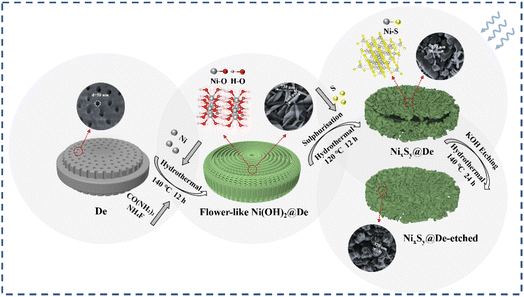
Three-dimensional biotemplate-loaded nickel sulfide vacancies engineered to promote the absorption of electromagnetic waves†
Wenrui
Zhu‡
 a,
Dashuang
Wang‡
a,
Zhilan
Du
a,
Yan
Liao
a,
Kai
Zhang
c,
Shuai
Xie
d,
Wenxin
Dong
e,
Jinsong
Rao
a,
Yuxin
Zhang
a,
Dashuang
Wang‡
a,
Zhilan
Du
a,
Yan
Liao
a,
Kai
Zhang
c,
Shuai
Xie
d,
Wenxin
Dong
e,
Jinsong
Rao
a,
Yuxin
Zhang
 *a and
Xiaoyin
Liu
*a and
Xiaoyin
Liu
 *b
*b
aCollege of Materials Science and Engineering, Chongqing University, Chongqing, 400044, China
bArmy Logistics Academy of PLA, Chongqing, 401331, China. E-mail: zhangyuxin@cqu.edu.cn; lxy_ctbu@163.com
cResearch Institute of Agricultural Engineering, Chongqing Academy of Agricultural Sciences, Chongqing, 401329, China
dState Key Laboratory of Green Building Materials, China Building Materials Academy, Beijing 100024, China
eSchool of Resources and Safety Engineering, Chongqing University, Chongqing, China
First published on 28th November 2023
Abstract
Vacancy engineering offers an appealing strategy for modifying the electronic structure of transition metals. Transition metals with abundant sulfur vacancies can significantly contribute to the microwave absorption capabilities of absorbers. In this study, an NixSy@De composite material was synthesized through a straightforward hydrothermal synthesis technique. The effective absorption bandwidth (EAB) of this composite material reached 9.86 GHz at 1.44 mm. A minimum reflection loss (RLmin) of −33.61 dB at 1 mm was achieved, and after mild etching, the RLmin further improved to −93.53 dB at 1.16 mm to achieve a high-attenuation microwave absorption. The exceptional performance of NixSy@De for the absorption of electromagnetic waves (EMWs) is based on its high dielectric loss, substantial magnetic loss, and excellent impedance matching. This work combines transition metal sulfides with three-dimensional biotemplated diatomite, providing valuable insights into the design of advanced EMW absorbing materials.
1. Introduction
With the development and progress of wireless communication technology, there has been a rapid and extensive increase in the use of various electronic devices and detection radars.1–4 These devices are being increasingly used in different frequency bands to address significant issues, such as electromagnetic radiation, pollution, and interference.5–8 Electromagnetic wave (EMW) absorption materials can effectively absorb EMWs and convert them into thermal energy or alternative forms of energy.9,10 Additionally, they can dissipate EMWs through interference, thereby reducing unnecessary electromagnetic radiation.11,12 This property is critical and of great importance in protecting people from radiation hazards and aiding in shielding electronic devices from electromagnetic interference.13,14 To date, researchers have actively explored and developed various EMW absorption materials, including ceramics,15 ferrites,16 carbon-based materials,17 and conductive polymers,18 and have made remarkable progress. However, most of the currently investigated EMW absorption materials have some drawbacks, such as a singular loss mechanism, suboptimal impedance matching, and relatively weak absorption capabilities.19,20 Among them, hierarchically engineered materials are considered to be efficient EMW absorption materials capable of meeting practical applications. In this way, hollow structures,21 pomegranate-like structures,22 and microsphere structures23 have been prepared. Although great progress has been made, the development of EMW absorbers with a small thickness, light weight, wide absorption bandwidth, and strong absorption capacity is still a necessary and urgent task.24–26Diatomite (De) is a type of siliceous biogenic sedimentary rock with a layered porous structure and naturally occurring large cavities, which is characterized by low density, lightweight, and a large surface area.27 The unique structure of diatomite grants it a photonic crystal effect, enhancing the surface ion resonance of loaded materials and thereby strengthening the absorption of incident EMWs.28,29 However, diatomite has poor electrical conductivity. To enhance the microwave absorption capacity of composite materials, materials with a higher electrical conductivity can be loaded onto diatomite. Consequently, diatomite has vast potential for applications in the field of microwave absorption. Li et al.30 prepared a ternary composite material with a MnFe2O4/rGO/De coated structure using a one-step hydrothermal method. The coated structure of the ternary composite material resulted in low-frequency microwave absorption capabilities. The minimum reflection loss (RLmin) of the composite material was −76.56 dB at 7.9 GHz and a thickness of 2.485 mm with an effective absorption bandwidth (EAB) of 3.61 GHz, demonstrating increased EMW absorption. Transition metal dichalcogenides (TMDs) are often used as loading materials owing to their high conductivity, high dielectric loss, small bandgap, excellent dielectric properties, and cost-effectiveness.31 The introduction of TMDs into microwave absorbers is beneficial for impedance matching, thus improving their absorption efficiency, and making them effective EMW materials.32 For example, Wang et al.33 prepared NixCo1S@De composite material using a two-step hydrothermal method, achieving an EAB of 6.16 GHz at 1.6 mm and 7.04 GHz at 4.1 mm. Chang et al.32 synthesized a NiS/MoS2/Ti3C2Tx hybrid material using a two-step method, with an EAB of 5.04 GHz at 2.1 mm and an RLmin of −58.48 dB at 2.4 mm. Through these studies, it is evident that TMDs possess strong microwave absorption capabilities. Besides, many reports show that vacancy engineering is an appealing strategy that can have a great impact on the design and development of high-performance EMW absorption materials, especially for the study of dielectric loss. Sulfur vacancies not only greatly improve the electrical conductivity,34 but also promote the formation of electric dipoles, increase the polarization loss caused by defects in the electromagnetic field environment, and greatly improve the absorption properties of EMW.35,36 Therefore, we aimed to construct an efficient composite microwave absorption material consisting of TMDs and diatomite by modulating the sulfur vacancy concentration.
In this study, we utilized a one-step hydrothermal approach to grow nickel hydroxide nanoflower precursors on diatomite. Subsequently, sulfurization was performed to improve the absorption characteristics of the nickel-based materials. Finally, we etched the sulfurized material to form the NixSy@De-etched composite material. As anticipated, NixSy@De demonstrated an EAB of 9.86 GHz with a thickness of 1.44 mm, the RLmin achieved was −33.61 dB at 1 mm. Mild etching resulted in reduced impurities in the sample, improved stability of the diatomite batch, and an RLmin of −93.53 dB at 1.16 mm.
Compared to previously designed De-based composite materials, the material prepared in this work showcased an extremely thin matching thickness, RLmin, and a broader EAB, indicating superior EMW absorption performance. This work provides a new idea to enhance the EMW of composite materials and demonstrates the feasibility of utilizing diatomite in EMW materials.
2. Experimental section
2.1. Materials and reagents
Nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O), potassium hydroxide (KOH) purchased from Shanghai Aladdin Co., Ltd, diatomite (white power), ammonium fluoride (NH4F) purchased from Chengdu Kelong Chemical Reagent Factory, anhydrous ethanol (C2H5OH), thioacetamide (C2H5NS), and urea (CO(NH2)2) were purchased from Chongqing Chuandong Chemical Co. Ltd. For this experiment, we prepared deionized water in the laboratory.2.2. Synthesis of Ni(OH)2@De
The synthetic process of the NixSy@De is depicted in Fig. 1. First, 4.8 mmol of Ni(NO3)2·6H2O and 800 mg of diatomite were completely dissolved in 300 ml of deionized water to form Solution A. Next, 324 mg of NH4F and 540 mg of CO(NH2)2 were added to 300 ml of deionized water to form Solution B. Solution A was then poured and thoroughly mixed with Solution B. The resulting mixture was transferred to a Teflon-lined stainless steel autoclave, sealed, and reacted in a rotary oven at 140 °C for 12 h in a homogeneous reactor. After the reaction was completed, the reaction kettle was cooled to room temperature, and the product was suction filtered. The filtered sample was then dried at 60 °C to obtain the precursor Ni(OH)2@De.2.3. Synthesis of NixSy@De
Ni(OH)2@De composite and thioacetamide powder were dissolved in deionized water at the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The solution was then transferred to a Teflon-lined stainless steel autoclave, sealed, and reacted in a rotary oven at 120 °C for 12 h in a homogeneous reactor. After the reaction was completed, the reaction kettle was cooled to room temperature and suction filtered. The filtered sample was then dried at 60 °C to obtain NixSy@De composite material.
10. The solution was then transferred to a Teflon-lined stainless steel autoclave, sealed, and reacted in a rotary oven at 120 °C for 12 h in a homogeneous reactor. After the reaction was completed, the reaction kettle was cooled to room temperature and suction filtered. The filtered sample was then dried at 60 °C to obtain NixSy@De composite material.
2.4. Etching of NixSy@De
An amount of NixSy@De composite, 56 ml of 3 mol L−1 KOH solution, and 14 ml of anhydrous ethanol were combined in a Teflon-lined stainless steel autoclave and reacted in a rotary oven at 140 °C for 24 h in a homogeneous reactor. After the reaction was completed, the reaction kettle was cooled to room temperature and suction filtered. The filtered sample was then dried at 60 °C to obtain the final sample NixSy@De-etched.2.5. Characterization
Morphological and structural characterization of the synthesized materials was conducted using scanning electron microscopy (SEM, Zeiss Auriga) and transmission electron microscopy (TEM, Talos F200S). The crystallographic data and chemical composition of the samples were analyzed by X-ray diffractometry (XRD, Rigaku Ultima IV, Cu/Kα) and X-ray photoelectron spectroscopy (XPS, ESCALAB Xi+). The vacancy levels of the samples were characterized by electron paramagnetic resonance (EPR, Bruker A300). The chemical bonding information of the samples was obtained by Fourier transform infrared spectroscopy (FTIR, Nicolet iS50) to determine their chemical structures. Thermogravimetric analysis (TGA, Mettler 1100LF, N2 atmosphere, 20 °C min−1 rate of heating). The prepared sample was mixed with paraffin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and its complex permittivity and complex permeability were measured using a vector network analyzer (E5071C) in the frequency range of 1–18 GHz using the coaxial method. The sample powders were measured for resistance (Cryoall CTA-3) by the four-probe method and resistivity and conductivity were calculated.
1, and its complex permittivity and complex permeability were measured using a vector network analyzer (E5071C) in the frequency range of 1–18 GHz using the coaxial method. The sample powders were measured for resistance (Cryoall CTA-3) by the four-probe method and resistivity and conductivity were calculated.
3. Discussion
Three samples were characterized to observe the surface morphology of the samples. Fig. 2(a–i) presents the SEM images showing the microstructures of three composite materials: Ni(OH)2@De, NixSy@De, and NixSy@De-etched. Firstly, it was observed that the base material, diatomite, remained unchanged in all three samples. Furthermore, we employed a one-step hydrothermal approach to load Ni(OH)2 nanoflowers onto diatomite and allowed the nanoflowers to grow vertically in situ on diatomite. After sulfurization, a significant change in the sample's morphology was apparent, and the sheet-like structures displayed a reduced length and an uneven loading. This implies that the crystal structure of the sample was changed. Following KOH etching, the flower-like structural morphology of the sample was once again rendered uniform and compact. This suggests that the etching process efficiently eliminated the impurities found in the sample, as well as other compounds created during the sulfurization process. By comparing the three samples, it is evident that the nanosheets grew uniformly and densely, completely encapsulating diatomite without noticeable aggregation. The sheet-like structures are arranged in a manner that forms a porous structure containing an air phase. The introduction of the sulfurization structure enables the effective design of a suitable absorber structure, fulfilling the requirements for EMW scattering, reflection conditions, and impedance-matching mechanisms. Simultaneously, at equivalent magnifications, differences in the interlayer spacing and the size of the sheets among the three samples were observed. The length of the Ni(OH)2 sheet-like structures was approximately 650 to 750 nm, which decreases to 250 to 350 nm after sulfurization (Fig. S1, ESI†). This reduction in the sheet length leads to increased reflection and scattering of the incident EMWs, facilitating the construction of a conductive network and promoting polarization losses of the incident EMWs,37 as validated by subsequent experimental results.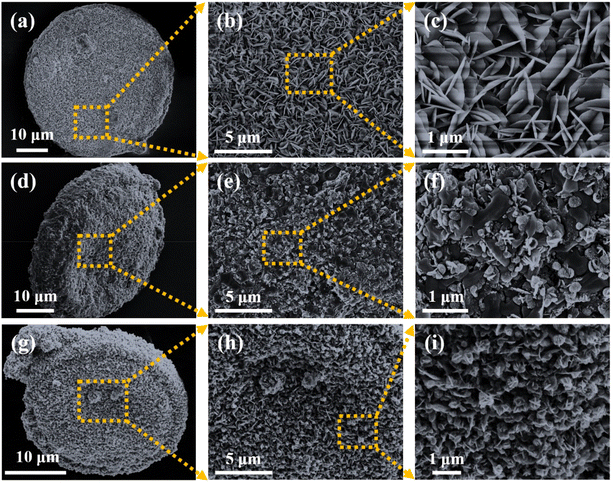 | ||
| Fig. 2 SEM images of (a–c) Ni(OH)2@De, (d–f) NixSy@De, and (g–i) NixSy@De-etched at different ratios. | ||
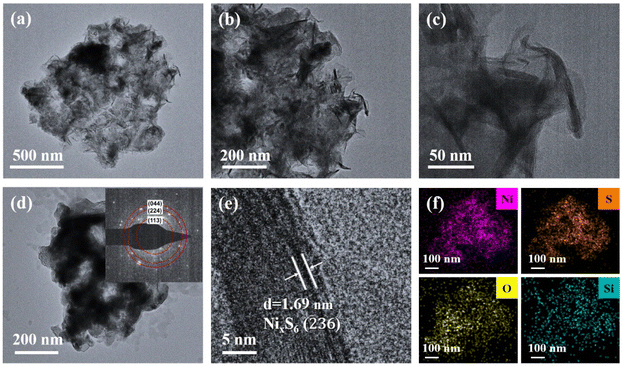
The microstructure and composition of the samples were analyzed in more detail using TEM. Fig. 3(a–d) present the morphology of the entire samples and partially magnified areas. The results align with the findings from the SEM images that the flower-like structure is still retained after sulfurization and etching, and the interface between the diatomite and NixSy nanoflowers can be observed under high magnification without any obvious voids or gaps at the boundaries, suggesting that the biphasic bond is tightly coupled to provide favorable conditions for scattering and reflection of the EMW and to optimize the impedance matching between the absorber and free space. The inset in Fig. 3d presents the diffraction pattern analysis, which displays recognizable polycrystalline patterns, with the (044), (224), and (113) crystal planes belonging to Ni3S4, aligning with the subsequent XRD results. As shown in Fig. 3e, a lattice spacing of 1.69 nm was observed, which can be attributed to the (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 36) crystal plane of NixS6. To verify their detailed vacancy features, the EPR images (Fig. S2, ESI†) confirmed that the NixSy@De samples obtained after sulfurization contained a large number of sulfide vacancies.35 The high-angle annular dark-field scanning transmission electron microscopy-energy-dispersive X-ray spectroscopy (HAADF-EDS) image shown in Fig. 3f reveals that NixSy@De is composed of Ni, S, O, and Si elements, further confirming the successful sulfurization process.
36) crystal plane of NixS6. To verify their detailed vacancy features, the EPR images (Fig. S2, ESI†) confirmed that the NixSy@De samples obtained after sulfurization contained a large number of sulfide vacancies.35 The high-angle annular dark-field scanning transmission electron microscopy-energy-dispersive X-ray spectroscopy (HAADF-EDS) image shown in Fig. 3f reveals that NixSy@De is composed of Ni, S, O, and Si elements, further confirming the successful sulfurization process.
XRD patterns of the three composite materials, Ni(OH)2@De, NixSy@De, and NixSy@De-etched are presented in Fig. 4a. The diffraction peaks at diffraction angles 2θ of 21.64°, 31.34°, 36.11°, 46.86°, and 56.48° can be attributed to the (101), (102), (200), (113), and (104) crystal planes of De (JCPDS 39-1425). The remaining diffraction peaks at 2θ angles peaks of the Ni(OH)2@De composite were 19.21°, 33.25°, 38.71°, 52.15°, 59.51°, 63.07°, and 73.29°, corresponding to the (001), (100), (011), (012), (110), (111), and (112) crystal planes of Ni(OH)2 (JCPDS 73-1520), indicating the presence of both diatomite and Ni(OH)2 phases in the sample Ni(OH)2@De. In the case of NixSy@De, besides the characteristic peaks of diatomite, the diffraction peaks at 2θ angles of 31.17°, and 61.51° correspond to the (113), (026) crystal planes of Ni3S4 (JCPDS 43-1469), respectively. The diffraction peaks at 2θ angles of 17.91°, 20.25°, 26.23°, 50.07°, and 54.49° are attributed to the (101), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 30), (
30), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 40), (
40), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 36), and (
36), and (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 36) crystal planes of NixS6 (JCPDS 51-0717), respectively. Compared to Ni(OH)2@De, the diffraction peaks of Ni(OH)2 completely disappeared, indicating the complete sulfurization of Ni(OH)2. The sulfurization product is composed of a mixed phase of Ni3S4 and NixS6, termed NixSy. In the case of NixSy@De-etched, the observation of an increased number of diffraction peaks for NixSy indicates a significant reduction in impurities in the sample after etching, highlighting a more pronounced sulfurization effect, consistent with the SEM results described earlier.
36) crystal planes of NixS6 (JCPDS 51-0717), respectively. Compared to Ni(OH)2@De, the diffraction peaks of Ni(OH)2 completely disappeared, indicating the complete sulfurization of Ni(OH)2. The sulfurization product is composed of a mixed phase of Ni3S4 and NixS6, termed NixSy. In the case of NixSy@De-etched, the observation of an increased number of diffraction peaks for NixSy indicates a significant reduction in impurities in the sample after etching, highlighting a more pronounced sulfurization effect, consistent with the SEM results described earlier.
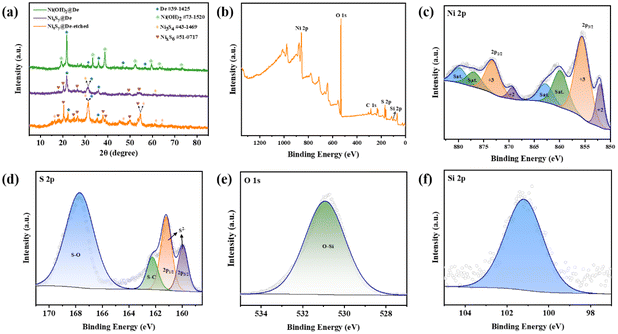 | ||
| Fig. 4 (a) XRD pattern of Ni(OH)2@De, NixSy@De, and NixSy@De-etched; (b) the total XPS spectrum of NixSy@De-etched; (c–f) the partial spectra of Ni, S, O, Si. | ||
To gain deeper insights into the elemental composition and chemical valence states of the material, XPS was utilized to analyze the NixSy@De-etched composite. The comprehensive XPS spectrum depicted in Fig. 4b unequivocally verifies the existence of Ni, O, S, and Si elements within the NixSy@De-etched sample. However, the presence of carbon peaks in the spectrum is ascribed to the adsorption of C-containing substances, such as CO2, from the surrounding environment. It was observed that the Si element content after etching was very low, indicating that the etching effect was very significant. As shown in Fig. 4c, the Ni 2p spectrum consists of two pairs of satellite peaks at 860.0 and 877.0 eV and 862.8 and 879.9 eV and two pairs of spin–orbit doublet peaks corresponding to Ni 2p3/2 and Ni 2p1/2 of Ni2+ (852.1 and 869.4 eV) and Ni3+ (855.6 and 873.4 eV) Ni2+ and Ni3+, respectively, distributed in Ni3S4 and NixS6.38,39 In Fig. 4d, the S 2p spectrum was analyzed, revealing four distinct peaks, with the peaks located at 160.0 and 161.1 eV corresponding to S 2p3/2 and S 2p1/2 of S2−, respectively, and the other two peaks at 162.2 and 167.7 eV are associated with the presence of S–C bonds and the oxidation of Ni3S4 and NixS6,40 respectively. The presence of S–O indicates the oxidation of sulfur in the sample, while the appearance of the S–C bond is probably due to the sulfurization of the trace amount of carbon present in the diatomite. In the O 1s high-resolution spectrum shown in Fig. 4e, the peak associated with the O element originates from the O–Si bond (530.8 eV).41
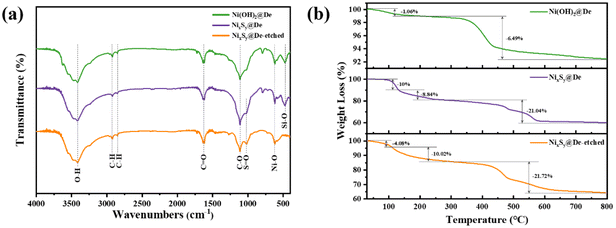
Furthermore, the chemical bond types within these three samples were investigated in greater detail through FTIR spectroscopy. In Fig. 5a, the absorption peak at 3412.96 cm−1 is attributed to the stretching vibration of O–H.42 The appearance of the absorption peaks at 2926.01, 2847.43, 1623.80, and 1110.82 cm−1 are attributed to asymmetric C–H stretching, symmetric C–H stretching vibration,43 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and C–O stretching vibration,42,44 respectively. A small peak is observed for NixSy@De and NixSy@De-etched samples around 1025.48 cm−1. This peak can be linked to the S
O stretching vibration, and C–O stretching vibration,42,44 respectively. A small peak is observed for NixSy@De and NixSy@De-etched samples around 1025.48 cm−1. This peak can be linked to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode, indicating the presence of sulfate ions resulting from the surface-adsorbed moisture.42 The absorption peak at 620.98 cm−1 is caused by the stretching vibration of the Ni–O bond. In both Ni(OH)2@De and NixSy@De samples, an absorption peak appeared at 470.55 cm−1, which was identified as the stretching vibration of the Si–O bond.45 This peak disappeared after etching, indicating the effective removal of diatomite from the samples. Therefore, a combination of XRD, XPS, and FTIR spectroscopy, it can be confirmed that Ni(OH)2 was successfully loaded onto diatomite and partially sulphurized, resulting in the formation of a NixSy composite consisting of two phases, NixS6 and Ni3S4. Thus, the NixSy@De composite was obtained.
O stretching mode, indicating the presence of sulfate ions resulting from the surface-adsorbed moisture.42 The absorption peak at 620.98 cm−1 is caused by the stretching vibration of the Ni–O bond. In both Ni(OH)2@De and NixSy@De samples, an absorption peak appeared at 470.55 cm−1, which was identified as the stretching vibration of the Si–O bond.45 This peak disappeared after etching, indicating the effective removal of diatomite from the samples. Therefore, a combination of XRD, XPS, and FTIR spectroscopy, it can be confirmed that Ni(OH)2 was successfully loaded onto diatomite and partially sulphurized, resulting in the formation of a NixSy composite consisting of two phases, NixS6 and Ni3S4. Thus, the NixSy@De composite was obtained.
To investigate the heat loss characteristics of the composite materials, TGA analyses were carried out on the three samples as shown in Fig. 5b. Specifically, for the Ni(OH)2@De sample, there was a 1.06% mass loss from room temperature to 213 °C, followed by a subsequent 6.49% mass loss from 213 °C to 800 °C. For the NixSy@De sample, there was a 10% mass loss from room temperature to 131 °C, followed by an additional 8.84% mass loss from 131 °C to 267 °C, and finally, a 21.04% mass loss from 267 °C to 800 °C. The thermal stability of the samples before and after etching remained essentially unchanged. The results show that all three samples exhibit good thermal stability up to 400 °C and the weight loss remains below 20%. The weight loss of the three samples below 120 °C is mainly due to the evaporation of the adsorbed water. The weight loss of Ni(OH)2@De at 230–800 °C is related to the decomposition of Ni(OH)2 and the dehydration of diatomite, and the weight loss of the sulphurized samples at 120–400 °C is related to the removal of the oxygen-containing functional groups and the dehydration of diatomite, and the weight loss above 400 °C is related to the oxidation of NixSy and the dehydration of diatomite.46,47 Considering the properties of Ni(OH)2, we speculate that the occurring reactions can be expressed by eqn (1) and (2):
| Ni(NO)3·6H2O → Ni(OH)2 + H2O + NO2↑ | (1) |
| Ni(OH)2 + CH3CSNH3 → NixS6 + Ni3S4 + CH3CONH2 + H2O | (2) |
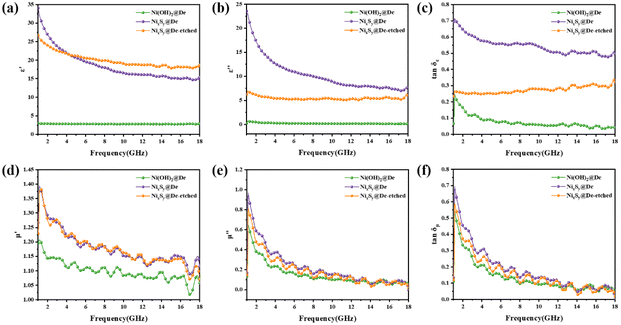
The electromagnetic parameters are fundamental factors that dictate the electromagnetic properties of the wave-absorbing materials, specifically the relative complex permittivity (εr = ε′ − jε′′) of Ni(OH)2@De, NixSy@De, and NixSy@De-etched are shown in Fig. 6a and b. In general, ε′ and ε′′ reflect the ability of the wave-absorbing material to store and dissipate electrical energy, respectively.48 The reduction in complex permittivity optimizes impedance matching, causing EMWs to enter the absorbers to the greatest extent possible for dissipation.49 For Ni(OH)2@De, the curves of ε′ and ε′′ hardly fluctuate and remain flat at 2.6 and 0.2, respectively, however, in general, the materials with stable real and imaginary parts are suitable to be used as the substrate materials. The ε′ of NixSy@De and NixSy@De-etched show a decreasing trend with increasing frequency, which can be attributed to the characteristic dispersion response due to the enhanced hysteresis of the dipole polarization response to the electric field, which also improves the impedance matching of the incident microwave.50,51 The ε′ and ε′′ values of the last two samples undergo a huge enhancement with respect to Ni(OH)2@De, suggesting that sulfide increases the dielectric properties of the samples in the form of phase components, vacancies, and defects.52 The presence of abundant defects and a large number of interfaces after sulfurization was demonstrated by XRD, EPR, and SEM, leading to significant polarization,53 thus synergistically contributing to the dielectric attenuation performance.54 According to the electronic free-electron theory, high conductivity leads to high ε values, which in turn increases the dielectric loss and leads to improved performance, which is in agreement with the later conclusions. For the magnetic permeability (μr = μ′ − jμ′′) of the three samples, the trend of the curves is almost the same, with a small enhancement occurring in the vulcanized samples, μ′ and μ′′, but not much increase in the contribution of the magnetic losses, which we will analyze in detail later. As far as tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε and tan
δε and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ are concerned, the dielectric loss tangent and the magnetic permeability tangent have the same values at low frequencies, but as the frequency increases, tan
δμ are concerned, the dielectric loss tangent and the magnetic permeability tangent have the same values at low frequencies, but as the frequency increases, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε remains stable and tan
δε remains stable and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ undergoes a sharp decrease, indicating that tan
δμ undergoes a sharp decrease, indicating that tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε dominates the losses.
δε dominates the losses.
By analyzing the electromagnetic parameters, we need to specifically discuss the dielectric loss of the samples, to further prove our inference, we tested the conductivity of the three samples, and the results are shown in Table 1.
| Sample | Electrical resistivity (μΩ m) | Average electrical resistivity (μΩ m) | Average electrical conductivity (S m−1) |
|---|---|---|---|
| Ni(OH)2@De | 976934051.962 | 932068294.342 | 0.00107288 |
| 914925151.340 | |||
| 904345679.724 | |||
| NixSy@De | 5616.026 | 5580.162 | 179.206267 |
| 5562.144 | |||
| 5562.316 | |||
| NixSy@De-etched | 5760.246 | 5689.778 | 178.897981 |
| 5695.880 | |||
| 5613.207 |
The electrical conductivities of Ni(OH)2@De, NixSy@De, and NixSy@De-etched were 0.0011 S m−1, 179.21 S m−1, and 178.90 S m−1, respectively, and their conductivity trends are consistent with ε′′, which is in line with the free-electron equation. Sulfurization has a great role in enhancing conductivity, and in the case of our samples, an order of magnitude increase in conductivity occurs after sulfurization, which is further demonstrated in the DFT calculations later on. The results show that NixSy@De-etched has good electrical conductivity, indicating excellent EMW absorption properties. In addition to analyzing the conductivity, the Debye relaxation is also an important way to analyze the dielectric loss of absorbing materials.55 Usually, eqn (5) can be derived from eqn (3), (4), and (5),54,56 and can represent the relationship between ε′ and ε′′, and the semicircular curve of ε′ − ε′′ proves the existence of the Debye relaxation process, also called Cole–Cole semicircles. Each semicircle represents a Debye relaxation process; therefore, the Cole–Cole semicircle can be used to identify the frequency bands in which polarization occurs. The conductivities of NixSy@De and NixSy@De-etched are close, but ε′′ is smaller because NixSy@De-etched has more semicircular arcs and irregularly dense regions (Fig. 7), which suggests that it has more polarization relaxation processes and stronger conductivity losses.
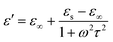 | (3) |
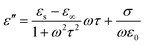 | (4) |
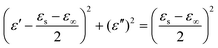 | (5) |
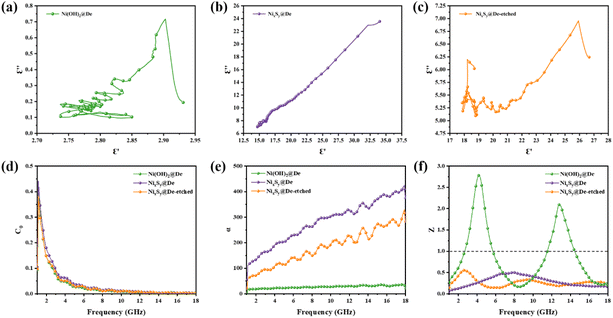 | ||
| Fig. 7 (a–c) Cole–Cole curves of three samples, (d) eddy current loss diagram, (e) attention constant diagram, and (f) impedance matching diagram. | ||
Therefore, we further analyze the magnetic loss, which in the microwave band is mainly manifested as hysteresis, domain wall resonance, eddy current loss, natural resonance, and exchange resonance.58 However, in a weak magnetic field, hysteresis arises from irreversible magnetization and can be ignored. Domain wall resonance occurs only in the 1–100 MHz range of multi-domain magnetic materials Eddy current loss is an important source of magnetic loss, and the effect of eddy current loss on the absorption effect can be initially analyzed based on calculations, and eqn (6) (ref. 59) is usually used to determine this effect:
| c0 = μ′′(μ′)−2μ′′(f)−1 = 2πμ0d2δ | (6) |
A high dielectric loss is essential for achieving exceptional performance in EMW absorbing materials. However, for effective loss mechanisms in the material, a high attenuation constant and favorable impedance matching are crucial prerequisites. The attenuation constant (α) serves as a key metric to assess the absorption capacity (eqn (7) (ref. 60)).
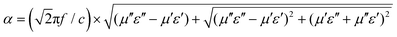 | (7) |
As depicted in Fig. 7e, the attenuation coefficient of NixSy@De exhibits a more prominent increase in frequency compared to the other samples. This observation suggests a superior attenuation of the incident EMW and better absorption performance for NixSy@De. The enhanced polarization relaxation and conductive loss contribute to this improved absorption capacity. It is important to emphasize that while a larger α is necessary, it is not solely sufficient for the absorber to achieve excellent absorption performance—it is also intricately linked to impedance matching. To mitigate EMW reflection, it is imperative to tailor the impedance-matching properties of the material. The introduction of NixSy on diatomite significantly enhanced both the impedance matching capability and the EMW attenuation capability. Usually, |Zin − Zo| should be as close to 0 as possible, i.e., |Zin/Zo| = 1, to facilitate better penetration of EMW into the material. Fig. 7f depicts |Zin/Zo| versus frequency. NixSy@De's |Zin/Zo| can be close to 1 in most of the region, which matches perfectly with our EAB results.
We analyzed the 1D, 2D, and 3D plots of the relationship between frequency, thickness, and reflection loss in the 1–18 GHz band for three samples by electromagnetic parameter calculations (Fig. 8). In general, RLmin values less than −10 dB are considered to have an absorption efficiency of 90% for EMW, and wave-absorbing materials on this basis are consistent with practical applications. As can be seen in Fig. 8, the RL values of Ni(OH)2@De do not reach −10 dB at any thickness and frequency; therefore, there is no effective absorption of EMWs. When Ni(OH)2 introduces NixSy on the surface of diatomite by reacting with thioacetamide, the RLmin increases dramatically, and for NixSy@De, the RLmin is −33.61 dB at 18.0 GHz, with a matching thickness of 1.0 mm. It is worth mentioning that, due to the dual effect of dielectric and magnetic losses, the impedance matching of NixSy@De also greatly improved due to both dielectric loss and magnetic loss, and the EAB is very good. With a thickness of 1.44 mm, the corresponding EAB is 9.86 GHz (8.14–18 GHz), which almost completely covers the X and Ku bands. Interestingly, when we adjust the thickness to 4.6 mm, the EAB expands to 10.55 GHz (1.85–4.39 GHz, 9.35–13.96 GHz, and 14.6–18 GHz), covering the complete C-band and most of the X, and Ku-bands. By adjusting the thickness, the purpose of realizing the absorption in different bands can be achieved, which has a strong practical application, and further illustrates that the performance of the samples is greatly improved after sulfurization of the samples due to the incorporation of vacancy engineering.52 To further improve the performance of the sample, we etched the sample and surprisingly, the RLmin is −93.53 dB and the EAB is 7.79 GHz at NixSy@De-etched with a thickness of only 1.16 mm. Its excellent broadband absorption can reach 7.79 GHz at an ultra-thin thickness of 1.16 mm (10.21–18.0 GHz). We infer that the etching process reduces the broken diatomite and some magazine elements produced during the sizing process, resulting in a more homogeneous loading of NixSy and better batch stability of diatomite, which not only increases the RLmin and reduces the matching thickness for optimal performance but also has an effect on the interaction between NixSy and diatomite at the material interface, which affects the impedance matching and slightly reduces the EAB, but the performance is still surprising and noteworthy.
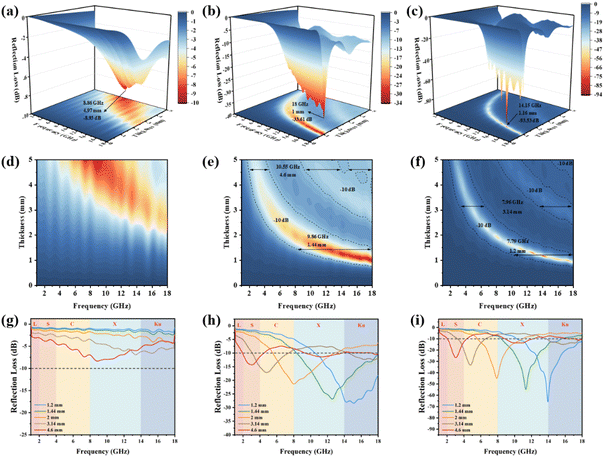 | ||
| Fig. 8 From left to right: Ni(OH)2@De, NixSy@De, and NixSy@De-etched. (a–c) the 3D loss diagram, (d–f) contour plot of RL with thickness and frequency, and (g–i) frequency-loss 1D diagram. | ||
For a more comprehensive understanding of how the absorption properties of EMW vary with thickness, (Fig. S3, ESI†) shows the quarter-wavelength model for both NixSy@De and NixSy@De-etched samples, where the frequency corresponding to RLmin shifts to lower frequencies with increasing thickness,61 which roughly agrees with the fitting curves given by eqn (8),62 further demonstrating the reliability of the data.
| tm = nc/[(4f)(|εr||μr|1/2)] (n = 1,3,5,…) | (8) |
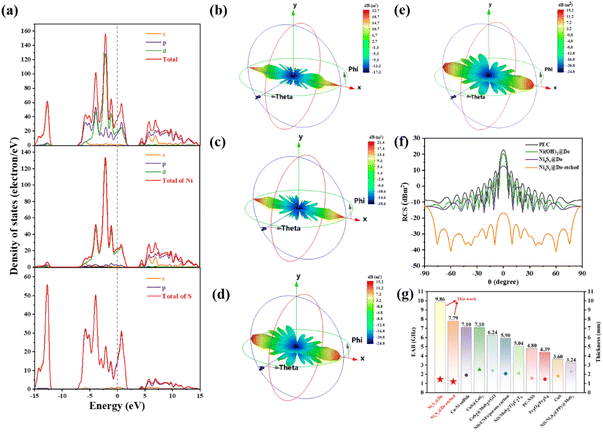
The density of states of theoretical models is calculated to investigate the excellent EMW absorption performance of NixSy@De-etched. The structural model of Ni3S4 is constructed based on the results of XRD. The model of NixSy@De-etched consists of the hexagonal Ni3S4, which is determined from the XRD data. From the analysis of the total and partial density of states (TDOS and PDOS) of Ni3S4 (Fig. 9a), the energy band of Ni3S4 near the Fermi level is primarily influenced by the Ni 3d state. To verify the promising application of NixSy@De-etched in practical applications, the simulation of the Radar Cross Section (RCS) was conducted utilizing the CST software. RCS is a physical metric quantifying the intensity of a target's echo when exposed to radar wave irradiation.63 The RCS area of a target can be likened to the projected area of a metal ball that produces a similar echo signal.64Fig. 9(b–e) show the 3D RCS simulation results for a perfect electric conductor (PEC), Ni(OH)2@De, NixSy@De, and NixSy@De-etched at different intensities, respectively. Fig. 9f shows the RCS values of the absorber at different incidence angles. NixSy@De-etched has the lowest RCS value in the −90° to 90° incidence angle range.
To visualize the impact of the coating on reducing the Radar Cross Section (RCS) value more intuitively, the absorber value is subtracted from the PEC value to obtain the reduction. The analysis shows that all absorbers contribute positively to reducing RCS values, with NixSy@De-etched exhibiting a more pronounced effect compared to other EMW absorbers (Fig. S4, ESI†). NixSy@De-etched has the highest noise reduction value at 10°, which reaches 44.49 dB m2. The simulation results of RCS align well with the EMW attenuation results, providing strong evidence for the significant potential of NixSy@De-etched in the fabrication of efficient EMW absorbers. A comparison of the EAB and thickness of some reported dielectric microwave absorbing composites is given in Fig. 9g,32,64–71 where it can be seen that the NixSy@De-etched and NixSy@De composites prepared in this study have a larger EAB and thinner thickness.
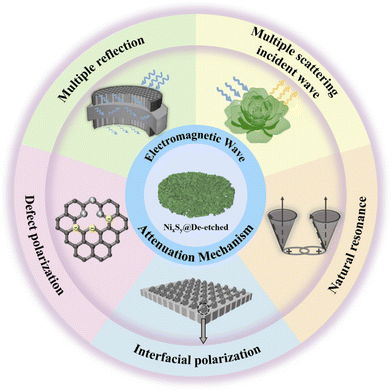
By analyzing the electromagnetic parameters and EMW absorption performance, we can elucidate the EMW attenuation mechanism of NixSy@De-etched composites that can be summarized as follows (Fig. 10). First, the combination of Ni3S4, NixS6, and diatomite optimizes the impedance matching of the absorber, reducing the surface reflection of the EMW, enhancing the attenuation capability, and ultimately obtaining excellent absorption performance. Second, the three-dimensional porous structure of diatomite as well as the two-dimensional small lamellar layer structure and the abundant specific surface area of NixSy cause multiple reflections and attenuation of the EMW entering the material, which enhances the EMW loss. Third, the abundant interfacial polarization formation between NixSy and diatomite, in conjunction with the paraffin wax, further contributes to the enhancement of EMW absorption, and the defective polarization caused by sulfurization, this flow of charge from the Ni–O bond to the Ni–S bond induces a variance in local electron density and disrupts the symmetry of the substituent sites. Consequently, spatial charge separation occurs. Under the influence of an applied electromagnetic field, this heterogeneous structure oscillates, generating dielectric polarization and significantly boosting macroscopic dielectric loss capability. The attenuation ability of the composite is improved. Finally, mild etching reduces the number of ineffective absorbers and increases the batch stability, which produces conduction losses while decreasing the dielectric properties to increase impedance matching, and the three-dimensional conductive network formed by diatomite's holes and NixSy lamellae facilitates the promotion of electron transfer. Under the combined effect of the above mechanisms, NixSy@De-etched composites obtained satisfactory EMW absorption properties.
4. Conclusion
In this study, uniform and dense Ni(OH)2 nanoflowers were grown on diatomite using a hydrothermal method. NixSy@De was prepared through sulfurization and the samples were mildly etched. The results indicate that sulfurization treatment significantly improved the dielectric loss, impedance matching characteristics, and absorption properties of the NixSy@De composites. At a thickness of 1.44 mm, the EAB was 9.86 GHz, and the RLmin at 1 mm was −33.61 dB. After mild etching, the RLmin at 1.16 mm further increases to −93.53 dB, resulting in high attenuation of microwave absorption. This composite material surpasses previous findings in terms of bandwidth and matched thickness, signifying its excellence. The exceptional ability to absorb EMW is mainly attributed to the high content of sulfur vacancy defect polarization and the synergistic effect of several mechanisms, such as multiple reflection and scattering incident waves, interfacial polarization, and generation of conduction loss. This study demonstrates the feasibility of diatomite as an EMW absorption material and provides new ideas for the development of new high-broadband EMW absorption materials.Conflicts of interest
The authors affirm that they have no known financial or interpersonal conflicts that would have appeared to have an impact on the research presented in this study.Acknowledgements
The authors gratefully acknowledge the financial support provided by Graduate Scientific Research and Innovation Foundation of Chongqing, China (CYB22007, CYS23071), Projects (No. 2020CDJXZ001) supported by the Fundamental Research Funds for the Central Universities, the Technology Innovation and Application Development Special Project of Chongqing (Z20211350 and Z20211351), Scientific Research Project of Chongqing Ecological Environment Bureau (No. CQEE2022-STHBZZ118). The authors also thank the Electron Microscopy Center of Chongqing University for its material characterizations.References
- Z. Wu, H.-W. Cheng, C. Jin, B. Yang, C. Xu, K. Pei, H. Zhang, Z. Yang and R. Che, Dimensional Design and Core–Shell Engineering of Nanomaterials for Electromagnetic Wave Absorption, Adv. Mater., 2022, 34(11), 2107538 CrossRef CAS PubMed.
- L. Liang, W. Gu, Y. Wu, B. Zhang, G. Wang, Y. Yang and G. Ji, Heterointerface Engineering in Electromagnetic Absorbers: New Insights and Opportunities, Adv. Mater., 2022, 34(4), 2106195 CrossRef CAS PubMed.
- A. Iqbal, F. Shahzad, K. Hantanasirisakul, M.-K. Kim, J. Kwon, J. Hong, H. Kim, D. Kim, Y. Gogotsi and C. M. Koo, Anomalous absorption of electromagnetic waves by 2D transition metal carbonitride Ti3CNTx (MXene), Science, 2020, 369(6502), 446–450 CrossRef CAS PubMed.
- P. Yi, Z. Yao, J. Zhou, B. Wei, L. Lei, R. Tan and H. Fan, Facile synthesis of 3D Ni@C nanocomposites derived from two kinds of petal-like Ni-based MOFs towards lightweight and efficient microwave absorbers, Nanoscale, 2021, 13(5), 3119–3135 RSC.
- L. Yang, Y. Wang, Z. Lu, R. Cheng, N. Wang and Y. Li, Construction of multi-dimensional NiCo/C/CNT/rGO aerogel by MOF derivative for efficient microwave absorption, Carbon, 2023, 205, 411–421 CrossRef CAS.
- Z. Xiang, Y. Shi, X. Zhu, L. Cai and W. Lu, Flexible and Waterproof 2D/1D/0D Construction of MXene-Based Nanocomposites for Electromagnetic Wave Absorption, EMI Shielding, and Photothermal Conversion, Nano-Micro Lett., 2021, 13(1), 150 CrossRef CAS PubMed.
- L. Sun, Q. Zhu, Z. Jia, Z. Guo, W. Zhao and G. Wu, CrN attached multi-component carbon nanotube composites with superior electromagnetic wave absorption performance, Carbon, 2023, 208, 1–9 CrossRef CAS.
- L. Wang, X. Li, X. Shi, M. Huang, X. Li, Q. Zeng and R. Che, Recent progress of microwave absorption microspheres by magnetic-dielectric synergy, Nanoscale, 2021, 13(4), 2136–2156 RSC.
- H. Lv, Z. Yang, B. Liu, G. Wu, Z. Lou, B. Fei and R. Wu, A flexible electromagnetic wave-electricity harvester, Nat. Commun., 2021, 12(1), 834 CrossRef CAS PubMed.
- M. Ling, F. Wu, P. Liu, Q. Zhang and B. Zhang, Fabrication of Graphdiyne/Graphene Composite Microsphere with Wrinkled Surface via Ultrasonic Spray Compounding and its Microwave Absorption Properties, Small, 2023, 19(7), 2205925 CrossRef CAS PubMed.
- Y. Cheng, J. Z. Y. Seow, H. Zhao, Z. J. Xu and G. Ji, A Flexible and Lightweight Biomass-Reinforced Microwave Absorber, Nano-Micro Lett., 2020, 12(1), 125 CrossRef CAS PubMed.
- X. Qiu, L. Wang, H. Zhu, Y. Guan and Q. Zhang, Lightweight and efficient microwave absorbing materials based on walnut shell-derived nano-porous carbon, Nanoscale, 2017, 9(22), 7408–7418 RSC.
- H. K. Choi, A. Lee, M. Park, D. S. Lee, S. Bae, S.-K. Lee, S. H. Lee, T. Lee and T.-W. Kim, Hierarchical Porous Film with Layer-by-Layer Assembly of 2D Copper Nanosheets for Ultimate Electromagnetic Interference Shielding, ACS Nano, 2021, 15(1), 829–839 CrossRef CAS PubMed.
- Q. Liu, X. Xu, W. Xia, R. Che, C. Chen, Q. Cao and J. He, Dependency of magnetic microwave absorption on surface architecture of Co20Ni80 hierarchical structures studied by electron holography, Nanoscale, 2015, 7(5), 1736–1743 RSC.
- M. Zhang, Z. Li, T. Wang, S. Ding, G. Song, J. Zhao, A. Meng, H. Yu and Q. Li, Preparation and electromagnetic wave absorption performance of Fe3Si/SiC@SiO2 nanocomposites, Chem. Eng. J., 2019, 362, 619–627 CrossRef CAS.
- D. Lan, M. Qin, J. Liu, G. Wu, Y. Zhang and H. Wu, Novel binary cobalt nickel oxide hollowed-out spheres for electromagnetic absorption applications, Chem. Eng. J., 2020, 382, 122797 CrossRef CAS.
- G. Liu, C. Wu, L. Hu, X. Hu, X. Zhang, J. Tang, H. Du, X. Wang and M. Yan, Anisotropy engineering of metal organic framework derivatives for effective electromagnetic wave absorption, Carbon, 2021, 181, 48–57 CrossRef CAS.
- Y. Jiao, F. Wu, A. Xie, L. Wu, W. Zhao, X. Zhu and X. Qi, Electrically conductive conjugate microporous polymers (CMPs) via confined polymerization of pyrrole for electromagnetic wave absorption, Chem. Eng. J., 2020, 398, 125591 CrossRef CAS.
- B. Wang, H. Chen, S. Wang, Y. Shi, X. Liu, Y. Fu and T. Liu, Construction of core-shell structured Co7Fe3@C nanocapsules with strong wideband microwave absorption at ultra-thin thickness, Carbon, 2021, 184, 223–231 CrossRef CAS.
- Q. Wu, B. Wang, Y. Fu, Z. Zhang, P. Yan and T. Liu, MOF-derived Co/CoO particles prepared by low temperature reduction for microwave absorption, Chem. Eng. J., 2021, 410, 128378 CrossRef CAS.
- C. Wei, M. He, M. Li, X. Ma, W. Dang, P. Liu and J. Gu, Hollow Co/NC@MnO2 polyhedrons with enhanced synergistic effect for high-efficiency microwave absorption, Mater. Today Phys., 2023, 36, 101142 CrossRef CAS.
- Z. Jiang, Y. Gao, Z. Pan, M. Zhang, J. Guo, J. Zhang and C. Gong, Pomegranate-like ATO/SiO2 microspheres for efficient microwave absorption in wide temperature spectrum, J. Mater. Sci. Technol., 2024, 174, 195–203 CrossRef.
- Z. Jiang, H. Si, Y. Li, D. Li, H. Chen, C. Gong and J. Zhang, Reduced graphene oxide@carbon sphere based metacomposites for temperature-insensitive and efficient microwave absorption, Nano Res., 2022, 15(9), 8546–8554 CrossRef CAS.
- Y. Zhang, Y. Huang, T. Zhang, H. Chang, P. Xiao, H. Chen, Z. Huang and Y. Chen, Broadband and Tunable High-Performance Microwave Absorption of an Ultralight and Highly Compressible Graphene Foam, Adv. Mater., 2015, 27(12), 2049–2053 CrossRef CAS PubMed.
- J. Yan, Y. Huang, Y. Yan, L. Ding and P. Liu, High-Performance Electromagnetic Wave Absorbers Based on Two Kinds of Nickel-Based MOF-Derived Ni@C Microspheres, ACS Appl. Mater. Interfaces, 2019, 11(43), 40781–40792 CrossRef CAS PubMed.
- X. Li, L. Wang, W. You, L. Xing, X. Yu, Y. Li and R. Che, Morphology-controlled synthesis and excellent microwave absorption performance of ZnCo2O4 nanostructures via a self-assembly process of flake units, Nanoscale, 2019, 11(6), 2694–2702 RSC.
- C. Z. Zhang, D. S. Wang, L. C. Dong, K. L. Li, Y. F. Zhang, P. A. Yang, S. Yi, X. J. Dai, C. Q. Yin, Z. L. Du, X. F. Zhang, Q. Zhou, Z. Y. Yi, J. S. Rao and Y. X. Zhang, Microwave Absorption of alpha-Fe2O3@diatomite Composites, Int. J. Mol. Sci., 2022, 23(16), 9362 CrossRef CAS PubMed.
- Y. F. Zhang, R. Cai, D. S. Wang, K. L. Li, Q. Sun, Y. T. Xiao, H. Teng, X. H. Huang, T. Sun, Z. H. Liu, K. X. Yao, Y. X. Zhang and P. A. Yang, Lightweight, Low-Cost Co2SiO4@diatomite Core-Shell Composite Material for High-Efficiency Microwave Absorption, Molecules, 2022, 27(3), 1055 CrossRef CAS PubMed.
- Z. Q. Yan, J. Cai, Y. G. Xu and D. Y. Zhang, Microwave absorption property of the diatomite coated by Fe-CoNiP films, Appl. Surf. Sci., 2015, 346, 77–83 CrossRef CAS.
- Q. Li, W. Guo, X. Kong, J. Xu, C. Xu, Y. e. Chen, J. Chen, X. Jia and Y. Ding, MnFe2O4/rGO/Diatomite composites with excellent wideband electromagnetic microwave absorption, J. Alloys Compd., 2023, 941, 168851 CrossRef CAS.
- Z. H. Xu, L. Tang, S. W. Zhang, J. Z. Li, B. L. Liu, S. C. Zhao, C. J. Yu and G. D. Wei, 2D MoS2/CuPc heterojunction based highly sensitive photodetectors through ultrafast charge transfer, Mater. Today Phys., 2020, 15, 100273 CrossRef.
- M. Chang, Z. Jia, S. He, J. Zhou, S. Zhang, M. Tian, B. Wang and G. Wu, Two-dimensional interface engineering of NiS/MoS2/Ti3C2Tx heterostructures for promoting electromagnetic wave absorption capability, Composites, Part B, 2021, 225, 109306 CrossRef CAS.
- D. S. Wang, Y. Z. Hu, Z. Y. Cui, P. X. Yang, Z. L. Du, Y. Hou, P. A. Yang, J. S. Rao, C. Wang and Y. X. Zhang, Sulfur vacancy regulation and multipolarization of NixCo1S nanowires-decorated biotemplated structures to promote microwave absorption, J. Colloid Interface Sci., 2023, 646, 991–1001 CrossRef CAS PubMed.
- J. Liu, L. Zhang and H. Wu, Anion-Doping-Induced Vacancy Engineering of Cobalt Sulfoselenide for Boosting Electromagnetic Wave Absorption, Adv. Funct. Mater., 2022, 32(26), 2200544 CrossRef CAS.
- S. Hui, L. Zhang and H. Wu, Cationic doping induced sulfur vacancy formation in polyionic sulfide for enhanced electromagnetic wave absorption, J. Colloid Interface Sci., 2023, 629, 147–155 CrossRef CAS PubMed.
- C. Li, D. Li, L. Zhang, Y. Zhang, L. Zhang, C. Gong and J. Zhang, Boosted microwave absorption performance of transition metal doped TiN fibers at elevated temperature, Nano Res., 2023, 16(2), 3570–3579 CrossRef CAS.
- Z. Du, D. Wang, X. Zhang, Z. Yi, J. Tang, P. Yang, R. Cai, S. Yi, J. Rao and Y. Zhang, Core-Shell Structured SiO2@NiFe LDH Composite for Broadband Electromagnetic Wave Absorption, Int. J. Mol. Sci., 2023, 24(1), 504 CrossRef CAS PubMed.
- W. Wei, Z. Guo, J. Xu, Z. Fang, J. Zhang, Y. Jia and L. Mi, Novel Ni3S4/NiS/NC composite with multiple heterojunctions synthesized through space-confined effect for high-performance supercapacitors, Int. J. Extreme Manuf., 2023, 5(1), 015504 CrossRef.
- D. Jiang, H. Liang, W. Yang, Y. Liu, X. Cao, J. Zhang, C. Li, J. Liu and J. J. Gooding, Screen-printable films of graphene/CoS2/Ni3S4 composites for the fabrication of flexible and arbitrary-shaped all-solid-state hybrid supercapacitors, Carbon, 2019, 146, 557–567 CrossRef CAS.
- D. Wu, X. Xie, J. Zhang, Y. Ma, C. Hou, X. Sun, X. Yang, Y. Zhang, H. Kimura and W. Du, Embedding NiS nanoflakes in electrospun carbon fibers containing NiS nanoparticles for hybrid supercapacitors, Chem. Eng. J., 2022, 446, 137262 CrossRef CAS.
- B. Zhou, Y. Li, Y. Zou, W. Chen, W. Zhou, M. Song, Y. Wu, Y. Lu, J. Liu, Y. Wang and S. Wang, Platinum Modulates Redox Properties and 5-Hydroxymethylfurfural Adsorption Kinetics of Ni(OH)2 for Biomass Upgrading, Angew. Chem., Int. Ed., 2021, 60(42), 22908–22914 CrossRef CAS PubMed.
- E. C. Linganiso, B. W. Mwakikunga, N. J. Coville and S. D. Mhlanga, Observation of the structural, optical and magnetic properties during the transformation from hexagonal NiS nano-compounds to cubic NiO nanostructures due to thermal oxidation, J. Alloys Compd., 2015, 629, 131–139 CrossRef CAS.
- G. I. Titelman, V. Gelman, S. Bron, R. L. Khalfin, Y. Cohen and H. Bianco-Peled, Characteristics and microstructure of aqueous colloidal dispersions of graphite oxide, Carbon, 2005, 43(3), 641–649 CrossRef CAS.
- H. Gu, Y. Huang, L. Zuo, W. Fan and T. Liu, Electrospun carbon nanofiber@CoS2 core/sheath hybrid as an efficient all-pH hydrogen evolution electrocatalyst, Inorg. Chem. Front., 2016, 3(10), 1280–1288 RSC.
- W. C. Geng, L. B. Duan and Q. Y. Zhang, Humidity Sensing Property of Al-Doped Mesoporous Silica, Appl. Mech. Mater., 2012, 249–250, 992–997 Search PubMed.
- L. Luo, S.-H. Chung and A. Manthiram, Rational Design of a Dual-Function Hybrid Cathode Substrate for Lithium–Sulfur Batteries, Adv. Energy Mater., 2018, 8(24), 1801014 CrossRef.
- Q. Chen, W. Chen, J. Ye, Z. Wang and J. Y. Lee, l-Cysteine-assisted hydrothermal synthesis of nickel disulfide/graphene composite with enhanced electrochemical performance for reversible lithium storage, J. Power Sources, 2015, 294, 51–58 CrossRef CAS.
- B. Li, Z. Ma, X. Zhang, J. Xu, Y. Chen, X. Zhang and C. Zhu, NiO/Ni Heterojunction on N-Doped Hollow Carbon Sphere with Balanced Dielectric Loss for Efficient Microwave Absorption, Small, 2023, 19(12), 2207197 CrossRef CAS PubMed.
- B. Zhao, Y. Li, Q. Zeng, L. Wang, J. Ding, R. Zhang and R. Che, Galvanic Replacement Reaction Involving Core–Shell Magnetic Chains and Orientation-Tunable Microwave Absorption Properties, Small, 2020, 16(40), 2003502 CrossRef CAS PubMed.
- B. Zhao, Y. Du, H. Lv, Z. Yan, H. Jian, G. Chen, Y. Wu, B. Fan, J. Zhang, L. Wu, D. W. Zhang and R. Che, Liquid-Metal-Assisted Programmed Galvanic Engineering of Core–shell Nanohybrids for Microwave Absorption, Adv. Funct. Mater., 2023, 33(34), 2302172 CrossRef CAS.
- M. Li, X. Song, J. Xue, F. Ye, L. Yin, L. Cheng and X. Fan, Construction of Hollow Carbon Nanofibers with Graphene Nanorods as Nano-Antennas for Lower-Frequency Microwave Absorption, ACS Appl. Mater. Interfaces, 2023, 15(26), 31720–31728 CrossRef CAS PubMed.
- W. Zhao, S. Yuan, S. Lei, Z. Zeng, J. Dong, F. Jiang, Y. Yang, W. Sun, X. Ji and P. Ge, Tailoring Rational Crystal Orientation and Tunable Sulfur Vacancy on Metal-Sulfides toward Advanced Ultrafast Ion-Storage Capability, Adv. Funct. Mater., 2023, 33(5), 2211542 CrossRef CAS.
- B. Zhao, Y. Du, Z. Yan, L. Rao, G. Chen, M. Yuan, L. Yang, J. Zhang and R. Che, Structural Defects in Phase-Regulated High-Entropy Oxides toward Superior Microwave Absorption Properties, Adv. Funct. Mater., 2023, 33(1), 2209924 CrossRef CAS.
- B. Zhao, Z. Yan, Y. Du, L. Rao, G. Chen, Y. Wu, L. Yang, J. Zhang, L. Wu, D. W. Zhang and R. Che, High-Entropy Enhanced Microwave Attenuation in Titanate Perovskites, Adv. Mater., 2023, 35(11), 2210243 CrossRef CAS PubMed.
- J. Wang, Z. Wu, Y. Xing, B. Li, P. Huang and L. Liu, Multi-Scale Design of Ultra-Broadband Microwave Metamaterial Absorber Based on Hollow Carbon/MXene/Mo2C Microtube, Small, 2023, 19(14), 2207051 CrossRef CAS PubMed.
- F. Pan, Z. Liu, B. Deng, Y. Dong, X. Zhu, C. Huang and W. Lu, Lotus Leaf-Derived Gradient Hierarchical Porous C/MoS2 Morphology Genetic Composites with Wideband and Tunable Electromagnetic Absorption Performance, Nano-Micro Lett., 2021, 13(43), 1–17 CAS.
- Z. X. Wang, X. F. Li, L. Y. Wang, Y. P. Li, J. Y. Qin, P. T. Xie, Y. P. Qu, K. Sun and R. H. Fan, Flexible multi-walled carbon nanotubes/polydimethylsiloxane membranous composites toward high-permittivity performance, Adv. Compos. Hybrid Mater., 2020, 3(1), 1–7 CrossRef.
- L. Gai, H. Zhao, F. Wang, P. Wang, Y. Liu, X. Han and Y. Du, Advances in core-shell engineering of carbon-based composites for electromagnetic wave absorption, Nano Res., 2022, 15(10), 9410–9439 CrossRef.
- M. Qin, H. Liang, X. Zhao and H. Wu, Filter paper templated one-dimensional NiO/NiCo2O4 microrod with wideband electromagnetic wave absorption capacity - ScienceDirect, J. Colloid Interface Sci., 2020, 566, 347–356 CrossRef CAS PubMed.
- H. Lv, G. Ji, X. H. Liang, H. Zhang and Y. Du, A novel rod-like MnO2@Fe loading on graphene giving excellent electromagnetic absorption properties, J. Mater. Chem. C, 2015, 3(19), 5056–5064 RSC.
- M. Li, W. Zhu, X. Li, H. Xu, X. Fan, H. Wu, F. Ye, J. Xue, X. Li, L. Cheng and L. Zhang, Ti3C2Tx/MoS2 Self-Rolling Rod-Based Foam Boosts Interfacial Polarization for Electromagnetic Wave Absorption, Adv. Sci., 2022, 9(16), 2201118 CrossRef CAS PubMed.
- X. Cui, X. Liang, W. Liu, W. Gu and Y. Du, Stable microwave absorber derived from 1D customized heterogeneous structures of Fe3N@C, Chem. Eng. J., 2019, 381(7), 122589 Search PubMed.
- Y. Wu, Y. Zhao, M. Zhou, S. J. Tan, R. Peymanfar, B. Aslibeiki and G. B. Ji, Ultrabroad Microwave Absorption Ability and Infrared Stealth Property of Nano-Micro CuS@rGO Lightweight Aerogels, Nano-Micro Lett., 2022, 14(1), 171 CrossRef CAS PubMed.
- H. T. Jiang, C. J. Wang, B. W. Cui, X. D. Xu, M. F. Li, Z. H. Xu, H. X. Tan, C. G. Wang and Y. X. Wang, Lotus Leaf Derived NiS/Carbon Nanofibers/Porous Carbon Heterogeneous Structures for Strong and Broadband Microwave Absorption, Small, 2023, 2304918 CrossRef PubMed.
- T. Zhu, W. Shen, X. Y. Wang, Y. F. Song and W. Wang, Paramagnetic CoS2@MoS2 core-shell composites coated by reduced graphene oxide as broadband and tunable high-performance microwave absorbers, Chem. Eng. J., 2019, 378, 122159 CrossRef CAS.
- B. Zhao, G. Shao, B. B. Fan, W. Y. Zhao, Y. J. Xie and R. Zhang, Synthesis of flower-like CuS hollow microspheres based on nanoflakes self-assembly and their microwave absorption properties, J. Mater. Chem. A, 2015, 3(19), 10345–10352 RSC.
- H. J. Wu, J. L. Liu, H. S. Liang and D. Y. Zang, Sandwich-like Fe3O4/Fe3S4 composites for electromagnetic wave absorption, Chem. Eng. J., 2020, 393, 124743 CrossRef CAS.
- P. B. Liu, S. Gao, X. D. Liu, Y. Huang, W. J. He and Y. T. Li, Rational construction of hierarchical hollow CuS@CoS2 nanoboxes with heterogeneous interfaces for high-efficiency microwave absorption materials, Composites, Part B, 2020, 192, 107992 CrossRef CAS.
- J. L. Liu, L. M. Zhang, D. Y. Zang and H. J. Wu, A Competitive Reaction Strategy toward Binary Metal Sulfides for Tailoring Electromagnetic Wave Absorption, Adv. Funct. Mater., 2021, 31(45), 2105018 CrossRef CAS.
- W. B. Huang, Z. Y. Tong, Y. X. Bi, M. L. Ma, Z. J. Liao, G. L. Wu, Y. Ma, S. Y. Guo, X. Y. Jiang and X. P. Liu, Synthesis and microwave absorption properties of coralloid core-shell structure NiS/Ni3S4@PPy@MoS2 nanowires, J. Colloid Interface Sci., 2021, 599, 262–270 CrossRef CAS.
- Y. N. Dong, X. J. Zhu, F. Pan, Z. Xiang, X. Zhang, L. Cai and W. Lu, Fire-retardant and thermal insulating honeycomb-like NiS2/SnS2 nanosheets @ 3D porous carbon hybrids for high-efficiency electromagnetic wave absorption, Chem. Eng. J., 2021, 426, 131272 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr05275c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |