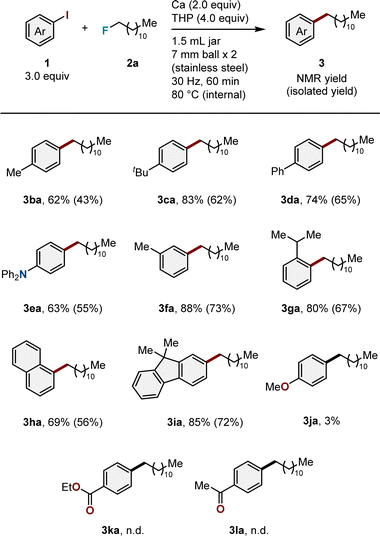
Using the obtained optimal conditions, the substrate scope was investigated. We first examined aryl iodides bearing different substituents (Table 2). The introduction of substituents such as methyl (1b), tert-butyl (1c), phenyl (1d), or diphenylamino (1e) groups at the para-position of the phenyl ring exhibited no effect on reactivity, resulting in the desired arylation products (3ba–3ea) in moderate to good yields (62–83%). Substrates with a methyl group at the meta position (1f) also reacted under mechanochemical conditions to produce the target product (3fa) in high yield (88%). The reaction with a substrate containing a sterically demanding substituent, such as the 2-isopropyl group (1g), proceeded smoothly, producing the corresponding product (3ga) in high yield (80%). We also discovered that arylation using polycyclic aryl iodides (1h and 1i) was feasible and produced the desired products (3ha and 3ia) in high yields (69 and 85%, respectively). However, the reactions failed when functional groups, including methoxy (1j), ester (1k), or carbonyl (1l) groups, were present.
| a Conditions: 1 (1.5 mmol), 2a (0.5 mmol), Ca (0.75 mmol), THP (2.0 mmol), in a stainless-steel ball-milling jar (1.5 mL) with two stainless-steel balls (diameter: 7 mm). b Yields determined by 1H NMR spectroscopy using dibromo methane as an internal standard. The isolated yields are shown in parentheses. |
|---|
|
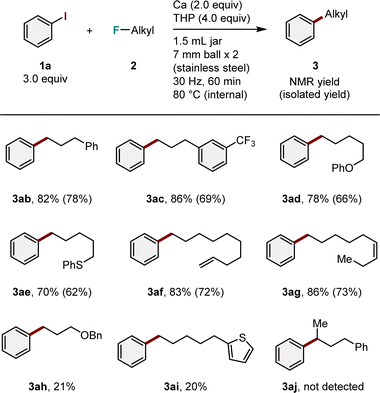
We then investigated the substrate scope in terms of alkyl fluorides (Table 3). Alkyl fluoride with a phenyl group 2b reacted smoothly to produce the desired product 3ab in good yield (82%). The presence of a trifluoromethyl group on the benzene ring (2c) had no effect on the reaction, and only the C–F bond of the monofluoromethyl group located at the end of the alkyl chain was activated to produce the corresponding arylation product 3ac in high yield (86%). Under the optimized conditions, functional groups, including phenoxy (2d), phenyl thioether (2e), and alkenes (2f and 2g), were tolerated, yielding the target products (3ad–3ag) in moderate to good yields (70–86%). However, it is important to note the current limitations of this method, including the difficulty of coupling with alkyl fluorides containing a benzyloxy group (2h) or a thiophene moiety (2i), resulting in low yields of the desired products (3ah and 3ai in 21 and 20% yields, respectively). Secondary alkyl fluoride 2j was used; however, the corresponding arylation product was not detected.
| a Conditions: 1a (1.5 mmol), 2 (0.5 mmol), Ca (0.75 mmol), and THP (2.0 mmol) in a stainless-steel ball-milling jar (1.5 mL) with two stainless-steel balls (diameter: 7 mm). b Yields determined by 1H NMR spectroscopy using dibromomethane as an internal standard. The isolated yields are shown in parentheses. |
|---|
|
Control experiments were performed to obtain mechanistic insights (Scheme 2). When iodonaphthalene (1h) was subjected to standard conditions in the absence of alkyl fluorides, protonation product 4 was obtained in 50% yield after quenching with 1.0 M aq. HCl, indicating that aryl calcium species formed following 1h under mechanochemical conditions (Scheme 2a).14 In contrast, the reaction using alkyl fluoride 2a in the absence of 1h yielded no protonation product 5, and 2a converted at a low rate (5% conversion, Scheme 2a). On the basis of protonation studies, we propose that C(sp3)–F bond arylation occurs most likely through the selective formation of aryl calcium nucleophiles via direct insertion of calcium metal into the C(sp2)–I bond, followed by nucleophilic substitution of alkyl fluorides.14 To further support our hypothesis, we prepared an aryl calcium reagent using pre-synthesized reagent, Rieke calcium, in solution and subjected it to nucleophilic substitution (Scheme 2b).10–12 We discovered that the prepared aryl calcium reagent reacted with 1-fluorododecane (2a) to produce the desired product 3ha (21%, Scheme 2b), supporting our hypothesis.
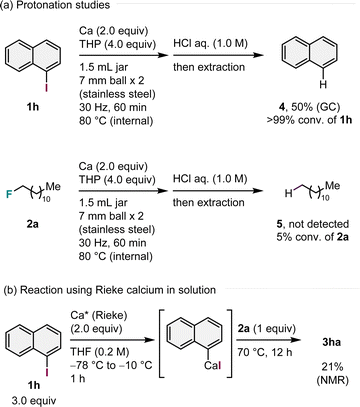 | ||
| Scheme 2 Control experiments.a aDetails of the reaction conditions are provided in the ESI.† | ||
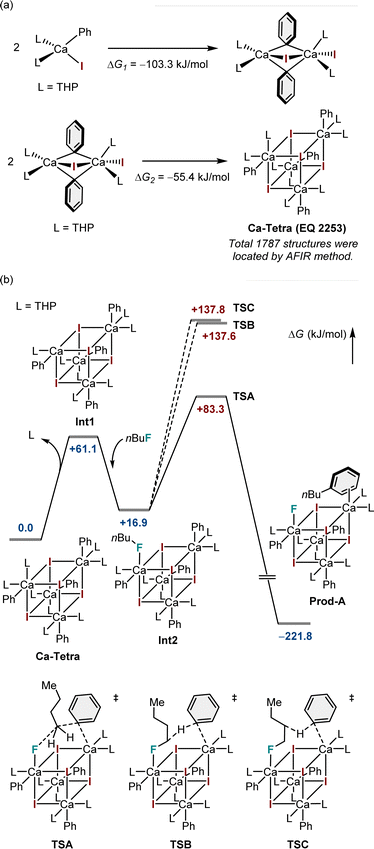
Furthermore, to gain insight into the mechanism of nucleophilic substitution, DFT calculations were performed on the model substrates, iodobenzene (1a) and n-butyl fluoride. Preliminary calculations indicated that the tetracoordinate mononuclear calcium complex was highly unstable owing to the presence of vacant coordination sites on the calcium atom. Meanwhile, the artificial force-induced reaction (AFIR)-based sampling calculation22 using the tetrameric model yielded 1787 possible structures, the most stable of which exhibits a highly symmetric cubic structure (Fig. 1a).23 The coordination vacancies of each calcium atom in this cubic structure are fulfilled, resulting in a coordination number of six. Furthermore, DFT calculations demonstrated that the tetramer was more stable than the dimer (Fig. 1a). Therefore, the tetramer with the composition formula (PhCaI)(THP)2 is proposed as the most likely structure in the reaction system.
After confirming the possible structure of the aryl calcium nucleophile, we focused on elucidating the detailed mechanism of defluoroarylation. Fig. 1b depicts three possible reaction pathways, beginning with the tetrameric species Ca-Tetra. To generate a vacant site for alkyl fluoride coordination, one of the THP ligands must be left in the first instance. This step increases free energy by 61.1 kJ mol−1. The alkyl fluoride substrate binds to the five-coordinate calcium atom through its fluorine atom, lowering the free energy by 44.2 kJ mol−1. Following the formation of Int2, three possible reaction pathways emerge: arylation (TSA), alpha-H abstraction reaction (TSB), and beta-H abstraction reaction (TSC). The arylation reaction has the lowest barrier among the three possible pathways, with an overall free energy barrier of +83.3 kJ mol−1. The calculated results are consistent with our experimental findings. In this transition state, the initial binding of the fluorine atom to the calcium atom significantly weakens the C(sp3)–F bond, as calcium(II) is regarded as a moderately strong Lewis acid for the fluorine atom.24 At the transition state, the phenyl anion from another calcium center directly attacks the alpha-carbon of the alkyl fluoride, while the previously mentioned calcium atom accepts the fluorine atom as a leaving group. The combination of these two effects causes the chemically inert C(sp3)–F bond to be cleaved, resulting in the formation of a new C(sp3)–C(sp2) bond. The ESI† incorporates the visualized geometries of the intermediate and transition states shown in Fig. 1.
In conclusion, we discovered a new reaction of calcium-based heavy Grignard reagents, which were generated in situ via a simple mechanochemical method, with unactivated alkyl fluorides in the absence of transition-metal catalysts, resulting in moderate to high yields of the corresponding C(sp3)–F bond arylation products. Notably, this is the first reported instance of an organocalcium species engaging in nucleophilic substitution of an inert C(sp3)–F bond. To elucidate the unprecedented reactivity of organocalcium nucleophiles in activating inert C(sp3)–F bonds, preliminary theoretical calculations were performed. The findings of this study validate that our operationally simple mechanochemical protocol for rapidly generating organocalcium nucleophiles using commercial, inactivated calcium metal provides a platform for discovering new reactions that can be performed using only calcium-based carbon nucleophiles.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Japan Society for the Promotion of Science (JSPS) via KAKENHI grants 22H00318, 22K18333, 24H00453, 24H01832 and 24H01050, by the JST via CREST grant JPMJCR19R1, by FOREST grant JPMJFR201I, and by the Institute for Chemical Reaction Design and Discovery (ICReDD) established by the World Premier International Re-search Initiative (WPI), MEXT, Japan. We thank Mr Mahiro Takahashi for his help in cross-checking experiments.Notes and references
- For selected reviews on organocalcium chemistry, see (a) T. P. Hanusa, Chem. Rev., 1993, 93, 1023 CrossRef CAS; (b) M. Westerhausen, Angew. Chem., Int. Ed., 2001, 40, 2975 CrossRef CAS; (c) J. S. Alexander, Eur. J. Inorg. Chem., 2002, 2002, 2761 CrossRef.
- Selected examples and reviews of the reactions using organocalcium compounds as catalysts: (a) S. Harder, Chem. Rev., 2010, 110, 3852 CrossRef CAS PubMed; (b) S. Kobayashi and Y. Yamashita, Acc. Chem. Res., 2011, 44, 58 CrossRef CAS PubMed; (c) M. S. Hill, D. J. Liptrota and C. Weetman, Chem. Soc. Rev., 2016, 45, 972 RSC; (d) M. Magre, S. Marcin and M. Rueping, Chem. Rev., 2022, 122, 8261 CrossRef CAS PubMed; (e) L. Zhao, P. Deng, X. Gong, X. Kang and J. Cheng, ACS Catal., 2022, 12, 7877 CrossRef CAS.
- Selected examples of reactions using organocalcium compounds as carbon nucleophiles: (a) A. S. Wilson, M. S. Hill, M. F. Mahon, C. Dinoi and L. Maron, Science, 2017, 358, 1168 CrossRef CAS PubMed; (b) B. M. Wolf, C. Stuhl, C. Maichle-Mössmer and R. Anwander, J. Am. Chem. Soc., 2018, 140, 2373 CrossRef CAS PubMed; (c) K. G. Pearce, C. Dinoi, M. S. Hill, M. F. Mahon, L. Maron, R. S. Schwamm and A. S. S. Wilson, Angew. Chem., Int. Ed., 2022, 61, e202200305 CrossRef CAS PubMed.
- Selected examples of organocalcium-mediated polymerization are as follows: (a) S. Harder, F. Feil and A. Weeber, Organometallics, 2001, 20, 1044 CrossRef CAS; (b) S. Harder and F. Feil, Organometallics, 2002, 21, 2268 CrossRef CAS; (c) M. Westerhausen, S. Schneiderbauer, A. N. Kneifel, Y. Söltl, P. Mayer, H. Nöth, Z. Zhong, P. J. Dijkstra and J. Feijen, Eur. J. Inorg. Chem., 2003, 2003, 3432 CrossRef; (d) Y. Li, H. Deng, W. Brittain and M. S. Chisholm, Polym. Bull., 1999, 42, 635 CrossRef CAS; (e) M.-W. Hsiao and C.-C. Lin, Dalton Trans., 2013, 42, 2041 RSC.
- Selected reviews of calcium-based heavy Grignard reagents: (a) M. Westerhausen, M. Gärtner, R. Fischer, J. Langer, L. Yu and M. Reiher, Chem.–Eur. J., 2007, 13, 6292 CrossRef CAS PubMed; (b) M. Westerhausen, M. Gärtner, R. Fischer and J. Langer, Angew. Chem., Int. Ed., 2007, 46, 1950 CrossRef CAS PubMed; (c) M. Westerhausen, Coord. Chem. Rev., 2008, 252, 1516 CrossRef CAS; (d) M. Westerhausen, A. Koch, H. Görls and S. Krieck, Chem.–Eur. J., 2017, 23, 1456 CrossRef CAS PubMed; (e) A. Koch, Q. Dufrois, M. Wirgenings, H. Görls, S. Krieck, M. Etienne, G. Pohnert and M. Westerhausen, Chem.–Eur. J., 2018, 24, 16840 CrossRef CAS PubMed; (f) S. Harder and J. Langer, Nat. Rev. Chem., 2023, 7, 843 CrossRef PubMed; (g) P. Schüler, S. Sengupta, S. Krieck and M. Westerhausen, Chem.–Eur. J., 2023, 29, e202300833 CrossRef PubMed.
- (a) Organometallics in Synthesis: A Manual, ed. M. Schlosser, Wiley, Chester, 2nd edn, 2013 Search PubMed; (b) T. Banno, Y. Hayakawa and M. Umeno, J. Organomet. Chem., 2002, 653, 288 CrossRef CAS; (c) Handbook of Grignard Reagents, ed. G. S. Silverman and P. E. Rakita, Marcel Dekker, 1996 Search PubMed; (d) The Chemistry of Organozinc Compounds, ed. Z. Rappoport and I. Marek, John Wiley & Sons, Chichester, U.K., 2006 Search PubMed; (e) U. Wietelmann and J. Klett, Z. Anorg. Allg. Chem., 2018, 644, 194 CrossRef CAS PubMed.
- (a) M. Gärtner, H. Görls and M. Westerhausen, Organometallics, 2007, 26, 1077 CrossRef; (b) M. Gärtner, H. Görls and M. Westerhausen, J. Organomet. Chem., 2008, 693, 221 CrossRef; (c) M. Westerhausen, Angew. Chem., Int. Ed., 2009, 48, 5741 CrossRef PubMed; (d) J. Langer, M. Köhler, H. Görls and M. Westerhausen, Chem.–Eur. J., 2014, 20, 3154 CrossRef CAS PubMed.
- (a) R. Fischer, M. Gärtner, H. Görls, L. Yu, M. Reiher and M. Westerhausen, Angew. Chem., Int. Ed., 2007, 46, 1618 CrossRef CAS PubMed; (b) P. Schüler, S. Sengupta, A. Koch, H. Görls, S. Krieck and M. Westerhausen, Chem.–Eur. J., 2022, 28, e202201897 CrossRef PubMed; (c) P. Schüler, S. Sengupta, S. Krieck and M. Westerhausen, Chem.–Eur. J., 2023, 29, e20230083 CrossRef PubMed.
- A. Koch, M. Wirgenings, S. Krieck, H. Görls, G. Pohnert and M. Westerhausen, Organometallics, 2017, 36, 3981 CrossRef CAS.
- (a) T.-C. Wu, H. Xiong and R. D. Rieke, J. Org. Chem., 1990, 55, 5045 CrossRef CAS; (b) K. Mochida and H. Ogawa, J. Organomet. Chem., 1983, 243, 131 CrossRef CAS; (c) R. D. Rieke, T.-C. Wu and L. I. Rieke, Org. Synth., 1995, 72, 147 CrossRef CAS; (d) D. Bryce-Smith and A. C. Skinner, J. Chem. Soc., 1963, 577 RSC; (e) M. L. Hays and T. P. Hanusa, Tetrahedron Lett., 1995, 36, 2435 CrossRef CAS.
- J. Langer, M. Köhler, H. Görls and M. Westerhausen, J. Organomet. Chem., 2014, 751, 563 CrossRef CAS.
- (a) R. Fischer, M. Gärtner, H. Görls and M. Westerhausen, Organometallics, 2006, 25, 3496 CrossRef CAS; (b) M. Gärtner, H. Görls and M. Westerhausen, Synthesis, 2007, 5, 725 Search PubMed; (c) M. Westerhausen, M. Gärtner, R. Fischer and J. Langer, Angew. Chem., Int. Ed., 2007, 46, 1950 CrossRef CAS PubMed; (d) N. Kawabata, A. Matsumura and S. Yamashita, Tetrahedron, 1973, 29, 1069 CrossRef CAS.
- (a) M. Köhler, J. Langer, H. Görls and M. Westerhausen, Organometallics, 2014, 33, 6381 CrossRef; (b) M. Gärtner, H. Görls and M. Westerhausen, J. Organomet. Chem., 2008, 693, 221 CrossRef.
- P. Gao, J. Jiang, S. Maeda, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2022, 61, e202207118 CrossRef CAS PubMed.
- For a tutorial review on the mechanical activation of zero-valent metals, A. C. Jones, J. A. Leitch, S. E. Raby-Buck and D. L. Browne, Nat. Synth., 2022, 1, 763 CrossRef.
- Selected examples of transition-metal-free nucleophilic substitution of alkyl fluorides using organometallic reagents: (a) K. Matsubara, T. Ishibashi and Y. Koga, Org. Lett., 2009, 11, 1765 CrossRef CAS PubMed; (b) J. Terao, S. A. Begum, Y. Shinohara, M. Tomita, Y. Naitoh and N. Kambe, Chem. Commun., 2007, 855 RSC; (c) L. Kane, B. C. Figula, K. Balaraman, J. A. Bertke and C. Wolf, Nat. Commun., 2024, 15, 1866 CrossRef PubMed.
- Selected examples and reviews of transition metal catalysed-cross-coupling reactions between alkyl fluorides and organometallic reagents: (a) J. Terao, A. Ikumi, H. Kuniyasu and N. Kambe, J. Am. Chem. Soc., 2003, 125, 5646 CrossRef CAS PubMed; (b) J. Terao, H. Todo, H. Watanabe, A. Ikumi and N. Kambe, Angew. Chem., Int. Ed., 2004, 43, 6180 CrossRef CAS PubMed; (c) J. Terao, H. Watabe and N. Kambe, J. Am. Chem. Soc., 2005, 127, 3656 CrossRef CAS PubMed; (d) J. Terao and N. Kambe, Acc. Chem. Res., 2008, 41, 11545 CrossRef PubMed; (e) Z. Mo, Q. Zhang and L. Deng, Organometallics, 2012, 31, 6518 CrossRef CAS; (f) L. W. Erickson, E. L. Lucas, E. J. Tollefson and E. R. Jarvo, J. Am. Chem. Soc., 2016, 138, 14006 CrossRef CAS PubMed; (g) T. Iwasaki, K. Yamashita, H. Kuniyasu and N. Kambe, Org. Lett., 2017, 19, 3691 CrossRef CAS PubMed; (h) T. Hatakeyama, S. Ito, M. Nakamura and E. Nakamura, J. Am. Chem. Soc., 2005, 127, 14192 CrossRef CAS PubMed.
- For selected reviews on C–F bond activation in organic synthesis, see: (a) H. Amii and K. Uneyama, Chem. Rev., 2009, 109, 2119 CrossRef CAS PubMed; (b) J.-D. Hamel and J.-F. Paquin, Chem. Commun., 2018, 54, 10224 RSC; (c) T. Stahl, H. F. T. Klare and M. Oestreich, ACS Catal., 2013, 3, 1578 CrossRef CAS; (d) G. Coates, F. Rekhroukh and M. R. Crimmin, Synlett, 2019, 30, 2233 CrossRef CAS; (e) T. Ahrens, J. Kohlmann, M. Ahrens and T. Braun, Chem. Rev., 2015, 115, 931 CrossRef CAS PubMed; (f) Q. Shen, Y.-G. Huang, C. Liu, J.-C. Xiao, Q.-Y. Chen and Y. Guo, J. Fluorine Chem., 2015, 179, 14 CrossRef CAS.
- H. Li, X.-Y. Wang, B. Wei, L. Xu, W.-X. Zhang, J. Pei and Z. Xi, Nat. Commun., 2014, 5, 4508 CrossRef CAS PubMed.
- T. Seo, N. Toyoshima, K. Kubota and H. Ito, J. Am. Chem. Soc., 2021, 143, 6165 CrossRef CAS PubMed.
- R. Takahashi, A. Hu, P. Gao, Y. Gao, Y. Pang, T. Seo, S. Maeda, J. Jiang, H. Takaya, K. Kubota and H. Ito, Nat. Commun., 2021, 12, 6691 CrossRef CAS PubMed.
- (a) S. Maeda, K. Ohno and K. Morokuma, Phys. Chem. Chem. Phys., 2013, 15, 3683 RSC; (b) S. Maeda and Y. Harabuchi, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2021, 11, e1538 CAS.
- For an example of the formation of organocalcium-based cubic structure, see: V. Knapp and G. Müller, Angew. Chem., Int. Ed., 2001, 40, 183 CrossRef CAS PubMed.
- For a review on calcium Lewis acid catalysis, see: J.-M. Begouin and M. Niggemann, Chem.–Eur. J., 2013, 19, 8030 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mr00067f |
| This journal is © The Royal Society of Chemistry 2024 |