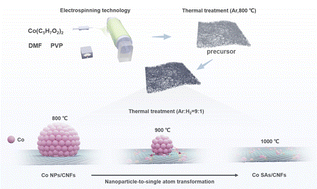
The rising top-down synthetic methodologies for transition metal single-atom catalysts (SACs) require controlled movement of metal atoms through the substrates; however, their direct transportation towards the ideal carrier remains a huge challenge. Herein, we showed a “top down” strategy for Co nanoparticles (NPs) to Co SA transformation by employing electrospun carbon nanofibers (CNFs) as atom carriers. Under high-temperature conditions, the Co atoms migrate from the surfaces of Co NPs and are then anchored by the surrounding carbon to form a Co-C3O1 coordination structure. The synthesized Co SAs/CNF electrocatalyst exhibits excellent electrocatalytic nitrate reduction reaction (NO3RR) activity with an NH3 yield of 0.79 mmol h−1 cm−2 and Faraday efficiency (FE) of 91.3% at −0.7 V vs. RHE in 0.1 M KNO3 and 0.1 M K2SO4 electrolytes. The in situ electrochemical characterization suggests that the NOH pathway is preferred by Co SAs/CNFs, and *NO hydrogenation and deoxygenation easily occur on Co SAs due to the small adsorption energy between Co SAs and *NO, as calculated by theoretical calculations. It is revealed that a small energy barrier (0.45 eV) for the rate determining step (RDS) ranges from *NO to *NOH and a strong capability for inhibiting hydrogen evolution (HER) significantly promotes the NH3 selectivity and activity of Co SAs/CNFs.