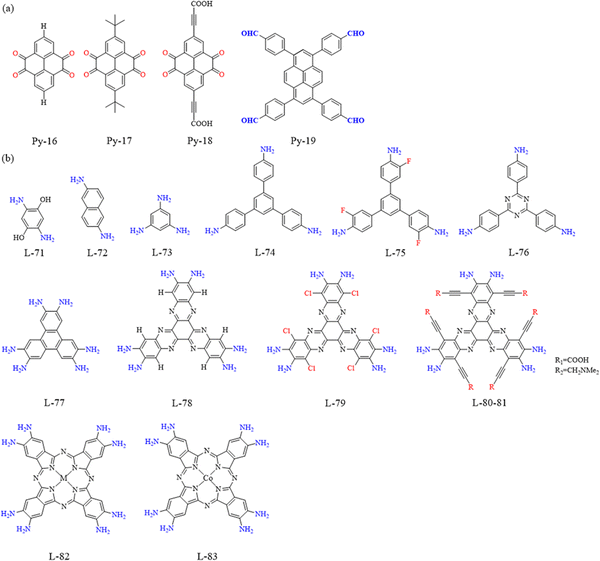
Pyrene-based covalent organic frameworks (PyCOFs): a review
Yao
Yang
 a,
Shiqiong
Peng
c,
Songhua
Chen
*b,
Fangyuan
Kang
a,
Shiqiong
Peng
c,
Songhua
Chen
*b,
Fangyuan
Kang
 d,
Jun
Fan
a,
Huan
Zhang
a,
Xianglin
Yu
c,
Junbo
Li
*a and
Qichun
Zhang
d,
Jun
Fan
a,
Huan
Zhang
a,
Xianglin
Yu
c,
Junbo
Li
*a and
Qichun
Zhang
 *d
*d
aSchool of Chemistry and Environmental Engineering, Wuhan Institute of Technology, Wuhan 430074, China. E-mail: jbliwit@163.com
bCollege of Chemistry and Material, Longyan University, Longyan 364000, China. E-mail: songhua@iccas.ac.cn
cSchool of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan 430074, China
dDepartment of Materials Science and Engineering, Department of Chemistry, Center of Super-Diamond and Advanced Films (COSDAF) & Hong Kong Institute of Clean Energy, City University of Hong Kong, 999077 Hong Kong, China. E-mail: qiczhang@cityu.edu.hk
First published on 20th September 2024
Abstract
Recently, pyrene-based covalent organic frameworks (PyCOFs) have aroused great interest because the large planar structure of the pyrene unit could effectively enhance the interlayer π–π interaction and promote the separation and migration of carriers, significantly improving the crystallinity and photoelectrical properties of PyCOFs. Since the first PyCOF-containing boroxate linkage was reported in 2008 by the Yaghi group, many PyCOFs with different kinds of linkages have been reported, exhibiting great potential applications in different fields such as adsorption/separation, chemical sensing, catalysis, energy storage, etc. However, as far as we know, the reviews related to PyCOFs are rare, although PyCOFs have been widely reported to show promising applications. Thus, it is right time and important for us to systematically summarize the research advance in PyCOFs, including the synthesis with different linkages and applications. Moreover, the prospects and obstacles facing the development of PyCOFs are discussed. We hope that this review will provide new insights into PyCOFs that can be explored for more attractive functions or applications.
1. Introduction
Covalent organic frameworks (COFs) are a class of crystalline polymeric materials made from small molecular building blocks connected through covalent bonds.1 They have the advantages of high crystallinity, rich pore structure, high stability, high functionality, structural designability, etc.2 Since the first COF was synthesized by the Yaghi group in 2005,3 the advances in diverse building units and different synthetic methods have greatly promoted the development of COFs, leading to rich types and various applications.4 Especially, topology-guided design of COFs can enable scientists to prepare targeted COFs with large specific surface area, tunable pore sizes, and functional pore environment.5 These features have led to the excellent application of COFs in the fields of adsorption and separation,6 catalysis,7 photovoltaics,8 sensing,9 and biology.10Pyrene is a highly symmetrical four-benzene-ring polycyclic aromatic hydrocarbon, which was discovered in the residue of the distillation of coal tar in 1837.11 Among diverse building units, pyrene derivatives12 have been widely employed as building units to construct framework materials due to their rich π-electrons,13 planar structure, high stability, and tightly stacking patterns through strong intermolecular π–π interactions in aggregated states.14 Besides, these factors are favorable for the preparation of porous framework materials.15 The formed large π conjugated system based on pyrene derivatives can widen the range of light absorption, help electron migration, and reduce photogenerated electron–hole recombination.16 Moreover, the conjugated nature of pyrene provides good chemical stability in energy storage and stimulation.17 A series of novel pyrene-based porous materials, such as pyrene-based metal–organic frameworks (PyMOFs), pyrene-based covalent organic frameworks (PyCOFs), pyrene-based hydrogen-bonded organic frameworks (PyHOFs) and pyrene-based polyporous organic polymers (PyPOPs), have been developed and utilized in various applications. Among them, PyMOFs constructed from metal ions/clusters and organic linkers through coordination bonds have the characteristics of periodic and well-defined structures large specific surface area, structural diversity, and customizability. However, they usually have relatively low chemical stability and poor electrical conductivity, which hinders their practical applications.18 PyHOFs are organic molecules with hydrogen bond interactions, well-defined structures and low density. However, their poor chemical stability limits their applications.19 PyPOPs are highly stable porous materials composed of strong covalent bonds based on organic molecules. Unfortunately, due to their unclear structures and irregular pores, it is difficult to gain a deep understanding of their structure–activity relationship.20 PyCOFs are a type of fully designed crystalline material made from organic building blocks polymerized through strong covalent bonds. Compared with PyMOFs, PyHOFs and PyPOPs, PyCOFs not only integrate their particular advantages but also remedy their drawbacks, and hence have attracted increasing interest in various applications.21 Furthermore, PyCOFs containing pyrene moieties exhibit extended π-conjugation and robust electronic interactions, allowing exceptional electron delocalization and transport. This enhances the capture and conversion of light energy, making them ideal for composite photocatalytic systems.22
The recent progress in PyCOFs strongly inspires us to summarize the linkages and applications of PyCOFs, and discuss the effects of different substitutes in pyrene-based linkages on the applications of PyCOFs, including photocatalysis (including hydrogen evolution, hydrogen peroxide production, reduction of carbon dioxide and conversion of organic matter), fluorescence detection, battery materials, etc. Finally, we will provide the fundamental issues and the future directions in the exploration of PyCOF-based applications.
2. Synthetic strategies of PyCOFs
In recent years, the formation of PyCOFs through polycondensation reactions among different building blocks has been widely observed to study how covalent bonds confer structural scalability and specific functional capabilities of COFs, and affect their crystallinity and chemical stability. Clearly, the linkages are very important because they not only connect the functional building blocks together, but also can extend the π-conjugation and facilitate the light absorption and electron transfer processes. Besides, according to different topological structures and connection modes, COFs with one-dimensional (1D),23 two-dimensional (2D) and three-dimensional (3D) structures are constructed. Because of their different structures, they can have different applications. In contrast to 1D and 2D COFs with π–π packing in the process of plane covalent bonding, 3D COFs are three-dimensional assembly through covalent bonding, and their synthesis mainly depends on the formation of covalent bonds. Because of the high bond energy of covalent bonds and the weak reversible bond assembly ability, it is difficult to accurately control the bonding process of three-dimensional frameworks at the atomic level, and crystallization is more difficult.24 The following is the summary of PyCOFs with different kinds of linkages.2.1 Boron–oxygen-linked PyCOFs
Boron–oxygen-linked PyCOFs can be constructed through the dehydration condensation of diboronic acid and o-diphenol or the dehydration self-condensation of diboronic acid to form boric anhydride (boroxane) (Fig. 1a). These materials have been extensively investigated and can be easily formed with high reversibility, high yield, and high crystallinity.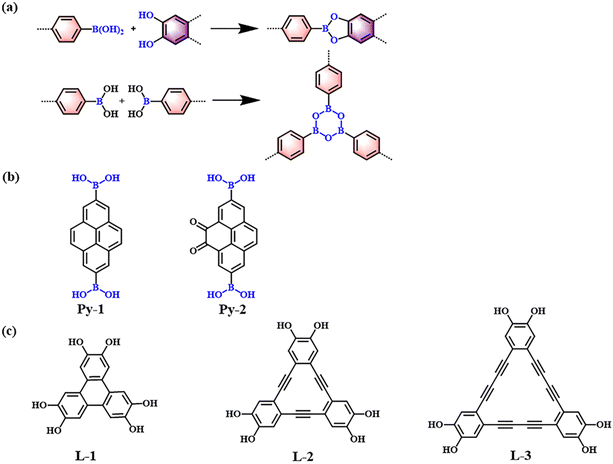 | ||
| Fig. 1 (a) Schematic illustration of boron–oxygen-linked PyCOFs. (b) Frequently used monomers containing boron hydroxyl. (c) Phenolic hydroxyl monomers. | ||
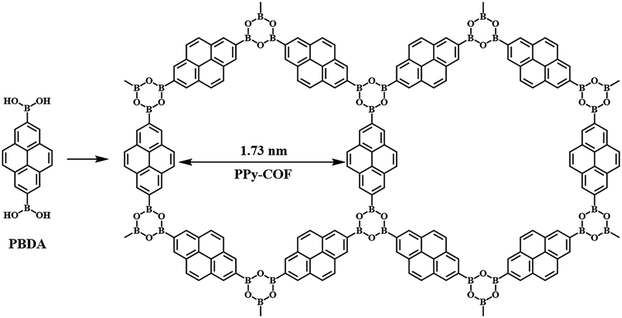
In 2008, Wan et al.25 synthesized TP-COF through a condensation reaction between 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP) and pyrene-2,7-diboronic acid (PDBA), which was the first example of PyCOFs containing boron–oxygen linkage. This TP-COF structure with hexagonal pores was designed to use D3h symmetric monomers as corners and D2h symmetric monomers as edges. Later on, Wan et al.26 employed the self-condensation of pyrene-2,7-diboronic acid to prepare PPy-COF (Fig. 2) with an eclipsed alignment. The authors found that this structure could significantly facilitate exciton migration and carrier transport. Moreover, PyCOFs can capture visible light and generate large photocurrent. Besides these, they also have been demonstrated to show rapid response to optical radiation. All these factors have driven the quick research progress of PyCOFs in optoelectronic and photovoltaic applications.
 | ||
| Fig. 2 Schematic representation of the synthesis of PPy-COF. Reproduced with permission.26 Copyright 2009, Wiley-VCH GmbH. | ||
Although the boron–oxygen-linked COFs could be prepared with high crystallinity using traditional solvothermal methods, their chemical stability is poor, and even they are unstable under weak acid, weak base, and relatively mild water vapor conditions. To enhance their stability, some of the boron–oxygen-linked PyCOFs were functionalized via post-modification. For example, in 2016, Salonen et al.27 prepared a new pyrene-4,5-dione building block to react with 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP), yielding a novel dione-COF (Fig. 3a). In 2022, the same group introduced o-phenylenediamine (Ph) and 2,3-diaminonaphthalene (Naph) into the pores of dione-COF, which could extend the π-system of the PyCOF backbone to produce pyrene-fused azaacene COFs, inhibiting the hydrolysis of COFs.28 In 2023, Frey et al.29 continued to graft (1R,2R)-(+)-1,2-diphenylethylenediamine onto dione-COF to obtain a shorter layer spacing while increasing the π-conjugated system, which could shift the absorption range to longer wavelengths (Fig. 3b).
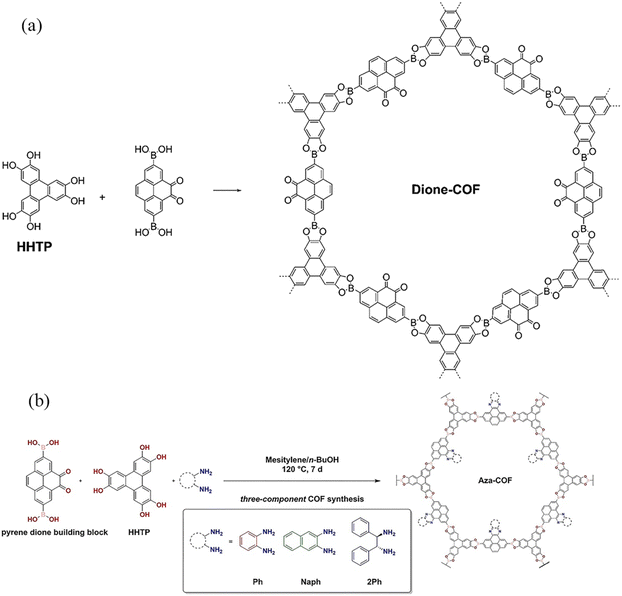 | ||
| Fig. 3 (a) Synthesis of dione-COF. Reproduced with permission.27 Copyright 2016, Royal Society of Chemistry. (b) Post-synthetic modification of dione-COF. Reproduced with permission.29 Copyright 2023, Wiley-VCH GmbH. | ||
It is possible to use the hybrid linker strategy for the creation of COFs with different pore channels or different degrees of conjugation. This factor is important to increase the structural diversity of COFs. For example, Zheng et al.30 synthesized two boron–oxygen-linked COFs by a solvothermal method using CTC as the apex unit and PDBA or BPDA as the linkage. The introduction of the pyrene group makes CTC-COF-3 a potential semiconducting π-conjugated material. In the same year, Crowe et al.31 reported three novel boron–oxygen-linked COFs (Fig. 4) (Py-DBA-COF 1, Py-DBA-COF 2, and Py-MV-DBA-COF) with π-conjugated DBA as the apex unit and PDBA as the connecting bridge. All three DBA-COFs are highly luminescent in the solid state, and the redshift in the emission wavelengths of Py-DBA-COF 1 and Py-MV-DBA-COF may be attributed to the symmetrically forbidden lowest energy jump in the ground state of the DBA[18] based materials compared to the DBA[12] based materials, facilitating the construction of materials with unique photovoltaics.
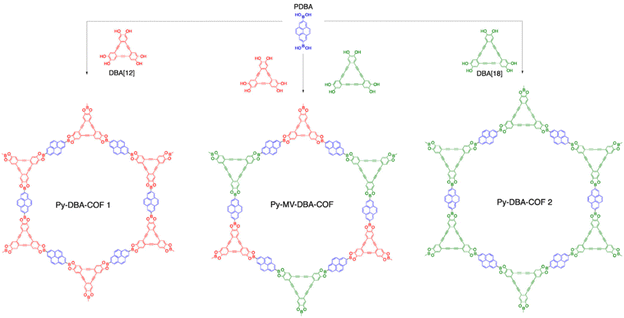 | ||
| Fig. 4 Synthesis of DBA-COFs. Reproduced with permission.31 Copyright 2016, American Chemical Society. | ||
2.2 sp2 carbon-linked PyCOFs
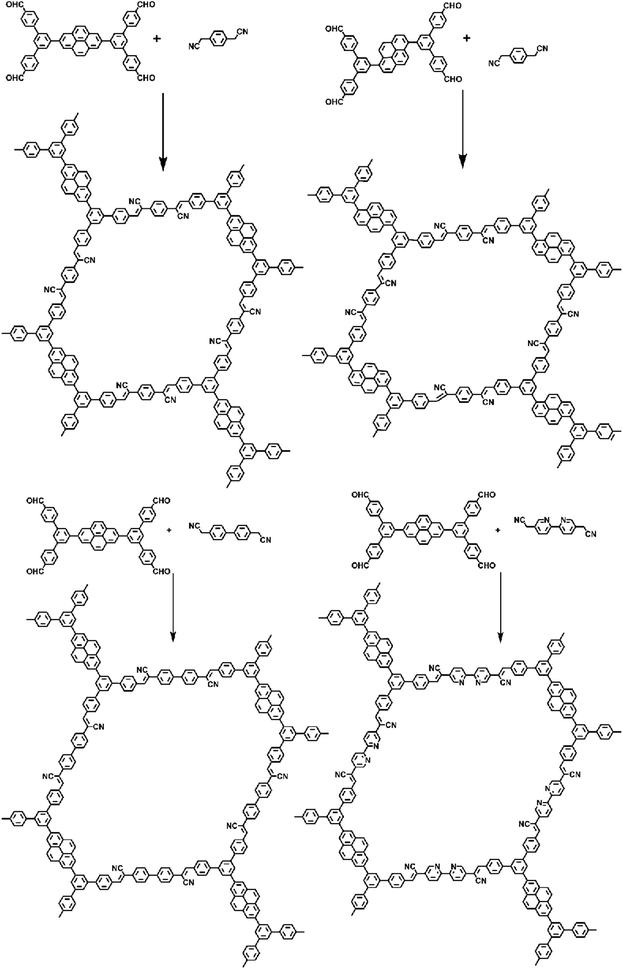
Knoevenagel condensation, a cascade nucleophilic addition-dehydration reaction between activated methylene groups and aldehydes or ketones, is one of the most common reactions to synthesize sp2-COFs. Cyano-substitution is of great importance in the strategy of synthesis of sp2c-COFs. As shown in Fig. 5a, sp2c-COFs can be obtained through topologically oriented polymerization reactions between benzyl cyanide and aryl aldehyde monomers, where pyrene derivatives are used as vertex units (Fig. 5b) and a series of cyanide derivatives are used as linking units (Fig. 5c).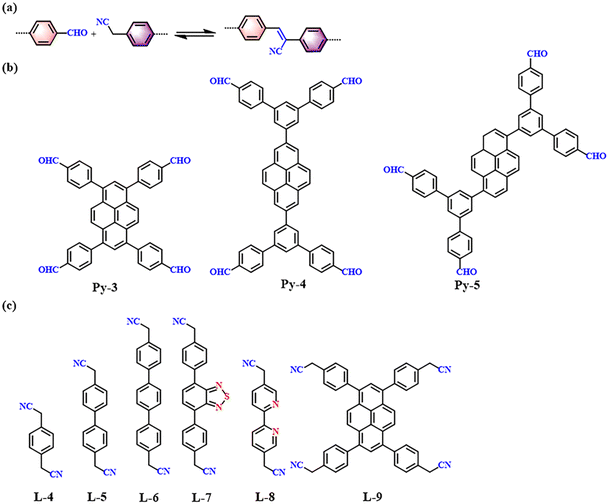 | ||
| Fig. 5 (a) Schematic illustration of sp2 carbon-conjugated-linked PyCOFs. (b) and (c) Frequently used monomers. | ||
Xiao et al.32 found that sp2c-PyCOFs as the fully π-conjugated systems could provide better electrical and magnetic properties. In 2017, Jiang et al.33 reported the synthesis of 2D crystalline COFs (sp2c-PyCOF) via the Knoevenagel condensation reaction between 1,3,6,8-tetrakis(4-formylphenyl)pyrene (TFFPy) and 1,4-phenylenediacetonitrile (PDAN) (Fig. 6a). The as-prepared sp2c-PyCOF has a surface area of 692 m2 g−1. The porous and ultrathin structure of sp2c-PyCOF effectively shortened the transport distance of electrons, ions, and co-reactants (S2O82−), which enhanced the utilization of luminophores. In 2023, Jiang et al.34 continued to study sp2c-PyCOF and found that sp2c-PyCOF could be used to degrade tetracycline hydrochloride in aqueous solution under visible light irradiation.
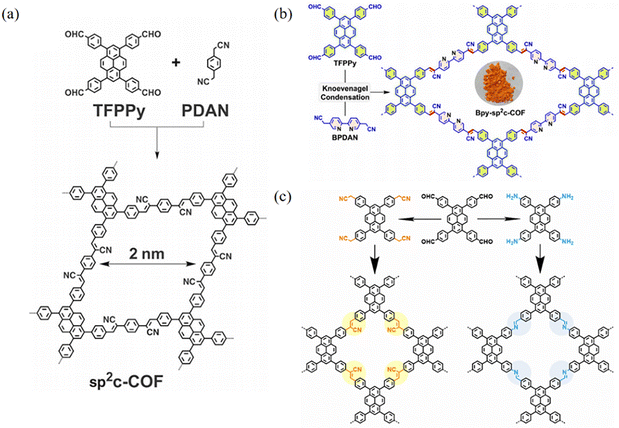 | ||
| Fig. 6 (a) Synthesis of sp2c-COF. Reproduced with permission.35 Copyright 2017, The American Association for the Advancement of Science. (b) Synthesis of Bpy-sp2c-COF. Reproduced with permission.36 Copyright 2023, Royal Society of Chemistry. (c) Schematic representation of the syntheses of imCOF and viCOF. Reproduced with permission.37 Copyright 2023, Wiley-VCH GmbH. | ||
The most common method for designing and synthesizing new sp2c-COFs is to increase the number of cyano-linked units and introduce heteroatoms. The introduction of heteroatoms modulates the pore size and electron cloud density of sp2c-COFs and improves the chemical stability and photoelectronic properties of sp2c-COFs through fully conjugated states. Nitrogen was introduced into the skeleton of the COF to form a pyrimidine structure, which significantly improved the electronic effect of sp2c-PyCOF. Bpy-sp2c-PyCOF was synthesized via the condensation reaction between TFPPy and 5,5′-diacetonitrile-2,2′-bipyridine (BPDAN) (Fig. 6b).36,38 Bpy-sp2c-PyCOF shortens the carrier transport distance, enhances electron–hole separation, and maintains high catalytic efficiency and reusability. In 2023, Lin et al.38a reported a sp2c-PyCOF containing bipyridyl and its post-synthetic modification through the introduction of Ni. Under light irradiation, the enhanced energy transfer in this COF facilitated the excitation of Ni centers to catalyze borylation and trifluoromethylation reactions of aryl halides. Mi et al.38b found that Bpy-sp2c-PyCOF showed excellent photocatalytic activity in aerobic hydroxylation and thiocyanation transformation in the visible range.
The photophysical properties of COFs are mainly affected by the structural features such as the conjugation degree, charge delocalization ability and exciton dynamics. In 2022, Jiang and co-workers39 developed a module-patterned polycondensation strategy for the efficient synthesis of crystalline porous COFs. They prepared a series of sp2c-COFs through polycondensation of two pyrene units with different substitution positions and three linkers (Fig. 7). Moreover, the C2-symmetric knot module can be generalized to possess different π backbones and enables polymerization with diverse linker units, producing a library of unprecedented 2D sp2-carbon polymers and open frameworks. In 2023, Han et al.37 reported that two PyCOFs with imine and vinylene linkages, named imCOF and viCOF (Fig. 6c). It was found that viCOF has a longer fluorescence lifetime and more prominent solid-state photoluminescence quantum yield, and is the preferred charge transfer pathway in the intralaminar mode due to the D–A structure, which increases the charge separation ability. sp2c-PyCOFs are not only of interest in the design of new 2D semiconducting frameworks but also provide insights into the development of π electronic functions.
 | ||
| Fig. 7 Synthesis of sp2c-PyCOFs. Reproduced with permission.39 Copyright 2022, Wiley-VCH GmbH. | ||
2.3 Imine-linked COFs
As shown in Fig. 8a, imine-linked COFs are constructed by the acid-catalyzed co-condensation of amine and aldehyde derivatives based on a Schiff base reaction. Imine-linked COFs are abundant and readily available. Imine-linked PyCOFs are usually synthesized by substitution reactions at the 1, 3, 6, and 8 positions of pyrene units, increasing the types of vertex units (Fig. 8b), where [1+1] condensation reactions occur with different kinds of linkages (Fig. 8c), leading to the rapid development of imine-linked COFs.40 The crystallinity and specific surface area of imine-linked COFs are lower than those of boron–oxygen-linked COFs with similar structures; however, their stability is significantly improved compared to boron–oxygen-linked COFs.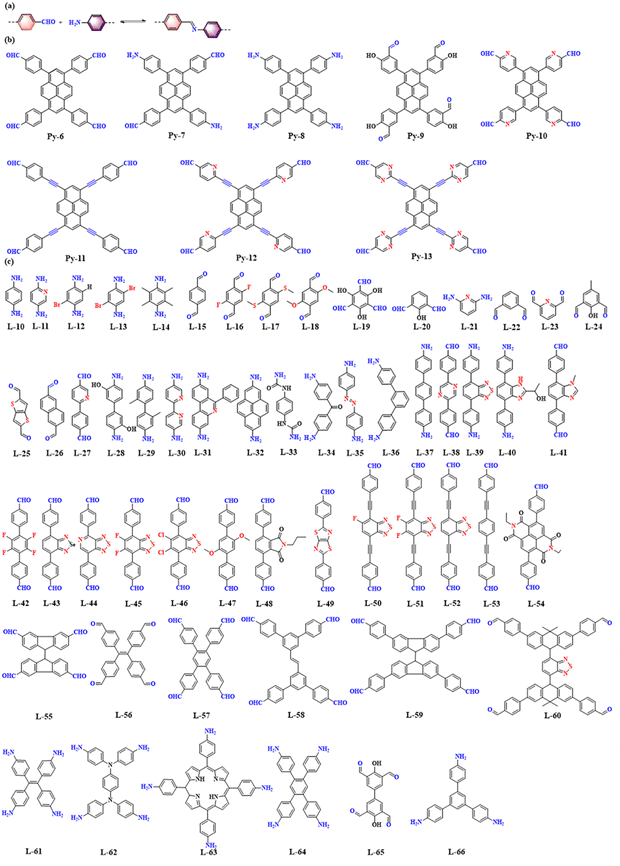 | ||
| Fig. 8 (a) Schematic illustration of imine-linked PyCOFs. (b) Frequently used pyrene-based building blocks. (c) Frequently used monomers. | ||
In 2019, Chen et al.41 synthesized a bifunctional building block, 1,6-bis(4-formylphenyl)-3,8-bis(4-aminophenyl)pyrene (BFBAPy), to construct highly crystalline and porous Py-COFs through self-condensation in different solvents such as CH2Cl2, CHCl3, tetrahydrofuran, methanol, ethanol, acetonitrile, and dimethylacetamide. This provides a ‘two-in-one’ molecular design strategy for readily preparing high-quality COFs. Self-condensation ensures that the optimal stoichiometric ratio during the polycondensation process to obtain COFs with good reproducibility and high crystallinity. In 2020, Py-COF films were used as stable acidic color change sensors with fast response times, low detection limits, and good reproducibility (Fig. 9a).42 In 2022, Gan et al.43 synthesized Py-COF via a one-pot polycondensation reaction using BFBAPy as a precursor to prepare amino-functionalized magnetic Fe3O4–NH2 nanoparticles and synthesized a magnetic self-assembled COF adsorbent (Fig. 9b).
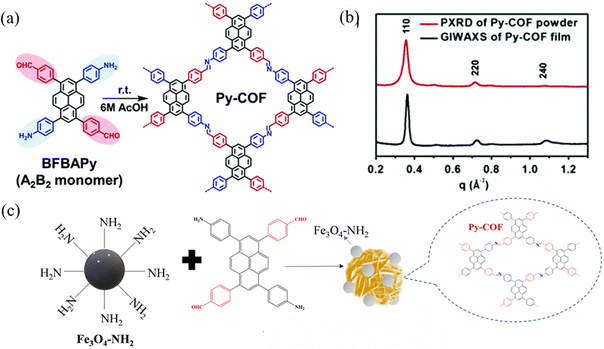 | ||
| Fig. 9 (a) Synthesis of Py-COF. Reproduced with permission.42 Copyright 2020, Royal Society of Chemistry. (b) Projection of in-plane GIWAXS data of the Py-COF film (black) and PXRD data of the Py-COF powder (red). (c) Synthesis of Fe3O4@Py-COF. Reproduced with permission.43 Copyright 2022, Elsevier. | ||
Currently, the mainstream of COF synthesis involves developing diverse strategies to construct different COFs through changing the length of the linkers, where the [1+1] strategy can also be used in two combinations of C4 + C2 and C4 + C4 so that 2D COFs with tetragonal apertures can be generated. By employing 1,3,6,8-tetrakis(4-aminophenyl)pyrene (PyTTA) as the building block to react with different linking units, a series of imine-linked COFs could be synthesized. Due to the high electron cloud density of pyrene units, the as-obtained imine-linked COFs are beneficial for charge separation and transfer, thus effectively improving the photoelectron absorption capacity of imine PyCOFs. In 2012, Nagao et al.44 reported that PyTTA-DHTA-COF was synthesized through the condensation reaction between PyTTA and 2,5-dihydroxyterephthalaldehyde under solvothermal conditions. PyTTA-DHTA-COF contains two hydroxyl groups, and the extension of the hydrogen bonding framework with the water molecule provides the mode of transport of protons and the efficiency of proton transfer.
The introduction of electron-absorbing groups and electron-donating groups on the pore wall surfaces of the linkers, respectively, resulted in COFs with significantly different photoelectronic properties and photocatalytic activities.45 In particular, this is solely due to the electron-donating conjugation effect of the electron-donor groups, which gives the COFs more charge separation and transport capabilities.46 In 2023, Ma et al.45b,47 prepared two new conjugated PyCOFs (PyDF-COF and PyBMT-COF) (Fig. 10a). The strong electron-donor conjugation effect of the thiomethyl group gives the PyBMT-COF wider visible absorption, narrower band gap, more negative reduction potential, and higher photocurrent response. Lin and co-workers48 reported the synthesis of the Py-TT COF with Py as the donor and TT as the acceptor. The as-obtained donor–acceptor Py-TT COF displayed a narrow bandgap and high efficiency of charge separation and transfer, which could greatly improve the photocatalytic activity (Fig. 10b). Chen et al.49 reported thickness tunable thienothiophene-pyrene COFs comprised of a carbon nanotube (CNT) core. The charge transfer potential barrier between the active catalytic site and the adsorbed oxygen intermediate was lowered, resulting in a significant increase in catalytic activity.
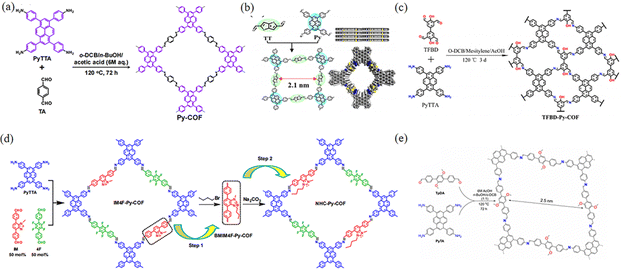 | ||
| Fig. 10 (a) Schematic illustration of the synthesis of Py-COF. Reproduced with permission.50 Copyright 2018, Royal Society of Chemistry. (b) Schematic of the synthesis of the Py-TT COF. Reproduced with permission.48 Copyright 2021, American Chemical Society. (c) Illustration of the synthesis and structure of TFBD-Py-COF. Reproduced with permission.51 Copyright 2022, Elsevier. (d) Synthesis of NHC-Py-COF. Reproduced with permission.52 Copyright 2021, MDPI. (e) Synthesis of COF-JLU25. Reproduced with permission.53 Copyright 2021, Wiley-VCH GmbH. | ||
With the continuous advances in organic chemistry, the synthetic methods to prepare COFs are constantly innovated, and the degree of conjugation between the building blocks and the connecting units can be changed from non-conjugated to partially conjugated or even to fully conjugated. In addition, the linkers of PyCOFs are development, leading to the large pores and surface area of COFs.54 In 2013, Liu et al.52 obtained an IM4F-Py-COF through the Schiff-base condensation reaction under solvothermal conditions (Fig. 10d), while in 2021, Wen et al.53 synthesized donor–acceptor-type COF-JLU25 through the reaction between (4-aminophenyl)pyrene (PyTA) and 4-[4-(4-formylmethyl)-2,5-dimethoxyphenyl] benzaldehyde (TpDA) (Fig. 10e). The as-obtained COF-JLU25 exhibits excellent photocatalytic activity with high efficiency, robust reusability, and low catalyst loading, demonstrating the previously under-exploited potential for application of COF-based photocatalysts consisting of only electron-rich units. In 2022, Gao et al.55 prepared a new ETTA-PyTTA-COF, constructed through the condensation reaction between 4,4′,4′′,4′′′-(ethane-1,1,2,2-tetrayl)-tetrabenzaldehyde (ETTA) and PyTTA. Furthermore, some COFs with regular pore structures have been demonstrated to show large π-conjugated structures with interesting photovoltaic properties (Fig. 8c).51
As to imine-linked PyCOFs, they have been synthesized with different linkers using TFPPy as a C4 node.56 In 2013, Kaderi and co-workers57 reported the synthesis of highly crystalline mesoporous TFPPy-PPD-COF through the condensation reaction between TFPPy and p-phenylenediamine, where strong π–π stacking interactions between the pyrene moieties could enhance the porosity and adsorption properties of COFs. In 2017, Wang et al.58 integrated TFPPy-PPD-COF with single-layered graphene (SLG) for application in the field of organic electronics and optoelectronics (Fig. 11a). In 2023, Hu and collages56e synthesized TFPPy-TMPD-COF through methyl substitution of TFPPy-PPD-COF, where they found that Me-substitution endowed the as-modified COFs with stronger charge transfer ability to increase the electron density of the backbone, enhancing the ability to interact with anions. Grafting methyl groups onto COFs changed the pore environment, promoted the formation of iodine anions, enhanced framework–iodine interactions, and facilitated the iodine trapping ability of TFPPy-TMPD-COF. In 2021, Yang et al.59 synthesized the DP-Py COF using TFPPy and 2,6-methyldiamino-pyridine (DP) as building blocks. The obtained DP-Py COF was employed to fabricate a novel electrochemical sensing platform for sensitively and selectively detecting theophylline (TP) and caffeine (CAF) simultaneously through compounding with AuNPs.
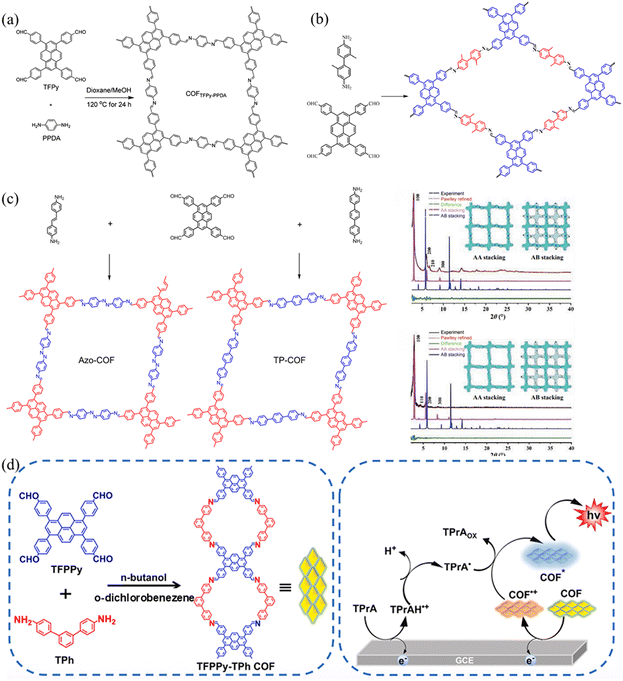 | ||
| Fig. 11 (a) The synthesis of COF TFPPy-PPDA. Reproduced with permission.58 Copyright 2017, American Chemical Society. (b) Schematic representation of the transformation of imine COFs into amide COF TFPPy-DP via oxidation by oxone. Reproduced with permission.56c Copyright 2022, Elsevier. (c) The synthesis of Azo-COF and Tp-COF. Reproduced with permission.60 Copyright 2022, Springer Nature. (d) Schematic diagram of TFPPy-TPh-COF. Reproduced with permission.61 Copyright 2024, Elsevier. | ||
COFs can allow atomically precise design and integration of nodes and linkers into ordered networks with permanent porosity through linkages.62 Developing novel linkers enables the design and synthesis of new COFs.3,63 In 2020, Guo and co-workers56d synthesized COF-TP based on TFPPy and 4,4′-diaminobenzophenone (DABP), exhibiting a rare 1D structure. The obtained COF-TP possessed a comparatively high BET surface area and relatively high chemical stability. The Liang group and Hou group used 4,4′-diamino-[1,1′-biphenyl]-3,3′-diol and 2,2′-dimethylbenzidine to synthesize two kinds of two-CoFs (TFPPy-BDOH and TFPPY-DM COF) with TFPPy, and the resulting COFs showed different properties (Fig. 11b).56c,64 TFPPy-BDOH has good selectivity for uranium due to the synergistic interaction between the nitrogen atom in the imine bond and the hydroxyl group in the conjugated framework, while the TF-DM COF is a metal-free catalyst for the hydroxylation of aryl boronic acids. In 2022, Yang et al.60 synthesized two COFs (Azo-COF and Tp-COF) using TFPPy as a building node and p-AA or DATP as the linear linkage through the Schiff base reaction (Fig. 11c). The two as-synthesized mesoporous COFs (Azo-COF and Ip-COF) adopt the AA stacking mode and have highly crystalline and stable frames. Because the lengths of the two chains did not show too much difference, the pore size of PyCOFs are slightly adjusted with high specificity for protein selection.
2.4 Hydrazone-linked PyCOFs
Hydrazones are a class of compounds containing the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNHCOR group, formed through the reaction between hydrazines or substituted hydrazines (such as phenylhydrazine) and carbonyl compounds (such as aldehydes or ketones) (Fig. 12). Due to the long non-aromatic portion of the hydrazone-linked COFs, such materials require the integration of ethoxy groups into the neighbour of the linear linker, to maintain the 2D conformation of the materials as well as facilitate the crystallizationprocess.65
NNHCOR group, formed through the reaction between hydrazines or substituted hydrazines (such as phenylhydrazine) and carbonyl compounds (such as aldehydes or ketones) (Fig. 12). Due to the long non-aromatic portion of the hydrazone-linked COFs, such materials require the integration of ethoxy groups into the neighbour of the linear linker, to maintain the 2D conformation of the materials as well as facilitate the crystallizationprocess.65
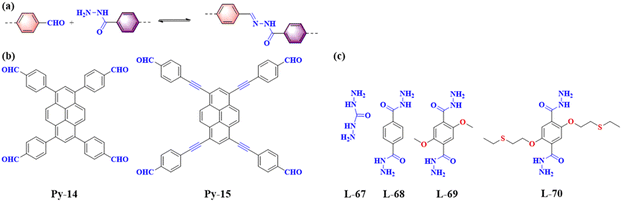 | ||
| Fig. 12 (a) Schematic illustration of hydrazone-linked PyCOFs. (b) Frequently used pyrene-based building blocks. (c) Frequently used monomers. | ||
Xu et al.66 constructed a hydrazone-linked Pythz-COF and a urea-linked Pyurea-COF based on TFPPy as the building block. Pythz-COF has a high specific surface area, with the pore size mainly concentrated at 2.34 nm and a small amount of pore size distributed around 1.36 nm, with good reusability. In 2012, Zhang et al.65c synthesized TFPPy-DMeTHz-COF with hydrazone bonds as the linkage and 2,5-dimethoxyterephthalohydrazide (DMeTHz) and TFPPy as the monomers. The π-conjugated skeleton formed in TFPPy-DMeTHz-COF results in a low bandgap. The COF showed stable photocurrent response and excellent photoelectronic behavior. In the same year, Liu and co-workers65b selected TEBPY with a complete planar structure and DHTH with the functional group (–OH) as the building blocks to prepare another COF (TD-COF) (Fig. 13a), where –OH is distributed throughout the molecular backbone, leading to dramatic changes in the orbital distribution and energy gap of TD-COF. In 2022, Qiu et al.67 discovered synergism between Pythz-COF neutral amide functional groups and Au(III) and hydrogen bonding between protonated amides and AuCl4− for fluorescence detection of Au(III) (Fig. 13b).
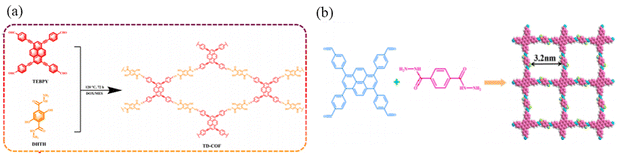 | ||
| Fig. 13 (a) Schematic representation of the synthesis of TD-COF. Reproduced with permission.65b Copyright 2023, Elsevier. (b) Synthetic diagram of DTF-ANDI-COF. Reproduced with permission.67 Copyright 2023, Elsevier. | ||
2.5 Heterocycle-linked PyCOFs
Heterocycle-linked COFs generally possess imine structures, which can be post-modified to produce cyclic compounds such as pyrazines, pyridines, pyrimidines, thiazoles, or oxazoles through redox reactions. Heterocyclic COFs have the advantages of structural stability and irreversibility and are commonly used in catalysis and energy storage (Fig. 14).Pyrazine-linked COFs are one of the common heterocycle-linked COFs, which are usually constructed through the reaction between 1,2-diketone and 1,2-diamine moieties. This makes pyrazine-connected COFs a promising material for optoelectronic applications. In 2018, Hoberg et al.68 reported a series of pyrazine-based PyCOFs with highly stable aromatic backbones and intrinsically ordered nanoscale pores (Fig. 15a). The nanoscale pores are modified to incorporate various desired functional groups. This new bottom-up approach provides an idiomatic way to achieve highly ordered and modifiable materials for design and synthesis. Jiang et al.69 reported a chemically stable, electronically conjugated 2D COF with topologically designed wire frameworks and open nanochannels (Fig. 15b). The conjugated COF permits inherent periodic ordering of conjugated chains in all three dimensions and displays a striking combination of physical properties: chemical stability, extended π-conjugation, and high carrier mobility. Fully π-conjugated COFs are useful for high on–off ratio photoswitches and photovoltaic cells.
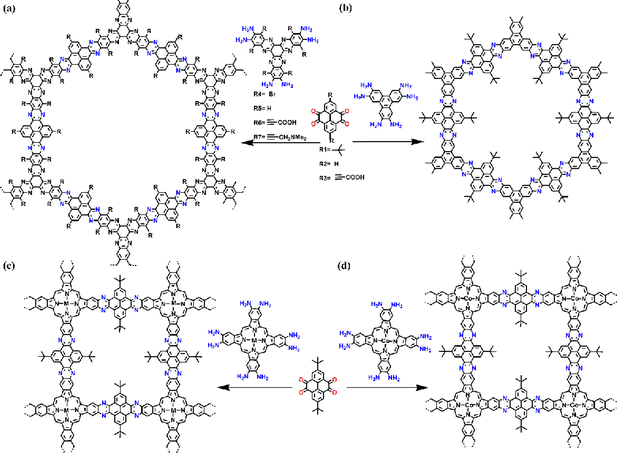 | ||
| Fig. 15 (a) Schematic representation of the synthesis of PyCOFs. Reproduced with permission.68 Copyright 2018, American Chemical Society. (b) Schematic representation of the synthesis of CS-COF. Reproduced with permission.69 Copyright 2013, Springer Nature. (c) Design and synthesis of the MPc-pz COF. Reproduced with permission.70,b Copyright 2019, American Chemical Society. (d) Design and synthesis of the CoPc-pz COF. Reproduced with permission.70,a Copyright 2020, Wiley-VCH GmbH. | ||
Porphyrin, possessing a highly conjugated core ring structure similar to chlorophyll and with flexible physical and chemical properties, has become one of the most investigated photosensitizers.71 Fully conjugated COFs were synthesized from porphyrin derivatives and pyrene-4,5,9,10-tetraketone, which can be complexed with metal ions to increase photocatalytic performance. In addition, tetragonal topology can be used to increase conjugated bonds for connecting catalytic sites.70 In 2020, Jiang and co-workers70,a integrated cobalt(II) phthalocyanine into 2D COFs via a robust phenazine bond, where the COFs displayed excellent stability, even under boiling water, acid, or base conditions (Fig. 15d). The MPc-pz COF can be used as an electrocatalyst due to its unique ability. By integrating the all-π conjugated stable backbone and catalytic sites into one single lattice, the MPc-pz COF exhibits excellent stability, high conductivity, and good catalytic activity in the reduction of CO2 to CO in water. Feng and co-workers70,b reported a series of pyrazine-connected metal-phthalocyanine-based conjugated 2D M-COFs (M = Zn, Cu, Ni) (Fig. 15c), which can provide a reliable way to design and develop semiconductor COF materials with enhanced charge transport properties.
Multicomponent reactions (MCRs) are widely used in organic synthesis to improve synthetic efficiency and versatility, especially for constructing ring structures. The classical synthesis of imidazole rings can be easily realized through the three-component Debus–Radziszewski reaction. In 2019, Wang et al.72 used this MCR strategy to construct a series of imidazolium-conjugated stable COFs (Fig. 16). A new approach to establish the precise composition of crystalline COFs is provided by the in situ formation of five covalent bonds in each cyclic imidazole ring. The synthesized pairs of imidazole-linked COFs are highly stable and also provide an additional driving force for crystallization.
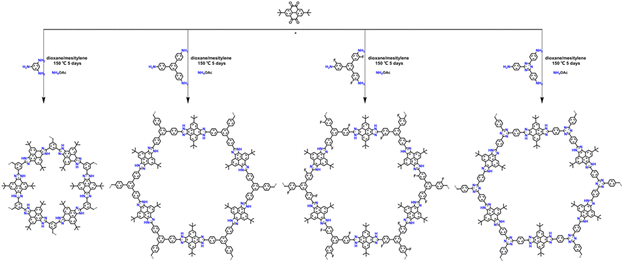 | ||
| Fig. 16 One-pot construction of imidazole-linked COFs by in situ formation of five-membered cyclic linkages. Reproduced with permission.72 Copyright 2019, American Chemical Society. | ||
A simple solution to improve the stability of COFs is to make covalent joints stable. Benzoxazole and benzothiazole structures are common solutions to achieve high stability. The classical synthesis of benzoxazole/benzothiazole structures is realized through a cascade reaction involving reversible imine bond formation followed by irreversible oxazole/thiazole ring formation. This reversible/irreversible sequence may be ideal for building robust COF structures. In 2018, Wang et al.73 synthesized highly crystalline benzobisoxazole-conjugated COFs using N-methyl-2-pyrrolidone/resorcinol as a solvent mixture and benzoxazole as an additive. It has been found that this COF is highly chemically stable. In addition, the binding of the benzothiazole portion in the π-conjugated COF backbone reduced the optical band gap and enhanced visible light absorption. Cooper and co-workers74 reported a simple and efficient three-component assembly reaction between readily available aldehydes, amines, and elemental sulfur via CH-functionalisation and oxidative cyclisation (Fig. 17a). Highly stable thiazole-conjugated COFs were successfully prepared through a one-pot method with a series of functionalized aldehydes and amines.
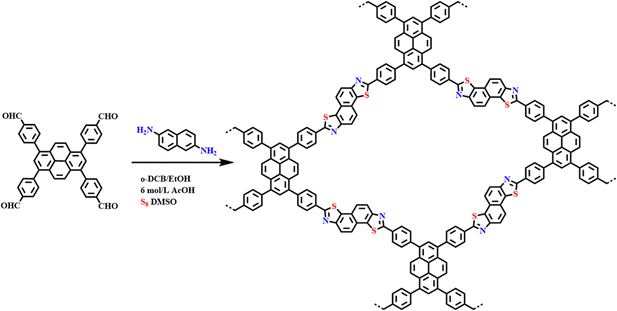 | ||
| Fig. 17 One-pot construction of benzoxazole-linked COFs via the cascade reactions. Reproduced with permission.74 Copyright 2018, American Chemical Society. | ||
Furthermore, fully conjugated COFs with enhanced chemical stability and optoelectronic properties can be achieved through post-synthetic modification. Imine-linked COFs can be post-oxidized to convert the imine bonds into heterocyclic linkages from cyclic units such as oxazole and thiazole linkages. In 2018, Yaghi et al.75 showed that imine-linked COFs could be transformed into two different heterogeneous COFs containing thiazole and oxazole bonds, respectively (Fig. 18). This conversion was achieved by successive substitution and oxidative cyclization procedures, and these materials exhibited higher chemical stability compared to the amine-substituted starting materials.
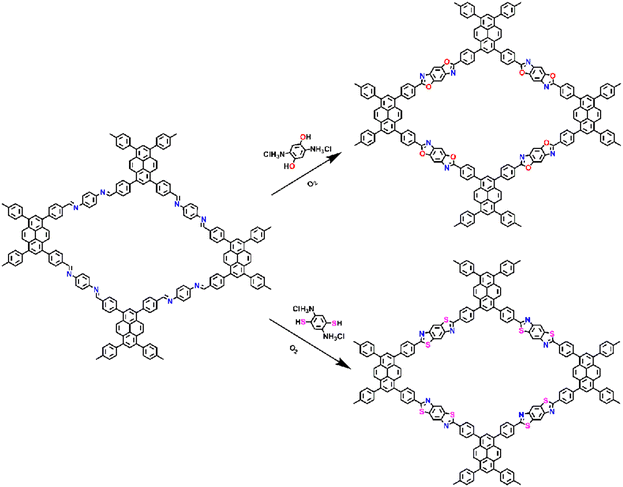 | ||
| Fig. 18 The synthesis and structures of heterocyclic linked PyCOFs through post-synthetic modification.73 Copyright 2020, American Chemical Society. | ||
2.6 Other linked COFs
These COFs, formed through the reaction of amine derivatives with anhydrides, are linked by an imide bond, where higher reaction temperatures and longer polymerization times are mostly required for such synthesis due to the lower degree of reversibility of the reaction when forming the imide bond. However, the introduction of the imide bond can increase the forces with some guest molecules resulting in unique properties (Fig. 19).In 2023, Cao and co-workers76 reported a stabilization strategy of D–A PyCOFs by intramolecular hydrogen (H)-bonds and a membrane-based mass transfer strategy for photocatalytic modulation (Fig. 20a). The high stability of these COFs comes from the strong π–π interactions and the H-bonds of adjacent PyTTA and naphthalene diimide units as well as the D–A charge transfer. Abbaspourrad and co-workers77 reported a new PEPy-COF, where the pyrene monomers with D4h symmetry were in the corners geometrically, and the D2h symmetric perylene units were located at the edges of the mesoporous tetragons (Fig. 20b).
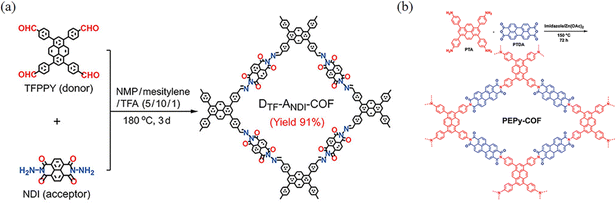 | ||
| Fig. 20 (a) Synthesis of DTF–ANDI-COF. Reproduced with permission.76 Copyright 2023, Wiley-VCH GmbH. (b) Synthesis of PEPy-COF. Reproduced with permission.77 Copyright 2023, Wiley-VCH GmbH. | ||
3. Application of PyCOFs
3.1 Photocatalytic applications of PyCOFs
3.1.1.1 Single-phase photocatalysis based on PyCOFs. In the development of photocatalytic catalysts, it has been observed that the structural adaptability of inorganic photocatalysts is limited.82 This limitation further impacts the bandgap, molecular orbital energy level, and photocatalytic activity. On the other hand, organic photocatalysts address these shortcomings and exhibit strong visible light absorption characteristics.83 COF materials are becoming ideal materials for photocatalytic hydrogen evolution.4,83c,84 The PyCOFs with a large conjugation degree provide a high density of surface active sites for mass transport, thus shortening the distance from the photogenerated carriers to the reaction center. Besides, PyCOFs exhibit large conjugated skeletons. Their band gaps could be finely tuned by changing the linkers. Actually, different linkers exhibit different conjugation and electronic properties, which affect the energy levels of the COFs.
Jiang and co-workers synthesized sp2c-COF and sp2c-COFERDN, which are entirely based on π-conjugation (Fig. 21a).85 3-Ethylrhodanine (ERDN) is electron-deficient unit, the push–pull effect of a 2D π-conjugated structure can enhance light trapping and narrow the band gap (Fig. 21b). The higher HOMO level of sp2c-COFERDN results in a narrower band gap, facilitating the excitation of electrons to the LUMO level, further enhancing the photocatalytic water decomposition capability. Using Pt as a co-catalyst to catalyze hydrogen production from water by shortening the electron transport distance and the photochemical processes of light harvesting, exciton migration and exciton splitting as well as electron transfer and harvesting were seamlessly integrated. In contrast, the photocatalytic hydrogen evolution performance of sp2c-COFERDN is better than that of sp2c-COF, with 2120 and 1360 μmol h−1 g−1, respectively (Fig. 21c).
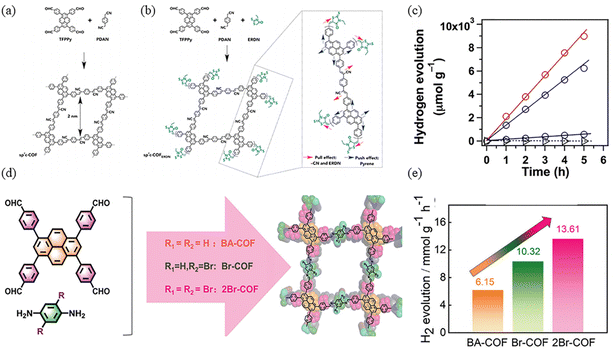 | ||
| Fig. 21 (a) Synthesis of the tetragonal sp2c-COF. (b) Synthesis of sp2c-COFERDN. (c) Hydrogen production monitored over 5 h with sp2c-COF (blue circles), sp2c-COFERDN (red circles), sp2c-CMP (black circles), and imine-linked pyrene COF (triangles) as photocatalysts under irradiation with wavelengths ≥420 nm. Reproduced with permission.85 Copyright 2019, Elsevier. (d) Schematic diagram of BA-COF. (e) Photocatalytic H2 evolution performance of as-prepared COFs. Reproduced with permission.86 Copyright 2024, Wiley-VCH GmbH. | ||
Sp2c-conjugated-linked COFs are highly rigid and well conjugated, but the sp2c-conjugated linkage is irreversible, leading to possible poor structural stability under photoexcitation. In order to improve the reversibility of COFs, Deng et al. introduced imide structure in PyCOFs for photocatalytic hydrogen evolution, and modified the linked unit with different contents of bromine atoms. It was found that the introduction of bromine atoms was helpful in the electron transfer process and increased the hydrogen production rate; the hydrogen production rate of 2Br-COF was also the highest at 13.61 mmol g−1 h−1 (Fig. 21e).
Symmetrical imine-linked PyCOFs have been reported. Compared to 2D COFs, 1D COFs can be constructed using V-shaped C2 connectors and planar C4 connectors to form a ribbon structure, where V-shaped C2 connectors promote the creation of ultra-small holes, enhancing the filling of micropores in the atmospheric water harvesting (AWH) and facilitating the cleavage of the optical water splitting. Gu et al.87 synthesized one-dimensional PyCOFs using asymmetric units, which have adsorption capacity for water to produce hydrogen. The exciton binding energy and band gap of Py-HMPA were found to be much lower than those of Py-MPA and Py-PDCA (Fig. 22a), respectively. The results suggest that the introduction of pyridine and –OH groups may facilitate the separation of electrons and holes. Appropriate hydrophilic groups such as –OH not only promoted the rapid nucleation of water molecules, but also enhanced the separation of photogenerated charges and improved the photocatalytic performance of COF The photocatalytic hydrogen evolution rate (HER) of Py-HMPA reached a value of 37.925 mmol g−1 h−1 at 7 wt% Pt, and its photocatalytic hydrogen evolution ability was much higher than that of Py-MPA and Py-PDCA (Fig. 22b–d).
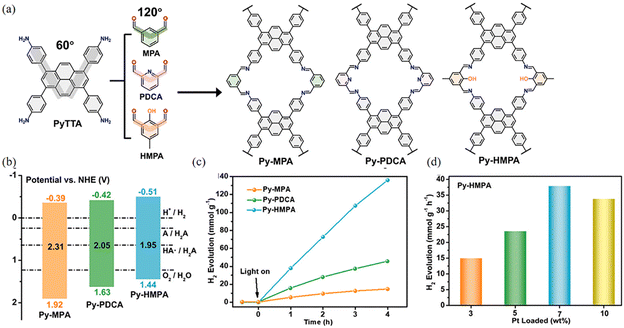 | ||
| Fig. 22 (a) Synthesis and molecular structure of Py-MPA, Py-PDCA and Py-HMPA. (b) Electronic band structure. (c) Time dependent H2 production in liquid water of three COFs. (d) The H2 evolution in liquid water for Py-HMPA with different Pt loading amount. Reproduced with permission.87 Copyright 2023, Elsevier. | ||
Elemental doping is an effective strategy to modulate the physicochemical properties of photocatalysts, which can broaden the light absorption range of photocatalysts and enhance the efficiency of photocatalytic energy conversion. The integration of benzidine and 4,4′-diaminoazobenzene together as connecting wires can produce two new COFs: Benzd-COF and Azod-COF (Fig. 23a).88 Using 4′-(pyrazine-2,5-diyl)dibenzaldehyde as the acceptor, Yan et al.89 synthesized imine-linked PyPz-COF with adjustable photocatalytic activity (Fig. 23b). The introduction of the pyrazine ring endows PyPz-COF with distinct optical, electrochemical and charge transfer properties. It also introduces abundant C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups, which could enhance proton transfer through hydrogen bonding. The bandgap of PyPz-COF is slightly smaller than that of PyTp-COF, which may be due to the introduction of the electron-deficient pyrazine ring that gives PyPz-COF the potential for photocatalytic cleavage of water to hydrogen (Fig. 23c). PyPz-COF demonstrates a significantly enhanced photocatalytic hydrogen generation rate of up to 7542 μmol g−1 h−1 with Pt as a cocatalyst, which is in stark contrast to that of PyTp-COF without pyrazine introduction (1714 μmol g−1 h−1) (Fig. 23d). The introduction of nitrogen effectively reduces the band gap and inhibits the complexation of photoexcited carriers, thus improving the photocatalytic performance.
N groups, which could enhance proton transfer through hydrogen bonding. The bandgap of PyPz-COF is slightly smaller than that of PyTp-COF, which may be due to the introduction of the electron-deficient pyrazine ring that gives PyPz-COF the potential for photocatalytic cleavage of water to hydrogen (Fig. 23c). PyPz-COF demonstrates a significantly enhanced photocatalytic hydrogen generation rate of up to 7542 μmol g−1 h−1 with Pt as a cocatalyst, which is in stark contrast to that of PyTp-COF without pyrazine introduction (1714 μmol g−1 h−1) (Fig. 23d). The introduction of nitrogen effectively reduces the band gap and inhibits the complexation of photoexcited carriers, thus improving the photocatalytic performance.
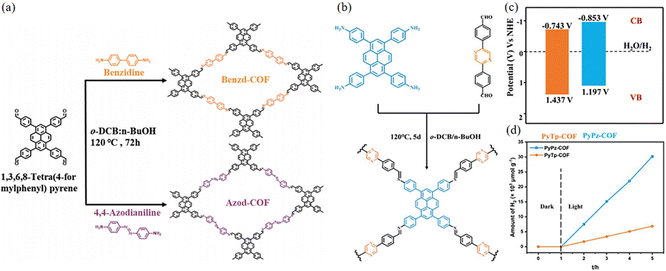 | ||
| Fig. 23 (a) Synthetic routes to Benzd-COF and Azod-COF. Reproduced with permission.88 Copyright 2023, Wiley-VCH GmbH. (b) Schematic diagram of the synthesis of PyPz-COF. (c) Band structure diagram of PyTp-COF and PyPz-COF. (d) The plots for time course photocatalytic H2 evolution of PyPz-COF and PyTp-COF under visible light irradiation. Reproduced with permission.89 Copyright 2023, Wiley-VCH GmbH. | ||
Strong electron-withdrawing groups (e.g., halogen, nitro, cyano) and electron-donating groups (e.g., methyl, methoxy) are often introduced into the building blocks of COFs as functional groups to regulate the light absorption range and bandgap through the electron push–pull effect, facilitating the separation and transfer of photoinduced charge carriers.
Chen et al.90 designed a strategy based on the well-established donor–acceptor (Py-XTP-BT-COF) integration to enhance charge separation efficiency and facilitate light absorption (Fig. 24a). The structure was finely tuned by introducing different halogen atoms to increase the rate of HER. Donor–acceptor (D–A) molecules are a family of functional compounds consisting of electron donors and acceptors. Integrating D–A moieties is a valid strategy to help electron–hole separation and charge transport of COFs (Fig. 24b and c).91 Adopting this strategy, Sun et al.92 reported two donor–acceptor COFs (TAPFy-PhI and TAPB-PhI) synthesized via a Schiff base reaction using phthalimide as the acceptor and TAPFy and TAPB as the donors (Fig. 24d). The D–A strategy can not only narrow the band gap and broaden photon absorption, but also enhance charge transport and separation, therefore, further improving the photocatalytic capacity for hydrogen evolution.
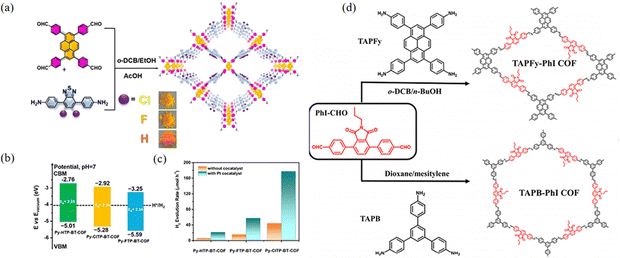 | ||
| Fig. 24 (a) Synthetic routes to Py-XTP-BT-COFs under solvothermal conditions. (b) Band structure diagram for Py-XTP-BT-COFs as determined from SRPES and UV-DRS. (c) The photocatalytic performances of Py-XTP-BT-COFs (20 mg) with and without co-catalyst under visible (λ > 420 nm) light irradiation. Reproduced with permission.90 Copyright 2020, Wiley-VCH GmbH. (d) Synthetic routes to the two COFs under solvothermal conditions. Reproduced with permission.92 Copyright 2023, American Chemical Society. | ||
A longer π-conjugation can improve the light absorption capacity and narrow the π–π* energy. This not only increases the photogenerated electron cloud density, but also improves the proton reduction capability. Therefore, adjusting the π-conjugation degree of the building blocks is a valid method to influence the photocatalytic performance. Chen and co-workers93 synthesized a series of donor–acceptor COFs (NKCOF-108, -109, -110, and -111) with V groups as donor groups. Benzothiadiazoles with different functional groups were introduced as an electron acceptor to tune the light-absorption ability of COFs (Fig. 25a). The bandgaps of NKCOF-108, -109, -110, and -111 were calculated by DFT to be 1.82, 1.89, 1.96, and 2.15 eV. With Pt as a cocatalyst, the trend of the HER was found to be NKCOF-108 > NKCOF-109 > NKCOF-110 > NKCOF-111. The HER of NKCOF-108 reached up to 120 μmol g−1 h−1, possibly attributed to the incorporation of fluorine groups. The larger conjugated system promotes the mobility and separation of photogenerated carriers.
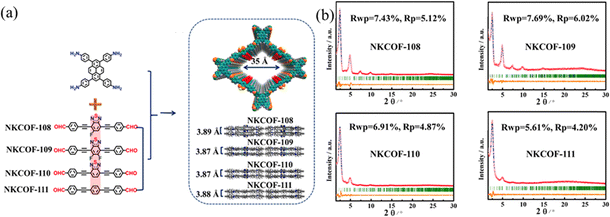 | ||
| Fig. 25 (a) Synthesis of NKCOFs. (b) Experimentally observed PXRD of NKCOFs. Reproduced with permission.93 Copyright 2021, American Chemical Society. | ||
Due to the incomplete delocalization of imine bonds, the stability of imine bonds is generally poor under acidic conditions. However, the hydrazone bond compensates for these disadvantages. Shen et al.94 designed and synthesized a pyrene-based S4-COF based on thioether group functionalization using the solvothermal method (Fig. 26a). It accelerates the separation of photogenerated carriers, enhances charge transfer, achieves long-term photostability, and uniformly distributes the ordered structure of the photocatalyst. The combination of COFs and NPs can adjust and modify the structure of COFs, and the surface of S4-COF exhibits strong hydrophilic properties. Under light excitation, photogenerated electrons in S4-COF can be transitioned to the conduction band. Then, these electrons are transferred and accumulated on the surface of Au NPs, effectively separating electrons and reducing water to H2. Under visible light (λ ≥ 420 nm) irradiation, the photocatalytic hydrogen production of pure S4-COF is 302 μmol g−1 h−1. The photocatalytic hydrogen production of S4-COF modified by Au NPs is 1377 μmol g−1 h−1, which is 4.5 times higher than that of pure S4-COF (Fig. 26b).
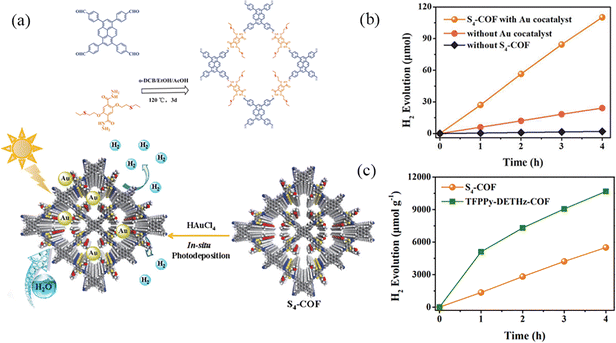 | ||
| Fig. 26 (a) Schematic synthesis of S4-COF and its application in photocatalytic H2 generation. (b) Photocatalytic H2 evolution of S4-COF under different conditions. (c) Time courses of photocatalytic H2 evolution of S4-COF and TFPPy-DETHz-COF. Reproduced with permission.94 Copyright 2024, Elsevier. | ||
COFs with rectangular pore structures were prepared by condensation reaction using building blocks with two-node (C2) and four-node (C4) symmetric structures. Meanwhile, due to the strong π-conjugated four-node (C4) symmetric structures, the COFs with these building block nodes usually have high stability, thus broadening the application scope of this kind of COF. In 2024, Li et al.95 reported the synthesis of PyAm-TpbAl-COF, where the pyrene molecule is electron-enriched and serves as the donor building block and benzene acts as the acceptor (Fig. 27). Although the structures of these COFs are similar, their performance in exciton dissociation and electron–hole pair separation differs significantly. The discrepancy may be attributed to the variation in the orientation of the imine bond. The photocatalytic hydrogen evolution performance of PyAl-TpbAm-COF is three times higher than that of PyAm-TpbAl-COF, with average hydrogen evolution rates of 2.1 mmol g−1 h−1 and 0.7 mmol g−1 h−1, respectively.
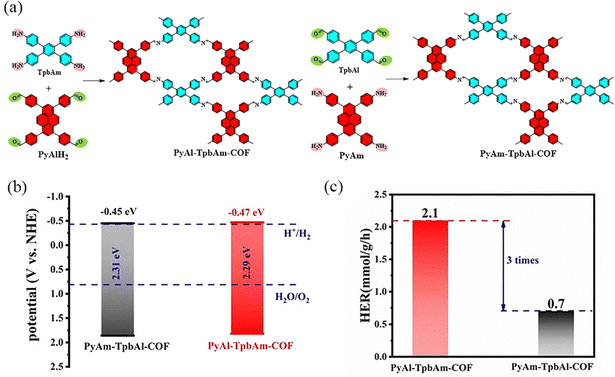 | ||
| Fig. 27 (a) Synthesis route to PyAl-TpbAm-COF and PyAm-TpbAl-COF. (b) Band levels of PyAl-TpbAm-COF and PyAm-TpbAl-COF. (c) Average hydrogen evolution rate. Reproduced with permission.95 Copyright 2023, Royal Society of Chemistry. | ||
PyCOFs have attracted significant attention because of their π–π stacked structure, excellent response to visible light, tunable band gap and high stability. Kuo et al.96 reported two tetraformyl carbazole species with different conjugation lengths as raw materials to prepare two ultrastable luminescent 2D COFs-PyTA-BC and PyTA-BC-Ph through the Schiff base reaction under solvothermal conditions (Fig. 28a). Increasing the conjugation length in the linker increased the degree of planarity and, thereby, increased the surface area and the d100 value of the 2D COF. Therefore, in the absence of a noble metal cocatalyst, the system of COFs and ascorbic acid could provide a photocatalytic H2 production of up to 1183 μmol g−1 h−1 (λ ≥ 420 nm) (Fig. 28b). Dong et al.97 employed [4+4] condensation methods to synthesize two ultrastable luminescent COFs, which designed and synthesized a novel imine-linked COF (DABT-Py-COF) by D–A–D type (Fig. 28c). Due to the ordered arrangement of positive and negative charges in the framework, the D–A–D strategy is utilized to inhibit the recombination of photogenerated charges and regulate the structure to enhance the photocatalytic activity.
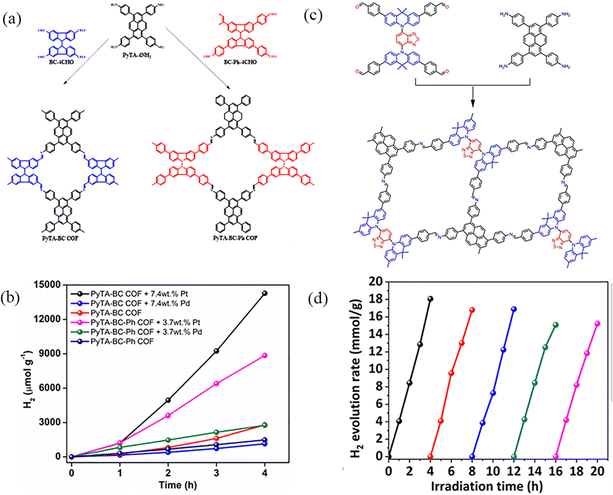 | ||
| Fig. 28 (a) Synthetic routes to PyTPA-BC and PyTA-BC-Ph COFs. (b) Effect of different co-catalysts for photocatalytic H2 production using visible light for PyTA-BC and PyTA-BC-Ph COFs. Reproduced with permission.96 Copyright 2022, Wiley-VCH GmbH. (c) Schematic diagram of the synthesis of DABT-Py-COF. (d) Photostability test of DABT-Py-COF for cycling runs over 20 h under visible-light irradiation (AM 1.5). Reproduced with permission.97 Copyright 2023, Royal Society of Chemistry. | ||
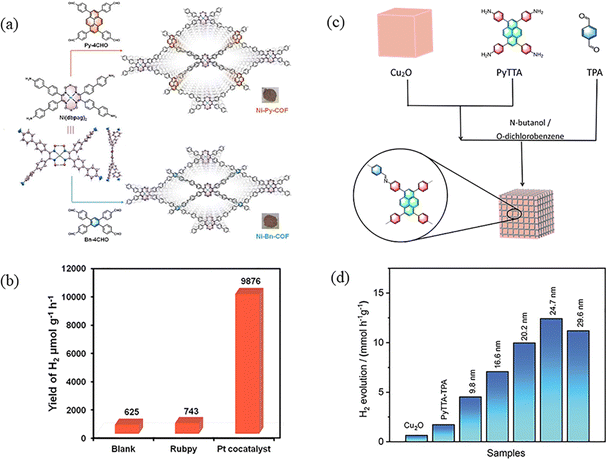
3.1.1.2 Composite photocatalysis. Due to the rapid recombination of photogenerated electron–hole pairs, the practical application of PyCOFs still faces great challenges: photogenerated carriers generated in the narrow bandgap of photocatalysts can easily recombine, while electron–hole pairs in wide bandgap photocatalysts are difficult to be generated, resulting in low quantum yields and low photocatalytic efficiencies. To address these issues, COF-based composite photocatalysts are considered to be the most promising materials on the verge of technological advancement, with significant improvements in charge separation and transfer. Excited electrons move from the higher CB to lower CB, and holes move from the higher VB in one semiconductor to the lower VB in the other. Thus, charge transfer between them effectively prevents photogenerated electron/hole complexation and improves the catalytic performance. Tight interfacial connections between metals and semiconductors or between different semiconductors can lead to efficient electron transfer, thus enhancing photocatalytic decomposition of water activity. The loading of suitable metal co-catalysts is an effective strategy to enhance the activity of semiconductor photocatalysts. For example, Chen et al.98 constructed two new MCOFs (Ni-Py-COF and Ni-Bn-COF) from nickel ethoxime based building blocks (Fig. 29a). MCOFs not only inherit the advantages of COFs, such as high crystallinity, porosity, and specific surface area, but also have the advantages of multiple active sites of the metal, and the mutual synergistic effect promotes charge transfer, effective separation, and inhibition of photogenerated electron–hole pair recombination, reducing the band gap and increasing the photocatalytic efficiency.99 Even without the addition of the Pt co-catalyst, the hydrogen evolution rates (HER) of Ni-Py-COF reached up to 626 μmol g−1 h−1 (Fig. 29b). Guo et al.100 designed and constructed a class of Cu2O/2D PyTTA-TPA COFs, which significantly improved the efficiency of photocatalytic hydrogen evolution (Fig. 29c). Driven by the electric field, the photogenerated electrons are transferred from the semiconductor with a more negative conduction-band potential to another semiconductor with a more positive conduction-band potential, and at the same time, the photogenerated holes are transferred from the semiconductor with a more positive valence-band potential to the other semiconductor with a more negative valence-band potential, which achieves the effective separation of photogenerated electrons and holes and improves the photocatalytic efficiency and hydrogen evolution and significantly inhibits the photocorrosion. Therefore, the as-made Cu2O/PyTTA-TPA COF core/shell nanocubes with a shell thickness of 24.7 nm possess an exceptional photocatalytic H2 evolution rate of 12.5 mmol h−1 g−1 (Fig. 29d).
 | ||
| Fig. 29 (a) Synthetic route to Ni-glyoximate-COFs. (b) H2 photogeneration control experiments of Ni-Py-COF. Reproduced with permission.98 Copyright 2022, Wiley-VCH GmbH. (c) Schematic diagram of the synthesis of Cu2O/PyTTA-TPA COFs core/shell nanocubes. (d) The photocatalytic H2 evolution rate of different photocatalysts. Reproduced with permission.100 Copyright 2023, American Chemical Society. | ||
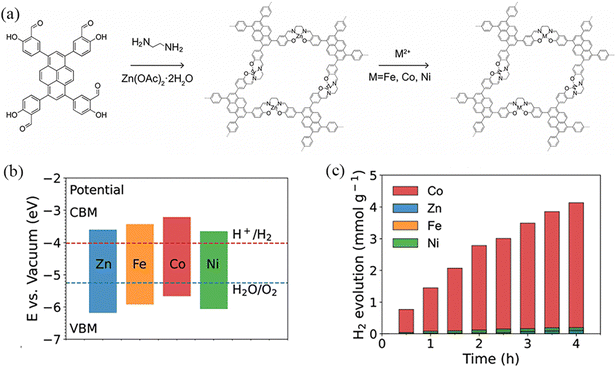
Metals and semiconductors have different Fermi energy levels, and when a metal is coupled onto a semiconductor, electrons can be transferred from the semiconductor to the metal. The surface energy band of a semiconductor bends upwards, so the Schottky barrier formed at the metal–semiconductor interface can act as an effective electron trap to inhibit electron–hole complexation, thus prolonging the lifetime of the photogenerated electrons and improving the photocatalytic efficiency. Deng et al.99 reported the heterogenization of a Salen metal-complex molecular catalyst into a rigid COF through covalent linkage with the light-harvesting unit of pyrene. The chemically conjugated bonds between two units contribute to the fast photogenerated electron transfer, thereby promoting the proton reduction reaction (Fig. 30a). Therefore, the Salen cobalt-based COF showed the best hydrogen evolution activity, which is 1378 μmol h−1 g−1 (Fig. 30b and c).
 | ||
| Fig. 30 (a) Synthesis and structure of Zn-Salen-COF and M/Zn-Salen-COF (M = Fe, Co, Ni). (b) Schematic energy band structures of Zn-Salen-COF and M/Zn-Salen-COF. (c) Photocatalytic H2 production on Zn-Salen-COF and M/Zn-Salen-COF. Reproduced with permission.99 Copyright 2023, Wiley-VCH GmbH. | ||
Immobilizing functional groups on the backbones of building units enables the modulation of the electron push–pull effect and narrows the bandgap, thus facilitating the separation and transfer of photoinduced excitons. Hence, strong electron-absorbing groups (like halogen atoms, heteroatomic atoms) and electron-donating groups (like methyl, methoxy) are often adopted to modify COF structures to boost photocatalytic hydrogen peroxide generation.
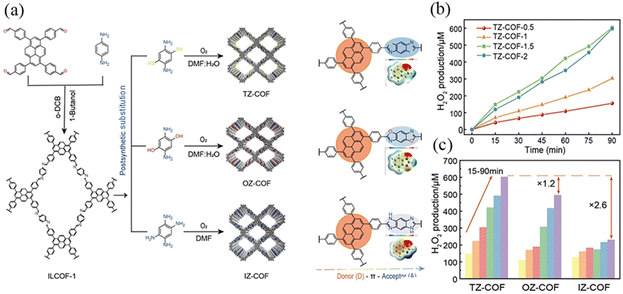
Wang et al.102 reported a series of D–A COFs (TZ-COF, OZ-COF, IZ-COF) with azoles as acceptors and pyrenes as donors to effectively enhance chemical stability and π-coupling, facilitating charge transfer and inhibiting photogenerated electron–hole recombination (Fig. 31a). Electronegativity follows the order O > N > S, and the difference in optical activity could be attributed to the distinct heteroatoms, the absorption of visible light, electron–hole separation, carrier migration, etc. At the optimal concentration, IZ-COF exhibited a H2O2 production rate of 102 μmol g−1 h−1, which was enhanced to 220 μmol g−1 h−1 for OZ-COF and 268 μmol g−1 h−1 for TZ-COF (Fig. 31b). According to DFT calculations, the linkage chemistry, which enhances the activities of neighboring benzene rings, promoted the formation of the necessary intermediates for the two-electron oxygen reduction reaction (2e− ORR), leading to H2O2 production (Fig. 31c).
 | ||
| Fig. 31 (a) Synthetic routes to and the D–π–A model and differences of COFs with azoles linkages. (b) H2O2 yield rates of TZ-COF with different concentrations. (c) H2O2 yields of COFs dispersed in H2O at a concentration of 1.5 g L−1. Reproduced with permission.102 Copyright 2023, Wiley-VCH GmbH. | ||
Pyrene units are the most active reduction centers over the other sites. Besides, the well distribution of active centers over a large surface area plays an important role in enhancing photocatalytic performance. Sun et al.103 synthesized four imine PyCOFs via a Schiff-base condensation reaction of Py-CHO with Da-NH2, 2,2-bipyridine-5,5′-diamine (Bpy-NH2), TAPD and Py-NH2 to obtain the corresponding Py-Da-COF, Py-Bpy-COF, Py-TAPD-COF, and Py-Py-COF. TAPD and Bpy are considered as electron-rich reduction centers for O2 binding, which is a crucial step for H2O2 formation (Fig. 32a). The photocatalytic performance is positively correlated with COFs BET surface area. The large BET surface area ensures a high surface availability of the active pyrene sites for ORR and accelerates the mass transfer. The nature of the charge transfer resistance and the efficiency of electron and hole separation showed the trend of Py-Da-COF > Py-Bpy-COF > Py-TAPD-COF > Py-Py-COF, and the amount of H2O2 generated by Py-Da-COF (868 μmol g−1) was much higher than that of the other three species in 3 h (Fig. 32c).
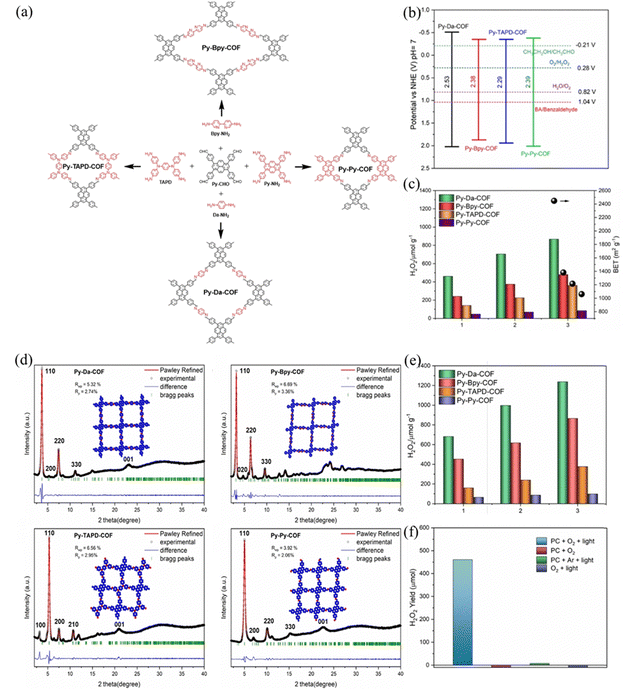 | ||
Fig. 32 (a) Schematic illustration of the PyCOFs. (b) Band gaps of the pyrene-based COFs. (c) Photoactivity of the COFs for H2O2 production in water. (e) Photoactivity of the COFs for H2O2 production in water–ethanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). (f) Photoactivity of the COFs for H2O2 production after three hours of irradiation in different systems. (d) The PXRD patterns and Pawley refinements of Py-Da-COF, Py-Bpy-COF, Py-TAPD-COF, and Py-Py-COF. Reproduced with permission.103 Copyright 2023, Wiley-VCH GmbH. 1). (f) Photoactivity of the COFs for H2O2 production after three hours of irradiation in different systems. (d) The PXRD patterns and Pawley refinements of Py-Da-COF, Py-Bpy-COF, Py-TAPD-COF, and Py-Py-COF. Reproduced with permission.103 Copyright 2023, Wiley-VCH GmbH. | ||
The absorbance range and intensity of COFs have very important effects on their photocatalytic performance. The π-conjugated unit in the framework structure of COFs plays an important role in their light absorption because the electron transition in the visible spectral range is π → π* transition. Chen et al.106 synthesized sp2c-COFs with excellent extended aromatic conjugation and potential embedding of redox active sites via simple base-catalyzed Knoevenagel polycondensation. Using TFPP and BPYDAN as the donor and acceptor, the fully π-conjugated system achieves intramolecular electron transfer (Fig. 33a) and the complete –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bridge in sp2c-COFdpy creates and unblocks donor–acceptor channels to achieve intramolecular electron delocalization and cascade effects. Using embedded bipyridine, a range of base metals (Fe, Co, Ni and Cu) can be anchored by sp2c-COFdpy to provide catalytic active sites for CO2 photoreduction. The absorbance and band gap of sp2c-COFdpy show significant changes, reaching up to 17.93 mmol g−1 CO in the photocatalytic reaction with a selectivity of 81.4% (Fig. 33d). The structural advantage of electron cascade transfer in sp2c-COFdpy-Co is theoretically calculated to allow excitons to easily reach a single Co site, thus promoting proton–electron coupled CO2 photoreduction.
C– bridge in sp2c-COFdpy creates and unblocks donor–acceptor channels to achieve intramolecular electron delocalization and cascade effects. Using embedded bipyridine, a range of base metals (Fe, Co, Ni and Cu) can be anchored by sp2c-COFdpy to provide catalytic active sites for CO2 photoreduction. The absorbance and band gap of sp2c-COFdpy show significant changes, reaching up to 17.93 mmol g−1 CO in the photocatalytic reaction with a selectivity of 81.4% (Fig. 33d). The structural advantage of electron cascade transfer in sp2c-COFdpy-Co is theoretically calculated to allow excitons to easily reach a single Co site, thus promoting proton–electron coupled CO2 photoreduction.
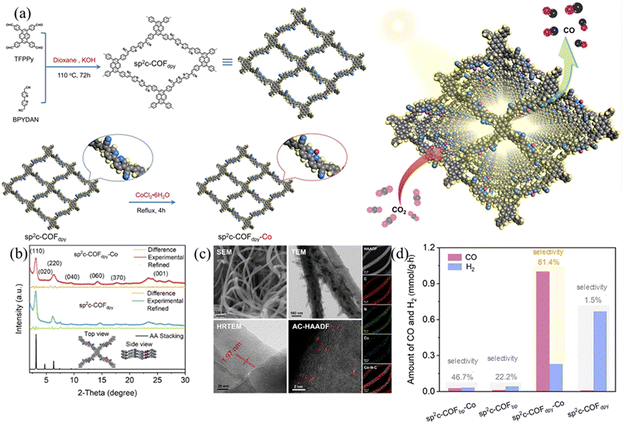 | ||
| Fig. 33 (a) Synthesis of sp2c-COFdpy and sp2c-COFdpy-Co. Schematic of CO2 photoreduction on the as-prepared sp2c-COFdpy-Co. (b) The PXRD of sp2c-COFdpy-Co and sp2c-COFdpy and calculated patterns for AA stacking. (c) The SEM image, TEM image, HR-TEM image with lattice, aberration-corrected HAADF-STEM and EDX elemental mapping of sp2c-COFdpy-Co. (d) Photocatalytic CO2 reduction over sp2c-COFdpy-Co, sp2c-COFdpy, sp2c-COFbpy-Co and sp2c-COFbpy under visible light in water using triethanolamine (TEOA) as the sacrificial agent. Reproduced with permission.106 Copyright 2020, Elsevier. | ||
Improving the separation and transfer efficiency of photogenerated electron–hole pairs by selecting suitable organic ligands is also conducive to enhancing the photocatalytic performance of COFs, and the orderly stacking of π units in COFs to form periodic columnar π arrays can provide an effective path for interlayer charge transfer. Mahdy et al.107 reported a new ethene-based COF (EPPT-COF), through polycondensation between electron-rich (E)-1,2-diphenylethene and 1,3,6,8-tetraphenyl pyrene units via a Schiff-base reaction (Fig. 34a). The π–π conjugated interactions between the EPPT-COF layers provides high thermal and chemical stability, while the π-electron delocalization leads to easier charge separation and transport. EPPT-COF has a narrower bandgap (1.7 eV) compared to the CN, which has a larger wavelength absorption range and produces more photogenerated electrons (Fig. 34e). The higher electron concentration on the surface of EPPT-COF is favorable for the catalytic reduction reaction. EPPT-COF absorbed higher absorption performance at 298 K produced CO2 (112 mg g−1), and produced CH4 (14.7 μmol g−1 h−1) consistently and constantly for 14 hours, and no other products were detected (Fig. 34b, c, e and f).
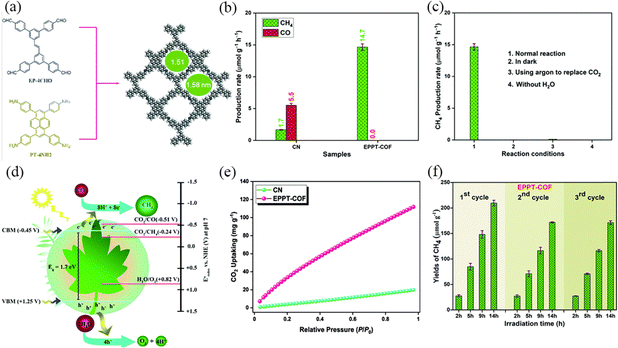 | ||
| Fig. 34 (a) Synthetic route to and top view of the AA-eclipsed model of EPPT-COF. (b) Photocatalytic performance for CO2 conversion over CN and EPPT-COF; each measurement was performed three times. (c) Control experiments of EPPT-COF were performed using various reaction conditions; each measurement was performed three times. (d) Proposed band structure diagram for EPPT-COF. (e) CO2 uptake of CN and EPPT-COF. (f) Cyclic performance measurement of EPPT-COF; each measurement was performed three times. Reproduced with permission.107 Copyright 2023, Elsevier. | ||
Gao et al.108 prepared 1D PyTTA-COF and 2D PyTTA-COF by Schiff base condensation, which have good chemical and thermal stability (Fig. 35a). 1D PyTTA-COF exhibited considerable CO2 reduction activity, with total CO release up to 1003 μmol g−1 over 8 hours, and no liquid carbon production such as HCOOH was found in the reaction system (Fig. 35b). For 1D PyTTA-COF, the highest occupied molecular orbital (HOMO) is mainly distributed on the PyTTA and extends slightly to the imine moiety. The lowest unoccupied molecular orbital (LUMO) is assigned to the phenyl moiety. The HOMO of 2D PyTTA-COF is similar to that of 1D PyTTA-COF, while the LUMO of 2D PyTTA-COF is distributed throughout the fragment, which may play a key role in local electron modulation. 1D PyTTA-COF has a narrower band gap, and this structural feature reduces the energy loss of excited photoelectrons, thus facilitating the transfer of photoelectrons to the catalytic active site via imine bonds (Fig. 35c).
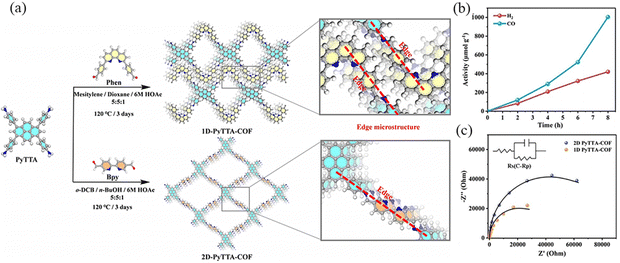 | ||
| Fig. 35 (a) Synthesis route to and the microstructures (enlarged regions) of 1D PyTTA-COF and 2D PyTTA-COF. (b) Kinetic profile for the evolution of CO and H2 over 1D PyTTA-COF-Co. (c) Nyquist plots of 1D PyTTA-COF and 2D PyTTA-COF. Reproduced with permission.108 Copyright 2023, Wiley-VCH GmbH. | ||
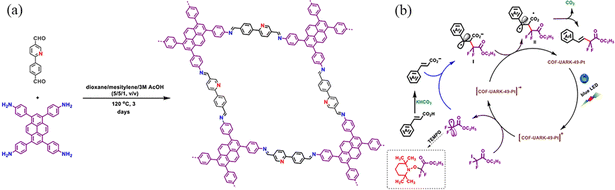 | ||
| Fig. 36 (a) Synthesis of COF-UARK-49 via imine condensation. (b) Proposed mechanism for the decarboxylative difluoroalkylation reaction. Reproduced with permission.109 Copyright 2021, American Chemical Society. | ||
Liu et al.110 synthesized imine COFs with electron donor and acceptor structures. COF-JLU22 was prepared through reacting 1,3,6,8-tetrakis(4-aminophenyl)pyrene with the highly electron-deficient longer linker 4,7-diphenylbenzo[c][1,2,5]thiadiazole. COF-JLU22 can be used as a metal-free and recyclable photocatalyst under visible light irradiation (Fig. 37a). Its red-shifted absorption can enhance the photocatalytic activity of the COF with high catalytic efficiency for reductive dehalogenation of benzoyl bromide derivatives and α-alkylation of aldehydes. The excited state of COF-JLU22 produces photogenerated holes and electrons through charge separation. The photogenerated holes extract electrons from the sacrificial agent N,N′-diisopropylethylamine (DIPEA). At the same time, the photogenerated electrons are transferred from the conduction band of COF-JLU22 (E1/2 = −0.86 V) to benzoyl bromide (E1/2 = −0.49 V), leading to the cleavage of C–Br and the formation of α-carbonyloxy radicals and bromine anions. The α-carbonyl radical can then take a proton and an electron from the Hantzsch ester to form the final product, acetophenone (Fig. 37b).
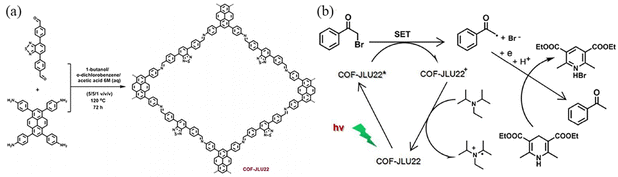 | ||
| Fig. 37 (a) Synthesis of COF-JLU22 by imine condensation reaction. (b) Proposed reaction mechanism for the photoreductive dehalogenation reaction with COF-JLU22. Reproduced with permission.110 Copyright 2018, Elsevier. | ||
Wen et al.53 reported COF-JLU25, where the D–A the electron-donor units in this overlapping stacked structure may be a source of abundant electrons to facilitate oxidation reactions (Fig. 38a). The COF-JLU25 sample exhibited two intense XRD peaks at 2.70° and 5.39°, and the BET surface area was measured to be 141.31 m−1 g−1 (Fig. 38c and d). COF-JLU25 was selected as a photocatalyst for the oxidative hydroxylation of arylboronic acids. The COF-JLU25-catalysed conversion is characterized by low catalyst loading, high efficiency, high reusability and compatibility with substrates bearing a wide range of functional groups.
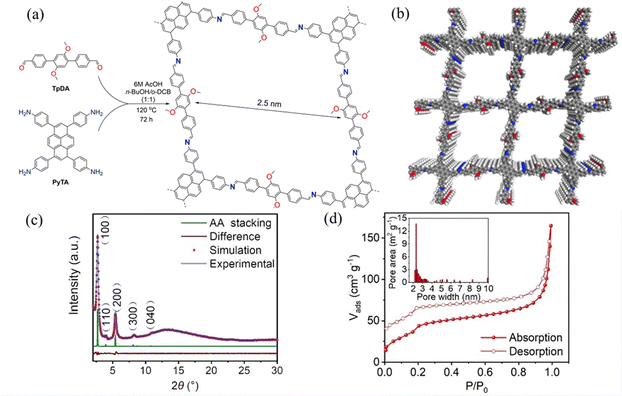 | ||
| Fig. 38 (a) Synthesis of COF-JLU25. (b) The structural model of COF-JLU25. (c) PXRD patterns of COF-JLU25. (d) N2 adsorption isotherms of activated COF-JLU25 at 77 K. Reproduced with permission.53 Copyright 2021, Wiley-VCH GmbH. | ||
Azine-linked COFs are constructed using two aldehyde derivatives to react with hydrazine monomers. Since hydrazine is the shortest diamino linker, azine-linked COFs have the smallest pore size among all COFs with the same building nodes. Lang et al.111 reported that Py-Azine-COF as the photocatalyst could selectively realize aerobic conversion using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) as an accelerator to facilitate hole transport and the formation of superoxide with oxygen for selective oxidation of organic sulfides (Fig. 39a). The partial charge density on pyrene was gradually transferred to the azine bond, and the interfacial transfer resistance of the charge carriers was decreased with the assistance of 2 mol% TEMPO, leading to a more efficient separation and migration of the charges on Py-Azine-COF. Under blue light irradiation, abundant photogenerated holes and electrons were generated on Py-Azine-COF, trapping the generated holes and converting TEMPO to TEMPO+ (Fig. 39b). TEMPO+ provides S-centred radical ions by acquiring electrons from methyl phenyl sulfide. The hole mediator TEMPO is capable of rapidly converting a variety of sulphides to sulphur oxides over the Py-Azine-COF photocatalyst in methanol.
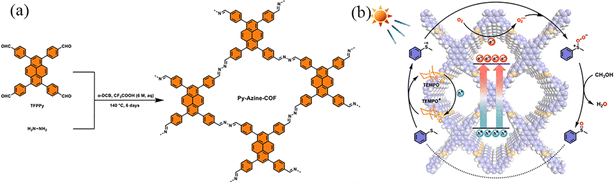 | ||
| Fig. 39 (a) Schematic of the construction of Py-Azine-COF. (b) A proposed mechanism for selective oxidation of methyl phenyl sulfide with O2 over the Py-Azine-COF photocatalyst with TEMPO. Reproduced with permission.111 Copyright 2023, Elsevier. | ||
2D sp2c COFs are characterized by ordered π stacks, abundant active sites, adjustable open nanoporous structures, customizable molecular building blocks and strong covalent bonds. In 2024, using a solvothermal method, Hu et al.34 prepared pyrene COF photocatalysts with different bond modes (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, N
C, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), and developed pyrene COFs (sp2c-COF and py-NH2-COF) to complete the photocatalytic degradation of tetracycline (TC) under visible light. The Eg of sp2c-COF is 1.98 eV. The sp2c-COF catalyst absorbs energy to produce electron transition, forming photogenerated electron–hole pairs, and TC is effectively attacked by the formed holes, increasing the degradation rate. The universality of sp2c-COF was analyzed by the degradation of tetracycline, chlortetracycline, oxytetracycline, and doxycycline under visible light irradiation. It was found that sp2c-COF was a recyclable and universal catalyst for degradation of various organic pollutants.
C), and developed pyrene COFs (sp2c-COF and py-NH2-COF) to complete the photocatalytic degradation of tetracycline (TC) under visible light. The Eg of sp2c-COF is 1.98 eV. The sp2c-COF catalyst absorbs energy to produce electron transition, forming photogenerated electron–hole pairs, and TC is effectively attacked by the formed holes, increasing the degradation rate. The universality of sp2c-COF was analyzed by the degradation of tetracycline, chlortetracycline, oxytetracycline, and doxycycline under visible light irradiation. It was found that sp2c-COF was a recyclable and universal catalyst for degradation of various organic pollutants.
3.2 Application of PyCOFs in battery based materials
COFs have recently attracted increasing attention in energy storage devices.112 In 2018, Wang et al.50 reported COF-based Li–S batteries by loading 70 wt% sulfur onto 2D PyCOF, which could be used as an electrode material to construct lithium–sulfur (Li–S) batteries with high multiplicative capacity and long-term stability (Fig. 40). The Py-COF/S electrode even outperforms some porous carbon-based sulfur body electrodes.113 The uniform distribution of sulfur in the pore volume of Py-COF/S nanopores was dramatically reduced (from 1.25 cm3 g−1 to 0. 07 cm3 g−1) along with a reduction in BET surface area (from 2093 to 10 m2 g−1) after sulphur loading; the diffusion rate of Li+ ions in the Py-COF/S electrode was faster than that of the BP2000/S electrode, and the difference in ion diffusion rate could be attributed to the pristine structure of Py-COF, which has a 1D ordered porous structure with a fast ion diffusion path.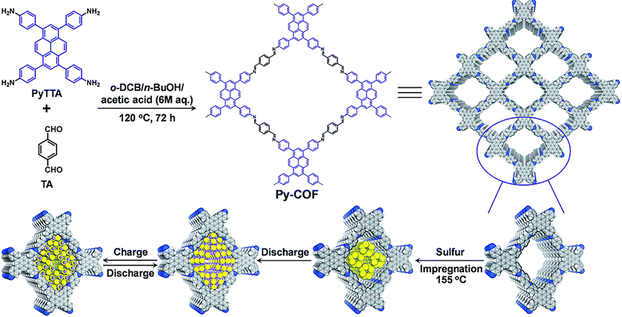 | ||
| Fig. 40 Schematic illustration of the synthesis of Py-COF and Py-COF/S composite as well as the discharge/charge process. Reproduced with permission.50 Copyright 2018, Royal Society of Chemistry. | ||
2D COFs usually form thick laminated sheets due to strong interlayer π–π interactions between 2D nanosheets, which impede the access of electrolytic ions to their redox active sites. In addition, most 2D COFs have a bandgap of a few electron volts and poor electrical conductivity, leading to poor rate capability at high charge/discharge current densities when tested as capacitive materials. Constructing heterostructures consisting of 2D and conductive materials can overcome the above challenges.58
In 2019, by tuning the mass ratio between COF precursors and CNTs (mprecursors: mCNTs¼x), Xu et al.114 prepared COF/CNT van der Waals heterostructures (vdWHs) with different COF mass loading (denoted as COF/CNT-x (x = 1, 0.5 and 0.2)) (Fig. 41a). The pyridine group in the TPP-Py COF undergoes two consecutive electron–proton transfer redox processes. All COF/CNT-x exhibited larger CV area with improved capacitive energy storage capacity compared to the TPP-Py COF and CNT (Fig. 41b). Chen et al.49 reported a thienothiophene-containing molecule TAPTt-COF. The carbon–sulfur region of the thiophene group in COFs is the active catalytic site for oxygen reduction and precipitation reactions, and coaxial 1D vdWHs are synthesized by π-electron interaction between 2D-COF and CNT. A strong π–π interaction occurs between CNT and pyrene groups in TTAP, and the interaction plays a key role in guiding the formation of 1D core–shell structure vdWHs.
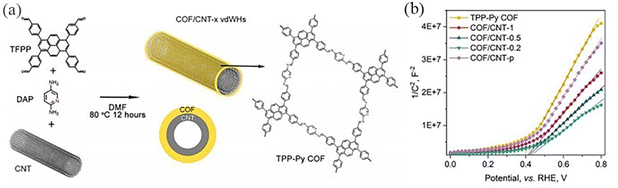 | ||
| Fig. 41 (a) Schematic illustration of the synthesis of 1D core–shell COF/CNT vdWHs. (b) M–S plots of COF/CNT vdWHs and reference samples. Reproduced with permission.114 Copyright 2021, AIP Publishing. | ||
The strong π–π interactions among the COF layers together with the extended structure can remarkably reduce the solubility of low molecular weight monomers in electrolytes. Zhang et al. (Fig. 42a) employed comprehensive characterization and theoretical calculations to confirm that pyrene-4,5,9,10-tetrone (PTO) was a carbonyl-rich compound, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group can provide a redox active site for Zn2+. The 4KT-Tp-COF electrode is theoretically capable of holding 8 electrons, and considering the additional Tp fragment in 4KT-Tp-COF, 4KT-Tp-COF is believed to be capable of storing 6 electrons. For the Tp fragments, Zn2+ tends to coordinate with the two adjacent carbonyls between the COF layers, whereas the two adjacent carbonyls in the PTO fragments can directly coordinate with the zinc ions, possibly because both Tp nodes near the PTO fragments have an effect on the PTO monomer (Fig. 42b). Imine-linked COFs have been extensively used for battery electrodes; however, the chemical instability of imine in acidic solutions has been widely known. For example, the β-ketoenamine-based COFs, prepared through irreversible keto–enol tautomerism, are the relatively stable type among the reported imine-based COFs.115
O group can provide a redox active site for Zn2+. The 4KT-Tp-COF electrode is theoretically capable of holding 8 electrons, and considering the additional Tp fragment in 4KT-Tp-COF, 4KT-Tp-COF is believed to be capable of storing 6 electrons. For the Tp fragments, Zn2+ tends to coordinate with the two adjacent carbonyls between the COF layers, whereas the two adjacent carbonyls in the PTO fragments can directly coordinate with the zinc ions, possibly because both Tp nodes near the PTO fragments have an effect on the PTO monomer (Fig. 42b). Imine-linked COFs have been extensively used for battery electrodes; however, the chemical instability of imine in acidic solutions has been widely known. For example, the β-ketoenamine-based COFs, prepared through irreversible keto–enol tautomerism, are the relatively stable type among the reported imine-based COFs.115
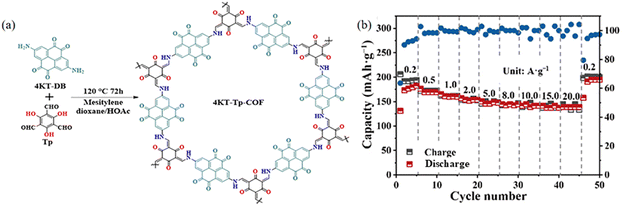 | ||
| Fig. 42 (a) Schematic illustration of the synthesis of 4KT-Tp-COF. (b) Rate performance of 4KT-Tp-COF. Reproduced with permission.116 Copyright 2024, Springer Nature. | ||
3.3 Application of PyCOFs in fluorescence detection
Fluorescence detection is an important method for the detection of ions and certain contaminants.117 COFs are a class of metal-free materials and therefore have superior biocompatibility and low biotoxicity.24a,117c,118 PyCOFs have large conjugated structures with designable active sites. From the structural point of view, the regularly arranged channels of PyCOFs facilitate the interaction between the contact active site and the target substance. In addition, PyCOFs have excellent structural stability; therefore, chemically stable PyCOFs are ideal candidates for the fabrication of highly stable and ultrasensitive sensors.Niu et al.64 synthesized a fluorescent PyCOF (TFPPy-BDOH) through integrating a biphenyl diamine and a pyrene unit into the π-conjugated framework (Fig. 43a). TFPPy-BDOH has an excellent selectivity to uranium due to the synergistic effect of the nitrogen atom in the imine bond and hydroxyl groups in the conjugated framework. In 2021, the Lin group48 designed photoactive donor–acceptor 2D COFs with enzyme-like catalytic properties as a robust colorimetric probe for inexpensive, highly sensitive and rapid colorimetric detection of GSH (Fig. 43b and c). Gu et al.51 reported that the O–H⋯N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C chelating unit can form a bidentate ligand, which could selectively bind metal ions.
C chelating unit can form a bidentate ligand, which could selectively bind metal ions.
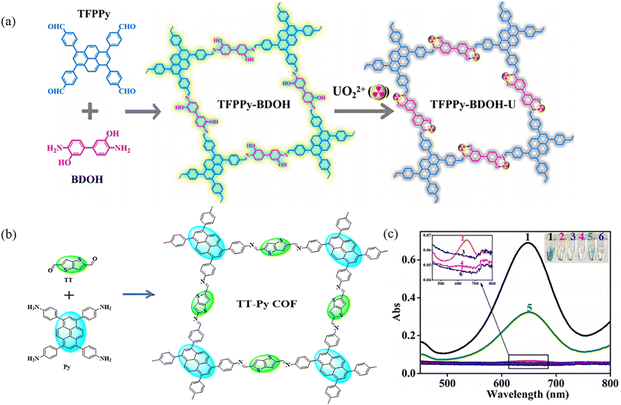 | ||
| Fig. 43 (a) Synthesis of TFPPy-BDOH for detection. Reproduced with permission.64 Copyright 2021, Elsevier. (b) Schematic synthesis of the Py-TT COF. (c) Typical absorption curves and color changes of TMB in different reaction systems. Reproduced with permission.48 Copyright 2021, American Chemical Society. | ||
Imine-bonded COFs are capable of transmitting extended π-conjugation effects via C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds. However, due to the highly polarized nature of C
N bonds. However, due to the highly polarized nature of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, these bonds usually lead to relatively weak electronic delocalisation, which affects the conjugation of COF layers; however, the full sp2-carbon linkage can increase the effectiveness of π-conjugation between the individual units in the 2D system. In 2021, Xiao et al.32 synthesized sp2-carbon-conjugated fluorescent COFs for detection of high-performance ECL emitters (Fig. 44a). Compared with imine-connected PyCOFs, Py-sp2c-CONs exhibited higher ECL emission, where Py-sp2c-CON was constructed with pyrene luminophores and sp2c connecting bonds to effectively reduce the ACQ effect and increase the mobility of the luminophores. At the same time, ultrathin pores in Py-sp2c-CONs could accelerate the migration of co-reactants, ions, and electrons to make more internal luminophores to be electrochemically activated, thus increasing the utilization of ECL luminophores and improving the ECL intensity (Fig. 44b).
N bonds, these bonds usually lead to relatively weak electronic delocalisation, which affects the conjugation of COF layers; however, the full sp2-carbon linkage can increase the effectiveness of π-conjugation between the individual units in the 2D system. In 2021, Xiao et al.32 synthesized sp2-carbon-conjugated fluorescent COFs for detection of high-performance ECL emitters (Fig. 44a). Compared with imine-connected PyCOFs, Py-sp2c-CONs exhibited higher ECL emission, where Py-sp2c-CON was constructed with pyrene luminophores and sp2c connecting bonds to effectively reduce the ACQ effect and increase the mobility of the luminophores. At the same time, ultrathin pores in Py-sp2c-CONs could accelerate the migration of co-reactants, ions, and electrons to make more internal luminophores to be electrochemically activated, thus increasing the utilization of ECL luminophores and improving the ECL intensity (Fig. 44b).
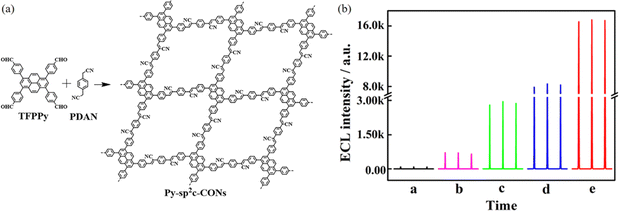 | ||
| Fig. 44 (a) Synthesis of Py-sp2c-CON. (b) ECL intensities of imine-linked pyrene COF/S2O82−. Reproduced with permission.32 Copyright 2021, American Chemical Society. | ||
The hydrazone bond is a type of flexible covalent bond that is easier to be constructed compared with the traditional Schiff base bond, thus allowing structural flexibility in the hydrazone-linked COF.119 In 2021, the Tian group constructed hydrazine-conjugated Pythz-COFs and urea-conjugated polyurea-COFs (Fig. 45a).66 These COFs have high specific surface area and good chemical stability, physical stability, and significant fluorescence-burst response to trace amounts of water in organic solvents. In 2022, Pythz-COFs were used for the detection and recovery of Au(III).67 The Pythz-COFs were found to be sensitive to Au(III), with electron transfer induced to burst the fluorescence and change the visual colour from bright yellow to dark green. The possible reason is the synergistic effect of hydrogen bonding, coordination bonding and redox reaction between the amide functional group and Au(III), leading to the efficient selectivity of Pythz-COFs. Qiu et al.65a designed a novel fluorescent COF, TFPPy-CHYD, by combining a pyrene-based building block with a flexible carbocyclic hydrazide linker, in which the nitrogen-based ligand permits reversible and highly selective binding of Hg2+, exhibiting excellent adsorption capacity (Fig. 45b).
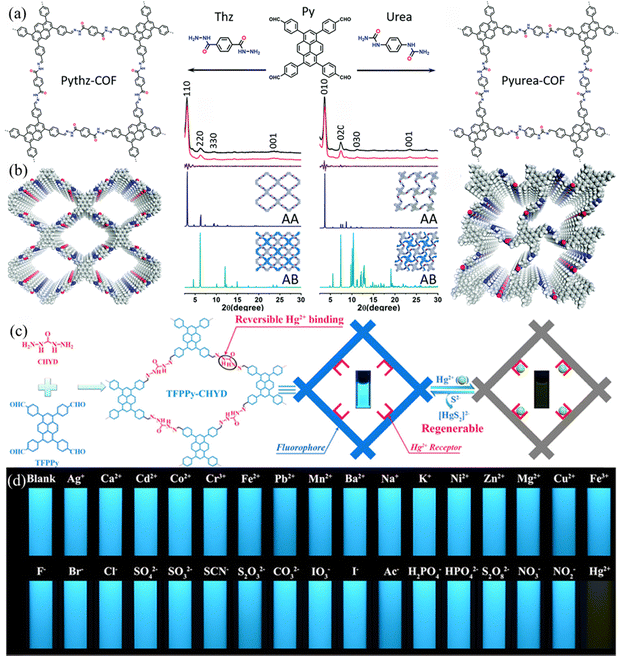 | ||
| Fig. 45 (a) Schematic representation of the synthesis of Pythz-COF and Pyurea-COF. (b) PXRD patterns of Pythz-COF and Pyurea-COF. Reproduced with permission.66 Copyright 2021, Royal Society of Chemistry. (c) Schematic diagram of preparation of the TFPPy-CHYD COF, its fluorescence detection and adsorption of Hg2+, and easy regeneration by adding Na2S. (d) Photographs showing the fluorescence emission change (under a portable 365 nm UV lamp) of TFPPy-CHYD with various ions. Fluorescence quenching of TFPPy-CHYD by Cu2+ was masked by glycine solution (100 μM). Reproduced with permission.65a Copyright 2020, American Chemical Society. | ||
4. Conclusion and outlook
Among various COFs, PyCOFs have high structural stability, strong designability, intrinsic charge-separation ability, and excellent visible-light-absorption ability. This review briefly summarizes the synthetic strategies of different PyCOF linkages, and the research progress of PyCOFs in photocatalysis (including hydrogen evolution, carbon dioxide reduction, hydrogen peroxide production, organic conversion, etc.), batteries, and fluorescence detection. These advances may provide insights into the design of new PyCOFs for future applications. However, to date, most PyCOFs have been constructed from PyTTA and TFFPy, and the remaining building units have been less reported. The practical application of PyCOFs is still in its infancy, and several issues are still required to be addressed.Pyrene is a kind of polycyclic aromatic hydrocarbon, which actually has certain toxicity. In addition, some methods for the preparation of pyrene derivatives produce toxic by-products; thus, more efficient syntheses and purification methods are highly desirable to be developed to reduce the cost of materials and remove residual toxic substances. PyCOFs have been widely used in the field of photocatalysis because of their high efficiency of light energy absorption, good charge transport performance, and diversified photocatalytic reactions. However, the applications of PyCOFs in electrocatalysis are relatively rare due to some issues such as poor conductivity and poor stability. Therefore, optimizing the synthetic conditions to regulate the structures of PyCOFs and provide more efficient electron transport channels is a common method to improve electrical conductivity. In some cases, the synthesis of PyCOFs might involve toxic chemicals and solvents, which increases the environmental burden. Meanwhile, the synthesis of PyCOFs mainly relies on traditional solvothermal methods with mg-scale synthesis, and the realization of large-scale, efficient and green synthesis remains to be solved. Therefore, during the experiment, it is necessary to screen and study chemical substances with similar reactivity but lower toxicity or even non-toxicity to participate in the synthetic reactions of PyCOFs, and reduce the introduction of toxic substances from the source. At the same time, it is important to explore the green solvents to replace the traditional toxic organic solvents and reduce the harm to the environment. Furthermore, the relatively high price and toxicity for PyCOFs impel scientists to find more simple methods for the preparation of COFs as well as more alternative low toxic Py-based monomers. We hope that this review would give readers new insights into PyCOFs and inspire scientists to explore more appealing functions or applications for the future development of PyCOFs.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Open Fund of Beijing National Laboratory for Molecular Sciences (No. BNLMS201842), and Postgraduate Innovation Foundation from Wuhan Institute of Technology. SH acknowledges the support from the collaborative Innovation Platform Project of Fu-Xia-Quan National Independent Innovation Demonstration Zone (No. 2022-P-021). Q. Z. acknowledges the funding support from the City University of Hong Kong (9380117 and 7020089) and the Innovation and Technology Fund (ITF, ITS/322/22), Hong Kong, P. R. China.References
- (a) J. Li, X. Jing, Q. Li, S. Li, X. Gao, X. Feng and B. Wang, Chem. Soc. Rev., 2020, 49, 3565–3604 Search PubMed; (b) Y. Yusran, H. Li, X. Guan, Q. Fang and S. Qiu, Energy Chem., 2020, 2, 100035 CrossRef; (c) J. Sun, Y. Xu, Y. Lv, Q. Zhang and X. Zhou, CCS Chem., 2023, 5, 1259–1276 Search PubMed; (d) J. Sun, F. Kang, D. Yan, T. Ding, Y. Wang, X. Zhou and Q. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202406511 CrossRef CAS PubMed; (e) Y. Shi, J. Yang, F. Gao and Q. Zhang, ACS Nano, 2023, 17, 1879–1905 CrossRef CAS PubMed; (f) S. Xu and Q. Zhang, Mater. Today Energy, 2021, 20, 100635 Search PubMed.
- (a) X. Chen, K. Geng, R. Liu, K. T. Tan, Y. Gong, Z. Li, S. Tao, Q. Jiang and D. Jiang, Angew. Chem., Int. Ed., 2020, 59, 5050–5091 CrossRef CAS PubMed; (b) S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 Search PubMed; (c) S. Xu, J. Wu, X. Wang and Q. Zhang, Chem. Sci., 2023, 14, 13601–13628 RSC; (d) Z. Chen, N. Li and Q. Zhang, Small Struct., 2024, 5, 2300495 Search PubMed; (e) J. Yang, Z. Chen, L. Zhang and Q. Zhang, ACS Nano, 2024, 18, 21804–21835 CrossRef CAS PubMed.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 Search PubMed.
- (a) Y. Li, X. Song, G. Zhang, L. Wang, Y. Liu, W. Chen and L. Chen, ChemSusChem, 2022, 15, e202200901 Search PubMed; (b) Z. Lin and J. Guo, Macromol. Rapid Commun., 2022, 44, 2200719 CrossRef PubMed; (c) N. Romero, R. Bofill, L. Francàs, J. García-Antón and X. Sala, Catalysts, 2021, 11, 754–771 Search PubMed; (d) S. S. Zhu, Z. Zhang, Z. Li and X. Liu, Mater. Chem. Front., 2024, 8, 1513–1535 RSC; (e) Q. Gu, X. Lu, C. Chen, X. Wang, F. Kang, Y. Y. Li, Q. Xu, J. Lu, Y. Han, W. Qin and Q. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202409708 CAS; (f) Q. Gu, J. Zha, C. Chen, X. Wang, W. Yao, J. Liu, F. Kang, J. Yang, Y. Y. Li, D. Lei, Z. Tang, Y. Han, C. Tan and Q. Zhang, Adv. Mater., 2024, 36, 2306414 CrossRef CAS PubMed; (g) F. Kang, X. Wang, C. Chen, C.-S. Lee, Y. Han and Q. Zhang, J. Am. Chem. Soc., 2023, 145, 15465–15472 Search PubMed; (h) Q. Gu, X. Lu, C. Chen, R. Hu, X. Wang, G. Sun, F. Kang, J. Yang, X. Wang, J. Wu, Y. Y. Li, Y.-K. Peng, W. Qin, Y. Han, X. Liu and Q. Zhang, ACS Nano, 2023, 17, 23903–23912 CrossRef CAS PubMed; (i) S. Zhang, X. Wang, F. Kang, Q. Gu, G. Sun, Y.-K. Peng and Q. Zhang, SmartMat, 2023, e1265 Search PubMed.
- (a) X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC; (b) N. Huang, P. Wang and D. Jiang, Nat. Rev. Mater., 2016, 1, 1–19 Search PubMed; (c) P. H. Kouwer, M. Koepf, V. A. L. Sage, M. Jaspers, A. M. V. Buul, Z. H. E. Akeroyd, T. Woltinge, E. Schwartz, H. J. Kitto, R. Hoogenboom, S. J. Picken, R. J. Nolte, E. Mendes and A. E. Rowan, Nature, 2013, 493, 651–655 Search PubMed; (d) D. Rodriguez-San-Miguel and F. Zamora, Chem. Soc. Rev., 2019, 48, 4375–4386 RSC; (e) J. L. Segura, M. J. Mancheno and F. Zamora, Chem. Soc. Rev., 2016, 45, 5635–5671 RSC.
- (a) Y. Wang, W. Wang, Z. Zhang and P. Li, Appl. Surf. Sci., 2022, 571, 151355 Search PubMed; (b) Y. X. Zhang, React. Funct. Polym., 2023, 184, 105516 Search PubMed; (c) E. A. Gendy, J. Ifthikar, J. Ali, D. T. Oyekunle, Z. Elkhlifia, I. I. Shahib, A. I. Khodair and Z. Chen, J. Environ. Chem. Eng., 2021, 9, 105687 CrossRef CAS.
- (a) X. L. Hu, H. G. Li and B. E. Tan, Chin. J. Polym. Sci., 2020, 38, 673–684 CrossRef CAS; (b) S. P. Qi, R. T. Guo, Z. X. Bi, Z. R. Zhang, C. F. Li and W. G. Pan, Small, 2023, 19, e2303632 CrossRef PubMed; (c) Y. Zhang, H. Liu, F. Gao, X. Tan, Y. Cai, B. Hu, Q. Huang, M. Fang and X. Wang, Energy Chem., 2022, 4, 100078 CrossRef CAS; (d) X. Chen, Y. Li, L. Jia, X. Yu, Q. Wang, Y. Zhang, Z. Zhang, E. Liang, B. Han and J. Li, J. Solid State Chem., 2023, 328, 124352 CrossRef CAS; (e) F. Kang, X. Wang, C. Chen, C. S. Lee, Y. Han and Q. Zhang, J. Am. Chem. Soc., 2023, 145, 15465–15472 Search PubMed; (f) Q. Wang, Y. Li, M. Zhou, J. Zhou, X. Liu, X. Yu, J. Gao, E. Liang, X. Chen, Y. Zhang, B. Han, J. Fan and J. Li, Microporous Mesoporous Mater., 2024, 364, 112872 CrossRef CAS.
- (a) P. H. Chang, M. C. Sil, K. S. K. Reddy, C. H. Lin and C. M. Chen, ACS Appl. Mater., 2022, 14, 25466–25477 CrossRef CAS PubMed; (b) Y. Li, M. Liu, J. Wu, J. Li, X. Yu and Q. Zhang, Front. Optoelectron., 2022, 15, 38 CrossRef PubMed; (c) F. Yu, W. Liu, S. W. Ke, M. Kurmoo, J. L. Zuo and Q. Zhang, Nat. Commun., 2020, 11, 5534 CrossRef CAS PubMed.
- (a) S. Zhang, D. Liu and G. Wang, Molecules, 2022, 27, 2586–2631 CrossRef CAS PubMed; (b) Y. Xiang, X. Yu, Y. Li, J. Chen, J. Wu, L. Wang, D. Chen, J. Li and Q. Zhang, Dyes Pigm., 2021, 195, 109710 CrossRef CAS; (c) Y. Zhang, J. Wu, J. Gao, X. Chen, Q. Wang, X. Yu, Z. Zhang, M. Liu and J. Li, J. Solid State Chem., 2023, 318, 123727 CrossRef CAS.
- (a) J. Lu, M. Wang, Y. Han, Y. Deng, Y. Zeng, C. Li, J. Yang and G. Li, Anal. Chem., 2022, 94, 5055–5061 CrossRef CAS PubMed; (b) X. Tao, Z. Wang, Q. P. Zhang, N. Liu, Y. L. Sun, R. X. Niu, R. Sun, X. Wang, B. Tan and C. Zhang, J. Am. Chem. Soc., 2023, 145, 25471–25477 CrossRef CAS PubMed.
- (a) J. Li, S. Chen, Z. Wang and Q. Zhang, Chem. Rec., 2016, 16, 1518–1530 CrossRef CAS PubMed; (b) S. E. A. M. Consuelo Cuquerella, M. A. Miranda and J. Pérez-Prieto, J. Org. Chem., 2009, 74, 3232–3235 CrossRef PubMed; (c) Z. Jiang, G. Huang, L. Sun, S. Wang and H. Chen, Energy Fuels, 2022, 36, 12201–12211 CrossRef CAS.
- (a) X. He, S. Zhang, S. Qi, P. Xu, B. Dong and B. Song, Dyes Pigm., 2023, 209, 110933 CrossRef CAS; (b) A. Kaplan, A. Erdem, C. Arslan, S. Savas, U. Tayfun and M. Dogan, Silicon, 2022, 15, 3165–3180 CrossRef; (c) W. Chen, F. Yu, Q. Xu, G. Zhou and Q. Zhang, Adv. Sci., 2020, 7, 1903766 CrossRef CAS PubMed; (d) W. Chen, X. Li, G. Long, Y. Li, R. Ganguly, M. Zhang, N. Aratani, H. Yamada, M. Liu and Q. Zhang, Angew. Chem., Int. Ed., 2018, 57, 13555–13559 CrossRef CAS PubMed; (e) Z. Zhang, Z. Wang, N. Aratani, X. Zhu and Q. Zhang, CCS Chem., 2022, 4, 3491–3496 CrossRef CAS; (f) Z. Zhang and Q. Zhang, Mater. Chem. Front., 2020, 4, 3419–3432 RSC; (g) Z. Wang, P. Gu, G. Liu, H. Yao, Y. Wu, Y. Li, G. Rakesh, J. Zhu, H. Fu and Q. Zhang, Chem. Commun., 2017, 53, 7772–7775 RSC; (h) P. Gu, Z. Wang, G. Liu, H. Yao, Z. Wang, Y. Li, J. Zhu, S. Li and Q. Zhang, Chem. Mater., 2017, 29, 4172–4175 CrossRef CAS.
- (a) T. Oyamada, H. Uchiuzou, S. Akiyama, Y. Oku, N. Shimoji, K. Matsushige, H. Sasabe and C. Adachi, J. Appl. Phys., 2005, 98, 074506 CrossRef; (b) P. Gu, Z. Wang and Q. Zhang, J. Mater. Chem. B, 2016, 4, 7060–7074 RSC; (c) J. Li and Q. Zhang, Synlett, 2013, 686–696 Search PubMed; (d) J. Li, S. Chen, Z. Wang and Q. Zhang, Chem. Rec., 2016, 16, 1518–1530 CrossRef CAS PubMed; (e) J. Xiao, Y. Divayana, Q. Zhang, H. M. Doung, H. Zhang, F. Boey, X. W. Sun and F. Wudl, J. Mater. Chem., 2010, 20, 8167–8170 RSC; (f) Y. Wu, Z. Yin, J. Xiao, Y. Liu, F. Wei, K. J. Tan, C. Kloc, L. Huang, Q. Yan, F. Hu, H. Zhang and Q. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 1883–1886 CrossRef CAS PubMed; (g) Q. Zhang, Y. Divayana, J. Xiao, Z. Wang, E. R. T. Tiekink, H. M. Doung, H. Zhang, F. Boey, X. W. Sun and F. Wudl, Chem. – Eur. J., 2010, 16, 7422–7426 CrossRef CAS PubMed.
- (a) M. Sang, S. Cao, J. Yi, J. Huang, W. Y. Lai and W. Huang, RSC Adv., 2016, 6, 6266–6275 RSC; (b) Y. R. Shi, H. L. Wei, Y. T. Shi and Y. F. Liu, Synth. Met., 2017, 223, 218–225 CrossRef CAS; (c) R. Das, S. S. Manna, B. Pathak and C. M. Nagaraja, ACS Appl. Mater., 2022, 14, 33285 CrossRef CAS PubMed.
- (a) L. Ascherl, T. Sick, J. T. Margraf, S. H. Lapidus, M. Calik, C. Hettstedt, K. Karaghiosoff, M. Döblinger, T. Clark, K. W. Chapman, F. Auras and T. Bein, Nat. Chem., 2016, 8, 310–316 CrossRef CAS; (b) T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260–7314 CrossRef CAS PubMed.
- X. Dong, H. Zhao, K. Zhang and X. Lang, Coord. Chem. Rev., 2024, 513, 215902 CrossRef CAS.
- (a) R. Das, R. Belgamwar, S. S. Manna, B. Pathak, V. Polshettiwar and C. M. Nagaraja, J. Colloid Interface Sci., 2023, 652, 480–489 CrossRef CAS PubMed; (b) R. Das, P. Kumar Verma and C. M. Nagaraja, Coord. Chem. Rev., 2024, 514, 215944 CrossRef CAS; (c) R. Das, S. Kamra and C. M. Nagaraja, Inorg. Chem. Front., 2023, 10, 2088–2099 RSC; (d) R. Das, T. Ezhil and C. M. Nagaraja, Cryst. Growth Des., 2021, 22, 598–607 CrossRef.
- (a) F. P. Kinik, A. Ortega-Guerrero, D. Ongari, C. P. Ireland and B. Smit, Chem. Soc. Rev., 2021, 50, 3143–3177 RSC; (b) D. Guo, H. Li, Z. Xu and Y. Nie, J. Alloys Compd., 2023, 968, 172004 CrossRef CAS; (c) J. Huang, Y. Ma, X. Jiang, J. Xian, Z. Fu and H. Ouyang, Anal. Chem., 2024, 37, 15042–15049 CrossRef PubMed; (d) Y. G. Li, J. J. Hu, J. L. Zhang, S. J. Liu, Y. Peng and H. R. Wen, CrystEngComm, 2022, 24, 2464–2471 RSC; (e) Y. Zhang, J. Pang, J. Li, X. Yang, M. Feng, P. Cai and H. C. Zhou, Chem. Sci., 2019, 10, 8455–8460 RSC.
- (a) J. Fang, L. Dai, X. Ren, D. Wu, W. Cao, Q. Wei and H. Ma, Biosens. Bioelectron., 2024, 266, 116726 CrossRef CAS PubMed; (b) Q. Wang, P. Li, H. M. Wen, K. J. Hu, Z. Y. Huang and J. Chen, Inorg. Chem. Commun., 2023, 156, 111213 CrossRef CAS; (c) R. Zhu, S. Yu, X. Yang, R. Zhu, H. Liu, K. Niu and L. Xing, Chin. Chem. Lett., 2024, 35, 109539 CrossRef CAS.
- (a) N. Baig, S. Shetty, S. Abdul Wahed, A. Hassan, N. Das and B. Alameddine, ACS Appl. Mater., 2024 DOI:10.1021/acsami.4c02948; (b) M. G. Mohamed, W. C. Chang, S. V. Chaganti, S. U. Sharma, J. T. Lee and S. W. Kuo, Polym. Chem., 2023, 14, 4589–4601 RSC; (c) M. G. Mohamed, B. X. Su and S. W. Kuo, ACS Appl. Mater., 2024, 16, 40858–40872 CrossRef CAS PubMed.
- (a) J. Wang, X. X. Tian, L. Yu, D. J. Young, W. B. Wang, H. Y. Li and H. X. Li, J. Mater. Chem. A, 2021, 9, 25474–25479 RSC; (b) X. Zhao, B. B. Qin, T. He, H. P. Wang and J. Liu, Inorg. Chem., 2023, 62, 18553–18562 CrossRef CAS PubMed.
- Y. N. Gong, X. Guan and H. L. Jiang, Coord. Chem. Rev., 2023, 475, 214889 CrossRef CAS.
- H. Yang, J. Wang, R. Zhao and L. Hou, Small, 2024, 20, 202400688 Search PubMed.
- (a) W. Li, B. Gui and C. Wang, Chin. Sci. Bull., 2023, 68, 3969–3978 Search PubMed; (b) Y. Zhang, Z. Qiao, R. Zhang, Z. Wang, H. J. Wang, J. Zhao, D. Cao and S. Wang, Angew. Chem., Int. Ed., 2023, 62, e202314539 CrossRef CAS PubMed.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2008, 47, 8826–8830 CrossRef CAS PubMed.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2009, 48, 5439–5442 CrossRef CAS PubMed.
- L. M. Salonen, D. D. Medina, E. Carbó-Argibay, M. G. Goesten, L. Mafra, N. Guldris, J. M. Rotter, D. G. Stroppa and C. Rodríguez-Abreu, Chem. Commun., 2016, 52, 7986–7989 RSC.
- S. P. S. Fernandes, L. Frey, K. M. Cid-Seara, O. Oliveira, N. Guldris, E. Carbó-Argibay, C. Rodríguez-Abreu, Y. V. Kolen'ko, A. M. S. Silva, D. D. Medina and L. M. Salonen, Microporous Mesoporous Mater., 2022, 343, 112162 CrossRef CAS.
- L. Frey, O. Oliveira, A. Sharma, R. Guntermann, S. P. S. Fernandes, K. M. C. Seara, H. Abbay, H. Thornes, J. Rocha, M. Döblinger, T. Kowalczyk, A. Rao, L. M. Salonen and D. D. Medina, Angew. Chem., Int. Ed., 2023, 62, e202302872 CrossRef CAS PubMed.
- J. Zhen, S. Ding, W. Wang, J. Liu, J. Sun, Z. Huang and Q. Zheng, Chin. J. Chem., 2016, 34, 783–787 CrossRef CAS.
- J. W. Crowe, L. A. Baldwin and P. L. McGrier, J. Am. Chem. Soc., 2016, 138, 10120–10123 CrossRef CAS PubMed.
- J. L. Zhang, Y. Yang, W. B. Liang, L. Y. Yao, R. Yuan and D. R. Xiao, Anal. Chem., 2021, 93, 3258–3265 CrossRef CAS PubMed.
- E. Jin, J. Li, K. Geng, Q. H. Jiang, H. Xu, Q. Xu and D. L. Jiang, Nat. Commun., 2018, 9, 4143 CrossRef PubMed.
- Z. Hu, Y. Luo, L. Wang, Y. Wang, Q. Wang, G. Jiang, Q. Zhang and F. Cui, ACS Appl. Polym., 2023, 5, 9263–9273 CrossRef CAS.
- M. Asada, E. Jin, Q. Xu, S. Dalapati, M. A. Addicoat, M. A. Brady, H. Xu, T. Nakamura, T. Heine, Q. H. Chen and D. L. Jiang, Science, 2017, 357, 673–676 CrossRef PubMed.
- A. Jati, S. Dam, S. Kumar, K. Kumar and B. Maji, Chem. Sci., 2023, 14, 8624–8634 RSC.
- Y. Wang, Y. Z. Cheng, K. M. Wu, D. H. Yang, X. F. Liu, X. Ding and B. H. Han, Angew. Chem., Int. Ed., 2023, 62, e202310794 CrossRef CAS PubMed.
- (a) Y. Fan, D. W. Kang, S. Labalme, J. Li and W. Lin, Angew. Chem., Int. Ed., 2023, 62, e202218908 CrossRef CAS PubMed; (b) B. Ma, X. Yang, J. Yuan, X. Yang, D. Han, K. Zhao, C. Lin, L. Wang, G. Liu and L. Mi, Appl. Catal., A, 2023, 666, 119403 CrossRef CAS.
- E. Jin, K. Geng, S. Fu, M. A. Addicoat, W. Zheng, S. Xie, J. S. Hu, X. Hou, X. Wu, Q. Jiang, Q. H. Xu, H. I. Wang and D. Jiang, Angew. Chem., Int. Ed., 2022, 61, e202115020 CrossRef CAS PubMed.
- B. Gui, G. Lin, H. Ding, C. Gao, A. Mal and C. Wang, Acc. Chem. Res., 2020, 53, 2225–2234 CrossRef CAS PubMed.
- Y. Li, Q. Chen, T. Xu, Z. Xie, J. Liu, X. Yu, S. Ma, T. Qin and L. Chen, J. Am. Chem. Soc., 2019, 141, 13822–13828 CrossRef CAS PubMed.
- B. Zhang, X. Song, Y. Li, Y. Li, Z. Peng, L. Ye and L. Chen, Chem. Commun., 2020, 56, 3253–3256 RSC.
- T. Wang, H. Xie, Y. Cao, Q. Xu and N. Gan, J. Chromatogr. A, 2022, 1685, 463614 CrossRef CAS PubMed.
- Y. Zhang, C. Li, Z. Liu, Y. Yao, M. M. Hasan, Q. Liu, J. Wan, Z. Li, H. Li and Y. Nagao, CrystEngComm, 2021, 23, 6234–6238 RSC.
- (a) X. Yuan, N. Wu, Z. Guo and H. Zhan, Microporous Mesoporous Mater., 2023, 355, 112573 CrossRef CAS; (b) B. Ma, F. Hu, X. Yang, Y. Huang, X. Yang, H. Qiao, Z. Wang, D. Yang, W. Ai and L. Mi, Appl. Catal., A, 2023, 662, 119269 CrossRef CAS; (c) H. Qiao, L. Yang, X. Yang, J. Wang, Y. Chen, L. Zhang, W. Sun, L. Zhai and L. Mi, Chem. – Eur. J., 2022, 28, e202200600 CrossRef CAS PubMed.
- X. Liu, H. Li, W. Zhang, Z. Yang, D. Li, M. Liu, K. Jin, L. Wang and G. Yu, Angew. Chem., Int. Ed., 2023, 62, e202308921 CrossRef CAS PubMed.
- Y. Meng, G. Lin, H. Ding, H. Liao and C. Wang, J. Mater. Chem. A, 2018, 6, 17186–17191 RSC.
- G. Li, W. Ma, Y. Yang, C. Zhong, H. Huang, D. Ouyang, Y. He, W. Tian, J. Lin and Z. Lin, ACS Appl. Mater., 2021, 13, 49482–49489 CrossRef CAS PubMed.
- C. Liu, F. Liu, H. Li, J. Chen, J. Fei, Z. Yu, Z. Yuan, C. Wang, H. Zheng, Z. Liu, M. Xu, G. Henkelman, L. Wei and Y. Chen, ACS Nano, 2021, 15, 3309–3319 CrossRef CAS PubMed.
- Y. Meng, G. Lin, H. Ding, H. Liao and C. Wang, J. Mater. Chem. A, 2018, 6, 17186–17191 RSC.
- X. Fang, Y. Liu, W. K. Han, X. Yan, Y. X. Shi, L. H. Chen, Y. Jiang, J. Zhang and Z. G. Gu, Dyes Pigm., 2022, 205, 110507 CrossRef CAS.
- Y. Chai, Y. Li, H. Hu, C. Zeng, S. Wang, H. Xu and Y. Gao, Catalysts, 2021, 11, 423–435 CrossRef CAS.
- G. Xiao, W. Li, T. Chen, W. B. Hu, H. Yang, Y. A. Liu and K. Wen, Eur. J. Org. Chem., 2021, 3986–3991 CrossRef CAS.
- Z. Li, S. Han, C. Li, P. Shao, H. Xia, H. Li, X. Chen, X. Feng and X. Liu, J. Mater. Chem. A, 2020, 8, 8706–8715 RSC.
- Z. Wen, S. Wang, S. Fu, J. Qian, Q. Yan, H. Xu, K. Zuo, X. Su, C. Zeng and Y. Gao, Chem. Res. Chin. Univ., 2022, 38, 472–477 CrossRef CAS.
- (a) S. Bhunia, S. K. Das, R. Jana, S. C. Peter, S. Bhattacharya, M. Addicoat, A. Bhaumik and A. Pradhan, ACS Appl. Mater., 2017, 9, 23843–23851 CrossRef CAS PubMed; (b) R. Chen, L. Kan, M. Xu, G. Zhang, M. Wang, J. Cui, N. Zhou and L. He, Microchim. Acta, 2022, 189, 229–238 CrossRef CAS PubMed; (c) Y. Chen, Y. Zhang and J. Huo, J. Solid State Chem., 2022, 310, 123047 CrossRef CAS; (d) Z. Chen, K. Wang, X. Hu, P. Shi, Z. Guo and H. Zhan, ACS Appl. Mater., 2020, 13, 1145–1151 CrossRef PubMed; (e) C. Gao, X. Guan, M. Zhang, H. Hu, L. Chen, C. Sun, C. Zhang, Y. Du and B. Hu, Macromol. Rapid Commun., 2023, 44, 2300311 CrossRef CAS PubMed; (f) A. Irfan, T. Wang, A. Wang, X. Jing, L. Yang and G. Zhu, Anal. Chim. Acta, 2022, 1209, 339876 CrossRef CAS PubMed.
- M. G. Rabbani, A. K. Sekizkardes, Z. Kahveci, T. E. Reich, R. Ding and H. M. E. Kaderi, Chem. – Eur. J., 2013, 19, 3324–3328 CrossRef CAS PubMed.
- B. Sun, C. H. Zhu, Y. Liu, C. Wang, L. J. Wan and D. Wang, Chem. Mater., 2017, 29, 4367–4374 CrossRef CAS.
- Q. Guan, H. Guo, R. Xue, M. Wang, N. Wu, Y. Cao, X. Zhao and W. Yang, Microchim. Acta, 2021, 188, 85–96 CrossRef CAS PubMed.
- T. Wang, I. Azhar, Y. Yang, Y. Lu, Y. Tian, N. Gao, F. Cui, L. Yang, X. Jing and G. Zhu, Nano Res., 2022, 15, 4569–4574 CrossRef CAS.
- L. Song, Q. Zhang, L. Min, X. Guo, W. Gao, L. Cui and C. Y. Zhang, Talanta, 2024, 266, 124964 CrossRef CAS PubMed.
- (a) Z. Zhou, X. H. Xiong, L. Zhang, Y. Li, Y. Yang, X. Dong, D. Lou, Z. Wei, W. Liu, C. Y. Su, J. Sun and Z. Zheng, J. Am. Chem. Soc., 2024, 146, 3449–3457 CrossRef CAS PubMed; (b) Z. H. He, S. D. Gong, S. L. Cai, Y. L. Yan, G. Chen, X. L. Li, S. R. Zheng, J. Fan and W. G. Zhang, Cryst. Growth Des., 2019, 19, 3543–3550 CrossRef CAS; (c) J. Kang, J. Hang, B. Chen, L. Chen, P. Zhao, Y. Xu, Y. Luo and C. Xia, ACS Appl. Mater., 2022, 14, 57225–57234 CrossRef CAS PubMed; (d) F. Yu, W. Liu, S.-W. Ke, M. Kurmoo, J.-L. Zuo and Q. Zhang, Nat. Commun., 2020, 11, 5534 CrossRef CAS PubMed; (e) F. Yu, W. Liu, B. Li, D. Tian, J.-L. Zuo and Q. Zhang, Angew. Chem., Int. Ed., 2019, 58, 16101–16104 CrossRef CAS PubMed; (f) J. Yang, F. Kang, X. Wang and Q. Zhang, Mater. Horiz., 2022, 9, 121–146 RSC; (g) M. Xue, J. Yang, F. Kang, X. Wang and Q. Zhang, J. Mater. Chem. C, 2022, 10, 17027–17047 RSC.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, 923–932 CrossRef CAS PubMed.
- C. P. Niu, C. R. Zhang, W. R. Cui, S. M. Yi, R. P. Liang and J. D. Qiu, J. Hazard. Mater., 2022, 425, 127951 CrossRef CAS PubMed.
- (a) W. R. Cui, W. Jiang, C. R. Zhang, R. P. Liang, J. Liu and J. D. Qiu, ACS Sustainable Chem. Eng., 2019, 8, 445–451 CrossRef; (b) Y. Zhang, X. Yuan, X. Zhu, D. Zhang, H. Liu and B. Sun, Anal. Chim. Acta, 2023, 1239, 340671 CrossRef CAS PubMed; (c) M. Zuo, L. Cui, S. Wang, W. Wei, W. Gao and C. Y. Zhang, Analyst, 2023, 148, 1764–1769 RSC.
- S. Jiang, L. Meng, W. Ma, G. Pan, W. Zhang, Y. Zou, L. Liu, B. Xu and W. Tian, Mater. Chem. Front., 2021, 5, 4193–4201 RSC.
- L. Zhang, J. Q. Fan, Q. Q. Zheng, S. J. Xiao, C. R. Zhang, S. M. Yi, X. Liu, W. Jiang, Q. G. Tan, R. P. Liang and J. D. Qiu, Chem. Eng. J., 2023, 454, 140212 CrossRef CAS.
- V. A. Kuehl, J. Yin, P. H. H. Duong, B. Mastorovich, B. Newell, K. D. Li-Oakey, B. A. Parkinson and J. O. Hoberg, J. Am. Chem. Soc., 2018, 140, 18200–18207 CrossRef CAS PubMed.
- J. Guo, Y. Xu, S. Jin, L. Chen, T. Kaji, Y. Honsho, M. A. Addicoat, J. Kim, A. Saeki, H. Ihee, S. Seki, S. Irle, M. Hiramoto, J. Gao and D. Jiang, Nat. Commun., 2013, 4, 2736 CrossRef PubMed.
- (a) N. Huang, K. H. Lee, Y. Yue, X. Xu, S. Irle, Q. Jiang and D. Jiang, Angew. Chem., Int. Ed., 2020, 59, 16587–16593 CrossRef CAS PubMed; (b) M. Wang, M. Ballabio, M. Wang, H. H. Lin, B. P. Biswal, X. Han, S. Paasch, E. Brunner, P. Liu, M. Chen, M. Bonn, T. Heine, S. Zhou, E. Cánovas, R. Dong and X. Feng, J. Am. Chem. Soc., 2019, 141, 16810–16816 CrossRef CAS PubMed; (c) M. Wang, M. Wang, H. H. Lin, M. Ballabio, H. Zhong, M. Bonn, S. Zhou, T. Heine, E. Cánovas, R. Dong and X. Feng, J. Am. Chem. Soc., 2020, 142, 21622–21627 CrossRef CAS PubMed.
- (a) D. S. García and J. L. Sessler, Chem. Soc. Rev., 2008, 37, 215–232 RSC; (b) M. Chen, H. Li, C. Liu, J. Liu, Y. Feng, A. G. H. Wee and B. Zhang, Coord. Chem. Rev., 2021, 435, 213778 CrossRef CAS; (c) L. Zhang, T. Wang, J. Jiang and M. Liu, Aggregate, 2022, 4, e198 CrossRef.
- P. L. Wang, S. Y. Ding, Z. C. Zhang, Z. P. Wang and W. Wang, J. Am. Chem. Soc., 2019, 141, 18004–18008 CrossRef CAS PubMed.
- P. F. Wei, M. Z. Qi, Z. P. Wang, S. Y. Ding, W. Yu, Q. Liu, L. K. Wang, H. Z. Wang, W. K. An and W. Wang, J. Am. Chem. Soc., 2018, 140, 4623–4631 CrossRef CAS PubMed.
- K. Wang, Z. Jia, Y. Bai, X. Wang, S. E. Hodgkiss, L. Chen, S. Y. Chong, X. Wang, H. Yang, Y. Xu, F. Feng, J. W. Ward and A. I. Cooper, J. Am. Chem. Soc., 2020, 142, 11131–11138 CrossRef CAS PubMed.
- P. J. Waller, Y. S. AlFaraj, C. S. Diercks, N. N. Jarenwattananon and O. M. Yaghi, J. Am. Chem. Soc., 2018, 140, 9099–9103 CrossRef CAS PubMed.
- J. Li, S. Y. Gao, J. Liu, S. Ye, Y. Feng, D. H. Si and R. Cao, Adv. Funct. Mater., 2023, 33, 2305735 CrossRef CAS.
- A. Zadehnazari, A. Khosropour, A. A. Altaf, S. Amirjalayer and A. Abbaspourrad, Adv. Opt. Mater., 2023, 11, 2300412 CrossRef CAS.
- T. He and Y. Zhao, Angew. Chem., Int. Ed., 2023, 62, 2005256 Search PubMed.
- (a) L. Qin, C. Ma, J. Zhang and T. Zhou, Adv. Funct. Mater., 2024, 2401562 CrossRef; (b) Q. Niu, L. Mi, W. Chen, Q. Li, S. Zhong, Y. Yu and L. Li, Chin. J. Catal., 2023, 50, 45–82 CrossRef CAS.
- (a) N. Khan, C. Azad, M. Luo, J. Chen, T. Kesharwani, A. Badshah and D. Wang, Energies, 2023, 16, 5888–5927 CrossRef CAS; (b) Y. I. A. Reyes, L. Y. Ting, X. Tu, H. Y. T. Chen, H. H. Chou and C. Coluccini, Appl. Sci., 2020, 10, 7017–7031 CrossRef CAS; (c) Q. Wang and K. Domen, Chem. Rev., 2019, 120, 919–985 CrossRef PubMed.
- (a) J. Zhang and X. Wang, Angew. Chem., Int. Ed., 2015, 54, 7230–7232 CrossRef CAS PubMed; (b) S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z. X. Guo and J. Tang, Energy Environ. Sci., 2015, 8, 731–759 RSC.
- (a) Y. Cao, P. Wang, J. Fan and H. Yu, Ceram. Int., 2021, 47, 654–661 CrossRef CAS; (b) D. E. Lee, M. Danish, U. Alam and W. K. Jo, J. Energy Chem., 2024, 92, 322–356 CrossRef CAS; (c) J. Sheng, C. Wang, F. Duan, S. Yan, S. Lu, H. Zhu, M. Du, X. Chen and M. Chen, Catal. Sci. Technol., 2021, 11, 7683–7693 RSC; (d) H. Yang, K. Dai, J. Zhang and G. Dawson, Chin. J. Catal., 2022, 43, 2111–2140 CrossRef CAS.
- (a) T. He, W. Zhen, Y. Chen, Y. Guo, Z. Li, N. Huang, Z. Li, R. Liu, Y. Liu, X. Lian, C. Xue, T. C. Sum, W. Chen and D. Jiang, Nat. Commun., 2023, 14, 329–330 CrossRef CAS PubMed; (b) M. Wang, Z. Wang, M. Shan, J. Wang, Z. Qiu, J. Song and Z. Li, Chem. Mater., 2023, 35, 5368–5377 CrossRef CAS; (c) L. J. Wang, P. Y. Dong, G. Zhang and F. M. Zhang, Energy Fuel., 2023, 37, 6323–6347 CrossRef CAS.
- (a) C. C. Gu, F. H. Xu, W. K. Zhu, R. J. Wu, L. Deng, J. Zou, B. C. Weng and R. L. Zhu, Chem. Commun., 2023, 59, 7302–7320 RSC; (b) S. Liu and J. Guo, Chem. Res. Chin. Univ., 2022, 38, 373–381 CrossRef CAS.
- E. Jin, Z. Lan, Q. Jiang, K. Geng, G. Li, X. Wang and D. Jiang, Chem., 2019, 5, 1632–1647 CAS.
- X. Deng, N. Gao and L. Bai, Small, 2024, 2311927 CrossRef CAS PubMed.
- Y. Liu, W. K. Han, W. Chi, J. X. Fu, Y. Mao, X. Yan, J. X. Shao, Y. Jiang and Z. G. Gu, Appl. Catal., B, 2023, 338, 123074 CrossRef CAS.
- H. Wu, X. He, X. Du, D. Wang, W. Li, H. Chen, W. Fang and L. Zhao, Small, 2023, 19, 2304367 CrossRef CAS PubMed.
- F. D. Wang, L. J. Yang, X. X. Wang, Y. Rong, L. B. Yang, C. X. Zhang, F. Y. Yan and Q. L. Wang, Small, 2023, 19, 2207421 CrossRef CAS PubMed.
- W. Chen, L. Wang, D. Mo, F. He, Z. Wen, X. Wu, H. Xu and L. Chen, Angew. Chem., Int. Ed., 2020, 59, 16902–16909 CrossRef CAS PubMed.
- W. Li, X. Huang, T. Zeng, Y. A. Liu, W. Hu, H. Yang, Y. B. Zhang and K. Wen, Angew. Chem., Int. Ed., 2020, 60, 1869–1874 CrossRef PubMed.
- G. Zhang, M. Zhao, L. Su, H. Yu, C. Wang, D. Sun and Y. Ding, ACS Appl. Mater., 2023, 15, 20310–20316 CrossRef CAS PubMed.
- Z. Zhao, Y. Zheng, C. Wang, S. Zhang, J. Song, Y. Li, S. Ma, P. Cheng, Z. Zhang and Y. Chen, ACS Catal., 2021, 11, 2098–2107 CrossRef CAS.
- Z. Zhou, C. Bie, P. Li, B. Tan and Y. Shen, Chin. J. Catal., 2022, 43, 2699–2707 CrossRef CAS.
- H. He, R. Shen, P. Zhang, G. Liang and X. Li, J. Mater. Chem. A, 2024, 12, 227–232 RSC.
- A. F. M. Mahdy, A. M. Elewa, S. W. Huang, H. H. Chou and S. W. Kuo, Adv. Opt. Mater., 2020, 8, 2000641 CrossRef.
- G. B. Wang, H. P. Xu, K. H. Xie, J. L. Kan, J. Fan, Y. J. Wang, Y. Geng and Y. B. Dong, J. Mater. Chem. A, 2023, 11, 4007–4012 RSC.
- L. Sun, M. Lu, Z. Yang, Z. Yu, X. Su, Y. Q. Lan and L. Chen, Angew. Chem., Int. Ed., 2022, 61, e202204326 CrossRef CAS PubMed.
- Q. Wang, D. W. Zhou, H. J. He, L. Yang, T. Y. Liu, X. Wang, D. Y. Zheng, Z. B. Dai, L. Sun, C. C. Liu, H. Wu, Z. Li and W. Q. Deng, Angew. Chem., Int. Ed., 2023, 62, e202214143 CrossRef PubMed.
- Y. X. Liu, Y. N. Wei, M. H. Liu, J. X. Hong, W. Q. Gao, S. Q. Zhao, S. P. Zhang and S. J. Guo, ACS Nano, 2023, 17, 5994–6001 CrossRef CAS PubMed.
- (a) F. Chen, T. Ma, T. Zhang, Y. Zhang and H. Huang, Adv. Mater., 2021, 33, 2005256 CrossRef CAS PubMed; (b) H. Hou, X. Zeng and X. Zhang, Angew. Chem., Int. Ed., 2020, 59, 17356–17376 CrossRef CAS PubMed; (c) D. Tan, R. Zhuang, R. Chen, M. Ban, W. Feng, F. Xu, X. Chen and Q. Wang, Adv. Funct. Mater., 2023, 34, 2311655 CrossRef.
- Y. Mou, X. Wu, C. Qin, J. Chen, Y. Zhao, L. Jiang, C. Zhang, X. Yuan, E. Huixiang Ang and H. Wang, Angew. Chem., Int. Ed., 2023, 62, e202309480 CrossRef CAS PubMed.
- J. Sun, H. S. Jena, C. Krishnaraj, K. S. Rawat, S. Abednatanzi, J. Chakraborty, A. Laemont, W. Liu, H. Chen, Y. Y. Liu, K. Leus, H. Vrielinck, V. V. Speybroeck and P. V. D. Voort, Angew. Chem., Int. Ed., 2023, 62, e202216719 CrossRef CAS PubMed.
- (a) P. D. Tran, L. H. Wong, J. Barber and J. S. C. Loo, Energy Environ. Sci., 2012, 5, 5902–5918 RSC; (b) Y. Amao, ChemCatChem, 2011, 3, 458–474 CrossRef CAS.
- (a) J. Albero, Y. Peng and H. García, ACS Catal., 2020, 10, 5734–5749 CrossRef CAS; (b) J. L. White, M. F. Baruch, J. E. Pander, Y. Hu, I. C. Fortmeyer, J. E. Park, T. Zhang, K. Liao, J. Gu, Y. Yan, T. W. Shaw, E. Abelev and A. B. Bocarsly, Chem. Rev., 2015, 115, 12888–12935 CrossRef CAS PubMed.
- (a) Y. Xiang, W. Dong, P. Wang, S. Wang, X. Ding, F. Ichihara, Z. Wang, Y. Wada, S. Jin, Y. Weng, H. Chen and J. Ye, Appl. Catal., B, 2020, 274, 119096 CrossRef CAS; (b) Z. Fu, X. Wang, A. M. Gardner, X. Wang, S. Y. Chong, G. Neri, A. J. Cowan, L. Liu, X. Li, A. Vogel, R. Clowes, M. Bilton, L. Chen, R. S. Sprick and A. I. Cooper, Chem. Sci., 2020, 11, 543–550 RSC.
- A. F. M. E. Mahdy, H. A. E. Omr, Z. A. Alothman and H. Lee, J. Colloid Interface Sci., 2023, 633, 775–785 CrossRef PubMed.
- L. Zou, Z. A. Chen, D. H. Si, S. L. Yang, W. Q. Gao, K. Wang, Y. B. Huang and R. Cao, Angew. Chem., Int. Ed., 2023, 62, e202309820 CrossRef CAS PubMed.
- Z. Almansaf, J. Hu, F. Zanca, H. R. Shahsavari, B. Kampmeyer, M. Tsuji, K. Maity, V. Lomonte, Y. Ha, P. Mastrorilli, S. Todisco, M. Benamara, R. Oktavian, A. Mirjafari, P. Z. Moghadam, A. R. Khosropour and H. Beyzavi, ACS Appl. Mater., 2021, 13, 6349–6358 CrossRef CAS PubMed.
- Z. Li, Y. Zhi, P. Shao, H. Xia, G. Li, X. Feng, X. Chen, Z. Shi and X. Liu, Appl. Catal., B, 2019, 245, 334–342 CrossRef CAS.
- X. Dong, F. Zhang, Y. Wang, F. Huang and X. Lang, Appl. Catal., B, 2024, 345, 123660 CrossRef CAS.
- (a) D. Guo, D. B. Shinde, W. Shin, E. A. Hamad, A. H. Emwas, Z. Lai and A. Manthiram, Adv. Mater., 2022, 34, 2201410 CrossRef CAS PubMed; (b) L. Liu, Y. Gong, Y. Tong, H. Tian, X. Wang, Y. Hu, S. Huang, W. Huang, S. Sharma, J. Cui, Y. Jin, W. Gong and W. Zhang, CCS Chem., 2024, 6, 1255–1263 CrossRef; (c) X. Wu, S. Zhang, X. Xu, F. Wen, H. Wang, H. Chen, X. Fan and N. Huang, Angew. Chem., Int. Ed., 2024, 63, e202319355 CrossRef CAS PubMed; (d) J. Sun, Y. Fei, H. Tang, J. Bao, Q. Zhang and X. Zhou, ACS Appl. Energy Mater., 2023, 7592–7602 Search PubMed; (e) M. Zhang, Y. Tong, Z. Sun, J. Wang, Y. Lin, F. Kang, Q. Zhang and W. Huang, Chem. Mater., 2023, 35, 4873–4881 CrossRef CAS; (f) Y. Tong, Z. Sun, J. Wang, W. Huang and Q. Zhang, SmartMat, 2022, 3, 685–694 CrossRef CAS; (g) S. Xu, J. Wu, X. Wang and Q. Zhang, Chem. Sci., 2023, 14, 13601–13628 RSC; (h) S. Xu, C. Wang, T. Song, H. Yao, J. Yang, X. Wang, J. Zhu, C.-S. Lee and Q. Zhang, Adv. Sci., 2023, 10, 2304497 CrossRef CAS PubMed.
- (a) Y. Song, X. Li and C. He, Chin. Chem. Lett., 2021, 32, 1106–1110 CrossRef CAS; (b) Y. Xiang, L. Lu, A. G. P. Kottapalli and Y. Pei, Carbon Energy, 2022, 4, 346–398 CrossRef CAS.
- F. Liu, C. Wang, C. Liu, Z. Yu, M. Xu, Y. Chen and L. Wei, Appl. Phys. Lett., 2021, 119, 211905 CrossRef CAS.
- M. K. Shehab, K. S. Weeraratne, O. M. E. Kadri, V. K. Yadavalli and H. M. E. Kaderi, Macromol. Rapid Commun., 2022, 44, 2200782 CrossRef PubMed.
- M. Cheng, S. Zheng, T. Sun, D. Li, W. Zhang, Z. Zha, Q. Sun, J. Tian, K. Zhang and Z. Tao, Nano Res., 2024, 17, 5095–5103 CrossRef CAS.
- (a) Z. Yan, Y. Cai, J. Zhang and Y. Zhao, Measurement, 2022, 187, 110355 CrossRef; (b) Z. Wang, B. Yao, Y. Xiao, X. Tian and Y. Wang, Chemosensors, 2023, 11, 405 CrossRef CAS; (c) D. Zhen, C. Liu, Q. Deng, S. Zhang, N. Yuan, L. Li and Y. Liu, Chin. Chem. Lett., 2024, 35, 109249 CrossRef CAS.
- (a) L. L. Wang, C. X. Yang and X. P. Yan, Sci. China: Chem., 2018, 61, 1470–1474 CrossRef CAS; (b) L. Li, X. Bi, M. Zhen, Y. Ren, L. Zhang and T. You, TrAC, Trends Anal. Chem., 2024, 171, 117488 CrossRef CAS.
- H. Zhuang, C. Guo, J. Huang, L. Wang, Z. Zheng, H. N. Wang, Y. Chen and Y. Q. Lan, Angew. Chem., Int. Ed., 2024, e202404941 CAS.
| This journal is © The Royal Society of Chemistry 2024 |