 Open Access Article
Open Access ArticleEffects of mechanical properties of carbon-based nanocomposites on scaffolds for tissue engineering applications: a comprehensive review
Reza
Eivazzadeh-Keihan
*a,
Zahra
Sadat
a,
Farnaz
Lalebeigi
a,
Nooshin
Naderi
a,
Leila
Panahi
a,
Fatemeh
Ganjali
 a,
Sakineh
Mahdian
a,
Zahra
Saadatidizaji
a,
Mohammad
Mahdavi
a,
Sakineh
Mahdian
a,
Zahra
Saadatidizaji
a,
Mohammad
Mahdavi
 b,
Elham
Chidar
a,
Erfan
Soleimani
a,
Azadeh
Ghaee
b,
Elham
Chidar
a,
Erfan
Soleimani
a,
Azadeh
Ghaee
 c,
Ali
Maleki
c,
Ali
Maleki
 *a and
Iman
Zare
*a and
Iman
Zare
 *d
*d
aDepartment of Chemistry, Catalysts and Organic Synthesis Research Laboratory, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: reza_eivazzadeh@alumni.iust.ac.ir; maleki@iust.ac.ir
bEndocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
cDepartment of Life Science Engineering, Faculty of New Sciences and Technologies, University of Tehran, P.O. Box 14395-1561, Tehran, Iran
dResearch and Development Department, Sina Medical Biochemistry Technologies Co. Ltd, Shiraz 7178795844, Iran. E-mail: imanzare146@gmail.com
First published on 22nd December 2023
Abstract
Mechanical properties, such as elasticity modulus, tensile strength, elongation, hardness, density, creep, toughness, brittleness, durability, stiffness, creep rupture, corrosion and wear, a low coefficient of thermal expansion, and fatigue limit, are some of the most important features of a biomaterial in tissue engineering applications. Furthermore, the scaffolds used in tissue engineering must exhibit mechanical and biological behaviour close to the target tissue. Thus, a variety of materials has been studied for enhancing the mechanical performance of composites. Carbon-based nanostructures, such as graphene oxide (GO), reduced graphene oxide (rGO), carbon nanotubes (CNTs), fibrous carbon nanostructures, and nanodiamonds (NDs), have shown great potential for this purpose. This is owing to their biocompatibility, high chemical and physical stability, ease of functionalization, and numerous surface functional groups with the capability to form covalent bonds and electrostatic interactions with other components in the composite, thus significantly enhancing their mechanical properties. Considering the outstanding capabilities of carbon nanostructures in enhancing the mechanical properties of biocomposites and increasing their applicability in tissue engineering and the lack of comprehensive studies on their biosafety and role in increasing the mechanical behaviour of scaffolds, a comprehensive review on carbon nanostructures is provided in this study.
1. Introduction
Various types of composites have different features, including physical, chemical, mechanical, thermal, electrical, magnetic, acoustic, and optical. Among them, one of the most important features is their mechanical properties. For example, materials used in tissue engineering must exhibit similar mechanical behaviour to the target tissue.1 The three general factors that determine the attributes of composite materials are as follows: (1) the constituent materials; (2) the geometric shapes of their constituent components, especially the reinforcing phase, together with the resulting structure of the materials; and (3) the interaction among the phases.2Mechanical effects are physical properties that a material exhibits upon the application of a force. Examples of mechanical properties are elasticity modulus, tensile strength, elongation, hardness, density, creep, creep rupture, corrosion and wear, a low coefficient of thermal expansion (CTE), and fatigue limit. The mechanical properties of composite materials usually depend on their structure.3 Composites are fabricated using various synthetic and natural materials, such as polymers,4 hydrogels,5 and nanomaterials (e.g., nanocarbons).6 Therefore, these properties usually depend on the shape of the inhomogeneities, the volume fraction occupied by the inhomogeneities, and the interfaces between the components.3 In the new era of research, carbon nanostructures are one of the most important components for the preparation of composites, which is owing to their amazing mechanical characteristics.7
There are many different types of carbon nanostructures, such as graphene, graphene oxide (GO), reduced graphene oxide (rGO), carbon nanotubes (CNTs), carbon dots (CDs), carbon quantum dots (CQDs), nanodiamonds (NDs), carbon black, fullerenes, and carbon fibers (CFs).8,9 These unique structures have been extensively studied in different biomedical applications, such as hyperthermia,10 gene and drug delivery,11 cancer therapy,12 wound healing,13,14 and tissue engineering.15,16 The mechanical attributes of carbon nanostructures can originate from their physical properties or interactions with the other components in the composite.17–19 These mechanical features usually affect the performance of the composite or its development (applications as absorbers, aerospace, marine, automotive, medical, construction infrastructure, and sports goods).20 For example, the combination of GO with other components makes it a strong adsorbent for the absorption and desorption of n-hexane vapor, which is due to both to the enhanced specific surface area and micropore volume of the composite.21 In the aerospace industry, CNTs have been used as a mechanical performance modifier due to their high specific surface area, mechanical strength, and inherent hardness.22
One of the most important applications of carbon structures is to the improve mechanical properties of materials in tissue engineering. In tissue engineering, despite the significant success achieved to date, tissue engineers face challenges in repairing or replacing tissues that principally have biomechanical functions. In tissue engineering, implanted cells, scaffolds, DNA, proteins, and/or protein fragments are used to repair, replace, and restore diseased or damaged tissue.23,24 In this case, the ideal scaffold should not only have the same mechanical traits as the host tissue, but should also be able to degrade properly over time, preferably with a controlled rate of resorption, which ultimately creates a suitable space for new tissue growth.25 Carbon nanomaterials have a large specific surface area, which makes them suitable for biomedical applications such as tissue engineering. Also, they show high drug loading and tissue loading capacities, together with good biocompatibility and long duration of action.26
Abundant evidence has shown that mechanical stress, together with other physical factors, may remarkably augment the biosynthetic activity of cells in biosynthetic matrices.27,28 Although strength and work at failure both define the mechanical reliability of biomaterials, the elastic modulus is remarkably related to cellular material interactions. For example, the increase in Young's modulus can be calculated from the linear part of the stress–strain curve, which was used to analyze the stiffness of a cryogel after it was blended with CNTs in a compression test for skeletal card tissue engineering. This illustrated that the hardness of the cryogel significantly indicated the in vivo similarity of its mechanical properties at a hardness of 12 kPa.29 Therefore, in general, it can be concluded that carbon structures increase the porosity, decrease the diameter, and accordingly cause increase the stability, Young's modulus, tensile strength, tensile modulus, tensile strength, compressive strength, compressive modulus, etc.30,31 These properties of nanocarbons increase the resistance of scaffolds until complete regeneration of the tissue, and also create a space similar to the host tissue for the differentiation of the desired tissue.32,33
2. Biosafety of carbon nanomaterials: crucial for biomedical applications
The biosafety of nanocarbons is an important consideration for their biomedical applications. Although nanocarbons offer promising benefits, it is crucial to evaluate their potential risks and ensure appropriate safety measures are in place. Several aspects need to be considered regarding the biosafety of nanocarbons. For example, the potential toxicity of nanocarbons is a crucial aspect to address for their safe use in biomedical applications.34 Their toxicity can vary depending on several factors, including the specific type of nanocarbon (e.g., carbon nanotubes and graphene), dimensions (e.g., length and diameter), surface characteristics (e.g., functionalization and charge), and the biological system that they interact with.35Some studies have indicated that certain forms of nanocarbon, particularly long and rigid carbon nanotubes, may induce inflammation or cellular damage under certain conditions.36 However, it is important to note that extensive research has also demonstrated the biocompatibility and low toxicity of many nanocarbons when used at appropriate concentrations and properly functionalized.37,38 Thorough toxicity assessments, including in vitro and in vivo studies, are necessary to understand the potential risks associated with specific nanocarbons and guide their safe application. Further, understanding the biodistribution and clearance pathways of nanocarbons is crucial for assessing their biosafety. Nanocarbons can be exposed to various routes in the body, such as systemic circulation, lymphatic drainage, and direct administration to specific sites. Thus, if nanocarbons persist in the body for extended periods or accumulate in specific organs, they can cause issues. Research has shown that the size, surface functionalization, and administration route can influence the biodistribution and clearance of nanocarbons.39
Nanocarbons, such as fullerenes, carbon nanotubes, graphene, and nanodiamonds, exhibit a remarkable array of attributes that distinguish them from conventional materials and make them highly attractive for a wide range of biomedical applications. One of the most intriguing aspects of nanocarbon materials is their unique structure and composition, which contribute to their exceptional virtues. For example, fullerenes are spherical carbon molecules with a hollow cage-like structure, while carbon nanotubes are cylindrical carbon structures. Graphene consists of a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice, while nanodiamonds are diamond particles with nanoscale dimensions.40
These distinctive structures result in a plethora of desirable attributes, including high surface area, excellent mechanical strength, exceptional thermal and electrical conductivity, and outstanding optical properties, making nanocarbon materials powerful tools in various biomedical applications. For instance, their high surface area and unique electronic properties make them excellent candidates for sensing applications.41
The biological activity of carbon nanostructures highly depends on their type or functionalization. For instance, it was demonstrated that hydrophobic graphene shows a significantly higher cell proliferation rate than nanocrystalline diamonds.42 Functionalized fullerenes have been reported to act as antioxidants in a variety of important biological applications. For example, polyhydroxylated fullerene, C60(OH), could prevent neuronal cell damage and death from radicals.43 In a study by Zhang et al., the observed that graphene at concentrations above 10 μg mL−1 shows a time- and concentration-dependent decrease in metabolic activity.44 In addition, the effect of GO on human red blood cells has been studied. It was indicated that individual and small sheets of GO show greater hemolytic activity compared to its aggregated sheets. Interestingly, by improving the dispersion of GO with the aid of chitosan, its hemolytic activity was minimized and almost eliminated.45 The cytotoxicity of CNTs limits their biomedical applications. However, their incorporation in hydrogel matrices, different functionalization, and size and dose alterations can severely alter their biocompatibility and solubility.46 Functionalizing the hydrophobic surface of the CNTs, usually with carboxylic acid groups, improves their hydrophilicity and biocompatibility and endows them the ability to achieve targeted delivery.47 In a study to determine the importance of the functionalization of CNTs on their cytotoxicity, phenyl-SO3H and phenyl-SO3 Na-functionalized SWCNTs exhibited no crucial mutilation on human dermal fibroblast cells even at high concentrations (>2 mg mL−1), while SWCNTs functionalized with phenyl-(COOH)2 manifested toxicity at low concentrations (80 μg mL−1), which indicates the importance of functionalization on the toxicity of these materials. Also, by increasing the degree of sidewall functionalization, the SWCNT samples showed less cytotoxicity.48 More examples of the different functionalization effects on carbon-based nanomaterials are provided throughout this manuscript.
Nanocarbon-based biosensors can detect specific molecules or biomarkers with high sensitivity, enabling the early diagnosis and monitoring of diseases. Additionally, their ability to interact with light at different wavelengths allows their use in advanced imaging techniques, such as fluorescence imaging and Raman spectroscopy, enabling the enhanced visualization of cells, tissues, and organs.49 Nanocarbons also show great promise in the field of drug delivery. Their large surface area and unique chemical properties enable efficient loading and controlled release of therapeutic molecules, including drugs and genes. By functionalizing the surface of nanocarbons with targeting ligands, these nanomaterials can be specifically delivered to diseased cells or tissues, enhancing the treatment efficacy, while minimizing side effects. For instance, nanodiamonds have been extensively used in reinforcing biopolymers, resulting in the formation of biocomposites that can be applied in bone tissue engineering, chemotherapeutic drug delivery, and wound healing.50 Besides, nanocarbon materials have shown great potential as photothermal agents for cancer therapy. Due to their exceptional light absorption and heat conversion activities, nanocarbons can be selectively heated upon exposure to near-infrared light. This localized heating effect can be harnessed for the targeted destruction of cancer cells, while minimizing damage to healthy tissues.51
In a study performed by Schrand and colleagues, it was reported that the biosafety of carbon-based nanomaterials is highly dependent on their type and concentration. In this study, different concentrations of NDs, CNTs, and carbon black (CB) were incubated with two different cell lines. The biocompatibility on both neuronal and lung cell lines followed the order of ND > CB > MWCNT > SWCNT. Apparently, NDs neither disturbed the macrophage mitochondrial membrane nor generated reactive oxygen species (ROS). In contrast, CNTs generated ROS and caused membrane leakage. Moreover, neuroblastoma cells lost their neurotic extension after being incubated with high concentrations of carbon nanomaterials.52
In summary, the advantage of nanocarbon materials is their biocompatibility, biodegradability, non-toxicity, and nonhemolytic nature, which are crucial for safe and effective biomedical applications. These properties reduce the risk of adverse reactions and allow their integration in biological systems without causing harm. Also, versatile functionalization of nanocarbon materials further expands their potential applications in biomedicine.
3. The role of mechanical properties of carbon-based nanomaterials in tissue engineering
The unique traits and applications of compounds are dependent on their physicochemical properties; therefore, the expression and measurement of these properties are considerable points in materials science. One of the most important physical features of compounds is their mechanical attributes, which define the behaviour of materials under the action of external forces.53The mechanical properties of materials determine their strength, hardness, toughness, brittleness, durability, stiffness, and elasticity. The strength of a material refers to the amount of load that it can bear before fracturing or deforming, which is divided into several subcategories including tensile, compressive, shear, yield, and fatigue strength. Generally, the stress–strain curve is used to determine the strength and stiffness of materials.54 The slope of the curve is called the Young's modulus and its value is typically large.55 Many synthetic and natural compounds have significant mechanical features. Also, composites of these compounds possess high mechanical properties.
Composites are produced using two or more constituent materials, which have special activities compared to their components. To date, various composites have been synthesized, and due to their unprecedented mechanical and physical features, they have been widely used in different fields. Carbon composites are one of the principal types of composites and many studies have focused on their physicochemical properties and biological applications.56,57 Carbon composites have high mechanical aspects due to the special structure of carbon-based nanomaterials and sp2 hybridization carbon atoms. Mainly, carbon nanomaterials can increase the mechanical characteristic of composites due to the following reasons: (1) easy chemical functionalization,58 (2) interaction of functional groups such as hydroxyl, carboxyl, ketone, and epoxy in various types of carbon nanomaterials with other functional groups in composite components,59 (3) formation of strong π–π interaction between carbon sheets and other composite components,60 and (4) formation of hydrogen bonding between the carboxyl and carbonyl groups of carbon nanomaterials and hydroxyl groups of other components in composites.61
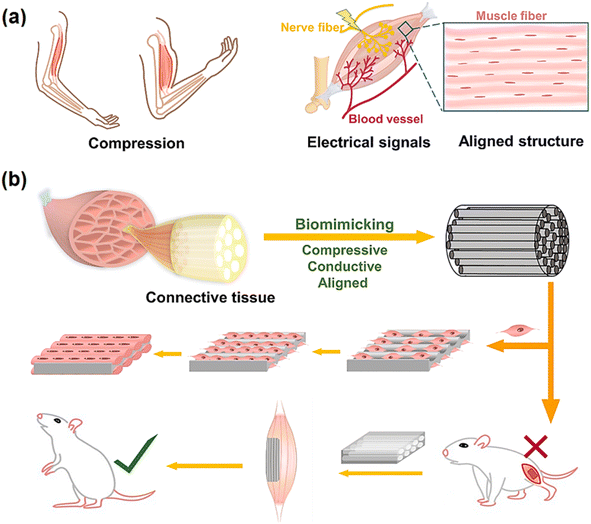
According to the information provided in the literature, carbon nanostructures and the aforementioned interactions enhance the mechanical properties including compressive strength,62 tensile strength,63 toughness,64 Young's modulus,65 and elastic modulus66 of composites. The effective mechanical properties of carbon nanomaterials in the composites facilitate their use in several biomedical applications, especially tissue engineering.67 Carbon-based nanomaterials such as GO, CNTs, fullerenes, CDs, carbon diamond, CFs, and their derivatives improve the mechanical stability of scaffolds,15 which play an important role in bone,9 skin,68 cartilage,69 neural,70 dental,71 articular72 and cardiac73 tissue engineering. The scaffolds employed in tissue engineering must have appropriate porosity compared to the size of desired cells for promoting cell infiltration. Most compounds are utilized to increase the strength of composites, reduce the porosity, and ultimately decrease cell proliferation in tissue engineering. Alternatively, the advantages of carbon-based nanomaterials are that they do not limit the pore sizes of scaffolds in cell growth, while simultaneously enhancing their strength (Fig. 1).74,75
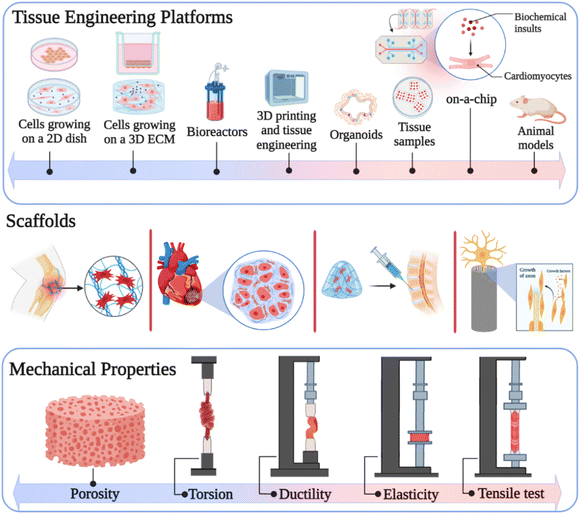 | ||
| Fig. 1 Mechanical properties of carbon-based nanomaterials in tissue engineering. Created with ChemDraw and https://www.BioRender.com. | ||
3.1. Graphene oxide and reduced graphene oxide
Go and rGO are members of the graphene family of carbon-based nanomaterials, which are synthesized using various methods. GO is the oxidized form of graphene, possessing hydroxyl, carboxylic, and epoxy functional groups, while rGO is a form of GO that has fewer oxygen molecules.76,77 The particular physicochemical properties of GO and rGO such as mechanical strength,78 large surface,79 abundant functional groups,80 and excellent hydrophilicity81 make them suitable for application in the biomedical field such as drug delivery,82 biosensing,83 bioimaging,84 cancer therapy,85 tissue engineering,86 and wound healing.87Also, GO and rGO promote the biomechanical properties of composites by forming covalent, non-covalent, and electrostatic interactions in the composite structure. Therefore, numerous studies have been performed on the mechanical properties of GO and rGO and their effects on scaffolds employed in various types of tissue engineering. For instance, Fu et al. reported the synthesis of a polydopamine-assisted gold nanoparticle–poly(lactide-co-glycolide)/L-lysine functionalized graphene oxide composite with mechanical strength, hydrophilicity, and antibacterial properties.88 This scaffold could significantly promote new bone formation, which was based on L-lysine-functionalized GO and polydopamine-assisted gold nanoparticle coating PLGA scaffold material. The in vivo bone regeneration assessment exhibited the repair of a defect in the radius of rabbits after 12 weeks of treatment, while PLGA alone had a lower repair effect score. The GO in the scaffold improved the hydrophilicity, cell adhesion, compressive strength (2.68 ± 0.21 MPa), and elastic modulus (7.2 ± 1.47 MPa) of the composite. The synergistic effect of these properties makes it a good candidate for bone defect repair.
Studies have indicated the importance of considering the concentration of GO in biomedical applications.89 For instance, in a study by Umar Aslam Khan and coworkers, by optimizing the concentration of GO, the cell adhesion and proliferation were enhanced, while cytotoxicity was avoided.90 Moreover, it was demonstrated that in the concentration range of 0.1–10 mg mL−1, sulfonated GO is non-toxic and biosafe.91 A recent study showed that 1.5 wt% of GO is the safest concentration for most cells, given that 3 wt% and 6 wt% exhibited significantly increased cytotoxicity on human bone marrow mesenchymal stem cells.92 The swelling ratio of the hydrogels also varied after the addition of GO nanoparticles in their structure. It was demonstrated that by the addition of GO nanoparticles to a poly(acrylic acid) hydrogel, its deswelling ratio decreased, while the neat hydrogel swelled faster. These ratios were also found to be relevant to the content of GO.93 The GO membrane and bulk GO swelling pressures were calculated in water and ethanol. It was demonstrated that under confinement conditions, the produced pressure could reach up to ∼226 bar in water and 194 bar in ethanol. This significant swelling pressure is a key factor in applications that require GO membrane confinement or encapsulation.94
Another scaffold that is widely used in tissue engineering is polymeric scaffolds. However, a disadvantage of polymeric scaffolds is their low mechanical strength for tissue regeneration, and thus the use of other compounds such as GO is essential to upgrade their mechanical features.95 Recently, Kadhim et al. reinforced polycaprolactone (PCL)–gelatin nanofiber scaffolds using bone morphogenetic protein (BMP)-modified GO.96 In this case, although gelatin has favorable biological properties, it does not possess good mechanical stability, and therefore it was combined with PCL and GO to achieve the desired mechanical properties. Comparing the mechanical properties of gel/PCL and gel/PCL–GO–BMP (2.5 wt% GO) proved that the ultimate tensile strength increased from 8.5 ± 0.3 MPa to 17.2 ± 0.3 MPa, but the strain at break was reduced from 116% ± 6.2% to 90% ± 2.9% due to its high stiffness. Moreover, the swelling ratio increased significantly from 150% for gel/PCL to 218% for gel/PCL–GO–PAA*BMP. The interactions formed between the composite components determined the unique mechanical properties and applications of the scaffold in different tissue engineering. For instance, Shuai and colleagues fabricated HAp nanorods on a GO nanosheet via a in situ strategy for reinforcement of poly-L-lactic acid (PLLA).97 The influence of the composite on cell growth and MG63 cell proliferation enabled its use in bone tissue engineering. The presence of abundant oxygen-containing groups in GO, which act as chelating sites, allowed HAp to be decorated on the GO in the form of nanorods. The special layer structure of HAp on the GO nanosheets induced the formation of chemical bonds between the composite and damaged bone. Specifically, 12% of GO–HAp increased the strength and modulus of the composite to 21.52 MPa and 4.99 GPa, respectively, through the formation of strong surface interactions with PLLA. One of the easy and appropriate strategies used in bone repairing is endogenous tissue engineering. In this method, hard cell culture is prevented and endogenous new stem cells are employed by implanting scaffolds. Therefore, Zhao et al. developed a bioinspired GO/chitosan/nHAP composite via a facile one-step in situ method for bone tissue engineering.98 The in vivo cranium bone repair indicated that the defects were recovered to new tissue after 2–3 months of implantation. The electrostatic interaction between the amine groups of chitosan and carboxyl radical of GO enhances the mechanical features of the composite, and thus 0.99% of GO induced the highest compressive strength (0.124 ± 0.022 MPa) and elastic modulus (0.413 ± 0.213 MPa) in the scaffold. Also, the strong π–π interaction between the GO layers prevented the fracture of the composite by distributing the external stress between the GO sheets and the chitosan/nHAP matrix, and therefore it is efficient in engineering hard tissues. In addition, due to the higher hydrophilicity of GO/CS/nHAP, it was able to absorb more water than CS/nHAP, and therefore had a higher swelling ratio (Fig. 2).98
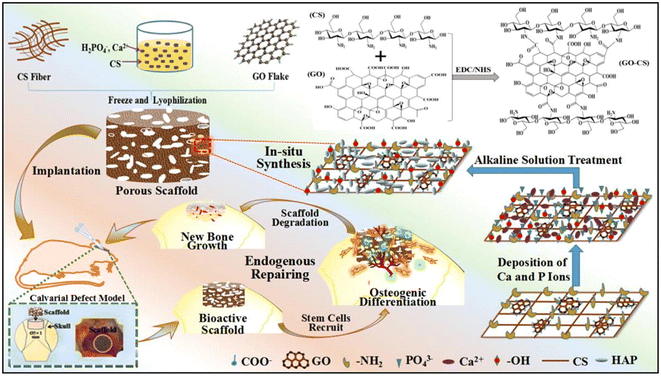 | ||
| Fig. 2 Synthesis processes for GO/chitosan/nHAp scaffold and its implantation in a cranium bone defect in a rat. Reprinted with permission from ref. 98 Elsevier, copyright 2019. | ||
The main point in tissue engineering is that scaffolds should be similar to the extracellular matrix (ECM) of the target tissue in terms of physicochemical properties. Thus, Bahrami et al. synthesized collagen and rGO-coated collagen composite (rGO–Col) via chemical cross-linking and freeze-drying methods.99 In this study, the regeneration of rabbit cranial defects after 12 weeks of implantation showed that rGO–Col can be used in implanting bone injuries. The addition of GO to the scaffold not only increased the proliferation of human bone marrow-derived mesenchymal stem cells (hBMSCs) but also compensated for the weak mechanical properties of collagen for bone structural support. According to the stress–strain curves from compression testing on Col–rGO and collagen, their elastic modulus was determined to be 325 ± 18 kPa and 115 ± 16 kPa, respectively, and thus the coated collagen with GO exhibited a 2.8-times increase in mechanical properties. Another challenge in tissue engineering, is articular cartilage regeneration. Accordingly, to repair this tissue, Gong and coworkers employed a natural acellular cartilage extracellular matrix (ACM) and cross-linked GO to ACM as a scaffold.100 The function of GO is to improve the biomechanical strength of the composite and the ACM retains the ECM components such as type II collagen and glucosaminoglycan. In vivo implantation in a mouse knee joint exhibited that the defect area was completely repaired after 12 weeks of GO-modified 3D ACM implantation with traditional seed cells. Increasing the concentration of GO enhanced the elastic modulus (0.1518 ± 0.0067 MPa) and reduced the porosity of the composite, and thus the best scaffold contained 2 mg mL−1 of GO, which promoted cell adhesion and proliferation.
Another composite that has attracted significant attention in cartilage tissue engineering due to its structural similarity to GAGs found in the cartilage extracellular matrix is chitosan-based scaffolds. In 2019, Shamekhi et al. developed a chitosan-based composite that was reinforced with different concentrations of GO nanoparticles.101 Increasing the mechanical properties of the scaffold by the addition of various amounts of GO prevented the scaffold from shrinking against the chondrocyte contraction and caused it to be completely placed on the defect side. The human articular chondrocyte culture on the scaffolds with different concentrations of GO exhibited that the enhancement in stiffness and modulus stimulated the proliferation of human articular chondrocytes. The stress–strain curves in this study demonstrated that an increase in the concentration of GO to 0.2% improved the Young's modulus, and therefore the use of compounds with high mechanical features such as GO is essential in cartilage tissue engineering.
One of the important mechanical characteristics determining the use of scaffolds in cartilage tissue engineering is their compressive strength. Cartilage tissue must have high compressive strength because it bears the weight of the body. In this regard, Nazar et al. reported the preparation of a gelatin hydrogel cross-linked with genipin and reinforced by GO-grafted chitosan.102 The composite containing 0.06% w/w CS-g-GO possessed appropriate mechanical features such as Young's modulus (134.18 Pa), toughness (0.1235 J m−3), and compressive stress (65 kPa). Also, the 90.2% viability of human chondrocyte cells, significant compressive stress, and high porosity (83.1 ± 2.6%) make this composite a favourable choice for cartilage repair. Interestingly, by increasing the CS-g-GO content, the swelling degree increased significantly from 800% to 1200%.
According to the ECM composition, density, and chondrocyte activity, cartilage is divided into different types, and thus repairing both superficial and deep parts of articular cartilage is noteworthy. In this case, Trucco and colleagues synthesized a bilayered hydrogel made of GO-doped gellan gum–poly(ethylene glycol) diacrylate with mechanical and lubrication features.103 This hydrogel could be injected into chondral defects as a surgically implantable substitute and sequentially cross-linked by UV light and ionic cross-linking method. Doping GO in the hydrogel not only improved its lubrication properties and chondrogenic differentiation but also significantly enhanced the average fracture stress and toughness values from 194 ± 27 to 246 ± 34 kPa and 36 ± 3 to 41 ± 9 kPa, respectively. Due to energy distribution by the GO nanosheets, the increase in toughness indicated that the GO-doped hydrogel had better mechanical properties as artificial cartilage than the bare hydrogel. However, with the addition of the GO nanofillers, the swelling ratio of this composite slightly decreased with no statistical significance.
Another tissue that has attracted significant attention in tissue engineering owning to its low regenerative capacity is cardiac tissue. Many injectable hydrogels can be used in heart tissue engineering, but the noteworthy point is that the desired hydrogel should have sufficient resistance to the surrounding applied forces after injection at the infarct site. In this regard, Mousavi and colleagues developed oxidized alginate/myocardial ECM injectable hydrogels with electromechanical properties for cardiac tissue engineering. The addition of 3-(2-aminoethyl amino) propyltrimethoxysilane (APTMS)-functionalized rGO to the hydrogel improved its electromechanical properties.104 The simultaneous increase in oxidized alginate from 3 to 4% w/v and 25 μg per mL rGO enhanced the Young's modulus from 22.1 kPa to 38.8 kPa. The Young's modulus of the native myocardium and cell viability of human umbilical vein endothelial cells indicate that this sample with an optimal concentration of rGO and oxidized alginate is a desirable hydrogel for cardiac tissue engineering.
One of the significant mechanical factors in heart pumping is the elastic property of the scaffold, which depends on the modulation of its failure. Thus, to investigate this mechanical feature, Nazari et al. incorporated two-dimensional nanomaterials, i.e., MoS2 and rGO nanosheets, in silk fibroin nanofibers via the electrospinning method for cardiac tissue engineering.105 The in vitro assessment showed that the c-TnT and α-MHC levels as cardiac mature markers of TBX18-hiPSC cells increased by the SF/MoS2 and SF/rGO composites. The addition of rGO increased the mechanical properties of SF including Young's modulus (561 ± 8 kPa), yield strength (1.27 ± 0.04 MPa), ultimate strength (3.65 ± 0.05 MPa), and fracture modulation (103 ± 5). Therefore, it can be concluded that the elasticity feature of the desired composite should be similar to heart tissue. Another mechanical property of a scaffold that affects the contraction and expansion of the heart is the elongation at break. Recently, Azizi and colleagues developed polyurethane/rGO composite nanofibrous scaffolds with different microstructures for cardiac tissue engineering.106 The rGO in the aligned scaffold increased the Young's modulus (496.4 kPa) and ultimate tensile strength (496.4 MPa) but decreased the elongation at break (52.07%) due to the small diameter of the fibers. The levels of troponin I as a cardiac gene indicated that the aligned composite enhanced the differentiation of satellite cells into cardiac prognostic cells. Consequently, to improve the efficiency of engineered tissue, all the mechanical properties of the tissue should be examined and a similar scaffold should be designed. Another serious muscle defect that affects movements is skeletal muscle defects. Recently, Zhao and coworkers designed a nanocomposite cryogel via the cryopolymerization of gelatin with poly(tannic acid)-reduced graphene oxide (rGO–PTA) for skeletal muscle tissue engineering. The 3D macroporous cryogel improved myogenic differentiation and C2C12 myoblast cells and also repaired volumetric muscle loss by integrating hemostasis. rGO/PTA as a cross-linked nanofiller in the composite network increased the compression modulus from 32 kPa to 75 kPa under 80% strain. In addition, the prepared cryogel could recover to its initial state after three compression–strain cycles due to its high elasticity and water adsorption. Thus, the presence of these excellent mechanical properties in the nanocomposite prevents the mechanical failure of the network (Fig. 3).107
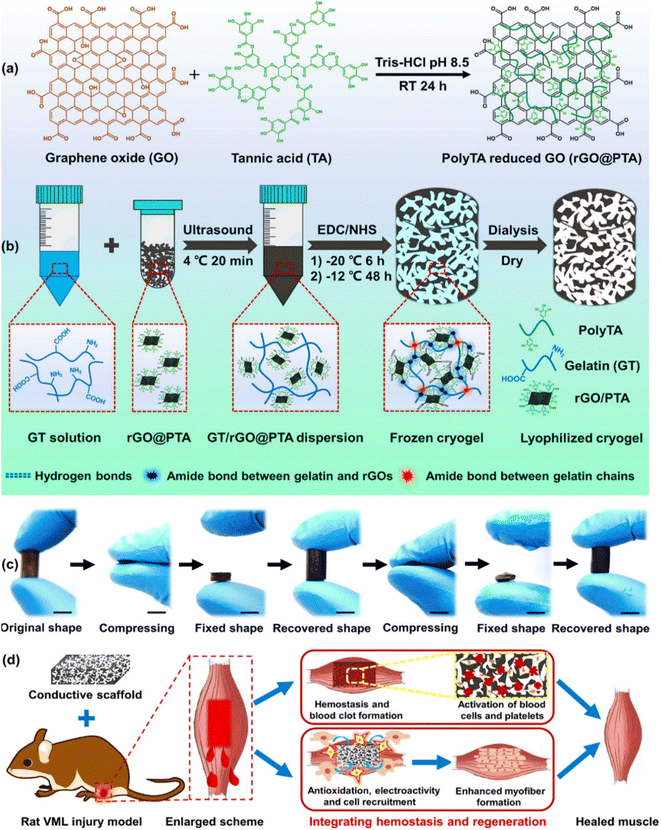 | ||
| Fig. 3 An overview of the composite preparation, mechanical properties, and its application in skeletal muscle tissue engineering. (a) Synthesis of rGO–PTA, (b) synthesis and formation mechanism of the GT/rGO–PTA cryogel, (c) photograph of fixing and recovering performance of the cryogel in both dry and wet states (scale bar: 1 cm), and (d) schematic illustration of GT/rGO–PTA cryogel application in skeletal muscle repair by an in situ strategy. Reprinted with permission from ref. 107 Elsevier, copyright 2020. | ||
Various occurrences such as accidents, bone fractures, trauma, and surgical complications lead to peripheral nerve damage, which may become long-term neurological disabilities, and thus artificial nerve guide conduits can be appropriate candidates for nerve regeneration. Recently, Abzan et al. developed a 3D polyvinylidene fluoride (PVDF)/GO scaffold via a non-solvent-induced phase separation method for nerve repairing.108 The employed GO improved the piezoelectricity of the composite and enhanced its hydrophilicity and mechanical properties such as toughness (2.27 times) and strength (1.3 times) by the formation of strong interaction between fluorine groups and carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of GO nanosheets. These particular properties endowed to PVDF by 0.5 wt% GO led to the promoted attachment, spreading, and proliferation of PC12 cells, and also enabled it to act as a nerve conduit with 4 internal longitudinally aligned channels. Moreover, to have appropriate mechanical properties, the scaffolds used in nerve repair must also have high porosity and surface area to provide neurite length growth. In this regard, Jaswal and colleagues investigated an electrospun scaffold based on rGO-enfolded AuNPs integrated with PCL.109 Due to the increased proliferation and differentiation of PC12 and S42 cells, this composite can be used for peripheral nerve regeneration. The integration of nano-sized rGO converted the composite into an excellent neural conduit with unique electrical and mechanical properties, which had a large surface area. The mechanical tests indicated that the ultimate tensile strength of rGO-AuNPs–PCL is 5.8 MPa, while pure PCL had a low tensile strength (3.7 MPa). Consequently, the used rGO not only provides suitable mechanical properties for nerve tissue engineering but also increases the porosity of the composite for cell growth and transmission of electrical stimuli.
O) of GO nanosheets. These particular properties endowed to PVDF by 0.5 wt% GO led to the promoted attachment, spreading, and proliferation of PC12 cells, and also enabled it to act as a nerve conduit with 4 internal longitudinally aligned channels. Moreover, to have appropriate mechanical properties, the scaffolds used in nerve repair must also have high porosity and surface area to provide neurite length growth. In this regard, Jaswal and colleagues investigated an electrospun scaffold based on rGO-enfolded AuNPs integrated with PCL.109 Due to the increased proliferation and differentiation of PC12 and S42 cells, this composite can be used for peripheral nerve regeneration. The integration of nano-sized rGO converted the composite into an excellent neural conduit with unique electrical and mechanical properties, which had a large surface area. The mechanical tests indicated that the ultimate tensile strength of rGO-AuNPs–PCL is 5.8 MPa, while pure PCL had a low tensile strength (3.7 MPa). Consequently, the used rGO not only provides suitable mechanical properties for nerve tissue engineering but also increases the porosity of the composite for cell growth and transmission of electrical stimuli.
Nowadays, tissue engineering as an effective solution also plays a key role in cutaneous wound healing and burn treatment. For instance, You and coworkers designed a poly(lactic-co-glycolic acid) (PLGA) copolymer incorporating GO for skin tissue engineering.110 According to the in vivo evaluations, the composite could enhance the skin wound healing rate in rats via exogenous electrical stimulation. The function of GO is to enhance the mechanical, electrical, and biological properties of the scaffold. The additional optimal concentration of GO (2%) increased the tensile strength (about 25 MPa) and surface roughness of the composite, improving the adhesion and proliferation of cells. In summary, the scaffolds based on GO and rGO nanomaterials play an important role in tissue engineering. The unparalleled mechanical strength, large specific surface area, abundant functional groups, and porosity of GO and rGO enable the use of different composites in various types of tissue regeneration. The mechanical properties of scaffolds are evaluated by different mechanical tests and these remarkable features provide appropriate conditions for cell growth, proliferation, adherence, and differentiation in various tissue repair including bone, cartilage, heart, nerve, and skin.
Table 1 summarizes the main characteristics and mechanical properties of GO and rGO composites proposed in the literature for tissue engineering.
| Composite | Type of tissue | Modulus | Strength | Porosity (%) | Optimum amount of GO/rGO | Cell viability (%) | Model(s) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PU-GO/HAp | Bone | Young's modulus: 56.25 ± 2.19 MPa/compressive modulus: 211.04 ± 12.94 | Tensile strength: 13.25 ± 1.19 MPa/flexural strength 18.50 ± 0.07 | 85.8% ± 1.5% | 2 wt% | ∼93% | In vitro | 111 |
| CO-g-GC-GO/AgNP | Skin | Young modulus: 2.19 ± 0.06 MPa/compressive modulus: 5.21 ± 0.65 MPa | Tensile strength: 4.64 ± 1.40 MPa | 59.29% ± 8.92% | Max 0.1 mg mL−1 | 94.83 ± 4.95% | In vitro | 112 |
| HAP/ZrO2/GO–PLA | Bone | Young's modulus: 0.211 ± 0.08 MPa | Tensile strength: 8.45 ± 1.1 MPa/fracture strength 0.43 ± 0.02 | 89.5% ± 2.5% | 0.01 g | 98.2 ± 5% | In vitro | 113 |
| ARX/GO/nHAp-NPs/PVA | Bone | Young's modulus: 236.22 ± 3.80 MPa | Ultimate compressive strength: 7.94 ± 3.10 MPa | 49.75% ± 3.10% | 0.3 mg | 98% | In vitro | 114 |
| PCL/rGO | Bone | Young's modulus: 20.11 ± 0.68 MPa | Ultimate tensile strength: 5.17 ± 0.95 MPa | 87.79% | 0.5 wt% | More than 100% | In vitro | 115 |
| HT-GO | Bone | Young's modulus: 71.77 ± 2.40 MPa | Compressive strength: 1.8 ± 016 | 89.7% ± 1.24% | 1 wt% | More than 90% | In vitro | 116 |
| PCL-PU/SV-loaded GO | Bone | Tensile modulus: 29.80 MPa | Tensile strength: 70.87 MPa | N.A. | 1.5 wt% | More than 85% | In vitro | 117 |
| CS-gel/GO/MMT | Bone | Young's modulus: 22.96 MPa | Compressive strength: 103.4 MPa | ∼90% | 1 wt% | More than 80% | In vitro | 118 |
| BC-g-(Fe3O4/GO) | Bone | Young's modulus: ∼200 MPa | Compressive strength: 11.66 MPa | 48.79% | 0.5 mg | More than 85% | In vitro | 119 |
| PLA/GO | Bone | Young's modulus: 2.6 GPa | Tensile strength 39 MPa | 30% | 0.3 wt% | N.A. | In vitro | 120 |
| SF/GO | Bone | Compressive modulus: 1.90 ± 0.084 MPa | Compression strength: 0.5 ± 0.052 MPa | 71% ± 2.41% | 10 wt% | OD value: about 1 (after 7 days) | In vitro | 121 |
| PCSG | Bone | Young's modulus: 72.4 ± 1.6 MPa | Tensile strength: 11.6 ± 0.5 MPa | 88.1% ± 1.7% | 2 wt% | N.A. | In vitro/in vivo | 122 |
| GG-AAc/GO/nHAp/TiO2 | Bone | Young's modulus: 300.81 MPa | Compression strength: 13.31 MPa | 44.32 ± 2.14% | 0.3 mg | ∼85% | In vitro | 123 |
| DT/GO | Skin | Young's modulus: 10.18 ± 1.77 kPa | N.A. | 51 ± 11% | 3 wt% | ∼70% (after 7 days) | In vitro | 124 |
| PCL-Sr/Se-HAp/GO | Bone | Young's modulus: 5.34 ± 0.53 MPa | Tensile strength: 9.74 ± 0.43 MPa | 47.7% | 0.22 g | 94.4% ± 3.1% | In vitro | 125 |
| SA–HA–GO | Bone | Elastic modulus: 149.4 ± 11.1 kPa | Compression stress: 165.5 ± 5.1 (60% strain) | 77.08 ± 3.00 | 1.0 wt% | N.A. | In vitro/in vivo | 126 |
| CMCS/PVA | Skin | Young's modulus: 1.68 MPa | Tensile strength: 1.93 MPa | N.A. | 5 wt% | 88.6% ± 2% | In vitro | 127 |
| HA/gelatin/GO | Bone | Young's modulus: 397.11 ± 98.51 MPa | Compression strength: 10.28 ± 1.08 MPa | 56.23 ± 1.07% | 0.5% (w/v) | N.A. | In vitro | 128 |
| Flexural strength: 12.58 ± 2.47 MPa | ||||||||
| X/C/HAp/GO | Bone | N.A. | Compression strength: 0.9–1.1 MPa | 90–93% | 0.5–1 wt% | N.A. | In vitro | 129 |
| PASS/ZnO/GO | Skin | N.A. | Tensile strength: 2.34 MPa | N.A. | 1 wt% | 100% (after 7 days) | In vitro | 130 |
| SF/SPI/GO/β-TCP | Bone | N.A. | Compression strength: 0.802 ± 0.065 MPa | 80.45 ± 2.04 | 10 mg | More than 100% (after 7 days) | In vitro | 131 |
| Gel-HA/VD-loaded GO | Bone | Young's modulus: 285.98 ± 90.2 MPa | N.A. | 40.8 ± 7.1% | 0.5 wt% | OD value: 0.16 (after 5 days) | In vitro | 132 |
| PB-GO | Cartilage | N.A. | Tensile strength: 2.08 ± 0.33 MPa | N.A. | 1.5% (w/v) | 108% ± 10.43% (after 7 days) | In vitro | 133 |
| HyA/gel/PEO-rGO | Skin | Young's modulus: 5176.62 MPa | Tensile strength: 108.53 MPa | N.A. | 20 vol% | N.A. | In vitro | 134 |
| GO-CMC/PEGDA | Bone | Young's modulus: 11.02 ± 0.01 kPa | N.A. | 87.4 ± 0.26% | 0.1 mg L−1 | N.A. | In vitro/in vivo | 135 |
| Gelatin/GO/MgO | Bone | Tensile modulus: 1778.76 MPa | Tensile strength: 11.653 ± 1.23 MPa | N.A. | 10 wt% | OD value: about 0.9 (after 7 days) | In vitro | 136 |
| np-HAp/GO/PEEK-p | Bone | Tensile modulus: ∼ 0.825 MPa | Tensile strength: 120 MPa | N.A. | 1 wt% | OD value: more than 1 (after 7 days) | In vitro | 137 |
| SF/nHAp/GO | Bone | Elastic modulus: 279.73 kPa | Compression strength: 83.86 kPa | 90.5 ± 1.1% | 0.5 wt% | OD value: more than 1.2 (after 7 days) | In vitro | 138 |
| PLL-PLGA/GO | Bone | N.A. | Tensile strength: 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 ± 0 65 ± 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 MPa 64 MPa |
N.A. | 2% (w/w) | N.A. | In vitro | 139 |
| RSF/rGO | Skin | Storage modulus: ∼ 18 kPa/Young's modulus: ∼ 1.77 MPa | N.A. | N.A. | 0.13 wt% | More than 86% (after 7 days) | In vitro | 140 |
| PCL/GO | Bone | Compressive modulus: 84.08 ± 3.93 MPa | Compression strength: 2.06 ± 0.11 MPa | N.A. | 3 wt% | N.A. | In vitro | 141 |
| V-GO-gel | Intestinal | Young's modulus: 83.74 ± 4.37 MPa | Tensile strength: ∼ 5 MPa | N.A. | 0.25% (w/v) | N.A. | In vitro/in vivo | 142 |
| PAmAA-Al3+/PVA/GO | Skin | N.A. | Tensile strength: 370.7 kPa | N.A. | 2 wt% | N.A. | In vivo | 143 |
| SMH/GO | Bone | Compressive modulus: 43 kPa | N.A. | N.A. | 0.04 wt% | In vitro/in vivo | 144 | |
| GO-CTS-CS | Skin | N.A. | Tensile strength: 10.10 MPa | N.A. | 30 wt% | More than 90% | In vitro/in vivo | 145 |
| PVA/GRGO | Skin | N.A. | Tensile strength: 2.58 ± 0.43 Pa | N.A. | 1.0 wt% | OD550–OD600: 0.4 (after 21 days) | In vitro | 146 |
| GelMA-PDA-rGO | Cardiac | Young's modulus: 23.6 ± 1.2 kPa | N.A. | N.A. | 1 mg mL−1 with PDA | OD value: about 2 (after 5 days) | In vitro | 147 |
| SF–GO-PPY | Neural | Compressive modulus: 7.73 ± 1.22 kPa | Compressive strength: 530.89 ± 30.39 kPa | N.A. | 5 mg mL−1 solution | N.A. | In vitro | 148 |
| RSF/GO | Nerve | Young's modulus: 122.7 ± 3.1 MPa | Breaking strength: 0.9 ± 0.1 MPa | N.A. | 1.5 wt% | N.A. | In vitro | 149 |
| PVA/nano-HA/CTI/GO | Bone | N.A. | Tensile strength: 19.05 ± 0.03 MPa | N.A. | 0.5 wt% | ∼110% (after 5 days) | In vitro | 150 |
| PCL–GO/PHBV-CaPO4 | Bone | Elastic modulus: 52.21 MPa | Tensile strength: 2.87 MPa | N.A. | 1 wt% | ∼110% (after 5 days) | In vitro | 151 |
| GelMA/GO | Neural | Storage modulus: 1.61 ± 0.06 kPa/compressive modulus: 3.64 ± 0.43 kPa/elastic modulus: 7.42 ± 0.29 kPa | N.A. | N.A. | 1.40 mg mL−1 | ∼100% | In vitro | 152 |
| GO/ATP/COL | Bone | N.A. | Compressive modulus: 4.3 ± 0.5 MPa | 56.0 ± 2.6% | 0.5 wt% | N.A. | In vitro/in vivo | 153 |
| PVA/CS/GO-PEG | Skin | Tensile modulus: 2.42 ± 0.12 GPa | Tensile strength: 79.49 ± 3.97 MPa | N.A. | 1 wt% | N.A. | In vitro | 154 |
| dECM–rGO | Cardiac | Compressive modulus: 17.5 ± 0.5 kPa | N.A. | N.A. | 0.3% (w/v) | More than 90% (after 35 days) | In vitro | 155 |
| GH/GO | Skin | N.A. | Tensile strength: 53 kPa/adhesive strength: 73 kPa | N.A. | 0.2 wt% | 661% (after 7 days) | In vitro | 156 |
| Col–rGO | Cardiac | Young's modulus: 340 ± 20 kPa | N.A. | N.A. | 400 μg mL−1 | OD value: ∼0.7 (after 96 h) | In vitro | 157 |
| Gel/PCL–GO–PAA*(DCP) | Bone | N.A. | Ultimate tensile strength: 17.8 ± 0.2 MPa | N.A. | N.A. | N.A. | In vitro | 158 |
| ZnO/GO–CA | Skin | Young's modulus: 0.37 ± 0.18 MPa | Tensile strength: 8.82 ± 1.2 MPa | N.A. | 0.5 g | 97.38% ± 3.9% | In vitro | 159 |
3.2. Carbon nanotubes
CNTs are thin and long tubes consisting of a rolled-up graphene sheet having a diameter of about 100 nm. Carbon nanotubes can be mainly divided into 3 categories, i.e., single-walled carbon nanotubes (SWNCTs), double-walled carbon nanotubes (DWNCTs), and multi-walled carbon nanotubes (MWNCTs). CNTs have attracted attention due to their phenomenal mechanical, thermal, and electrical properties.9 Accordingly, CNTs and their related composites have been widely studied for tissue engineering applications.160 The mechanical properties of carbon-based nanostructures have been of interest to scientists for a long time to strengthen the mechanical specification of composites or directly use these mechanical properties for their desired work. The mechanical effects of CNTs have been greatly noticed and used in applications such as medical,161,162 pharmaceutical,163 wound dressings,164 various industries (namely aerospace, nano, and optics165–168), strengthening of electrical devices,169,170 and tissue engineering.27 In tissue engineering, the appearance of the materials used, which originate from their mechanical traits, plays an important role.Based on previous studies, oriented CNTs exist in the form of sheets and yarns, which act as suitable substrates for the growth of various types of cells. For instance, the roughness and large exposed surface of CNTs may play an important role in their ability to favor neuronal adhesion.171,172 In this regard, Li and coworkers designed and developed a nerve guidance conduit (including CNTs/sericin) with electrical conductivity, suitable mechanical features, and desirable swelling capacity for sciatic nerve repair. CNTs were added to strengthen the mechanical virtues of the composite nerve guidance conduit. The mechanical properties including tensile modulus (kPa), compressive modulus (kPa), tensile strength (kPa), compressive strength (kPa), and electrical conductivity (S cm−1) with the addition of CNTs increased to 76.43 ± 11.01, 7.69 ± 0.59, 67.56 ± 2.47, 5.46 ± 0.88, (3.90 ± 0.26) × 10−4, respectively. The porosity was also 85% with 0.5 mg per mL CNT, which is proportional to the optimal porosity for nerve regeneration (80%). The addition of these physical traits and improvement of mechanical properties by CNTs created an interconnected porous microstructure guiding channel for the axon longitudinal outgrowth exit and facilitated the exchange of nutrients and needed.173 Nanofibrous nerve conduits manufactured with diverse synthetic and natural materials have enormous potential for back nerve regeneration as a bridge between adjoining ends. Interestingly, Wang and coworkers developed PLGA–MWCNT aligned nanofibers coated with poly-L-lysine for nerve tissue engineering. After the addition of MWCNTs, the scaffold possessed the improved mechanical properties of L-PC_A− and L-PC_A+, including ultimate tensile strength (MPa), elongation at break (%), and Young's modulus (MPa) of up to 2.92 ± 0.12, 127.81 ± 2.92 and 6.82, 58.03 ± 2.94 and 22.47 ± 1.36, 31.96 ± 8.65, 485.05 ± 49.55, respectively. Consequently, the scaffold could guide PC12 cells and grow DRG neurons along the fiber direction, which is beneficial for neurite outgrowth, making it a potential candidate for nerve regeneration in tissue engineering.174
Biocompatible scaffolds in tissue engineering offer an appropriate micro-environment for the regeneration of injured tissue and the attachment and growth of cells. CNTs, as promising materials, can create a similar micro environment for bone repair in osteonecrosis and the natural function of bone and cartilage such as mechanical strength.175 In another study, Shuai et al., after bonding 3D hybrid montmorillonite (MMT)/CNT (MMT–CNT) with an optimal weight ratio of MMT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNT 1
CNT 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, and embedding it in PLLA by the SLS method, prepared a self-assembly scaffold. The addition of CNTs, besides reducing the accumulation of MMT by being sandwiched between its middle layer, increased the mechanical effects of the scaffold due to the tolerance of the stress transferred from the matrix, which was high due to their high strength and modulus and large specific surface area. Adequate mechanical traits are essential during the induction of bone scaffolds. The results of the mechanical tests (tensile strength and modulus 113.04% and 111.46% and compressive strength and modulus 58.20% and 63.27%, respectively) showed that the addition of CNTs to the scaffold structure in this bone tissue engineering further facilitated the transfer of nutrients due to the uniform distribution of pores on the scaffold and its rapid degradation created space for the growth of bone tissue.176 Because of their special mechanical properties, CNTs can act as an interface for strong interfacial bonding and increased adhesion. In this respect, hydroxyapatite–carbon nanotube–wood derived carbon (HA–CNTs–WDC) composites have been developed as scaffolds for bone engineering by electrochemical deposition and chemical vapor deposition methods. The CNTs present in the structure, in addition to promoting HA/WDC interfacial adhesion, with their porous structure, increased the HA nucleation points and uniformity, and consequently increased the mechanical attributes of the scaffold. The improvement in mechanical properties with the addition of CNTs was proven by the increase in the values of compressive strength (10.54 MPa) and compressive stress (almost 12 MPa).177
0.1, and embedding it in PLLA by the SLS method, prepared a self-assembly scaffold. The addition of CNTs, besides reducing the accumulation of MMT by being sandwiched between its middle layer, increased the mechanical effects of the scaffold due to the tolerance of the stress transferred from the matrix, which was high due to their high strength and modulus and large specific surface area. Adequate mechanical traits are essential during the induction of bone scaffolds. The results of the mechanical tests (tensile strength and modulus 113.04% and 111.46% and compressive strength and modulus 58.20% and 63.27%, respectively) showed that the addition of CNTs to the scaffold structure in this bone tissue engineering further facilitated the transfer of nutrients due to the uniform distribution of pores on the scaffold and its rapid degradation created space for the growth of bone tissue.176 Because of their special mechanical properties, CNTs can act as an interface for strong interfacial bonding and increased adhesion. In this respect, hydroxyapatite–carbon nanotube–wood derived carbon (HA–CNTs–WDC) composites have been developed as scaffolds for bone engineering by electrochemical deposition and chemical vapor deposition methods. The CNTs present in the structure, in addition to promoting HA/WDC interfacial adhesion, with their porous structure, increased the HA nucleation points and uniformity, and consequently increased the mechanical attributes of the scaffold. The improvement in mechanical properties with the addition of CNTs was proven by the increase in the values of compressive strength (10.54 MPa) and compressive stress (almost 12 MPa).177
The biocompatibility of the composites based on CNTs is highly dependent on the concentration of these nanoparticles in the composite. Sang et al. proved that by increasing the MWCNT concentration in a composite from 1% to 5%, its cytotoxicity increased. Also, by increasing the MWCNT concentration, the swelling ratio decreased.178 Other studies also indicated the biocompatibility of the CNTs at low and controlled concentrations.179 Besides concentration, the size and shape of CNTs play a decisive in the biocompatibility of the composite and are related to cell damage and cell destruction.52
The incorporation of CNTs in the hydrogel matrix can effectively increase its swelling capacity. In the study by Tong and coworkers, it was indicated that by adding CNTs to a polyvinyl alcohol (PVA) hydrogel matrix, the swelling properties were enhanced as well as the tensile modulus, tensile strength, and strain at break. The final swelling ratios increased by 35.7% and 44.9% at room temperature and 310 K, respectively.180 As another important aspect of materials used in tissue engineering, the biodegradation of CNTs has been studied. For example, Zhang and coworkers investigated the relationship between the physicochemical properties of CNTs and biodegradation. It is believed that the key oxidizer of CNTs in macrophages is sodium hypochlorite. Therefore, it was used for investigating the biodegradation of CNTs. It was indicated that the biodegradation of CNTs is generally related to their diameter and degradation followed the order of SWCNT ≥ carbon nanohorns > thinner MWCNT ≥ thicker MWCNTs.181
In terms of structural–chemical, biological, and mechanical properties, MWCNTs simulate the ECM and participate in bone regeneration as a composite reinforcing material. In the designed and developed scaffold of dendrimer-MWCNT reinforced SrHAP composite, the presence of MWCNTs resulted in: (1) 30-fold increase in curcumin loading, (2) 1.7-fold delay in its burst release, (3) creation of porosity of 89.63% ± 2.4% in the composite, which is comparable to human cortical bone, (4) creating mechanical strength of 0.415 ± 0.04 GPa, (5) guiding bone repair due to homology and 3D structure similar to spiral collagen, and (6) a pattern for apatite nucleation due to anionic chemical properties in nanotubes. Thus, due to all these reasons, especially in creating the appropriate mechanical features, the differentiation of MG-63 human bone-like cells improved and increased.182
In the report by Kaur et al., the addition of COOH-SWCNTs to the CS-Coll/COOH-SWCNTs composite changed the structural morphology of the hydrogel into a parallel network with a porosity of 88% ± 3%. In addition, it has caused a 63% increase in mechanical properties (storage modulus (almost 110 Pa), loss modulus (around 5 Pa), and Young's modulus (around 106–107 Pa)), which increased the mechanical strength of the hydrogel from kPa to MPa, making it closer to the mechanical strength of bone. Thus, the composite was proven to be suitable as an injectable scaffold capable of cell differentiation and proliferation. The swelling ratio and degradation rate were found to be dependent on the composition with the optimal PBS absorbance through Fickian diffusion and stability in a liquid environment.19 In another study, they prepared a scaffold that included PCL membranes, β-glycerol phosphate (β-GP), and functionalized multiwall carbon nanotubes (f-MWCNTs), which provided a potential alternative for bone tissue regeneration with viability and proliferation of HDPSCs in vitro. The addition of f-MWCNTs increased the Young's modulus and the maximum tensile strength to 405.9 MPa and 25, respectively, which greatly contributed to the production of a highly efficient bone biomaterial with the ability to release bioactive factors and stimulate bone formation.183
The purpose of tissue engineering is to repair, replace, and restore unhealthy or damaged tissues, where the mechanical properties of scaffolds, especially the ability to mimic natural tissue, are significant. In bone tissue engineering, the ECM consists of collagen and inorganic bone minerals, which are hierarchically organized on multiple-length scales. In this direction, in an investigation, a highly biomimetic 3D printed porous scaffold comprised of MWCNTs with the same dimensions as collagen fibers, which were mainly aligned along the printing direction, was coupled with nHA and blended with a PCL matrix to produce scaffolds using a production system based on extrusion with the help of screws. Also, besides increasing the storage modulus, the elastic modulus of the filament (compressive modulus was almost 90 MPa) and the yield strength (almost 3 MPa) of the scaffold, the surface roughness increased, which promoted bone differentiation, helped to adjust the path of the hard surface transfer mechanism and enabled high protein absorption (Fig. 4).184
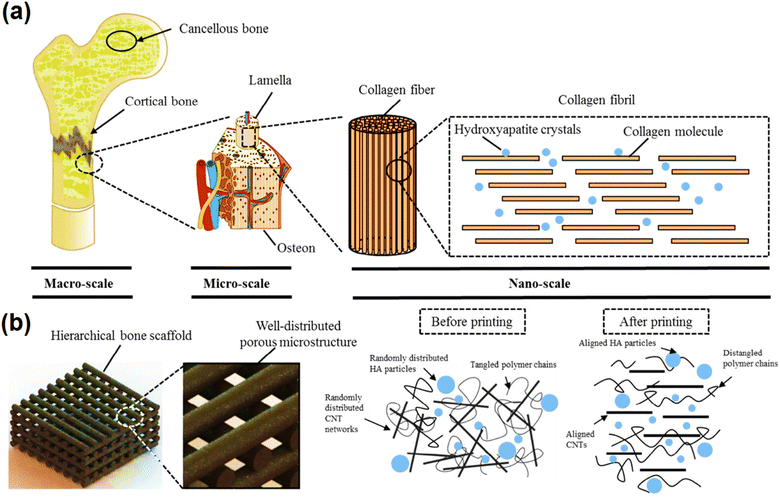 | ||
| Fig. 4 Schematic of the hierarchical scaffold system with uniform geometry at the microscale and highly aligned structures at the nanoscale that mimics the bone structure from the macroscale to the nanoscale (a and b). Reprinted with permission from ref. 184 Elsevier, copyright 2020. | ||
Tissue engineering is a promising method to regenerate damaged tissue by inducing the ability of natural tissue to heal using a cell scaffold that is transplanted into the host tissue. For example, Miyanji et al. designed and synthesized chitosan–gelatin/single-walled carbon nanotube electrospun composite scaffolds for cartilage tissue engineering, which is a connective tissue with a slow healing rate. CNTs functionalized with COOH provide interaction between themselves and biopolymers, which leads to better interface bonding and improved mechanical specs. Thus, their addition as a polymer matrix reinforcement due to their special properties, including high inherent mechanical properties, reduced the fiber diameter, contact angle, and elongation at break (3.26% ± 0.02%), and also increased the tensile strength (3.26 ± 0.02 MPa), Young's modulus (187.56 ± 9.31 MPa) and hydrophilicity of the scaffold, which enhanced the stability, cell viability, and cell behaviour to repair cartilage tissue to absorb growth factor. Their tubular structure allows them to transfer the external load from the matrix (the outermost layer) to the inner layers as a bridge in the polymer matrix. In addition, they create a porosity of over 80% (necessary for tissue engineering) in the scaffold, which plays an important role in the penetration of oxygen, humidity, and the transfer of nutrients to the cells and the removal of waste materials from them.185
Synthetic materials that are used for bone grafting in fracture must be attached to the porous bone, and thus the synthesized scaffold, besides biodegradability, must have sufficient compressive strength that is more than 2 MPa. For example, Akbari-Aghdam and coworkers fabricated a scaffold of polymer nanoceramic materials with controlled porosity that is similar to cancellous bone tissue and includes components such as resin polymer, SWCNT, and hydroxyapatite (HA) made by the digital light processing method. SWCNT having a suitable porosity of nearly 55% made the scaffold similar to the porosity of human bone, with acceptable strength. It also increased the mechanical properties of the sample up to 36 MPa and increased protein conversion and absorption in human bone.186
In skeletal muscle tissue engineering, the engineered scaffold must have the ability to endure periodic contractions and rest in daily life, which, in addition to having the same structure as the original tissue, requires excellent mechanical features to withstand continuous movements. Skeletal muscle tissue has compressive properties and a regular structure that is sensitive to electrical signals. Alternatively, platforms using biomimetic 3D aligned conductive tubular cryogel scaffolds consisting of gelatin and polydopamine-coated carbon nanotubes (PCNTs) by unidirectional casting technology by anisotropic compression as a framework, with a parallel structure were developed for 3D cell alignment and differentiation of C2C12 myoblasts and skeletal muscle regeneration in vivo in a rat tibialis anterior muscle defect model. The combined CNTs not only increased the conductivity, but also enhanced the mechanical attributes of the cryogel, including the compressive modulus in the longitudinal section to 66.3 ± 9.42 kPa, enabling the cryogel able to withstand repeated contraction behaviour and dynamic contraction similar to native skeletal muscle (Fig. 5).187 In a report on the design of scaffolds for use in heart and nerve tissue engineering, a CNT/PCL/gelatin composite was prepared by “sandwich” (sCNT) and dual deposition (DD CNT) methods. According to the report, the presence of CNTs, in addition to increasing the fiber diameter, pore size, and hydrophobicity of the scaffold, electrical conductivity up to 5.22 ± 0.49 kS, 80% increase in Young's modulus and 28% in tensile strength, and decrease in the rate of degradation, the scaffold also showed antimicrobial activity. Consequently, cell migration into the scaffold was promoted, making it a potential scaffold for the differentiation of heart and nerve cells (spinal cord and neural growth substrate).188
 | ||
| Fig. 5 (a) Properties of skeletal muscle tissue. (b) Schematic of the process of using a gelatin cryogel scaffold based on a PCNT in desired skeletal muscle tissue engineering. Reprinted with permission from ref. 187 Elsevier, copyright 2022. | ||
Cardiovascular tissue is exposed to continuous pulsating flow and mechanical stress, and blood vessels always faces pulsating shear stress of blood flow. Therefore, in cardiovascular tissue engineering, Tondnevis et al. prepared a scaffold consisting of gelatin, SWCNT, and polyurethane fiber. The addition of SWCNT increased the Young's modulus and ultimate strength of the scaffolds up to 16.47 ± 0.5 and 23.73 ± 0.5 MPa, respectively, reduced the average diameter to 140 nm, increasing the porosity to 89% ± 5% and hydrophilicity, and improved and increased the mechanical properties of the scaffold for it to withstand the above-mentioned physiological conditions. Studies indicated that the incorporation of CWCNTs in the composite structure reduced the degradation rate of the samples. The Young's modulus and ultimate stress were comparable to native myocardium tissue due to the strengthening effect of SWCNTs.189 In another study, a three-layer hybrid nanobiocomposite based on ultrahigh-molecular-weight-polyethylene (UHMWPE), MWCNTs, and nanosized hydroxyapatite (nHA) was developed for biomedical applications in tissue engineering of bone/blood cells. According to the mechanical tests, the addition of MWCNT had effects such as increasing the yield strength, work of fracture, and porosity up to ∼16.9 ± 0.6 MPa, 4.7 ± 0.2 J, and 8.6%, respectively. These properties strengthened the composite to enable it to resist the physiological environment and support the growth of stem cells, osteoblasts for the articulation of the femoral head, and the expression of rouleaux formation of RBCs.190
Conductive scaffolds are a suitable option for cardiovascular tissue engineering due to their similarity to the ECM of native tissue. For example, Mombini et al. fabricated chitosan–polyvinyl alcohol (PVA)–CNT nanofiber scaffolds with different percentages of CNTs with an optimal diameter of 255 ± 3.5 nm for cardiomyocyte differentiation. The presence of CNTs, in addition to increasing the conductivity, caused a significant increase in porosity (70% to 80% on average) and an increase in elastic modulus and tensile strength to 130 ± 3.605 and 4.9 ± 1.961, respectively. This led to the creation of a 3D platform for the proliferation and growth of cells and increased the adhesion of MSCs to the scaffold.191 The sufficient strength of biomaterials is essential in the field of tissue engineering because high strength damages soft tissues and tissue regeneration, while low strength causes rapid collapse, and thus the mechanical properties of the scaffold are vital. Finally, it can be said that CNTs, as biologically active molecules, have unique potential for different types of tissue engineering because by introducing their inherent mechanical properties in the scaffold, they can promote ECM expression and improve the substrate to stimulate cell growth and repair.192–194
Table 2 presents some reports concerning the mechanical features of CNT-based composites used in tissue engineering.
| Composite structure | Type of tissue | Modulus | Strength | Porosity, % | Optimum amount of CNTs | Cell viability, % | Model(s) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ACNTs-doped PMMA composites | Bone | Elastic modulus: almost 175 GPa | Compressive strength: almost 130 MPa | N.A. | 6 wt% | N.A. | In vitro | 195 |
| COL/MWNT composite film | Nerve tissue | Young's modulus: almost 5 MPa | Tensile stress–strain curves: almost 40 MPa-8% | N.A. | 0.15% | Almost 80 | In vitro | 196 |
| CNT-reinforced PCL | Non-soft tissues (e.g., cartilage, tendons, and meniscus) | Elastic modulus: almost 1200 MPa | N.A. | N.A. | 0.01% | 95% | In vitro | 194 |
| PHB-Cs/MWCNTs coating deposited on nBG/nTiO | Bone | Compressive modulus: 0.33 ± 0.004 MPa | Compressive strength: 11.14 MPa | 79.08 ± 0.8 | 1 wt% | OD: around 2.5 | In vitro | 197 |
| PLGA/SiO2NT nanocomposite | Bone | Young's modulus: around 64.15 GPa, shear modulus, G and bulk modulus, B: around 0.0000 GPa | N.A. | N.A. | N.A. | N.A. | In vitro | 198 |
| HA/HDPE/MWCNT bio-composites | Bone | N.A. | Tensile strength: 135.56 MPa | N.A. | 1% | N.A. | In vitro | 199 |
| HA–alginate–SWCNT composite | Bone | Young's modulus E: 0.645 ± 0.028 GPa | Compressive strength σc: 202 ± 9 MPa | N.A. | 0.5% | N.A. | In vitro | 200 |
| SWNT-COOH/Coll | Cartilage | N.A. | Compressive strength: almost 14 kPa | N.A. | 0.5 wt% | N.A. | In vivo | 28 |
| PAAm/SA/CNTs/CaCl2 hybrid DN hydrogels | N.A. | Young's modulus: 9.16 kPa | Tensile strength: 271.68 ± 6.04 kPa, compressive strength: 801.95 ± 284.63 kPa, compressive strain: 763.39% ± 45.42% | Almost 70 | 0.5 wt% | N.A. | N.A. | 201 |
| PCL/MWCNT | Bone | Compressive modulus: almost 80 MPa | N.A. | N.A. | 3 wt% | N.A. | In vitro | 202 |
| HA-CNT nanofibers | Nerve tissue | Bulk modulus: 1.769 ± 0.26, local modulus: 174.85 ± 31.9 kPa | Strength: 0.583 ± 0.08 MPa | N.A. | 0.01% | N.A. | In vitro | 203 |
| AG-Col-o-CNT scaffolds | Bone | Tensile modulus: almost 240 kPa | N.A. | N.A. | 200 mg | N.A. | In vivo | 204 |
| CS/PVA/CS-g-CNO nanocomposite films | Bone | Young's modulus: 2.26 ± 0.15 GPa | Tensile strength: 28.0 ± 2.27 MPa | N.A. | 500 mg Cl–CNO | N.A. | In vivo | 205 |
| c.p.Ti-CNT nanocomposite | Bone | Young's modulus: 7.19 GPa ± 0.40 GPa, elastic modulus: 112.3 GPa ± 3.7 GPa | N.A. | 1.29 ± 0.83 μm2 | 5% | 90% | In vitro | 206 |
| P3HB-chitosan-MWNTs/silk nano-micro-scaffold | Cartilage | N.A. | Ultimate tensile strength (mean ± SD): 46.68 ± 2.20 MPa, ultimate tensile strain (mean ± SD): 33.19% ± 1.18% | 83.78 | 1 wt% | N.A. | In vitro | 207 |
| PEDOT/CNT scaffolds | C8-astrocytes | Young's modulus: almost 60 kPa | N.A. | Almost 60 (pore size mostly 50–100 μm) | 30% | N.A. | In vitro | 208 |
| N.A. | Hard tissue | N.A. | Tensile strength: around 10 MPa | 83 | 0.5% | N.A. | In vitro | 209 |
| Gel/Akr/Fe3O4/MWNT | Bone | Elastic modulus: 0.45 ± 0.1 GPa | Compressive strength: 3.82 ± 0.2 MPa | 76 ± 3 | 10% w/w | Around 90 | In vitro | 210 |
| CMX-g-PAA/Fe3+/HCNTs hydrogel | N.A. | N.A. | Compressive strength: 10.4 MPa | N.A. | 0.15 | N.A. | In vitro | 211 |
| MWCNT-containing PCL-based scaffold | Cartilage | N.A. | Tensile strength: around 2.4 MPa | Around 75 | 10% | Around 120 | In vitro | 212 |
| ALG in situ gel-forming PLGA–CNT microspheres | Nerve | Loss modulus: 103 Pa | N.A. | N.A. | 10% | 70% | In vitro | 213 |
| PCL/CNT scaffolds | Bone | Compressive modulus: 45.47 ± 1.12 MPa | Compressive strength: 3.83 ± 0.28 MPa | N.A. | 3 wt% | Fluorescence intensity around 5800 | In vitro | 214 |
| PU/silk-fMWCNTs scaffold | Nerve tissue | N.A. | Tensile stress: ∼16.21 MPa | N.A. | 3 wt% | N.A. | In vitro | 215 |
| CNT-TES-PAMAM-G3-collagen scaffolds | Bone | Young's modulus: 369.62 ± 5.2 MPa | Tensile strength: 17.15 ± 1.7 MPa | 96 | 100 mg | Around 90 | In vivo | 68 |
| PLA/PCL/MWCNTs nanocomposites | N.A. | Modulus: 2.81 ± 1.00 GPa | N.A. | N.A. | 0.5% | N.A. | N.A. | 216 |
| PNIPAM/L/CNT hydrogel | Human motion sensing | Modulus: 46.8 kPa | Adhesive strength: almost 7 kPa, tensile stress: 0.53 ± 0.02 | N.A. | 4 mg | N.A. | In vitro | 217 |
| CS/PVA/ox-CNO biodegradable scaffold | Skin | Young's modulus: 3.86 ± 0.77 GPa | N.A. | N.A. | 1% | N.A. | In vivo | 218 |
| MWCNTS/BG/PEEK | Bone | Tensile modulus: 6.1 GPa | Tensile strength: 93 MPa, bending strength: 170 MPa | N.A. | 4% | OD: 0.7 | In vitro | 219 |
| Ag/MWCNTs | N.A. | Elastic moduli: around 1030 G′ and viscous moduli: around 130 G′′ | N.A. | N.A. | 20 mg | N.A. | In vitro | 220 |
| PCL/PEG/MWCNT | Myocardial tissue | Elastic modulus: almost 55 MPa | Tensile strength: almost 8 MPa | Around 52 | 1% (w/w) | N.A. | In vitro and in vivo | 221 |
| TPU/MWNT nanofibrous scaffolds | Rat mesenchymal stem cells | Elastic modulus: 10.01 MPa | Tensile strength: 17.21 MPa, tensile strain: 125% | N.A. | 2.5 wt% | N.A. | In vivo | 222 |
| Poly(NIPAM-co-β-CD)/CNT/PPY hydrogel | N.A. | Storage modulus: 357 Pa, modulus: 93.0 ± 9.0 | Tensile stress: 0.18 ± 0.02 MPa | N.A. | 100 mg | N.A. | In vitro | 223 |
| Poly(3-hydroxybutyrate)-chitosan-MWNT/silk scaffold | N.A. | N.A. | Tensile strength of aligned: 48.13 ± 2.2 MPa | Above 80 | 1 wt% | N.A. | In vitro | 224 |
| Tensile strength (random): 46.68 ± 5.95 MPa | ||||||||
| MWCNTs and SWCNTs/PHBV composites | Bone | N.A. | Flexural strength: SWCNT: around 60 MPa/MWCNTs: around 58 MPa | N.A. | 4 wt% | N.S (0.4) | In vitro and in vivo | 225 |
| MWCNTs/GEL-CS nanocomposites | Bone or cartilage | Tensile modulus: around 330 MPa | Tensile strength: 55 MPa | N.A. | 1.0 g | N.A. | In vitro | 226 |
| HA/HDPE/MWCNT | Bone | N.A. | Flexural strength: around 150 MPa | N.A. | 1% | N.A. | In vitro | 227 |
| CS/gel/nHAp/0.6%MWCNTs scaffolds | Bone | Elastic modulus: around 6.5 MPa | N.A. | Around 90 | 0.6% | OD: around 1.5 | In vitro | 228 |
| CS–HAp–MWCNT | Bone | Young's modulus ca.: 4.88 GPa | Tensile strength ca. 76.21 MPa | N.A. | 0.5 wt% | Around 90 | In vitro | 229 |
| PVA/BCP scaffold | Bone | Elasticity modulus: 681 kPa | Compressive strength 81 ± 6 kPa | 80% ± 0.6% | 0.25% | N.A. | In vitro | 230 |
| MWCNTs/nHA/PCL composite | Bone | Modulus: nearly 95% | Compressive strength: 32.4 ± 1.6 MPa, tensile strength: 30.0 ± 1.4 MPa | N.A. | 1 wt% | N.A. | In vitro | 231 |
G/Ch scaffolds with two ratios (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3 1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Bone | Compressive modulus: 18.7 MPa, elastic modulus: 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 723 kPa 723 kPa |
Compressive strength: 411 kPa | ∼91 | 0.05 wt% | N.A. | In vitro | 232 |
| BMP-6/CCM scaffold | Calvarial bone | Compressive modulus: 0.84 ± 0.08 MPa | Compressive strength: 10.06 ± 0.07 MPa | 80.66 ± 1.99 | 1.5% | N.A. | In vivo | 233 |
3.3. Fibrous carbon nanostructures
Fibrous carbon nanostructures are considered one of the most important structures in enhancing physical attributes including mechanical properties due to their exceptional morphology.234 The most common way to produce fibrous structures is electrospinning.235 CFs are also known for their good electrical and thermal conductivity.236 Together with CFs, other types of fibrous carbon nanostructures, such as CNTs, multi-wall CNTs (MWCNT), and GO, are also investigated in this section.237,238CFs exhibit excellent mechanical, thermal, and electrical properties, and therefore have great potential for a variety of biomedical applications, including tissue engineering. Carbon nanofibers (CNFs), as a category of CFs, have a higher length-to-diameter ratio than CNTs. They also show significant mechanical properties and can act as reinforcements to increase the tensile and compressive strain limits of materials.236 In addition, nanofibrous structures can serve as an efficient framework for cell adhesion, proliferation, and differentiation, which differentiates them from other forms of nanomaterials.239
Improved mechanical property is an important factor in many materials used in biological applications, such as dental and orthopedic implants. In this regard, Han et al. combined the mechanical properties of polyether-ether-ketone (PEEK) with CFs using fused deposition modeling (FDM) printing technology and prepared CF-reinforced PEEK (CFR-PEEK) composites. The mechanical tensile, bending, and compressive strength improved from 95.21 MPa, 140.83 MPa, and 138.63 MPa to 101.41 MPa, 159.25 MPa, and 137.11 MPa respectively, which indicates that compared to pure PEEK, CFR-PEEK has much higher mechanical strength and can be used as a great candidate for dental and orthopedic implants in bone grafting and tissue engineering applications. Moreover, biological tests exhibited good biocompatibility for biological applications.240
Generally, PEEK is considered a suitable polymer for soft and hard tissue engineering due to its physical and chemical properties such as stability and radiolucency. However, it has a lower elastic modulus in the range of 3.7–4 GPa compared to that of human cortical bone of 7–30 GPa.241 In this case, CFs can be used to increase the stiffness of PEEK and enhance its utilization. For instance, Uddin and coworkers designed highly porous PEEK foams with CFs, carbon nanotubes, and hydroxyapatite (HA) particles through cast melting and salt porogen leaching techniques and used them as bone scaffold materials. The results showed that with the incorporation of these nanoparticles in the composites, the modulus and yield strength significantly increased, making them good candidates for biological applications, including bone regeneration and scaffolding.242 For instance, Naskar et al. fabricated bio-nanocomposites based on silk protein fibroin and reinforced them with CFs. These composites where further used for bone-tissue engineering applications. The addition of CF increased the compressive modulus by about 4.3-times compared to the control fibroin sponges. Besides having good mechanical properties, these composites were shown to be biocompatible and non-hemolytic.243 Using the electrospinning technique, other types of carbon nano-structures can be incorporated in fibrous systems. Mahmoodi and coworkers designed a PCL/keratin (Kr)/CNT scaffold using electrospinning to study the effect of CNTs-COOH on the fibrous scaffold in hard tissue engineering applications. The incorporation of nano carbon led to a decrease in the fiber diameter from 123 to 55 nm and an increase in porosity and mechanical tensile strength from 3 MPa for PCL/Kr to 8 MPa for PCL/Kr/CNT. The CNT-containing scaffolds showed more cell adhesion and growth due to their higher special surface area and hydrophilicity compared to the untreated PCL/Kr scaffolds.25
Generally, PCL is considered a frequently used polymer in biological applications.244 Tohidlou and coworkers embedded an amine-functionalized single-walled carbon nano-tube (aSWCNT) in PCL scaffolds for bone tissue engineering applications. By adding 0.2% of aSWCNT to PCL, the tensile strength increased from 1.6 MPa for pure PCL to 9.3 MPa for aSWCNT/PCL, which is very considerable. Also, the incorporation of aSWCNT led to an increase in cell proliferation, adhesion, and stem cell differentiation, together with the bioactivity and biodegradation rate of the biomaterial.33 In a different study, Abdal-hay and colleagues used air jet spinning to fabricate PCL nanofibers incorporated with MWCNTs. The tensile strength increased from 0.382 ± 0.1 for pure PCL to 0.483 ± 0.12 for PCL/1% MWCNT. Also, the tensile elastic modulus increased from 17 ± 3.5 for PCL alone to 110 ± 41.8 for PCL/0.5% MWCNT and 650 ± 74.5 for PCL/1% MWCNT nanofibers.245
Mechanical strength is an important factor in cardiac tissue engineering as well as hard tissue engineering. The implanted scaffolds used in this specific application must be able to recapitulate the environment of the heart.246 To achieve an effective scaffold for cardiac tissue engineering, Abedi and coworkers fabricated electro-conductive scaffolds based on chitosan (CS), PVA, and MNCNTs using electrospinning. Further, USSC stem cells were cultured on these scaffolds and were pushed into desired differentiation path using small molecules and electrical stimulation. The addition of 2% of MWCNT to this fibrous structure caused a decrease in the fiber dimeter by 115 nm and an improvement in tensile strength and their conductivity from 8 × 10−5 S m−1 to 9 × 10−3 S m−1, which are considered crucial factors for this application (Fig. 6).32
 | ||
| Fig. 6 (1–3) The preparation of electrospun scaffolds based on CS, PVA, and MWCNTs; (4) culturing USSC stem cells; (5) applying small molecules and electrical stimulation; and (6) the preparation of cardiac patches by combining the above-mentioned factors. Reprinted with permission from ref. 32 Elsevier, copyright 2021. | ||
In cardiac tissue engineering, the scaffold must mimic the native environment of the body with factors such as similar conductivity and mechanical behaviour.247 Therefore, incorporating conductive materials in scaffold structures can be beneficial to their behaviour. Considering their conductivity and appropriate mechanical properties, CNTs have shown interesting potential for these applications. To overcome limitations with the incorporation of these particles, Jiang and coworkers electrospun PCL/gelatin into a CNT bath and fabricated textile-based scaffolds using the resulting yarns. The test results showed that the incorporation of CNTs influenced the thermal and mechanical properties of the scaffolds. The yarns where also biocompatible and could guide cell elongation and alignment. The textile fabric resulting from these yarns was tested as a vascular scaffold, showing mechanical properties similar to native vessels.30
Another carbon nano-structure that can be incorporated in a fibrous structure is GO. GO is known for its hydrophilicity and conductivity and once combined with the appropriate material, it shows potential for different types of tissue engineering. For instance, Öztatlı et al. designed a PLLA/GO scaffold using electrospinning and tested its potential for nerve regeneration applications. PLLA is biocompatible and easy to shape but it also is hydrophobic and lacks proper binding sites. Accordingly, the addition of GO improved the physical, chemical, and mechanical properties of the scaffold including tensile and water contact angle, making it appropriate for nerve tissue engineering. By adding 10% of GO to the composite, the tensile strength increased up to 12.93 MPa, which was significantly higher than that of the neat PLLA nanofibers with a tensile strength of 2.25 MPa.248
Cartilage tissue, as a connecting tissue, plays an important role in protecting joints and bones. However, its injury is very common and it does not possess high regeneration capability. Therefore, it is necessary to develop new technologies and methods for the treatment of this important tissue.249 In this regard, Golshayan et al. used the electrospinning method to prepare poly(3-hydroxybutyrate)–chitosan/functionalized MWCNT (PHB–CS/f-MWCNT) with glucosamine sulfate (GAS) and tested its capabilities for cartilage tissue engineering. GAS is considered an important factor in cartilage tissue extracellular matrix and it improved the hydrophilicity of the fiber. Furthermore, the addition of CS to PHP decreased the Young's modulus from ∼4.71 to ∼4.20 MPa, while after the addition of nanotubes, this number increased to ∼7.24 MPa. The combination of the scaffolds with GAS decreased the tensile modulus slightly but the composite still had sufficient strength for cartilage tissue engineering application.250
Generally, one of the most important factors in designing new composites for different types of tissue engineering is that the designed composite must show similar mechanical properties with the target native tissue. For example, Deng and coworkers designed a novel composite consisting of CF, HA, and polyamide46 (PA46) and tested its mechanical properties and biocompatibility for bone repair applications. The addition of CF increased the bending strength from ∼116 to 159–223 MPa, tensile strength from ∼95 to 127–199 MPa, and tensile modulus from ∼4.6 to 7.7–10.8 GPa. The incorporation of CF also had positive effects on the biocompatibility of MG63 cells, indicating the potential of this composite for bone tissue engineering application.251 Using the electrospinning technique, Zhang and coworkers prepared CS/PVA/astaxanthin (ASTA) nanofibers and evaluated their morphological and mechanical properties together with biocompatibility and cytotoxicity. The addition of GO led to a decrease in the diameter of the nanofibers and mechanical tensile strength from 2.57 MPa to 2.24 MPa, which was further elevated up to 3.39 by adding ASTA to the fibrous structure. Bacteriostatic and cytotoxicity tests also showed Escherichia coli growth inhibition and good cell compatibility.252
Interestingly, the size differentiation appears to be non-decisive in the biocompatibility rate of fibrous carbons. For instance, Grabinski et al. prepared CFs and carbon nanofibers with different sizes and diameters (10 μm to 100 nm), which showed no significant cytotoxicity in a cell viability test.253
In conclusion, CFs can be beneficial in increasing the mechanical properties of composites used in different types of tissue engineering.254 They can be added to different materials to overcome their mechanical limitations.255 Moreover, by incorporating different types of carbon nanostructures in fibrous systems, their mechanical properties can be altered as desired for specific tissue engineering applications.256,257
3.4. Nanodiamonds
NDs, which are carbon-based nanomaterials with a tetrahedral structure and sp3 hybridization, are gaining traction in the biomedical field due to their high biocompatibility, simple surface functionalization, bright fluorescence, antimicrobial activity, chemical inertness, and superior mechanical properties including hardness, Young's modulus, high-pressure resistance, and great fracture toughness.258–260 ND composites include polymer components and types of NDs. The use of NDs increased the mechanical strength, thermal stability, elastic modulus, and tensile strength of biodegradable polymers.261 The fabrication of an appropriate biocompatible and biodegradable scaffold with mechanical strength similar to human bone is the primary challenge in bone tissue engineering. Recently, among the common bone implant materials (titanium), the ability of coatings containing diamond to increase the bone integrity, reinforce the mechanical properties of implants, ease the surface chemistry, and provide antimicrobial properties has been studied in the field of orthopedic implants.258Furthermore, recent studies have revealed the effect of various designing methods of diamond lattice on penetrability, successful cell culture, proper elastic modulus, etc.262 Following this investigation, Timercan et al. prepared lattice systems of gyroid and diamond that were mechanically flexible and penetrable to liquids, thus encouraging osseointegration and decreasing complexity risks, for instance, a lack of fixation, device migration, and subsidence in current intervertebral fusion devices. These structures were manufactured using Ti–6Al–4V and laser powder bed fusion. The Sy/E of the lattice structures (25–33 × 10−3) was considerably better than that of the bulk metals (∼10 × 10−3 for Ti–6Al–4V). Compared to Ti–6Al–4V, the modulus of elasticity and mechanical resistance were 1.9–4.8 GPa and 52–160 MPa (i.e., a 24–60-fold decline in Young's modulus, E, and 7–21-fold decline in yield stress), respectively.263
Besides, the surface of ND has an influential function in the design of utility and biocompatibility in the biological field. A combination of covalently functionalized UNCD surface, biocompatibility, and superior mechanical properties led to the use of UNCD as a hermetic coating for implantable artificial retina devices and as a guide to improve neural stem cell transplantation and neural tissue regeneration.264 In an important study, Liskova et al. revealed that O-terminated NCD films with high mechanical hardness and chemical resistance increased osteogenic cell differentiation and cell adhesion and growth.265 Element doping is the primary method for solving insufficient adhesion and bioinertness in carbon films, particularly DLC and a-C films. The design of a multifunctional Cu/a-C:H thin coating deposited on the Ti–6Al–4V alloy (TC4) via magnetron sputtering in the presence of Ar and CH4 for applications in bone implants was reported by Milan and coworkers. The results of the test revealed that with an increase in the copper concentration in a-C:H coatings, the ratio of sp2/sp3 also increased, and the interior stress intensity of the Cu/a-C:H bio-films decreased. Furthermore, with an increase in Cu concentration (from 30 wt% to 37 wt%), the Young's modulus decreased significantly from 88 GPa to 25 GPa; however, the hardness increased considerably from 7.8 GPa to 10 GPa. In Cu/a-C:H films with an increasing Cu content, arteries develop on the titanium alloy surface, which increases the likelihood of implants succeeding.266
Investigations on the shear modulus variations as a function of the volume of ND in hybrid substances indicated that the shear modulus of new nanocomposites comprised of pHEMA and NDs at concentrations 2% to 5% volume as a biomimetic biomaterial was comparable to that of the cortical bone (10–20 GPa). In the case of nano silica hybrids, similar results were obtained in higher concentrations of about 15% to 30% volume. Therefore, it is expected that hybrid nanocomposites possessing higher mechanical strength, biomimetic, osteoconductivity, and osteoinductivity properties, while overcoming the main weakness of hydrogels (i.e., poor mechanical characteristics) are suitable candidates for applications in cartilage cell tissue engineering.267,268
Nanocellulose/nanocarbon composites are another type of new hybrid materials proposed for biomedical applications, particularly for wound dressing. A CS/BC composite was prepared using chitosan-based biopolymers containing 33 wt% bacterial cellulose and 1 to 3 wt% MND. The mechanical properties of mats significantly increased by adding 1 wt% MND particles. Also, the ductility loss, elastic modulus (458 MPa), and yield strength (25.3 MPa) of the fibers increased and were close to that of natural skin. The results of the MTT assay demonstrated on L929 fibroblasts cultured on electrospun composite nanofibrous mats that the cell viability after a one-day incubation was higher than 90% compared with the control.259,269
A summary of the reports on ND-based composites and their mechanical properties in tissue engineering is shown in Table 3.
| Composite Structure | Type of tissue | Modulus | Strength | Porosity, % | Optimum amount of NDs | Cell viability, % | Model(s) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ND-OAD/PLLA | Bone marrow | Young's modulus: 7.9 GPa | N.A. | N.A. | 10 wt% | N.A. | In vitro | 260 |
| n-DP-PLA/poly (LLA-co-CL) | Bone | 112.1 MPa | N.A. | 92.2% | 10 wt% | N.A. | In vitro | 261 |
| Nb-C film | Bone mouse calvaria | Young's modulus: 191.7 GPa | N.A. | N.A. | 52.1 wt% | N.A. | In vitro | 270 |
| PVDF/BG scaffold | Bone | N.A. | Tensile strength: 59.61 MPa | N.A. | 1 wt% | N.A. | In vitro | 271 |
3.5. Other carbon-based nanocomposites
Other nanocarbon materials that can reinforce and modify the mechanical properties of nanocomposites include fullerenes, carbon black, active carbon (also called activated charcoal), CDs, and CQDs. Recently, a considerable number of studies focused on the use of nanocomposites based on these nanocarbons, which is ascribed to their extraordinary structural, electronic properties and high mechanical stability. Due to their features such as unique porosity with a large surface area/volume ratio, higher tensile strength and electrical conductivity, excellent solubility in water, high cell penetration ability, and acceptable biocompatibility, nanocarbon materials have been widely used in nanocomposites operated for a variety of biomedical purposes including drug delivery, wound dressing, bioimaging, and tissue engineering. Among these materials, one of the most attractive is fullerenes. Fullerenes are allotropic forms of carbon and exist in various forms, depending on the number of carbon atoms present in the molecule such as C60, C70, C80, and multi-layer fullerenes, which are described as carbon nano-onions (CNOs). The biological application of fullerene nanomaterials expands new scientific frontiers in tissue engineering. The biodegradable CS/PVA/ox-CNO scaffolds with potent biomedical applications were developed by Tovar et al. In this study, they used five different formulations of CS/PVA/ox-CNOs and evaluated their properties to achieve a targeted scaffold for tissue engineering. Studying the degradation of samples in an SBF showed that the samples without ox-CNOs lost 75% and 85% of their weight after 15 days, while the in vivo tests showed that after 90 days of subcutaneous implantation of films containing ox-CNOs in the tissue of Wistar rats, only partial degradation occurred without any immune responses and allergic reactions. All the results illustrated the biocompatibility of CS/PVA/ox-CNOs and strongly assured that the presence of ox-CNOs not only stabilized the films through chemical interactions with the CS/PVA chains, but also delayed the degradation. Alternatively, mechanical tests were performed for five different as-fabricated formulas, which showed an ascending trend in Young's modulus and tensile strength of the CS/PVA/ox-CNOs samples compared to CS/PVA composites. The hydrogen bond between the chitosan amino groups and carboxyl groups on the ox-CNO surface increased the tensile strength of the nanocomposite. Consequently, ox-CNO acts as an absorber of part of the mechanical stress imposed on the nanocomposite and amends the tensile strength. Incorporating low amounts of ox-CNOs (0.25, 0.50, and 0.75 wt%) in the CS/PVA composite created disorder and resulted in the loss of crystallinity and decreased Young's modulus. Besides, increasing the content of ox-CNO, caused an adequate and uniform dispersion of ox-CNO, stress harmonious distribution, modification of crystallinity (confirmed by XRD analysis), and finally an increase in Young's modulus. Thus, based on the results, the CS/PVA/ox-CNOs nanocomposite can be considered a conducive scaffold for skin tissue engineering.272 Following this investigation, Vedhanayagam and coworkers functionalized CBNs (including GO, rGR, fullerene C60, and CNT) with TES-PAMAM-G3, and then cross-linked collagen in them. These scaffolds were investigated as candidates for wound healing and tissue engineering in soft- and high-load bearing application. Compared to pure collagen, the CBN-TES-PAMAM-G3 cross-linked collagen showed a higher Young's modulus (close to natural bone tissue) and tensile strength, biocompatibility, controlled swelling, and enhanced degradability.273 In addition to fullerenes, other carbon nanoparticles utilized in biomedical applications include CB and AC. CB is a type of paracrystalline carbon that has a large surface-to-volume ratio, although this ratio is lower than AC. Activated carbon is a graphite-like and processed form of carbon with high porosity.259,274 Besides mechanical properties, conductive scaffolds based on CBNs have received significant attention due to the transmission of the electrical signal necessary to regulate cell behaviour in the engineering of tissues such as nerves, bone, and heart, which are electrically active. For example, Kai and coworkers developed a porous, hybrid memory-shape scaffold using PDMS and PCL blended with various amounts of CB. The results indicated that the addition of CB to the poly(PCL/PDMS urethane) nanofibers slowly increased the tensile strength (i.e., from 4.22 MPa for neat polymer to 4.81 MPa for CB-based nanofiber), while considerably decreasing the elongation and Young's modulus. Furthermore, the good biocompatibility, low degradation rate, intact morphology, increased electrical conductivity with an increase in CB content in the fibers and biostability make this a promising scaffold for nerve tissue engineering.275 In a recent study, the fabrication of the PAN/nCB/HA nanocomposite via electrospinning was presented by Haider et al. as a bioactive platform in bone tissue engineering. A substantial improvement in Young's modulus and tensile strength was observed in PAN/HA by incorporating 1 wt% nCB. This is because the polymer chains and CB nanoparticles interact strongly. Although there was a downward trend in Young's modulus and tensile strength with a change in the content of HA (from 5 wt% to 15 wt%), these values were still higher for PAN/nCB/HA than that without nCB. With the addition of nCB and HA to the PAN membrane, the swelling ratio increased, which can be related to the higher porosity of the scaffold, and also more hydroxyl groups on the surface. Overall, scaffolds based on nCB/HA can be successful in the regeneration of bone and other hard tissue.276,277 The in vitro biological assay of porous PVA-AC nanocomposites in osteoblast MG 63 cells showed that the PVA-AC scaffold facilitated osteoblast differentiation, cell proliferation, and cell growth without the addition of any extra growth factor. The findings of this research revealed that the modification of the polymer matrix with AC reinforcing agent improves the mechanical properties without disrupting the scaffold porosity as well as biological properties. Interestingly, the prepared scaffold has advantages in terms of morphology and porosity, which are essential for handling cell migration and transport of nutrients to the cells by allowing osteoblast growth inside the scaffold without cytotoxic effects. According to the mechanical behaviour assay, the presence of 2.5 wt% AC in the polymer matrix resulted in more than a 207% enhancement in tensile strength and 349% in Young's modulus, which was related to the homogeneous dispersion of AC with an increase in its concentration (from 0.5 wt% to 2.5 wt%) and effective stress transfer in the polymer matrix. Consequently, the obtained innovative nanocomposite demonstrated the applicable role of AC in bone tissue engineering.278 C-dots have emerged as a new class of fluorescent nano-sized carbon-based materials (<10 nm in size). These zero-dimensional carbon nanoparticles consist of carbon atoms with sp2 and sp3 hybridization in a quasi-spherical shape.279,280 Recently, the applications of C-dots as highly potential bone tissue engineering scaffolds and in diagnosis and therapies of bone-related diseases have been widely examined.281 Accordingly, the fabrication of a CD-decorated HAp/PU nanocomposite using waste and bio-based resources was designed as a tremendous potential bone regenerating scaffold by Gogoi et al. The CD-decorated HAp resulted in increased cell proliferation and deep cell adhesion and had no negative effect on osteoblast cells according to the cytotoxicity evaluations. The uniform dispersion of CD–HAp and strong interactions between the surface substitutions of the CDs and PVA chains through hydrogen bonding without any agglomeration in the matrix led to an increase in tensile strength, scratch hardness, and toughness, as calculated using the stress–strain curve of the CD–HAp/PU nanocomposites. Also, the layered structure of CD added to the polymer matrix improved the elongation at break of CD–HAp/PUs, followed by good flexibility, which is one of the requirements of bone implant materials.282 Numerous researchers have incorporated CBNs in hydrogels to enhance their biological, mechanical properties, and electrical conductivity, making them targeted nanoplatforms in biomedical applications.283 For example, the mechanical properties of as-prepared CD/HA/PVA double-network (DN) hydrogels were investigated based on the changes in three factors, including CD to PVA mass ratio (at 0.0, 1.5, 3.0, 4.5 and 6.0 wt% from CDs), HA to PVA (at 0.0, 0.2, 0.4, 0.6 and 0.8 wt% from HA) and the number of freezing/thawing cycles (from 0 to 9 cycles). Generally, after achieving the optimal conditions based on the above-mentioned parameters, the CDs3.0/HA0.6/PVA DN9 hydrogel exhibited excellent compression mechanical properties, i.e., compression strength of 3.462 MPa (about 477% increase) at a strain of 80%, and a notable enhancement of 429% in Young's modulus (4.5 kPa), introducing it as an outstanding potential cartilage/bone replacement in the field of biomedical engineering.284 Considering the presence of –OH groups in the structure of CDs, they highly influence the hydrophilicity and swelling ratio of the gel. Therefore, the CD/HA/PVA DN hydrogels with more freezing/thawing cycles exhibited a higher crosslinking density, and therefore decreased crosslinking ratio. In an interesting study, Ghanbari et al. developed chemically cross-linked OA/GEL/CNQD hydrogels based on OA, GEL, and CNQDs (with 0.06% CNQDs) as conducive scaffolds for injection in the regeneration of damaged cartilage tissue due to their cell viability (>97%), enhanced cell adhesion on MG 63 cells, and mechanical durability.285 Another study by Rastegar and coworkers described a notable improvement in tensile strength (5 MPa), Young's modulus (11 MPa), and elongation at break (10 mm) of a multifunctional PGS/PCL/CQD scaffold with the optimized weight ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) compared to PGS/PCL. Thus, the enhancement in mechanical and biological properties and electrical conductivity mainly created by the addition of CQDs make the PGS/PCL/CQD nanocomposite an immensely promising material for cardiac tissue engineering.286
0.5) compared to PGS/PCL. Thus, the enhancement in mechanical and biological properties and electrical conductivity mainly created by the addition of CQDs make the PGS/PCL/CQD nanocomposite an immensely promising material for cardiac tissue engineering.286
4. Advantages and disadvantages of carbon-based nanocomposites regarding mechanical properties in tissue engineering applications
The proliferation and migration of cells are dependent on the physicochemical interplay between cells and the surrounding extracellular matrix.287 Consequently, the mechanical properties of carbon-based nanomaterials used in tissue engineering play a vital role in affecting cellular behaviour. It was observed that cells cultured on substrates possessing greater stiffness exhibited a greater modulus, as well as a more elongated and well-structured actin cytoskeleton,288 and therefore the carbon-based nanocomposites utilized in the engineering of this tissue must exhibit sufficient mechanical strength to facilitate cellular growth and proliferation. In addition to possessing good mechanical properties, the scaffold used in tissue engineering must also provide sufficient porosity for cell growth. In this case, besides excellent mechanical strength, carbon-based nanomaterials exhibit notable porosity, which can accommodate cells and facilitate cellular infiltration.289 Furthermore, carbon-based nanoscale scaffolds possess a high surface-to-volume ratio, thereby revealing a heightened probability of molecular interactions with cells.290 Furthermore, the electrical property of carbon-based nanomaterials is an added advantage in tissue engineering, especially neural and cardiac regeneration. Electrical stimulation has the potential to activate numerous intracellular signaling pathways, thereby interfacing with the intracellular microenvironment, and subsequently affecting cellular processes such as migration, proliferation, and differentiation.72 However, the presence of impurities, non-uniform morphology and structure, hydrophobicity, and insolubility of carbon-based nanomaterials represent impediments that pose significant challenges for tissue engineering and biomedical applications.291In the field of biomedicine, the importance of water solubility and dispersibility cannot be overstated. Henceforth, it becomes indispensable to augment the hydrophilicity of carbon-based nanomaterials via diverse functionalization techniques, including the induction of covalent and non-covalent interactions.292,293 Another disadvantage of carbon-based nanomaterials is the production of reactive oxygen species and oxidative stress, which increases their toxicity to living systems. There are various methods to decrease the toxicity of nanomaterials, and one of them is surface functionalization. This technique involves altering the surface of nanomaterials to minimize their interaction with biological systems.294 Another method is encapsulation, which involves coating nanomaterials with a biocompatible substance to prevent them from interacting with biological systems. Additionally, managing the size and shape of nanomaterials can also minimize their toxicity.295 Thus, considering the advantages and disadvantages of using carbon-based nanomaterials in tissue engineering, it can be concluded that carbon nanomaterials can be utilized as novel compounds in biomedical applications.
5. Conclusions
Carbon-based nanocomposites appear well-suited as biomaterials and may become useful tools in tissue engineering to combine with the host body. They can be utilized in applications such as cellular imaging, biological and chemical sensing, bioactive agent delivery, and matrix engineering. However, although the novel uses of carbon-based nanocomposites for biomedical applications are being expanded, there are concerns about their cytotoxicity, which can be mitigated by chemical functionalization. Engineered scaffolds made of carbon-based nanocomposites have effectively overcome many limitations of existing treatments owing to their useful attributes including biocompatibility, biodegradability, porosity, NIR optical features, and mechanical and electrical properties. Mechanical properties including elasticity modulus, tensile strength, elongation, hardness, density, creep, toughness, brittleness, durability, stiffness, creep rupture, corrosion and wear, low CTE, and fatigue limit, are considered one of the key issues in tissue engineering. Because engineered scaffolds made of carbon-based nanocomposites should accommodate long-term tissue regeneration and ensure the stability of local physiological structures via controlled biodegradability and the maintenance of appropriate mechanical performance during degradation, surgery is no longer required to remove them. The porosity of these carbon-based nanocomposites also creates a suitable microenvironment for cell adhesion and proliferation, which is vital for tissue regeneration.Besides, these characteristics have shown a mutually restrictive relationship in in vivo and in vitro research. For instance, excessive porosity in carbon-based scaffolds may reduce mechanical strength. Conversely, too fast or too slow degradation may affect the repair. Therefore, the use of the appropriate dosage of carbon nanomaterials in composites and choosing the optimal production technique can lead to the preparation of scaffolds containing carbon nanomaterials that are more favorable for application in tissue engineering. Overall, carbon-based nanocomposites can be used as an important and unique component to impart the best mechanical properties to biomaterial to guide cell growth in combination with other biomaterials to potentially achieve tissue engineering goals.
Author contributions
R. Eivazzadeh-Keihan: conceptualization; visualization; validation; writing – original draft; writing – review & editing; project administration; supervision. Zahra Sadat: conceptualization; visualization; validation; writing – original draft; writing – review & editing. Farnaz Lalebeigi: formal analysis; data curation; conceptualization; visualization; validation; writing – original draft; writing – review & editing. Nooshin Naderi: formal analysis; data curation; visualization; validation; writing – original draft; writing – review & editing. Leila Panahi: conceptualization; visualization; validation; writing – original draft; writing – review & editing. Fatemeh Ganjali: visualization; validation; writing – original draft; writing – review & editing. Sakineh Mahdian: formal analysis; data curation; validation; writing – original draft; writing – review & editing. Z. Saadatidizaji: data curation; validation; writing – original draft; writing – review & editing. M. Mahdavi: visualization; writing – original draft; and writing – review & editing. Elham Chidar: visualization; writing – original draft; and writing – review & editing. Erfan Soleimani: visualization; writing – original draft; and writing – review & editing. Azadeh Ghaee: formal analysis; validation; writing – original draft; writing – review & editing. A. Maleki: conceptualization; writing – original draft; validation; and writing – review & editing; project administration; supervision. Iman Zare: formal analysis; writing – review & editing; validation; project administration; supervision.Conflicts of interest
The authors have reported no potential conflicts of interest.Acknowledgements
The authors express their gratitude for the partial support received from the Research Council of the Iran University of Science and Technology.Notes and references
- W. Wang, Y. Shi, G. Lin, B. Tang, X. Li, J. Zhang, X. Ding and G. Zhou, Macromol. Biosci., 2023, 2200539 CrossRef CAS PubMed.
- M. Kutz, Mechanical Engineers' Handbook, Volume 4: Energy and Power, John Wiley & Sons, 2015 Search PubMed.
- L. A. Carlsson, D. F. Adams and R. B. Pipes, Experimental Characterization of Advanced Composite Materials, CRC Press, 2014 Search PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, S. Asgharnasl, A. Maleki and H. Bahreinizad, J. Mater. Res. Technol., 2020, 9, 12244–12259 CrossRef CAS.
- H. Zhao, M. Liu, Y. Zhang, J. Yin and R. Pei, Nanoscale, 2020, 12, 14976–14995 RSC.
- R. Eivazzadeh-Keihan, F. Radinekiyan, H. Madanchi, H. A. M. Aliabadi and A. Maleki, Carbohydr. Polym., 2020, 248, 116802 CrossRef CAS PubMed.
- G. Wang, L. Liu and Z. Zhang, Composites, Part A, 2021, 141, 106212 CrossRef CAS.
- Á. Serrano-Aroca, K. Takayama, A. Tuñón-Molina, M. Seyran, S. S. Hassan, P. Pal Choudhury, V. N. Uversky, K. Lundstrom, P. Adadi and G. Palù, ACS Nano, 2021, 15, 8069–8086 CrossRef PubMed.
- R. Eivazzadeh-Keihan, A. Maleki, M. De La Guardia, M. S. Bani, K. K. Chenab, P. Pashazadeh-Panahi, B. Baradaran, A. Mokhtarzadeh and M. R. Hamblin, J. Adv. Res., 2019, 18, 185–201 CrossRef CAS PubMed.
- N. Naderi, F. Lalebeigi, Z. Sadat, R. Eivazzadeh-Keihan, A. Maleki and M. Mahdavi, Colloids Surf., B, 2023, 113430 CrossRef CAS PubMed.
- A. Gholami, S. A. Hashemi, K. Yousefi, S. M. Mousavi, W.-H. Chiang, S. Ramakrishna, S. Mazraedoost, A. Alizadeh, N. Omidifar and G. Behbudi, J. Nanomater., 2020, 2020, 1–24 Search PubMed.
- N. Saini, P. Pandey, M. Shirolkar, A. Kulkarni, S.-H. Moh and A. A. Kulkarni, Handbook of Porous Carbon Materials, 2023, pp. 1151–1172 Search PubMed.
- P. Singh, K. R. Singh, A. K. Yadav, J. Singh, P. R. Solanki and R. P. Singh, in Nanotechnological Aspects for Next-Generation Wound Management, Elsevier, 2024, pp. 193–218 Search PubMed.
- Z. Sadat, F. Farrokhi-Hajiabad, F. Lalebeigi, N. Naderi, M. G. Gorab, R. A. Cohan, R. Eivazzadeh-Keihan and A. Maleki, Biomed. Sci., 2022, 10, 6911–6938 CAS.
- Z. Peng, T. Zhao, Y. Zhou, S. Li, J. Li and R. M. Leblanc, Adv. Healthcare Mater., 2020, 9, 1901495 CrossRef CAS PubMed.
- M. Adel, P. Keyhanvar, I. Zare, Z. Tavangari, A. Akbarzadeh and M. Zahmatkeshan, J. Drug Delivery Sci. Technol., 2023, 90, 105130 CrossRef CAS.
- A. Haroun, Z. Gospodinova and N. Krasteva, Nano Biomed. Eng, 2021, 13, 380–393 CAS.
- L. S. Ulloa, F. Perissinotto, I. Rago, A. Goldoni, R. Santoro, M. Pesce, L. Casalis and D. Scaini, Nanomater, 2021, 11, 2724 CrossRef CAS PubMed.
- K. Kaur, S. Sa'Paiva, D. Caffrey, B. L. Cavanagh and C. M. Murphy, Mater. Sci. Eng., C, 2021, 128, 112340 CrossRef CAS PubMed.
- F. Chowdhury, M. Hosur and S. Jeelani, Mater. Sci. Eng., A, 2006, 421, 298–306 CrossRef.
- J. Zhu, W. Huang, L. Fu, B. Zhu, X. Li, X. Wang, Y. Wang and W. Chen, ACS Appl. Nano Mater., 2021, 4, 12453–12460 CrossRef CAS.
- R. Wazalwar, M. Sahu and A. M. Raichur, Nanoscale Adv., 2021, 3, 2741–2776 RSC.
- D. L. Butler, S. A. Goldstein and F. Guilak, J. Biomech. Eng., 2000, 122, 570–575 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, K. K. Chenab, R. Taheri-Ledari, J. Mosafer, S. M. Hashemi, A. Mokhtarzadeh, A. Maleki and M. R. Hamblin, Mater. Sci. Eng., C, 2020, 107, 110267 CrossRef CAS PubMed.
- M. Mahmoodi, V. Haghighi, M. Mirhaj, M. Tavafoghi, F. Shams and A. Darabi, Mater. Today Commun., 2021, 27, 102401 CrossRef CAS.
- S. Zheng, Y. Tian, J. Ouyang, Y. Shen, X. Wang and J. Luan, Front. Chem., 2022, 10, 990362 CrossRef CAS PubMed.
- A. A. Al-Allaq, J. S. Kashan, M. T. El-Wakad and A. M. Soliman, Mater. Technol., 2021, 55, 673–680 CAS.
- J. Wang, C. Huang, Y. Wang, Y. Chen, Z. Ding, C. Yang and L. Chen, Colloids Surf., A, 2020, 606, 125520 CrossRef CAS.
- F. Velasco-Mallorquí, J. M. Fernández-Costa, L. Neves and J. Ramón-Azcón, Nanoscale Adv., 2020, 2, 2885–2896 RSC.
- C. Jiang, K. Wang, Y. Liu, C. Zhang and B. Wang, ACS Biomater. Sci. Eng., 2021, 7, 2627–2637 CrossRef CAS PubMed.
- E. Díaz, J. Martín and J. León, Compos. Interfaces, 2021, 28, 511–525 CrossRef.
- A. Abedi, B. Bakhshandeh, A. Babaie, J. Mohammadnejad, S. Vahdat, R. Mombeiny, S. R. Moosavi, J. Amini and L. Tayebi, Mater. Chem. Phys., 2021, 258, 123842 CrossRef CAS.
- H. Tohidlou, S. S. Shafiei, S. Abbasi, M. Asadi-Eydivand and M. Fathi-Roudsari, Fibers Polym., 2019, 20, 1869–1882 CrossRef CAS.
- N. Saito, H. Haniu, K. Aoki, N. Nishimura and T. Uemura, Adv. Sci., 2022, 9, 2201214 CrossRef CAS PubMed.
- S. Loeve, in Between Nature and Society: Biographies of Materials, World Scientific, 2022, pp. 129–142 Search PubMed.
- R. S. Wils, N. R. Jacobsen, U. Vogel, M. Roursgaard and P. Møller, Toxicol. Sci., 2021, 183, 184–194 CrossRef CAS PubMed.
- S. Shamsi, A. A. Alagan, S. N. E. Sarchio and F. Md Yasin, Int. J. Nanomed., 2020, 8311–8329 CrossRef CAS PubMed.
- M. d'Amora, M. Rodio, J. Bartelmess, G. Sancataldo, R. Brescia, F. Cella Zanacchi, A. Diaspro and S. Giordani, Sci. Rep., 2016, 6, 33923 CrossRef PubMed.
- S. Bettini and G. Giancane, Nanomaterials, 2022, 12, 2738 CrossRef CAS PubMed.
- N. Dhiman, S. Ghosh, Y. K. Mishra and K. M. Tripathi, Mater. Adv., 2022, 3, 3101–3122 RSC.
- N. Panwar, A. M. Soehartono, K. K. Chan, S. Zeng, G. Xu, J. Qu, P. Coquet, K.-T. Yong and X. Chen, Chem. Rev., 2019, 119, 9559–9656 CrossRef CAS PubMed.
- M. Verdanova, B. Rezek, A. Broz, E. Ukraintsev, O. Babchenko, A. Artemenko, T. Izak, A. Kromka, M. Kalbac and M. Hubalek Kalbacova, Small, 2016, 12, 2499–2509 CrossRef CAS PubMed.
- A. Isakovic, Z. Markovic, B. Todorovic-Markovic, N. Nikolic, S. Vranjes-Djuric, M. Mirkovic, M. Dramicanin, L. Harhaji, N. Raicevic and Z. Nikolic, Toxicol. Sci., 2006, 91, 173–183 CrossRef CAS PubMed.
- L. Zhang, J. Xia, Q. Zhao, L. Liu and Z. Zhang, Small, 2010, 6, 537–544 CrossRef CAS PubMed.
- K.-H. Liao, Y.-S. Lin, C. W. Macosko and C. L. Haynes, ACS Appl. Mater. Interfaces, 2011, 3, 2607–2615 CrossRef CAS PubMed.
- S. Vardharajula, S. Z. Ali, P. M. Tiwari, E. Eroğlu, K. Vig, V. A. Dennis and S. R. Singh, Int. J. Nanomed., 2012, 5361–5374 CAS.
- J. Wang, Z. Hu, J. Xu and Y. Zhao, NPG Asia Mater., 2014, 6, e84 CrossRef CAS.
- C. M. Sayes, F. Liang, J. L. Hudson, J. Mendez, W. Guo, J. M. Beach, V. C. Moore, C. D. Doyle, J. L. West and W. E. Billups, Toxicol. Lett., 2006, 161, 135–142 CrossRef CAS PubMed.
- M. Marzana, M. M. A. Khan, A. Ahmed, M. A. Jalil and M. M. Hossain, in Nanomaterials for Biocatalysis, Elsevier, 2022, pp. 689–714 Search PubMed.
- A. Rehman, S. Houshyar and X. Wang, J. Biomed. Mater. Res., Part A, 2020, 108, 906–922 CrossRef CAS PubMed.
- Z. Xu, Y. Zhang, W. Zhou, L. Wang, G. Xu, M. Ma, F. Liu, Z. Wang, Y. Wang and T. Kong, J. Nanobiotechnol., 2021, 19, 1–11 CrossRef PubMed.
- A. M. Schrand, L. Dai, J. J. Schlager, S. M. Hussain and E. Osawa, Diamond Relat. Mater., 2007, 16, 2118–2123 CrossRef CAS.
- Y. Kang, C. Wang, Y. Qiao, J. Gu, H. Zhang, T. Peijs, J. Kong, G. Zhang and X. Shi, Biomacromolecules, 2019, 20, 1765–1776 CrossRef CAS PubMed.
- H. Mokhtari, M. Kharaziha, F. Karimzadeh and S. Tavakoli, Carbohydr. Polym., 2019, 214, 234–249 CrossRef CAS PubMed.
- B. Farshid, G. Lalwani, M. S. Mohammadi, J. S. Sankaran, S. Patel, S. Judex, J. Simonsen and B. Sitharaman, J. Biomed. Mater. Res., Part A, 2019, 107, 1143–1153 CrossRef CAS PubMed.
- G. Choe, S. Oh, J. M. Seok, S. A. Park and J. Y. Lee, Nanoscale, 2019, 11, 23275–23285 RSC.
- R. Eivazzadeh-Keihan, F. Radinekiyan, A. Maleki, M. S. Bani, Z. Hajizadeh and S. Asgharnasl, Int. J. Biol. Macromol., 2019, 140, 407–414 CrossRef CAS PubMed.
- K. Krukiewicz, D. Putzer, N. Stuendl, B. Lohberger and F. Awaja, Materials, 2020, 13, 2991 CrossRef CAS PubMed.
- M. Sabzevari, D. E. Cree and L. D. Wilson, Mater. Chem. Phys., 2019, 234, 81–89 CrossRef CAS.
- Z. Wright, A. Arnold, B. Holt, K. Eckhart and S. Sydlik, Regener. Eng. Transl. Med., 2019, 5, 190–209 CrossRef CAS.
- A. M. Díez-Pascual, in Materials for Biomedical Engineering, Elsevier, 2019, pp. 417–447 Search PubMed.
- C. S. Cabral, S. P. Miguel, D. de Melo-Diogo, R. O. Louro and I. J. Correia, Carbon, 2019, 146, 513–523 CrossRef CAS.
- M. Li, Y. Miao, X. Zhai, Y. Yin, Y. Zhang, Z. Jian, X. Wang, L. Sun and Z. Liu, Mater. Des., 2019, 181, 107961 CrossRef.
- A. Najafinezhad, H. Bakhsheshi-Rad, A. Saberi, A. Nourbakhsh, M. Daroonparvar, A. F. Ismail, S. Sharif, S. RamaKrishna, Y. Dai and F. Berto, Biomed. Mater., 2022, 17, 035011 CrossRef PubMed.
- M. M. H. Rumon, S. D. Sarkar, M. M. Uddin, M. M. Alam, S. N. Karobi, A. Ayfar, M. S. Azam and C. K. Roy, RSC Adv., 2022, 12, 7453–7463 RSC.
- K. N. Alagarsamy, S. Mathan, W. Yan, A. Rafieerad, S. Sekaran, H. Manego and S. Dhingra, Bioact. Mater., 2021, 6, 2261–2280 CAS.
- P. Yılmaz, E. öztürk Er and S. Bakırdere, J. Mater. Res. Technol., 2019, 8, 5201–5216 CrossRef.
- M. Vedhanayagam, B. U. Nair and K. J. Sreeram, Materialia, 2019, 7, 100354 CrossRef CAS.
- M. K. Satapathy, Y. B. Manga, K. K. Ostrikov, W.-H. Chiang, A. Pandey, B. Nyambat, E.-Y. Chuang and C.-H. Chen, ACS Appl. Mater. Interfaces, 2019, 12, 86–95 CrossRef PubMed.
- X. Yao, Z. Yan, X. Wang, H. Jiang, Y. Qian and C. Fan, Regener. Biomater., 2021, 8, rbab032 CrossRef PubMed.
- X. Qi, F. Jiang, M. Zhou, W. Zhang and X. Jiang, Smart Mater. Med., 2021, 2, 280–291 CrossRef.
- M. Islam, A. D. Lantada, D. Mager and J. G. Korvink, Adv. Healthcare Mater., 2022, 11, 2101834 CrossRef CAS PubMed.
- S. Ghosh and K. Chatterjee, Int. J. Nanomed., 2020, 15, 5991 CrossRef CAS PubMed.
- S. D. Purohit, R. Bhaskar, H. Singh, I. Yadav, M. K. Gupta and N. C. Mishra, Int. J. Biol. Macromol., 2019, 133, 592–602 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, E. Bahojb Noruzi, K. Khanmohammadi Chenab, A. Jafari, F. Radinekiyan, S. M. Hashemi, F. Ahmadpour, A. Behboudi, J. Mosafer and A. Mokhtarzadeh, J. Tissue Eng. Regener. Med., 2020, 14, 1687–1714 CrossRef CAS PubMed.
- H. Jodati, B. Yilmaz and Z. Evis, Ceram. Int., 2021, 47, 29535–29549 CrossRef CAS.
- R. Eivazzadeh-Keihan, F. Alimirzaloo, H. Aghamirza Moghim Aliabadi, E. Bahojb Noruzi, A. R. Akbarzadeh, A. Maleki, H. Madanchi and M. Mahdavi, Sci. Rep., 2022, 12, 6205 CrossRef CAS PubMed.
- M. Z. I. Nizami, S. Takashiba and Y. Nishina, Appl. Mater. Today, 2020, 19, 100576 CrossRef.
- L. Shang, Y. Qi, H. Lu, H. Pei, Y. Li, L. Qu, Z. Wu and W. Zhang, in Theranostic Bionanomaterials, Elsevier, 2019, pp. 165–185 Search PubMed.
- A. Maio, I. Pibiri, M. Morreale, F. P. L. Mantia and R. Scaffaro, Nanomaterials, 2021, 11, 1717 CrossRef CAS PubMed.
- A. Sadeghianmaryan, Y. Karimi, S. Naghieh, H. Alizadeh Sardroud, M. Gorji and X. Chen, Appl. Biochem. Biotechnol., 2020, 191, 567–578 CrossRef CAS PubMed.
- J. Li, H. Zeng, Z. Zeng, Y. Zeng and T. Xie, ACS Biomater. Sci. Eng., 2021, 7, 5363–5396 CrossRef CAS PubMed.
- J. Sun, S. Shakya, M. Gong, G. Liu, S. Wu and Z. Xiang, ChemistrySelect, 2019, 4, 5745–5754 CrossRef CAS.
- M. G. Tupone, G. Panella, M. d'Angelo, V. Castelli, G. Caioni, M. Catanesi, E. Benedetti and A. Cimini, Int. J. Mol. Sci., 2021, 22, 13047 CrossRef CAS PubMed.
- T. V. Patil, D. K. Patel, S. D. Dutta, K. Ganguly and K.-T. Lim, Molecules, 2021, 26, 2797 CrossRef CAS PubMed.
- M. A. J. Maras, S. F. Rahman and G. W. Hardi, AIP Conf. Proc., 2021, 2344, 020014 CrossRef CAS.
- A. Montazeri, F. Saeedi, Y. Bahari and A. Ahmadi Daryakenari, Polym. Polym. Compos., 2021, 29, S926–S936 CrossRef CAS.
- C. Fu, Y. Jiang, X. Yang, Y. Wang, W. Ji and G. Jia, Int. J. Nanomed., 2021, 16, 6693 CrossRef PubMed.
- C. Chen, Y. Xi and Y. Weng, Materials, 2022, 15, 2164 CrossRef CAS PubMed.
- M. U. A. Khan, S. I. A. Razak, A. Hassan, S. Qureshi and G. M. Stojanović, Front. Bioeng. Biotechnol., 2022, 10, 865059 CrossRef PubMed.
- S. J. Corr, M. Raoof, B. T. Cisneros, O. Kuznetsov, K. Massey, W. D. Kaluarachchi, M. A. Cheney, E. W. Billups, L. J. Wilson and S. A. Curley, Nanoscale Res. Lett., 2013, 8, 1–10 CrossRef PubMed.
- M. Mazaheri, O. Akhavan and A. Simchi, Appl. Surf. Sci., 2014, 301, 456–462 CrossRef CAS.
- J. Shen, B. Yan, T. Li, Y. Long, N. Li and M. Ye, Soft Matter, 2012, 8, 1831–1836 RSC.
- A. Iakunkov, N. Boulanger, A. Nordenström and A. V. Talyzin, Adv. Mater. Interfaces, 2021, 8, 2100552 CrossRef CAS.
- M. U. A. Khan, S. I. Abd Razak, H. Mehboob, M. R. Abdul Kadir, T. J. S. Anand, F. Inam, S. A. Shah, M. E. Abdel-Haliem and R. Amin, ACS Omega, 2021, 6, 4335–4346 CrossRef CAS PubMed.
- M. M. Kadhim, D. O. Bokov, M. J. Ansari, W. Suksatan, M. A. Jawad, S. Chupradit, M. N. Fenjan and M. Kazemnejadi, Bioprocess Biosyst. Eng., 2022, 45, 981–997 CrossRef CAS PubMed.
- C. Shuai, B. Peng, P. Feng, L. Yu, R. Lai and A. Min, J. Adv. Res., 2022, 35, 13–24 CrossRef CAS PubMed.
- Y. Zhao, J. Chen, L. Zou, G. Xu and Y. Geng, J. Chem. Eng., 2019, 378, 122174 CrossRef CAS.
- S. Bahrami, N. Baheiraei and M. Shahrezaee, Sci. Rep., 2021, 11, 1–10 CrossRef PubMed.
- M. Gong, J. Sun, G. Liu, L. Li, S. Wu and Z. Xiang, Mater. Sci. Eng., C, 2021, 119, 111603 CrossRef CAS PubMed.
- M. A. Shamekhi, H. Mirzadeh, H. Mahdavi, A. Rabiee, D. Mohebbi-Kalhori and M. B. Eslaminejad, Int. J. Biol. Macromol., 2019, 127, 396–405 CrossRef CAS PubMed.
- V. Nazar, M. Kashi, M. Haghbin Nazarpak, E. Shahryari and M. Mehrjoo, Int. J. Polym. Mater. Polym. Biomater., 2022, 1–12 Search PubMed.
- D. Trucco, L. Vannozzi, E. Teblum, M. Telkhozhayeva, G. D. Nessim, S. Affatato, H. Al-Haddad, G. Lisignoli and L. Ricotti, Adv. Healthcare Mater., 2021, 10, 2001434 CrossRef CAS PubMed.
- A. Mousavi, S. Mashayekhan, N. Baheiraei and A. Pourjavadi, Int. J. Biol. Macromol., 2021, 180, 692–708 CrossRef CAS PubMed.
- H. Nazari, A. Heirani-Tabasi, M. Hajiabbas, M. Khalili, M. Shahsavari Alavijeh, S. Hatamie, A. Mahdavi Gorabi, E. Esmaeili and S. H. Ahmadi Tafti, Polym. Adv. Technol., 2020, 31, 248–259 CrossRef CAS.
- M. Azizi, M. Navidbakhsh, S. Hosseinzadeh and M. Sajjadi, J. Polym. Res., 2019, 26, 1–9 CrossRef.
- X. Zhao, Z. Zhang, J. Luo, Z. Wu, Z. Yang, S. Zhou, Y. Tu, Y. Huang, Y. Han and B. Guo, Appl. Mater. Today, 2022, 26, 101365 CrossRef.
- N. Abzan, M. Kharaziha and S. Labbaf, Mater. Des., 2019, 167, 107636 CrossRef CAS.
- R. Jaswal, S. Shrestha, B. K. Shrestha, D. Kumar, C. H. Park and C. S. Kim, Mater. Sci. Eng., C, 2020, 116, 111213 CrossRef CAS PubMed.
- D. You, K. Li, W. Guo, G. Zhao and C. Fu, Int. J. Nanomed., 2019, 14, 7039 CrossRef CAS PubMed.
- A. Singh, S. L. Banerjee, V. Dhiman, S. K. Bhadada, P. Sarkar, M. Khamrai, K. Kumari and P. P. Kundu, Polymer, 2020, 195, 122436 CrossRef CAS.
- A. Salleh, N. Mustafa, Y. H. Teow, M. N. Fatimah, F. A. Khairudin, I. Ahmad and M. B. Fauzi, Biomedicines, 2022, 10, 816 CrossRef CAS PubMed.
- R. Al-Wafi, S. Mansour, M. AlHammad and M. Ahmed, Int. J. Pharm., 2021, 601, 120517 CrossRef CAS PubMed.
- M. U. Aslam Khan, A. Haider, S. I. Abd Razak, M. R. Abdul Kadir, S. Haider, S. A. Shah, A. Hasan, R. Khan, S. u. d. Khan and I. Shakir, J. Tissue Eng. Regener. Med., 2021, 15, 322–335 CrossRef CAS PubMed.
- P. H. M. Gohari, M. H. Nazarpak and M. Solati-Hashjin, Mater. Today Commun., 2021, 27, 102287 CrossRef.
- M. Azizi, M. Kalantar, N. Nezafati and A. Zamanian, J. Aust. Ceram. Soc., 2021, 57, 947–960 CrossRef CAS.
- H. Rezaei, M. Shahrezaee, M. J. Monfared, S. F. Karkan and R. Ghafelehbashi, J. Polym. Eng., 2021, 41, 375–386 CrossRef CAS.
- A. Olad, H. B. K. Hagh, A. Mirmohseni and F. F. Azhar, Ceram. Int., 2019, 45, 15609–15619 CrossRef CAS.
- M. U. A. Khan, M. Rizwan, S. I. A. Razak, A. Hassan, T. Rasheed and M. Bilal, J. Biomater. Sci., Polym. Ed., 2022, 1–20 Search PubMed.
- H. Belaid, S. Nagarajan, C. Teyssier, C. Barou, J. Barés, S. Balme, H. Garay, V. Huon, D. Cornu and V. Cavailles, Mater. Sci. Eng., C, 2020, 110, 110595 CrossRef CAS PubMed.
- M. Narimani, A. Teimouri and Z. Shahbazarab, Polym. Bull., 2019, 76, 725–745 CrossRef CAS.
- Y. Du, M. Yu, W. Lu and J. Kong, Composites, Part B, 2021, 225, 109270 CrossRef CAS.
- M. U. Aslam Khan, W. S. Al-Arjan, M. S. Binkadem, H. Mehboob, A. Haider, M. A. Raza, S. I. Abd Razak, A. Hasan and R. Amin, Nanomaterials, 2021, 11, 1319 CrossRef PubMed.
- M. Jafarkhani, Z. Salehi, Z. Bagheri, Z. Aayanifard, A. Rezvan, H. Doosthosseini and M. A. Shokrgozar, Can. J. Chem. Eng., 2020, 98, 62–68 CrossRef CAS.
- M. Ahmed, S. Mansour and R. Al-Wafi, Biomed. Mater., 2021, 16, 045030 CrossRef CAS PubMed.
- X. Li, J. Chen, Z. Xu, Q. Zou, L. Yang, M. Ma, L. Shu, Z. He and C. Ye, J. Mater. Sci.: Mater. Med., 2021, 31, 125 CrossRef PubMed.
- A. Pourjavadi, Z. Mazaheri Tehrani, H. Salami, F. Seidi, A. Motamedi, A. Amanzadi, E. Zayerzadeh and M. Shabanian, Polym. Eng. Sci., 2020, 60, 889–899 CrossRef CAS.
- H. Lee, J. M. Yoo, N. K. Ponnusamy and S. Y. Nam, Ceram. Int., 2022, 48, 10155–10163 CrossRef CAS.
- A. Ali, A. Hasan and Y. S. Negi, Int. J. Biol. Macromol., 2022, 197, 1–11 CrossRef CAS PubMed.
- X. Lang, Z. Tang, Z. Wei, X. Wang, S. Long, G. Zhang, J. Yang and Q. Lin, J. Innovative Opt. Health Sci., 2022, 15, 2250007 CrossRef CAS.
- F. Liu, C. Liu, B. Zheng, J. He, J. Liu, C. Chen, I.-s. Lee, X. Wang and Y. Liu, Polymers, 2020, 12, 69 CrossRef CAS PubMed.
- R. Mahdavi, G. Belgheisi, M. Haghbin-Nazarpak, M. Omidi, A. Khojasteh and M. Solati-Hashjin, J. Mater. Sci.: Mater. Med., 2020, 31, 1–14 CrossRef PubMed.
- N. More, A. Srivastava and G. Kapusetti, ACS Appl. Bio Mater., 2020, 3, 6823–6835 CrossRef CAS PubMed.
- D. Kaya and N. Alemdar, J. Appl. Polym. Sci., 2019, 136, 46905 CrossRef.
- M. Zou, J. Sun and Z. Xiang, Adv. Healthcare Mater., 2021, 10, 2001502 CrossRef CAS PubMed.
- M. R. Mahdavi, M. Kehtari, A. Mellati, R. N. Mansour, M. Mahdavi, M. Mahdavi and S. E. Enderami, Cell Biochem. Funct., 2022, 40, 203–212 CrossRef PubMed.
- Z. Huang, Y. Wan, X. Zhu, P. Zhang, Z. Yang, F. Yao and H. Luo, Mater. Sci. Eng., C, 2021, 123, 111967 CrossRef CAS PubMed.
- L. Wang, M. Fang, Y. Xia, J. Hou, X. Nan, B. Zhao and X. Wang, RSC Adv., 2020, 10, 10118–10128 RSC.
- J. Zhu, Z. Qi, C. Zheng, P. Xue, C. Fu, S. Pan and X. Yang, J. Nanomater., 2020, 2020, 1–15 Search PubMed.
- P. Dorishetty, R. Balu, A. Gelmi, J. P. Mata, A. Quigley, N. K. Dutta and N. R. Choudhury, Mater. Today Adv., 2022, 14, 100233 CrossRef CAS.
- Y. Hou, W. Wang and P. Bártolo, Int. J. Bioprint., 2020, 6, 266 CrossRef CAS PubMed.
- C. Shen, J. Liu, Q. Lu, G. Wang, Z. Wang and L. Liu, Int. J. Nanomed., 2022, 17, 681 CrossRef CAS PubMed.
- Z. Jing, X. Xian, Q. Huang, Q. Chen, P. Hong, Y. Li and A. Shi, New J. Chem., 2020, 44, 10390–10403 RSC.
- C. Qi, Y. Deng, L. Xu, C. Yang, Y. Zhu, G. Wang, Z. Wang and L. Wang, Theranostics, 2020, 10, 741 CrossRef CAS PubMed.
- J. Xue, X. Wang, E. Wang, T. Li, J. Chang and C. Wu, Acta Biomater., 2019, 100, 270–279 CrossRef CAS PubMed.
- K. B. Narayanan, G. T. Park and S. S. Han, Colloids Surf., B, 2020, 191, 110994 CrossRef CAS PubMed.
- X.-P. Li, K.-Y. Qu, B. Zhou, F. Zhang, Y.-Y. Wang, O. D. Abodunrin, Z. Zhu and N.-P. Huang, Colloids Surf., B, 2021, 205, 111844 CrossRef CAS PubMed.
- Y. Wang, H. Yu, H. Liu and Y. Fan, J. Bioact. Compat. Polym., 2020, 35, 216–227 CrossRef CAS.
- C. Zhang, X. Wang, S. Fan, P. Lan, C. Cao and Y. Zhang, Colloids Surf., B, 2021, 197, 111444 CrossRef CAS PubMed.
- S. Rethinam, A. W. Aruni, S. Vijayan, C. Munusamy and N. Gobi, J. Drug Delivery Sci. Technol., 2019, 53, 101163 CrossRef CAS.
- V. Kheiri Mollaqasem, A. Asefnejad, M. R. Nourani, V. Goodarzi and M. R. Kalaee, J. Appl. Polym. Sci., 2021, 138, 49797 CrossRef CAS.
- A. Xavier Mendes, S. Moraes Silva, C. D. O'Connell, S. Duchi, A. F. Quigley, R. M. Kapsa and S. E. Moulton, ACS Biomater. Sci. Eng., 2021, 7, 2279–2295 CrossRef CAS PubMed.
- W. Qin, C. Li, C. Liu, S. Wu, J. Liu, J. Ma, W. Chen, H. Zhao and X. Zhao, J. Biomater. Appl., 2022, 36, 1838–1851 CrossRef CAS PubMed.
- S. Mohammadi and A. Babaei, Int. J. Biol. Macromol., 2022, 201, 528–538 CrossRef CAS PubMed.
- J. H. Tsui, A. Leonard, N. D. Camp, J. T. Long, Z. Y. Nawas, R. Chavanachat, A. S. Smith, J. S. Choi, Z. Dong and E. H. Ahn, Biomaterials, 2021, 272, 120764 CrossRef CAS PubMed.
- S. B. Ryu, K. M. Park and K. D. Park, Biochem. Biophys. Res. Commun., 2022, 592, 24–30 CrossRef CAS PubMed.
- M. H. Norahan, M. Pourmokhtari, M. R. Saeb, B. Bakhshi, M. S. Zomorrod and N. Baheiraei, Mater. Sci. Eng., C, 2019, 104, 109921 CrossRef CAS PubMed.
- H. S. Budi, M. J. Ansari, S. A. Jasim, W. K. Abdelbasset, D. Bokov, Y. F. Mustafa, M. A. Najm and M. Kazemnejadi, Inorg. Chem. Commun., 2022, 139, 109336 CrossRef.
- A. A. Aly and M. Ahmed, Int. J. Pharm., 2021, 598, 120325 CrossRef CAS PubMed.
- B. Huang, Biomanuf. Rev., 2020, 5, 3 CrossRef.
- P. Yupapin, C. Mahesha, H. Fouladi, A. Hamidi and A. Farmani, Silicon, 2022, 1–7 Search PubMed.
- N. Anzar, R. Hasan, M. Tyagi, N. Yadav and J. Narang, Sens. Int., 2020, 1, 100003 CrossRef.
- V. Kumar and H. Kumar, Environ. Appl. Carbon Nanomater.-Based Devices, 2021, 423–438 Search PubMed.
- T. V. Patil, D. K. Patel, S. D. Dutta, K. Ganguly, A. Randhawa and K.-T. Lim, Appl. Sci., 2021, 11, 9550 CrossRef.
- J. M. Schnorr and T. M. Swager, Chem. Mater., 2011, 23, 646–657 CrossRef CAS.
- A. Kumar, K. Sharma and A. R. Dixit, Carbon Lett., 2021, 31, 149–165 CrossRef.
- J. N. Coleman, U. Khan, W. J. Blau and Y. K. Gun’ko, Carbon, 2006, 44, 1624–1652 CrossRef CAS.
- A. Raunika, S. A. Raj, K. Jayakrishna and M. Sultan, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 270, 012027 Search PubMed.
- B. Portillo-Rodríguez, J. Sanchez-Vasquez, M. Reyes-Reyes and R. Lopez-Sandoval, Diamond Relat. Mater., 2022, 121, 108720 CrossRef.
- R. Maheswaran and B. P. Shanmugavel, J. Electron. Mater., 2022, 1–15 Search PubMed.
- S. Bosi, L. Ballerini and M. Prato, Making and Exploiting Fullerenes, Graphene, and Carbon Nanotubes, 2013, pp. 181–204 Search PubMed.
- R. Sorkin, A. Greenbaum, M. David-Pur, S. Anava, A. Ayali, E. Ben-Jacob and Y. Hanein, Nanotechnology, 2008, 20, 015101 CrossRef PubMed.
- X. Li, W. Yang, H. Xie, J. Wang, L. Zhang, Z. Wang and L. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 36860–36872 CrossRef CAS PubMed.
- J. Wang, L. Tian, N. Chen, S. Ramakrishna and X. Mo, Mater. Sci. Eng., C, 2018, 91, 715–726 CrossRef CAS PubMed.
- T. Zhu, Y. Cui, M. Zhang, D. Zhao, G. Liu and J. Ding, Bioact. Mater., 2020, 5, 584–601 Search PubMed.
- C. Shuai, B. Peng, M. Liu, S. Peng and P. Feng, Mater. Today Adv., 2021, 11, 100158 CrossRef CAS.
- Z. Wang, L. Zhang, R. Zhang and M. Wang, Mater. Lett., 2022, 315, 131974 CrossRef CAS.
- S. Sang, R. Cheng, Y. Cao, Y. Yan, Z. Shen, Y. Zhao and Y. Han, J. Zhejiang Univ., Sci., B, 2022, 23, 58–73 CrossRef CAS PubMed.
- M. E. David, R. M. Ion, R. M. Grigorescu, L. Iancu, A. M. Holban, F. Iordache, A. I. Nicoara, E. Alexandrescu, R. Somoghi and S. Teodorescu, Nanomaterials, 2022, 12, 239 CrossRef CAS PubMed.
- X. Tong, J. Zheng, Y. Lu, Z. Zhang and H. Cheng, Mater. Lett., 2007, 61, 1704–1706 CrossRef CAS.
- M. Zhang, M. Yang, H. Nakajima, M. Yudasaka, S. Iijima and T. Okazaki, ACS Appl. Nano Mater., 2019, 2, 4293–4301 CrossRef CAS.
- E. Murugan, C. Akshata, V. Yogaraj, G. Sudhandiran and D. Babu, Ceram. Int., 2022, 48, 16000–16009 CrossRef CAS.
- M. L. Flores-Cedillo, R. Rosales-Ibáñez, M. Martin-del-Campo-Fierro, A. Reyes-Matute, J. V. Cauich-Rodríguez, L. A. Oros-Méndez, Á. L. Rodríguez-Morales and M. O. L. Flores-Sánchez, Polym. Bull., 2021, 1–15 Search PubMed.
- B. Huang, C. Vyas, J. J. Byun, M. El-Newehy, Z. Huang and P. Bártolo, Mater. Sci. Eng., C, 2020, 108, 110374 CrossRef CAS PubMed.
- P. Bahrami Miyanji, D. Semnani, A. Hossein Ravandi, S. Karbasi, A. Fakhrali and S. Mohammadi, Polym. Adv. Technol., 2022, 33, 81–95 CrossRef CAS.
- H. Akbari-Aghdam, A. Bagherifard, M. Motififard, J. Parvizi, E. Sheikhbahaei, S. Esmaeili, S. Saber-Samandari and A. Khandan, Arch. Bone Jt. Surg., 2021, 9, 445 Search PubMed.
- T. Hu, M. Shi, X. Zhao, Y. Liang, L. Bi, Z. Zhang, S. Liu, B. Chen, X. Duan and B. Guo, J. Chem. Eng., 2022, 428, 131017 CrossRef CAS.
- T. C. Suh, J. Twiddy, N. Mahmood, K. M. Ali, M. M. Lubna, P. D. Bradford, M. A. Daniele and J. M. Gluck, ACS Omega, 2022, 7, 20006–20019 CrossRef CAS PubMed.
- F. Tondnevis, H. Keshvari and J. A. Mohandesi, J. Biomed. Mater. Res., Part B, 2020, 108, 2276–2293 CrossRef CAS PubMed.
- S. Naskar, A. K. Panda, A. Jana, S. Kanagaraj and B. Basu, J. Biomed. Mater. Res., Part B, 2020, 108, 2320–2343 CrossRef CAS PubMed.
- S. Mombini, J. Mohammadnejad, B. Bakhshandeh, A. Narmani, J. Nourmohammadi, S. Vahdat and S. Zirak, Int. J. Biol. Macromol., 2019, 140, 278–287 CrossRef CAS PubMed.
- P. Falamarzpour and S. R. Ghaffarian Anbaran, J. Polym. Res., 2022, 29, 1–10 CrossRef.
- F. Luo, R. Li, H. Zheng, Y. Xu, L. Yang, C. Qu, G. Hong and Q. Wan, Front. Bioeng. Biotechnol., 2022, 10, 913080 CrossRef PubMed.
- A. A. Mieloch, J. A. Semba and J. D. Rybka, Int. J. Bioprint., 2022, 8, 548 CrossRef CAS PubMed.
- M. Li, Q. Zhou, H. Song, Y. Lu, M. Lu, X. Lu, K. Duan and J. Weng, Mater. Lett., 2022, 314, 131780 CrossRef CAS.
- Q. Li, C. Yue, T. Chen, C. Ding and H. Zhang, AIP Adv., 2022, 12, 055124 CrossRef CAS.
- M. Parvizifard and S. Karbasi, Int. J. Biol. Macromol., 2020, 152, 645–662 CrossRef CAS PubMed.
- M. Masoumi, M. Jahanshahi, M. G. Ahangari and G. N. Darzi, Appl. Surf. Sci., 2021, 546, 148894 CrossRef CAS.
- A. A. Al-Allaq, J. S. Kashan, M. T. El-Wakad and A. M. Soliman, J. Ceram. Process. Res., 2021, 22, 446–454 Search PubMed.
- L. Sukhodub, L. Sukhodub, M. Kumeda, Y. I. Prylutskyy, M. Pogorielov, M. Evstigneev, V. Kostjukov, N. Strutynska, L. Vovchenko and S. Khrapatiy, Prog. Biomater., 2020, 9, 1–14 CrossRef CAS PubMed.
- J. Zhang, L. Zeng, Z. Qiao, J. Wang, X. Jiang, Y. S. Zhang and H. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 30247–30258 CrossRef CAS PubMed.
- B. Huang, C. Vyas, I. Roberts, Q.-A. Poutrel, W.-H. Chiang, J. J. Blaker, Z. Huang and P. Bártolo, Mater. Sci. Eng., C, 2019, 98, 266–278 CrossRef CAS PubMed.
- E. M. Steel, J.-Y. Azar and H. G. Sundararaghavan, Materialia, 2020, 9, 100581 CrossRef CAS.
- L. Liu, B. Yang, L.-Q. Wang, J.-P. Huang, W.-Y. Chen, Q. Ban, Y. Zhang, R. You, L. Yin and Y.-Q. Guan, J. Mater. Chem. B, 2020, 8, 558–567 RSC.
- C. D. Grande Tovar, J. I. Castro, C. H. Valencia, D. P. Navia Porras, J. Herminsul Mina Hernandez, M. E. Valencia Zapata and M. N. Chaur, Molecules, 2020, 25, 1203 CrossRef PubMed.
- J. J. Pavón, D. López, F. Mondragón, J. Gallego, S. L. Arias, K. Luitjohan, B. Holybee, Y. Torres, J. A. Rodríguez and M. Echeverry-Rendón, J. Biomed. Mater. Res., Part A, 2019, 107, 719–731 CrossRef PubMed.
- M. H. Mirmusavi, P. Zadehnajar, D. Semnani, S. Karbasi, F. Fekrat and F. Heidari, Int. J. Biol. Macromol., 2019, 132, 822–835 CrossRef CAS PubMed.
- A. Dominguez-Alfaro, N. Alegret, B. Arnaiz, J. M. González-Domínguez, A. Martin-Pacheco, U. Cossío, L. Porcarelli, S. Bosi, E. Vázquez and D. Mecerreyes, ACS Biomater. Sci. Eng., 2019, 6, 1269–1278 CrossRef PubMed.
- M. Zarei, N. Tanideh, S. Zare, F. Sari Aslani, O. Koohi-Hosseinabadi, R. Muthuraj, I. Jamhiri, A. Rowshanghias and P. Mehryar, J. Bioact. Compat. Polym., 2019, 34, 386–400 CrossRef CAS.
- S. Saber-Samandari, M. Mohammadi-Aghdam and S. Saber-Samandari, Int. J. Biol. Macromol., 2019, 138, 810–818 CrossRef CAS PubMed.
- X. Liu, M. Chang, B. He, L. Meng, X. Wang, R. Sun, J. Ren and F. Kong, J. Compos. Sci., 2019, 538, 507–518 CAS.
- A. M. Deliormanlı and H. Atmaca, Int. J. Polym. Mater. Polym. Biomater., 2019, 68, 1154–1166 CrossRef.
- A. Shafiee, M. Kehtari, Z. Zarei, M. Soleimani, R. Varshochian, A. Ahmadi, F. Atyabi and R. Dinarvand, Mater. Sci. Eng., C, 2021, 120, 111739 CrossRef CAS PubMed.
- W. Wang, B. Huang, J. J. Byun and P. Bártolo, J. Mech. Behav. Biomed. Mater., 2019, 93, 52–60 CrossRef CAS PubMed.
- S. Shrestha, B. K. Shrestha, J. Lee, O. K. Joong, B.-S. Kim, C. H. Park and C. S. Kim, Mater. Sci. Eng., C, 2019, 102, 511–523 CrossRef CAS PubMed.
- S. Zhang, H. Liu, J. Gou, J. Ying, Y. Wang, C. Liu and C. Shen, Polym. Test., 2019, 77, 105904 CrossRef CAS.
- Z. Deng, T. Hu, Q. Lei, J. He, P. X. Ma and B. Guo, ACS Appl. Mater. Interfaces, 2019, 11, 6796–6808 CrossRef CAS PubMed.
- C. D. Grande Tovar, J. I. Castro, C. H. Valencia, D. P. Navia Porras, J. H. Mina Hernandez, M. E. Valencia, J. D. Velasquez and M. N. Chaur, Biomolecules, 2019, 9, 684 CrossRef PubMed.
- J. Cao, Y. Lu, H. Chen, L. Zhang and C. Xiong, Int. J. Polym. Mater. Polym. Biomater., 2019, 68, 433–441 CrossRef CAS.
- P. Makvandi, M. Ashrafizadeh, M. Ghomi, M. Najafi, H. H. S. Hossein, A. Zarrabi, V. Mattoli and R. S. Varma, Prog. Biomater., 2021, 10, 77–89 CrossRef CAS PubMed.
- M. Mehdikhani and S. Ghaziof, Appl. Phys. A, 2018, 124, 1–15 CrossRef CAS.
- F. Pouladzadeh, A. A. Katbab, N. Haghighipour and E. Kashi, Eur. Polym. J., 2018, 105, 286–296 CrossRef CAS.
- Z. Deng, Y. Guo, X. Zhao, P. X. Ma and B. Guo, Chem. Mater., 2018, 30, 1729–1742 CrossRef CAS.
- M. H. Mirmusavi, S. Karbasi, D. Semnani, M. Rafienia and A. Zargar Kharazi, Micro Nano Lett., 2018, 13, 829–834 CrossRef CAS.
- W. Pan, X. Xiao, J. Li, S. Deng, Q. Shan, Y. Yue, Y. Tian, N. R. Nabar, M. Wang and L. Hao, J. Mater. Sci.: Mater. Med., 2018, 29, 1–14 CrossRef CAS PubMed.
- S. Sharmeen, A. M. Rahman, M. M. Lubna, K. S. Salem, R. Islam and M. A. Khan, Bioact. Mater., 2018, 3, 236–244 Search PubMed.
- A. A. Al-allaq, J. S. Kashan, M. T. El-Wakad and A. M. Soliman, Period. Eng. Nat. Sci., 2021, 9, 930–939 Search PubMed.
- J. Xu, X. Hu, S. Jiang, Y. Wang, R. Parungao, S. Zheng, Y. Nie, T. Liu and K. Song, Polymers, 2019, 11, 230 CrossRef PubMed.
- A. G. Sanchez, E. Prokhorov, G. Luna-Barcenas, J. Hernández-Vargas, R. Román-Doval, S. Mendoza and H. Rojas-Chávez, Mater. Today Commun., 2021, 28, 102615 CrossRef CAS.
- W. Lan, X. Zhang, M. Xu, L. Zhao, D. Huang, X. Wei and W. Chen, RSC Adv., 2019, 9, 38998–39010 RSC.
- H. Yang, J. Li, Q. Liao, H. Guo, H. Chen, Y. Zhu, M. Cai and H. Lv, J. Mater. Res. Technol., 2019, 34, 532–544 CrossRef CAS.
- A. S. Mesgar, Z. Mohammadi and S. Khosrovan, Int. J. Polym. Mater. Polym. Biomater., 2018, 67, 267–276 CrossRef CAS.
- R. Zhang, X. Li, M. Yang, X. Gao, T. Zhu and L. Lu, J. Hard Tissue Biol., 2019, 28, 217–224 CrossRef CAS.
- F. Tondnevis, H. Keshvari and J. A. Mohandesi, J. Polym. Res., 2019, 26, 1–16 CrossRef.
- A. Kausar, J. Plast. Film Sheeting, 2021, 37, 70–92 CrossRef CAS.
- C. M. Das, L. Kang, G. Yang, D. Tian and K.-T. Yong, J. Compos. Sci., 2020, 4, 117 CrossRef CAS.
- M. Nikbakht, S. Karbasi, S. M. Rezayat, S. Tavakol and E. Sharifi, Polym.-Plast. Technol. Mater., 2019, 58, 2031–2040 CAS.
- T. D. Stocco, E. Antonioli, M. L. Romagnolli, G. F. Sousa, M. Ferretti and A. O. Lobo, Mater. Lett., 2020, 264, 127351 CrossRef CAS.
- R. Vasita and D. S. Katti, Int. J. Nanomed., 2006, 1, 15–30 CrossRef CAS PubMed.
- X. Han, D. Yang, C. Yang, S. Spintzyk, L. Scheideler, P. Li, D. Li, J. Geis-Gerstorfer and F. Rupp, J. Clin. Med., 2019, 8, 240 CrossRef CAS PubMed.
- C. Liao, Y. Li and S. C. Tjong, Polymers, 2020, 12, 2858 CrossRef CAS PubMed.
- M. Uddin, P. S. Dhanasekaran and R. Asmatulu, Prog. Biomater., 2019, 8, 211–221 CrossRef CAS PubMed.
- D. Naskar, A. K. Ghosh, M. Mandal, P. Das, S. K. Nandi and S. C. Kundu, Biomaterials, 2017, 136, 67–85 CrossRef CAS PubMed.
- G. P. Awasthi, V. K. Kaliannagounder, J. Park, B. Maharjan, M. Shin, C. Yu, C. H. Park and C. S. Kim, Colloids Surf., A, 2021, 622, 126584 CrossRef CAS.
- A. Abdal-hay, M. Taha, H. M. Mousa, M. Bartnikowski, M. L. Hassan, M. Dewidar and S. Ivanovski, Ceram. Int., 2019, 45, 15736–15740 CrossRef CAS.
- P. R. Schmitt, K. D. Dwyer and K. L. Coulombe, ACS Appl. Bio Mater., 2022, 5, 2461–2480 CrossRef CAS PubMed.
- A. Savchenko, R. T. Yin, D. Kireev, I. R. Efimov and E. Molokanova, Front. Bioeng. Biotechnol., 2021, 9, 797340 CrossRef PubMed.
- H. Öztatlı and D. Ege, MRS Adv., 2017, 2, 1291–1296 CrossRef.
- L. Roseti and B. Grigolo, J. Exp. Orthop., 2022, 9, 1–7 CrossRef PubMed.
- N. S. Golshayan, S. Karbasi, E. Masaeli, E. Bahremandi-Toloue, M. H. Nasr-Esfahani and M. Rafienia, Polym.-Plast. Technol. Mater., 2022, 61, 1244–1264 CAS.
- Z. Deng, H. Han, J. Yang, Y. Li, S. Du and J. Ma, Med. Sci. Monit., 2017, 23, 2479 CrossRef CAS PubMed.
- H. Zhang, L. Lin, L. Ren, Y. Liu and R. Song, J. Appl. Polym. Sci., 2021, 138, 50166 CrossRef CAS.
- C. Grabinski, S. Hussain, K. Lafdi, L. Braydich-Stolle and J. Schlager, Carbon, 2007, 45, 2828–2835 CrossRef CAS.
- J. S. Czarnecki, K. Lafdi, R. M. Joseph and P. A. Tsonis, Tissue Eng., Part A, 2012, 18, 946–956 CrossRef CAS PubMed.
- Y. Han, Y. Lv, J. Chen, M. Yao, C. Wang, P. Hu and Y. Liu, ACS Appl. Polym. Mater., 2021, 3, 4195–4202 CrossRef CAS.
- P. Zadehnajar, B. Akbari, S. Karbasi and M. H. Mirmusavi, Int. J. Polym. Mater. Polym. Biomater., 2020, 69, 326–337 CrossRef CAS.
- P. Zadehnajar, S. Karbasi, B. Akbari and L. Ghasemi, Mater. Technol., 2020, 35, 39–49 CrossRef CAS.
- J. S. L. Fong, M. A. Booth, A. Rifai, K. Fox and A. Gelmi, Adv. Healthcare Mater., 2021, 10, e2100007 CrossRef PubMed.
- L. Bacakova, J. Pajorova, M. Tomkova, R. Matejka, A. Broz, J. Stepanovska, S. Prazak, A. Skogberg, S. Siljander and P. Kallio, Nanomaterials, 2020, 10, 196 CrossRef CAS PubMed.
- Q. Zhang, V. N. Mochalin, I. Neitzel, I. Y. Knoke, J. Han, C. A. Klug, J. G. Zhou, P. I. Lelkes and Y. Gogotsi, Biomaterials, 2011, 32, 87–94 CrossRef CAS PubMed.
- Y. Sun, A. Finne-Wistrand, T. Waag, Z. Xing, M. Yassin, A. Yamamoto, K. Mustafa, D. Steinmüller-Nethl, A. Krueger and A.-C. Albertsson, Macromol. Mater. Eng., 2015, 300, 436–447 CrossRef CAS.
- D. Karaman and H. Ghahramanzadeh Asl, Proc. Inst. Mech. Eng., Part H, 2022, 236, 794–810 CrossRef PubMed.
- A. Timercan, V. Sheremetyev and V. Brailovski, Sci. Technol. Adv. Mater., 2021, 22, 285–300 CrossRef CAS PubMed.
- Y. C. Chen, D. C. Lee, N. H. Tai and I. M. Chiu, Diamond-Based Materials for Biomedical Applications, 2013, pp. 171–185 Search PubMed.
- J. Liskova, O. Babchenko, M. Varga, A. Kromka, D. Hadraba, Z. Svindrych, Z. Burdikova and L. Bacakova, Int. J. Nanomed., 2015, 10, 869–884 CrossRef PubMed.
- P. B. Milan, S. Khamseh, P. Zarrintaj, B. Ramezanzadeh, M. Badawi, S. Morisset, H. Vahabi, M. R. Saeb and M. Mozafari, Heliyon, 2020, 6, e03798 CrossRef PubMed.
- R. Aversa, R. V. V. Petrescu, A. Apicella and F. I. T. Petrescu, Eng. Rev., 2019, 39, 81–89 CrossRef.
- R. Aversa, R. V. V. Petrescu, A. Apicella and F. I. T. Petrescu, Am. J. Biochem. Biotechnol., 2017, 13, 34–41 CrossRef CAS.
- M. Mahdavi, N. Mahmoudi, F. Rezaie Anaran and A. Simchi, Mar. Drugs, 2016, 14, 128 CrossRef PubMed.
- L. Yate, L. E. Coy, D. Gregurec, W. Aperador, S. E. Moya and G. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 6351–6358 CrossRef CAS PubMed.
- C. Shuai, W. Huang, P. Feng, C. Gao, D. Gao, Y. Deng, Q. Wang, P. Wu and X. Guo, J. Porous Mater., 2016, 24, 249–255 CrossRef.
- C. D. Grande Tovar, J. I. Castro, C. H. Valencia, D. P. Navia Porras, J. H. Mina Hernandez, M. E. Valencia, J. D. Velasquez and M. N. Chaur, Biomolecules, 2019, 9, 684 CrossRef PubMed.
- M. Vedhanayagam, B. U. Nair and K. J. Sreeram, Materialia, 2019, 7, 100354 CrossRef CAS.
- A. Kausar, J. Plast. Film Sheeting, 2020, 36, 409–429 CrossRef CAS.
- D. Kai, M. J. Tan, M. P. Prabhakaran, B. Q. Y. Chan, S. S. Liow, S. Ramakrishna and X. J. Loh, Colloids Surf., B, 2016, 148, 557–565 CrossRef CAS PubMed.
- M. K. Haider, L. Sun, A. Ullah, S. Ullah, Y. Suzuki, S. Park, Y. Kato, Y. Tamada and I. S. Kim, Mater. Today Commun., 2021, 27, 102259 CrossRef CAS.
- S. Yousefiasl, M. Imani, I. Zare, S. Samaei, R. E. Ashtiani and E. Sharifi, Electrically Conducting Polymers and Their Composites for Tissue Engineering, 2023, pp. 109–136 Search PubMed.
- T. Kaur and A. Thirugnanam, J. Mater. Sci. Technol., 2017, 33, 734–743 CrossRef CAS.
- N. C. Jeremy Kong Yoong Lee, S. Peng, L. Li, L. Tian, N. Thakor and S. Ramakrishna, Prog. Polym. Sci., 2018, 86, 40–84 CrossRef.
- M. Vedhanayagam, I. S. Raja, A. Molkenova, T. S. Atabaev, K. J. Sreeram and D. W. Han, Int. J. Mol. Sci., 2021, 22, 5378 CrossRef CAS PubMed.
- Z. Peng, T. Zhao, Y. Zhou, S. Li, J. Li and R. M. Leblanc, Adv. Healthcare Mater., 2020, 9, e1901495 CrossRef PubMed.
- S. Gogoi, M. Kumar, B. B. Mandal and N. Karak, RSC Adv., 2016, 6, 26066–26076 RSC.
- S. K. Kailasa, D. J. Joshi, M. R. Kateshiya, J. R. Koduru and N. I. Malek, Mater. Today Chem., 2022, 23, 100746 CrossRef CAS.
- Y. Wang, Y. Xue, J. Wang, Y. Zhu, Y. Zhu, X. Zhang, J. Liao, X. Li, X. Wu, Y. X. Qin and W. Chen, Polymers, 2019, 11, 1112 CrossRef CAS PubMed.
- M. Ghanbari, M. Salavati-Niasari and F. Mohandes, RSC Adv., 2021, 11, 18423–18431 RSC.
- S. Rastegar, M. Mehdikhani, A. Bigham, E. Poorazizi and M. Rafienia, Mater. Chem. Phys., 2021, 266, 124543 CrossRef CAS.
- A. Vedadghavami, F. Minooei, M. H. Mohammadi, S. Khetani, A. R. Kolahchi, S. Mashayekhan and A. Sanati-Nezhad, Acta Biomater., 2017, 62, 42–63 CrossRef CAS PubMed.
- K. Ghosh, Z. Pan, E. Guan, S. Ge, Y. Liu, T. Nakamura, X.-D. Ren, M. Rafailovich and R. A. Clark, Biomaterials, 2007, 28, 671–679 CrossRef CAS PubMed.
- G. Lalwani, A. Gopalan, M. D'Agati, J. Srinivas Sankaran, S. Judex, Y. X. Qin and B. Sitharaman, J. Biomed. Mater. Res., Part A, 2015, 103, 3212–3225 CrossRef CAS PubMed.
- T. D. Nguyen, O. E. Kadri, V. I. Sikavitsas and R. S. Voronov, Appl. Sci., 2019, 9, 2381 CrossRef CAS.
- M. Porwal, V. Rastogi and A. Kumar, MOJ Bioequiv. Bioavailab., 2017, 3, 114–116 Search PubMed.
- J. V. Veetil and K. Ye, Biotechnol. Prog., 2009, 25, 709–721 CrossRef CAS PubMed.
- M. Alavi, E. Jabari and E. Jabbari, Expert Rev. Anti-Infect. Ther., 2021, 19, 35–44 CrossRef CAS PubMed.
- H. Mohammaddokht, S. M. Mahdizadeh, E. Khodeir, M. Baniadam, J. Sargolzaei, M. Maghrebi, M.E. Karkhanechin, M. Mokhtarifar and H. Arab, Mater. Res. Express, 2019, 6, 095010 CrossRef CAS.
- A. Thakor, J. Jokerst, C. Zavaleta, T. Massoud and S. Gambhir, Nano Lett., 2011, 11, 4029–4036 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
